Abstract
Hypothesis:
The presence and distribution of ionized calcium binding adaptor 1 and CD68 macrophages in the human cochlea is altered in cochlear implantation (CI) compared with the normative or nonimplanted cochlea.
Background:
It has been hypothesized that CI induces an immunological response in macrophages leading to implant failure or reduced hearing. Macrophages are resident immune cells in human cochlea and have been shown to phagocytize implant material. In animal models, macrophage populations increase with surgical stress and with the introduction of a foreign body. However, the function and response of inner ear macrophages to CI are only beginning to be understood. This study seeks to investigate the inflammatory response to CI by comparing cochlear macrophages in implanted and nonimplanted human temporal bones.
Methods:
Nineteen temporal bones from nine implanted ears, seven contralateral controls, and three normal control ears were evaluated for the presence and distribution of CD68 and Iba1 expressing positive macrophages.
Results:
Three types of macrophage populations were detected 1) CD68 positive macrophages, 2) Iba1 positive macrophages, and 3) CD68 and Iba1 colocalizing macrophages. Macrophage distribution was ubiquitous: the stria vascularis, Rosenthal canal, and the mid-modiolus intermingled in the spiral ganglia. Iba1 and CD68 macrophages were found in the CI and non-CI contralateral and normal human cochlea. Most ionized calcium binding adaptor 1 expressing macrophages were ramified/amoeboid cells, while CD68 expressing macrophages were round shaped with foamy appearance in some areas. In the CI cochlea, both types of macrophages were detected in the fibrous sheath surrounding the CI path and within fibrotic areas within the scala tympani and the scala vestibuli in the case of CI translocation. In four cases, the density of macrophages was unchanged in the CI compared with the contralateral nonimplanted side, and in three cases, there was an increased number of macrophages in the implanted CI side compared with the nonimplanted side.
Conclusion:
Multiple populations of macrophages exist within the cochlea which are present at baseline and in response to trauma from CI. These results further support evidence for a macrophage response to cochlear implantation. Further studies are indicated to evaluate whether these macrophages have a beneficial, detrimental, or a mixed effect in CI patients.
Keywords: CD68, Cochlea, Cochlear implant, Histopathology, Immune cells, Macrophage, Otopathology, Temporal bone anatomy
Cochlear implantation surgery commonly causes direct injury from traumatic forces which may induce a cellular inflammatory response, contributing to damage within the cochlea and potentially to the loss of residual hearing or decrement in cochlear implantation (CI) function (1,2). previous temporal bone studies have demonstrated a biologic reaction in the cochlea causing significant fibrosis (3,4) and inflammation (5). The inflammatory reaction has been proposed to result in a delayed loss of residual hearing (T). Further studies demonstrated that the resultant fibrosis near the ductus reuniens secondary to reaction to cochleostomy may also be associated with cochlear hydrops (4) and vestibular hydrops (6) causing a delayed loss of residual hearing and dizziness. A previous human temporal bone study has demonstrated an increase in density of macrophages in the vestibular endorgans of the implanted inner ear compared with the contralateral nonimplanted ear in unilateral CI (7). Steroids are sometimes used during and after surgery to protect against the inflammatory response to inner ear insults, yet the underlying mechanism of this reaction is poorly understood (8,9). Therefore, cellular mediators of the inflammatory cellular response to cochlear implantation are an active field of research (5,10).
Macrophages are leukocytes that serve as effector cells of the innate immune system. They play a role in the development and maintenance of tissues including hemostasis, injury response, and phagocytosis. It was previously thought that the macrophages in the inner ear migrate from the vasculature rather than being mobilized from inner ear resident macrophages. However, recent studies have demonstrated evidence for the presence of resident cells in the cochlea (5,10). Multiple different macrophage populations exist within the inner ear and they are thought to incite both beneficial and detrimental effects (10,11). Macrophages can be recruited to sites of tissue injury, and secrete cytokines and phagocytize dying cells (10–16).
Published studies have demonstrated that immune cells including macrophages are ubiquitous in the normal cochlea (5,16). The response of these cells to surgical trauma and their contribution to the inflammatory process in the human cochlea is beginning to be elucidated. Previous animal studies have demonstrated macrophage response to otologic insults including noise (14), aminoglycoside antibiotics (17,18), infection (12), and aging. To understand the inflammatory response to cochlear implantation, this study investigates the distribution of macrophages in the implanted cochlea and compares the results to the distribution of macrophages in the nonimplanted cochlea. The macrophages are identified by antibodies against CD68 and the Ionized Calcium Binding Adaptor 1 (Ib1a), using both fluorescence tagged secondary antibodies and traditional immunohistochemistry.
METHODS
The Institutional Review Board of University of California Los Angeles (UCLA) approved this study (Institutional Review Board protocol # 10-001449) (Table 1). The temporal bone donors were part of a National Institute of Health funded National Temporal Bone Laboratory at UCLA through the National Institute on Deafness and Other Communication Disorders. The archival human temporal bones were from eight cochlear implant patients, human temporal bone 1 (HTB 1) was from a bilateral CI recipient, and HTBs 2–8 were from unilateral CI recipients yielding nine implanted ears (CI) and seven contralateral not implanted ears (NI). Serving as controls (HTB 9–11) were human temporal bones from three subjects with normal hearing and no vestibular disorders (C) (ages 26, 38, and 50). Table 1 contains additional demographic details on the temporal bones used as well as age at collection and duration of cochlear implant.
TABLE 1.
Demographic characteristics of temporal bones used and qualitative immunoreactive macrophage presence
| HTB | Side | Age | Yr With CI | Macrophage Localization | Iba1 | CD68 | Histopathology |
|---|---|---|---|---|---|---|---|
| 1-CI | Left | 89 | 16 | Around CI, SV, SG, MM | +++ | +++ | Translocation and lateral wall. Cochleostomy Fibrosis, mild hydrops |
| 1-CI | Right | 89 | 18 | Around CI SV, SG, MM | +++ | +++ | Translocation, cochleostomy. Fibrosis, Stria atrophy |
| 2-NI | Left | 61 | NA | SV, SG, MM | ++ | ++ | Mild hydrops |
| 2-CI | Right | 61 | 1 | Around CI | ++ | ++ | Translocation, no cochleostomy. Fibrosis, hydrops |
| 3-NI | Left | 87 | NA | SV, SG, MM | + | + | Normal |
| 3-CI | Right | 87 | 19 | SV, SG, MM | + | + | Translocation, cochleostomy. Fibrosis, strial atrophy, hydrops |
| 4-NI | Left | 65 | NA | Minimum SV, SG, MM | + | + | Normal |
| 4-CI | Right | 65 | 2 | Minimum SV, SG, MM | + | + | No translocation, cochleostomy. Fibrosis. Hydrops with rupture |
| 5-CI | Left | 92 | 11 | Minimum | + | + | No translocation no cochleostomy. Mild fibrosis |
| 5-NI | Right | 92 | NA | Minimum | + | + | Stria atrophy |
| 6-CI | Left | 75 | 10 | Around CI, SV, SG, MM | +++ | +++ | Translocation, cochleostomy. Fibrosis, mild hydrops |
| 6-NI | Right | 75 | NA | SV, SG, MM | + | + | Strial atrophy |
| 7-CI | Left | 73 | 3 | Around CI, SV, SG, MM | +++ | +++ | Translocation and lateral wall, cochleostomy fibrosis, atrophy. |
| 7-NI | Right | 73 | NA | Minimum | + | + | Stria atrophy |
| 8-CI | Left | 87 | 10 | SV, SG, MM | +++ | +++ | Translocation, cochleostomy, fibrosis. Ossified, hydrops |
| 8-NI | Right | 87 | NA | SV, SG, MM | + | + | Normal |
| HTB: Normal controls with normal hearing | |||||||
| 9-C | Left | 26 | NA | SV, SG, MM | ++ | ++ | Normal |
| 10-C | Left | 38 | NA | SV, SG, MM | ++ | ++ | Normal |
| 11-C | Right | 50 | NA | SV, SG, MM | ++ | ++ | Normal |
HTB 1 had bilateral CI and HTBs 2 to 8 had unilateral CI and contralateral nonimplanted side (NI). HTBs 9 to 11 are from normal hearing (C).
C indicates controls with normal hearing; CI, cochlear implant; HTB, human temporal bone; MM, mid-modiolus; NA, not applicable; NI, nonimplanted contralateral side; SG, spiral ganglia; SV, stria vascularis.
Qualitative presence: +: minimum, ++: medium, +++ strong.
Macrophage Immunofluorescence (IF)
Celloidin-embedded sections from temporal bones were stained with mouse monoclonal antibodies against human CD68 (Bio-Rad, Hercules CA) and rabbit polyclonal antibodies against Iba1 (Abcam, Cambridge, MA). Detailed methods for celloidin removal and antigen retrieval are described in detail by Lopez et al. (19). Both primary antibodies were diluted 1:1,000 in a solution of phosphate buffered saline (PBS). After celloidin removal cochlea sections were incubated at room temperature for 1 hour with a blocking solution containing 0.5% bovine serum albumin (Faction V, Sigma, St Louis, MO) diluted in PBS solution, pH 7.4. Next, the tissue sections were incubated with a mixture of the two primary antibodies (preparation described below) in a humidity chamber at room temperature for 16 hours. At the end of the incubation period, tissue sections were washed three times for 5 minutes each wash in PBS. The secondary antibody mixture was prepared using goat anti mouse (Alexa 594) for CD68, and goat anti rabbit (Alexa 488) for Iba1 antibodies. This antibody mixture was applied to the cochlea sections for 1 hour. Thereafter, tissue sections were washed three times for 5 minutes each using PBS and then mounted with Vectashield solution (Vector Labs) containing DAPI (4′,6-diamidino-2-phenylindole) stain to visualize cells. Negative control sections were incubated with all reagents except for the primary antibodies and the primary antibody preabsorbed with the corresponding antigen (19).
Digital fluorescent images were obtained using a Leica (SP8) high-resolution light sheet laser confocal microscope, located in the Advanced Microscopy Laboratory and Spectroscopy of California Nanosystems Institute at UCLA. Images were taken individually of each fluorescent marker for the two macrophage markers, and the DAPI nuclei stain for the same tissue cross-section at ×400 magnification. These images were then merged into a single image using computer software ImageJ to visualize the two markers and DAPI simultaneously. All the images were prepared using the Adobe Photoshop software program run in a Dell OptiPlex 3020 computer.
Macrophage Immunohistochemistry (IHC)
Secondary antibodies against rabbit (to detect IBA1 antibody) and mouse (to detect CD68 antibody) labeled with HRP (Vector labs) were used, the antigen–antibody reaction was visualized with diaminobenzidine (Vector Labs). The IHC protocol has been described in detail (5,19). The IHC images were acquired using a Zeiss Axio Observer Z1 microscope. The images were prepared using the Adobe Photoshop software program run on a Mac-Pro computer.
Qualitative immunoreactivity was assessed by two independent observers, to minimize bias in the analysis, one observer was “blinded” to the identity of the immunostained celloidin sections. Qualitative assessment was categorized as mild (+), moderate (++), or strong (+++) presence of immunofluorescent cells for all the sections. A second person not blinded to the immunostained sections, coded each sample. Systematic observations were made at the apical, middle, and basal portions of the cochlea (×400) to determine whether there were regional variations in the localization of CD68 and Iba1 immunoreactive cells. The contralateral nonimplanted and normal cochlear were also examined and used for comparison.
RESULTS
Iba1 and CD68 Immunofluorescence (IF) in the CI Cochlea and the Contralateral Non-CI Side
Cochlea in Subject With CI: Iba1 and CD68 Expression in the Organ of Corti
Iba1-IF and CD68-IF macrophage distribution was investigated in celloidin-embedded sections of the CI cochlea. Figure 1 shows the CI cochlea from a 73-year-old female using IF staining for Iba1 and CD68. Figure 1A shows a low magnification view of the CI cochlea to demonstrate the localization of IF macrophages. Figure 1B shows Iba1-IF and CD68-IF cells in the organ of Corti (OC) and osseous spiral lamina (OSL). Both Iba1 and CD68 expressing macrophages were present in the OC and OSL. The macrophages were more predominant within the OSL and a few macrophages were noted in the OC, mainly in the basilar membrane. A minority of macrophages exhibited colocalization (double-labeling) of Iba1 and CD68 in the OSL.
FIG. 1.
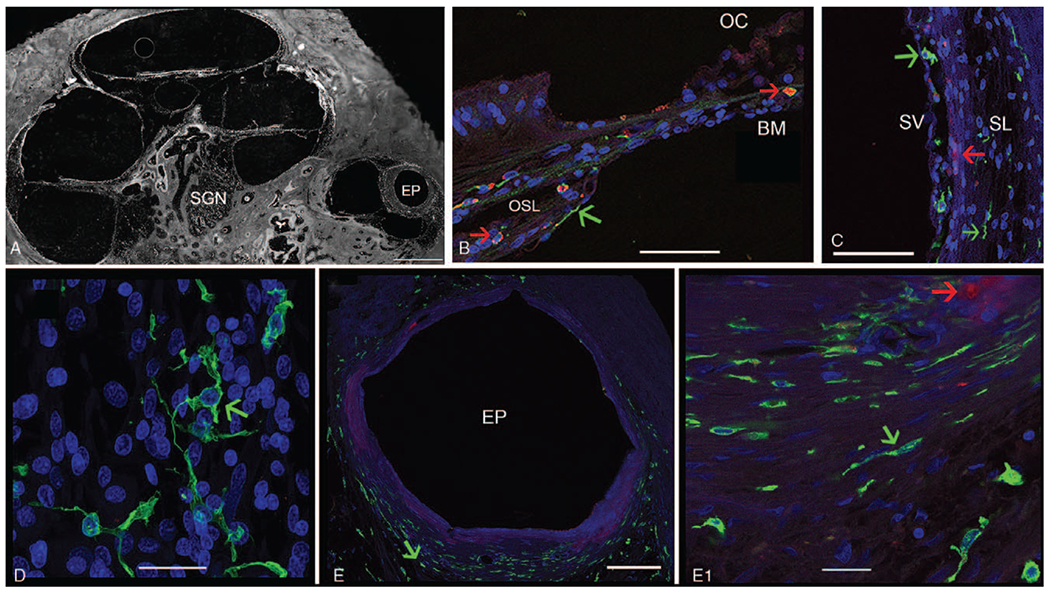
Immunofluorescence (IF) for Iba1 (green color) and CD68 (red color) in a CI cochlea. (Seventy-three-year-old female with a history of CI for 3 yr). A, Low magnification view of the implanted cochlea. EP indicates electrode path can be seen within the scala tympani; SGN, spiral ganglia neurons. B, Iba1-IF and CD68-IF macrophages are seen in the osseous spiral lamina (OSL) and basilar membrane (BM) underlying the organ of Corti (OC), with evidence of colocalization. C, IF macrophages in the stria vascularis (SV) and spiral ligament (SL). D, Iba1-IF macrophages in the spiral ganglia, exhibiting a ramified morphology E, Fibrous tissue encapsulation around the electrode path (EP) in the base-hook region of the cochlea (from (A)). Iba1-IF macrophages of elongated shape (green color) are seen surrounding the fibrous encapsulation. (E1) High magnification view from E, showing Iba1-IF macrophages (green short arrow), CD68-IF cells macrophages are visualized in red IF with a round morphology, and foamy appearance (red short arrow). DAPI indicates delineates cell nuclei. Counterstaining with Alcian blue shows the bony labyrinth. Bar in A= is 500 μm, E= 100 μm, E1= 20 μm, B to D= 40 μm.
Cochlea in Subject With CI: Iba1 and CD68 in the Lateral Wall
Figure 1C shows Iba1-IF and CD68-IF cells in the stria vascularis (SV) and spiral ligament (SL). Iba1-IF and CD68-IF macrophages were present in the SV. However, in contrast, the SL contains mainly Iba1-IF cells. Figure 1D shows Iba1-IF cells in the spiral ganglia, CD68-IF macrophages were almost absent.
CI Specific Areas of Fibrosis
Both CD68-IF and Iba1-IF macrophage cells were noted in the fibrotic sheath surrounding the CI. Figure 1E shows Iba1-IF and CD68-IF macrophages around the fibrotic capsule formed by the implant. Higher magnification view (Fig. 1E1) shows that Iba1-IF macrophages were more abundant that CD68-IF cells. Iba1-IF macrophages showed an elongated and spider morphology. CD68-IF macrophages showed a foamy morphology. Both Iba1-IF and CD68-IF macrophages were observed in the fibrotic areas present in the scala tympani and scala vestibuli (not shown). The macrophage distribution was similar in all the CI sections examined. Table 1 summarizes the IF distribution in the cochlea examined. Iba1-IF and CD68-IF in the non-CI contralateral side showed a similar distribution in the cochlea with variable degrees of density of Iba1 and CD68 macrophage cells (Table 1).
Iba1 and CD68 Immunofluorescence (IF) in the Normative Cochlea From Subjects With Normal Hearing
In the cochlea from a 26-year-old with normal hearing, both Iba1-IF and CD68-IF macrophages were present but to a lesser extent than the cochlea from patients with a history of CI. Figure 2 shows the cochlea from a 26-year-old female with normal hearing. Figure 2A shows Iba1-IF and CD68-IF macrophages in the OC and OSL. Both Iba1-IF and CD68-IF macrophages in the normative cochlea were present predominantly in the osseous spiral lamina and a few cells were present in the basilar membrane of the OC. Fewer macrophages exhibited double labeling. Figure 2B demonstrates the distribution of Iba1-IF and CD68-IF macrophages in the stria vascularis and spiral ligament. Both Iba1-IF and CD68-IF macrophages were present in the stria vascularis; however, the spiral ligament contains fewer Iba1-IF macrophages and almost no CD68-IF cells. Figure 2C shows the Iba1-IF cells in the spiral ganglia which had predominantly a ramified morphology with spider-like extensions and branching; CD68-IF cells were nearly absent in the spiral ganglia.
FIG. 2.
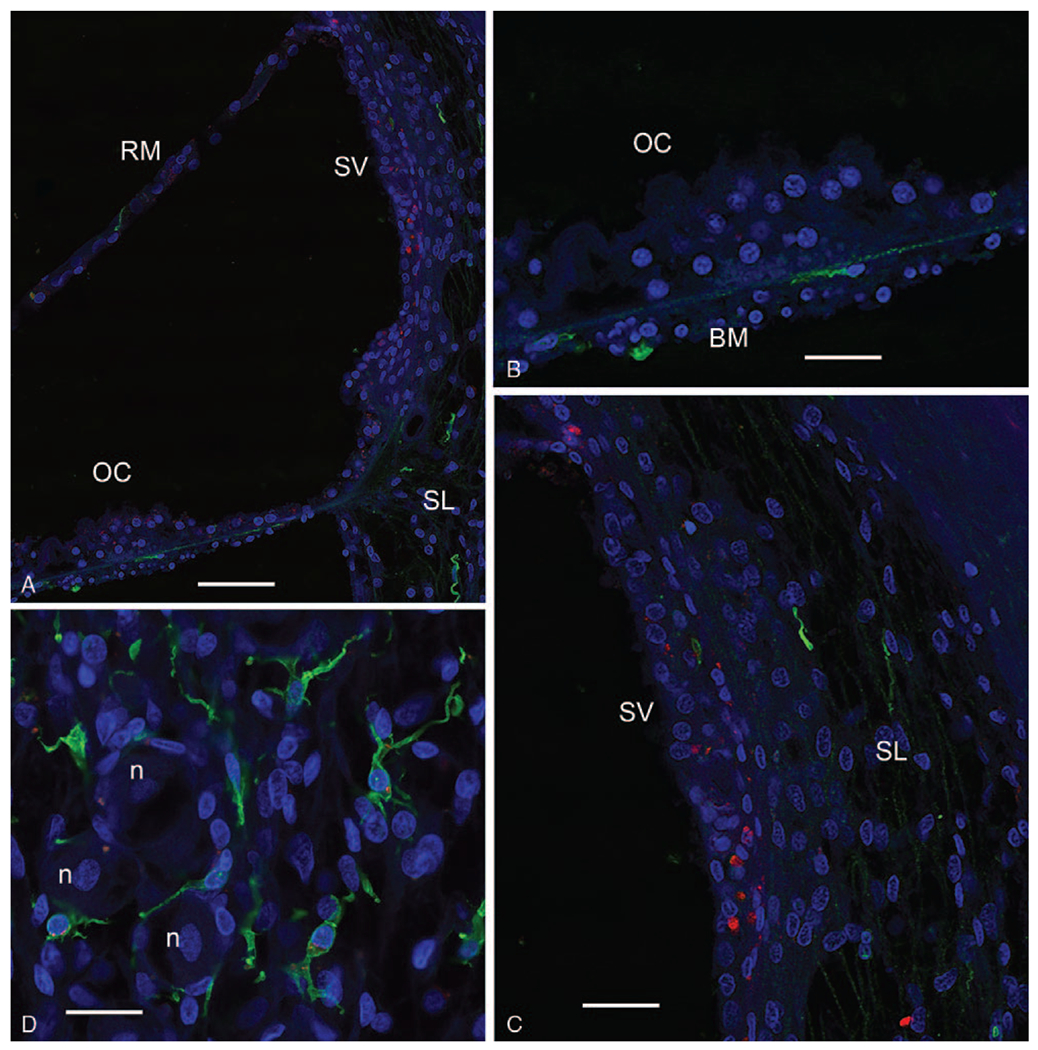
Iba1-F (green color) and CD68-IF (red color) in a normal cochlea (26-yr-old female). A, Low magnification view showing the organ of Corti (OC), visualizing Reissner’s membrane (RM), and the stria vascularis (SV), and spiral ligament (SL) of the lateral wall. B, High magnification view of the OC (same as (A)) demonstrates that Iba1-IF macrophages are present beneath the basilar membrane (BM). C, Iba1-IF and CD68 in the stria vascularis (SV) and spiral ligament (SL). CD68-IF macrophages are more predominant in the stria vascularis (SV), and Iba1-IF was more predominant in the spiral ligament (SL). D, Iba1-IF macrophages are noted in the spiral ganglia with a ramified morphology with spider-like extensions near spiral ganglia neurons (n). Bar in A = 250 μm, B and C = 100 μm, D = 100 μm. Iba1 indicates ionized calcium binding adaptor 1.
Iba1 and CD68 Immunohistochemistry (IHC) in the CI Cochlea and the Contralateral Non-CI Side
CD68 IHC in the Cochlea of a Subject With CI
To corroborate the CD68-IF macrophage distribution, IHC staining was performed for both CD68 and Iba1 in the CI cochlea. Figure 3 shows CD68 immunoreactive (IR) macrophages in another human temporal bone section from the same CI cochlea used in Figure 1 for CD68-IF. In Figure 3A, which is a low magnification view of the cochlea, the localization of the CD68-IR macrophages is similar to the observed using CD68-IF staining. CD68-IR macrophages were observed in the OC (Fig. 3B), in the fibrotic area in the scala vestibuli (Fig. 3C), in the SV and the SL (Fig. 3D). In the spiral ganglia neuron region (SG), there was near absence of CD68 expressing macrophages (Fig. 3E). CD68-IR macrophages were also present in the fibrotic area where the electrode was inserted (Fig. 3F). CD68-IR macrophages in the areas of fibrosis were associated with a foamy, round morphology.
FIG. 3.
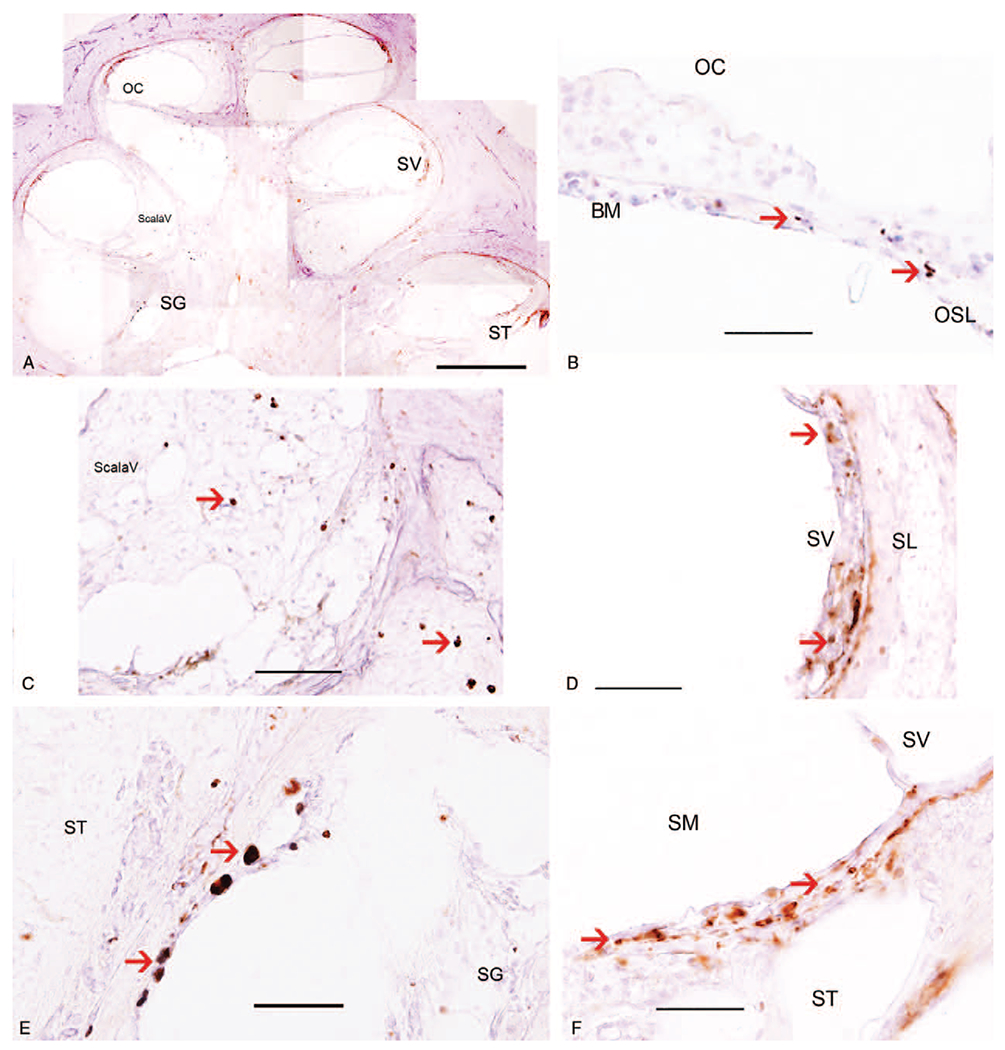
Immunohistochemistry for CD68 in a CI temporal bone (same temporal bone used for Figure 1 but different CI section). A, Low magnification view to indicate the location of CD68 immunoreactive (IR) macrophages in the cochlea. B, CD68-IR macrophages were seen in Organ of Corti (OC). C, CD-68 IR macrophages are noted within the fibrotic area in the scala vestibuli (ScalaV). D, CD-68 macrophages are notable within the stria vascularis (SV). However, only a few CD68-IR macrophages are seen in the spiral ligament (SL). E, CD68-IR macrophages in the spiral ganglia area were almost absent. F, CD68-IR macrophages are seen in the area of fibrosis within the scala tympani (ST). The scala media (SM) is noted to exhibit hydrops. Magnification bar in A = 500 μm, in B to F = 40 μm. CI indicates cochlear implant.
Iba1 IHC in the Cochlear of a Subject With CI
To corroborate the Iba1 macrophage distribution, IHC staining for Iba1 is demonstrated in Figure 3 in another celloidin section from the same HTB with CI used for Figure 1: immunofluorescence of Iba1. Iba1-IR macrophages were ubiquitous throughout the CI cochlea, in an identical distribution as the Iba-IF macrophages, corroborating the distribution. Figure 4A shows a low magnification view of the cochlea of a CI patient. The Iba1-IR cells were seen in the OC (Fig. 1B), the OSL (Fig. 1C), and in the spiral ligament (Fig. 1D) and in the stria vascularis (Fig. 1E). Iba1-IR macrophages were also noted in the fibrotic area of the scala tympani (Fig. 1F) and in macrophages within the spiral ganglia area (Fig. 1G). The Iba1-IR macrophages were associated with an ameboid, ramified appearance with spider-like extensions.
FIG. 4.
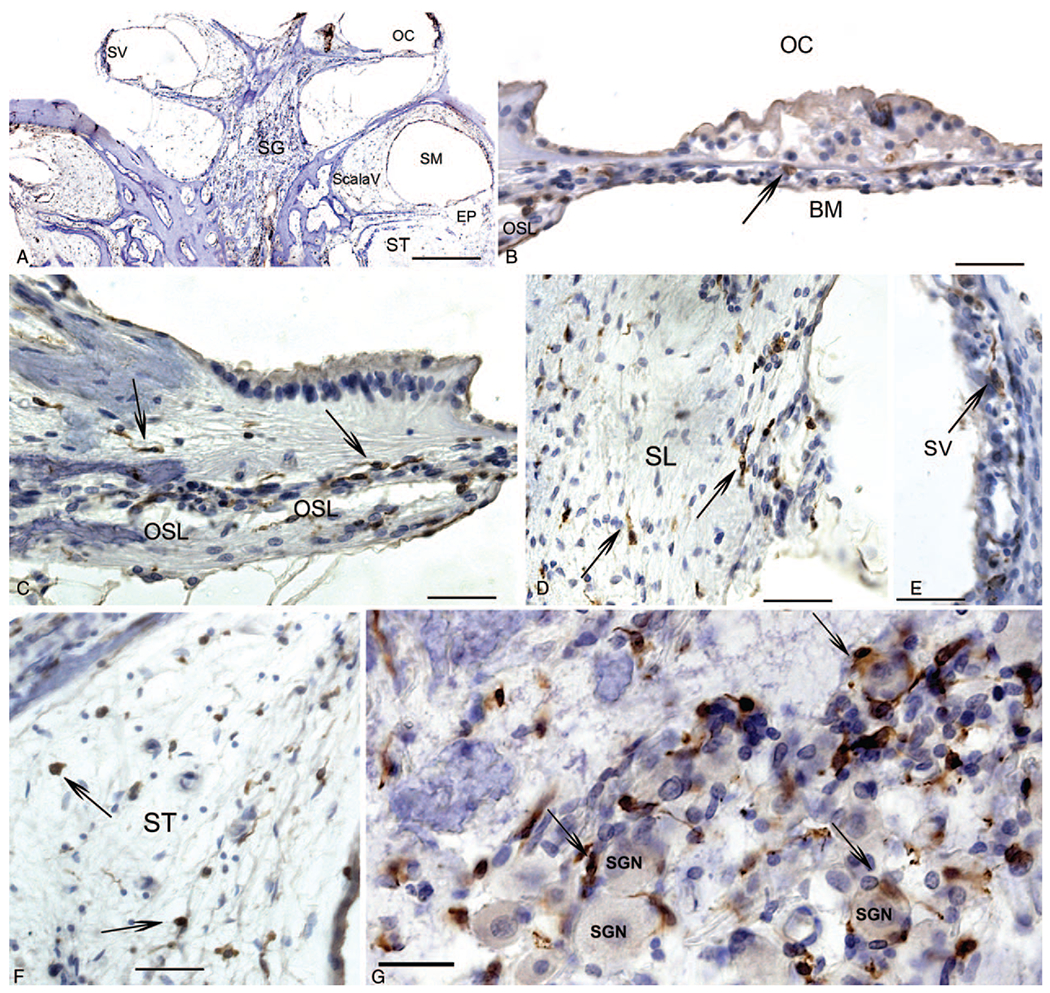
Immunohistochemistry for Iba1 macrophages in another CI cochlea section. A, Low magnification view to indicate the location of Iba1 IR macrophages in the cochlea: visualizing the stria vascularis (SV), the organ of Corti (OC), Scala Vestibuli (Scala V), the Scala Media (SM) which exhibits hydrops, and the Scala tympani (ST). B, Organ of Corti (OC), thin arrows point to Iba1-IR round macrophages, within the basilar membrane (BM). C, Iba1-IR elongated macrophages in the osseous spiral lamina (OSL). D, Iba1-IR macrophages in the spiral ligament (SL). E, Iba1-IR macrophages in the stria vascularis (SV). F, Iba1-IR macrophages within the areas of fibrosis within the scala tympani (ST). G, Iba1-IR macrophages in the spiral ganglia region, some surrounding spiral ganglia neurons (SGN) showing a ramified morphology with spider-like extensions (long thin arrows). Magnification bar in A = 500 μm, in B to F = 40 μm. CI indicates cochlear implant; Iba1, ionized calcium binding adaptor 1; IR, immunoreactive.
The relative distribution of Iba1-IR and CD68-IR macrophages in the cochlea is summarized in Table 1. The relative presence of the Iba1-IR and CD68-IR macrophages by age was also examined; however, no comparison could be made due to the small sample size. In HTBs 2-5, the nonimplanted side had a similar distribution and density of Iba1 and CD68 expressing macrophages. However, in HTBs 6 -8, there was a significantly greater amount of Iba1 and CD68 expressing macrophages in the implanted cochlea.
DISCUSSION
In the present study, the presence of CD68 and Ib1a expressing macrophages was identified in human cochlea using celloidin-embedded archival human temporal bones from patients with a history of CI and the results were compared with the unimplanted contralateral side and the cochlea from normal hearing subjects. In the CI cochlea, there were CD68 and Ib1a macrophages lining the fibrous sheath surrounding the CI and in areas of fibrosis in the scala tympani and scala vestibuli in cases of cochleostomy and CI translocation. There was a ubiquitous distribution of both CD68 and Iba1 expressing macrophages throughout the cochlea in the implanted cochlea, the contralateral nonimplanted cochlea, and the normative cochlea from a normal hearing patient. There were no significant differences in the implanted cochlea compared with the nonimplanted cochlea other than the areas of fibrosis related to the implant electrode. Notably, the stria vascularis exhibited many CD68 expressing macrophages, and the spiral ligament exhibited many Iba1 expressing macrophages. These findings are in alignment with previous studies describing the distribution of CD163, Iba1, and CD68 expressing macrophages in human temporal bone from normative (16). Similarly, previous studies have demonstrated a distribution of Iba1 expressing macrophages of varying morphologies was reported in close association with spiral ganglion cells in normative human cochlea (from petroclival meningioma surgery) (10). Although it was once thought the cochlea was an immune-privileged region, we now acknowledge that several distinct populations of macrophages reside within the cochlea (5). It has been suggested that they play a critical role in the innate immune response and the multiple distinct populations have varied roles in other tissues (20).
When analyzing the relative presence of each macrophage type, this study found that the relative density of cells was not consistently altered in the implanted cochlea compared with the contralateral unimplanted cochlea. However, it was consistently noted that the fibrous capsule surrounding the electrode arrays and areas of fibrosis possibly due to translocation and/or cochleostomy trauma, were populated by both Iba1 and CD68 macrophages. In four of seven pairs of HTBs, the relative density and number of macrophages was similar in the implanted ear and the contralateral nonimplanted ear. In three of seven pairs of HTBs, the implanted ear had more fibrosis and tissue reaction when compared with the contralateral nonimplanted ear. The relative density of cells may correlate more closely with individual patient factors or innate differences, as the relative density of cells varied by patient. Animal studies have reported a macrophage density level with a peak at 14 days after an insult and higher levels persisting for months (12). The density of cells did not correlate with duration of implantation in this study; however, due to the small number of temporal bones studied, a comparison cannot be made. No long-term studies have previously been conducted looking at macrophage populations over time. Macrophage populations may also vary with patient age; however, the small sample size hampers making a comparison.
Using immunofluorescence, it was possible to identify apparent subtypes of macrophages expressing only CD68 or only Ib1a, and macrophages expressing both CD68 and Iba1. Immunohistochemistry verified the presence of CD68 expressing and Ib1a expressing macrophages. Heterogeneity of the macrophage lineage is common in many microenvironments of the body and therefore it is not unexpected to find this in the inner ear (20). Liu et al. (10) studied human macrophages and reported macrophage populations with differing morphology dependent on localization. It has been hypothesized that the auditory nerve is under surveillance and receives neurotrophic stimulation from the macrophage population and that the varied macrophage types play a role in immune modulation, nerve regeneration, and waste disposal (10).
CONCLUSION
Iba1 only macrophages, CD 68 only macrophages, and colocalized Iba1 and CD68 macrophages were ubiquitously identified in the CI and non-CI temporal bones. These results further support previous studies (5,10) and provide evidence for a macrophage response to CI implantation. There was colocalization of Iba1 and CD68 in the macrophages within the fibrotic capsule surrounding the CI, and also in areas of fibrosis in the scala tympani and in the scala vestibuli in cases where cochleostomy insertion trauma and/or translocation of the CI had taken place. The ubiquitous presence of Iba1 and CD68 expressing macrophages was also noted in normative cochlea from normal hearing subjects as in previous studies (16), and the distribution within the cochlea was unchanged in four of seven CI patients, other than the areas of fibrosis and surrounding the CI sheath, suggestive of a surveillance role of those macrophages. In contrast, in three of seven of the HTB pairs from subjects with unilateral CI, the implanted side had increased numbers of Iba1 and CD68 expressing macrophages, consistent with previous studies reporting an increased number of macrophages and/or fibrosis in the inner ear after CI related to surgical trauma or reaction to the CI (1–7). However, the role of macrophages in the function of CI, and whether there is a beneficial, deleterious, or mixed effects remains to be further investigated.
Acknowledgments
Funding from NIDCD grant # 1U24DC015910-01
(AI) paid to UCLA. PI Akira Ishiyama.
Footnotes
The authors disclose no conflicts of interest.
REFERENCES
- 1.Seyyedi M, Nadol JB. Intracochlear inflammatory response to cochlear implant electrodes in humans. Otol Neurotol 2014;35: 1545–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eshraghi AA, Van De Water TR. Cochlear implantation trauma and noise-induced hearing loss: Apoptosis and therapeutic strategies. Anat Rec A Discov Mol Cell Evol Biol 2006;288:473–81. [DOI] [PubMed] [Google Scholar]
- 3.Linthicum FH, Fayad J, Otto SR, Galey FR, House WF. Cochlear implant histopathology. Am J Otol 1991;12:245–311. [PubMed] [Google Scholar]
- 4.Ishiyama A, Doherty J, Ishiyama G, Quesnel AM, Lopez I, Linthicum FH. Post hybrid cochlear implant hearing loss and endolymphatic hydrops. Otol Neurotol 2016;37:1516–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nadol JB, O’Malley JT, Burgess BJ, Galler D. Cellular immunologic responses to cochlear implantation in the human. Hear Res 2014;318:11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Su-Velez BM, Lopez IA, Ishiyama A, Ishiyama G. Human temporal bone study of vestibular histopathology in cochlear implant patients with cochlear hydrops. Otol Neurotol 2020;41:e607–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okayasu T, O’Malley JT, Nadol JB Jr. Density of macrophages immunostained with anti-Iba1 antibody in the vestibular endorgans after cochlear implantation in the human. Otol Neurotol 2019;40:e774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rajan GP, Kuthubutheen J, Hedne N. The role of preoperative, intratympanic glucocorticoids for hearing preservation in cochlear implantation: A prospective clinical study. Laryngoscope 2012; 122:190–5. [DOI] [PubMed] [Google Scholar]
- 9.Cho HS, Lee KY, Choi H, Jang JH, Lee SH. Dexamethasone is one of the factors minimizing the inner ear damage from electrode insertion in cochlear implantation. Audiol Neurotol 2016;21:178–86. [DOI] [PubMed] [Google Scholar]
- 10.Liu W, Molnar M, Garnham C, Benav H, Rask-Andersen H. Macrophages in the human cochlea: Saviors or predators — a study using super-resolution immunohistochemistry. Front in Immunol 2018;9:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kämpfe Nordström C, Danckwardt-Lillieström N, Laurell G, Liu W, Rask-Andersen H. The Human endolymphatic sac and inner ear immunity: Macrophage interaction amd molecular expression. Front Immunol 2018;9:3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaur T, Zamani D, Tong L, et al. Fractalkine signaling regulates macrophage recruitment into the cochlea and promotes the survival of spiral ganglion neurons after selective hair cell lesion. J Neurosci 2015;35:15050–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okano T, Nakagawa T, Kita T, et al. Bone marrow-derived cells expressing Iba1 are constitutively present as resident tissue macrophages in the mouse cochlea. J Neurosci Res 2008;1767: 1758–67. [DOI] [PubMed] [Google Scholar]
- 14.Mizushima Y, Fujimoto C, Kashio A, Kondo K, Yamasoba T. Macrophage recruitment, but not interleukin 1 beta activation, enhances noise-induced hearing damage. Biochem Biophys Res Commun 2017;493:894–900. [DOI] [PubMed] [Google Scholar]
- 15.Frye MD, Zhang C, Hu BH. Lower level noise exposure that produces only TTS modulates the immune homeostasis of cochlear macrophages. J Neuroimmunol 2018;323:152–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Malley JT, Nadol JB Jr, McKenna MJ. Anti CD163+, Iba1+, and CD68+ cells in the adult human inner ear: Normal distribution of an unappreciated class of macrophages/microglia and implications for inflammatory otopathology in humans. Otol Neurotol 2016;37: 99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Warchol ME. Macrophage activity in organ cultures of the avian cochlea: Demonstration of a resident population and recruitment to sites of hair cell lesions. J Neurobiol 1997;33:724–34. [PubMed] [Google Scholar]
- 18.Warchol ME, Schwendener RA, Hirose K. Depletion of resident macrophages does not alter sensory regeneration in the avian cochlea. PLoS One 2012;7:e51574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopez IA, Ishiyama G, Hosokawa S, et al. Immunohistochemical techniques for the human inner ear. Histochem Cell Biol 2016;146:367–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol 2005;5:953–64. [DOI] [PubMed] [Google Scholar]


