Abstract
Background:
Aspects of intraoperative management (e.g., hypotension) are associated with acute kidney injury (AKI) in non-cardiac surgery patients. However, it is unclear if and how the addition of intraoperative data affects a baseline risk prediction model for postoperative AKI.
Methods:
With IRB approval, an institutional cohort (2005–2015) of inpatient intraabdominal surgery patients without preoperative AKI was identified. Data from the American College of Surgeons National Surgical Quality Improvement Program (preoperative and procedure data), Anesthesia Information Management System (intraoperative data), and electronic health record (postoperative laboratory data) were linked. The sample was split into derivation/validation (70%/30%) cohorts. AKI was defined as an increase in serum creatinine ≥0.3 mg/dL within 48 hours or >50% within 7 days of surgery. Forward logistic regression fit a baseline model incorporating preoperative variables and surgical procedure. Forward logistic regression fit a second model incorporating the previously selected baseline variables as well as additional intraoperative variables. Intraoperative variables reflected the following aspects of intraoperative management: anesthetics, beta blockers, blood pressure, diuretics, fluids, operative time, opioids, and vasopressors. The baseline and intraoperative models were evaluated based on statistical significance and discriminative ability (c-statistic). The risk threshold equalizing sensitivity and specificity in the intraoperative model was identified.
Results:
Of 2691 patients in the derivation cohort, 234 (8.7%) developed AKI. The baseline model had c-statistic 0.77 (95% CI 0.74–0.80). The additional variables added to the intraoperative model were significantly associated with AKI (P<0.0001) and the intraoperative model had c-statistic 0.81 (95% CI 0.78–0.83). Sensitivity and specificity were equalized at a risk threshold of 9.0% in the intraoperative model. At this threshold, the baseline model had sensitivity and specificity of 71% (95% CI 65%−76%) and 69% (95% CI 67%−70%), respectively, and the intraoperative model had sensitivity and specificity of 74% (95% CI 69%−80%) and 74% (95% CI 73%−76%), respectively. The high risk group had an AKI risk of 18% (95% CI 15%−20%) in the baseline model and 22% (95% CI 19%−25%) in the intraoperative model.
Conclusions:
Intraoperative data, when added to a baseline risk prediction model for postoperative AKI in intraabdominal surgery patients, improves the performance of the model.
Introduction
Postoperative acute kidney injury (AKI) is a significant clinical problem leading to greater morbidity in surgical patients, including increased hospital readmission, progression to chronic kidney disease, higher costs, and mortality.1 Preoperative factors, such as patient comorbidities and the planned surgical procedure, provide a baseline assessment of the risk of AKI.2,3 The increasing adoption of Anesthesia Information Management Systems (AIMS) has allowed various aspects of intraoperative management to be evaluated in the context of postoperative AKI risk. In particular, the association between intraoperative hypotension and AKI has been widely studied in non-cardiac surgery patients,4–7 but other factors such as fluid balance8 and urine output9 have also been evaluated. Despite these reported associations with postoperative AKI, it is not entirely clear how these findings should be interpreted. Intraoperative hemodynamic stability is an important consideration as organ hypoperfusion contributes to AKI risk,10 but some analyses focus on one aspect of intraoperative management (e.g., hypotension) without accounting for other aspects that may be strongly intertwined and provide context on a patient’s relative hemodynamic stability (e.g., vasopressor use).6 Additionally, intraoperative factors are not often evaluated in the context of preoperative risk and studies of how risk prediction models change with the addition of intraoperative data are limited.11
To that end, in a cohort of intraabdominal surgery patients at a single academic medical center, we developed risk prediction models for postoperative AKI to evaluate if and how intraoperative data could improve a baseline model consisting of preoperative variables and surgical procedure. Rather than focusing on a specific intraoperative exposure (e.g., mean arterial pressure [MAP] <60 mm Hg), we developed an intraoperative model with candidate variables related to hemodynamic stability (e.g., anesthetics, beta blockers, blood pressure, diuretics, fluids, opioids, and vasopressors). Our analyses aimed to determine if intraoperative data improved our ability to identify patients with the greatest risk for postoperative AKI.
Methods
Patient selection
This study was approved by the Columbia University Medical Center (CUMC; New York, NY) IRB, including waiver of consent. This is a retrospective study of intraabdominal surgery patients at CUMC from 2005–2015. The study was guided by the TRIPOD framework. We used a subset of patients participating in the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP). ACS NSQIP provides data on patient demographics and comorbidities. There were 14,606 patients in the ACS NSQIP at CUMC (Figure 1). Patients with missing or incomplete AIMS (CompuRecord, Philips Medical Systems, Eindhoven, The Netherlands) data were excluded. Intraabodminal procedures were identified using the Clinical Classifications Software for Services and Proceduresa (Agency for Healthcare Research and Quality, Rockville, MD) (Supplemental Table 1). Outpatient procedures were excluded as they have a low risk for AKI. Patients with preoperative acute renal failure, as defined by ACS NSQIP, or dialysis were excluded. Patients missing either a preoperative (within 30 days prior to procedure) or postoperative serum creatinine (mg/dL) measurement were also excluded. Patients across all years were randomly assigned to derivation or validation cohorts with probabilities of 70% and 30%, respectively. Of the 3,834 patients meeting the inclusion criteria, 2,691 were assigned to the derivation cohort (70.2%) and 1,143 were assigned to the validation cohort (29.8%).
Figure 1.
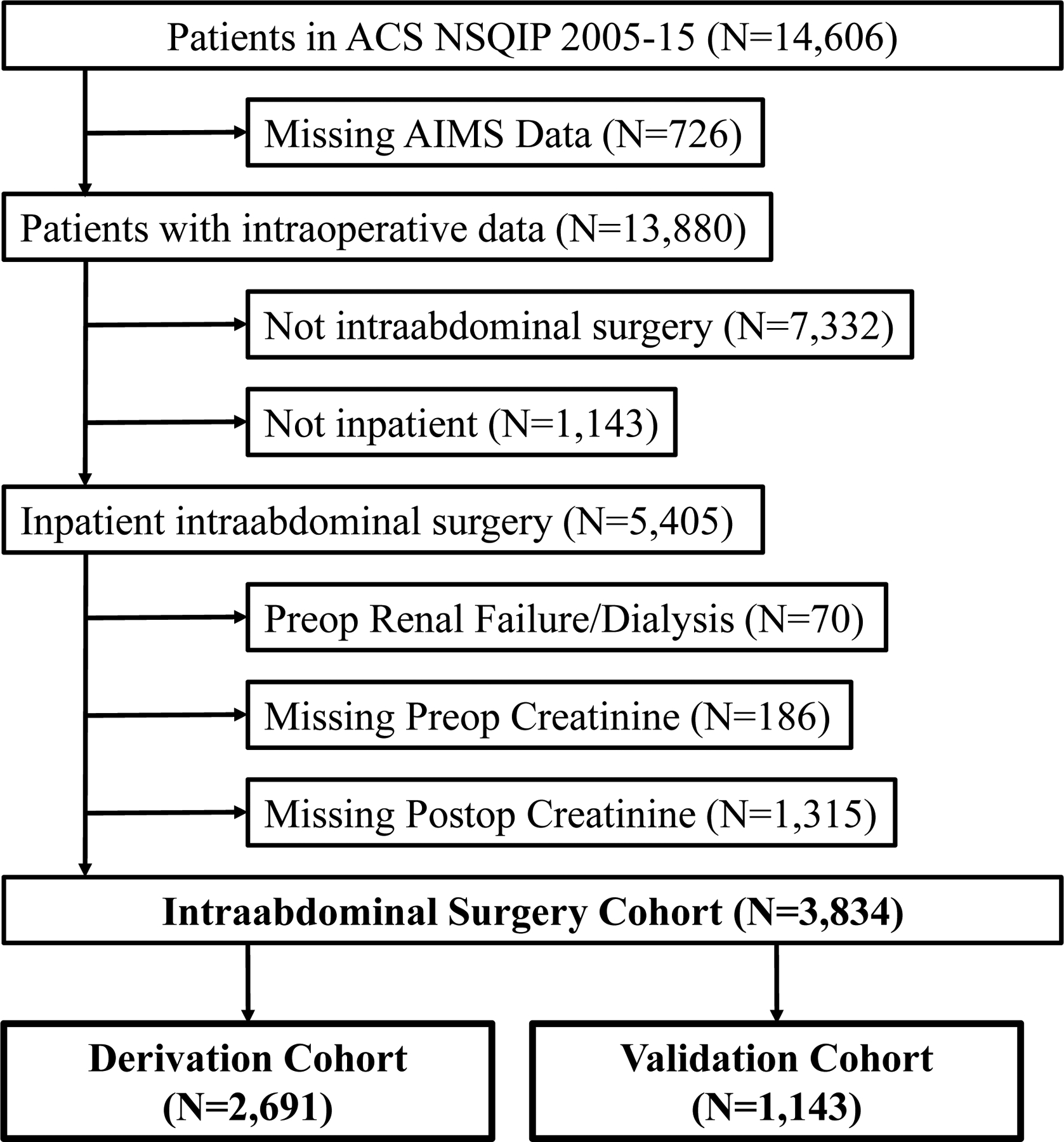
Selection of inpatient intraabdominal surgery procedures, 2005–15. ACS NSQIP, American College of Surgeons National Surgical Quality Improvement Program; AIMS, anesthesia information management system.
Intraoperative variables
Intraoperative variables were obtained from the AIMS. Intraoperative management and documentation reflect routine clinical care. AIMS data were stored in a relational database (Microsoft SQL Server Enterprise 12.0, Redmond, WA) and extracted using structured query language queries. Continuously streamed variables (e.g., HR, anesthetic agent concentration, invasive blood pressures [IBPs], etc.) were recorded every 15 seconds. Non-invasive blood pressures (NIBPs) were recorded when measured. Other variables were manually entered by the anesthesia provider (e.g., medications, IV fluids, etc.).
We identified intraoperative variables related to hemodynamic management that could potentially be associated with AKI: anesthetics, beta blockers, blood pressure, diuretics, fluids, opioids, and vasopressors. In addition, operative time was included as a candidate variable. Variables for medications and fluids reflect cumulative doses or total volumes. For continuous variables, the univariable relationship between the deciles of each variable and the log-odds of AKI risk was visualized. Variables with linear relationships were entered as continuous variables while variables with non-linear relationships were categorized based on the plots. Variable specification (continuous or categorical) is summarized in Supplemental Table 2.
Medications
The cumulative exposures to inhaled anesthetics (sevoflurane, desflurane, isoflurane, and nitrous oxide; %-hours) were calculated as the average end-tidal (ET) concentration (%) multiplied by the duration of exposure (hours).
The cumulative total dose of IV medications, both bolus doses and continuous infusion doses, were determined for 1) anesthetics (propofol [mg]), 2) beta blockers (esmolol, metoprolol, and labetalol [mg]), 3) opioids (fentanyl [mcg], morphine [mg], and hydromorphone [mg]), and 4) vasopressors (phenylephrine [mcg], ephedrine [mg], norepinephrine [mcg], epinephrine [mcg], and vasopressin [units]). Furosemide was recorded as a binary variable (Yes vs. No). Other diuretics were infrequently used (e.g., mannitol) and were not recorded.
Blood pressure
Blood pressure measurements from the operating room (‘Patient in Room’ until ‘Patient Out of Room’) were captured by the AIMS. Both invasive blood pressure (IBP) and/or non-invasive blood pressure (NIBP) measurements were included. Artifactual measurements were removed as previously described.7 For our analysis, a blood pressure measurement was recorded for every 15-second interval throughout the intraoperative period for every patient. If an interval had both an IBP and an NIBP measurement, the IBP value took precedence. If an interval had a missing value due to artifact or because it was not measured (i.e., NIBP not measured during that interval), the value was linearly interpolated between the previous measured value and the next measured value.
For each component of blood pressure (systolic blood pressure [SBP], diastolic blood pressure [DBP], and mean arterial pressure [MAP]), the mean, SD, and coefficient of variation (CV) were calculated. Of CV and SD, the variability metric with the smallest P-value in univariable logistic regression was used in multivariable analyses; CV was used for MAP and DBP, while SD was used for SBP.
The number of 15-second intervals with MAP values below or above specific thresholds were determined and divided by 4 to obtain the minutes of exposure to the given threshold. We chose the duration of time (min) with MAP <60 mm Hg as our metric for hypotension as described previously.6 As there is no standard threshold to define intraoperative hypotension,12 sensitivity analyses evaluated alternate thresholds (e.g., MAP <55 mm Hg or MAP <65 mm Hg). The duration with MAP >90 mm Hg was used as a measure of intraoperative hypertension.
Fluids
The volumes of IV fluids (mL) were determined as recorded in the AIMS: Ringer’s lactate (LR), normal saline, colloids (hydroxyethyl starch and dextran solutions), albumin 5%, plasma, and red blood cells. Hydroxyethyl starch solutions include hetastarches and pentastarches. Red blood cells include both packed red blood cells and intraoperative cell salvage.
The volume of fluid outputs (mL) were determined as recorded in the AIMS: estimated blood loss and urine output.
Clinical end points
The primary outcome of AKI was determined based on serum creatinine changes. The preoperative value was the most recent measurement within 30 days prior to surgery and all postoperative measurements during the hospitalization were recorded. AKI was defined, based on KDIGO criteria,13 as an increase in creatinine ≥0.3 mg/dL within 48 hours of the end of surgery or >50% within 7 days of surgery. Urine output was not used as a criterion for diagnosing AKI due to poor specificity14 and incomplete measurement in the sample. KDIGO AKI severity was determined: Stage 1 – creatinine 1.5–1.9 times baseline OR increase in creatinine ≥0.3 mg/dL within 48 hours; Stage 2 – creatinine 2.0–2.9 times baseline; and Stage 3 – creatinine ≥3.0 times baseline OR increase in creatinine ≥4.0 mg/dL OR initiation of renal replacement therapy.
Secondary postoperative outcomes were obtained from the ACS NSQIP dataset: 30-day mortality, mechanical ventilation, dialysis, sepsis/septic shock, and days from procedure to discharge. Additionally, postoperative location was determined: post-anesthesia care unit (PACU) vs. intensive care unit (ICU).
Statistical analysis
Differences in baseline and intraoperative variables between patients with and without AKI were analyzed with the t-test or chi-square test. Univariable relationships were also assessed using logistic regression. Logistic regression modelled the risk of AKI in the derivation cohort. First, we fit a baseline model with preoperative characteristics and procedure category. Forward logistic regression to maximize the Akaike information criterion was used to identify variables for inclusion in this model.15
We then fit a second model incorporating both baseline and intraoperative variables. Baseline variables identified in the previous step and all intraoperative variables were entered into a forward selection logistic regression model with the same criteria as above (N.B. Baseline variables were not fixed but were subject to selection in the intraoperative model.) The likelihood ratio test assessed statistical significance of the additional intraoperative variables. Model discrimination (area under the curve [AUC] of the receiver operator characteristic [ROC] curve) and calibration plots were evaluated.16
The risk threshold (e.g., at a 5% threshold, low risk = predicted risk <5% and high risk = predicted AKI risk ≥5%) that equalized sensitivity and specificity in the intraoperative model was identified.17,18 Sensitivity, specificity, positive predictive value, negative predictive value, and reclassification proportion were calculated.16 Reclassification proportion was evaluated separately for patients with and without AKI and indicates the net proportion of patients with improved risk classification using the intraoperative model vs. the baseline model. For patients with AKI, this can be interpreted as the increase in the true positive rate, and for patients without AKI, this can be interpreted as the decrease in the false positive rate.16 Internal validation of the models was assessed by applying the parameters estimated from the derivation cohort to the validation cohort. We evaluated ROC curves and calibration curves in the validation cohort.
Statistical analyses were performed using SAS Software version 9.4 (SAS Institute, Cary, NC, USA) and GraphPad Prism 7.05 (GraphPad Software, Inc, La Jolla, CA, USA). SAS macros were used to calculate reclassification proportion.b The baseline α for statistical significance was set to 0.05.
Results
Baseline preoperative characteristics of patients with and without AKI
Of 2,691 patients in the derivation cohort, 234 (8.7%) developed postoperative AKI (Table 1). Of patients with AKI, there were 270 (73%), 37 (16%), and 27 (12%) patients who developed Stage 1, Stage 2, and Stage 3 AKI, respectively. Patients with AKI were more likely to be older, male, and have increased rates of comorbidities such as diabetes, hypertension, functional dependence, and disseminated cancer. In addition, patients with AKI had higher rates of preoperative anemia and reduced estimated glomerular filtration rate; the type of surgery was associated with postoperative AKI. Univariable logistic regression analyses of baseline variables were consistent these results (data not shown).
Table 1.
Preoperative characteristics of intraabdominal surgery patients with and without postoperative acute kidney injury.
| AKI | No AKI | P-Value | |||
|---|---|---|---|---|---|
| Variable | 234 | (8.7) | 2,457 | (92) | |
| Age (years) | 64.2 | (15.7) | 55.4 | (17.4) | <0.0001 |
| Age (years) | <0.0001 | ||||
| Age ≤40 | 22 | (9.4%) | 557 | (23%) | |
| Age >40 and ≤50 | 19 | (8.1%) | 397 | (16%) | |
| Age >50 and ≤60 | 33 | (14%) | 451 | (18%) | |
| Age >60 and ≤70 | 74 | (32%) | 499 | (20%) | |
| Age >70 and ≤80 | 59 | (25%) | 399 | (16%) | |
| Age >80 | 27 | (12%) | 154 | (6.3%) | |
| Female | 115 | (49%) | 1,464 | (60%) | 0.002 |
| Current Smoker | 37 | (16%) | 313 | (13%) | 0.2 |
| Emergency | 52 | (22%) | 415 | (17%) | 0.04 |
| Hypertension | 155 | (66%) | 1,147 | (47%) | <0.0001 |
| Diabetes | 72 | (31%) | 469 | (19%) | <0.0001 |
| Congestive Heart Failure | 5 | (2.1%) | 10 | (0.4%) | 0.007 |
| Ascites | 5 | (2.1%) | 19 | (0.8%) | 0.051 |
| Functionally Dependent | 30 | (13%) | 91 | (3.7%) | <0.0001 |
| Mechanical Ventilation | 7 | (3.0%) | 13 | (0.5%) | 0.001 |
| Dyspnea | 26 | (11%) | 223 | (9.1%) | 0.3 |
| Chronic Obstructive Pulmonary Disease | 7 | (3.0%) | 42 | (1.7%) | 0.2 |
| Preoperative Steroid | 21 | (9.0%) | 98 | (4.0%) | <0.001 |
| Sepsis/Septic Shock | 24 | (10%) | 119 | (4.8%) | <0.001 |
| Wound Infection | 3 | (1.3%) | 17 | (0.7%) | 0.2 |
| Disseminated Cancer | 16 | (6.8%) | 74 | (3.0%) | 0.002 |
| Bleeding Disorder | 23 | (10%) | 95 | (3.9%) | <0.0001 |
| Preoperative Transfusion | 7 | (3.0%) | 12 | (0.5%) | <0.001 |
| Body Mass Index (kg/m2) | 29.0 | (8.3) | 31.8 | (10.4) | <0.0001 |
| Body Mass Index (kg/m2) | <0.001 | ||||
| BMI Missing | 0 | (0.0%) | 4.0 | (0.2%) | |
| BMI <18.5 | 10 | (4.3%) | 73 | (3.0%) | |
| BMI ≥18.5 and <25 | 72 | (31%) | 626 | (25%) | |
| BMI ≥25 and <30 | 67 | (29%) | 666 | (27%) | |
| BMI ≥30 and <35 | 43 | (18%) | 313 | (13%) | |
| BMI ≥35 | 42 | (18%) | 775 | (32%) | |
| Hematocrit (%) | 35.8 | (5.7) | 38.5 | (5.1) | <0.0001 |
| Hematocrit (%) | <0.0001 | ||||
| HCT Missing | 0 | (0.0%) | 17 | (0.7%) | |
| HCT ≤38 | 154 | (66%) | 1,057 | (43%) | |
| HCT >38 | 80 | (34%) | 1,383 | (56%) | |
| Estimated glomerular filtration rate (mL/min/1.73 m2) | 83.0 | (37.0) | 86.6 | (27.9) | 0.15 |
| Estimated glomerular filtration rate (mL/min/1.73 m2) | <0.0001 | ||||
| eGFR <30 | 15 | (6.4%) | 23 | (0.9%) | |
| eGFR ≥30 and <60 | 49 | (21%) | 352 | (14%) | |
| eGFR ≥60 and <90 | 81 | (35%) | 1,100 | (45%) | |
| eGFR ≥90 | 89 | (38%) | 982 | (40%) | |
| Procedure | <0.001 | ||||
| Procedures on spleen | 4 | (1.7%) | 14 | (0.6%) | |
| Colostomy, temporary and permanent | 0 | (0.0%) | 14 | (0.6%) | |
| Ileostomy and other enterostomy | 43 | (18.4%) | 313 | (13%) | |
| Gastrectomy, partial and total | 1 | (0.4%) | 61 | (2.5%) | |
| Small bowel resection | 12 | (5.1%) | 90 | (3.7%) | |
| Colorectal resection | 47 | (20%) | 296 | (12%) | |
| Local excision of large intestine lesion (not endoscopic) | 0 | (0.0%) | 1 | (0.0%) | |
| Appendectomy | 7 | (3.0%) | 99 | (4.0%) | |
| Cholecystectomy and common duct exploration | 20 | (8.6%) | 202 | (8.2%) | |
| Other hernia repair | 17 | (7.3%) | 186 | (7.6%) | |
| Exploratory laparotomy | 14 | (6.0%) | 74 | (3.0%) | |
| Excision, lysis peritoneal adhesions | 4 | (1.7%) | 48 | (2.0%) | |
| Other operating room lower gastrointestinal therapeutic procedures | 14 | (6.0%) | 115 | (4.7%) | |
| Other operating room gastrointestinal therapeutic procedures | 38 | (16%) | 343 | (14%) | |
| Gastric bypass and volume reduction | 13 | (5.6%) | 601 | (24%) | |
Continuous variables, displayed on a single row, are expressed as mean (standard deviation), and compared with the t-test. Categorical variables, displayed with one row per level of the variable, are expressed as counts (%) and compared with chi-square test.
Age, body mass index, hematocrit, and estimated glomerular filtration rate are displayed as both continuous and categorical variables.
AKI, acute kidney injury.
Baseline logistic regression model for AKI using preoperative risk factors and surgical procedure
Forward logistic regression using preoperative variables and surgical procedure identified 12 variables for inclusion in the baseline model (Table 2). The AUC was 0.77 (95% confidence interval {CI} 0.74–0.80; ROC curve in Figure 2) and the model was well-calibrated (Supplemental Figure 1A).
Table 2.
Baseline and intraoperative multivariable logistic regression models for postoperative acute kidney injury in intraabdominal surgery patients.
| Baseline Model | Intraoperative Model | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | OR | 95% CI | P-Value | OR | 95% CI | P-Value | ||
| Age (years) | 0.052 | 0.1 | ||||||
| Age ≤40 | 0.94 | [0.49,1.79] | 0.95 | [0.49,1.85] | ||||
| Age >40 and ≤50 [Ref] | 1.00 | 1.00 | ||||||
| Age >50 and ≤60 | 0.95 | [0.51,1.75] | 0.95 | [0.51,1.79] | ||||
| Age >60 and ≤70 | 1.81 | * | [1.03,3.19] | 1.73 | [0.96,3.12] | |||
| Age >70 and ≤80 | 1.55 | [0.85,2.82] | 1.57 | [0.83,2.96] | ||||
| Age >80 | 1.53 | [0.75,3.11] | 1.69 | [0.79,3.61] | ||||
| Female | 0.70 | * | [0.52,0.94] | 0.02 | 0.77 | [0.57,1.04] | 0.09 | |
| Current Smoker | 1.56 | * | [1.04,2.34] | 0.03 | 1.45 | [0.96,2.21] | 0.08 | |
| Hypertension | 1.56 | * | [1.11,2.18] | 0.01 | 1.45 | * | [1.02,2.06] | 0.04 |
| Diabetes | 1.37 | [0.98,1.91] | 0.06 | 1.38 | [0.98,1.94] | 0.07 | ||
| Functionally Dependent | 2.04 | * | [1.25,3.33] | 0.004 | 2.05 | * | [1.24,3.38] | 0.005 |
| Preoperative Steroid | 1.69 | [0.99,2.91] | 0.06 | 1.94 | * | [1.12,3.37] | 0.02 | |
| Disseminated Cancer | 1.71 | [0.92,3.15] | 0.09 | 1.71 | [0.91,3.21] | 0.10 | ||
| Preoperative Transfusion | 2.90 | * | [1.03,8.18] | 0.04 | 3.31 | * | [1.12,9.84] | 0.03 |
| Hematocrit (%) | <0.001 | <0.001 | ||||||
| HCT Missing | NE | NE | ||||||
| HCT ≤38 | 1.85 | * | [1.35,2.52] | 2.02 | * | [1.45,2.80] | ||
| HCT >38 [Ref] | 1.00 | 1.00 | ||||||
| Estimated glomerular filtration rate (mL/min/1.73 m2) | <0.001 | <0.001 | ||||||
| eGFR <30 | 3.29 | * | [1.54,7.03] | 3.42 | * | [1.54,7.57] | ||
| eGFR ≥30 and <60 | 0.89 | [0.58,1.36] | 0.86 | [0.55,1.33] | ||||
| eGFR ≥60 and <90 | 0.69 | * | [0.49,0.97] | 0.68 | * | [0.48,0.96] | ||
| eGFR ≥90 [Ref] | 1.00 | 1.00 | ||||||
| Procedure | 0.03 | 0.054 | ||||||
| Appendectomy | 0.73 | [0.31,1.75] | 1.21 | [0.49,2.97] | ||||
| Cholecystectomy and common duct exploration | 0.74 | [0.41,1.33] | 1.12 | [0.61,2.08] | ||||
| Colorectal resection [Ref] | 1.00 | 1.00 | ||||||
| Colostomy, temporary and permanent | NE | NE | ||||||
| Excision, lysis peritoneal adhesions | 0.49 | [0.16,1.48] | 0.52 | [0.17,1.67] | ||||
| Exploratory laparotomy | 0.91 | [0.45,1.85] | 1.04 | [0.49,2.19] | ||||
| Gastrectomy, partial and total | 0.08 | * | [0.01,0.66] | 0.08 | * | [0.01,0.66] | ||
| Gastric bypass and volume reduction | 0.28 | * | [0.14,0.56] | 0.37 | * | [0.18,0.76] | ||
| Ileostomy and other enterostomy | 0.86 | [0.54,1.39] | 0.60 | [0.36,1.01] | ||||
| Local excision of large intestine lesion (not endoscopic) | NE | NE | ||||||
| Other hernia repair | 0.69 | [0.37,1.27] | 0.94 | [0.49,1.79] | ||||
| Other operating room gastrointestinal therapeutic procedures | 0.83 | [0.51,1.35] | 0.82 | [0.50,1.36] | ||||
| Other operating room lower gastrointestinal therapeutic procedures | 1.04 | [0.53,2.04] | 1.23 | [0.61,2.49] | ||||
| Procedures on spleen | 2.92 | [0.88,9.69] | 2.08 | [0.53,8.19] | ||||
| Small bowel resection | 0.86 | [0.42,1.76] | 0.85 | [0.40,1.81] | ||||
| Red Blood Cells (L) | 0.5 | |||||||
| None [Ref] | 1.00 | |||||||
| ≤1 L | 0.98 | [0.61,1.56] | ||||||
| >1 L | 1.60 | [0.73,3.50] | ||||||
| Colloid (Yes vs. No) | 1.84 | * | [1.08,3.14] | 0.03 | ||||
| Fentanyl (100 mcg) | 1.07 | [1.00,1.14] | 0.053 | |||||
| SBP Mean (10 mm Hg) | 0.87 | [0.75,1.01] | 0.07 | |||||
| DBP CV (0.05) | 1.24 | * | [1.01,1.50] | 0.04 | ||||
| Minutes with MAP >90 mm Hg (5 min) | 1.02 | * | [1.01,1.03] | 0.004 | ||||
| Phenylephrine (>400 mcg vs ≤400 mcg) | 1.41 | * | [1.01,1.96] | 0.04 | ||||
| Ephedrine (≥30 mg vs <30 mg) | 1.76 | * | [1.08,2.86] | 0.02 | ||||
| Esmolol (50 mg) | 1.39 | * | [1.00,1.94] | 0.048 | ||||
| Metoprolol (5 mg) | 1.36 | * | [1.03,1.79] | 0.03 | ||||
| Furosemide (Yes vs. No) | 2.08 | * | [1.10,3.90] | 0.02 | ||||
| AUC | 0.77 | [0.74,0.80] | 0.81 | [0.78,0.83] | ||||
OR, odds ratio; CI, confidence interval; NE, non-estimable; AUC, area under the curve of the receiver operator characteristic curve.
P<0.05
Figure 2.
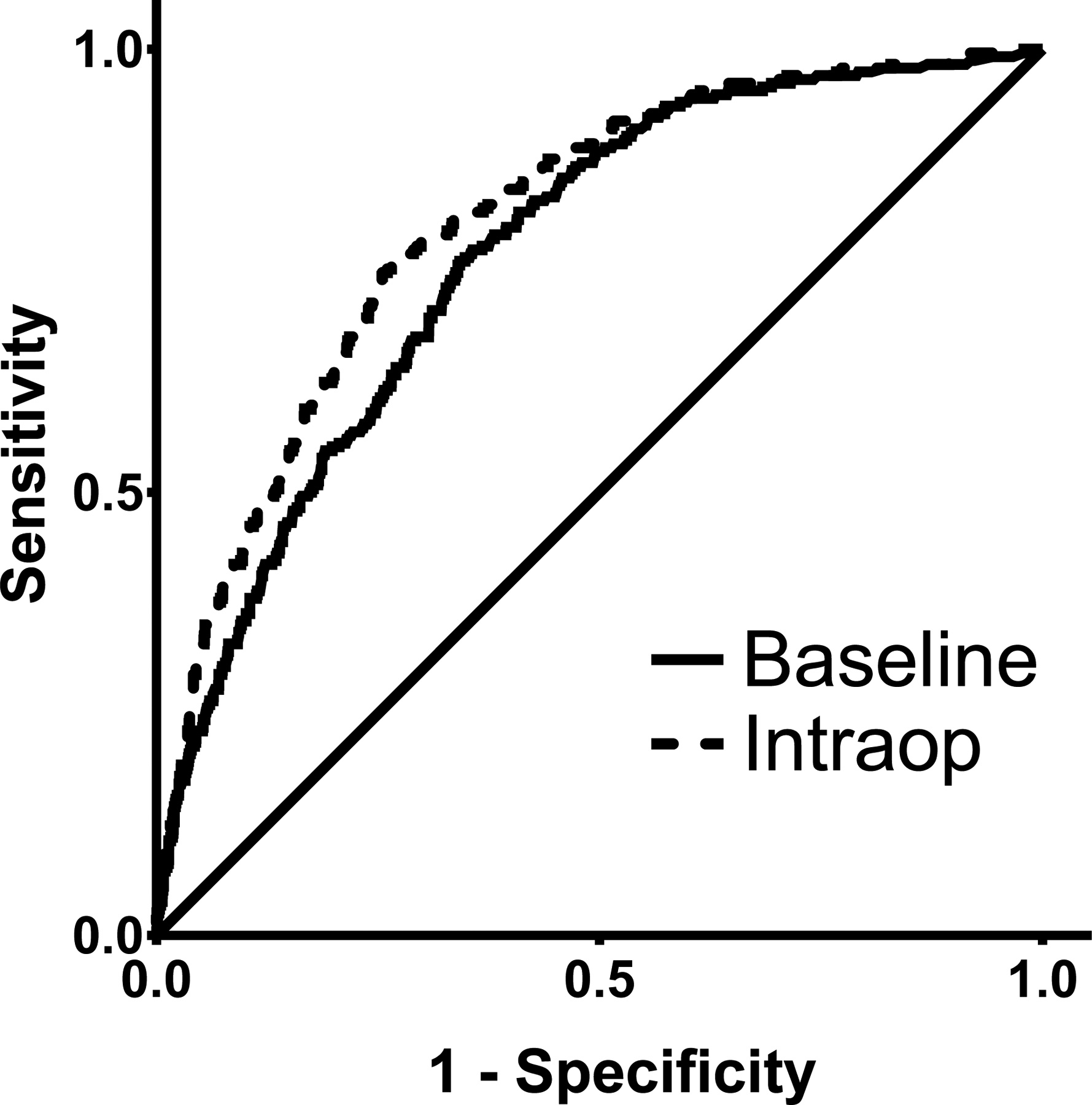
Receiver operator characteristic (ROC) curves of the baseline and intraoperative (Intraop) risk prediction models for the postoperative acute kidney injury in intraabdominal surgery patients.
Differences in intraoperative variables for patients with and without AKI
There were differences in intraoperative variables for patients with and without AKI (Table 3). Patients with AKI received more vasopressors, such as phenylephrine, ephedrine, and norepinephrine. Patients with AKI received larger volumes of IV fluids (e.g., LR, red blood cells, albumin, and plasma) and had higher estimated blood loss. Patients with AKI received greater doses of fentanyl. Patients with AKI had greater blood pressure (SBP, DBP, and MAP) variability (CV or SD) and longer periods of time with a MAP >90 mm Hg, but no difference in the duration of time with a MAP <60 mm Hg. (There were no differences in the duration of time with a MAP <55 mm Hg or <MAP 65 mm Hg.) Patients with AKI had greater exposure to sevoflurane and isoflurane but no differences in exposure to desflurane or nitrous oxide. In addition, patients with AKI were more likely to not have received propofol. Finally, patients with AKI received more metoprolol, furosemide, and had longer procedures. Univariable logistic regression analyses revealed similar relationships between intraoperative variables and postoperative AKI (data not shown).
Table 3.
Intraoperative variables in intraabdominal surgery patients with and without postoperative acute kidney injury.
| AKI | No AKI | P-Value | |||
|---|---|---|---|---|---|
| 234 | (8.7) | 2,457 | (92) | ||
| Anesthetics | |||||
| Desflurane (%-Hours) | 2.1 | (6.0) | 2.8 | (6.2) | 0.1 |
| Isoflurane (%-Hours) | 1.0 | (1.9) | 0.5 | (1.4) | <0.001 |
| Sevoflurane | 0.04 | ||||
| None | 44 | (19%) | 415 | (17%) | |
| <5.5%-Hours | 117 | (50%) | 1,432 | (58%) | |
| ≥5.5%-Hours | 73 | (31%) | 610 | (25%) | |
| Nitrous Oxide (%-Hours) | 11.8 | (25) | 9.2 | (22) | 0.12 |
| Propofol | <0.0001 | ||||
| None | 25 | (11%) | 103 | (4.2%) | |
| <200 mg | 118 | (50%) | 1,083 | (44%) | |
| ≥200 mg | 91 | (39%) | 1,271 | (52%) | |
| Beta Blockers | |||||
| Esmolol (mg) | 7.6 | (30) | 2.8 | (14) | 0.02 |
| Metoprolol (mg) | 0.9 | (3.0) | 0.3 | (1.6) | 0.002 |
| Labetalol (mg) | 3.3 | (10) | 2.6 | (8.8) | 0.26 |
| Blood Pressure | |||||
| MAP Mean (mm Hg) | 84.3 | (9.7) | 83.9 | (9.9) | 0.5 |
| MAP CV (Unitless) | 0.155 | (0.037) | 0.146 | (0.035) | <0.001 |
| SBP Mean (mm Hg) | 121 | (13) | 119 | (13) | 0.12 |
| SBP SD (mm Hg) | 18.6 | (5.0) | 16.9 | (5.2) | <0.0001 |
| DBP Mean (mm Hg) | 65.1 | (9.3) | 67.1 | (9.2) | 0.002 |
| DBP CV (Unitless) | 0.162 | (0.043) | 0.155 | (0.037) | 0.03 |
| Minutes with MAP <60 mm Hg | 8.7 | (13) | 8.2 | (15) | 0.5 |
| Minutes with MAP >90 mm Hg | 97 | (84) | 74 | (69) | <0.0001 |
| Diuretics | |||||
| Furosemide | <0.0001 | ||||
| No | 214 | (91%) | 2,409 | (98%) | |
| Yes | 20 | (8.6%) | 48 | (2.0%) | |
| Fluids | |||||
| Lactated Ringer’s | <0.0001 | ||||
| ≤3.6 L | 136 | (58%) | 1,754 | (71%) | |
| >3.6 L | 98 | (42%) | 703 | (29%) | |
| Red Blood Cells | <0.0001 | ||||
| None | 180 | (77%) | 2,252 | (92%) | |
| ≤1 L | 40 | (17%) | 174 | (7%) | |
| >1 L | 14 | (6.0%) | 31 | (1.3%) | |
| Albumin | <0.0001 | ||||
| None | 151 | (65%) | 2,015 | (82%) | |
| ≤250 mL | 13 | (5.6%) | 105 | (4.3%) | |
| >250 mL | 70 | (30%) | 337 | (14%) | |
| Plasma | <0.0001 | ||||
| No | 216 | (92%) | 2,408 | (98%) | |
| Yes | 18 | (7.7%) | 49 | (2.0%) | |
| Colloids | <0.0001 | ||||
| No | 209 | (89%) | 2,351 | (96%) | |
| Yes | 25 | (11%) | 106 | (4%) | |
| Normal Saline (mL) | 167 | (635) | 49 | (254) | 0.01 |
| Estimated Blood Loss (mL) | 622 | (1125) | 303 | (679) | <0.0001 |
| Urine Output | 0.002 | ||||
| ≤250 mL | 104 | (44%) | 1,352 | (55%) | |
| 250–600 mL | 77 | (33%) | 730 | (30%) | |
| >600 mL | 53 | (23%) | 375 | (15%) | |
| Operative Time | |||||
| Operative Time (min) | 231 | (138) | 184 | (106) | <0.0001 |
| Opioids | |||||
| Fentanyl (mcg) | 342 | (322) | 276 | (174) | 0.002 |
| Morphine (mg) | 2.5 | (5.3) | 2.0 | (4.7) | 0.1 |
| Hydromorphone (mg) | 0.7 | (1.1) | 0.7 | (1.0) | 0.002 |
| Vasopressors | |||||
| Phenylephrine | <0.0001 | ||||
| ≤400 mcg | 137 | (59%) | 1,809 | (74%) | |
| >400 mcg | 97 | (41%) | 648 | (26%) | |
| Ephedrine | <0.001 | ||||
| <30 mg | 205 | (88%) | 2,306 | (94%) | |
| ≥30 mg | 29 | (12%) | 151 | (6%) | |
| Norepinephrine | <0.0001 | ||||
| No | 193 | (82%) | 2,323 | (95%) | |
| Yes | 41 | (18%) | 134 | (5.5%) | |
| Epinephrine | <0.001 | ||||
| None | 225 | (96%) | 2,422 | (99%) | |
| ≤150 mcg | 5 | (2.1%) | 30 | (1.2%) | |
| >150 mcg | 4 | (1.7%) | 5 | (0.2%) | |
| Vasopressin (Units) | 1.2 | (4.6) | 0.3 | (1.6) | 0.003 |
Continuous variables, displayed on a single row, are expressed as mean (standard deviation), and compared with the t-test. Categorical variables, displayed with one row per level of the variable, are expressed as counts (%) and compared with chi-square test.
AKI, acute kidney injury; MAP, mean arterial pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; CV, coefficient of variation.
Intraoperative logistic regression model for AKI
In addition to the 12 previously selected baseline variables, forward logistic regression selected 11 intraoperative variables (Table 2) and these additional variables were significantly associated with AKI as compared to the baseline model (P<0.0001). Of note, all 12 baseline variables remained in the intraoperative model. The AUC of the intraoperative model was 0.81 (95% CI 0.78–0.83) (ROC curve in Figure 2) and the model was well-calibrated (Supplemental Figure 1B).
Comparison of baseline and intraoperative models at the threshold equalizing sensitivity and specificity
In the intraoperative model, sensitivity and specificity were equalized at a threshold of 9.0%. At this threshold, the sensitivity and specificity of the baseline model were 71% (95% CI 65%−76%) and 69% (95% CI 67%−70%), respectively, and 74% (95% CI 69%−80%) and 74% (95% CI 73%−76%) in the intraoperative model, respectively (Supplemental Table 3). In the baseline model, 1755 (65%) patients were in the low risk group, of which 69 (3.9%) developed AKI, while 936 (35%) were in the high risk group, of which 165 (18%) developed AKI. In the intraoperative model, 1890 (70%) patients were in the low risk group, of which 60 (3.2%) developed AKI, while 801 (30%) were in the high risk group, of which 174 (22%) developed AKI.
Reclassification tables demonstrate that of the 234 patients with AKI, 25 had an appropriate change in risk classification with the intraoperative model (low⟶high) while 16 had an inappropriate change (high⟶low), for a net reclassification proportion of 0.038 (P=0.16) (Supplemental Table 4). For the 2457 patients without AKI, 239 had an appropriate change in risk classification (high⟶low) while 95 had an inappropriate change, for a reclassification proportion of 0.059 (P<0.0001).
Postoperative outcomes by AKI risk status
Using the intraoperative model and a 9.0% risk threshold, high risk patients had more severe postoperative outcomes compared to low risk patients, including dialysis, mechanical ventilation, sepsis/septic shock, and 30-day mortality (Table 4). In addition, high risk patients were more likely to be transferred to an ICU after surgery and had more days in the hospital following surgery. There were no statistically significant differences in KDIGO AKI stage among patients developing AKI between the low and high risk groups.
Table 4.
Postoperative outcomes of intraabdominal surgery patients by postoperative acute kidney injury risk category at a risk threshold of 9.0%.
| High AKI Risk | Low AKI Risk | P-Value | |||
|---|---|---|---|---|---|
| Outcome | 801 | (30%) | 1,890 | (70%) | |
| Acute Kidney Injury | 174 | (22%) | 60 | (3.2%) | <0.0001 |
| Acute Kidney Injury by KDIGO Stage | 0.07 | ||||
| Stage 1 | 123 | (71%) | 47 | (78%) | |
| Stage 2 | 26 | (15%) | 11 | (18%) | |
| Stage 3 | 25 | (14%) | 2 | (3.3%) | |
| Dialysis | 22 | (2.8%) | 0 | (0.0%) | <0.0001 |
| 30-Day Mortality | 32 | (4.0%) | 2 | (0.1%) | <0.0001 |
| Mechanical Ventilation | 43 | (5.4%) | 14 | (0.7%) | <0.0001 |
| Sepsis/Septic Shock | 61 | (7.6%) | 32 | (1.7%) | <0.0001 |
| Postoperative Location | <0.0001 | ||||
| Recovery Room | 618 | (78%) | 1791 | (95%) | |
| Intensive Care Unit | 173 | (22%) | 97 | (5.1%) | |
| Days to Discharge (Median [IQR]) | 7 | (4, 11) | 3 | (2, 6) | <0.0001 |
AKI, acute kidney injury; KDIGO, Kidney Disease: Improving Global Outcomes; IQR, interquartile range.
Predicted acute kidney injury risk was determined using the intraoperative model. Patients with predicted risk <9.0% were designated “low” risk, while those with predicted risk ≥9.0% were designated “high” risk.
Categorical variables expressed as counts (%) and compared with the chi-square test or Fisher’s test. Days to discharge expressed as median (IQR) and analyzed with Kruskall-Wallis test.
Internal validation of risk prediction models for postoperative acute kidney injury
There were no statistically significant differences between the derivation and validation cohorts with respect to AKI risk, preoperative characteristics, and type of surgery (data not shown). There were no statistically significant differences between the derivation and validation cohorts with respect to intraoperative variables with the exceptions of CV MAP (derivation mean 0.147 [SD 0.035] vs. validation mean 0.150 [SD 0.036]; P=0.02) and SD SBP (derivation mean 17.0 mm Hg [SD 5.2] vs. validation mean 17.5 mm Hg [SD 5.4]; P=0.02), but these differences were not clinically meaningful.
For both the baseline and intraoperative models, parameters estimated from the derivation cohort were applied to the validation cohort to obtain predicted probabilities for AKI. The AUCs in the validation cohort were 0.72 (95% CI 0.67–0.78) for the baseline model and 0.74 (95% CI 0.69–0.79). ROC curves are displayed in Supplemental Figure 2 and calibration plots in Supplemental Figure 1C–D.
Discussion
We sought to evaluate the impact of intraoperative data on postoperative AKI risk stratification in intraabodminal surgery patients in a clinically meaningful context. Our framework mirrored the clinical flow in the perioperative period, with a preoperative assessment followed by reassessment after accounting for the intraoperative course. The baseline model confirmed the importance of patient factors and surgical procedure in predicting AKI risk. Variables related to intraoperative factors were then added to this baseline model, and these were significantly associated with AKI risk, demonstrating that intraoperative data could meaningfully contribute to our understanding of a patient’s risk for AKI.
A critical aspect remains, which is to determine how best to apply the models to clinical risk prediction. Our framework involved choosing a risk threshold with which to dichotomize patients as low or high risk. Using a criterion that equalized sensitivity and specificity,17 we found the optimal threshold to be 9.0% where the sensitivity and specificity were both 74% in the intraoperative model. Using this threshold, the high risk cohort was 30% of the sample with an AKI risk of ~22%, while the low risk cohort was 70% of the sample with an AKI risk of ~3%. In addition, this threshold was able to differentiate patients with respect to the risk for other major complications, such as mortality, mechanical ventilation, and sepsis/septic shock. This threshold did not differentiate patients based on the severity of AKI based on KDIGO stage, but our study was underpowered to detect these differences.
Our analyses differ from prior work5–8,19–23 in that we did not choose a specific exposure of interest (e.g., hypotension) but instead examined the overall impact of intraoperative data in the context of AKI risk. To that end, we used a method to empirically determine the variables to be included in the models. The baseline model was consistent with prior studies,2,3 identifying risk factors such as age, hypertension, baseline renal function, and category of procedure as important for predicting AKI risk. Interestingly, all 12 baseline variables remained in the intraoperative model, suggesting that their importance did not diminish when intraoperative factors were considered.
The intraoperative model added an additional 11 variables and the model had higher discrimination (AUC) compared to the baseline model. A critical aspect in clinical application is determining the appropriate threshold with which to label a patient as low or high risk for AKI as there are no standards to guide this choice. Many factors must be considered, including characteristics of the disease and those of the prediction model, such as the tradeoffs between sensitivity and specificity.
Our approach focused on overall prediction rather than on statistical significance of selected variables.15 Therefore, we are cautious in interpreting individual variables, but this may provide intuition as to the relative importance of these variables. Intraoperative hypotension is widely studied and associated with AKI in prior studies,6,7,24 but we did not find a similar association and the reasons for this are unclear. Intraoperative hypotension is a concept with no clear definition,12 but many studies evaluate the duration of time with MAP below specific thresholds.4,6,7 Our models evaluated MAP <60 mm Hg, but there were also no clear relationships with other thresholds (<55 or <65 mm Hg). Possible reasons for this may include differences in institutions, surgical populations (intraabdominal surgery vs. broader group of non-cardiac surgery), and modeling approach. We did not model hypotension in terms of time-weighted average below the threshold, as in previous studies,7 and this may play a role.
Hypertension may be important as the duration with MAP >90 mm Hg was modestly associated with increased AKI risk. Indeed, in elderly hypertensive patients undergoing gastrointestinal surgery, patients managed at low (65–79 mm Hg) and high (96–110 mm Hg) MAP targets had greater AKI risk than patients managed in the middle MAP range (80–95 mm Hg).25 Blood pressure variability21,26 may also be relevant as the DBP CV was a variable in our model. Rather than arbitrary cutoff values, individualized blood pressure management may also be important,27 and these relationships will need further exploration. The relationship between blood pressure, end-organ perfusion, and AKI is complex and likely cannot be reduced to simple metrics.
Fluid homeostasis is critically important for adequate renal perfusion and oxygenation.19 Red blood cells and colloids were selected in the intraoperative model and their administration likely identifies patients with challenging fluid management. Transfusions are associated with postoperative AKI and preoperative anemia may also be relevant.22,28 Both components of the colloid variable (hydroxyethyl starch and dextran solutions) are associated with increased AKI risk29 but their use has decreased over time in our cohort, from a peak usage of 18% in 2007 to <2% in 2010. Fluid balance is associated with AKI23,30 and in a sensitivity analysis, fluid balance was significantly associated with postoperative AKI, but overall model performance would not have changed compared to the current model (data not shown).
Other selected intraoperative variables include the use of furosemide, which was associated with a doubling of the odds of AKI, consistent with a prior study.31 Esmolol and metoprolol were also associated with increased AKI risk; beta-blockers reduce renal blood flow32 but may be beneficial in the setting of sympathetic activation. Vasopressors present a concern for reductions in renal blood flow33 but there is no clear empiric evidence of harm from their use in the operative34 or intensive care unit setting35 with respect to AKI. However, phenylephrine and ephedrine were associated with AKI in our models. As this is a retrospective analysis, these associations may reflect the indication (e.g., hypotension, fluid overload, hypertension) rather than the direct effects of the exposure (e.g., phenylephrine, furosemide, esmolol, and labetalol). We did not capture data on preoperative medications but the preoperative use of diuretics and beta-blockers may be relevant. Interestingly, although perioperative opioids appear to be safe with respect to the kidney,36 fentanyl was a selected variable in our model.
There were no anesthetic variables included in intraoperative model. Certain anesthetic agents may have protective effects in renal ischemia-reperfusion injury37 but we did not find evidence for this association. In addition, operative time was not included in the model, although there was a significant association with AKI in univariable analysis, suggesting that length of surgery is not of direct importance but is correlated with other important factors. Our results highlight the complex nature of the relationship between intraoperative variables and postoperative AKI risk.
We used a selection method to identify variables for inclusion that optimized prediction performance and included variables that did not meet traditional criteria for statistical significance.15 While this provided a model with good performance characteristics, it is possible that an alternate model could have better performance. Our selection method also did not allow variables to be removed after they were entered in the model. An interesting result is that red blood cells was the first variable to be selected in the intraoperative model as it had a strong univariable association with AKI (P<0.0001) and it remained despite the lack of association in the multivariable model (P=0.5). Alternate selection methods, such as penalized regression methods, could be of potential benefit.38
We internally validated our models using a random derivation/validation split of the data. There may be some degree of overfitting in our models, and while calibration was good in the derivation cohort, there was slight miscalibration in the validation cohort at high predicted AKI risk. There may be better approaches to internal validation than a single random derivation/validation split, and future analyses may benefit from methods such as cross-validation and bootstrap validation.39 External validation will also be required to determine the applicability of our models to other settings.
Our analyses are subject to other limitations. There may be complex, non-linear relationships among our predictor variables and AKI risk, and future analyses may benefit from alternate approaches that are able to account for these relationships, such as spline functions.38 Our retrospective, observational study design was aimed at risk prediction and causal relationships between intraoperative variables and AKI risk cannot be determined. There may be inaccuracies in the AIMS with manually entered data (e.g., medications, fluids) while automatically collected variables (e.g., blood pressure) are subject to artifactual values. Our analysis focused on specific intraoperative variables, but there may be other relevant factors that need to be considered, such as temperature.40 When developing our intraoperative model, we allowed previously selected baseline variables to be removed if they no longer met statistical criteria for inclusion after intraoperative variables were added. Although, all baseline variables remained in the intraoperative model, it is possible that fixing the baseline variables in the intraoperative model may have led to slightly different results. In addition, it is possible that alternate risk stratification schemas might be clinically informative (e.g., low, medium, and high risk) and these may require further exploration. Finally, our risk threshold was selected using statistical criteria, but clinical criteria (e.g., prioritizing high sensitivity) may lead to alternate thresholds being selected.
In conclusion, we found that intraoperative data, when added to a baseline prediction model, improves overall model performance and can aid in identifying patients with the greatest risk of postoperative AKI. As there are no specific treatments to prevent or treat postoperative AKI,41 appropriate risk stratification and clinical management is of utmost importance to optimize the care of patients at the greatest risk of this serious and devastating complication.
Supplementary Material
Supplemental Table 1: Lists Current Procedural Terminology Codes within each surgical procedure category.
Supplemental Table 2: Lists intraoperative variables used in the analyses.
Supplemental Figure 1: Details the calibration curves.
Supplemental Table 4: Details the reclassification tables comparing the baseline and intraoperative models using a risk threshold of ?9%.
Supplemental Table 3: Details model characteristics at a risk threshold of ?9%.
Supplemental Figure 2: Presents the receiver operator characteristic curves for the validation cohort.
Key Points.
Question:
Do intraoperative data improve a baseline risk prediction model for postoperative acute kidney injury in intraabdominal surgery patients?
Findings:
Intraoperative data, when added to a baseline model for acute kidney injury, improves overall model performance and can aid in the detection of high-risk patients who ultimately developed the complication.
Meaning:
Intraoperative data meaningfully contributes to an understanding of which patients have the greatest risk for postoperative AKI.
Acknowledgements
The authors would like to thank Mitchell F. Berman, MD (Columbia University Medical Center) and Brian Thumm (CUMC) for their assistance with data from the Anesthesia Information Management System; Nadine M. Thomas (New York Presbyterian Hospital) for her assistance with ACS NSQIP data; and Alla Babina (NYPH) for her assistance with electronic health record data.
Funding: Supported by the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (NIH; Bethesda, MD) through Grant Number KL2TR001874 (MK). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Glossary of Terms
- ACS NSQIP
American College of Surgeons National Surgical Quality Improvement Program
- AIMS
Anesthesia Information Management System
- AKI
acute kidney injury
- AUC
area under the curve
- CUMC
Columbia University Medical Center
- CV
coefficient of variation
- DBP
diastolic blood pressure
- ET
end-tidal
- IBP
invasive blood pressure
- ICU
intensive care unit
- KDIGO
Kidney Disease: Improving Global Outcomes
- MAP
mean arterial pressure
- NIBP
non-invasive blood pressure
- PACU
post-anesthesia care unit
- ROC
receiver operator characteristic
- SBP
systolic blood pressure
Footnotes
Presented at the International Anesthesia Research Society’s Annual Meeting, May 19, 2019, Montreal, QC, Canada.
Conflicts of Interest: None.
Available at: http://www.hcup-us.ahrq.gov/toolssoftware/ccs_svcsproc/ccssvcproc.jsp, Accessed June 15, 2012.
Kennedy KF. Evaluation of Novel Markers in Risk Prediction. Midwest SAS User’s Group. Available from: http://www.mwsug.org/proceedings/2011/stats/MWSUG-2011-SA17.pdf; Accessed 2/14/19.
References
- 1.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 2005;16:3365–70. [DOI] [PubMed] [Google Scholar]
- 2.Kheterpal S, Tremper KK, Heung M et al. Development and validation of an acute kidney injury risk index for patients undergoing general surgery: results from a national data set. Anesthesiology 2009;110:505–15. [DOI] [PubMed] [Google Scholar]
- 3.Park S, Cho H, Park S et al. Simple Postoperative AKI Risk (SPARK) Classification before Noncardiac Surgery: A Prediction Index Development Study with External Validation. J Am Soc Nephrol 2019;30:170–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walsh M, Devereaux PJ, Garg AX et al. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: toward an empirical definition of hypotension. Anesthesiology 2013;119:507–15. [DOI] [PubMed] [Google Scholar]
- 5.An R, Pang QY, Liu HL. Association of Intraoperative Hypotension with Acute Kidney Injury, Myocardial Injury and Mortality in Noncardiac Surgery: A Meta-analysis. Int J Clin Pract 2019:e13394. [DOI] [PubMed] [Google Scholar]
- 6.Sun LY, Wijeysundera DN, Tait GA, Beattie WS. Association of intraoperative hypotension with acute kidney injury after elective noncardiac surgery. Anesthesiology 2015;123:515–23. [DOI] [PubMed] [Google Scholar]
- 7.Salmasi V, Maheshwari K, Yang D et al. Relationship between Intraoperative Hypotension, Defined by Either Reduction from Baseline or Absolute Thresholds, and Acute Kidney and Myocardial Injury after Noncardiac Surgery: A Retrospective Cohort Analysis. Anesthesiology 2017;126:47–65. [DOI] [PubMed] [Google Scholar]
- 8.Weinberg L, Li M, Churilov L et al. Associations of fluid amount, type, and balance and acute kidney injury in patients undergoing major surgery. Anaesth Intensive Care 2018;46:79–87. [DOI] [PubMed] [Google Scholar]
- 9.Shiba A, Uchino S, Fujii T, Takinami M, Uezono S. Association Between Intraoperative Oliguria and Acute Kidney Injury After Major Noncardiac Surgery. Anesth Analg 2018;127:1229–35. [DOI] [PubMed] [Google Scholar]
- 10.Goren O, Matot I. Update on perioperative acute kidney injury. Curr Opin Crit Care 2016;22:370–8. [DOI] [PubMed] [Google Scholar]
- 11.Adhikari L, Ozrazgat-Baslanti T, Ruppert M et al. Improved predictive models for acute kidney injury with IDEA: Intraoperative Data Embedded Analytics. PLoS One 2019;14:e0214904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bijker JB, van Klei WA, Kappen TH et al. Incidence of intraoperative hypotension as a function of the chosen definition: literature definitions applied to a retrospective cohort using automated data collection. Anesthesiology 2007;107:213–20. [DOI] [PubMed] [Google Scholar]
- 13.National Kidney Foundation: Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2012;2:19–36. [Google Scholar]
- 14.Lei VJ, Luong T, Shan E et al. Risk Stratification for Postoperative Acute Kidney Injury in Major Noncardiac Surgery Using Preoperative and Intraoperative Data. JAMA Netw Open 2019;2:e1916921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shtatland ES, Kleinman K, Cain EM. Stepwise Methods in Using SAS PROC LOGISTIC and SAS Enterprise Miner for Prediction. SAS Users Group International 28, 2003: Paper 258–28. [Google Scholar]
- 16.Kerr KF, Wang Z, Janes H et al. Net reclassification indices for evaluating risk prediction instruments: a critical review. Epidemiology 2014;25:114–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mascha EJ. Identifying the Best Cut-Point for a Biomarker, or Not. Anesth Analg 2018;127:820–2. [DOI] [PubMed] [Google Scholar]
- 18.Zhou X-h, McClish DK, Obuchowski NA. Statistical methods in diagnostic medicine. 2nd ed Hoboken, N.J.: Wiley, 2011. [Google Scholar]
- 19.Ding X, Cheng Z, Qian Q. Intravenous Fluids and Acute Kidney Injury. Blood Purif 2017;43:163–72. [DOI] [PubMed] [Google Scholar]
- 20.Frenette AJ, Bouchard J, Bernier P et al. Albumin administration is associated with acute kidney injury in cardiac surgery: a propensity score analysis. Crit Care 2014;18:602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jinadasa SP, Mueller A, Prasad V et al. Blood Pressure Coefficient of Variation and Its Association With Cardiac Surgical Outcomes. Anesth Analg 2018;127:832–9. [DOI] [PubMed] [Google Scholar]
- 22.Transfusion Karkouti K. and risk of acute kidney injury in cardiac surgery. Br J Anaesth 2012;109 Suppl 1:i29–i38. [DOI] [PubMed] [Google Scholar]
- 23.Shin CH, Long DR, McLean D et al. Effects of Intraoperative Fluid Management on Postoperative Outcomes: A Hospital Registry Study. Ann Surg 2018;267:1084–92. [DOI] [PubMed] [Google Scholar]
- 24.Mathis MR, Naik BI, Freundlich RE et al. Preoperative Risk and the Association between Hypotension and Postoperative Acute Kidney Injury. Anesthesiology 2020;132:461–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu X, Jiang Z, Ying J, Han Y, Chen Z. Optimal blood pressure decreases acute kidney injury after gastrointestinal surgery in elderly hypertensive patients: A randomized study: Optimal blood pressure reduces acute kidney injury. J Clin Anesth 2017;43:77–83. [DOI] [PubMed] [Google Scholar]
- 26.Xie Z, Liao X, Yin W et al. Relationship Between Short-Term Blood Pressure Variability and Incidence of Acute Kidney Injury in Critically Ill Patients. Kidney Blood Press Res 2017;42:1238–46. [DOI] [PubMed] [Google Scholar]
- 27.Futier E, Lefrant JY, Guinot PG et al. Effect of Individualized vs Standard Blood Pressure Management Strategies on Postoperative Organ Dysfunction Among High-Risk Patients Undergoing Major Surgery: A Randomized Clinical Trial. JAMA 2017;318:1346–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karkouti K, Grocott HP, Hall R et al. Interrelationship of preoperative anemia, intraoperative anemia, and red blood cell transfusion as potentially modifiable risk factors for acute kidney injury in cardiac surgery: a historical multicentre cohort study. Can J Anaesth 2015;62:377–84. [DOI] [PubMed] [Google Scholar]
- 29.Schortgen F, Brochard L. Colloid-induced kidney injury: experimental evidence may help to understand mechanisms. Crit Care 2009;13:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Myles PS, Bellomo R, Corcoran T et al. Restrictive versus Liberal Fluid Therapy for Major Abdominal Surgery. N Engl J Med 2018;378:2263–74. [DOI] [PubMed] [Google Scholar]
- 31.Tagawa M, Ogata A, Hamano T. Pre- and/or Intra-Operative Prescription of Diuretics, but Not Renin-Angiotensin-System Inhibitors, Is Significantly Associated with Acute Kidney Injury after Non-Cardiac Surgery: A Retrospective Cohort Study. PLoS One 2015;10:e0132507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilkinson R Beta-blockers and renal function. Drugs 1982;23:195–206. [DOI] [PubMed] [Google Scholar]
- 33.Richer M, Robert S, Lebel M. Renal hemodynamics during norepinephrine and low-dose dopamine infusions in man. Crit Care Med 1996;24:1150–6. [DOI] [PubMed] [Google Scholar]
- 34.Farag E, Makarova N, Argalious M et al. Vasopressor Infusion During Prone Spine Surgery and Acute Renal Injury: A Retrospective Cohort Analysis. Anesth Analg 2019. [DOI] [PubMed] [Google Scholar]
- 35.Bellomo R, Wan L, May C. Vasoactive drugs and acute kidney injury. Crit Care Med 2008;36:S179–86. [DOI] [PubMed] [Google Scholar]
- 36.Mallappallil M, Sabu J, Friedman EA, Salifu M. What Do We Know about Opioids and the Kidney? Int J Mol Sci 2017;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Motayagheni N, Phan S, Eshraghi C, Nozari A, Atala A. A Review of Anesthetic Effects on Renal Function: Potential Organ Protection. Am J Nephrol 2017;46:380–9. [DOI] [PubMed] [Google Scholar]
- 38.Hastie T, Tibshirani R, Friedman JH. The elements of statistical learning : data mining, inference, and prediction. 2nd ed New York, NY: Springer, 2009. [Google Scholar]
- 39.Steyerberg EW. Validation in prediction research: the waste by data splitting. J Clin Epidemiol 2018;103:131–3. [DOI] [PubMed] [Google Scholar]
- 40.Oh TK, Ryu JH, Sohn HM, Jeon YT. Intraoperative Hypothermia is Associated With Reduced Acute Kidney Injury After Spine Surgery Under General Anesthesia: A Retrospective Observational Study. J Neurosurg Anesthesiol 2018. [DOI] [PubMed] [Google Scholar]
- 41.Zacharias M, Mugawar M, Herbison GP et al. Interventions for protecting renal function in the perioperative period. Cochrane Database Syst Rev 2013;9:CD003590. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1: Lists Current Procedural Terminology Codes within each surgical procedure category.
Supplemental Table 2: Lists intraoperative variables used in the analyses.
Supplemental Figure 1: Details the calibration curves.
Supplemental Table 4: Details the reclassification tables comparing the baseline and intraoperative models using a risk threshold of ?9%.
Supplemental Table 3: Details model characteristics at a risk threshold of ?9%.
Supplemental Figure 2: Presents the receiver operator characteristic curves for the validation cohort.


