Abstract
Long acquisition times due to intrinsically low signal-to-noise and the need for highly homogeneous B0 field make magnetic resonance spectroscopy (MRS) particularly susceptible to motion or scanner instability compared to MRI. Motion induced changes in both localization and shimming (i.e., B0 homogeneity) degrade MRS data quality. To mitigate the effects of motion three approaches can be employed: 1) subject immobilization, 2) retrospective correction, and 3) prospective real-time correction using internal and/or external tracking methods. Prospective real-time correction methods can simultaneously update localization and the B0 field to improve MRS data quality. While localization errors can be corrected with both internal (navigators) and external (optical camera, NMR probes) tracking methods, the B0 field correction requires internal navigator methods to measure the B0 field inside the imaged volume and the possibility to update the scanner shim hardware in real time. Internal and external tracking can rapidly update the MRS localization with sub-millimeter and sub-degree precision, while scanner frequency and 1st order shims of scanner hardware can be updated by internal methods every sequence repetition. These approaches are most well-developed for neuroimaging, for which rigid transformation is primarily applicable. Real-time correction greatly improves the stability of MRS acquisition and quantification as shown in clinical studies on subjects prone to motion, including children and patients with movement disorders, enabling robust measurement of metabolite signals including those with low concentrations, such as gamma-aminobutyric acid (GABA) and glutathione (GSH). Thus, motion correction is recommended for MRS users and calls for tighter integration and wider availability of such methods by MR scanner manufacturers.
Keywords: Magnetic resonance spectroscopy, Motion correction, Shim correction, Real-time, Navigator, Optical tracking, NMR probes, Metabolites, Neurochemistry, GABA, GSH
1. INTRODUCTION
The requirements of long scan time to compensate the inherent low signal-to-noise ratio (SNR) of metabolites and stringent B0 homogeneity to resolve spectral overlaps among metabolite signals make magnetic resonance spectroscopy (MRS) particularly susceptible to subject motion and hardware instability. Motion artifacts and incorrect signal localization in MRS are not always apparent,1 especially in single voxel spectroscopy (SVS) that has no anatomical information, unlike MRI. Motion during acquisition of SVS may be suspected sometimes by the presence of signals that originate outside the localized volume, such as from extracranial lipids. However, even in the case of MR spectroscopic imaging (MRSI), motion artifacts are hard to recognize visually at the spatial resolution typically achieved in MRSI with limited structural information (anatomical edges), which makes it difficult to correct motion artifacts in MRSI through post-processing as may be done in MRI.2
Furthermore, the correct fitting and estimation of metabolite concentrations depend on having adequate spectral resolution, which requires spectral linewidth narrower than the separation of neighboring signals. Improving linewidth is achieved by B0 shimming of the volume of interest before MRS measurement.3 A change in the pose will be accompanied by a change in the B0 field, rendering the original B0 shimming suboptimal for the new pose. This results in degradation of spectral quality that manifests either as line splitting or broadening depending on the speed and nature of head motion.
Due to the strong relationship between B0 shimming and spectral quality, undoing the effects of motion in MRS requires the correction of both localization and B0 shimming. Hence, the problem of motion correction in MRS includes the problem of B0 shimming, and the experts’ recommendations for B0 shimming apply in the case of MRS motion correction. This combined problem in MRS is more demanding than in anatomical MRI where correction of spatial encoding may be sufficient to mitigate the effect of motion. Furthermore, even in the absence of motion, B0 field changes occur due to extrinsic sources such as magnet field drifts or heating and cooling of the shim iron, which causes slow drifts in the B0 field.4 Such scanner instability leads to broadening and distortion of spectral lineshape, or inefficient water suppression and spectral editing. Since MRI scanners do not have a frequency lock channel such as NMR spectrometers, other methods are needed to correct frequency drifts. Furthermore, in the case of MRSI motion causes inconsistencies between k-space points, which result in signal smearing in image space along the directions of motion. This becomes particularly problematic when artifacts originate from the large nuisance signals of water and lipid that can overwhelm metabolite signal. Because these artifacts do not obey the rules predicted by spatial response functions (SRF), improving SRF does not eliminate them. Efficient lipid/water suppression5,6 and lipid/water removal7,8 are necessary to mitigate them.9 Lipid/water suppression that use frequency selective methods benefits from real time shim update that can maintain the frequency stability and efficiency of these suppression methods. Note that due to the complexity of signal localization in MRS similar spectral artifacts could result not only from motion, but also in stationary conditions with a sequence that has poor localization due to chemical shift displacement error or for a scanner with magnet drift. For example, presence of large lipid signal in spectra of healthy brain could be due to motion but also due to chemical shift error in an otherwise stationary subject. Broad spectral peaks could be due motion but also due to B0 drift of the magnet in a stationary subject. Hence, by inspection of spectral appearance it cannot be confidently decided if degraded quality is due to subject motion or experimental failures related to the instrumentation. This is quite different from MRI where motion signs are obvious in the image.
While various motion correction methods are commonly used for MRI,10–13 currently these have not been widely used in the MRS community outside of a few research laboratories, despite the fact that MRS is more susceptible to motion degradation compared to MRI. This is mainly due to the limited availability of methods that are easy to integrate and use with the MRS acquisition and processing, or they are suboptimal because they do not mitigate B0 field changes that are critical in MRS. Both retrospective14 (post-processing) and prospective15–17 (real-time) motion correction methods for MRI have been proposed and reviewed. The present paper focuses uniquely on methods for prospective motion and B0 shim correction as these approaches offer the most comprehensive solution for MRS applications. The paper aims to (i) provide minimum recommendations that may be adopted by the MR community at large, and (ii) advocate for their implementation by MR scanner manufacturers.
Although the simplest method to mitigate motion is to immobilize the subject using head restraints and bite bars, this strategy is often unsatisfactory or impossible because it reduces patient comfort and still permits some movement. In body imaging, respiratory and cardiac related movement cannot be suppressed. Even under ideal conditions with a non-moving sample or subject, scanner instabilities might arise that compromise data quality. Dynamic B0 field changes can result from changes in magnetic susceptibility of the air in the lungs during respiration, which can modulate the B0 field in the brain at ultra-high field (≥ 7 T).18 In cardiac and abdominal applications, periodic movements of organs associated with cardiac pulsation and breathing are also accompanied by periodic B0 field changes. In such cases, cardiac and respiratory gating will be necessary to obtain reliable spectra although it reduces data acquisition efficiency.19
In practice, perfect correction of motion may not be possible under various hardware and software limitations, and it is necessary to specify the required level of performance to make motion correction worthwhile. Recommendations for acceptable performance of motion correction methods can be made by considering the precision and accuracy of MRS measurements under ideal conditions and research questions that needs to be answered by an MRS study. Research studies under ideal conditions e.g., by experienced MRS operators with well optimized protocols in compliant subjects, showed that the intrinsic variability of metabolite concentrations measured by MRS is on the order of 5–15% for primary metabolites (e.g., N-acetylaspartate (NAA), creatine (Cr) and choline (Cho)), though it may be higher for metabolites with lower SNR and more complicated spectra (e.g., GABA, GSH, taurine).20–23 On the other hand, clinical applications often require measuring metabolite changes either cross-sectionally between healthy controls and patients, or longitudinal time courses in patients. Well-controlled studies24–26 have shown that MRS can measure 5–20% change in metabolite levels due to disease conditions. Hence, as a rule of thumb an advanced motion correction method should be able to provide at least 5% stability or better for primary metabolites in patient populations in the clinical context.
Numerical specifications for performance of motion correction for MRS could be proposed considering the worst-case scenario for motion that causes a 5% change in metabolite concentrations. Considering a voxel in SVS that is at the edge of the brain compartment (either next to the skull or ventricles), if motion occurs at the beginning of an MRS acquisition causing a displacement of the voxel volume by 5%, if maintained until the end of the acquisition, this would cause 5% change in the metabolite concentrations in the given voxel. For a voxel size of 1 cm3, this means that motion would cause a displacement of 0.05 cm3 in volume localization. Since position is a combination of both translation and rotation, it is hard to visualize the amount of motion for the most general case. However, for the 0.05 cm3 volume change, in the simple case of pure translation this would amount to an average 0.17 mm displacement along three orthogonal axes, or in the case of pure rotation this would mean an angle between 0.26–2.9 degrees, depending on the distance of the voxel to a rotation axis through the center of the brain and the atlanto-axial joint. A correction method that would provide 95% stability of metabolite estimates in the presence of motion should be able to detect translations and rotations with a precision better than previous examples in order to update the voxel position. However, these examples do not consider the motion effects on B0 field homogeneity that leads to changes in spectral linewidth and lineshape, resulting in errors in metabolite fitting. In brain locations where B0 has large spatial variation, e.g., prefrontal and temporal areas, even small motions as defined above for the 5% volume displacement may lead to a larger change in metabolite quantification, and B0 shim update is necessary to restore spectral quality and reduce variability of metabolite quantification. More information regarding specifications and requirements on B0 homogeneity and spectral linewidth for adequate MRS quality is presented in the B0 shimming consensus paper of this special issue.3
2. MOTION CORRECTION METHODS
2.1. Retrospective post-processing methods
Post-processing methods have historically been the easiest to implement for SVS data, however, they are limited in their ability to reverse all the changes caused by motion. When motion artifacts corrupt only a few of the transients in SVS with multiple transients, those corrupted transients could be discarded selectively, while frequency and phase changes could be corrected by spectral alignment of the remaining transients. Though this approach may improve the spectral quality, it cannot correct the fact that averaged spectra are collected from different voxel locations or the fact that narrow-band selective pulses used for, e.g., water suppression or spectral editing will be out of band due to frequency shifts caused by changes in the voxel position. In addition, spectral distortion due to higher-order spatial B0 inhomogeneities that accompany position changes cannot be corrected in post-processing and result in imperfect spectral averaging.
While prospective motion correction approaches require hardware/software adjustments that may be difficult to implement on all scanners/software platforms, retrospective motion correction by post-processing is simpler to perform and requires minimum adjustments to the scanner. The retrospective motion correction methods are primarily based upon correcting for motion-induced frequency drift and incoherent phasing, as well as discarding motion corrupted data sets. Shot-to-shot phase and frequency offsets reduce the SNR of the averaged spectrum – the theoretical loss of SNR has been shown to be a factor of sinc(σφ√3/π), where σφ is the standard deviation of the phases of the individual transients.27
MRS data can be phase-corrected on a shot-by-shot basis using an interleaved water FID navigator signal acquisition during metabolite recovery time (within a single TR), and by performing a deconvolution of individual metabolite signals with the navigator information. Phase-coherent averaging of the individual transients has been demonstrated to be effective in correcting for motion-induced frequency drifts in SVS28. Degraded quality of water suppression has also been used to detect and discard severely motion corrupted data in SVS.28,29 Using a scheme similar to that by Thiel et al.,28 signal fluctuation of the water navigator data has been shown to be a good indicator of subject motion. Motion corrupted data have been analyzed retrospectively in J-difference edited SVS after discarding datasets with water signal fluctuation beyond a certain threshold (>3%).30 Ernst and colleagues used a phase and frequency navigator with water suppression cycling (resulting in positive and negative water signals).31 In this method, motion correction during SVS scans was achieved by shot-to-shot frequency and phase alignment of residual water, with the average spectrum resulting in almost complete elimination of the residual water signal, thus minimizing baseline distortion. Performing shot-to-shot phase correction using the phase of a high SNR resonance peak with subsequent constructive averaging of phase-coherent data has been shown to improve the SNR of SVS by ~15%.27 Phase- and frequency-correction using residual water of the individual transients as reference has been shown to restore SNR and remove lineshape distortion in SVS.32 Using spectral registration in time domain by fitting each transient to a reference scan, efficient phase- and frequency-correction is possible33.
However, in order to eliminate mis-localization and other motion effects, it is necessary to use real-time correction methods to update both the localization and B0 shimming for each voxel position relative to the organ during acquisition as described in the next section.
2.2. Prospective real-time methods
Prospective real-time methods track the organ throughout the acquisition and correct for changes in position or gate the acquisition in response to the estimated motion. There are two major classes of such methods, namely (i) internal navigator methods that use the MR signal itself to discern the position of the organ, and (ii) external tracking systems that use external hardware such as a camera and some sort of marker, which may be a marker on the face, or the face itself, to discern the patient’s head position. Both systems have advantages and disadvantages. A comparison of different systems and methods is provided in Table 1.
Table 1.
Technical requirements, performance, and costs/availability for motion correction methods.
| METHOD | System Requirements | Motion tracking (temporal and spatial resolution) |
B0 field tracking (temporal and spatial resolution) |
Performance, costs and availability |
|---|---|---|---|---|
| Retrospective: Post-processing | Custom post-processing software | No localization update | Temporal resolution is determined by the TR of MRS sequence | Lower cost of software solution |
| Prospective: Navigators | Custom MR sequence, on-line reconstruction and real-time feedback | 0.05–0.1 mm & 0.05–0.1 deg tracking accuracy, 300–1000 ms update rate | 0.05–0.1 mm & 0.05–0.1 deg tracking accuracy, 300–1000 ms update rate | Lower cost of software solution |
| Prospective: External tracking (optical and NMR probes | Additional hardware and software, integration with MR scanner | 0.005–0.1 mm & 0.003–0.1 deg tracking accuracy, 12–30 ms update rate | No field update is possible without additional frequency navigators | Higher costs for commercial and custom-made systems |
| Prospective: NMR probes | Additional hardware and software, integration with MR scanner | 0.01–0.1 mm & 0.01–0.1 deg tracking accuracy, 300–1000 ms update rate | Field is measured outside the head, and computational EM modelling can approximate the internal field with limited accuracy. | Higher costs for commercial and custom-made systems |
i) Navigator sequences34–36 can update position and some are capable of updating B0 shimming, however, they require additional pulse sequence elements that have to be physically compatible with the timing of the MRS sequence, estimating position and shim parameters at a limited rate (e.g., typically once per TR, hence ~1 s), and possibly extending the total acquisition time. Some navigators have been designed only for frequency or shim correction, to be used in combination with external camera that provide position correction.
ii) External cameras37–39 estimate position independent of the MR sequence at rates typically much greater than a TR (e.g., up to 60 Hz) and can track motion continuously even when no active MRI sequence is played. While positions are estimated independent of the sequence, corrections must be applied in a manner consistent with the physics of the acquisition, reducing the correction rate. Nevertheless, the availability of a rapid stream of position estimates may reduce the lag time between position estimates and corrections. However, these systems cannot directly estimate the B0 field inside the body. In MRS, dynamic shimming (real-time B0 shim correction) during motion is critical to ensure spectral quality.
i)+ii) Hybrid systems may also be realized, where motion is corrected using an external camera, while B0 shim is corrected using an internal navigator.17 The schematic in Figure 1 illustrates the concept of prospective real-time methods and provides examples of motion artifacts and correction of these artifacts in MRS data.
Figure 1.
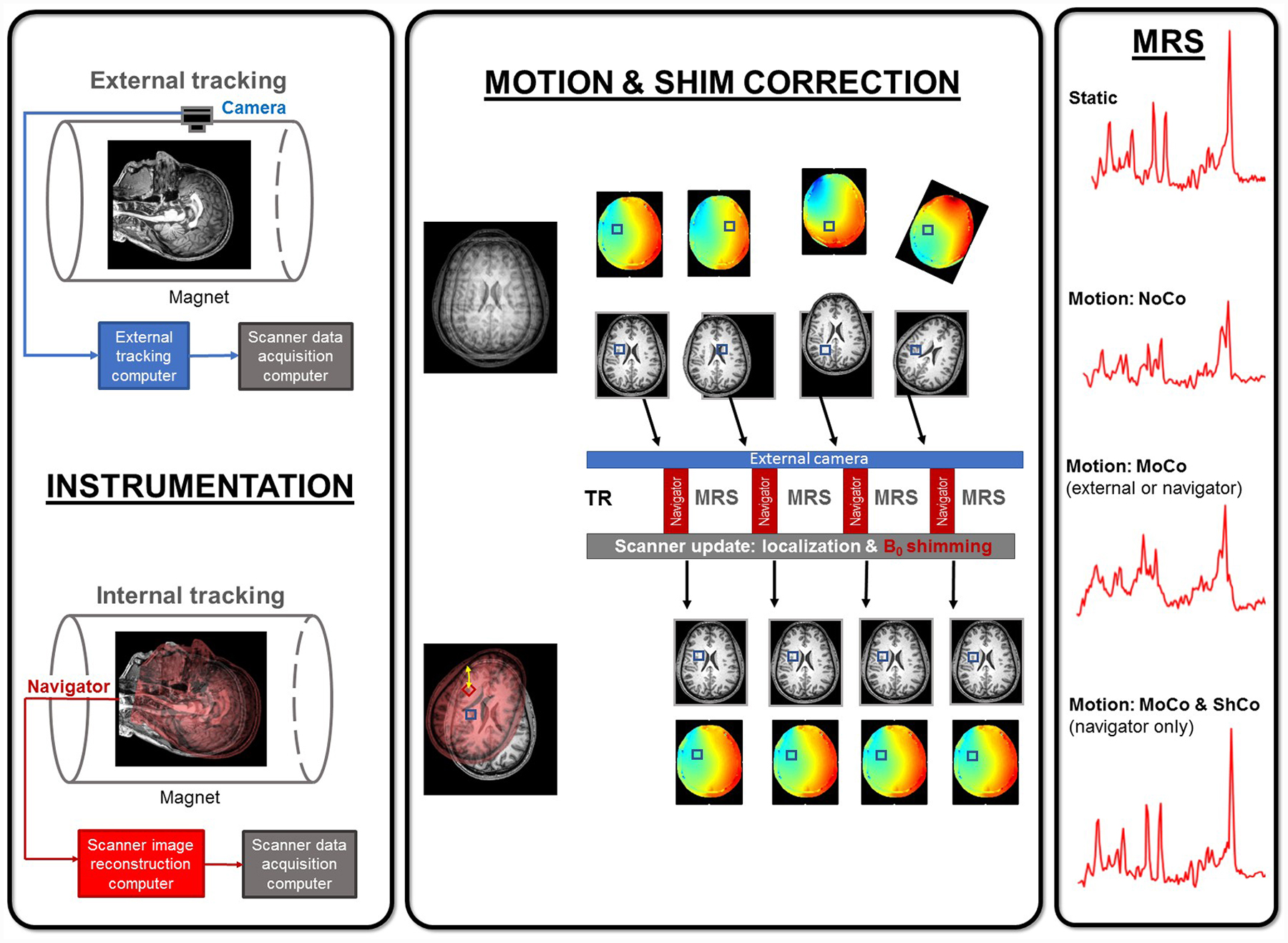
Prospective real-time motion correction for MRS. Left panel: External (optical camera) and internal (navigator) tracking systems. Note that NMR probes use a combination of external and internal tracking. Middle Panel: Localization update is possible with both external and internal tracking, however, B0 shimming is possible only with navigators with B0 field mapping. Right panel: Examples of MR spectra under stationary conditions and in the presence of motion with no correction (NoCo), motion correction only (MoCo), and with both motion and shim correction (ShMoCo). Decreased SNR and line broadening/splitting are noticed in NoCo spectra. MoCo recovers partially the SNR and linewidth, while the full spectral quality is restored only if both motion and shim corrections (ShMoCo) are performed.
2.2.1. Internal MRI tracking with navigator sequences
Internal MRI tracking methods interleave navigator sequence modules with a slower parent modality such as MRS. Typically, the position is estimated once per TR, in accordance with the physics of the MRS sequence, which cannot usually be corrected more than once per TR given that a long readout period follows a single excitation pulse or train of preparatory pulses (e.g., pre-localization and suppression), and given that motion correction requires modifying the properties of the pulse and gradient encoding to adapt the region of excitation and encoded orientation. Most navigators excite the water signal with a very low flip angle pulse that can be made water or lipid selective, to minimize disturbing metabolite magnetization. Navigators may also be acquired after a block of multiple TRs of the parent sequence to reduce their time requirement, although every TR is preferred for precise localization/shim update and spin history. The navigator can measure position12 (e.g., position and orientation of the brain) and some even the B0 field.40 Hence, ideally the navigators can update both the localization and the B0 field, which may range from simple frequency update to full shim update depending on the navigator type and the shimming hardware.
Three types of navigators exist: 1) FID navigators that encode spatial information without gradients by using multiple spatially distributed RF receive coils,4 2) k-space navigators that acquire constrained k-space trajectories,36 and 3) image navigators that acquire entire images.12,41 First, FID navigators are used mostly for frequency update without localization update as described in the previous section, but have recently been extended to exploit phased array coil technology to estimate head position.42 Second, k-space navigators are shorter, occupying less time in the MRS sequence, at the expense of navigator SNR and resolution, leading to greater variance on the position estimates. Current k-space navigator implementations do not estimate spatial B0 field components beyond the first order. Third, image navigators are longer but provide full position and B0 field characterization of the entire organ.
2.2.1.1. FID navigators
Traditionally, the use of FIDs have been limited to estimate the average off-resonance frequency of everything within the profile of the RF excitation pulse inducing the FID4,43. With the advent of multi-channel phased arrays, the sensitivity profiles of the multiple coils can be used to add spatial information to the gradient encoding in MRI sequences to accelerate image encoding using parallel imaging techniques42, and to accelerate RF slice encoding in multiband/multislice encoding44. In the lowest limit, gradient and/or RF encoding is unnecessary with array coils to produce a low-resolution image and even a field map. This approach has been used for FID navigators to track the head position43 and even to obtain a field map. These techniques are extremely efficient, but rather ill-posed, requiring mapping or modeling to obtain the images with limited resolution. Hence, they have not been used in real time or MRS applications for position update. On the other hand, FID navigators have been used for frequency45 and phase correction46 caused by motion or magnet field drift4, as detailed in the sub-section 2.2.1.4.
2.2.1.2. k-Space navigators.
Acquisition of navigator echoes is a popular technique to detect and correct for subject motion in real-time in MRI.34 Different k-space trajectories have been employed in k-space based approaches, e.g. orbital, spherical35 and cloverleaf.36 The orbital navigator provides rotation information in a plane, while the spherical trajectory provides 3D rotation information but takes substantially longer time to acquire and analyze than other k-space navigators, therefore finding applications in inter-scan registration. The cloverleaf navigators provide position information with 6 degrees of freedom (i.e., 3 translations and 3 rotations) as well as 0th- and 1st-order shim information in a few milliseconds, but require an initial map of k-space and those estimates are relatively noisy. With k-space navigators, rotation is typically obtained from the magnitude information, since rotations in image space are manifest as rotations in k-space, while translations are obtained from the navigator phase information since translations in image space result in a phase roll across k-space. Frequency information is obtained from the phase accumulation across the navigator with time from excitation, while higher-order field information requires spatial encoding using the gradients or physical locations of the RF coils. Position estimates are fed back from the image reconstruction system to the RF and gradient control systems. RF pulse frequencies and accompanying gradients are modified to adapt the excitation orientation and position, while gradient pulse allocation across the physical gradient X, Y and Z coils determines the orientation of the spatial encoding by the gradients. Translations are corrected by adjusting the phase of each k-space sample consistent with its position in k-space. This doesn’t have to be done in real-time as long as it precedes the transformation from k-space to image space.
2.2.1.3. Image based navigators.
One of the earliest navigators, the pencil beam navigator,47 is a method to obtain the respiratory phase using a columnar excitation across the diaphragm and combine it with frequency encoding along the column (i.e., pencil). This may be considered to be a one-dimensional image navigator, since it is the Fourier transform of the signal, which provides the data for tracking the diaphragm motion. Pencil beam navigators are used for respiratory gating, which in combination with an electrocardiogram (ECG) has been shown to significantly improve the quality of SVS in the heart.48
With image navigators, motion of organs can be deduced directly from the images. For example, cardiac motion can be assessed with redundant radial sampling, a self-navigating technique.49 In MRS, estimation of head motion followed by updates of rotations and translations has been shown for three orthogonal navigator images right before the MRS water suppression module50.
While image-based navigators take longer to acquire than k-space navigators, the acquisition of a full image volume provides the flexibility to define the whole brain for motion correction while selecting a sub-region for B0 shimming. Selecting a sub-region even for frequency tracking alone is very important if motion occurs since the average frequency over the entire head may substantially differ from the average frequency in the volume of interest.51 A full B0 map also facilitates fitting of the field beyond linear shim terms, although this capability cannot be exploited with the shim hardware of commonly available MR scanners, which does not allow higher-order (≥ 2nd) shims to be changed in real time. Since typical TRs in MRS are on the order of 1 s or longer, with some dead time for signal recovery, whole-brain navigator imaging can be performed without prolonging the acquisition time of MRS. Image-based navigators like PROspective MOtion correction (PROMO)52 or EPI navigators12,40,53 make use of low-resolution images or volumes so that the imaging volume follows the object. Neither of these techniques provides B0 maps by default, which require modifications of the framework to incorporate phase images for B0 mapping besides magnitude images for position tracking. EPI volume navigators (“vNavs”) can easily be extended to interleave two echo times with the corresponding phase images from which a B0 map may be derived.16,40,54,55 The double-echo navigators take a longer time (~600 ms), which limits the minimum TR, however, recently B0 mapping has been demonstrated with shorter single-echo navigators by using the coil-dependent phase offsets.56 Also, image-based spiral navigators in combination with PROMO have been used in SVS to update the position of MRS voxels as well as the B0 shims.57 Since the brain is enclosed by the rigid frame of the skull, the motion displacement can be rapidly determined through rigid image registration, which can be computed within <0.1 s by linear transformations using six degrees of freedom (3 translations and 3 rotations) running on image reconstruction computers on commonly available MR scanners. Phase unwrapping for both echo times and fitting of the spherical shim harmonics to the obtained B0 maps can also be done in the same time frame. Another advantage of image-based navigators is the capability to correct for changes in the B1− receive field changes due to motion, hence to update the weights for coil combination of data acquired with phased arrays, thus improving SNR and reducing potential variability arising from coil combination.58
Several other image-based navigator techniques have been proposed, including fat navigators,59 collapsed fat navigators,60 and iNavs,61 but these techniques have not yet been extended to include field maps.
The use of image-based navigators may be more challenging at ultra-high field. For instance, vNavs are based on fast EPI readout, which may suffer geometric distortions and other imaging artifacts. The B0 field changes due to organ motion or respiration are more severe at ultra-high field and correct estimation of those changes becomes more challenging. Hence, vNavs at 7 T have so far been implemented only without real-time B0 shim updates.62 Another useful approach for MRSI could be to reduce effects of motion by employing acquisition strategies that null or minimize gradient moments used for k-space encoding.11 Spiral and radial trajectories that start at the center of k-space can achieve gradient moment nulling, which reduces phase errors between consecutive readouts. Such an approach using spiral out-in trajectories for gradient moment compensation has been shown to be effective in reducing the effect of respiratory motions in liver MRSI.63 In addition, repeated sampling of the k-space center can be used for self-navigation correction methods.64
2.2.1.4. Frequency/shim-only navigators
Motion is accompanied by B0 field changes, including frequency (0th order spatial) changes and higher-order spatial changes, which adversely affect the resonance behavior and hence the spectral quality with broader/distorted lines and reduced SNR. Navigators that update only frequency or shimming have been used in combination with other methods such as external cameras that update position for correction of motion effects in MRS. Such navigators acquire only limited information about the B0 field using a global FID for the main frequency,4,45,46,51 or 1D projections along three orthogonal directions for 1st order shims.17,65 Their main advantage is that they are faster than full B0 field imaging sequences. These FID navigators for frequency update can be used for both SVS and MRSI sequences. However, acquiring three orthogonal projections is sufficient to sample the B0 field only for small volumes in SVS, while it is too sparse to sample adequately the B0 field pattern for larger volumes used in MRSI, which requires full mapping of the B0 field by imaging sequences. Hence, projection-based shim navigators have been limited only to SVS and 1st order shimming.
Real-time frequency update has been achieved by following the position of the residual water signal in each FID, subtracting the frequency from the center frequency and subsequently correcting for the drift in real time.57 Linear shim offsets are typically implemented by applying a fixed offset current on the linear gradients. Modifying the shim induces a mean frequency change that must be corrected in a similar fashion.57 A field-frequency lock has been effectively used to update frequency in real time with a whole-body spectrometer.45 This was achieved by acquiring a reference scan interleaved in the PRESS scan, measuring the frequency shift of the reference water signal in real time and adjusting the Z0 shim coil current of the spectrometer by applying a negative feedback. A feedback monitoring system based on fluorine NMR probes has been used for SVS at 7T for stabilization of B0 due to respiratory motions.66 Real-time frequency adjustment during J-difference edited SVS has been performed by reading the FID signal during water suppression and using that to track and update the system frequency during each TR cycle.67 It should be noted that detrimental effects of scanner center frequency drift from gradient heating following heavy duty echo-planar imaging (EPI) scans or various forms of spatial-spectral encoding in MRSI can also be corrected prospectively using these methods.4,51,68 However, besides frequency update, in the case of motion, it is necessary to update other B0 shim terms, such as 1st and 2nd spherical harmonics, which require more complex navigators for B0 fieldmapping16,40,54.
2.2.2. External tracking methods.
External tracking systems use methods other than the MR signal of internal water to determine the position of the organ. Clinical scanners typically include physiologic sensors that enable cardiac and respiratory gating (e.g., ECG sensors, pulse oximeters and respiratory belts), and these have been used to gate MRS acquisitions in body applications.48 Since the brain moves rigidly with the head, tracking a marker attached to the head is a good proxy for tracking motion of the brain, provided that the marker remains rigidly attached to the head.
In addition to the optical and NMR probe tracking methods reviewed in detail below, other technologies were also used in the past. The EndoScout (Robin Medical Inc., Baltimore, MD, USA) electromagnetic position sensor uses a small sensor cube to detect gradient activity in the bore and determine position and orientation at a very high rate69. Several sensors have been developed purely for research purposes, including a small wireless device, which uses gravity and the main B0 field to determine its orientation in the scanner70. Similarly, active markers (tuned coils) that provide fiducial points for position and orientation estimation along the B0 field have been demonstrated.71 However, these methods do not provide B0 field estimates.
2.2.2.1. Optical tracking.
Optical tracking methods use one or more cameras to observe a marker on the head or the face to track the brain. Kineticor (Honolulu, HI, USA) provides a multi-camera system that observes a marker attached to the face and tracks the head inside the scanner. Together with Siemens, they provided the first FDA-approved prospective motion correction solution for MRI on the market. Hobbitview (San Jose, CA, USA) provides a similar system targeted at GE MRI & PET/MR scanners. Metria Innovation (Wauwatosa, WI, USA) provides camera systems based on the Moiré Phase Tracking (MPT) principle, which are very sensitive to rotations.15,46,72,73 Most recently, the TCL system (TracInnovations, Copenhagen, Denmark) provides a markerless tracking system that eliminates possible marker attachment problems using surface tracking of the face and computer vision algorithms to directly track head motion.13,74 All these systems provide rapid and continuous position updates independent of the MR sequence, and these positions can be fed back to the scanner to provide real-time correction, or the time-synchronized information can be used to correct MR data offline. A comparison between optical tracking and MR-based tracking methods discovered a high degree of correlation between the motion estimates provided by both tracking modalities.75 Optical tracking has been used for motion correction of SVS and MRSI,15,46,72 however as was recognized early on, none of these systems provides B0 field information which needs to be updated to restore full spectral quality. Since the stability of B0 is critical in MRS, external tracking for localization updates was combined with interleaved water FID, which was initially used for retrospective frequency update,15,46,72 without higher-order shim updates.
2.2.2.2. NMR probes
NMR probes have been used extensively in tracking catheters and surgical instruments during interventional MRI.76 Inductively coupled wireless NMR probes resonating at proton Larmor frequency can be attached to the head and used together with low flip angle interleaved navigators to detect head motion and update the localization of MRI sequences.77 Essentially, this approach combines the use of external markers (NMR probes) with internal tracking measurement (navigators). Comparison between NMR probes and optical cameras revealed slightly higher precision for optical cameras,78 but more than sufficient precision in both cases to track head motion with an accuracy of tens of microns. However, as with optical systems no information about the B0 field inside the head is obtained, and no such systems have been demonstrated yet for motion correction of MRS. Alternatively, fluorine resonating NMR probes can be used to monitor the B0 field over time and such a system was demonstrated for feedback control of B0 field to minimize the linewidth modulation of SVS in the brain at 7 T due to respiratory motion.66 Advanced field cameras have been built by research groups or commercially (Skope, Zurich, Switzerland) for rapid dynamic B0 field information at multiple discrete points in space.79 However, such systems probe B0 field outside the head, and it has been shown for the brain that measuring the B0 field just outside the head does not suffice to predict accurately the B0 field inside the brain.18,80 Currently no technique exists for measuring the B0 field inside the body without using the MR signal. Predicting the B0 field after motion based on computational electromagnetic (EM) models, head structure and an initial B0 map has been proposed,81 and with fast enough computation might offer an alternative to real-time measurements of B0 field.
2.3. Hybrid methods
While external systems are expensive and require additional hardware, they provide some desirable properties for motion tracking, such as speed and independence from the pulse sequence. On the other hand, navigators can be implemented entirely in software and use the existing MR scanner hardware, and they may provide the missing B0 field information that cannot be measured with external tracking devices. Therefore, a hybrid approach leveraging the advantages of both approaches may be attractive. In fact, all demonstrations of motion correction for MRS based on external tracking have used some simple form of field correction with retrospective frequency update. In the absence of scanner instability, the requirements of monitoring B0 field can be relaxed since the B0 field update is only required when motion actually occurs. A B0 map could be collected only when necessary, triggered by the external device or an efficient internal navigator such as an FID.43,82 A combination of external tracking, structural head imaging and B0 field computational EM modeling might also be implemented.81 Recently, a combination of prospective motion correction using optical tracking and prospective shim correction using an interleaved FAST(EST)MAP navigator has been reported for SVS measurements in the human brain at 7 T.17 Gradient and RF pulses used for localization were updated based on the feedback from an external camera, while the navigator measured the B0 field along three orthogonal projections, which were used to update frequency and linear shim terms.
3. Applications
Motion correction methods have been used in clinical studies of MRS beyond technical proof of concept, and proven to be especially beneficial in subjects who have a predisposition to move such as children or patients with movement disorders. In pediatric studies of HIV and fetal alcohol syndrome, SVS measurements with image based navigators were used.83,84 In the children in whom PRESS in deep cerebellar nuclei was acquired, navigators resulted in an increase in success rate from 50% to 73%, where success was defined as usable data with linewidths below 0.08 ppm and SNR > 8.85 In a pediatric study, 73% of children moved between the anatomical data acquisition and MRS and 32% moved during the acquisition, with the predominant movement directions being sliding out of the coil and nodding (up-–down rotation). First-order B0 corrections were significant (>10 μT/m) in 18% of acquisitions.86
When the MRS acquisition is not a single shot method and the transient spectra have different magnitude and phase modulations, motion correction is extremely important. J-difference editing is particularly susceptible to measurement instabilities arising from subject motion or scanner frequency drifts. MEGA editing is one of the most used techniques for editing gamma-aminobutyric acid (GABA)87 as well as other important metabolites such as the oncometabolite D-2-hydroxyglutarate (2HG) in mutant isocitrate dehydrogenase (IDH) mutated gliomas,88 and glutathione (GSH).89 The MEGA-edited spectrum is the difference of spectra acquired in two consecutive TRs, and large subtraction artifacts can preclude quantification of edited signals in the presence of motion or B0 field changes. Because of this extreme motion sensitivity, the real-time reacquisition of corrupted TRs can be performed in addition to motion and B0 shim correction (ReShMoCo) to further improve spectral quality and achieve the minimum subtraction error.54 Reacquisition was demonstrated also to be beneficial in MRI,12 where damaged k-space regions affect the entire reconstructed image. Reacquisition is not mandatory in SVS if every transient is the same (i.e., in the absence of phase cycling or editing), since the corrupted transients can be discarded but at the cost of lower SNR. In contrast, reacquisition is critical in MRSI because motion or B0 field differences between spatial encoding steps during MRSI encoding between navigator blocks affect the entire MRSI data. Examples of motion subtraction artifacts and real-time correction with navigators are shown in Figure 2.
Figure 2.
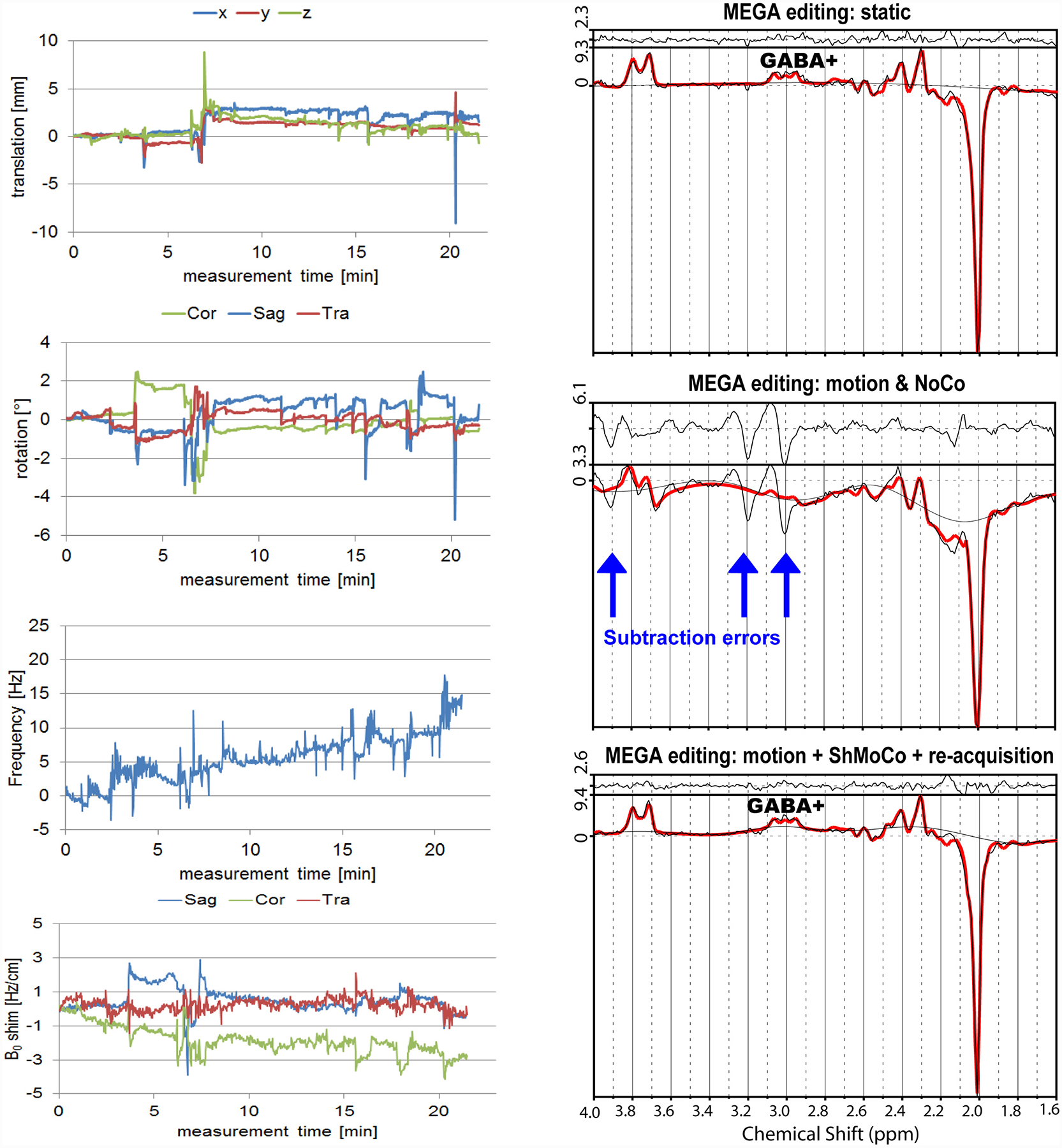
Real-time motion and B0 field correction for MEGA edited MRS of GABA. Subtraction artifacts are eliminated with re-acquisition of corrupted TRs.
Real-time motion correction with shim update has been used in clinical MRSI studies with adult subjects, including clinical studies of patients with brain tumors,90 amyotrophic lateral sclerosis (ClinicalTrials.gov/NCT02288091),91 multiple sclerosis and neurodegenerative disorders (e.g., Parkinson and mild cognitive impairment).92 Cancer IDH mutations (IDH 1&2) have high de novo production of the oncometabolite D-2-hydroxyglutarate (2HG), and in vivo imaging of 2HG is a key application of MRSI in neuro-oncology. Robust 3D MRSI of 2HG with real-time ReShMoCo MEGA-editing was used in mutant IDH glioma patients to assess tumor burden93, response to standard radiochemotherapy90 and targeted therapy with novel mutant-IDH inhibitors90,94. The same methodology was used to image GSH in the brain of patients with amyotrophic lateral sclerosis (ClinicalTrials.gov/NCT02288091),91 including the motor cortex and corticospinal tracts, or in patients with mutant IDH glioma tumors.94 GABA was imaged using the same methods in patients with Parkinson’s disease.92 Figure 3 shows examples of such metabolite maps using edited MRSI with real-time motion and shim correction.
Figure 3.
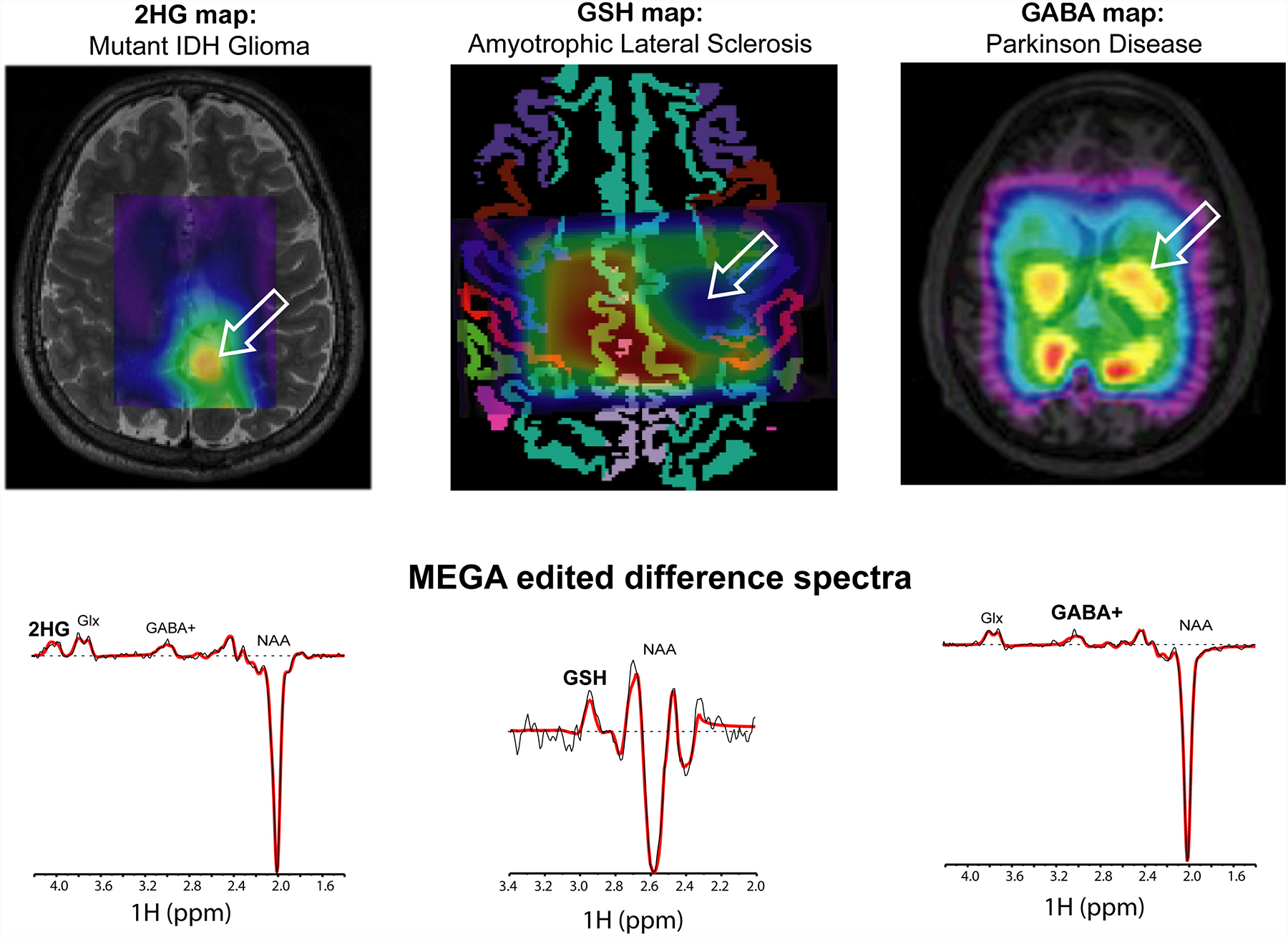
Applications of real-time motion and shim correction for edited MRSI of metabolites: i) oncometabolite D-2-hydroxyglutarate (2HG) in mutant IDH glioma; ii) antioxidant glutathione (GSH) in amyotrophic lateral sclerosis; iii) inhibitory neurotransmitter gamma-aminobutyric acid (GABA) in Parkinson’s disease. Arrows indicate regions with abnormal metabolite levels in metabolic maps. Examples of edited spectra are shown at the bottom.
4. Recommendations
Recommendations regarding technical performance for prospective real-time correction of motion effects in MRS may be specified based on the stability and precision that is needed for metabolite quantification. Ideally the most advanced and effective motion correction should be used when possible. However, if this is not available or there are other limitations precluding it, practical guidelines for acceptable performance can be established based on the question/hypothesis to be answered/tested by MRS. Different levels of MRS precision may be required depending on the effect size of each study. For example, a larger estimation error may be acceptable in diseases where there are large metabolic differences between the healthy tissue and pathological lesion such as cancer,95 while smaller errors are needed in diffuse diseases such as neurodegenerative or psychiatric conditions,96,97 where there are subtle metabolic differences compared to healthy controls. In the case of more demanding research paradigms, such as functional MRS (fMRS), the smallest estimation errors are necessary to measure dynamic metabolite changes.98
Simple guidelines for precision of MRS motion correction (Table 2) may be derived assuming an isotropic voxel in the case of SVS for either pure translation or rotation under the most unfavorable movement, i.e., motion starting at the beginning of the acquisition, which moves the voxel outside the brain. For an isotropic SVS cube of side L, in order to have volumetric error less than E, the precision of motion correction for pure translation is given by , which is proportional to the side of the cube. For head rotations, the axis of rotation is given by the atlanto-axial joint and the same rotation angle will affect voxels more the farther away they are from the axis. For the farthest voxel located at the frontal pole, a cubic voxel of side L, to have a volumetric error less than E the precision of motion correction for pure rotation is given by: , where is the anterior-posterior diameter of the brain and we assume that the axis of the atlanto-axial joint passes through the center of the brain.
Table 2.
Recommendations for the technical performance of prospective real-time motion and shim correction for various required levels of precision in MRS. Note: 1) precision has been derived for either pure translation or rotation considering the case of an isotropic SVS voxel; 2) for MRSI, similar precision thresholds are hard to derive theoretically and the acceptable performance level for motion correction should be empirically evaluated by test-retest. An SVS voxel of 10 mm × 10 mm × 10 mm and anterior-posterior brain diameter of 220 mm were considered. Translation for any voxel and rotation angle for a frontal pole voxel, which is displaced the most by rotation around an axis through the center of the atlanto-axial joint are calculated according to the formulas given above. All reported real-time methods have higher translation and rotation precisions than the values listed in the table. The guidelines for homogeneity of the B0 field are the same as recommended in the B0 shimming consensus paper.3
| Basic | Moderate | Advanced |
|---|---|---|
20% error in estimation of metabolite concentration
|
10% error in estimation of metabolite concentrations
|
5% error in estimation of metabolite concentrations
|
However, for the case of MRSI and simultaneous translation and rotation, such limits are harder to derive theoretically. In such cases, a more practical way is to empirically assess the performance and precision of a certain motion correction method with a test-retest paradigm, in which the variability of metabolite quantification between stationary and motion conditions should be less than the desired precision for metabolite quantification either in vivo or in structural phantoms with metabolite compartments of size comparable to the MRSI resolution. Conversely, under perfect stationary conditions any correction method should provide data that are undistinguishable from data acquired without correction.
Experts’ recommendations
Motion correction is particularly beneficial for MRS and should be applied whenever possible. Because of the long acquisition time that is typical in MRS, motion and scanner instability is likely to occur during the scan. Because of low SNR and the need for narrow spectral linewidth to resolve metabolite signals, any instability arising from subject motion or hardware can degrade the quality of MRS data.
B0 field changes secondary to motion and/or scanner instabilities substantially affect spectral quality, and B0 shim correction for these effects is recommended. While the effects of motion and hardware instability are easy to recognize in MRI through blurring of anatomical structures, these effects are harder to identify in MRS, because the anatomical information is not directly incorporated in the SVS data and the low spatial resolution in typical MRSI does not sufficiently resolve anatomical structures. Motion can be suspected when artifacts are present in the spectra such as line splitting or if signal from outside the organ of interest (e.g., subcutaneous lipid signals for brain MRS) is present. However, these spectral artifacts can be confounded by poor B0 shimming and poor localization (e.g. chemical shift displacement error) that can happen even under stationary conditions. In particular, for MRS there is a strong coupling between localization and B0 homogeneity that makes MRS very susceptible to motion-related degradation. Both localization and the B0 field must be corrected to adequately compensate for the effects of motion on MRS.
The approaches recommended for MRS, similar to MRI, to minimize and mitigate effects of motion and scanner instability are: i) head immobilization; ii) retrospective correction; and iii) prospective real-time correction. However, the main difference is that correction of B0 field is absolutely necessary to restore spectral quality in addition to localization update in MRS.
For retrospective correction, transients need to be stored separately, or raw data need to be used. Such corrections can improve data quality by correcting for frequency drifts or discarding badly corrupted transients. However, they cannot correct for localization error or the failure of frequency selective sequence blocks such as water suppression and spectral editing.
Prospective real-time correction is the method of choice for MRS if available. Methods that simultaneously track organ position and B0 field inside the organ provide the best data quality restoration for MRS. Scanner manufacturers such as Siemens have moved toward supporting external camera-based tracking systems.37,72 At present such certified solutions are only available for a limited set of imaging sequences, although the underlying software provides support for real-time position update in any pulse sequence. However, such systems cannot measure the B0 field.
Motion correction alone is insufficient, and at a minimum, frequency update has to be performed in real time or retrospectively. Manufacturers should support dynamic field correction methods, including high-order (≥ 2nd) shims, as part of the product approved for clinical use. Retrospective correction cannot be performed for 1st or higher-order spherical harmonics. Higher-order (>1st) shim updates should be performed in real-time to provide significantly more data improvement compared to frequency and 1st-order shim update. While commercial scanners are limited to real-time update of 1st-order shims, the possibility of either real-time update of 2nd- or 3rd-order shims or shim arrays99 should be considered by manufacturers if technically feasible to allow best data quality in MRS. To incentivize such efforts, there is a high return as other MRI modalities could also benefit substantially from better B0 field management such as fMRI, DTI, SWI and CEST.
The best available method for prospective motion correction should be used with the highest spatial and temporal resolution and B0 shim updates. Criteria for minimal acceptable technical performance of prospective correction can be derived based on the effect size that needs to be measured by MRS. Variability of 5% or less in quantification of the primary metabolite concentrations is sufficient for most clinical applications. The lowest possible variability is necessary for functional MRS studies.
Spectral editing methods based on subtraction or multiple TE averaging are highly sensitive to motion compared to non-edited MRS, and reacquisition of corrupted transients is necessary for full data restoration.
Oversampling of the center of k-space is recommended for motion robustness in MRSI. Trajectories that repeatedly sample the central part of k-space in the shortest possible time frame facilitate self-navigation as an alternative to interleaved navigation.
In MRSI, motion causes signal smearing and large image artifacts from nuisance signals of water and lipid that can overwhelm metabolite signals. Lipid/water suppression that use frequency selective methods benefit largely from B0 field stability, and shim update is recommended to maintain efficiency of these suppression methods in the presence of motion and scanner instabilities.
At ultra-high field (≥ 7 T), controlling the B0 field stability is even more important, with the added challenge that frequency modulation in the brain can result from changes in lung magnetic susceptibility due to respiration18 even if the head is perfectly stationary. For this reason, B0 field update is especially recommended in addition to motion correction at ultra-high field.
Acknowledgements.
Support from NIH/NCI grants R01CA211080 and K22CA178269 (O.C.A.); NIH/NICHD grants R01HD085813, R01HD093578 and R01HD099846 (A.v.d.K. and E.M.); the BHF Centre of Research Excellence, Oxford (RE/13/1/30181); the Austrian Science Fund (FWF) via projects KLI 718 and P 30701 (W.B.).
Abbreviations.
- NoCo
No Motion Correction
- MoCo
Motion Correction
- ShMoCo
Shim and Motion Correction
- ReShMoCo
Reacquisition of corrupted TRs with ShMoCo
- PROMO
PROspective Motion
- vNAV
volumetric Navigator
- iNav
image Navigators
- EPI
Echo planar imaging
- FAST(EST)MAP
Fast, Automatic Shim Technique (using Echo-planar Signal readout) for Mapping Along Projections
- FID
Free Induction Decay
- SVS
Single Voxel Spectroscopy
- PRESS
Point Resolved Spectroscopic Sequence
- MRS
Magnetic Resonance Spectroscopy
- MRSI
Magnetic Resonance Spectroscopic Imaging
- GABA
gamma-amino-butyric acid
- GSH
glutathione
- 2HG
D-2-hydroxyglutarate
- fMRI
functional MRI
- DTI
Diffusion Tensor Imaging
- SWI
Susceptibility Weighted Imaging
- CEST
Chemical Exchange Saturation Transfer
- EM
Electro-Magnetic
References
- 1.Kreis R Issues of spectral quality in clinical 1H-magnetic resonance spectroscopy and a gallery of artifacts. NMR in biomedicine. 2004;17(6):361–381. [DOI] [PubMed] [Google Scholar]
- 2.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17(2):825–841. [DOI] [PubMed] [Google Scholar]
- 3.Juchem C, Cudalbu C, de Graaf RA, et al. B0 Shimming for In Vivo Magnetic Resonance Spectroscopy: Experts’ Consensus Recommendations. Nmr Biomed. 2020:in press. [DOI] [PubMed] [Google Scholar]
- 4.Ebel A, Maudsley AA. Detection and correction of frequency instabilities for volumetric 1H echo-planar spectroscopic imaging. Magnetic resonance in medicine. 2005;53(2):465–469. [DOI] [PubMed] [Google Scholar]
- 5.Ogg RJ, Kingsley PB, Taylor JS. Wet, a T-1-Insensitive and B-1-Insensitive Water-Suppression Method for in-Vivo Localized H-1-Nmr Spectroscopy. Journal of Magnetic Resonance Series B. 1994;104(1):1–10. [DOI] [PubMed] [Google Scholar]
- 6.Esmaeili M, Bathen TF, Rosen BR, Andronesi OC. Three-dimensional MR spectroscopic imaging using adiabatic spin echo and hypergeometric dual-band suppression for metabolic mapping over the entire brain. Magnetic resonance in medicine. 2017;77(2):490–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bilgic B, Gagoski B, Kok T, Adalsteinsson E. Lipid suppression in CSI with spatial priors and highly undersampled peripheral k-space. Magn Reson Med. 2013;69(6):1501–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naressi A, Couturier C, Devos JM, et al. Java-based graphical user interface for the MRUI quantitation package. Magnetic Resonance Materials in Physics Biology and Medicine. 2001;12(2–3):141–152. [DOI] [PubMed] [Google Scholar]
- 9.Tkáč I, Deelchand D, Dreher W, et al. Water and lipid suppression techniques for advanced 1H MRS and MRSI: Experts’ Consensus Recommendations. Nmr Biomed. 2020:in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maclaren J, Herbst M, Speck O, Zaitsev M. Prospective motion correction in brain imaging: A review. Magnetic resonance in medicine. 2013;69(3):621–636. [DOI] [PubMed] [Google Scholar]
- 11.Zaitsev M, Maclaren J, Herbst M. Motion artifacts in MRI: A complex problem with many partial solutions. Journal of magnetic resonance imaging : JMRI. 2015;42(4):887–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tisdall MD, Hess AT, Reuter M, Meintjes EM, Fischl B, van der Kouwe AJW. Volumetric navigators for prospective motion correction and selective reacquisition in neuroanatomical MRI. Magn Reson Med. 2012;68(2):389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slipsager JM, Ellegaard AH, Glimberg SL, et al. Markerless motion tracking and correction for PET, MRI, and simultaneous PET/MRI. PloS one. 2019;14(4):e0215524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson M Robust retrospective frequency and phase correction for single-voxel MR spectroscopy. Magnetic resonance in medicine. 2019;81(5):2878–2886. [DOI] [PubMed] [Google Scholar]
- 15.Andrews-Shigaki BC, Armstrong BS, Zaitsev M, Ernst T. Prospective motion correction for magnetic resonance spectroscopy using single camera Retro-Grate reflector optical tracking. Journal of magnetic resonance imaging : JMRI. 2011;33(2):498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hess AT, Andronesi OC, Tisdall MD, van der Kouwe AJW, Sorensen AG, Meintjes E. Real-time Motion and B0 correction for LASER MRSI using EPI volumetric navigators. NMR Biomedicine. 2012;25(2):347–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deelchand DK, Joers JM, Auerbach EJ, Henry PG. Prospective motion and B0 shim correction for MR spectroscopy in human brain at 7T. Magnetic resonance in medicine. 2019;82(6):1984–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andersen M, Hanson LG, Madsen KH, et al. Measuring motion-induced B0 -fluctuations in the brain using field probes. Magnetic resonance in medicine. 2016;75(5):2020–2030. [DOI] [PubMed] [Google Scholar]
- 19.Gastl M, Peereboom SM, Fuetterer M, et al. Cardiac- versus diaphragm-based respiratory navigation for proton spectroscopy of the heart. Magma (New York, NY). 2019;32(2):259–268. [DOI] [PubMed] [Google Scholar]
- 20.Li BSY, Babb JS, Soher BJ, Maudsley AA, Gonen O. Reproducibility of 3D proton spectroscopy in the human brain. Magnetic resonance in medicine. 2002;47(3):439–446. [DOI] [PubMed] [Google Scholar]
- 21.Kirov II, George IC, Jayawickrama N, Babb JS, Perry NN, Gonen O. Longitudinal inter- and intra-individual human brain metabolic quantification over 3 years with proton MR spectroscopy at 3 T. Magnetic resonance in medicine. 2012;67(1):27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gasparovic C, Bedrick EJ, Mayer AR, et al. Test-retest reliability and reproducibility of short-echo-time spectroscopic imaging of human brain at 3T. Magnetic resonance in medicine. 2011;66(2):324–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Terpstra M, Cheong I, Lyu T, et al. Test-retest reproducibility of neurochemical profiles with short-echo, single-voxel MR spectroscopy at 3T and 7T. Magnetic resonance in medicine. 2016;76(4):1083–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheong I, Marjanska M, Deelchand DK, Eberly LE, Walk D, Oz G. Ultra-High Field Proton MR Spectroscopy in Early-Stage Amyotrophic Lateral Sclerosis. Neurochemical research. 2017;42(6):1833–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donadieu M, Le Fur Y, Lecocq A, et al. Metabolic voxel-based analysis of the complete human brain using fast 3D-MRSI: Proof of concept in multiple sclerosis. Journal of magnetic resonance imaging : JMRI. 2016;12(10):doi: 10.1002/jmri.25139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu X, Schuff N, Kornak J, et al. Effects of Alzheimer disease on fronto-parietal brain N-acetyl aspartate and myo-inositol using magnetic resonance spectroscopic imaging. Alzheimer disease and associated disorders. 2006;20(2):77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gabr RE, Sathyanarayana S, Schar M, Weiss RG, Bottomley PA. On restoring motion-induced signal loss in single-voxel magnetic resonance spectra. Magn Reson Med. 2006;56(4):754–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thiel T, Czisch M, Elbel GK, Hennig J. Phase coherent averaging in magnetic resonance spectroscopy using interleaved navigator scans: compensation of motion artifacts and magnetic field instabilities. Magn Reson Med. 2002;47(6):1077–1082. [DOI] [PubMed] [Google Scholar]
- 29.Hock A, Henning A. Motion correction and frequency stabilization for MRS of the human spinal cord. NMR in biomedicine. 2016;29(4):490–498. [DOI] [PubMed] [Google Scholar]
- 30.Bhattacharyya PK, Lowe MJ, Phillips MD. Spectral quality control in motion-corrupted single-voxel J-difference editing scans: An interleaved navigator approach. Magn Reson Med. 2007;58(4):808–812. [DOI] [PubMed] [Google Scholar]
- 31.Ernst T, Li JK. A Novel Phase and Frequency Navigator for Proton Magnetic Resonance Spectroscopy Using Water-Suppression Cycling. Magn Reson Med. 2011;65(1):13–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Helms G, Piringer A. Restoration of motion-related signal loss and line-shape deterioration of proton MR spectra using the residual water as intrinsic reference. Magn Reson Med. 2001;46(2):395–400. [DOI] [PubMed] [Google Scholar]
- 33.Near J, Edden R, Evans CJ, Paquin R, Harris A, Jezzard P. Frequency and phase drift correction of magnetic resonance spectroscopy data by spectral registration in the time domain. Magnetic resonance in medicine. 2015;73(1):44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maclaren J, Herbst M, Speck O, Zaitsev M. Prospective motion correction in brain imaging: a review. Magnetic resonance in medicine. 2013;69(3):621–636. [DOI] [PubMed] [Google Scholar]
- 35.Welch EB, Manduca A, Grimm RC, Ward HA, Jack CR. Spherical navigator echoes for full 3D rigid body motion measurement in MRI. Magn Reson Med. 2002;47(1):32–41. [DOI] [PubMed] [Google Scholar]
- 36.van der Kouwe AJ, Benner T, Dale AM. Real-time rigid body motion correction and shimming using cloverleaf navigators. Magnetic resonance in medicine. 2006;56(5):1019–1032. [DOI] [PubMed] [Google Scholar]
- 37.Maclaren J, Armstrong BS, Barrows RT, et al. Measurement and correction of microscopic head motion during magnetic resonance imaging of the brain. PloS one. 2012;7(11):e48088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dold C, Zaitsev M, Speck O, Firle EA, Hennig J, Sakas G. Advantages and limitations of prospective head motion compensation for MRI using an optical motion tracking device. Academic radiology. 2006;13(9):1093–1103. [DOI] [PubMed] [Google Scholar]
- 39.Olesen OV, Paulsen RR, Hojgaard L, Roed B, Larsen R. Motion tracking for medical imaging: a nonvisible structured light tracking approach. IEEE transactions on medical imaging. 2012;31(1):79–87. [DOI] [PubMed] [Google Scholar]
- 40.Hess AT, Tisdall MD, Andronesi OC, Meintjes EM, van der Kouwe AJW. Real-time motion and B0 corrected single voxel spectroscopy using volumetric navigators. Magnetic resonance in medicine. 2011;66(2):314–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.White N, Roddey C, Shankaranarayanan A, et al. PROMO: Real-time prospective motion correction in MRI using image-based tracking. Magnetic resonance in medicine. 2010;63(1):91–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blaimer M, Breuer F, Mueller M, Heidemann RM, Griswold MA, Jakob PM. SMASH, SENSE, PILS, GRAPPA: how to choose the optimal method. Topics in magnetic resonance imaging : TMRI. 2004;15(4):223–236. [DOI] [PubMed] [Google Scholar]
- 43.Kober T, Marques JP, Gruetter R, Krueger G. Head motion detection using FID navigators. Magnetic resonance in medicine. 2011;66(1):135–143. [DOI] [PubMed] [Google Scholar]
- 44.Barth M, Breuer F, Koopmans PJ, Norris DG, Poser BA. Simultaneous multislice (SMS) imaging techniques. Magnetic resonance in medicine. 2016;75(1):63–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Henry PG, van de Moortele PF, Giacomini E, Nauerth A, Bloch G. Field-frequency locked in vivo proton MRS on a whole-body spectrometer. Magnetic resonance in medicine. 1999;42(4):636–642. [DOI] [PubMed] [Google Scholar]
- 46.Lange T, Maclaren J, Buechert M, Zaitsev M. Spectroscopic imaging with prospective motion correction and retrospective phase correction. Magnetic resonance in medicine. 2012;67(6):1506–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nehrke K, Bornert P, Groen J, Smink J, Bock JC. On the performance and accuracy of 2D navigator pulses. Magnetic resonance imaging. 1999;17(8):1173–1181. [DOI] [PubMed] [Google Scholar]
- 48.Schar M, Kozerke S, Boesiger P. Navigator gating and volume tracking for double-triggered cardiac proton spectroscopy at 3 Tesla. Magnetic resonance in medicine. 2004;51(6):1091–1095. [DOI] [PubMed] [Google Scholar]
- 49.Bhat H, Ge L, Nielles-Vallespin S, Zuehlsdorff S, Li D. 3D radial sampling and 3D affine transform-based respiratory motion correction technique for free-breathing whole-heart coronary MRA with 100% imaging efficiency. Magn Reson Med. 2011;65(5):1269–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Keating B, Deng WR, Roddey JC, et al. Prospective Motion Correction for Single-Voxel H-1 MR Spectroscopy. Magnetic resonance in medicine. 2010;64(3):672–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee CY, Choi IY, Lee P. Prospective frequency correction using outer volume suppression-localized navigator for MR spectroscopy and spectroscopic imaging. Magnetic resonance in medicine. 2018;80(6):2366–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.White N, Roddey C, Shankaranarayanan A, et al. PROMO: Real-Time Prospective Motion Correction in MRI Using Image-Based Tracking. Magn Reson Med. 2010;63(1):91–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alhamud A, Tisdall MD, Hess AT, Hasan KM, Meintjes EM, van der Kouwe AJW. Volumetric navigators for real-time motion correction in diffusion tensor imaging. Magn Reson Med. 2012;68(4):1097–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bogner W, Gagoski B, Hess AT, et al. 3D GABA imaging with real-time motion correction, shim update and reacquisition of adiabatic spiral MRSI. NeuroImage. 2014;103:290–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bogner W, Hess AT, Gagoski B, et al. Real-time motion- and B-correction for LASER-localized spiral-accelerated 3D-MRSI of the brain at 3T. NeuroImage. 2013;88C:22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moser P, Eckstein K, Hingerl L, et al. Intra-session and inter-subject variability of 3D-FID-MRSI using single-echo volumetric EPI navigators at 3T. Magnetic resonance in medicine. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Keating B, Ernst T. Real-time dynamic frequency and shim correction for single-voxel magnetic resonance spectroscopy. Magn Reson Med. 2012;68(5):1339–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Papp D, Callaghan MF, Meyer H, Buckley C, Weiskopf N. Correction of inter-scan motion artifacts in quantitative R1 mapping by accounting for receive coil sensitivity effects. Magnetic resonance in medicine. 2016;76(5):1478–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gallichan D, Marques JP, Gruetter R. Retrospective correction of involuntary microscopic head movement using highly accelerated fat image navigators (3D FatNavs) at 7T. Magnetic resonance in medicine. 2016;75(3):1030–1039. [DOI] [PubMed] [Google Scholar]
- 60.Engstrom M, Martensson M, Avventi E, Norbeck O, Skare S. Collapsed fat navigators for brain 3D rigid body motion. Magnetic resonance imaging. 2015;33(8):984–991. [DOI] [PubMed] [Google Scholar]
- 61.Addy NO, Ingle RR, Luo J, et al. 3D image-based navigators for coronary MR angiography. Magnetic resonance in medicine. 2017;77(5):1874–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moser P, Hingerl L, Strasser B, et al. Whole-slice mapping of GABA and GABA(+) at 7T via adiabatic MEGA-editing, real-time instability correction, and concentric circle readout. NeuroImage. 2019;184:475–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim DH, Gu M, Spielman DM. Gradient moment compensated magnetic resonance spectroscopic imaging. Magnetic resonance in medicine. 2009;61(2):457–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim DH, Adalsteinsson E, Spielman DM. Spiral readout gradients for the reduction of motion artifacts in chemical shift imaging. Magn Reson Med. 2004;51(3):458–463. [DOI] [PubMed] [Google Scholar]
- 65.Keating B, Ernst T. Real-time dynamic frequency and shim correction for single-voxel magnetic resonance spectroscopy. Magnetic resonance in medicine. 2012;68(5):1339–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wilm BJ, Duerst Y, Dietrich BE, et al. Feedback field control improves linewidths in in vivo magnetic resonance spectroscopy. Magn Reson Med. 2014;71(5):1657–1662. [DOI] [PubMed] [Google Scholar]
- 67.Ahn S, Meyerhoff D, Laub G. MEGA-PRESS Single-voxel Spectroscopy for GABA J-editing with Real-time Frequency Adjustment. Proc Intl Soc Mag Reson Med. 2016;24(4):4008. [Google Scholar]
- 68.Lange T, Zaitsev M, Buechert M. Correction of frequency drifts induced by gradient heating in 1H spectra using interleaved reference spectroscopy. Journal of magnetic resonance imaging : JMRI. 2011;33(3):748–754. [DOI] [PubMed] [Google Scholar]
- 69.Gholipour A, Polak M, van der Kouwe A, Nevo E, Warfield SK. Motion-robust MRI through real-time motion tracking and retrospective super-resolution volume reconstruction. Conference proceedings : Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Annual Conference. 2011;2011:5722–5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van Niekerk A, van der Kouwe A, Meintjes E. A Method for Measuring Orientation Within a Magnetic Resonance Imaging Scanner Using Gravity and the Static Magnetic Field (VectOrient). IEEE transactions on medical imaging. 2017;36(5):1129–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van Niekerk A, Meintjes E, van der Kouwe A. A Wireless Radio Frequency Triggered Acquisition Device (WRAD) for Self-Synchronised Measurements of the Rate of Change of the MRI Gradient Vector Field for Motion Tracking. IEEE transactions on medical imaging. 2019;38(7):1610–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zaitsev M, Speck O, Hennig J, Buchert M. Single-voxel MRS with prospective motion correction and retrospective frequency correction. NMR in biomedicine. 2010;23(3):325–332. [DOI] [PubMed] [Google Scholar]
- 73.Zaitsev M, Dold C, Sakas G, Hennig J, Speck O. Magnetic resonance imaging of freely moving objects: prospective real-time motion correction using an external optical motion tracking system. NeuroImage. 2006;31(3):1038–1050. [DOI] [PubMed] [Google Scholar]
- 74.Olesen OV, Sullivan JM, Mulnix T, et al. List-mode PET motion correction using markerless head tracking: proof-of-concept with scans of human subject. IEEE transactions on medical imaging. 2013;32(2):200–209. [DOI] [PubMed] [Google Scholar]
- 75.Gumus K, Keating B, White N, et al. Comparison of optical and MR-based tracking. Magnetic resonance in medicine. 2015;74(3):894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Coutts GA, Gilderdale DJ, Chui M, Kasuboski L, DeSouza NM. Integrated and interactive position tracking and imaging of interventional tools and internal devices using small fiducial receiver coils. Magnetic resonance in medicine. 1998;40(6):908–913. [DOI] [PubMed] [Google Scholar]
- 77.Sengupta S, Tadanki S, Gore JC, Welch EB. Prospective real-time head motion correction using inductively coupled wireless NMR probes. Magnetic resonance in medicine. 2014;72(4):971–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Eschelbach M, Aghaeifar A, Bause J, et al. Comparison of prospective head motion correction with NMR field probes and an optical tracking system. Magnetic resonance in medicine. 2019;81(1):719–729. [DOI] [PubMed] [Google Scholar]
- 79.Dietrich BE, Brunner DO, Wilm BJ, et al. A field camera for MR sequence monitoring and system analysis. Magnetic resonance in medicine. 2016;75(4):1831–1840. [DOI] [PubMed] [Google Scholar]
- 80.Wezel J, Boer VO, van der Velden TA, et al. A comparison of navigators, snap-shot field monitoring, and probe-based field model training for correcting B0 -induced artifacts in T2*-weighted images at 7 T. Magn Reson Med. 2017;78(4):1373–1382. [DOI] [PubMed] [Google Scholar]
- 81.Liu J, de Zwart JA, van Gelderen P, Murphy-Boesch J, Duyn JH. Effect of head motion on MRI B0 field distribution. Magnetic resonance in medicine. 2018;80(6):2538–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Babayeva M, Kober T, Knowles B, et al. Accuracy and Precision of Head Motion Information in Multi-Channel Free Induction Decay Navigators for Magnetic Resonance Imaging. IEEE transactions on medical imaging. 2015;34(9):1879–1889. [DOI] [PubMed] [Google Scholar]
- 83.du Plessis L, Jacobson JL, Jacobson SW, et al. An in vivo 1H magnetic resonance spectroscopy study of the deep cerebellar nuclei in children with fetal alcohol spectrum disorders. Alcoholism, clinical and experimental research. 2014;38(5):1330–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mbugua KK, Holmes MJ, Cotton MF, et al. HIV-associated CD4+/CD8+ depletion in infancy is associated with neurometabolic reductions in the basal ganglia at age 5 years despite early antiretroviral therapy. AIDS (London, England). 2016;30(9):1353–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hess AT, Jacobson SW, Jacobson JL, Molteno CD, van der Kouwe AJ, Meintjes EM. A comparison of spectral quality in magnetic resonance spectroscopy data acquired with and without a novel EPI-navigated PRESS sequence in school-aged children with fetal alcohol spectrum disorders. Metabolic brain disease. 2014;29(2):323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hess AT, van der Kouwe AJ, Mbugua KK, Laughton B, Meintjes EM. Quality of 186 child brain spectra using motion and B0 shim navigated single voxel spectroscopy. Journal of magnetic resonance imaging : JMRI. 2014;40(4):958–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR in biomedicine. 1998;11(6):266–272. [DOI] [PubMed] [Google Scholar]
- 88.Andronesi OC, Kim GS, Gerstner E, et al. Detection of 2-Hydroxyglutarate in IDH-Mutated Glioma Patients by In Vivo Spectral-Editing and 2D Correlation Magnetic Resonance Spectroscopy. Science Translational Medicine. 2012;4(116):116ra114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Terpstra M, Henry PG, Gruetter R. Measurement of reduced glutathione (GSH) in human brain using LCModel analysis of difference-edited spectra. Magnetic resonance in medicine. 2003;50(1):19–23. [DOI] [PubMed] [Google Scholar]
- 90.Andronesi OC, Loebel F, Bogner W, et al. Treatment response assessment in IDH-mutant glioma patients by non-invasive 3D functional Spectroscopic Mapping of 2-Hydroxyglutarate. Clinical Cancer Research. 2016;22(7):1632–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nicholson K, Chan J, Macklin EA, et al. Pilot trial of inosine to elevate urate levels in amyotrophic lateral sclerosis. Annals of clinical and translational neurology. 2018;5(12):1522–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Heckova E, Povazan M, Strasser B, et al. Real-time Correction of Motion and Imager Instability Artifacts during 3D gamma-Aminobutyric Acid-edited MR Spectroscopic Imaging. Radiology. 2018;286(2):666–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jafari-Khouzani K, Loebel F, Bogner W, et al. Volumetric relationship between 2-Hydroxyglutarate and FLAIR hyperintensity has potential implications for radiotherapy planning of mutant IDH glioma patients. Neuro-oncology. 2016;18(11):1569–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Andronesi OC, Arrillaga-Romany IC, Ly KI, et al. Pharmacodynamics of mutant-IDH1 inhibitors in glioma patients probed by in vivo 3D MRS imaging of 2-hydroxyglutarate. Nature communications. 2018;9(1):1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11(2):85–95. [DOI] [PubMed] [Google Scholar]
- 96.Kantarci K, Petersen RC, Boeve BF, et al. 1H MR spectroscopy in common dementias. Neurology. 2004;63(8):1393–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Birur B, Kraguljac NV, Shelton RC, Lahti AC. Brain structure, function, and neurochemistry in schizophrenia and bipolar disorder—a systematic review of the magnetic resonance neuroimaging literature. npj Schizophrenia. 2017;3(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Boillat Y, Xin L, van der Zwaag W, Gruetter R. Metabolite concentration changes associated with positive and negative BOLD responses in the human visual cortex: A functional MRS study at 7 Tesla. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2019:271678×19831022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Arango N, Stockman J, Strasser B, et al. Dynamically Switched B0 Field Control for Separate Optimization of Tailored Volume Lipid Suppression and B0 Homogeneity for Brain Chemical Shift Imaging at 3T using Multi-Coil Shim Array. Paper presented at: ISMRM 26th Annual Meeting 2018; Paris. [Google Scholar]


