Abstract
Background
Although intensive blood pressure (BP)-lowering treatment reduces risk for cardiovascular disease, there are concerns that it might cause orthostatic hypotension (OH).
Purpose
To examine the effects of intensive BP-lowering treatment on OH in hypertensive adults. (PROSPERO: CRD42020153753)
Data Sources
MEDLINE, EMBASE, and Cochrane CENTRAL from inception through 7 October 2019, without language restrictions.
Study Selection
Randomized trials of BP pharmacologic treatment (more intensive BP goal or active agent) that involved more than 500 adults with hypertension or elevated BP and that were 6 months or longer in duration. Trial comparisons were groups assigned to either less intensive BP goals or placebo, and the outcome was measured OH, defined as a decrease of 20 mm Hg or more in systolic BP or 10 mm Hg or more in diastolic BP after changing position from seated to standing.
Data Extraction
Two investigators independently abstracted articles and rated risk of bias.
Data Synthesis
Five trials examined BP treatment goals, and 4 examined active agents versus placebo. Trials examining BP treatment goals included 18 466 participants with 127 882 follow-up visits. Trials were open-label, with minimal heterogeneity of effects across trials. Intensive BP treatment lowered risk for OH (odds ratio, 0.93 [95% CI, 0.86 to 0.99]). Effects did not differ by prerandomization OH (P for interaction = 0.80). In sensitivity analyses that included 4 additional placebo-controlled trials, overall and subgroup findings were unchanged.
Limitations
Assessments of OH were done while participants were seated (not supine) and did not include the first minute after standing. Data on falls and syncope were not available.
Conclusion
Intensive BP-lowering treatment decreases risk for OH. Orthostatic hypotension, before or in the setting of more intensive BP treatment, should not be viewed as a reason to avoid or de-escalate treatment for hypertension.
Primary Funding Source
National Heart, Lung, and Blood Institute, National Institutes of Health.
Orthostatic hypotension (OH) is an important risk factor for falls, syncope, and death. It is common among adults with hypertension (1–3) and is associated with multiple classes of antihypertensive medications (4). These associations with hypertension and its treatment have led to the widespread concern that OH is a complication of intensive blood pressure (BP) therapy, harming vulnerable groups (such as elderly persons) (5, 6). Thus, many view OH detected in the setting of BP treatment as a reason to relax BP treatment.
Our objectives were to determine the effects of BP treatment on OH and to use individual participant data from trials to assess for effect modification by demographic characteristics or related comorbidities. We hypothesized that contrary to widespread concerns, more intensive BP treatment would reduce patients’ risk for OH, regardless of their baseline characteristics.
Methods
We followed standard procedures for conducting reviews (7) and registered the protocol for this review at PROSPERO (CRD42020153753) on 19 September 2019 (Supplement 1, available at Annals.org, shows the submitted proposal and amendments).
Data Sources and Search Strategy
We searched (without language restrictions) MEDLINE (PubMed), EMBASE, and the Cochrane Central Register of Controlled Trials (CENTRAL) databases through 7 October 2019 by using a strategy collated by an experienced librarian (C.M.). Search terms focused on hypertension, BP treatment, OH, and randomized trials (Supplement 2, available at Annals.org). Duplicate abstracts were removed in Microsoft EndNote and imported into Covidence for screening. Two investigators (J.L.B., S.P.J.) independently reviewed the abstracts. Discrepancies were adjudicated by consensus with a third investigator (A.I.).
We also reviewed the bibliography of a recent meta-analysis of trials of intensive BP control and cardiovascular disease (CVD) (8) and attempted to contact investigators of each to ask whether or not the trial obtained standing BP measurements. One trial of BP treatment goal included in our primary meta-analysis was identified through this approach (Supplement Table 1, available at Annals.org) (9).
Study Selection
Prespecified inclusion criteria were 1) population: 500 or more adults with hypertension or elevated BP; 2) intervention: randomized trials of BP pharmacologic treatment (BP goal or active agent) that lasted 6 months or longer; 3) comparison: at least 2 BP goals (one less than the other) or placebo; and 4) outcome: OH measured during postrandomization study visits. Trials of pregnant women or children, observational studies, and studies with self-reported or claim-based OH were excluded. We made an a priori decision to include trials comparing 2 treatment goals (a more intensive goal versus a less intensive goal) in our primary pooled analysis and to include placebo-controlled trials in a secondary sensitivity analysis.
Data Extraction and Risk of Bias Assessment
Two reviewers (J.R.H. and A.I.) independently extracted information from published papers about publication characteristics, trial characteristics, and OH measurement details, which was confirmed by a third reviewer (S.P.J.). One reviewer (J.R.H.) assessed each trial’s risk of bias (confirmed by a second reviewer [S.P.J.]), considering the following factors: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and reporting bias. Risk of bias was characterized as low, high, or uncertain on the basis of published descriptions of trial design. Disagreements were adjudicated by a third reviewer (K.J.M.).
Data Requests
We requested the following data from investigators or data repositories: age, sex (female or male), race (Black or non-Black), prerandomization seated and standing systolic BP (SBP) and diastolic BP (DBP), baseline creatinine concentration or estimated glomerular filtration rate (eGFR) or chronic kidney disease status, body mass index, diabetes status, prior stroke, and history of any CVD. Obesity was defined as a body mass index (BMI) of 30 kg/m2 or greater. Stage 3 chronic kidney disease was defined as an eGFR less than 60 mL/min per 1.73 m2 on the basis of the CKD-EPI equation (10). Definitions of diabetes, stroke, and CVD varied among studies (Supplement Tables 2 and 3, available at Annals.org).
Outcomes
Orthostatic hypotension was derived by taking the difference between standing and seated BP. Seated BP was based on the convention reported by each trial (1 measurement or the average of 2 to 3 measurements, sometimes excluding the first measurement). Standing BP was also based on trial conventions, which included various numbers and timing of measurement (details are summarized in the Results section). Orthostatic hypotension was defined by using the consensus definition as a decrease in SBP of at least 20 mm Hg or a decrease in DBP of at least 10 mm Hg (11). Baseline OH was based on the seated and standing BP measured at the visit just before randomization.
Secondary outcomes were postural change in SBP or DBP (standing minus seated BP), systolic OH (a decrease in SBP ≥20 mm Hg), diastolic OH (a decrease in DBP ≥10 mm Hg), the occurrence of low standing SBP or DBP (standing SBP ≤110 mm Hg or DBP ≤60 mm Hg), and the occurrence of high standing SBP or DBP (standing SBP ≥180 mm Hg or DBP ≥100 mm Hg). These thresholds were based on the fifth and 95th percentiles for the pooled, postrandomization SBP and DBP of all 9 trials, respectively.
Statistical Analysis
Details of the analytical approach are found in Supplement 3 (available at Annals.org). Data from all trials and all prerandomization and postrandomization follow-up visits were combined into a single analytic data set before pooled analyses. To address outliers, analyses were restricted to BP, BMI, and eGFR measures between the 0.01st and 99.99th percentiles of all measurements (baseline and follow-up) from all 9 trials. We used means and proportions to characterize population characteristics according to each study.
Given that OH is a recurrent clinical phenomenon and that surveillance for OH only occurred during scheduled follow-up visits, we used a generalized estimating equation (GEE) to account for repeated within-person measurement (12, 13). We used this approach in prior studies of OH (14–16). This model is advantageous because it allows for the use of the Huber–White sandwich estimator of variance, which can generate valid SEs even if the correlations within group are not as hypothesized (12).
Mean (SE) seated and standing BP as well as the mean difference were determined by low and high BP treatment goal for each study and in pooled analyses overall (GEE with normal family, identity link). We also used kernel density plots to examine the distribution of SBP and DBP according to the prerandomization visit, follow-up visits among participants assigned to a more intensive BP treatment goal, and follow-up visits among those assigned to a standard BP treatment goal.
We examined the effect of the intensive compared with the standard treatment goals on the odds of OH during follow-up visits, using GEE (binomial family, logit link) for the 5 BP treatment goal trials and the 4 placebo-controlled trials, individually and overall. Models were repeated for change in postural SBP or DBP (GEE with normal family, identity link) and low and high SBP or DBP (GEE with binomial family, logit link). We also performed models examining OH in the following subgroups: age (≤75 or >75 years), sex (male or female), race (non-Black or Black), prerandomization seated SBP of 140 mm Hg or greater or DBP of 90 mm Hg or greater, diabetes (no or yes), prior stroke (no or yes), eGFR (<60 or ≥60 mL/min per 1.73 m2), BMI (<30 or ≥30 kg/m2), history of CVD (no or yes), standing SBP before randomization (≥110 or <110 mm Hg), and prerandomization OH (no or yes). Interaction terms were used to make comparisons across strata.
Finally, we examined the effects of treatment on OH according to follow-up window. These follow-up windows for visits were categorized as prerandomization, less than 1 month, 1 month or longer, more than 1 month to 6 months or less, more than 6 months to 12 months or less, more than 12 months to 24 months or less, more than 24 months to 36 months or less, more than 36 months to 48 months or less, and more than 48 months. We determined the association of time interval with OH in strata of treatment (GEE with binomial family, logit link), using interaction terms to assess for differences at different time windows. We also examined time trends within strata of treatment, using a continuous variable defined by the median follow-up days in each time window.
All primary analyses were performed among the 5 trials, comparing 2 BP treatment goals. In secondary analyses, we included 4 placebo-controlled trials (for a total of 9 trials). Sensitivity analyses included removing SPRINT (Systolic Blood Pressure Intervention Trial, which was the trial with the largest effect on OH) and pooling findings by use of Hawksley random-zero sphygmomanometers. We assessed trial heterogeneity using the I2 statistic, which provides the proportion of total variation in study estimates that is due to heterogeneity (17). Statistical analyses were performed by using Stata, version 15.1.
Role of the Funding Source
This review and analysis was funded by the National Institutes of Health (grant 7K23HL135273). The funder did not play any role in the design, conduct, and analysis of the study or in the decision to submit the manuscript for publication.
RESULTS
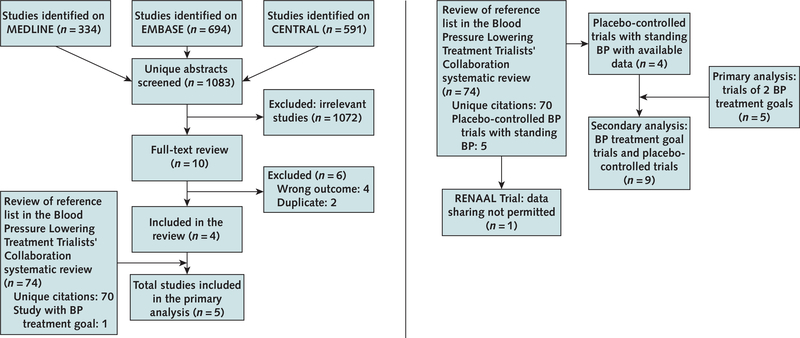
Details of our systematic review are shown in the Appendix Figure (available at Annals.org). Of 1619 references from the database searches, we screened 1083 unique records, identifying 4 trials of different BP treatment goals that measured OH. One additional trial (9) with OH data was identified from the Blood Pressure Lowering Treatment Trialists’ Collaboration systematic review (8), for a total of 5 trials (9, 18 –21). We also identified 4 eligible placebo-controlled BP treatment trials that measured OH (22–25).
Appendix Figure. Study search and selection.
Left. Search process for trials comparing BP treatment goals. These 5 trials were included in the primary analysis. Right. Results of contact of investigators of BP trials, leading to the identification of the 4 placebo-controlled trials included in secondary analyses. BP = blood pressure.
Trial size ranged from 1090 to 9361 participants in our primary grouping of trials (Table 1). Intensive BP goal varied, with 2 trials targeting a SBP less than 120 mm Hg (versus <140 mm Hg), 1 trial targeting a SBP less than 130 mm Hg (versus 130 to 149 mm Hg), 1 trial targeting a mean arterial pressure of 92 mm Hg or less (versus 102 to 107 mm Hg), and 1 trial targeting a SBP less than 150 mm Hg and DBP less than 85 mm Hg (versus <180/<105 mm Hg). Trial duration varied from a median of 3.3 years to 8.4 years. Among the primary studies, OH was measured 1 to 3 times after standing, at time points ranging from immediately after standing to after 2 minutes 45 seconds of standing. Frequency of measurements ranged from monthly to yearly.
Table 1.
Characteristics of Included Trials
| Inclusion Status | Study, Year (Reference) | Participants, n | Study Population | Standard BP Treatment (Goal or Placebo), mm Hg | Intensive BP Treatment (Goal or Agent), mm Hg |
|---|---|---|---|---|---|
| Primary: BP treatment goal | AASK, 2002 (19) | 1094 | African American patients aged 18–70 y with hypertensive renal disease, without diabetes Seated DBP ≤95 |
MAP 102–107 | MAP ≤92 |
| Primary: BP treatment goal | ACCORD BP, 2010 (20) | 4733 | Patients aged ≥40 y with diabetes and CVD or aged ≥55 y with diabetes with CV risk factors Seated SBP 130–180 mm Hg* |
SBP <140 | SBP <120 |
| Primary: BP treatment goal | SPRINT, 2015 (18) | 9361 | Patients aged ≥50 y at high risk for CVD but who do not have stroke or DM Seated SBP 130–180 mm Hg* and standing SBP < 110 mm Hg |
SBP <140 | SBP <120 |
| Primary: BP treatment goal | SPS3, 2013 (21) | 3020 | Patients aged ≥30 y who had had a recent lacunar stroke Seated SBP ≥140 mm Hg or seated DBP ≥90 mm Hg and diagnosis of hypertension |
SBP 130–149 | SBP <130 |
| Primary: BP treatment goal | UKPDS, 1998 (9) | 1148 | Patients aged 25–65 y with diabetes and hypertension Seated SBP ≥150 mm Hg or seated DBP ≥85 mm Hg (≥160/≥85 mm Hg if receiving hypertension medication) |
BP <180/105 | BP <150/85 |
| Secondary: placebo-controlled | HYVET, 2008 (22) | 3845 | Patients aged ≥80 y with hypertension Seated SBP 160–199 mm Hg, standing SBP ≥140 mm Hg, seated DBP 90–109 mm Hg‡ |
Placebo | <150/<80 |
| Secondary: placebo-controlled | SHEP, 1991 (23) | 4736 | Patients aged ≥60 y with isolated systolic hypertension Seated SBP 160–219 mm Hg§, standing SBP ≥140 mm Hg, seated DBP <90 mm Hg |
Placebo | SBP <160 if baseline SBP was >180 20 mm Hg reduction if baseline SBP was 160–179 |
| Secondary: placebo-controlled | Syst-Eur, 1997 (24) | 4695 | Patients aged ≥60 y with isolated systolic hypertension Seated SBP <220 mm Hg, standing SBP ≥140 mm Hg, seated DBP <95 mm Hg |
Placebo | SBP <150 (a reduction ≥20 mm Hg) |
| Secondary: placebo-controlled | TOMHS, 1993 (25) | 902 | Patients aged 45–69 y with mild hypertension DBP 90–99 mm Hg |
Nutritional-hygienic intervention and placebo | Nutritional-hygienic intervention and 1 of 5 arms∥: acebutolol, amlodipine, chlorthalidone, doxazosin, or enalapril |
| Range, 3–6.4 | First line: 1 of 3 agents: metoprolol (50–200 mg/d), ramipril (2.5–10 mg/d), or amlodipine (5–10 mg/d) | Hawksley random-zero sphygmomanometer | Mean of the last 2 of 3 measurements | 1 measure obtained after2 min 45 s of standing | Pre-randomization and follow-up visits (monthly for the first 6 mo and then every 2 mo forthe trial duration) |
| Median, 4.7 | First line: a combination of a diuretic and either an ACE inhibitor or a β-blocker | Omron HEM-907 | Mean of 3 measurements | Mean of 3 measures obtained 1 min after standing, each measure separated by 1 min | Baseline for newly enrolled, 12 mo, 48 mo, and exit visits (in participants with no study events) |
| Median, 3.3 | First line: thiazide-type diuretic encouraged, loop diuretics (advanced CKD), and β-adrenergic blockers (coronary artery disease) Chlorthalidone was encouraged as the primary thiazide-type diuretic, and amlodipine as the preferred calcium-channel blocker |
Omron HEM-907 | Mean of 3 measurements | 1 measurement obtained 1 min after standing | Baseline,1, 6, 12, 24, 36, 48, 60-mo, and exit visit (in participants with no study events) |
| Mean, 3.7 (SD, 2.0) | Clinician directed the antihypertensive regimen | Colin Press-Mate BP-8800C | Mean of 3 measurements | 1 measurement obtained 2 min after standing | Baseline and quarterly during follow-up |
| Median, 8.4 | First line: captopril (25 mg/d to 50 mg twice daily) or atenolol (50–100 mg/d) | Copal UA-251, Takeda UA-751, or Hawksley Random Zero | Mean of the last 3 of 4 measurements† | 1 measurement obtained 1 minute after standing | Baseline and every 3 y of follow-up |
| Median, 1.8 | First line: indapamide (sustained release, 1.5 mg) or matching placebo alone | Mercury sphygmomanometer or a validated automated device | Mean of 2 measurements | Mean of 2 measurements obtained after 2 min of standing | Pre-randomization and yearly |
| Median, 4.0 | Step 1: chlorthalidone, 12.5–25 mg/d Step 2: atenolol, 25–50 mg/d, or reserpine, 0.05–0.1 mg/d |
Hawksley random zero sphygmomanometer | Mean of 2 measurements | 2 measurements obtained after 1 and 3 min of standing | Baseline and every 1–2 mo during follow-up |
| Median, 2.0 | Nitrendipine (10 mg/d to 20 mg twice daily), combined with or replaced by enalapril (5–20 mg/d), hydrochlorothiazide (12.5–25 mg/d), or both. Goal to reduce the sitting SBP by ≥20 mm Hg to <150 mm Hg Placebos were identical to the study drugs, with a similar schedule |
Unspecified, conventional sphygmomanometers | Mean of 2 measurements | 2 measurements obtained after 2 min of standing | Run-in and every3 mo during follow-up |
| Median, 4.4 | Chlorthalidone, 15–30 mg/d Acebutolol, 400–800 mg/d Doxazosin mesylate, 2–4 mg/d Amlodipine maleate, 5–10 mg/d Enalapril maleate, 5–10 mg/d Doses were doubled or chlorthalidone/enalapril was added if DBP was ≥95 mm Hg (3 successive visits or ≥105 mm Hg during a single visit) Participants assigned to the placebo group were given chlorthalidone if BP was not controlled with nutritional-hygienic intervention alone |
Hawksley random-zero sphygmomanometer | Mean of 2 measurements | 1 measurement obtained 2 min after standing | Pre-randomization screening visit and every 3 mo during follow-up |
AASK = African American Study of Kidney Disease and Hypertension; ACCORD BP = Action to Control Cardiovascular Risk in Diabetes: Blood Pressure; ACE = angiotensin-converting enzyme; BP = blood pressure; CKD = chronic kidney disease; CV = cardiovascular; CVD = cardiovascular disease; DBP = diastolic blood pressure; DM = diabetes mellitus; MAP = mean arterial pressure; SPRINT = Systolic Blood Pressure Intervention Trial; SPS3 = Secondary Prevention of Small Subcortical Strokes; UKPDS = U.K. Prospective Diabetes Study; HYVET = Hypertension in the Very Elderly Trial; SHEP = Systolic Hypertension in the Elderly Program; Syst-Eur = Systolic Hypertension in Europe; TOMHS = Treatment of Mild Hypertension Study; SBP = systolic blood pressure.
Range varied by baseline antihypertension use: SBP of 130 to 160 mm Hg while receiving 0 to 3 antihypertensive agents, SBP of 161 to 170 while receiving 0 to 2 antihypertensive agents, or SBP of 171 to 180 mm Hg while receiving 0 or 1 antihypertensive agents.
Of 4 measures, the first was discarded and the mean of the next 3 consecutive readings with a coefficient of variation <15% was used in the study.
The average seated DBP was later changed to <110 mm Hg to be able to recruit participants with isolated systolic hypertension.
Range was 130 to 219 mm Hg and DBP <85 mm Hg if receiving antihypertensive medications.
These arms were combined to represent the “intensive blood pressure treatment group” in our extended pooled meta-analysis, which included the 4 placebo-controlled studies.
Participants (N = 18 466) in the primary 5 trials had a mean age of 64.5 years (SD, 9.9) and 38.9% were women (Table 2), and they had a total of 127 882 follow-up visits. The overall mean baseline seated SBP was 141.4 mm Hg (SD, 17.6) (n = 18 431), and mean DBP was 79.1 mm Hg (SD, 12.2) (n = 18 437). Of 14 846 participants with baseline OH measurements, 8.5% had OH in the visit just before randomization. Characteristics of participants in the 4 placebo-controlled trials are shown in Supplement Table 4 (available at Annals.org).
Table 2.
Population Characteristics of the 5 Blood Pressure Treatment Goal Trials Included in the Primary Meta-analysis*
| Characteristic | AASK (19) |
ACCORD BP (20) |
SPRINT (18) |
SPS3 (21) |
UKPDS (9) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Participants, n | Value | Participants, n | Value | Participants, n | Value | Participants, n | Value | Participants, n | Value | |
| Mean age (SD), y | 1090 | 54.6 (10.7) | 4196 | 62.6 (6.6) | 9221 | 67.9 (9.4) | 2887 | 62.8 (10.7) | 1072 | 56.8 (8.0) |
| Age >75y, % | 1090 | 0.0 | 4196 | 4.8 | 9221 | 24.9 | 2887 | 13.8 | 1072 | 0.0 |
| Women, % | 1090 | 38.9 | 4196 | 46.4 | 9221 | 35.5 | 2887 | 36.6 | 1072 | 45.3 |
| Black, % | 1090 | 100.0 | 4196 | 22.3 | 9221 | 31.3 | 2887 | 16.0 | 1072 | 7.6 |
| Prerandomization seated SBP (SD), mm Hg | 1087 | 143.8 (22.3) | 4195 | 139.1 (15.7) | 9219 | 139.7 (15.6) | 2886 | 143.1 (18.8) | 1044 | 158.1 (21.9) |
| Prerandomization seated DBP (SD), mm Hg | 1088 | 88.7 (13.5) | 4195 | 75.9 (10.3) | 9220 | 78.1 (11.9) | 2887 | 78.4 (10.6) | 1047 | 92.8 (11.2) |
| Prerandomization standing SBP (SD), mm Hg | 1087 | 143.2 (23.4) | 1218 | 139.3 (16.8) | 9204 | 140.3 (17.9) | 2294 | 144.3 (22.4) | 1047 | 155.0 (22.0) |
| Prerandomization standing DBP (SD), mm Hg | 1086 | 91.5 (14.5) | 1218 | 79.1 (11.2) | 9206 | 81.8 (12.9) | 2298 | 81.1 (12.9) | 1047 | 93.7 (10.9) |
| eGFR (SD), mL/min per 1.73 m2† | 1088 | 42.4 (13.8) | 4181 | 84.6 (17.6) | 9184 | 72.4 (20.0) | 2520 | 80.7 (18.9) | 0 | Not released |
| Stage 3 CKD (SD), %‡ | 1088 | 89.5 | 4181 | 10.1 | 9184 | 27.1 | 2520 | 15.2 | 1045 | 25.3 |
| Body mass index (SD), kg/m2 | 1090 | 30.6 (6.6) | 4196 | 32.1 (5.4) | 9158 | 29.9 (5.8) | 2879 | 28.9 (5.6) | 1042 | 29.6 (5.5) |
| Obese, % | 1090 | 46.7 | 4196 | 61.3 | 9158 | 43.0 | 2879 | 35.0 | 1042 | 39.6 |
| Diabetes, % | 1090 | 6.7 | 4196 | 100.0 | 9220 | 1.6 | 2887 | 33.2 | 1072 | 100.0 |
| Prior stroke, % | NR | NR | 9218 | 0.5 | 2887 | 100.0 | 1072 | 0.3 | ||
| History of CVD, % | NR | 4196 | 32.9 | 9221 | 20.0 | 2887 | 10.6 | 1072 | 0.4 | |
| Standing SBP <110 mm Hg before randomization, % | 1087 | 5.8 | 1218 | 3.6 | 9204 | 3.2 | 2294 | 4.1 | 1047 | 0.6 |
| Pre-randomization OH, % | 1081 | 9.1 | 1218 | 7.5 | 9203 | 7.2 | 2293 | 10.2 | 1044 | 16.6 |
AASK = African American Study of Kidney Disease and Hypertension; ACCORD BP = Action to Control Cardiovascular Risk in Diabetes: Blood Pressure; CKD = chronic kidney disease; CVD = cardiovascular disease; DBP = diastolic blood pressure; eGFR = estimated glomerular filtration rate; SPRINT = Systolic Blood Pressure Intervention Trial; SPS3 = Secondary Prevention of Small Subcortical Strokes; UKPDS = U.K. Prospective Diabetes Study; NR = not reported; OH = orthostatic hypotension; SBP = systolic blood pressure.
This sample was restricted to participants with a follow-up visit. The number cited in the table is not always equal to the number of participants in the original trial who underwent randomization.
Based on the CKD-EPI equation.
Defined as eGFR <60 mL/min per 1.73 m2.
Trials had a low risk of bias with regard to random sequence generation, allocation concealment, and outcome reporting and a higher risk for performance and detection bias, given that study personnel were aware of the treatment assignment and performed the OH assessment (Supplement Table 5, available at Annals.org). One of the 5 trials had unclear evidence of attrition bias, given potential imbalances in follow-up between treatment groups (21).
Pooled Effects on Blood Pressure
There was minimal heterogeneity (I2 = 0.0%; P = 0.84) across the 5 primary studies. Among the primary group of studies, the mean postural increase in SBP was 1.82 mm Hg (95% CI, 1.65 to 2.00 mm Hg) in 63 630 follow-up visits among participants assigned to a standard BP goal and 1.84 mm Hg (CI, 1.67 to 2.01 mm Hg) in 64 252 follow-up visits among those assigned an intensive BP treatment goal (Table 3). Whereas the distributions of seated and standing SBP shifted to the left among those assigned the intensive BP treatment goal, the distributions of postural change were nearly identical among baseline, standard BP goal, and intensive BP treatment goal (Supplement Figure 1, available at Annals.org). Findings were similar in secondary pooled analyses of the 5 BP treatment goal trials and the 4 placebo-controlled trials and in analyses of DBP (Supplement Figure 2 and Supplement Table 6, available at Annals.org).
Table 3.
Effect of Intensive Therapy on Seated, Standing, and Orthostatic SBP
| Trial |
Standard Goal (or Placebo) |
Low Goal (or Active Treatment) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BP treatment goal | Participants, n | Visits, n* | Mean Seated SBP (SD), mm Hg | Mean Standing SBP (SD), mm Hg | Difference (95% CI) | Participants, n | Visits, n* | Mean Seated SBP (SD), mm Hg | Mean Standing SBP (SD), mm Hg | Difference (95% CI) |
| AASK (19) | 553 | 23 986 | 142.1 (0.45) | 141.2 (0.50) | −0.87 (−1.42 to −0.32) | 537 | 24 785 | 131.7 (0.52) | 130.9 (0.54) | −0.80 (−1.33 to −0.27) |
| ACCORD BP (20) | 2119 | 3590 | 133.9 (0.26) | 136.7 (0.30) | 2.81 (2.40 to 3.23) | 2077 | 3572 | 120.1 (0.27) | 122.9 (0.30) | 2.79 (2.38 to 3.21) |
| SPRINT (18) | 4606 | 25 482 | 135.5 (0.12) | 137.2 (0.16) | 1.73 (1.49 to 1.96) | 4615 | 25 745 | 123.3 (0.13) | 125.3 (0.16) | 2.00 (1.77 to 2.22) |
| SPS3 (21) | 1443 | 9673 | 137.1 (0.27) | 140.2 (0.32) | 3.18 (2.73 to 3.62) | 1444 | 8420 | 129.9 (0.28) | 133.1 (0.33) | 3.24 (2.78 to 3.70) |
| UKPDS (9) | 359 | 899 | 160.7 (0.95) | 159.2 (0.99) | −1.64 (−2.52 to −0.76) | 713 | 1730 | 150.8 (0.65) | 149.0 (0.66) | −1.79 (−2.51 to −1.07) |
| Pooled effect of 5 treatment goal trials | 9080 | 63 630 | 136.9 (0.11) | 138.7 (0.13) | 1.82 (1.65 to 2.00) | 9386 | 64 252 | 126.3 (0.13) | 128.1 (0.14) | 1.84 (1.67 to 2.01) |
| Placebo-controlled | ||||||||||
| HYVET (22) | 1171 | 2219 | 159.6 (0.50) | 155.7 (0.49) | −3.91 (−4.25 to −3.57) | 1233 | 2513 | 145.3 (0.39) | 141.8 (0.40) | −3.57 (−3.88 to −3.27) |
| SHEP (23) | 2345 | 46 222 | 155.5 (0.26) | 154.2 (0.27) | −1.22 (−1.48 to −0.97) | 2336 | 43 134 | 142.4 (0.23) | 140.8 (0.25) | −1.66 (−1.92 to −1.40) |
| Syst-Eur (24) | 2244 | 18 377 | 162.7 (0.30) | 160.2 (0.31) | −2.41 (−2.63 to −2.19) | 2351 | 20 952 | 152.3 (0.22) | 149.8 (0.24) | −2.49 (−2.70 to −2.28) |
| TOMHS (25) | 234 | 3526 | 132.0 (0.76) | 130.3 (0.77) | −1.59 (−2.13 to −1.06) | 663 | 10 273 | 124.3 (0.43) | 122.1 (0.43) | −2.17 (−2.47 to −1.88) |
| Pooled effect of all 9 trials | 15 074 | 133 974 | 145.5 (0.14) | 145.7 (0.13) | 0.18 (0.05 to 0.30) | 15 969 | 141 124 | 134.2 (0.13) | 134.2 (0.13) | −0.00 (−0.12 to 0.11) |
AASK = African American Study of Kidney Disease and Hypertension; ACCORD BP = Action to Control Cardiovascular Risk in Diabetes: Blood Pressure; SPRINT = Systolic Blood Pressure Intervention Trial; SPS3 = Secondary Prevention of Small Subcortical Strokes; UKPDS = U.K. Prospective Diabetes Study; HYVET = Hypertension in the Very Elderly Trial; SHEP = Systolic Hypertension in the Elderly Program; Syst-Eur = Systolic Hypertension in Europe; TOMHS = Treatment of Mild Hypertension Study; SBP = systolic blood pressure.
Postrandomization follow-up visits.
Orthostatic Hypotension
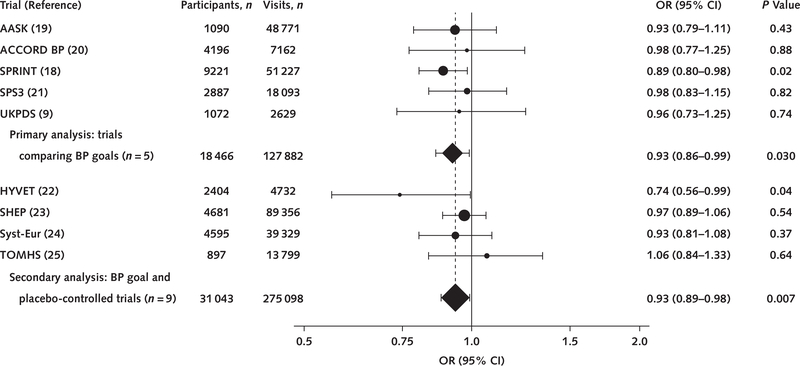
All trials comparing different BP treatment goals demonstrated reductions in the odds of OH, with SPRINT demonstrating the strongest relationship (odds ratio [OR], 0.89 [CI, 0.80 to 0.98]) (Figure). In the pooled analysis of the primary 5 trials of BP treatment goals, assignment to a more intensive versus standard BP goal lowered the odds of OH (OR, 0.93 [CI, 0.86 to 0.99]). Together, there was no effect among placebo-controlled BP trials on OH (OR, 0.95 [CI, 0.88 to 1.02]; P = 0.13) (Supplement Table 7, available at Annals.org). When these placebo-controlled trials were pooled with the 5 trials of different BP treatment goals, more intensive BP treatment was still associated with a significantly lower risk for OH (OR, 0.93 [CI, 0.89 to 0.98]).
Figure. Effects of BP treatment on risk for orthostatic hypotension, by study.
Pooled effects are organized by the 5 primary studies (primary effect) and with the 4 additional trials identified through our search. The size of each point estimate is weighted by the number of follow-up visits with orthostatic hypotension assessments. AASK = African American Study of Kidney Disease and Hypertension; ACCORD BP = Action to Control Cardiovascular Risk in Diabetes: Blood Pressure; BP = blood pressure; SPRINT = Systolic Blood Pressure Intervention Trial; SPS3 = Secondary Prevention of Small Subcortical Strokes; UKPDS = U.K. Prospective Diabetes Study; HYVET = Hypertension in the Very Elderly Trial; OR = odds ratio; SHEP = Systolic Hypertension in the Elderly Program; Syst-Eur = Systolic Hypertension in Europe; TOMHS = Treatment of Mild Hypertension Study.
We also examined the effects of more intensive BP treatment on postural change in BP, systolic OH, diastolic OH, low standing BP (≤110/≤60 mm Hg), and high standing BP (≥180/≥100 mm Hg) (Supplement Tables 8 and 9, available at Annals.org). More intensive BP treatment was not associated with postural change in BP, systolic OH, or diastolic OH, but did increase the risk for low standing BP and decrease the risk for high standing BP.
Stratified Analyses
There was no difference in the primary pooled effect of more intensive versus standard BP treatment on OH on the basis of age, sex, or Black race (Table 4).
Table 4.
Subgroup Analyses from the 5 Primary Blood Pressure Treatment Goal Trials: Effects of More Intensive Treatment Versus Standard Treatment on OH
| Characteristic | Participants, n | Visits, n* | OR (95% CI) | P Value | P Value for Interaction |
|---|---|---|---|---|---|
| Age | |||||
| ≤75 y | 15 571 | 112 897 | 0.96 (0.89–1.04) | 0.34 | 0.70 |
| >75 y | 2895 | 14985 | 0.92 (0.79–1.09) | 0.35 | |
| Sex | |||||
| Male | 11 278 | 80 568 | 0.97 (0.88–1.06) | 0.48 | 0.71 |
| Female | 7188 | 47 314 | 0.94 (0.85–1.04) | 0.25 | |
| Race | |||||
| Non-Black | 13 015 | 58 515 | 1.00 (0.91–1.09) | 0.94 | 0.068 |
| Black | 5451 | 69 367 | 0.87 (0.78–0.98) | 0.02 | |
| Pre-randomization seated SBP ≥140 mm Hg or DBP ≥90 mm Hg† | |||||
| No | 3635 | 22 187 | 0.96 (0.81–1.14) | 0.67 | 0.99 |
| Yes | 14 804 | 105 631 | 0.96 (0.89–1.03) | 0.26 | |
| Diabetes | |||||
| No | 12 018 | 107 648 | 0.90 (0.83–0.98) | 0.01 | 0.015 |
| Yes | 6447 | 20 231 | 1.10 (0.96–1.27) | 0.17 | |
| Prior stroke | |||||
| No | 10 240 | 53 577 | 0.95 (0.86–1.04) | 0.25 | 0.76 |
| Yes | 2937 | 18 358 | 0.98 (0.83–1.15) | 0.77 | |
| Stage 3 CKD‡ | |||||
| No | 13 483 | 64 980 | 0.95 (0.87–1.04) | 0.27 | 0.65 |
| Yes | 4535 | 60 718 | 0.97 (0.87–1.08) | 0.59 | |
| Body mass index | |||||
| <30 kg/m2 | 9921 | 69 848 | 0.97 (0.88–1.06) | 0.49 | 0.69 |
| ≥30 kg/m2 | 8444 | 57 579 | 0.94 (0.85–1.05) | 0.27 | |
| History of CVD | |||||
| No | 13 837 | 64 543 | 0.96 (0.88–1.05) | 0.35 | 0.98 |
| Yes | 3539 | 14 568 | 0.96 (0.81–1.14) | 0.65 | |
| Standing BP before randomization | |||||
| ≥110 mm Hg | 14 352 | 113 247 | 0.96 (0.89–1.04) | 0.35 | 0.02 |
| <110 mm Hg | 498 | 4915 | 0.66 (0.48–0.91) | 0.010 | |
| Prerandomization OH | |||||
| No | 13 577 | 107 395 | 0.94 (0.87–1.02) | 0.11 | 0.75 |
| Yes | 1262 | 10 462 | 0.91 (0.77–1.09) | 0.33 |
CKD = chronic kidney disease; CVD = cardiovascular disease; DBP = diastolic blood pressure; OH = orthostatic hypotension; OR = odds ratio; SBP = systolic blood pressure.
Postrandomization follow-up visits.
Based on the visit in closest temporal proximity before randomization.
Defined as an estimated glomerular filtration rate < 60 mL/min per 1.73 m2 based on the CKD-EPI equation.
Adults without diabetes had a lower odds of OH (OR, 0.90 [CI, 0.83 to 0.98]) with an intensive versus standard BP goal, whereas intensive treatment in adults with diabetes demonstrated a nonsignificant higher risk for OH (OR, 1.10 [CI, 0.96 to 1.27]; P for interaction = 0.015). Effects of intensive versus standard BP treatment goal did not differ by prerandomized seated SBP of 140 mm Hg or greater or DBP of 90 mm Hg or greater, history of stroke, eGFR less than 60 mL/min per 1.73 m2, obesity status, or history of CVD. There was evidence that the odds of OH were lower among adults with a standing SBP less than 110 mm Hg at baseline (OR, 0.66 [CI, 0.48 to 0.91]; P for interaction = 0.02). Effects did not differ by prerandomization OH (P for interaction = 0.80).
Time Effects
Follow-up time was associated with a lower odds of OH, regardless of being assigned to a standard (P for trend < 0.001) or intensive BP treatment goal (P for trend < 0.001) (Supplement Figure 3 and Supplement Table 10, available at Annals.org). There was no consistent evidence that the temporal association between trial follow-up and OH differed by treatment assignment, even after the 4 placebo-controlled trials were incorporated (Supplement Figures 4 and 5 and Supplement Table 11, available at Annals.org).
Sensitivity Analyses
Effects were nearly identical after the 4 placebo-controlled trials were included (Supplement Table 12, available at Annals.org) and were attenuated after SPRINT (the trial with the largest effect on OH) was excluded (Supplement Table 13, available at Annals.org). Effects were attenuated in the subgroup of trials that measured BP with a Hawksley random-zero sphygmomanometer (Supplement Tables 14 and 15, available at Annals.org).
DISCUSSION
In this individual participant data meta-analysis of 18 466 adults with hypertension enrolled in randomized trials, in which sitting and standing BPs were measured to assess for OH, a lower BP treatment goal decreased the odds of OH. The odds of OH were even lower in adults without diabetes and in adults with low standing SBP before treatment initiation but were not significantly altered by age, sex, or Black race. These findings confirm that a more intensive BP treatment regimen does not increase risk for OH in most patients.
Over the past several years, secondary analyses of trials have suggested that more intensive BP treatment lowers risk for OH, although results of individual trials were not always statistically significant (14 –16, 18, 26). To our knowledge (based on English-language literature searches through August 2020), our study is the first attempt to systematically pool these trials. Our pooled effects are striking in that they demonstrate that mean postural change is conserved with more intensive BP treatment. This modification in the distribution of postural change at the population level results in a lower (not higher) risk for OH. Of note, these findings were even greater among persons with a standing SBP less than 110 mm Hg before randomization, a group excluded from SPRINT (18). It is possible that intensive treatment improves BP regulation upon standing by improving baroreflex function (27) and diastolic filling while reducing left ventricular hypertrophy and arterial stiffness (28), but confirmation of these mechanisms goes beyond the scope of our study.
Our findings may seem at odds with clinical experience because many clinicians have observed hypotension, syncope, and falls while treating their hypertensive patients (29). This observation may be due to the acute effects of antihypertensive therapy (30, 31) before baroreflex sensitivity, vascular stiffness, ventricular diastolic filling, and other blood pressure regulatory mechanisms have had a chance to adapt. The long-term treatment of hypertension has been shown to improve many of these mechanisms (28, 32), which may explain the beneficial effect of treatment shown here. It is also possible that the medication regimens or the titration procedures in most of these trials may have different effects on OH incidence than other medications or regimens used in clinical practice. This hypothesis is illustrated by TOMHS (Treatment of Mild Hypertension Study), the only trial suggesting some increase in OH, in that it included an α-blocker among its active treatments, which is known to cause more OH or orthostatic lightheadedness (25). Finally, we noted that intensive treatment did increase risk for low standing BP. Whether falling below low standing BP thresholds predict adverse events independently of orthostatic changes in BP is an important topic for subsequent research.
Our study has limitations. First, we only identified data from 5 clinical trials for our primary analysis. Although we attempted to contact investigators of other trials that compared BP treatment goals, many could not be reached and publications of most of these trials did not describe whether standing OH was assessed. As a result, it is possible that we missed some hypertension trials that measured OH. Second, trials differed in their interventions, duration and frequency of follow-up, and study populations. Nevertheless, statistical heterogeneity was minimal despite these differences, which attests to the robustness of our findings. Third, subgroups based on medical history involved different definitions across studies, which could result in misclassification, introducing a conservative bias in subgroup analyses. Fourth, we did not have consistent access to orthostatic symptoms or notable sequelae of OH (such as syncope and falls). Fifth, most studies assessed OH based on measurements occurring after 1 minute of standing (versus within 1 minute) and based on transitions from seated to standing positions (versus transitions from supine to standing). Such measurements may miss the important first minute after standing (1) and blunt the effects of gravity, reducing the opportunity to detect OH (33). Sixth, despite the number of trials included, the large sample, and consistency of findings, the strict entry criteria utilized in trials may limit generalizability of our findings, especially to more frail populations. Moreover, trial safety protocols, particularly in the open-label studies, may unduly influence response to OH identified among the intensive BP treatment group. This extra attention (especially in the form of medication titration) could affect the subsequent occurrence of OH. The careful observation experienced by these participants may not generalize well to clinic practices where visits are less frequent, BP is not measured appropriately, and medications are not carefully titrated. Seventh, the 5 BP treatment goal trials were open-label and thus are at risk of performance and detection bias. Although use of standard protocols and objective BP monitors mitigate this risk, it cannot be ruled out. Finally, we did not conduct a search to examine BP medication class, although this would be of interest for subsequent studies.
Our study also has several strengths. First, this review is one of the only attempts to synthesize individual studies of BP treatment and OH. It is the largest such analysis to date. Second, the large sample allowed us to examine the effects of treatment in groups that may have been excluded or represented in small numbers in the individual trials (for example, standing SBP <110 mm Hg). Third, the meta-analysis was conducted at the individual participant data level rather than the study level. Finally, statistical heterogeneity was limited, which speaks to the consistency of the observed effects of lower BP treatment goal on OH across populations and study designs.
Our study has direct clinical implications. Hypertension affects over 45% of the U.S. adult population (34) and 31% of the world population (35) and is a major contributor to premature death (36). Despite revised guidelines and public health initiatives, treatment of hypertension remains suboptimal with over 32% of adults with known hypertension being uncontrolled (37). One of the primary obstacles to effective BP management is the concern that treatment causes adverse side effects that increases risk for disability in vulnerable populations, especially older adults. Orthostatic hypotension is a known risk factor for adverse events, such as falls and syncope, that is prevalent among older adults with hypertension (38) and is frequently attributed to BP treatment (39). Our study, incorporating a diverse range of treatment agents and goals and a large population older than 75 years, strongly affirms that more intensive BP treatment usually does not induce OH even among older adults. Furthermore, our study suggests that intensive BP treatment may potentially improve postural regulation of BP upon standing particularly, among adults with lower standing BP before treatment.
In conclusion, in this large, individual participant–level synthesis of data from BP treatment trials, more intensive BP treatment did not increase risk for OH, regardless of age, and may even improve BP regulation in adults with standing hypotension. Although individual patients may have unique reactions to specific agents requiring changes in therapy, our aggregate findings support the growing body of evidence that OH identified in the setting of intensive BP treatment should not be viewed as a reason to downtitrate or discontinue BP treatment.
Supplementary Material
Acknowledgement
The authors thank the BioLINCC repository for data from the following studies: ACCORD BP, SPRINT, and SHEP. They also thank Dr. James Neaton, original investigator of the TOMHS trial, for facilitating data sharing, and Sahalie Martin, who assembled contact information for nearly 70 investigators who conducted blood pressure treatment trials.
Grant Support: By grant 7K23HL135273-02 from the National Heart, Lung, and Blood Institute, National Institutes of Health, to Dr. Juraschek. Dr. Holman is an Emeritus UK National Institute for Health Research Senior Investigator.
Footnotes
Disclosures: Authors have disclosed no conflicts of interest. Forms can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M20-4298.
Reproducible Research Statement: Study protocol: See Supplement 1 (available at Annals.org). Statistical code: See Supplement 3 (available at Annals.org). Data set: The data sets used in this study were obtained through data use agreements and may be obtained by contacting the NHBLI BioLINCC repository or the original investigators of the trials.
Contributor Information
Stephen P. Juraschek, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, Massachusetts.
Jiun-Ruey Hu, Vanderbilt University Medical Center, Nashville, Tennessee.
Jennifer L. Cluett, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, Massachusetts.
Anthony Ishak, Healthcare Associates, Beth Israel-Lahey Health System, Boston, Massachusetts.
Carol Mita, Countway Library, Harvard University, Boston, Massachusetts.
Lewis A. Lipsitz, Beth Israel Deaconess Medical Center, Hebrew SeniorLife, Hinda and Arthur Marcus Institute for Aging Research, and Harvard Medical School, Boston, Massachusetts.
Lawrence J. Appel, Johns Hopkins University, Baltimore, Maryland.
Nigel S. Beckett, Guy’s and St Thomas’ NHS Foundation Trust, London, United Kingdom.
Ruth L. Coleman, Diabetes Trials Unit, Radcliffe Department of Medicine, University of Oxford, Oxford, United Kingdom.
William C. Cushman, University of Tennessee Health Science Center, Memphis, Tennessee.
Barry R. Davis, Coordinating Center for Clinical Trials, The University of Texas School of Public Health, Houston, Texas.
Greg Grandits, School of Public Health, University of Minnesota, Minneapolis, Minnesota.
Rury R. Holman, Diabetes Trials Unit, Radcliffe Department of Medicine, University of Oxford, Oxford, United Kingdom.
Edgar R. Miller, 3rd, Johns Hopkins University, 2024 East Monument Street, Baltimore, MD 21205..
Ruth Peters, Neuroscience Research Australia, Margarete Ainsworth Building, Barker Street, Sydney, NSW 2031, Australia.
Jan A. Staessen, University of New South Wales, Sydney, and Neuroscience Research Australia, Randwick, New South Wales, Australia (); Studies Coordinating Centre, Research Unit Hypertension and Cardiovascular Epidemiology, University of Leuven, Leuven, and NPA Alliance for the Promotion of Preventive Medicine (APPREMED), Mechelen, Belgium.
Addison A. Taylor, Michael E. DeBakey VA Medical Center and Baylor College of Medicine, Houston, Texas.
Lutgarde Thijs, Studies Coordinating Centre, Research Unit Hypertension and Cardiovascular Epidemiology, University of Leuven, Leuven, Belgium.
Jackson T. Wright, Jr., Case Western Reserve University, University Hospitals Cleveland Medical Center, Cleveland, Ohio.
Kenneth J. Mukamal, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, Massachusetts.
References
- 1.Juraschek SP, Daya N, Rawlings AM, et al. Association of history of dizziness and long-term adverse outcomes with early vs later orthostatic hypotension assessment times in middle-aged adults. JAMA Intern Med. 2017;177:1316–1323. doi: 10.1001/jamainternmed.2017.2937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strogatz DS, Keenan NL, Barnett EM, et al. Correlates of postural hypotension in a community sample of elderly blacks and whites. J Am Geriatr Soc. 1991;39:562–6. [DOI] [PubMed] [Google Scholar]
- 3.Shin C, Abbott RD, Lee H, et al. Prevalence and correlates of orthostatic hypotension in middle-aged men and women in Korea: the Korean Health and Genome Study. J Hum Hypertens. 2004;18:71723. [DOI] [PubMed] [Google Scholar]
- 4.Kamaruzzaman S, Watt H, Carson C, et al. The association between orthostatic hypotension and medication use in the British Women’s Heart and Health Study. Age Ageing. 2010;39:51–6. doi: 10.1093/ageing/afp192 [DOI] [PubMed] [Google Scholar]
- 5.Berry SD, Kiel DP, Colón-Emeric C. Hip fractures in older adults in 2019. JAMA. 2019;321:2231–2232. doi: 10.1001/jama.2019.5453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Wijnen VK, Harms MPM, Wieling W. Orthostatic hypotension in the first minute after standing up: what is the clinical relevance and do symptoms matter? [Editorial]. Hypertension. 2018;71:816–818. doi: 10.1161/HYPERTENSIONAHA.118.10609 [DOI] [PubMed] [Google Scholar]
- 7.Robinson KA, Saldanha IJ, Mckoy NA. Frameworks for Determining Research Gaps During Systematic Reviews. Agency for Healthcare Research and Quality; 2011. Accessed at www.ncbi.nlm.nih.gov/books/NBK62478/ on 15 December 2019. [PubMed] [Google Scholar]
- 8.Brunström M, Carlberg B. Association of blood pressure lowering with mortality and cardiovascular disease across blood pressure levels: a systematic review and meta-analysis. JAMA Intern Med. 2018; 178:28–36. doi: 10.1001/jamainternmed.2017.6015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317:703–13. [PMC free article] [PubMed] [Google Scholar]
- 10.Levey AS, Stevens LA, Schmid CH, et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaufmann H Consensus statement on the definition of orthostatic hypotension, pure autonomic failure and multiple system atrophy. Clin Auton Res. 1996;6:125–6. [DOI] [PubMed] [Google Scholar]
- 12.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–30. [PubMed] [Google Scholar]
- 13.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 14.Juraschek SP, Lipsitz LA, Beach JL, et al. Association of orthostatic hypotension timing with clinical events in adults with diabetes and hypertension: results from the ACCORD trial. Am J Hypertens. 2019;32:684–694. doi: 10.1093/ajh/hpz015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Juraschek SP, Taylor AA, Wright JT Jr, et al. ; SPRINT Research Group. Orthostatic hypotension, cardiovascular outcomes, and adverse events: results from SPRINT. Hypertension. 2020;75:660–667. doi: 10.1161/HYPERTENSIONAHA.119.14309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Juraschek SP, Appel LJ, Miller ER 3rd, et al. Hypertension treatment effects on orthostatic hypotension and its relationship with cardiovascular disease. Hypertension. 2018;72:986–993. doi: 10.1161/HYPERTENSIONAHA.118.11337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- 18.Wright JT Jr, Williamson JD, Whelton PK, et al. ; SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103–16. doi: 10.1056/NEJMoa1511939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wright JT Jr, Bakris G, Greene T, et al. ; African American Study of Kidney Disease and Hypertension Study Group. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421–31. [DOI] [PubMed] [Google Scholar]
- 20.Cushman WC, Evans GW, Byington RP, et al. ; ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–85. doi: 10.1056/NEJMoa1001286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benavente OR, Coffey CS, Conwit R, et al. ; SPS3 Study Group. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet. 2013;382:507–15. doi: 10.1016/S0140-6736(13)60852-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beckett NS, Peters R, Fletcher AE, et al. ; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887–98. doi: 10.1056/NEJMoa0801369 [DOI] [PubMed] [Google Scholar]
- 23.Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension: final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group. JAMA. 1991;265:3255–64. [PubMed] [Google Scholar]
- 24.Staessen JA, Fagard R, Thijs L, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet. 1997;350:757–64. [DOI] [PubMed] [Google Scholar]
- 25.Neaton JD, Grimm RH Jr, Prineas RJ, et al. Treatment of mild hypertension study: final results. Treatment of Mild Hypertension Study Research Group. JAMA. 1993;270:713–24. [PubMed] [Google Scholar]
- 26.Fleg JL, Evans GW, Margolis KL, et al. Orthostatic hypotension in the ACCORD (Action to control cardiovascular risk in diabetes) blood pressure trial: prevalence, incidence, and prognostic significance. Hypertension. 2016;68:888–95. doi: 10.1161/HYPERTENSIONAHA.116.07474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ylitalo A, Airaksinen KE, Sellin L, et al. Effects of combination antihypertensive therapy on baroreflex sensitivity and heart rate variability in systemic hypertension. Am J Cardiol. 1999;83:885–9. [DOI] [PubMed] [Google Scholar]
- 28.Lipsitz LA, Gagnon M, Vyas M, et al. Antihypertensive therapy increases cerebral blood flow and carotid distensibility in hypertensive elderly subjects. Hypertension. 2005;45:216–21. [DOI] [PubMed] [Google Scholar]
- 29.Lipsitz LA, Habtemariam D, Gagnon M, et al. Reexamining the effect of antihypertensive medications on falls in old age. Hypertension. 2015;66:183–9. doi: 10.1161/HYPERTENSIONAHA.115.05513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shimbo D, Barrett Bowling C, Levitan EB, et al. Short-term risk of serious fall injuries in older adults initiating and intensifying treatment with antihypertensive medication. Circ Cardiovasc Qual Outcomes. 2016;9:222–9. doi: 10.1161/CIRCOUTCOMES.115.002524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Juraschek SP, Simpson LM, Davis BR, et al. Effects of antihypertensive class on falls, syncope, and orthostatic hypotension in older adults: the ALLHAT trial. Hypertension. 2019;74:1033–1040. doi: 10.1161/HYPERTENSIONAHA.119.13445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Masuo K, Mikami H, Ogihara T, et al. Changes in frequency of orthostatic hypotension in elderly hypertensive patients under medications. Am J Hypertens. 1996;9:263–8. [DOI] [PubMed] [Google Scholar]
- 33.van Wijnen VK, Finucane C, Harms MPM, et al. Noninvasive beat-to-beat finger arterial pressure monitoring during orthostasis: a comprehensive review of normal and abnormal responses at different ages. J Intern Med. 2017;282:468–483. doi: 10.1111/joim.12636 [DOI] [PubMed] [Google Scholar]
- 34.Muntner P, Carey RM, Gidding S, et al. Potential U.S. population impact of the 2017 ACC/AHA High Blood Pressure Guideline. J Am Coll Cardiol. 2018;71:109–118. doi: 10.1016/j.jacc.2017.10.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mills KT, Bundy JD, Kelly TN, et al. Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation. 2016;134:441–50. doi: 10.1161/CIRCULATIONAHA.115.018912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Woolf SH, Schoomaker H. Life expectancy and mortality rates in the United States, 1959–2017. JAMA. 2019;322:1996–2016. doi: 10.1001/jama.2019.16932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Derington CG, King JB, Herrick JS, et al. Trends in antihypertensive medication monotherapy and combination use among US adults, national health and nutrition examination survey 2005–2016. Hypertension. 2020;75:973–981. doi: 10.1161/HYPERTENSIONAHA.119.14360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gangavati A, Hajjar I, Quach L, et al. Hypertension, orthostatic hypotension, and the risk of falls in a community-dwelling elderly population: the maintenance of balance, independent living, intellect, and zest in the elderly of Boston study. J Am Geriatr Soc. 2011; 59:383–9. doi: 10.1111/j.1532-5415.2011.03317.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Margolis KL. Antihypertensive medication use in older adults at risk for hip fracture [Letter]. JAMA. 2019;322:1608–1609. doi: 10.1001/jama.2019.13951 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.