Abstract
Delirium is a debilitating form of brain dysfunction frequently encountered in the intensive care unit (ICU). It is associated with increased morbidity and mortality, longer lengths of stay, higher hospital costs, and cognitive impairment that persists long after hospital discharge. Predisposing factors include smoking, hypertension, cardiac disease, sepsis, and premorbid dementia. Precipitating factors include respiratory failure and shock, metabolic disturbances, prolonged mechanical ventilation, pain, immobility, and sedatives and adverse environmental conditions impairing vision, hearing, and sleep. Historically, antipsychotic medications were the mainstay of delirium treatment in the critically ill. Based on more recent literature, the current Society of Critical Care Medicine (SCCM) guidelines suggest against routine use of antipsychotics for delirium in critically ill adults. Other pharmacologic interventions (e.g., dexmedetomidine) are under investigation and their impact is not yet clear. Nonpharmacologic interventions thus remain the cornerstone of delirium management. This approach is summarized in the ABCDEF bundle (Assess, prevent, and manage pain; Both SAT and SBT; Choice of analgesia and sedation; Delirium: assess, prevent, and manage; Early mobility and exercise; Family engagement and empowerment). The implementation of this bundle reduces the odds of developing delirium and the chances of needing mechanical ventilation, yet there are challenges to its implementation. There is an urgent need for ongoing studies to more effectively mitigate risk factors and to better understand the pathobiology underlying ICU delirium so as to identify additional potential treatments. Further refinements of therapeutic options, from drugs to rehabilitation, are current areas ripe for study to improve the short- and long-term outcomes of critically ill patients with delirium.
Keywords: delirium, ABCDEF bundle, cognitive impairment, critical illness, intensive care, early mobility, antipsychotics, dementia
Delirium is a common organ dysfunction encountered in critically ill adults and a significant cause of morbidity and mortality. Delirium has been identified with critical illness as far back as the ancient Roman empire, where the nobleman and encyclopedist Aulus Cornelius Celsus described the manifestations of delirium in patients with wound infections and head trauma in his seminal work, De Medicina.1 More than half of all patients in modern intensive care units (ICUs) will go on to develop delirium at some point during their admission.2–5 This is of particular importance, as delirium is independently associated with an increased risk of death.6–8 Additionally, duration of delirium is the major risk factor for cognitive impairment after critical illness, a form of ICU-acquired dementia that is particularly debilitating.4,9–11 Lastly, the development of delirium is associated with increased hospital length of stay and health care costs.12–15
The mechanism of delirium is unclear and most likely a result of multiple pathways that are affected during critical illness that alters normal cognition. Numerous pathological mechanisms have been proposed, ranging from genetic defects to worsening brain inflammation and poor cerebral blood flow, and to neurotransmitter imbalance.16–19 Multiple concurrent insults are likely responsible and vary depending on individual patients’ physiologic reserve and their severity of illness. Given the complex nature of the disease, a multifaceted approach to delirium is warranted.
The management of ICU delirium has historically been challenging, as there have been very few pharmacological options that have demonstrated efficacy in treating delirium once it develops. For example, antipsychotics have consistently shown little benefit in treating the disease.20,21 The prevailing approach to management is focused on prevention and early recognition. Prevention includes strategies to limit risk factors, such as catheter removal and promoting a healthy sleep environment. It also includes targeted approach to analgosedation, such as avoidance of benzodiazepine-based sedation and focusing on providing light sedation in addition to encouraging patient mobilization. Early recognition of delirium involves the use of well-validated instruments to screen for the disease, and when it is identified, a focused search for potential etiologies such as occult infection should be pursued. A bundle of care, incorporating these evidence-based care processes for delirium management such as goal-directed light sedation and early mobilization, has demonstrated significant improvements in multiple important ICU outcomes, including delirium, days of mechanical ventilation, and mortality.22,23 This synergistic bundle, called the ABCDEF bundle (Assess, prevent, and manage pain; Both SAT and SBT; Choice of analgesia and sedation; Delirium: assess, prevent, and manage; Early mobility and exercise; Family engagement and empowerment), represents the best management strategy for critically ill patients with delirium. The future of delirium management will be focused on optimizing these processes of care as well as identifying the basic, mechanistic underpinnings of the disease to help develop novel treatment strategies to improve the care of the critically ill.
Manifestations and Outcomes of ICU Delirium
Manifestations
Delirium is a form of acute brain dysfunction that manifests as fluctuating attention and impaired cognitive function. It can present with a variety of symptoms, including significant psychomotor agitation, depressed level of consciousness, or both. According to the American Psychiatric Association’s Diagnostic and Statistical Manual—5th edition (DSM-5) guidelines, delirium is an acute confusional state defined by acute disturbances in attention, awareness, or cognition developing over hours to days due to disease or sedation that is not better explained by an alternative diagnosis or a comatose state.24 Notably, delirium can and often does coexist with underlying neurological disease such as dementia, traumatic brain injury, and stroke and so is not precluded from developing in these patients.25,26
The incidence of delirium varies among individual studies but is a frequent diagnosis in all inpatient care settings. Vasilevskis and colleagues estimated that approximately one-third of patients who were hospitalized ultimately developed delirium.27 Delirium is especially common in the ICU, with three-quarters of mechanically ventilated patients suffering with disease and up to half of those not receiving mechanical ventilation developing delirium.2,3,28,29
The symptomatology of delirium varies as well, particularly with respect to its psychomotor manifestations. Delirium is classified into hyperactive, hypoactive, and mixed subtypes. Hypoactive and mixed delirium are the most common presentations seen in the ICU, accounting for over 90% of cases.30 Patients with hypoactive delirium are predominantly lethargic with reduced motor activity, in contrast to patients with hyperactive delirium who are often agitated and restless. Patients with mixed delirium have symptoms of both hypoactive and hyperactive delirium that can change over the course of the disease.
Outcomes
Advances in critical care medicine have substantially improved survival rates, yet delirium remains a frequent and significant cause of morbidity and mortality with significant downstream impairments in cognitive and physical functioning (Fig. 1). Patients with delirium have increased mortality, both in the hospital and in the year following hospitalization, as compared with nondelirious patients.31,32 The presence of delirium within 24 hours of a critical care admission is strongly associated with increased in-hospital mortality.33 Mechanically ventilated patients who develop delirium had greater 6-month mortality and a significantly longer length of stay in the hospital as compared with their counterparts without delirium.7,12 Higher mortality has also been linked to delirium out to 1 year from admission among older critically ill adults.6,34,35 Notably, hypoactive delirium is associated with higher mortality as compared with hyperactive delirium or mixed delirium.36,37
Fig. 1.
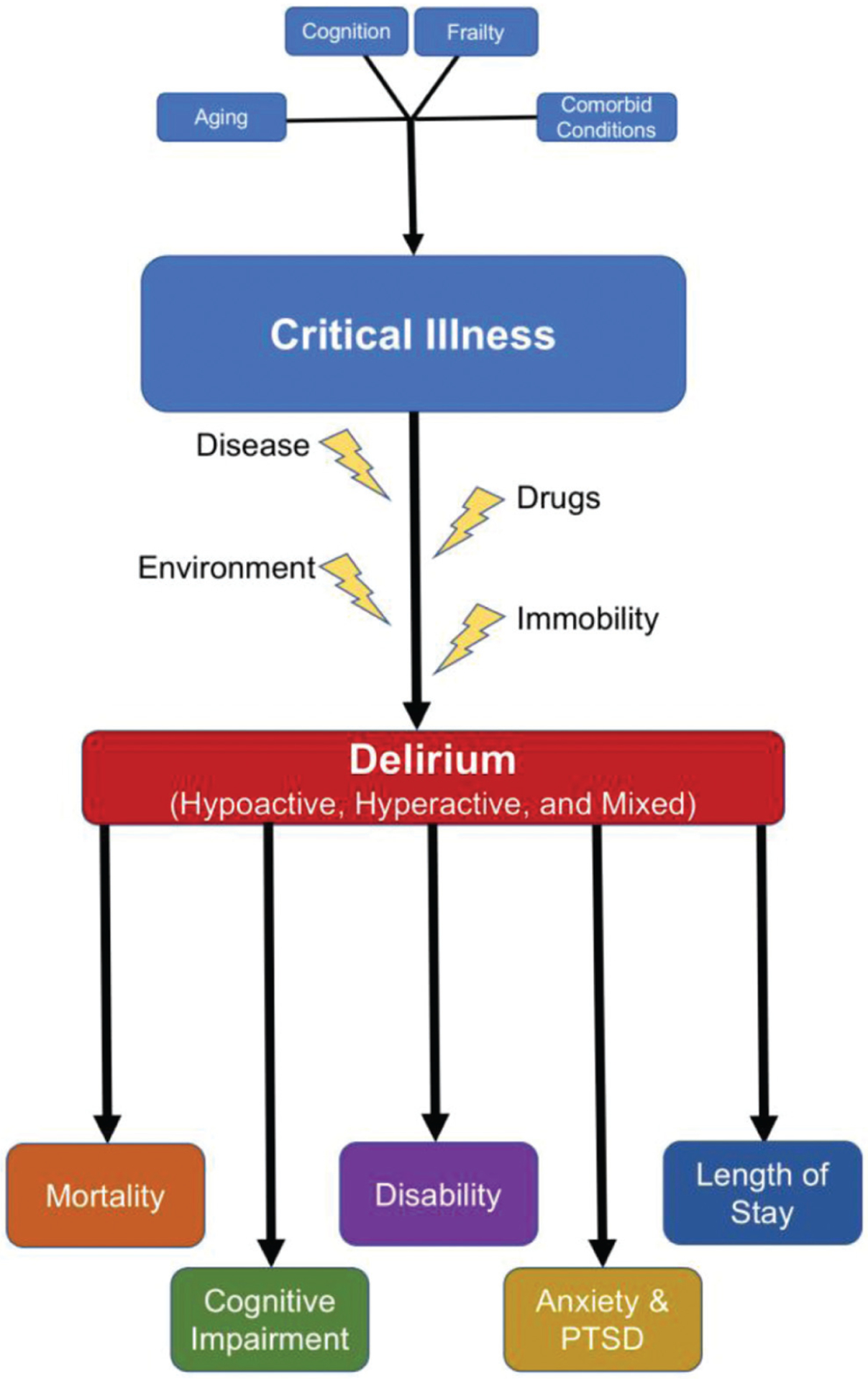
Risk factors for and outcomes after ICU delirium. Pre-existing conditions, such as frailty or comorbid diseases, contribute to critical illness, and in combination with modifiable risks, such as immobility and drug use, lead to the development of delirium and its long-term complications. ICU, intensive care unit.
In addition to higher mortality, delirious patients suffer from other physical complications. Delirium has been consistently associated with both longer ICU and hospital length of stay.7,34 Patients with delirium also spend a longer time on mechanical ventilation and have more respiratory complications with increased difficulty weaning.34,38,39 Delirium has also been associated with an increased likelihood of being discharged to a long-term care facility, and patients with blood stream infections were also less likely to return to their baseline functional status.34,40 Brummel and colleagues demonstrated that critically ill patients on mechanical ventilation who develop delirium had substantial impairments in their basic activities of daily living as well as worse sensory-motor function at 3 and 12 month follow-up.41 Prolonged mechanical ventilation and disability secondary to delirium prevents patients from returning to their baseline and reduces their overall quality of life.
The neurocognitive impact of delirium extends beyond the acute illness. Older delirious adults both with and without dementia demonstrated symptoms of delirium for months following their initial episode, including symptoms of inattention and disorientation.42 Among the critically ill, delirious patients have greater cognitive impairment at hospital discharge than those who were not delirious, with more severe delirium associated with greater cognitive impairment.43 Girard and colleagues demonstrated that delirium was independently associated with cognitive impairment in ICU survivors at 3 and 12 months after hospitalization with over 70% of ICU survivors experiencing cognitive impairment at 12 months.9 This finding was confirmed by Pandharipande and colleagues in the BRAIN-ICU study.4 Cognitive impairment was seen across all age ranges in this cohort with similar incidence to the Girard study. Of these, over one-third of patients had evidence of moderate to severe cognitive impairment at 12 months. In a related study, delirium was associated with significant cognitive impairment at 18-months post-ICU.44 Additionally, patients who experience delirium also experience an increased risk of dementia and greater cognitive decline. In older adults over the age 85 followed for 10 years, delirium was a strong independent predictor of incident dementia.45 And in patients with pre-existing Alzheimer’s dementia, delirium was associated with a significantly worse cognitive trajectory as compared to those without delirium, adjusting for baseline disease severity and comorbidity.46 In addition to the clinical manifestations, neuroimaging of delirium suggests important structural brain changes, such as atrophy and volume loss as well as white matter lesions.47,48 Just as delirium worsens cognitive function in those with baseline dementia, pre-existing neurologic disease also increases the risk of developing delirium.49,50 The persistent cognitive dysfunction related to delirium has significant socioeconomic impact in that it is also associated with reduced employment in ICU survivors.51 The link between delirium and cognitive impairment represents an unfortunate reciprocal relationship that impacts the most vulnerable critically ill patients.
Along with cognitive impairment and increased incidence of dementia, there is increasing recognition of the psychological sequelae of delirium. Approximately three-quarters of critically ill patients who were interviewed 2 weeks after surviving their initial illness reported delusions, and those with limited factual recall of their hospitalizations had significantly increased anxiety and symptoms of posttraumatic stress disorder (PTSD).52 Jackson and colleagues found that 37 and 33% of ICU survivors reported depressive symptoms at 3 and 12 months after critical illness, respectively, with 7% of all survivors having symptoms consistent with PTSD.53 Patel et al found that one in 10 patients following critical illness had symptoms consistent with PTSD attributable to their ICU admission, and that pre-existing psychiatric diagnoses such as anxiety, depression, or military-service-related PTSD were associated with greater likelihood of ICU-related PTSD.54
The important downstream sequelae of delirium remain a significant challenge for the world of critical care medicine. These complications of delirium range from higher in-hospital mortality and increased time of mechanical ventilation to long-term cognitive impairment, disability, and psychiatric disease. Such problems mandate urgency in further elucidating both the underlying pathophysiology and improving the clinical management of the disease. What is clear is that focusing on the prevention of delirium in combination with aggressive management of risk factors, as well as with other evidence-based practices to prevent iatrogenesis, will be the mainstay of treatment and research going forward.
Risk Factors and Proposed Mechanisms
Delirium is triggered by an acute medical event.55,56 Despite its frequency and detrimental outcomes, the condition is often underdiagnosed,55,57 and its pathophysiology is still poorly understood.55 Identification of precipitating risk factors for delirium is important to implement, where possible, preventative strategies in patients who are most at risk. Multiple factors appear to contribute to the acute syndrome, and over 100 of them have been studied for a potential link with an increased risk for developing delirium in the ICU.27 These risks can be grouped into two categories: risks that can predispose the patient to delirium (modifiable and nonmodifiable) and risks related to the treatment received while in the ICU or the environment (Fig. 2).58
Fig. 2.
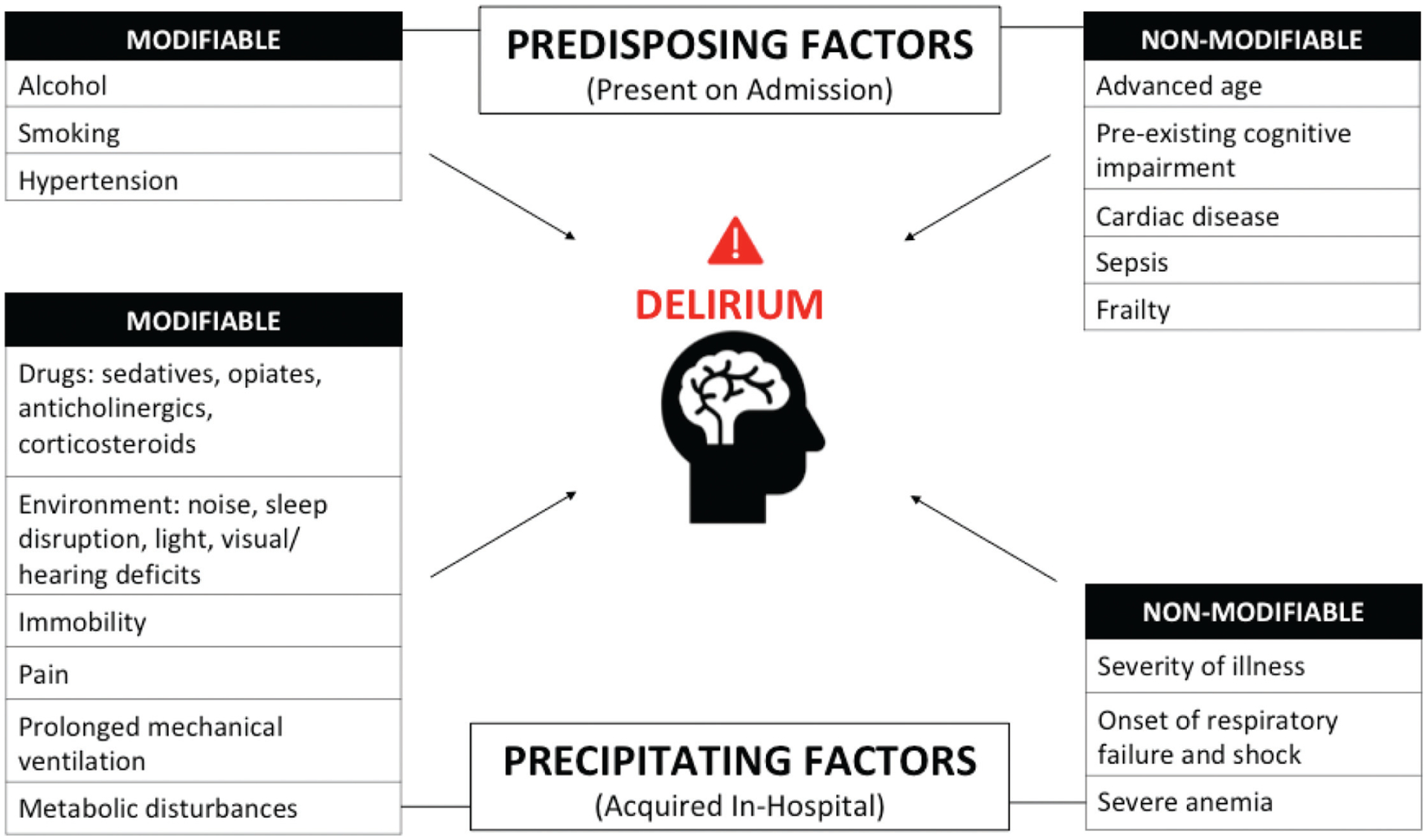
Predisposing and precipitating factors for developing ICU delirium. Delirium is a result of both baseline, or predisposing, risk factors (top) and acquired, or precipitating, risk factors (bottom). These may be modifiable (left) or nonmodifiable (right). ICU, intensive care unit.
Predisposing Factors
Patient Characteristics
Characteristics of the patient that have been consistently found to increase the delirium risk include advanced age,59 pre-existing cognitive impairment,5,60 and history of hypertension.61 Some studies have found that cigarette smoking2,62 and alcohol use60,61 increase the risk of delirium incidence, although the current evidence is insufficient to determine if they are independently associated with ICU delirium.59 Of these predisposing factors, only uncontrolled hypertension, alcohol use, and cigarette smoking are potentially modifiable.
A high number of comorbidities,63 cardiac disease,64 and frailty65,66 also appear to increase the risk, although the evidence is still inconclusive.59 Patients with multiple comorbidities and frailty have a lower physical and cognitive physiological reserve,67 which impairs the capacity to sustain normal brain functioning in response to the stress of critical illness and may ultimately lead to delirium.68
Pharmacological Treatment
There are other risks that are associated with the treatment provided in the ICU. Studies have shown that the use of benzodiazepines (especially lorazepam and midazolam) is independently associated with increased risk of delirium.29,69–71 In addition, these studies have demonstrated a dose-dependent relationship, whereby the risk is higher with higher benzodiazepine doses. This is particularly the case when benzodiazepines are used as sedatives for mechanical ventilation.72–74
Opiates, especially morphine, have also been linked with delirium risk,2,29,69,75 and there seems to be a correlation between administration of opiates with benzodiazepines and increased delirium duration.69 The link between these medications and delirium may be related to the duration of action of these agents, which increases the risk of drug accumulation in the setting of altered organ function. Furthermore, administration of epidural analgesia2 and sedation with propofol76 also show some association, although the evidence is still inconclusive.
Anticholinergic agents can also precipitate delirium,77 and systemic corticosteroids have been shown to be significantly associated with transitioning to delirium from a nondelirious or noncomatose state.78 The connection between delirium and psychopharmacological agents is likely due to their effect on neurotransmitters that appear to be critical to the emergence of delirium, in particular gamma-amino butyric acid (GABA), acetylcholine, dopamine, and serotonin.79 An imbalance in the synthesis, release, and inactivation of these neurotransmitters seems to be one of the mechanisms of delirium.80
ICU Course
Critical illness constitutes a severe systemic insult, and patients admitted to intensive care with a high severity of illness or high Acute Physiology and Chronic Health Evaluation II (APACHE II) score,61,69 polytrauma,75,81,82 organ failure,81 and patients admitted to the ICU after emergency surgery64,82,83 have a higher risk of delirium than patients with a less severe systemic insult.27 Coma has also been shown to be a risk factor for delirium, in particular iatrogenic coma induced pharmacologically.60,61
Moreover, studies have shown that prolonged mechanical ventilation,60,84 emergence from delirium,59,85,86 requirement of blood transfusions,81,83,87 immobility,60,71,88–90 metabolic acidosis,5,75 and pain29,61 are all independent risk factors for higher delirium risk. It has been suggested that acute systemic inflammation, aging, and ischemic injury lead to the release of proinflammatory cytokines, which can threaten the integrity of the brain–blood barrier (BBB).91,92 The disruption of the BBB in turns activates a series of events that can lead to delirium. There is increasing evidence suggesting that hypoxia can lead to increased brain dysfunction in critically ill patients, potentially contributing to long-term cognitive impairment.93–95 Chronic intermittent hypoxia can induce neurodegenerative changes to brain tissue that may predispose patients to delirium.96,97
Finally, research has shown that the presence of plasma tryptophan,86,98 a precursor of the neurotransmitter serotonin, and inflammatory biomarkers (procalcitonin and C-reactive protein)99–101 may be linked with the development of delirium. The mechanism behind C-reactive protein and delirium appears to be associated with a disruption in the BBB due to generation of reactive oxygen species.102 Research into biomarkers is a promising development in the field of delirium, as their identification could constitute a useful diagnostic tool which would enable early diagnosis and risk stratification.
Environment
Despite its importance for recovery, the quality of sleep in the ICU is known to be generally poor.103 Factors that play a significant role include noise, frequent disruptions, administration of medications that alter sleep architecture,104 and disturbance of the light–dark cycle due to decreased exposure to natural light.80 Poor quality of sleep has been suggested as a potential modifiable risk factor for delirium105,106; although this association has not yet been definitively established,107 sleep promotion is considered important and is part of the strategy to prevent delirium proposed by the Society of Critical Care Medicine (SCCM).
Overall, there seems to be a complex relationship between predisposing and precipitating factors leading to delirium, involving structural characteristics, several neurotransmitters, immunologic, physiologic and genetic factors, as well as iatrogenic and environmental exposures. Thus, a highly fragile patient may develop delirium despite a lesser physiological insult, and equally a patient with less comorbidities and good functional state may require a more severe insult to develop delirium. A good understanding of these interrelated factors is important to stratify patients in different risk categories, so that preventive strategies can be developed leading to a reduction in the incidence of delirium.
Historical Perspective on Management
The last few decades have seen a dramatic shift in the prevailing perspective on effective management of delirium. For context, we provide a brief description of the historical observations and practices that led to the current state of the field.
Early Perspectives and the Use of Antipsychotics
The early literature on delirium management focused primarily on alcohol-related disturbances, in particular delirium tremens. For delirium in hospitalized psychiatric patients, a combination of chloral hydrate and potassium bromide was recommended.108 During the mid-20th century, various treatments were proposed for “acute delirium” in association with hospitalization, systemic illness, or postoperative states. Such treatments included paraldehyde and sodium chloride,109 physostigmine (particularly for delirium related to surgery and anticholinergic administration),110 phenothiazine drugs,111 and even electroconvulsive therapy.112 In 1978 the use of intravenous haloperidol was reported in a series of 15 delirious patients during recovery from cardiac surgery113 and this became the mainstay of treatment for delirium in the critically ill. The next several decades saw an increasing reliance on antipsychotic medications for this purpose. In 2002 the SCCM guidelines for use of sedatives and analgesics in ICU recommended haloperidol as the preferred agent for treatment of delirium.114
Changing Perspectives on Antipsychotics
The appropriateness of first-generation antipsychotics as treatment for delirium was challenged in the mid-2000s by reports that these medications increased mortality in the elderly.115 Pursuant to this, second-generation (“atypical”) antipsychotics such as quetiapine and risperidone began to gain popularity. The rationale was that these carry less risk of adverse effects such as cardiac arrhythmias and neuroleptic malignant syndrome. A systematic review of atypical antipsychotics versus haloperidol revealed that atypical antipsychotics were at least as efficacious as haloperidol for the treatment of delirium.116 In the past 10 years, however, it has become evident that antipsychotics do not diminish risk of ICU delirium nor do they improve the negative outcomes associated with this state of acute brain failure. The Hope-ICU study randomized 142 mechanically ventilated patients to receive intravenous haloperidol or normal saline until delirium-free or ICU discharge, but found no difference in number of coma-free and delirium-free days, delirium duration, or survival between the two groups.21 The HARPOON study randomized 245 acutely hospitalized elderly patients to prophylactic haloperidol versus placebo and found no clear differences in delirium incidence, duration, severity, or 3-month mortality.117 The MIND-USA study compared haloperidol, ziprasidone, and placebo in a randomized, double-blind trial and found no effect of either antipsychotic medication on the number of coma-free and delirium-free days (Fig. 3). Thirty day and 90-day survivals were also similar among the three groups.20 Another randomized, double-blind study of haloperidol, risperidone, and placebo for delirium in inpatient hospice and palliative care patients found an increase in delirium symptom severity among patients receiving haloperidol or risperidone compared with placebo.118 A recent systematic review of antipsychotics for treating delirium in hospitalized adults found no difference among haloperidol, atypical antipsychotics, and placebo in terms of delirium duration, hospital length of stay, or mortality.119 A second review of antipsychotics for prevention of delirium found no evidence that haloperidol lowers incidence or duration of delirium, hospital length of stay, or mortality compared with placebo. The same review did, however, suggest a possibility that atypical antipsychotics may decrease the incidence of delirium specifically in postoperative patients.120 The current SCCM guidelines suggest against routine use of haloperidol or atypical antipsychotics for prevention or treatment of delirium in critically ill adults.121
Fig. 3.
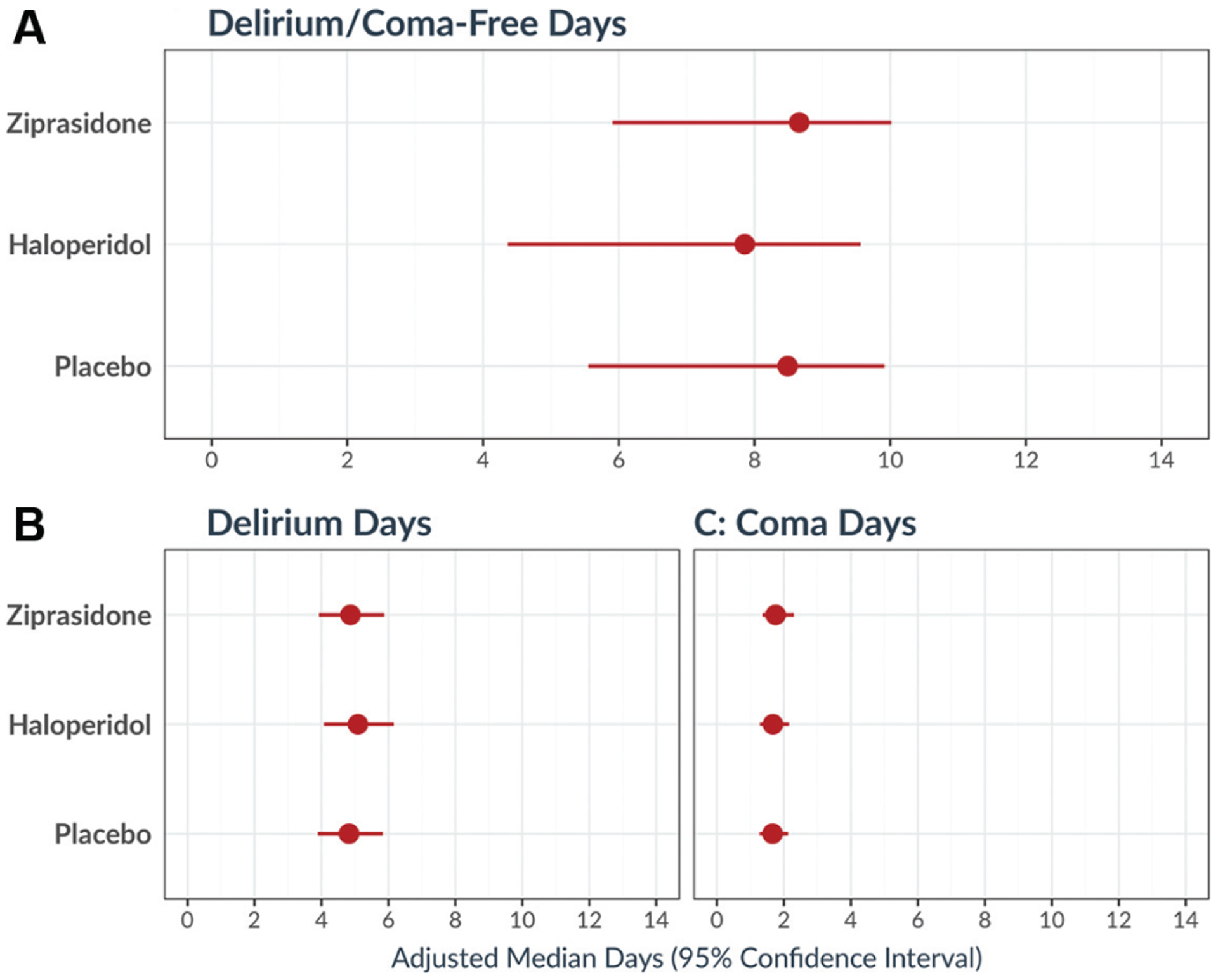
Effects of haloperidol, ziprasidone, and placebo on ICU delirium or coma. In a randomized clinical trial of 566 ICU patients receiving haloperidol, ziprasidone, or placebo for treatment of hypoactive or hyperactive delirium, there were no significant differences among groups in days with delirium, days with coma, or days alive without delirium or coma. These analyses were adjusted for age, pre-existing cognitive impairment, Clinical Frailty Score and Charlson Comorbidity Index score at baseline, and modified Sequential Organ Failure Assessment score and Richmond Agitation–Sedation Scale score at randomization. ICU, intensive care unit. Reproduced with permission from Girard et al.20
Current Knowledge of Management and Prevention
The surprising results of systematic studies of haloperidol and other antipsychotics, once thought to be the mainstay of delirium management, have led clinicians and researchers alike to consider alternative therapies to prevent and treat delirium. However, the field is far from identifying a “silver bullet.” This section describes the most promising pharmacologic and nonpharmacologic therapies, the current state of investigation, and barriers or challenges impeding widespread implementation.
Pharmacological Management and Prevention
Dexmedetomidine
Dexmedetomidine is a selective α−2 adrenoreceptor agonist that may promote sleep–wake cycle regulation in addition to providing anxiolysis and sedation.122 Use of dexmedetomidine for the prevention of delirium is controversial. In two double-blind, randomized controlled trials, mechanically ventilated patients sedated with dexmedetomidine had a 23% lower risk of delirium compared with those sedated with midazolam,73 and more than twice as many coma-free and delirium-free days compared with those sedated with lorazepam.72 An open-label, randomized controlled trial comparing dexmedetomidine to usual care for first choice of sedation in mechanically ventilated patients also found a slight increase in the number of coma-free and delirium-free days in the dexmedetomidine group. There was, however, no difference in 90-day mortality between the groups.123 This study was limited by the fact that a large percentage of the dexmedetomidine group ultimately received other drugs such as propofol and fentanyl. There is a randomized controlled trial in progress that compares dexmedetomidine versus propofol in mechanically ventilated patients with sepsis.124 Another randomized controlled trial will compare the incidence of delirium in elderly cardiac surgery patients with or without a single postoperative sleep-inducing dose of dexmedetomidine.125 The results of these studies are eagerly awaited and will inform future potential uses of dexmedetomidine in the prevention of delirium.
Regarding pharmacologic treatmentonce delirium develops, a recent Cochrane analysis found evidence that dexmedetomidine may shorten duration of delirium, mechanical ventilation, and ICU stay. That study found no evidence of difference in coma-free or delirium-free days, long-term cognitive impairment, or mortality.126
Statins
As described previously, one of the postulated mechanisms of delirium pathogenesis involves inflammation within the central nervous system causing breakdown of the BBB. 3-Hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors (statins) have been observed to exert an anti-inflammatory effect in humans and animals, and thus a protective effect of statins in ICU delirium has been postulated.127 In support of this hypothesis, preoperative statins are associated with decreased risk of postoperative delirium in elderly patients undergoing cardiac surgery.128 Observational studies suggest a protective effect of statins with respect to delirium development during critical illness129 and that this effect may be mediated in part by the effect of statins on systemic inflammation as measured by the C-reactive protein levels.130 However, an ancillary study of a prospective randomized controlled trial comparing rosuvastatin and placebo in acute respiratory distress syndrome found no effect on the incidence of delirium or on long-term cognitive impairment overall. Patients in the rosuvastatin group had slightly worse delayed memory scores at 6-month follow-up (mean difference in scores: −1.2; 95% confidence interval [CI]: −2.2 to −0.2, p = 0.017).131
Ketamine
Ketamine is an intravenous N-methyl-D-aspartate (NMDA) receptor antagonist with anesthetic, analgesic, antidepressant, and anti-inflammatory properties. Intraoperative administration of ketamine significantly decreases postoperative inter-leukin-6 concentrations132 as well as postoperative opiate requirements.133 Hudetz et al found that ketamine administration during induction was also associated with an improvement in post-cardiac surgery cognitive dysfunction. The C-reactive protein concentration was also significantly lower in the ketamine group (median: 7.9 vs. 11.6 mg/dL, p < 0.01).134 These findings suggest that ketamine may hold promise as a prophylactic or therapeutic drug for postoperative or critical illness delirium. Nevertheless, a randomized controlled trial comparing a single intraoperative subanesthetic dose of ketamine to placebo did not find a difference in incidence of postoperative delirium. The ketamine groups did, on the other hand, suffer an increased rate of hallucinations and nightmares.135 Although this study used similar doses of ketamine to the Hudetz study, the studies differed with respect to timing and premedication protocols.
Thus far no pharmacologic agent has demonstrated efficacy in treating or preventing delirium. The current SCCM guidelines suggest against routine use of dexmedetomidine, statins, or ketamine for prevention of delirium in critically ill adults.121 One or more of these agents may turn out to be useful for delirium management, but given the heterogeneous nature of the disorder, optimal treatment will likely depend on the prevailing risk factors, the neurologic and systemic comorbidities, and the metabolic and physiologic profile of each patient.
Nonpharmacologic Interventions
For decades, nonpharmacologic interventions have been the cornerstone of delirium management and treatment.109 Interventions such as promoting regular sleep–wake cycles, avoiding unnecessary invasive sensory stimulation, and regular reorientation have been refined over the decades and have become standard of care in ICUs worldwide. These interventions have largely been conceptualized in the continually evolving ABCDEF bundle.136,137 Improvements in compliance with the ABCDEF bundle are associated with a reduction in mortality as well as a reduction in number of coma-free and delirium-free ICU days (Fig. 4).22,23 The principal components of this approach can be summarized in the ABCDEF bundle, described in the sections below.
Fig. 4.

Association between proportional performance of the ABCDEF bundle and patient outcomes. Adjusted hazard ratio and 95% confidence interval for ICU discharge, hospital discharge, and death, comparing patients with a given proportion of eligible ABCDEF bundle elements performed on a given day with patients with none of the bundle elements performed that day. Hazard ratios are adjusted for baseline, ICU admission characteristics, and daily covariates, measured the same day as bundle performance. ICU, intensive care unit. Reproduced with permission from Pun et al.22
Assess, Prevent, and Manage Pain
Critically ill patients experience pain at rest and with routine procedures. Inadequately treated pain can result in delirium as well as several other complications. Pain should be monitored routinely in all adult ICU patients. This can be done by self-report in awake, communicative patients, or using validated behavioral pain scales such as the Behavioral Pain Scale or the Critical Care Pain Observation Tool138 in those who are unable to communicate pain.
Both SAT and SBT
Spontaneous awakening trials (SATs) are pauses of intravenous narcotics and sedatives. When appropriate, these medications are restarted at half the prior dose. Spontaneous breathing trials (SBTs) are periods of minimal ventilator support. A randomized, controlled trial comparing daily SBTs to standard of care in mechanically ventilated patients revealed that those receiving SBTs had shorter time on the ventilator (4.5 vs. 6 days, p = 0.003). The intervention arm also had less ventilator-related complications and lower costs of ICU care.139 A randomized controlled trial comparing a daily SAT and SBT protocol with daily SBT plus routine sedation found that patients on the SAT plus SBT protocol spent more days breathing without assistance and less time in the ICU. Patients in the SAT arm were also less likely to die during the 12-month follow-up period. Reintubation rates were similar in the two groups.28 The daily coordination of SATs and SBTs is a key part of the timely cessation of mechanical ventilation and leads to improved ICU outcomes.
Choice of Analgesia and Sedation
Effective management of pain, anxiety, and delirium is a primary objective in the ICU. This management needs to be based on agreed-upon, patient-oriented goals and standardized assessment measures. There are several validated scales published for assessment of sedation level in the ICU, for example the Richmond Agitation–Sedation Scale (RASS) or the Riker Sedation–Agitation Scale.
The most effective medication and titration protocol for sedation and analgesia is not yet clear, and likely depends on the clinical context and patient characteristics. The MENDS randomized controlled trial compared lorazepam and dexmedetomidine for sustained sedation in mechanically ventilated patients and found that the dexmedetomidine groups experienced more days alive without delirium or coma (median: 7.0 vs. 3.0 days, p = 0.01). Costs of care were similar between the two groups.72 The MENDS2 study will compare dexmedetomidine and propofol in septic patients on mechanical ventilation. Results from that study are pending as of the writing of this review.
Delirium: Assess, Prevent, and Manage
A key component of delirium management is monitoring for early identification and risk factor modification. The most widely used tool for delirium assessment in the ICU is the Confusion Assessment Method for the ICU (CAM-ICU).3 The CAM-ICU comprises four features: acute onset of mental status change or fluctuating curse; inattention; disorganized thinking; and altered level of consciousness. Its pooled values as a diagnostic test are 80% sensitivity and 95.9% specificity.140 Other tools include the Intensive Care Delirium Screening Checklist (ICDSC).141 The ICDSC comprises eight items, including assessment of altered level of consciousness, inattention, hallucination/delusions/psychosis, psychomotor agitation or retardation, inappropriate speech or mood, sleep wake/cycle disturbance, and symptom fluctuation. Its pooled sensitivity and specificity are 74 and 81.9%, respectively.140 It is important to assess patients regularly to reduce the risk of overlooking hypoactive delirium, and the optimal time for this assessment is during SATs.142
Once delirium is identified, management entails revisiting primary prevention measures, including reorientation, an appropriate sleep environment, and adequate pain management. Many of these nonpharmacological interventions are outlined in Table 1. Since the duration of delirium predicts worse long-term outcomes,4 implementation of these nonpharmacologic measures can be expected to improve patient care.
Table 1.
Primary delirium prevention principles
| Primary delirium prevention principles |
|---|
|
Early Mobility and Exercise
Early mobility consists of a range of activities from passive range of motion to ambulation with assistance. It is safe and feasible in critically ill patients, and decreases days of delirium, duration of mechanical ventilation, ICU length of stay, and overall hospital length of stay. Any member of the care team can perform early mobility; the appropriate level of activity is determined based on the patient’s level of sedation. Early mobilization during SATs was associated with improved odds of return to independent functional status by discharge (odds ratio: 2.7; 95% CI: 1.2–6.1) in a series of critically ill adults on mechanical ventilation.143 Early combined cognitive and physical therapy has been shown to be feasible in a similar population, and outcomes are currently under study.144
Family Engagement and Empowerment
Empowering family members to be equal participants in patient care can improve ICU team performance and communication, reveal key insights into the patient condition, and keep providers focused on the most salient goals of care for each patient. This intervention may also lead to early identification of and reduction in the burden of ICU-related psychological and emotional stress among family members. Key elements of strategic communication with families include using simplified speech, being concrete, and avoiding rapid communication. A recent systematic review and meta-analysis of protocolized family support interventions found that such interventions reduce ICU length of stay without impacting mortality.137 Additionally, specific programs have been developed to reproduce familiar environments for high-risk patients or to augment the presence of family members in person or online to decrease the cognitive deficit of critically ill patients at ICU discharge.145 The importance of family involvement in cognitive rehabilitation has been recognized in other types of brain injury146 and may provide a promising avenue for reducing the burden of brain injury due to delirium in ICU patients.144,147 Other methods for creating comforting environments include music-based therapies, which can improve physiologic measures associated with delirium148 and have been recognized as potentially beneficial during critical illness and at the end of life.149 Such interventions are the subject of ongoing study.150
Implementation Challenges and Difficulties
Delirium is often underrecognized57,151; therefore the use of validated tools for its identification is essential to improve patients’ outcomes.151,152 As previously mentioned, the Pain, Agitation/Sedation, Delirium Immobility, and Sleep Disruption (PADIS) guidelines by the SCCM recommend the use of either the CAM-ICU or the ICDSC to assess delirium in critically ill adult patients.121 Despite the excellent reliability of these tools, systematic screening for delirium is lacking in many ICUs151,153–155 due to several barriers.
Nurses often reported that delirium was difficult to evaluate in intubated patients,154,156–158 whereas others felt that sedated patients could not be assessed.154,157 However, the CAM-ICU was specifically designed to assess nonverbal ICU patients receiving mechanical ventilation, and the current CAM-ICU training manual159 indicates that the only condition to be tested with the CAM-ICU is responsiveness to verbal stimulation irrespective of sedative use (RASS ≤ 3).
Other studies156,160 have noted that some clinicians believe that delirium can be identified without an assessment tool. This is contrary to the evidence found by Spronk et al,57 which suggests that delirium was very often missed without a validated screening tool such as the CAM-ICU: nurses only recognized 35% of delirious days, while attending intensivists performed even worse (only 28% of days with delirium were recognized). In another study by van Eijk et al,161 almost three out of four patients of all ICU delirium were missed by doctors not using a validated assessment tool. Missed diagnosis may lead to lack of treatment, whereas false positives due to lack of objectivity may expose patients to unnecessary pharmacological therapy despite its unproven efficacy56 and safety risks.162,163
In some units delirium screening is perceived as too complex154 or too time consuming156,164 although assessment with the CAM-ICU or the ICDSC takes 2 to 5 minutes to complete.158,165 Some clinicians feel that CAM-ICU is unreliable,164,166 or unnecessary, often choosing to use clinical observation instead of validated tools to monitor agitation or to assess the ability to follow commands.154 This is especially the case in the neurocritical care setting, where there is a perception that the CAM-ICU and ICDSC are unsuitable. This in contrast to the findings by Mitasova et al,167 who have shown that the CAM-ICU has a sensitivity of 76% and a specificity of 98% in patients who have suffered a stroke. Moreover, Frenette et al168 found that both the CAM-ICU and the ICDSC had good sensitivity and specificity in evaluating delirium in patients who had suffered a traumatic brain injury.167,168 Despite these findings, the assessment of neurocritically ill patients is challenging especially in those with extreme depression, severe catatonia, and receptive aphasia. Therefore, more research is needed to find suitable tools to diagnose and prevent delirium in this population.
Other elements that have been reported as barriers for delirium assessment include lack of knowledge about delirium or training to use the assessment,156,160,164 perception that it cannot be prevented or that assessment is futile as there is no treatment for it157,158,164,166; lack of confidence performing the CAM-ICU158; belief that the test is embarrassing for the patient166; and low familiarity with the guidelines, lack of motivation to follow them, and perception that following guidelines regarding delirium screening would not impact positively on patient’s care.164
More broadly, the implementation of the ABCDEF bundle as a whole has been shown to substantially improve outcomes, including reducing the odds of developing delirium and reducing the chances of needing mechanical ventilation.23,136 Barriers for implementing the ABCDEF as a bundle are similar to the ones encountered when using a delirium assessment tool. Some are related to the patient, including safety concerns in terms of hemodynamical instability or lack of cooperation.169 The majority are related to the clinician implementing the bundle, in particular lack of knowledge about its benefits, reluctance to follow guidelines, preference for autonomy, perceived increased workload,170 and lack of confidence in the bundle’s efficacy.169–171 Finally, there are barriers related to the structure of the ICU, including culture and organization, teamwork, physical environment, lack of resources, and inadequate management/leadership.169,171,172
Practical Considerations for the Treatment of Delirium
At the individual level, for the clinician at the bedside, the management of delirium can be difficult. The physician must understand delirium as a form of acute organ failure, in this instance, a form of acute brain failure. One must be familiar with and look for the various manifestations of delirium, from hypoactive to hyperactive disease. Additionally, similar to other organ failures, a search for the cause is warranted. Whether in the ICU or on the medical/surgical wards, acute deliriogenic insults due to disease or treatment should be evaluated when evaluating a patient. There are several mnemonics that have been developed to remind the clinician of potential etiologies. One commonly used mnemonic is “Dr. DRE,” where “DRE” refers to categories of causes of delirium including diseases, drugs that need to be removed, and environmental risk factors (Table 2). This particular tool is useful for any patient that is hospitalized, not just the critically ill patient in the ICU. One should look for diseases, such as sepsis or heart failure, as potential etiologies, as well as sequelae of acute and chronic diseases, such as metabolic abnormalities. The clinician should also look for potentially harmful drugs, such as benzodiazepines or antihistamines, that could be contributing. In addition to evaluating for new or worsening disease processes and removing offending drugs, evaluation of the patient’s environment is paramount to addressing delirium. For example, is the patient restrained or immobile in bed with limited day time stimulus that orients the patient to place and time? Simple interventions, such as providing the patient’s eyeglasses and hearing aids and encouraging mobility during the day, are effective at reducing delirium and represent good clinical care. The clinician could also assess and make changes to night time vital checks or other bedside interventions in an effort to promote restful and restorative sleep.
Table 2.
“Dr. DRE” mnemonic for clinicians to address delirium at the bedside
| Mnemonic | Clinical causes and interventions |
|---|---|
| Diseases | Evaluate the patient for new or worsening disease, such as congestive heart failure or sepsis, as well as other disease findings such as metabolic abnormalities |
| Drug Removal | Look for and stop deliriogenic medications including benzodiazepines, antihistamines, and inappropriate opioids |
| Environment | Encourage daytime mobilization and remove restraints, provide frequent reorientation as well as cues such as clocks and windows to the outside, reduce night-time interventions to promote restful sleep |
Implementing the above assessments and interventions is the front-line of bedside management of delirium. While the majority of the scientific literature does not demonstrate any benefit to the use of medications such as antipsychotics to treat or reduce the duration of delirium, there may be uncommon indications for their use. For example, for the patient with significant hyperactive delirium who is a danger to themselves due to removing medical devices or for falls at the bedside, discrete, limited use of antipsychotics may be indicated to prevent harm. The clinician should understand that such use is not to treat or improve delirium but is instead to reduce agitation that may result in harm. If such an intervention is needed, one should also continue to look for reversible etiologies and nonpharmacological interventions to manage and reduce delirium. Using this judicious approach, in addition to systemic implementation of evidence-based bundles such as the ABCDEF bundle, will provide the best care for patients.
Conclusion and Future Directions
Delirium is a devastating condition in critically ill patients with wide-ranging impacts, from cognitive impairment to psychological distress to short-term, and perhaps long-term, mortality. It is common, with studies indicating that the majority of patients receiving routine critical care interventions such as mechanical ventilation develop the disease at some point in their hospitalization. The downstream consequences of delirium go beyond the ICU to impact patients for months to years after critical illness.
These facts have increased the urgency to better understand the disease and its pathobiology as well as identify potential treatments and mitigate risk factors. There has been substantial interest in pharmacological treatments for delirium, with widespread use of antipsychotics. It has been estimated that 1 in 10 critically ill patients are given antipsychotics in the setting of delirium,173 with up to 30% of all adult, nonpsychiatric admissions to the hospital receiving antipsychotics.174 Yet multiple, prospective studies of antipsychotics have shown that they are ineffective, and they are no longer recommended in care guidelines.20,21,121 The most effective treatment, and the standard of care for critically ill patients, is the ABCDEF bundle. It has demonstrated a significant reduction in delirium in a dose-responsive manner, in addition to improving other important outcomes such as days of mechanical ventilation and readmission rates.22,23 The future picture of the critically ill patient is no longer one of a deeply sedated, immobile patient on mechanical ventilation, but instead one of a patient up and out of bed, walking, interacting with family and the care team, even with invasive interventions such as mechanical ventilation or continuous renal replacement therapy. Going forward, all ICU patients should be managed utilizing the guidelines and framework as outlined in the ABCDEF bundle to reduce delirium, limit iatrogenesis, and optimize patient-centered outcomes.
There remain numerous areas of investigation to tackle the scourge of delirium in the most critically ill patients. Ongoing mechanistic studies are needed to further understand the complex and inter-related pathways that are at work in the development of delirium. Such studies will provide other potential targets for therapeutic interventions and also help elucidate other risk factors that could be mitigated in the critical care environment. For example, future medications that can selectively modify the neuroinflammation and neurotransmitter disturbances may be developed from ongoing basic science studies of delirium, and care practices that impact delirium such as optimizing the sleep environment and limiting noxious stimuli will be increasingly understood at the molecular level. There also remain questions regarding our optimal practices in the ICU to prevent or reduce delirium—what is the best sedation choice for patients requiring mechanical ventilation? How, and to what extent, should we mobilize and exercise our critically ill patients? The data surrounding the ABCDEF bundle are convincing and represent a significant advance in delirium care and for ICU care as a whole. Further investigation into the best practices to implement the bundle widely, in both large and small ICUs, will be needed to optimize care universally. Following critical illness, questions regarding care abound—for example, how can primary care physicians and post-ICU recovery clinics work together to reduce the untoward sequelae of critical illness? Each of these ongoing areas of investigation is important and necessary to address in the future to improve patient care and outcomes.
As the care of the critically ill patient improves, so does our understanding of delirium and its consequences. As knowledge advances, the importance of unraveling both the pathways of disease and improving its prevention and treatment increases exponentially. While no single agent has been identified yet that prevents and/or treats delirium, significant advances have been made. Increasingly, the standard of care in the ICU for managing delirium centers upon integrated, symbiotic care processes such as those outlined in the ABCDEF bundle, which both prevents delirium and limits its duration. Further refinements of therapeutic options, from drugs to rehabilitation, are needed and are current areas ripe for study to improve the lives of critically ill, delirious patients.
Footnotes
Conflict of Interest
None declared.
References
- 1.Celsus AC, Spencer WG. On Medicine [electronic resource]/with an English Translation by Spencer WG. Cambridge, MA: Harvard University Press; 2014 [Google Scholar]
- 2.Dubois MJ, Bergeron N, Dumont M, Dial S, Skrobik Y. Delirium in an intensive care unit: a study of risk factors. Intensive Care Med 2001;27(08):1297–1304 [DOI] [PubMed] [Google Scholar]
- 3.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the Confusion Assessment Method for the intensive care unit (CAM-ICU). JAMA 2001;286(21):2703–2710 [DOI] [PubMed] [Google Scholar]
- 4.Pandharipande PP, Girard TD, Jackson JC, et al. ; BRAIN-ICU Study Investigators. Long-term cognitive impairment after critical illness. N Engl J Med 2013;369(14):1306–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pisani MA, Murphy TE, Van Ness PH, Araujo KL, Inouye SK. Characteristics associated with delirium in older patients in a medical intensive care unit. Arch Intern Med 2007;167(15):1629–1634 [DOI] [PubMed] [Google Scholar]
- 6.Pisani MA, Kong SY, Kasl SV, Murphy TE, Araujo KL, Van Ness PH. Days of delirium are associated with 1-year mortality in an older intensive care unit population. Am J Respir Crit Care Med 2009;180(11):1092–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA 2004;291(14):1753–1762 [DOI] [PubMed] [Google Scholar]
- 8.Lin SM, Liu CY, Wang CH, et al. The impact of delirium on the survival of mechanically ventilated patients. Crit Care Med 2004;32(11):2254–2259 [DOI] [PubMed] [Google Scholar]
- 9.Girard TD, Jackson JC, Pandharipande PP, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med 2010;38(07):1513–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolters AE, van Dijk D, Pasma W, et al. Long-term outcome of delirium during intensive care unit stay in survivors of critical illness: a prospective cohort study. Crit Care 2014;18(03):R125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakusic A, O’Horo JC, Dziadzko M, et al. Potentially modifiable risk factors for long-term cognitive impairment after critical illness: a systematic review. Mayo Clin Proc 2018;93(01):68–82 [DOI] [PubMed] [Google Scholar]
- 12.Ely EW, Gautam S, Margolin R, et al. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med 2001;27(12):1892–1900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomason JW, Shintani A, Peterson JF, Pun BT, Jackson JC, Ely EW. Intensive care unit delirium is an independent predictor of longer hospital stay: a prospective analysis of 261 non-ventilated patients. Crit Care 2005;9(04):R375–R381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milbrandt EB, Deppen S, Harrison PL, et al. Costs associated with delirium in mechanically ventilated patients. Crit Care Med 2004;32(04):955–962 [DOI] [PubMed] [Google Scholar]
- 15.Leslie DL, Marcantonio ER, Zhang Y, Leo-Summers L, Inouye SK. One-year health care costs associated with delirium in the elderly population. Arch Intern Med 2008;168(01):27–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Gool WA, van de Beek D, Eikelenboom P. Systemic infection and delirium: when cytokines and acetylcholine collide. Lancet 2010;375(9716):773–775 [DOI] [PubMed] [Google Scholar]
- 17.Inouye SK, Ferrucci L. Elucidating the pathophysiology of delirium and the interrelationship of delirium and dementia. J Gerontol A Biol Sci Med Sci 2006;61(12):1277–1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet 2014;383(9920):911–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marcantonio ER. In the clinic. Delirium. Ann Intern Med 2011;154(11):ITC6–ITC1, ITC6–ITC2, ITC6–ITC3, ITC6–ITC4, ITC6–ITC5, ITC6–ITC6, ITC6–ITC7, ITC6–ITC8, ITC6–ITC9, ITC6–ITC10, ITC6–ITC11, ITC6–ITC12, ITC6–ITC13, ITC6–ITC14, ITC6–ITC15, quiz ITC6–ITC16 [DOI] [PubMed] [Google Scholar]
- 20.Girard TD, Exline MC, Carson SS, et al. ; MIND-USA Investigators. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med 2018;379(26):2506–2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Page VJ, Ely EW, Gates S, et al. Effect of intravenous haloperidol on the duration of delirium and coma in critically ill patients (Hope-ICU): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med 2013;1(07):515–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pun BT, Balas MC, Barnes-Daly MA, et al. Caring for critically ill patients with the ABCDEF bundle: results of the ICU liberation collaborative in over 15,000 adults. Crit Care Med 2019;47(01):3–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barnes-Daly MA, Phillips G, Ely EW. Improving hospital survival and reducing brain dysfunction at seven california community hospitals: implementing PAD guidelines via the ABCDEF bundle in 6,064 patients. Crit Care Med 2017;45(02):171–178 [DOI] [PubMed] [Google Scholar]
- 24.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders: DSM-5. Washington, DC: American Psychiatric Association; 2013 [Google Scholar]
- 25.Abengaña J, Chong MS, Tay L. Delirium superimposed on dementia: phenomenological differences between patients with and without behavioral and psychological symptoms of dementia in a specialized delirium unit. Int Psychogeriatr 2017;29(03):485–495 [DOI] [PubMed] [Google Scholar]
- 26.Patel MB, Bednarik J, Lee P, et al. Delirium monitoring in neurocritically ill patients: a systematic review. Crit Care Med 2018;46(11):1832–1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vasilevskis EE, Han JH, Hughes CG, Ely EW. Epidemiology and risk factors for delirium across hospital settings. Best Pract Res Clin Anaesthesiol 2012;26(03):277–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet 2008;371(9607):126–134 [DOI] [PubMed] [Google Scholar]
- 29.Pandharipande P, Cotton BA, Shintani A, et al. Prevalence and risk factors for development of delirium in surgical and trauma intensive care unit patients. J Trauma 2008;65(01):34–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peterson JF, Pun BT, Dittus RS, et al. Delirium and its motoric subtypes: a study of 614 critically ill patients. J Am Geriatr Soc 2006;54(03):479–484 [DOI] [PubMed] [Google Scholar]
- 31.Siddiqi N, House AO, Holmes JD. Occurrence and outcome of delirium in medical in-patients: a systematic literature review. Age Ageing 2006;35(04):350–364 [DOI] [PubMed] [Google Scholar]
- 32.Witlox J, Eurelings LS, de Jonghe JF, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA 2010;304(04):443–451 [DOI] [PubMed] [Google Scholar]
- 33.van den Boogaard M, Peters SAE, van der Hoeven JG, et al. The impact of delirium on the prediction of in-hospital mortality in intensive care patients. Crit Care 2010;14(04):R146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Z, Pan L, Ni H. Impact of delirium on clinical outcome in critically ill patients: a meta-analysis. Gen Hosp Psychiatry 2013;35(02):105–111 [DOI] [PubMed] [Google Scholar]
- 35.Leslie DL, Zhang Y, Holford TR, Bogardus ST, Leo-Summers LS, Inouye SK. Premature death associated with delirium at 1-year follow-up. Arch Intern Med 2005;165(14):1657–1662 [DOI] [PubMed] [Google Scholar]
- 36.Stagno D, Gibson C, Breitbart W. The delirium subtypes: a review of prevalence, phenomenology, pathophysiology, and treatment response. Palliat Support Care 2004;2(02):171–179 [DOI] [PubMed] [Google Scholar]
- 37.Meagher D Motor subtypes of delirium: past, present and future. Int Rev Psychiatry 2009;21(01):59–73 [DOI] [PubMed] [Google Scholar]
- 38.Salluh JI, Wang H, Schneider EB, et al. Outcome of delirium in critically ill patients: systematic review and meta-analysis. BMJ 2015;350:h2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dessap AM, Roche-Campo F, Launay J-M, et al. Delirium and circadian rhythm of melatonin during weaning from mechanical ventilation: an ancillary study of a weaning trial. Chest 2015;148(05):1231–1241 [DOI] [PubMed] [Google Scholar]
- 40.Dittrich T, Tschudin-Sutter S, Widmer AF, Rüegg S, Marsch S, Sutter R. Risk factors for new-onset delirium in patients with bloodstream infections: independent and quantitative effect of catheters and drainages-a four-year cohort study. Ann Intensive Care 2016;6(01):104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brummel NE, Jackson JC, Pandharipande PP, et al. Delirium in the ICU and subsequent long-term disability among survivors of mechanical ventilation. Crit Care Med 2014;42(02):369–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCusker J, Cole M, Dendukuri N, Han L, Belzile E. The course of delirium in older medical inpatients: a prospective study. J Gen Intern Med 2003;18(09):696–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sakuramoto H, Subrina J, Unoki T, Mizutani T, Komatsu H. Severity of delirium in the ICU is associated with short term cognitive impairment. A prospective cohort study. Intensive Crit Care Nurs 2015;31(04):250–257 [DOI] [PubMed] [Google Scholar]
- 44.van den Boogaard M, Schoonhoven L, Evers AW, van der Hoeven JG, van Achterberg T, Pickkers P. Delirium in critically ill patients: impact on long-term health-related quality of life and cognitive functioning. Crit Care Med 2012;40(01):112–118 [DOI] [PubMed] [Google Scholar]
- 45.Davis DH, Muniz Terrera G, Keage H, et al. Delirium is a strong risk factor for dementia in the oldest-old: a population-based cohort study. Brain 2012;135(Pt 9):2809–2816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gross AL, Jones RN, Habtemariam DA, et al. Delirium and long-term cognitive trajectory among persons with dementia. Arch Intern Med 2012;172(17):1324–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gunther ML, Morandi A, Krauskopf E, et al. ; VISIONS Investigation, VISualizing Icu SurvivOrs Neuroradiological Sequelae. The association between brain volumes, delirium duration, and cognitive outcomes in intensive care unit survivors: the VISIONS cohort magnetic resonance imaging study. Crit Care Med 2012;40(07):2022–2032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morandi A, Gunther ML, Vasilevskis EE, et al. Neuroimaging in delirious intensive care unit patients: a preliminary case series report. Psychiatry (Edgmont Pa) 2010;7(09):28–33 [PMC free article] [PubMed] [Google Scholar]
- 49.Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol 2009;5(04):210–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fong TG, Jones RN, Shi P, et al. Delirium accelerates cognitive decline in Alzheimer disease. Neurology 2009;72(18):1570–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Norman B, Jackson J, Graves J, et al. Employment changes after critical illness. Am J Respir Crit Care Med 2014;44(11):2003–2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jones C, Griffiths RD, Humphris G, Skirrow PM. Memory, delusions, and the development of acute posttraumatic stress disorder-related symptoms after intensive care. Crit Care Med 2001;29(03):573–580 [DOI] [PubMed] [Google Scholar]
- 53.Jackson JC, Pandharipande PP, Girard TD, et al. ; Bringing to light the Risk Factors And Incidence of Neuropsychological dysfunction in ICU survivors (BRAIN-ICU) study investigators. Depression, post-traumatic stress disorder, and functional disability in survivors of critical illness in the BRAIN-ICU study: a longitudinal cohort study. Lancet Respir Med 2014;2(05):369–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patel MB, Jackson JC, Morandi A, et al. Incidence and risk factors for intensive care unit-related post-traumatic stress disorder in veterans and civilians. Am J Respir Crit Care Med 2016;193(12):1373–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Page VJ, Ely EW. Delirium in Critical Care. 2nd ed. Cambridge: Cambridge University Press; 2015 [Google Scholar]
- 56.Barr J, Fraser GL, Puntillo K, et al. ; American College of Critical Care Medicine. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med 2013;41(01):263–306 [DOI] [PubMed] [Google Scholar]
- 57.Spronk PE, Riekerk B, Hofhuis J, Rommes JH. Occurrence of delirium is severely underestimated in the ICU during daily care. Intensive Care Med 2009;35(07):1276–1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA 1996;275(11):852–857 [PubMed] [Google Scholar]
- 59.Zaal IJ, Devlin JW, Peelen LM, Slooter AJ. A systematic review of risk factors for delirium in the ICU. Crit Care Med 2015;43(01):40–47 [DOI] [PubMed] [Google Scholar]
- 60.Van Rompaey B, Elseviers MM, Schuurmans MJ, Shortridge-Baggett LM, Truijen S, Bossaert L. Risk factors for delirium in intensive care patients: a prospective cohort study. Crit Care 2009;13(03):R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ouimet S, Kavanagh BP, Gottfried SB, Skrobik Y. Incidence, risk factors and consequences of ICU delirium. Intensive Care Med 2007;33(01):66–73 [DOI] [PubMed] [Google Scholar]
- 62.Santos FS, Velasco IT, Fráguas R Jr. Risk factors for delirium in the elderly after coronary artery bypass graft surgery. Int Psychogeriatr 2004;16(02):175–193 [PubMed] [Google Scholar]
- 63.Van Rompaey B, Schuurmans MJ, Shortridge-Baggett LM, Truijen S, Bossaert L. Risk factors for intensive care delirium: a systematic review. Intensive Crit Care Nurs 2008;24(02):98–107 [DOI] [PubMed] [Google Scholar]
- 64.Abelha FJ, Fernandes V, Botelho M, et al. Apolipoprotein E e4 does not increase the risk of early post-operative delirium after major surgery. J Anesth 2012;26:412–421 [DOI] [PubMed] [Google Scholar]
- 65.Jung P, Pereira MA, Hiebert B, et al. The impact of frailty on postoperative delirium in cardiac surgery patients. J Thorac Cardiovasc Surg 2015;149(03):869–75.e1, 2 [DOI] [PubMed] [Google Scholar]
- 66.Leung JM, Tsai TL, Sands LP. Brief report: preoperative frailty in older surgical patients is associated with early postoperative delirium. Anesth Analg 2011;112(05):1199–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jones RN, Fong TG, Metzger E, et al. Aging, brain disease, and reserve: implications for delirium. Am J Geriatr Psychiatry 2010;18(02):117–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Quinlan N, Marcantonio ER, Inouye SK, Gill TM, Kamholz B, Rudolph JL. Vulnerability: the crossroads of frailty and delirium. J Am Geriatr Soc 2011;59(Suppl 2):S262–S268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pisani MA, Murphy TE, Araujo KL, Slattum P, Van Ness PH, Inouye SK. Benzodiazepine and opioid use and the duration of intensive care unit delirium in an older population. Crit Care Med 2009;37(01):177–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pandharipande P, Shintani A, Peterson J, et al. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology 2006;104(01):21–26 [DOI] [PubMed] [Google Scholar]
- 71.McPherson JA, Wagner CE, Boehm LM, et al. Delirium in the cardiovascular ICU: exploring modifiable risk factors. Crit Care Med 2013;41(02):405–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pandharipande PP, Pun BT, Herr DL, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA 2007;298(22):2644–2653 [DOI] [PubMed] [Google Scholar]
- 73.Riker RR, Shehabi Y, Bokesch PM, et al. ; SEDCOM (Safety and Efficacy of Dexmedetomidine Compared With Midazolam) Study Group. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA 2009;301(05):489–499 [DOI] [PubMed] [Google Scholar]
- 74.Kamdar BB, Niessen T, Colantuoni E, et al. Delirium transitions in the medical ICU: exploring the role of sleep quality and other factors. Crit Care Med 2015;43(01):135–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van den Boogaard M, Pickkers P, Slooter AJ, et al. Development and validation of PRE-DELIRIC (PREdiction of DELIRium in ICU patients) delirium prediction model for intensive care patients: observational multicentre study. BMJ 2012;344:e420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Seymour CW, Pandharipande PP, Koestner T, et al. Diurnal sedative changes during intensive care: impact on liberation from mechanical ventilation and delirium. Crit Care Med 2012;40(10):2788–2796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Burry LD, Williamson DR, Mehta S, et al. Delirium and exposure to psychoactive medications in critically ill adults: a multicentre observational study. J Crit Care 2017;42:268–274 [DOI] [PubMed] [Google Scholar]
- 78.Schreiber MP, Colantuoni E, Bienvenu OJ, et al. Corticosteroids and transition to delirium in patients with acute lung injury. Crit Care Med 2014;42(06):1480–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gunther ML, Morandi A, Ely EW. Pathophysiology of delirium in the intensive care unit. Crit Care Clin 2008;24(01):45–65, viii [DOI] [PubMed] [Google Scholar]
- 80.Figueroa-Ramos MI, Arroyo-Novoa CM, Lee KA, Padilla G, Puntillo KA. Sleep and delirium in ICU patients: a review of mechanisms and manifestations. Intensive Care Med 2009;35(05):781–795 [DOI] [PubMed] [Google Scholar]
- 81.Angles EM, Robinson TN, Biffl WL, et al. Risk factors for delirium after major trauma. Am J Surg 2008;196(06):864–869, discussion 869–870 [DOI] [PubMed] [Google Scholar]
- 82.Serafim RB, Dutra MF, Saddy F, et al. Delirium in postoperative nonventilated intensive care patients: risk factors and outcomes. Ann Intensive Care 2012;2(01):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Veiga D, Luis C, Parente D, et al. Postoperative delirium in intensive care patients: risk factors and outcome. Rev Bras Anestesiol 2012;62(04):469–483 [DOI] [PubMed] [Google Scholar]
- 84.Guillamondegui OD, Richards JE, Ely EW, et al. Does hypoxia affect intensive care unit delirium or long-term cognitive impairment after multiple trauma without intracranial hemorrhage? J Trauma 2011;70(04):910–915 [DOI] [PubMed] [Google Scholar]
- 85.Agarwal V, O’Neill PJ, Cotton BA, et al. Prevalence and risk factors for development of delirium in burn intensive care unit patients. J Burn Care Res 2010;31(05):706–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pandharipande PP, Morandi A, Adams JR, et al. Plasma tryptophan and tyrosine levels are independent risk factors for delirium in critically ill patients. Intensive Care Med 2009;35(11):1886–1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Whitlock EL, Torres BA, Lin N, et al. Postoperative delirium in a substudy of cardiothoracic surgical patients in the BAG-RECALL clinical trial. Anesth Analg 2014;118(04):809–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Needham DM, Korupolu R, Zanni JM, et al. Early physical medicine and rehabilitation for patients with acute respiratory failure: a quality improvement project. Arch Phys Med Rehabil 2010;91(04):536–542 [DOI] [PubMed] [Google Scholar]
- 89.Hopkins RO, Suchyta MR, Farrer TJ, Needham D. Improving post-intensive care unit neuropsychiatric outcomes: understanding cognitive effects of physical activity. Am J Respir Crit Care Med 2012;186(12):1220–1228 [DOI] [PubMed] [Google Scholar]
- 90.Vasilevskis EE, Ely EW, Speroff T, Pun BT, Boehm L, Dittus RS. Reducing iatrogenic risks: ICU-acquired delirium and weakness–crossing the quality chasm. Chest 2010;138(05):1224–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zeevi N, Pachter J, McCullough LD, Wolfson L, Kuchel GA. The blood-brain barrier: geriatric relevance of a critical brain-body interface. J Am Geriatr Soc 2010;58(09):1749–1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.de Rooij SE, van Munster BC, Korevaar JC, Levi M. Cytokines and acute phase response in delirium. J Psychosom Res 2007;62(05):521–525 [DOI] [PubMed] [Google Scholar]
- 93.Turon M, Fernández-Gonzalo S, de Haro C, Magrans R, López-Aguilar J, Blanch L. Mechanisms involved in brain dysfunction in mechanically ventilated critically ill patients: implications and therapeutics. Ann Transl Med 2018;6(02):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sasannejad C, Ely EW, Lahiri S. Long-term cognitive impairment after acute respiratory distress syndrome: a review of clinical impact and pathophysiological mechanisms. Crit Care 2019;23(01):352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mikkelsen ME, Christie JD, Lanken PN, et al. The adult respiratory distress syndrome cognitive outcomes study: long-term neuropsychological function in survivors of acute lung injury. Am J Respir Crit Care Med 2012;185(12):1307–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Macheda T, Roberts K, Lyons DN, et al. Chronic intermittent hypoxia induces robust astrogliosis in an alzheimer’s disease-relevant mouse model. Neuroscience 2019;398:55–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Snyder B, Shell B, Cunningham JT, Cunningham RL. Chronic intermittent hypoxia induces oxidative stress and inflammation in brain regions associated with early-stage neurodegeneration. Physiol Rep 2017;5(09):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Adams Wilson JR, Morandi A, Girard TD, et al. The association of the kynurenine pathway of tryptophan metabolism with acute brain dysfunction during critical illness*. Crit Care Med 2012;40(03):835–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Macdonald A, Adamis D, Treloar A, Martin F. C-reactive protein levels predict the incidence of delirium and recovery from it. Age Ageing 2007;36(02):222–225 [DOI] [PubMed] [Google Scholar]
- 100.Vasunilashorn SM, Dillon ST, Inouye SK, et al. High C-reactive protein predicts delirium incidence, duration, and feature severity after major noncardiac surgery. J Am Geriatr Soc 2017;65(08):e109–e116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.McGrane S, Girard TD, Thompson JL, et al. Procalcitonin and C-reactive protein levels at admission as predictors of duration of acute brain dysfunction in critically ill patients. Crit Care 2011;15(02):R78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ali S, Patel M, Jabeen S, et al. Insight into delirium. Innov Clin Neurosci 2011;8(10):25–34 [PMC free article] [PubMed] [Google Scholar]
- 103.Rotondi AJ, Chelluri L, Sirio C, et al. Patients’ recollections of stressful experiences while receiving prolonged mechanical ventilation in an intensive care unit. Crit Care Med 2002;30(04):746–752 [DOI] [PubMed] [Google Scholar]
- 104.Hardin KA, Seyal M, Stewart T, Bonekat HW. Sleep in critically ill chemically paralyzed patients requiring mechanical ventilation. Chest 2006;129(06):1468–1477 [DOI] [PubMed] [Google Scholar]
- 105.Weinhouse GL, Schwab RJ, Watson PL, et al. Bench-to-bedside review: delirium in ICU patients - importance of sleep deprivation. Crit Care 2009;13(06):234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kamdar BB, King LM, Collop NA, et al. The effect of a quality improvement intervention on perceived sleep quality and cognition in a medical ICU. Crit Care Med 2013;41(03):800–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Boyko Y, Ording H, Jennum P. Sleep disturbances in critically ill patients in ICU: how much do we know? Acta Anaesthesiol Scand 2012;56(08):950–958 [DOI] [PubMed] [Google Scholar]
- 108.Beadles CF. The treatment of acute maniacal delirium: means directed to relieve the acute symptoms and the subsequent treatment. Hospital (Lond 1886) 1893;15(375):137–138 [PMC free article] [PubMed] [Google Scholar]
- 109.Doty EJ. The incidence and treatment of delirious reactions in later life. Geriatrics 1946;1:21–26 [PubMed] [Google Scholar]
- 110.Greene LT. Physostigmine treatment of anticholinergic-drug depression in postoperative patients. Anesth Analg 1971;50(02):222–226 [PubMed] [Google Scholar]
- 111.Blachly PH, Starr A. Treatment of delirium with phenothiazine drugs following open heart surgery. Dis Nerv Syst 1966;27(02):107–110 [PubMed] [Google Scholar]
- 112.Roberts AH. The value of E.C.T. in delirium. Br J Psychiatry 1963;109:653–655 [DOI] [PubMed] [Google Scholar]
- 113.Cassem N Intravenous use of haloperidol for acute delirium in intensive care settings In: Continuing Medical education syllabus and scientific proceedings in summary form from the 131st Annual Meeting of the American Psychiatric Association. Washington, DC: No. 394; 1978:204–205 [Google Scholar]
- 114.Jacobi J, Fraser GL, Coursin DB, et al. ; Task Force of the American College of Critical Care Medicine (ACCM) of the Society of Critical Care Medicine (SCCM), American Society of Health-System Pharmacists (ASHP), American College of Chest Physicians. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med 2002;30(01):119–141 [DOI] [PubMed] [Google Scholar]
- 115.Wang PS, Schneeweiss S, Avorn J, et al. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med 2005;353(22):2335–2341 [DOI] [PubMed] [Google Scholar]
- 116.Rea RS, Battistone S, Fong JJ, Devlin JW. Atypical antipsychotics versus haloperidol for treatment of delirium in acutely ill patients. Pharmacotherapy 2007;27(04):588–594 [DOI] [PubMed] [Google Scholar]
- 117.Schrijver EJM, de Vries OJ, van de Ven PM, et al. Haloperidol versus placebo for delirium prevention in acutely hospitalised older at risk patients: a multi-centre double-blind randomised controlled clinical trial. Age Ageing 2018;47(01):48–55 [DOI] [PubMed] [Google Scholar]
- 118.Agar MR, Lawlor PG, Quinn S, et al. Efficacy of oral risperidone, haloperidol, or placebo for symptoms of delirium among patients in palliative care: a randomized clinical trial. JAMA Intern Med 2017;177(01):34–42 [DOI] [PubMed] [Google Scholar]
- 119.Nikooie R, Neufeld KJ, Oh ES, et al. Antipsychotics for treating delirium in hospitalized adults: a systematic review. Ann Intern Med 2019;171(07):485–495 [DOI] [PubMed] [Google Scholar]
- 120.Oh ES, Needham DM, Nikooie R, et al. Antipsychotics for preventing delirium in hospitalized adults: a systematic review. Ann Intern Med 2019;171(07):474–484 [DOI] [PubMed] [Google Scholar]
- 121.Devlin JW, Skrobik Y, Gélinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med 2018;46(09):e825–e873 [DOI] [PubMed] [Google Scholar]
- 122.Nelson LE, Lu J, Guo T, Saper CB, Franks NP, Maze M. The alpha2-adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effects. Anesthesiology 2003;98(02):428–436 [DOI] [PubMed] [Google Scholar]
- 123.Shehabi Y, Howe BD, Bellomo R, et al. ; ANZICS Clinical Trials Group and the SPICE III Investigators. Early sedation with dexmedetomidine in critically ill patients. N Engl J Med 2019;380(26):2506–2517 [DOI] [PubMed] [Google Scholar]
- 124.The MENDS II Study. Available at: http://clinicaltrials.gov/ct2/show/NCT01739933. Accessed December 4, 2013
- 125.Shelton KT, Qu J, Bilotta F, et al. Minimizing ICU Neurological Dysfunction with Dexmedetomidine-induced Sleep (MINDDS): protocol for a randomised, double-blind, parallel-arm, placebo-controlled trial. BMJ Open 2018;8(04):e020316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Burry L, Hutton B, Williamson DR, et al. Pharmacological interventions for the treatment of delirium in critically ill adults. Cochrane Database Syst Rev 2019;9:CD011749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Morandi A, Hughes CG, Girard TD, McAuley DF, Ely EW, Pandharipande PP. Statins and brain dysfunction: a hypothesis to reduce the burden of cognitive impairment in patients who are critically ill. Chest 2011;140(03):580–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Katznelson R, Djaiani GN, Borger MA, et al. Preoperative use of statins is associated with reduced early delirium rates after cardiac surgery. Anesthesiology 2009;110(01):67–73 [DOI] [PubMed] [Google Scholar]
- 129.Morandi A, Hughes CG, Thompson JL, et al. Statins and delirium during critical illness: a multicenter, prospective cohort study. Crit Care Med 2014;42(08):1899–1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Page VJ, Davis D, Zhao XB, et al. Statin use and risk of delirium in the critically ill. Am J Respir Crit Care Med 2014;189(06):666–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Needham DM, Colantuoni E, Dinglas VD, et al. Rosuvastatin versus placebo for delirium in intensive care and subsequent cognitive impairment in patients with sepsis-associated acute respiratory distress syndrome: an ancillary study to a randomised controlled trial. Lancet Respir Med 2016;4(03):203–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dale O, Somogyi AA, Li Y, Sullivan T, Shavit Y. Does intraoperative ketamine attenuate inflammatory reactivity following surgery? A systematic review and meta-analysis. Anesth Analg 2012;115(04):934–943 [DOI] [PubMed] [Google Scholar]
- 133.Elia N, Tramèr MR. Ketamine and postoperative pain–a quantitative systematic review of randomised trials. Pain 2005;113(1–2):61–70 [DOI] [PubMed] [Google Scholar]
- 134.Hudetz JA, Iqbal Z, Gandhi SD, et al. Ketamine attenuates postoperative cognitive dysfunction after cardiac surgery. Acta Anaesthesiol Scand 2009;53(07):864–872 [DOI] [PubMed] [Google Scholar]
- 135.Avidan MS, Maybrier HR, Abdallah AB, et al. ; PODCAST Research Group. Intraoperative ketamine for prevention of postoperative delirium or pain after major surgery in older adults: an international, multicentre, double-blind, randomised clinical trial. Lancet 2017;390(10091):267–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Balas MC, Vasilevskis EE, Olsen KM, et al. Effectiveness and safety of the awakening and breathing coordination, delirium monitoring/management, and early exercise/mobility bundle. Crit Care Med 2014;42(05):1024–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lee HW, Park Y, Jang EJ, Lee YJ. Intensive care unit length of stay is reduced by protocolized family support intervention: a systematic review and meta-analysis. Intensive Care Med 2019;45(08):1072–1081 [DOI] [PubMed] [Google Scholar]
- 138.Kanji S, MacPhee H, Singh A, et al. Validation of the critical care pain observation tool in critically ill patients with delirium: a prospective cohort study. Crit Care Med 2016;44(05):943–947 [DOI] [PubMed] [Google Scholar]
- 139.Ely EW, Baker AM, Dunagan DP, et al. Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously. N Engl J Med 1996;335(25):1864–1869 [DOI] [PubMed] [Google Scholar]
- 140.Gusmao-Flores D, Salluh JI, Chalhub RA, Quarantini LC. The Confusion Assessment Method for the intensive care unit (CAM-ICU) and intensive care delirium screening checklist (ICDSC) for the diagnosis of delirium: a systematic review and meta-analysis of clinical studies. Crit Care 2012;16(04):R115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bergeron N, Dubois MJ, Dumont M, Dial S, Skrobik Y. Intensive care delirium screening checklist: evaluation of a new screening tool. Intensive Care Med 2001;27(05):859–864 [DOI] [PubMed] [Google Scholar]
- 142.Patel SB, Poston JT, Pohlman A, Hall JB, Kress JP. Rapidly reversible, sedation-related delirium versus persistent delirium in the intensive care unit. Am J Respir Crit Care Med 2014;189(06):658–665 [DOI] [PubMed] [Google Scholar]
- 143.Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet 2009;373(9678):1874–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Brummel NE, Girard TD, Ely EW, et al. Feasibility and safety of early combined cognitive and physical therapy for critically ill medical and surgical patients: the Activity and Cognitive Therapy in ICU (ACT-ICU) trial. Intensive Care Med 2014;40(03):370–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hung L, Au-Yeung A, Helmer C, et al. Feasibility and acceptability of an iPad intervention to support dementia care in the hospital setting. Contemp Nurse 2018;54(4–5):350–361 [DOI] [PubMed] [Google Scholar]
- 146.Foster AM, Armstrong J, Buckley A, et al. Encouraging family engagement in the rehabilitation process: a rehabilitation provider’s development of support strategies for family members of people with traumatic brain injury. Disabil Rehabil 2012;34(22):1855–1862 [DOI] [PubMed] [Google Scholar]
- 147.Wilson JE, Collar EM, Kiehl AL, et al. Computerized cognitive rehabilitation in intensive care unit survivors: returning to everyday tasks using rehabilitation networks-computerized cognitive rehabilitation pilot investigation. Ann Am Thorac Soc 2018;15(07):887–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Johnson K, Fleury J, McClain D. Music intervention to prevent delirium among older patients admitted to a trauma intensive care unit and a trauma orthopaedic unit. Intensive Crit Care Nurs 2018;47:7–14 [DOI] [PubMed] [Google Scholar]
- 149.Salas B Crossing the River Styx: the power of music, spirituality and religion at the end of life. Music Med 2019;11(4):226–235 [Google Scholar]
- 150.Khan SH, Wang S, Harrawood A, et al. Decreasing delirium through music (DDM) in critically ill, mechanically ventilated patients in the intensive care unit: study protocol for a pilot randomized controlled trial. Trials 2017;18(01):574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Pun BT, Gordon SM, Peterson JF, et al. Large-scale implementation of sedation and delirium monitoring in the intensive care unit: a report from two medical centers. Crit Care Med 2005;33(06):1199–1205 [DOI] [PubMed] [Google Scholar]
- 152.Ely EW, Stephens RK, Jackson JC, et al. Current opinions regarding the importance, diagnosis, and management of delirium in the intensive care unit: a survey of 912 healthcare professionals. Crit Care Med 2004;32(01):106–112 [DOI] [PubMed] [Google Scholar]
- 153.Soja SL, Pandharipande PP, Fleming SB, et al. Implementation, reliability testing, and compliance monitoring of the Confusion Assessment Method for the intensive care unit in trauma patients. Intensive Care Med 2008;34(07):1263–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Devlin JW, Fong JJ, Howard EP, et al. Assessment of delirium in the intensive care unit: nursing practices and perceptions. Am J Crit Care 2008;17(06):555–565, quiz 566 [PubMed] [Google Scholar]
- 155.Patel RP, Gambrell M, Speroff T, et al. Delirium and sedation in the intensive care unit: survey of behaviors and attitudes of 1384 healthcare professionals. Crit Care Med 2009;37(03):825–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Riekerk B, Pen EJ, Hofhuis JG, Rommes JH, Schultz MJ, Spronk PE. Limitations and practicalities of CAM-ICU implementation, a delirium scoring system, in a Dutch intensive care unit. Intensive Crit Care Nurs 2009;25(05):242–249 [DOI] [PubMed] [Google Scholar]
- 157.Scott P, McIlveney F, Mallice M. Implementation of a validated delirium assessment tool in critically ill adults. Intensive Crit Care Nurs 2013;29(02):96–102 [DOI] [PubMed] [Google Scholar]
- 158.Andrews L, Silva SG, Kaplan S, Zimbro K. Delirium monitoring and patient outcomes in a general intensive care unit. American journal of critical care: an official publication. Am J Crit Care 2015;24:48–56 [DOI] [PubMed] [Google Scholar]
- 159.Ely EW. Confusion Assessment Method for the ICU (CAM-ICU): The Complete Training Manual Vanderbilt University Medical Center: Vanderbilt University; 2014 [Google Scholar]
- 160.Elliott SR. ICU delirium: a survey into nursing and medical staff knowledge of current practices and perceived barriers towards ICU delirium in the intensive care unit. Intensive Crit Care Nurs 2014;30(06):333–338 [DOI] [PubMed] [Google Scholar]
- 161.van Eijk MM, van Marum RJ, Klijn IA, de Wit N, Kesecioglu J, Slooter AJ. Comparison of delirium assessment tools in a mixed intensive care unit. Crit Care Med 2009;37(06):1881–1885 [DOI] [PubMed] [Google Scholar]
- 162.Pun BT, Devlin JW. Delirium monitoring in the ICU: strategies for initiating and sustaining screening efforts. Semin Respir Crit Care Med 2013;34(02):179–188 [DOI] [PubMed] [Google Scholar]
- 163.Guenther U, Weykam J, Andorfer U, et al. Implications of objective vs subjective delirium assessment in surgical intensive care patients. Am J Crit Care 2012;21(01):e12–e20 [DOI] [PubMed] [Google Scholar]
- 164.Trogrlić Z, Ista E, Ponssen HH, et al. Attitudes, knowledge and practices concerning delirium: a survey among intensive care unit professionals. Nurs Crit Care 2017;22(03):133–140 [DOI] [PubMed] [Google Scholar]
- 165.van den Boogaard M, Pickkers P, van der Hoeven H, Roodbol G, van Achterberg T, Schoonhoven L. Implementation of a delirium assessment tool in the ICU can influence haloperidol use. Crit Care 2009;13(04):R131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Oxenbøll-Collet M, Egerod I, Christensen V, Jensen J, Thomsen T. Nurses’ and physicians’ perceptions of Confusion Assessment Method for the intensive care unit for delirium detection: focus group study. Nurs Crit Care 2018;23(01):16–22 [DOI] [PubMed] [Google Scholar]
- 167.Mitasova A, Kostalova M, Bednarik J, et al. Poststroke delirium incidence and outcomes: validation of the Confusion Assessment Method for the intensive care unit (CAM-ICU). Crit Care Med 2012;40(02):484–490 [DOI] [PubMed] [Google Scholar]
- 168.Frenette AJ, Bebawi ER, Deslauriers LC, et al. Validation and comparison of CAM-ICU and ICDSC in mild and moderate traumatic brain injury patients. Intensive Care Med 2016;42(01):122–123 [DOI] [PubMed] [Google Scholar]
- 169.Costa DK, White MR, Ginier E, et al. Identifying barriers to delivering the awakening and breathing coordination, delirium, and early exercise/mobility bundle to minimize adverse outcomes for mechanically ventilated patients: a systematic review. Chest 2017;152(02):304–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Boehm LM, Dietrich MS, Vasilevskis EE, et al. Perceptions of workload burden and adherence to ABCDE bundle among intensive care providers. American journal of critical care: an official publication. American Association of Critical-Care Nurses 2017;26:e38–e47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Balas MC, Pun BT, Pasero C, et al. Common challenges to effective ABCDEF bundle implementation: the ICU liberation campaign experience. Crit Care Nurse 2019;39(01):46–60 [DOI] [PubMed] [Google Scholar]
- 172.Carrothers KM, Barr J, Spurlock B, Ridgely MS, Damberg CL, Ely EW. Contextual issues influencing implementation and outcomes associated with an integrated approach to managing pain, agitation, and delirium in adult ICUs. Crit Care Med 2013;41(09, Suppl 1):S128–S135 [DOI] [PubMed] [Google Scholar]
- 173.Swan JT, Fitousis K, Hall JB, Todd SR, Turner KL. Antipsychotic use and diagnosis of delirium in the intensive care unit. Crit Care 2012;16(03):R84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Herzig SJ, Rothberg MB, Guess JR, Gurwitz JH, Marcantonio ER. Antipsychotic medication utilization in nonpsychiatric hospitalizations. J Hosp Med 2016;11(08):543–549 [DOI] [PMC free article] [PubMed] [Google Scholar]


