Abstract
HIV infection and drugs of abuse induce oxidative stress and redox imbalance, which cause neurodegeneration. The mechanisms by which HIV infection and cocaine consumption affect astrocyte energy metabolism, and how this leads to neurodegenerative dysfunction, remain poorly understood. Presently, we investigated how oxidative injury causes the depletion of energy resources and glutathione synthetase (GSS), which in turn activates AMPK, glycolytic enzymes, and mitochondrial biogenesis, finally resulting in NRF transcription in astrocytes. Both human primary astrocytes incubated with HIV-1 Tat protein in vitro and HIV-inducible Tat (iTat) mice exposed to cocaine showed decreased levels of GSS and increased superoxide dismutase (SOD) levels. These changes, in turn, significantly activated AMPK and raised the concentrations of several glycolytic enzymes, along with oxidative phosphorylation, the mitochondrial biogenesis of PGC-1α and TFAM, and Nrf1 and Nrf2 gene transcription and protein expression. Moreover, neurons exposed to HIV-1Tat/cocaine-conditioned media showed reductions in dendritic formation, spine density, and neuroplasticity compared to control neurons. These results suggest that redox inhibition of GSS altered AMPK activation and mitochondrial biogenesis to influence Nrf transcription. These processes are important components of the astrocyte signaling network regulating brain energy metabolism in HIV-positive cocaine users. In conclusion, HIV-1 Tat alters redox inhibition, thus increasing glycolytic metabolic profiles and mitochondrial biogenesis, leading to Nrf transcription, and ultimately impacting astrocyte energy resource and metabolism. Cocaine exacerbated these effects, leading to a worsening of neurodegeneration.
Keywords: HIV-1 Tat, Cocaine, oxidative stress, energy deficits, mitochondria, astrocytes
Introduction
Human immunodeficiency virus (HIV) is a major cause of human death. HIV infection either directly or indirectly affects the central nervous system (CNS), causing neurological impairments such as HIV-associated neurocognitive disorders (HAND), which are manifested by dysfunction and death of neurons in brain regions [1]. HIV-1 infection primarily affects immune cells such as microglial cells and subsequently impacts astrocytes and neurons [2]. The HIV-infected cells and activated immune cells in the brain release viral proteins, including HIV-1 transactivator protein (Tat). HIV-1 Tat is a 14–16 kDa protein and a key activator of HIV transcription in host cells. HIV-1 Tat induces viral replication but also induces cellular dysfunction [3] such as oxidative stress, modifying redox state, increasing inflammation, and increasing the production of various cytokines, chemokines, neurotransmitters and metabolites [4–6]. In addition, HIV-1 Tat regulates apoptosis and activates intracellular signaling cascade-mediated neurodegeneration [7]. In astrocytes, HIV-1 Tat induces oxidative stress and impairs mitochondrial function, ultimately leading to cell death [8]. Studies have shown that HIV-1 Tat mRNA expression is increased in HIV brain dementia. However, we do not yet understand the mechanism by which HIV-1 Tat impacts energy resource and oxidative metabolic dysfunction or how mitochondrial biogenesis and Nrf transcription impact astrocytic energy network-led neurodegeneration.
We are currently facing a global epidemic of psychostimulant abuse, including cocaine abuse. A significant proportion of cocaine users develop neuronal impairments. Cocaine use can deteriorate CNS cells, including astrocytes, oligodendrocytes, and microglia [9]. Cocaine causes numerous cellular dysfunctions and leads to immune and neuronal disorders, which can subsequently contribute to neurodegeneration and cognitive impairments. Cocaine causes inflammation, increases cytokine and chemokine production, and alters cellular metabolism, all of which affect CNS function [10, 11]. Cocaine use is often associated with HIV infection and can enlarge the major reservoir of viral replication and accelerate the progression of acquired immunodeficiency syndrome (AIDS) [12, 13], which also leads to neuronal impairment mediated by oxidative stress induction, altered energy resources, and metabolic fuel transfer [14]. Clinical observations suggest that cocaine use in conjunction with HIV infection may be associated with energy deficits in adenosine triphosphate (ATP) metabolic resources; this results in neuronal impairments in neuroplasticity and decreases the formation of axons and dendrites [15–17].
Brain energy requirements are very high, and astrocytes are active players in maintaining brain energy resources, as they are critically involved in metabolic functions such as producing and storing energy and supplying energy to neurons [18–20]. Metabolic enzymes have several cellular functions, including proteolysis and digestion, cellular respiration, energy storage, transcription, and responding to changes in the environment of CNS cells. These energy-intensive life-sustaining pathways are vital for the growth and maintenance of cellular integrity [21]. An increased level of oxidative stress, reactive oxygen species (ROS) production, and redox modification—such as a modified GSH/GSSG ratio—are all associated with metabolic dysfunction [22]. Glutathione (GSH) is a tripeptide that acts as a cell protectant in intracellular antioxidant defense mechanisms. Lower GSH levels have been correlated with an impaired metabolic system [23]. However, oxidative stress and redox modification re-entry seem counterintuitive to oxidative metabolism. Studies show that oxidative stress and redox changes increase ROS-mediated metabolic alterations and are correlated with energy deficits [24]. However, whether the oxidative stress response leads to activation of the energy sensor 5’ adenosine monophosphate-activated protein kinase (AMPK) may impact energy deficits.
Several studies have demonstrated that cocaine abuse and HIV infection inhibit energy metabolism and promote neuronal dysfunction [16, 25, 26]. Cocaine users with HIV show synergistically potentiated viral replication and disease progression compared to HIV patients who do not use cocaine [13, 27, 28]. Despite mounting evidence suggesting that cocaine use aggravates HIV infection, there has been no mechanistic study to determine the mutual role of cocaine and HIV infection in energy metabolism and their role in neurodegeneration. Here, we investigated the mechanisms by which HIV-1 Tat-induced oxidative stress alters the energy dysfunction and cocaine-accelerated effects in primary astrocytes. We then validated these mechanistic studies in a doxycycline-inducible astrocyte-specific HIV-1 Tat transgenic mouse (iTat) model.
In this study, we investigated the effects of HIV-1 Tat coupled with cocaine-mediated oxidative stress on the redox balance via glutathione synthetase (GSS), super oxide dismutase (SOD), catalase (CAT), energy sensor AMPKs and acetyl-CoA (ACC). We sought to examine the effects on the metabolic profiles of hexokinase (HK-I, HK-II), phosphofructokinase (PFK), lactate dehydrogenase A (LDHA), the mitochondrial biogenesis of peroxisome proliferator-activated receptor-γ coactivator (PGC)-1α, and mitochondrial transcription factor (TFAM). We found that the levels of nuclear factor erythroid (Nrf) transcription were inhibited, which in turn led to associated neuropathogenesis both in vitro and in vivo in the HIV-1 iTat transgenic mouse model. Furthermore, HIV-1 Tat and cocaine altered the astrocytic energy metabolic dysfunction, possibly affecting neuronal growth and neuroplasticity. Testing this, we collected conditioned medium from astrocytes exposed to HIV-Tat plus cocaine for 24 h. This conditioned medium was then used to treat SK-N-MC human neuroblastoma cells to identify impacts on neuronal development, maturation, and plasticity. Cocaine in combination with HIV-1 Tat exacerbated oxidative metabolism and the use of energy resources. This metabolic switch affected energy transfer in astrocyte-mediated neurodegeneration, both in vitro and in cocaine-exposed HIV-1 iTat mice.
Materials and Methods
Cell culture and Reagents
Cell culture reagents were purchased from ScienCell (Carlsbad, CA, USA). Antibodies against GSS, SOD, CAT, AMPK, PGC-1α, and TFAM and the goat anti-rabbit IgG and goat anti-mouse IgG antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies against ACC, HK-I, HK-II, PFK, LDHA, MCT-1 and MCT-4 were from Cell Signaling Technology (San Jose, CA, USA). Nrf-1 and Nrf-2 antibodies were purchased from Proteintech (Rosemont, IL, USA). Propidium iodide was acquired from BD bioscience (San Jose, CA, USA), and the electrophoresis reagents were from Bio-Rad (Richmond, CA, USA). The nitrocellulose membranes were purchased from Amersham Scientific (Piscataway, NJ, USA). All other reagents were purchased from Sigma–Aldrich (St. Louis, MO, USA).
HIV-1 Tat proteins
HIV-1 trans-activator of transcription (Tat) protein was obtained from the National Institutes of Health AIDS Research and Reference Reagent Program (Cat # 2222). The recombinant Tat protein had a purity level of >95%.
Human Primary Astrocytes
We used primary cell astrocytes isolated from human cerebellum, procured from ScienCell (Carlsbad, CA, USA). Astrocytes were maintained in astrocyte basal medium supplemented with, 10 ml of fetal bovine serum (FBS, Cat. No. 0010), 5 ml of astrocyte growth supplement (AGS, Cat. No. 1852) and 5 ml of penicillin/streptomycin solution (P/S, Cat. No. 0503) to a final concentration of 10% and 1% antibiotic/antimycotic solution.
Animals and Housing
Adult male HIV-1-inducible transgenic mice (iTat, formerly known as GT-tg bigenic mice [29]) were bred in a colony established at the University of Florida and founded by progenitors that were generously gifted by Dr. Johnny He. All mice (8–10 weeks of age) were maintained at the University of Florida (Gainesville, FL, USA) animal facility and used in experiments according to protocols approved by the Institutional Animal Care and Use Committee, in accordance with the 2011 National Institute of Health Guide for the Care and Use of Laboratory Animals. In conducting research using animals, the investigators adhere to the laws of the United States and regulations of the Department of Agriculture. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010). Initial sample sizes were approximated by Power analysis, with animals assigned to groups randomly. Drug treatment experiments were conducted in a blinded fashion. Note that no subjects were excluded from presentation and data analysis. Procedures for creating HIV-1 iTat mice and confirming the genotype of the inducible and brain-targeted HIV-1 Tat protein are described in detail elsewhere [29, 30].
Drug Treatment
Brain-targeted Tat protein was induced with doxycycline (Dox) treatment, both with and without exposure to cocaine. To express HIV-1 Tat 1–86, iTat mice received Dox via intraperitoneal (i.p.) injections administered daily over 14 days. A single daily dose of 100 mg/kg Dox dissolved in 0.9% saline (iTat-Dox; n=6) was administered in a volume of 0.3 ml/30 g body weight. The control group (n=6) consisted of iTat mice that received saline injections, as described in detail elsewhere [30]. A subset of mice received subcutaneous (s.c.) administrations of cocaine at a dose of 10 mg/kg/d in 0.9% saline either directly (n=6) or 2 min after the Dox treatment (n=6).
Isolating brain specimens for gene and immunoblotting analysis
After the 14-day treatment period, mice were anesthetized with isoflurane (4%) until they were euthanized and then tissues were harvested. We collected brains from three euthanized mice from each treatment set, flash froze the tissue in liquid nitrogen, and stored it at −80°C for gene and protein modification analyses, detailed below.
RNA Extraction and Real-time quantitative PCR (qRT-PCR)
For the in vitro experiment, we extracted total RNA from primary astrocytes (1 × 106) exposed to HIV-1 Tat (50 ng/ml) and cocaine (0.5 μM) for 24 h based on our previous report [31]. At the end of the incubation period, the cells were split using 0.05% trypsin-EDTA and washed with PBS. The cells were pelleted by centrifugation (1000 × g for 10 min) at room temperature (RT) and suspended in a mitochondrial assay buffer. The cells were incubated in the solution on ice for 2 min and then homogenized with a glass homogenizer using 30 up-down strokes. Total RNA was extracted from primary astrocytes and from the HIV-Tat (iTat) mice frontal lobe brain tissues using a Qiagen kit (Invitrogen Life Technologies, Carlsbad, CA, USA), following the manufacturer’s instructions. cDNA was synthesized using the total RNA (5 μg) and was amplified using specific primers for GSS (assay ID Hs00609286_m1), AMPK (Hs00272166_m1), and catalase (Hs00156308_m1). The housekeeping gene β-actin (Hs99999903_m1; Applied Biosystems, Foster City, CA, USA) served as a control for quantifying the real-time PCR results. We assessed the relative abundance of each mRNA species using a Q-PCR master mix from Stratagene. This master mix leverages an Mx3000P instrument to detect and plot the increase in fluorescence versus the PCR cycle number to produce a continuous measure of PCR amplification. We quantitated the relative mRNA species expression and calculated the mean fold change in expression of the target gene using the comparative CT method (transcript accumulation index, TAI = 2_ΔΔCT). The data were assessed for the quantity of RNA input measuring an endogenous reference gene, β-actin. In addition, the results from RNA obtained from treated samples were normalized to the results from RNA obtained from the control (untreated sample).
Mitochondrial Isolation
We used a Mitochondrial Isolation Kit (Abcam, CA, USA) to isolate mitochondria from astrocytes. Briefly, for the in vitro experiment, astrocytes (3 × 108) were grown and treated with HIV-1 Tat (50 ng/ml) and cocaine (0.5 μM) for 24 h. At the end of the incubation period, cells were split using 0.05% trypsin-EDTA and washed with PBS. The cells were pelleted by centrifugation (1000 × g for 10 min) at RT and suspended in a mitochondrial assay buffer. The cells were incubated in the solution on ice for 2 min and then homogenized with a glass homogenizer using 30 up-down strokes. We confirmed cellular disruption by microscopy. For the in vivo experiment, four groups of mice brain frontal lobe tissue (50 mg each) were homogenized: saline, cocaine-exposed, HIV-iTat, and cocaine-exposed HIV-iTat. The extracts were centrifuged at 800 × g for 10 min. The resulting pellet contained the nuclear fraction, and the supernatant was centrifuged at 12,000 × g for 15 min at 4°C. The mitochondrial pellet was then resuspended in lysis buffer for Western blot analysis according to the manufacture’s protocol.
Western blot analysis
We performed Western blotting to determine the contents of the redox-related proteins GSS, SOD, catalase, AMPK, and ACC. In addition, we examined the downstream effect of the glycolytic metabolic profiles of HK-I, HK-II, PMK, LDHA, MCT-1, MCT-4, mitochondrial PGC-1α, TFAM, and Nrf alterations in primary astrocytes and in HIV-iTat mice. Equal amounts of astrocytes and total cellular protein in the brain tissue were resolved on a 4–15% gradient sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gel. The gels were then transferred to a nitrocellulose membrane and incubated with their respective primary antibodies. We visualized the immunoreactive bands using a chemiluminescence Western blotting system from Amersham (GE Healthcare Life Sciences, Pittsburgh, PA, USA), following the manufacturer’s instructions.
Measuring SK-N-MC spine density and dendrite morphology
DiI staining
In brief, SK-N-MC neuroblastoma cells were grown in Eagle’s minimal essential medium containing 10% fetal bovine serum, 5 mM sodium pyruvate, 100 units/ml penicillin, 100 mg/ml streptomycin, and retinoic acid at 37°C with 5% CO2. SK-N-MC cells were grown on 22 mm×50 mm glass coverslips that were placed in a Petri dish. The cells were treated with the conditioned medium for 48 h. At the end of the incubation, the cells were fixed with 4% formaldehyde in PBS for 30 min at RT. The fluorescent membrane tracer 1, 1′-dioctadecyl-3, 3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI) at 7.5 μg/ml (in PBS) was directly added onto the fixed cells and allowed to incubate for 90 min at RT. The stained coverslips were placed in Petri dishes containing PBS and stored overnight at 4°C before proceeding with confocal microscopy.
Dendrite formation and spine density analysis
The dendrite formation and spine density were quantified by DiI-labeled cells using the ImageJ software program. Dendritic segments were chosen randomly from the apical and basal regions and at least one soma length away from the cell soma. Six parameters were measured: total dendrite area, dendrite diameter, spine density (the number of spines divided by the dendrite length), spine area and spine length [32].
Statistical analysis
Statistical analysis was performed using GraphPad Prism version 6 (GraphPad Software, San Diego, CA). We calculated the differences between the HIV-1 Tat, cocaine, HIV-1 Tat plus cocaine, and control groups using one-way ANOVA. Values are expressed as the mean ± standard error (SE), with a significance level of p < 0.05 utilized.
Results
HIV-1 Tat and cocaine shuttle astrocyte energy resources and promote neurodegeneration
HIV infection and HIV-1 proteins are known to induce oxidative stress and redox imbalance, which ultimately contributes to neurodegeneration [33, 34]. Illicit drugs such as cocaine also induce oxidative stress and affect cellular homeostasis. However, we do not yet understand how HIV-1 Tat and cocaine-induced redox modification leads to energy deficits, how energy metabolism affects astrocytic networks, or how these changes lead to neurodegeneration. Therefore, we determined whether HIV-1 Tat induces redox modification, energy metabolism, and mitochondrial biogenesis, leading to neurodegeneration. Ultimately, we sought to determine how these effects are accelerated by cocaine.
Our findings demonstrated that HIV-1 Tat and cocaine 1) significantly affects GSS and CAT and increased the level of SOD; and 2) activated AMPK expression, leading to the metabolic enzymes and mitochondrial biogenesis in human primary astrocytes culture. In addition, the validation analysis using total and mitochondrial fractions of HIV-iTat mice brain showed similar results and confirmed the in vitro pattern.
HIV-1 Tat and cocaine affect redox modification, energy metabolic profile genes, and protein expression in human primary astrocytes
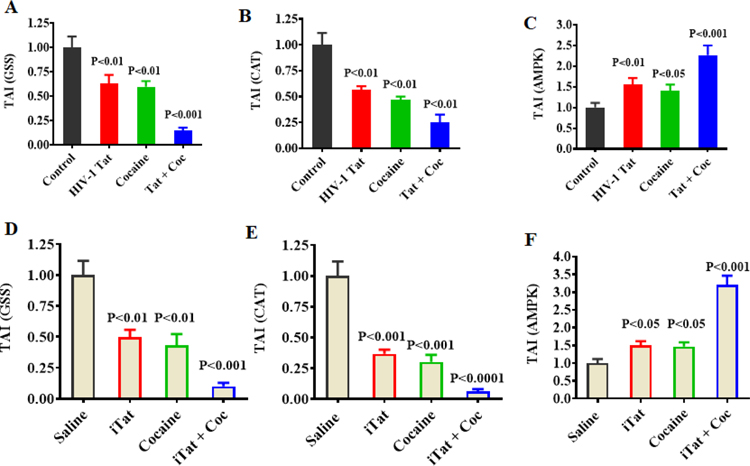
Figure 1 shows exposure to HIV-1 Tat, cocaine, and HIV-1 Tat plus cocaine impact the gene expression of GSS (A), CAT (B), and AMPK (C). These results indicated that HIV-1 Tat and cocaine exposure inhibited the levels of GSS and CAT, whereas these exposures significantly increased the gene expression of AMPK. Moreover, the combination of HIV-1 Tat plus cocaine accelerated these effects compared to control. These results suggest that HIV-1 Tat plus cocaine 1) increases oxidative stress by decreasing GSS and CAT, and 2) increases AMPK compared to either HIV-1 Tat or cocaine exposure alone in vitro (Figure 1 A–C) and in vivo (Figure 1 D–F).
Figure 1.
Effect of HIV-1 Tat and cocaine on redox-related gene expression and AMPK gene expression in in vitro and in vivo experiments. Primary human astrocytes (A, B and C; 1 × 106 cells/ml) were treated with HIV-1 Tat (50 ng), cocaine (0.5 μM) or a combination of HIV-1 Tat and cocaine for 24 h. The controls were maintained in drug-free medium. HIV-1 transgenic (iTat) mice (D, E and F) were treated for 14 days with a daily i.p. injection of either saline or iTat [doxycycline (Dox)-induced iTat expression] 100 mg/kg/d, with or without s.c. injection of 10 mg/kg/d cocaine, administered consecutively at 24 h intervals for 14 days. At the end of treatment, astrocytes and brain tissues were harvested, and RNA was extracted and reverse-transcribed. Next, quantitative real-time PCR was conducted for in vitro GSS (A), CAT (B), and AMPK (C), and in vivo GSS (D), CAT (E) and AMPK (F). Data are expressed as the mean ± SE of TAI values of five independent experiments.
We examined whether HIV-1 Tat-induced oxidative stress and modification of redox protein regulation are accelerated by cocaine. Figure 2 shows the effects of three treatment groups— HIV-1 Tat, cocaine alone, and HIV-1 Tat plus cocaine—on GSS (A), SOD (B), CAT (C), AMPK (D), and ACC (I). The densitometry analysis is shown in Figure 2 for GSS (E), SOD (F), CAT (G), P-AMPK (H), and P-ACC (M). These results confirmed that HIV-1 Tat-induced redox changes were accelerated by cocaine. Taken together, these findings suggest that HIV infection alters the redox imbalance and activates the energy sensor AMPK. Moreover, the addition of cocaine affects astrocytic gene and protein expression signaling cascades.
Figure 2.
Effect of HIV-1 Tat and cocaine on redox-related proteins and energy metabolism glycolytic profiles. Primary human astrocytes (1 × 106 cells/ml) were treated for 24 h with either HIV-1 Tat (50 ng), cocaine (0.5 μM), or a combination of HIV-1 Tat and cocaine. Controls were maintained in drug-free medium. At the end of the incubation, equal amounts of protein lysate were resolved by 4–15% SDS-PAGE. We analyzed protein expression by Western blot: GSS (A), SOD (B), CAT (C), AMPK (D), ACC (I), HK-I (J), HK-II (K), PKM1/2 (L), LDH (Q), MCT-1 (R), and MCT-4 (S). Panels E, F, G, H, M, N, O, P, T, U and V represent the percent densitometric value of the protein levels (as % control). Data are expressed as the mean ± SE of five independent experiments.
HIV-1 Tat and cocaine impact oxidative metabolism and glycolytic profiles in astrocytes
Reduced levels of GSH are known to affect cellular functions, such as intracellular signaling networks, which are mediated by several cellular neurotransmitters and ion channels. Furthermore, metabolic profiles control the energy resources of glucose storage and energy transfer levels [35]. Therefore, we investigated whether exposure to HIV-1 Tat influences astrocytic energy resources and leads to cellular oxidative metabolism in glycolytic enzyme-mediated neurodegeneration. In Figure 2, primary astrocytes treated with HIV-1 Tat and cocaine (for 24 h) showed significant reductions in HK-I. In contrast, HK-II, PKM1/2, LDHA, MCT-1 and MCT-4 showed significantly increased protein expression levels at 24 h compared with treatment with either HIV-1 Tat or cocaine alone. Figure 2 shows the effects of HIV-1 Tat plus cocaine on HK-I (J), HK-II (K), PKM1/2 (L), LDHA (Q), MCT-1 (R) and MCT-4 (S) protein expression. Directly comparison of these results with the control is reported by densitometry analysis in Figure 2 (see HK-I (N), HK-II (O), PKM1/2 (P), LDH (T), MCT-1 (U), and MCT-4 (V), respectively). These protein activations confirmed changes in the metabolic profile of glycolytic enzymes, underscoring that cocaine accelerates the impact on astrocytic energy networks.
HIV-1 Tat and cocaine accelerate energy deficits and lead to mitochondrial biogenesis and Nrf transcription
HIV infection affects astrocyte energy storage, utilization, and transfer of energy resources, ultimately contributing to CNS dysfunction [25, 36–38]. Astrocytes are the major energy storage sites of glycogen granules in the CNS. HIV infection is known to target the brain energy environment and subsequently affects cellular functions in the CNS. Therefore, we examined how HIV-1 Tat plus cocaine affects energy resources and oxidative metabolism, and the downstream impact on mitochondrial biogenesis-mediated astrocyte signaling networks. Figure 3 shows that astrocytes treated with HIV-1 Tat and cocaine display significant upregulation of levels of PGC-1α and the mitochondrial transcriptional factor TFAM compared with either HIV-1 Tat or cocaine treatment alone. This result was consistent in the cytoplasm of primary astrocytes (Figure 3A and B), the mitochondrial fraction (Figure 3E and F), the densitometry analysis of cytoplasmic PGC-1α and TFAM (Figure 3C and D), and in the mitochondria (Figure 3G and H). In addition, HIV-1 Tat plus cocaine increased the level of oxidative phosphorylation (OXPHOS; Figure 3I and J), which impacts oxidative metabolism and is led by PGC-1α and TFAM, which target Nrf transcription. However, Nrf transcriptional factors bind to antioxidant gene elements and control oxidative stress, which is mediated by Nrf transcription. Therefore, we investigated Nrf transcriptional factors 1 and 2 by exposing astrocytes to the combination of HIV-1 Tat and cocaine. Interestingly, our results showed that Nrf-1 and Nrf-2 were differentially regulated; that is, Nrf-1 was significantly downregulated, and Nrf-2 was significantly upregulated in the cytoplasm. Nrf-2 was subsequently decreased in mitochondria fractions (Figure 3K–N).
Figure 3.
Impact of HIV-1 Tat and cocaine on mitochondrial biogenesis. Primary human astrocytes (1 × 106 cells/ml) were treated with HIV-1 Tat (50 ng), cocaine (0.5 μM), or a combination of HIV-1 Tat and cocaine for 24 h. Controls were maintained in drug-free medium. At the end of incubation, equal amounts of protein lysate were resolved by SDS-PAGE, and protein expression was analyzed by Western blot: cytoplasmic PGC-1α (A), TFAM (B), mitochondrial PGC-1α (E), AFTM (F), OXPHOS (I), cytoplasmic NRF1 and 2 (K), and mitochondrial NRF1 and 2 (L). Panels C, D, G, H, J, M and N represent the % densitometric value.
HIV-1 Tat and cocaine impact neuroplasticity and spine density
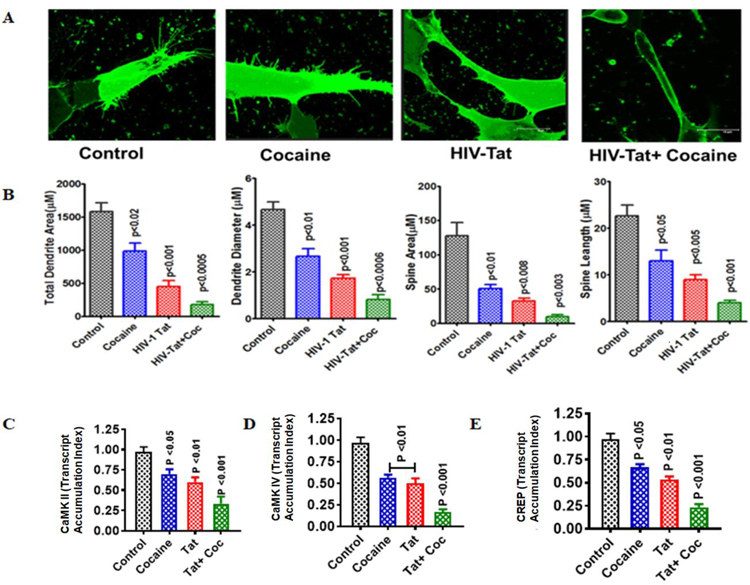
SK-N-MC neuroblastoma cells were exposed to conditioned medium collected from astrocytes exposed to HIV-1 Tat plus cocaine for 48 h in vitro, and the expression levels of neuroplasticity genes CaMK and CREP were analyzed. The inhibition of CaMK II and IV and CREP ultimately impacted dendrite formation, growth, space diameter, and spine density (Figure 4). These effects lead to neuroplasticity dysfunction and demolished the formation of neuronal growth and spine density (Figure 4A and B). The activated gene expression of CaMK II and IV and CREP is shown in Figure 4C, D and E.
Figure 4.
Impact of HIV-1 Tat and cocaine on neuroplasticity. SK-N-MC human neuroblastoma cells (1 × 106 cells/ml) were grown on glass coverslips for microscopy studies and gene expression experiments. The cells were treated for 48 h with conditioned medium [collected from HIV-1 Tat (50 ng), cocaine (0.5 μM), or a combination of HIV-1 Tat and cocaine exposure for 24 h]. Cover slips for microcopy observations were stained with DiI and observed under a confocal microscope. (A) Randomly selected images from each group (control, HIV-Tat, cocaine, and HIV-Tat plus cocaine). (B) ImageJ software was used to analyze the total dendrite area and diameter and the total spine area and length. At the end of incubation, we extracted the RNA and reverse-transcribed it, followed by quantitative real-time PCR for CaMK II (C), CaMK IV (D), and CREB (E). Data are expressed as the mean ± SE of three or more independent experiments.
HIV-1 iTat mice with cocaine exposure showed altered oxidative metabolism and glycolytic profiles
Our validation analysis (shown in Figure 5) of the in vivo HIV-1 iTat mice model suggested that oxidative stress-induced redox imbalance inhibits GSS (A) and catalase (E), which subsequently increased the level of SOD1 (C) and the astrocytic marker GFAP (G); it also activated the energy sensor AMPK (I). Figure 5 panels B, D, F, H, and J show the densitometry analyses of GSS, SOD1, catalase, GFAP, and AMPK expression, respectively. The energy sensor AMPK is known to alter glycolytic metabolic enzymes, which was validated in the analysis from cocaine-exposed HIV-iTat mouse brain. The data in Figure 6 demonstrate that HK-II (A), LDH (C), PYK (E) and PMK1/2 (G) were increased in HIV-iTat mice. Moreover, these effects were accelerated by cocaine. Figure 6 panels B, D, F, and H show the densitometry analysis of HK-II, LDH, PYK, and PMK1/2, respectively.
Figure 5.
Impact of HIV-1 Tat and cocaine on redox-related protein expression and energy sensor protein in vivo. HIV-1-inducible Tat transgenic (iTat) or saline-administered (control) mice received daily intraperitoneal injections of saline or doxycycline (Dox; 100 mg/kg/d) over 14 days, with or without s.c. injection of cocaine (10 mg/kg/d). Following treatment, equal amounts of protein lysate from harvested brain tissue were resolved, and protein expression was analyzed by Western blot: GSS (A), SOD (C), CAT (E), GFAP (G), and AMPK (I). Figure B, D, F H, and J represent the percent densitometric value protein levels (% control). Data are expressed as the mean ± SE of three independent experiments.
Figure 6.
Effects of cocaine and HIV on the energy metabolic profiles of glycolytic enzymes in HIV-transgenic (iTat) mice. HIV-1-inducible Tat transgenic (iTat) mice received daily i.p. injections of saline or doxycycline (Dox; 100 mg/kg/d) over 14 days, with or without s.c. injection of cocaine (10 mg/kg/d). Following treatment, equal amounts of protein lysate from harvested brain tissue were resolved by 4–15% SDS-PAGE. Protein expression was analyzed by Western blot: HK-II (A), LDH (C), PYK (E), and PMK1/2 (G). Figure B, D, F and H represent percent densitometric value protein levels (as % control). The data are expressed as the mean ± SE of three independent experiments.
HIV-1 iTat mice with cocaine exposure lead to oxidative phosphorylation, mitochondrial biogenesis and NRF transcription
Energy resources, transfer, and utilization are maintained by oxidative metabolism. Glycolytic enzymes are the major players in oxidative metabolism; changes in glycolytic enzymes are known to activate OXPHOS-mediated mitochondrial biogenesis. In vivo validation analysis of the cocaine-exposed HIV-iTat mice identified a synergistic acceleration of OXPHOS compared to control mice (Figure 7A and B) which led to mitochondrial biogenesis. Figure 7 shows that unlike either HIV-iTat mice or mice exposed to cocaine alone, the combination of cocaine and HIV-iTat upregulated PGC-1α and TFAM in the cytoplasm and mitochondrial fraction compared to control samples (Figure 7C–F). These data confirm that the mitochondrial biogenesis of PGC-1α and TFAM is activated by OXPHOS, which may impact Nrf transcription. However, Nrf transcription is known to control the antioxidant responsive elements (ARE) of mitochondrial genes [39]. Figure 7 (G–J) suggests that the inhibition of Nrf-1 and Nrf-2 in the cytoplasm and mitochondria may promote neurodegeneration. This effect might be accelerated by the presence of cocaine. However, these two isoforms (Nrf-1 and Nrf-2) were similarly altered in the cells exposed in vitro to HIV-Tat plus cocaine. In fact, our in vivo validation analysis in HIV-1 iTat mice with cocaine exposure confirmed these effects. The overall analysis performed with in vitro and in vivo models is summarized in Table 1.
Figure 7.
Impact of HIV-1 Tat and cocaine on mitochondrial biogenesis and OXPHOS-impaired Nrf transcription. HIV-1 Transgenic (iTat) mice received daily i.p. injections of saline or doxycycline (Dox; 100 mg/kg/d) over 14 days, with or without s.c. injection of cocaine (10 mg/kg/d). After the treatment course, equal amounts of protein lysate were resolved by 4–15% SDS-PAGE. Protein expression was analyzed by Western blot: cytoplasmic PGC-1α, TFAM (C) and mitochondrial PGC-1α, TFAM (E), OXPHOS (A), cytoplasmic NRF1 and 2 (G) and mitochondrial NRF1 and 2 (I). Figure B, D, F, H, and J represent % densitometric value protein levels (as % control). Data are expressed as the mean ± SE of three independent experiments.
Table 1.
Results summary of the impacts of HIV-Tat/iTat and cocaine on neurodegeneration in in vitro and in vivo models.
| Protein and gene expression profile | Expression levels of poteins involving in HIV and cocaine mediated neurodegeneration | In vitro | In vivo |
|---|---|---|---|
| Redox and energy sensor | GSS, CAT | ↓ | ↓ |
| SOD-1 | ↑ | N.S. | |
| AMPK | ↑ | ↑ | |
| ACC | ↑ | – | |
| Glycolytic Profile | HK-1 | N.S. | – |
| HK-II, PKM1/2, LDHA | ↑ | ↑ | |
| MCT-1, MCT-4 | ↑ | – | |
| PYK GFAP | – | ↑ | |
| Mitochondrial biogenesis | PGC-1 alpha | ↑ | N.S. |
| TFAM | ↑ | ↑ | |
| OXPHOS | ↑ | ↑ | |
| NFR-1 | ↓ | ↓ | |
| NRF-2 | ↓ | ↓ | |
| Neuroplasticity | CaK II. CaK IV, CREB | ↓ | – |
Discussion
HIV infection and illicit drug use are known to induce oxidative stress and ROS production, which can ultimately impact cellular functions such as oxidative metabolism and cause energy deficits [25, 40–42]. GSH plays an important cell-protectant role by exerting an antioxidant defense mechanism and closely linked to the energy source of ATP production. Low levels of GSH have been associated with an impaired immune response and neuronal dysfunction; its metabolites also interface with energy and neurotransmitter syntheses through several prominent metabolic pathways [43, 44]. HIV infection affects cellular redox by altering GSS, SOD and catalase, as evidenced in HIV-infected patients and cocaine users [45–47]. However, the inhibition of redox through an imbalanced GSH/GSSG ratio [48] activates the energy sensor AMPK and subsequently impacts the energy profile machinery of metabolic enzymes such as HK-1, HK-II, PKM, LDH, MCT-1, and MCT-4, all of which play vital roles in neuronal dysfunction and in the disease progression of HIV-associated dementia (HAD) patients [49]. In the CNS, astrocytes are the major reservoirs of energy storage, and they enable the maintenance of cellular energy metabolism. Astrocyte function is modulated by HIV infection and drug use, both of which lead to neurodegeneration [12, 34, 50]. This study showed that HIV-1 iTat mice had increased levels of the astrocyte marker GFAP, which is associated with the formation of GFAP aggregates and with HIV-1 iTat-mediated neurotoxicity [51]. HIV-1 Tat impacts several cellular functions, but we do not yet understand the effects of HIV-1 Tat and cocaine-induced redox imbalance on energy deficits, oxidative metabolism, and ultimately, neurodegeneration. Our observations provide new insights into the functional roles of redox imbalance. This imbalance induced by the combined exposure to HIV-1 Tat and cocaine can alter the energy profiles of glycolytic enzymes and mitochondrial biogenesis, leading to Nrf transcription, which is a potential sign of neurodegeneration.
We demonstrated for the first time that exposure to HIV-1 Tat, cocaine, and HIV-1 Tat plus cocaine leads to redox inhibition of GSS and catalase and subsequently increases the levels of SOD and activates the energy sensor AMPK. Redox imbalance is known to disturb cellular homeostasis [35]. Our study suggests that cocaine combined with HIV–1 Tat may play an enhanced role in oxidative metabolism. Our results are consistent with earlier reports of HIV-gp120-induced changes in microglia and HIV-Tat-induced changes in astrocytes, where activation of the redox and oxidative pathways were observed [25, 26]. Furthermore, studies have shown that oxidative stress and redox impairment are associated with deficits in oxidative metabolism and energy levels, leading to metabolic changes associated with neuronal impairments, such as the decreased formation and density of dendritic spines, and neuroplasticity of dendrites [52, 53, 54]. AMPK is a key regulator of metabolism and survival during energy stress. The dysregulation of AMPK is strongly associated with oxidative injury, which impacts redox modifications. Defective oxidative metabolism and a reduced level of ATP are both known to activate AMPK [55]. AMPK plays a critical role in controlling both cellular and whole-body metabolic responses. However, it is not clear how HIV-1 Tat and cocaine regulate AMPK, which in turn impacts the intracellular redox status (i.e., the levels of GSS, CAT and SOD). These studies confirm that redox imbalance and energy dysfunction affect glycolytic enzymes and mitochondrial biogenesis. These factors, in turn, activate OXPHOS and impair Nrf transcription, which ultimately leads to astrocytic signaling-mediated neurodegeneration.
Our results also show that exposure to cocaine, HIV-1 Tat, and the combination of cocaine and HIV-1 Tat, causes the induction of redox inhibition and the activation of AMPK associated with altered glycolytic enzymes and with increased mitochondrial biogenesis. Importantly, HIV-1 Tat, cocaine, and cocaine with HIV-Tat all led to oxidative metabolism, which in turn impaired mitochondrial biogenesis and subsequently reduced the level of energy resource and transfer. However, HIV-positive cocaine users have higher energy demands, which increases ATP utilization and subsequently affect glycolytic enzymes. This study suggests that HIV-1 Tat-induced redox inhibition and energy demand are accelerated by cocaine use compared with either cocaine use or HIV infection alone. These results confirm previous reports of HIV-1 Tat-induced metabolic profiles [26].
Previous studies have also demonstrated that HIV infection and HIV proteins affect oxidative metabolism and glycolytic enzyme regulation, which may impact mitochondrial biogenesis [56]. The first step in the breakdown of glucose extracts energy for cellular metabolism. Glucose phosphorylation is catalyzed by the enzyme HK, which is the predominant isoform of HK I and HK-II. Importantly, HK-I and HK-II contain a hydrophobic terminal mitochondrial-binding motif. Energy metabolism in glycolysis is mainly thought of as a cytosolic process, and HK-I is predominantly associated with mitochondria in ATP production. In contrast, HK-II is located in the cytosol and at the mitochondrial outer membrane [57]. Interestingly, our results demonstrated that HIV–1 Tat and cocaine exposure independently downregulated HK-I and upregulated HK-II expression; moreover, these effects were augmented when cocaine exposure was combined with HIV-1 Tat. These results confirm that increased HK-II expression and its binding to mitochondria facilitate and increase the levels of aerobic glycolysis and lactate production. However, the increased level of HK-II also induces mitochondria-mediated cell death, which is known to activate LDH. Furthermore, monocarboxylic acid transport (MCT) is a metabolic target of HK-II, wherein the flux of small ketone bodies such as lactic acid and pyruvic acid occurs to support metabolic demands. The predominant role of MCTs 1–4 is the transport of L-lactate, pyruvate, and ketone bodies in and out of cells; where L-lactate is quantitatively the most important substrate [58]. MCT-1 is responsible for the efflux of lactic acid when the oxygen supply is compromised, stimulating glycolysis under this condition. However, glycolytic profiles in astrocytes treated with HIV-Tat and cocaine displayed significant upregulation of MCT-1 and MCT-4, which serve to increase lactic acid levels and initiate neurodegeneration. Recent studies have found that in the brain, MCT-1 and MCT-4 export astrocyte-produced lactic acid, which is then taken up by MCT-1 or MCT-2 into the neurons for oxidation as an important respiratory fuel [59].
The energy sensor AMPK is a key regulator that activates PGC-1α, a member of the peroxisome proliferator-activated receptor-gamma (PGC) family of transcriptional coactivators, and the PGC family is the master regulator of mitochondrial biogenesis [60]. AMPK activates different transcriptional factors that promote the expression of TFAM, including Nrf-1 and Nrf-2. The oxidative stress response is increased by antioxidant defenses through the activation of Nrf, an important transcription factor [61, 62]. Nrf is the main player in the controlled antioxidant-response element (ARE) found in the promoter regions of many genes that encode antioxidant and detoxification enzymes (e.g., SOD1, CAT, and GPx1) [39]. In addition, Nrf-1 and Nrf-2 are important contributors to the sequence of events that increase the transcription of key mitochondrial enzymes. Nrf-1 and −2 can interact with TFAM, which drives the transcription and replication of mtDNA [63]. TFAM is a downstream target gene of PGC-1α and controls the transcription of mitochondrial DNA-encoded genes as well as DNA replication during biogenesis [64].
In the present study, exposure to HIV-Tat plus cocaine led to the activation of PGC-α1 (unlike the control group). The level of TFAM protein in the total and mitochondrial fractions was increased after exposure to HIV-1 Tat or cocaine alone. Compared to control samples, these effects were increased by HIV-Tat combined with cocaine in total cell lysates and in the mitochondrial fraction. Other studies have confirmed that the amount of mitochondrial damage induced by increasing mitochondrial biogenesis depends on upregulated PGC-1α expression [65, 66]. In addition, increased oxidative damage and increased levels of PGC-1α and TFAM in astrocytes might enhance the susceptibility of mtDNA to fragmentation, which can potentially cause neuronal dysfunction and result in neurodegeneration. Therefore, it is possible that the impact of energy consumption during HIV infection inhibits redox-related gene expression and dysregulates AMPK-mediated metabolic function and mitochondrial impairment, thus leading to Nrf transcription. Several studies have shown that interrupting glycolysis can contain oxidative metabolism, but has an adverse effect on CNS function that is out of proportion with any change in total energy status [67, 68]. In addition, a clinically significant energy imbalance may also occur as a result of disrupting important energy-generating enzyme systems in mitochondria. In support of our current results, a recent report demonstrated that mitochondrial biogenesis is differentially regulated in neurons and astroglia in the brains of patients with HIV-associated neurocognitive disorders (HANDs,) and that astroglial bioenergetics processes could be tagged [69]. The HAND-associated activation of glia cells releases neurotoxic substances such as inflammatory cytokines, chemokines, glutamate, and nitric oxide radicals, which leads to disturbed bioenergetic homeostasis and neuroinflammation. Our recent studies also showed that HIV-associated accumulation of mtDNA damage and inflammatory stimulation contributes to HANDs [70, 71]. Taken together, these findings indicate that PGC-1α plays a crucial role in linking HIV infection and cocaine abuse to the internal metabolic response of mitochondrial biogenesis through, among other routes, the level, and actions of NRF transcription factors.
Overall, our findings suggest a connection between redox genes and protein enhancement, the activation of AMPK, and altered intracellular signaling mechanisms in cells treated with HIV-Tat plus cocaine. The enhanced redox gene inhibition and altered oxidative metabolism by cocaine and HIV-Tat may lead to increased energy utilization and ultimately affect the storage of energy resources and the transfer of metabolic fuel, potentially contributing to cell death. The present study supports the idea that oxidative stress and redox-dependent gene expression are associated with energy dysfunction, particularly with the reduction of glutathione (GSH) and the activation of AMPK, glycolytic enzymes, and mitochondrial biogenesis. SK-N-MC neuroblastoma cells exposed to conditioned medium had a decreased number of spines and a decrease in dendrite diameter, dendrite area, and spine area compared to control cells. These results suggest that either cocaine or HIV-infected astrocytes play a vital role in energy storage, metabolic dysfunction, and neurodegeneration.
Summary and Conclusion
This study reveals that HIV-Tat-induced redox imbalance activates the energy sensor AMPK, which increases the levels of energy deficit, mitochondrial biogenesis, and Nrf transcription. The metabolic switch, in turn, affects the process of energy transfer to neurons. Based on these results, a redox imbalance potentiates HIV viral replication and disease progression by impairing the metabolic function of astrocytes, which may lead to neurodegeneration. The present study extends insights from earlier reports of energy metabolism dysfunction and increased neurodegeneration in HIV patients exposed to cocaine and to HIV-derived proteins [25, 26].
Acknowledgments
Funding
The present study was supported by a grant from the National Institutes of Health (NIH): R01DA 044872 to S. Thangavel.
Abbreviations
- iTat
HIV-1 inducible Tat (transgenic mice)
- HIV-1 Tat
Trans activator protein
- ROS
Reactive oxygen species
- GFAP
glial acidic fibril protein
- GSS
glutathione synthetase
- CAT
Catalase
- SOD
super oxide dismutase
- HAND
HIV-associated neurocognitive disorders
- AMPK
5' AMP-activated protein kinase
- HK
Hexo kinase
- ACC
Acetyl coenzyme A
- PFK
Phosphofructokinase
- LDHA
Lactate dehydrogenase
- MCT
Monocarboxylate transporters
- PGC1α
Peroxisome proliferator-activated receptor gamma coactivator 1-alpha
- TFAM
transcription factor A, mitochondrial
- CaMK
Ca2+/calmodulin-dependent protein kinase
- CREB
cAMP response element-binding protein
- ACC
Acetyl-CoA carboxylase
- OXPHOS
Oxidative phosphorylation
- NRF
Nuclear respiratory factor
- ARE
Antioxidant response element
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Ethics Statement
The studies involving mice were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Florida, Gainesville, Florida, in accordance with the 2011 National Institute of Health Guide for the Care and Use of Laboratory Animals.
Conflict of interest
The authors declare that they have no conflicts of interest.
References:
- 1.Kaul M, Zheng J, Okamoto S, et al. (2005) HIV-1 infection and AIDS: Consequences for the central nervous system. Cell Death Differ 12:878–892. 10.1038/sj.cdd.4401623 [DOI] [PubMed] [Google Scholar]
- 2.Minagar A, Shapshak P, Fujimura R, et al. (2002) The role of macrophage/microglia and astrocytes in the pathogenesis of three neurologic disorders: HIV-associated dementia, Alzheimer disease, and multiple sclerosis. J Neurol Sci 202:13–23. 10.1016/s0022-510x(02)00207-1 [DOI] [PubMed] [Google Scholar]
- 3.Amarapal P, Tantivanich S, Balachandra K, et al. (2005) The role of the TAT gene in the pathogenesis of HIV infection. Southeast Asian J Trop Med Public Health 36:352–361 [PubMed] [Google Scholar]
- 4.Sabatier JM, Mabrouk K, Vives E, et al. (1992) Evidence for neurotoxic activity of Tat from human immunodeficiency virus type 1. J Virol 65: 961–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peruzzi F (2006) The multiple functions of HIV-1 Tat: proliferation versus apoptosis. Front Biosci 11:708 10.2741/1829 [DOI] [PubMed] [Google Scholar]
- 6.Chauhan A, Turchan J, Pocernich C, et al. (2003) Intracellular human immunodeficiency virus Tat expression in astrocytes promotes astrocyte survival but induces potent neurotoxicity at distant sites via axonal transport. J Biol Chem 278:13512–13519. 10.1074/jbc.M209381200 [DOI] [PubMed] [Google Scholar]
- 7.Bartz SR, Emerman M (1999) Human immunodeficiency virus type 1 Tat induces apoptosis and increases sensitivity to apoptotic signals by up-regulating FLICE/caspase-8. J Virol 73:1956–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Simone FI, Darbinian N, Amini S, et al. (2016) HIV-1 Tat and Cocaine Impair Survival of Cultured Primary Neuronal Cells via a Mitochondrial Pathway. J Neuroimmune Pharmacol 11:358–368. 10.1007/s11481-016-9669-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Badisa RB, Kumar SS, Mazzio E, et al. (2015) N-acetyl cysteine mitigates the acute effects of cocaine-induced toxicity in astroglia-like cells. PLoS One 10: e0114285 10.1371/journal.pone.0114285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Narvaez JCM, Magalhães PV, Fries GR, et al. (2013) Peripheral toxicity in crack cocaine use disorders. Neurosci Lett 544:80–84. 10.1016/j.neulet.2013.03.045 [DOI] [PubMed] [Google Scholar]
- 11.Castilla-Ortega E, Ladrón de Guevara-Miranda D, Serrano A, et al. (2017) The impact of cocaine on adult hippocampal neurogenesis: Potential neurobiological mechanisms and contributions to maladaptive cognition in cocaine addiction disorder. Biochem Pharmacol 141:100–117. 10.1016/j.bcp.2017.05.003 [DOI] [PubMed] [Google Scholar]
- 12.Nath A, Hauser KF, Wojna V, et al. (2002) Molecular basis for interactions of HIV and drugs of abuse. In: Journal of Acquired Immune Deficiency Syndromes. J Acquir Immune Defic Syndr 31:62–9. 10.1097/00126334-200210012-00006 [DOI] [PubMed] [Google Scholar]
- 13.Samikkannu T, Rao KVK, Arias AY, et al. (2013) HIV infection and drugs of abuse: Role of acute phase proteins. J Neuroinflammation 10:113 10.1186/1742-2094-10-113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bélanger M, Allaman I, Magistretti PJ (2011) Brain energy metabolism: Focus on Astrocyte-neuron metabolic cooperation. Cell Metab 4:724–738. 10.1016/j.cmet.2011.08.016 [DOI] [PubMed] [Google Scholar]
- 15.Valcour V, Shiramizu B (2004) HIV-associated dementia, mitochondrial dysfunction, and oxidative stress. Mitochondrion 4:119–129. 10.1016/j.mito.2004.05.009 [DOI] [PubMed] [Google Scholar]
- 16.Chang E, Sekhar R, Patel S, Balasubramanyam A (2007) Dysregulated Energy Expenditure in HIV-Infected Patients: A Mechanistic Review. Clin Infect Dis 44:1509–1517. 10.1086/517501 [DOI] [PubMed] [Google Scholar]
- 17.Schweinsburg BC, Taylor MJ, Alhassoon OM, et al. (2005) Brain mitochondrial injury in human immunodeficiency virus-seropositive (HIV+) individuals taking nucleoside reverse transcriptase inhibitors. J Neurovirol 11:356–364. 10.1080/13550280591002342 [DOI] [PubMed] [Google Scholar]
- 18.Araque A (2006) Astrocyte-neuron signaling in the brain--implications for disease. Curr Opin Investig Drugs 7:619–24. [PubMed] [Google Scholar]
- 19.Sidoryk-Wegrzynowicz M, Wegrzynowicz M, Lee E, et al. (2011) Role of Astrocytes in Brain Function and Disease. Toxicol Pathol 39:115–123. 10.1177/0192623310385254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allaman I, Bélanger M, Magistretti PJ (2011) Astrocyte-neuron metabolic relationships: For better and for worse. Trends Neurosci 34:76–87. 10.1016/j.tins.2010.12.001 [DOI] [PubMed] [Google Scholar]
- 21.van der Knaap JA, Verrijzer CP (2016) Undercover: Gene control by metabolites and metabolic enzymes. Genes Dev 30:2345–2369. 10.1101/gad.289140.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forrester SJ, Kikuchi DS, Hernandes MS, et al. (2018) Reactive oxygen species in metabolic and inflammatory signaling. Circ Res 22:877–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dringen R (2000) Metabolism and functions of glutathione in brain. Prog Neurobiol 62:649–671. 10.1016/s0301-0082(99)00060-x [DOI] [PubMed] [Google Scholar]
- 24.Cobley JN (2018) Synapse Pruning: Mitochondrial ROS with Their Hands on the Shears. BioEssays 40:1800031 10.1002/bies.201800031 [DOI] [PubMed] [Google Scholar]
- 25.Samikkannu T, Atluri VSR, Nair MPN (2016) HIV and cocaine impact glial metabolism: Energy sensor AMP-activated protein kinase role in mitochondrial biogenesis and epigenetic remodeling. Sci Rep 6:31784 10.1038/srep31784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Natarajaseenivasan K, Cotto B, Shanmughapriya S, et al. (2018) Astrocytic metabolic switch is a novel etiology for Cocaine and HIV-1 Tat-mediated neurotoxicity article. Cell Death Dis 9:415 10.1038/s41419-018-0422-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baum MK, Rafie C, Lai S, et al. (2009) Crack-cocaine use accelerates HIV disease progression in a cohort of HIV-positive drug users. J Acquir Immune Defic Syndr 50:93–99. 10.1097/QAI.0b013e3181900129 [DOI] [PubMed] [Google Scholar]
- 28.Cofrancesco J, Scherzer R, Tien PC, et al. (2008) Illicit drug use and HIV treatment outcomes in a US cohort. AIDS 22:357–365. 10.1097/QAD.0b013e3282f3cc21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hee JK, Martemyanov KA, Thayer SA (2008) Human immunodeficiency virus protein Tat induces synapse loss via a reversible process that is distinct from cell death. J Neurosci 28:12604–12613. 10.1523/JNEUROSCI.2958-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paris JJ, Carey AN, Shay CF, et al. (2014) Effects of conditional central expression of HIV-1 tat protein to potentiate cocaine-mediated psychostimulation and reward among male mice. Neuropsychopharmacology 39:380–388. 10.1038/npp.2013.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Samikkannu T, Rao KVK, Pilakka Kanthikeel S, et al. (2014) Immunoneuropathogenesis of HIV-1 clades B and C: Role of redox expression and thiol modification. Free Radic Biol Med 69:136–144. 10.1016/j.freeradbiomed.2013.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith DL, Pozueta J, Gong B, et al. (2009) Reversal of long-term dendritic spine alterations in Alzheimer disease models. Proc Natl Acad Sci USA 106:16877–16882. 10.1073/pnas.0908706106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samikkannu T, Agudelo M, Gandhi N, et al. (2011) Human immunodeficiency virus type 1 clade B and C gp120 differentially induce neurotoxin arachidonic acid in human astrocytes: Implications for neuroAIDS. J Neurovirol 17:230–238. 10.1007/s13365-011-0026-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buch S, Yao H (2011) HIV-1 Tat Toxin In: Reproductive and Developmental Toxicology. Elsevier Inc; pp 773–780 [Google Scholar]
- 35.Halliwell B (1992) Reactive Oxygen Species and the Central Nervous System. J Neurochem 59:1609–1623. 10.1111/j.1471-4159.1992.tb10990.x [DOI] [PubMed] [Google Scholar]
- 36.Dickens AM, Anthony DC, Deutsch R, et al. (2015) Cerebrospinal fluid metabolomics implicate bioenergetic adaptation as a neural mechanism regulating shifts in cognitive states of HIV-infected patients. AIDS 29:559–569. 10.1097/QAD.0000000000000580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cotto B, Natarajanseenivasan K, Langford D (2019) HIV-1 infection alters energy metabolism in the brain: Contributions to HIV-associated neurocognitive disorders. Prog Neurobiol 181:101616 10.1016/j.pneurobio.2019.101616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ratai EM, Annamalai L, Burdo T, et al. (2011) Brain creatine elevation and N-acetylaspartate reduction indicates neuronal dysfunction in the setting of enhanced glial energy metabolism in a macaque model of NeuroAIDS. Magn Reson Med 66:625–634. 10.1002/mrm.22821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Itoh K, Chiba T, Takahashi S, et al. (1997) An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun 236:313–322. 10.1006/bbrc.1997.6943 [DOI] [PubMed] [Google Scholar]
- 40.Perfeito R, Cunha-Oliveira T, Rego AC (2013) Reprint of: Revisiting oxidative stress and mitochondrial dysfunction in the pathogenesis of Parkinson disease - Resemblance to the effect of amphetamine drugs of abuse. Free Radic Biol Med 62:186–201. 10.1016/j.freeradbiomed.2013.05.042 [DOI] [PubMed] [Google Scholar]
- 41.Valente MJ, Carvalho F, Bastos M d. L, et al. (2012) Contribution of Oxidative Metabolism to Cocaine-Induced Liver and Kidney Damage. Curr Med Chem 19:5601–5606. 10.2174/092986712803988938 [DOI] [PubMed] [Google Scholar]
- 42.Dutta R, Roy S (2012) Mechanism(s) Involved in Opioid Drug Abuse Modulation of HAND. Curr HIV Res 10:469–477. 10.2174/157016212802138805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sen CK (1998) Glutathione: A key role in skeletal muscle metabolism In: Oxidative Stress in Skeletal Muscle. Birkhäuser Basel, pp 127–139. [Google Scholar]
- 44.Cacciatore I, Baldassarre L, Fornasari E, et al. (2012) Recent advances in the treatment of neurodegenerative diseases based on GSH delivery systems. Oxid Med Cell Longev 2012: 240146 10.1155/2012/240146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pace GW, Leaf CD (1995) The role of oxidative stress in HIV disease. Free Radic Biol Med 19:523–528. 10.1016/0891-5849(95)00047-2 [DOI] [PubMed] [Google Scholar]
- 46.Walker J, Winhusen T, Storkson JM, et al. (2014) Total antioxidant capacity is significantly lower in cocaine-dependent and methamphetamine-dependent patients relative to normal controls: Results from a preliminary study. Hum Psychopharmacol 29:537–543. 10.1002/hup.2430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thangavel S, Mulet CT, Atluri VSR, et al. (2018) Oxidative Stress in HIV Infection and Alcohol Use: Role of Redox Signals in Modulation of Lipid Rafts and ATP-Binding Cassette Transporters. Antioxid Redox Signal 28:324–337. 10.1089/ars.2016.6830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cardaci S, Ciriolo MR (2012) TCA cycle defects and cancer: When metabolism tunes redox state. Int. J. Cell Biol 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu SB, Wu YT, Wu TP, Wei YH (2014) Role of AMPK-mediated adaptive responses in human cells with mitochondrial dysfunction to oxidative stress. Biochim. Biophys. Acta - Gen. Subj 1840:1331–1344. 10.1016/j.bbagen.2013.10.034 [DOI] [PubMed] [Google Scholar]
- 50.Buch S, Yao H, Guo M, et al. (2012) Cocaine and HIV-1 Interplay in CNS: Cellular and Molecular Mechanisms. Curr HIV Res 10:425–428. 10.2174/157016212802138823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fan Y, Gao X, Chen J, et al. (2016) HIV Tat impairs neurogenesis through functioning as a notch ligand and activation of notch signaling pathway. J Neurosci 36:11362–11373. 10.1523/JNEUROSCI.1208-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harris JJ, Jolivet R, Attwell D (2012) Synaptic Energy Use and Supply. Neuron 75:762–777. 10.1016/j.neuron.2012.08.019 [DOI] [PubMed] [Google Scholar]
- 53.Rangaraju V, Calloway N, Ryan TA (2014) Activity-driven local ATP synthesis is required for synaptic function. Cell 156:825–835. 10.1016/j.cell.2013.12.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pathak D, Shields LY, Mendelsohn BA, et al. (2015) The role of mitochondrially derived ATP in synaptic vesicle recycling. J Biol Chem 290:22325–22336. 10.1074/jbc.M115.656405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hardie DG (2011) AMP-activated protein kinase-an energy sensor that regulates all aspects of cell function. Genes Dev 25:1895–1908. 10.1101/gad.17420111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Valle-Casuso JC, Angin M, Volant S, et al. (2019) Cellular Metabolism Is a Major Determinant of HIV-1 Reservoir Seeding in CD4 + T Cells and Offers an Opportunity to Tackle Infection. Cell Metab 29:611–626.e5. 10.1016/j.cmet.2018.11.015 [DOI] [PubMed] [Google Scholar]
- 57.Nederlof R, Eerbeek O, Hollmann MW, et al. (2014) Targeting hexokinase II to mitochondria to modulate energy metabolism and reduce ischaemia-reperfusion injury in heart. Br J Pharmacol 171:2067–2079. 10.1111/bph.12363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Halestrap AP, Wilson MC (2012) The monocarboxylate transporter family-Role and regulation. IUBMB Life 64:109–119. 10.1002/iub.572 [DOI] [PubMed] [Google Scholar]
- 59.Bergersen LH, Gjedde A (2012) Is lactate a volume transmitter of metabolic states of the brain? Front Neuroenergetics 4:5 10.3389/fnene.2012.00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jäer S, Handschin C, St-Pierre J, Spiegelman BM (2007) AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1α. Proc Natl Acad Sci U S A 104:12017–12022. 10.1073/pnas.0705070104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang S, Sun H, Ma J, et al. (2013) Target analysis by integration of transcriptome and ChIP-seq data with BETA. Nat Protoc 8:2502–2515. 10.1038/nprot.2013.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dinkova-Kostova AT, Abramov AY (2015) The emerging role of Nrf2 in mitochondrial function. Free Radic Biol Med 88:179–188. 10.1016/j.freeradbiomed.2015.04.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Virbasius JV, Scarpulla RC (1994) Activation of the human mitochondrial transcription factor A gene by nuclear respiratory factors: A potential regulatory link between nuclear and mitochondrial gene expression in organelle biogenesis. Proc Natl Acad Sci USA 91:1309–1313. 10.1073/pnas.91.4.1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Larsson NG, Wang J, Wilhelmsson H, et al. (1998) Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nat Genet 18:231–236. 10.1038/ng0398-231 [DOI] [PubMed] [Google Scholar]
- 65.Rasbach KA, Schnellmann RG (2007) PGC-1α over-expression promotes recovery from mitochondrial dysfunction and cell injury. Biochem Biophys Res Commun 355:734–739. 10.1016/j.bbrc.2007.02.023 [DOI] [PubMed] [Google Scholar]
- 66.Rohas LM, St-Pierre J, Uldry M, et al. (2007) A fundamental system of cellular energy homeostasis regulated by PGC-1α. Proc Natl Acad Sci USA 104:7933–7938. 10.1073/pnas.0702683104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dirks B, Hanke J, Krieglstein J, et al. (1980) Studies on the Linkage of Energy Metabolism and Neuronal Activity in the Isolated Perfused Rat Brain. J Neurochem 35:311–317. 10.1111/j.1471-4159.1980.tb06266.x [DOI] [PubMed] [Google Scholar]
- 68.Nabetani M, Okada Y, Kawai S, Nakamura H (1995) Neural activity and the levels of high energy phosphates during deprivation of oxygen and/or glucose in hippocampal slices of immature and adult rats. Int J Dev Neurosci 13:3–12. 10.1016/0736-5748(95)95839-V [DOI] [PubMed] [Google Scholar]
- 69.Swinton MK, Carson A, Telese F, et al. (2019) Mitochondrial biogenesis is altered in HIV+ brains exposed to ART: Implications for therapeutic targeting of astroglia. Neurobiol Dis 130:104502 10.1016/j.nbd.2019.104502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Samikkannu T, Atluri VSR, Arias AY, et al. (2014) HIV-1 subtypes B and C Tat differentially impact synaptic plasticity expression and implicates HIV-associated neurocognitive disorders. Curr HIV Res 12:397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Atluri VSR, Kanthikeel SP, Reddy PVB, et al. (2013) Human Synaptic Plasticity Gene Expression Profile and Dendritic Spine Density Changes in HIV-Infected Human CNS Cells: Role in HIV-Associated Neurocognitive Disorders (HAND). PLoS One 8:e61399 10.1371/journal.pone.0061399 [DOI] [PMC free article] [PubMed] [Google Scholar]