Abstract
Despite its importance, understanding the early phases of human development has been significantly limited by availability of human samples. The recent emergence of stem cell-derived embryo models, a new field aiming to use stem cells to construct in vitro models to recapitulate snapshots of the development of the mammalian conceptus, opens up exciting opportunities to promote fundamental understanding of human development and advance reproductive and regenerative medicine. This review provides a summary of the current knowledge of early mammalian development, using mouse and human conceptuses as models, and emphasizes their similarities and critical differences. We then highlight existing embryo models that mimic different aspects of mouse and human development. We further discuss bioengineering tools used for controlling multicellular interactions and self-organization critical for the development of these models. We conclude with a discussion of the important next steps and exciting future opportunities of stem cell-derived embryo models for fundamental discovery and translation.
Summary:
This Review highlights the recent emergence of stem cell-derived embryo models and opportunities of using these models for advancing human embryology and reproductive and regenerative medicine.
The development of a multicellular organism from a single fertilized egg is a brilliant triumph of evolution that has fascinated generations of scientists (Box 1). Understanding our own development is of particular fundamental and practical interest; however, it poses a unique set of technical and ethical challenges. Our current knowledge of embryonic development is derived from a number of animal species, chosen because they are convenient to study and amenable to experimental manipulation or genetic analysis1. These studies have revealed developmental principles (Box 2) and signaling and transcriptional networks that underlie cell fate patterning and tissue morphogenesis. In particular, most of our knowledge of mammalian development derives from the mouse model. However, it is becoming evident that there are morphological and genetic differences between mice and humans that make cross-species comparisons problematic2.
Box 1. Glossary.
-
Conceptus
The products of conception at all stages of development from zygote to birth. These include the embryo proper, the fetus, the placenta, and all extraembryonic membranes. The term “embryo proper” refers to those parts of the conceptus that will form the new body and excludes extraembryonic tissues. Often, the terms “embryo” and “conceptus” are used interchangeably.
-
Pre-implantation development
The first few days of development, from fertilization to implantation, during which the conceptus travels down the oviduct toward the uterus. It encompasses the first 7 – 9 days after fertilization in humans.
-
Morula
The very early stage in a conceptus when cleavage has resulted in a sold ball of cells.
-
Implantation
The process of attachment and invasion of the conceptus to the uterine tissues that occurs around day 7–9 after fertilization in humans. Implantation establishes the fetal-maternal interface leading to later placental development.
-
Blastula and blastocyst
The stage of the conceptus prior to implantation is termed blastula. At this stage, the conceptus is call a blastocyst.
-
Peri-implantation development
The development of the conceptus in the uterine tissues prior to gastrulation.
-
Gastrula and gastrulation
Gastrulation describes the process by which the three definitive germ layers of the embryonic compartment of the conceptus are formed. Gastrulation begins around day 14 in humans. The gastrulation stage conceptus is termed gastrula.
-
Primitive streak
The embryonic structure that breaks radially symmetry by establishing the anterior-posterior axis and establishes bilateral symmetry (alignment of equivalent structures on both sides of the anterior-posterior axis), the site of gastrulation, and the formation of the germ layers. In the human embryo, the primitive streak appears around day 14 after fertilization.
-
Neurula and neurulation
Neurulation describes the process by which the neural tube is formed from the ectodermal neural plate. The neural tube will give rise to the brain and spinal cord. The neurulation stage conceptus is termed neurula.
-
Organogenesis
The development of specific organs in the embryo such as the brain and heart. Organogenesis starts soon after gastrulation. In humans, organogenesis commences during the 4th week after fertilization.
-
Embryonic and fetal stages
The embryonic stage begins with the division of the zygote and encompasses the development of the body plan and formation of the organs. This is followed by the fetal stage, during which growth and maturation of tissues and organs occurs. In humans, the fetal stage begins during the 9th week after fertilization and continues to birth.
Box 2. Classic developmental concepts and principles.
-
Developmental potency
Developmental potency describes the ability of a cell in the embryo to differentiate into other cell types. There is a continuum of developmental potency following progressive development of the embryo, from totipotency, pluripotency, multipotency, oligopotency, and finally unipotency.
-
Pattern formation
Pattern formation is the process by which initially equivalent cells in a developing tissue acquire identities that lead to a well-ordered spatial pattern of cell activities. Pattern formation can involve positional information or cell sorting (see below).
-
Positional information
The concept of positional information proposes that cells in a developing tissue acquire positional values as in a coordinate system, which they interpret by developing in particular ways to give rise to spatial patterns. Positional information often involves diffusion of morphogens, leading to signaling gradients and differential gene expression in a morphogen concentration-dependent manner. A key feature of positional information being the basis for pattern formation is that there is no pre-pattern in the developing tissue.
-
Cell sorting
Pattern formation in a developing tissue can initiate from the specification of different cell types in the tissue in a salt-and-pepper fashion, which is followed by sorting of different cell types into distinct domains from where different tissues are formed. Cell sorting involves a morphogenetic process during which individual cells exchange neighbors, increasing the number of homotypic contacts and decreasing the number of heterotypic contacts.
-
Symmetry breaking
Symmetry breaking is the process by which an initially homogeneous system acquires an asymmetry along an axis. While external cues can induce or assist symmetry breaking, asymmetries can emerge spontaneously without such input, guided by self-organization (see below).
-
Self-organization
Self-organization, as a non-equilibrium process, can be defined as the formation of complex patterned structures from units of less complexity by collective, non-linear interactions, without referring to an external blueprint or template. These local internal interactions typically form feedback loops, thereby conferring robustness to the system. Other common features found in self-organizing systems are non-linearity, symmetry breaking, and the emergence of patterns through amplifications of stochastic fluctuations.
-
Embryonic induction
Embryonic induction is an interaction between one (inducing) tissue and another (responding) tissue, as a result of which the responding tissue undergoes a change in its direction of differentiation.
-
Signaling center
A localized region of the embryo that exerts a special influence on surrounding cells, usually by means of secreted signaling molecules, and thus instructs how those cells develop.
-
Organizer
A signaling center that directs the development of the whole embryo or of part of the embryo.
-
Community effect
Community effect describes cell-cell communication among a group of nearby cells in a developing tissue, which is necessary for them to maintain coordinated behaviors.
-
Morphogenetic regulation
Tissue-scale morphogenetic changes, including changes in cell shape, number, position and force, can work in concert with classic developmental signaling events mediated by diffusible signals to mediate gene expression and cell fate specification.
Knowledge of human embryogenesis, which is critical for assisted reproductive technologies and prevention of pregnancy loss, birth defects and teratogenesis (Box 3), should ideally be learned from studying the human embryo per se; however, such studies have been challenging, due to limited access to and bioethical constraints on human embryo specimens. Excess pre-implantation human embryos generated in in vitro fertilization (IVF) clinics are available for research3,4; however, once a human embryo implants into the uterus, subsequent development is hidden from direct observation. Recent progress in prolonged in vitro culture of IVF human embryos has opened the door for genetic and molecular studies of human embryos directly5–7. However, international guidelines prohibit in vitro culture of human embryos beyond 14 days post-fertilization (embryonic day 14, E14) or reaching the onset of primitive streak (PS) development (“the 14-day rule”)8,9, which marks the outset of gastrulation. The bioethical regulation of human embryo culture has significantly limited studies of IVF human embryos for understanding post-implantation human development. There is significant progress in studying non-human primate (NHP) monkey embryos10–12, whose developments are similar to humans. However, NHP monkey models remain costly, are difficult to modify genetically, and have their own ethical challenges.
Box 3. Clinical benefits of human embryology.
Advancing fundamental understanding of human embryogenesis can provide a scientific foundation for improving assisted human reproduction and prevention of pregnancy loss, birth defects and teratogenesis. It will also advance the biology of germ cells and treatment of infertility. Advancing understanding of human implantation will help develop effective contraception technologies and treatments of recurrent implantation failure. Detailed understanding of the widespread epigenetic programming during human embryonic development can provide important insights for disease progression in later life. Studying human development is critical for improving stem cell differentiation protocols to mimic embryogenesis, in order to achieve desired cell functions for research and therapy.
Recent advances in mammalian embryology, stem cell biology, organoid technology, and bioengineering have contributed to a significant interest in the development of multicellular systems based on emergent self-organization and tissue patterning. Importantly, different models of the mammalian conceptus have been developed using mouse and human stem cells13–28. This emerging field aims to use stem cell cultures to create organized embryo-like structures (or embryoids), whose development and architecture bear significant similarities to their in vivo counterparts. Embryoids are distinct from organoids, as organoids are organized multicellular structures that mimic the development, regeneration and homeostasis of a single tissue or organ. In contrast, embryoids aim to model integrated development of the entire conceptus or a significant portion thereof. In general, embryoids have a more reproducible cellular organization and architecture than organoids, as bioengineering approaches are commonly deployed to guide their development and culture time is limited to a few days. In addition, the stem cells used in embryoids have been well established and their cultures are robust. This review discusses the developmental principles manifested in the development of embryoids and their applications for advancing human embryology (particularly at the post-implantation stages) and reproductive and regenerative medicine. Bioengineering tools used for controlling multicellular interactions and self-organization critical for embryoid development are highlighted. We conclude with a discussion of important next steps to leverage advanced bioengineering controls of multicellular interactions to promote the continuous, progressive development of this exciting nascent field.
Mammalian development as a reference framework
The development of embryoids, like that of embryos, involves the emergence of organized multicellular structures, through coordinated cellular processes including pattern formation, morphogenesis, cell differentiation and growth. Here we first discuss the principles of early mouse and human development before turning to how these are manifest in embryoids.
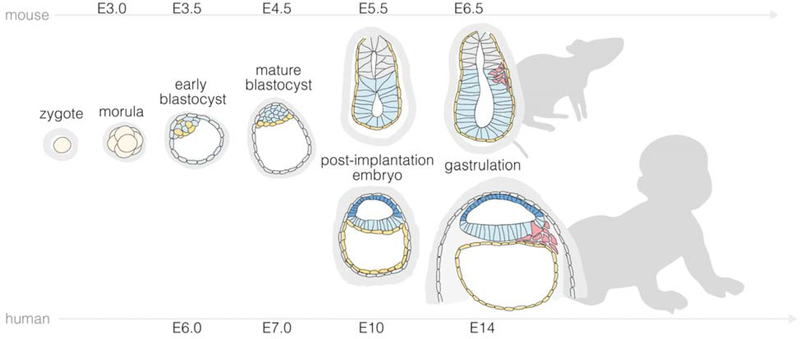
During early development, both mouse and human embryos develop from a zygote and proceed through recognizable stages of morula, blastula, gastrula, neurula and organogenesis (Figure 1, Box 1, Box 4). The overall program of pre-implantation development from a zygote to a blastocyst is conserved between mice and humans, leading to the formation of the blastocyst, containing an outer trophectoderm (TE) layer surrounding a cavity (blastocoel) and an inner cell mass (ICM) on one side of the cavity29,30 (Figure 1, Box 4). As the blastocyst develops, the ICM becomes further segregated into two cell populations: the pluripotent epiblast (EPI) and primitive endoderm (PE; or hypoblast in human)29,30.
Figure 1. Overview of mouse and human development from pre-implantation to the onset of gastrulation.
Prior to implantation, both mouse and human embryos undergo cell divisions culminating in the development of a blastocyst, comprising an outer trophectoderm (TE) layer and an inner cell mass (ICM) that further segregates into epiblast (EPI) and primitive endoderm (PE; hypoblast in humans). The timing of blastocyst implantation differs between mice and humans (E5 in mouse and E7 in human). Furthermore, morphogenesis and lineage developments during peri-implantation mouse and human development show distinct features. Mouse development from E5 - E6.5 leads to the formation of a cup-shaped EPI juxtaposed with TE-derived extraembryonic ectoderm (ExE), enclosing the pro-amniotic cavity. Concurrently, the PE forms the visceral endoderm (VE) that envelops both EPI and ExE. In contrast, soon after human blastocyst implantation, while the EPI undergoes lumenogenesis to form the pro-amniotic cavity, EPI cells adjacent to polar TE cells become specified into the amniotic ectoderm (AM), with remaining pluripotent EPI cells forming the embryonic disc. By E6.5 for mice and E14 for humans, gastrulation is initiated in the posterior EPI compartment. Mouse primordial germ cells (PGCs) emerge at the boundary between posterior EPI and ExE at the onset of gastrulation. Data on primate PGC specification remain sparse. Existing data suggest that human PGCs may emerge in the nascent AM prior to the gastrulation. Human PGC specification requires additional studies for clarification. For peri-implantation mouse and human embryos, only their embryonic regions are shown.
Box 4. Early mouse and human development.
Pre-implantation development
Pre-implantation mouse and human development displays intricate self-organization and autonomy. After fertilization, the one-cell zygote undergoes cleavage cell divisions to form a solid ball of cells resembling a mulberry (and hence the name morula). Cells of the morula begin to differentiate, leading to blastocyst formation. In the blastocyst, the trophectoderm (TE) surrounds a fluid-filled cavity (blastocoel) with an inner cell mass (ICM) on one side. The TE is an extraembryonic tissue and will give rise to the placenta. As the blastocyst develops, the ICM becomes segregated into two distinct cell populations: the embryonic epiblast (EPI), which will give rise to the embryo proper, and a second extraembryonic tissue known as the primitive endoderm (PE; or hypoblast in human). Pre-implantation development has been extensively studied using the mouse embryo, revealing important cellular and morphogenetic events including position-dependent TE / ICM patterning, the blastocoelic cavity formation, and lineage segregation and sorting of EPI and PE in the ICM. The readers are referred to some excellent reviews on the mouse blastocyst development29,30. Human blastocyst formation remains incompletely understood3,109. Existing data suggests that there are differences in timing, in gene expression and potentially in mechanisms of lineage development and function between mice and humans during pre-implantation development2.
Peri-implantation development
Successful implantation involves a bilateral interaction between a competent blastocyst and a receptive uterus. Implantation of the blastocyst (E5 in mouse and E7 in human) triggers major morphological reorganization and lineage developments. Upon implantation of the mouse blastocyst, the TE adjacent to the EPI (polar TE) forms the extraembryonic ectoderm (ExE) and ectoplacental cone. Concomitantly, the EPI and ExE each undergo lumenogenesis so that separate apical lumens are formed in each compartment82,103. The two lumenal cavities soon fuse to establish the pro-amniotic cavity, leading to the formation of a cup-shaped EPI juxtaposed with the ExE at E6 (the egg cylinder). From E5 to E6, the PE forms the parietal endoderm and visceral endoderm (VE). By E6, the VE envelops both the EPI and ExE, setting the stage for gastrulation.
Morphogenesis and lineage development in the peri-implantation human embryo show distinct features compared with mice32. Upon implantation, the EPI undergoes lumenogenesis to form the pro-amniotic cavity5–7, similar to the mouse EPI. Distinctly, the lumenal EPI soon breaks symmetry and resolves into the bipolar patterned EPI-amnion sac7,31. Specifically, EPI cells adjacent to invading polar TE cells differentiate into the amniotic ectoderm (AM)7,31, an extraembryonic tissue involved in future fetal membrane development. The EPI cells at the opposite pole adjacent to the hypoblast remain pluripotent and become thickened and more columnar, forming the embryonic disc. Thus, the pre-gastrulation EPI displays distinct topologies between humans and mice: discoid in human and cup-shaped in mouse. The mouse embryo does not develop the bipolar EPI-amnion structure. In mice, the AM emerges as amniotic folds at the junction of the EPI and ExE during gastrulation110,111.
Gastrulation
Prior to mouse gastrulation, reciprocal interactions between EPI, ExE and VE lead to regionalized patterning in these tissues33,34. Regionalization of VE is particularly important, leading to the formation of the anterior VE or AVE at the prospective anterior side of the embryo35,36. The AVE secrets antagonists to shield overlying EPI cells from differentiation. Development of the AVE thus breaks radial symmetry and marks anterior-posterior (A-P) axis formation in the mouse embryo. Soon after, gastrulation is initiated at the proximal, posterior end of the EPI by a convergence of BMP-WNT-NODAL signaling interactions between EPI, ExE and VE37–39. The antagonists secreted by the AVE block signaling and impart neuroectoderm characters at the anterior pole of the EPI36, whereas signals at the proximal, posterior end of the EPI instruct cells to undergo an epithelial–mesenchymal transition (EMT) and ingress through the PS to acquire mesendodermal fates37. During mouse gastrulation, primordial germ cells (PGCs) emerge at the proximal, posterior end of the EPI40,41. Experimental evidence supports that prospective PGCs are selected from somatic, gastrulating EPI cells by dose-dependent BMP signals that originate from the ExE112.
Gastrulation remains one of the most mysterious phases of human development. Recent studies of NHP monkey embryos suggest conserved mechanisms are likely in play for A-P patterning during human gastrulation12,43. Limited data from NHP monkey43 and in vitro cultured human42 embryos suggest that PGCs may emerge in the dorsal nascent AM soon after implantation. This unexpected finding will require additional confirmation.
After gastrulation, the EPI in mouse and human embryos transforms into a trilaminar structure consisting of ectoderm, mesoderm and endoderm. As gastrulation proceeds, it brings subpopulations of cells in the three germ layer linages into proximity so that they can undergo inductive interactions to pattern layers and specify new cell types, driving the development of organ rudiments.
Neurulation
Gastrulation is followed by neurulation. During neurulation, the ectoderm is first patterned into the neuroectoderm (neural plate) in the medial portion of the embryo flanked by future epidermis. At the border between these regions, the neural crest and placodes form in response to BMP and WNT signals emanated from surrounding tissues113,114. The neural plate soon folds into the neural tube (NT)44,45, with its anterior and posterior regions giving rise to the brain and spinal cord, respectively. Cells in the NT continue to differentiate under the influence of inductive factors emanating from surrounding tissues. Sonic hedgehog (Shh)-mediated transcriptional networks that control ventral patterning of the mouse spinal cord have been well elucidated46,47.
Human neurulation remains challenging to study, even though NT closure defects remain one of the most common birth defects44,45. Recent studies suggest that late-manifesting neurodegenerative disorders, such as Huntington’s disease, may have a neurodevelopmental component25,115. The role of early nervous system development in late-onset neurodegenerative disorders remains a debated topic.
While the ectoderm undergoes neurulation, the mesoderm and endoderm also become further specified. Specifically, mesodermal cells are organized into cardiogenic mesoderm, axial mesoderm of the prechordal plate and notochord, paraxial mesoderm, intermediate mesoderm and lateral plate mesoderm. Each of these mesodermal regions undergoes some form of segmentation. The most evident and complete segmentation occurs in the paraxial trunk mesoderm, where each segment becomes an entirely separate somite. Much of the other mesodermal regions develop into embryonic connective tissues, cardiovascular and lymphatic systems, skeletal muscle cells, most of the urogenital system, and the lining of the pericardial, pleural and peritoneal cavities. Following gastrulation, the endoderm folds to form the primitive gut tube consisting of three subdivisions: foregut, midgut, and hindgut, which subsequently give rise to the epithelial lining of the digestive and respiratory systems.
The timing of blastocyst implantation differs between mice and humans (E5 in mouse and E7 in human). Furthermore, morphogenesis and lineage developments during early post-implantation mouse and human development show distinct features2,31 (Figure 1, Box 4). Mouse development from E5 - E6.5 leads to the formation of a cup-shaped EPI juxtaposed with TE-derived extraembryonic ectoderm (ExE), enclosing the pro-amniotic cavity. Concurrently, the PE forms the visceral endoderm (VE) that envelops both EPI and ExE. In contrast, soon after human blastocyst implantation, while the EPI undergoes epithelization and lumenogenesis to form the pro-amniotic cavity5–7, EPI cells closer to invading TE cells become specified into the amniotic ectoderm (AM)32, with remaining pluripotent EPI cells forming the embryonic disc. Thus, in pre-gastrulation human embryo, the pro-amniotic cavity is surrounded by a continuous epithelium with AM cells on one side and EPI cells on the other.
Studies of mouse gastrulation support the importance of extraembryonic tissues33,34 (Figure 1, Box 4). In particular, regional patterning of VE in pre-gastrulation mouse embryo leads to a gradient of WNT and NODAL signaling and the establishment of the anterior (A) -posterior (P) axis of the embryo35,36. Importantly, developmental signals involving WNT, NODAL and BMP at the proximal, posterior end of the EPI instruct EPI cells to form the PS by E6.5 and ingress through the PS to acquire mesoderm and endoderm fates37–39. Human gastrulation initiates around E14. However, given limited access to post-implantation human tissues, gastrulation remains one of the most mysterious phases of human development.
During mouse gastrulation, primordial germ cells (PGCs), precursors of sperm and egg, emerge at the boundary between posterior EPI and ExE40,41. Data on human PGC specification remain sparse42. Existing data from NHP monkey embryos43 suggest that primate PGCs may emerge in the nascent AM prior to the gastrulation. Additional studies are required to determine whether the same is true for human PGCs.
In mouse and human embryos, gastrulation transforms the EPI into a trilaminar structure consisting of definitive ectoderm, mesoderm and endoderm. The three germ layers undergo inductive interactions to pattern layers and specify new cell types, driving organ rudiment development (Box 4). Following gastrulation, the ectoderm undergoes neurulation, in which the neural plate is first patterned in the dorsal ectoderm before folding into the neural tube (NT)44,45 (Box 4). Cells in the NT continue to differentiate into different classes of neuronal progenitors46,47. Concomitantly, mesodermal cells are organized into different regions to from the primordia of major organ systems including cardiovascular and lymphatic systems and skeletal muscle cells. Simultaneously, the endoderm will fold to form the primitive gut tube, which will produce the digestive and respiratory systems.
Embryonic and extraembryonic stem cells as building blocks
As a bottom-up approach, the development of embryoids uses embryonic and extraembryonic stem cells, including those derived from embryos, as building blocks to construct models to recapitulate embryonic development (Figure 2).
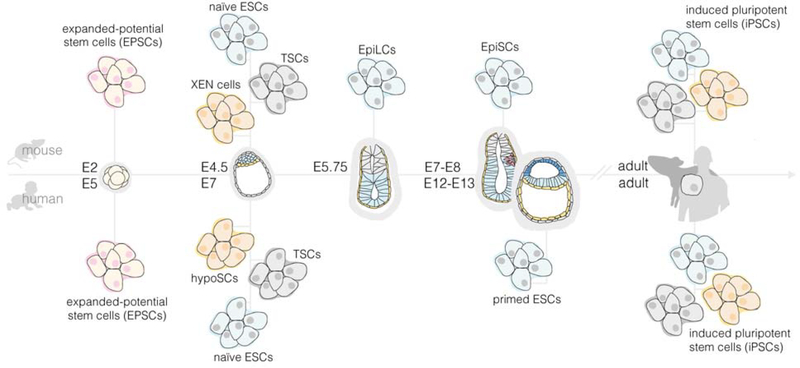
Figure 2. Mouse and human embryonic and extraembryonic stem cells and their corresponding developmental potencies.
Expanded potential stem cells (EPSCs) are established by isolating individual cells (or blastomeres) from eight-cell stage mouse and human embryos (or morula). By isolating cells from mouse blastocysts, mouse embryonic stem cells (mESCs) with naïve pluripotency, trophoblast stem cells (mTSCs), and extraembryonic endoderm (mXEN) cells representing the stem cell population of the primitive endoderm (PE) have been established. Similarly, human trophoblast stem cells (hTSCs) have been derived from human blastocyst. Primed mouse ESCs, known as mouse epiblast stem cells (mEpiSCs), are derived from the late post-implantation, pre-gastrulation mouse epiblast (EPI). Mouse EPI-like cells (EpiLCs) with an intermediate or formative state between naïve and primed pluripotency have been generated from mESCs in vitro, with a transcriptional profile similar to the early post-implantation mouse EPI. Human ESCs (hESCs) with primed pluripotency have also been derived from pre-implantation human blastocysts. Using strategies such as reprogramming, differentiated somatic mouse and human cells can be converted to a pluripotent state to establish induced pluripotent stem cells, or iPSCs. Using chemical cocktails, primed hESCs can be reverted into a naïve-like pluripotent state. Human hypoblast stem cells (hypoSCs) can be generated using these chemically reset naïve hESC lines.
Mouse stem cells
The EPI cells of the mouse blastocyst are pluripotent, and their functional, epigenetic, and signaling properties have been extensively characterized. These studies reveal that pluripotency is dynamic and progressive. As the mouse embryo develops from blastula to gastrula, EPI cells transit from a naïve state, in which they do not respond to inductive signals, to a primed state in which they readily differentiate48. The transition between naïve and primed states has been referred to as formative pluripotency during which EPI cells gain the capacity to make lineage decisions49.
Mouse pluripotent stem cells (mPSCs) in culture display a similar continuum of states. Mouse embryonic stem cells (mESCs) with naïve pluripotency can be isolated directly from the ICM of the mouse blastocyst and maintained in culture50,51. In contrast, mouse stem cells corresponding to the primed state, known as mouse epiblast stem cells (mEpiSCs), are derived from the post-implantation mouse EPI52,53. mEpiSCs exhibit more advanced developmental features consistent with the early-gastrulation EPI54. Mouse EPI-like cells (mEpiLCs) with formative pluripotency have been generated from mESCs in vitro, with a transcriptional profile consistent with early post-implantation mouse EPI55.
Stem cell lines representative of extraembryonic lineages in the mouse blastocyst have also been established, including mouse trophoblast stem cells (mTSCs)56 and extraembryonic endoderm (XEN) cells representing the stem cell population of the PE57.
Human stem cells
Human ESCs (hESCs) have also been successfully derived from human blastocysts58. However, hESCs have transcriptome and methylome different from the EPI of the human blastocyst59,60, suggesting that the conditions in which hESCs are cultured fail to capture the pre-implantation developmental program of the human embryo. Instead, hESCs are developmentally similar to the post-implantation, pre-gastrulation EPI in cynomolgus monkey embryos12. Consistently, hESCs more closely resemble mEpiSCs than mESCs in terms of molecular properties, lineage potency, and culture conditions48,52,53. However, there are still some notable differences in gene expression61 and in the propensity for PGC formation between hESCs and mEpiSCs, as hESCs can initiate PGC formation whereas mEpiSCs cannot62,63. There are recent reports showing derivations of hESC lines from human blastocysts with naïve pluripotency features64,65, and of chemical reprogramming cocktails capable of converting hESCs from primed to naïve pluripotency66,67. However, functional validations of naïve pluripotency including chimera formation and germline transmission as well as tetraploid complementation, which have been used for mESCs, cannot be implement with human cells for ethical reasons. To address this issue, there are ongoing discussions of a testable functional framework to assess naïve pluripotency in human cells31.
Somatic human cells can also be converted to a pluripotent state by cell fusion, somatic cell nuclear transfer, transcription factor-based reprogramming, and chemical reprogramming68. Pluripotent stem cells generated by these reprogramming strategies are called induced pluripotent stem cells, or iPSCs. Human iPSCs (hiPSCs) are considered molecularly and functionally equivalent to hESCs69.
Recently, through chemical screening, individual blastomeres isolated from eight-cell stage mouse morula were successfully cultured to establish mouse expanded potential stem cells (mEPSCs) that appear to possess developmental potency for all embryonic and extraembryonic lineages in blastocyst chimaera assays70,71. Using similar approaches, human EPSCs (hEPSCs) were also derived from primed hESCs and hiPSCs, and hEPSCs are shown to have the potency to form trophoblast stem cells72.
In contrast to mouse extraembryonic stem cells, human extraembryonic stem cells have only emerged recently. Using chemical screening, human trophoblast stem cells (hTSCs) were first derived from human blastocysts and first-trimester placental tissues73. Human hypoblast stem cells (hypoSCs) were also recently reported using chemically reset naïve hESC lines74. Recent work showed that chemically reprogrammed naïve hESCs could give rise to hTSCs when cultured in appropriate conditions75, and that both chemically reset and embryo-derived naïve hESCs could be used to derive hTSC lines76. With the emergence of these human extraembryonic cell lines as well as hEPSCs, it becomes imperative for additional molecular and functional characterizations for authentication and establishing their developmental identities compared to their in vivo counterparts76,77.
A rapidly growing toolbox of embryoids
Stem cells serve as building blocks for the development of embryoids that recapitulate different stages of mammalian development, from blastula through gastrula or early neurula and organogenesis (Figure 3). Development of embryoids use the same developmental principles that manifest in mammalian development. Importantly, embryoids have already generated new insights into early mammalian development.
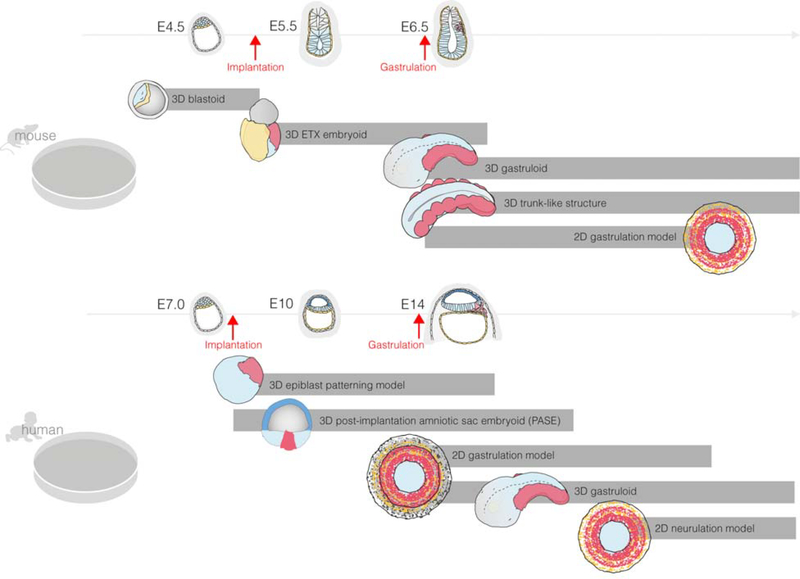
Figure 3. Existing embryoids that recapitulate different stages of mouse (top) and human development (bottom), from pre-implantation through gastrulation or early neurulation and organogenesis.
3D blastoid: Embryoid to model pre-implantation blastocyst development. 3D ETX embryoid: Embryoid to model post-implantation embryo development up to early gastrulation. 3D gastruloid and trunk-like structure: Embryoid to model post-gastrulation development of the posterior portion of the embryo. 2D gastrulation model: Embryoid to model germ layer patterning during gastrulation. 3D epiblast patterning model: Embryoid to model epiblast morphogenesis and patterning during early post-implantation development. 3D post-implantation amniotic sac embryoid (PASE): Embryoid to model post-implantation human development up to early gastrulation. 2D neurulation model: Embryoid to model the neurulation process, leading to neural tube development and patterning.
Embryoid to model blastocyst development
The first embryoid to model blastocyst formation (or blastoid) was successfully developed by mixing mESCs with mTSCs at a defined ratio under appropriate culture conditions, leading to their self-assembly into a tissue organization reminiscent of the mouse blastocyst22. As in vivo, mouse blastoids possess an outer TE layer surrounding a compact ICM-like compartment, and their transcriptome is more similar to mouse blastocyst than is achieved by simply combining mESC and mTSC transcriptomes. Mouse blastoids have been used to dissect interactions between embryonic and extraembryonic compartments, revealing that NODAL and BMP signals from the ICM-like compartment are important for growth and morphogenesis of TE cells22. This insight has proven useful for improving culture conditions of mTSCs78. In the initial blastoid protocol, further cell segregation and sorting of ICM-like cells into PE-like cells was inefficient. Optimization studies yielded improved conditions in which the relative proportions of the three cell lineages (EPI-, TE- and PE-like cells) more closely resemble that of mouse blastocyst79. Remarkably, culture of mEPSCs in appropriate conditions yields self-organized blastoids consisting of EPI-, TE- and PE-like cells26. Another recent work mixing mEPSCs with mTSCs also led to the formation of blastoids that showed developmental progression from the pre- to post-implantation egg cylinder morphology in vitro80, similar to the mouse ETX embryoid described below. Although capable of implanting in the mouse uterus, all of the mouse blastoids fail to develop much further than the blastocyst stage either in vitro or in vivo. The reasons for this are not currently understood and may point to a requirement for greater organization than currently achieved in mouse blastoids. It remains to be determined whether human blastoids can be generated by using either naïve hPSCs or hEPSCs with or without hTSCs or hypoSCs under suitable culture environments.
Human amniotic sac embryoid
During early post-implantation human development, a patterned bipolar EPI-AM structure arises from the EPI. It was recently shown that culturing primed hPSCs on a soft culture surface together with native extracellular matrix (ECM) proteins (i.e., Geltrex) diluted in culture medium leads to the formation of a spherical lumenal hPSC colony20. This observation is consistent with the intrinsic lumenogenic property associated with primed but not naïve hPSCs81,82. Interesting, hPSCs in the colony lose pluripotency and differentiate into amniotic cells, even without exogenous inductive factors in the culture medium20. If only one of these culture elements is present, either a soft substrate or diluted gel in the culture medium, primed hPSCs form lumenal sacs but retain pluripotency, suggesting that amniotic differentiation of hPSCs is mechanosensitive20. Amniotic differentiation of hPSCs requires endogenous BMP signaling, and its inhibition under amnion-differentiation conditions is sufficient for rescuing hPSC pluripotency20.
A small fraction of lumenal sacs, rather than differentiating entirely into squamous amniotic tissues, spontaneously break symmetry and form a bipolar structure with columnar pluripotent cells on one side and squamous amniotic cells on the other, mimicking EPI-AM patterning in the pre-gastrulation human embryo20. This model is termed the post-implantation amniotic sac embryoid (PASE). Symmetry breaking in the PASE also depends on BMP activity, and active BMP signaling is only evident at the prospective AM-like pole20. Progressive development of the PASE results in EPI-like cells further differentiating into PS-like cells. This spontaneous symmetry breaking occurs in only 5 – 10% of lumenal hPSC sacs. To increase efficiency of PASE formation, a microfluidic PASE model has recently been developed27. This microfluidic device allows small clusters of primed hPSCs to be grown in small indentations with separate channels supplying culture medium to each side27. Flowing BMP4 in only one of these channels leads reproducible pattering of amniotic cells only on the side exposed to BMP427. The opposite side remains pluripotent but soon goes on to differentiate into PS-like cells27. The identity of PS derivatives can be modulated by stimulating this side of the cell clusters with additional ligands: WNT stimulation together with either BMP4 or ACTIVIN-A leads to posterior and anterior PS derivatives, respectively27. Importantly, human PGC-like cells (hPGCLCs) emerge in the microfluidic PASE before it initiates gastrulation-like events27, suggesting applications of the microfluidic PASE model for studying the origin and specification of hPGCs.
The PASE represents the first embryoid to model early post-implantation development of the EPI and AM compartments of the human embryo. It also suggests the inductive role of AM in triggering human gastrulation. Although the microfluidic PASE model significantly improves the controllability of EPI-AM patterning, further asymmetries are not demonstrated and the EPI-like compartment is either entirely anterior or posterior in character. Prolonged culture of the PASE is also limited by the confined space in the microfluidic device. Furthermore, disseminating cells from the PASE mimicking gastrulation would lead to its disassembly. Future efforts should be devoted to identifying a strategy to prolong the culture of the PASE and promote self-organization of gastrulating cells. It is possible that adding human extraembryonic stem cells including hypoSCs to the PASE will be helpful for these efforts.
2D models of gastrulation
Treatment of primed hPSCs confined to micropatterned colonies with BMP4 reproducibly leads to organized differentiation with putative TE-like cells on the colony outer edge, ectodermal cells in the colony center, and mesodermal and endodermal cells forming two layers in between18,83,84. This multicellular pattern is consistent as in the gastrulating mammalian embryo. However, the fate territories in the 2D gastrulation model are adjacent on a 2D surface rather than layered one on top of the other as in in vivo. Although the 2D geometry is artificial and distinct from the 3D topology of mammalian embryos, the reproducibility and compatibility with live imaging of the 2D gastrulation model has allowed quantification of the self-organized signaling dynamics that drive these patterning events85–88. These studies have revealed that rather than creating stable gradients, cells generate dynamic expanding fronts of endogenous WNT and NODAL signaling that are interpreted combinatorially to pattern different germ layers. These studies can serve as a template for investigating the mechanisms of patterning through signaling dynamics in other embryoids. Interestingly, in colonies treated with WNT rather than BMP4, similar cell fate patterns are observed but with a different mechanism, which involves a wave of EMT that sensitizes cells to the exogenous WNT signal89. Mouse gastrulation has also been successfully modeled using the 2D gastrulation embryoid with mouse EpiLCs90. Importantly, findings from this mouse embryoid have been compared to the mouse embryo, providing a more direct validation, which for obvious reasons is not possible for human gastrulation embryoids. Micropatterning can be readily combined with other bioengineering approaches. For example, recent work has shown that overlaying gradients of exogenous ligands on micropatterned 2D gastrulation embryoids can bias the resulting fate pattern in a reproducible way91. Specifically, a gradient of BMP4 (and in some cases a counteracting gradient of BMP antagonist NOGGIN) generated in a microfluidic device induced axially organized patterning of the germ layers along the gradient, breaking the radial symmetry of 2D circular colonies91.
3D models of gastrulation
In addition to blastoids, mESCs and mTSCs can be cultured together in conditions that promote their self-organization to model post-implantation mouse development. In such models, rather than forming structures that morphologically resemble the mouse blastocyst, mESCs and mTSCs form separate compartments before fusing together. Each compartment undergo lumenogenesis, with the resulting lumens merging together, resulting in a structure resembling the egg cylinder stage mouse embryo (referred to as the ETS embryoid)19. Remarkably, the ETS embryoid initiates developmental events mimicking both germ cell formation and PS development in an asymmetric manner19. This is surprising as VE, which is critical for A-P symmetry breaking in vivo, is not present in the ETS embryoid, although further studies show that adding XEN cells into the ETS embryoid improves this model (called the ETX embryoid)23.
A 3D embryoid for modeling symmetry breaking of the EPI has also been developed starting from primed hPSCs26. In this model, hPSCs grown in 3D and treated with a low dose of BMP4 form lumenal sacs before polarizing into two opposing regions displaying gene expression patterns associated with ectoderm and mesoderm induction26. This observation is similar to cell fate patterning that emerges from the 2D human gastrulation embryoid18. However, as the initial degree of symmetry is higher in 3D (sphere vs. disk), the development of the 3D human gastrulation embryoid involves spontaneous symmetry breaking while in 2D it does not.
Finally, an embryoid model beginning from only mESCs or primed hPSCs has been shown to be able to model the post-gastrulation development of the posterior portion of the mouse and human embryos, respetively17,21,28,92. Growing these cells in aggregates of defined size and exposing them to a properly timed pulse of WNT activation leads to the formation of a tail-bud-like structure on one end of the aggregate that continues with axial organization, somitogenesis, PGC specification and even NT formation17,21,28,92. These structures, known as gastruloids, recapitulate some essential features of A-P axial patterning of the post-gastrulation mouse and human embryos as revealed by HOX gene expression and somite formation21,28,92. The mouse gastruloid can even be coaxed to generate a primitive beating heart following pathways similar to those of the mouse embryo93. These mouse and human gastruloids all lack anterior structures, such as the forebrain, likely due to the posteriorizing effect of WNT signaling.
While the 3D embryoid models of gastrulation show remarkable emergence of patterning and morphogenesis, they lack the controllability and reproducibility of the 2D gastrulation models. Bioengineering approaches, which can control cell-cell interactions and modulate symmetry breaking and patterning, as in the microfluidic PASE27, will be useful for improving the controllability and reproducibility of the 3D gastrulation models.
Models of neurulation
The nervous system acquires its form and pattern during the neurulation stage. Several stem cell-based neurulation models have been developed, which focus on the ectodermal germ layer, the source of the nervous system. One of the earliest studies showed that 3D spherical lumenal sacs composed of NE cells, reminiscent of the NT, could be grown from single mESCs under appropriate neural differentiation conditions15. These neural sacs were entirely dorsal in character but could be patterned by exogenous ventralizing or posteriorizing signals. Optimizing the gel matrix in which neural sacs were embedded also improved their dorsal (D)-ventral (V) patterning, showing the power of bioengineering approaches to optimize conditions for embryoid self-organization94. D-V patterned neural sacs mimicking human NT development were recently demonstrated using primed hPSCs under a culture condition similar to that used for mESCs27.
Recently, several 2D models have been reported to recapitulate patterning of a significant portion of the ectodermal germ layer. One study with primed hPSCs showed that regional patterning of neural crest and NE cells could be created on micropatterned surfaces, mimicking neural induction as the first step of the neurulation process, and that this emergent regional patterning was regulated by mechanical control of BMP-SMAD signaling24. Other recent studies have recapitulated patterning of all four major fates within the ectoderm during neural induction – neural, neural crest, placode and epidermis25,95. Modulating both BMP and WNT signaling enabled control over the fates that emerged at the border between neural and non-neural ectoderm95, and one of these models was shown to be useful for modeling developmental effects of the mutations that cause Huntington’s disease25. In the future, models that recapitulate not only regional fate patterning, but also morphogenesis involved in neural plate folding and neural fold closure, could both lead to new fundamental knowledge of this stage of human development and provide essential systems for research into human NT closure defects.
Bioengineering tools to control embryoid development
Mammalian embryogenesis is a context-dependent process, involving interactions between multiple, co-emerging embryonic and extraembryonic cell lineages that are intricately organized in 3D. This 3D context provides spatial boundary conditions, as well as biochemical and biomechanical inputs and positional information that are often absent in conventional 2D culture vessels. Although mouse and human stem cells can be efficiently differentiated into specialized cell types under classical 2D cultures, poor control over initial seeding conditions and tissue growth lead to disorganized cell fate patterns and tissue shapes. Bioengineering tools such as microfluidics or microfabricated cell culture substrates have been proven highly effective to ‘reconstruct’, in a bottom-up fashion, the missing 3D physical and biochemical context of the early embryo (Figure 4). Indeed, recent advances in bioengineering and biomaterials not only promote the reproducibility of embryoids, e.g. to facilitate the development of quantitative assays, but also enable systematic studies of how the complex array of extrinsic inputs influences embryonic development.
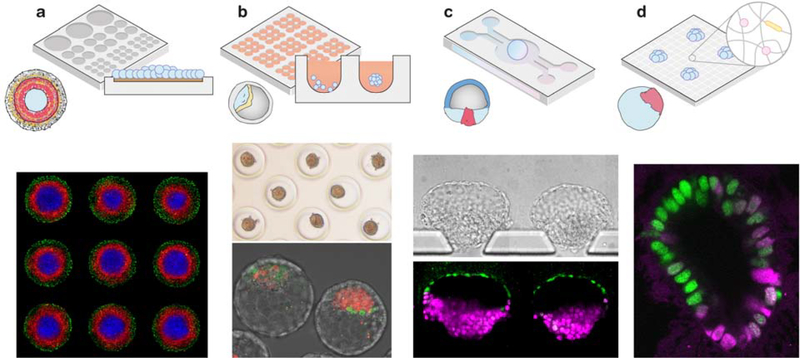
Figure 4. Bioengineering tools to promote multicellular interaction and self-organization in embryoid development.
(a) Micropatterning to generate 2D circular colonies of hPSCs to model germ layer patterning during gastrulation. Immunofluorescence image shows emergence of concentric gene expression regions, mimicking development of the germ layers (SOX2+ ectoderm, blue; TBXT+ mesendoderm, red) as well as a GATA3+ extraembryonic layer (green). Image from A. Yoney and E.D. Siggia. (b) Microwell array to promote cell aggregation and development of mouse blastoids. Top: Microwell arrays composed of agarose hydrogels to promote aggression of mESCs and mTSCs. Bottom: Merged image showing two blastoids, with a layer of mTSCs surrounding a cavity and a cluster of mESCs mimicking the inner cell mass. Immunostaining: NANOG (red) and GATA6 (green). Images from N. Rivron. (c) Microfluidics to control spatiotemporal morphogen signaling and tissue patterning. Bright-field (top) and immunofluorescence (bottom) images of an array of post-implantation amniotic sac embryoids (PASEs), showing molecular asymmetry and tissue patterning, with TFAP2A+ amniotic cells on one pole (green) and TBXT+ gastrulating cells (magenta) on the opposite pole. Images from Y. Zheng. (d) Chemically and physically defined hydrogels for 3D embryoid development. Immunofluorescence image of a 3D human gastrulation embryoid for modeling epiblast morphogenesis and patterning (SOX2, green; TBXT, magenta). Image from M. Simunovic.
Micropatterning to control tissue shape
The simplest and perhaps most adopted approach to influence multicellular self-organization is based on cultures of cell colonies selectively on cell adhesive substrates that are designed to spatially control tissue size and shape. This can be readily achieved through micropatterning, a classical microfabrication technique widely used to study how cell or tissue shape affects cellular phenotypes96. As illustrated in 2D embryoid models, 2D micropatterning provides significant advantages as an assay technology, given their scalability and reproducibility, coupled with the ability to manipulate culture conditions and the simplicity of live imaging. Importantly, 2D micropatterning can be integrated with microfluidics91 and cell mechanics tools (such as micropost force sensors or traction force microscopy97) for dynamic, quantitative measurements and perturbations of soluble biochemical signals and insoluble biophysical cues. These unique features have not been fully exploited to date, but are important for future studies to examine the roles of tissue geometry and mechanical forces in influencing cell signaling and cell-cell communication to regulate patterning in 2D embryoids.
Using similar microfabrication approaches, micropatterning can be extended from 2D to 3D, such as to embed cells within microscale cavities in soft materials such as hydrogels or viscoelastic polymers. This approach has been demonstrated for tubular mammary epithelia, shedding light on mechanisms of branching morphogenesis98. When single primed hPSCs are grown in microcavities overlaid with Matrigel, the cells self-organize and form a single central lumen with a defined geometry81. Such tissues might serve as precursors for the generation of new embryoids, e.g. for modeling NT patterning along the A-P and/or D-V axes.
Microwell arrays for controlling initial cell aggregation
Another simple yet useful microfabrication approach for improving the consistency of embryoid development is the microwell array. The microwell array can be used to trap cells in suspension to promote their initial aggregation into spheroids of controlled sizes and multicellular compositions. This approach, for example, has been exploited to reproducibly generate blastoids from mESC / mTSC aggregates in microwell arrays composed of agarose hydrogels fabricated by micromolding with PDMS stamps22. Along similar lines, the AggreWell™ plate, a commercial microwell array, was used to improve the reproducibility and efficiency in generating ETX embryoids and mEPSC-based blastoids23,26,80.
For both micropatterning and microwell arrays, their impacts on embryoid development seem to derive from their influences on setting up the initial number of cells in each cell colony and colony geometrical boundaries. Colony geometry can directly influence cell signaling and cell-cell communication through regulatory mechanisms involving dynamic morphogenetic cues and diffusible signals24,84,85. It remains elusive how the initial number of cells (or cell density) in each cell colony affects progressive embryoid development. Existing data suggest its effect on cell polarity, paracrine signaling, the actin cytoskeleton, and mechanotransduction, which are known to regulate classic developmental signaling events.
Microfluidics for establishing signaling centers and gradients and stem cell niches
Whereas micropatterned substrates and microwell arrays offer an effective means to control cell colony shape and aggregate composition, the environment in which the cells are grown is typically isotropic and static, and thus poorly suited to recapitulate the spatiotemporal dynamics of morphogen signaling operating in vivo. Because of its ability to precisely manipulate tiny quantities of fluids and establish dynamic chemical gradients, microfluidics offers exciting opportunities to control morphogen signaling in space and time such as to establish artificial signaling centers to direct multicellular self-organization and patterning.
An example along this line reported the development of microfluidic devices to expose micropatterned 2D gastrulation embryoids to linear morphogen gradients generated via passive diffusion (i.e. a “source and sink” type gradient system)91. Beyond establishing controlled biomolecular gradients, microfluidics offers a powerful way to optimize and standardize advanced embryoid cultures, as demonstrated by the microfluidic PASE27. This and similar microengineered 3D culture systems should be particularly useful for designing multicellular embryoid systems with the robustness and scalability needed in translational applications such as high-content screening.
It is worth noting that artificial signaling centers in multicellular self-organization and development can also be established using microbeads loaded with signaling molecules99, optogenetics100, or through co-culture with morphogen-secreting cells101. A recent work demonstrated optogenetic stimulation for local activation of WNT signals in both 2D and 3D human gastrulation models to drive mesendoderm differentiation100. In another work, an inducible Shh-producing cell aggregate was embedded at one pole of an hPSC spheroid, mimicking a developmental organizer, to promote ordered self-organization along D-V and A-P axes in a forebrain organoid model101.
Advanced biomaterials to mimic ECM
Mammalian development involves not only cell-cell interactions, but also cell-ECM interactions that guide embryonic organization, cellular differentiation and morphogenesis. The ECM is synthesized and secreted by embryonic cells beginning at the earliest stages of development. Providing adhesive substrates in a 3D context, the ECM further defines tissue boundaries to guide cell migration and functions as a dynamic repository for growth factors to regulate morphogen signaling. Importantly, embryonic cells sense and respond to the 3D organization and physical properties of the ECM through mechanotransductive processes involving integrin-mediated adhesions and the intracellular actin cytoskeleton102. An exciting contemporary research question is how these mechanotransduction processes interact with growth-factor-mediated developmental signaling to regulate cellular differentiation and patterning102.
The importance of basement membrane-mediated integrin signaling in transforming amorphous EPI cells into an apico-basally polarized lumenal EPI sac was first shown in the peri-implantation mouse embryo103. Indeed, the use of 3D ECM cultures to promote the development of mESCs and primed hPSCs into lumenal EPI-like structures has been an important first step for the development of different embryoids19,20,23,26. In view of these data, how can one then explain the remarkable level of self-organization and patterning seen in the mouse and human gastruloids grown in suspension, i.e. without a surrounding 3D matrix support?13,17,21 Two recent papers that report the exposure of mouse gastruloids to low percentage Matrigel at a later culture time point might shed light on the role of ECM in morphogenesis in the gastruloid. Intriguingly, instead of observing gene expression patterns in the absence of any visible morphogenesis, as in mouse gastruloids derived in suspension culture17,21, the provision of Matrigel resulted in the development of somites and a NT28,92, suggesting that fate patterns could arise even in morphologically rather disorganized tissues and elaborate morphogenesis and tissue formation might be dependent on physical contacts with ECM. However, whether Matrigel exerts its function in the mouse gastruloid through adhesive signaling or mechanical interactions or both remains to be elucidated.
All of the above examples have relied on native ECM isolated from animal tissues, in particular Matrigel and Geltrex, basement membrane extracts derived from mouse tumor tissues. The main limitations of these native ECM, e.g. its batch-to-batch variability and potential immunogenicity, are widely documented. However, despite sizeable efforts in biomaterial development over the last decade, it has not yet been possible to identify synthetic alternatives that can completely replace native ECM in 3D cultures. However, some progress has been reported in the use of Matrigel alternatives for embryoid cultures. For example, Poh and colleagues utilized fibrin matrices, a clinically approved biomaterial generated from fibrinogen, to coax mESC colonies to differentiate and form spatially organized germ layers16. Interestingly, the authors reported the roles of matrix dimensionality, stiffness, as well as cell-cell adhesion in promoting germ layer self-organization16.
An approach based on chemically defined, poly(ethylene glycol) (PEG)-based hydrogels was explored in the context of 3D NT models generated from mESCs15,94. By systematically screening PEG matrices of variable stiffness, degradability, and ECM composition, the authors identified a parameter window in which apico-basally polarized NE sacs with proper D-V patterning robustly emerged, with an efficiency greater than achieved in Matrigel94. More recently, synthetic hydrogels were applied to the 3D human gastrulation embryoid, by using two commercially available hydrogel systems (physically crosslinked PNIPAAm-PEG gel and an Fmoc-based supramolecular gel) that were admixed with Matrigel26. Beyond assisting translational applications of embryoids, the exquisite modularity of such chemically and physically defined hydrogel systems will facilitate a systematic dissection of the independent roles of extrinsic ECM factors (including matrix stiffness, porosity, degradability, and ECM composition) in early development.
Conclusions and future directions
Understanding human development has been one of the central goals of modern biology. To circumvent the limited availability of human samples, conventional mammalian developmental biology studies have relied heavily on animal models, including NHP monkeys. In all of these models, the need for in utero development prevents precise manipulations and high-resolution observation. As a matter of fact, there will never be sufficient embryonic materials - from humans, NHP monkeys, or other mammalian species - available for quantitative assays with a level of resolution offered by synthetic in vitro embryoid systems. As a bottom-up approach using stem cells to model embryonic development without using intact embryos, embryoids are quickly becoming an essential experimental tool for advancing mammalian embryology. In particular, human embryoids are the only method available to study human embryological events between the onset of gastrulation and 4 – 5 weeks post-fertilization when the earliest fetal tissues from elective terminations are available. By this time point, the primordia of most of the major organ systems have formed in the recognizable fetal body, so it is imperative to develop alternative models to understand the origins of the human body plan.
The development of embryoids integrates knowledge and methodologies from stem cell biology, developmental biology, synthetic biology, tissue engineering, and bioengineering. Coupled with the ease of genetically modifying stem cell lines, the ability to manipulate culture conditions and the simplicity of live imaging, embryoids are becoming robust and attractive systems to disentangle cellular behaviors and signaling interactions that drive mammalian embryogenesis. Using lineage and signaling reporter lines, embryoids offer exciting trackable systems to study pattern formation, morphogenesis, cell differentiation, and growth and how these developmental processes are dynamically regulated and coordinated during embryonic development. Embryoids are also useful for elucidating intracellular signaling dynamics and gene regulatory networks and their cross-regulation with cell mechanics and morphogenetic signals during embryonic development and for studying classic developmental biology questions, such as symmetry breaking, scaling and induction.
Directed differentiations of hPSCs towards clinically relevant cell lineages using conventional 2D cultures have made significant progress over the last two decades and are largely based on developmental biology knowledge generated from model organisms to optimize growth and differentiation factors to modulate relevant developmental signaling pathways. However, intricate cell-cell interactions involved in embryonic development, which are important for lineage specification and functional maturation, are often missing and difficult to recapitulate in conventional 2D cultures. Thus, it is possible that continuous development of human embryoids can lead to advanced 3D cultures in which human stem cells can undergo successive developmental stages to produce tissue progenitors and fully differentiated cells with better fidelity to their in vivo counterparts in terms of gene expression, epigenetics, and function.
Nonetheless, the widespread utility of embryoids and their broad impact on human embryology and reproductive medicine will depend upon continuous improvements of their controllability, scalability, reproducibility and standardization and the commercial availability of culture platforms used for embryoid development. More sophisticated embryoid platforms, such as the microfluidic PASE, will require additional collaborative efforts between bioengineers and stem cell and developmental biologists for their dissemination to the broad research community. In principle, embryoids can be integrated with multi-well plate formats to achieve highly parallelized assays compatible with existing automation workflows and screening infrastructure.
Mouse embryoids, which contain all embryonic and extraembryonic lineages and their correct organizations to mimic mouse development from pre-implantation to early gastrulation, have been successfully developed19,22,23,26. Since culture protocols are available to enable whole mouse embryos to develop in vitro from the pre-gastrula to the early organogenesis stages104, it is conceivable that mouse embryoids will eventually be able to mimick whole mouse embryonic development to the organogenesis stages. Compared to mouse embryoids, human embryoids developed so far have only used primed hPSCs to model post-implantation developmental events associated with the EPI lineage. It is foreseeable that as hTSCs and human hypoblast stem cell lines become available and further authenticated, these extraembryonic cells will be integrated into existing human embryoids (as in the mouse blastoids and ETS and ETX embryoids19,22,23), allowing their prolonged and organized development and studies of the roles of embryonic-extraembryonic interactions in guiding implantation, placentation, embryonic patterning and gastrulation. Another future direction will be to leverage hPSCs possessing developmental potency for both embryonic and extraembryonic cell lineages (such as naïve hPSCs and hEPSCs) and identify suitable culture conditions to guide their development into embryoids that contain organized embryonic and extraembryonic structures26. Architecturally and functionally competent endometrial culture systems are available using both human primary cultures and established cell lines105. In the future, it will be important to use human embryoids containing TE-like cells coupled with endometrial culture systems to model human implantation and placentation, in the hope of understanding the interrelationship between embryonic and placental development.
We envision that continuous developments of mouse and human embryoids in the next 5 – 10 years will incorporate new advances of developmental biology, stem cell biology and bioengineering and will lead to new understanding of intracellular signaling and cell fate dynamics at single-cell resolution, extracellular movement of developmental signals at cellular and tissue scales, and embryonic-extraembryonic interactions and their critical roles in guiding embryonic development. It is foreseeable that advanced approaches integrating different bioengineering tools will further promote the controllability and reproducibility of different embryoids. Bioengineering tools to dynamically control the cellular environment, such as by modulating morphogen gradients, symmetry breaking and local signaling centers, will be essential for improving embryoids and gleaning new insights in their developments. Next-generation 3D human embryoids (such as human ETX embryoids) or even new human embryoid systems mimicking organogenesis (including the brain, heart, blood and gut) will likely emerge. We also envision that in the next 5 – 10 years initial translational applications of human embryoids will allow studying genetic and environmental causes of recurrent implantation failure (by combining human embryoids with endometrial culture systems) and early birth structural defects such as NT defects (NTDs) and congenital heart defects. Embryoids also have the potential to replace in vivo teratoma assays commonly used for establishing stem cell pluripotency. ‘Organism-level’, high-throughput, embryoid-based screening pipelines will likely emerge in the near future. We also envision that an important next technological milestone is to achieve a human embryoid that can recapitulate the entire gastrulation process and the development of the trilaminar germ disc containing the three organized definitive germ layers.
Validation of findings from embryoids using in vivo controls will be important to evaluate their developmental relevance90. However, this is challenging for human embryoids that aim to recapitulate post-gastrulation human development, given the scarcity of relevant human embryo data106. This challenge will be partially addressed by the recent progress of NHP monkey embryo studies10–12, which provide quantitative transcriptomic and epigenomic profiles of monkey cells at post-gastrulation developmental stages. Nonetheless, it becomes imperative to establish a molecular and cellular standard to assess the authenticity and equivalency and establish developmental identities of human embryoids compared to their in vivo counterparts. This might require the current 14-day rule governing the in vitro human embryo research to be extended to a post-gastrulation developmental stage9.
Accompanying the emergence of different human embryoids, there are ongoing discussions and recommendations from the bioethics community on their regulation107,108. Even though the existing human embryoids are far from being equivalent to human embryos, as we continue to improve and generate new human embryoids, they are expected to more closely mimic human embryos, in terms of cell organization, morphogenesis and gene expression. The continuous development and progression of this nascent field will inevitably lead to important bioethical questions: What should the ethical status of human embryoids be and how should they be regulated? What does determine the developmental potential of human embryoids in culture and equally as important, can it be functionally assessed? Discussions about these questions are clearly out of the scope of this review, and the readers are referred to recent commentaries elsewhere107,108. Currently there is little explicit regulation of human embryoid research. However, a consensus among researchers working in this field (including the authors) has urged regulators to prohibit implantation of human embryoids into mammalian uterus and ban the use of human embryoids for reproductive purposes107,108. As this nascent field moves forward, we should keep in mind social responsibility as an essential part of the responsible conduct of research. Transparency and effective engagement with all stakeholders including the public is essential to ensure that promising avenues for research proceed with due caution, especially given the complexity and rapid progress of this field.
Acknowledgements
The authors thank S. Vianello for invaluable work on illustrations and N. Rivron, A. Yoney, E.D. Siggia, M. Simunovic and Y. Zheng for microscopic images shown in Fig. 4. J.F.’s research is supported by the University of Michigan Mechanical Engineering Department, the Michigan-Cambridge Research Initiative, the University of Michigan Mcubed Fund, the National Institutes of Health (R21 NS113518 and R21 HD100931), and the National Science Foundation (CMMI 1917304 and CBET 1901718). A.W.’s work is supported by the Rice University, the Welch Foundation (C-2021), the Simons Foundation (511709), the National Institutes of Health (R01 GM126122), and the National Science Foundation (MCB-1553228). M.P.L.’s work is supported by the École Polytechnique Fédérale de Lausanne, the National Center of Competence in Research (NCCR) “Bio-Inspired Materials”, and the Swiss National Science Foundation Sinergia Grant (#3189956). The authors apologize to colleagues whose work they could not cite owing to space restrictions.
Footnotes
Competing Interests Statement
The authors declare no competing interests.
REFERENCES
- 1.Gastrulation: From Embryonic Pattern to Form. (Academic Press, 2020). [Google Scholar]
- 2.Rossant J & Tam PPL New insights into early human development: Lessons for stem cell derivation and differentiation. Cell Stem Cell 20, 18–28 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Fogarty NME et al. Genome editing reveals a role for OCT4 in human embryogenesis. Nature 550, 67–73 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma H et al. Correction of a pathogenic gene mutation in human embryos. Nature 548, 413–419 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Deglincerti A et al. Self-organization of the in vitro attached human embryo. Nature 533, 251–254 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Shahbazi MN et al. Self-organization of the human embryo in the absence of maternal tissues. Nature Cell Biology 18, 700–708 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiang L et al. A developmental landscape of 3D-cultured human pre-gastrulation embryos. Nature 577, 537–542 (2020). [DOI] [PubMed] [Google Scholar]
- 8.Daley GQ et al. Setting global standards for stem cell research and clinical translation: The 2016 ISSCR guidelines. Stem Cell Reports 6, 787–797 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hyun I, Wilkerson A & Johnston J Revisit the 14-day rule. Nature 533, 169–171 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Ma H et al. In vitro culture of cynomolgus monkey embryos beyond early gastrulation. Science 366, eaax7890 (2019). [DOI] [PubMed] [Google Scholar]
- 11.Niu Y et al. Dissecting primate early post-implantation development using long-term in vitro embryo culture. Science 366, eaaw5754 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Nakamura T et al. A developmental coordinate of pluripotency among mice, monkeys and humans. Nature 537, 57–62 (2016). [DOI] [PubMed] [Google Scholar]
- 13.ten Berge D et al. Wnt signaling mediates self-organization and axis formation in embryoid bodies. Cell Stem Cell 3, 508–518 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuchs C et al. Self-organization phenomena in embryonic stem cell-derived embryoid bodies: Axis formation and breaking of symmetry during cardiomyogenesis. Cells Tissues Organs 195, 377–391 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meinhardt A et al. 3D reconstitution of the patterned neural tube from embryonic stem cells. Stem Cell Reports 3, 987–999 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poh Y-C et al. Generation of organized germ layers from a single mouse embryonic stem cell. Nature Communications 5, 4000 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van den Brink SC et al. Symmetry breaking, germ layer specification and axial organisation in aggregates of mouse embryonic stem cells. Development 141, 4231–4242 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warmflash A et al. A method to recapitulate early embryonic spatial patterning in human embryonic stem cells. Nature Methods 11, 847–854 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrison SE et al. Assembly of embryonic and extra-embryonic stem cells to mimic embryogenesis in vitro. Science 356, eaal1810 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Shao Y et al. A pluripotent stem cell-based model for post-implantation human amniotic sac development. Nature Communications 8, 208 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beccari L et al. Multi-axial self-organization properties of mouse embryonic stem cells into gastruloids. Nature 562, 272–276 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Rivron NC et al. Blastocyst-like structures generated solely from stem cells. Nature 557, 106–111 (2018). [DOI] [PubMed] [Google Scholar]
- 23.Sozen B et al. Self-assembly of embryonic and two extra-embryonic stem cell types into gastrulating embryo-like structures. Nature Cell Biology 20, 979–989 (2018). [DOI] [PubMed] [Google Scholar]
- 24.Xue X et al. Mechanics-guided embryonic patterning of neuroectoderm tissue from human pluripotent stem cells. Nature Materials 17, 633–641 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haremaki T et al. Self-organizing neuruloids model developmental aspects of Huntington’s disease in the ectodermal compartment. Nature Biotechnology 37, 1198–1208 (2019). [DOI] [PubMed] [Google Scholar]
- 26.Simunovic M et al. A 3D model of a human epiblast reveals BMP4-driven symmetry breaking. Nature Cell Biology 21, 900–910 (2019). [DOI] [PubMed] [Google Scholar]
- 27.Zheng Y et al. Controlled modelling of human epiblast and amnion development using stem cells. Nature 573, 421–425 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van den Brink SC et al. Single-cell and spatial transcriptomics reveal somitogenesis in gastruloids. Nature 582, 405–409 (2020). [DOI] [PubMed] [Google Scholar]
- 29.Rossant J & Tam PPL Blastocyst lineage formation, early embryonic asymmetries and axis patterning in the mouse. Development 136, 701–713 (2009). [DOI] [PubMed] [Google Scholar]
- 30.Wennekamp S, Mesecke S, Nédélec F & Hiiragi T A self-organization framework for symmetry breaking in the mammalian embryo. Nature Reviews Molecular Cell Biology 14, 452–459 (2013). [DOI] [PubMed] [Google Scholar]
- 31.Boroviak T & Nichols J Primate embryogenesis predicts the hallmarks of human naïve pluripotency. Development 144, 175–186 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Rahilly R & Müller F Developmental Stages in Human Embryos: Revised and New Measurements. Cells Tissues Organs 192, 73–84 (2010). [DOI] [PubMed] [Google Scholar]
- 33.Tam PPL & Loebel DAF Gene function in mouse embryogenesis: get set for gastrulation. Nature Reviews Genetics 8, 368–381 (2007). [DOI] [PubMed] [Google Scholar]
- 34.Arnold SJ & Robertson EJ Making a commitment: cell lineage allocation and axis patterning in the early mouse embryo. Nature Reviews Molecular Cell Biology 10, 91–103 (2009). [DOI] [PubMed] [Google Scholar]
- 35.Rivera-Pérez JA, Mager J & Magnuson T Dynamic morphogenetic events characterize the mouse visceral endoderm. Developmental Biology 261, 470–487 (2003). [DOI] [PubMed] [Google Scholar]
- 36.Thomas P & Beddington R Anterior primitive endoderm may be responsible for patterning the anterior neural plate in the mouse embryo. Current Biology 6, 1487–1496 (1996). [DOI] [PubMed] [Google Scholar]
- 37.Yamamoto M et al. Nodal antagonists regulate formation of the anteroposterior axis of the mouse embryo. Nature 428, 387–392 (2004). [DOI] [PubMed] [Google Scholar]
- 38.Yoon Y et al. Extra-embryonic Wnt3 regulates the establishment of the primitive streak in mice. Developmental Biology 403, 80–88 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tortelote GG et al. Wnt3 function in the epiblast is required for the maintenance but not the initiation of gastrulation in mice. Developmental Biology 374, 164–173 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohinata Y et al. Blimp1 is a critical determinant of the germ cell lineage in mice. Nature 436, 207–213 (2005). [DOI] [PubMed] [Google Scholar]
- 41.Saitou M, Barton SC & Surani MA A molecular programme for the specification of germ cell fate in mice. Nature 418, 293–300 (2002). [DOI] [PubMed] [Google Scholar]
- 42.Chen D et al. Human primordial germ cells are specified from lineage-primed progenitors. Cell Reports 29, 4568–4582.e4565 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sasaki K et al. The germ cell fate of cynomolgus monkeys is specified in the nascent amnion. Developmental Cell 39, 169–185 (2016). [DOI] [PubMed] [Google Scholar]
- 44.Colas J-F & Schoenwolf GC Towards a cellular and molecular understanding of neurulation. Developmental Dynamics 221, 117–145 (2001). [DOI] [PubMed] [Google Scholar]
- 45.Vijayraghavan DS & Davidson LA Mechanics of neurulation: From classical to current perspectives on the physical mechanics that shape, fold, and form the neural tube. Birth Defects Research 109, 153–168 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ribes V & Briscoe J Establishing and interpreting graded sonic hedgehog signaling during vertebrate neural tube patterning: The role of negative feedback. Cold Spring Harbor Perspectives in Biology 1, a002014 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Briscoe J & Small S Morphogen rules: Design principles of gradient-mediated embryo patterning. Development 142, 3996–4009 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nichols J & Smith A Naive and Primed Pluripotent States. Cell Stem Cell 4, 487–492 (2009). [DOI] [PubMed] [Google Scholar]
- 49.Smith A Formative pluripotency: the executive phase in a developmental continuum. Development 144, 365–373 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boroviak T et al. The ability of inner-cell-mass cells to self-renew as embryonic stem cells is acquired following epiblast specification. Nature Cell Biology 16, 513–525 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ying Q-L et al. The ground state of embryonic stem cell self-renewal. Nature 453, 519–523 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brons IGM et al. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature 448, 191–195 (2007). [DOI] [PubMed] [Google Scholar]
- 53.Tesar PJ et al. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature 448, 196–199 (2007). [DOI] [PubMed] [Google Scholar]
- 54.Kojima Y et al. The Transcriptional and Functional Properties of Mouse Epiblast Stem Cells Resemble the Anterior Primitive Streak. Cell Stem Cell 14, 107–120 (2014). [DOI] [PubMed] [Google Scholar]
- 55.Hayashi K et al. Reconstitution of the Mouse Germ Cell Specification Pathway in Culture by Pluripotent Stem Cells. Cell 146, 519–532 (2011). [DOI] [PubMed] [Google Scholar]
- 56.Tanaka S et al. Promotion of Trophoblast Stem Cell Proliferation by FGF4. Science 282, 2072–2075 (1998). [DOI] [PubMed] [Google Scholar]
- 57.Niakan KK, Schrode N, Cho LTY & Hadjantonakis A-K Derivation of extraembryonic endoderm stem (XEN) cells from mouse embryos and embryonic stem cells. Nature Protocols 8, 1028–1041 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thomson JA et al. Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147 (1998). [DOI] [PubMed] [Google Scholar]
- 59.Guo H et al. The DNA methylation landscape of human early embryos. Nature 511, 606–610 (2014). [DOI] [PubMed] [Google Scholar]
- 60.Yan L et al. Single-cell RNA-Seq profiling of human preimplantation embryos and embryonic stem cells. Nature Structural & Molecular Biology 20, 1131–1139 (2013). [DOI] [PubMed] [Google Scholar]
- 61.Chia N-Y et al. A genome-wide RNAi screen reveals determinants of human embryonic stem cell identity. Nature 468, 316–320 (2010). [DOI] [PubMed] [Google Scholar]
- 62.Irie N et al. SOX17 is a critical specifier of human primordial germ cell fate. Cell 160, 253–268 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sasaki K et al. Robust in vitro induction of human germ cell fate from pluripotent stem cells. Cell Stem Cell 17, 178–194 (2015). [DOI] [PubMed] [Google Scholar]
- 64.Theunissen Thorold W. et al. Systematic Identification of Culture Conditions for Induction and Maintenance of Naive Human Pluripotency. Cell Stem Cell 15, 471–487 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guo G et al. Naive Pluripotent Stem Cells Derived Directly from Isolated Cells of the Human Inner Cell Mass. Stem Cell Reports 6, 437–446 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Theunissen, Thorold W et al. Molecular Criteria for Defining the Naive Human Pluripotent State. Cell Stem Cell 19, 502–515 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Takashima Y et al. Resetting Transcription Factor Control Circuitry toward Ground-State Pluripotency in Human. Cell 158, 1254–1269 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yamanaka S & Blau HM Nuclear reprogramming to a pluripotent state by three approaches. Nature 465, 704–712 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Choi J et al. A comparison of genetically matched cell lines reveals the equivalence of human iPSCs and ESCs. Nature Biotechnology 33, 1173–1181 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Quadrato G et al. Cell diversity and network dynamics in photosensitive human brain organoids. Nature 545, 48–53 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang Y et al. Derivation of Pluripotent Stem Cells with In Vivo Embryonic and Extraembryonic Potency. Cell 169, 243–257.e225 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gao X et al. Establishment of porcine and human expanded potential stem cells. Nature Cell Biology 21, 687–699 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Okae H et al. Derivation of Human Trophoblast Stem Cells. Cell Stem Cell 22, 50–63.e56 (2018). [DOI] [PubMed] [Google Scholar]
- 74.Linneberg-Agerholm M et al. Naïve human pluripotent stem cells respond to Wnt, Nodal and LIF signalling to produce expandable naïve extra-embryonic endoderm. Development 146, dev180620 (2019). [DOI] [PubMed] [Google Scholar]
- 75.Dong C et al. Derivation of trophoblast stem cells from naïve human pluripotent stem cells. eLife 9, e52504 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guo G et al. Trophectoderm Potency is Retained Exclusively in Human Naïve Cells. bioRxiv, DOI: 10.1101/2020.1102.1104.933812 (2020). [DOI] [Google Scholar]
- 77.Posfai E et al. Defining totipotency using criteria of increasing stringency. bioRxiv, DOI: 10.1101/2020.1103.1102.972893 (2020). [DOI] [PubMed] [Google Scholar]
- 78.Frias-Aldeguer J et al. Embryonic signals perpetuate polar-like trophoblast stem cells and pattern the blastocyst axis. bioRxiv, DOI: 10.1101/510362 (2019). [DOI] [Google Scholar]
- 79.Vrij EJ et al. Chemically-defined induction of a primitive endoderm and epiblast-like niche supports post-implantation progression from blastoids. bioRxiv, DOI: 10.1101/510396 (2019). [DOI] [Google Scholar]
- 80.Sozen B et al. Self-Organization of Mouse Stem Cells into an Extended Potential Blastoid. Developmental Cell 51, 698–712.e698 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Taniguchi K et al. Lumen formation is an intrinsic property of isolated human pluripotent stem cells. Stem Cell Reports 5, 954–962 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shahbazi MN et al. Pluripotent state transitions coordinate morphogenesis in mouse and human embryos. Nature 552, 239 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Minn KT et al. High-resolution transcriptional and morphogenetic profiling of cells from micropatterned human embryonic stem cell gastruloid cultures. bioRxiv, DOI: 10.1101/2020.1101.1122.915777 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tewary M et al. A stepwise model of reaction-diffusion and positional-information governs self-organized human peri-gastrulation-like patterning. Development 144, 4298–4312 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Etoc F et al. A balance between secreted inhibitors and edge sensing controls gastruloid self-organization. Developmental Cell 39, 302–315 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Heemskerk I et al. Rapid changes in morphogen concentration control self-organized patterning in human embryonic stem cells. eLife 8, e40526 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nemashkalo A, Ruzo A, Heemskerk I & Warmflash A Morphogen and community effects determine cell fates in response to BMP4 signaling in human embryonic stem cells. Development 144, 3042–3053 (2017). [DOI] [PubMed] [Google Scholar]
- 88.Chhabra S et al. Dissecting the dynamics of signaling events in the BMP, WNT, and NODAL cascade during self-organized fate patterning in human gastruloids. PLOS Biology 17, e3000498 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Martyn I, Brivanlou AH & Siggia ED A wave of WNT signaling balanced by secreted inhibitors controls primitive streak formation in micropattern colonies of human embryonic stem cells. Development 146, dev172791 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Morgani SM et al. Micropattern differentiation of mouse pluripotent stem cells recapitulates embryo regionalized cell fate patterning. eLife 7, e32839 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Manfrin A et al. Engineered signaling centers for the spatially controlled patterning of human pluripotent stem cells. Nature Methods 16, 640–648 (2019). [DOI] [PubMed] [Google Scholar]
- 92.Veenvliet JV et al. Mouse embryonic stem cells self-organize into trunk-like structures with neural tube and somites. bioRxiv, DOI: 10.1101/2020.1103.1104.974949 (2020). [DOI] [PubMed] [Google Scholar]
- 93.Rossi G et al. Embryonic organoids recapitulate early heart organogenesis. bioRxiv, DOI: 10.1101/802181 (2019). [DOI] [Google Scholar]
- 94.Ranga A et al. Neural tube morphogenesis in synthetic 3D microenvironments. Proceedings of the National Academy of Sciences 113, E6831–E6839 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Britton G et al. A novel self-organizing embryonic stem cell system reveals signaling logic underlying the patterning of human ectoderm. Development 146, dev179093 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McBeath R et al. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Developmental Cell 6, 483–495 (2004). [DOI] [PubMed] [Google Scholar]
- 97.Lam RHW, Sun Y, Chen W & Fu J Elastomeric microposts integrated into microfluidics for flow-mediated endothelial mechanotransduction analysis. Lab on a Chip 12, 1865–1873 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nelson CM et al. Tissue Geometry Determines Sites of Mammary Branching Morphogenesis in Organotypic Cultures. Science 314, 298–300 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Habib SJ et al. A Localized Wnt Signal Orients Asymmetric Stem Cell Division in Vitro. Science 339, 1445–1448 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Repina NA et al. Optogenetic control of Wnt signaling for modeling early embryogenic patterning with human pluripotent stem cells. bioRxiv, DOI: 10.1101/665695 (2019). [DOI] [Google Scholar]
- 101.Cederquist GY et al. Specification of positional identity in forebrain organoids. Nature Biotechnology 37, 436–444 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sun Y, Chen CS & Fu J Forcing stem cells to behave: A biophysical perspective of the cellular microenvironment. Annual Review of Biophysics 41, 519–542 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bedzhov I & Zernicka-Goetz M Self-organizing properties of mouse pluripotent cells initiate morphogenesis upon implantation. Cell 156, 1032–1044 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tam PP Postimplantation mouse development: whole embryo culture and micro-manipulation. The International journal of developmental biology 42, 895–902 (1998). [PubMed] [Google Scholar]
- 105.Wang H et al. A novel model of human implantation: 3D endometrium-like culture system to study attachment of human trophoblast (Jar) cell spheroids. Molecular Human Reproduction 18, 33–43 (2011). [DOI] [PubMed] [Google Scholar]
- 106.Tyser RCV et al. A spatially resolved single cell atlas of human gastrulation. bioRxiv, DOI: 10.1101/2020.1107.1121.213512 (2020). [DOI] [Google Scholar]
- 107.Rivron Nicolas et al. Debate ethics of embryo models from stem cells. Nature 564, 183–185 (2018). [DOI] [PubMed] [Google Scholar]
- 108.Hyun I et al. Toward Guidelines for Research on Human Embryo Models Formed from Stem Cells. Stem Cell Reports 14, 169–174 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Petropoulos S et al. Single-Cell RNA-Seq Reveals Lineage and X Chromosome Dynamics in Human Preimplantation Embryos. Cell 165, 1012–1026 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dobreva MP et al. Amniotic ectoderm expansion in mouse occurs via distinct modes and requires SMAD5-mediated signalling. Development 145, dev157222 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pereira PNG et al. Amnion formation in the mouse embryo: the single amniochorionic fold model. BMC Developmental Biology 11, 48 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lawson KA et al. Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes & Development 13, 424–436 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Munoz-Sanjuan I & Brivanlou AH Neural induction, the default model and embryonic stem cells. Nature Reviews Neuroscience 3, 271–280 (2002). [DOI] [PubMed] [Google Scholar]
- 114.Stern CD Neural induction: Old problem, new findings, yet more questions. Development 132, 2007–2021 (2005). [DOI] [PubMed] [Google Scholar]
- 115.Barnat M et al. Huntington’s disease alters human neurodevelopment. Science, eaax3338 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]