Abstract
The high prevalence of rotator cuff tears poses challenges to individual patients and the health care system at large. This orthopedic injury is complicated further by high rates of re-tear after surgical repair. Outcomes following repair are highly dependent upon the quality of the injured rotator cuff muscles, and it is therefore crucial that the pathophysiology of rotator cuff degeneration continue to be explored. Fibro-adipogenic progenitors, a major population of resident muscle stem cells, have emerged as the main source of intramuscular fibrosis and fatty infiltration, both of which are key features of rotator cuff muscle degeneration. Improvements to rotator cuff repair outcomes will likely require addressing the muscle pathology produced by these cells. The aim of this review is to summarize the current rotator cuff degeneration assessment tools, the effects of poor muscle quality on patient outcomes, the role of fibro-adipogenic progenitors in mediating muscle pathology, and how these cells could be leveraged for potential therapeutics to augment current rotator cuff surgical and rehabilitative strategies.
Keywords: rotator cuff, fatty infiltration, fibrosis, fibro-adipogenic progenitors
Graphical abstract
Fibro-adipogenic progenitors have emerged as the main source of intramuscular fibrosis and fatty infiltration in rotator cuff muscle degeneration. This review summarizes the current rotator cuff degeneration assessment tools, the effects of poor muscle quality on patient outcomes, the role of fibro-adipogenic progenitors in mediating muscle pathology, and how these cells could be leveraged for potential therapeutics to augment current rotator cuff surgical and rehabilitative strategies.
Introduction
Rotator cuff (RC) tears are among the most common musculoskeletal injuries encountered by the health care system, financially burdening society as a whole and negatively impacting patients’ quality of life.1, 2 In the United States alone, there is an estimated 4.5 million patient visits related to shoulder pain and a total of 250,000 RC repairs annually, with these numbers expected to increase due to the aging US population.3, 4 A study conducted by Yamamoto et al. found that the overall prevalence of RC tears in subjects aged 22–87 was 20.7%.5 This number increased with age, with 25.6% of people in their 60’s presenting with RC tears, 45.8% of people in their 70’s, and 50.0% of people in their 80’s.5 In addition to age, hand dominance, history of trauma, nicotine use, hypercholesterolemia, and genetics have all been found to be either correlated with or strongly implicated in increasing the risk of RC tears.5–7
In order to understand the total lifetime societal costs (LSC) associated with RC tears it is important to consider direct medical costs and indirect costs for both operative and non-operative treatments. Li et al.8 evaluated 40,618 RC repair cases in 6 different US states, and found that the average cost per procedure was $25,353. Considering the estimated cost per procedure of $25,353 and that approximately 250,000 rotator cuffs are repaired annually, the economic burden from direct medical costs alone is substantial.4, 8 In addition to direct medical costs, there are indirect costs to society, including deficits in household income, missed workdays, and increases in disability payments. When considering the total LSC associated with nonrepaired RC tears, Mather et al. found that these costs exceeded that of the aforementioned operative group.4 In the United States, the total LSC associated with annual non-operative treatment exceeds that of the operative treatment by $3.44 billion. This highlights just how economically burdensome RC tears are, whether or not they undergo repair. In addition to levying a heavy societal cost, RC tears cause patients to experience a decrease in quality-of-life, as has been demonstrated by the significant improvements in patient-reported physical and mental health parameters, such as depression, anxiety, and insomnia, after undergoing successful repair.1, 2
The substantial societal and personal costs of RC injuries are complicated further by high rates of re-tears after surgical repair.9–12 The probability of re-tear has been highly correlated with the degenerative features that are often present in RC tears: muscle atrophy and fatty infiltration.10, 11 These re-tear injuries inject additional costs into an already expensive endeavor and introduce additional morbidity for this patient population. Therefore, understanding the ways in which surgeons assess RC degeneration and include these assessments into surgical and rehabilitation planning are of importance. Furthermore, an understanding of the cellular mechanisms and actors involved in the development of RC degeneration are crucial for discovering new therapeutic approaches to improve muscle quality and augment current surgical and rehabilitation techniques. In this review, we discuss current assessment tools of muscle quality, the impact of RC degeneration on patient function and surgical outcomes, the role of fibro-adipogenic progenitor cells in RC degeneration, and future directions for novel therapeutics that leverage these resident muscle stem cells.
RC muscle quality and degeneration
Consideration of RC muscle quality and degeneration has emerged as an important factor when investigating RC tear progression, related shoulder pain, and surgical and functional outcomes for patients. Historically, characterization of RC degeneration has focused on muscle atrophy and intramuscular fatty infiltration (FI).10, 13, 14 More recently, studies have also examined whether the development of intramuscular fibrosis plays a significant role in RC pathology.15–18
Assessment of RC muscle quality
Efforts have been made to create classification systems to characterize the degree of FI and muscle atrophy. Goutallier introduced the first system, which employed a global fatty degeneration index based on axial computer tomography (CT) imaging of the scapula and surrounding musculature.14, 19 With the onset of magnetic resonance imaging (MRI) technology, modifications were made to the scoring system to simplify it, but kept consistent with comparing the fat content with muscle content (Fig. 1).20 There have also been multiple scoring systems for muscle atrophy over the years. Warner et al. developed a system evaluating the amount of muscle above or below lines drawn from the coracoid to the edge of the scapular spine and to the inferior border of the scapula (Fig. 2).21 Earlier systems developed by Thomazeau et al.22 and Zanetti et al.23 used similar principles that employed tangent sign measurement. The scoring system developed by Patte et al. focused on the amount of RC retraction after tear injury.24
Figure 1.

Representative proton density–weighted images using the Goutallier classification (top row) and fat fraction maps (bottom row) for different degrees of fatty infiltration of the supraspinatus muscle. Images were obtained in the sagittal-oblique plane. Gray scale bar corresponds to fat fraction values (in %). Reprinted with permission from Nardo et al. (2014).41
Figure 2.

Warner RC atrophy classification. Measurement of muscle atrophy is based the amount of muscle (gray area) above or below a line drawn from the edge of the coracoid to the inferior scapular tip, from the inferior tip of the scapula to the spine, and from the scapular spine to the coracoid process. Reprinted with permission from Warner et al. (2001).21
These assessment tools can be useful when attempting to stratify patient presentation as well as guide surgical interventions; however, many of these classification systems have shown poor inter-observer reliability. Spencer et al. demonstrated that of 19 RC MRI assessment parameters, the Goutallier grade had the lowest observer agreement, with a kappa of 0.1. The degree of muscle retraction fared moderately better at 0.63.25 In a study by Lippe et al., inter-observer agreement of MR RC images was 65%, 77%, and 28% for the Patte, Goutallier, and Warner classification systems, respectively.26 Oh et al. showed significant variability in inter-observer reliability in grading FI in both preoperative and postoperative imaging of RC tears. They also noted differences in reliability between radiologists and orthopedic surgeons, and between those with less and more years of experience.27 Intra-observer reliability has also been problematic as demonstrated by a study from Slabaugh et al. in which surgeons were asked to review the same set of images two months apart, resulting in a kappa of only 0.56.28 Other studies have further highlighted these issues with observer reliability.29–31
Attempts have been made to improve upon the reliability of these grading systems. Some studies have demonstrated that simplifying classifications by shrinking the number of grades can improve reliability, although these strategies may risk reducing the precision and clinical utility of these measurments.20, 28, 32 Alternatively, new MRI methods and sequences have been adapted to give more precise measurement of FI. Spectroscopic FLASH sequence has been used to quantitatively measure the fat/water ratio of a given RC region of interest.33, 34 Other quantitative MRI techniques, such as iterative decomposition of echoes of asymmetric length (IDEAL) and Dixon, which utilize chemical-shift based fat/water separation to identify distinct spectroscopic peaks from fat and water molecules, allow for water–fat separation and calculation of more accurate fat fraction.35–39 Horiuchi et al. found an increased inter-observer kappa of 0.89 using Dixon quantification of RC FI as opposed to 0.51 with Goutallier classification.40 Nardo et al. compared IDEAL fat fractions and Goutallier grading of 57 shoulders and found excellent reproducibility of the fat fraction using the IDEAL method.41 Furthermore, their comparisons of fat content assessed by IDEAL and Goutallier showed that for a given RC, Goutallier classification often overestimated fat content (Fig. 3). These advanced imaging techniques do not require substantial additional costs or time, and are therefore feasible for the clinical setting.42
Figure 3.
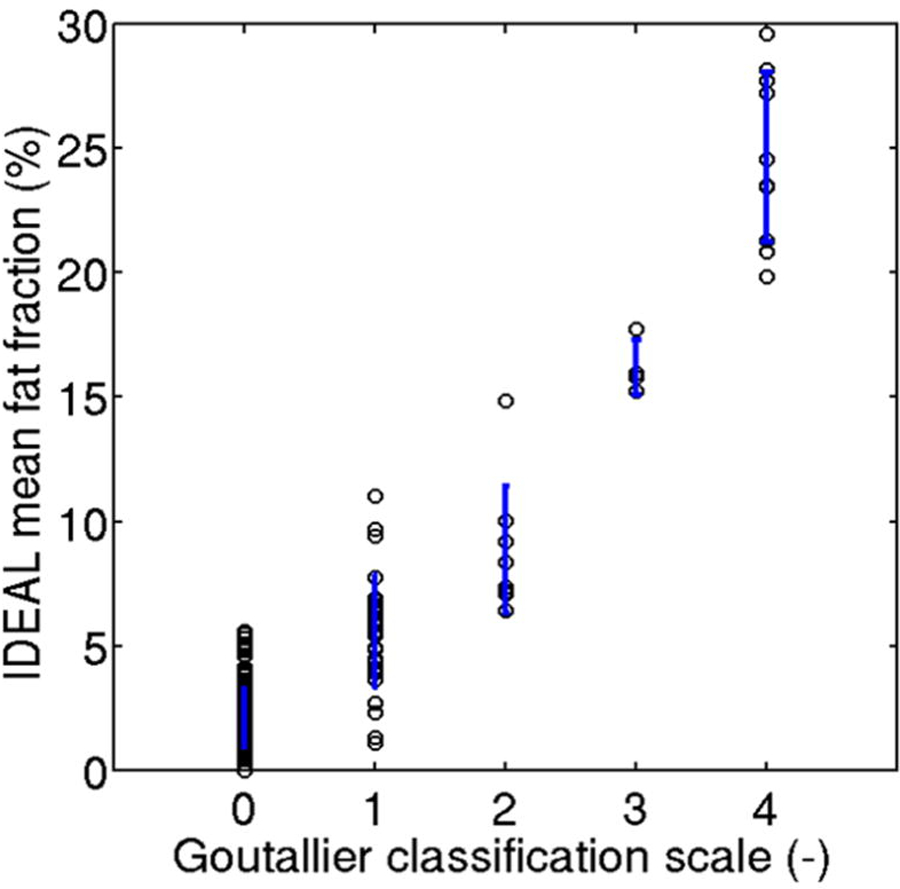
Comparison of fat fraction measurements of a set of RC MRI images as assessed by quantitative IDEAL MRI techniques (y-axis) versus qualitative Goutallier classification (x-axis). Blue vertical lines represent standard deviation for IDEAL fat fraction measurements at each Goutallier grade. Reprinted with permission from Nardo et al. (2014).41
In addition to more accurately and reliably assessing fat content through advanced noninvasive imaging techniques, characterizing the presence of specific fat subtypes, such as brown and beige fat, in the RC may further inform patient care. Although FI is mostly comprised of white adipose tissue, there is emerging evidence that the more metabolically active brown and beige fat phenotypes have pro-myogenic functions beyond their basic thermogenic role.43–49 Thus far, the prevailing method of brown fat quantification has been positron emission tomography-computed tomography (PET/CT) with 2-deoxy-2-[18F]fluoroglucose (18F-FDG) as a tracer.50–55 This procedure is performed under cold exposure due to the increased activation of brown fat under these conditions, and it relies on increased glucose uptake by activated brown fat.50–56 In addition to the feasibility concerns of this technique in a clinical setting, this method’s signal reliance on metabolically active brown adipose tissue may underestimate fat that failed to activate with cold exposure.50, 56, 57 Alternatively, xenon-enhanced CT (XECT) is a newly explored brown fat quantification tool that utilizes xenon as a probe for brown fat that is undergoing thermogenesis.51 Following a period of xenon and oxygen inhalation, CT scans are performed prior to and during induction of non-shivering thermogenesis (NST) via norepinephrine administration.51 In order to test the efficacy of XECT in distinguishing brown fat content, Branca et al. examined both obese and lean mice.51 During stimulation of NST there were no significant increases in tissue radiodensity of white fat or skeletal muscle but significant increases were observed in brown fat from both obese and lean mice. However, the risk of radiation and norepinephrine infusion would need to be considered given the age demographics of the RC tear population.
Although there are emerging quantitative MRI assessments of general FI in the RC using chemical-shift based MRIs, quantification of brown and beige fat in the RC using this method has not been heavily explored.58, 59 Chemical shift–based MRIs may hold some promise in facilitating the quantification of BAT in a noninvasive and more affordable fashion compared with PET/CT using 18F-FDG.50, 52, 54–57, 60, 61 The multilocular nature of brown adipocytes and their increased water content give brown fat protons a distinct spectral profile compared with white fat.55, 57 The difference in spectral profiles can be used to create fat fraction maps that distinguish fat subtypes (Fig. 4). However, there could still be issues with this technique in terms of inter-subject and intra-subject variability secondary to variance in tissue thermogenic activity. Future studies are warranted to validate brown and beige fat quantification techniques in the RC, such as the ones mentioned here, that would also be suited for the clinical setting. The ability to distinguish between fat subtypes may provide finer detail of RC muscle quality and help assess the response to novel interventions aimed at enhancing the brown fat phenotype in RC injures.
Figure 4.

(A) Chemical-shift MRI technique demonstrating the ability to differentiate between interscapular brown fat (white arrows) and white adipose tissue (WAT) more dorsally in an adult (top) and juvenile (bottom) mouse. (B) Anatomic reference for MRI using axial cryosection of mouse demonstrating the location of interscapular brown adipose tissue (IBAT) and WAT. Reprinted with permission from Hu et al. (2010).57
Impacts of poor RC muscle quality
Despite the issues of inter-observer reliability of qualitative systems and ongoing refinement of newer quantitative methods of assessing RC degeneration, many studies have been performed to better characterize these degenerative features and their relationship to each other. Gladstone et al. noted strong correlations between preoperative RC FI and muscle atrophy, both of which were also shown to increase with tear size.10 Barry et al. demonstrated that 41.4% of patients with complete RC tears had signs of FI on MRI compared with just 6.5% of patient without tears.62 In addition, the prevalence of supraspinatus atrophy was twice as high in patients with complete tears than in those without evidence of tear. Other studies have corroborated the association of increased muscle atrophy and fatty infiltration with larger tear sizes, although many of these studies do report evidence of FI in smaller tears.13, 63–66
The development of FI and atrophy in these muscles has important clinical ramifications, which include degree of pain, function, and surgical outcomes. Gladstone et al. found that increased muscle atrophy and FI were the only predictors of worse shoulder functional scores and strength testing.10 Importantly, they demonstrated no reversal of FI or atrophy after surgical repair, and showed that those with moderate or severe FI and atrophy had re-tear rates of 67%–70%, compared with 22%–29% in those with no or mild FI and atrophy. Mall et al. showed that patients with larger tears had more pain, more fatty degeneration, and worse functional scores.67 The presence of FI was a strong predictor of worse shoulder pain and functional scores (P = 0.0009) in a study from Jain et al. that evaluated 70 non-operatively managed RC tear patients over an 18-month period.68 Lansdown et al. recently used IDEAL imaging and showed increased fat fraction in the supraspinatus and infraspinatus six months after surgical repair, and that baseline fat fractions in the supraspinatus were significantly higher in patients who eventually failed repair (11.7% ± 6.8% versus 7.1% ± 4.8%; P = 0.037).12 Multiple other studies have also detailed the poor functional and surgical outcomes based on the degree of degenerative features.9,11, 69–71
Fibrosis is a definite pathologic feature in a number of other musculotendinous diseases, such as contractures, volumetric muscle injury, and muscular dystrophies.72–75 However, the clinical effects of fibrosis in RC injury have not been well defined. Previous studies have found increased stiffness of muscle tissue when large amounts of fibrosis are present.18, 76 Significant retraction of the RC muscle after large tears is associated with increased passive stiffness, which may impede surgical efforts to re-position the muscle for repair as well as affect tendon-bone healing.77–80 The role of fibrosis is further confused by its often co-presence with significant FI in RC tears, in which the biomechanical effects of each likely compete.81, 82 It is, therefore, possible that increased stiffness in the RC affects patient outcomes, but this has been difficult to quantify given the lack of non-invasive modalities to assess RC fibrosis content, which could then be correlated with specific outcomes.
Role of fibro-adipogenic progenitors
Achieving improvements in patient outcomes following RC tears will likely not be possible without addressing the underlying biology and pathophysiology of these degenerative features which often progress after repair. Therefore, it is crucial to understand the main stem cell population that mediates these effects, fibro-adipogenic progenitors (FAPs). Further investigation of these resident muscle stem cells may provide insights into novel treatment approaches to mitigate RC degeneration and improve patient outcomes.
FAP contribution to RC FI and fibrosis
Fibro-adipogenic progenitors were first described by Uezumi et al. as a distinct stem cell population residing within skeletal muscle.83, 84 These cells were characterized as expressing the cell surface marker PDGFRα/β. They lacked Pax7 expression, a marker of myogenic satellite cells (SCs). In adipogenic conditions, these cells readily differentiated into adipocytes with increased expression of fat markers such as CEBPα and PPARγ; however, they did not possess the ability to differentiate into myotubes. These cells were located in the interstitial space between muscle fibers as opposed to beneath the basal lamina where SCs reside (Fig. 5).85 In addition, these cells have been shown to differentiate into fibroblasts and intramuscular adipocytes, colocalizing with fat deposits.16, 17, 43, 86–88
Figure 5.

(A) Immunostaining and histologic images showing the relative position of FAPs (PDGFRα-positive) and SCs (M-cad–positive) in skeletal muscle tissue. FAPs are located in the interstitial space between muscle fibers while SCs reside beneath the basal lamina of the muscle fiber. (B) Schematic of the image above. Reprinted with permission from Uezumi et al. (2014).85
Subsequent studies have further elucidated the role of FAPs in skeletal muscle injury. After muscle injury, FAPs rapidly proliferate and have been shown to initially provide pro-differentiation signaling for myogenic precursors.84, 89, 90 In a myotoxin injury model, Joe et al. found rapid expansion of FAPs and increased expression of pro-myogenic factors such as IL-6 and IGF-1, which increased myoblast terminal differentiation and myotube formation.43 Mozzetta et al. showed that FAPs expressed greater levels of follistatin, an important inhibitor of myostatin, than SCs.91 FAP Wnt gene and protein expression were significantly increased in injured muscle, which induced SC differentiation.92 Quarta et al. found increased muscle cross sectional area and SC engraftment using FAP-infused bioconstructs transplanted in a mouse model of volumetric muscle injury.93 Based on these studies, the functional role of FAPs during their initial rapid expansion phase appears to be beneficial for regenerating muscle. After the expansion, these cells rapidly contract in number via apoptosis.43, 84, 94 However, the FAPs that persist after this reduction become the main pool of cells destined for adipogenic and fibroblastic differentiation.
It is the eventual differentiation into fibroblasts and adipocytes that complicates FAPs’ transient pro-myogenic role, as they become the main source of intramuscular fibrosis and FI.85, 86 FAPs have been implicated as the main contributors of these degenerative features in a range of musculotendinous conditions.94–97 In the RC, these cells are particularly important for understanding the natural history of RC tears and the degenerative features that develop. Liu et al. used PDGFRα reporter mice to confirm colocalization of RC FAPs with over 83% of cells staining positive for the fat markers adiponectin and PPARγ.98 Jensen et al. also demonstrated similar findings of colocalization with in vitro differentiation of FAPs into adipocytes and fibroblasts.17 Promotion of FAP apoptosis using a TGFβ small molecule inhibitor resulted in a significant reduction of RC fibrosis and FI in a mouse RC injury model.16 These studies strongly suggest that FAPs are the main mediators of RC FI and fibrosis.
The exact mechanism by which FAPs are pushed towards RC adipogenesis and fibrogenesis is not well defined at this time, but likely includes changes in local signaling, gene expression, and stem cell epigenetics, as well as the presence of baseline differences in FAP subpopulations. For example, Itoigawa et al. found increased levels of the fat markers PPARγ and CEBPα in a rat RC tear model.99 The correlation between increased FAP number and FI with larger tear sizes suggests that different tear states can result in epigenetic changes in FAPs that alter their proliferative and differentiation behaviors.100 Lee et al. found that of the FAPs taken from multiple uninjured muscle groups in mice, those harvested from the RC were of the highest quantity and possessed the greatest proliferative and adipogenic potential. In addition, there was significantly more FI in the RC than in the tibialis anterior (12.6% ± 3.9% versus 1.5% ± 1.0%; P < 0.05) two weeks after each muscle received an intramuscular injection of glycerol, suggestive of different baseline characteristics of FAPs depending on anatomic location.101 Studies that detail increased FAP quantity in disuse and tendon transection injury models indicate that changes in mechanical load may influence FAPs as well.17, 98, 102
Other mechanisms that may contribute to FAP adipogenesis and fibrogenesis include denervation injury and interactions with inflammatory mediators. FAP number, fibrosis, and muscle atrophy were all increased in an amyotrophic lateral sclerosis mouse model that resulted in diffuse denervation.103 Madaro et al. found that denervated hind limb muscle possessed increased fibrosis and FAP percentages.104 In addition, Wang et al. showed increased FI in the RC after suprascapular nerve compression in mice.44 Therefore, suprascapular neuropathy, which can be seen as a result of significant RC retraction in large tears,105, 106 is likely another factor in RC FAP-related degeneration. Recent studies have also highlighted the role of FAP–immune cell interactions and how the inflammatory milieu within injured muscle can influence FAP differentiation.89, 107 Moratal et al. found that FAPs tended to cluster near macrophages and that factors secreted by IL-4–polarized macrophages increased FAP adipogenesis.108 TGFβ signaling, a known stimulator of FAP fibrosis, is upregulated in injured muscle, with macrophages being identified as a main source.16, 94, 109, 110 Additional studies are required to confirm and better define the relative contributions of each underlying mechanism of RC FAP-derived FI and fibrosis.
Although there has been convincing evidence of the presence of FAPs in the RC and their connection to RC degeneration in animal models, the confirmation of these cells within the human RC is relatively recent. Kang et al. found that supraspinatus biopsies of patients with chronic RC tears had significantly greater amounts of FAPs and fibrosis compared with those with normal RCs (Fig. 6).111 Feeley et al. demonstrated that RC FI, fibrosis, and FAP number increased with tear size and thickness in human subjects.100 Furthermore, the FAPs from full thickness tears displayed greater fibrogenic and adipogenic differentiation capacity in vitro than those harvested from partial tears, suggestive of FAP epigenetic changes across tear states. These studies represent important steps in confirming the cellular actors involved in human RC FI and fibrosis.
Figure 6.

(A) Immunofluorescence for FAPs (PDGFRα-positive) and laminin in muscles from human subjects with normal rotator cuff muscle (RCN) versus those with rotator cuff tears (RCT). (B) Quantification of number of FAPs in muscles from subjects with RCT. (C) Immunofluorescence for collagen I in muscles from subjects with RCT. (D) Quantification of percentage of collagen deposition area in muscles from subjects with RCT. (E) qPCR analysis of mRNA expression of IL-15 in samples from patients with RCT. (F) Pearson’s correlation analysis for mRNA level of IL-15 and number of FAPs in samples from patients with RCT. (G) Pearson’s correlation analysis for mRNA level of IL-15 and percentage of area of collagen deposition in samples from patients with RCT. Reprinted with permission from Kang et al. (2018).111
FAP contribution to RC muscle atrophy
The role of FAPs in RC muscle atrophy is multifactorial, likely consisting of changes to the stem cell–signaling microenvironment as well as the extracellular matrix (ECM). When considering cellular actors involved in muscle atrophy, SCs are usually implicated as these are the main myogenic precursors responsible for regenerating muscle.112, 113 A recent study evaluating human supraspinatus biopsies from patients undergoing RC repair found no differences in SC number across different tear sizes, suggesting that RC atrophy is not simply due to a lack of SCs.100 Thus, there are likely other forces influencing atrophy progression. This is consistent with studies showing that the ability of SCs to regenerate muscle is highly dependent on the characteristics of their stem cell niche, which in turn is influenced by the surrounding cells and matrix.114 SCs rely heavily on ECM signaling to direct their expansion and differentiation.115, 116 For example, the ECM glycoprotein fibronectin has been shown to stimulate SC expansion.117 Therefore, changes in the ECM may alter the accessibility of SCs to different growth factors, as well as alter the concentration of these factors within the niche.103, 118–121 Alterations in muscle stiffness as a byproduct of ECM changes may also alter SC pathways, pushing them towards differentiation versus proliferation and replenishing their stem pool.122 Gilbert et al. demonstrated a reduction in SC engraftment after transplantation within stiff constructs compared with constructs mirroring normal muscle tissue.123 Borisov et al. found impaired terminal differentiation of myoblasts constricted within fibrotic ECM of degenerating muscle.124, 125 Taken together, FAP production of fibrosis and FI can significantly alter the composition of the SC niche, which may negatively impact the regenerative capacity of SCs.
Beyond indirectly influencing the SC activity via ECM modification, FAPs in injured muscle may mediate anti-myogenic signaling more directly. One candidate mechanism could be the TGFβ pathway. Injection of TGFβ into mice hind limbs resulted in decreased muscle fiber size, reduced contractile force, and increased expression of atrogin-1, a muscle ubiquitin ligase protein involved in proteolysis and muscle atrophy.126, 127 In a mouse model of RC injury, administration of a TGFβ inhibitor that promoted FAP apoptosis demonstrated 4-fold reduction in atrogin-1 and a 50% increase in supraspinatus wet weight.16 The TGFβ pathway has been shown to partially interact and interfere with mammalian target of rapamycin (mTOR), a critical signaling pathway in preserving of muscle mass.106, 128–130 In addition to TGFβ signaling, FAPs harvested from denervated muscle displayed increased expression of genes associated with muscle atrophy, and their co-culture with myoblasts resulted in reduced fusion and myotube diameter.104 Furthermore, their transplantation into normal muscle in vivo led to smaller cross-sectional area of surrounding muscle fibers. This suggests that nerve injury, such as in the case of suprascapular neuropathy often seen in RC tears, could induce a FAP phenotype that drives muscle atrophy.
Potential therapeutic role of FAPs in RC regeneration
Because of the fibroblast and adipocyte differentiation pathways of FAPs, it plausible that modulating FAP number and activity could affect the amount of fibrosis and FI that develop after muscle injury. Indeed, many studies have examined this premise. Lemos et al. found that blocking TNF signaling halted FAP apoptosis, resulting in double the FAP quantity and twice as much fibrosis after muscle injury.94 They also demonstrated that use of nilotinib, a tyrosine kinase inhibitor that targets the TGFβ signaling pathway, led to increased FAP apoptosis and a reduction in fibrosis. In a study of dystrophic mice, administration of the similar small molecule inhibitor imatinib reduced PDGFRα signaling, decreased muscle fibrosis, and improved hind limb grip strength.109 Similar results were seen in a study by Ito et al.131 In regard to the RC, the small molecule inhibitor CWHM-12 was shown to decrease FAP-derived fibrosis in vitro.17 Furthermore, Davies et al. used a TGFβ inhibitor that promoted RC FAP apoptosis in vivo, resulting in significant reductions in FI and fibrosis in a mouse RC injury model.16
Although it appears that reducing the overall FAP number and activity can mitigate their downstream pathology, removing them from muscle tissue through pharmacologic means may run the risk of silencing any beneficial role they may have. It has been shown that FAPs are initially helpful in muscle regeneration.43, 84, 89–92 However, these early positive effects are arguably eclipsed by their contribution to muscle pathology in their latter fibroblast and adipocyte forms. However, this may suggest that modifying their behavior towards a more useful phenotype, rather than simply depleting them, could be an approach that both preserves and augments their initial beneficial functions. The use of histone deacetylase inhibitors (HDACIs) and microRNAs are approaches that have been tested with encouraging results in mice. Increased expression of FAP myogenic genes such as MYOD and follistatin, adoption of a myogenic transcription signature, and promotion of myotube formation in SC co-culture experiments have been shown with the use of HDACIs and microRNAs.91, 132, 133.
Along similar lines, manipulating FAP-specific receptor signaling is another strategy which may prove beneficial. Mosich et al. generated a novel mesenchymal stem cell population from human embryonic stem cell precursors that displayed PDGFRβ expression but no PDGFRα expression.134 They showed that this novel stem cell lineage, which lacks the classical PDGFRα expression of FAPs, did not contribute to fatty infiltration or fibrosis and resulted in a four-fold decrease in myofiber atrophy when transplanted into a mouse RC injury model. This study and others suggest that FAP fibroblast and adipocyte differentiation may be specifically dependent on PDGFRα signaling.109 Therefore, investigation of strategies that inhibit FAP PDGFRα expression and activity are warranted as this could be an approach that retains the positive effects of FAPs while simultaneously reducing their negative ones.
A more recently explored approach to mitigating FI and fibrosis while simultaneously promoting muscle regeneration has been to investigate the ability of FAPs to differentiate into a more beneficial fat phenotype. Brown fat is known for its thermogenic capacity via expression of uncoupling protein 1 (UCP1), which disrupts cellular respiration. Beige fat is similar in this respect to brown fat in that it can also express UCP1, but it a shares a common origin with white adipose tissue.135–138 The vast majority of FI in skeletal muscle is comprised of white adipocytes, which represents a much less metabolically active form of fat than the beige or brown phenotype.43–45 Beyond its thermogenic role, beige fat has also been shown to influence muscle quality.46 This includes paracrine signaling and secretion of anabolic and myogenic factors, such as follistatin and IL-6.47, 48, 139–142 For example, Meyer et al. demonstrated that RC epimuscular fat exhibited a beige fat phenotype and was able to increase myotube formation in co-culture experiments with myogenic progenitors.49
Because of the possibility that beige fat may be more beneficial to muscle health than white fat, successful attempts have been made to push FAPs towards a beige fat phenotype.44, 45 In a mouse RC muscle cardiotoxin injury model, transplantation of brown fat reduced muscle atrophy and increased contractile force and fiber cross-sectional area.143 Transplantation of beige-like FAPs into the supraspinatus in a delayed RC repair model resulted in reduced muscle atrophy, FI, and fibrosis, while improving vascularity and shoulder gait function (Fig. 7).144 Similar findings were reported in a massive tear and suprascapular nerve transection model without repair.145 Wang et al. found that reversal of RC FI after suprascapular nerve compression and release involved a process of “browning” white fat cells.44 Recently, FAPs from the human RC have been shown to adopt a beige fat phenotype in vitro after treatment with the beta-3 agonist amibegron.100 Furthermore, these beige like FAPs displayed markedly increased gene expression of IGF-1 and follistatin. Beta-3 agonists like amibegron have been shown to enhance brown fat characteristics, which may point to a potential clinical use in RC injury.146–148 These compounds are of interest in treating metabolic syndrome and overactive bladder conditions (e.g., FDA-approved Mirabegron), and multiple studies have demonstrated encouraging safety profiles with very low rates of mild adverse side effects.148–151 Additional studies that investigate transplantation and pharmacologic techniques to increase RC FAP transition to a beige fat phenotype may elucidate whether this approach is viable in reducing FI and fibrosis while supporting RC regeneration via secretion of myogenic factors.
Figure 7.
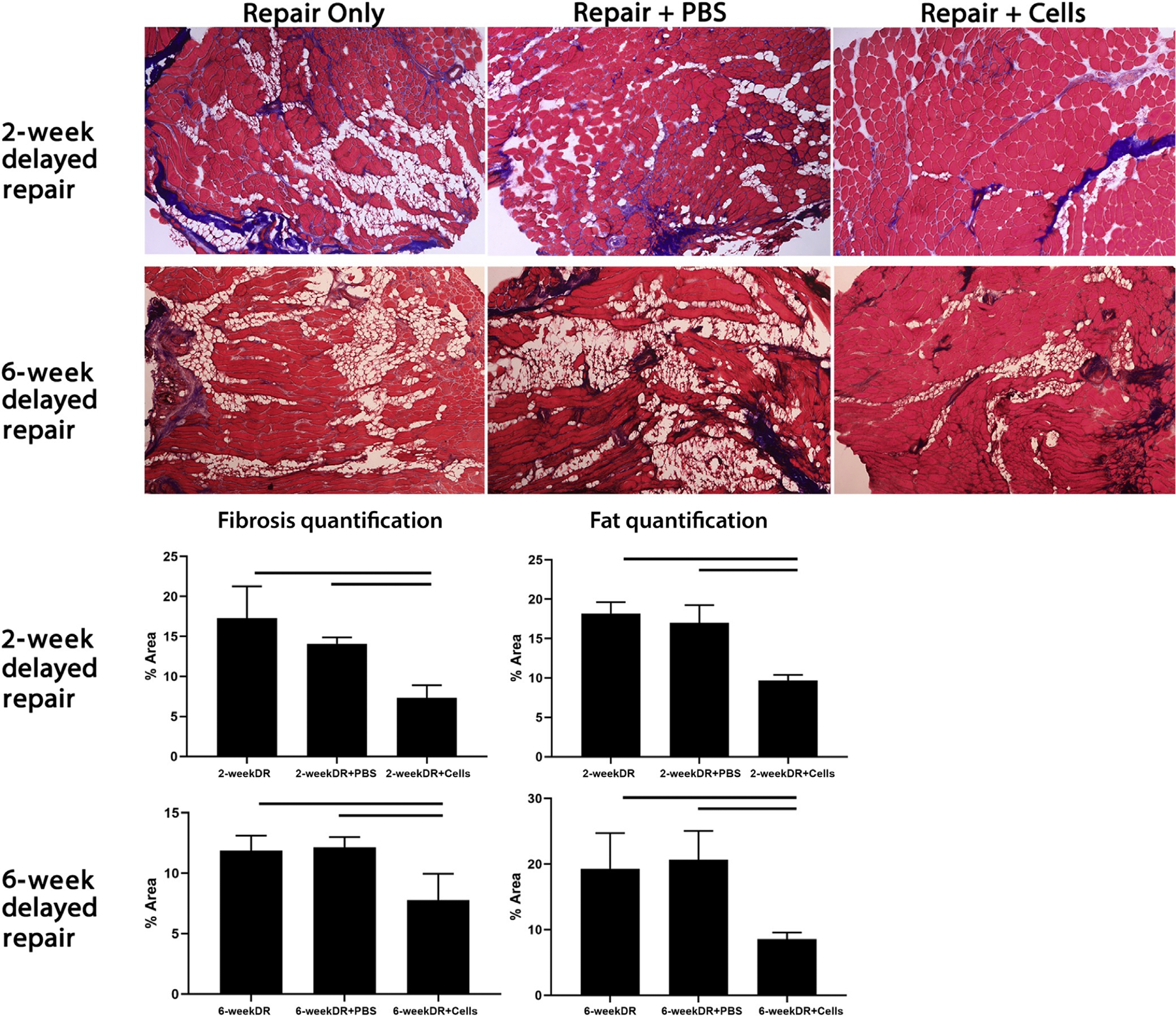
Top: Trichrome staining of mouse supraspinatus after tendon transection injury and delayed repair with specific treatment at the time of repair. Three treatment groups were included: repair only, repair plus intra-supraspinatus injection of phosphate-buffered saline (PBS) vehicle, and repair plus intra-supraspinatus injection of 250,000 beige-like FAPs. Mice were sacrificed and supraspinatus muscles harvested 6 weeks after the procedure. Bottom: Fibrosis and fat quantification comparing different treatment arms. Solid line denotes P < 0.05. Reprinted with permission from Lee et al. (2019).144
Conclusion and future directions
In summary, RC tears are an extremely common orthopedic injury encountered by the health care system. These injuries involve significant health care expenditures and represent a substantial source of morbidity for patients. Current assessment tools of RC degeneration have historically focused on FI and muscle atrophy as these degenerative features have significant influence on patient function and rates of successful RC repair; however, these tools possess significant inter-observer reliability concerns that undermine their clinical utility. New quantitative imaging techniques, such as IDEAL MRI, may improve assessment reliability across different observers. In terms of the cellular mechanism underlying RC degeneration, FAPs have emerged as a consequential resident stem cell source, characterized by their dichotomous role in muscle quality over time. After muscle injury, FAPs rapidly proliferate and initially provide pro-differentiation signaling for SCs to assist in regeneration. The FAPs that persist after initial expansion and contraction differentiate into adipocytes and fibroblasts, becoming the main contributors of intramuscular FI and fibrosis. Furthermore, FAP-related changes in the ECM and signaling microenvironment likely impact SC function, which may contribute to muscle atrophy. Additional studies are needed to assess the efficacy of different strategies of FAP modification to aid in RC regeneration as attempts to improve patient outcomes will likely require addressing the degenerative pathology caused by these cells. These potential strategies include reducing FAP number, activity, and driving them towards a more beneficial phenotype, such as beige fat. Concurrent advancements in the ability to differentiate fat subtypes through non-invasive imaging techniques may aid in the development and evaluation of these novel cellular approaches to reducing RC degeneration.
Acknowledgments
H.T.K. was funded by the U.S. Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development Merit Review Grant (1I01BX002680, PI: Kim). B.T.F. was funded by a NIH/NIAMS Research Grant (1R01AR072669-01A1, PI: Feeley), and by U.S. Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development Merit Review Grant (1I01BX002680, PI: Kim).
Footnotes
Competing interests
B.T.F. has received hospitality payments from Zimmer Biomet Holdings that were unrelated to the submitted work, and he is a Basic Science Editor for the Journal of Shoulder and Elbow Surgery and Current Reviews in Musculoskeletal Medicine. All other authors declare no competing interests.
References
- 1.Cho CH, Song KS, Hwang I, et al. 2015. Does Rotator Cuff Repair Improve Psychologic Status and Quality of Life in Patients With Rotator Cuff Tear? Clin Orthop Relat Res. 473: 3494–3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chung SW, Park JS, Kim SH, et al. 2012. Quality of life after arthroscopic rotator cuff repair: evaluation using SF-36 and an analysis of affecting clinical factors. Am J Sports Med. 40: 631–639. [DOI] [PubMed] [Google Scholar]
- 3.Yelin E, Weinstein S & King T. 2016. The burden of musculoskeletal diseases in the United States. Semin Arthritis Rheum. 46: 259–260. [DOI] [PubMed] [Google Scholar]
- 4.Mather RC 3rd, Koenig L, Acevedo D, et al. 2013. The societal and economic value of rotator cuff repair. J Bone Joint Surg Am. 95: 1993–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamamoto A, Takagishi K, Osawa T, et al. 2010. Prevalence and risk factors of a rotator cuff tear in the general population. J Shoulder Elbow Surg. 19: 116–120. [DOI] [PubMed] [Google Scholar]
- 6.Galatz LM, Silva MJ, Rothermich SY, et al. 2006. Nicotine delays tendon-to-bone healing in a rat shoulder model. J Bone Joint Surg Am. 88: 2027–2034. [DOI] [PubMed] [Google Scholar]
- 7.Tashjian RZ 2012. Epidemiology, natural history, and indications for treatment of rotator cuff tears. Clin Sports Med. 31: 589–604. [DOI] [PubMed] [Google Scholar]
- 8.Li L, Bokshan SL, Ready LV, et al. 2019. The primary cost drivers of arthroscopic rotator cuff repair surgery: a cost-minimization analysis of 40,618 cases. J Shoulder Elbow Surg. 28: 1977–1982. [DOI] [PubMed] [Google Scholar]
- 9.Goutallier D, Postel JM, Gleyze P, et al. 2003. Influence of cuff muscle fatty degeneration on anatomic and functional outcomes after simple suture of full-thickness tears. J Shoulder Elbow Surg. 12: 550–554. [DOI] [PubMed] [Google Scholar]
- 10.Gladstone JN, Bishop JY, Lo IK, et al. 2007. Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am J Sports Med. 35: 719–728. [DOI] [PubMed] [Google Scholar]
- 11.Liem D, Lichtenberg S, Magosch P, et al. 2007. Magnetic resonance imaging of arthroscopic supraspinatus tendon repair. J Bone Joint Surg Am. 89: 1770–1776. [DOI] [PubMed] [Google Scholar]
- 12.Lansdown DA, Lee S, Sam C, et al. 2017. A Prospective, Quantitative Evaluation of Fatty Infiltration Before and After Rotator Cuff Repair. Orthop J Sports Med. 5: 2325967117718537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melis B, DeFranco MJ, Chuinard C, et al. 2010. Natural history of fatty infiltration and atrophy of the supraspinatus muscle in rotator cuff tears. Clin Orthop Relat Res. 468: 1498–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goutallier D, Postel JM, Bernageau J, et al. 1994. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res. 78–83. [PubMed]
- 15.Gigliotti D, Xu MC, Davidson MJ, et al. 2017. Fibrosis, low vascularity, and fewer slow fibers after rotator-cuff injury. Muscle Nerve. 55: 715–726. [DOI] [PubMed] [Google Scholar]
- 16.Davies MR, Liu X, Lee L, et al. 2016. TGF-beta Small Molecule Inhibitor SB431542 Reduces Rotator Cuff Muscle Fibrosis and Fatty Infiltration By Promoting Fibro/Adipogenic Progenitor Apoptosis. PLoS One. 11: e0155486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jensen AR, Kelley BV, Mosich GM, et al. 2018. Neer Award 2018: Platelet-derived growth factor receptor alpha co-expression typifies a subset of platelet-derived growth factor receptor beta-positive progenitor cells that contribute to fatty degeneration and fibrosis of the murine rotator cuff. J Shoulder Elbow Surg. 27: 1149–1161. [DOI] [PubMed] [Google Scholar]
- 18.Sato EJ, Killian ML, Choi AJ, et al. 2014. Skeletal muscle fibrosis and stiffness increase after rotator cuff tendon injury and neuromuscular compromise in a rat model. J Orthop Res. 32: 1111–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goutallier D, Postel JM, Bernageau J, et al. 1995. Fatty infiltration of disrupted rotator cuff muscles. Rev Rhum Engl Ed. 62: 415–422. [PubMed] [Google Scholar]
- 20.Fuchs B, Weishaupt D, Zanetti M, et al. 1999. Fatty degeneration of the muscles of the rotator cuff: assessment by computed tomography versus magnetic resonance imaging. J Shoulder Elbow Surg. 8: 599–605. [DOI] [PubMed] [Google Scholar]
- 21.Warner JJ, Higgins L, Parsons I.M.t., et al. 2001. Diagnosis and treatment of anterosuperior rotator cuff tears. J Shoulder Elbow Surg. 10: 37–46. [DOI] [PubMed] [Google Scholar]
- 22.Thomazeau H, Boukobza E, Morcet N, et al. 1997. Prediction of rotator cuff repair results by magnetic resonance imaging. Clin Orthop Relat Res. 275–283. [PubMed]
- 23.Zanetti M, Gerber C & Hodler J. 1998. Quantitative assessment of the muscles of the rotator cuff with magnetic resonance imaging. Invest Radiol. 33: 163–170. [DOI] [PubMed] [Google Scholar]
- 24.Patte D 1990. Classification of rotator cuff lesions. Clin Orthop Relat Res. 81–86. [PubMed]
- 25.Spencer EE, Dunn WR, Wright RW, et al. 2008. Interobserver Agreement in the Classification of Rotator Cuff Tears Using Magnetic Resonance Imaging. The American Journal of Sports Medicine. 36: 99–103. [DOI] [PubMed] [Google Scholar]
- 26.Lippe J, Spang JT, Leger RR, et al. 2012. Inter-Rater Agreement of the Goutallier, Patte, and Warner Classification Scores Using Preoperative Magnetic Resonance Imaging in Patients With Rotator Cuff Tears. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 28: 154–159. [DOI] [PubMed] [Google Scholar]
- 27.Oh JH, Kim SH, Choi JA, et al. 2010. Reliability of the grading system for fatty degeneration of rotator cuff muscles. Clin Orthop Relat Res. 468: 1558–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Slabaugh MA, Friel NA, Karas V, et al. 2012. Interobserver and Intraobserver Reliability of the Goutallier Classification Using Magnetic Resonance Imaging. The American Journal of Sports Medicine. 40: 1728–1734. [DOI] [PubMed] [Google Scholar]
- 29.Kuhn JE, Dunn WR, Ma B, et al. 2007. Interobserver Agreement in the Classification of Rotator Cuff Tears. The American Journal of Sports Medicine. 35: 437–441. [DOI] [PubMed] [Google Scholar]
- 30.Khazzam M, Kuhn JE, Mulligan E, et al. 2012. Magnetic resonance imaging identification of rotator cuff retears after repair: interobserver and intraobserver agreement. Am J Sports Med. 40: 1722–1727. [DOI] [PubMed] [Google Scholar]
- 31.Yazigi Junior JA, Anauate Nicolao F, Archetti Netto N, et al. 2019. Magnetic resonance imaging reproducibility for rotator cuff partial tears in patients up to 60 years. BMC Musculoskelet Disord. 20: 383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams MD, Lädermann A, Melis B, et al. 2009. Fatty infiltration of the supraspinatus: A reliability study. Journal of Shoulder and Elbow Surgery. 18: 581–587. [DOI] [PubMed] [Google Scholar]
- 33.Kenn W, Hm DB, Gohlke F, et al. 2004. 2D SPLASH: a new method to determine the fatty infiltration of the rotator cuff muscles. European Radiology. 14: 2331–2336. [DOI] [PubMed] [Google Scholar]
- 34.Köstler H, Kenn W, Hümmer C, et al. 2002. [2D-SPLASH spectroscopy to determine the fat/water ratio in the muscle of the rotator cuff]. Rofo. 174: 991–995. [DOI] [PubMed] [Google Scholar]
- 35.Reeder SB, McKenzie CA, Pineda AR, et al. 2007. Water-fat separation with IDEAL gradient-echo imaging. J Magn Reson Imaging. 25: 644–652. [DOI] [PubMed] [Google Scholar]
- 36.Burakiewicz J, Sinclair CDJ, Fischer D, et al. 2017. Quantifying fat replacement of muscle by quantitative MRI in muscular dystrophy. J Neurol. 264: 2053–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mankodi A, Bishop CA, Auh S, et al. 2016. Quantifying disease activity in fatty-infiltrated skeletal muscle by IDEAL-CPMG in Duchenne muscular dystrophy. Neuromuscul Disord. 26: 650–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Del Grande F, Santini F, Herzka DA, et al. 2014. Fat-Suppression Techniques for 3-T MR Imaging of the Musculoskeletal System. RadioGraphics. 34: 217–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hines CD, Yu H, Shimakawa A, et al. 2010. Quantification of hepatic steatosis with 3-T MR imaging: validation in ob/ob mice. Radiology. 254: 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horiuchi S, Nozaki T, Tasaki A, et al. 2017. Reliability of MR Quantification of Rotator Cuff Muscle Fatty Degeneration Using a 2-point Dixon Technique in Comparison with the Goutallier Classification: Validation Study by Multiple Readers. Acad Radiol. 24: 1343–1351. [DOI] [PubMed] [Google Scholar]
- 41.Nardo L, Karampinos DC, Lansdown DA, et al. 2014. Quantitative assessment of fat infiltration in the rotator cuff muscles using water-fat MRI. Journal of Magnetic Resonance Imaging. 39: 1178–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Madhuranthakam AJ, Yu H, Shimakawa A, et al. 2010. T(2)-weighted 3D fast spin echo imaging with water-fat separation in a single acquisition. J Magn Reson Imaging. 32: 745–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Joe AW, Yi L, Natarajan A, et al. 2010. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 12: 153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Z, Feeley BT, Kim HT, et al. 2018. Reversal of Fatty Infiltration After Suprascapular Nerve Compression Release Is Dependent on UCP1 Expression in Mice. Clin Orthop Relat Res. 476: 1665–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gorski T, Mathes S & Krutzfeldt J. 2018. Uncoupling protein 1 expression in adipocytes derived from skeletal muscle fibro/adipogenic progenitors is under genetic and hormonal control. J Cachexia Sarcopenia Muscle. 9: 384–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kajimura S, Spiegelman BM & Seale P. 2015. Brown and Beige Fat: Physiological Roles beyond Heat Generation. Cell Metab. 22: 546–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Braga M, Reddy ST, Vergnes L, et al. 2014. Follistatin promotes adipocyte differentiation, browning, and energy metabolism. J Lipid Res. 55: 375–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singh R, Braga M & Pervin S. 2014. Regulation of brown adipocyte metabolism by myostatin/follistatin signaling. Front Cell Dev Biol. 2: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meyer GA, Gibbons MC, Sato E, et al. 2015. Epimuscular Fat in the Human Rotator Cuff Is a Novel Beige Depot. Stem Cells Transl Med. 4: 764–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Branca RT, He T, Zhang L, et al. 2014. Detection of brown adipose tissue and thermogenic activity in mice by hyperpolarized xenon MRI. Proc Natl Acad Sci U S A. 111: 18001–18006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Branca RT, McCallister A, Yuan H, et al. 2018. Accurate quantification of brown adipose tissue mass by xenon-enhanced computed tomography. Proc Natl Acad Sci U S A. 115: 174–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Branca RT, Zhang L, Warren WS, et al. 2013. In vivo noninvasive detection of Brown Adipose Tissue through intermolecular zero-quantum MRI. PLoS One. 8: e74206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chondronikola M, Beeman SC & Wahl RL. 2018. Non-invasive methods for the assessment of brown adipose tissue in humans. J Physiol. 596: 363–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chondronikola M, Porter C, Ogunbileje JO, et al. 2017. Identification and Quantification of Human Brown Adipose Tissue. Methods Mol Biol. 1566: 159–176. [DOI] [PubMed] [Google Scholar]
- 55.Zhang Y, Hu X, Hu S, et al. 2018. Non-invasive Imaging Methods for Brown Adipose Tissue Detection and Function Evaluation. Intern Med Open Access. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sampath SC, Sampath SC, Bredella MA, et al. 2016. Imaging of Brown Adipose Tissue: State of the Art. Radiology. 280: 4–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hu HH, Smith DL Jr., Nayak KS, et al. 2010. Identification of brown adipose tissue in mice with fat-water IDEAL-MRI. J Magn Reson Imaging. 31: 1195–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Khanna R, Saltzman MD, Elliott JM, et al. 2019. Development of 3D method to assess intramuscular spatial distribution of fat infiltration in patients with rotator cuff tear: reliability and concurrent validity. BMC Musculoskelet Disord. 20: 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Matsumura N, Oguro S, Okuda S, et al. 2017. Quantitative assessment of fatty infiltration and muscle volume of the rotator cuff muscles using 3-dimensional 2-point Dixon magnetic resonance imaging. J Shoulder Elbow Surg. 26: e309–e318. [DOI] [PubMed] [Google Scholar]
- 60.Deng J, Neff LM, Rubert NC, et al. 2018. MRI characterization of brown adipose tissue under thermal challenges in normal weight, overweight, and obese young men. J Magn Reson Imaging. 47: 936–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hu HH, Perkins TG, Chia JM, et al. 2013. Characterization of human brown adipose tissue by chemical-shift water-fat MRI. AJR Am J Roentgenol. 200: 177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barry JJ, Lansdown DA, Cheung S, et al. 2013. The relationship between tear severity, fatty infiltration, and muscle atrophy in the supraspinatus. J Shoulder Elbow Surg. 22: 18–25. [DOI] [PubMed] [Google Scholar]
- 63.Melis B, Wall B & Walch G. 2010. Natural history of infraspinatus fatty infiltration in rotator cuff tears. Journal of Shoulder and Elbow Surgery. 19: 757–763. [DOI] [PubMed] [Google Scholar]
- 64.Cheung S, Dillon E, Tham SC, et al. 2011. The presence of fatty infiltration in the infraspinatus: its relation with the condition of the supraspinatus tendon. Arthroscopy. 27: 463–470. [DOI] [PubMed] [Google Scholar]
- 65.Beeler S, Ek ET & Gerber C. 2013. A comparative analysis of fatty infiltration and muscle atrophy in patients with chronic rotator cuff tears and suprascapular neuropathy. J Shoulder Elbow Surg. 22: 1537–1546. [DOI] [PubMed] [Google Scholar]
- 66.Nozaki T, Tasaki A, Horiuchi S, et al. 2015. Quantification of Fatty Degeneration Within the Supraspinatus Muscle by Using a 2-Point Dixon Method on 3-T MRI. AJR Am J Roentgenol. 205: 116–122. [DOI] [PubMed] [Google Scholar]
- 67.Mall NA, Kim HM, Keener JD, et al. 2010. Symptomatic progression of asymptomatic rotator cuff tears: a prospective study of clinical and sonographic variables. J Bone Joint Surg Am. 92: 2623–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jain NB, Ayers GD, Fan R, et al. 2018. Predictors of Pain and Functional Outcomes After the Nonoperative Treatment of Rotator Cuff Tears. Orthopaedic Journal of Sports Medicine. 6: 232596711878853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nozaki T, Tasaki A, Horiuchi S, et al. 2016. Predicting Retear after Repair of Full-Thickness Rotator Cuff Tear: Two-Point Dixon MR Imaging Quantification of Fatty Muscle Degeneration-Initial Experience with 1-year Follow-up. Radiology. 280: 500–509. [DOI] [PubMed] [Google Scholar]
- 70.Shen PH, Lien SB, Shen HC, et al. 2008. Long-term functional outcomes after repair of rotator cuff tears correlated with atrophy of the supraspinatus muscles on magnetic resonance images. J Shoulder Elbow Surg. 17: 1s–7s. [DOI] [PubMed] [Google Scholar]
- 71.Mellado JM, Calmet J, Olona M, et al. 2005. Surgically repaired massive rotator cuff tears: MRI of tendon integrity, muscle fatty degeneration, and muscle atrophy correlated with intraoperative and clinical findings. AJR Am J Roentgenol. 184: 1456–1463. [DOI] [PubMed] [Google Scholar]
- 72.Smith LR & Barton ER. 2018. Regulation of fibrosis in muscular dystrophy. Matrix Biol. 68–69: 602–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Garg K, Ward CL, Hurtgen BJ, et al. 2015. Volumetric muscle loss: persistent functional deficits beyond frank loss of tissue. J Orthop Res. 33: 40–46. [DOI] [PubMed] [Google Scholar]
- 74.Corona BT, Wenke JC & Ward CL. 2016. Pathophysiology of Volumetric Muscle Loss Injury. Cells Tissues Organs. 202: 180–188. [DOI] [PubMed] [Google Scholar]
- 75.Smith LR, Lee KS, Ward SR, et al. 2011. Hamstring contractures in children with spastic cerebral palsy result from a stiffer extracellular matrix and increased in vivo sarcomere length. J Physiol. 589: 2625–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Silldorff MD, Choo AD, Choi AJ, et al. 2014. Effect of supraspinatus tendon injury on supraspinatus and infraspinatus muscle passive tension and associated biochemistry. J Bone Joint Surg Am. 96: e175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Safran O, Derwin KA, Powell K, et al. 2005. Changes in rotator cuff muscle volume, fat content, and passive mechanics after chronic detachment in a canine model. J Bone Joint Surg Am. 87: 2662–2670. [DOI] [PubMed] [Google Scholar]
- 78.Gerber C, Meyer DC, Schneeberger AG, et al. 2004. Effect of tendon release and delayed repair on the structure of the muscles of the rotator cuff: an experimental study in sheep. J Bone Joint Surg Am. 86: 1973–1982. [DOI] [PubMed] [Google Scholar]
- 79.Gimbel JA, Mehta S, Van Kleunen JP, et al. 2004. The tension required at repair to reappose the supraspinatus tendon to bone rapidly increases after injury. Clin Orthop Relat Res. 258–265. [DOI] [PubMed]
- 80.Gimbel JA, Van Kleunen JP, Lake SP, et al. 2007. The role of repair tension on tendon to bone healing in an animal model of chronic rotator cuff tears. J Biomech. 40: 561–568. [DOI] [PubMed] [Google Scholar]
- 81.Giambini H, Hatta T, Gorny KR, et al. 2018. Intramuscular fat infiltration evaluated by magnetic resonance imaging predicts the extensibility of the supraspinatus muscle. Muscle Nerve. 57: 129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Giambini H, Hatta T, Rezaei A, et al. 2018. Extensibility of the supraspinatus muscle can be predicted by combining shear wave elastography and magnetic resonance imaging-measured quantitative metrics of stiffness and volumetric fat infiltration: A cadaveric study. Clin Biomech (Bristol, Avon). 57: 144–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Uezumi A, Fukada S, Yamamoto N, et al. 2014. Identification and characterization of PDGFRalpha+ mesenchymal progenitors in human skeletal muscle. Cell Death Dis. 5: e1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Uezumi A, Fukada S, Yamamoto N, et al. 2010. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol. 12: 143–152. [DOI] [PubMed] [Google Scholar]
- 85.Uezumi A, Ikemoto-Uezumi M & Tsuchida K. 2014. Roles of nonmyogenic mesenchymal progenitors in pathogenesis and regeneration of skeletal muscle. Front Physiol. 5: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Uezumi A, Ito T, Morikawa D, et al. 2011. Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. J Cell Sci. 124: 3654–3664. [DOI] [PubMed] [Google Scholar]
- 87.Wang Z, Liu X, Davies MR, et al. 2018. A Mouse Model of Delayed Rotator Cuff Repair Results in Persistent Muscle Atrophy and Fatty Infiltration. Am J Sports Med. 46: 2981–2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Davies MR, Garcia S, Tamaki S, et al. 2018. Muscle stem cell activation in a mouse model of rotator cuff injury. J Orthop Res. 36: 1370–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Farup J, Madaro L, Puri PL, et al. 2015. Interactions between muscle stem cells, mesenchymal-derived cells and immune cells in muscle homeostasis, regeneration and disease. Cell Death Dis. 6: e1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Heredia JE, Mukundan L, Chen FM, et al. 2013. Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell. 153: 376–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mozzetta C, Consalvi S, Saccone V, et al. 2013. Fibroadipogenic progenitors mediate the ability of HDAC inhibitors to promote regeneration in dystrophic muscles of young, but not old Mdx mice. EMBO Mol Med. 5: 626–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lukjanenko L, Karaz S, Stuelsatz P, et al. 2019. Aging Disrupts Muscle Stem Cell Function by Impairing Matricellular WISP1 Secretion from Fibro-Adipogenic Progenitors. Cell Stem Cell. 24: 433–446.e437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Quarta M, Cromie M, Chacon R, et al. 2017. Bioengineered constructs combined with exercise enhance stem cell-mediated treatment of volumetric muscle loss. Nature Communications. 8: 15613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lemos DR, Babaeijandaghi F, Low M, et al. 2015. Nilotinib reduces muscle fibrosis in chronic muscle injury by promoting TNF-mediated apoptosis of fibro/adipogenic progenitors. Nat Med. 21: 786–794. [DOI] [PubMed] [Google Scholar]
- 95.Lemos DR, Paylor B, Chang C, et al. 2012. Functionally Convergent White Adipogenic Progenitors of Different Lineages Participate in a Diffused System Supporting Tissue Regeneration. STEM CELLS. 30: 1152–1162. [DOI] [PubMed] [Google Scholar]
- 96.Paylor B, Joe AW, Rossi FM, et al. 2014. In vivo characterization of neural crest-derived fibro/adipogenic progenitor cells as a likely cellular substrate for craniofacial fibrofatty infiltrating disorders. Biochem Biophys Res Commun. 451: 148–151. [DOI] [PubMed] [Google Scholar]
- 97.Fiore D, Judson RN, Low M, et al. 2016. Pharmacological blockage of fibro/adipogenic progenitor expansion and suppression of regenerative fibrogenesis is associated with impaired skeletal muscle regeneration. Stem Cell Res. 17: 161–169. [DOI] [PubMed] [Google Scholar]
- 98.Liu X, Ning AY, Chang NC, et al. 2016. Investigating the cellular origin of rotator cuff muscle fatty infiltration and fibrosis after injury. Muscles Ligaments Tendons J. 6: 6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Itoigawa Y, Kishimoto KN, Sano H, et al. 2011. Molecular mechanism of fatty degeneration in rotator cuff muscle with tendon rupture. J Orthop Res. 29: 861–866. [DOI] [PubMed] [Google Scholar]
- 100.Feeley BT, Liu M, Ma CB, et al. 2020. Human Rotator Cuff Tears have an Endogenous, Inducible Stem Cell Source Capable of Improving Muscle Quality and Function after Rotator Cuff Repair. Am J Sports Med. In Press. [DOI] [PMC free article] [PubMed]
- 101.Lee C, Agha O, Liu M, et al. 2019. Rotator Cuff Fibro-Adipogenic Progenitors Demonstrate Highest Concentration, Proliferative Capacity, and Adipogenic Potential Across Muscle Groups. J Orthop Res. [DOI] [PMC free article] [PubMed]
- 102.Pagano AF, Brioche T, Arc-Chagnaud C, et al. 2018. Short-term disuse promotes fatty acid infiltration into skeletal muscle. J Cachexia Sarcopenia Muscle. 9: 335–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gonzalez D, Contreras O, Rebolledo DL, et al. 2017. ALS skeletal muscle shows enhanced TGF-beta signaling, fibrosis and induction of fibro/adipogenic progenitor markers. PLoS One. 12: e0177649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Madaro L, Passafaro M, Sala D, et al. 2018. Denervation-activated STAT3-IL-6 signalling in fibro-adipogenic progenitors promotes myofibres atrophy and fibrosis. Nat Cell Biol. 20: 917–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kong BY, Kim SH, Kim DH, et al. 2016. Suprascapular neuropathy in massive rotator cuff tears with severe fatty degeneration in the infraspinatus muscle. Bone Joint J. 98-b: 1505–1509. [DOI] [PubMed] [Google Scholar]
- 106.Laron D, Samagh SP, Liu X, et al. 2012. Muscle degeneration in rotator cuff tears. J Shoulder Elbow Surg. 21: 164–174. [DOI] [PubMed] [Google Scholar]
- 107.Biferali B, Proietti D, Mozzetta C, et al. 2019. Fibro–Adipogenic Progenitors Cross-Talk in Skeletal Muscle: The Social Network. Frontiers in Physiology. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Moratal C, Raffort J, Arrighi N, et al. 2018. IL-1β- and IL-4-polarized macrophages have opposite effects on adipogenesis of intramuscular fibro-adipogenic progenitors in humans. Sci Rep. 8: 17005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Huang P, Zhao XS, Fields M, et al. 2009. Imatinib attenuates skeletal muscle dystrophy in mdx mice. Faseb j. 23: 2539–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Davies MR, Lee L, Feeley BT, et al. 2017. Lysophosphatidic acid-induced RhoA signaling and prolonged macrophage infiltration worsens fibrosis and fatty infiltration following rotator cuff tears. J Orthop Res. 35: 1539–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kang X, Yang MY, Shi YX, et al. 2018. Interleukin-15 facilitates muscle regeneration through modulation of fibro/adipogenic progenitors. Cell Commun Signal. 16: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Morgan JE & Partridge TA. 2003. Muscle satellite cells. Int J Biochem Cell Biol. 35: 1151–1156. [DOI] [PubMed] [Google Scholar]
- 113.Sambasivan R, Yao R, Kissenpfennig A, et al. 2011. Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development. 138: 3647–3656. [DOI] [PubMed] [Google Scholar]
- 114.Yin H, Price F & Rudnicki MA. 2013. Satellite cells and the muscle stem cell niche. Physiol Rev. 93: 23–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sanes JR 2003. The Basement Membrane/Basal Lamina of Skeletal Muscle. 278: 12601–12604. [DOI] [PubMed] [Google Scholar]
- 116.Urciuolo A, Quarta M, Morbidoni V, et al. 2013. Collagen VI regulates satellite cell self-renewal and muscle regeneration. Nat Commun. 4: 1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bentzinger CF, Wang YX, von Maltzahn J, et al. 2013. Fibronectin regulates Wnt7a signaling and satellite cell expansion. Cell Stem Cell. 12: 75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hynes RO 2009. The extracellular matrix: not just pretty fibrils. Science. 326: 1216–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Brizzi MF, Tarone G & Defilippi P. 2012. Extracellular matrix, integrins, and growth factors as tailors of the stem cell niche. Curr Opin Cell Biol. 24: 645–651. [DOI] [PubMed] [Google Scholar]
- 120.Fallon JR & McNally EM. 2018. Non-Glycanated Biglycan and LTBP4: Leveraging the extracellular matrix for Duchenne Muscular Dystrophy therapeutics. Matrix Biol. 68–69: 616–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ahmad S, Jan AT, Baig MH, et al. 2017. Matrix gla protein: An extracellular matrix protein regulates myostatin expression in the muscle developmental program. Life Sci. 172: 55–63. [DOI] [PubMed] [Google Scholar]
- 122.Shefer G, Van de Mark DP, Richardson JB, et al. 2006. Satellite-cell pool size does matter: defining the myogenic potency of aging skeletal muscle. Dev Biol. 294: 50–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gilbert PM, Havenstrite KL, Magnusson KE, et al. 2010. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 329: 1078–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Borisov AB, Dedkov EI & Carlson BM. 2005. Differentiation of activated satellite cells in denervated muscle following single fusions in situ and in cell culture. Histochemistry and Cell Biology. 124: 13–23. [DOI] [PubMed] [Google Scholar]
- 125.Borisov AB, Dedkov EI & Carlson BM. 2005. Abortive myogenesis in denervated skeletal muscle: differentiative properties of satellite cells, their migration, and block of terminal differentiation. Anat Embryol (Berl). 209: 269–279. [DOI] [PubMed] [Google Scholar]
- 126.Mendias CL, Gumucio JP, Davis ME, et al. 2012. Transforming growth factor-beta induces skeletal muscle atrophy and fibrosis through the induction of atrogin-1 and scleraxis. Muscle & Nerve. 45: 55–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gomes MD, Lecker SH, Jagoe RT, et al. 2001. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci U S A. 98: 14440–14445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sartori R, Milan G, Patron M, et al. 2009. Smad2 and 3 transcription factors control muscle mass in adulthood. American Journal of Physiology-Cell Physiology. 296: C1248–C1257. [DOI] [PubMed] [Google Scholar]
- 129.Viel S, Marçais A, Guimaraes FS, et al. 2016. TGF-β inhibits the activation and functions of NK cells by repressing the mTOR pathway. Sci Signal. 9: ra19. [DOI] [PubMed] [Google Scholar]
- 130.Ko DY, Shin JM, Um JY, et al. 2016. Rapamycin inhibits transforming growth factor beta 1 induced myofibroblast differentiation via the phosphorylated-phosphatidylinositol 3-kinase mammalian target of rapamycin signal pathways in nasal polyp-derived fibroblasts. Am J Rhinol Allergy. 30: 211–217. [DOI] [PubMed] [Google Scholar]
- 131.Ito T, Ogawa R, Uezumi A, et al. 2013. Imatinib attenuates severe mouse dystrophy and inhibits proliferation and fibrosis-marker expression in muscle mesenchymal progenitors. Neuromuscul Disord. 23: 349–356. [DOI] [PubMed] [Google Scholar]
- 132.Saccone V, Consalvi S, Giordani L, et al. 2014. HDAC-regulated myomiRs control BAF60 variant exchange and direct the functional phenotype of fibro-adipogenic progenitors in dystrophic muscles. Genes Dev. 28: 841–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Giordani L, Sandoná M, Rotini A, et al. 2014. Muscle-specific microRNAs as biomarkers of Duchenne Muscular Dystrophy progression and response to therapies. Rare Dis. 2: e974969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mosich GM, Husman R, Shah P, et al. 2019. Non-fibro-adipogenic pericytes from human embryonic stem cells attenuate degeneration of the chronically injured mouse muscle. JCI Insight. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bartesaghi S, Hallen S, Huang L, et al. 2015. Thermogenic activity of UCP1 in human white fat-derived beige adipocytes. Mol Endocrinol. 29: 130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Keipert S & Jastroch M. 2014. Brite/beige fat and UCP1 - is it thermogenesis? Biochim Biophys Acta. 1837: 1075–1082. [DOI] [PubMed] [Google Scholar]
- 137.Tseng YH, Kokkotou E, Schulz TJ, et al. 2008. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature. 454: 1000–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sidossis L & Kajimura S. 2015. Brown and beige fat in humans: thermogenic adipocytes that control energy and glucose homeostasis. J Clin Invest. 125: 478–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rahman S, Lu Y, Czernik PJ, et al. 2013. Inducible brown adipose tissue, or beige fat, is anabolic for the skeleton. Endocrinology. 154: 2687–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yamashita H, Sato N, Kizaki T, et al. 1995. Norepinephrine stimulates the expression of fibroblast growth factor 2 in rat brown adipocyte primary culture. Cell Growth Differ. 6: 1457–1462. [PubMed] [Google Scholar]
- 141.Sharples AP, Al-Shanti N, Hughes DC, et al. 2013. The role of insulin-like-growth factor binding protein 2 (IGFBP2) and phosphatase and tensin homologue (PTEN) in the regulation of myoblast differentiation and hypertrophy. Growth Horm IGF Res. 23: 53–61. [DOI] [PubMed] [Google Scholar]
- 142.Wang G-X, Zhao X-Y & Lin JD. 2015. The brown fat secretome: metabolic functions beyond thermogenesis. Trends in Endocrinology & Metabolism. 26: 231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bryniarski AR & Meyer GA. 2019. Brown Fat Promotes Muscle Growth During Regeneration. J Orthop Res. [DOI] [PMC free article] [PubMed]
- 144.Lee C, Liu M, Agha O, et al. 2019. Beige fibro-adipogenic progenitor transplantation reduces muscle degeneration and improves function in a mouse model of delayed repair of rotator cuff tears. J Shoulder Elbow Surg. [DOI] [PMC free article] [PubMed]
- 145.Lee C, Liu M, Agha O, et al. 2019. Beige FAP transplantation improves muscle quality and shoulder function after massive rotator cuff tears. J Orthop Res. [DOI] [PMC free article] [PubMed]
- 146.Manara L, Badone D, Baroni M, et al. 1996. Functional identification of rat atypical beta-adrenoceptors by the first beta 3-selective antagonists, aryloxypropanolaminotetralins. Br J Pharmacol. 117: 435–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Williams CA, Shih MF & Taberner PV. 1999. Sustained improvement in glucose homeostasis in lean and obese mice following chronic administration of the beta 3 agonist SR 58611A. Br J Pharmacol. 128: 1586–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Nisoli E, Tonello C & Carruba MO. 1994. SR 58611A: a novel thermogenic beta-adrenoceptor agonist. Eur J Pharmacol. 259: 181–186. [DOI] [PubMed] [Google Scholar]
- 149.Rosa GM, Baccino D, Valbusa A, et al. 2018. Cardiovascular effects of antimuscarinic agents and beta3-adrenergic receptor agonist for the treatment of overactive bladder. Expert Opin Drug Saf. 17: 487–497. [DOI] [PubMed] [Google Scholar]
- 150.Christ T, Molenaar P, Klenowski PM, et al. 2011. Human atrial β(1L)-adrenoceptor but not β3-adrenoceptor activation increases force and Ca(2+) current at physiological temperature. Br J Pharmacol. 162: 823–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Nozawa Y, Kato D, Tabuchi H, et al. 2018. Safety and Effectiveness of Mirabegron in Patients with Overactive Bladder in a Real-World Clinical Setting: A Japanese Post-Marketing Study. Low Urin Tract Symptoms. 10: 122–130. [DOI] [PubMed] [Google Scholar]


