Abstract
Depression is a major cause of disease burden and is linked to poor quality of life (QOL) among adolescents. We examined the roles of sexual behaviors, HIV risk perception, and anticipated HIV stigma on depressive symptomatology and QOL among 4096 adolescents in a rural region of western Kenya with a high burden of HIV. Participants were aged 15–19 years, had not been tested for HIV in the previous 6 months, and had never been diagnosed with HIV. Anticipated stigma and risk perception were directly associated with depressive symptomatology and QOL. There was evidence of small indirect effects -- through stigma -- of risk perception on depressive symptomatology and QOL. Gender moderated relationships between sexual behavior and risk perception, depressive symptomatology, and QOL. Results suggest that developing effective gender-based interventions to address stigma, sexual behavior, and risk perception may be important for improving adolescent well-being in high HIV prevalence contexts.
Keywords: Adolescents, Depression, Quality of Life, Anticipated HIV stigma, Structural Equation Modeling
Resumen
La depresión es una de las principales causas de carga de morbilidad y se asocia con una pobre calidad de vida (CdV) de los adolescentes. Nosotros estudiamos el papel de los comportamientos sexuales, la percepción de riesgo del VIH, y el estigma anticipado del VIH en relación con la sintomatología depresiva y la CdV de 4 096 adolescentes provenientes de una región rural del oeste de Kenia con alta carga de VIH. Los participantes, adolescentes de entre 15 y 19 años, no se habían hecho la prueba de detección del VIH en los últimos 6 meses y, además, nunca habían sido diagnosticados con VIH. El estigma anticipado y la percepción de riesgo estaban asociados directamente con la sintomatología depresiva y la CdV. Hubo evidencia de pocos efectos indirectos de percepción de riesgo–generados por el estigma–en la sintomatología depresiva y la CdV. El género moderó las relaciones entre el comportamiento sexual y la percepción de riesgo, la sintomatología depresiva y la CdV. Los resultados sugieren que desarrollar intervenciones con enfoque de género para abordar el tema del estigma, los comportamientos sexuales y la percepción de riesgo, puede ser importante para mejorar el bienestar de los adolescentes que viven en un contexto con alta prevalencia de VIH.
INTRODUCTION
Depression is a major cause of suicide (1–3), disease burden (4–6), and poor quality of life (QOL) (7, 8) globally among children and adolescents. According to the World Health Organization (WHO), an estimated 10–20% of children and adolescents worldwide have a mental disorder (9). In 2017, having a mental disorder was one of the top ten causes of years lived with disability among global youth under 20 years old (10). Recent research among children and adolescents in the US and China indicates that 21 – 26% report having current depressive symptoms (11–13).
However, there is currently a dearth of data about prevalence of mental disorders, including depression, among children and adolescents in sub-Saharan Africa (4, 14). The limited available data suggest that the region has high rates of mental illness burden among young people (5), with increasing concerns about depression (4). We reviewed recent community–based (e.g., non–clinic) research in 11 sub-Saharan African countries that reported prevalence of depressive symptoms among young people aged 13–26 years (3, 15–24). In these studies, the median prevalence of depressive symptoms was 30.5%. Like depressive symptom estimates in other regions (12, 25), the estimates varied widely (range = 9 – 64%; interquartile range = 25.5 – 39.5%), which may reflect differences in measurement tools, depression severity or recall period assessed, population or sample characteristics, or other factors. Moreover, the literature suggests that among youth, risk factors for mental disorders, such as depression, may be multifactorial (biological, psychological, and social) (26).
In this paper, we build on previous research among adults that indicates that anticipated HIV stigma (the belief that an HIV diagnosis will result in vulnerability to stigma due to community norms) may be an important risk factor for poor mental health in high risk populations or among individuals in high prevalence regions (27–29). Studies among adolescents living with HIV and AIDS orphans also suggest an association between HIV-related stigma and poor mental health (30–32). Similarly, adolescents who are either uninfected or unaware of their status residing in high burden regions may be vulnerable to anticipated HIV stigma because they may consider themselves to be at heightened risk for infection. This risk perception may be due to a number of factors including risky sexual behavior; persistent communication (in school and at home) about the elevated risk for HIV among youth; research, programmatic, and media focus on HIV infection among youth in sub-Saharan African countries; and the likelihood that adolescents know (or knew, if now deceased) someone living with HIV (33).
Moreover, these adolescents are likely to be familiar with long-standing consequences of an HIV diagnosis in their communities, including negative perceptions and stigmatizing norms, often directed at individuals living with HIV. They may anticipate being similarly stigmatized if they were to receive an HIV diagnosis (34). In turn, chronic stress, fear, and anxiety due to anticipated HIV stigma may have a deleterious effect on the mental health and well-being of these adolescents (35).
The primary mode of HIV transmission among adolescents in sub-Saharan Africa is unsafe heterosexual intercourse, e.g., unprotected sex with multiple partners or with someone who has had multiple partners (36). It is possible that adolescents who identify as being at higher risk for HIV because of their risky sexual behavior are likely to experience anticipated stigma and consequently poorer psychosocial outcomes. Thus, sexual behavior and HIV risk perceptions may affect adolescents’ mental health indirectly through anticipated stigma. Although these relationships may be important among youth in high burden sub-Saharan African countries, they have not been examined in previous research.
Sexual behavior may also have a direct effect on psychological outcomes among youth. For example, a longitudinal study among adolescents in the United States found that engaging in sexual behaviors increased their risk for depression, especially for girls (37). Additionally, cross-sectional studies among youth in sub-Saharan Africa have shown associations between feelings of sadness or hopelessness, sexual activity, and number of sexual partners (3) and between psychological distress and sexual health risks (38, 39).
In this study, we examine the interrelationships between risky sexual behavior, perception of risk for HIV, and anticipated HIV stigma. We also examine the effects of these factors on adolescent well-being (i.e., depression and QOL). Further, we investigate whether risky sexual behavior and perception of risk are associated with well-being directly or indirectly via stigma as a mediator, or both (See Figure 1). We hypothesize that risky sexual behavior is positively associated with perception of HIV risk. In turn, HIV risk perception has an indirect effect on adolescent well-being via anticipated HIV stigma. Our conceptual framework is informed by Starks et al. (29), specifically their finding of higher anticipated stigma among groups of men who have sex with men (MSM) who perceive themselves at greater risk for infection. Additionally, we hypothesize an inverse relationship between sexual behavior and well-being. We test a moderated mediation model because previous research suggests that the pathway that is most salient may depend on gender and age (18, 38).
Figure 1.
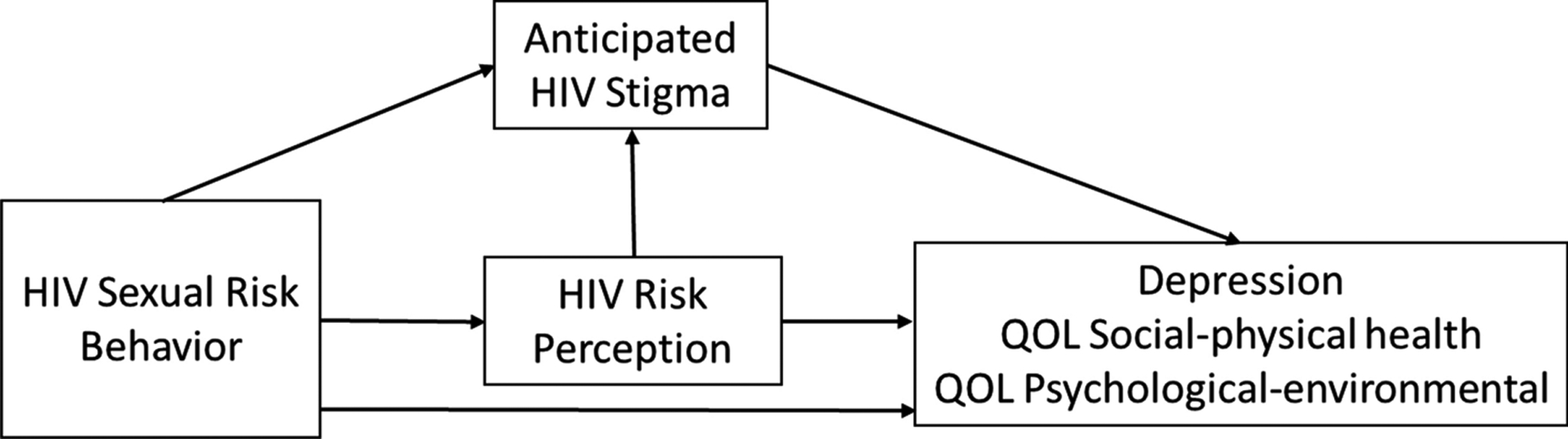
Conceptual Framework
Understanding the relationships among psychosocial factors (depression, QOL, and stigma), sexual behaviors, and HIV risk perceptions is important for developing effective mental health interventions for adolescents in high HIV prevalence regions. Our conceptual model depicts two potential paths linking risk perception to adolescent well-being: the social path and the health consequences path. In the social pathway, adolescents who perceive themselves at risk for acquiring HIV may fear that living with the disease will weaken social ties that provide important sources of emotional support and economic opportunity. This is the indirect path whereby anticipated HIV-related stigma connects risk perception to mental health. The direct path, on the other hand, may be formed by concerns about the personal health consequences of HIV infection, such as poor health and a shorter life. While approaches that address sexual behaviors and stigma may also improve well-being among adolescents, knowing the relative saliency of these two pathways would lead to more effective interventions.
METHODS
Design, participants, and procedures
Data presented here are from the baseline survey of an observational, cohort study designed to examine four ethical issues: (1) effects of HIV test result disclosure on adolescent psychosocial well-being, health-seeking, and risk behavior; (2) minimizing harms in the recruitment of adolescents; (3) comprehension of informed consent among parents and adolescents; and (4) the appropriate use of compensatory payments for youth and parent participation. At the baseline data collection, conducted from June 2016 through December 2017, participating adolescents completed a survey and were tested for HIV. The study design included follow-up assessments at two- and 12-months post baseline.
The study was conducted in one county in the Nyanza region of western Kenya, where estimated HIV prevalence and incidence are highest in the country at 13% and 6 per 1000 population, respectively. The national averages are estimated at 5% and 2 per 1000 population (40, 41). Three sub-counties comprised strata and villages or wards within sub-counties were randomly selected to participate. Village clusters or wards of participants were randomly assigned to be administered the baseline survey and HIV test in either a nearby clinic or the participant’s home (i.e., clinic arm versus home arm).
We sought to enroll equal numbers of adolescents by gender, sub-county, and age group (15–17 vs. 18–19). Our study goal was to identify 60–70 HIV-positive adolescents during baseline activities for follow-up procedures. Thus, we determined that a target baseline sample size of 4200 would be adequate based on available epidemiological data (42, 43), an expected refusal rate of 10%, anticipated loss to follow–up between recruitment and baseline data collection of 34%, and an attrition rate after HIV testing of 20%.
Staff conducted community awareness activities as well as recruitment and information meetings with potential youth participants and parent/guardians. Eligibility criteria were being 15–19 years old, not having been tested for HIV in the past six months, and never having tested positive for HIV. Study staff screened individuals for eligibility using computer–assisted interviewing techniques.
The baseline survey was administered individually using audio computer–assisted self–interview (ACASI) in the participant’s choice of language (Luo, English, or Swahili). On completion of the survey, trained counselors conducted HIV testing, pre-/post-test counseling, and disclosure of test results. Blood sample specimens were collected by finger prick and sequential testing was done using rapid HIV test kits (Determine [Determine™, Abbott Laboratories, United States] for screening and, if positive, First Response [Premier Medical Corporation, Kachigam, India] for confirmatory testing). Participants with positive or indeterminate results were referred to treatment, support, and further testing services as appropriate. At the conclusion of baseline activities, adolescents (and their parent/guardian if a minor) received a t-shirt; those in the clinic arm also received KSh300 (for travel costs).
Human subjects protections
All study participation was voluntary. Adolescents provided verbal consent to answer the screening questionnaire items. Before baseline data collection, adolescents ages 18–19 provided written informed consent for study participation; for adolescents ages 15–17, we obtained written consent from their parent or guardian and child assent. A waiver of parent/guardian consent was used in the case of emancipated minors (e.g., married or cohabiting, pregnant or a parent, or living in a child-headed household).
Study protocols were approved by the ethics review boards of the Pacific Institute for Research and Evaluation (PIRE) and Kenya Medical Research Institute (KEMRI). The study was conducted in accordance with the US Common Rule and the Guidelines for Ethical Conduct of Biomedical Research Involving Human Subjects in Kenya.
Measures
Data used in the present analyses were from the eligibility screening questionnaire and baseline survey. We also report the HIV test results.
Depressive symptoms were based on responses to the Center for Epidemiological Studies Depression Scale Revised (CESD-R), an instrument measuring current depression symptomatology and dysphoria found to have good psychometric properties in a U.S. general population (44). Versions of the CES-D have been used with youth in sub-Saharan Africa (18, 20). A composite CESD-R score was created by summing the 20 items (e.g., I felt sad and I lost interest in my usual activities), which are coded from 0–3 (lowest to highest symptom frequency), with a possible range for the overall score of 0–60. Higher scores indicate higher levels of depression. Reliability for the scale in our sample was Cronbach’s alpha = 0.92 (see supplementary material). We created a dichotomous variable (has depressive symptoms = score of 16 and above, no depressive symptoms = score below 16), which has been found in an African setting to have good sensitivity and utility in identifying depression (45).
Quality of Life (QOL) items were from the 26-item World Health Organization Quality of Life Questionnaire abbreviated version (WHOQOL-BREF) (46, 47) which has been found acceptable and feasible with adolescents (8) and been used with adolescents in sub-Saharan Africa (48) and adults in rural Kenya (49). Response options were on a 5-point Likert scale with the respondent being asked to think about their life in the last four weeks. Two QOL scales (possible range = 1–5, with higher scores indicating better QOL) were created based on factor analysis results: social-physical health QOL (mean of 10 items, e.g., How satisfied are you with your ability to perform your daily living activities?; Cronbach’s alpha = 0.83) and psychological-environmental QOL (9 items, e.g., How well are you able to concentrate? and How safe do you feel in your daily life?; Cronbach’s alpha = 0.76) (see supplementary materials).
Anticipated HIV stigma was a composite of the mean of 5 items (e.g., If you were HIV positive and people found out, do you think that you would be verbally abused?; coded 0 = “no”, 1 = “don’t know”, 2 = “yes”; Cronbach’s alpha = 0.72) (see supplementary material) selected from survey questions used with adults in Malawi that asked about community reactions if the respondent were HIV-positive and other people found out (50, 51). HIV risk perception was from a survey item asking if participants considered themselves at risk for HIV infection (yes, no). HIV sexual risk behavior was a composite variable that included sexual activity, condom use, and number of sexual partners. An ordinal 3-category variable was used in analyses (coded 0=never had sex [Never had sex], 1=used condom at last sex and had fewer than two partners in the past 12 months [Less risky], and 2=no condom use at last sex and/or had two or more partners in past 12 months [More risky]).
Sociodemographics.
These measures included gender (male, female) and age (dichotomized as ages 15–17 or 18–19). To test our moderated mediation hypothesis, we combined the binary age and gender categories into a single four category variable. Other measures were ever been pregnant or impregnated a partner (yes, no), orphan status (one or both parents deceased versus neither deceased), currently enrolled in school or completed secondary school (yes/no), and ever been married (yes, no). Religiosity was based on religious service attendance of once a week or more frequently (yes) versus less often (no). Religious affiliation was coded Roman Catholic; Protestant or other Christian; or Muslim, no religion, or other. Region was based on the three sub-counties (labeled “1,” “2”, and “3”).
HIV test result was coded negative, positive, or indeterminate (inconclusive results) based on HIV antibody testing done at baseline.
Analyses
We described sample characteristics of the participants and examined differences between the four age-gender categories using chi-squared and F tests. Also, we examined the association between baseline HIV test result and the age-gender categories using Fisher’s Exact Test. We tested the hypothesized associations between our three outcomes (i.e. depression and two dimensions of QOL), HIV risk perception, anticipated HIV stigma, and sexual risk behavior using structural equation modeling (SEM). The model containing the primary exogenous and endogenous variables of interest is shown in Figure 1. In total, there were 5 equations: three for our adolescent well-being outcome measures and two for anticipated stigma and HIV risk perception. We omitted from Figure 1 the set of subject background characteristics that served as control variables in each of the structural equations implied by our hypothesized model. These variables, along with sexual behavior, were assumed to be exogenous. They included orphan status, current schooling, religious affiliation (Protestant, Catholic, Muslim), religious service attendance, and region (sub-counties: 1, 2, and 3). Although some of our endogenous variables were binary (e.g. depression and HIV risk perception), we assumed pair-wise linear associations among all analytic variables. We tested the moderated mediation hypothesis using multigroup analysis (MGA) – allowing the estimated path coefficients involving our primary analytic variables of interest to vary discreetly across the four age–gender categories. Although this amounted to estimating the SEM model separately for each of the four categories, we had to hold some parameter estimates constant across groups for identification purposes.
Rather than using the data matrix, the classic SEM method fits a model to the sample variance covariance matrix of the variables involved in the analysis. Although the accuracy of this method rests on a general assumption that the data are sampled from a multivariate normal distribution, a key component of this assumption is that bivariate associations among the variables are roughly linear. Since linearity is unlikely to capture associations between variables that are highly skewed, it is advisable to assess univariate normality among all variables used in the analyses. In our case, univariate normality is a particular concern among our three endogenous variables that are measured on a continuous scale: anticipated HIV stigma, social-physical QOL, and psychological-environmental QOL. To assess univariate normality in each case, we constructed histograms and overlaid them with empirical kernel density plots and normal density plots. In all cases, the range of these variables was limited by response categories available in the survey. For this reason, there was no issue with outliers. Both of the QOL measures were very symmetric around their sample means and well approximated a normal distribution. The distribution of the stigma variable, on the other hand, was multi-modal - with one mode located at the lowest level of perceived stigma and the other model close to the center of the distribution. Although this is not ideal from the standpoint of fitting linear associations, we do not think that the departure from univariate normality was extreme enough to merit departing from the default SEM model fitting procedures. With respect to our categorical measures, none of the measures employed in our analyses involved variables where a large majority of the sample fell into a single category. The models were estimated using the lavaan package in the statistical computing software R using maximum likelihood (52). Model fit was evaluated using non-significant model χ2, Comparative Fit Index (CFI) ≥ 0.95, root mean square error of approximation (RMSEA) ≤ 0.05, and standardized root mean square residual (SRMR) ≤ 0.05.
RESULTS
Sample characteristics and measures
As shown in Figure 2, of the 6726 people who participated in the screening interview, 4799 (71%) were found to be eligible for the study and recruited. Of those individuals, 4096 (85%) were enrolled in the study and completed baseline procedures.
Figure. 2.
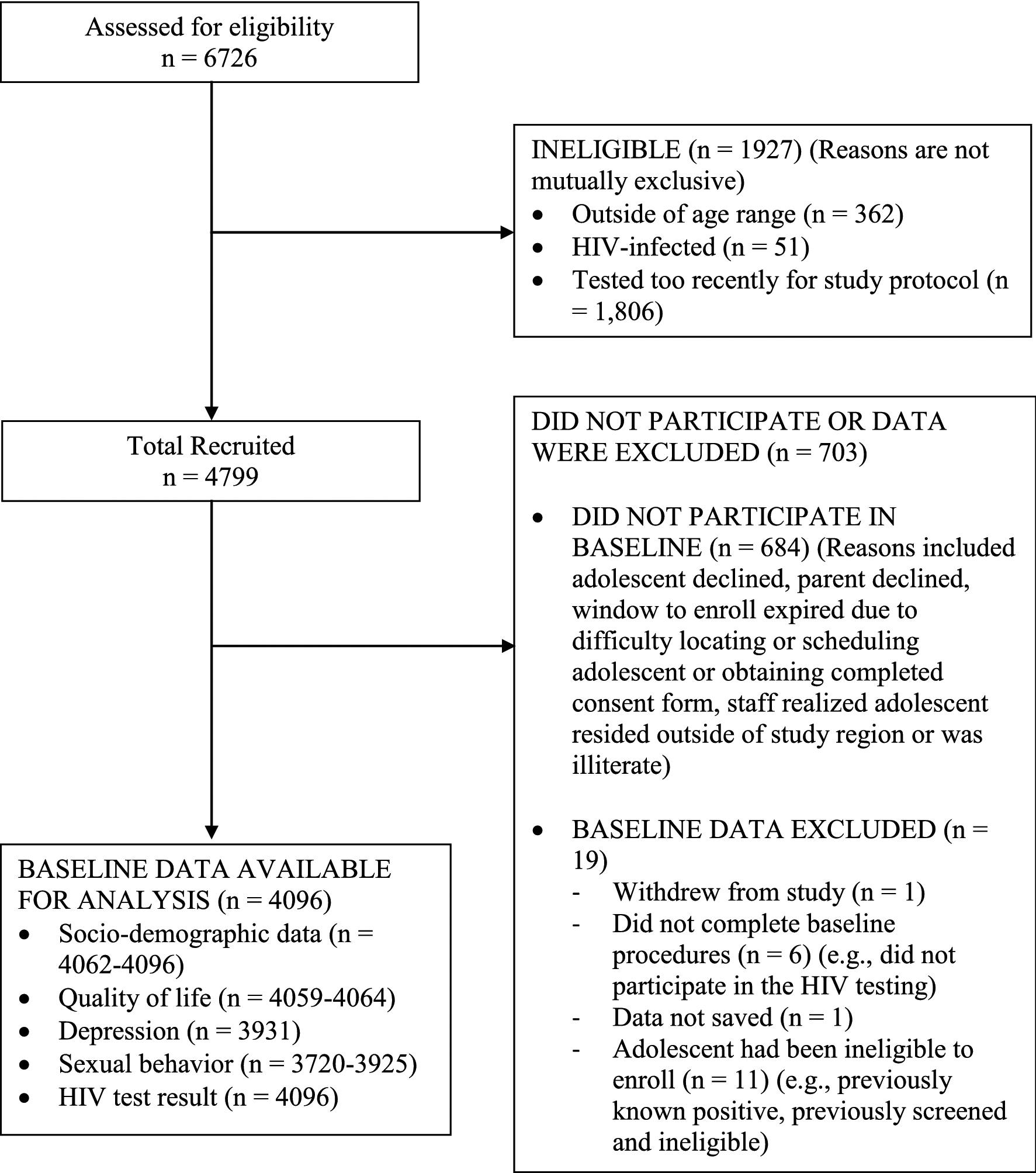
Participant flow of baseline sample
Descriptive statistics are presented for the overall sample and by age-gender groups in Table 1. About 39% of the overall sample met the depressive symptomatology threshold. There were significant differences across the age-gender groups on the endogenous (depression, QOL, anticipated stigma, and HIV risk perception) and primary exogenous (sexual risk behavior) variables. Within each age group, females had higher scores than males on the depression scale. Within each gender, depression scores were higher and QOL scores were lower among adolescents aged 18–19 than those aged 15–17. Older participants were more likely to consider themselves at risk for HIV infection. About 35% of males aged 18–19, 34% of females aged 18–19, 71% of males aged 15–17, and 75% of females aged 15–17 reported never having had sex. For both genders, the proportion of adolescents aged 18–19 reporting risky sex (as opposed to never having had sex) was more than double that of those aged 15–17. About one-third of females 18–19 years old reported having ever been pregnant and 7% of males in that age group said they had ever impregnated someone. Nearly all of the overall sample had a negative HIV test result (99.0%), 0.4% had a positive result, and 0.6% had an indeterminate result (data not shown in table). The HIV test result did not significantly differ by age-gender group (N = 4096, p = 0.09), with 99% testing HIV-negative within each group (Data not shown).
Table 1.
Characteristics of the sample (percentages and means) at baseline by age-gender group
| Variable | Overall (N=4096) % or mean (SD) | Male 15–17 (N=1170, 28.6%) % or mean (SD) | Female 15–17 (N=1359, 33.2%) % or mean (SD) | Male 18–19 (N=895, 21.9%) % or mean (SD) | Female 18–19 (N=672, 16.4%) % or mean (SD) | Statistic F or chi-square, (df) |
|---|---|---|---|---|---|---|
| Endogenous Variables | ||||||
| Has depressive symptoms (% meeting threshold) | 38.62 | 35.37 | 40.79 | 38.02 | 40.74 | χ2(3,N = 3931) = 8.94* |
| Mean depression composite score (CESD-R) (range = 0–60, sum of 20 items, high scores | 14.42 (12.47) | 13.56 (11.91) | 14.96 (12.82) | 14.17 (12.38) | 15.18 (12.77) | F(3,3927) = 3.53* |
| indicate higher symptomatology) | ||||||
| Mean QOL social-physical health factor (mean of 10 items on a 5-point scale 1=poor, 5=good) | 3.80 (0.76) | 3.89 (0.77) | 3.88 (0.72) | 3.65 (0.78) | 3.64 (0.76) | F(3,4060) = 32.56*** |
| Mean QOL psychological-environmental factor (mean of 9 items on a 5-point scale 1=poor, 5=good) | 3.09 (0.70) | 3.13 (0.70) | 3.08 (0.68) | 3.06 (0.70) | 3.06 (0.71) | F(3,4055) = 2.77* |
| Mean anticipated HIV stigma (range=0 – 2, mean of 5 items coded 0 =”no”, 1=”don’t know”, 2=”yes”) | 0.78 (0.52) | 0.79 (0.54) | 0.80 (0.51) | 0.77 (0.54) | 0.71 (0.51) | F(3,4040) = 4.54** |
| Considers oneself at risk for HIV infection (% yes) | 22.85 | 19.30 | 18.46 | 29.81 | 28.50 | χ2(3,N = 3869) = 56.80*** |
| Primary Exogenous Variable | ||||||
| Sexual risk behavior (%) | χ2(6, N = 3804) = 585.98*** | |||||
| Never had sex | 58.60 | 70.61 | 75.30 | 35.33 | 33.98 | |
| Less riskya | 15.93 | 8.41 | 10.72 | 22.49 | 31.24 | |
| More risky | 25.47 | 20.98 | 13.98 | 42.18 | 34.78 | |
| Other Exogenous Variables | ||||||
| You or partner ever been pregnant (% yes) | 9.45 | 2.07 | 5.86 | 6.88 | 32.98 | χ2(3, N = 4062) = 532.57*** |
| Orphan (% any type of orphanb) | 41.70 | 40.79 | 36.92 | 45.32 | 48.13 | χ2(3, N = 4062) = 29.14*** |
| Currently in school or completed secondary (% yes) | 83.25 | 92.74 | 92.13 | 69.39 | 67.26 | χ2(3, N = 4096) = 398.88*** |
| Ever married (% yes) | 1.99 | 0.17 | 0.67 | 0.68 | 9.60 | χ2(3, N = 4064) = 237.06*** |
| Attends religious service once a week or more frequently (% yes) | 53.35 | 51.15 | 61.75 | 42.23 | 55.01 | χ2(3, N = 4090) = 85.94*** |
| Religious affiliation (%) | χ2(6, N = 4092) = 66.98*** | |||||
| Roman Catholic | 24.95 | 22.16 | 24.01 | 26.62 | 29.51 | |
| Protestant or other Christian | 68.99 | 71.00 | 72.46 | 62.98 | 66.47 | |
| Muslim, other, or none | 6.06 | 6.84 | 3.53 | 10.40 | 4.02 | |
| Region (%) | χ2(6, N = 4096) = 9.03 | |||||
| Subcounty 1 | 26.81 | 29.57 | 25.17 | 25.92 | 26.49 | |
| Subcounty 2 | 35.55 | 34.10 | 35.47 | 37.77 | 35.27 | |
| Subcounty 3 | 37.65 | 36.32 | 39.37 | 36.31 | 38.24 |
Notes. CESD-R = Center for Epidemiological Studies Depression Scale Revised. QOL = Quality of Life. F-statistic was from one-way ANOVAs testing differences in means. Chi-square was from frequency cross-tabulations of categorical variables.
”Less risky” refers to having used a condom at last sex and having had fewer than two partners in the past 12 months. “More risky” refers to no condom use at last sex and/or having had two or more partners in the past 12 months.
”Any type of orphan” includes maternal, paternal or double/total orphans.
p < 0.05;
p < 0.01;
p < 0.001
Relationships between sexual risk behaviors, perceived HIV risk, and anticipated HIV stigma
Bivariate associations between risk factors and anticipated stigma with perceiving oneself at risk for HIV are shown by gender in Table 2. Among both males and females, sexual risk behavior was related to perceived risk (χ2 = 103.82[2], p < 0.001 among males and 94.63[2], p < 0.001 among females), with never having had sex being associated with lowest frequency of perceived risk. Females who had ever been pregnant were more likely than other females to perceive themselves at risk for HIV (χ2 = 11.36[2], p < 0.001). Among both sexes, individuals perceiving themselves at risk for HIV had higher mean level of anticipated HIV stigma (t = −4.59, p < 0.001 among males and t = −2.77, p < 0.01 among females).
Table 2.
Bivariate associations between risk factors and anticipated HIV stigma with perceived HIV risk, stratified by sex (Total N=3869)a
| Males (N=1961) | Females (N=1908) | |||
|---|---|---|---|---|
| Risk factor variable | % replied “at risk” | Chi-square or t-value (df) | % replied “at risk” | Chi-square or t-value (df) |
| Overall, by sex (Chi-square = 2.33, NS) | 23.9 | 21.8 | ||
| Sexual risk behavior | χ2(2,N = 1866) = 103.82*** | χ2(2, N = 1817) = 94.63*** | ||
| Never had sex | 14.7 | 14.6 | ||
| Less riskyb | 34.0 | 28.0 | ||
| More risky | 35.2 | 37.4 | ||
| You or partner ever been pregnant | χ2(1, N = 1944) = 0.41 | χ2(2, N = 1279) = 11.36*** | ||
| No | 23.7 | 20.4 | ||
| Yes | 26.8 | 29.3 | ||
| Ever married | χ2(2, N = 1475) = 0.57 | χ2(2, N = 1431) = 1.81 | ||
| No | 23.9 | 21.5 | ||
| Yes | 12.5 | 28.2 | ||
| Mean (SD) | t-value | Mean (SD) | t-value | |
| Anticipated HIV stigma by risk perception | t(725.75) = −4.59*** | t(617.79) = −2.77** | ||
| Perceive “at risk” | 0.88 (0.58) | 0.83 (0.55) | ||
| Perceive “not at risk” | 0.74 (0.52) | 0.75 (0.50) | ||
Notes: Categorical variables were evaluated by cross-tabulation. HIV stigma was evaluated by t-test.
Total number of participants with non-missing data for the perceived HIV risk survey item.
”Less risky” refers to having used a condom at last sex and having had fewer than two partners in the past 12 months. “More risky” refers to no condom use at last sex and/or having had two or more partners in the past 12 months.
p < 0.001,
p < 0.01
Structural model of predictors of well-being outcomes by age-gender groups
To summarize our model fitting process, our initial approach was to impose equality constraints for the control variables across the four age-gender groups for identification purposes. However, the model fit indices indicated a poor fit and we used modification indices to relax equality constraints in order to allow for different associations between the control and endogenous variables. After a few iterations of imposing a set of parameter restrictions and examining modification indices, we stopped when the fit of the model was deemed acceptable: (χ2[54, N=3544] = 60.399, p = 0.256, CFI = 0.997, RMSEA = 0.012, SRMR = 0.012).
The SEM estimated path coefficients for the primary analytic variables across all five structural equations and four age-gender groups are presented in Table 3. These results represent analysis for the complete moderated mediation hypothesis of the primary associations of interest by the age and gender categories. In order to test this hypothesis, we compared the difference in model chi-square between the complete moderated mediation model and the no moderated mediation model to the difference in their respective degrees of freedom. The result led us to reject the hypothesis of complete moderated mediation in favor of the null hypothesis of no moderated mediation (Δχ2[51] = 63.381, p = 0.114). An examination of Table 3 reveals why this result was obtained. There was remarkable stability in the magnitudes of many of the estimated path coefficients across the age and gender categories. This is certainly the case for the associations between perception of HIV risk, anticipated stigma, and the three adolescent well-being outcomes.
Table 3.
Regression coefficients (confidence interval) in structural equation model of adolescent well-being depicting complete moderation by age and gender (Total N = 4096)
| Early/Young Adolecents (age 15–17) | Late/Older Adolecents (age 18–19) | ||||
|---|---|---|---|---|---|
| Dependent Variable | Independent Variable | Male (n = 1170) | Female (n= 1359) | Male (n = 895) | Female (n = 672) |
| Depression (no/yes) | Perceive self at risk (ref = no) | 0.12[0.04,0.19]** | 0.16[0.08,0.23]*** | 0.14[0.07,0.21]*** | 0.12[0.04,0.21]** |
| Anticipated HIV stigma | 0.12[0.07,0.17]*** | 0.14[0.08,0.21]*** | 0.12[0.06,0.17]*** | 0.15[0.08,0.22]*** | |
| Sexual risk behavior (ref = never had sex) | |||||
| Less risky sex | 0.02[−0.08,0.13] | −0.07[−0.16,0.03] | 0.10[0.01,0.19]* | −0.02[−0.11,0.08] | |
| More risky sex | 0.12[0.05,0.19]** | 0.02[−0.06,0.1] | 0.17[0.08,0.25]*** | 0.11[0.01,0.21]* | |
| QOL social-physical health | Perceive self at risk (ref = no) | −0.24[−0.35,−0.12]*** | −0.30[−0.42,−0.18]*** | −0.20[−0.3,−0.1]*** | −0.36[−0.50,−0.22]*** |
| Anticipated HIV stigma | −0.26[−0.35,−0.18]*** | −0.23[−0.33,−0.13]*** | −0.22[−0.3,−0.14]*** | −0.20[−0.32,−0.09]*** | |
| Sexual risk behavior (ref = never had sex) | |||||
| Less risky sex | −0.11[−0.27,0.06] | 0.03[−0.11,0.18] | −0.10[−0.23,0.03] | 0.00[−0.14,0.15] | |
| More risky sex | −0.33[−0.44,−0.22]*** | −0.13[−0.25,0.00]* | −0.29[−0.4,−0.17]*** | −0.17[−0.33,−0.02]* | |
| QOL psychological-environmental | Perceive self at risk (ref = no) | −0.04[−0.14,0.07] | −0.03[−0.14,0.08] | −0.07[−0.17,0.03] | −0.20[−0.33,−0.07]** |
| Anticipated HIV stigma | −0.12[−0.2,−0.05]** | −0.21[−0.3,−0.12]*** | −0.15[−0.23,−0.08]*** | −0.16[−0.27,−0.05]** | |
| Sexual risk behavior (ref = never had sex) | |||||
| Less risky sex | 0.08[−0.07,0.23] | 0.15[0.02,0.28]* | −0.04[−0.16,0.08] | 0.02[−0.12,0.16] | |
| More risky sex | −0.16[−0.26,−0.05]** | −0.11[−0.22,0.01] | −0.22[−0.33,−0.11]*** | −0.14[−0.28,0.01] | |
| Anticipated HIV stigma | Perceive self at risk (ref = no) | 0.22[0.13,0.30]*** | 0.11[0.02,0.19]* | 0.09[0.01,0.16]* | 0.08[−0.02,0.18] |
| Sexual risk behavior (ref = never had sex) | |||||
| Less risky sex | 0.03[−0.08,0.15] | −0.12[−0.23,−0.02]* | 0.00[−0.09,0.09] | −0.02[−0.13,0.08] | |
| More risky sex | 0.04[−0.04,0.13] | 0.05[−0.04,0.13] | 0.14[0.06,0.23]** | 0.11[0,0.21] | |
| Risk perception (no/yes) | Sexual risk behavior (ref = never had sex) | ||||
| Less risky sex | 0.13[0.04,0.21]** | 0.22[0.13,0.3]*** | 0.14[0.07,0.21]*** | 0.14[0.06,0.23]** | |
| More risky sex | 0.17[0.11,0.23]*** | 0.26[0.19,0.34]*** | 0.19[0.12,0.25]*** | 0.30[0.21,0.39]*** | |
Note. All of the effects are allowed to vary across the age-sex groups. Models included control variables of region, religiosity, religious affiliation, in school/completed schooling, orphan status, ever pregnant (partner or self), and ever married.
p < 0.05,
p < 0.01,
p < 0.001
Nonetheless, an examination of Table 3 also reveals evidence of more limited forms of moderation. For example, the magnitudes of the estimated path coefficients linking sexual risk behavior to perceiving oneself at risk for HIV appear to be larger for girls than boys. Compared to never having had sex, the impact of reporting the highest level of risky sexual behavior is associated with an increase of 0.26 (95% CI 0.19, 0.34; p < 0.001) and 0.30 (95% CI 0.21, 0.39; p < 0.001) in the binary HIV risk perception measure for younger and older adolescent girls, respectively. For younger and older adolescent boys this increase is considerably smaller: 0.17 (95% CI 0.11, 0.23; p < 0.001) and 0.19 (95% CI 0.12, 0.25; p < 0.001), respectively. A similar pattern can be observed when shifting to the moderate level of risky sexual behavior. Moderation by gender is also evident in the association between risky sexual behavior and the three adolescent well-being outcomes, although in this case the patterns observed across gender are reversed. For each well-being outcome, older and younger adolescent boys who report engaging in riskier sexual behavior are worse off than their peers who report not having sex. For girls, the relationship between sexual behavior and the well-being outcomes appears to be more attenuated. Finally, Table 3 does provide evidence for at least one isolated three-way interaction between gender, age, and perception of HIV risk in their associations with anticipated HIV stigma. The association between risk perception and anticipated stigma is modest, bordering on statistical insignificance for three out of the four age-gender groups. This is not the case for young adolescent boys (age 15–17), where the estimated coefficient is double in magnitude.
We tested a limited version of the moderated mediation hypothesis by gender and found that to be statistically significant (χ2[8] = 18.599, p = 0.017). We then tested the three-way interaction between age, gender, and the association between risk perception and anticipated stigma. This result was not statistically significant (χ2[3] = 6.08, p = 0.108). Thus, the model containing only the previously discussed path coefficients moderated by gender constitutes our final structural model for the associations hypothesized in this study. According to selected fit indices, overall fit of our final model to the data was acceptable (CFI = 0.996, RMSEA= 0.010, SRMR = 0.016), and we restrict the remaining presentation and discussion of our findings to this model.
As shown in Figure 3, anticipated HIV stigma has consistent and statistically significant associations with all three measures of adolescent well-being, and an examination of the standardized estimates indicate that these effects are similar in magnitude and in the expected direction (i.e. stigma is inversely related to well-being). The estimated associations between HIV risk perception and adolescent well-being are less consistent in magnitude, although all three are statistically significant. The strongest association appears to involve the QOL social-physical health outcome while the weakest involves the QOL psychological-environmental outcome.
Figure 3.
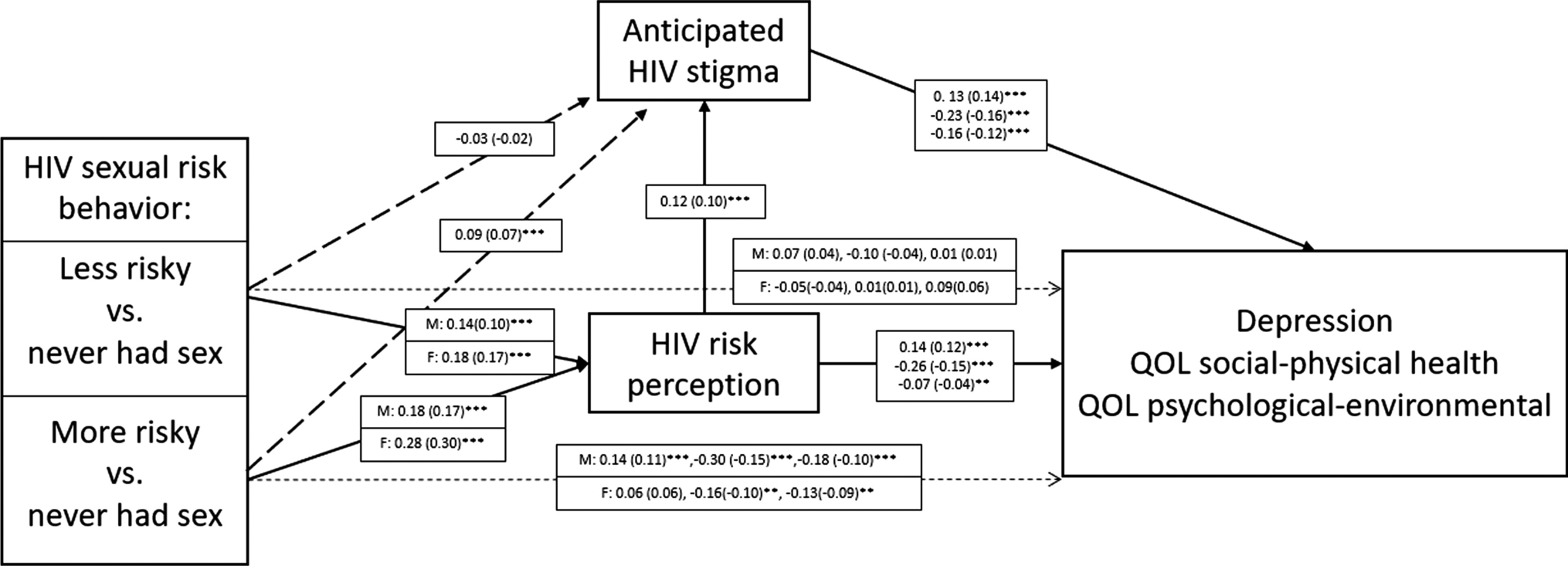
Structural model for influences of anticipated stigma, risk behavior and risk perception on well-being outcomes among Kenyan adolescents with moderation by gender, 2016–2017 Note. : Numerical values are the unstandardized coefficient estimates (standardized estimate). Where three pairs of coefficients are listed, they are for Depression, QOL social-physical health, and QOL psychological-environmental health, respectively. <p/>M = Male, F = Female <p/>*p < 0.05; **p < 0.01; ***p < 0.001
During the model fitting process, we found evidence of moderation by gender with respect to HIV sexual risk behavior and its association with both HIV risk perception and the adolescent well-being outcomes. The pattern of moderation can be gleaned from Figure 3. For girls, risk behavior was a stronger predictor of risk perception than it was for boys. Regarding well-being, however, we obtained the opposite result: stronger associations between behavior and well-being were observed among boys, although this association is only statistically significant in the contrast between the highest and lowest risk behavior categories. We also found some evidence of mediation by stigma with respect to sexual behavior and its association with well-being across both genders indicated by the statistically significant and positive path coefficient linking the highest category of sexual risk behavior and anticipated stigma (0.09, p < 0.001)
We examined the relative saliencies of the social and health consequences pathways linking HIV risk perception to well-being by estimating the indirect effects that link risk perception to each of the three well-being outcomes through anticipated stigma (i.e. HIV risk perception → Anticipated HIV stigma → Well-being). In Table 4, we report the direct, indirect, and total effects of HIV risk perception on each well-being measure.
Table 4.
Total, indirect (through anticipated HIV stigma), and direct effects of perceived HIV risk on well-being outcomes (Total N = 4096)
| Outcome variable | Total effect estimate (CI) | Total effect standardized coefficient | Indirect effect estimate (CI) | Indirect effect standardized coefficient | Direct effect estimate (CI) | Direct effect standardized coefficient (averaged) |
|---|---|---|---|---|---|---|
| Depression | 0.15 [0.11,0.19] | 0.12 | 0.02 [0.01,0.02]* | 0.01 | 0.14 (0.10,0.18)*** | 0.12 |
| QOL Social-physical health | −0.29 [−0.36,−0.23] | −0.15 | −0.03 [−0.04,−0.02]* | −0.02 | −0.26 (−0.33,−0.19)*** | −0.15 |
| QOL Psychological-environmental | −0.09 [−0.15,−0.04] | −0.05 | −0.02 [−0.03,−0.01]* | −0.01 | −0.07 (−0.13,−0.02)** | −0.04 |
Notes: Results are for genders combined. Models included control variables of region, religiosity, religious affiliation, in school/completed schooling, orphan status, ever pregnant (partner or self), and ever married. CI = confidence interval.
CI are the 95% intervals using the adjusted bootstrap percentile (BCa) method but with no correction for acceleration (only for bias).
p < 0.05,
p < 0.01,
p < 0.001
Examining the total effects in Table 4, it appears that largest deleterious effects of HIV risk perception on adolescent well-being is observed with respect to depression and the social-physical QOL measure. The estimates of direct and indirect effects indicate that most of these effects flow through what we have termed the “health consequences” pathways (i.e. the direct effect). Although statistically significant, the magnitudes of the estimates for the indirect effects are much smaller, suggesting that the social pathway linking HIV risk perception to our health outcomes through stigma plays a smaller role in shaping adolescent well-being.
DISCUSSION
Our study is among the first to explicate the interrelationships between sexual risk behavior, HIV risk perception, anticipated stigma, and well-being (i.e., depression symptoms and QOL) among adolescents in a high prevalence rural setting in sub-Saharan Africa. The results partially confirmed our hypotheses. First, risky sexual behavior was positively associated with HIV risk perception, with evidence of moderation by gender. Second, sexual behavior and risk perception were both positively associated with anticipated HIV stigma. Additionally, the impact of risk perception on adolescent well-being was stable across gender and age and largely direct; indirect effects through stigma were minimal. Third, sexual risk behavior was inversely related to adolescent well-being, with evidence of moderation by gender.
Not unlike previous studies, about 39% of adolescents in our sample met the CESD-R threshold for depressive symptomatology, (3, 15–24) providing additional evidence of the need for mental health services to improve well-being among youth in this setting. Our study findings suggest that while fears of the social consequences of an HIV diagnosis (i.e., anticipated stigma) may affect adolescent well-being, that relationship is not driven by risky sexual behavior or heightened perceptions of HIV risk. Indeed, anticipated stigma, risk perception, and sexual behavior each appear to independently affect mental health and quality of life among adolescents. In contrast, in their study among MSM in the U.S., Starks et al. did not find a direct effect of perceived HIV risk (measured by sexual role identification) on negative affect. (29) However, like our study, they found a statistically significant association between perceived risk and anticipated stigma, and a small albeit significant indirect effect of perceived risk on negative affect via anticipated HIV stigma.
Our findings suggest that Kenyan adolescents who anticipate stigma from an HIV diagnosis fare worse in terms of their well-being even if they are not engaged in risky sexual behaviors or do not perceive themselves to be personally at risk of infection. Additionally, adolescents who are engaged in risk behaviors or perceive themselves at heightened risk for HIV are also more likely to report depression symptoms and poorer QOL. Like other research, we found older adolescents were more likely to engage in risky sex and to have poorer psychological health compared to younger adolescents (3, 38, 53, 54). Identifying adolescents who are at risk for poor mental health is key for reaching those who are most in need of interventions. Future studies should explore the contextual factors that may explain the relationship between risk behaviors and mental health among adolescents. Additionally, they should investigate whether screening for risky sexual behavior, risk perception, and perceptions of stigma in high HIV prevalence settings is an effective approach to identifying vulnerable adolescents in need of mental health services.
Moreover, like other sub-Saharan African countries, Kenya faces important challenges to addressing mental disorders, especially in rural areas. Impediments include lack of government spending on mental health, shortages of trained mental health professionals, low capacity among non-specialist health workers to provide quality mental health services to young people, and stigma associated with mental disorder. (26, 55–58) Strategies that circumvent these challenges and attenuate risk factors or promote protective factors for mental health among adolescents are critical in the short- to medium-term, while policymakers and healthcare providers identify effective approaches to address needs in the long term. Prior research in resource-rich settings has indicated that stigma and risk behavior prevention interventions can improve adolescent mental health. (59, 60) Future studies should examine whether interventions that address stigma and sexual risk behaviors reduce depression and increase quality of life among youth in resource-limited settings with high HIV prevalence.
Our study is the first to show that the relationship between sexual risk behavior and HIV risk perception may be moderated by gender. (3, 61) Specifically, we found that compared to the lowest risk category, riskier sexual behavior was a stronger predictor of perceiving oneself to be at risk for HIV among females than among males. Our study is also the first to suggest that gender may moderate the association between risk behavior and well-being. (62) In this case, however, riskier behavior was a stronger predictor of poorer well-being among adolescent males than among females in the highest risk category. Young women in sub-Saharan Africa have been the primary focus of HIV prevention and mental health efforts because of higher rates of infection and mental disorders among them than their male counterparts (17, 41). Our results highlight the need to expand this focus to include adolescent males and to conduct research to determine whether and how approaches to address risk behavior and mental health may differ between adolescent females and males.
It bears highlighting that even though substantial proportions of adolescents reported risky sexual behaviors (25% of our sample were in the higher risk category) and perceived themselves to be at risk for HIV (23% of our sample), HIV prevalence among our study participants was low at less than 1%, with no difference by gender. The problem of inconsistency in self-reported sexual behavior among adolescents is well known. (63, 64) Indeed, a previous study in the same region of Kenya similarly reported low HIV prevalence and discordance between biomarker data and self-reported questionnaire responses among orphan adolescents. (65, 66) However, the finding that adolescents perceive themselves to be at risk for HIV despite the low prevalence is new and warrants further investigation. It is also possible that as HIV incidence in the region decreases, risky sexual behaviors among youth may be on the rise, which in turn, is heightening perceptions of risk. (41, 67)
Potential limitations of our study include that our data were cross-sectional. Thus, despite our large sample size, we were limited in our ability to establish causality and it is possible that our proposed conceptual framework has alternative causal mechanisms or that causality was bidirectional for some pathways (e.g., between anticipated stigma and depression and QOL). Second, because our study was observational and our survey questionnaire brief, we were limited in our ability to adjust our estimates for potential confounding or to test for additional mediators or moderators to fully explain the pathways that link sexual risk behavior, HIV risk perception, anticipated HIV stigma, and well-being among adolescents. For example, family dynamics and poverty are known determinants of psychosocial distress among adolescents in sub-Saharan Africa that we were unable to adjust for due to data limitations. (20, 68) Additionally, due to lack of data, we were unable to account for key relationship factors such as power dynamics, intimate partner violence, and lack of trust in partners, which have been shown to play an important role in risky sexual behavior, risk perception, and HIV stigma, especially among women in sub-Saharan Africa (69, 70). Third, because our data are from a community-based, rural sample rather than a random or probability-based sample, generalizability of our findings to settings outside of the study region is limited. Nevertheless, our attribution of causal order is similar to preceding SEM studies and informed by prior research. (27, 29, 37, 38) Although preliminary, we believe that the evidence presented here will inform future longitudinal studies with representative samples to examine the mechanisms linking sexual behavior, HIV risk perception, anticipated stigma, and mental health.
CONCLUSIONS
We examine associations among depression symptoms, QOL, anticipated HIV stigma, risk perception, and sexual behaviors. The results highlight the importance of anticipated stigma, risk perception, and sexual behaviors in the mental health and QOL of adolescents in a region of Kenya with high HIV prevalence. Additionally, the results suggest a need to expand HIV and mental health research to include adolescent males and to develop effective gender-specific interventions to improve adolescent well-being.
Supplementary Material
Acknowledgments
We thank all participants for their contributions to this study. We are also grateful to the research staff at PIRE and KEMRI for their work and dedication to this research. Research reported in this publication was supported by funding from the National Institute of Mental Health and the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number R01MH102125 (Luseno, W.K., PI). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The research used the World Health Organization Quality of Life Questionnaire (WHOQOL-BREF). We are grateful to Shane Hartman and research assistants at KEMRI for their important contributions to this research.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- 1.Page RM, West JH. Suicide ideation and psychosocial distress in sub-Saharan African youth. Am J Health Behav. 2011;35(2):129–41. [DOI] [PubMed] [Google Scholar]
- 2.Swahn MH, Palmier JB, Kasirye R, Yao H. Correlates of suicide ideation and attempt among youth living in the slums of Kampala. Int J Environ Res Public Health. 2012;9(2):596–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.James S, Reddy SP, Ellahebokus A, Sewpaul R, Naidoo P. The association between adolescent risk behaviours and feelings of sadness or hopelessness: a cross-sectional survey of South African secondary school learners. Psychol Health Med. 2017;22(7):778–89. [DOI] [PubMed] [Google Scholar]
- 4.Erskine HE, Moffitt TE, Copeland WE, et al. A heavy burden on young minds: The global burden of mental and substance use disorders in children and youth. Psychol Med. 2015;45(7):1551–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kassebaum N, Kyu HH, Zoeckler L, et al. Child and Adolescent Health From 1990 to 2015: Findings From the Global Burden of Diseases, Injuries, and Risk Factors 2015 Study. JAMA Pediatr. 2017;171(6):573–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel V Reducing the burden of depression in youth: what are the implications of neuroscience and genetics on policies and programs? J Adolesc Health. 2013;52(2):S36–S8. [DOI] [PubMed] [Google Scholar]
- 7.Kagee A, Coetzee B, Toit S, Loades M. Psychosocial predictors of quality of life among South Africa adolescents receiving antiretroviral therapy. Qual Life Res. 2019;28(1):57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skevington SM, Dehner S, Gillison FB, McGrath EJ, Lovell CR. How appropriate is the WHOQOL-BREF for assessing the quality of life of adolescents? Psychol Health. 2014;29(3):297–317. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. Child and adolescent mental health [webpage]. Available at https://www.who.int/mental_health/maternal-child/child_adolescent/en/. Accessed on May 28, 2020.
- 10.GBD 2017 Child and Adolescent Health Collaborators, Reiner RC Jr, Olsen HE, et al. Diseases, Injuries, and Risk Factors in Child and Adolescent Health, 1990 to 2017: Findings From the Global Burden of Diseases, Injuries, and Risk Factors 2017 Study. JAMA Pediatr. 2019;173(6):e190337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haroz EE, Ybarra ML, Eaton WW. Psychometric evaluation of a self-report scale to measure adolescent depression: The CESDR-10 in two national adolescent samples in the United States. J Affect Disord. 2014;158:154–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li JY, Li J, Liang JH, Qian S, Jia RX, Wang YQ, Xu Y. Depressive symptoms among children and adolescents in China: a systematic review and meta-analysis. Med Sci Monit. 2019;25:7459–7470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang L, Feng Z, Yang G, et al. Depressive symptoms among children and adolescents in western China: an epidemiological survey of prevalence and correlates. Psychiatry Res. 2016;246:267–274. [DOI] [PubMed] [Google Scholar]
- 14.Erskine HE, Baxter AJ, Patton G, et al. The global coverage of prevalence data for mental disorders in children and adolescents. Epidemiol Psychiatr Sci. 2017;26(4):395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barhafumwa B, Dietrich J, Closson K, et al. High prevalence of depression symptomology among adolescents in Soweto, South Africa associated with being female and cofactors relating to HIV transmission. Vulnerable Child Youth Stud. 2016;11(3):263–73. [Google Scholar]
- 16.Khasakhala LI, Ndetei DM, Mutiso V, Mbwayo VAW, Mathai M. The prevalence of depressive symptoms among adolescents in Nairobi public secondary schools: Association with perceived maladaptive parental behavior. Afr J Psychiatry. 2012;15(2):106–13. [DOI] [PubMed] [Google Scholar]
- 17.Kilburn K, Prencipe L, Hjelm L, Peterman A, Handa S, Palermo T. Examination of performance of the Center for Epidemiologic Studies Depression Scale Short Form 10 among African youth in poor, rural households. BMC Psychiatry. 2018;18(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nduna M, Jewkes RK, Dunkle KL, Shai NPJ, Colman I. Prevalence and factors associated with depressive symptoms among young women and men in the Eastern Cape Province, South Africa. J Child Adolesc Ment Health. 2013;25(1):43–54. [DOI] [PubMed] [Google Scholar]
- 19.Otiende M, Abubakar A, Mochamah G, et al. Psychometric evaluation of the Major Depression Inventory among young people living in Coastal Kenya. Wellcome Open Res. 2017;2:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng Y, Li X, Lou C, et al. The association between social support and mental health among vulnerable adolescents in five cities: findings from the study of the well-being of adolescents in vulnerable environments. J Adolesc Health. 2014;55(6 Suppl):S31–S8. [DOI] [PubMed] [Google Scholar]
- 21.Mngoma NF, Ayonrinde OA, Fergus S, Jeeves AH, Jolly RJ. Distress, desperation and despair: anxiety, depression and suicidality among rural South African youth [published online ahead of print, 2020 Apr 20]. Int Rev Psychiatry. 2020;1–11. [DOI] [PubMed] [Google Scholar]
- 22.Nalugya-Sserunjogi J, Rukundo GZ, Ovuga E, Kiwuwa SM, Musisi S, Nakimuli-Mpungu E. Prevalence and factors associated with depression symptoms among school-going adolescents in Central Uganda. Child Adolesc Psychiatry Ment Health. 2016;10(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nyundo A, Manu A, Regan M, et al. Factors associated with depressive symptoms and suicidal ideation and behaviours amongst sub-Saharan African adolescents aged 10–19 years: cross-sectional study. Trop Med Int Health. 2020;25(1):54–69. [DOI] [PubMed] [Google Scholar]
- 24.Osborn TL, Venturo-Conerly KE, Wasil AR, Schleider JL, Weisz JR. Depression and anxiety symptoms, social support, and demographic factors among Kenyan high school students. J Child Fam Stud. 2020;29(5):1432–1443. [Google Scholar]
- 25.Grover S, Raju VV, Sharma A, Shah R. Depression in children and adolescents: a review of Indian studies. Indian J Psychol Med. 2019;41(3):216–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel V, Flisher AJ, Hetrick S, McGorry P. Mental health of young people: a global public-health challenge. Lancet. 2007;369(9569):1302–13. [DOI] [PubMed] [Google Scholar]
- 27.Gupta R, Dandu M, Packel L, et al. Depression and HIV in Botswana: A population-based study on gender-specific socioeconomic and behavioral correlates. PLoS ONE. 2010;5(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamen C, Arganbright J, Kienitz E, et al. HIV-related stigma: Implications for symptoms of anxiety and depression among Malawian women. Afr J AIDS Res. 2015;14(1):67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Starks TJ, Rendina HJ, Breslow AS, Parsons JT, Golub SA. The psychological cost of anticipating HIV stigma for HIV-negative gay and bisexual men. AIDS Behav. 2013;17(8):2732–41. [DOI] [PubMed] [Google Scholar]
- 30.Dow DE, Turner EL, Shayo AM, Mmbaga B, Cunningham CK, O’Donnell K. Evaluating mental health difficulties and associated outcomes among HIV-positive adolescents in Tanzania. AIDS care. 2016;28(7):825–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boyes ME, Cluver LD. Relationships among HIV/AIDS orphanhood, stigma, and symptoms of anxiety and depression in South African youth: a longitudinal investigation using a path analysis framework. Clin Psychol Sci. 2013;1(3):323–30. [Google Scholar]
- 32.Cluver LD, Gardner F, Operario D. Effects of stigma on the mental health of adolescents orphaned by AIDS. J Adolesc Health. 2008;42(4):410–7. [DOI] [PubMed] [Google Scholar]
- 33.Anderson KG, Beutel AM, Maughan-Brown B. HIV risk perceptions and first sexual intercourse among youth in Cape Town, South Africa. Int Fam Plan Perspect. 2007:98–105. [DOI] [PubMed] [Google Scholar]
- 34.Luseno WK, Iritani BJ, Maman S, et al. “If the mother does not know, there is no way she can tell the adolescent to go for drugs”: Challenges in promoting health and preventing transmission among pregnant and parenting Kenyan adolescents living with HIV. Child Youth Serv Rev 2019;103:100–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Travaglini LE, Himelhoch SS, Fang LJ. HIV stigma and its relation to mental, physical and social health among Black women living with HIV/AIDS. AIDS Behav. 2018;22(12):3783–3794. [DOI] [PubMed] [Google Scholar]
- 36.Ng’eno BN, Kellogg TA, Kim AA, et al. Modes of HIV transmission among adolescents and young adults aged 10–24 years in Kenya. Int J STD AIDS. 2018;29(8):800–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hallfors DD, Waller MW, Bauer D, Ford CA, Halpern CT. Which comes first in adolescence—sex and drugs or depression? Am J Prev Med. 2005;29(3):163–70. [DOI] [PubMed] [Google Scholar]
- 38.Cluver L, Orkin M, Boyes ME, Sherr L, Makasi D, Nikelo J. Pathways from parental AIDS to child psychological, educational and sexual risk: developing an empirically-based interactive theoretical model. Soc Sci Med. 2013;87:185–93. [DOI] [PubMed] [Google Scholar]
- 39.Nyamukapa CA, Gregson S, Lopman B, et al. HIV-associated orphanhood and children’s psychosocial distress: theoretical framework tested with data from Zimbabwe. Am J Public Health. 2008;98(1):133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Government of Kenya MoH, National AIDS Control Council (NACC). Kenya AIDS Response Progress Report 2016. Nairobi: NACC; 2016. [Google Scholar]
- 41.Joint United Nations Programme on AIDS (UNAIDS). AIDS Info 2018. Available at: http://aidsinfo.unaids.org/.
- 42.Cho H, Deming ME, Park J-H, Iritani B. Gender Differences in HIV/HSV-2: Evidence from a School Support Randomized Controlled Trial Among Orphaned Adolescents in Kenya. AIDS Behav. 2019;23(9):2396–2406. [DOI] [PubMed] [Google Scholar]
- 43.National AIDS and STI Control Programme (NASCOP) Kenya. Regional Fact Sheet (Nyanza). Kenya AIDS Indicator Survey 2012. Nairobi: NASCOP; 2014. [Google Scholar]
- 44.Van Dam NT, Earleywine M. Validation of the Center for Epidemiologic Studies Depression Scale--Revised (CESD-R): pragmatic depression assessment in the general population. Psychiatry Res. 2011;186(1):128–32. [DOI] [PubMed] [Google Scholar]
- 45.Olagunju AT, Aina OF, Fadipe B. Screening for depression with Centre for Epidemiological Studies Depression Scale Revised and its implication for consultation–liaison psychiatry practice among cancer subjects: A perspective from a developing country. Psychooncology. 2013;22(8):1901–6. [DOI] [PubMed] [Google Scholar]
- 46.Skevington SM, Lotfy M, O’Connell KA. The World Health Organization’s WHOQOL-BREF quality of life assessment: Psychometric properties and results of the international field trial A Report from the WHOQOL Group. Qual Life Res. 2004;13(2):299–310. [DOI] [PubMed] [Google Scholar]
- 47.World Health Organization (WHO). The World Health Organization Quality of Life (WHOQOL)-BREF. Geneva: World Health Organization; 2004. [Google Scholar]
- 48.Enimil A, Nugent N, Amoah C, et al. Quality of life among Ghanaian adolescents living with perinatally acquired HIV: A mixed methods study. AIDS Care. 2016;28(4):460–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ongeri L, McCulloch CE, Neylan TC, et al. Suicidality and associated risk factors in outpatients attending a general medical facility in rural Kenya. J Affect Disord 2018;225:413–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.MacPherson P, Webb EL, Choko AT, et al. Stigmatising attitudes among people offered home-based HIV testing and counselling in Blantyre, Malawi: construction and analysis of a stigma scale. PLoS One. 2011;6(10):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Musyimi CW, Mutiso VN, Nayak SS, Ndetei DM, Henderson DC, Bunders J. Quality of life of depressed and suicidal patients seeking services from traditional and faith healers in rural Kenya. Health Qual Life Outcomes. 2017;15(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosseel Y lavaan: An R Package for Structural Equation Modeling. 2012 2012;48(2):36. [Google Scholar]
- 53.MacQuarrie KLD, Mallick L, Allen C. Sexual and Reproductive Health in Early and Later Adolescence: DHS Data on Youth Age 10–19. DHS Comparative Reports No. 45 Rockville, Maryland, USA: ICF; 2017. [Google Scholar]
- 54.Obeng Gyimah S, Kodzi I, Emina J, Adjei J, Ezeh A. Adolescent sexual risk-taking in the informal settlements of Nairobi, Kenya: understanding the contributions of religion. J Relig Health. 2014;53(1):13–26. [DOI] [PubMed] [Google Scholar]
- 55.Kamau JW, Omigbodun OO, Bella-Awusah T, Adedokun B. Who seeks child and adolescent mental health care in Kenya? A descriptive clinic profile at a tertiary referral facility. Child Adolesc Psychiatry Ment Health. 2017;11:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kiima D, Jenkins R. Mental health policy in Kenya -an integrated approach to scaling up equitable care for poor populations. Int J Ment Health Syst. 2010;4:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.National Academies of Sciences, Engineering, and Medicine. Providing sustainable mental and neurological health care in Ghana and Kenya: Workshop summary. Washington, DC: The National Academies Press; 2016. [PubMed] [Google Scholar]
- 58.Morrison-Beedy D, Mazurek Melnyk B. Making a case for integrating evidence-based sexual risk reduction and mental health interventions for adolescent girls. Issues Ment Health Nurs. 2019;40(11):932–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Burk J, Park M, Saewyc EM. A media-based school intervention to reduce sexual orientation prejudice and its relationship to discrimination, bullying, and the mental health of lesbian, gay, and bisexual adolescents in Western Canada: a population-based evaluation. Int J Environ Res Public Health. 2018;15(11):2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brown CH, Brincks A, Huang S, et al. Two-Year impact of prevention programs on adolescent depression: an integrative data analysis approach. Prev Sci. 2018;19(Suppl 1):74–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Price JT, Rosenberg NE, Vansia D, et al. Predictors of HIV, HIV risk perception, and HIV Worry among adolescent girls and young women in Lilongwe, Malawi. J Acquir Immune Defic Syndr. 2018;77(1):53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hill LM, Gottfredson NC, Kajula LJ, et al. Changes in anxiety and depression symptoms predict sexual risk behaviors among young men living in Dar es Salaam, Tanzania. AIDS Behav 2018;22(5):1435–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Plummer ML, Ross DA, Wight D, et al. “A bit more truthful”: the validity of adolescent sexual behaviour data collected in rural northern Tanzania using five methods. Sex Transm Infect. 2004;80 Suppl 2:ii49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Beguy D, Kabiru CW, Nderu EN, Ngware MW. Inconsistencies in self-reporting of sexual activity among young people in Nairobi, Kenya. J Adolesc Health. 2009;45(6):595–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cho H, Luseno W, Halpern C, et al. Discordance of HIV and HSV-2 biomarkers and self-reported sexual behaviour among orphan adolescents in Western Kenya. Sex Transm Infect. 2015;91(4):260–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cho H, Mbai I, Luseno WK, Hobbs M, Halpern C, Hallfors DD. School support as structural HIV prevention for adolescent orphans in Western Kenya. J Adolesc Health. 2018;62(1):44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Borgdorff MW, Kwaro D, Obor D, et al. HIV incidence in Western Kenya during scale-up of antiretroviral therapy and voluntary medical male circumcision: a population-based cohort analysis. Lancet HIV. 2018;5(5):e241–e9. [DOI] [PubMed] [Google Scholar]
- 68.Hatcher AM, Gibbs A, Jewkes R, McBride R-S, Peacock D, Christofides N. Effect of childhood poverty and trauma on adult depressive symptoms among young men in Peri-urban South African settlements. J Adolesc Health. 2019;64(1):79–85. [DOI] [PubMed] [Google Scholar]
- 69.Teitelman AM, Jemmott JB, Bellamy SL, et al. Partner violence, power, and gender differences in South African adolescents’ HIV/sexually transmitted infections risk behaviors. Health Psychol. 2016;35(7):751–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maughan-Brown B, Evans M, George G. Sexual behaviour of men and women within age-disparate partnerships in South Africa: implications for young women’s HIV risk. PloS One. 2016;11(8):e0159162. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


