Abstract
Objective(s):
To understand the association between children’s anthropometric measures and maternal HIV status in Zimbabwe and to determine whether these relationships changed over time.
Design:
Data from Demographic Health Surveys in Zimbabwe rounds 2005, 2010, and 2015 were used to conduct cross-sectional analyses of child anthropometric measures (stunting, underweight, and wasting).
Methods:
Using separate logistic regression models for each of the anthropometric measures, we estimated the adjusted prevalence odds ratio of stunting, underweight, and wasting in children according to maternal HIV status. Moreover, we evaluated an interaction by survey year to evaluate change over time.
Results:
Children of mothers with HIV had 32% greater odds (OR = 1.32, 95% CI 1.16 to 1.5) of stunting, 27% greater odds (OR= 1.27, 95% CI 1.1 to 1.48) of underweight status and 7% greater odds (OR = 1.07, 95% CI 0.81 to 1.42) of wasting status, than children of mothers without HIV. These associations between maternal HIV status and child undernutrition did not differ by year (p > 0.05 for all interaction terms).
Conclusion:
In Zimbabwe, having a mother who tested positive for HIV at the time of the survey has been associated with greater child undernutrition over the last two decades with no significant change by survey round. This emphasizes the need for continued programming to address nutritional deficiencies, sanitation, and infectious disease prevention in this high-risk population. The greatest impact of maternal HIV status has been on child stunting and underweight, associated with poor long-term child development.
Keywords: HIV, undernutrition, Zimbabwe, child development, epidemiology
Introduction
As of 2018, 1.3 million people in Zimbabwe were living with HIV [1]. HIV disproportionately affects Zimbabwean women in peak childbearing ages (15-24 years), who experience double the number of new HIV infections compared to Zimbabwean men [1]. There is increased morbidity and mortality among children whose mothers are living with HIV, particularly if children are infected at an early developmental stage [2]. Infants who acquire HIV are more likely to experience growth failure due to the infection [3], and low-grade inflammation in the mother or child is strongly associated with stunting among children [4].
The introduction of widespread antiretroviral therapy (ART), has largely reduced the mother to child transmission (MCTC) of HIV [6]. However, even in the absence of vertical transmission of HIV, positive maternal HIV status is associated with negative child growth outcomes [7]. Impaired child growth has been detected in utero among pregnant women living with HIV in Zimbabwe as a consequence of low maternal growth hormone levels at birth [4]. After birth, maternal undernutrition influences breastfeeding behavior and maternal morbidity, which impacts infant and child growth through maternal and household socio-economic status (SES) [8]. While the relationship between maternal nutrition and HIV status and child undernourishment has been well established [9–11], the relationship between maternal HIV status and child growth over time has not been examined at a population level in Zimbabwe.
The period from 2005 to 2015 was marked by increasing access to ART for pregnant women and children in Zimbabwe. In 2005, routine HIV testing and counselling was added to standard of care in antenatal care (ANC) visits [12], and in 2012 Zimbabwe’s Ministry of Health and Child Care (MoHCC) implemented national rollout of point-of-care CD4 testing and extended lifelong antiretroviral therapy (ART) regimens (Options A or B) to more women infected with HIV [13–15]. In 2014, MoHCC rolled out lifelong ART for all pregnant and breastfeeding women living with HIV, treatment for all children under the age of five years living with HIV, and treatment for older children and adults (Option B+) [16,17]. As a result, 94% of pregnant women living with HIV in Zimbabwe were accessing ART in 2018, an increase from 29% in 2010 [18].
Despite progress over this period in ART access, MTCT and food security challenges remain. The MTCT rate in Zimbabwe in 2016 was about 621 new HIV infections per 100,000 live births, well above the MTCT elimination target of 50 per 100,000 live births [15]. While exclusive breastfeeding increased nationally from 22% in 2005 to 46% in 2015, the overall food insecurity situation in Zimbabwe worsened over time [19,20]. As of 2012, almost half of Zimbabwe’s population experienced food insecurity, including 33% of moderately food insecure households and 18% of severely food insecure households [13,21,22]. In response, public and private sector actors, nongovernmental organizations, and intergovernmental organizations instituted child nutrition programs have focused on provision of health and water and sanitation services during this period [10,23].
Few studies have analyzed the dynamic association between mother’s HIV status and child undernutrition over time on the national level [4,7,24]. Therefore, this study examines this relationship in Zimbabwe between 2005 and 2015 to capture changes over time in a period of increased implementation of policies to reduce MTCT, expansion of CD4 testing and ART, and increased action to address food and nutrition security in the country.
Methods
We analyzed data from the Zimbabwe Demographic & Health Survey (DHS) rounds in 2005-2006, 2010-2011, and 2015 [11,20]. DHS Surveys provide nationally representative household-level surveys of health and nutrition variables of interest, including HIV biomarker modules for household members. These surveys are cross-sectional, and the same respondents are not surveyed in each round. Based on DHS’s sampling method using a stratified two-stage cluster design, we assume independence of household selection across rounds. We linked the Standard DHS Surveys to HIV/Other Biomarkers Datasets by case identifiers to capture the relationship between mother’s HIV status and child growth. Several UN studies of HIV-related mortality in Zimbabwe on the national level use DHS surveys to validate their models, which supports use of the DHS as a generalizable data source [25,26].
Datasets were restricted to children whose mothers consented to HIV testing. According to DHS documentation, children under the age of four were linked to the mother dataset, and the median child age in each survey round was 2 years. Our target exposure was the mother’s HIV-positive status from the HIV/Other Biomarkers dataset. This variable captures mother’s HIV status at the time of the survey, but not necessarily at the time of the child’s birth. Collection of blood samples for HIV biomarker testing was conducted during household surveys, and specimens were transported to the National Microbiology Reference Laboratory (NMRL) in Harare, Zimbabwe, for analysis [20].
We evaluated anthropometric outcomes among children whose mothers were living with HIV at the time of the survey. We ascertained our outcome, prevalence of anthropometric measures of undernutrition among children, using three binary metrics: stunting, underweight, and wasting. Each measure was defined as more than two standard deviations below the 2007 WHO Child Growth Standards (CGS) median height-for-age (stunting), weight-for-age (underweight), and weight-for-height (wasting) for the reference population [27].
Statistical Analysis
Survey-weighted multivariable logistic regression
Prevalence odds ratios for each outcome by mother’s HIV status and survey year were calculated using survey-weighted multivariable logistic regression. We estimated prevalence odds ratios for stunting, underweight, and wasting status by mother’s HIV status in three main effects regression models. We used a directed acyclic graph (DAG) to select confounders identified in a literature review (Figure 1). We controlled for the following confounders: survey year, mother’s age, mother’s BMI, mother’s height to account for genetic or ethnic variation in children’s height, household wealth index, and number of living children (Figure 1). Moreover, we incorporated child’s sex and age as precision variables in the models. We did not control for the following mediators identified in Figure 1: child birthweight, any vaccination of the child, feeding nutritious food to the child, comorbidities, and currently breastfeeding. We omitted mother’s anemia level (32% missing), and any tobacco use of the mother, as fewer than 30 women reported smoking in all rounds of the survey.
Figure 1.
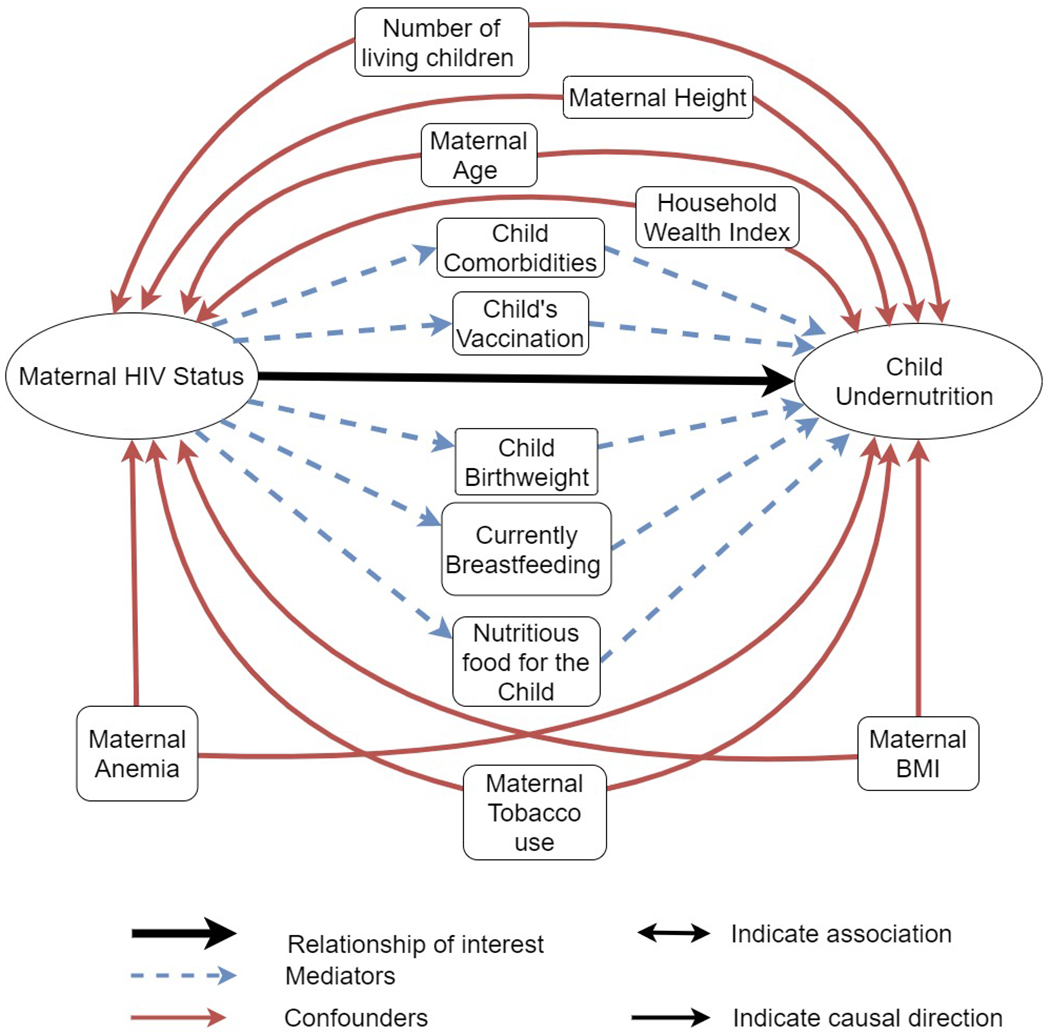
Direct Acyclic Graph of the association between Mother’s HIV status and Child Undernutrition
Blue arrows indicate mediators and red arrows indicate confounders accounted for in the association between mother’s HIV status and child undernutrition. Black arrows indicate the association of interest. Bidirectional arrows indicate associations, while unidirectional indicate the direction of causation.
We also assessed the modification of the association between mother’s HIV status and child growth outcomes by year of the survey [24]. Furthermore, we tested three-way interactions of HIV status with child’s sex (maternal HIV status by year by child’s sex) and child’s age (maternal HIV status by year by child’s age) to see if the relationship between mother’s HIV status and child anthropometric outcomes varied over time and by child age or sex. These logistic regression models are found in the Appendix.
Using the margins command in Stata [28], we estimated prevalence differences (with 95% CI) for each outcome by mother’s HIV positive status from the main effects logistic regression models. Data cleaning and analyses were done in Stata 15.1 (StataCorp.2017). We applied DHS primary sampling unit (clusters), strata, and sampling weights using the svy suite of commands in Stata 15.1. All hypothesis tests were conducted with a significance level of 0.05.
Results
Description of survey participants by year
DHS survey respondents increased in each survey round in terms of number of eligible households, mothers, and children (Table 1). In this study, mothers living with HIV made up 22%, 16% and 15% of the women who agreed to HIV testing in the 2005-2006, 2010-2011, and 2015 rounds of the DHS survey, respectively. Access to preventive medical care in terms of child vaccination increased from 75% vaccinated children in 2005-2006, to 88% in 2010-2011, and 92% in 2015. The percentage of children who experienced a recent illness was much greater in 2015 (51%) compared to 2005-2006 (31%) and 2010-2011 (35%). Parity, defined as the number of living children, was lower in 2010-2011 compared to the other two surveys with an average of 2.6 children per mother in 2010-2011 compared to 2.7 in both 2005-2006 and 2015.
Table 1.
Baseline characteristics of the samples by DHS survey
| DHS Year | 2005-06 | 2010-11 | 2015-16 |
|---|---|---|---|
| Mean or Prevalence (95% CI) | Mean or Prevalence (95% CI) | Mean or Prevalence (95% CI) | |
| Child Characteristics | |||
| Number of children | 4,496 | 5,563 | 5,577 |
| Male | 0.51 (0.48-0.53) | 0.50 (0.48-0.51) | 0.49 (0.48-0.50) |
| Age (years) | 1.95 (1.9-2.01) | 1.80 (1.76-1.83) | 2.02 (1.98-2.07) |
| Recent illness | 0.31 (0.29-0.34) | 0.35 (0.33-0.37) | 0.51 (0.49-0.53) |
| Any vaccinations | 0.75 (0.72-0.77) | 0.88 (0.87-0.90) | 0.92 (0.91-0.94) |
| Mother Characteristics | |||
| Number of mothers | 3,495 | 4,397 | 4,437 |
| Age (years) | 27.5 (27.3-27.7) | 27.4 (27.2-27.6) | 28.6 (28.4-28.9) |
| BMI | 22.7 (22.5-23) | 23.5 (23.3-23.7) | 24.4 (24.2-24.6) |
| HIV-positive | 0.22 (0.20-0.25) | 0.16 (0.15-0.18) | 0.15 (0.14-0.17) |
| Number of living children | 2.71 (2.63-2.8) | 2.56 (2.5-2.63) | 2.74 (2.68-2.8) |
| Any tobacco use | 0.004 (0.002-0.006) | 0.004 (0.002-0.006) | 0.002 (0.001-0.005) |
| Height (cm) | 159.8 (159.5-160.1) | 159.8 (159.5-160.1) | 160.3 (160.1-160.6) |
| Currently Breastfeeding | 0.47 (0.45-0.49) | 0.50 (0.48-0.52) | 0.41 (0.39-0.43) |
| Household Characteristics | |||
| Number of households in DHS Round | 3,232 | 4,130 | 4,147 |
| Household Wealth Index* | |||
| Quintile 1 - Poorest | 0.25 (0.20-0.30) | 0.25 (0.22-0.28) | 0.23 (0.20-0.26) |
| Quintile 2 - Poorer | 0.21 (0.19-0.24) | 0.22 (0.21-0.25) | 0.19 (0.17-0.21) |
| Quintile 3 - Middle | 0.17 (0.15-0.20) | 0.20 (0.18-0.22) | 0.17 (0.15-0.19) |
| Quintile 4 - Wealthier | 0.21 (0.18-0.24) | 0.20 (0.17-0.22) | 0.24 (0.21-0.28) |
| Quintile 5 - Wealthiest | 0.16 (0.13-0.19) | 0.13 (0.11-0.15) | 0.17 (0.14-0.20) |
All statistics are survey-weighted;
Wealth quintile percentiles applied population weights.
Round-Specific Prevalence
The prevalence of child stunting, underweight and wasting for each survey round are displayed in Figure 2, disaggregated by child sex and maternal HIV status. In 2005-2006, the prevalence of child stunting was 0.34 (95% CI: 0.31-0.38) among children whose mothers were living with HIV, and 0.28 (95% CI 0.26-0.31) among those whose mothers were living without HIV. In 2010-2011, stunting prevalence was 0.31 (95% CI: 0.28-0.36) among children whose mothers were living with HIV, and 0.25 (95% CI: 0.23-0.27) among those with mothers living without HIV. In 2015, the prevalence of child stunting further decreased to 0.25 (95% CI: 0.21-0.30) among children whose mothers were living with HIV and 0.20 (95% CI: 0.19-0.22) among those with mothers living without HIV. Prevalence estimates of child underweight and wasting are shown in Figure 2 and in Appendix Table 1.
Figure 2a-2c:
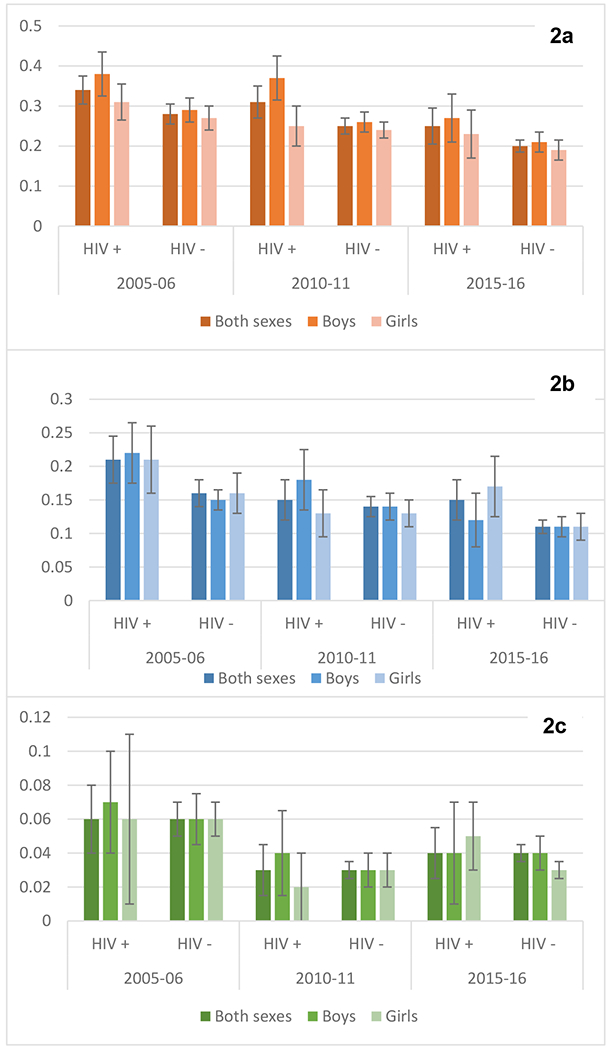
Adjusted Prevalence of Outcomes by Mother’s HIV Status
Children’s Outcome Prevalence and 95% CI by Mothers’ HIV Status: Stunting Prevalence (2a), Underweight Prevalence (2b), and Wasting Prevalence (2c). Adjusted for: mother’s HIV status, survey year, child sex, child age, mother’s BMI, mother stunting, household wealth index, mother’s age, and parity.
Multivariable logistic regression results
Results of the main effect logistic regression model indicate 32% greater odds of stunting among children whose mothers were living with HIV compared to children whose mothers were living without HIV (OR = 1.32, 95% CI 1.16 to 1.5). The second model testing for effect modification by survey year did not find a statistically significant difference in stunting over time (interaction of maternal HIV status*year, joint Wald test p=0.808). Models testing effect modification of mother’s HIV status and stunting by year and child’s sex or by year and child’s age were also not statistically significant (see Appendix Table 2).
In terms of underweight status, children of mothers with HIV had 27% greater odds of underweight compared to children of mothers without HIV (OR= 1.27, 95% CI 1.1 to 1.48). Effect modification of mother’s HIV status and child underweight status by year was not supported by the inclusion of an interaction term (interaction of maternal HIV status*year, joint Wald test p=0.24). For child wasting status, the effect of maternal HIV status was much smaller and not as precise as on other outcomes in the main effect model (OR = 1.07, 95% CI 0.81 to 1.42). There was insufficient evidence of effect modification of maternal HIV status and child wasting status by year (interaction of maternal HIV status*year, joint Wald test p= 0.94). Three-way interactions (HIV status*year*child’s sex and HIV status*year*child’s age) for underweight and wasting status were not statistically significant (see Appendix Table 2).
Prevalence Differences within Study Rounds
We estimated prevalence differences (PD) for each outcome by mother’s HIV status, using Stata’s margins command. Children of mothers living with HIV were more likely to be stunted than children of mothers without HIV (PD=0.05, 95% CI 0.03 to 0.08)(Figure 3a). Moreover, children of mothers living with HIV were more likely to be underweight than children of mothers without HIV (PD=0.03, 95% CI 0.01 to 0.05) (Figure 3b). There were not significant differences in the prevalence of wasting between children whose mothers were living with or without HIV after standardization (PD=0.003, 95% CI −0.009 to 0.01) (Figure 3c).
Figure 3a-3c:
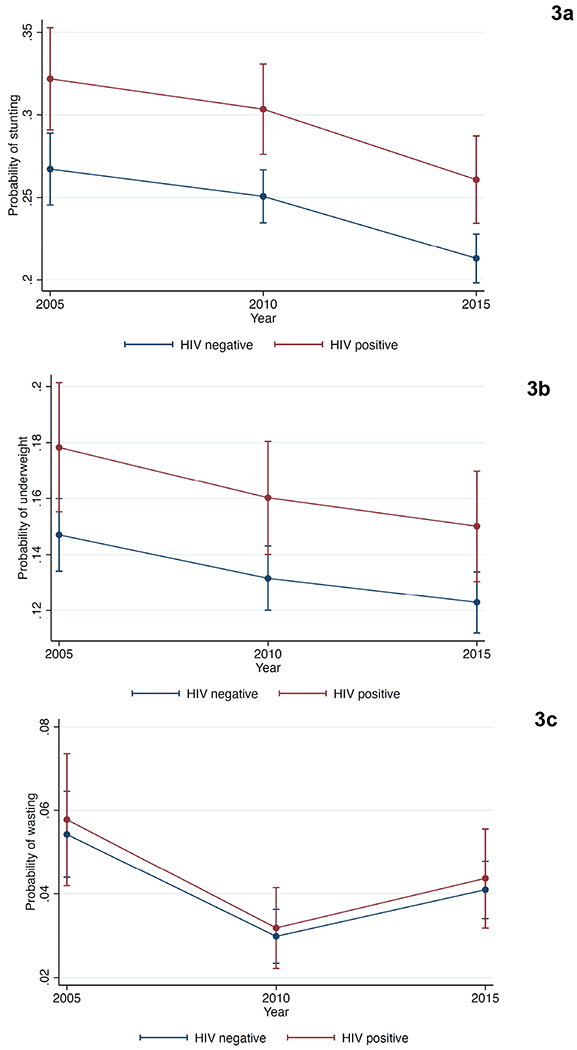
Adjusted Prevalence of Outcomes by Mother’s HIV Status
Trends in the adjusted prevalence of stunted children (3a), underweight children (3b), and children with wasting(3c) according to mother’s HIV status.
Discussion
Maternal HIV status is an important risk factor for undernutrition and child development, both by the social and food security realities of having a parent with HIV as well as the biological relationship between HIV exposure and growth restrictions among exposed children [29]. Our work builds upon research carried out by Omoni et al., who found an association between stunting and HIV exposure for children in Zimbabwe among 14,110 mother-child pairs enrolled in the ZVITAMBO vitamin A supplementation and co-trimoxazole trial between 1997 and 2007 [31]. They found children with HIV had higher odds of stunting than children without HIV, and children exposed to HIV but uninfected had higher odds of stunting compared with children unexposed to HIV [31]. We see the same patterns emerge among children of mothers living with HIV in the DHS data, which were robust over time despite an overall decline in stunting. Our findings did not show a gender disparity in growth outcomes that were found in the ZVITAMBO trial, which could be due to unmeasured confounding or differences in study populations; the ZVITAMBO population was drawn from Harare, the capital city, whereas the DHS is nationally representative.
Our study also confirms findings from the Development of AntiRetroviral Therapy in Africa (DART) trial in Uganda and Zimbabwe [24]. In this trial, women taking Tenofovir Disoproxil Fumarate were monitored before and throughout pregnancy to test management strategies for monitoring ART, and their children were followed up in a separate longitudinal study. Children born to mothers living with HIV had lower than average weight and height at birth, and greater stunting compared to national averages, while infants born to uninfected mothers had comparable growth measures to the general population (mean weight, mid-upper-arm circumference (MUAC), and head circumference) [24]. Our findings also indicated greater odds of stunting among children of mothers living with HIV compared to children of mothers living without HIV on the national level, and our study further builds on their work by assessing the change in these associations over time.
While this analysis makes a valid contribution, it has its limitations. Due to DHS sampling methods, we were unable to model the data hierarchically by cluster and year to follow the same individuals over time. Furthermore, some potentially important variables, such as maternal anemia, were excluded from our analysis due to missingness. Missingness of outcome measures may also be a source of bias, as 21.4% of all children surveyed did not have anthropometric measurements taken. However, we assume missingness does not vary by exposure status because sampling of women tested for HIV was independent of child health outcomes. There is also potential for survivor bias because only children who survived up to the time of the DHS survey were included. Orphans would not have been included because the DHS surveys mothers and their living children. However, our study complements the rich body of literature on the role of maternal HIV-status and mortality in Zimbabwe by analyzing anthropometric outcomes among survivors [2,30,32,33].
Another primary limitation is that the HIV-status of the child was not ascertainable for all years. HIV test results were collected for women starting at age 15 in 2005-2006 and 2010-2011 samples, while results were reported for women and their children in the 2015 sample [20]. In 2015, 4,274 children from mothers tested for HIV also received testing for HIV. Of these children, 1.1% tested positive for HIV, and only 6.4% of children whose mothers tested positive for HIV were infected. Moreover, antenatal care visits are one of the principal opportunities for HIV testing in Zimbabwe; in 2007, 71% of pregnant women who tested positive for HIV received their results as part of antenatal care, and by 2015, 99% of all women tested positive as part of the national PMTCT program [10]. For the 2015 round, we can assume that a good proportion of children of mothers with HIV were HIV-exposed but uninfected (HEU) at the time of the survey. Thus, other socio-economic factors may be as important in the association between maternal HIV status and a child’s anthropometric outcomes as the child’s HIV positive status.
The long-term effects of HIV infection or HIV exposure on children is an important area for future research, given the improved survival rates of people living with HIV. This research could inform targeting of nutrition programs toward families impacted by HIV/AIDS and could be a metric of impact for current HIV programming. Food insecurity and HIV/AIDS can be a vicious cycle, as food insecurity may result in reduced baseline CD4 cell count, incomplete virologic suppression, decreased survival, and interruption of ART [35]; this is a particular concern for pregnant and postpartum women due to the increased risk of MTCT and other impacts on children’s health [36]. Unfortunately, our findings indicate that changes in guidelines did not overcome the effect of maternal HIV status on child growth outcomes in the period of our analysis, posing a significant challenge for policymakers and researchers.
Conclusion
These results confirm that the relationship between maternal HIV-positive status and child undernutrition persisted between 2005 and 2015 in Zimbabwe. Maternal HIV-positivity was most significantly related to stunting. This evidence should inform health and food security program implementation in Zimbabwe and other Sub-Saharan African countries by focusing on mothers with HIV. Despite the implementation of guidelines establishing ART access to increase breastfeeding and prevent MTCT, we still see an effect of maternal HIV status on child anthropometric outcomes. More work is needed to support the health of children impacted by HIV. Future research using prospective cohort designs could test the impact of health programming on anthropometric outcomes on the national level to address this persistent public health problem.
Supplementary Material
Appendix - Table 1: *Prevalences are survey-weighted proportions.
Appendix – Table 2: POR = Prevalence Odds Ratio; 1Child age in years; 2mother’s height in centimeters; 3mother’s age in year; 4Parity is defined as the number of living children.
Table 2.
Prevalence Odds Ratios (POR) and 95% CI of Children Stunted, Underweight, and Wasted by Mother’s HIV Status from 2005-2015
| Stunting | Underweight | Wasting | ||||
|---|---|---|---|---|---|---|
| Covariates | Main Effects Model | Interaction Model | Main Effects Model | Interaction Model | Main Effects Model | Interaction Model |
| POR (95% CI) | POR (95% CI) | POR (95% CI) | POR (95% CI) | POR (95% CI) | POR (95% CI) | |
| Mother HIV positive | 1.32 (1.16, 1.51) | 1.36 (1.12, 1.64) | 1.27 (1.1, 1.48) | 1.48 (1.19, 1.83) | 1.07 (0.81, 1.42) | 1.11 (0.70, 1.76) |
| Year (ref =2005) | ||||||
| 2010 | 0.91 (0.79, 1.05) | 0.91 (0.78, 1.07) | 0.87 (0.76, 1.01) | 0.92 (0.79, 1.08) | 0.53 (0.40, 0.71) | 0.54 (0.39, 0.75) |
| 2015 | 0.73 (0.63, 0.84) | 0.74 (0.64, 0.87) | 0.81 (0.7, 0.93) | 0.84 (0.72, 0.99) | 0.74 (0.57, 0.96) | 0.75 (0.56, 1.00) |
| HIV Status * Year (ref =2005) | ||||||
| 2010 | - | 1.01 (0.76, 1.34) | - | 0.76 (0.54, 1.06) | - | 0.88 (0.43, 1.83) |
| 2015 | - | 0.91 (0.66, 1.25) | - | 0.82 (0.57, 1.17) | - | 0.97 (0.50, 1.88) |
| Child sex (ref=male) | 0.86 (0.78, 0.95) | 0.86 (0.78, 0.95) | 0.96 (0.84, 1.08) | 0.96 (0.84, 1.08) | 0.93 (0.75, 1.15) | 0.93 (0.75, 1.15) |
| Child age1 | 1.12 (1.09, 1.16) | 1.12 (1.09, 1.16) | 1.04 (0.99, 1.08) | 1.04 (0.99, 1.08) | 0.81 (0.75, 0.86) | 0.80 (0.75, 0.86) |
| Mother’s BMI | 0.95 (0.93, 0.96) | 0.95 (0.93, 0.96) | 0.89 (0.87, 0.91) | 0.89 (0.87, 0.91) | 0.90 (0.87, 0.93) | 0.90 (0.87, 0.93) |
| Mother’s height2 | 0.93 (0.92, 0.94) | 0.93 (0.92, 0.94) | 0.94 (0.93, 0.95) | 0.94 (0.93, 0.95) | 0.99 (0.98, 1.01) | 0.99 (0.98, 1.01) |
| HH Wealth Index (ref = Poorest) | ||||||
| Poorer | 0.96 (0.82, 1.13) | 0.96 (0.82, 1.13) | 1.05 (0.87, 1.28) | 1.05 (0.87, 1.27) | 0.93 (0.69, 1.24) | 0.92 (0.69, 1.24) |
| Middle | 0.98 (0.82, 1.17) | 0.98 (0.82, 1.16) | 0.84 (0.69, 1.03) | 0.84 (0.69, 1.02) | 0.92 (0.64, 1.30) | 0.91 (0.65, 1.30) |
| Wealthier | 0.88 (0.74, 1.04) | 0.88 (0.74, 1.04) | 0.89 (0.73, 1.08) | 0.89 (0.73, 1.08) | 1.00 (0.72, 1.40) | 1.00 (0.72, 1.40) |
| Wealthiest | 0.74 (0.6, 0.9) | 0.73 (0.6, 0.9) | 0.7 (0.54, 0.9) | 0.69 (0.54, 0.9) | 0.63 (0.40, 0.97) | 0.63 (0.40, 0.97) |
| Mother’s age3 | 0.99 (0.98, 1.00) | 0.99 (0.98, 1.00) | 1.00 (0.98, 1.01) | 1.00 (0.98, 1.01) | 1.00 (0.97, 1.03) | 1.00 (0.97, 1.03) |
| Parity4 | 1.08 (1.02, 1.14) | 1.08 (1.02, 1.14) | 1.09 (1.02, 1.16) | 1.09 (1.03, 1.16) | 1.00 (0.91, 1.11) | 1.00 (0.91, 1.11) |
POR = Prevalence Odds Ratio;
Child age in years;
mother’s height in centimeters;
mother’s age in year;
Parity is defined as the number of living children.
Acknowledgements
E.A.G drafted the manuscript. E.A.G., R.J.V.C., and A.M.S. participated in study design implementation, and statistical analysis, and results interpretation. S.E.S. and S.C. provided guidance on methods and analysis. All authors edited and approved the final manuscript.
Funding: The authors gratefully acknowledge support from the Minnesota Population Center (P2C HD041023) funded through a grant from the Eunice Kennedy Shriver National Institute for Child Health and Human Development (NICHD). This research was also supported by the United States Fogarty International Center (K01TW010268, R25TW009345) and the National Institutes of Health’s National Center for Advancing Translational Sciences (TL1TR002493, UL1TR002494).
Footnotes
Conflicts of interest: There are no conflicts of interest.
Meetings Presented: Population Association of America, Austin, Texas, April 10-13, 2019.
Supplementary Digital Content: Appendix.doc
References:
- 1.UNAIDS. Zimbabwe Overview. UNAIDS; 2020. https://www.unaids.org/en/regionscountries/countries/zimbabwe#:~:text=In Zimbabwe in 2018%3A,49 years) was 12.7%25. (accessed 27 Sep2020). [Google Scholar]
- 2.Marinda E, Humphrey JH, Iliff PJ, Mutasa K, Nathoo KJ, Piwoz EG, et al. Child Mortality According to Maternal and Infant HIV Status in Zimbabwe. 2007; 26:519–526. [DOI] [PubMed] [Google Scholar]
- 3.Mason JB, Chotard S, Bailes A, Mebrahtu S, Hailey P. Impact of Drought and HIV on Child Nutrition in Eastern and Southern Africa. Food Nutr Bull 2010; 31:S209–S218. [DOI] [PubMed] [Google Scholar]
- 4.Prendergast AJ, Rukobo S, Chasekwa B, Mutasa K, Ntozini R, Mbuya MNN, et al. Stunting Is Characterized by Chronic Inflammation in Zimbabwean Infants. 2014; 9. doi: 10.1371/journal.pone.0086928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.HHS Panel on Treatment of Pregnant Women with HIV Infection and Prevention of Perinatal Transmission. Recommendations for the Use of Antiretroviral Drugs in Pregnant Women with HIV Infection and Interventions to Reduce Perinatal HIV Transmission in the United States. AIDSinfo. 2020. [Google Scholar]
- 6.Chi BH, Stringer JSA, Moodley D. Antiretroviral Drug Regimens to Prevent Mother-To-Child Transmission of HIV: A Review of Scientific, Program, and Policy Advances for Sub-Saharan Africa. Curr HIV/AIDS Rep 2013; 10:124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muhangi L, Lule SA, Mpairwe H, Ndibazza J, Kizza M, Nampijja M, et al. Maternal HIV infection and other factors associated with growth outcomes of HIV-uninfected infants in Entebbe, Uganda. Public Health Nutr 2013; 16:1548–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Na M, Shamim AA, Mehra S, Labrique A, Ali H, Wu L, et al. Maternal nutritional status mediates the linkage between household food insecurity and mid-infancy size in rural Bangladesh. Br J Nutr 2020; 53:1–29. [DOI] [PubMed] [Google Scholar]
- 9.Piwoz EG, Bentley ME. Women’s Voices, Women’s Choices: The Challenge of Nutrition and HIV/AIDS. https://watermark.silverchair.com/z4w00405000933.pdf?token=AQECAHi208BE49Ooan9kkhW_Ercy7Dm3ZL_9Cf3qfKAc485ysgAAAaswggGnBgkqhkiG9w0BBwagggGYMIIBlAIBADCCAY0GCSqGSIb3DQEHATAeBglghkgBZQMEAS4wEQQMDAVDkzFDh6JHnHXhAgEQgIIBXjgLiqg_W5Fo6mWzZmgZPB0bsUA_kixIEXWe6yGf (accessed 10 Feb2018). [DOI] [PubMed]
- 10.UNAIDS. Global AIDS Response Progress Report 2016: Zimbabwe Contry Report. ; 2016. http://www.unaids.org/sites/default/files/country/documents/ZWE_narrative_report_2016.pdf [Google Scholar]
- 11.Zimbabwe National Statistics Agency - ZIMSTAT - and ICF International. Zimbabwe Demographic and Health Survey 2010-11. Calverton, Maryland: ; 2012. [Google Scholar]
- 12.Ministry of Health & Child Welfare. Zimbabwe National Guidelines on Hiv Testing and Counselling. 2005. https://www.who.int/hiv/topics/vct/ZIM_HIVTesting Guidelines Oct2005.pdf [Google Scholar]
- 13.Buzdugan R, McCoy SI, Watadzaushe C, Kang Dufour M-S, Petersen M, Dirawo J, et al. Evaluating the Impact of Zimbabwe’s Prevention of Mother-to-Child HIV Transmission Program: Population-Level Estimates of HIV-Free Infant Survival Pre-Option A. PLoS One 2015; 10:e0134571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization. Programmatic Update Use of Antiretroviral Drugs for Treating Pregnant Women and Preventing Hiv Infection in Infants Executive Summary. World Heal Organ 2012; 45:5. [Google Scholar]
- 15.Id BK, Id SS, Takarinda KC, Mukungunugwa H, Mugurungi O, Chonzi P, et al. Identifying high or low risk of mother to child transmission of HIV : How Harare City, Zimbabwe is doing? 2019; 32:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ford D, Muzambi M, Nkhata MJ, Abongomera G, Joseph S, Ndlovu M, et al. Implementation of antiretroviral therapy for life in pregnant/breastfeeding HIV+ women (option B+) alongside rollout and changing guidelines for ART initiation in Rural Zimbabwe: The lablite project experience. J Acquir Immune Defic Syndr 2017; 74:508–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization. The use of anti retro-viral drugs for treatment and prevention of HIV infection. 2013; :176–180. [Google Scholar]
- 18.Joint United Nations Programme on HIV/AIDS. UNAIDS Data 2019. Geneva, Switzerland: ; 2019. [Google Scholar]
- 19.Ndaimani A, Chitsike I, Haruzivishe C, Stray-Pedersen B, Ndaimani H. Mother to child transmission of HIV and its option B+ cascade predictors: An ecological study. Ann Trop Med Public Heal 2018; 11:87. [Google Scholar]
- 20.Zimbabwe National Statistics Agency - ZIMSTAT - and ICF International. Zimbabwe Demographic and Health Survey 2015 - Final Report. Rockville, Maryland: ; 2016. [Google Scholar]
- 21.Church JA, Rukobo S, Govha M, Carmolli MP, Diehl SA, Chasekwa B, et al. Immune responses to oral poliovirus vaccine in HIV-exposed uninfected Zimbabwean infants. 2017; 5515. doi: 10.1080/21645515.2017.1359454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahomed K, Makuyana D, Moyo S, Mbidzo M, Tswana S. Seroprevalence of HIV infection amongst antenatal women in greater Harare, Zirrtbabwe. J Cent African Med 1991; 37:322–325. [PubMed] [Google Scholar]
- 23.Mason JB, Bailes A, Mason KE, Yambi O, Jonsson U, Hudspeth C, et al. AIDS, drought, and child malnutrition in southern Africa. doi: 10.1079/PHN2005726 [DOI] [PubMed] [Google Scholar]
- 24.Gibb DM, Kizito H, Russell EC, Chidziva E, Zalwango E, Nalumenya R, et al. Pregnancy and infant outcomes among HIV-infected women taking long-term art with and without tenofovir in the DART trial. PLoS Med 2012; 9. doi: 10.1371/journal.pmed.1001217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robertson L, Gregson S, Madanhire C, Walker N, Mushati P, Garnett G, et al. Discrepancies between UN models and DHS survey estimates of maternal orphan prevalence : insights from analyses of survey data from Zimbabwe. 2008; 84:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Place N, Grassly NC, Lewis JJC, Mahy M, Walker N. Comparison of household survey estimates with projections of mortality and orphan numbers in sub-Saharan Africa in the era of HIV / AIDS. [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization. WHO Global Database on Child Growth and Malnutrition - Zimbabwe. Glob. Database Child Growth Malnutrition. 2015. https://www.who.int/nutgrowthdb/database/countries/who_standards/zwe_dat.pdf?ua=1 [Google Scholar]
- 28.Williams R Using the margins command to estimate and interpret adjusted predictions and marginal effects. Stata J 2012; 12:308–331. [Google Scholar]
- 29.Blaney S, Beaudry M, Latham M. Determinants of undernutrition in rural communities of a protected area in Gabon. Public Health Nutr 2009; 12:1711–1725. [DOI] [PubMed] [Google Scholar]
- 30.Prendergast A, Bwakura-dangarembizi MF, Cook AD, Bakeera-kitaka S, Natukunda E, Nahirya P, et al. Hospitalization for severe malnutrition among HIV-infected children starting antiretroviral therapy. 2011; :951–956. [DOI] [PubMed] [Google Scholar]
- 31.Omoni AO, Ntozini R, Evans C, Bch MB, Prendergast AJ, Moulton LH, et al. Child Growth According to Maternal and Child HIV Status in Zimbabwe. 2017; 36:869–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kurewa EN, Gumbo FZ, Munjoma MW, Mapingure MP, Chirenje MZ, Rusakaniko S. Effect of maternal HIV status on infant mortality : evidence from a 9-month follow-up of mothers and their infants in Zimbabwe. 2010; :88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schultz K, Chapman G, Hansen KS, Jelsma J, Ndhlovu C. Københavns Universitet The burden of disease in Zimbabwe in 1997 as measured by disability-adjusted life years lost. 2020; 11:660–671. [DOI] [PubMed] [Google Scholar]
- 34.Irigoyen C Tackling Malnutrition in Zimbabwe. ; 2017. https://www.centreforpublicimpact.org/case-study/tackling-malnutrition-zimbabwe/ [Google Scholar]
- 35.Anema A, Vogenthaler N, Frongillo EA, Kadiyala S, Weiser SD. Food insecurity and HIV/AIDS: Current knowledge, gaps, and research priorities. Curr HIV/AIDS Rep 2009; 6:224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mccoy SI, Buzdugan R, Mushavi A, Mahomva A, Cowan FM, Padian NS. Food insecurity is a barrier to prevention of mother-to-child HIV transmission services in Zimbabwe : a cross-sectional study. 2015; :1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix - Table 1: *Prevalences are survey-weighted proportions.
Appendix – Table 2: POR = Prevalence Odds Ratio; 1Child age in years; 2mother’s height in centimeters; 3mother’s age in year; 4Parity is defined as the number of living children.


