Abstract
BACKGROUND/OBJECTIVES:
APOL1 high-risk genotypes confer an increased risk for kidney disease, but their clinical significance among older adults remains unclear. We aimed to determine whether APOL1 genotype status (high risk = 2 risk alleles; low risk = 0–1 risk alleles) and self-reported race (Black; White) are associated with number of hospitalizations, incident chronic kidney disease (CKD), end-stage renal disease (ESRD), and mortality among older adults participating in a community-based cohort study.
DESIGN:
Observational longitudinal cohort study.
SETTING:
The Atherosclerosis Risk in Communities (ARIC) study.
PARTICIPANTS:
Community-dwelling older adults (mean age = 75.8 years; range = 66–90 years).
RESULTS:
Among 5,564 ARIC participants (78.2% White, 19.1% APOL1 low-risk Black, and 2.7% APOL1 high-risk Black), the proportion with creatinine and cystatin C–based estimated glomerular filtration rate (eGFRCrCys) below 60 mL/min/1.73 m2 at baseline was 40.6%, 34.8%, and 43.2%, respectively. Over a mean follow-up of 5.1 years, APOL1 high-risk Blacks had a 2.67-fold higher risk for ESRD compared with low-risk Blacks (95% confidence interval [CI] = 1.05–6.79) in models adjusted for age and sex. This association was no longer significant upon further adjustment for baseline eGFRCrCys and albuminuria (hazard ratio [HR] = 1.08; 95% CI = .39–2.96). Rate of hospitalizations and risks of mortality and incident CKD did not differ significantly by APOL1 genotype status. Compared with Whites, Blacks had 1.85-fold and 3.45-fold higher risks for incident CKD and ESRD, respectively, in models adjusted for age, sex, eGFRCrCys, and albuminuria. These associations persisted after additional adjustments for clinical/socioeconomic factors and APOL1 genotype (incident CKD: HR = 1.38; 95% CI = 1.06–1.81; ESRD: HR = 3.20; 95% CI = 1.16–8.86).
CONCLUSION:
Among older Black adults, APOL1 high-risk genotypes were associated with lower kidney function and therefore higher risk of ESRD. Racial disparities in incident kidney disease persisted in older age and were not fully explained by APOL1.
Keywords: APOL1, apolipoprotein L1, end-stage renal disease, chronic kidney disease, mortality
Race and age differences in the progression of chronic kidney disease (CKD) have been well described.1–3 In the United States, the risk of incident end-stage renal disease (ESRD) is 2.9-fold higher in Blacks compared with Whites and 2.7-fold higher in individuals aged 75 and older compared with individuals aged 45 to 64.2 In Markov models using data from the National Health and Nutrition Examination Survey and the U.S. Renal Data System (USRDS), Blacks were shown to have a greater lifetime risk of advanced CKD (stage ≥4 or ESRD) compared with Whites, with this difference in risk more pronounced in older age.4 Given the high morbidity and mortality associated with ESRD, the complex interplay of race, aging, and kidney disease warrants further investigation.2
Traditionally, the excess burden of kidney disease among Blacks was attributed to inequalities in social determinants of health; however, more recent research suggests that genetic factors likely also play a role.3,5–7 The APOL1 gene encodes for apolipoprotein L1, a protein that confers protection against African sleeping sickness but at the cost of an increased risk for kidney disease. Studies to date have demonstrated that the APOL1 high-risk genotypes, present in 13% of Black Americans, are associated with the development and/or progression of focal segmental glomerulosclerosis, HIV-associated nephropathy, and hypertension-attributed CKD.8–11 These risk variants have also been associated with kidney disease in the general population.12,13 To date, few studies have examined the natural history of APOL1 risk variants in older age, and their results have been conflicting.14,15 Thus whether the APOL1 risk variants are associated with worse kidney and other adverse outcomes among individuals who survive to older age remains to be determined.
Using longitudinal data beginning at the fifth visit of the Atherosclerosis Risk in Communities (ARIC) study, in which the mean age of participants was 76 years, we aimed to study the clinical implications of the APOL1 risk variants and their impact on racial disparities in older age. We further evaluated whether the risk of ESRD and mortality associated with APOL1 high-risk genotypes over the full 30-year follow-up in the ARIC study varied with age.
METHODS
Study Population
ARIC is a prospective community-based cohort study of 15,792 individuals, ages 45 to 64 years, who were recruited from 1987 to 1989 from four field centers across the United States (Forsyth County, NC; Jackson, MS; Minneapolis, MN; and Washington County, MD).16 Since the baseline examination (Visit 1: 1987–1989), there have been five follow-up examinations: Visit 2 (1990–1992), Visit 3 (1993–1995), Visit 4 (1996–1998), Visit 5 (2011–2013), and Visit 6 (2016–2017). All study sites obtained approval from their institutional review board and informed consent from study participants. Our study population was derived from participants who attended Visit 5 (n = 6,538). We excluded individuals who did not self-report Black or White race (n = 18), did not have data on APOL1 genotyping (among Blacks only; n = 157), or were missing data on creatinine and cystatin C–based estimated glomerular filtration rate (eGFR) (eGFRCrCys, n = 97) or albuminuria (n = 702) at Visit 5, resulting in a final sample size of 5,564 participants (Supplementary Figure S1).
Genotyping of APOL1
As described in prior studies, TaqMan assays were used to directly genotype the APOL1 risk alleles, G1 and G2, from Black participants who had provided informed consent for genetic studies.12,13 The G1 allele is defined by missense mutations at rs73885319 and rs60910145 that are in near-absolute linkage disequilibrium. The G2 allele is defined by a six base pair deletion at rs71785313.8,9 Using a recessive genetic model, the APOL1 high-risk genotypes were specified by the presence of two risk alleles (G1/G1, G1/G2, or G2/G2), whereas the APOL1 low-risk genotypes were specified by the presence of one or no risk alleles (G1/G0, G2/G0, or G0/G0). Given that the frequency of the APOL1 risk variants is very low among individuals of European ancestry, White participants were considered as having no APOL1 risk variants.17,18
Ascertainment of Outcomes
Hospitalizations until December 31, 2017, were captured via active surveillance of ARIC community hospitals and self-report by patients during annual phone follow-ups. Mortality was ascertained through linkage with the National Death Index as well as active surveillance methods described previously.13,19,20 Cases of ESRD were identified through linkage with the USRDS national registry. Serum creatinine was measured using the Roche enzymatic method (Roche Diagnostics, Indianapolis, IN) on the Roche Modular P Chemistry Analyzer at Visit 5 and on the Roche Cobas 6,000 Chemistry Analyzer at Visit 6. Serum cystatin C was measured using the Gentian Cystatin C reagent (Gentian AS, Moss, Norway) on the Roche Modular P Chemistry Analyzer at Visit 5 and on the Roche Cobas 6,000 Chemistry analyzer at Visit 6.12,13 The GFR was estimated by the Chronic Kidney Disease Epidemiology Collaboration equation.21 Consistent with prior ARIC studies and clinical practice, incident CKD was defined among individuals with a baseline creatinine-based eGFR (eGFRCr) above 60 mL/min/1.73 m2 by (1) a more than 25% decline in eGFRCr from Visit 5 to a value below 60 mL/min/1.73 m2 at subsequent study visit; (2) a USRDS-identified ESRD event; or (3) a hospitalization or death with an International Classification of Diseases (ICD-9-CM/ICD-10) CKD diagnostic code of CKD.22
Measurement of Covariates
Blood pressure was measured in a standardized manner, as a series of three measurements following 5 minutes of rest. The average of the second and third measurements was used.13 Hypertension was defined by a systolic blood pressure of 140 mm Hg or above, diastolic blood pressure of 90 mm Hg or above, or use of medication for high blood pressure. Diabetes mellitus was defined by a fasting glucose value of 126 mg/dL or above, non-fasting glucose of 200 mg/dL or above, or use of medication for diabetes. Body mass index was calculated from height and weight, and waist-to-hip ratio was calculated from waist girth and hip girth measured at Visit 5. Coronary heart disease was defined as a self-reported history of myocardial infarction, coronary bypass surgery, or angioplasty of a coronary artery at Visit 1, myocardial infarction from adjudicated Visit 1 electrocardiogram data, or adjudicated events (definite or probable myocardial infarction, fatal coronary heart disease, cardiac procedure, or myocardial infarction on electrocardiogram) between Visit 1 and Visit 5. Total cholesterol, high-density lipoprotein, and triglycerides were measured using the Beckman Coulter Olympus AU400 analyzer. Low-density lipoprotein was calculated using the Friedewald formula. Albuminuria was estimated by the urine albumin-to creatinine ratio (ACR), with albumin measured from urine samples at Visit 5 using an immunoturbidometric method on the ProSpec nephelometric analyzer. Creatinine was measured from urine samples at Visit 5 using the Roche enzymatic method. Percentage of European ancestry was estimated based on approximately 1,350 ancestry informative markers and using ANCESTRYMAP.12
Statistical Analyses
Baseline characteristics at ARIC Visit 5 were compared by APOL1 genotype status (among Black participants only) and race (each APOL1 genotype status vs White as the reference group; among all participants) using a two-sample t test or Wilcoxon rank sum test for continuous variables and Pearson chi-square tests for categorical variables. We performed two sets of analyses: (1) among Black participants, with the primary exposure being APOL1 genotype status; and (2) among all participants, with the exposure of interest as self-reported race. Incident rate ratios (IRRs) for the number of hospitalizations during follow-up were estimated using negative binomial regression; hazard ratios (HRs) for times to all-cause mortality, incident CKD, and incident ESRD were estimated using Cox proportional hazards models. Participants were censored at death or last known status up to December 31, 2017. For each outcome, a series of models were constructed: Model 1 was unadjusted; Model 2 adjusted for sociodemographic factors including age and sex; Model 3a further adjusted for baseline eGFRCrCys; and Model 3b further adjusted for natural log-transformed urine ACR. We considered Model 3b as our primary model.
In additional analyses among Blacks with APOL1 genotype status as the exposure, Model 4 further adjusted for percentage of European ancestry. Multiple imputation, assuming a multivariate normal distribution and using an iterative Markov chain Monte Carlo method, was used to impute missing data on European ancestry (approximately 11% of Black participants). Among all participants with self-reported race as the exposure, Model 4 further adjusted for additional clinical factors including prevalent hypertension, diabetes mellitus, coronary heart disease, and heart failure; Model 5 further adjusted for socioeconomic factors including baseline income and education; and Model 6 further adjusted for APOL1 genotype status.
We also investigated whether associations of APOL1 risk status with incident ESRD and all-cause mortality were modified by age. For these analyses, we used all data from genotyped Black participants at Visit 1 (n = 3,757) and divided their follow-up time into five age strata (ie, ≥45 to <55 years, ≥55 to <65 years, ≥65 to <75 years, ≥75 to <85 years, and ≥ 85 to <95 years). As an example, an individual participating in ARIC from ages 50 through 82 would contribute 5 years to strata “≥45 to <55 years,” 10 years to strata “≥55 to <65 years” and “≥65 to <75 years,” and 7 years to strata “≥75 to <85 years.” Ages <45 and ≥ 95 years were excluded from these analyses given few observations in these age ranges. We then constructed Poisson models that included APOL1 risk status, the five age strata, sex, and interaction terms between APOL1 risk status and each age strata.
Analyses were performed using STATA v.15 (College Station, TX), and P values <.05 were considered to be statistically significant.
RESULTS
Baseline Characteristics
Our study population consisted of 5,564 individuals, of whom 78.2% were White, 19.1% were Black with the APOL1 low-risk genotypes, and 2.7% were Black with the APOL1 high-risk genotypes (Table 1). The mean age at baseline was 75.8 ± 5.3 years. Among Black participants, those with the APOL1 high-risk genotypes had a lower mean eGFRCrCys (61.9 vs 67.7 mL/min/1.73 m2; P = .001) and more albuminuria (median = 16.6 vs 10.8 mg/g Cr; P = .002) compared with those with the low-risk genotypes. Of note, prevalence of hypertension, coronary heart disease, atrial fibrillation/flutter, stroke, and heart failure did not differ significantly by APOL1 genotype status. However, several differences were observed when comparing Black participants (APOL1 high or low risk) with White participants including higher prevalence of hypertension, diabetes mellitus, and cardiovascular disease among Blacks. There was also substantial overlap in the distributions of baseline eGFRCrCys among the three APOL1/race risk groups (Supplementary Figure S2).
Table 1.
Baseline Characteristics of Study Population by APOL1 Risk Status and Race at ARIC Visit 5
| Characteristic | White n = 4,351 | Black APOL1 low risk n =1,065 | Black APOL1 high risk n = 148 |
|---|---|---|---|
| Age, y | 76.1 ± 5.3 | 75.1 ± 5.2a | 74.2 ± 4.8a |
| European ancestry, % | — | 18.1 ± 10.3 | 15.7 ± 7.4b |
| Female, n (%) | 2,332 (53.6) | 716 (67.2)a | 94 (63.5)a |
| Study center, n (%) | |||
| Forsyth County | 1,212 (27.9) | 65 (6.1)a | 7 (4.7)a,b |
| Jackson | 2 (.0) | 1,000 (93.9) | 140 (94.6) |
| Minneapolis | 1,622 (37.3) | 0(.0) | 1 (.7) |
| Washington County | 1,515 (34.8) | 0 (.0) | 0(.0) |
| Annual family income <$25,000, n (%) | 842 (21.2) | 530 (56.2)a | 81 (60.0)a |
| Non-high school graduate, n (%) | 435 (10.0) | 320 (30.1)a | 51 (34.5)a |
| Current smoking, n (%) | 222 (5.4) | 74 (7.3)a | 10 (7.0) |
| Hypertension, n (%) | 3,101 (72.1) | 927 (87.5)a | 130 (89.0)a |
| Diabetes mellitus, n (%) | 1,117 (26.4) | 405 (40.7)a | 61 (42.7)a |
| Systolic blood pressure, mm Hg | 130 ± 18 | 136 ± 20a | 135 ±19a |
| Diastolic blood pressure, mm Hg | 65 ± 11 | 70 ± 11a | 70 ± 10a |
| Body mass index, kg/m2 | 28.3 ± 5.2 | 30.6 ± 7.0a | 31.4 ± 7.8a |
| Waist-to-hip ratio | .95 ± .08 | .91 ± .08a | .91 ± .08a |
| Hemoglobin A1c, % | 5.9 ± .8 | 6.3 ± 1.1a | 6.4 ± 1.2a |
| Total cholesterol, mg/dL | 179 ± 43 | 183 ± 40a | 187 ± 45a |
| HDL cholesterol, mg/dL | 51 ± 14 | 53 ± 13a | 54 ± 14a |
| LDL cholesterol, mg/dL | 102 ± 35 | 109 ± 35a | 110 ± 36a |
| Triglycerides, mg/dL | 132 ± 68 | 108 ± 49a | 115 ± 53a |
| High sensitive C-reactive protein, mg/L | 1.9 (.9 to 4.0) | 2.7 (1.3 to 6.3)a | 3.1 (1.4 to 7.0)a |
| Serum creatinine, mg/dL | .98 ± .33 | 1.07 ± .49a | 1.26 ± .79a,b |
| Serum cystatin C, mg/L | 1.22 ± .39 | 1.19 ± .47a | 1.38 ± .75a,b |
| eGFRCrCys, ml/min/1.73 m2 | 63.9 ± 17.3 | 67.7 ± 20.3a | 61.9 ± 23.3b |
| eGFRCrCys <60 mL/min/1.73 m2, n (%) | 1,766 (40.6) | 371 (34.8)a | 64 (43.2)b |
| Urine ACR, mg/g Cr | 10.8 (6.5 to 22.2) | 10.8(6.1 to 29.1) | 16.6 (7.1 to 60.8)a,b |
| Urine ACR ≥30 mg/g Cr | 828 (19.0) | 258 (24.2)a | 51 (34.5)a,b |
| Prevalent CHD, n (%) | 752 (17.6) | 110 (10.5)a | 11 (7.4)a |
| Prevalent atrial fibrillation/flutter, n (%) | 383 (8.8) | 48 (4.5)a | 4 (2.7)a |
| Prevalent stroke, n (%) | 155 (3.6) | 59 (5.6)a | 11 (7.4)a |
| Prevalent heart failure, n (%) | 511 (11.7) | 230 (21.6)a | 29 (19.6)a |
Note: Values presented as mean ± standard deviation, number (%), or median (Q1 to Q3).
Number missing for the following variables: European ancestry (n = 133); hypertension (n = 58); diabetes mellitus (n = 190); prevalent CHD (n = 86); prevalent stroke (n = 8); systolic blood pressure (n = 21); diastolic blood pressure (n = 21); body mass index (n = 170); waist-to-hip ratio (n = 229); hemoglobin A1c (n = 50); high sensitive C-reactive protein (n = 18); total cholesterol (n = 16); triglycerides (n = 16); HDL (n = 16); LDL (n = 57); family income (n = 517); education (n = 10); current smoking (n = 296).
Abbreviations: ACR, albumin-to-creatinine ratio; ARIC, Atherosclerosis Risk in Communities study; CHD, coronary heart disease; Cr, creatinine; eGFRCrCys, estimated glomerular filtration rate based on serum creatinine and cystatin C; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
P < .05 for comparison with White subgroup.
P < .05 for comparison of APOL1 high risk versus low risk in Black subgroups.
Incident Events by APOL1 Risk Status
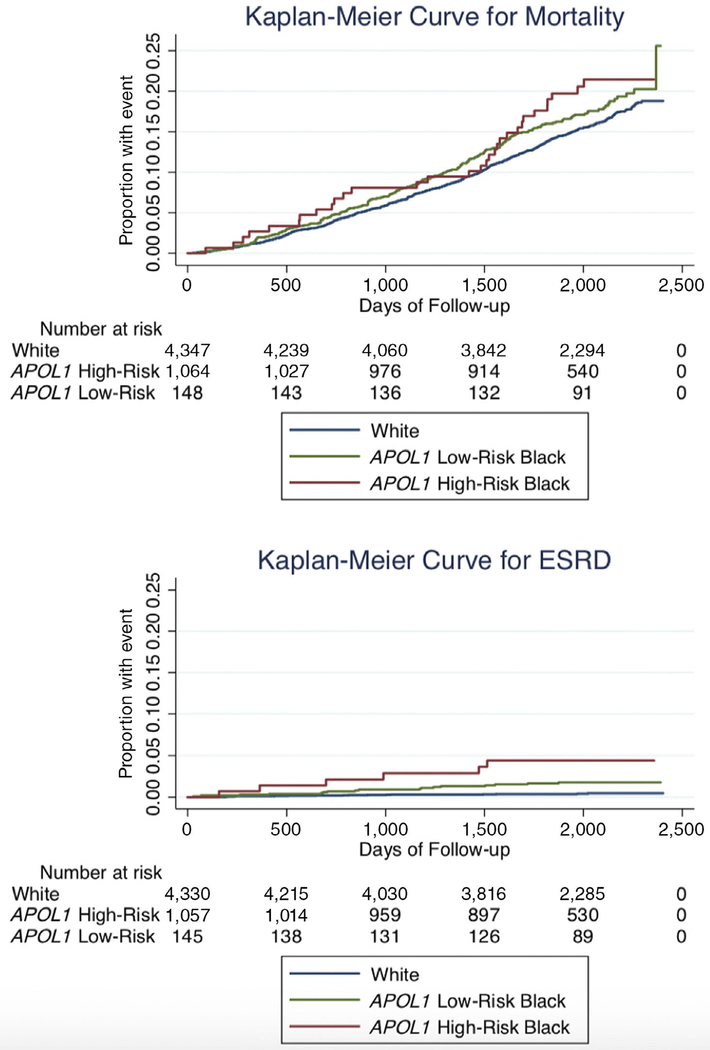
Over a mean follow-up of 5.1 years, there were 2,174 total hospitalizations, 122 incident CKD events, 23 incident ESRD events, and 220 deaths among Black ARIC Visit 5 participants with available APOL1 genotyping and kidney function measures (Supplementary Table S1). The number of hospitalizations per participant ranged from 0 to 23. In crude and adjusted models, risks of hospitalization, all-cause mortality, and incident CKD did not differ significantly when comparing APOL1 high- versus low-risk genotypes (Table 2 and Figure 1). For the outcome of incident ESRD, the APOL1 high-risk genotypes were associated with a 2.67-fold higher risk compared with the low-risk genotypes after adjusting for age and sex (Model 2: HR = 2.67; 95% confidence interval [CI] = 1.05–6.79; P = .04). When baseline measures of eGFRCrCys and log-transformed urine ACR were added to the model, this association was greatly attenuated and no longer statistically significant (Model 3b: HR = 1.08; 95% CI = .39–2.96; P = .88). Mean eGFRCrCys of APOL1 high-risk individuals at Visit 5 who developed ESRD was 23.4 mL/min/1.73 m2 (range = 10.6–35.2 mL/min/1.73 m2).
Table 2.
Associations of APOL1 Risk Status with Adverse Outcomes Among Black ARIC Visit 5 Participants
| IRR, APOL1 high risk vs low risk | 95% CI | P value | |
| No. of hospitalizations | |||
| No. of events | 2,174 | ||
| No. at risk | 1,212 | ||
| Model 1: Unadjusted | 1.21 | (.90–1.63) | .21 |
| Model 2: Adjusted for age and sex | 1.27 | (.94–1.71) | .12 |
| Model 3a: Further adjusted for baseline eGFRCrCys | 1.18 | (.83–1.66) | .36 |
| Model 3b: Further adjusted for | 1.07 | (.82–1.40) | .62 |
| log-transformed urine ACR | |||
| Model 4: Further adjusted for European ancestry | 1.04 | (.80–1.36) | .76 |
| HR, APOL1 high risk vs low risk | 95% CI | P value | |
| All-cause mortality | |||
| No. of events | 220 | ||
| No. at risk | 1,212 | ||
| Model 1: Unadjusted | 1.16 | (.79–1.69) | .46 |
| Model 2: Adjusted for age and sex | 1.24 | (.85–1.82) | .26 |
| Model 3a: Further adjusted for baseline eGFRCrCys | 1.04 | (.71–1.53) | .83 |
| Model 3b: Further adjusted for log-transformed urine ACR | 1.03 | (.70–1.51) | .90 |
| Model 4: Further adjusted for European ancestry | 1.02 | (.69–1.49) | .94 |
| Incident chronic kidney disease | |||
| No. of events | 122 | ||
| No. at risk | 858 | ||
| Model 1: Unadjusted | .68 | (.35–1.29) | .24 |
| Model 2: Adjusted for age and sex | .71 | (.37–1.35) | .30 |
| Model 3a: Further adjusted for baseline eGFRCrCys | .66 | (.34–1.26) | .21 |
| Model 3b: Further adjusted for log-transformed urine ACR | .67 | (.35–1.28) | .23 |
| Model 4: Further adjusted for European ancestry | .65 | (.34–1.24) | .19 |
| Incident end-stage renal disease | |||
| No. of events | 23 | ||
| No. at risk | 1,202 | ||
| Model 1: Unadjusted | 2.56 | (1.01–6.50) | .05 |
| Model 2: Adjusted for age and sex | 2.67 | (1.05–6.79) | .04 |
| Model 3a: Further adjusted for baseline eGFRCrCys | 1.28 | (.50–3.32) | .61 |
| Model 3b: Further adjusted for log-transformed urine ACR | 1.08 | (.39–2.96) | .88 |
| Model 4: Further adjusted for European ancestry | 1.18 | (.42–3.29) | .76 |
Abbreviations: ACR, albumin-to-creatinine ratio; ARIC, Atherosclerosis Risk in Communities study; CI, confidence interval; eGFRCrCys, estimated glomerular filtration rate based on serum creatinine and cystatin C; HR, hazard ratio; IRR, incident rate ratio.
Figure 1.
APOL1 high-risk genotypes and Black race were associated with end-stage renal disease (ESRD) but not mortality among older adults in unadjusted analyses.
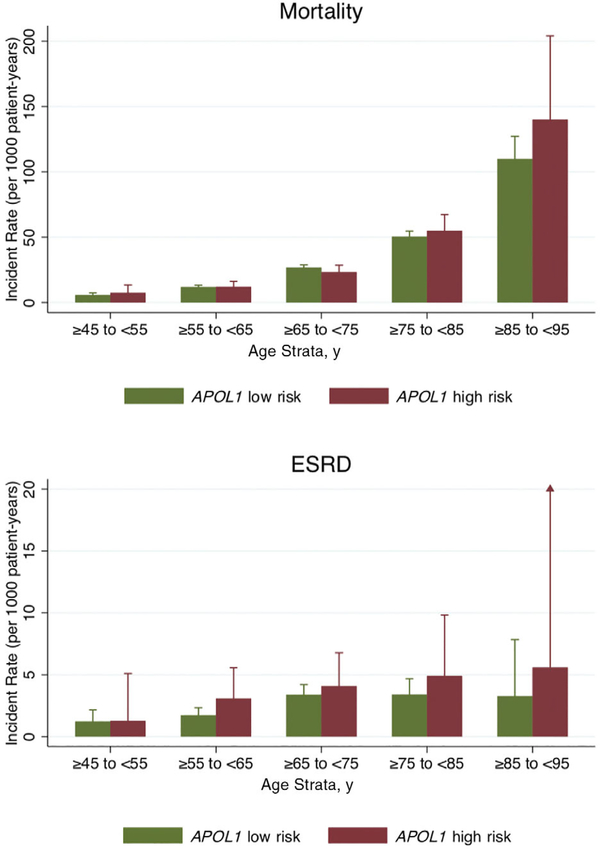
In analyses that included data from Visits 1 through 6, there were 218 ESRD events occurring over 82,118 person-years and 2,051 deaths over 83,553 person-years of follow-up time. The incidence of ESRD plateaued after age 65 to 75 years; mortality was highest in the oldest age stratum (85–95 years). There was no evidence of interaction between APOL1 risk genotypes and each age strata on rates of incident ESRD or all-cause mortality (P interaction >.05 for all; Figure 2).
Figure 2.
The incidence rates of end-stage renal disease (ESRD) but not mortality were consistently higher in Black individuals with APOL1 high-risk genotypes compared with those with APOL1 low-risk genotypes, irrespective of age strata (P for interaction >.05 for both outcomes). *P interaction >.05 for incident rate ratio comparing APOL1 high-risk versus low-risk groups within each age strata, adjusted for sex. Bars represent upper limit of 95% confidence interval. For mortality, n = 3,757 with 2,051 events. For ESRD, n = 3,746 with 218 events.
Incident Events by Race
Over a mean follow-up of 5.2 years, there were 8,953 total hospitalizations, 416 incident CKD events, 40 incident ESRD events, and 915 deaths among the total study population (Supplementary Table S2). The number of hospitalizations per participant ranged from 0 to 34. Rates of hospitalization were higher in Black versus White participants (Model 3b: IRR = 1.26; 95% CI = 1.13–1.40; P < .001). Black participants also had a 30% higher risk for death compared with White participants (Model 3b: HR = 1.30; 95% CI = 1.11–1.52; P = .001). Both of these associations persisted after adjustment for kidney function but attenuated and lost statistical significance after adjusting for additional clinical (prevalent hypertension, diabetes mellitus, coronary heart disease, and heart failure) and socioeconomic (income and education) factors (Table 3). Racial differences in the risk for adverse kidney outcomes were also observed. Compared with White participants, Black participants had 1.85-fold and 3.45-fold increased risks for incident CKD and ESRD, respectively. These associations persisted in additional models that accounted for clinical and socioeconomic factors as well as APOL1 genotype status (HR = 1.38; 95% CI = 1.06–1.81; P = .02 for incident CKD and HR = 3.20; 95% CI = 1.16–8.86; P = .03 for ESRD).
Table 3.
Associations of Race with Adverse Outcomes Among all ARIC Visit 5 Participants
| No. of events, | No. at risk, | IRR, Black vs White | 95% CI | P value | |
| No. of hospitalizations | |||||
| Model 1: Unadjusted | 8,953 | 5,556 | 1.23 | (1.10–1.38) | <.001 |
| Model 2: Adjusted for age and sex | 8,953 | 5,556 | 1.33 | (1.19–1.49) | <.001 |
| Model 3a: Further adjusted for baseline eGFRCrCys | 8,953 | 5,556 | 1.37 | (1.23–1.52) | <.001 |
| Model 3b: Further adjusted for log-transformed urine ACR | 8,953 | 5,556 | 1.26 | (1.13–1.40) | <.001 |
| Model 4: Further adjusted for prevalent hypertension, diabetes mellitus, coronary heart disease, and heart failure | 8,381 | 5,231 | 1.12 | (1.01–1.25) | .04 |
| Model 5: Further adjusted for baseline income and education | 7,607 | 4,782 | 1.00 | (.89–1.12) | 1.00 |
| Model 6: Further adjusted for APOL1 risk status | 7,607 | 4,782 | .98 | (.87–1.11) | .79 |
| No. of events | No. at risk | HR, Black vs White | 95% CI | P value | |
| All-cause mortality | |||||
| Model 1: Unadjusted | 915 | 5,559 | 1.16 | (1.00–1.35) | .06 |
| Model 2: Adjusted for age and sex | 915 | 5,559 | 1.38 | (1.18–1.61) | <.001 |
| Model 3a: Further adjusted for baseline eGFRCrCys | 915 | 5,559 | 1.44 | (1.24–1.68) | <.001 |
| Model 3b: Further adjusted for log-transformed urine ACR | 915 | 5,559 | 1.30 | (1.11–1.52) | .001 |
| Model 4: Further adjusted for prevalent hypertension, diabetes mellitus, coronary heart disease, and heart failure | 840 | 5,233 | 1.16 | (.97–1.37) | .10 |
| Model 5: Further adjusted for baseline income and education | 748 | 4,784 | 1.08 | (.90–1.31) | .41 |
| Model 6: Further adjusted for APOL1 risk status | 748 | 4,784 | 1.10 | (.90–1.34) | .35 |
| Incident chronic kidney disease | |||||
| Model 1: Unadjusted | 416 | 3,876 | 1.51 | (1.22–1.87) | <.001 |
| Model 2: Adjusted for age and sex | 416 | 3,876 | 1.63 | (1.32–2.02) | <.001 |
| Model 3a: Further adjusted for baseline eGFRCrCys | 416 | 3,876 | 1.97 | (1.59–2.44) | <.001 |
| Model 3b: Further adjusted for log-transformed urine ACR | 416 | 3,876 | 1.85 | (1.50–2.30) | <.001 |
| Model 4: Further adjusted for prevalent hypertension, diabetes mellitus, coronary heart disease, and heart failure | 386 | 3,660 | 1.57 | (1.24–1.98) | <.001 |
| Model 5: Further adjusted for baseline income and education | 355 | 3,358 | 1.33 | (1.02–1.72) | .04 |
| Model 6: Further adjusted for APOL1 risk status | 355 | 3,358 | 1.38 | (1.06–1.81) | .02 |
| Incident end-stage renal disease | |||||
| Model 1: Unadjusted | 40 | 5,532 | 4.97 | (2.65–9.30) | <.001 |
| Model 2: Adjusted for age and sex | 40 | 5,532 | 5.39 | (2.85–10.18) | <.001 |
| Model 3a: Further adjusted for baseline eGFRCrCys | 40 | 5,532 | 4.65 | (2.42–8.93) | <.001 |
| Model 3b: Further adjusted for log-transformed urine ACR | 40 | 5,532 | 3.45 | (1.77–6.70) | <.001 |
| Model 4: Further adjusted for prevalent hypertension, diabetes mellitus, coronary heart disease, and heart failure | 40 | 5,208 | 3.69 | (1.79–7.61) | <.001 |
| Model 5: Further adjusted for baseline income and education | 28 | 4,764 | 4.14 | (1.60–10.72) | .003 |
| Model 6: Further adjusted for APOL1 risk status | 28 | 4,764 | 3.20 | (1.16–8.86) | .03 |
Abbreviations: ACR, albumin-to-creatinine ratio; ARIC, Atherosclerosis Risk in Communities study; CI, confidence interval; eGFRCrCys, estimated glomerular filtration rate based on serum creatinine and cystatin C; HR, hazard ratio; IRR, incident rate ratio.
DISCUSSION
In this community-based cohort, we describe the clinical implications of the APOL1 high-risk genotypes in older age. We found that among older adults with a mean age of 76 years, those with the APOL1 high-risk genotypes were not more likely to be hospitalized or die compared with their counterparts with the low-risk genotypes. However, more individuals with the APOL1 high-risk genotypes had reduced kidney function or albuminuria, which resulted in a higher risk of ESRD. For people who had not already developed reduced kidney function, the APOL1 high-risk genotypes were not associated with incident CKD or ESRD. We also report that, even in older age, Blacks were more likely to develop adverse kidney outcomes compared with Whites, and this difference in risk by race was not explained by traditional clinical factors, socioeconomic factors, or the APOL1 risk variants.
This study advances the literature on the natural history of APOL1 and health disparities in older age. In the Cardiovascular Health Study (CHS; mean age about 74 years), there was no difference in mean eGFR or rate of eGFR decline by APOL1 genotype status; however, the APOL1 high-risk genotypes were associated with twofold higher levels of albuminuria and a trend toward increased mortality risk.14 In the Reasons for Geographic and Racial Differences in Stroke study (REGARDS; mean age about 64 years), levels of albuminuria and prevalence of CKD did not differ significantly by APOL1 genotype status, although the APOL1 high-risk genotypes were associated with a lower mean baseline eGFR. Among REGARDS participants without diabetes mellitus, those with the APOL1 high-risk genotypes had a sixfold higher risk of ESRD compared with their counterparts with the low-risk genotypes.15 In the current study (mean age = 76 years), we also reported that APOL1 high-risk individuals were more likely to develop ESRD; however, these individuals already had advanced kidney disease. Moreover, no significant association was found between APOL1 genotype status and incident CKD among people without extant eGFR below 60 mL/min/1.73 m2. Taken together, our findings suggest that among older adults who have not yet developed kidney disease, APOL1 genotype status may not portend worse renal outcomes.
We noted that the APOL1 high-risk genotypes were not associated with an increased risk for all-cause mortality. This is in line with findings from the African American Study of Kidney Disease and Hypertension (AASK; mean age = 54 years), the Women’s Health Initiative (mean age = 62 years), and the Systolic Blood Pressure Intervention Trial (mean age about 64 years), all of which reported a lack of association between APOL1 and all-cause mortality.23–25 In contrast, the CHS (mean age about 74 years) reported a borderline increased risk for total mortality associated with the APOL1 high-risk genotypes (adjusted HR = 1.3; 95% CI = 1.0–1.7; P = .05),14 whereas the African American Diabetes Heart Study (mean age about 56 years) reported a survival advantage associated with the APOL1 risk variants.26 Although these discrepant results could be due to differences in study populations, additional studies are needed to clarify whether the APOL1 risk variants confer an increased risk for death, particularly in the context of diabetes mellitus and older age.
We found no evidence that age modified the associations of APOL1 genotype status with incident ESRD. These findings are in contrast with a recent study by Wei et al. in AASK who reported a threshold age of 54 years, below which the APOL1 high-risk genotypes were associated with a nearly 3-fold higher HR of kidney replacement therapy and above which the APOL1 high-risk genotypes were not associated with risk of kidney replacement therapy.27 Importantly, participants of AASK consisted of African Americans with hypertension-attributed CKD with baseline 123I-iothalamate GFR of 20 to 65 mL/min,27–29 whereas our study population was derived from a community-based general population cohort with approximately 2% having a baseline eGFR below 60 mL/min/1.73 m2.13,16
Our study shows that, even in older age, racial disparities in health outcomes continue to exist, and that many of these disparities, particularly in hospitalizations and all-cause mortality, are, in part, explained by social determinants of health. Notably, the associations of Black race with incident CKD and ESRD remained robust to further adjustments for socioeconomic and APOL1 risk status. These findings stress the importance of ongoing policy efforts that might improve equity in kidney health30 and a need to investigate additional environmental and/or genetic risk factors that might also contribute to the excess burden of kidney disease in Black individuals.
Our study has several strengths. First, we used data from a large prospective cohort study in which outcomes were captured via active surveillance methods and linkage to the USRDS registry and the National Death Index. Second, our study sample was relatively large, with approximately 3,700 Black participants at Visit 1 and 1,200 Black participants at Visit 5. Third, we considered both APOL1 risk status and race as exposures of interest. Limitations include the relatively small number of incident CKD and ESRD events, perhaps limiting our power to detect an association between APOL1 and these outcomes. Moreover, our findings may not be generalizable to younger individuals. Still, our study is informative and characterizes a population that has not been extensively studied regarding the APOL1 risk variants.
In conclusion, the APOL1 risk variants were risk factors for ESRD but not for mortality, and this association did not appear to vary by age. For individuals in their mid70s with the APOL1 high-risk genotypes and no signs of kidney damage, the risk of ESRD was similar to their low-risk counterparts. Nevertheless, racial disparities in kidney disease were present, even in older age. Further investigation on how to reduce the excess burden of kidney disease in individuals of African ancestry is of utmost importance.
Supplementary Material
Supplementary Table S1: Number of events and incidence rates, by APOL1 risk status.
Supplementary Table S2: Number of events and incidence rates, by race.
Supplementary Figure S1: Flow diagram of ARIC Visit 5 participants included in current study.
Supplementary Figure S2: The distribution of eGFR (creatinine and cystatin C–based) in older adults varies by race/APOL1 risk status.
ACKNOWLEDGMENTS
The authors thank the staff and participants of the Atherosclerosis Risk in Communities (ARIC) study for their important contributions.
Financial Disclosure: Teresa K. Chen was supported by a Clinician Scientist Career Development Award from Johns Hopkins University and is supported by a George M. O’Brien Center for Kidney Research Pilot and Feasibility Grant from Yale University (under Award No. NIH/NIDDK P30DK079310) and NIH/NIDDK K08DK117068. Morgan E. Grams is supported by NIH/NIDDK R01DK108803. The ARIC study has been funded in whole or in part with federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health (NIH), Department of Health and Human Services, under Contract Nos. HHSN268 201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, and HHSN268201700004I. Funding for laboratory testing and biospecimen collection at ARIC Visit 6 was supported by Grant No. R01DK089174 from the National Institute of Diabetes and Digestive and Kidney Diseases of the NIH. Some of the data reported here were supplied by the U.S. Renal Data System. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the U.S. government.
Sponsor’s Role: The NIH funded the ARIC study but had no role in the design, analysis, interpretation, or preparation of this manuscript.
Footnotes
Conflict of Interest: The authors have declared no conflicts of interest for this article.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article.
REFERENCES
- 1.Crews DC, Liu Y, Boulware LE. Disparities in the burden, outcomes, and care of chronic kidney disease. Curr Opin Nephrol Hypertens. 2014;23: 298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.United States Renal Data System. 2016 USRDS annual data report: Epidemiology of kidney disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2016. [Google Scholar]
- 3.Norton JM, Moxey-Mims MM, Eggers PW, et al. Social determinants of racial disparities in CKD. J Am Soc Nephrol. 2016;27:2576–2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grams ME, Chow EK, Segev DL, Coresh J. Lifetime incidence of CKD stages 3–5 in the United States. Am J Kidney Dis. 2013;62:245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McClellan W, Tuttle E, Issa A. Racial differences in the incidence of hypertensive end-stage renal disease (ESRD) are not entirely explained by differences in the prevalence of hypertension. Am J Kidney Dis 1988;12:285–290. [DOI] [PubMed] [Google Scholar]
- 6.Cowie CC, Port FK, Wolfe RA, Savage PJ, Moll PP, Hawthorne VM. Disparities in incidence of diabetic end-stage renal disease according to race and type of diabetes. N Engl J Med. 1989;321:1074–1079. [DOI] [PubMed] [Google Scholar]
- 7.Lipworth L, Mumma MT, Cavanaugh KL, et al. Incidence and predictors of end stage renal disease among low-income blacks and whites. PLoS One. 2012;7:e48407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Genovese G, Friedman DJ, Ross MD, et al. Association of trypanolytic APOL1 variants with kidney disease in African Americans. Science. 2010; 329:841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tzur S, Rosset S, Shemer R, et al. Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Hum Genet. 2010;128:345–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kopp JB, Nelson GW, Sampath K, et al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol. 2011;22:2129–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lipkowitz MS, Freedman BI, Langefeld CD, et al. Apolipoprotein L1 gene variants associate with hypertension-attributed nephropathy and the rate of kidney function decline in African Americans. Kidney Int. 2013;83:114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foster MC, Coresh J, Fornage M, et al. APOL1 variants associate with increased risk of CKD among African Americans. J Am Soc Nephrol. 2013; 24:1484–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grams ME, Rebholz CM, Chen Y, et al. Race, APOL1 risk, and eGFR decline in the general population. J Am Soc Nephrol. 2016;27:2842–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mukamal KJ, Tremaglio J, Friedman DJ, et al. APOL1 genotype, kidney and cardiovascular disease, and death in older adults. Arterioscler Thromb Vasc Biol. 2016;36:398–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gutierrez OM, Judd SE, Irvin MR, et al. APOL1 nephropathy risk variants are associated with altered high-density lipoprotein profiles in African Americans. Nephrol Dial Transplant. 2015;31:602–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 17.Limou S, Nelson GW, Kopp JB, Winkler CA. APOL1 kidney risk alleles: population genetics and disease associations. Adv Chronic Kidney Dis. 2014; 21:426–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.OʼSeaghdha CM, Parekh RS, Hwang SJ, et al. The MYH9/APOL1 region and chronic kidney disease in European-Americans. Hum Mol Genet. 2011; 20:2450–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rebholz CM, Coresh J, Ballew SH, et al. Kidney failure and ESRD in the Atherosclerosis Risk in Communities (ARIC) Study: comparing ascertainment of treated and untreated kidney failure in a cohort study. Am J Kidney Dis. 2015; 66:231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu B, Heiss G, Alexander D, Grams ME, Boerwinkle E. Associations between the serum metabolome and all-cause mortality among African Americans in the Atherosclerosis Risk in Communities (ARIC) Study. Am J Epidemiol. 2016;183:650–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grams ME, Rebholz CM, McMahon B, et al. Identification of incident CKD stage 3 in research studies. Am J Kidney Dis. 2014;64:214–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parsa A, Kao WH, Xie D, et al. APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med. 2013;369:2183–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franceschini N, Kopp JB, Barac A, et al. Association of APOL1 with heart failure with preserved ejection fraction in postmenopausal African American women. JAMA Cardiol. 2018;3:712–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freedman BI, Rocco MV, Bates JT, et al. APOL1 renal-risk variants do not associate with incident cardiovascular disease or mortality in the systolic blood pressure intervention trial. Kidney Int Rep. 2017;2:713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freedman BI, Langefeld CD, Lu L, et al. APOL1 associations with nephropathy, atherosclerosis, and all-cause mortality in African Americans with type 2 diabetes. Kidney Int. 2015;87:176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei J, Johansen KL, McCulloch CE, et al. Association between APOL1 genotype and need for kidney replacement therapy in patients without diabetes: does age matter? Am J Kidney Dis. 2020;75:294–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wright JT Jr, Bakris G, Greene T, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421–2431. [DOI] [PubMed] [Google Scholar]
- 29.Appel LJ, Wright JT Jr, Greene T, et al. Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med. 2010;363:918–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crews DC, Novick TK. Achieving equity in dialysis care and outcomes: the role of policies. Semin Dial 2020;33:43–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1: Number of events and incidence rates, by APOL1 risk status.
Supplementary Table S2: Number of events and incidence rates, by race.
Supplementary Figure S1: Flow diagram of ARIC Visit 5 participants included in current study.
Supplementary Figure S2: The distribution of eGFR (creatinine and cystatin C–based) in older adults varies by race/APOL1 risk status.