Abstract
Regulatory properties of macrophages associated with alternative activation serve to limit the exaggerated inflammatory response during pneumonia caused by Pseudomonas aeruginosa infection. Arginase-1 is an important effector of these macrophages believed to play an essential role in decreasing injury and promoting repair. We investigated the role of arginase-1 in the control of inflammatory immune responses to P. aeruginosa pneumonia in mice that exhibit different immunologic phenotypes. C57BL/6 mice with conditional knockout of the arginase-1 (Arg1) gene from myeloid cells (Arg1ΔM) or BALB/c mice treated with small molecule inhibitors of arginase were infected intratracheally with P. aeruginosa. Weight loss, mortality, bacterial clearance, and lung injury were assessed and compared, as were the characterization of immune cell populations over time post-infection. Myeloid arginase-1 deletion resulted in greater morbidity along with more severe inflammatory responses compared to littermate control mice. Arg1ΔM mice had greater numbers of neutrophils, macrophages, and lymphocytes in their airways and lymph nodes compared to littermate controls. Additionally, Arg1ΔM mice recovered from inflammatory lung injury at a significantly slower rate. Conversely, treatment of BALB/c mice with the arginase inhibitor S-(2-boronoethyl)-L-cysteine hydrochloride (BEC) did not change morbidity as defined by weight loss, but mice at day 10 post-infection treated with BEC had gained significantly more weight back than controls. Neutrophil and macrophage infiltration were similar between groups in the lung parenchyma, and neutrophil migration into the airways was reduced by BEC treatment. Differences seem to lie in the impact on T cell subset disposition. Arg1ΔM mice had increased total CD4+ T cell expansion in the lymph nodes, and increased T cell activation, IFNγ production, and IL-17 production in the lymph nodes, lung interstitium, and airways, while treatment with BEC had no impact on T cell activation or IL-17 production, but reduced the number of T cells producing IFNγ in the lungs. Lung injury scores were increased in the Arg1ΔM mice, but no differences were observed in the mice treated with pharmacologic arginase inhibitors. Overall, myeloid arginase production was demonstrated to be essential for control of damaging inflammatory responses associated with P. aeruginosa pneumonia in C57BL/6 mice, in contrast to a protective effect in the Th2-dominant BALB/c mice when arginase activity is globally inhibited.
Keywords: Arginase-1, Pseudomonas aeruginosa, Pneumonia, Alternative macrophages, Neutrophils, Cystic fibrosis, Inflammatory lung injury
Introduction
Macrophages are important sentinel cells of the immune system that exert highly complex and coordinated functions during infection. In response to extracellular bacterial pathogens in the lung, both tissue resident and infiltrating macrophages take on a classical inflammatory (M1) macrophage phenotype. These cells initiate a neutrophil-driven inflammation through up-regulation of a gene expression program that includes pro-inflammatory cytokines and chemokines, along with reactive oxygen species including nitric oxide (NO) in order to facilitate killing and clearance of the infectious organism (Bogdan, 2001a). As these processes can be damaging to host tissues, macrophages also regulate inflammation and coordinate tissue repair. The balance between the inflammatory and regulatory aspects of macrophage function is essential for proper activation and termination of the immune response and for maintaining tissue homeostasis. The distinct alternatively activated (M2) functional phenotype describes macrophages that participate in Th2-driven inflammatory responses and produce anti-inflammatory cytokines and other mediators that control inflammation and coordinate tissue repair processes (Gordon, 2003; Gordon and Taylor, 2005). M1 and M2 macrophages have distinct metabolic properties, including the pathway in which each metabolizes arginine, a conditionally-essential amino acid. M1 macrophages primarily express inducible nitric oxide synthase (iNOS) which generates NO from arginine, which is important for bacterial clearance (Bogdan, 2001a; Bogdan, 2001b; Williams et al., 2010). Conversely, M2 macrophages express arginase-1 which metabolizes arginine into proline and polyamines essential for cell proliferation and wound healing (Murray and Wynn, 2011). This dualistic interpretation of macrophage activation, however, is not entirely reflective of the complex in vivo microenvironment, and macrophage responses are radically different depending on a number of factors including organism type and organism location (Murray, 2017).
Arginine metabolism in macrophages has been evaluated as a key regulator of the immune response (Rath et al., 2014). Under physiological conditions, arginine primarily participates in the urea cycle and the elimination of toxic ammonia compounds. However, under certain inflammatory conditions, arginine becomes semi-essential and is metabolized by arginase-1, arginase-2, or iNOS (Wijnands et al., 2015). Arginase-1 expression is induced in macrophages, neutrophils, and other immune cells where it competes with iNOS, thereby regulating NO generation and limiting NO-mediated inflammatory injury (Munder et al., 1998; Rath et al., 2014). However, recent studies evaluating the immunomodulatory role of arginase-1 suggest that its beneficial properties reach beyond its NO-limiting effects. In contrast to iNOS which does not exhaust the arginine pool due to the citrulline recycling pathway, arginase-1 depletion of arginine exerts important regulatory effects on T cell proliferation and function (Bronte et al., 2003; Kang et al., 2014; Munder et al., 1998; Rodriguez et al., 2003).
Enhancing our understanding of arginine metabolism during the inflammatory response to P. aeruginosa pneumonia is critical in light of the importance of this pathogen in patients with chronic inflammatory lung conditions including cystic fibrosis (CF). P. aeruginosa colonizes the lungs of 80% of patients with CF who are greater than 18 years of age and causes repeated acute exacerbations which result in neutrophil-dominant responses (Bruscia et al., 2009; Elizur et al., 2008). As these cells die in large numbers they release massive amounts of DNA, neutrophil elastase, and proteases which increase mucus viscosity and oxidative stress thereby destroying structural airway proteins leading to bronchiectasis (Elizur et al., 2008). Additionally, Th1 and Th17 subsets of CD4+ T cells are disproportionately activated, which adds to the excessive neutrophil influx and inflammation through the production of IFNγ and IL-17 (Elizur et al., 2008; Kushwah et al., 2013; Lavoie et al., 2011; Tiringer et al., 2013). This exaggerated response has been mechanistically attributed to abnormal NF-κB activation, and decreased negative feedback mechanisms among immune cells in the lungs (Blackwell et al., 2001; Elizur et al., 2008).
Our group has previously demonstrated that polarization of macrophages to an alternative-like phenotype early after inoculation can control damaging aspects of the immune response in mice infected with P. aeruginosa (Feola et al., 2010; Murphy et al., 2008). This has been described in part through the study of the immunomodulatory properties of the antimicrobial agent azithromycin, an agent used to longitudinally decrease inflammation in patients with CF. Azithromycin treatment polarizes macrophages towards a regulatory, M2-like phenotype, blunts neutrophil influx, and shifts the T cell response away from Th1 dominance, resulting in decreased IFNγ production in C57BL/6 mice in a model of chronic P. aeruginosa infection (Feola et al., 2010; Murphy et al., 2008). Azithromycin treatment significantly increases arginase-1 expression and activity in vitro and in vivo through its impacts on NF-κB signanling (Feola et al., 2010; Haydar et al., 2019). In mice treated with azithromycin, peak arginase expression occurs at day 7 post-infection and is associated with reduced morbidity and decreased lung damage (Feola et al., 2010).
In this study, we evaluated the role of arginase-1 in the regulation of inflammation in response to P. aeruginosa pneumonia in mice. Conditional deletion of the Arg1 gene in myeloid cells of C57BL/6 mice resulted in greater morbidity and a more severe, neutrophil-dominated inflammatory lung injury. This was accompanied by a dysregulation of T cell activation and an overabundance of T cells producing IFNγ and IL-17. To determine whether similar results would be obtained in a Th2-dominant mouse strain, we treated BALB/c mice with small molecule inhibitors of arginase and found that this had little impact on morbidity or lung injury score, and instead decreased airway neutrophil infiltration and the number of IFNγ-producing T cells in the lungs.
Material and methods
Mice
To generate arginase-1 conditional knock-out mice, C57BL/6-Arg1 tm1Pmu/J (Arg1flox) mice were bred with B6.129P2-Lyz2 tm1(cre)Ifo/J (LysMcre) mice (The Jackson Laboratory, Bar Harbor, ME). All animals were bred in-house starting with the homozygous loxP mice (Arg1flox) and the transgenic Cre mice. These generate heterozygous loxP, hemizygous Cre mice; the latter were backcrossed with the homozygous loxP mice to generate homozygous loxP, hemizygous Cre mice (arginase-1 conditional knock-out mice, Arg1ΔM), and homozygous loxP, non-carrier mice (littermate controls that do not have Cre recombinase to excise the flanked allele, Arg1flox/flox). For experiments with pharmacological arginase inhibitors, BALB/c mice (Jackson Laboratory, Bar Harbor, ME) were used due to their Th2-bias and significant expression of macrophage arginase-1 after infection (Duleu et al., 2004; Iniesta et al., 2005). All mice were 6–8 weeks of age and were randomized into different treatment groups. All studies were approved by the University of Kentucky Institutional Animal Care and Use Committee as guided by the National Institutes of Health guide for the care and use of laboratory animals. The mice were housed under conditions of pathogen-free isolation and were transferred to a biosafety level 2 housing unit after infection.
Murine infection and drug dosing
Pseudomonas-laden agarose beads:
Mice were infected with P. aeruginosa incorporated into agarose beads to cause a prolonged infection similar to that in patients with CF (Nacucchio et al., 1984; Nau et al., 2002). PA M57–15 is a clinical mucoid strain of P. aeruginosa isolated from a patient with CF and obtained from Case Western University (van Heeckeren and Schluchter, 2002). PA M57–15 was grown overnight in Trypticase Soy Broth (TSB) to reach the late log phase or early stationary phase. According to the growth curves and the optical density (OD) analysis of the PA M57–15 cultures, the starting OD of the bacterial culture that would result in optimal infectious inoculum was selected to be incorporated into agarose beads as described previously (Nacucchio et al., 1984). Briefly, bacterial cultures with appropriate OD were slowly incorporated into warmed agarose solution and then transferred to mineral oil stirring at high speed, thus creating bacteria-incorporated beads within different size ranges. A sample of the bead stock was then homogenized and plated on dry trypticase soy agar plates to determine the inoculum of P. aeruginosa. The bead stock was then diluted to the desired inoculum of 2×105 CFU in 100 μl for Arg1ΔM and 2×106 CFU in 100 μl for BALB/c mice. This inoculum was determined to result in significant pneumonia without causing severe morbidity or mortality for each strain. The target inoculum was calculated as the 10% of the lethal CFU or LD10.
For infection, mice were lightly anesthetized using inhaled isoflurane and beads were instilled intra-tracheally using a blunted 24-gauge curved needle. The experimental bead stock was again homogenized and plated on Pseudomonas isolation agar (PSA) plates to confirm the inoculation used for each experiment.
Arginase inhibitor dosing:
The arginase inhibitors BEC or L-norvaline were given to mice to evaluate the immunomodulatory effects of pharmacological arginase inhibition in P. aeruginosa pneumonia. Either agent was dosed via oral gavage starting 1 day prior to infection and daily thereafter. L-Norvaline was purchased from Sigma-Aldrich (St. Louis, MO). BEC was synthesized in the lab of Dr. Sylvie Garneau-Tsodikova at our institution. The thiolene reaction strategy was used to synthesize BEC. The single step reaction between di-(n-butyl)-vinylboronate and L-cysteine in the presence of a radical initiator 2,2′-azobis(2-methylpropionitrile) resulted in the formation of BEC at a 53% yield (Busnel et al., 2005). The detailed synthesis procedure and NMR verification is provided in the Supplemental Materials (Fig. S1).
The dose of BEC was calculated based on previous studies that produced a 50% reduction in arginase activity in cells isolated from treated animals (Anthony et al., 2006; McLarren et al., 2011). A daily dose of 0.012 g of BEC was administered in 100 μl of water via oral gavage to match the dosing equivalent in studies that exposed mice to BEC in their drinking water. The mouse dose of L-Norvaline was estimated from the rat dose of 50 mg/kg through animal dose equivalent calculations based upon allometric scaling to 100 mg/kg in mice (El-Bassossy et al., 2012; Nair and Jacob, 2016). Based on these studies, dosing of each agent was achieved through daily administration in 100 μl of water via oral gavage to ensure precise exposure.
Tissue harvesting
Mouse body weight was monitored daily to assess morbidity post infection. Mice were euthanized and excluded from the experiment if they lose 20% or more of their body weight along with 1 sign of morbidity (immobility, hunched posture, or lack of response to handling). If mice suffered from cyanosis, dyspnea, or loss of righting reflex, they were euthanized immediately. A representative sample of the infected mice was humanely killed at different timepoints post-infection and tissues were harvested and processed as described elsewhere (Feola et al., 2010). Briefly, lung lavage was performed on each mouse to collect cells from the airways and alveolar spaces. A flexible catheter was inserted into a small incision in the trachea and 5ml of PBS (with 30 μM EDTA) was instilled and removed in 1 ml aliquots. Tracheobronchial lymph nodes draining the site of infection were excised and pushed through 70 μm cell strainers with a syringe plunger to create single cell suspensions. Lung tissues were then collected and digested followed by incubation with 1 mg/ml of collagenase and 50 U/ml of DNase. After digestion, the lung tissues were then pushed through a 70 μm cell strainer. An aliquot of lung digest was plated on PSA plates to determine bacterial burden. Red blood cells were lysed from all samples and cells were counted and processed for flow cytometry analysis.
Flow cytometry
Processed samples from lung lavage, lung digest, and lymph nodes were stained for surface and intracellular proteins to characterize immune populations over time post-infection. Cells were incubated with fluorochrome-conjugated antibodies (myeloid panel: PerCpCy5.5-F4/80, PE Cy7-CD11c, APC Cy7-Ly6G, PE-CD206, APC-CD11b; lymphoid panel: PE Cy7-CD4, FITC-CD44, PE-CD62L, APC-CD25, APC Cy7-CD3) at 4°C in the dark for 20 minutes. For intracellular staining, cells were stimulated with ionomycin (1 μg/ml final concentration) and PMA (Phorbol 12-myristate 13-acetate, 50 ng/ml final concentration) for 4 hours at 37 °C. Subsequently, brefeldin A was added (2 μl of 1000x) in order to block protein transport. Cells were then stained for surface proteins as described above followed by fixation with 5% formalin and permeabilization using 0.5% saponin. Fc block was then added followed by incubation with fluorochrome-conjugated antibodies against intracellular proteins (FITC-TNFα, PE-IL10, FITC-IFNγ, APC-IL17, PE-CXCR3, PerCpCy5.5-RORγt, and APC-ARG1).
Samples were analyzed using either an Attune Flow Cytometer (Applied Biosystems, Foster City, CA) or a BD LSR II Flow Cytometer (BD Biosciences, Franklin Lakes, NJ) at the University of Kentucky Flow Cytometry Core Facility. Each sample was analyzed using fluorescence gating schemes defined by control samples and compensation to record a minimum of 50,000 events per sample. Further analysis of the recorded events was performed using FlowJo software (FlowJo, LLC, Ashland, Oregon).
Histology
Lungs were gently inflated with 2–3 ml of 10% formalin and then fixed and processed for paraffin embedding according to standard procedures (Morton and Snider, 2017). Lungs were trimmed in from the ventral face until all 5 lobes were visible. Paraffin embedding and hematoxylin and eosin (H&E) staining were performed at the COBRE Pathology Core of the University of Kentucky which is supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM103527.
Lung injury scoring was calculated according to the American Thoracic Society guidelines by an independent pathologist (Matute-Bello et al., 2011). Briefly, 20 random high-power fields were independently and blindly scored through assessment of the number of neutrophils in the alveolar and interstitial spaces, the presence of hyaline membranes and proteinaceous debris, and septal wall thickening. The scoring system is summarized in Supplemental Table S1. To generate a lung injury score, the five independent variables were weighted according to the significance factor of each variable and then normalized to the number of fields evaluated. The following formula was used to calculate the lung injury score: Score = [(20 x A) + (14 x B) + (7 x C) + (7 x D) + (2 x E)] / (number of fields x 100).
Statistical analysis
Differences measured between treatment groups were analyzed for statistical significance. Based on previous in vivo data, a sample size of at least 10 mice per group were used to achieve a power of 80% and a within-mouse intra-correlation coefficient of 0.50 (Deckman et al., 2017; Feola et al., 2010). All experiments included both male and female mice, although differences in response related to sex were not studied. Quantitative data (means, standard deviations, and 95% confidence intervals) were analyzed using GraphPad Prism (GraphPad Software, La Jolla, CA). Comparison between groups was made via one-way ANOVA with Tukey’s test multiple comparisons, paired sample T-test with McNemar’s test, or via two-way ANOVA with Sidak’s multiple comparisons test. Repeated-measures ANOVA with Tukey’s multiple comparison tests were utilized for time course experiments. Kaplan-Meier survival curves were compared using Log-rank (Mantel-Cox) and Gehan-Breslow-Wilcoxon tests. A p-value <0.05 was considered as a statistically significant difference.
Results
Myeloid arginase-1 deletion results in increased morbidity and lung damage after infection
To evaluate the impact of arginase deletion from myeloid cells upon the acute inflammatory response to P. aeruginosa pneumonia, C57BL/6 mice with conditionally deleted arginase-1 in myeloid cells (Arg1ΔM) and littermate controls (Arg1flox/flox) were infected intratracheally with P. aeruginosa-laden agarose beads. Arginase production was examined to confirm deletion in alveolar macrophages, infiltrating monocytes, and neutrophils, as well as confirming that lymphocytes or lymphoid cells retained the production of arginase (shown in Supplemental Fig. S2). Morbidity in infected mice was evaluated by measuring body weight over time post-infection. There was a significantly faster and greater decline in murine body weight at days 1 and 2 post-infection in the Arg1ΔM group (Fig. 1A). Increased morbidity in the Arg1ΔM mice was associated with an average weight loss of 13.83% of their baseline body weight versus an average weight loss of 8.03% in the control mice (day 2, p-value <0.0001). Control mice recovered faster than the Arg1ΔM mice which slowly regained their weight over time (day 3, control Arg1flox/flox mice at 96.02% of their baseline weight versus 88.84% in the Arg1ΔM mice, p-value <0.0001). However, there were no significant differences in terms of bacterial clearance kinetics or mortality between the two groups (Fig. 1B and C). Arg1ΔM mice cleared P. aeruginosa at a comparable rate similar to the Arg1flox/flox mice, and both groups failed to completely clear the infection, as the organism was still present in the lungs by day 10 post-infection (Fig. 1B). Across experimental replicates, mortality rates were comparable between groups at approximately 10–15% (Fig. 1C).
Fig. 1. Myeloid arginase-1 deletion worsens morbidity and lung damage after infection.
Arg1ΔM and Arg1flox/flox mice were infected intratracheally with 2×105 CFU P. aeruginosa incorporated into agarose beads. (A) Weight loss over time post-infection expressed as the percent weight of baseline value at day 0. (B) CFU counts from harvested lungs of infected mice at post-infection timepoints. (C) Kaplan-Meier curves depicting the percent survival in each group infected mice over time. (D) Inflammatory lung injury scores assessed by histological examination over time post-infection. (E) Hematoxylin-eosin staining of representative lung sections from Arg1flox/flox and Arg1ΔM mice harvested on day 0 (uninfected) and at day 5 post-infection. Magnification is indicated by the scale bars representing 100, 200, and 50 μm, respectively from left to ring. Data represents mean ± SD of at least 4 mice per group per timepoint, and statistical significance was defined for a P-value < 0.05 (*) for differences between groups at each time point analyzed using repeated-measures ANOVA with Tukey’s test for multiple comparisons and are representative of three independent replicates. CFU, colony forming units; SD, standard deviation; ANOVA, analysis of variance.
Arg1ΔM mice display increased inflammatory lung damage when infected with P. aeruginosa
Inflammatory lung injury was evaluated by H&E staining of lung sections from infected mice over time post-infection. Lung injury scoring was assessed according to the American Thoracic Society guidelines as described in the Materials and methods section. Mean lung injury scores are depicted (Fig. 1D), with representative lung sections from day 0 and 5 post-infection shown in Fig. 1E. Arg1ΔM mice responded to the infection in an exaggerated manner with dilated bronchi plugged with mucous and inflammatory cells. The majority of inflammatory cells infiltrating the lungs in the Arg1ΔM mice consisted of neutrophils at early timepoints. By day 5 post-infection, immune infiltrates consisted of mostly monocytes and lymphoid cells. Conversely, the Arg1flox/flox mice responded with an intra-alveolar monocytic predominance and lower numbers of neutrophils. Importantly, both groups of mice suffered from increasing lung injury scores by day 3 post-infection, after which scores diverged to a significantly higher peak in the Arg1ΔM mice while the littermate controls recovered to near baseline scores on day 5 post-infection (p-value = 0.0003).
Myeloid arginase deletion alters recruitment of innate immune cells to the lungs
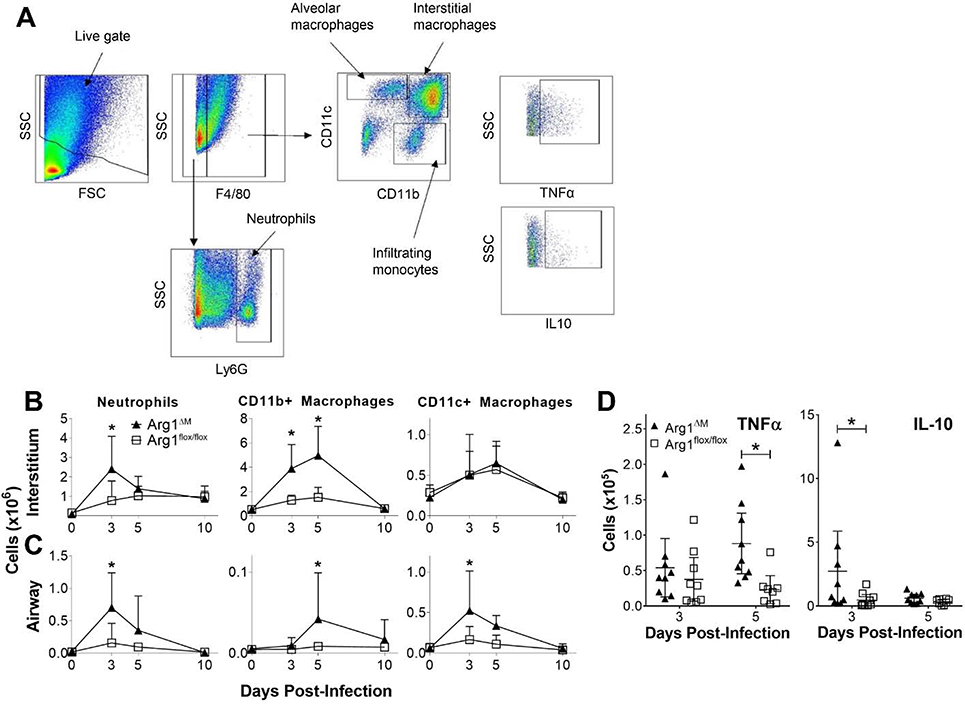
Influx of innate inflammatory cells into the lungs of Arg1ΔM mice infected with P. aeruginosa was evaluated (Fig. 2). The flow cytometry gating scheme for myeloid immune cell subsets is depicted in Fig. 2A. A greater peak in neutrophil counts at day 3 post-infection was observed in Arg1ΔM mice compared to their littermate controls (Fig. 2B, p-value = 0.001 and 0.0033 in the lung interstitium and airway, respectively). Arg1ΔM mice also had significantly larger numbers of CD11b+ infiltrating macrophages into the lung interstitium at days 3 and 5 post-infection (Fig. 2B, day 3, p-value = 0.0003; day 5 p-value <0.0001), with the infiltrating macrophage populations reaching a slightly higher peak in the airways on day 5 post-infection. CD11c+ macrophages peaked significantly higher at day 3 post-infection in the Arg1ΔM group (p-value = 0.0042).
Fig. 2. Myeloid arginase-1 deletion associated with increased cellular influx and inflammation after infection.
Arg1ΔM and Arg1flox/flox mice were infected intratracheally with 2×105 CFU P. aeruginosa incorporated into agarose beads. Lungs were lavaged to collect cells from the airways, and excised and digested for assessment of interstitial cellularity. (A) Gating scheme for identification of different myeloid cell populations analyzed by flow cytometry. (B-C) Mean cell counts for neutrophils (F4/80- Ly6G+), infiltrating monocyte/macrophages (F4/80+ CD11b+), and resident/alveolar macrophages (F4/80+ CD11c+) in the lung interstitium (B) and airways (C) over time post-infection. (D) Numbers of airway monocyte/macrophages (F4/80+ CD11b/c+) producing TNFα or IL-10 at days 3 and 5 post-infection as assessed by intracellular cytokine staining and flow cytometry. Mean ± SD in panels B and C (at least 4 mice per group per timepoint), and mean ± 95% CI along with individual mouse values in panel D depicted, with statistical significance defined for P < 0.05 (*) for differences between groups at each timepoint analyzed using repeated-measures ANOVA with Tukey’s test for multiple comparisons. Results are representative of three independent replicates. CFU, colony forming units; TNFα, tumor necrosis factor-α; IL, interleukin; SD, standard deviation; CI, confidence interval; ANOVA, analysis of variance; SSC, side scatter; FSC, forward scatter.
The expression of macrophage cytokines was assessed to determine the phenotype of macrophages recruited in response to the infection. Intracellular cytokine staining and evaluation by flow cytometry revealed significantly higher numbers of TNFα-producing macrophages in the airways of the Arg1ΔM mice on day 5 post-infection (p-value = 0.0063), and a significantly higher number of macrophages producing IL-10 on day 3 post-infection (p-value = 0.0225). No significant differences were detected in the number of macrophages producing these cytokines in the lung interstitium. The time courses for both CD11b+ and CD11c+ macrophage expression of TNFα, IL-10, and M2-associated surface protein mannose receptor (MR) from both the airways and lung interstitium are shown in Supplemental Fig. S3.
Arginase conditional knock-out mice respond to bacterial pneumonia with greater T cell recruitment and activation
T cell responses were also evaluated over time in Arg1ΔM mice. T cell phenotypes were examined over time post-infection in the lung interstitium, the tracheobronchial lymph nodes that drain the site of infection, and in the airways. Fig. 3A shows the gating scheme used to identify different T cell populations. Arg1ΔM mice responded with comparable T cell recruitment and activation in lung interstitial spaces compared to littermate controls with no significant differences in total or activated (CD44hiCD62Llo) CD4+ T cell counts on day 5 post-infection (Fig. 3B). Conversely, Arg1ΔM mice had higher numbers of total and activated CD4+ T cells in the lymph nodes and the airways at this timepoint (Fig. 3C and D).
Fig. 3. Lack of myeloid arginase-1 leads to greater T cell recruitment and activation in response to P. aeruginosa pneumonia.
Arg1ΔM and Arg1flox/flox mice were infected intratracheally with 2×105 CFU P. aeruginosa incorporated into agarose beads. Lungs were lavaged to collect cells from the airways, and excised and digested for assessment of interstitial cellularity. Tracheobronchial lymph nodes draining the site of infection were harvested and processed into single cell suspensions. (A) Gating scheme for identification of T lymphocyte populations analyzed by flow cytometry. (B-D) Numbers of total CD4+ T cells, activated CD4+ (CD44hi CD62Llo) T cells, Th17 (CD4+ IL-17+ RORγt+) lymphocytes, and Th1 (CD4+ CXCR3+ IFNγ+) lymphocytes in the lung interstitium (B), lymph nodes (C), and airways (D) of infected mice on day 5 post-infection. Mean ± 95% CI along with individual mouse values are depicted, with statistical significance defined for P < 0.05 (*) for differences between groups analyzed using repeated-measures ANOVA with Tukey’s test for multiple comparisons. Results are representative of three independent replicates. CFU, colony forming units; IL, interleukin; ROR, RAR-related orphan nuclear receptor; CXCR3, C-X-C motif chemokine receptor 3; IFNγ, interferon-γ; SD, standard deviation; CI, confidence interval; ANOVA, analysis of variance; SSC, side scatter; FSC, forward scatter.
Analysis of CD4+ T cell subsets revealed striking increases in the numbers of Th17 (defined by RORγt and IL-17 expression) and Th1 (defined by CXCR3 and IFNγ expression) phenotypes in the lung interstitium, lymph nodes, and airways (Fig. 3B, C, and D) on day 5 post-infection in the Arg1ΔM mice. These increases in pro-inflammatory T cells correspond with the increase in lung damage and neutrophil influx observed in the Arg1ΔM animals. T cell subset population kinetics over time through day 10 post-infection are depicted in the supplemental materials (Supplemental Fig. S4) for all 3 tissue compartments. While Th1 and Th17 subsets were also increased on day 3 post-infection in the lungs in the Arg1ΔM mice, all other timepoints revealed similar numbers.
Arginase inhibition in BALB/c mice did not impact morbidity and acute inflammatory responses to P. aeruginosa pneumonia
Th2-dominant mouse strains differ greatly in the role that arginine metabolism plays in response to infection. To address this, BALB/c mice were treated with one of two pharmacological arginase inhibitors (BEC or L-Norvaline) via oral gavage starting 1 day prior to infection and daily thereafter. Control mice were gavaged with vehicle (water) only. To induce comparable morbidity as in Arg1ΔM mice, we selected an inoculum of 2×106 CFU/mL for these experiments. For simplicity, data is shown for the BEC and control treatment groups, as results generated using L-Norvaline were comparable.
Morbidity was evaluated by measuring weight loss as compared to the baseline body weight prior to infection. There was a sharp decline in body weight through day 3 post-infection in both groups, and there was no significant difference in acute weight loss as a result of arginase inhibition (Fig. 4A). However, global arginase inhibition led to a more rapid and complete recovery of body weight as compared to control mice by day 10 post-infection. BEC-treated mice recovered by day 10 post-infection to an average of 98.21% of their baseline body weight, while control mice regained weight to a significantly lower mean percentage body weight of 88.5% at 10 days post-infection (p-value = 0.025). Bacterial clearance kinetics were similar between groups (Fig. 4B). Of note, on day 10 post-infection mice carried a significant bacterial burden despite recovery from a majority of lung injury and most of the immune cell subset numbers having returned to baseline, except T cell populations in the lymph nodes as expected. No significant differences in mortality were observed, with comparable survival rates among BEC-treated and control mice (Fig. 4C).
Fig. 4. Pharmacological arginase inhibition in BALB/c mice has little impact on acute morbidity or survival during P. aeruginosa pneumonia.
BALB/c mice were infected intratracheally with 2×106 CFU P. aeruginosa incorporated into agarose beads. Mice were either dosed with 0.012g of BEC daily via oral gavage starting 1 day prior to infection or with vehicle control (water). (A) Weight loss over time post-infection expressed as the percent weight of baseline value at day 0. (B) CFU counts from harvested lungs of infected mice at post-infection timepoints. (C) Kaplan-Meier curves depicting the percent survival in each group infected mice over time. (D) Inflammatory lung injury scores assessed by histological examination over time post-infection. (E) Hematoxylin-eosin staining of representative lung sections from BEC-treated and control mice harvested on day 5 post-infection. Magnification is indicated by the scale bars representing 100, 200, and 50 μm, respectively from left to right. Data represents mean ± SD of at least 4 mice per group per timepoint, and statistical significance was defined for a P-value < 0.05 (*) for differences between groups at each time point analyzed using repeated-measures ANOVA with Tukey’s test for multiple comparisons and are representative of three independent replicates. BEC, S-(2-Boronoethyl)-L-cysteine hydrochloride; CFU, colony forming units; SD, standard deviation; ANOVA, analysis of variance.
We then evaluated the impact of global arginase inhibition on the development of and recovery from inflammatory lung injury in these mice. H&E stained lung sections were again analyzed with resultant mean lung injury scores that were comparable between treatment groups at all post-infection timepoints evaluated (Fig. 4D). Lung images from BEC-treated and control mice revealed comparable dilation of the bronchi along with mucoid plugging and infiltration of neutrophils and other immune cells at day 5 post-infection (Fig. 4E). However, close evaluation of the inflammatory processes in the lungs of BEC-treated mice revealed a striking perivascular and peribronchial inflammation. While infection in both groups was associated with early infiltration of immune cells into the alveolar spaces by day 2 post-infection, in the BEC-treated mice this was followed by migration of immune cells to the areas surrounding blood vessels and bronchioles. Representative images show a predominantly intrabronchial infiltration along with increased perivascular and peribronchial inflammatory infiltrates in the BEC-treated mice. Additionally, uninfected mice (day 0) that were treated with BEC also displayed a noticeable vasodilation.
Arginase inhibition impacts recruitment of innate and adaptive immune cells to the lungs of BALB/c mice infected with P. aeruginosa
The impact of arginase inhibition on immune cell recruitment was then evaluated in the lungs of infected mice. Acute neutrophil recruitment into the lung interstitium was comparable among BEC-treated and control mice, while BEC treatment significantly reduced the number of neutrophils recruited into their alveolar spaces on day 2 post-infection (Fig. 5B, p-value = 0.0264). Similar patterns emerged for CD11b+ infiltrating monocytes although statistically significant differences were not attained. CD11c+ tissue macrophages displayed a delayed peak in the BEC-treated mice, with significantly lower numbers on day 2 followed by significantly higher numbers on day 5 post-infection in the lung interstitium (Fig. 5A, p-values = 0.0136 and 0.015, respectively).
Fig. 5. Pharmacologic arginase inhibition in BALB/c mice infected with P. aeruginosa results in comparable recruitment of innate immune cells to the lungs but blunted early influx into the airways.
BALB/c mice were infected intratracheally with 2×106 CFU P. aeruginosa incorporated into agarose beads. Mice were either dosed with 0.012g of BEC daily via oral gavage starting 1 day prior to infection or with vehicle control (water). Lungs were lavaged to collect cells from the airways, and excised and digested for assessment of interstitial cellularity. Immune cell populations were analyzed by flow cytometry as depicted in the gating scheme in Fig. 2. (A-B) Mean cell counts for neutrophils (F4/80−Ly6G+), infiltrating monocyte/macrophages (F4/80+ CD11b+), and resident/alveolar macrophages (F4/80+ CD11c+) in the lung interstitium (A) and airways (B) over time post-infection. (C) Numbers of airway monocyte/macrophages (F4/80+ CD11b/c+) producing TNFα or IL-10 at days 2 and 5 post-infection as assessed by intracellular cytokine staining and flow cytometry. Mean ± SD in panels A and B (at least 4 mice per group per timepoint), and mean ± 95% CI along with individual mouse values in panel C depicted, with statistical significance defined for P < 0.05 (*) for differences between groups at each timepoint analyzed using repeated-measures ANOVA with Tukey’s test for multiple comparisons. Results are representative of three independent replicates. CFU, colony forming units; BEC, S-(2-Boronoethyl)-L-cysteine hydrochloride; TNFα, tumor necrosis factor-α; IL, interleukin; SD, standard deviation; CI, confidence interval; ANOVA, analysis of variance.
To evaluate the phenotype of macrophages recruited into the lungs of mice treated with pharmacologic arginase inhibitors we stained for M1 and M2 macrophage effectors (Fig. 5C). There were no statistically significant differences in the number of airway macrophages expressing IL-10 or TNFα (Fig. 5C) among treatment groups. The kinetics of MR, IL-10, and TNFα producing macrophage infiltration into the lungs and airways of infected mice had no significant differences observed at any post-infection timepoint examined (not shown).
We then evaluated the impact of arginase inhibition on T cell responses. The total number of CD4+ T cells and the numbers of CD4+ T cell subsets were quantified and depicted at day 2 post-infection (Fig. 6) and at all timepoints examined through day 10 post-infection (Supplemental Fig. S5). There were no significant differences in terms of the number of total or activated CD4+ T cells the lung interstitium, lymph nodes, or airways between the groups at day 2 post-infection (Fig. 6A, B, and C). However, BEC treatment significantly increased the numbers of total and activated CD4+ T cells at day 10 post-infection in the tracheobronchial lymph nodes compared to the control mice (Supplemental Fig. S5).
Fig. 6. Effects of global arginase inhibition on different lymphocyte subsets in BALB/c mice infected with P. aeruginosa.
BALB/c mice were infected intratracheally with 2×106 CFU P. aeruginosa incorporated into agarose beads. Mice were either dosed with 0.012g of BEC daily via oral gavage starting 1 day prior to infection or with vehicle control (water). Lungs were lavaged to collect cells from the airways, and excised and digested for assessment of interstitial cellularity. Tracheobronchial lymph nodes draining the site of infection were harvested and processed into single cell suspensions. T lymphocyte populations were analyzed by flow cytometry as depicted in the gating scheme in Fig. 3. (A-C) Numbers of total CD4+ T cells, activated CD4+ (CD44hi CD62Llo) T cells, Th17 (CD4+ IL-17+ RORγt+) lymphocytes, and Th1 (CD4+ CXCR3+ IFNγ+) lymphocytes in the lung interstitium (A), lymph nodes (B), and airways (C) of infected mice on day 2 post-infection. Mean ± 95% CI along with individual mouse values are depicted, with statistical significance defined for P < 0.05 (*) for differences between groups analyzed using repeated-measures ANOVA with Tukey’s test for multiple comparisons. Results are representative of three independent replicates. CFU, colony forming units; BEC, S-(2-Boronoethyl)-L-cysteine hydrochloride; IL, interleukin; ROR, RAR-related orphan nuclear receptor; CXCR3, C-X-C motif chemokine receptor 3; IFNγ, interferon-γ; SD, standard deviation; CI, confidence interval; ANOVA, analysis of variance.
Next, we sought to determine the phenotype of T cells activated in the lymph nodes and lungs of mice treated with BEC. There were no significant differences in the number of Th17 lymphocytes in the lung interstitium, lymph nodes, or airways between groups of mice at day 2 post-infection (Fig. 6). However, mice treated with BEC had significantly lower numbers of Th17 cells in their lymph nodes at day 10 post-infection (Supplemental Fig. S5, p-value = 0.0079). Interestingly, treatment with the arginase inhibitors caused a decreased number of Th1 lymphocytes to migrate to both the lung interstitium and to the airways on day 2 post-infection (Fig. 6A and C, p-values = 0.0002 and 0.0066, respectively). However, there was no significant difference in Th1 cell numbers in the lymph nodes at this timepoint. These results are in contrast to the increases observed in the Th1 and Th17 subsets in the Arg1ΔM mice.
Discussion
Our results show that arginase production by myeloid cells is essential to regulate inflammation in response to P. aeruginosa pneumonia. Arginase deletion from myeloid cells in the Th1-dominant mouse strain C57BL/6 resulted in increased morbidity and weight loss, but no change in survival or bacterial clearance kinetics. Arg1ΔM mice responded to the infection with an amplified influx of neutrophils and pro-inflammatory macrophages into the lungs and alveolar spaces, an increase in Th1 and Th17 responses, and worsened lung damage. Conversely, arginase inhibition in a Th2-dominant mouse strain by small molecule inhibitors did not impact morbidity or lung damage, as mice treated with BEC responded with comparable influx of neutrophils and macrophages in their interstitial spaces and reduced neutrophil counts in the airways. The difference between absence or inhibition of arginase-1 in a Th1- and Th2-dominant models appears to primarily be driven by the impact on T cells, as BALB/c mice treated with arginase inhibitors, unlike the C57BL/6 Arg1ΔM mice, displayed no change in Th17 cell numbers and decreased Th1 cells in the airways and lung interstitium.
In addition to its role in coordinating tissue repair, arginase-1 expression controls excessive inflammation in a number of disease models through limiting the conversion of arginine to NO. M1 macrophages up-regulate iNOS to produce NO in large quantities in response to inflammatory signals, a process in which the rate-limiting step is arginine uptake (Yeramian et al., 2006). Alternatively, arginase-1 expression is induced in M1 macrophages, neutrophils, and other immune cells where it competes with iNOS thereby regulating NO generation and limiting NO-mediated inflammatory injury (Modolell et al., 1995). Additionally, the polyamines that are produced from ornithine products also inhibit macrophage effector functions and NO synthesis (Mossner et al., 2001; Zhang et al., 1997). In response to Th1-dominant infections, arginase-1 expression can contribute to the pathology by countering effective responses through suppressing NO production, as shown in mouse models of infection with the intracellular pathogens Mycobacterium tuberculosis and Toxoplasma gondii (El Kasmi et al., 2008). Conversely, while arginase-1 regulates the pathologic aspects of Th2-driven inflammation and fibrosis in S. mansoni infection (Herbert et al., 2010; Pesce et al., 2009), myeloid arginase-1 expression does not impact Th2-driven inflammation in lung infection or asthma (Barron et al., 2013). Instead, type-2 innate lymphoid cell (ILC2) expression of arginase-1 drives its influence here, highlighting the specificity in the coordination of inflammation in different pathologic processes (Monticelli et al., 2016).
Exploration of the immunomodulatory role of arginase-1 in response to extracellular bacterial infection has been limited. NO production and killing by neutrophils are critically important for the clearance of P. aeruginosa (Williams et al., 2010; Yu et al., 2000). In accordance with this, in response to P. aeruginosa pneumonia, our previous work and the work of others show that there is an early dramatic influx of M1 macrophages producing pro-inflammatory cytokines and iNOS, along with Th1 and Th17 cytokines shortly after infection. However, by day 3 post-infection these responses dissipate and macrophages take on M2 characteristics that includes elevated arginase-1 production (Bielen et al., 2017; Feola et al., 2010; Mehl et al., 2014). A study by Mehl and colleagues examined the impact of arginine metabolism in a mouse model of P. aeruginosa pneumonia (Mehl et al., 2014). Impairment of iNOS through arginine restriction occurred after P. aeruginosa infection as arginase-1 was up-regulated. Additionally, the inhibition of arginase-1, which led to increased production of NO, did not impact the concentrations of pro-inflammatory cytokines in the lungs on day 3 post-infection (Mehl et al., 2014). Of note, similar results were observed in response to P. aeruginosa wound infection in a burn model in mice (Everett et al., 2017). Our results are therefore consistent with these observations as limiting myeloid arginase-1 is associated with excessive morbidity while in mice with macrophages capable of producing arginase-1, inflammation in response to P. aeruginosa was well-controlled.
While the studies described above demonstrate the control of NO production by arginase-1 in the lungs, the impact on the bacterial burden was not evaluated (Bielen et al., 2017; Mehl et al., 2014). Importantly, neither in our previous work examining early polarization to an M2-dominant macrophage response, nor in the current study with the absence of myeloid arginase-1, was the clearance rate of P. aeruginosa impacted (Feola et al., 2010). This suggests that the suppression of NO-mediated killing of P. aeruginosa is not significantly altered by increased or decreased arginase activity in the lungs. This coincides with clinical studies using azithromycin to impact the dysregulated inflammation associated with chronic P. aeruginosa colonization in patients with CF, as long-term azithromycin therapy does not seem to suppress bacterial clearance in these patients (Equi et al., 2002; Nichols et al., 2020; Saiman et al., 2003; Saiman et al., 2010; Wolter et al., 2002).
In addition to its control of NO production, arginase-1 immunoregulates by controlling T cell activation and proliferation (Munder et al., 2006; Rodriguez et al., 2007). T cells are highly sensitive to the depletion of arginine and other amino acids essential for their proliferation and function (Fletcher et al., 2015; Rodriguez et al., 2003; Rodriguez et al., 2007). Arginase-mediated arginine depletion impacts T cell-mediated responses in a variety of infection models and inflammatory processes including trauma, asthma, glomerulonephritis, and malignancy (Munder, 2009). The mechanisms vary and include the modulation of Th1 and Th2 cytokine expression, suppression of immune recognition and rejection of tumor cells, suppression of overall T cell proliferation, and modulation of regulatory T cell (Treg) and Th17 balance (Das et al., 2010; Herbert et al., 2010; Pesce et al., 2009; Rodriguez and Ochoa, 2008; Taylor et al., 2006; Zea et al., 2004). Much of this suppression is executed by myeloid derived suppressor cells (MDSC), which control T cells either by depriving them of arginine or indirectly through induction of Treg differentiation or the induction of tumor associated macrophages (Makarenkova et al., 2006; Rodriguez and Ochoa, 2008; Ugel et al., 2015). However, investigation of the relationship between arginine metabolism and Th17 responses has produced disparate results depending on the disease model. MDSCs producing high amounts of arginase-1 and have been shown to promote and suppress Th17-mediated immunity, depending on the disease being studied (Herbert et al., 2010; Jianjun et al., 2013; Obermajer et al., 2013; Wu et al., 2016). Our data indicate that in extracellular bacterial pneumonia, the impact of myeloid arginase-1 expression is to suppress Th17 responses. While this result is in agreement with previous studies that demonstrate a protective effect from M2 macrophage polarization through control of neutrophil influx, additional study of the interaction to P. aeruginosa is required.
Responses driven by IL-17 during P. aeruginosa pneumonia have been investigated and may contribute to pulmonary pathology in the chronic phase. IL-17 is critical for the clearance of P. aeruginosa, and the main source during the acute phase of infection is γδ T cells and NKT cells (Coquet et al., 2008; Sutton et al., 2012). In a study of C57BL/6 mice infected with P. aeruginosa incorporated into agarose beads, the absence of IL-17RA hampered bacterial clearance (Bayes et al., 2016). However, while there were no differences in lung histological scores, IL-17RA knockout mice lost less weight than WT mice (Bayes et al., 2016). This suggests that while IL-17 signaling promotes efficient bacterial clearance, the response comes at a cost. Similar results demonstrating that IL-17-associated responses contribute more to lung damage than control of the infection were observed IL-23p19 knockout mice infected with P. aeruginosa (Dubin and Kolls, 2007). IL-17 was not produced in these mice, and while bacterial clearance was not affected, neutrophil influx and intraluminal and peribronchial inflammation were dramatically reduced (Dubin and Kolls, 2007). While no direct link has been made establishing a relationship between arginase-1 expression and Th17 proliferation or activation in extracellular bacterial pneumonia, the timing of increased arginase-1 and decreased IL-17-mediated gene expression in the chronic phase of P. aeruginosa pneumonia in mice is compelling (Bielen et al., 2017; Feola et al., 2010). Additionally, our results demonstrating excessive numbers of Th17 cells in both the lungs and lymph nodes after P. aeruginosa infection in mice that lack myeloid arginase-1 support this interaction as a possible mechanism for future study.
Dysregulation of T cell immunity plays a major role in the neutrophil-driven chronic inflammation of the CF lung, and the Th17 response is highly contributory (McAllister et al., 2005; Tan et al., 2011; Way et al., 2013). As a result of many factors, inflammation in patients with CF continues to be neutrophil-driven over time, described as a “sustained acute response” in contrast to a maturing, chronic inflammation (Elizur et al., 2008; Nichols and Chmiel, 2015). Additionally, efferocytosis of neutrophils is impaired in the CF airway, which leads to increased inflammatory cytokines including IL-17, thereby contributing to additional neutrophil recruitment (Dubin et al., 2007; Liu et al., 2008; McAllister et al., 2005). Lavage and sputum samples from patients with CF infected with P. aeruginosa show elevated concentrations of IL-17 and IFNγ, which are associated with poor lung function and prognosis (Kushwah et al., 2013; Tan et al., 2011). Conversely, proliferation and function of Treg are impaired in CF patients with chronic P. aeruginosa infection (Hector et al., 2015). Taken in the context of this literature, our results demonstrating disproportionate T cell responses in the absence of myeloid arginase-1 support that Th17 and Th1 phenotypes are likely driving the exaggerated immune response and pulmonary injury observed in the infected mice.
Critical differences in macrophage responses between mouse strains is one factor in the investigation of macrophage biology that makes translating findings to humans difficult (Murray, 2017). Aspects of arginine metabolism in macrophages are different in typical Th1-dominant mouse strains (C57BL/6) compared to those observed in typical Th2-dominant strains (BALB/c) (Mills et al., 2000). Seminal work defining macrophage phenotypes demonstrated differences in the activation of arginine metabolism in M1 versus M2 cells in response to the same stimuli, including LPS (Mills et al., 2000). Macrophages from C57BL/6 mice preferentially produce NO and macrophages from BALB/c mice preferentially produce ornithine when stimulated with LPS, although IFNγ equalizes the responses somewhat. This impacts subsequent lymphocyte proliferation and differences in Th1- and Th2-related cytokine production (Mills et al., 2000). Studies show that BALB/c mice are more resistant to infections with P. aeruginosa whereas the C57BL/6 mice are more susceptible. Robust neutrophil influx in C57BL/6 mice is associated with extensive bronchiectasis, while BALB/c mice have a monocyte-dominant response associated with little tissue damage (Sapru et al., 1999; Tam et al., 1999). Indeed, our studies required a log-fold increase in the inoculum of BALB/c mice compared to that used to infect C57BL/6 mice to elicit similar morbidity and lung inflammation.
Arginase inhibition using the small molecule inhibitor BEC in BALB/c mice did not recapitulate the detrimental effects from exaggerated inflammation observed in the Arg1ΔM mice (on the C57BL/6 background). The major difference between the 2 models was reflected in the impact that the lack of arginase activity had on T cells. Systemic arginase inhibition in BALB/c mice did not lead to exaggerated Th17 populations as observed in the Arg1ΔM mice, and had the opposite effect of decreasing the number of Th1 cells in the lungs compared to the Th1 increase in the Arg1ΔM mice. This is congruent with the work described above that demonstrated that macrophages from C57BL/6 mice stimulated in vitro with LPS and IFNγ cause lymphocytes to produce high amounts of IFNγ, whereas macrophages from BALB/c mice cause them instead to increase production of TGFβ, which promotes a reparative role (Mills et al., 2000). Reflected in these differences is the impact we observed on bacterial clearance, as C57BL/6 mice cleared P. aeruginosa to lower levels by day 10 post-infection as compared to the BALB/c mice, which still had substantial mean CFU counts. The 10-fold higher inoculum in the BALB/c mice caused similar lung injury scores in both the BEC-treated and control groups as the WT C57BL/6 mice, with the Arg1ΔM mice experiencing significantly more damage. Another potential cause for the difference in effect could be due to arginase production by ILC2s. In pathologies driven by Th2-mediated responses, it is arginase-1 expressed by ILC2s and not myeloid cells that governs inflammation and reduces cytokine release (Monticelli et al., 2016). While it is unknown whether ILC2 production of arginase-1 is involved in pulmonary responses to P. aeruginosa, a significant number of cells in the lymphocyte/lymphoid gate in the flow cytometric analysis of the lungs from Arg1ΔM mice were producing arginase (Supplemental Fig. S2).
The reduction of neutrophil infiltration of the airways in mice treated with BEC could be explained by an inhibition of arginase-1 expressed by airway epithelial cells and vascular endothelial cells. These cells produce arginase-1 that reduces eNOS-derived NO thereby causing a loss in barrier function, as both low and high NO levels will destabilize endothelial barriers (Lucas et al., 2013). Although not directly tested, the increased immune cell influx in the Arg1ΔM mice could be partially due to increased barrier dysfunction in the airways because arginase-1 was still active in endothelial and epithelial cells. Treatment with the arginase inhibitors would have countered this response by inhibiting the enzyme in all cells. Indeed, BEC has been demonstrated to blunt endothelial hyper-permeability during lung injury that normally occurs due to the reduction of NO (Huynh et al., 2009; Lucas et al., 2012). However, other studies suggest that high levels of NO, by impairing pulmonary vascular permeability, increase fluid content in the lung tissues and alveoli thus impeding adhesion and migration of immune cells across the destabilized junctions (Ckless et al., 2008; Worrall et al., 1997). The fact that P. aeruginosa infected mice treated with BEC had increased perivascular and peribronchial inflammation may be due to high NO levels, although this was not directly measured. These results reflect the complexity of arginine metabolism and arginase-1 production by multiple cell types, making arginase-1 a difficult therapeutic target. More study is needed to examine the interactions between immune cells and endothelial and epithelial cells during infection, especially in the CF lungs where airway integrity is compromised (Nichols and Chmiel, 2015).
Conclusions
Our results demonstrate that myeloid arginase-1 regulates inflammation in chronic P. aeruginosa pneumonia in mice prone to M1-mediated responses. We show that arginase production by myeloid cells is essential in protecting against excessive morbidity and inflammation. To our knowledge, we are the first to show that deletion of arginase-1 from myeloid cells is associated with an exaggerated influx of neutrophils and pro-inflammatory macrophages, and a T cell polarization that favors Th1 and Th17 phenotypes in response to P. aeruginosa pneumonia. Arginase inhibition in BALB/c mice, in contrast, produces a different effect on immune cell recruitment, activation, and chemotaxis. Targeting arginase-1 for the treatment of patients with CF in whom P. aeruginosa infection drives pulmonary functional decline is complicated by the production of the enzyme by multiple cell types. However, it does appear that manipulation of arginine metabolism could lessen the damage associated with excessive inflammation without significantly impacting the protective microbial killing effects governed by NO.
Supplementary Material
Acknowledgements
The authors wish to thank Melissa Hollifield and Cynthia Mattingly for their technical support. We would like to also acknowledge the Flow Cytometry and the Pathology core facilities at the University of Kentucky for their services.
Funding
This work was supported by the National Institutes of Health via grant number R01AI095307 to D.J.F.
Abbreviations:
- BEC
S-(2-boronoethyl)-l-cysteine hydrochloride
- MDSC
myeloid derived suppressor cells
- MR
mannose receptor
- ILC2
group-2 innate lymphoid cells
Footnotes
Declaration of Competing Interest
None.
Declaration of Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anthony RM, Urban JF Jr., Alem F, Hamed HA, Rozo CT, Boucher JL, et al. , 2006. Memory T(H)2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nat. Med 12 (8), 955–960. 10.1038/nm1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron L, Smith AM, El Kasmi KC, Qualls JE, Huang X, Cheever A, et al. , 2013. Role of arginase 1 from myeloid cells in th2-dominated lung inflammation. PLoS One 8 (4), e61961 10.1371/journal.pone.0061961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayes HK, Ritchie ND, Evans TJ, 2016. Interleukin-17 Is required for control of chronic lung infection caused by Pseudomonas aeruginosa. Infect. Immun 84 (12), 3507–3516. 10.1128/IAI.00717-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielen K, s Jongers B, Boddaert J, Raju TK, Lammens C, Malhotra-Kumar S, et al. , 2017. Biofilm-induced type 2 innate immunity in a cystic fibrosis model of Pseudomonas aeruginosa . Front. Cell Infect. Microbiol 7 274 10.3389/fcimb.2017.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell TS, Stecenko AA, Christman JW, 2001. Dysregulated NF-kappaB activation in cystic fibrosis: evidence for a primary inflammatory disorder. Am. J. Physiol. Lung. Cell Mol. Physiol 281 (1), L69–70. 10.1152/ajplung.2001.281.1.L69. [DOI] [PubMed] [Google Scholar]
- Bogdan C, 2001a. Nitric oxide and the immune response. Nat. Immunol 2 (10), 907–916. 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- Bogdan C, 2001b. Nitric oxide and the regulation of gene expression. Trends Cell. Biol 11 (2), 66–75. 10.1016/s0962-8924(00)01900-0. [DOI] [PubMed] [Google Scholar]
- Bronte V, Serafini P, Mazzoni A, Segal DM, Zanovello P, 2003. L-arginine metabolism in myeloid cells controls T-lymphocyte functions. Trends Immunol 24 (6), 302–306. 10.1016/s1471-4906(03)00132-7. [DOI] [PubMed] [Google Scholar]
- Bruscia EM, Zhang PX, Ferreira E, Caputo C, Emerson JW, Tuck D, et al. , 2009. Macrophages directly contribute to the exaggerated inflammatory response in cystic fibrosis transmembrane conductance regulator−/− mice. Am. J. Respir. Cell. Mol. Biol 40 (3), 295–304. 10.1165/rcmb.2008-0170OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busnel O, Carreaux F, Carboni B, Pethe S, Goff SV, Mansuy D, et al. , 2005. Synthesis and evaluation of new omega-borono-alpha-amino acids as rat liver arginase inhibitors. Bioorg. Med. Chem 13 (7), 2373–2379. 10.1016/j.bmc.2005.01.053. [DOI] [PubMed] [Google Scholar]
- Ckless K, Lampert A, Reiss J, Kasahara D, Poynter ME, Irvin CG, et al. , 2008. Inhibition of arginase activity enhances inflammation in mice with allergic airway disease, in association with increases in protein S-nitrosylation and tyrosine nitration. J. Immunol 181 (6), 4255–4264. 10.4049/jimmunol.181.6.4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coquet JM, Chakravarti S, Kyparissoudis K, McNab FW, Pitt LA, McKenzie BS, et al. , 2008. Diverse cytokine production by NKT cell subsets and identification of an IL-17-producing CD4-NK1.1- NKT cell population. Proc. Natl. Acad. Sci. U. S. A 105 (32), 11287–11292. 10.1073/pnas.0801631105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das P, Lahiri A, Lahiri A, Chakravortty D, 2010. Modulation of the arginase pathway in the context of microbial pathogenesis: a metabolic enzyme moonlighting as an immune modulator. PLoS Pathog 6 (6), e1000899 10.1371/journal.ppat.1000899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckman JM, Kurkjian CJ, McGillis JP, Cory TJ, Birket SE, Schutzman LM, et al. , 2017. Pneumocystis infection alters the activation state of pulmonary macrophages. Immunobiology 222 (2), 188–197. 10.1016/j.imbio.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin PJ, Kolls JK, 2007. IL-23 mediates inflammatory responses to mucoid Pseudomonas aeruginosa lung infection in mice. Am. J. Physiol. Lung Cell. Mol. Physiol 292 (2), L519–528. 10.1152/ajplung.00312.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin PJ, McAllister F, Kolls JK, 2007. Is cystic fibrosis a TH17 disease? Inflamm. Res 56 (6), 221–227. 10.1007/s00011-007-6187-2. [DOI] [PubMed] [Google Scholar]
- Duleu S, Vincendeau P, Courtois P, Semballa S, Lagroye I, Daulouede S, et al. , 2004. Mouse strain susceptibility to trypanosome infection: an arginase-dependent effect. J. Immunol 172 (10), 6298–6303. 10.4049/jimmunol.172.10.6298. [DOI] [PubMed] [Google Scholar]
- El-Bassossy HM, El-Fawal R, Fahmy A, 2012. Arginase inhibition alleviates hypertension associated with diabetes: effect on endothelial dependent relaxation and NO production. Vascul. Pharmacol 57 (5–6), 194–200. 10.1016/j.vph.2012.01.001. [DOI] [PubMed] [Google Scholar]
- El Kasmi KC, Qualls JE, Pesce JT, Smith AM, Thompson RW, Henao-Tamayo M, et al. , 2008. Toll-like receptor-induced arginase 1 in macrophages thwarts effective immunity against intracellular pathogens. Nat. Immunol 9 (12), 1399–1406. 10.1038/ni.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elizur A, Cannon CL, Ferkol TW, 2008. Airway inflammation in cystic fibrosis. Chest 133 (2), 489–495. 10.1378/chest.07-1631. [DOI] [PubMed] [Google Scholar]
- Equi A, Balfour-Lynn IA, Bush A, Rosenthal M, 2002. Long term azithromycin in children with cystic fibrosis: a randomised, placebo-controlled crossover trial. Lancet 360 (9338), 978–984. 10.1016/s0140-6736(02)11081-6. [DOI] [PubMed] [Google Scholar]
- Everett J, Turner K, Cai Q, Gordon V, Whiteley M, Rumbaugh K, 2017. Arginine is a critical substrate for the pathogenesis of Pseudomonas aeruginosa in burn wound infections. mBio 8 (2), e02160–02116. 10.1128/mBio.02160-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feola DJ, Garvy BA, Cory TJ, Birket SE, Hoy H, Hayes D Jr., et al. , 2010. Azithromycin alters macrophage phenotype and pulmonary compartmentalization during lung infection with Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 54 (6), 2437–2447. 10.1128/AAC.01424-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher M, Ramirez ME, Sierra RA, Raber P, Thevenot P, Al-Khami AA, et al. , 2015. l-Arginine depletion blunts antitumor T-cell responses by inducing myeloid-derived suppressor cells. Cancer Res 75 (2), 275–283. 10.1158/0008-5472.CAN-14-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S, 2003. Alternative activation of macrophages. Nat. Rev. Immunol 3 (1), 23–35. 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- Gordon S, Taylor PR, 2005. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol 5 (12), 953–964. 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- Haydar D, Cory TJ, Birket SE, Murphy BS, Pennypacker KR, Sinai AP, et al. , 2019. Azithromycin polarizes macrophages to an M2 phenotype via inhibition of the STAT1 and NF-kappaB signaling pathways. J. Immunol 203 (4), 1021–1030. 10.4049/jimmunol.1801228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hector A, Schafer H, Poschel S, Fischer A, Fritzsching B, Ralhan A, et al. , 2015. Regulatory T-cell impairment in cystic fibrosis patients with chronic Pseudomonas infection. Am. J. Respir. Crit. Care Med 191 (8), 914–923. 10.1164/rccm.201407-1381OC. [DOI] [PubMed] [Google Scholar]
- Herbert DR, Orekov T, Roloson A, Ilies M, Perkins C, O’Brien W, et al. , 2010. Arginase I suppresses IL-12/IL-23p40-driven intestinal inflammation during acute schistosomiasis. J. Immunol 184 (11), 6438–6446. 10.4049/jimmunol.0902009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh NN, Harris EE, Chin-Dusting JF, Andrews KL, 2009. The vascular effects of different arginase inhibitors in rat isolated aorta and mesenteric arteries. Br. J. Pharmacol 156 (1), 84–93. 10.1111/j.1476-5381.2008.00036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iniesta V, Carcelen J, Molano I, Peixoto PM, Redondo E, Parra P, et al. , 2005. Arginase I induction during Leishmania major infection mediates the development of disease. Infect. Immun. 73 (9), 6085–6090. 10.1128/IAI.73.9.6085-6090.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jianjun Y, Zhang R, Lu G, Shen Y, Peng L, Zhu C, et al. , 2013. T cell-derived inducible nitric oxide synthase switches off Th17 cell differentiation. J. Exp. Med 210 (7), 1447–1462. 10.1084/jem.20122494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang K, Shu XL, Zhong JX, Yu TT, 2014. Effect of L-arginine on immune function: a meta-analysis. Asia Pac. J. Clin. Nutr 23 (3), 351–359. 10.6133/apjcn.2014.23.3.09. [DOI] [PubMed] [Google Scholar]
- Kushwah R, Gagnon S, Sweezey NB, 2013. Intrinsic predisposition of naive cystic fibrosis T cells to differentiate towards a Th17 phenotype. Respir. Res 14 (1), 138 10.1186/1465-9921-14-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie EG, Wangdi T, Kazmierczak BI, 2011. Innate immune responses to Pseudomonas aeruginosa infection. Microbes Infect 13 (14–15), 1133–1145. 10.1016/j.micinf.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Wang J, Park YJ, Tsuruta Y, Lorne EF, Zhao X, et al. , 2008. High mobility group protein-1 inhibits phagocytosis of apoptotic neutrophils through binding to phosphatidylserine. J. Immunol 181 (6), 4240–4246. 10.4049/jimmunol.181.6.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas R, Yang G, Gorshkov BA, Zemskov EA, Sridhar S, Umapathy NS, et al. , 2012. Protein kinase C-alpha and arginase I mediate pneumolysin-induced pulmonary endothelial hyperpermeability. Am. J. Respir Cell. Mol. Biol 47 (4), 445–453. 10.1165/rcmb.2011-0332OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas R, Czikora I, Sridhar S, Zemskov EA, Oseghale A, Circo S, et al. , 2013. Arginase 1: an unexpected mediator of pulmonary capillary barrier dysfunction in models of acute lung injury. Front. Immunol 4, 228 10.3389/fimmu.2013.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarenkova V, Bansal V, Matta B, Perez L, Ochoa J, 2006. CD11b+/Gr−1+ myeloid suppressor cells cause T cell dysfunction after traumatic stress. J. Immunol 176 (4), 2085–2094. 10.4049/jimmunol.176.4.2085. [DOI] [PubMed] [Google Scholar]
- Matute-Bello G, Downey G, Moore BB, Groshong SD, Matthay MA, Slutsky AS, et al. , 2011. An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals. Am. J. Respir. Cell. Mol. Biol 44 (5), 725–738. 10.1165/rcmb.2009-0210ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister F, Henry A, Kreindler JL, Dubin PJ, Ulrich L, Steele C, et al. , 2005. Role of IL-17A, IL-17F, and the IL-17 receptor in regulating growth-related oncogene-alpha and granulocyte colony-stimulating factor in bronchial epithelium: implications for airway inflammation in cystic fibrosis. J. Immunol 175 (1), 404–412. 10.4049/jimmunol.175.1.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLarren KW, Cole AE, Weisser SB, Voglmaier NS, Conlin VS, Jacobson K, et al. , 2011. SHIP-deficient mice develop spontaneous intestinal inflammation and arginase-dependent fibrosis. Am. J. Pathol 179 (1), 180–188. 10.1016/j.ajpath.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehl A, Ghorbani P, Douda D, Huang H, Palaniyar N, Ratjen F, et al. , 2014. Effect of arginase inhibition on pulmonary L-arginine metabolism in murine Pseudomonas pneumonia. PLoS One 9 (3), e90232 10.1371/journal.pone.0090232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM, 2000. M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol 164 (12), 6166–6173. 10.4049/jimmunol.164.12.6166. [DOI] [PubMed] [Google Scholar]
- Modolell M, Corraliza IM, Link F, Soler G, Eichmann K, 1995. Reciprocal regulation of the nitric oxide synthase/arginase balance in mouse bone marrow-derived macrophages by TH1 and TH2 cytokines. Eur. J. Immunol 25 (4), 1101–1104. 10.1002/eji.1830250436. [DOI] [PubMed] [Google Scholar]
- Monticelli LA, Buck MD, Flamar AL, Saenz SA, Tait Wojno ED, Yudanin NA, et al. , 2016. Arginase 1 is an innate lymphoid-cell-intrinsic metabolic checkpoint controlling type 2 inflammation. Nat. Immunol 17 (6), 656–665. 10.1038/ni.3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton J, Snider TA, 2017. Guidelines for collection and processing of lungs from aged mice for histological studies. Pathobiol. Aging Age Relat. Dis 7 (1), 1313676 10.1080/20010001.2017.1313676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossner J, Hammermann R, Racke K, 2001. Concomitant down-regulation of L-arginine transport and nitric oxide (NO) synthesis in rat alveolar macrophages by the polyamine spermine. Pulm. Pharmacol. Ther 14 (4), 297–305. 10.1006/pupt.2001.0297. [DOI] [PubMed] [Google Scholar]
- Munder M, Eichmann K, Modolell M, 1998. Alternative metabolic states in murine macrophages reflected by the nitric oxide synthase/arginase balance: competitive regulation by CD4+ T cells correlates with Th1/Th2 phenotype. J. Immunol 160 (11), 5347–5354. [PubMed] [Google Scholar]
- Munder M, Schneider H, Luckner C, Giese T, Langhans CD, Fuentes JM, et al. , 2006. Suppression of T-cell functions by human granulocyte arginase. Blood 108 (5), 1627–1634. 10.1182/blood-2006-11-010389. [DOI] [PubMed] [Google Scholar]
- Munder M, 2009. Arginase: an emerging key player in the mammalian immune system. Br. J. Pharmacol. 158 (3), 638–651. 10.1111/j.1476-5381.2009.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy BS, Sundareshan V, Cory TJ, Hayes D Jr, Anstead MI, Feola DJ, 2008. Azithromycin alters macrophage phenotype. J. Antimicrob. Chemother 61 (3), 554–560. 10.1093/jac/dkn007. [DOI] [PubMed] [Google Scholar]
- Murray PJ, Wynn TA, 2011. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol 11 (11), 723–737. 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PJ, 2017. Macrophage Polarization. Ann. Rev. Physiol 79 541–566. 10.1146/annurev-physiol-022516-034339. [DOI] [PubMed] [Google Scholar]
- Nacucchio MC, Cerquetti MC, Meiss RP, Sordelli DO, 1984. Short communication. Role of agar beads in the pathogenicity of Pseudomonas aeruginosa in the rat respiratory tract. Pediatr. Res 18 (3), 295–296. 10.1203/00006450-198403000-00018. [DOI] [PubMed] [Google Scholar]
- Nair AB, Jacob S, 2016. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm 7 (2), 27–31. 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nau GJ, Richmond JF, Schlesinger A, Jennings EG, Lander ES, Young RA, 2002. Human macrophage activation programs induced by bacterial pathogens. Proc. Natl. Acad. Sci. U. S. A 99 (3), 1503–1508. 10.1073/pnas.022649799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols DP, Chmiel JF, 2015. Inflammation and its genesis in cystic fibrosis. Pediatr. Pulmonol 50 Suppl 40, S39–56. 10.1002/ppul.23242. [DOI] [PubMed] [Google Scholar]
- Nichols DP, Odem-Davis K, Cogen JD, Goss CH, Ren CL, Skalland M, et al. , 2020. Pulmonary outcomes associated with long-term azithromycin therapy in cystic fibrosis. Am. J. Respir. Crit. Care Med 201 (4), 430–437. 10.1164/rccm.201906-1206OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermajer N, Wong JL, Edwards RP, Chen K, Scott M, Khader S, et al. , 2013. Induction and stability of human Th17 cells require endogenous NOS2 and cGMP-dependent NO signaling. J. Exp. Med 210 (7), 1433–1445. 10.1084/jem.20121277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesce JT, Ramalingam TR, Mentink-Kane MM, Wilson MS, El Kasmi KC, Smith AM, et al. , 2009. Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis. PLoS Pathog 5 (4), e1000371 10.1371/journal.ppat.1000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rath M, Muller I, Kropf P, Closs EI, Munder M, 2014. Metabolism via arginase or nitric oxide synthase: two competing arginine pathways in macrophages. Front. Immunol 5, 532 10.3389/fimmu.2014.00532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez PC, Zea AH, DeSalvo J, Culotta KS, Zabaleta J, Quiceno DG, et al. , 2003. L-arginine consumption by macrophages modulates the expression of CD3 zeta chain in T lymphocytes. J. Immunol 171 (3), 1232–1239. 10.4049/jimmunol.171.3.1232. [DOI] [PubMed] [Google Scholar]
- Rodriguez PC, Quiceno DG, Ochoa AC, 2007. L-arginine availability regulates T-lymphocyte cell-cycle progression. Blood 109 (4), 1568–1573. 10.1182/blood-2006-06-031856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez PC, Ochoa AC, 2008. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: mechanisms and therapeutic perspectives. Immunol. Rev 222, 180–191. 10.1111/j.1600-065X.2008.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiman L, Marshall BC, Mayer-Hamblett N, Burns JL, 2003. Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trial. J. Am. Med. Assoc 290 (13), 1749–1756. 10.1001/jama.290.13.1749. [DOI] [PubMed] [Google Scholar]
- Saiman L, Anstead M, Mayer-Hamblett N, Lands LC, Kloster M, Hocevar-Trnka J, et al. , 2010. Effect of azithromycin on pulmonary function in patients with cystic fibrosis uninfected with Pseudomonas aeruginosa: a randomized controlled trial. J. Am. Med. Assoc 303 (17), 1707–1715. 10.1001/jama.2010.563. [DOI] [PubMed] [Google Scholar]
- Sapru K, Stotland PK, Stevenson MM, 1999. Quantitative and qualitative differences in bronchoalveolar inflammatory cells in Pseudomonas aeruginosa-resistant and -susceptible mice. Clin. Exp. Immunol 115 (1), 103–109. 10.1046/j.1365-2249.1999.00762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton CE, Mielke LA, Mills KH, 2012. IL-17-producing gammadelta T cells and innate lymphoid cells. Eur. J. Immunol 42 (9), 2221–2231. 10.1002/eji.201242569. [DOI] [PubMed] [Google Scholar]
- Tam M, Snipes GJ, Stevenson MM, 1999. Characterization of chronic bronchopulmonary Pseudomonas aeruginosa infection in resistant and susceptible inbred mouse strains. Am. J. Respir. Cell. Mol. Biol 20 (4), 710–719. 10.1165/ajrcmb.20.4.3223. [DOI] [PubMed] [Google Scholar]
- Tan HL, Regamey N, Brown S, Bush A, Lloyd CM, Davies JC, 2011. The Th17 pathway in cystic fibrosis lung disease. Am. J. Respir. Crit. Care Med 184 (2), 252–258. 10.1164/rccm.201102-0236OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MD, Harris A, Nair MG, Maizels RM, Allen JE, 2006. F4/80+ alternatively activated macrophages control CD4+ T cell hyporesponsiveness at sites peripheral to filarial infection. J. Immunol 176 (11), 6918–6927. 10.4049/jimmunol.176.11.6918. [DOI] [PubMed] [Google Scholar]
- Tiringer K, Treis A, Fucik P, Gona M, Gruber S, Renner S, et al. , 2013. A Th17- and Th2-skewed cytokine profile in cystic fibrosis lungs represents a potential risk factor for Pseudomonas aeruginosa infection. Am. J. Respir. Crit. Care Med 187 (6), 621–629. 10.1164/rccm.201206-1150OC. [DOI] [PubMed] [Google Scholar]
- Ugel S, De Sanctis F, Mandruzzato S, Bronte V, 2015. Tumor-induced myeloid deviation: when myeloid-derived suppressor cells meet tumor-associated macrophages. J. Clin. Invest 125 (9), 3365–3376. 10.1172/JCI80006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heeckeren AM, Schluchter MD, 2002. Murine models of chronic Pseudomonas aeruginosa lung infection. Lab. Anim 36 (3), 291–312. 10.1258/002367702320162405. [DOI] [PubMed] [Google Scholar]
- Way EE, Chen K, Kolls JK, 2013. Dysregulation in lung immunity - the protective and pathologic Th17 response in infection. Eur. J. Immunol 43 (12), 3116–3124. 10.1002/eji.201343713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijnands KA, Castermans TM, Hommen MP, Meesters DM, Poeze M, 2015. Arginine and citrulline and the immune response in sepsis. Nutrients 7 (3), 1426–1463. 10.3390/nu7031426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BJ, Dehnbostel J, Blackwell TS, 2010. Pseudomonas aeruginosa: host defence in lung diseases. Respirology 15 (7), 1037–1056. 10.1111/j.1440-1843.2010.01819.x. [DOI] [PubMed] [Google Scholar]
- Wolter J, Seeney S, Bell S, Bowler S, Masel P, McCormack J, 2002. Effect of long term treatment with azithromycin on disease parameters in cystic fibrosis: a randomised trial. Thorax 57 (3), 212–216. 10.1136/thorax.57.3.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worrall NK, Chang K, LeJeune WS, Misko TP, Sullivan PM, Ferguson TB Jr., et al. , 1997. TNF-alpha causes reversible in vivo systemic vascular barrier dysfunction via NO-dependent and -independent mechanisms. Am. J. Physiol 273 (6), H2565–2574. 10.1152/ajpheart.1997.273.6.H2565. [DOI] [PubMed] [Google Scholar]
- Wu H, Zhen Y, Ma Z, Li H, Yu J, Xu ZG, et al. , 2016. Arginase-1-dependent promotion of TH17 differentiation and disease progression by MDSCs in systemic lupus erythematosus. Sci. Transl. Med 8 (331), 331ra340 10.1126/scitranslmed.aae0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeramian A, Martin L, Serrat N, Arpa L, Soler C, Bertran J, et al. , 2006. Arginine transport via cationic amino acid transporter 2 plays a critical regulatory role in classical or alternative activation of macrophages. J. Immunol 176 (10), 5918–5924. 10.4049/jimmunol.176.10.5918. [DOI] [PubMed] [Google Scholar]
- Yu H, Nasr SZ, Deretic V, 2000. Innate lung defenses and compromised Pseudomonas aeruginosa clearance in the malnourished mouse model of respiratory infections in cystic fibrosis. Infect. Immun 68 (4), 2142–2147. 10.1128/iai.68.4.2142-2147.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zea AH, Rodriguez PC, Culotta KS, Hernandez CP, DeSalvo J, Ochoa JB, et al. , 2004. L-Arginine modulates CD3zeta expression and T cell function in activated human T lymphocytes. Cell. Immunol 232 (1–2), 21–31. 10.1016/j.cellimm.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Zhang M, Caragine T, Wang H, Cohen PS, Botchkina G, Soda K, et al. , 1997. Spermine inhibits proinflammatory cytokine synthesis in human mononuclear cells: a counterregulatory mechanism that restrains the immune response. J. Exp. Med 185 (10), 1759–1768. 10.1084/jem.185.10.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.