Abstract
Background:
Major gaps exist in the routine initiation and dose up-titration of guideline-directed medical therapies (GDMT) for patients with heart failure with reduced ejection fraction (HFrEF). Without novel approaches to improve prescribing, the cumulative benefits of HFrEF treatment will be largely unrealized. Direct-to-consumer marketing and shared decision making reflect a culture where patients are increasingly involved in treatment choices, creating opportunities for prescribing interventions that engage patients.
Methods:
The Electronically delivered, Patient-activation tool for Intensification of medications for Chronic Heart Failure with reduced ejection fraction (EPIC-HF) trial randomized patients with HFrEF from a diverse health system to usual care versus patient-activation tools—a 3-minute video and 1-page checklist—delivered electronically 1 week prior, 3 days prior and 24 hours prior to a cardiology clinic visit. The tools encouraged patients to work collaboratively with their clinicians to “make one positive change” in HFrEF prescribing. The primary endpoint was the percent of patients with GDMT medication initiations and dose intensifications from immediately preceding the cardiology clinic visit to 30 days, compared to usual care during the same period.
Results:
EPIC-HF enrolled 306 patients, 290 of whom attended a clinic visit during the study period: 145 were sent the patient-activation tools and 145 were controls. Median age was 65 years, 29% female, 11% black, 7% Hispanic, median ejection fraction 32%. Pre-clinic data revealed significant GDMT opportunities, with no patients on target doses of beta-blocker, sacubitril/valsartan, and mineralocorticoid receptor antagonists. From immediately preceding the cardiology clinic visit to 30 days later, 49.0% in the intervention and 29.7% in control experienced an initiation or intensification of their GDMT (p=0.001). The majority of these changes were made at the clinician encounter itself and involved dose uptitrations. There were no deaths, and no significant differences in hospitalization or emergency department visits at 30 days between groups.
Conclusion:
A patient-activation tool delivered electronically prior to a cardiology clinic visit improved clinician intensification of GDMT.
(ClinicalTrials.gov Identifier: NCT03334188)
Keywords: heart failure, quality, clinical trial, outcomes
Introduction
Advances in medicine have revolutionized the care of patients with heart failure with reduced ejection fraction (HFrEF). Multiple medications—evidence-based beta-blockers (EVBB), angiotensin converting enzyme inhibitors (ACEI), angiotensin receptor blockers (ARB), angiotensin receptor neprilysin inhibitors (ARNI), mineralocorticoid receptor agonists (MRA), hydralazine/isosorbide dinitrate (H/ISDN), and ivabradine—have been shown to improve cardiac function, quality of life, and survival for patients with HFrEF.1–4 Current HFrEF clinical practice guidelines recommend the use of these medications in combination and at target doses that have been shown to be beneficial in clinical trials, i.e., GDMT. Unfortunately, multiple studies have shown that prescribing of GDMT in routine clinical practice is suboptimal.5–8 While EVBB and ACEI/ARB are usually prescribed, few patients receive target doses.5 Meanwhile, a minority of patients receive MRA and ARNI.5
Gaps in the prescribing of GDMT are likely due to a variety of factors. While patient intolerance and physiological factors related to HFrEF or other comorbidities account for some portion of submaximal GDMT,9 clinical inertia is common. Achieving GDMT demands polypharmacy, iterative dose escalation, and active monitoring of patient symptoms, vital signs, and blood chemistries.3 Furthermore, clinicians have few ambulatory quality measures for GDMT and payers’ remuneration for clinic visits are relatively independent of prescribing. Consequently, continuation of current medical therapy is often the path of least resistance. Traditional quality improvement interventions designed to improve GDMT, such as clinician reminders, have been met with limited success.10–12 Without novel approaches to improve prescribing, the cumulative benefits of HFrEF treatment will be largely unrealized.
Both direct-to-consumer advertising (DTCA) and shared decision making (SDM) are promising strategies for improving prescribing of GDMT for HFrEF. Patients increasingly cite DTCA from pharmaceutical companies as the motivator for asking clinicians for drug information or new prescriptions,13, 14 and the majority of clinicians say exposure to DTCA prompts higher quality discussions between clinician and patient about treatment options.15 However, industry-based DTCA is generally limited to therapies still on-patent, may be hampered by real or perceived biases, and does not provide a global view of treatment options for a disease. At the same time, SDM—a communication process by which patients and clinicians work together to make optimal health care decisions that align with patient values and preferences—has become increasingly recognized as a key component of patient-centered care.16 Formal patient decision aids can support SDM;17 however, existing patient decision aids tend to focus on treatment decisions identified by a clinician, rather than directly prompting patients to identify treatment opportunities themselves.
In this context, we developed EPIC-HF18 which incorporates aspects of DTCA and SDM. The EPIC-HF intervention utilizes a patient-activation tool that combines a 3-minute video with a 1-page medication checklist. This study used an randomized control trial design to test the effectiveness of this patient-activation tool delivered before a cardiology-based clinic appointment to encourage patients to independently ask about opportunities for medication optimization, in turn prompting their prescribing clinicians to appropriately intensify GDMT.
Methods
The EPIC-HF trial (ClinicalTrials.gov Identifier: NCT03334188) is an American Heart Association-funded, Heart Failure Strategically Focused Research Network study designed to test the implementation, effectiveness, and safety of the EPIC-HF intervention. The study was a randomized clinical trial with patients randomized 1:1 to receive the intervention or usual care. A detailed description of the rationale, intervention development, and trial methods were previously published.18 In order to protect participant personal health information, data for this study are available from the corresponding author only upon reasonable request. Many of the supporting materials used in the study are present in the previously-published rationale, or are available upon request.
Patients and Setting
The EPIC-HF trial was conducted across the UCHealth system, involving patients with HFrEF and their cardiology clinicians. UCHealth services approximately 3.5 million individuals from 3 regions of the state (northern Colorado, southern Colorado, and metro Denver). The health system includes 6 cardiology clinics with a mix of academic and private-practice community-based delivery models and vary in size, demographic composition of patients served, and capture of urban, suburban, and rural populations. All UCHealth facilities use a single instance of the Epic electronic health record (Epic Systems, Verona, WI).
Enrollment occurred from January 2018 to January 2020. Subjects were required to be 18 years and older, have a left ventricular ejection fraction (LVEF) of <=40% on their most recent cardiac imaging study, and a history of HF. Due to the nature of the intervention, patients also had to be English-speaking, have cognitive capacity to engage in a prescribing discussion, and have either an active email address or a smartphone with texting capabilities. Patients with an estimated glomerular filtration rate <15 mL/min, listed for transplant, or enrolled in hospice were excluded. Prisoners and pregnant women were also excluded. During screening, 699 patients were initially identified through an automated list using field-coded LVEF measures in the electronic health record who also had a clinic visit with a cardiology clinician in the UCHealth system. Of these, 306 provided written informed consent, either in-person or by phone, and were enrolled (Figure 1). Screen failures were older and less likely to be seeing an advanced heart failure specialist, but otherwise were similar by gender, race/ethnicity, and LVEF. To be included in the study, patients needed to make a clinic visit following enrollment since delivery of the intervention required a patient-clinician interaction and because the pre-clinic medication data required for the outcome analysis was only available for patients that made a clinic visit. A relatively small number of enrolled patients (8 study and 8 control) were excluded because they did not make a clinic visit during the study period. There were no significant differences in baseline characteristics between the 8 study patients and 8 control patients who were excluded. All phases of the study procedures and personnel were approved by the Colorado Multiple Institutional Review Board.
Figure 1.
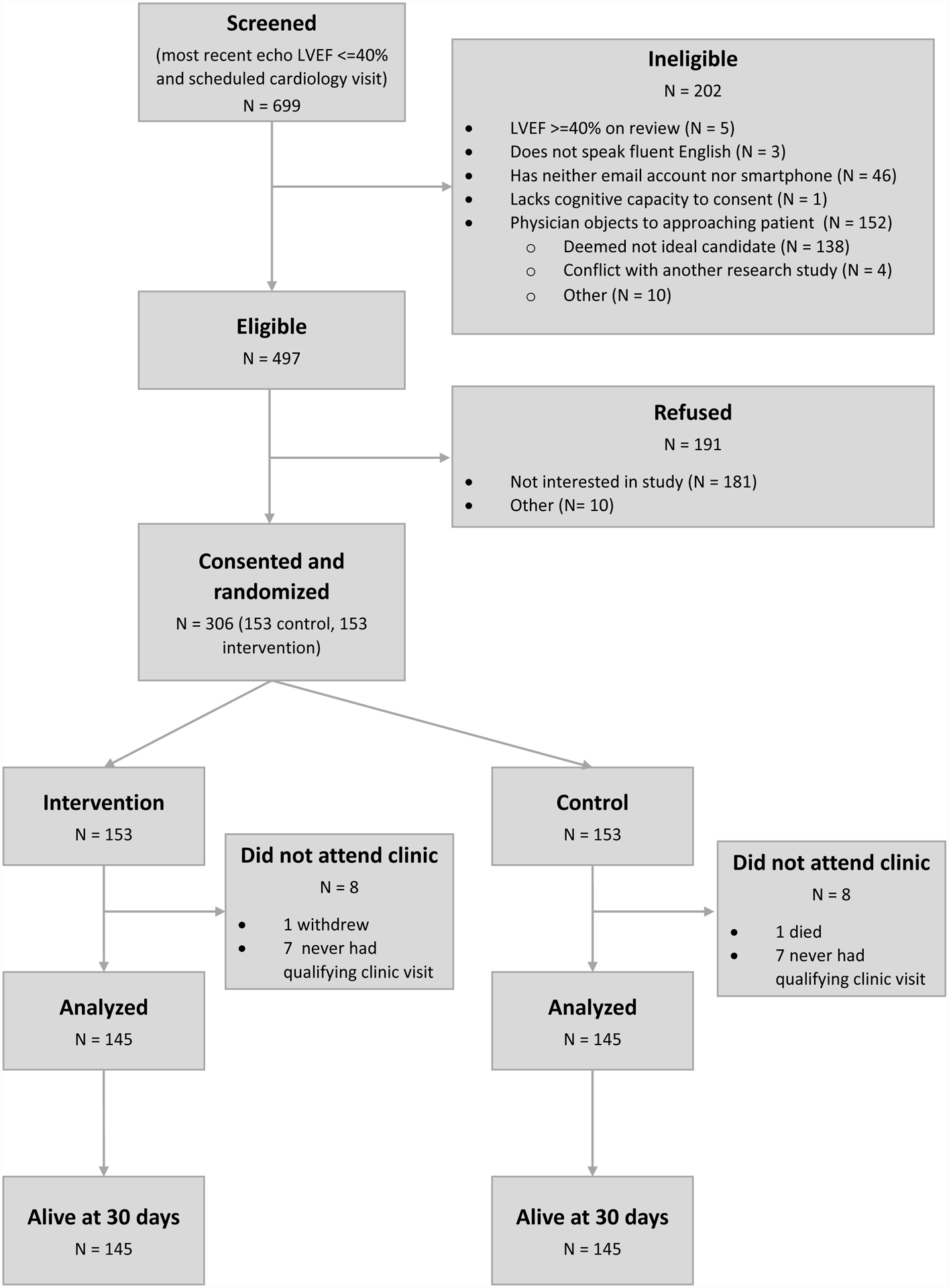
Consort diagram.
Intervention
The EPIC-HF patient-activation tool included a 3-minute video combined with a 1-page checklist. The design and pilot testing of the tool was previously published.18 In addition to following prior standards19 and incorporating extensive multi-stakeholder input into a user-centered design, development combined aspects of DTCA and SDM. The result was a novel intervention designed to address real-world informational needs, time constraints, and interactions found in most clinic appointments, while challenging clinicians to step out of the mindset of “not rocking the boat”. Using a “flipped classroom” model, the EPIC-HF tool was designed for electronic delivery before the visit and then asks the patient to use the information provided to engage their cardiology clinician in a conversation during the clinic visit. The final tool provided the flexibility for the patient and clinician to discuss as few or as many changes to the medication plan as appropriate for that patient at that time.
The short-animated video was electronically delivered to patients as a URL hyperlink that explains in lay terms the benefits of GDMT intensification, gaps in prescribing, reasons for clinical inertia, and rationale for patients engaging in prescribing discussions. The 1-page tool is a list of drug classes, each with associated medication names (generic and brand) and target doses for each medication (https://patientdecisionaid.org/heart_medications/). The checklist allows patients to fill in their current medications and dosing, and contrast optimal GDMT against their current medication regimen. Patients were encouraged to bring the checklist to the visit to promote a conversation about changing at least “one thing” to optimize medication management. GDMT was based off of the most recent American Heart Association / American College of Cardiology1, 2 and European Society of Cardiology4 guidelines for the diagnosis and treatment of heart failure. At enrollment, patients were given the choice of whether they would like to receive the materials by email, text message, or both. Their next upcoming cardiology-based ambulatory clinic visit was identified by automated notifications to the study team generated by the electronic health record. The time between enrollment and the clinic visit was naturalistic, determined by usual clinical care, and variable for each patient. Intervention delivery occurred at 3 time points for all patients: 1 week, 3 days, and 24 hours prior to the next cardiology clinic visit. A brief note regarding the intervention materials and their delivery to the patient was sent through the electronic health record to clinicians 24 hours prior to the clinic visit for intervention patients; no such notification was sent to clinicians before a control visit.
Outcomes Measures
The primary effectiveness endpoint was the percent of patients with a GDMT intensification from immediately preceding the study-qualifying cardiology clinic visit to 30 days later. GDMT intensification was defined as 1) initiation of EVBB, ACEI/ARB, ARNI, MRA, H/ISDN, or ivabradine, 2) a switch from ACEI/ARB to ARNI, or 3) dose intensification of these medications. Secondary outcomes included intensification type (initiation versus dose up-titration), intensification timing (at the clinic visit versus those occurring in the days to weeks after the visit), and total intensifications. Medication data was extracted from the electronic health record through review of medication reconciliation data, medication orders, and clinical notes. Safety was assessed by comparing the rate occurrence of emergency department visits, non-elective hospitalizations, and death at 30 days between the study and control groups. A survey was administered either electronically or mail to study patients, usually the day after the clinic visit, to collect self-reported use of the intervention.
Analysis
The percent of subjects with a GDMT intensification, and other dichotomous secondary outcomes, were compared between the control and intervention group using a 2-sided Fisher’s exact test. Risk ratios for the outcome were calculated from a log binomial model; risk ratios were calculated rather than odds ratios for ease of interpretation, as this study does not meet the rare outcomes assumption where the odds ratio approximates relative risk. Region-level effects were accounted for through the inclusion of region as a fixed effect (i.e., indicator variables for region in the model). Clinician-level clustering within region was tested through random effects but did not improve model fit and was not included in the final models. Number of intensifications was compared using a Poisson model, also with a fixed effect for site. The number of intensifications was confirmed to fit the Poisson distribution through an examination of within-group means and variances, and a test of the overdispersion parameter in a negative binomial (NB) model; the overdispersion parameter in the NB model was estimated to be zero for both count outcomes, and therefore we proceeded with the Poisson model for these outcomes. Primary and secondary analyses did not have any missing data, as one month of follow-up data was able to be obtained on 290 all patients from the electronic health record. Missing demographic information was excluded from Table 1. All analyses were performed in SAS version 9.4.
Table 1.
Patient characteristics.
| Control Median(IQR) or %(N) n=145 | Intervention Median(IQR) or %(N) n=145 | |
|---|---|---|
| Age, years | 64 (5–72) | 66 (58–74) |
| Female | 29.7% (43) | 28.3% (41) |
| Race | ||
| Black or African American | 11.3% (16) | 10.0% (14) |
| White | 85.1% (120) | 85.0% (119) |
| Another race or multiracial | 3.5% (5) | 5.0% (7) |
| Hispanic or Latino ethnicity | 6.3% (9) | 8.0% (11) |
| Employment | ||
| Employed | 31.5% (45) | 32.6% (46) |
| Retired | 41.3% (59) | 51.1% (72) |
| Unemployed | 27.3% (39) | 16.3% (23) |
| Insurance | ||
| Medicare, Tricare | 49.0% (71) | 50.4% (73) |
| Medicaid | 15.9% (23) | 14.5% (21) |
| Private | 33.1% (48) | 33.1% (48) |
| None | 2.1% (3) | 2.1% (3) |
| Income | ||
| Less than or equal to $20,000 | 22.8% (31) | 17.9% (24) |
| $20,001–$40,000 | 22.8% (31) | 12.7% (17) |
| $40,001–$60,000 | 11.0% (15) | 14.9% (20) |
| $60,001–$80,000 | 19.9% (27) | 21.6% (29) |
| Greater than $80,000 | 23.5% (32) | 32.8% (44) |
| Single relationship status | 41.0% (59) | 38.6% (56) |
| Can receive text messages | 59.3% (86) | 53.1% (77) |
| Systolic blood pressure, mmHg | 110 (102–124) | 112.5 (104–124) |
| Diastolic blood pressure, mmHg | 70 (62–80) | 70 (64–78) |
| Pulse, bpm | 74.5 (65.5–83) | 72.5 (68–80) |
| LVEF, percent | 32.5 (27–37.5) | 32.5 (25.2–37.5) |
| BNP, pg/mL | 266 (126–728) | 161.5 (90–444.5) |
| Serum creatinine, mg/dL | 1.1 (0.9–1.4) | 1.0 (0.9–1.2) |
| Serum potassium | 4.3 (3.9–4.6) | 4.2 (4.0–4.5) |
BNP=brain natriuretic peptide; BPM=beats per minute; LVEF=left ventricular ejection fraction
Data from day of cardiology clinic visit or closest to that day but not after.
Results
Cohort Characteristics
Overall 290 patients completed a cardiology clinic visit, 145 randomized to intervention and 145 to control (Figure 1). Overall, median age was 65 years, 29% were female, 11% were black, 7% were Hispanic, median LVEF was 32%, 134 patients were from the academic metropolitan-Denver region, and 171 patients from the community-practice regions in northern and southern Colorado. Cardiology clinic visits involved 56 physicians and 17 advanced practice providers. Brain natriuretic peptide and serum creatinine levels were lower in the intervention group; otherwise, there were no significant differences between intervention and control patients (Table 1).
Pre-Clinic Visit Medications
At the start of the clinic visit, there were many potential opportunities for GDMT maximization (Figure 2). No subject was simultaneously receiving target doses of EVBB, ARNI, and MRA.
Figure 2.
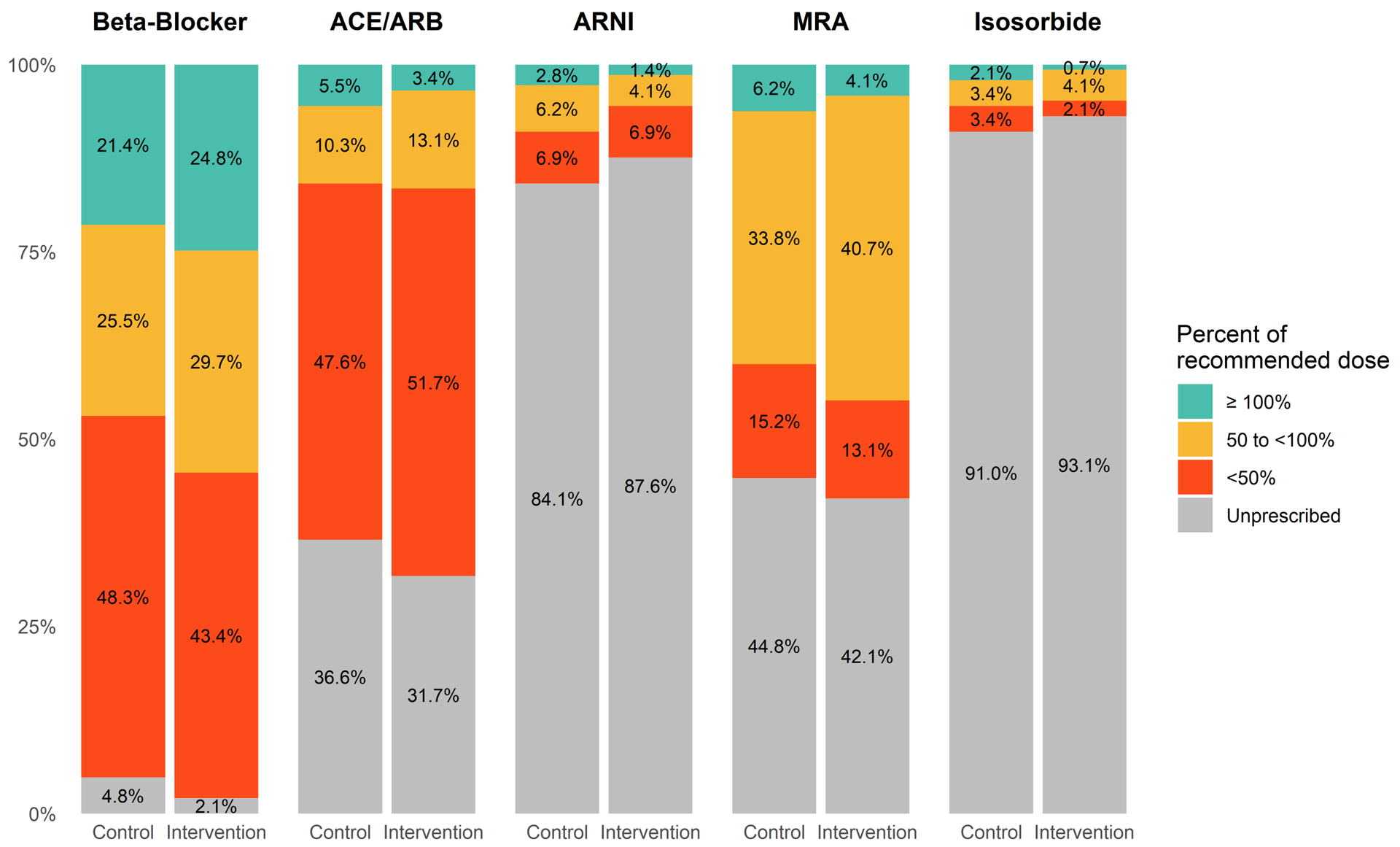
Baseline medication prescribing pre-clinic visit.
“Isosorbide” includes isosorbide dinitrate and mononitrate (hydralazine was assessed separately)
GDMT Intensification
From the start of the qualifying cardiology clinic visit to 30 days later, 49.0% of patients in the intervention group and 29.7% of patients in the control group experienced an intensification of their GDMT (p=0.001, RR(95% CI)= 1.6 (1.2, 2.2)) (Figure 3, Supplemental Table I). When counting multiple intensifications over the 30 days, there were 0.61 intensifications per patient in the intervention group versus 0.36 intensifications per patient in control (p=0.002, Incidence Rate Ratio (95% CI)= 1.7 (1.2, 2.4)) (Table 2). The vast majority of these changes, including the effect of the intervention, were reflected by dose intensifications; initiation of medications was much less common, and switch to ARNI was rare and not different between intervention and control. Intensification of loop diuretics and digoxin (not considered part of GDMT intensification) were less frequent than changes to EVBB or ACEI/ARB/ARNI (Supplemental Table I). The most common dose intensification, and where our intervention showed the strongest effect, was among beta blockers; median(IQR) carvedilol dose increased from 18.75 (12.5, 25.0) to 37.5mg (25.0, 50.0) and median(IQR) metoprolol succinate dose increased from 50 (25, 100) to 75mg (50, 150) (Supplemental Table II).
Figure 3.
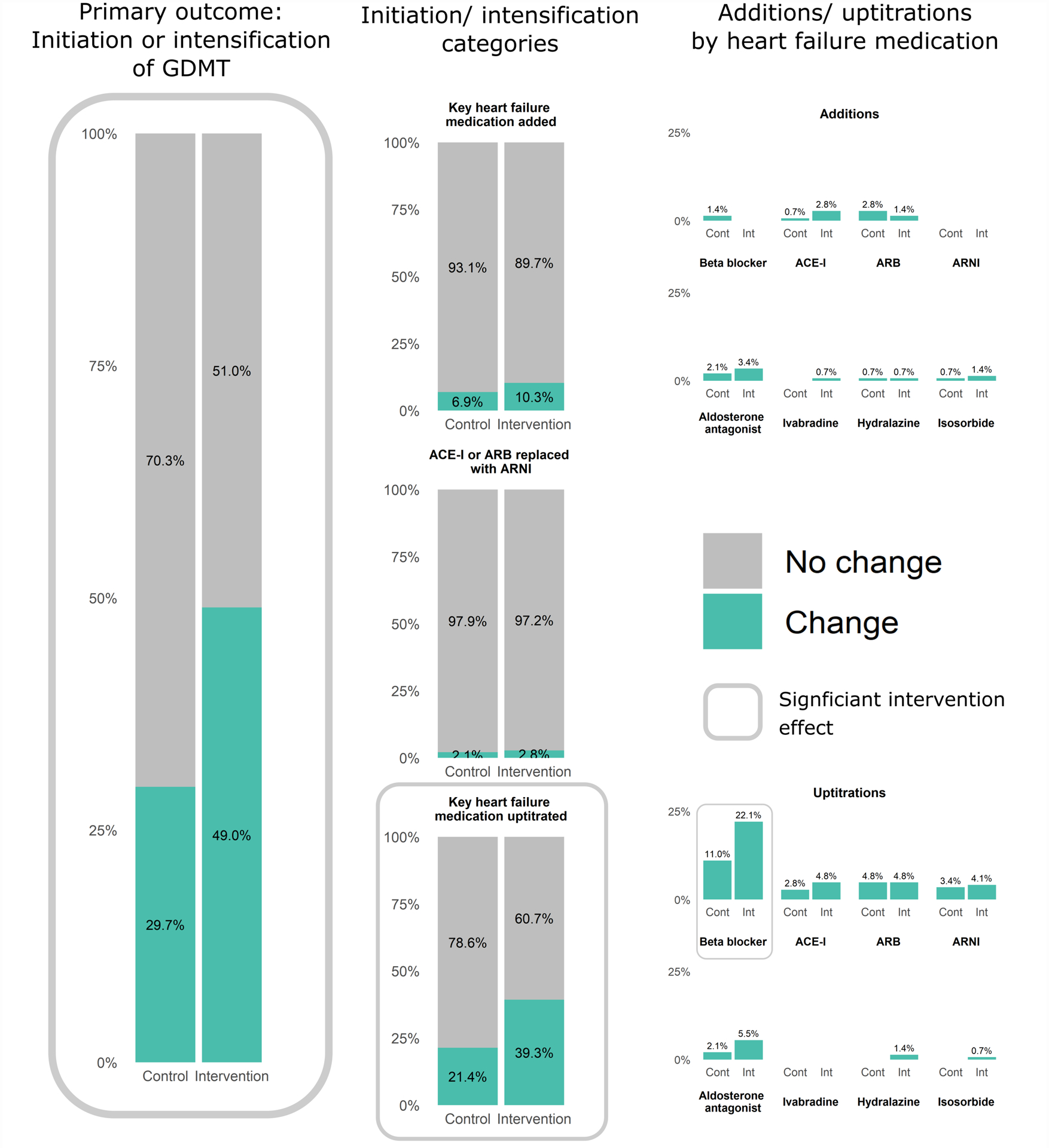
Medication intensification from pre-clinic to 30-days later, stratified by treatment assignment (further details in Supplemental Table I).
Table 2.
Total guideline-directed medical therapy intensifications per patient from pre-clinic visit to 30 days later.
| Treatment mean(sd) intensifications N = 145 | Control mean(sd) intensifications N = 145 | Estimate (95% CI) incidence rate ratio | p-value | |
|---|---|---|---|---|
| Number of GDMT intensifications (initiation or dose increase EVBB, ACEI, ARB, ARNI, MRA, H/ISDN, or ivabradine) | 0.61 (0.73) | 0.36 (0.61) | 1.7 (1.2, 2.4) | 0.002 |
| Number of all intensifications (including GDMT plus initiation or uptitration of loop diuretic or digoxin) | 0.63 (0.73) | 0.38 (0.62) | 1.7 (1.2, 2.3) | 0.002 |
ACE-I=angiotensin converting enzyme inhibitors; ARB= angiotensin receptor blockers; ARNI= angiotensin receptor neprilysin inhibitors; EVBB=evidence-based beta blocker; GDMT=guideline-directed medical therapy; H/ISDN=hydralazine/isosorbide dinitrate; MRA=mineralocorticoid receptor agonists
P values from Poisson model with fixed effect for clinic.
Safety Events
No patients died between the cardiology clinic visit and 30 days. Numerically there were more non-elective hospitalizations and emergency department visits in the intervention group than the control group, but this was not statistically significant. Detailed chart review showed that the majority of these events were not related to hypotension, syncope, bradycardia, worsening heart failure, acute renal failure, or hyperkalemia (Table 3).
Table 3.
Safety outcomes.
| Treatment N = 145 | Control N = 145 | p-value | Relative Risk | |
|---|---|---|---|---|
| Deaths at 30 days | 0 | 0 | - | - |
| Unplanned hospitalization at 30 days | 6 (4.1%) | 4 (2.8%) | 0.75 | 1.5 (0.4, 5.1) |
| Emergency department visit without hospitalization at 30 days | 9 (6.2%) | 5 (3.4%) | 0.41 | 1.8 (0.6, 5.3) |
| Death, hospitalization, or emergency department visit at 30 days | 15 (10.3%) | 9 (6.2%) | 0.29 | 1.6 (0.7, 3.6) |
Hospitalizations and emergency department visits analyzed as yes/no per patient. Only 1 of 10 patients had 2 hospitalizations, and 1 of 14 patients had 2 emergency department visits. No patients had both a hospitalization and emergency department visit. P values using Fisher exact. Relative risk from log-binomial model with fixed site effect.
Intervention Delivery, Use, and Patient Impressions
Survey results of patients in the intervention group showed gradual loss of exposure from tool delivery to tool viewing to tool use, noting that more than half of patients reported reviewing the materials and more than a third of patients reported bringing the checklist to the cardiology clinic visit (Figure 4).
Figure 4.
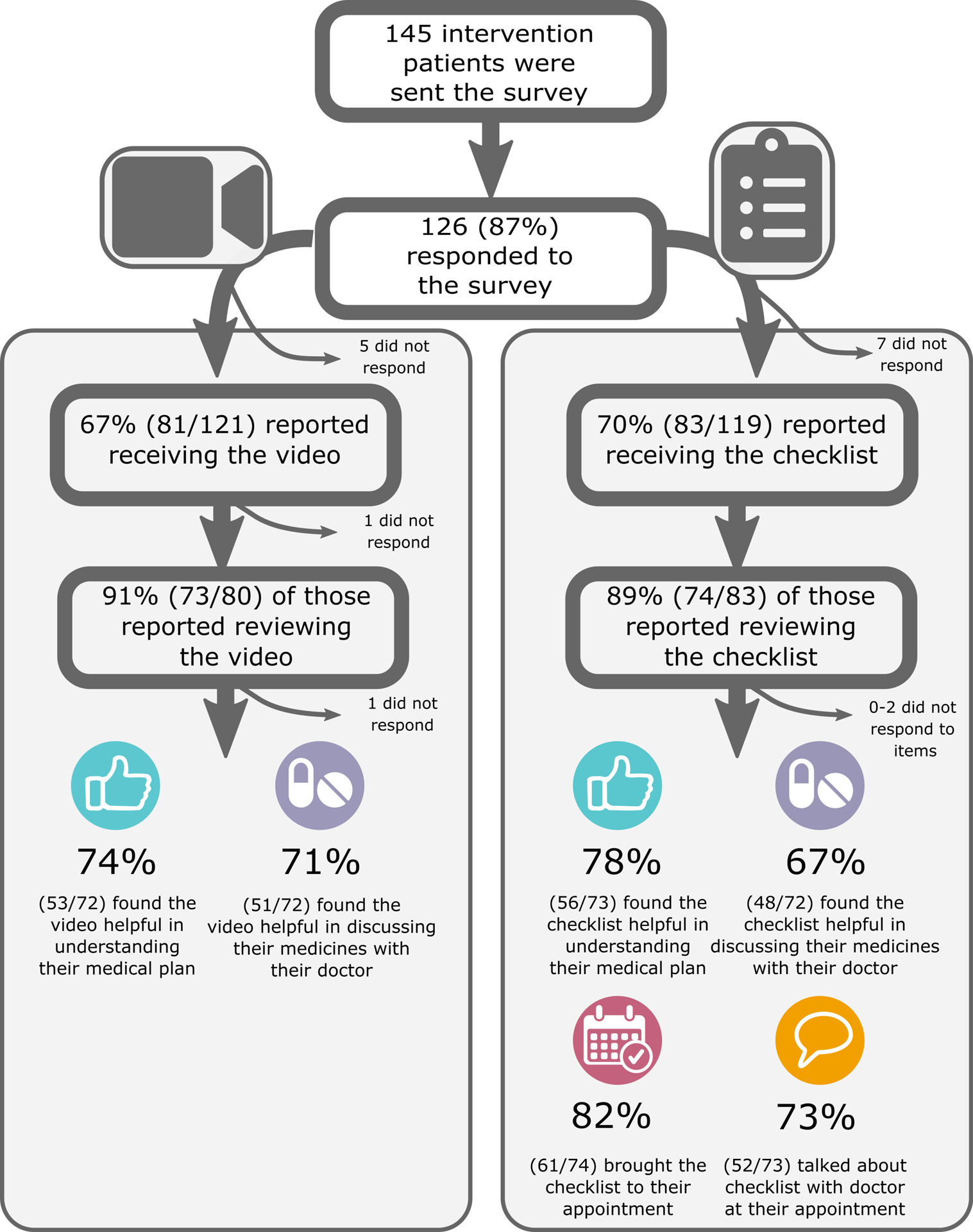
Survey results following patient-activation tool delivery and cardiology clinic visit.
Discussion
In this randomized, controlled trial of patients with HFrEF and an upcoming regularly-scheduled cardiology clinic visit, delivery of a patient-activation tool consisting of a 3-minute video and 1-page checklist before the visit was associated with a 19% absolute increase (1.6 RR) in GDMT intensification over the subsequent month. The majority of these increases occurred during the clinic visit itself and mostly involved dose intensification of previously prescribed generic medications. Patient medical records reported relatively low serious adverse events in this population, which were not significantly increased with the use of the intervention. This relatively simple patient-activation tool was deployed in the routine care of patients, suggesting opportunities for broader validation and dissemination.
The findings of our study expand upon prior research on GDMT prescribing and optimization. Consistent with a number of HFrEF registries,5–8, 20 the vast majority of patients enrolled had multiple potential opportunities to improve GDMT. Consistent with prior research documenting clinical inertia,21 we also showed that among those in the usual care group, less than a third of patients experienced any change to their HFrEF prescribing. Real-world use of optimal GDMT has been suboptimal due to a combination of patient and clinician factors. At the patient level, medications for HFrEF often do not produce immediate improvements in symptoms, and many are associated with short-term side effects. Out-of-pocket costs for newer on-patent medications can be a burden.22 Also not surprisingly, patient adherence initiatives have had relatively little impact on clinical outcomes.23–25 On the clinician level, limited clinic time, complexity of care, and the need for extensive patient medication education, make keeping medication doses at the status quo a practical option. Clinical inertia can become a necessity if uptitration is met with patient skepticism and uncertainty.
The EPIC-HF intervention bridged these gaps between patients and clinicians. Unlike many prior trials designed to influence GDMT use through clinician notifications,10, 11, 26, 27 and patient education,23 the combined DTCA, SDM, and flipped-classroom approach engaged and activated patients prior to the clinical encounter and increased GDMT use more effectively. Others have reported successful mechanisms for improving GDMT use, but few studies have employed randomized controlled study design that remove the healthy user effects and treatment selection bias that naturally accompany GDMT intensification.28
Our results help counter the common argument, often from clinicians, that lack of GDMT intensification is an appropriate response to physiological limitations or drug intolerance.9 In the closely monitored GUIDE-IT trial, medication adjustments were made in only 54.6% of qualified visits.29 The most common reasons in GUIDE-IT for not adjusting were “clinically stable” and “already at maximally tolerated therapy;” yet at 6 months, only 16% achieved optimal GDMT. In our study, a simple tool activating patients to ask their clinicians about opportunities for GDMT intensification resulted in substantial increase in GDMT use. It is clear that not all patients should have GDMT increased; but the data here make it clearer that a good portion of them should at least try. The need for iterative dose escalation and active monitoring of patient symptoms, vital signs, and blood tests does not constitute intolerance, nor does it constitute a reasonable excuse not to deploy these high-value therapies.30 With recent positive trials of sodium glucose transport 2 inhibitors,31, 32 and stimulators of soluble guanylate cyclase,33 and possibly myosin activators,34 the options for, and thus gaps in, GDMT are growing. Innovative and pragmatic approaches to care will be required to reap the majority of benefit these advances offer.
Engaging patients in their healthcare treatment decisions and creating patient-clinician partnerships have emerged as major priorities in American health care. The 2001 publication of the Institute of Medicine’s 6 domains of healthcare quality includes patient centeredness. Since that time, multiple healthcare organizations have called for engagement of patients in treatment decisions. These include Patient Centered Outcomes Research Institute’s research agenda,35 as well as Medicare national coverage decisions requiring the use of patient decision aids prior to implantable cardioverter-defibrillator and left atrial appendage occluder device placement.36, 37 Furthermore, a growing body of evidence suggests that successful strategies to improve medication use do not place the onus for change on merely one party, but rather involve patient, clinician, and healthcare system working collaboratively.38 Our trial and the EPIC-HF intervention were grounded in this principle, which may partially explain its success.
Perhaps as important as its patient-focus is the user-centered, pragmatic design of the EPIC-HF trial and intervention. Based on experiences with clinical operations and care delivery for patients with chronic HFrEF, this tool was designed using input from patients and caregivers, as well as physicians, advanced practice providers, nurses, pharmacists, hospital administrators, and experts in implementation science. This trial not only tested the EPIC-HF video and checklist, but it also leveraged years of work around field-coded LVEF, automated alerts around upcoming appointments, and patient-centered design following International Patient Decision Aid Standards. Our intervention is not solely a video and checklist, but requires the presence of timely, automated delivery that is seen by patients, clinicians, and the health system as not overly burdensome. A recent report from the Pew Research Center found that 81% of all Americans now own a smartphone, and the majority use the internet regularly,39 such that we were able to deliver the intervention electronically. The success of the EPIC-HF tool is likely a combination of a variety of factors—video, checklist, notification to the clinician—which were all integrated into existing workflows using familiar technologies.
The study has a number of limitations. First, we did not measure intolerance and contraindications to GDMT. However, these are often vague and subjective, and absolute cut offs for heart rate, blood pressure, and creatinine elevation are not well defined. What we found is that if patients and clinicians are asked to try, GDMT can be intensified more often, and safely. Second, randomization occurred at the patient-level rather than the clinician- or site-level. This opens the potential for contamination, as clinicians who see intervention patients may subsequently be more active in prescribing and titrating medications for patients in the control arm. However, this would bias the result towards the null. Third, only cardiology specialists were included in this study, given that within the UCHealth system most HFrEF prescribing is managed by specialists. As such, the external validity of the tool for patients whose HFrEF is managed primary care clinicians is less certain. Finally, the use of a single, regional health system may further limit the generalizability. However, UCHealth is a diverse system spanning a range of local care approaches, and care was taken to over-enroll under-represented patient groups where possible. Further, the study tools can be modified for future use in a larger number of contexts: we have discussed the possibility of translating the video to a paper, comic-strip style format, which could then be delivered to patients who do not have access to, or the ability to use, a smartphone or email address. In total, we believe the insights gleaned from this study are likely to apply to a broad range of settings. At a minimum, the pragmatic approach should be further tested in different clinical contexts.
Conclusion
A myriad of medications can improve clinical outcomes in patients with HFrEF, but they remain widely underutilized. The EPIC-HF intervention improves suboptimal GDMT prescribing in HFrEF patients by using principles of DTCA and SDM. The result is a practical, yet novel tool that encourages collaboration between patients and clinicians, while still leaving room for the variability in clinical encounters and patient-clinician relationships. The positive results of the EPIC-HF trial beg for validation in other populations with HFrEF and suggest an approach that may be used in other chronic diseases that benefit from combination therapy.
Supplementary Material
Clinical Perspectives:
What is new?
A 3-minute patient activation video plus a 1-page medication checklist delivered directly to patients with heart failure with reduced ejection fraction (HFrEF) before a visit with a cardiology clinician resulted in a 19% absolute increase those who had their guideline-directed medical therapies (GDMT) intensified.
The majority of these intensifications involved dose increases of beta-blockers.
What are the clinical implications?
Clinical inertia accounts for some portion of underuse of GDMT in HFrEF.
A brief tool delivered to patients electronically before the visit encouraging patients to ask about opportunities to enhance their medical therapy led to improved GDMT.
Funding
This work was funded entirely by a grant from the American Heart Association’s Strategically Focused Research Network, Award # 16SFRN29640000 https://professional.heart.org/professional/ResearchPrograms/StrategicallyFocusedResearchPrograms/UCM_454438_Strategically-Focused-Research-Networks.jsp.
Non-standard Abbreviations and Acronyms
- GDMT
guideline-directed medical therapies
- HFrEF
heart failure with reduced ejection fraction
- EPIC-HF
Electronically delivered, Patient-activation tool for Intensification of medications for Chronic Heart Failure with reduced ejection fraction
- EVBB
evidence-based beta-blockers
- ACEI
angiotensin converting enzyme inhibitors
- ARB
angiotensin receptor blockers
- ARNI
angiotensin receptor neprilysin inhibitors
- MRA
mineralocorticoid receptor agonists
- H/ISDN
hydralazine/isosorbide dinitrate
- DTCA
direct-to-consumer advertising
- SDM
shared decision making
- LVEF
left ventricular ejection fraction
Footnotes
Conflicts of Interest
Dr. Allen reports grant funding from AHA, NIH, and PCORI, and reports consulting fees from Abbott, ACI Clinical, Amgen, Boston Scientific, Cytokinetics, and Novartis.
Ms. Venechuk reports no disclosures.
Dr. McIlvennan reports no disclosures.
Dr. Page reports no disclosures.
Dr. Knoepke is supported by the NIH (1K23HL153892) and the American Heart Association (18CDA34110026).
Ms. Helmkamp reports no disclosures.
Dr. Khazanie is supported by the NIH and NIH Ethics Supplement (K23HL145122) and the Doris Duke-University of Colorado FRCS Award.
Dr. Peterson reports grant funding from NHLBI (4R33HL143324–02).
Mr. Pierce reports no disclosures.
Mr. Harger reports no disclosures.
Ms. Thompson reports no disclosures.
Dr. Dow reports no disclosures.
Dr. Richards reports no disclosures.
Dr. Huang reports no disclosures.
Dr. Strader reports no disclosures.
Dr. Trinkley is supported by the NIH (K12HL137862).
Dr. Kao reports grant funding from NIH, AHA and CDC and reports an advisory agreement with Codex, Inc.
Dr. Magid reports grant funding form NIH, AHA, and CMS.
Dr. Buttrick reports grant funding from AHA.
Dr. Matlock reports funding from AHA, NIH, and PCORI.
Supplemental Materials
Supplemental Tables I – II
REFERENCES
- 1.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–327. [DOI] [PubMed] [Google Scholar]
- 2.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137–e161. [DOI] [PubMed] [Google Scholar]
- 3.Yancy CW, Januzzi JL Jr., Allen LA, Butler J, Davis LL, Fonarow GC, Ibrahim NE, Jessup M, Lindenfeld J, Maddox TM, et al. 2017 ACC Expert Consensus Decision Pathway for Optimization of Heart Failure Treatment: Answers to 10 Pivotal Issues About Heart Failure With Reduced Ejection Fraction: A Report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol. 2018;71:201–230. [DOI] [PubMed] [Google Scholar]
- 4.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 5.Greene SJ, Butler J, Albert NM, DeVore AD, Sharma PP, Duffy CI, Hill CL, McCague K, Mi X, Patterson JH, et al. Medical Therapy for Heart Failure With Reduced Ejection Fraction: The CHAMP-HF Registry. J Am Coll Cardiol. 2018;72:351–366. [DOI] [PubMed] [Google Scholar]
- 6.Brunner-La Rocca HP, Linssen GC, Smeele FJ, van Drimmelen AA, Schaafsma HJ, Westendorp PH, Rademaker PC, van de Kamp HJ, Hoes AW, et al. Contemporary Drug Treatment of Chronic Heart Failure With Reduced Ejection Fraction: The CHECK-HF Registry. JACC Heart Fail. 2019;7:13–21. [DOI] [PubMed] [Google Scholar]
- 7.Chang HY, Wang CC, Wei J, Chang CY, Chuang YC, Huang CL, Chong E, Lin JL, Mar GY, Chan KC, et al. Gap between guidelines and clinical practice in heart failure with reduced ejection fraction: Results from TSOC-HFrEF registry. J Chin Med Assoc. 2017;80:750–757. [DOI] [PubMed] [Google Scholar]
- 8.Teng TK, Tromp J, Tay WT, Anand I, Ouwerkerk W, Chopra V, Wander GS, Yap JJ, MacDonald MR, Xu CF, et al. Prescribing patterns of evidence-based heart failure pharmacotherapy and outcomes in the ASIAN-HF registry: a cohort study. Lancet Glob Health. 2018;6:e1008–e1018. [DOI] [PubMed] [Google Scholar]
- 9.Jarjour M, Henri C, de Denus S, Fortier A, Bouabdallaoui N, Nigam A, O’Meara E, Ahnadi C, White M, Garceau P, et al. Care Gaps in Adherence to Heart Failure Guidelines: Clinical Inertia or Physiological Limitations? JACC Heart Fail. 2020;8:725–738. [DOI] [PubMed] [Google Scholar]
- 10.Ansari M, Shlipak MG, Heidenreich PA, Van Ostaeyen D, Pohl EC, Browner WS, Massie BM. Improving guideline adherence: a randomized trial evaluating strategies to increase beta-blocker use in heart failure. Circulation. 2003;107:2799–804. [DOI] [PubMed] [Google Scholar]
- 11.Wadhwa R, Fridsma DB, Saul MI, Penrod LE, Visweswaran S, Cooper GF, Chapman W. Analysis of a failed clinical decision support system for management of congestive heart failure. AMIA Annu Symp Proc. 2008:773–777. [PMC free article] [PubMed] [Google Scholar]
- 12.Caraballo PJ, Naessens JM, Klarich MJ, Leutink DJ, Peterson JA, Wagie AE, Manning DM, Qian Q. Decline in ACEI/ARB Prescribing as Heart Failure Core Metrics Improve During Computer-Based Clinical Decision Support. Am J Med Qual. 2014;29:300–307. [DOI] [PubMed] [Google Scholar]
- 13.Bell RA, Kravitz RL, Wilkes MS. Direct-to-consumer prescription drug advertising and the public. Journal of General Internal Medicine. 1999;14:651–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi SM, Lee WN. Understanding the impact of direct-to-consumer (DTC) pharmaceutical advertising on patient-physician interactions - Adding the web to the mix. J Advertising. 2007;36:137–149. [Google Scholar]
- 15.Weissman JS, Blumenthal D, Silk AJ, Newman M, Zapert K, Leitman R, Feibelmann S. Physicians report on patient encounters involving direct-to-consumer advertising. Health Affair. 2004;23:W4219–W4233. [DOI] [PubMed] [Google Scholar]
- 16.Barry MJ, Edgman-Levitan S. Shared decision making--pinnacle of patient-centered care. N Engl J Med. 2012;366:780–781. [DOI] [PubMed] [Google Scholar]
- 17.Stacey D, Legare F, Col NF, Bennett CL, Barry MJ, Eden KB, Holmes-Rovner M, Llewellyn-Thomas H, Lyddiatt A, Thomson R, Trevena L, Wu JH. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2014:CD001431. [DOI] [PubMed] [Google Scholar]
- 18.Venechuk GE, Khazanie P, Page RL, Knoepke CE, Helmkamp LJ, Peterson PN, Pierce K, Thompson JS, Huang J, Strader JR, et al. An Electronically delivered, Patient-activation tool for Intensification of medications for Chronic Heart Failure with reduced ejection fraction: Rationale and design of the EPIC-HF trial. Am Heart J. 2020;229:144–155. [DOI] [PubMed] [Google Scholar]
- 19.Matlock DD, Spatz ES. Design and testing of tools for shared decision making. Circ Cardiovasc Qual Outcomes. 2014;7:487–492. [DOI] [PubMed] [Google Scholar]
- 20.Allen LA, Fonarow GC, Liang L, Schulte PJ, Masoudi FA, Rumsfeld JS, Ho PM, Eapen ZJ, Hernandez AF, Heidenreich PA, et al. Medication Initiation Burden Required to Comply With Heart Failure Guideline Recommendations and Hospital Quality Measures. Circulation. 2015;132:1347–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allen LA, Tang F, Jones P, Breeding T, Ponirakis A, Turner SJ. Signs, symptoms, and treatment patterns across serial ambulatory cardiology visits in patients with heart failure: insights from the NCDR PINNACLE(R) registry. BMC Cardiovasc Disord. 2018;18:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith GH, Shore S, Allen LA, Markham DW, Mitchell AR, Moore M, Morris AA, Speight CD, Dickert NW. Discussing Out-of-Pocket Costs With Patients: Shared Decision Making for Sacubitril-Valsartan in Heart Failure. J Am Heart Assoc. 2019;8:e010635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Powell LH, Calvin JE Jr., Richardson D, Janssen I, Mendes de Leon CF, Flynn KJ, Grady KL, Rucker-Whitaker CS, Eaton C, Avery E, et al. Self-management counseling in patients with heart failure: the heart failure adherence and retention randomized behavioral trial. JAMA. 2010;304:1331–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bekelman DB, Plomondon ME, Carey EP, Sullivan MD, Nelson KM, Hattler B, McBryde CF, Lehmann KG, Gianola K, Heidenreich PA, Rumsfeld JS. Primary Results of the Patient-Centered Disease Management (PCDM) for Heart Failure Study: A Randomized Clinical Trial. JAMA Intern Med. 2015;175:725–732. [DOI] [PubMed] [Google Scholar]
- 25.Kini V, Ho PM. Interventions to Improve Medication Adherence: A Review. JAMA. 2018;320:2461–2473. [DOI] [PubMed] [Google Scholar]
- 26.Roshanov PS, Fernandes N, Wilczynski JM, Hemens BJ, You JJ, Handler SM, Nieuwlaat R, Souza NM, Beyene J, Van Spall HG, Garg AX, Haynes RB. Features of effective computerised clinical decision support systems: meta-regression of 162 randomised trials. BMJ. 2013;346:f657. [DOI] [PubMed] [Google Scholar]
- 27.Blecker S, Austrian JS, Horwitz LI, Kuperman G, Shelley D, Ferrauiola M, Katz SD. Interrupting providers with clinical decision support to improve care for heart failure. Int J Med Inform. 2019;131:103956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Desai AS, Maclean T, Blood AJ, Bosque-Hamilton J, Dunning J, Fischer C, Fera L, Smith KV, Wagholikar K, Zelle D, Gaziano T, Plutzky J, Scirica B, MacRae CA. Remote Optimization of Guideline-Directed Medical Therapy in Patients With Heart Failure With Reduced Ejection Fraction. JAMA Cardiol. 2020;16:e203757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fiuzat M, Ezekowitz J, Alemayehu W, Westerhout CM, Sbolli M, Cani D, Whellan DJ, Ahmad T, Adams K, Pina IL, et al. Assessment of Limitations to Optimization of Guideline-Directed Medical Therapy in Heart Failure From the GUIDE-IT Trial: A Secondary Analysis of a Randomized Clinical Trial. JAMA Cardiol. 2020;5:757–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaziano TA, Fonarow GC, Velazquez EJ, Morrow DA, Braunwald E, Solomon SD. Cost-effectiveness of Sacubitril-Valsartan in Hospitalized Patients Who Have Heart Failure With Reduced Ejection Fraction. JAMA Cardiol. 2020. DOI: 10.1001/jamacardio.2020.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med. 2019;381:1995–2008. [DOI] [PubMed] [Google Scholar]
- 32.Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N Engl J Med. 2020;383:1413–1424. [DOI] [PubMed] [Google Scholar]
- 33.Armstrong PW, Pieske B, Anstrom KJ, Ezekowitz J, Hernandez AF, Butler J, Lam CSP, Ponikowski P, Voors AA, Jia G, et al. Vericiguat in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med. 2020;382:1883–1893. [DOI] [PubMed] [Google Scholar]
- 34.Teerlink JR, Diaz R, Felker GM, McMurray JJV, Metra M, Solomon SD, Legg JC, Büchele G, Varin C, Kurtz CE, et al. Honarpour N. Omecamtiv Mecarbil in Chronic Heart Failure With Reduced Ejection Fraction: Rationale and Design of GALACTIC-HF. JACC Heart Fail. 2020;8:329–340. [DOI] [PubMed] [Google Scholar]
- 35.National Priorities for Research and Research Agenda. Patient-Centered Research Institute. 2014. https://www.pcori.org/sites/default/files/PCORI-National-Priorities-and-Research-Agenda.pdf Accessed October 28, 2020.
- 36.Decision Memo for Implantable Cardioverter Defibrillators (CAG-00157R4). 2018. https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=288 Accessed October 28, 2020.
- 37.Decision Memo for Percutaneous Left Atrial Appendage (LAA) Closure Therapy (CAG-00445N). 2016. https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=281 Accessed October 28, 2020.
- 38.Lauffenburger JC, Choudhry NK. A Call for a Systems-Thinking Approach to Medication Adherence: Stop Blaming the Patient. JAMA Intern Med. 2018;178:950–951. [DOI] [PubMed] [Google Scholar]
- 39.Anderson M. Mobile Technology and Home Broadband 2019. Pew Research Center; 2019. https://www.pewresearch.org/internet/2019/06/13/mobile-technology-and-home-broadband-2019/ Accessed October 28, 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


