Abstract
Background:
Critically shortened telomeres contribute to chromosomal instability and neoplastic transformation and are associated with early death of patients with certain cancer types. Shorter leukocyte telomere length (LTL) has been associated with higher risk for pancreatic ductal adenocarcinoma (PDAC) development and might be associated also with survival of patients with PDAC. We investigated the association between treatment-naïve LTL and overall survival of patients with incident PDAC.
Methods:
The study included 642 consecutively enrolled PDAC patients in the Mayo Clinic Biospecimen Resource for Pancreas Research. Blood samples were obtained at the time of diagnosis, before the start of cancer treatment, from which LTL was assayed by real-time quantitative polymerase chain reaction. LTL was modeled as a continuous variable (per-interquartile range decrease in LTL) and as a categorized variable (short, medium, long). Multivariable-adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated for overall mortality using Cox proportional hazard models.
Results:
Shorter treatment-naïve LTL was associated with higher mortality among PDAC patients (HRcontinuous = 1.13, 95% CI: 1.01-1.28, p-value=0.03; HRshortest vs. longest LTL = 1.29, 95% CI: 1.05-1.59, Ptrend=0.01). There was evidence of a difference in the association between LTL and overall mortality by tumor stage at diagnosis; resectable tumors (HRcontinuous = 0.91, 95% CI: 0.73-1.12), locally advanced tumors (HRcontinuous = 1.29, 95% CI: 1.07-1.56), and metastatic tumors (HRcontinuous = 1.17, 95% CI: 0.96-1.42), Pinteraction=0.04.
Conclusion:
Shorter treatment-naïve LTL is associated with poorer overall survival of incident PDAC patients.
Impact:
Peripheral blood LTL might be a prognostic marker for PDAC.
Keywords: Telomere length, pancreatic cancer, pancreatic ductal adenocarcinoma, mortality, survival
Introduction
Despite substantial progress in understanding the etiology of pancreatic ductal adenocarcinoma (PDAC), it remains a rapidly fatal malignancy with the highest mortality rate of all gastrointestinal malignancies (1,2). The five-year mortality rate for PDAC is about 90% (2), due mainly to its frequently late stage of detection, aggressive clinical course, and treatment resistance in many patients despite the availability of newer therapies (3–6). The poor prognosis of PDAC highlights the need for better understanding of the factors associated with survival, toward the goal of improving outcomes for persons with PDAC. Initial evidence suggesting that telomeres may be associated with survival of PDAC patients has been reported (7).
Telomeres, the hexanucleotide sequence repeats (TTAGGG)n and an associated protein complex (shelterin/telosome) that cap the ends of eukaryotic chromosomes are essential for maintaining chromosomal and genomic stability (8–10). Telomeres shorten progressively with each successive cell division due to incomplete replication by DNA polymerases (10). Consequently, telomere length is considered a marker of cumulative cellular aging, replicative capacity, and senescence across human cell types (8,9,11). In addition to aging, other host factors, such as cigarette smoking, obesity, and chronic health conditions (e.g., diabetes) tend to accelerate telomere shortening further to a point where telomeres become critically short and dysfunctional (8,10). Because fully functional telomeres are essential for maintaining genomic stability, they tend to protect against cancer development (12). Cells with critically short telomere length undergo cell cycle arrest, apoptosis, or replicative senescence (8,9,11). Cells that evade these cell cycle checkpoint mechanisms and continue to divide despite dysfunctional telomeres lead to the accumulation of aberrant cells, ultimately contributing to genomic instability and age-related pathologies, such as cancer (8,9,11–13). In line with this understanding, tissue-based studies have reported progressive telomere shortening during the early stages of PDAC development (14,15). Additionally, PDAC tissues have been found to have shorter telomeres than normal pancreatic duct epithelium (15). These observations together suggest that telomere shortening might be a fundamental process in PDAC development, and perhaps, might play a role in the prognosis of patients with PDAC. Indeed, studies suggest that critically shortened telomeres and associated chromosomal abnormalities facilitate cancer progression (11–13), and shortened telomeres have been associated with early death of patients with certain cancer types (16–18), including PDAC (7).
Specifically, epidemiological studies have found that shorter peripheral blood leukocyte telomere length (LTL), a reflection of overall telomere status, is associated with poorer survival of patients with bladder cancer (19), hematological malignancies (20), and renal cell carcinoma (18). To our knowledge, two studies have investigated the association between LTL and survival of patients with PDAC. One of these studies reported a null finding (16), and the other found that shorter LTL measured in blood samples collected a median of six years before PDAC diagnosis is associated with poorer overall survival after PDAC diagnosis (7). No study has yet examined the association between LTL assayed in blood samples collected at the time of PDAC diagnosis and survival of patients. To clarify the relationship between LTL and survival of PDAC patients, we performed a large, clinic-based study to test the hypothesis that shorter treatment-naïve LTL in blood samples obtained at diagnosis is associated with poorer overall survival of patients with incident PDAC. We further explored whether treatment-naïve LTL interacts with PDAC patient characteristics (e.g., tumor stage at diagnosis) to influence overall survival.
Materials and Methods
Study Sample and Data Collection
The study was reviewed and approved by the Mayo Clinic Institutional Review Board (IRB) under protocol number 06-004892. Mayo Clinic patients included in this study had previously provided written informed consent under IRB protocol number 354-06. The Mayo Clinic IRB is compliant with the requirements of the U.S. Food and Drug Administration (FDA) regulations 21 CFR Parts 50 and 56 and the U.S. Department of Health and Human Services (HHS) regulations 45 CFR 46, which are guided by the Belmont Report. In addition, the Mayo Clinic IRB complies with the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) guidelines on Good Clinical Practices and are consistent with FDA and HHS regulations.
We obtained data and biospecimen from the Mayo Clinic Biospecimen Resource for Pancreas Research, a prospective patient registry supported by the Mayo Clinic Specialized Program of Research Excellence (SPORE) in pancreatic cancer (21–23). The registry utilizes an ultra-rapid case finding and recruitment process that ensures that about 86% of patients diagnosed with PDAC at Mayo Clinic are recruited within one month of diagnosis, with an overall two-week average between first contact and recruitment. The patients were recruited between October 2000 and June 2016, and all patients provided blood samples at the time of diagnosis before starting cancer treatment. The participation rate among those approached was approximately 70% (21). In our experience, the main reasons for non-participation in the patient registry are the rapid demise of patients following PDAC diagnosis and severe debilitation caused by PDAC (22). Included in this study were data and biospecimens from 642 consecutively enrolled patients with pathologically confirmed (95%) or clinically confirmed PDAC.
At the time of enrollment, the patients were asked to complete a standardized risk factor questionnaire that solicited information on demographics (age, sex, race), smoking history, personal history of diabetes, and usual adult weight and height. We categorized smoking history as never, former, and current, and calculated body mass index (BMI) as weight in kilograms divided by height in meters squared (kg/m2). Information on cancer stage at diagnosis was obtained from medical records by medical oncologist in the pancreatic cancer SPORE, and were grouped as surgically resected, locally advanced, or metastatic. Vital status was ascertained from multiple sources including medical records, periodic correspondence with patients, direct contact with next of kin, linkage with Mayo Clinic tumor registry data, and use of online sources (e.g., LexisNexis Accurint®)(24). We were able to ascertain vital status for all patients included in this study.
LTL measurement
The methods used for measuring LTL have been described previously (23). In brief, the patients donated peripheral blood samples that were fractionated into plasma, serum, and leukocytes (buffy coat). DNA was extracted from buffy coat leukocytes using the Maxwell RSC Instrument with appropriate kit (Promega) and quantitated with a Qubit fluorometer. LTL was measured by real-time quantitative polymerase chain reaction (qPCR). The qPCR assay measures telomere length as the relative ratio of telomere copy number to single gene copy number (T/S ratio)(25). Each sample was assayed in triplicate in relation to a reference sample. The final T/S ratio was based on the average of the three measurements. The coefficient of variation (CV) within triplicate samples was less than 3% and the median CV among all samples was 5.5%, which are indicative of high assay reproducibility. Laboratory personnel were blinded to all clinical data (age, sex, vital status, etc.).
Statistical Analysis
Descriptive statistics were performed using means and medians for continuous variables and proportions for categorical variables. The outcome of interest was overall survival. Overall survival was determined starting with the date of PDAC diagnosis to date of death or date of last follow-up, whichever came first. Cox proportional hazard models were used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs). The proportional hazards assumption was assessed for all variables using residual plots and was determined to have been satisfied. In the Cox model, we modeled LTL as the predictor and time-to-death as outcome, with initial adjustment for age at diagnosis (continuous), sex, and tumor stage at diagnosis (resectable, locally advanced, metastatic). Subsequently, we performed additional adjustments for BMI (continuous), self-reported personal history of diabetes (yes, no), and smoking status (never, former, current). In the primary analysis, we treated LTL as a continuous variable, with HRs reflecting one interquartile range decrease in LTL. We assessed potential nonlinearity of the association between the continuous LTL variable and overall survival with restricted cubic splines in the Cox proportional hazard model (26). Based on residual analysis (plots of residuals against continuous LTL variable) and the Bayesian information criterion, we determined that there was no evidence of a nonlinear relationship between LTL and overall survival. Hence, for parsimony and ease of interpretation, we retained LTL as a continuous variable in the primary analyses.
In secondary analyses and for visualization of association trends, we treated LTL as a categorical variable grouped into tertiles (short, medium, long) based on the distribution in the entire cohort. The Kaplan-Meier estimator was used to calculate median survival time with corresponding 95% CIs. For the categorical LTL variable, we used the longest LTL group as the reference to calculate HRs and 95% CIs for the medium and shortest LTL groups. Linear trend across categories was assessed using the ordinal method.
In exploratory analyses, we assessed whether the association between LTL and overall survival differed by tumor stage at diagnosis, sex, usual adult BMI (≤ 24.9, 25-29.9, ≥30 kg/m2), personal history of diabetes mellitus (yes, no), and smoking status (never, former, current) by assessing a multiplicative interaction between the stratifying variable and LTL. For these analyses, the continuous LTL variable was used to conserve statistical power. A forest plot was generated to visualize an interaction observed between tumor stage at diagnosis and LTL on overall survival using the continuous LTL variable. We also used the categorical LTL variable to glean additional insights into the interaction between tumor stage at diagnosis and LTL. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC). A two-sided p-value < 0.05 was considered statistically significant.
Results
Characteristics of the 642 incident PDAC patients are summarized in Table 1. The patients were predominantly White (98%) and majority were male (56%). The average age at PDAC diagnosis was 67 years. Twenty-nine percent of the patients self-reported a personal history of diabetes mellitus, 17% were current smokers, and 32% were obese (BMI ≥30 kg/m2). The proportion of patients with resectable, locally advanced, and metastatic tumors at diagnosis were 28%, 38%, and 33%, respectively. The quantitated LTL (T/S ratio) ranged from 0.341 to 1.280, with a mean of 0.775 and standard deviation of 0.162. The LTLs correlated inversely with age at diagnosis (Spearman r = −0.31, p-value <0.001). By the end of follow-up, 605 (94%) deaths had occurred. Overall median survival time was 10.8 months. Median survival was longer for patients with resectable PDAC (20.5 months) than patients with locally advanced (9.5 months) or metastatic (7.8 months) PDAC. When patients’ characteristics were stratified by categorical LTL, patients in the shortest LTL category were found to be older than those in the longest LTL category (mean age: 70 yrs. vs. 63 yrs.). Additionally, patients in the shortest LTL category included greater proportions of males, former and current smokers, self-reported diabetics, and obese individuals compared to those in longest LTL. The patients in the longest LTL category had longer overall survival than those in the shortest LTL category (12.7 months vs. 8.7 months).
Table 1.
Characteristics of the pancreatic ductal adenocarcinoma patients in the study
| Telomere length categories a | ||||
|---|---|---|---|---|
| Characteristics | Total n = 642 |
Long n = 215 |
Medium n = 212 |
Short n = 215 |
| Age at diagnosis | ||||
| Mean (SD) | 66.7 (10.3) | 63.4 (10.4) | 66 (10.0) | 70.4 (9.2) |
| Range | 38-89 | 38-87 | 43-87 | 41-89 |
| Male | 360 (56.1%) | 114 (53.0%) | 107 (50.5%) | 139 (64.7%) |
| Whites | 632 (98.4%) | 213 (99.1%) | 208 (98.1%) | 211 (98.1%) |
| Smoking status | ||||
| Never | 248 (38.6%) | 91 (42.3%) | 84 (39.6%) | 73 (34.0%) |
| Former | 284 (44.2%) | 92 (42.8%) | 89 (42.0%) | 103 (47.9%) |
| Current | 110 (17.1%) | 32 (14.9%) | 39 (18.4%) | 39 (18.1%) |
| Self-reported history of DM | 187 (29.1%) | 55 (25.6%) | 64 (30.2%) | 68 (31.6%) |
| BMI, kg/m2 | ||||
| ≤ 24.9 | 167 (26.0%) | 60 (27.9%) | 50 (23.6%) | 57 (26.5%) |
| 25-29.9 | 272 (42.4%) | 95 (44.2%) | 92 (43.4%) | 85 (39.5%) |
| ≥30 | 203 (31.6%) | 60 (27.9%) | 70 (33.0%) | 73 (34.0%) |
| Mean (SD) | 28.5 (5.3) | 27.875 (6.0) | 28.7 (5.2) | 28.8 (5.4) |
| Range | 17.6-53.0 | (17.6-53.0) | (18.2-48.8) | (18.0-49.9) |
| Tumor stage at diagnosis | ||||
| Resectable | 181 (28.2%) | 61 (28.4%) | 61 (28.8%) | 59 (27.4%) |
| Locally advanced | 247 (38.5%) | 73 (34.0%) | 94 (44.3%) | 80 (37.2%) |
| Metastatic | 214 (33.3%) | 81 (37.7%) | 57 (26.9%) | 76 (35.3%) |
| Telomere length | ||||
| Mean (SD) | 0.755 (0.162) | 0.937 (0.100) | 0.742 (0.040) | 0.587 (0.070) |
| Range | 0.341-1.280 | (0.817-1.280) | (0.678-0.815) | (0.341-0.677) |
| Median survival, months (95% CI) | ||||
| Overall (all stages combined) | 10.8 (10.1-12.5) | 12.7 (11.4-13.9) | 11.3 (10.1-13.3) | 8.7 (8.2-10.0) |
| Resectable | 20.5 (16.7-23.5) | 20.4 (16.8-24.1) | 22.8 (16.7-24.0) | 20.5 (16.4-23.7) |
| Locally advanced | 9.5 (8.2-10.6) | 10.7 (9.6-13.2) | 10.6 (9.2-13.0) | 7.9 (6.9-8.8) |
| Metastatic | 7.8 (6.8-8.6) | 10.3 (8.1-11.6) | 8.0 (7.3-9.3) | 6.3 (5.2-7.7) |
Leukocyte telomere length was measured as the ratio of telomere to single-copy gene (T/S ratio) and categorized as short (≤0.677), medium (0.678-0.815), and long (>0.815).
Abbreviations: CI, confidence interval, BMI, body mass index; DM, diabetes mellitus; SD, standard deviation.
The association between treatment-naïve LTL and overall survival is summarized in Table 2. For analyses that were based on continuous LTL variable, the HRs reflect an interquartile range (0.216 T/S ratio) decrease in LTL. Shorter treatment-naïve LTL was associated with a higher risk of death after adjusting for age, sex, and tumor stage at diagnosis (HR continuous LTL = 1.15, 95% CI: 1.02-1.29, p-value = 0.02; HR shortest vs. longest LTL = 1.23, 95% CI: 1.01-1.50, P trend = 0.04). After additional adjustment for BMI, diabetes and smoking history, the association between shorter treatment-naïve LTL and higher risk of death remained significant (HR continuous LTL = 1.13, 95% CI: 1.01-1.28, p-value = 0.03; HR shortest vs. longest LTL = 1.29, 95% CI: 1.05-1.59, P trend = 0.01). Survival probabilities plotted by Kaplan-Meier survival curves also show poorer overall survival for patients in the shortest LTL groups, compared to those in the longest LTL group (Figure 1).
Table 2.
Association between treatment-naïve leukocyte telomere length and overall survival of patients with pancreatic ductal adenocarcinoma
| Telomere length (T/S ratio)a | Deaths/No. at risk | Median survival, months (95% CI) | Adjustedd HR (95% CI) | p-value | Adjustede HR (95% CI) | p-value |
|---|---|---|---|---|---|---|
| Continuousb | 605/642 | 10.8 (10.1-12.5) | 1.15 (1.02-1.29) | 0.02 | 1.13 (1.01-1.28) | 0.03 |
| Categoricalc | ||||||
| Long | 197/215 | 12.7 (11.4-13.9) | 1.00 (ref) | 0.04f | 1.00 (ref) | 0.01f |
| Medium | 198/212 | 11.3 (10.1-13.3) | 0.98 (0.80-1.19) | 0.97 (0.79-1.18) | ||
| Short | 210/215 | 8.7 (8.2-10.0) | 1.23 (1.01-1.50) | 1.29 (1.05-1.59) |
Telomere length was measured as the ratio of telomere to single-copy gene (T/S ratio).
Hazard ratios for continuous telomere length reflects per interquartile range (0.216 T/S ratio) decrease in leukocyte telomere length.
Categorical telomere length was based on distribution in the entire cohort, categorized as short (≤0.677), medium (0.678-0.815), and long (>0.815).
Adjusted for age at diagnosis (continuous), sex, and tumor stage at diagnosis (resectable, locally advanced, metastatic).
Additional adjustment for body mass index (continuous), diabetes (yes, no), and smoking status (never, former, current).
Trend p-value across tertiles
Abbreviations: CI, confidence interval; HR, hazard ratio
Figure 1.
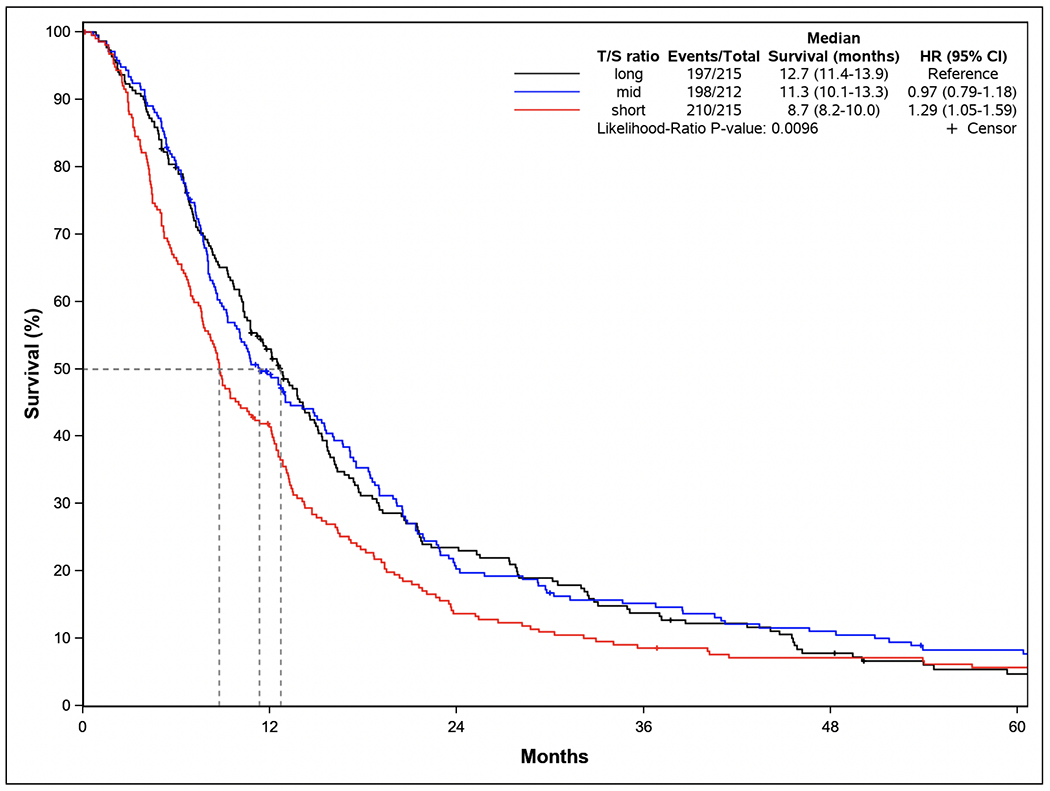
Kaplan-Meier survival curves, multivariable-adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for the association between treatment-naive leukocyte telomere length and pancreatic ductal adenocarcinoma. Telomere length was measured as the ratio of telomere to single-copy gene (T/S ratio) and categorized as short (≤0.677), medium (0.678-0.815), and long (>0.815). HRs and 95% CIs were calculated from Cox proportional hazard models, adjusting for age at diagnosis (continuous), sex, tumor stage at diagnosis (resectable, locally advanced, metastatic), body mass index (continuous), personal history of diabetes mellitus (yes, no), and smoking history (never, former, current).
We observed an interaction between treatment-naïve LTL and tumor stage at diagnosis (P interaction = 0.04) (Table 3). The interaction was such that shorter LTL was significantly associated with higher mortality among patients with locally advanced PDAC (HR continuous LTL = 1.29, 95% CI: 1.07-1.56), but not those with resectable PDAC (HR continuous LTL = 0.91, 95% CI: 0.73-1.12) or metastatic PDAC (HR continuous LTL = 1.17, 95% CI: 0.96-1.42), as illustrated graphically in Supplementary Figure 1. We further explored the interaction by tumor stage using the categorical LTL variable (Table 3). While no association was found among patients with resectable PDAC (HR shortest vs. longest LTL = 0.92, 95% CI: 0.62-1.35), shorter LTL was significantly associated with higher mortality among patients with locally advanced PDAC (HR shortest vs. longest LTL = 1.42, 95% CI: 1.01-1.98) and those with metastatic PDAC (HR shortest vs. longest LTL = 1.46, 95% CI: 1.06-2.01). However, interaction p-value based on the categorical LTL was not significant (P interaction = 0.16).
Table 3.
Association between treatment-naïve leukocyte telomere length and overall survival, stratified by tumor stage at diagnosis
| Telomere length by tumor stage | Deaths/No. at risk | Median survival, months (95% CI) | HR (95% CI)c | Interaction p-valued |
|---|---|---|---|---|
| Continuous LTLa | 0.04 | |||
| Resectable | 159/181 | 20.5 (16.7-23.5) | 0.91 (0.73-1.12) | |
| Locally advanced | 233/247 | 9.5 (8.2-10.6) | 1.29 (1.07-1.56) | |
| Metastatic | 213/214 | 7.8 (6.8-8.6) | 1.17 (0.96-1.42) | |
| Categorical LTLb | 0.16 | |||
| Resectable | ||||
| long | 50/61 | 20.4 (16.8-24.1) | 1.00 (ref) | |
| medium | 54/61 | 22.8 (16.7-24.0) | 0.96 (0.65-1.42 | |
| short | 55/59 | 20.5 (16.4-23.7) | 0.92 (0.62-1.35) | |
| Locally advanced | ||||
| long | 67/73 | 10.7 (9.6-13.2) | 1.00 (ref) | |
| medium | 87/94 | 10.6 (9.2-13.0) | 0.86 (0.62-1.18) | |
| short | 79/80 | 7.9 (6.9-8.8) | 1.42 (1.01-1.98) | |
| Metastatic | ||||
| long | 80/81 | 10.3 (8.1-11.6) | 1.00 (ref) | |
| medium | 57/57 | 8.0 (7.3-9.3) | 1.06 (0.75-1.50) | |
| short | 76/76 | 6.3 (5.2-7.7) | 1.46 (1.06-2.01) |
Leukocyte telomere length (LTL) was measured as the ratio of telomere to single-copy gene (T/S ratio), with hazard ratio for continuous LTL reflecting per interquartile range (0.216 T/S ratio) decrease in leukocyte telomere length.
Categorical leukocyte telomere length was based on the distribution in the entire cohort, categorized as short (≤0.677), medium (0.678-0.815), or long (>0.815).
Adjustment for age (continuous), sex, body mass index (continuous), diabetes (yes, no), and smoking status (never, former, current).
For continuous leukocyte telomere length, interaction p-value was calculated using a continuous T/S ratio and a categorical stage variable (resectable, locally advanced, metastatic). For categorical leukocyte telomere length, interaction p-value was calculated using a categorical T/S ratio variable (short, medium, long) and a categorical stage variable (resectable, locally advanced, metastatic).
Abbreviations: CI, confidence interval; HR, hazard ratio; LTL, leukocyte telomere length.
There also were instances where no statistically significant interaction was observed, but associations were observed in stratified analyses among certain subgroup (Supplementary Table 1), such as patients with BMI ≥30 kg/m2 (HR continuous LTL = 1.31, 95% CI: 1.07-1.61) and those with personal history of diabetes mellitus (HR continuous LTL = 1.25, 95% CI: 1.01-1.54). A marginal association was also observed among never smokers (HR continuous LTL = 1.20, 95% CI: 0.99-1.45). These associations were not observed among patients with BMI < 30 kg/m2, non-diabetics, and former or current smokers (Supplementary Table 1).
Discussion
PDAC is a rapidly fatal malignancy; hence, improved understanding of the molecular processes associated with survival of patients with PDAC could offer insights into the pathobiology of the tumor for prognostication, treatment selection, and response monitoring (27,28). In the present study, we investigated the association between treatment-naïve LTL and overall survival of 642 PDAC patients consecutively enrolled in the Mayo Clinic Biospecimen Resource for Pancreas Research. Our results show that shorter treatment-naïve LTL is associated with poorer overall survival of patients with PDAC. In interaction analyses, we found that shorter treatment-naïve LTL was associated with poorer survival among patients with locally advanced PDAC, and possibly patients with metastatic PDAC, but not those with resectable PDAC. Collectively, our findings suggest that LTL plays a plausible role in the survival of patients with PDAC.
In previous studies, we and others found that shorter LTL is associated with higher risk for PDAC (23,29). Here we provide additional evidence that shorter LTL is associated also with poorer survival of patients with PDAC. Consistent with our findings are the results of a prospective study of pooled data from four independent cohorts, in which investigators assessed the association between prediagnostic LTL and overall mortality among 423 patients (HR shortest vs. longest LTL = 1.39, 95% CI: 1.01-1.93, P trend = 0.04)(7). In addition to having a smaller sample size than our study, the blood samples used in this prior study were collected a median of six years before cancer diagnosis (7). In our study, blood samples were collected at the time of cancer diagnosis, before cancer treatment. Hence, our results are more germane to clinical settings during initial evaluation of PDAC patients, seeking to aid in the assessment of patient prognosis and decisions regarding patient management. In another prospective study in Denmark, the association between LTL and overall survival was assessed among 124 PDAC patients and a null finding was reported (per kilobase decrease in LTL, HR=1.02, 95% CI: 0.83-1.25)(20). However, this study was limited substantially by small sample size and lack of control of confounding by lifestyle factors, such as obesity and cigarette smoking history (16).
Our study further suggests that among patients with locally advanced PDAC, shorter LTL is associated with poorer survival based on a continuous LTL variable, whereas no association was found among patients with resectable PDAC or metastatic PDAC. When a categorical LTL variable was used, we found a significant association between shorter LTL and poorer survival among patients with locally advanced PDAC and patients with metastatic PDAC, but not those with resectable PDAC. However, statistical interaction was not significant when using categorical LTL, which may be due to reduced statistical power associated with categorization of continuous variables (30,31). There also was a suggestion that shorter LTL is associated with poorer overall survival among patients BMI ≥30 kg/m2, diabetic patients, and never smokers, but no significant interaction was found by these factors, and therefore require verification in larger studies.
Importantly, having a shorter LTL has been associated with higher mortality in the general population, with much higher mortality among individuals with certain age-related health conditions (32–34), lending support to the hypothesis that telomere shortening contributes to premature death from certain age-related diseases (16,32). It is thought that the presence of fully functional telomeres may delay the progression of age-related pathologies and thereby prevent early death (12), which may explain our finding that shorter LTL is associated with poorer survival of patients with PDAC. Alternatively, telomere shortening may not contribute directly to mortality but may be a marker for a higher burden of terminally arrested senescent cells. Excessive accumulation of dysfunctional senescent cells tends to alter gene expression patterns, disrupt tissue renewal processes, and obstruct the functions of normal cells (12,35–37). Telomere shortening may also compromise the capacity to repair double-strand DNA breaks (38), which has multiple phenotypic consequences, including susceptibility to cancer and poor prognosis after cancer diagnosis. Additionally, senescent cells often secrete deleterious substances, such as pro-inflammatory cytokines, epithelial growth factors, and extracellular matrix remodeling enzymes that can alter tissue microenvironment thereby compromising tissue structure and function, culminating to deleterious effects on health and limiting longevity (10,12).
Our study has several important strengths and some limitations. Strengths of our study include the relatively larger sample size compared to prior studies, and the prospective assessment of LTL in blood samples collected before the start of treatment and therefore avoids confounding by cancer therapy, particularly chemotherapeutic agents, which are known to influence telomere length (39–41). Biospecimens were processed and stored by standard protocol in a core lab, ensuring quality, and each sample was assayed in triplicate with an overall high level of reproducibility. The assessment of LTL at the time of diagnosis is reflective of telomere dynamics in the setting of PDAC and has potential clinical application for prognostication, disease monitoring, and future development of targeted therapies, as discussed elsewhere (42,43). Additionally, we used data from a prospective patient registry that utilizes an ultra-rapid case ascertainment process with the majority of patients recruited within one month of diagnosis with a high participation rate (~70%). We also controlled for potential confounding by multiple clinical and patient factors that have been associated with both LTL and PDAC survival.
Limitations of our study include the predominantly White population, limiting racial generalizability of the findings. Furthermore, we did not have data on PDAC-specific mortality. However, because PDAC is a rapidly fatal cancer with most patients (~90%) dying within five years of diagnosis, overall survival is a reasonable surrogate for PDAC-specific survival and is widely used in PDAC survival studies (7,44). Vital status was ascertained from multiple sources. This could have introduced some level of bias into the study, influencing effect estimates to some extent, either toward or away from null. Moreover, we measured average telomere length among all leukocyte subtypes. Because telomere length varies by leukocyte subtype (20), the average telomere length among all subtypes may be influenced by differential proportions of certain subtypes in the sample. Future studies that are based on cell type-specific telomere length will avoid this limitation. Moreover, we had limited data on treatments administered to the patients, which precluded its use in our study. However, blood samples were collected before cancer treatment, and treatment-naïve LTL is unlikely to have been affected by treatment choice. It is also worth noting that tumor stage at diagnosis is a major determinant of treatment choice. We had reasonable numbers of patients with resectable, locally advanced, and metastatic tumor, reflective of what is commonly seen in most clinics (1), and we adjusted for stage at diagnosis as a surrogate for treatment. We also acknowledge that peripheral blood LTL may not faithfully represent telomere length of pancreatic tumor epithelium. However, studies have shown that telomere length of peripheral blood leukocytes correlates strongly with telomere length of matching somatic tissues, including the skin, subcutaneous fat, and skeletal muscle (Pearson’s r = 0.83-0.84, P <0.0001)(45).
In conclusion, our study shows that shorter treatment-naïve LTL is associated with poorer overall survival of patients with PDAC. The findings suggest that treatment-naïve LTL may have prognostic relevance in PDAC and could be useful for patient stratification for treatment selection and prioritization toward the goal of improving outcomes for patients with PDAC. Additional investigations into leukocyte cell type-specific telomere length and PDAC survival would advance our understanding of telomere kinetics in PDAC.
Supplementary Material
Acknowledgments
Grant support: The study was supported by funding from the National Cancer Institute (P50CA102701 to G.M. Petersen, R01 CA204013 to L.A. Boardman, and K01 CA237875 to S.O. Antwi)
Abbreviations:
- BMI
Body mass index
- CI
Confidence intervals
- FDA
Food and Drug Administration
- HR
Hazard ratio
- HHS
Health and Human Services
- ICH
International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use
- IPMN
Intraductal papillary mucinous neoplasia
- LTL
Leukocyte telomere length
- PDAC
Pancreatic ductal adenocarcinoma
- TL
Telomere length
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest
Disclosures: Dr. Richard M. Cawthon and the University of Utah hold a patent for the technology used herein to measure average telomere length by quantitative PCR, and have licensed the commercial use of this technology to Telomere Diagnostics, Inc. of Menlo Park, California. However, Dr. Cawthon was blind regarding all clinical data (sex, age, clinical outcomes, etc.) for the subjects studied here, at the time he performed and reported the results of the telomere length assays.
Data availability statement: The data may be made available to researchers upon request to Dr. Gloria Petersen (Petersen.gloria@mayo.edu). Ethical and legal restrictions apply to these data.
References
- 1.Antwi SO, Jansen RJ, Petersen GM. Cancer of the pancreas Schottenfeld and Fraumeni Cancer Epidemiology and Prevention, Fourth Edition: Oxford University Press; 2017. p 611–34. [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70(1):7–30 doi 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 3.Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet 2011;378(9791):607–20 doi 10.1016/s0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maitra A, Hruban RH. Pancreatic cancer. Annu Rev Pathol 2008;3:157–88 doi 10.1146/annurev.pathmechdis.3.121806.154305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369(18):1691–703 doi 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364(19):1817–25 doi 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 7.Hamada T, Yuan C, Bao Y, Zhang M, Khalaf N, Babic A, et al. Prediagnostic Leukocyte Telomere Length and Pancreatic Cancer Survival. Cancer Epidemiol Biomarkers Prev 2019;28(11):1868–75 doi 10.1158/1055-9965.Epi-19-0577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blackburn EH, Epel ES, Lin J. Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science 2015;350(6265):1193–8 doi 10.1126/science.aab3389. [DOI] [PubMed] [Google Scholar]
- 9.Lin J, Kaur P, Countryman P, Opresko PL, Wang H. Unraveling secrets of telomeres: one molecule at a time. DNA Repair (Amst) 2014;20:142–53 doi 10.1016/j.dnarep.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Antwi SO, Petersen GM. Leukocyte Telomere Length and Pancreatic Cancer Risk: Updated Epidemiologic Review. Pancreas 2018;47(3):265–71 doi 10.1097/mpa.0000000000000995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Artandi SE, DePinho RA. A critical role for telomeres in suppressing and facilitating carcinogenesis. Curr Opin Genet Dev 2000;10(1):39–46 doi 10.1016/s0959-437x(99)00047-7. [DOI] [PubMed] [Google Scholar]
- 12.Campisi J Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell 2005;120(4):513–22 doi 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Shay JW. Role of Telomeres and Telomerase in Aging and Cancer. Cancer Discov 2016;6(6):584–93 doi 10.1158/2159-8290.Cd-16-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hashimoto Y, Murakami Y, Uemura K, Hayashidani Y, Sudo T, Ohge H, et al. Telomere shortening and telomerase expression during multistage carcinogenesis of intraductal papillary mucinous neoplasms of the pancreas. J Gastrointest Surg 2008;12(1):17–28; discussion -9 doi 10.1007/s11605-007-0383-9. [DOI] [PubMed] [Google Scholar]
- 15.Matsuda Y, Ishiwata T, Izumiyama-Shimomura N, Hamayasu H, Fujiwara M, Tomita K, et al. Gradual telomere shortening and increasing chromosomal instability among PanIN grades and normal ductal epithelia with and without cancer in the pancreas. PLoS One 2015;10(2):e0117575 doi 10.1371/journal.pone.0117575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weischer M, Nordestgaard BG, Cawthon RM, Freiberg JJ, Tybjærg-Hansen A, Bojesen SE. Short telomere length, cancer survival, and cancer risk in 47102 individuals. J Natl Cancer Inst 2013;105(7):459–68 doi 10.1093/jnci/djt016. [DOI] [PubMed] [Google Scholar]
- 17.Willeit P, Willeit J, Mayr A, Weger S, Oberhollenzer F, Brandstätter A, et al. Telomere Length and Risk of Incident Cancer and Cancer Mortality. JAMA 2010;304(1):69–75 doi 10.1001/jama.2010.897. [DOI] [PubMed] [Google Scholar]
- 18.Callahan CL, Schwartz K, Ruterbusch JJ, Shuch B, Graubard BI, Lan Q, et al. Leukocyte telomere length and renal cell carcinoma survival in two studies. Br J Cancer 2017;117(5):752–5 doi 10.1038/bjc.2017.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Russo A, Modica F, Guarrera S, Fiorito G, Pardini B, Viberti C, et al. Shorter leukocyte telomere length is independently associated with poor survival in patients with bladder cancer. Cancer Epidemiol Biomarkers Prev 2014;23(11):2439–46 doi 10.1158/1055-9965.Epi-14-0228. [DOI] [PubMed] [Google Scholar]
- 20.Aubert G, Baerlocher GM, Vulto I, Poon SS, Lansdorp PM. Collapse of telomere homeostasis in hematopoietic cells caused by heterozygous mutations in telomerase genes. PLoS Genet 2012;8(5):e1002696 doi 10.1371/journal.pgen.1002696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Antwi SO, Fagan SE, Chaffee KG, Bamlet WR, Hu C, Polley EC, et al. Risk of Different Cancers Among First-degree Relatives of Pancreatic Cancer Patients: Influence of Probands’ Susceptibility Gene Mutation Status. J Natl Cancer Inst 2019;111(3):264–71 doi 10.1093/jnci/djx272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antwi SO, Oberg AL, Shivappa N, Bamlet WR, Chaffee KG, Steck SE, et al. Pancreatic cancer: associations of inflammatory potential of diet, cigarette smoking and long-standing diabetes. Carcinogenesis 2016;37(5):481–90 doi 10.1093/carcin/bgw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Antwi SO, Bamlet WR, Rabe KG, Cawthon RM, Umudi I, Druliner BR, et al. Leukocyte Telomere Length and Its Interaction with Germline Variation in Telomere-Related Genes in Relation to Pancreatic Adenocarcinoma Risk. Cancer Epidemiol Biomarkers Prev 2020. doi 10.1158/1055-9965.Epi-19-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LexisNexis Accurint record locator service. Available at www.accurint.com.
- 25.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res 2002;30(10):e47 doi 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med 1989;8(5):551–61 doi 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 27.Wong HH, Lemoine NR. Pancreatic cancer: molecular pathogenesis and new therapeutic targets. Nat Rev Gastroenterol Hepatol 2009;6(7):412–22 doi 10.1038/nrgastro.2009.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zavoral M, Minarikova P, Zavada F, Salek C, Minarik M. Molecular biology of pancreatic cancer. World J Gastroenterol 2011;17(24):2897–908 doi 10.3748/wjg.v17.i24.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bao Y, Prescott J, Yuan C, Zhang M, Kraft P, Babic A, et al. Leucocyte telomere length, genetic variants at the TERT gene region and risk of pancreatic cancer. Gut 2017;66(6):1116–22 doi 10.1136/gutjnl-2016-312510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bennette C, Vickers A. Against quantiles: categorization of continuous variables in epidemiologic research, and its discontents. BMC Med Res Methodol 2012;12:21 doi 10.1186/1471-2288-12-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Royston P, Altman DG, Sauerbrei W. Dichotomizing continuous predictors in multiple regression: a bad idea. Stat Med 2006;25(1):127–41 doi 10.1002/sim.2331. [DOI] [PubMed] [Google Scholar]
- 32.Cawthon RM, Smith KR, O’Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet 2003;361(9355):393–5 doi 10.1016/s0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- 33.Needham BL, Rehkopf D, Adler N, Gregorich S, Lin J, Blackburn EH, et al. Leukocyte telomere length and mortality in the National Health and Nutrition Examination Survey, 1999–2002. Epidemiology 2015;26(4):528–35 doi 10.1097/ede.0000000000000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rode L, Nordestgaard BG, Bojesen SE. Peripheral blood leukocyte telomere length and mortality among 64,637 individuals from the general population. J Natl Cancer Inst 2015;107(6):djv074 doi 10.1093/jnci/djv074. [DOI] [PubMed] [Google Scholar]
- 35.Kipling D. Telomeres, replicative senescence and human ageing. Maturitas 2001;38(1):25–37; discussion -8 doi 10.1016/s0378-5122(00)00189-4. [DOI] [PubMed] [Google Scholar]
- 36.Faragher RG, Kipling D. How might replicative senescence contribute to human ageing? Bioessays 1998;20(12):985–91 doi . [DOI] [PubMed] [Google Scholar]
- 37.Campisi J. Cancer, aging and cellular senescence. In Vivo 2000;14(1):183–8. [PubMed] [Google Scholar]
- 38.Kroupa M, Polivkova Z, Rachakonda S, Schneiderova M, Vodenkova S, Buchler T, et al. Bleomycin-induced chromosomal damage and shortening of telomeres in peripheral blood lymphocytes of incident cancer patients. Genes Chromosomes Cancer 2018;57(2):61–9 doi 10.1002/gcc.22508. [DOI] [PubMed] [Google Scholar]
- 39.Diker-Cohen T, Uziel O, Szyper-Kravitz M, Shapira H, Natur A, Lahav M. The effect of chemotherapy on telomere dynamics: clinical results and possible mechanisms. Leuk Lymphoma 2013;54(9):2023–9 doi 10.3109/10428194.2012.757765. [DOI] [PubMed] [Google Scholar]
- 40.Unryn BM, Hao D, Glück S, Riabowol KT. Acceleration of telomere loss by chemotherapy is greater in older patients with locally advanced head and neck cancer. Clin Cancer Res 2006;12(21):6345–50 doi 10.1158/1078-0432.Ccr-06-0486. [DOI] [PubMed] [Google Scholar]
- 41.Lee JJ, Nam CE, Cho SH, Park KS, Chung IJ, Kim HJ. Telomere length shortening in non-Hodgkin’s lymphoma patients undergoing chemotherapy. Ann Hematol 2003;82(8):492–5 doi 10.1007/s00277-003-0691-4. [DOI] [PubMed] [Google Scholar]
- 42.Jafri MA, Ansari SA, Alqahtani MH, Shay JW. Roles of telomeres and telomerase in cancer, and advances in telomerase-targeted therapies. Genome Med 2016;8(1):69 doi 10.1186/s13073-016-0324-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arndt GM, MacKenzie KL. New prospects for targeting telomerase beyond the telomere. Nat Rev Cancer 2016;16(8):508–24 doi 10.1038/nrc.2016.55. [DOI] [PubMed] [Google Scholar]
- 44.Couch FJ, Wang X, Bamlet WR, de Andrade M, Petersen GM, McWilliams RR. Association of mitotic regulation pathway polymorphisms with pancreatic cancer risk and outcome. Cancer Epidemiol Biomarkers Prev 2010;19(1):251–7 doi 10.1158/1055-9965.Epi-09-0629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Daniali L, Benetos A, Susser E, Kark JD, Labat C, Kimura M, et al. Telomeres shorten at equivalent rates in somatic tissues of adults. Nat Commun 2013;4:1597 doi 10.1038/ncomms2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


