Abstract
Cell-to-cell transfer of α-synuclein (αS) is increasingly thought to play an important role in propagation of αS pathology, but mechanisms responsible for formation of initial αS seeds and factors facilitating their propagation remain unclear. We previously demonstrated that αS aggregates are formed rapidly in apoptotic neurons and that interaction between cytoplasmic αS and proaggregant nuclear factors generates seed-competent αS. We also provided initial evidence that histones have proaggregant properties. Since histones are released from cells undergoing apoptosis or cell stress, we hypothesized that internalization of histones into αS expressing cells could lead to intracellular αS aggregation. Here using mCherry-tagged histone we show that nuclear extracts from apoptotic cells can induce intracellular αS inclusions after uptake into susceptible cells, while extracts from non-apoptotic cells did not. We also demonstrate that nuclear extracts from apoptotic cells contained histone-immunoreactive amyloid fibrils. Moreover, recombinant histone-derived amyloid fibrils are able to induce αS aggregation in cellular and animal models. Induction of αS aggregation by histone amyloid fibrils are associated with endocytosis-mediated rupture of lysosomes, and this effect can be enhanced in cells with chemically-induced lysosomal membrane defects. These studies provide initial descriptions of the contribution of histone amyloid fibrils to αS aggregation.
Keywords: Aggregation, histone, nuclear amyloid fibrils, Parkinson’s disease, α-Synuclein
INTRODUCTION
The concept of cell-to-cell transfer of α-synuclein (αS) is increasingly thought to play an important role in propagation of αS pathology in Parkinson’s disease (PD) and related disorders.[2, 17, 26] The factors responsible for formation of initial transmissible αS species (or seeds) and factors facilitating subsequent propagation remain unclear. We previously demonstrated that αS aggregates can be rapidly formed in apoptotic neurons by interaction between cytoplasmic αS and proaggregant nuclear factors associated with disruption of nuclear envelope, and that histones are candidate proaggregant factors.[10, 12] Given that histones can be released from cells undergoing apoptosis or cell stress,[1, 14, 19] we hypothesized that extracellular histones could be internalized and then induce αS aggregation in adjacent neurons. Cell-based experiments were designed to test the hypothesis and results showed that exogenous histone-labeled nuclear extracts from apoptotic cells could induce intracellular αS inclusions after internalization into recipient cells, while extracts from non-apoptotic cells would not. We also explored differences between nuclear extracts of apoptotic and non-apoptotic cells, with a focus on histones to better understand the nature of their proaggregant properties. We used both cellular and animal models to study the role of histones in seeding and spread of αS aggregates and roles of endocytosis and lysosomal integrity play in these processes. We provide evidence that during cell apoptosis, histones form amyloid fibrils that facilitate αS aggregation and that this process is greatly enhanced in cells with lysosomal membrane defects.
Methods and Materials
Cell culture and maintenance
Cells were grown in a cell culture incubator 37°C, 5% CO2, 100% humidity, and maintained in OPTI-MEM (Invitrogen) medium containing 10% fetal bovine serum (Invitrogen). For confocal microscopic live cell imaging, cells were cultured in Nunc® Lab-Tek® II chambered coverglasses (Sigma-Aldrich). For differentiation of cells derived from the human dopaminergic cell line BE(2)-M17 (CRL-2267, Manassas, VA, USA), media were replaced with Neurobasal medium (Invitrogen, Thermo Fisher, Waltham, MA) with 2% B-27 supplement (Invitrogen, Thermo Fisher), 2 mM l-glutamine (Sigma-Aldrich) and 10 μM retinoic acid (Sigma-Aldrich).
Lentiviral plasmids and virus preparation
Lentiviral plasmids carrying LAMP1-eCFP and mCherry-Galectin3 were described previously.[11] The lentiviral vector for CRISPR-Cas9 knockout of αS was designed by VectorBuilder, Inc. The guide sequences of SgRNAs were “GGAGAGGACCTCCTGTTAGC”, “GTGGTGCATGGTGTGGCAAC”. The preparation of Lentivirus carrying genes of interest were the same as described previously.[9]
Nuclear extract preparation
Nuclear extracts were prepared using NE-PER nuclear and cytoplasmic extraction reagents (Thermo Scientific, Waltham, MA). Briefly, cells were resuspended in cytoplasmic extraction reagent-I and incubated on ice for 10 minutes. After that, cytoplasmic extraction reagent-II was added to cell suspensions, which were briefly vortexed, and then centrifuged at 16000×g for 5 minutes. The resultant supernatant (“cytoplasmic fraction”) was removed and the pellet (“isolated nuclei”) was used to make nuclear extracts for immediate use or storage at −80°C for future use. To prepare nuclear extracts, the nuclear pellet was mixed with nuclear extraction reagent and subjected to homogenization for 1 min using a homogenizer (IKA T10 basic, Ultra-Turrax) followed by centrifugation at 16000×g for 10 min. The supernatant was saved as “nuclear extract.” The whole process was done on ice or at 4°C. Protein concentration of each group was measured by the bicinchoninic acid (BCA) assay. Both cell counting and BCA methods were used to assure that equivalent amounts of nuclear extracts were used in cell treatments and other analyses for both experimental and control groups.
Preparation of in vitro formed histone amyloid fibrils
Recombinant human histone was purchased from Active Motif (Catalog No: 81826). The H1 fibrils for induction of αS aggregation were prepared in accordance with previous reports,[27] with minor modifications. Briefly, fresh recombinant histone was diluted in 20 mM HEPES and 0.1 mM EDTA (pH 7.4) to a 1 mg/ml stock solution, then added to brain phosphatidylserine/1-stearoyl-2-oleoyl-sn-glycero-3-phosphocholine (PS/SOPC) liposomes (1:4 molar ratio) in the same buffer, to make a mixed solution with a final concentrations of 100 μM phospholipids and 0.2 mg/mL H1 protein. The solution was incubated at 37°C overnight. The formed fibrils were concentrated by ultracentrifugation (110,000 ×g, 20 min) and resuspended in phosphate buffered saline (PBS). For confirmation of amyloid fibrillar structure, a portion of the solution was subjected to thioflavin T assays and to electron microscopic examination, as described previously.[13] The remaining solution was aliquoted and stored at −80°C. Prior to use, the aliquots were briefly sonicated using Sonicator 3000 (Misonix).
Electron microscopy (EM) and immunoelectron microscopy (IEM)
Cells were fixed with 2% glutaraldehyde, 2% paraformaldehyde (PFA) in 0.1 M PBS for transmission EM and 4% PFA in in 0.1 M PBS for IEM. For EM, the cells were post-fixed in 1% OsO4, washed in distilled water for 3 times, stained with 1% uranyl acetate in 50% ethanol, dehydrated with 70%, 80%, 95% and 100% ethanol then propylene oxide, infiltrated and embedded in Epon 812 (Polysciences, Warrington, PA, USA). For IEM, cells were exposed in 30%, 50%, 70% and 90% ethanol then 90% ethanol-LR White (1:1), 90% ethanol-LR White (1:2), and infiltrated and embedded in pure LR White. Ultrathin sections were cut from the Epon 812 or LR White embedded samples with a Leica Ultramicrotome. Tissue sections on EM grids were subjected to immunogold labeling and counterstained with uranyl acetate and lead citrate. Results were examined and photographed with a Philips 208S electron microscope.
Induction and quantification of intracellular αS aggregates
For induction of intracellular αS aggregation, culture media were replaced with Opti-MEM (Invitrogen) mixed with histones of different preparations (soluble or fibrillar histones). After incubation for 3 days at 37°C, induced intracellular αS inclusions were monitored with confocal fluorescence microscopy (Zeiss LSM 510, Carl Zeiss MicroImaging). For quantitation of the ratio of cells containing αS inclusions, five fields (upper right, upper left, center, lower right, and lower left) with at least 90 cells were selected from each group and used for counts.
Stereotaxic brain injection
All animal procedures are approved by Mayo Clinic IACUC and IBC, in accordance with the NIH Guide for the Care and Use of Laboratory Animals and for Research Involving Recombinant DNA Molecules. A mixture of 3 male and 3 female FVB mice were used for brain injection in each group to avoid the influence of sex on the outcome. Ten month-old mice were chosen for experiment because such age is about 50 years old for human age [7] and age is the most prominent risk factor for PD.[4] Mice were anesthetized with 3% isoflurane and stereotaxically injected with soluble or fibrillar histone preparations at a dose of 10 μg per brain. A single-needle insertion (coordinates: X = 2.0 mm; Y = 0.2 mm; Z = 0.8 mm) was used to deliver the inoculum into the somatosensory cortex of the left forebrain as previously described.[12] Material was injected with a Hamilton syringe at a rate of 0.5 μl per minute. After recovery from surgery, animals were maintained for one week, and then subjected to transcardial perfusion with PBS. Brains were fixed in 10% formaldehyde prior to processing for paraffin embedding using standard histologic procedures. Coronal sections were cut at 5-micron thickness and used for immunohistochemical and immunofluorescent studies.
Immunohistochemical and immunofluorescent staining
Paraffin sections of mouse brain were subjected to deparaffinization, rehydration, steaming in DAKO target retrieval solution pH 6.1 for 30 minutes, and blocking with Protein Block (X0909, DAKO) at room temperature for 1 h. For immunoperoxidase labeling, sections were treated with 3% hydrogen peroxide to block endogenous peroxidase, followed by 20-minute incubation with 5% normal goat serum (G9023, Sigma-Aldrich) to reduce non-specific labeling. Subsequently, sections were sequentially incubated with antibody to αS (610787, 1:100, BD Biosciences) for 45 min and Envision-Plus labeled polymer HRP (DAKO) for 30 min. Peroxidase labeling was visualized with a solution containing 3, 3’ - diaminobenzidine (DAB-Plus). Lerner 1 hematoxylin (14–930-11, Fisher Scientific) and cytoseal mounting medium (8310–16, Richard-Allan Scientific, Kalamazoo, MI) was used for counterstaining and coverslipping, respectively. For immunofluorescent staining, the sections were incubated with primary antibodies to αS (610787, 1:100, BD Biosciences ) and H1 (ab125027, 1:100, Abcam) at 4°C overnight, followed by 90 minutes of incubation with secondary antibodies (1:500) after washing. Sudan Black was used to block non-specific fluorescence. Sections were coverslipped with Vectashield mounting media (H-1200, Vector Laboratories, Burlingame, CA) and imaged with confocal microscopy (Zeiss LSM 510, Carl Zeiss MicroImaging).
Statistical analysis
Data from at least 3 sets of independent experiments were analyzed by one-way ANOVA with Dunnett’s post hoc test or Student’s t test for comparison of groups >3 and =2, respectively, to determine statistical significance.
Results
Apoptotic neuron-derived nuclear extracts induce αS aggregation upon cellular uptake
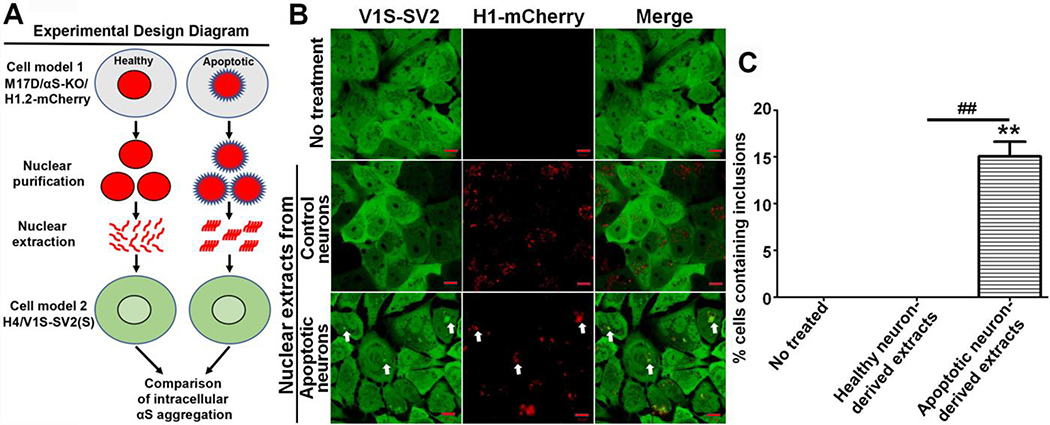
We previously showed that apoptosis-induced nuclear membrane disruption causes translocation of nuclear histones to the cytoplasm, and that this was associated with aggregation of cytoplasmic αS.[10, 12] In the present experiments, we addressed if histones derived from apoptotic neurons are able to induce αS aggregation in adjacent cells after cellular uptake. If proven, this would identify a novel pathway for propagation of αS pathology. For these studies, we generated two cell models. One model was derived from the H4 neuroglioma cell line (HTB-148 - ATCC, Manassas, VA, USA), which stably (S) expresses the N-terminal half of venus YFP tagged to αS (V1S) and the C-terminal half of venus YFP tagged αS (SV2), referred to as H4/V1S-SV2(S). This cell line is used to monitor αS aggregation in real-time in living cells since close proximity of V1S and SV2 reconstitutes YFP fluorescence indicative of αS aggregation, as described previously.[11] The other model, derived from the BE(2)-M17D neuroblastoma cell line, does not express αS due to knockout via lentiviral delivery of vector carrying CRISPR/Cas9 and gRNA of Snca gene, referred to as M17D/αS-KO. This cell line was further transfected with vector carrying mCherry-tagged histone H1.2 (H1.2-mCherry) to derive another cell line, named as M17D/αS-KO/H1.2-mCherry. Cells expressing H1-mCherry were used to monitor histones in live cells in which deletion of αS expression excludes contribution of endogenous αS. The use of the two different cell models were briefly depicted in an experimental design diagram shown as Fig. 1A. M17D/αS-KO/H1.2-mCherry cells were differentiated for 7 days, after which the cells have a neuronal phenotype.[15] They were then they exposed to 500 μM 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP,) a PD related neurotoxin[16] that induced apoptosis.[6, 21] MPTP-treated cells were compared to M17D/αS-KO/H1.2-mCherry cells exposed to only vehicle. After 2 days of MPTP treatment, both sets of cells were harvested and cell number was assessed by counting. Nuclei were isolated as described above, aliquoted and stored at −80°C. To test the capability of nuclear extracts to induce αS aggregation upon cellular uptake, nuclear extracts derived from MPTP- or vehicle (water)-treated nuclei were added to H4/V1S-SV2(S) cells growing in 8-well cell culture slides. In parallel, cells without any treatment were included as a control. After 3 days, we observed intracellular αS inclusions in cells treated with apoptotic neuron-derived nuclear extracts, and the inclusions were positive for H1-mCherry (Fig. 1B & 1C). In contrast, no αS inclusions were found in cells of the two control groups (Fig. 1B & 1C). These results raised the question as to why only histone-associated nuclear extracts from apoptotic neurons were competent in inducing αS aggregation. Therefore, we investigated difference between nuclear extracts of MPTP-induced apoptotic cells and control cells.
Fig. 1. Apoptotic neuron-derived nuclear extracts induce αS aggregation upon cellular uptake.
A) Experimental design diagram. B) M17D/αS-KO/H1-mCherry cells (M17D cells with αS knockout and expression of mCherry-tagged histone H1) were differentiated for 7 days then subjected to MPTP to induce apoptosis (apoptotic neurons) or vehicle (control neurons) for 2 days followed by purification of nuclear extracts, which were subsequently added to H4/V1S-SV2 cells for 3 days. H4/V1S-SV2 cells without any treatment were used as another negative control. White arrows denote internalized H1-mCherry-labeled nuclear extract and its-associated αS inclusion. αS-KO: αS knockout; H1-mCherry: mCherry tagged histone H1; H4/V1S-SV2: a H4 neuroglioma cell line co-expressing the N-terminal half of Venus YFP tagged αS (V1S) and C-terminal half of Venus YFP tagged αS (SV2) for visualization of αS aggregation. C) Bar graph shows the comparison of the 3 groups, with ratio of cells with inclusions to total cells. Error bars represent standard error of the mean (*p < 0.05, **p < 0.01, comparing subsets linked by line, n = 3).
Nuclear components of apoptotic neurons contain histone amyloid fibrils
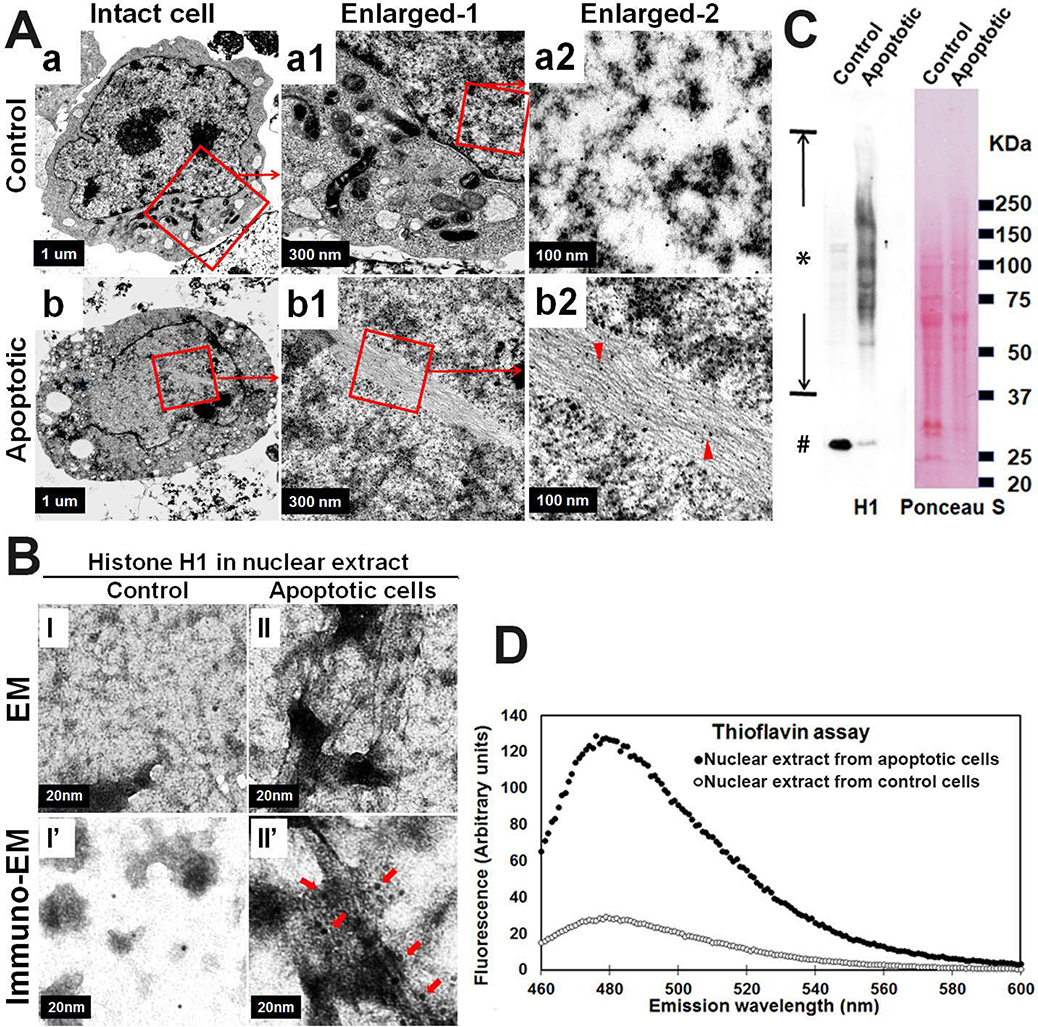
First, we wanted to know if there is any observable difference in nucleus at ultrastructural level between MPTP-induced apoptotic and the control healthy neurons. M17D/αS-KO cells were differentiated for 7 days, then exposed to MPTP or vehicle for 2 days. Subsequently, neurons with and without treatment were harvested and divided into 2 parts. One part was fixed and processed for immune-EM; the other part was used for isolation of nuclei and preparation of nuclear extracts. Unexpectedly, we observed filamentous structures in nuclei of MPTP-induced apoptotic neurons, as well as the nuclear extracts. No similar filamentous structures were observed in control neurons or their nuclear extracts (Fig. 2A & 2B). Thioflavin T assay, a sensitive method for detecting a wide range of amyloids, demonstrated that nuclear extracts from apoptotic cells contained significantly more signal than control cells (Fig. 2D). Previous studies have reported that histones are capable of forming amyloid fibrils.[20, 24, 27] Given prior work suggesting that histones may be proaggregant factors in αS [10], they were a reasonable candidate for the amyloid observed in these nuclear extracts. To determine if histone was a constituent of the observed amyloid fibrils, we used immuno-EM and antibodies to H1 histone. We found H1 labeling of filamentous structures in both nuclei of MPTP-treated cells and in nuclear extracts from these apoptotic cells (Fig. 2A & 2B). In addition, western blotting revealed that nuclear extracts from MPTP-treated cells, but not control neurons, contain a series of histone H1 immunopositive bands and a diffuse smear ranging from low to high molecular weights, consistent with SDS-resistant histone H1 multimers (Fig. 2C). The results demonstrated that histones can form amyloid fibrils in nuclei of apoptotic neurons induced by MPTP, while histones in control neurons do not.
Fig. 2. Nuclear histone amyloid fibrils form in apoptotic neuron cells.
Neuroblastoma cells M17D with αS knockout (M17D/αS-KO) were differentiated for 7 days then subjected to MPTP for 2 days to induce apoptosis. Sibling cells treated by vehicle were used as control. Cells from each group were harvested and divided into 2 fractions. (A) One fraction was processed for immuno-EM, which demonstrated histone H1-immunopositive filamentous structures in nuclei from apoptotic neurons (see “a, a1, a2” & “b, b1, b2”). Arrow heads in (b2) denote histone H1-immunopositive amyloid fibrils in an intact nucleus of an apoptotic cell (organelles are decreased compared with control cells); (B, C &D) The other fraction was used for isolation of nuclei and then generation of nuclear extracts. Nuclear extracts were further divided into three sub-fractions: (B) One sub-fraction was subjected to negative staining EM (I and II) and immuno-EM (I’ and II’) to demonstrate histone H1 immunopositive filamentous structure in nuclear extracts isolated from apoptotic neurons. Arrows in (II’) denote histone H1-immunopositive amyloid fibrils in nuclear extracts isolated from apoptotic neurons; (C) The second sub-fraction from apoptotic neurons was subjected to SDS-PAGE and western blotting to demonstrate a series of histone H1 immunopositive bands, ranging from monomers (denoted by number sign #) to aggregated high molecular weight species (denoted by asterisk sign). Ponceau S staining of the blot was used to confirm that comparable protein was loaded in the two groups; (D) The third sub-fraction was subjected to a Thioflavin T assay to measure the content of beta-sheet (amyloid) in the samples. Primary antibody for detection of histone H1: sc-8030, Santa Cruz Biotechnology. Colloidal Gold Conjugate: GA1003, Boster Bio.
Exogenous soluble histones don’t induce intracellular αS aggregation upon internalization into recipient cells
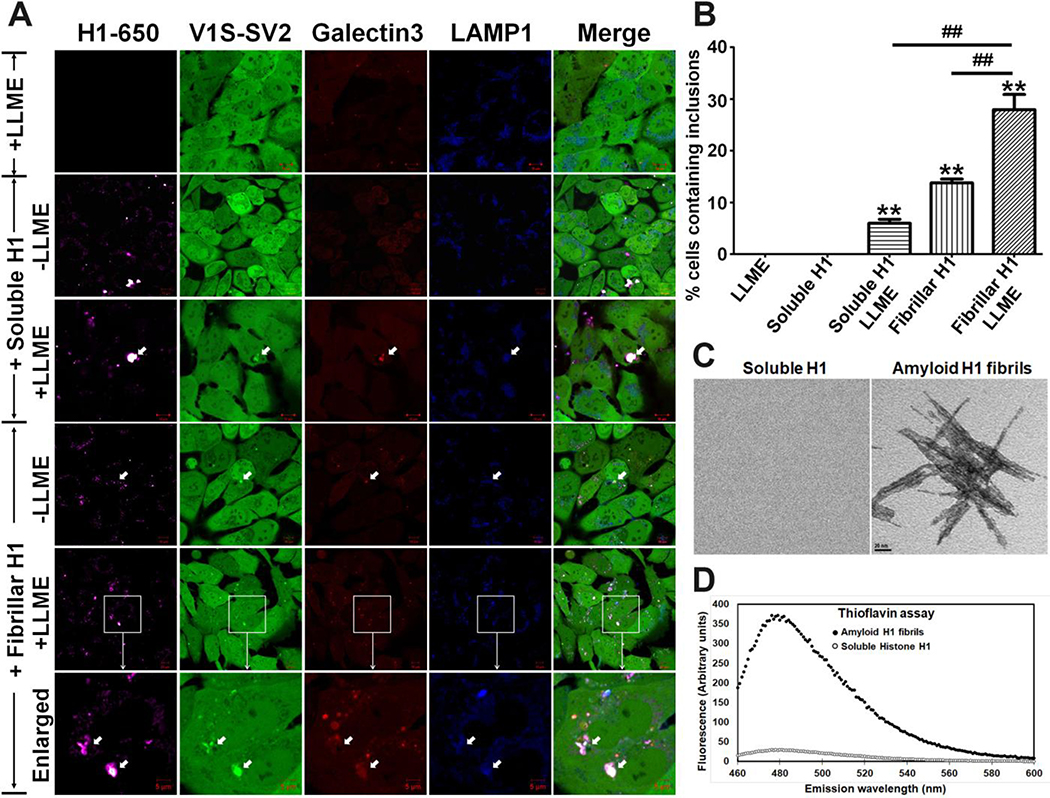
Given that αS can be cross-seeded to aggregate by other types of fibrils,[22, 23] we hypothesized that histone amyloid fibrils in nuclear extracts would be capable of inducing αS aggregation. To test this hypothesis, we prepared histone H1-derived amyloid fibrils following methods described previously,[27] labeled them with Lightning-Link® Rapid DyLight® 650 (Innova Biosciences) and directly treated H4/V1S-SV2(S) cells with the histone amyloid fibrils. Sibling cells treated with soluble recombinant histones labeled with same dye were used as a control. After 3 days of treatment, histone-associated αS inclusions could be observed in the cells exposed to histone amyloid fibrils, but not in cells exposed to soluble histones (Fig. 3). This result strongly supported the hypothesis that an amyloid fibrillar form of histone is competent in inducing αS aggregation upon internalization into recipient cells, while soluble forms of histone do not have this property. Since soluble histone induces αS to rapidly aggregate upon in vitro interaction[8, 10], it is intriguing why this is not the case in living cells.
Fig. 3. Amyloid fibrillar, but not soluble, histones induce intracellular αS aggregation upon internalization into recipient cells.
H4/V1S-SV2(S)/LAMP1-eCFP/mCherry-Galectin-3 cells were treated with the same amount of soluble recombinant histone H1 and histone H1 amyloid fibrils, respectively. After 3 days of treatment, sibling cultures of each group were exposed to 1 mM LLME for 1–2 hours. In parallel, a group of cells without histone, but with LLME treatment for the same time, were used as a control. (A) Cells in the individual groups were imaged under confocal microscope to detected intracellular αS inclusions; (B) Bar graph shows the comparison among different groups for the ratio of cells with inclusions to total cells. Error bars represent standard error of the mean (*p < 0.05, **p < 0.01, comparing subsets linked by line, n = 3). (C) & (D) For confirmation of histone H1 amyloid fibrils, samples were subjected to negative staining to demonstrate amyloid filaments with EM (C). Thioflavin T assay was also used to measure formation of beta-sheet structure (D). The same concentration of soluble histone H1 solution was used as a negative control. Scale bar in (C): 20 nm.
Exogenous histone amyloid fibrils induce aggregation of intracellular αS via endocytosis-mediated lysosome rupture
We previously reported that exogenous PD brain-derived αS fibrils seed αS aggregation in cultured cells mainly through endocytosis-mediated lysosomal rupture, and the capability of αS fibrils in seeding αS to aggregate is highly dependent on the membrane penetration ability of the seeds.[11] We therefore hypothesized that exogenous histone amyloid fibrils might induce αS aggregation in our cell model through the same mechanism. The hypothesis to be tested is that only amyloid fibrillar histones, but not soluble histones, are able to penetrate lysosomal membrane to interact with cytoplasmic αS leading to its aggregation. To test this speculation, we introduced H4/V1S-SV2(S) cells with two plasmids carrying LAMP-eCFP and mCherry-Galectin-3, respectively, to establish a new cell line, referred to as H4/V1S-SV2(S)/LAMP1-eCFP/mCherry-Galectin-3. This cell line was used to demonstrate the distribution of lysosomes and sites of rupture on lysosomal membranes.[11] Cells were treated for 3 days with soluble H1 and amyloid fibrillar H1 labeled with Lightning-Link® Rapid DyLight® 650 (Innova Biosciences), then examined under confocal microscopy. As expected, both the internalized soluble and amyloid fibrillar forms of H1 were associated with lysosomes, as reflected by colocalization between H1–650 and LAMP1-eCFP; however, only intracellular αS inclusions induced by histone amyloid fibrils had co-localization of Galectin-3, indicative of lysosomal membrane rupture. To further confirm a critical effect of lysosome membrane rupture on the formation of αS inclusions, we exposed cells treated with soluble and amyloid fibrillar H1 to a lysosomotropic detergent (L-Leucyl-L-Leucine methyl ester (LLME)) for 1–2 hours. LLME is internalized by cells through endocytosis, and then converted into (LeuLeu)n-OMe (n > 3) by dipeptidyl peptidase I (Cathepsin C) in lysosomes, which leads to lysosome rupture.[25] We found that exposure of cells to LLME significantly promoted formation of αS inclusions in cells treated with either soluble H1 or amyloid fibrillar H1 compared to cells only exposed to LLME (Fig. 3). These results strongly support the hypothesis that endocytosis-mediated lysosome rupture plays a critical role in histone-induced αS aggregation within living cells.
Amyloid fibrillar H1 but not soluble H1 can induce αS aggregation in vivo upon inoculation into mouse brain
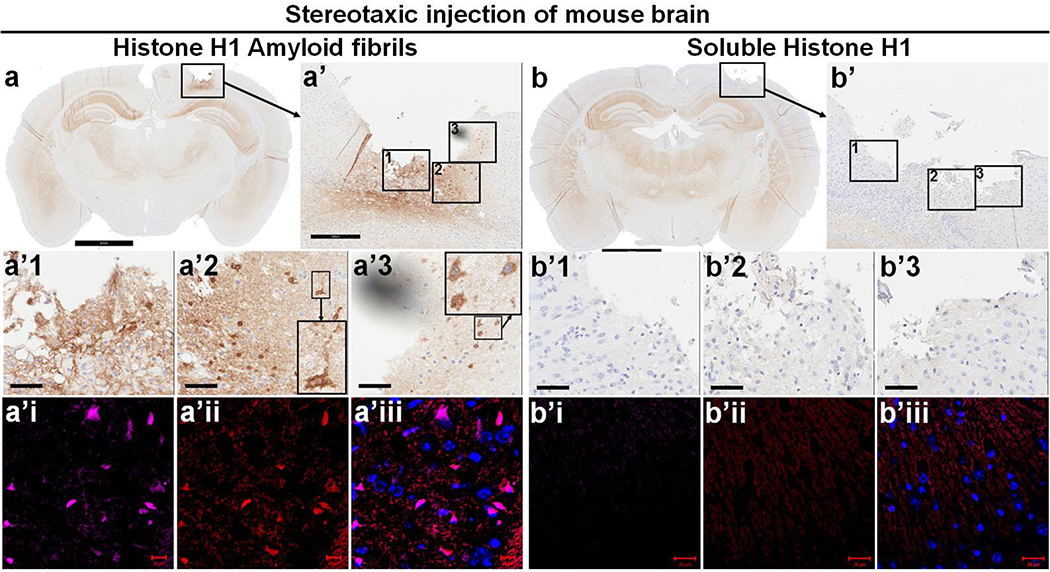
Given these in vitro cell-based findings, we wanted to see if we could confirm the effects of amyloid fibrillar histone on αS aggregation in vivo. For this purpose, freshly prepared soluble H1 or amyloid fibrillar histone H1 were stereotaxically injected into somatosensory cortex of wild type FVB mice. After one week, mouse brains were harvested, fixed and embedded in paraffin for immunohistochemical studies. Coronal sections were double labeled and observed with confocal immunofluorescence microscopy. Results showed that αS pathology appeared surrounding the injection site of mice injected with histone amyloid fibrils. There were also αS-positive dot-like deposits and intraneuronal αS deposits in the subjacent cortex (Fig. 4). Dual immunofluorescence demonstrated that αS pathology colocalized with H1 immunoreactivity. In contrast, sections from mice injected with soluble histone did not show neuronal or neuritic αS pathology. This suggested an association of histone amyloid fibrils with formation of αS deposits.
Fig. 4. Amyloid fibrillar, but not soluble histone H1, can induce αS aggregation in vivo upon inoculation into mouse brain.
Soluble recombinant human histone H1 and derived histone amyloid fibrils were inoculated into somatosensory cortex of wild-type FVB mice at 10 months of age via stereotaxic injection. Brains were harvested a week later. Paraffin sections of brain injected with amyloid fibrillar histone H1 (a) or soluble histone H1 (b) were subjected to immunohistochemical staining with antibody to αS (610787, BD Biosciences) to demonstrate αS pathology. Scale bar 2 mm. Panels a’ and b’ show enlarges framed areas in a and b. Scale bar 300 μm. The framed areas in a’ and b’ were further enlarged to a’1, a’2, a’3, b’1, b’2 and b’3 to show that amyloid histone H1 fibrils could induce intracellular αS deposits and thread- or dot-like αS pathology in the brain. Brain regions corresponding to a’ and b’ in adjacent sections were subjected to immunofluorescence staining with primary antibodies to human histone H1 (ab125027, Abcam, a’i & b’i) and αS (610787, BD Biosciences, a’ii & b’ii) with goat secondary antibodies to rabbit (Alexa Fluor 647) and mouse (Alexa Fluor 568). Scale bar 10 μm. Note: There are two inset pictures in both a’2 and a’3 in which the picture of small size is enlarged aside.
Discussion
We previously reported that nuclear proaggregant factors can interact with cytoplasmic αS to rapidly induce αS aggregation upon nuclear membrane disruption, such as occurs during apoptosis. We also identified histones as one of the potential nuclear proaggregant factors and showed that histone is associated with Lewy pathology in human brain tissues.[10, 12] In those studies, we focused on the effect of histones on αS aggregation in neurons undergoing apoptosis. It was uncertain if extracellular histone could induce aggregation of intracellular αS upon internalization into recipient cells. This question is important because histones can be detected in interstitial fluid of the brain and that levels of extracellular histone can be significantly elevated in response to brain injury. It is well documented that brain cells release histones into extracellular fluids under sub-lethal stress.[3, 5, 14] In the present study we found that apoptotic-neuron-derived histones are associated with αS aggregation upon cellular uptake and that this effect was greater if the recipient cells have lysosome membrane defects.
We found that nuclear extracts from MPTP-induced apoptotic neurons were capable of inducing intracellular αS aggregation after internalization into recipient cells, while nuclear extracts from healthy neurons did not have this property. These findings suggested that nuclear extracts, which contain histone, from neurons under apoptosis, are potent in inducing αS aggregation. Nuclei of apoptotic neurons and nuclear extracts from cells undergoing apoptosis contained filamentous amyloid structures that were immunoreactive for histone H1. Moreover, these extracts had strong thioflavin T binding and they contained SDS-resistant histone multimers on western blots. Extracts from control cells had none of these properties. The evidence strongly suggests that histone amyloid fibrils are formed in apoptotic neurons. Previously unrelated studies have shown that histones have structural properties that make them prone to form amyloid fibrils.[20, 24, 27] We hypothesized that histone amyloid fibrils may be one of the factors that contribute to the observed ability of nuclear extracts from apoptotic cells to induce αS aggregation. In addition to in vitro experiments, mouse brain injection models showed that amyloid fibrillar histone H1, but not soluble histone H1, could induce detectable αS aggregates in the vicinity of the site of injection. In these experiments, we did not show propagation of αS pathology, and this remains an important objective of future investigations.
The existence of histone amyloid fibrils capable of inducing αS aggregation upon cellular uptake is of particular importance in understanding the formation of initial αS aggregates in PD and related disorders. It is increasingly accepted that cell-to-cell propagation play a significant role in progression of αS pathology. Evidence in support of this hypothesis comes from both in vivo and in vitro studies. [17, 18, 26] Most of these studies, particularly those that use invasive methods such as brain injections of fibrillar or aggregated forms of αS, do not account for formation of initial αS aggregates in the human disease that they purport to model. Most of in vitro and in vivo studies on αS propagation use exogenous αS aggregates prepared from recombinant hαS or lysates of post-mortem PD brains.[17, 18, 26] Our previous studies provided evidence of interaction between proaggregant nuclear substances (e.g., histones) and cytoplasmic αS upon the disruption of nuclear membrane, which may be a trigger for formation of initial αS aggregates.[10, 12] The present study revealed that proaggregant nuclear factors, particularly those from apoptotic cells, may contribute to induction and propagation of αS pathology, while nuclear factors from non-apoptotic cells were considerably weaker or entirely lacked the ability to induce αS pathology. A particularly novel observation in the current studies is that one of the proaggregant nuclear factors derived from cells under stress or undergoing apoptosis is a form of amyloid composed of histone. Of note, histone amyloid fibrils have been previously demonstrated in other experimental paradigms, but their biological significance to neurodegenerative diseases is largely unexplored.[20, 24, 27]
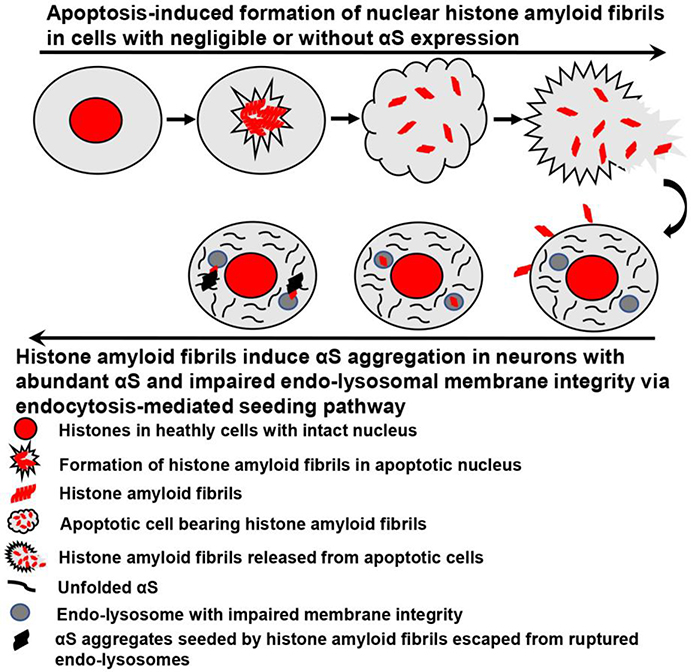
Although soluble histone can induce αS aggregation in vitro in buffer system,[8, 10] this has not been shown in vivo at the cellular or tissue level. Such difference should be due to the complexity for the two proteins to interact with each other in different systems. Our cell-based experiments showed that exogenous histone amyloid fibrils can induce aggregation of intracellular αS and that endocytosis-mediated lysosome rupture accelerates this process. These findings are in accord with our previous studies showing that αS aggregation can be induced by PD brain-derived αS seeds.[11] In contrast, no lysosomal membrane rupture or αS inclusions were observed in cells treated with exogenous soluble histone protein. Therefore, defects in lysosomal membrane integrity may be an important factor in disease pathogenesis. It may also explain differences in potency of amyloid fibrillar histone and soluble histone in ability to induce intracellular αS aggregation upon cellular uptake. Moreover, these results also highlight the importance of lysosomal integrity in protecting cells from αS pathology given that fully functioned lysosomes can act as a natural barrier to block cell-to-cell transmission of proaggregant nuclear factors (e.g., histones). For a better understanding of the process we proposed for the formation and spreading of apoptosis-induced histone amyloid fibrils and subsequent induction of αS aggregation in recipient neurons, we made a schematic diagram shown as in Fig. 5.
Fig. 5. The formation and spreading of apoptosis-induced histone amyloid fibrils and subsequent induction of αS aggregation in recipient neurons.
This schematic diagram only focuses on formation of nuclear histone amyloid fibrils in cells with negligible or without αS expression. For apoptotic cells bearing abundant αS, histones can directly interact with αS to rapidly form αS aggregates, leading to the propagation of αS pathology in surrounding cells, which was discussed in our previous studies[10, 12].
It is worth noting that although we observed αS inclusions in both soluble and filamentous histones-treated cells when cells were exposed to LLME leading to lysosome rupture, the capability of histone amyloid fibrils to induce αS inclusions was significantly greater than soluble histone. This could be related to the fact that amyloid fibrillar histone has a higher charge density per molecule. The potential for cross-seeding of histone amyloid fibrils and αS fibrils is another consideration. Further investigation is warranted to clarify roles of histone amyloid fibrils in α-synucleinopathies.
A finding of uncertain significance was that histone amyloid fibrils were better formed in later stages of apoptosis (Fig. S1). Although it is not clear how these amyloid fibrils are formed in neurons, drastic changes in the physiochemical milieu of the nucleus during apoptosis likely contributes. While the focus of our studies was on histone H1, it is not likely to be the only constituent of nuclear amyloid fibrils. Other histones, in particular H3, can be detected in nuclear extracts from apoptotic neurons (Fig. S1), and histone H3-derived amyloid fibrils are also capable of inducing αS aggregation (Fig. S2).
Overall, the present findings indicate a role of histones in formation of αS aggregates, and the importance of lysosome membrane integrity in limiting propagation of αS pathology. Finally, the studies revealed novel findings of nuclear histone amyloid fibrils and their role in PD pathogenesis, which opens up potentially novel therapeutic targets for α-synucleinopathies.
Supplementary Material
Supplementary Fig. 1. Nuclear histone amyloid fibrils form in neuron cells at late stages of apoptosis. Neuroblastoma cells M17D with αS knockout (M17D/αS-KO) were differentiated for 7 days then subjected to apoptosis induction by MPTP for 3 days. Cells were harvested and processed for EM and immuno-EM to demonstrate histone-immunopositive amyloid fibrils in nuclei from neurons at late stages of apoptosis as reflected by disruption of nuclear and cell membranes and loss of morphological detail in the nuclei. EM shows the morphology of amyloid fibrils (arrows) within neurons at late stages of apoptosis, and immuno-EM demonstrates that the amyloid fibrils (arrows) are immunopositive to both histone H1 and histone H3. Primary antibody for detection of histone H1: sc-8030, Santa Cruz Biotechnology; for histone H3: ab1791, Abcam. Colloidal Gold Conjugate: 111-215-144 (Rb), 115-215-146 (Ms), Jackson ImmunoResearch.
Supplementary Fig. 2. Amyloid fibrillar, but not soluble forms of histone H3, induce intracellular αS aggregation upon internalization into recipient cells. H4/V1S-SV2(S)/LAMP1-eCFP/mCherry-Galectin-3 cells were treated with the same amount of soluble recombinant histone H3 and H3-derived amyloid fibrils, respectively. After 3 days of treatment, sibling cultures were exposed to 1 mM LLME for 1-2 hours. In parallel, a group of cells without histone, but with LLME treatment for the same time, were used as a control. (A) Cells in the individual groups were imaged under confocal microscope to evaluate the presence of intracellular αS inclusions; (B) Bar graph shows a comparison between different groups of cells for the ratio of cells with inclusions to the total number of cells. Error bars represent standard error of the mean (*p < 0.05, **p < 0.01, comparing subsets linked by line, n = 3).
Acknowledgements
The authors thank Monica Castanedes-Casey, Ariston Librero and Virginia Phillips for histopathology support. This study was supported by the National Institute of Health (U54 NS110435, UG3 NS104095 and R21 NS099757), the Mangurian Foundation Lewy Body Dementia Program at Mayo Clinic (Dickson & Jiang), and The American Parkinson Disease Association Center for Advanced Research.
Footnotes
Conflict of interest
All authors have no actual or potential conflicts of interest.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Bolton SJ, Perry VH (1997) Histone H1; a neuronal protein that binds bacterial lipopolysaccharide. J Neurocytol 26: 823–831 [DOI] [PubMed] [Google Scholar]
- 2.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24: 197–211 Doi 10.1016/s0197-4580(02)00065-9 [DOI] [PubMed] [Google Scholar]
- 3.Chen R, Kang R, Fan XG, Tang D (2014) Release and activity of histone in diseases. Cell Death Dis 5: e1370 Doi 10.1038/cddis.2014.337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collier TJ, Kanaan NM, Kordower JH (2011) Ageing as a primary risk factor for Parkinson’s disease: evidence from studies of non-human primates. Nat Rev Neurosci 12: 359–366 Doi 10.1038/nrn3039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Meyer SF, Suidan GL, Fuchs TA, Monestier M, Wagner DD (2012) Extracellular chromatin is an important mediator of ischemic stroke in mice. Arterioscler Thromb Vasc Biol 32: 1884–1891 Doi 10.1161/ATVBAHA.112.250993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eberhardt O, Schulz JB (2003) Apoptotic mechanisms and antiapoptotic therapy in the MPTP model of Parkinson’s disease. Toxicol Lett 139: 135–151 Doi 10.1016/s0378-4274(02)00428-9 [DOI] [PubMed] [Google Scholar]
- 7.Flurkey K CJ, Harrison DE (2007) The Mouse in Aging Research In: Fox JG ea (ed) The Mouse in Biomedical Research 2nd Edition American College Laboratory Animal Medicine (Elsevier), City, pp 637–672 [Google Scholar]
- 8.Goers J, Manning-Bog AB, McCormack AL, Millett IS, Doniach S, Di Monte DA, Uversky VN, Fink AL (2003) Nuclear localization of alpha-synuclein and its interaction with histones. Biochemistry 42: 8465–8471 Doi 10.1021/bi0341152 [DOI] [PubMed] [Google Scholar]
- 9.Jiang P, Gan M, Ebrahim AS, Castanedes-Casey M, Dickson DW, Yen SH (2013) Adenosine monophosphate-activated protein kinase overactivation leads to accumulation of alpha-synuclein oligomers and decrease of neurites. Neurobiol Aging 34: 1504–1515 Doi 10.1016/j.neurobiolaging.2012.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang P, Gan M, Yen SH, McLean PJ, Dickson DW (2017) Histones facilitate alpha-synuclein aggregation during neuronal apoptosis. Acta Neuropathol 133: 547–558 Doi 10.1007/s00401-016-1660-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang P, Gan M, Yen SH, McLean PJ, Dickson DW (2017) Impaired endo-lysosomal membrane integrity accelerates the seeding progression of alpha-synuclein aggregates. Sci Rep 7: 7690 Doi 10.1038/s41598-017-08149-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang P, Gan M, Yen SH, Moussaud S, McLean PJ, Dickson DW (2016) Proaggregant nuclear factor(s) trigger rapid formation of alpha-synuclein aggregates in apoptotic neurons. Acta Neuropathol: Doi 10.1007/s00401-016-1542-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang P, Ko LW, Jansen KR, Golde TE, Yen SH (2008) Using leucine zipper to facilitate alpha-synuclein assembly. FASEB J 22: 3165–3174 Doi 10.1096/fj.08-108365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein B, Lutz-Meindl U, Kerschbaum HH (2014) From the nucleus to the plasma membrane: translocation of the nuclear proteins histone H3 and lamin B1 in apoptotic microglia. Apoptosis 19: 759–775 Doi 10.1007/s10495-014-0970-7 [DOI] [PubMed] [Google Scholar]
- 15.Ko LW, Ko HH, Lin WL, Kulathingal JG, Yen SH (2008) Aggregates assembled from overexpression of wild-type alpha-synuclein are not toxic to human neuronal cells. J Neuropathol Exp Neurol 67: 1084–1096 Doi 10.1097/NEN.0b013e31818c3618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kostrzewa RM (2014) Handbook of neurotoxicity. Springer Reference, City [Google Scholar]
- 17.Luk KC, Kehm V, Carroll J, Zhang B, O’Brien P, Trojanowski JQ, Lee VM (2012) Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 338: 949–953 Doi 10.1126/science.1227157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masuda-Suzukake M, Nonaka T, Hosokawa M, Oikawa T, Arai T, Akiyama H, Mann DM, Hasegawa M (2013) Prion-like spreading of pathological alpha-synuclein in brain. Brain 136: 1128–1138 Doi 10.1093/brain/awt037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mishra B, von der Ohe M, Schulze C, Bian S, Makhina T, Loers G, Kleene R, Schachner M (2010) Functional role of the interaction between polysialic acid and extracellular histone H1. J Neurosci 30: 12400–12413 Doi 10.1523/JNEUROSCI.6407-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Munishkina LA, Fink AL, Uversky VN (2004) Conformational prerequisites for formation of amyloid fibrils from histones. J Mol Biol 342: 1305–1324 Doi 10.1016/j.jmb.2004.06.094 [DOI] [PubMed] [Google Scholar]
- 21.Nicotra A, Parvez S (2002) Apoptotic molecules and MPTP-induced cell death. Neurotoxicol Teratol 24: 599–605 Doi 10.1016/s0892-0362(02)00213-1 [DOI] [PubMed] [Google Scholar]
- 22.Ono K, Takahashi R, Ikeda T, Yamada M (2012) Cross-seeding effects of amyloid beta-protein and alpha-synuclein. J Neurochem 122: 883–890 Doi 10.1111/j.1471-4159.2012.07847.x [DOI] [PubMed] [Google Scholar]
- 23.Ren B, Zhang Y, Zhang M, Liu Y, Zhang D, Gong X, Feng Z, Tang J, Chang Y, Zheng J (2019) Fundamentals of cross-seeding of amyloid proteins: an introduction. J Mater Chem B 7: 7267–7282 Doi 10.1039/c9tb01871a [DOI] [PubMed] [Google Scholar]
- 24.Topping TB, Gloss LM (2011) The impact of solubility and electrostatics on fibril formation by the H3 and H4 histones. Protein Sci 20: 2060–2073 Doi 10.1002/pro.743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uchimoto T, Nohara H, Kamehara R, Iwamura M, Watanabe N, Kobayashi Y (1999) Mechanism of apoptosis induced by a lysosomotropic agent, L-Leucyl-L-Leucine methyl ester. Apoptosis 4: 357–362 Doi 10.1023/a:1009695221038 [DOI] [PubMed] [Google Scholar]
- 26.Volpicelli-Daley LA, Luk KC, Patel TP, Tanik SA, Riddle DM, Stieber A, Meaney DF, Trojanowski JQ, Lee VM (2011) Exogenous alpha-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron 72: 57–71 Doi 10.1016/j.neuron.2011.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao H, Tuominen EK, Kinnunen PK (2004) Formation of amyloid fibers triggered by phosphatidylserine-containing membranes. Biochemistry 43: 10302–10307 Doi 10.1021/bi049002c [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1. Nuclear histone amyloid fibrils form in neuron cells at late stages of apoptosis. Neuroblastoma cells M17D with αS knockout (M17D/αS-KO) were differentiated for 7 days then subjected to apoptosis induction by MPTP for 3 days. Cells were harvested and processed for EM and immuno-EM to demonstrate histone-immunopositive amyloid fibrils in nuclei from neurons at late stages of apoptosis as reflected by disruption of nuclear and cell membranes and loss of morphological detail in the nuclei. EM shows the morphology of amyloid fibrils (arrows) within neurons at late stages of apoptosis, and immuno-EM demonstrates that the amyloid fibrils (arrows) are immunopositive to both histone H1 and histone H3. Primary antibody for detection of histone H1: sc-8030, Santa Cruz Biotechnology; for histone H3: ab1791, Abcam. Colloidal Gold Conjugate: 111-215-144 (Rb), 115-215-146 (Ms), Jackson ImmunoResearch.
Supplementary Fig. 2. Amyloid fibrillar, but not soluble forms of histone H3, induce intracellular αS aggregation upon internalization into recipient cells. H4/V1S-SV2(S)/LAMP1-eCFP/mCherry-Galectin-3 cells were treated with the same amount of soluble recombinant histone H3 and H3-derived amyloid fibrils, respectively. After 3 days of treatment, sibling cultures were exposed to 1 mM LLME for 1-2 hours. In parallel, a group of cells without histone, but with LLME treatment for the same time, were used as a control. (A) Cells in the individual groups were imaged under confocal microscope to evaluate the presence of intracellular αS inclusions; (B) Bar graph shows a comparison between different groups of cells for the ratio of cells with inclusions to the total number of cells. Error bars represent standard error of the mean (*p < 0.05, **p < 0.01, comparing subsets linked by line, n = 3).