Abstract
Background.
Medicaid recipients have a high burden of opioid overdose and opioid use disorder (OUD). Opioid agonist therapies are an effective treatment for OUD, but there is a wide and persisting gap between those who are indicated and those who receive treatment. The objective of this study was to identify the predictors of enrollment in opioid agonist therapy within 6 months of an opioid overdose or OUD diagnosis in a cohort of Medicaid recipients.
Methods.
Using multiple linked, state-level databases, we conducted a retrospective cohort study of 17,449 Medicaid recipients in Rhode Island who had an opioid overdose or an OUD diagnosis between July 2013 and June 2018.
Results.
The majority (58%) of Medicaid recipients did not enroll in opioid agonist therapy within 6 months. In adjusted models, having one or more prior overdose (adjusted risk ratio [ARR]=0.33, 95% CI: 0.28, 0.38), alcohol use disorder (ARR=0.56, 95% CI: 0.52, 0.60), or back problems (ARR=0.58, 95% CI: 0.55, 0.61) were strong predictors of non-enrollment. Conversely, emergency department (ARR=1.31, 95% CI: 1.28–1.34) and primary care provider (ARR=1.03, 95% CI: 1.01–1.34) visit frequency above the 75th percentile were associated with timely enrollment in opioid agonist therapy.
Conclusions.
Our findings underscore the need to enhance pathways to treatment for OUD through varied nodes of engagement with healthcare systems. Interventions to improve screening for OUD and referrals to opioid agonist therapies should include high-impact settings, such as treatment programs for alcohol and substance use disorders, pain clinics, and outpatient behavioral care settings.
Keywords: opioid agonist therapy, overdose, opioid use disorder, Medicaid
1. INTRODUCTION
Opioid use disorder (OUD) and opioid-related overdose are persisting epidemics in the United States (US). In 2018, an estimated 2 million people in the US had an OUD (Substance Abuse and Mental Health Services Administration, 2019). In the same year, over 67,000 overdose deaths occurred in the US, and 70% of overdose deaths were opioid-involved (Wilson, 2020). The burden of OUD and opioid-related overdose has been particularly high among individuals belonging to Medicaid-eligible groups. In 2017, 38% of non-elderly adults with an OUD were Medicaid recipients (Orgera and Tolbert, 2019), and prior studies have documented an increased risk of opioid-related overdose among Medicaid recipients (Garg et al., 2017; Mack et al., 2015).
Opioid agonist therapies, such as treatment with methadone or buprenorphine, are Food and Drug Administration (FDA)-approved and efficacious in the treatment of OUD and in mitigating opioid-related morbidity and mortality (National Academies of Sciences Engineering and Medicine, 2019). Medicaid expansion has been associated with reductions in opioid overdose and increased provision of opioid agonist therapy (Kravitz-Wirtz et al., 2020; Saloner et al., 2018); however, there is a wide and persisting gap between those indicated for opioid agonist therapy and those who ultimately receive treatment, with significant disparities by race, rurality, provider availability, and income (Andrilla et al., 2019; Goedel et al., 2020; Jones et al., 2015; Joudrey et al., 2019; Lagisetty et al., 2019; Wen et al., 2018; Williams et al., 2019). Patient preferences also influence enrollment in opioid agonist therapies. Stigma, awareness of treatment options, treatment expectations, and prior experience with methadone or buprenorphine all influence whether a patient will choose to enroll in opioid agonist therapy and which medication is preferred (Yarborough et al., 2016).
Rhode Island has been highly affected by the opioid overdose crisis, and had the 12th highest rate of opioid overdose death in 2018 (CDC/NCHS, 2020). The state also has among the highest capacity for provision of methadone and buprenorphine in the nation. However, qualitative research has shown that a substantial number of persons with OUD continue to experience barriers to accessing opioid agonist therapy, including stigma and discrimination, inability to locate providers that accept Medicaid or other insurance plans, and out-of-pocket treatment costs (Carroll et al., 2018; Liebling et al., 2016).
Prior research has documented predictors of timely initiation of opioid agonist therapy among veterans (Manhapra et al., 2020; Wyse et al., 2019), but the predictors of enrollment in opioid agonist therapy among Medicaid recipients are not yet well understood. The purpose of this study was to identify predictors of enrollment in opioid agonist therapy within 6 months following an opioid overdose or diagnosis of OUD in a statewide population of Medicaid recipients. A secondary goal of this analysis was to identify approaches to enhance existing points-of-care for key populations to increase enrollment in opioid agonist therapy.
2. MATERIAL AND METHODS
2.1. Study Design and Data Source
We conducted a retrospective cohort study using data available through the Rhode Island Executive Office of Health and Human Services (EOHHS) Data Ecosystem. The EOHHS Data Ecosystem is a centralized analytics database comprised of anonymized person-level data from EOHHS and other state agencies (Sokol, 2019). This data environment was developed by the Rhode Island EOHHS to provide a mechanism to access integrated data, tied to operational purpose, in a user-friendly way that enables self-service analytics across state agencies. The EOHHS Data Ecosystem permits the development of programs that respond to the lived experience of Rhode Island residents, as well as changing policy and operational needs. Data across agencies are linked within the EOHHS Data Ecosystem using a robust anonymization and person matching process. Additional details regarding the anonymization and person matching process are provided in the Supplemental Appendix. In the present study, we utilized linked Rhode Island databases from 4 sources: Medicaid claims and enrollment, the Department of Labor and Training, the Department of Human Services, and the Department of Corrections. Medicaid claims and enrollment data included medical and pharmacy claims for Medicaid beneficiaries, as well as Medicaid enrollment and suspension while under supervision of the state Department of Corrections. This evaluation was conducted in collaboration with the Rhode Island EOHHS. The Rhode Island Department of Health Institutional Review Board determined that this study was not human subjects research, as this study involved analysis of preexisting, deidentified data.
2.2. Cohort Selection
We identified persons in the Medicaid population who had a healthcare encounter for an opioid overdose or a diagnosis of OUD between July 2013 and June 2018, hereafter referred to collectively as an index event. This combined index event measure was used to capture a broad cohort of Medicaid recipients who may be indicated for treatment with opioid agonist therapy (Rhode Island Department of Health, 2017). Data prior to July 2013 were not available for analysis due to changes in the format of Medicaid claims data. Opioid overdose was defined as having one or more emergency department (ED) claims containing a diagnosis code for opioid poisoning from the International Classification of Diseases, Ninth and Tenth Revisions Clinical Modification (ICD-9, ICD-10). Having a diagnosis of OUD was defined as having one or more medical claims containing an ICD-9 or ICD-10 diagnosis code for OUD (see Supplemental Appendix, Table S4). For individuals who had multiple healthcare encounters for an opioid overdose and/or a diagnosis of OUD between July 2013 and June 2018, the earliest claim for either is classified as the index event.
We identified 18,926 Medicaid recipients who had an index event and at least 6 months of continuous Medicaid enrollment. Individuals with less than 6 months of continuous Medicaid enrollment were not considered eligible for these analyses. Our primary outcome was enrollment in opioid agonist therapy within 6 months of the index event. Individuals who had matching index event and treatment enrollment dates were excluded from the study cohort to control for the effect of Medicaid Expansion, which took effect in January 2014, and brought many long-time, but previously uninsured methadone patients into the Medicaid population. We excluded a total of 1,363 persons whose index event date matched their initial date of enrollment in opioid agonist therapy.
Of the 17,817 remaining persons with an index event and with at least 6 months of continuous Medicaid enrollment, we excluded an additional 114 who were less than 18 years of age. We restricted our cohort to persons 18 years of age and older because access to opioid agonist therapy differs greatly between adult and adolescent populations (Wu et al., 2016). The final cohort for these analyses contained 17,449 persons (study inclusion flow diagram; Supplemental Appendix Figure S1).
2.3. Key Variables
Our primary outcome was enrollment in opioid agonist therapy (methadone or buprenorphine) within 6 months of index event. We measured enrollment in opioid agonist therapy within 6 months of an index event because timely enrollment is associated with improved long-term treatment outcomes and reduced morbidity and mortality (Wyse et al., 2019). Moreover, provided that many provider, institutional, regulatory, financial, and other barriers to enrolling in opioid agonist therapy have been documented (Madras et al., 2020), we chose to measure enrollment within 6 months of an index event in our main analysis, rather than a more proximal period of 1- or 3-months post-index event to permit ample time for enrollment despite these challenges. Predictors of enrollment in treatment with opioid antagonists (e.g., naltrexone) were not examined due to sample size limitations; in 2019, fewer than 160 individuals in the general Rhode Island population were enrolled in treatment with opioid antagonists (Prevent Overdose RI, 2020). Enrollment in treatment with methadone and/or buprenorphine was ascertained using Medicaid claims data. Specifically, enrollment in treatment with methadone was identified using the Healthcare Common Procedure Coding Systems code for a medical claim for methadone administration (H0020). Enrollment treatment with buprenorphine was identified using the National Drug Code List (Supplemental Appendix Table S4).
We examined potential predictors of enrollment in opioid agonist therapy within 6 months of the index event. We obtained patient sex, race (categorized as white vs. nonwhite), and age (categorized: 18–29, 30–39, 40–60, and >60) from Medicaid claims data. The following diagnoses were also obtained using Medicaid claims data: alcohol use disorder (AUD), another substance use disorder (non-opioid), depression, anxiety, any other mental illness or mental health treatment (including inpatient and outpatient), back problems, one or more prior overdose, hepatitis C virus infection, ED and primary care provider (PCP) visit frequency above the 75th percentile, pregnancy and pregnancy complications, and incarceration (see Supplemental Appendix Table S4 for definitions and code details). Department of Corrections data were used to ascertain incarceration status. Department of Labor and Training data were used to ascertain wage information and employment type. Department of Human Services data were used to ascertain eligibility for cash assistance programs, such as the federal Temporary Assistance for Needy Families program. Covariates were assessed during the period spanning 6 months prior to the index event until either 6 months following the index event or initiation or opioid agonist therapy.
2.5. Sensitivity Analyses
After the index event, we followed Medicaid recipients until initiation of opioid agonist therapy or the end of the observation period (June 2018). For the purpose of these analyses, individuals were classified as either receiving opioid agonist therapy within 6 months of the index event or not receiving treatment within 6 months of the index event. In our main analyses, those who eventually enrolled in opioid agonist therapy after 6 months from the index event were included in the non-enrolled within 6 months group. In additional sensitivity analyses, those who enrolled in treatment >6 months after index event were administratively censored (i.e., removed from the study population; see below).
While our primary outcome was enrollment in opioid agonist therapy within 6 months of index event, we also examine enrollment in opioid agonist therapy at more proximal (i.e., 1- and 3-months) and distal periods (i.e., 12-months) post-index event in additional sensitivity analyses to examine the robustness of our findings to varied observation period duration. Additional sensitivity analyses were also included to assess the robustness of our findings to type of index event (i.e., opioid overdose vs. diagnosis of OUD). Finally, we examined whether opioid agonist therapy enrollment varied by year of index event to better understand trends in timely enrollment and by continuous Medicaid enrollment status to assess for risk of selection bias.
2.5. Statistical Analysis
We compared Medicaid recipient characteristics and potential predictors of enrollment in opioid agonist therapy between those receiving opioid agonist therapy within 6 months of index event and those who did not using χ2 tests and independent t-tests for continuous variables. We considered a comprehensive set of variables from the linked EOHHS Data Ecosystem described above. Content expert feedback, the results from our unadjusted relative risk analysis, and machine learning approaches were used to inform our final model. Machine learning was used to identify the subset of variables most predictive of enrollment in opioid agonist therapy within 6 months of the index event. Additional details about our machine learning approach are provided in the Supplemental Appendix. All statistical analyses were performed using R version 3.6.1.
3. RESULTS
We identified 17,449 Medicaid recipients meeting inclusion criteria, with an opioid overdose (n = 1,426; 8.2%), a diagnosis of OUD (n = 16,023; 91.8%) or both occurring on the same day (n = 161, 0.9%) between July 2013 and June 2018. The majority of the sample was male (57%), white (72%), and 40–59 years of age (44%). Following the index event, 58% (n = 10,112) did not enroll in opioid agonist therapy within 6 months. Characteristics of Rhode Island Medicaid recipients, overall and by opioid agonist therapy enrollment status, are presented in Table 1. Among the 7,337 who enrolled in opioid agonist therapy within 6 months of index event, 53% initiated methadone and 47% initiated buprenorphine.
Table 1.
Characteristics of Rhode Island Medicaid recipients, overall and by opioid agonist therapy enrollment status (i.e., non-enrolled vs. enrolled within 6 months of index event), July 2013 – June 2018*
| Characteristic | Overall (N = 17,449) | Non-enrolled† (n = 10,112) | Enrolled† (n = 7,337) |
|---|---|---|---|
| Age, years | |||
| 18–29 | 2,420 (14%) | 1,575 (16%) | 845 (12%) |
| 30–39 | 5,254 (30%) | 2,695 (27%) | 2,559 (35%) |
| 40–59 | 7,750 (44%) | 4,457 (44%) | 3,293 (45%) |
| ≥60 | 2,025 (12%) | 1,385 (14%) | 640 (9%) |
| Gender (male) | 9,894 (57%) | 5,716 (57%) | 4,178 (57%) |
| Race (non-white) | 4,806 (28%) | 3,160 (31%) | 1,646 (22%) |
| Prior diagnoses | |||
| Anxiety | 7,145 (41%) | 5,526 (55%) | 1,619 (22%) |
| Depression | 5,909 (34%) | 4,480 (44%) | 1,429 (19%) |
| Any other mental illness | 7,128 (41%) | 5,509 (54%) | 1,619 (22%) |
| Mental health treatment (outpatient) | 8,428 (48%) | 6,418 (63%) | 2,010 (27%) |
| Mental health treatment (inpatient) | 2,280 (13%) | 1,913 (19%) | 367 (5%) |
| Mental health treatment (outpatient or inpatient) | 8,657 (50%) | 6,578 (38%) | 2,079 (28%) |
| Alcohol use disorder | 4,693 (27%) | 3,875 (38%) | 818 (11%) |
| Substance use disorder (non-opioid) | 8,360 (48%) | 6,418 (63%) | 1,942 (26%) |
| Hepatitis C | 1,097 (6%) | 815 (8%) | 282 (4%) |
| Back problems | 6,174 (35%) | 4,817 (48%) | 1,357 (18%) |
| Pregnancy | 1,149 (7%) | 641 (6%) | 508 (7%) |
| Complicated pregnancy | 268 (2%) | 191 (2%) | 77 (1%) |
| One or more prior overdose | 1,634 (9%) | 1,467(15%) | 167(2%) |
| Subsequent diagnoses | |||
| Subsequent overdose | 284 (2%) | 259 (3%) | 31 (0.4%) |
| Subsequent diagnosis of OUD | 7,788 (45%) | 5322 (53%) | 2466 (34%) |
| Health care utilization | |||
| PCP visits above 75th percentile | 4,722 (27%) | 2,680 (27%) | 2,042 (28%) |
| ED visits above 75th percentile | 5,179 (30%) | 2,690 (27%) | 2,489 (34%) |
| Socioeconomic factors | |||
| Zero wages | 13,324 (76%) | 7,573 (75%) | 5,661 (77%) |
| Employed at temp agency | 1,767 (10%) | 1,141 (11%) | 626 (9%) |
| Employed in construction | 428 (2%) | 243 (2%) | 185 (3%) |
| Cash assistance eligibility | 1,080 (6%) | 561 (6%) | 519 (7%) |
| Prior incarceration | 3,614 (21%) | 2,404 (24%) | 1,210 (16%) |
PCP = primary care provider; ED = emergency department; OUD = opioid use disorder
Data are presented as number (percentage) of patients. Index event was defined as a patient’s initial opioid overdose or diagnosis of OUD between July 2013 and June 2018.
p < 0.0001 for χ2 comparison of each characteristic category except gender, age 40–59, pregnancy, and employed in construction (all p > 0.05); Zero wages (p <0.001), PCP visits above 75th percentile (p = 0.05)
The unadjusted relative risks for non-enrollment in opioid agonist therapy within 6 months of index event among Medicaid recipients in Rhode Island are presented in Table 2. One or more prior opioid overdose (unadjusted risk ratio [RR] = 0.23, 95% confidence interval [CI]: 0.20, 0.26), subsequent overdose (RR = 0.25, 95% CI: 0.18, 0.35), alcohol use disorder (RR = 0.34, 95% CI: 0.32, 0.37), and inpatient mental health treatment (RR = 0.35, 95% CI: 0.32, 0.38) were strong predictors of non-enrollment in opioid agonist therapy within six months of an index event. In contrast, being age 30–39 (RR = 1.24, 95% CI: 1.19, 1.28), having ED visit frequency above the 75th percentile (RR = 1.21, 95% CI: 1.16, 1.25), and cash assistance eligibility (RR = 1.15, 95% CI: 1.08, 1.23) were associated with enrollment in opioid agonist therapy within six months.
Table 2.
Unadjusted risk ratios for enrollment in opioid agonist therapy within 6 months of index event among Medicaid recipients in Rhode Island, July 2013 – June 2018 (N = 17,449)*
| Characteristic | Unadjusted risk ratio (RR) (95% CI) |
|---|---|
| Age, years† | |
| 18–29 | 0.81 (0.76, 0.86) |
| 30–39 | 1.24 (1.19, 1.28) |
| 40–59 | 1.02 (0.99, 1.06) |
| ≥60 | 0.73 (0.68, 0.78) |
| Gender (male) | 1.01 (0.97, 1.05) |
| Race (non-white) | 0.76 (0.73, 0.79) |
| Prior diagnoses | |
| Anxiety | 0.41 (0.39, 0.43) |
| Depression | 0.48 (0.45, 0.50) |
| Any other mental illness | 0.41 (0.39, 0.43) |
| Mental health treatment (outpatient) | 0.40 (0.39, 0.42) |
| Mental health treatment (inpatient) | 0.35 (0.32, 0.38) |
| Mental health treatment (outpatient or inpatient) | 0.40 (0.38, 0.42) |
| Alcohol use disorder | 0.34 (0.32, 0.37) |
| Substance use disorder (non-opioid) | 0.43 (0.41, 0.45) |
| Hepatitis C | 0.61 (0.55, 0.67) |
| Back problems | 0.42 (0.40, 0.44) |
| Pregnancy | 1.06 (0.99, 1.14) |
| Complicated pregnancy | 1.03 (0.76, 1.39) |
| One or more prior overdose | 0.23 (0.20, 0.26) |
| Subsequent diagnoses | |
| Subsequent overdose | 0.25 (0.18,0.35) |
| Subsequent diagnosis of OUD | 0.63 (0.60,0.65) |
| Health care utilization | |
| PCP visits above 75th percentile | 1.03 (0.99, 1.07) |
| ED visits above 75th percentile | 1.21 (1.16, 1.25) |
| Socioeconomic factors | |
| Zero wages | 0.88 (0.84, 0.91) |
| Employed at temp agency | 0.82 (0.77, 0.88) |
| Employed in construction | 1.03 (0.93, 1.15) |
| Cash assistance eligibility | 1.15 (1.08, 1.23) |
| Prior incarceration | 0.75 (0.72, 0.79) |
PCP = primary care provider; ED = emergency department; OUD = opioid use disorder
Index event was defined as a patient’s initial opioid overdose or diagnosis of OUD between July 2013 and June 2018.
As compared to those who did not belong to a given age category.
In log binomial regression models that adjusted for several potential confounding influences, having one or more prior overdose (adjusted risk ratio [ARR]=0.33, 95% CI: 0.28, 0.38), alcohol use disorder (ARR=0.56, 95% CI: 0.52, 0.60), or back problems (ARR=0.58, 95% CI: 0.55, 0.61) were strong predictors of non-enrollment in opioid agonist therapy within six months, among several other variables (Table 3). Conversely, ED visit frequency above the 75th percentile (ARR=1.31, 95% CI: 1.28–1.34) and PCP visit frequency above the 75th percentile (ARR=1.03, 95% CI: 1.01–1.34) were associated with timely enrollment in opioid agonist therapy.
Table 3.
Adjusted risk ratios from the final multivariable model for enrollment in opioid agonist therapy within 6 months of index event among Medicaid recipients in Rhode Island, July 2013 – June 2018 (N = 17,449)*
| Characteristic | Adjusted risk ratio (ARR) (95% CI) |
|---|---|
| Age, years† | |
| 30–39 | 1.00 (0.99, 1.01) |
| ≥60 | 0.94 (0.89, 0.99) |
| Prior diagnoses | |
| Anxiety | 0.75 (0.71, 0.78) |
| Depression | 0.92 (0.89, 0.97) |
| Any other mental illness | 0.80 (0.75, 0.84) |
| Mental health treatment (inpatient or outpatient) | 0.85 (0.80, 0.89) |
| Alcohol use disorder | 0.56 (0.52, 0.60) |
| Substance use disorder (non-opioid) | 0.76 (0.73, 0.80) |
| Back problems | 0.58 (0.55, 0.61) |
| One or more prior overdose | 0.33 (0.28, 0.38) |
| Health care utilization | |
| PCP visits above 75th percentile | 1.03 (1.01, 1.04) |
| ED visits above 75th percentile | 1.31 (1.28, 1.34) |
PCP = primary care provider; ED = emergency department; OUD = opioid use disorder
Index event was defined as a patient’s initial opioid overdose or diagnosis of OUD between July 2013 and June 2018.
As compared to those who did not belong to a given age category.
In sensitivity analyses in which those who enrolled in treatment >6 months after index event were administratively censored rather than included in the study population, key findings were comparable. The strongest predictors of non-enrollment in opioid agonist therapy were having one or more prior overdose (adjusted risk ratio [ARR]=0.32, 95% CI: 0.28, 0.37), alcohol use disorder (ARR=0.54, 95% CI: 0.51, 0.58), or back problems (ARR=0.58, 95% CI: 0.55, 0.61). In addition, as in the main analyses, ED visit frequency above the 75th percentile (ARR = 1.22, 95% CI: 1.20, 1.25) and PCP visit frequency above the 75th percentile (ARR = 1.02, 95% CI: 1.01, 1.03) were associated with timely enrollment in opioid agonist therapy (Supplemental Appendix Table S3).
In sensitivity analyses in which we examined enrollment in opioid agonist therapy at more proximal (i.e., 1- and 3-months) and distal periods (i.e., 12-months) post-index event, estimates were broadly similar to main findings (Supplemental Appendix Table S4). Notably, having one or more prior overdose was more strongly predictive of non-enrollment at 1 month post-index event (ARR=0.18, 95% CI: 0.14, 0.22) than at 12 months post-index event (ARR=0.38, 95% CI: 0.34, 0.43). In sensitivity analyses in which we assess the robustness of our findings to type of index event (i.e., opioid overdose vs. diagnosis of OUD), only one variable differed qualitatively (Supplemental Appendix Table S5). Among those whose index event was a diagnosis of OUD, having a prior diagnosis of substance use disorder (non-opioid) was predictive of non-enrollment at 6 months post-index event (ARR=0.74, 95% CI: 0.71, 0.78), while among those whose index event was an opioid overdose, diagnosis of substance use disorder (non-opioid) was predictive of enrollment at 6 months post-index event (ARR=2.12, 95% CI: 1.30, 3.47).
In additional sensitivity analyses, we examined whether opioid agonist therapy enrollment varied by year of index event to better understand trends in timely enrollment. The majority (64%) of Medicaid recipients with an index event in 2013 enrolled in opioid agonist therapy within 6 months of index event, while a minority (35%) of those with an index event enrolled in opioid agonist therapy within 6 months during years thereafter (Supplemental Appendix Table S6). Finally, individuals who had less than 6 months of continuous Medicaid enrollment were not included in main analyses; when we examined enrollment in opioid agonist therapy at varied time points post-index event (e.g., within 6 months, 7–12 months, 13–36 months, etc.), enrollment was broadly similar between those who were continuously enrolled in Medicaid for at least 6 months and those who were not (Supplemental Appendix Table S7). Additional consideration of the results of these sensitivity analyses is supplied in the Supplemental Appendix.
4. DISCUSSION
In a cohort of over 17,000 Medicaid recipient in Rhode Island who had an opioid overdose or a diagnosis of OUD between July 2013 and June 2018, the majority (58%) did not enroll in opioid agonist therapy within 6 months. In adjusted models, having one or more prior overdose, alcohol and substance use disorders, back problems, mental health-related treatment and diagnoses, and being age 60 or older were significant predictors of non-enrollment in opioid agonist therapy. Conversely, having ED or PCP visit frequency above the 75th percentile were associated with timely enrollment in opioid agonist therapy. To our knowledge, this study is among the first to explore correlates of enrollment in treatment with methadone or buprenorphine following an opioid overdose or diagnosis of OUD.
Our finding that the majority of Medicaid patients did not enroll in opioid agonist therapy within six months of an index event is consistent with prior literature. In a study of Medicaid recipients in Pennsylvania who had an overdose event, 63% of those with a heroin overdose and 84% of those with a prescription opioid overdose did not enroll in opioid agonist therapy within 6 months of the overdose event (Frazier et al., 2017). Higher uptake of opioid agonist therapy in the Rhode Island Medicaid population relative to the Pennsylvania Medicaid population may be attributable to Rhode Island’s earlier Medicaid expansion, which occurred early in the period of observation in the present study and after the period of observation in Pennsylvania, and associated with increased enrollment in opioid agonist therapy (Mojtabai et al., 2019). Nonetheless, additional efforts are urgently needed to increase uptake of opioid agonist therapies in at-risk populations.
In the present study, several markers of poor health status were found to be associated with increased risk of non-enrollment, including having one or more prior overdose, alcohol and substance use disorders, back problems, mental health-related treatment and diagnoses, and older age. Prior evidence has documented a myriad of barriers to enrolling in opioid agonist therapy, including provider, institutional, regulatory, financial, and logistical barriers (Madras et al., 2020). Prior research has also identified several barriers to accessing healthcare among people who use drugs, including inability to miss work, lack of transportation, and competing needs (e.g., appointment would interfere with opportunity for a meal or drug acquisition) (Miller-Lloyd et al., 2020). We hypothesize that persons with one or more prior overdose, alcohol and substance use disorders, back problems, mental health-related treatment and diagnoses, and those who are older age have additional barriers that reduce their ability or agency to enroll in opioid agonist therapy as compared to others. Several of our findings are also consistent with prior research. In a study of Medicaid recipients in Oregon with substance use disorder diagnoses, rates of treatment initiation were lower among those with diagnosis codes for chronic or acute pain (e.g., back problems) and psychiatric diagnoses (Lind et al., 2019). In a study of young adults in Rhode Island who use prescription opioids non-medically, history of overdose was associated with an increased likelihood of unsuccessfully accessing treatment (Liebling et al., 2016). Taken together, our findings indicate a strong need to expand referrals to opioid agonist therapy and improved treatment pathways in healthcare settings that engage those populations with a recent overdose or diagnosis of an OUD. These settings may include inpatient psychiatric hospitals, community mental health organizations, alcohol detoxification and residential programs, treatment programs for substance use disorders, and general outpatient behavioral care settings. Given that those with previously diagnosed back pain were at greater risk of non-enrollment in opioid agonist therapy after an overdose or diagnosis of an OUD, improved care coordination and linkage to opioid agonist therapy in pain management clinics and primary care facilities treating patients with chronic lower back problem should be a priority.
Overall, our findings underscore the need to enhance pathways to treatment for OUD through varied nodes of engagement with existing healthcare systems. Based on our results and prior literature, we created a 2-tiered approach for enhancements to existing points-of-care for these populations to ensure greater utilization of treatment for OUD (Figure 1). First, we outline potential strategies to deepen integration of opioid agonist therapies across a broad base of behavioral healthcare and primary care providers. Baseline enhancements across larger systems of care include leveraging secure information sharing technology across State Agencies to identify at-risk patients (Massachusetts Department of Public Health, 2016; Sokol, 2019), engaging low-volume buprenorphine prescribers to increase treatment capacity for opioid agonist therapies (Brooklyn and Sigmon, 2017; Haffajee et al., 2018; Montanaro et al., 2015), integrating care coordination standards in contracts and licensure conditions (Rhode Island Department of Health, 2017; Rhode Island General Assembly, 2016; Samuels et al., 2019), and establishing on-demand buprenorphine induction guidelines and support (D’Onofrio et al., 2015). The EOHHS Data Ecosystem in Rhode Island might be used as a model for increased capacity for secure information sharing in other states (Sokol, 2019).
Figure 1:
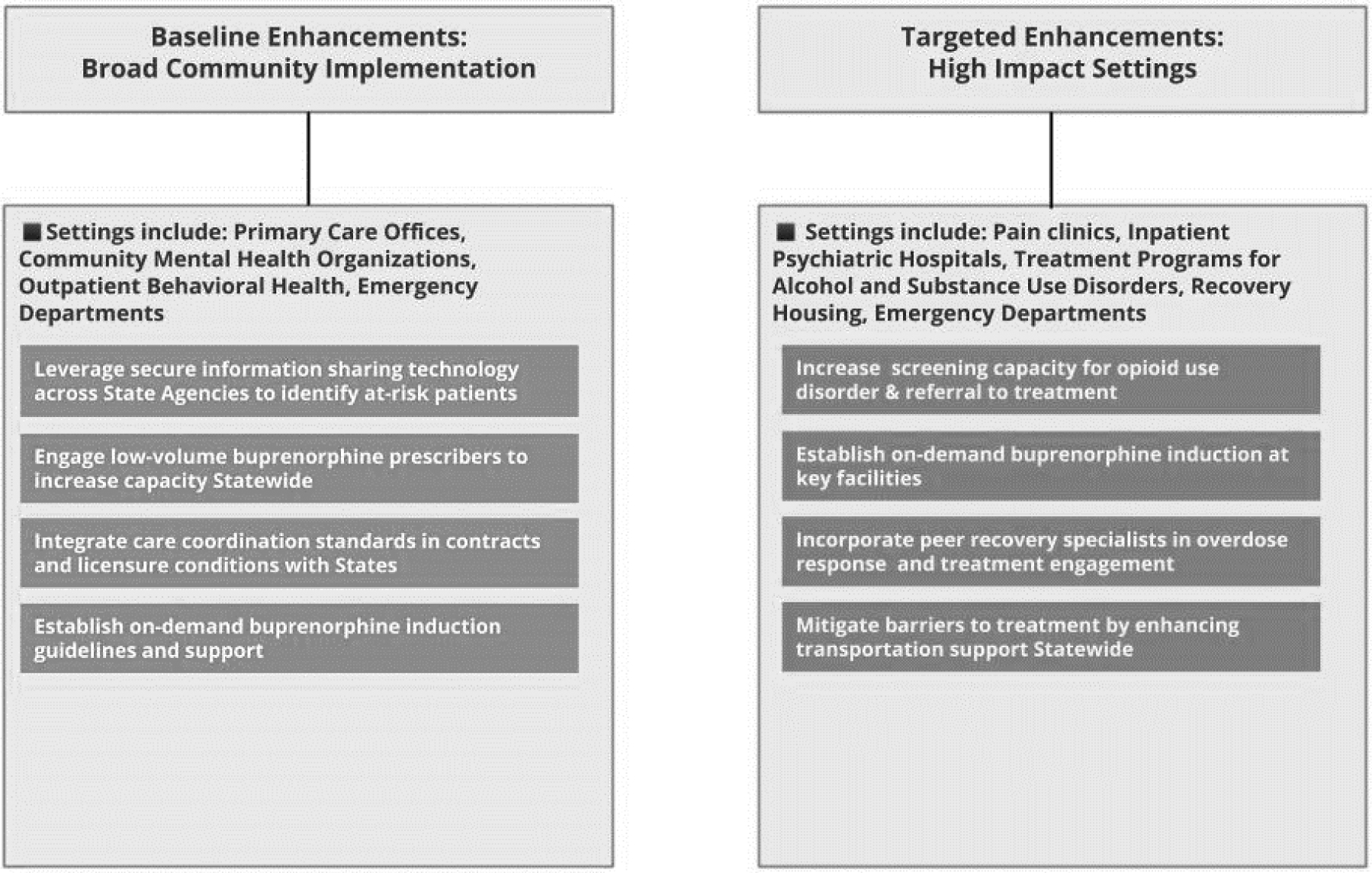
Enhancing Point-of-Care Pathways To Treatment for States
Next, we suggest targeted opportunities for increased screening for OUD and referrals to opioid agonist therapies in high impact settings such as treatment programs for alcohol and substance use disorders, pain clinics, inpatient psychiatric hospitals, emergency departments, and outpatient behavioral care settings. Evidence based approaches for high impact settings could include increasing screening capacity for OUD and referral to treatment (Brooklyn and Sigmon, 2017), establishing on-demand buprenorphine induction at key facilities (Dunkley et al., 2019), incorporating peer recovery specialists into care settings (Waye et al., 2019), and mitigating barriers to treatment by enhancing transportation support (Chatterjee et al., 2018).
Our findings also underscore several protective factors that should be taken into consideration. Having ED and PCP visit frequency above the 75th percentile was found to be positively associated with timely enrollment in opioid agonist therapy in the present study. These findings suggest that repeated interaction with ED and primary care settings improves uptake of opioid agonist therapy, which underscore the recommendations and enhancements for existing point-of-care systems as outlined in Figure 1. Prior research has documented that primary care and ED settings are feasible and effective venues to initiate individuals with OUD into evidence-based treatment (D’Onofrio et al., 2015). In Rhode Island, statewide treatment standards for emergency and inpatient care of adult patients with OUD have increased ED-based initiation in opioid agonist therapy and additional services for comprehensive treatment (Samuels et al., 2019). Since 2016, enrollment in treatment with methadone has increased by 20%, and enrollment in treatment with buprenorphine has increased by 26% (Prevent Overdose RI, 2020). Findings in the present study underscore the critical role of primary care and ED settings in engaging and retaining individuals with OUD in evidence-based care.
Our study is subject to limitations. First, as in any observational study, it is possible that there are unmeasured patient characteristics that were associated with both our exposures and outcome of interest, which would bias our estimates of characteristics associated with non-enrollment in opioid agonist therapy. While we were able to control for many patient characteristics, including prior diagnoses and health care utilization, residual confounding may be present. Second, key variables pertaining to our exposure, outcome, and cohort inclusion may have been misclassified through either incomplete data or data linkage error. While the data collection and linkage systems used for the present study were robust, error due to misclassification is possible and would bias our estimates toward the null. Third, our findings may have limited generalizability due to characteristics that are unique to Rhode Island, including higher prevalence of insurance coverage relative to the US average and expanded treatment capacity—both of which may translate to higher rates of enrollment in opioid agonist therapy. Fourth, persons whose index event date matched their initial date of enrollment in opioid agonist therapy were excluded from the current study; while this approach was necessary to control for the effect of Medicaid expansion, this adjustment may also exclude a small number of clients with same-day index event and enrollment in opioid agonist therapy, and the experiences of these individuals are not represented in the present study.
The EOHHS Data Ecosystem is also subject to limitations. First, given the current structure of the EOHHS Data Ecosystem, we are not able to report frequency, dose, or duration of opioid agonist therapy, which would permit us to distinguish between short term detoxification and maintenance treatment. Second, we do not have access to patient data pertaining to severity of opioid use disorder or prior treatment for substance use disorders, which may be important risk factors for overdose or OUD diagnosis. Third, we are not currently able to determine the healthcare setting in which OUD diagnoses occurred. The setting or type of healthcare encounter in which an OUD diagnosis occurs may influence individuals’ trajectory toward enrollment in opioid agonist therapy, and future research should seek to examine this potential effect moderation. Correspondingly, the present study lacks detail about the healthcare encounter in which index events occurred (e.g., whether a referral was made), which limits our ability to characterize pathways to opioid agonist therapy following an OUD diagnosis or overdose.
5. CONCLUSIONS
In a statewide sample of Medicaid patients in Rhode Island, the majority did not enroll in opioid agonist therapy within 6 months of an opioid overdose or a diagnosis of OUD. Having one or more prior overdose, alcohol and substance use disorders, back problems, mental health-related treatment and diagnoses, and older age were predictors of non-enrollment in opioid agonist therapy in adjusted analyses. We identified a two-tiered approach to enhance existing systems of care and ensure maximum exposure to pathways to treatment for OUD at the state level, suggesting a targeted focus on inpatient psychiatric hospitals, community mental health organizations, alcohol detoxification and residential programs, as well as outpatient behavioral care settings.
Supplementary Material
HIGHLIGHTS.
The majority (58%) did not enroll in opioid agonist therapy within 6 months
Prior overdose, alcohol use disorder, and back problems predicted non-enrollment
ED and PCP visits above the 75th percentile were associated with timely enrollment
We identified high-impact healthcare settings to enhance pathways to treatment
ACKNOWLEDGEMENTS
We would like to give special thanks to those who contributed to this project, including Annice Correia Gabel, Lisa Tse, and Jessica Hole.
ROLE OF FUNDING SOURCE
This project was supported by the State Opioid Response (SOR) grants program administered by the Substance Abuse and Mental Health Services Administration (SAMHSA). AM is supported by a Presidential Fellowship from Brown University. JY and BDLM and are supported in part by the Center for Biomedical Research Excellence (COBRE) on Opioids and Overdose, funded by the National Institute of General Medical Sciences (P20-GM125507). The funding sources had no role in the design or conduct of the study; collection, management, analysis, or interpretation of data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication. The views and opinions outlined herein do not necessarily reflect those of SAMHSA, the U.S. Department of Health and Human Services, or the state of Rhode Island & Providence Plantations, and should not be construed as such.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST STATEMENT
No conflicts declared.
REFERENCES
- Andrilla CHA, Moore TE, Patterson DG, Larson EH, 2019. Geographic distribution of providers with a DEA waiver to prescribe buprenorphine for the treatment of opioid use disorder: A 5- year update. The Journal of Rural Health. 35(1), 108–112. [DOI] [PubMed] [Google Scholar]
- Brooklyn JR, Sigmon SC, 2017. Vermont hub-and-spoke model of care for opioid use disorder: development, implementation, and impact. Journal of Addiction Medicine. 11(4), 286–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JJ, Rich JD, Green TC, 2018. The more things change: buprenorphine/naloxone diversion continues while treatment remains inaccessible. Journal of Addiction Medicine. 12(6), 459–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC/NCHS, 2020. National Vital Statistics System, Mortality. CDC WONDER, Atlanta, GA: US Department of Health and Human Services, CDC. [Google Scholar]
- Chatterjee A, Yu EJ, Tishberg L, 2018. Exploring opioid use disorder, its impact, and treatment among individuals experiencing homelessness as part of a family Drug and Alcohol Dependence. 188, 161–168. [DOI] [PubMed] [Google Scholar]
- D’Onofrio G, O’Connor PG, Pantalon MV, Chawarski MC, Busch SH, Owens PH, Bernstein SL, Fiellin DA, 2015. Emergency department–initiated buprenorphine/naloxone treatment for opioid dependence: a randomized clinical trial. JAMA. 313(16), 1636–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkley CA, Carpenter JE, Murray BP, Sizemore E, Wheatley M, Morgan BW, Moran TP, Steck A, 2019. Retrospective review of a novel approach to buprenorphine induction in the emergency department. The Journal of Emergency Medicine. 57(2), 181–186. [DOI] [PubMed] [Google Scholar]
- Frazier W, Cochran G, Lo-Ciganic W-H, Gellad WF, Gordon AJ, Chang C-CH, Donohue JM, 2017. Medication-assisted treatment and opioid use before and after overdose in Pennsylvania Medicaid. JAMA. 318(8), 750–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg RK, Fulton-Kehoe D, Franklin GM, 2017. Patterns of opioid use and risk of opioid overdose death among Medicaid patients. Medical Care. 55(7), 661–668. [DOI] [PubMed] [Google Scholar]
- Goedel WC, Shapiro A, Cerdá M, Tsai JW, Hadland SE, Marshall BD, 2020. Association of racial/ethnic segregation with treatment capacity for opioid use disorder in counties in the United States. JAMA Network Open. 3(4), e203711–e203711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffajee RL, Bohnert AS, Lagisetty PA, 2018. Policy pathways to address provider workforce barriers to buprenorphine treatment. American Journal of Preventive Medicine. 54(6), S230–S242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CM, Campopiano M, Baldwin G, McCance-Katz E, 2015. National and state treatment need and capacity for opioid agonist medication-assisted treatment. American Journal of Public Health. 105(8), e55–e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joudrey PJ, Edelman EJ, Wang EA, 2019. Drive times to opioid treatment programs in urban and rural counties in 5 US states. JAMA. 322(13), 1310–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz-Wirtz N, Davis CS, Ponicki WR, Rivera-Aguirre A, Marshall BD, Martins SS, Cerdá M, 2020. Association of Medicaid expansion with opioid overdose mortality in the United States. JAMA Network Open. 3(1), e1919066–e1919066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagisetty PA, Ross R, Bohnert A, Clay M, Maust DT, 2019. Buprenorphine treatment divide by race/ethnicity and payment. JAMA Psychiatry. 76(9), 979–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebling EJ, Yedinak JL, Green TC, Hadland SE, Clark MA, Marshall BD, 2016. Access to substance use treatment among young adults who use prescription opioids non-medically. Substance Abuse Treatment, Prevention, and Policy. 11(1), 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind BK, McCarty D, Gu Y, Baker R, McConnell KJ, 2019. Predictors of substance use treatment initiation and engagement among adult and adolescent Medicaid recipients. Substance Abuse. 40(3), 285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack KA, Zhang K, Paulozzi L, Jones C, 2015. Prescription practices involving opioid analgesics among Americans with Medicaid, 2010. Journal of Health Care for the Poor and Underserved. 26(1), 182–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madras BK, Ahmad NJ, Wen J, Sharfstein J, 2020. Improving access to evidence-based medical treatment for opioid use disorder: Strategies to address key barriers within the treatment system, NAM Perspectives. Discussion Paper, Washington, DC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manhapra A, Stefanovics E, Rosenheck R, 2020. Initiating opioid agonist treatment for opioid use disorder nationally in the Veterans Health Administration: Who gets what? Substance Abuse. 41(1), 110–120. [DOI] [PubMed] [Google Scholar]
- Massachusetts Department of Public Health, 2016. An assessment of opioid-related deaths in Massachusetts (2013–2014). Massachusetts Department of Public Health. [Google Scholar]
- Miller-Lloyd L, Landry J, Macmadu A, Allard I, Waxman M, 2020. Barriers to healthcare for people who inject drugs: A survey at a syringe exchange program. Substance Use & Misuse. 55(6), 896–899. [DOI] [PubMed] [Google Scholar]
- Mojtabai R, Mauro C, Wall MM, Barry CL, Olfson M, 2019. The Affordable Care Act and opioid agonist therapy for opioid use disorder. Psychiatric Services. 70(7), 617–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montanaro M, Alexander-Scott N, Green T, Rich J, Marshall B, Bratbertg J, Goyer J, McCance-Katz E, Macmadu A, Kinnard E, 2015. Rhode Island’s strategic plan on addiction and overdose: four strategies to alter the course of an epidemic Rhode Island Department of Health, Rhode Island Department of Behavioral Healthcare Developmental Disabilities Hospitals. Providence, RI: The Governor’s Task Force on Overdose Prevention Intervention. [Google Scholar]
- National Academies of Sciences Engineering and Medicine, 2019. Medications for Opioid Use Disorder Save Lives. Washington, DC: The National Academies Press. [PubMed] [Google Scholar]
- Orgera K, Tolbert J, 2019. The opioid epidemic and Medicaid’s role in facilitating access to treatment, Kaiser Family Foundation.
- Prevent Overdose RI, 2020. Medication-Assisted Treatment Data. Available at: https://preventoverdoseri.org/medication-assisted-therapy/.
- Rhode Island Department of Health, 2017. Levels of care for Rhode Island emergency departments and hospitals for treating overdose and opioid use disorder. Available at: https://health.ri.gov/publications/guides/LevelsOfCareForTreatingOverdoseAndOpioidUseDisorder.pdf.
- Rhode Island General Assembly, 2016. An Act Relating to Health and Safety—Comprehensive Discharge Planning. Available at: http://webserver.rilin.state.ri.us/BillText16/senateText16/S2546.htm.
- Saloner B, Levin J, Chang H-Y, Jones C, Alexander GC, 2018. Changes in buprenorphine-naloxone and opioid pain reliever prescriptions after the Affordable Care Act Medicaid expansion. JAMA Network Open. 1(4), e181588–e181588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels EA, McDonald JV, McCormick M, Koziol J, Friedman C, Alexander-Scott N, 2019. Emergency department and hospital care for opioid use disorder: implementation of statewide standards in Rhode Island, 2017–2018. American Journal of Public Health. 109(2), 263–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol E, 2019. Data Integration, Analytics Support Public Health in Rhode Island. Available at: https://healthitanalytics.com/news/data-integration-analytics-support-public-health-in-rhode-island.
- Substance Abuse and Mental Health Services Administration, 2019. Key substance use and mental health indicators in the United States: Results from the 2018 National Survey on Drug Use and Health (HHS Publication No. PEP19–5068, NSDUH Series H-54). Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration; Retrieved from https://www.samhsa.gov/data/. [Google Scholar]
- Waye KM, Goyer J, Dettor D, Mahoney L, Samuels EA, Yedinak JL, Marshall BD, 2019. Implementing peer recovery services for overdose prevention in Rhode Island: An examination of two outreach-based approaches. Addictive Behaviors. 89, 85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen H, Hockenberry JM, Pollack HA, 2018. Association of buprenorphine-waivered physician supply with buprenorphine treatment use and prescription opioid use in Medicaid enrollees. JAMA Network Open. 1(5), e182943–e182943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A, Nunes EV Jr, Bisaga A, Levin FR, Olfson M, 2019. Development of an Opioid Use Disorder Cascade of Care to Address the Addiction Treatment Gap. American Journal of Drug and Alcohol Abuse. 45(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson N, 2020. Drug and Opioid-Involved Overdose Deaths—United States, 2017–2018. MMWR. Morbidity and Mortality Weekly Report. 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L-T, Zhu H, Swartz MS, 2016. Treatment utilization among persons with opioid use disorder in the United States. Drug and Alcohol Dependence. 169, 117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyse JJ, Robbins JL, McGinnis KA, Edelman EJ, Gordon AJ, Manhapra A, Fiellin DA, Moore BA, Korthuis PT, Gaither JR, 2019. Predictors of timely opioid agonist treatment initiation among veterans with and without HIV. Drug and Alcohol Dependence. 198, 70–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarborough BJH, Stumbo SP, McCarty D, Mertens J, Weisner C, Green CA, 2016. Methadone, buprenorphine and preferences for opioid agonist treatment: a qualitative analysis. Drug and Alcohol Dependence. 160, 112–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


