Abstract
Objective:
To compare the prefrontal cortex (PFC) activation and task performance during single- and dual-task conditions between typically developing (TD) children and children with hemiplegic cerebral palsy (HCP).
Design:
A prospective, comparative design.
Setting:
Research laboratory.
Participants:
Participants (N=21) included 12 TD children (age, 6.0±1.1y) and 9 children with HCP (age, 7.2±3.1).
Interventions:
Not applicable.
Main Outcome Measures:
PFC activation was assessed by measuring the concentration of oxygenated hemoglobin while the children performed a shape-matching task with their more affected arm while sitting on a stable (single task) vs dynamic surface (dual task). The task performance was assessed with the total number of shapes matched, dual-task cost, and reaction time (RT).
Results:
For both conditions, the children with HCP exhibited greater PFC activation, matched a fewer shapes, and had slower RT than the TD children. These differences were accentuated during the dual-task condition and the dual-task cost was greater. An increase in the PFC activation during the dual-task condition was tightly correlated with a higher dual-task cost in children with HCP (r=0.77, P=.01).
Conclusions:
Children with HCP appear to have a heightened amount of PFC activity while performing a dual task. The greater cortical activity may be a result of the finite attentional resources that are shared between both the motor as well as cognitive demands of the task. The cognitive-motor interference is likely exacerbated in children with HCP because of the structural and functional brain changes as a result of an insult to the developing brain.
Keywords: Cerebral palsy, Cognition, Rehabilitation
Numerous activities of daily living are dual task in nature. Dual task is defined as concurrent performance of 2 tasks, usually a cognitive and motor task, that can be performed independently and have distinct and separate goals.1 The performance of 1 or both tasks deteriorates while performing the 2 tasks concurrently, which is termed dual-task interference.2–4 Because attention needs to be divided between both the cognitive and motor tasks, there is limited central processing capacity5 or competing cognitive-motor resources that result in a dual-task interference.6 In rehabilitation, dual-task interference has an important consideration because it affects functional task performance.
Several studies have demonstrated dual-task interference in healthy and clinical populations.4,7 Because of deficits in motor or cognitive processing, dual-task interference is heightened in neurologic populations and has been widely acknowledged in individuals with neurologic disorders such as stroke,8 Parkinson disease,9 and multiple sclerosis.10 However, very few studies have investigated dual-task paradigms in children with cerebral palsy (CP). The experimental paradigms used in these few investigations have primarily focused on the effects of dual tasks on walking and standing postural stability of children with CP.11–15 The findings from these studies demonstrated that dual task reduces walking performance and interferes with postural control. None of the prior studies to date have assessed upper limb dual-task paradigms in children with CP. Investigation of upper limb dual-task paradigms is important specifically in children with hemiplegic cerebral palsy (HCP) because the loss of upper extremity motor control affects activities of daily living and restricts the child’s participation in various educational, social, and vocational roles.
Because of various sensorimotor dysfunctions, motor actions such as reaching, grasping, releasing, object manipulations, and bimanual coordination are impaired in children with HCP.16 However, compelling evidence indicates that the difficulty in performing a motor task is not only because of deficits in the sensorimotor system but also arises from central deficits in cognitive processing17,18 and visuomotor dysfunctions.19–22 Our prior experimental work showed that the reduced motor task performance in children with HCP is partially related to a greater cognitive load imposed for controlling motor actions with the affected arm.17,23 Clearly, motor control of the affected upper extremity likely requires greater attentional or cognitive resources. Such competing cognitive-motor resources for controlling the affected upper extremity may potentially contribute to the reduced task performance.
Neuroimaging studies reveal the effect of the cognitive-motor interference associated with performing a dual task on the activation of the prefrontal cortex (PFC).24–26 These studies indicate that the PFC activation increases in a dual-task condition in healthy young and older adults as well as in individuals with neurologic injuries.3,27–31 It is currently unknown if these findings extend to children with HCP. It is plausible that the accentuated activity seen in the PFC of children with HCP might become further hyperactivated during a dual task because of the additional attentional resources required for performing the cognitive and motor components concurrently.23 Moreover, these additional challenges of dual task might affect the performance of goal-directed motor task with the affected upper extremity in children with HCP. Although this conjecture is plausible, this scientific premise has yet to be tested.
The purposes of this investigation were (1) to assess differences in PFC activation while performing a goal-directed, shape-matching task with the more affected upper limb during single- (sitting on a stable surface) vs dual-task (sitting on dynamic surface) conditions between typically developing (TD) children and children with HCP and (2) to compare the differences in shape-matching task performance, dual-task cost, and reaction times (RT) during single- vs dual-task conditions between TD children and children with HCP. We hypothesized that because of the greater cognitive-motor interference in children with HCP, the PFC activation would markedly increase, the task performance would deteriorate, and information processing would be delayed in the dual-task condition compared with the single-task condition.
Methods
Study design
The study was a prospective, comparative design. The Institutional Review Board of the University of Nebraska Medical Center approved the study, and we obtained parental consent and child assent to participate in this investigation.
Participants
The sample size for this study was based on our previous experimental work that assessed PFC activation between TD and children with HCP while performing a shape-matching task with the more affected arm.23 With the effect size (2.33) for the difference in the oxygenated hemoglobin (HbO) between the TD children and children with CP seen in this prior study, a sample size of 12 (6 in each group) would provide >86% power to detect a similar difference at a 0.01 α level. A total of 9 children with HCP (age, 7.2±3.1y; male=5; Manual Ability Classification System Levels I-III) and 12 TD children (age, 6.0±1.1y; male=7) were recruited to participate in this investigation. The children with HCP were recruited from the physical therapy clinic at the University of Nebraska Medical Center, and the TD children were recruited through word of mouth and targeted e-mails to University employees. The physical therapists, who had access to the children’s health records, prescreened the children based on the inclusion and exclusion criteria to qualify for the study. The inclusion criteria were (1) children with hemiplegia diagnosed by a pediatric neurologist, (2) children who were able to reach and grasp the objects, and (3) children who were able to comprehend and follow instructions. Age-equivalent TD children were also included. The exclusion criteria were (1) the presence of frontal cortical lesions; (2) children with HCP with cognitive impairments (lack of age-appropriate intelligence, verbal comprehension, visuospatial orientation, and working memory indices based on Wechsler Preschool and Primary Scale of Intelligence-Fourth Edition)32; (3) visual deficits; (4) musculoskeletal deformity of the hand and arm such as any fixed contractures of hand or wrist, which would limit the ability to grasp and manipulate objects; (5) Manual Ability Classification System levels IV (handles a limited selection of easily managed objects in adapted situations) and V (does not handle objects and has severely limited ability to perform simple actions)33; and (6) arm weakness due to neurologic impairments such as brachial plexus injuries. Table 1 presents the characteristics of the participants that were recruited for this investigation. There were no differences in the age (P=.653) and sex (P=.071) between TD children and children with HCP.
Table 1.
Participant characteristics
| Variables | Children With HCP (n = 9) | TD Children (n = 12) | P Value |
|---|---|---|---|
| Age (y), mean ± SD | 6.0±1.1 | 7.2±3.1 | .653 |
| Sex (n), M/F | 5/4 | 7/5 | .071 |
| Side of hemiplegia (n), L/R | 6/3 | ||
| Manual Ability Classification level | I = 1 | ||
| II = 1 | |||
| III = 7 |
Abbreviations: F, female; L, left; M, male; R, right.
Experimental paradigm
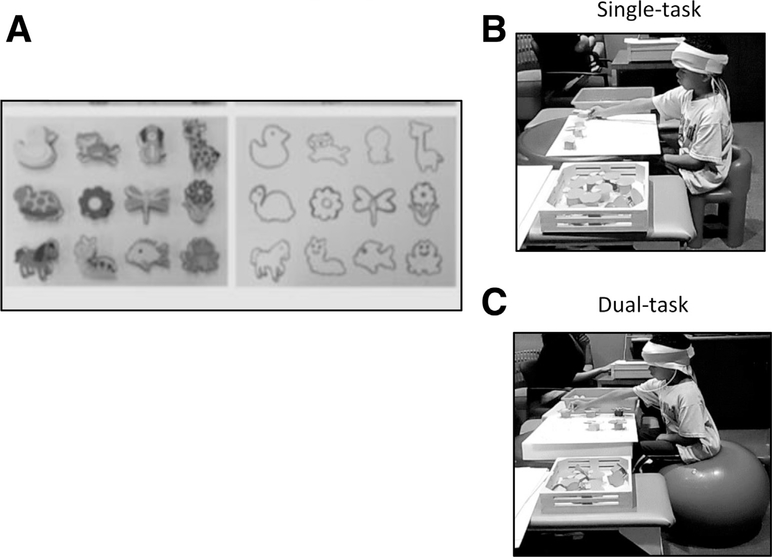
The experimental task consisted of a shape-matching task, which was tested as a valid cognitive-motor task and has previously been shown to elicit changes in HbO concentration within the PFC (fig 1A).23 The children were asked to match the shapes by selecting an appropriate shape and placing it accurately on a template. The children with HCP performed the task with their more affected hand, while the TD children performed the task with their nondominant hand.
Fig 1.
(A) Experimental task and protocol. The shapes template was presented to the child just before beginning the task. Children were asked to match maximum number of shapes with the corresponding template for 30 s. The task was repeated 4 times. (B) Performance of the shape-matching task in single-task condition. In a single-task condition, children performed the shape-matching task while sitting on a stable surface (chair). (C) Performance of the shape-matching task in dual-task condition. In dual-task condition, children performed the shape-matching task while sitting on a dynamic surface (therapy ball).
The shape-matching task was performed in a block paradigm, which consisted of a 30-second rest period where the child sat still and a 30-second active period where the child matched the shapes. For a single-task condition, the children performed the shape-matching task while sitting on a stable surface (chair) (fig 1B), whereas for a dual-task condition, the children performed the task while sitting on a dynamic surface (therapy ball) (fig 1C). The single-task and dual-task conditions were performed in random order. The assessor presented the template and shapes at time 0, and the children were instructed to match as many shapes as they could within the 30-second active period. Typically, the children could not match all 12 shapes in this time frame. However, in the rare instance, if the child matched all 12 shapes, the assessor removed the shapes from the template and the child continued matching the shapes on the same template until the 30 seconds of the active period was over. The children performed a total of 4 blocks of the shape-matching task during the entire session with an alternating pattern of the 30-second active and rest periods. The total duration of the data collection for each condition was 4 minutes.
Functional near-infrared spectroscopy data acquisition and data analysis
A continuous wave functional near-infrared spectroscopy (fNIRS) systema that used 2 different wavelengths (730 and 850nm) was used to measure the concentration of HbO and deoxygenated hemoglobin in the PFC. The fNIRS system was composed of a flexible head piece that contained a series of 2 infrared-emitting optodes and 2 detector optodes. These optodes comprised the 4 measurement channels that were used in this investigation. According to 10–20 electroencephalography system, the optodes were positioned lateral to the Fpz on the left and the right side of the forehead. The sensors had 2 Hz sampling rate and measured the hemodynamic change in approximately 5–10 mm of the outermost cortical tissue.34–36 All optodes were connected to fiber-optic cables that allowed the transmission of infrared light to the fNIRS system. We used cognitive optical brain imaging studio software (fNIRSoft software v 3.1a) for data acquisition and visualization.
The fNIRSoft software was used to calculate the optical density of the raw light intensity signals and was used to implement a modified Beer-Lambert Law to determine the changes in the HbO concentration (H. Ayaz, unpublished data, 2010). Waveforms that were saturated or had motion artifacts were visually identified and excluded from the analysis. A fourth-order digital filter was applied to low- and high-pass filter the HbO time series with 0.1- and 10-Hz cutoffs, respectively. These filter settings were applied to reduce the potential effects of the physiological noise (eg, equipment noise, respiration, cardiac cycle effects) that often accompanies the measured cortical hemodynamics.37–39 The epochs of each trial were 60 seconds in duration (−30 to +30s), with the presentation of the shape-matching task defined as 0.0 seconds. The HbO hemodynamic waveforms for each channel were corrected based on the average HbO seen in the baseline period (−10 to 0s), and the 4 trials performed in each condition were averaged to create the time course of the concentration of the HbO for the right and left PFC, respectively. The average of the HbO concentration in the right and left PFC was calculated because it has been previously shown that there are no hemispheric differences in the activation of the PFC for the shape-matching task that was used in this investigation.23 We used the average HbO as a marker for regional brain activation because previous study findings have shown that HbO is more sensitive to neural changes than deoxygenated hemoglobin.40
Behavioral data analysis
The video-recorded behavioral data for the shape-matching task was used for the analysis of the behavioral performance. The total number of shapes matched across 4 shape-matching trials and dual-task cost (percentage of change in shape matching task from single- to dual-task condition) were outcomes for task performance.41 In addition, we assessed RT, which was determined as time to initiate the hand movement after the shape-matching board was presented. The exploratory sequential data analysis software (Datavyub) was used to calculate the RT, and the average RT across all 4 trials was considered for the final analysis. A trained coder calculated the RT with an intrarater reliability of 0.98.
Statistical analysis
All the data were normally distributed. Therefore, parametric tests were used to determine the group and condition differences. Separate mixed-model analysis of variance (ANOVA) (group-×task conditions) with group (TD and HCP) as the between-subject and task conditions (single- vs dual-task) as the within-subject factor were used to determine if there were significant differences in HbO, total number of shapes matched, and RT. The data conformed to the assumptions of normality, homogeneity, and sphericity. Significant interaction effects were followed up with a Bonferroni post hoc analysis. An independent t test was also used to determine between-group differences in dual-task cost. Lastly, a Pearson correlation was performed to assess the relationship between PFC activation and dual-task cost. All statistical analysis was performed using SPSSc and P values equal to or less than the corrected 0.01 α levels were considered significant. Results in the text and graphs are presented as a mean ± SD of the mean.
Results
fNIRS PFC activation
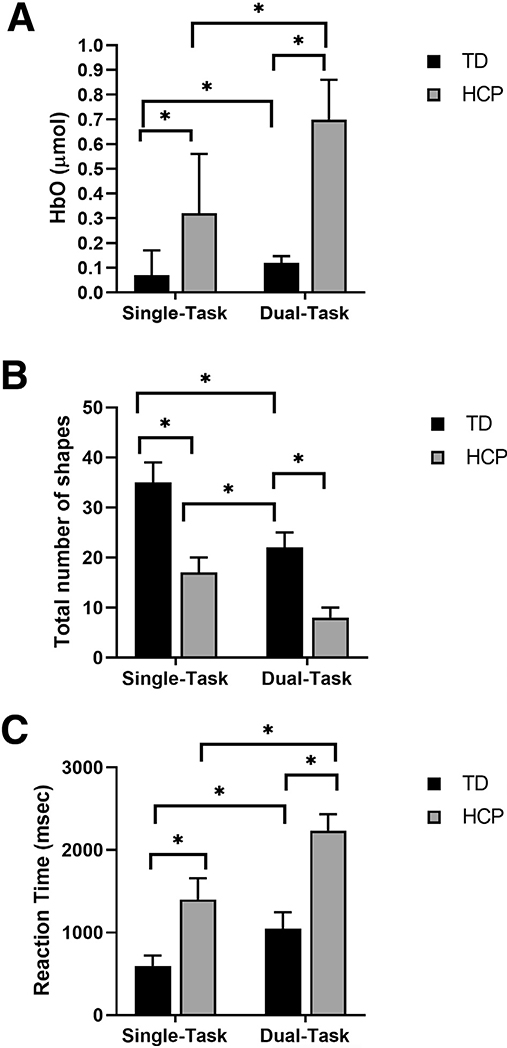
There was a significant condition main effect (P=.001) indicating overall greater PFC activation in the dual-task condition (0.41±0.11μmol) than the single-task condition (0.19±0.06μmol) for all the participating children (ie, TD children as well as children with HCP). There was also a significant group main effect (P=.001) indicating that overall the children with HCP (0.51±0.14μmol) had greater PFC activation than the TD children (0.10±0.03μmol). In addition, there was a significant group-by-condition interaction (P=.001). Post hoc analyses revealed greater PFC activation in children with HCP during the dual-task condition (P=.001) as well as the single-task condition (P=.019) when compared with the TD children (fig 2A). Furthermore, there was greater PFC activation in the dual-task condition (P=.001) than the single-task condition (P=.013) for both groups (see fig 2A). Altogether these results reveal that the dual-task condition resulted in greater PFC activation and that the children with HCP had greater PFC activation during the dual-task and single-task conditions than the TD children.
Fig 2.
(A) PFC activation: comparison of the average HbO between TD children (n=12) and children with HCP (n=9) while performing the shape-matching task during single- vs dual-task conditions. Mixed-model ANOVA and Bonferroni post hoc analysis shows that the concentration of HbO was highest during dual-task condition followed by single-task condition in children with HCP compared with dual- and single-task conditions in TD children. (B) Total number of shapes matched: comparison of the task performance between TD children (n=12) and children with HCP (n=9) while performing the shape-matching task during single- vs dual-task conditions. Mixed-model ANOVA and Bonferroni post hoc analysis shows maximum deterioration of the task performance was observed in dual-task condition followed by single-task condition in children with HCP compared with dual- and single-task conditions in TD children. (C) Reaction time: comparison of RT between TD children (n=12) and children with HCP (n=9) during single- vs. dual-task conditions. Mixed-model ANOVA and Bonferroni post hoc analysis shows that the RT was most delayed during dual-task condition followed by single-task condition in children with HCP compared with dual- and single-task conditions in TD children. NOTE. The error bars are standard error of mean. *P≤.01.
Number of shapes matched
There was a significant condition main effect (P=.001) indicating that overall fewer shapes were matched in dual-task condition (17±8 shapes) than with the single-task condition (27±10 shapes) for all the participating children. There also was a significant group main effect for the number of shapes matched (P=.001) indicating that the children with HCP (13±6 shapes) matched fewer shapes overall than the TD children (30±7 shapes). Moreover, there was a significant group-by-condition interaction (P=.036). Post hoc analyses revealed the children with HCP matched fewer shapes in the single-task (P=.011) and dual-task conditions (P=.001) than the TD children (fig 2B). Similarly, fewer shapes were matched during the dual-task condition (P=.001) than the single-task condition (P=.001) for both groups (see fig 2B). Overall these results imply that fewer shapes were matched during the dual-task condition, and the children with HCP matched fewer shapes during the dual-task condition because of a greater dual task-cost for both the single- and dual-task conditions.
Reaction time
For reaction time, there was a significant condition main effect (P=.001) indicating that overall the RT was slower for the dual-task condition (1558±663 msec) compared to single-task condition (941±448ms) for all the participating children. In addition, we found a significant group main effect for RT (P=.001) indicating that the children with HCP (1819±484ms) had a slower RT than TD children (822±282ms). Finally, there also was a significant group-by-condition interaction (P=.01). Post hoc analyses revealed that the RT for the single-task condition as well the dual-task condition was slower for the children with HCP than TD children (fig 2C). Similarly, the RT was also slower for the dual-task condition than the single-task condition for both groups (see fig 2C). Altogether these results imply that the children with HCP required a longer time to process the information and plan their motor actions during the dual-task as well as the single-task condition than TD children.
Dual-task cost
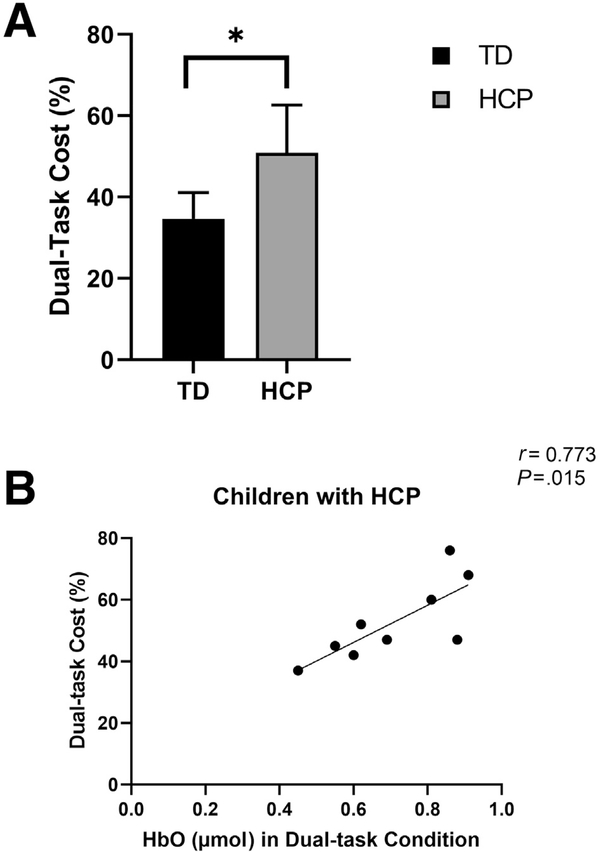
The children with HCP had greater dual-task cost on the number of shapes matched than TD children (P=.011) (fig 3A). There was a strong positive correlation between the HbO concentration and dual-task cost in children with HCP (r=0.773, P=.007) (fig 3B) and a moderate positive but nonsignificant correlation in TD children (r=0.39, P=.104). Altogether these results suggest that a greater burden on PFC increases the cost of task performance in dual-task condition.
Fig 3.
(A) Dual-task cost: dual-task cost=(single-task–dual-task)/single-task×100. Dual-task cost was measured with the total number of shapes matched. Independent t test results show higher dual-task cost indicates greater cognitive-motor interference. Children with HCP (n=9) had higher dual-task cost than TD children (n=12). (B) Correlations between PFC activation and dual-task cost: scatter plot of the PFC activation while performing dual-task condition and dual-task cost in children with HCP (n=9). Abscissa represents average HbO concentration and the ordinate represents percentage of dual-task cost. Pearson correlation analysis shows a strong positive correlation (r= 0.77, P=.01) in children with HCP, which suggests that children who had greater PFC activation tended to have greater cost of performing a dual task.
Discussion
This study evaluated the neural substrate of the cognitive-motor interference that occurs during an upper extremity dual task as performed by children with HCP with their more affected hand. Our results support our core hypothesis that the dual task would have greater cognitive-motor interference in children with HCP and that this interference would be reflected by hyperactivation of the PFC. Overall our results had 3 key findings: (1) the PFC activation was higher in children with HCP during the single and dual tasks than TD children (2) the dual-task cognitive-motor interference resulted in slower RT and diminished ability to match the shapes for the children with HCP compared with the TD children, and (3) the dual-task noticeably increased dual-task cost and was associated with greater PFC activation in children with HCP compared with TD children. The implications of these findings are further discussed in the following sections.
The children with HCP had greater PFC activation during the single-task condition, which corroborates our prior results showing similar changes in the cortical activation while performing a similar shape-matching task.23 Experimental results presented elsewhere indicated that performing a motor task with the affected arm requires greater attentional resources for children with HCP.23,42,43 Combined with the results presented here, the heightened PFC activation seen in the children with HCP during the single-task condition might reflect the additional attentional resources that are required to plan a motor action with the affected arm.
The additional motor challenges imposed by the dynamic surface during the dual-task condition exacerbated the hyperactivation seen in the PFC for the children with HCP. Maintaining dynamic postural control requires attentional resources44 and is associated with increased activation within the PFC.45,46 Therefore, during the dual-task condition the competing streams of cognitive and motor demands were likely increased during the dual task for the children with HCP. This perspective is consistent with other neuroimaging studies that have also shown an increased activation within the PFC as individuals post stroke perform a dual task.29,47,48
For both the TD children and children with HCP, fewer shapes were matched during the dual-task condition. Furthermore, the dual-task cost increased and was associated with greater PFC activation, indicating prominent interference between the cognitive and motor demands of the task. These behavioral and associated changes in cortical activation support the idea that the dual-task condition required more attentional resources than the single-task condition. Furthermore, they substantiate the notion that the heighted PFC activity reflects the cortical demands associated with performing the concurrent cognitive and motor demands of the dual task. Therefore, based on the capacity-sharing theory of a dual task there was likely a capacity overload.5 We suspect that a “posture first” strategy was implied, which allows prioritization of motor over the cognitive task.49 Hence, it is plausible that the degraded shape-matching performance is partially related to the children primarily focusing on maintaining and stabilizing their dynamic posture as opposed to matching the shapes. However, we do not have any postural control measures, which limits our ability to infer that the attentional resources were allocated for a “posture first” strategy.
Another indicator of dual-task interference was the slower RT during the dual-task condition compared with the single-task condition. When 2 tasks that share common domains are performed concurrently, this can create a bottleneck that slows down the processing of the respective tasks because of limited processing capacity.6,50–52 There was likely a bottleneck for the dual task because maintaining postural control on the dynamic surface and executing the shape-matching task both require sufficient attentional or cognitive resources. Such a bottleneck might have resulted in a delay in information processing associated with performing the shape-matching task, which was exhibited by a slower RT.
From clinical and rehabilitation perspective, it is important to understand that impaired performance of goal-directed movements with the affected upper extremity does not only result from weakness or paresis of the affected arm but potentially because of amalgamation of motor weakness, impaired sensorimotor processing, and greater cognitive-motor interferences. Our study results specifically indicate that the greater cognitive-motor interference resulted from motor weakness combined with limited attentional resources, which further reduced the motor task performance. Therefore, while assessing performance of the affected extremity, it would be crucial to assess the cognitive demands of the task because greater cognitive demands would likely interfere with the motor outcome or goal-directed task performance. Similarly, while designing the rehabilitation strategies, practicing the motor tasks combined with slowly progressing or appropriately challenging cognitive demands in variable environments and incorporated with various postural challenges would enhance the rehabilitation outcomes.
Study limitations
We recognize several limitations of this study. First, only the activation of the PFC was assessed. It is highly likely that other areas associated with dual-task activation such as parietal cortex, insula, and cerebellum3,53 were likely active, and their assessment would further enhance our understanding about the neural underpinnings of the cognitive-motor interference seen in children with HCP. Second, we did not record any kinesiological data such as center of pressure measures or electromyography, which limited our ability to fully evaluate the movements seen in the participating children. Having this data would have helped us in partitioning whether children used a “motor/posture first” or a “cognition first” strategy. Third, our findings are limited to children with HCP who had mild to moderate impairments. Therefore, we do not know the degree of burden on the PFC in children with severe impairments. It would also be an interesting next step to assess whether these results extend to children with other types of CP such as diplegia and quadriplegia.
Conclusions
Our results indicate that children with HCP have greater activation in the PFC while performing dual as well as single task with their more affected arm. This burden on the PFC may result from finite attentional resources that need to be shared between both the motor as well as cognitive demands of the task. Moreover, the reduced task performance in the children with HCP might partially stem from exacerbated cognitive-motor interference due to structural and functional changes that result from the initial insult to the developing brain. Therefore, it is imperative to understand that the reduced performance of the goal-oriented task with the affected arm in children with HCP may not be only because of motor impairments but also cognitive-motor interferences resulting from developmental brain damage. These results clearly point to the notion that the perinatal brain insults seen in children with HCP are not purely sensorimotor but rather can have cascading effects that affect other cortical networks that involve in cognitive processing. Clinical assessments and therapeutic interventions should consider the interaction between cognitive and motor components of children with HCP to improve rehabilitation outcomes.
Acknowledgments
Supported in part by the National Institutes of Health (grant nos. R01HD086245 and R01HD101833), the World Bank Margaret McNamara Fund Education Grant, the American Association of University Women International Doctoral Fellowship, and the North American Society for the Psychology of Sports and Physical Activity.
List of abbreviations:
- ANOVA
analysis of variance
- CP
cerebral palsy
- fNIRS
functional near-infrared spectroscopy
- HbO
oxygenated hemoglobin
- HCP
hemiplegic cerebral palsy
- PFC
prefrontal cortex
- RT
reaction time
- TD
typically developing
Footnotes
Disclosures: none.
Supplier
a. fNIR Devices LLC.
b. Datavyu: A Video Coding Tool, Databrary Project; New York University.
c. SPSS version 24.0; IBM.
References
- 1.McIsaac TL, Lamberg EM, Muratori LM. Building a framework for a dual task taxonomy. Biomed Res Int 2015;2015:591475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Plummer P, Eskes G. Measuring treatment effects on dual-task performance: a framework for research and clinical practice. Front Hum Neurosci 2015;9:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leone C, Feys P, Moumdjian L, D’Amico E, Zappia M, Patti F. Cognitive-motor dual-task interference: a systematic review of neural correlates. Neurosci Biobehav Rev 2017;75:348–60. [DOI] [PubMed] [Google Scholar]
- 4.Al-Yahya E, Dawes H, Smith L, Dennis A, Howells K, Cockburn J. Cognitive motor interference while walking: a systematic review and meta-analysis. Neurosci Biobehav Rev 2011;35:715–28. [DOI] [PubMed] [Google Scholar]
- 5.Tombu M, Jolicoeur P. A central capacity sharing model of dual-task performance. J Exp Psychol Hum Percept Perform 2003;29:3–18. [DOI] [PubMed] [Google Scholar]
- 6.Pashler H Dual-task interference in simple tasks: data and theory. Psychol Bull 1994;116:220–44. [DOI] [PubMed] [Google Scholar]
- 7.Plummer P, Eskes G, Wallace S, et al. Cognitive-motor interference during functional mobility after stroke: state of the science and implications for future research. Arch Phys Med Rehabil 2013;94: 2565–2574.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plummer-D’Amato P, Altmann LJ, Saracino D, Fox E, Behrman AL, Marsiske M. Interactions between cognitive tasks and gait after stroke: a dual task study. Gait Posture 2008;27:683–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raffegeau TE, Krehbiel LM, Kang N, et al. A meta-analysis: Parkinson’s disease and dual-task walking. Parkinsonism Relat Disord 2019;62:28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wajda DA, Sosnoff JJ. Cognitive-motor interference in multiple sclerosis: a systematic review of evidence, correlates, and consequences. Biomed Res Int 2015;2015:720856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carcreff L, Fluss J, Allali G, et al. The effects of dual tasks on gait in children with cerebral palsy. Gait Posture 2019;70:148–55. [DOI] [PubMed] [Google Scholar]
- 12.Hung YC, Meredith GS. Influence of dual task constraints on gait performance and bimanual coordination during walking in children with unilateral cerebral palsy. Res Dev Disabil 2014;35: 755–60. [DOI] [PubMed] [Google Scholar]
- 13.Katz-Leurer M, Rotem H, Meyer S. Effect of concurrent cognitive tasks on temporo-spatial parameters of gait among children with cerebral palsy and typically developed controls. Dev Neurorehabil 2014;17:363–7. [DOI] [PubMed] [Google Scholar]
- 14.Reilly DS, Woollacott MH, van Donkelaar P, Saavedra S. The interaction between executive attention and postural control in dual-task conditions: children with cerebral palsy. Arch Phys Med Rehabil 2008;89:834–42. [DOI] [PubMed] [Google Scholar]
- 15.Tramontano M, Morone G, Curcio A, et al. Maintaining gait stability during dual walking task: effects of age and neurological disorders. Eur J Phys Rehabil Med 2017;53:7–13. [DOI] [PubMed] [Google Scholar]
- 16.Bleyenheuft Y, Gordon AM. Precision grip control, sensory impairments and their interactions in children with hemiplegic cerebral palsy: a systematic review. Res Dev Disabil 2013;34: 3014–28. [DOI] [PubMed] [Google Scholar]
- 17.Surkar SM, Hoffman RM, Harbourne R, Kurz MJ. Neural activation within the prefrontal cortices during the goal-directed motor actions of children with hemiplegic cerebral palsy. Neurophotonics 2018;5: 011021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steenbergen B, Gordon AM. Activity limitation in hemiplegic cerebral palsy: evidence for disorders in motor planning. Dev Med Child Neurol 2006:48780–3. [DOI] [PubMed] [Google Scholar]
- 19.Surkar SM, Hoffman RM, Davies B, Harbourne R, Kurz MJ. Impaired anticipatory vision and visuomotor coordination affects action planning and execution in children with hemiplegic cerebral palsy. Res Dev Disabil 2018;80:64–73. [DOI] [PubMed] [Google Scholar]
- 20.Rodrigo AH, Domenico SI, Ayaz H, Gulrajani S, Lam J, Ruocco AC. Differentiating functions of the lateral and medial prefrontal cortex in motor response inhibition. Neuroimage 2014;85:423–31. [DOI] [PubMed] [Google Scholar]
- 21.VerMaas JR, Gehringer JE, Wilson TW, Kurz MJ. Children with cerebral palsy display altered neural oscillations within the visual MT/V5 cortices. Neuroimage Clin 2019;23:101876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurz MJ, Proskovec AL, Gehringer JE, Heinrichs-Graham E, Wilson TW. Children with cerebral palsy have altered oscillatory activity in the motor and visual cortices during a knee motor task. Neuroimage Clin 2017;15:298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Surkar SM, Hoffman RM, Willett S, Flegle J, Harbourne R, Kurz MJ. Hand-arm bimanual intensive therapy improves prefrontal cortex activation in children with hemiplegic cerebral palsy. Pediatr Phys Ther 2018;30:93–100. [DOI] [PubMed] [Google Scholar]
- 24.Stelzel C, Brandt SA, Schubert T. Neural mechanisms of concurrent stimulus processing in dual tasks. Neuroimage 2009;48:237–48. [DOI] [PubMed] [Google Scholar]
- 25.Atsumori H, Kiguchi M, Katura T, et al. Noninvasive imaging of prefrontal activation during attention-demanding tasks performed while walking using a wearable optical topography system. J Biomed Opt 2010;15:046002. [DOI] [PubMed] [Google Scholar]
- 26.Tachibana A, Noah JA, Bronner S, et al. Activation of dorsolateral prefrontal cortex in a dual neuropsychological screening test: an fMRI approach. Behav Brain Funct 2012;8:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beurskens R, Helmich I, Rein R, Bock O. Age-related changes in prefrontal activity during walking in dual-task situations: a fNIRS study. Int J Psychophysiol 2014;92:122–8. [DOI] [PubMed] [Google Scholar]
- 28.Holtzer R, Mahoney JR, Izzetoglu M, Izzetoglu K, Onaral B, Verghese J. fNIRS study of walking and walking while talking in young and old individuals. J Gerontol A Biol Sci Med Sci 2011;66: 879–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al-Yahya E, Johansen-Berg H, Kischka U, Zarei M, Cockburn J, Dawes H. Prefrontal cortex activation while walking under dual-task conditions in stroke: a multimodal imaging study. Neurorehabil Neural Repair 2016;30:591–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mori T, Takeuchi N, Izumi SI. Prefrontal cortex activation during a dual task in patients with stroke. Gait Posture 2018;59:193–8. [DOI] [PubMed] [Google Scholar]
- 31.Ohsugi H, Ohgi S, Shigemori K, Schneider EB. Differences in dual-task performance and prefrontal cortex activation between younger and older adults. BMC Neurosci 2013;14:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wechsler D Wechsler preschool and primary scale of intelligence. 4th ed. San Antonio: The Psychological Corporation; 2012. [Google Scholar]
- 33.Morris C, Kurinczuk JJ, Fitzpatrick R, Rosenbaum PL. Reliability of the manual ability classification system for children with cerebral palsy. Dev Med Child Neurol 2006;48:950–3. [DOI] [PubMed] [Google Scholar]
- 34.Fukui Y, Ajichi Y, Okada E. Monte Carlo prediction of near-infrared light propagation in realistic adult and neonatal head models. Appl Opt 2003;42:2881–7. [DOI] [PubMed] [Google Scholar]
- 35.Okada E, Delpy DT. Near-infrared light propagation in an adult head model. I. Modeling of low-level scattering in the cerebrospinal fluid layer. Appl Opt 2003;42:2906–14. [DOI] [PubMed] [Google Scholar]
- 36.Okada E, Delpy DT. Near-infrared light propagation in an adult head model. II. Effect of superficial tissue thickness on the sensitivity of the near-infrared spectroscopy signal. Appl Opt 2003;42:2915–22. [DOI] [PubMed] [Google Scholar]
- 37.Gentili RJ, Shewokis PA, Ayaz H, Contreras-Vidal JL. Functional near-infrared spectroscopy-based correlates of prefrontal cortical dynamics during a cognitive-motor executive adaptation task. Front Hum Neurosci 2013;7:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ayaz H, Shewokis PA, Curtin A, Izzetoglu M, Izzetoglu K, Onaral B. Using MazeSuite and functional near infrared spectroscopy to study learning in spatial navigation. J Vis Exp 2011;56:e3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Izzetoglu M, Bunce SC, Izzetoglu K, Onaral B, Pourrezaei K. Functional brain imaging using near-infrared technology. IEEE Eng Med Biol Mag 2007;26:38–46. [DOI] [PubMed] [Google Scholar]
- 40.Suzuki M, Miyai I, Ono T, et al. Prefrontal and premotor cortices are involved in adapting walking and running speed on the treadmill: an optical imaging study. Neuroimage 2004;23:1020–6. [DOI] [PubMed] [Google Scholar]
- 41.Yogev-Seligmann G, Hausdorff JM, Giladi N. The role of executive function and attention in gait. Mov Disord 2008;23:329–42 [quiz: 472]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Houwink A, Aarts PB, Geurts AC, Steenbergen B. A neurocognitive perspective on developmental disregard in children with hemiplegic cerebral palsy. Res Dev Disabil 2011;32:2157–63. [DOI] [PubMed] [Google Scholar]
- 43.Zielinski IM, Jongsma ML, Baas CM, Aarts PB, Steenbergen B. Unravelling developmental disregard in children with unilateral cerebral palsy by measuring event-related potentials during a simple and complex task. BMC Neurol 2014;14:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Woollacott M, Shumway-Cook A. Attention and the control of posture and gait: a review of an emerging area of research. Gait Posture 2002; 16:1–14. [DOI] [PubMed] [Google Scholar]
- 45.Mihara M, Miyai I, Hatakenaka M, Kubota K, Sakoda S. Role of the prefrontal cortex in human balance control. Neuroimage 2008;43: 329–36. [DOI] [PubMed] [Google Scholar]
- 46.Ferrari M, Bisconti S, Spezialetti M, et al. Prefrontal cortex activated bilaterally by a tilt board balance task: a functional near-infrared spectroscopy study in a semi-immersive virtual reality environment. Brain Topogr 2014;27:353–65. [DOI] [PubMed] [Google Scholar]
- 47.Hawkins KA, Fox EJ, Daly JJ, et al. Prefrontal over-activation during walking in people with mobility deficits: interpretation and functional implications. Hum Mov Sci 2018;59:46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu YC, Yang YR, Tsai YA, Wang RY, Lu CF. Brain activation and gait alteration during cognitive and motor dual task walking in stroke-a functional near-infrared spectroscopy study. IEEE Trans Neural Syst Rehabil Eng 2018;26:2416–23. [DOI] [PubMed] [Google Scholar]
- 49.Schaefer S The ecological approach to cognitive-motor dual-tasking: findings on the effects of expertise and age. Front Psychol 2014;5: 1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Jong R Multiple bottlenecks in overlapping task performance. J Exp Psychol Hum Percept Perform 1993;19:965–80. [DOI] [PubMed] [Google Scholar]
- 51.Schubert T Processing differences between simple and choice reactions affect bottleneck localization in overlapping tasks. J Exp Psychol Hum Percept Perform 1999;25:408. [Google Scholar]
- 52.Szameitat AJ, Schubert T, Muller K, Von Cramon DY. Localization of executive functions in dual-task performance with fMRI. J Cogn Neurosci 2002;14:1184–99. [DOI] [PubMed] [Google Scholar]
- 53.Papegaaij S, Hortobagyi T, Godde B, Kaan WA, Erhard P, Voelcker-Rehage C. Neural correlates of motor-cognitive dual-tasking in young and old adults. PLoS One 2017;12:e0189025. [DOI] [PMC free article] [PubMed] [Google Scholar]