Abstract
Objective:
Cognitive impairment is prevalent among individuals with Parkinson’s disease (PD). Effort has been made to identify individuals at risk for cognitive decline and dementia. Objectively-defined subtle cognitive decline (Obj-SCD) is a novel classification that may identify individuals at risk for cognitive decline prior to a diagnosis of mild cognitive impairment (MCI). We examined the utility of Obj-SCD criteria to predict future cognitive decline and difficulties with activities of daily living (ADLs) among individuals with PD.
Method:
The sample included 483 individuals newly diagnosed with PD. Participants were followed for a five-year span with yearly visits where they completed neuropsychological tests. Participants were categorized as cognitively normal (CN), the newly proposed Obj-SCD, PD-MCI or Parkinson’s disease dementia (PDD). Analyses determined if utilization of Obj-SCD criteria predicted subsequent cognitive impairment and difficulties with ADLs.
Results:
At baseline, 372 (77%) participants were classified as CN, 40 (8.3%) classified as Obj-SCD, and 71 (14.7%) classified as PD-MCI. Analyses revealed that relative to the CN group, participants classified as Obj-SCD at baseline, were more likely to develop PD-MCI or PDD within 5 years (Odds Ratio = 2.413; 95% confidence interval = 1.215 to 4.792). Furthermore, the Obj-SCD represented an intermediate level of impairment, relative to the CN and PD-MCI groups, on an independent measure of cognition (Montreal Cognitive Assessment) and ADL.
Conclusions:
Findings provide evidence that Obj-SCD criteria can identify individuals at risk for cognitive decline and impairments in ADL. Obj-SCD criteria may identify individuals at risk for cognitive impairment who are not detected by PD-MCI criteria.
Keywords: Parkinson’s disease, mild cognitive impairment, dementia, early detection, cognitive dysfunction, neuropsychology
INTRODUCTION
Individuals with Parkinson’s Disease (PD) frequently experience cognitive impairments [1]. Cognitive impairments can be heterogeneous but commonly include slow processing speed, working memory difficulties, visual-spatial impairments, and impairments in learning and memory [2,3]. The concepts of Parkinson’s disease, mild cognitive impairment (PD-MCI), and Parkinson’s disease dementia (PDD) are commonly used for classifying the severity of cognitive impairment. In PDD, cognitive impairments are severe and impair functioning in activities of daily living (ADL). The risk of developing PDD increases as PD progresses, and up to 80% of patients develop PDD within 15–20 years of initial diagnosis [4].
PD-MCI is a popular research construct due to the hypothesis that early identification of individuals at risk for PDD may lead to earlier interventions and better treatment outcomes [5,6]. In PD-MCI, cognitive impairments are detected with standard neuropsychological tests, but the cognitive impairments do not significantly impair daily functioning. Approximately 20%–33% of PD patients experience PD-MCI at the time of PD diagnosis [7]. Research generally supports the clinical utility of PD-MCI [6]. PD-MCI is a risk factor for future PDD, depression, reduced quality of life, and higher mortality rates [8–10]. However, a recent review of PD-MCI criteria suggested that future research should consider the concept of a pre-PD-MCI stage [11].
The hypothesis that cognitive impairments can be detected prior to a diagnosis of MCI has already been supported with research. One approach has focused on subjective cognitive complaints (SCC) reported by the patient. Indeed, studies have followed cognitively intact individuals with and without SCC and have found SCC to be predictive of future PD-MCI and PDD [12,13]. However, findings should be interpreted with caution because both studies were relatively limited in terms of the sample size of participants with PD (n < 50). Additionally, the relatively higher frequency of depression and anosognosia among individuals with PD may limit the utility of a subjective self-reported measure of cognition [14]. There is more research on SCC within the context of Alzheimer’s disease (AD). Indeed, a past meta-analysis of SCC revealed poor sensitivity and positive predictive value to detect MCI or AD [15]. Admittedly, there are significant differences between AD and PD in terms of the characterization of cognitive impairment; however, this meta-analysis raises concerns about criteria that solely rely on subjective complaints.
An alternative approach utilizing objective criteria, as opposed to subjective complaints, was proposed in the context of preclinical Alzheimer’s disease (AD). Objectively-defined subtle cognitive decline (Obj-SCD) is a phase of the disease in which individuals are cognitively unimpaired, but may have biomarker evidence of AD or show very minimal cognitive changes that are not sufficient to warrant a diagnosis of MCI or dementia [16]. Thomas and colleagues proposed that “process scores” (quantifiable measures of an individual’s approach to completing a neuropsychological task) on neuropsychological tests of memory could be used to predict progression to MCI/AD and objectively classify individuals with Obj-SCD, prior to a diagnosis of MCI [17,18]. Relative to cognitively normal (CN) controls, individuals with Obj-SCD transition to MCI/AD 2–3 times faster. Additionally, individuals with Obj-SCD displayed an intermediate amount of amyloid and tau relative to the CN and MCI groups and exhibited faster rates of amyloid accumulation and entorhinal cortex thinning relative to participants [17,19]. These findings suggest that Obj-SCD defined by alternative neuropsychological metrics (i.e. process scores) can identify individuals at risk for cognitive decline prior to a diagnosis of MCI or AD.
The utility of alternative neuropsychological metrics to predict future cognitive decline has received little attention among individuals with PD; however, the limited existing research is promising. Intra-individual variability (IIV; a measure of variability across multiple neuropsychological scores) has been shown to predict incident PD-MCI and PDD among individuals newly diagnosed with PD [20]. Additionally, IIV predicted cognitive decline independent of standard neuropsychological metrics (i.e., normative scores on tests of attention, memory, language, etc.). Similar to the AD literature, process scores on memory tests, including false positive errors and learning curves (i.e. the ability to learn more information across multiple trials), have been shown to be impaired among individuals with PD [21–23]. Furthermore, both PDD patients and non-demented PD patients demonstrated greater deficits on metrics accessing false-positive errors, relative to traditional memory metrics (e.g. short delay and long delay free recall) [21,22]. These findings suggest that process scores or alternative neuropsychological metrics may be used to objectively identify individuals at risk for cognitive decline prior to a diagnosis of PD-MCI.
The current study examined the utility of Obj-SCD criteria among individuals with PD. Specifically, there are two study aims/questions: 1) are individuals classified as Obj-SCD, relative to CN, at increased risk for developing PD-MCI or PDD, and 2) does Obj-SCD represents an intermediate stage of cognitive impairment and functional difficulties relative to individuals classified as CN or PD-MCI.
METHODS
This current study utilized data from the Parkinson’s Markers Initiative (PPMI) database (www.ppmi-info.org/data). The PPMI is a longitudinal multisite study of untreated and newly diagnosed PD patients. A secondary data analysis of 483 newly diagnosed PD patients was conducted. Data was downloaded from the repository on February 2019. Participants were followed for up to 5 years (baseline and 5 annual follow-ups). The study was approved by the institutional review board at each site and participants provided informed consent.
Neurocognitive Tests
At each visit, all participants completed a neuropsychological assessment. Tests assessed attention (Letter–Number Sequencing), processing speed (Symbol Digit Modalities Test), visuospatial functioning (Judgement of Line Orientation), verbal fluency (Animal Fluency), verbal learning and verbal delayed recall (Hopkins Verbal Learning Test-Revised; Trials 1–3 and delayed recall). Scores were normed on demographic variables and converted into z-scores.
We computed three neuropsychological alternative/process scores that were not used in the classification of PD-MCI, but were used to classify Obj-SCD. Learning slope (Trial 3 raw score minus Trial 1 raw score) and recognition false positives errors were computed from the HVLT-R. Intraindividual variability (IIV) was calculated as the standard deviation of the five neuropsychological scores (Letter-Number Sequencing, SDMT, JOLO Animal fluency, HVLT-R Learning, and Delayed Recall;[20]). Obj-SCD criteria have previously involved process scores from memory tests [17,19]. Past Obj-SCD criteria have not utilized IIV. However numerous studies have shown that IIV is an early marker of cognitive impairment for various neurological disorders (e.g., PD, HIV and Alzheimer’s disease), which is consistent with the conceptualization of Obj-SCD [10,20,24,25]. Normative z-scores for learning slope, recognition of false-positive errors, and IIV were calculated from the baseline non-PD control sample of the PPMI [26].
Participants also completed the Montreal Cognitive Assessment (MoCA). The MoCA was not used to calculate Obj-SCD, PD-MCI or PDD, but was utilized as a measure of global cognitive functioning that is independent of measures used for cognitive classifications.
Cognitive Classifications
Participants were categorized as CN, Obj-SCD, PD-MCI, or PDD. For all classifications, an “impaired” score was defined as performance >1.5 SD below the mean. PDD was defined as impaired performance at least two “standard” neuropsychological tests (Letter-Number Sequencing, SDMT, JOLO Animal fluency, HVLT-R Learning, and Delayed Recall) and subjective report of functional difficulties due to cognitive symptoms. PD-MCI was defined as impaired performance on at least two standard neuropsychological tests, but, no endorsement of functional difficulties due to cognitive symptoms. Participants were classified as Obj-SCD if they: 1) had one impaired standard test and also had one impaired process score (learning slope, recognition, false positives, and IIV); or 2) had two or more impaired process scores. Participants were classified as CN if there was no evidence of cognitive impairment on standard tests or process scores.
Activities of Daily Living & Motor Severity
Difficulties with activities of daily living were measured using the clinician-rated Schwab and England Activities of Daily Living Scale (ADL). Scores on the ADL scale range from 0% to 100%, with 100% representing complete independence and no disability.
Motor severity was assessed with the Unified Parkinson’s Disease Rating Scale- part III (UPDRS-III). The UPDRS-III is a clinician-rated measure of motor symptoms (e.g., tremor, slowness, rigidity). Higher scores indicate greater motor severity.
Statistical Analyses
For aim 1, an ordinal multilevel model (MLM) was used to examine if Obj-SCD at baseline was predictive of subsequent PD-MCI or PDD. Therefore, the model was only conducted among individuals who met criteria for Obj-SCD or CN at baseline. The dependent variable was the development of a more severe stage of cognitive impairment (PD-MCI or PDD). Independent variables included age, years of education, gender, motor severity (UPDRS-III), and baseline cognitive classification (CN or Obj-SCD).
Aim 2 examined longitudinal group differences (CN, Obj-SCD, PD-MCI) in global cognition and ADL. MoCA and Schwab and England ADL scores were entered as the dependent variable in separate analyses. Independent variables included age, years of education, gender, motor severity (UPDRS-III), and the main effect of group. Group was treated as an ordinal variable (CN<Obj-SCD<PD-MCI), which is consistent with the conceptualization of Obj-SCD as an intermediate stage of cognitive impairment that predates MCI [17,19]. Additionally, a Group X Occasion interaction term was computed. By modeling both the main effect of group and the separate Group X Occasion interaction term we will be able to detect: 1) the presence of group differences (CN<Obj-SCD<PD-MCI) in global cognition and ADL (i.e. main effect of group), and 2) if group differences become larger or smaller over time (i.e. the Group X Occasion interaction term).
RESULTS
Demographic and Clinical Characteristics
At baseline, 372 (77%) participants were classified as CN, 40 (8.3%) classified as Obj-SCD, 71 (14.7%) classified as PD-MCI, and no participants met criteria for PDD. The rates of CN, Obj-SCD, PD-MCI, and PDD at each occasion are displayed in Figure 1. The stability and conversion of Obj-SCD and PD-MCI are displayed in Supplemental Figures 1 and 2.
Fig. 1.
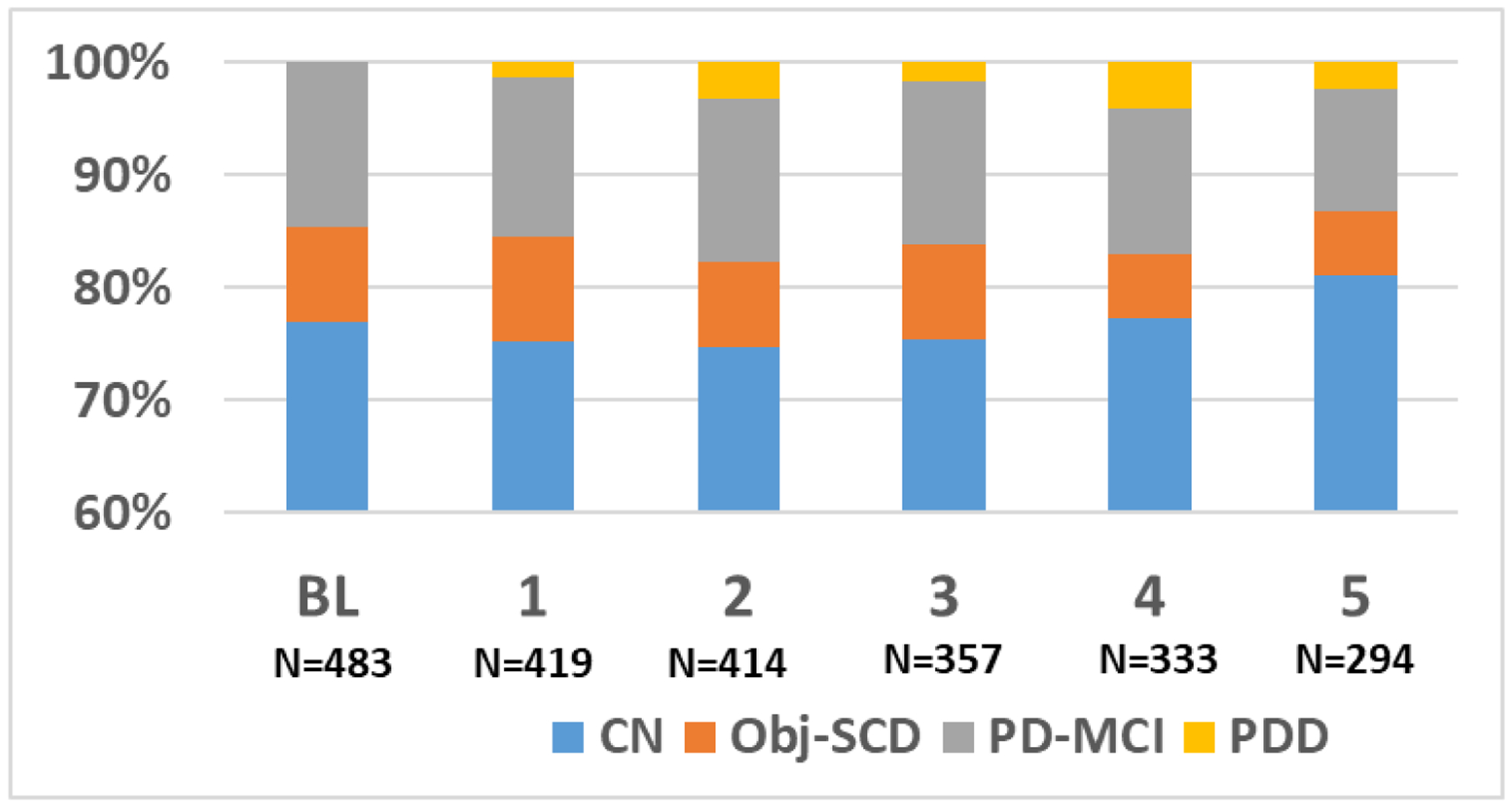
Rates of Cognitive Classifications at Each Annual Assessment. BL= Base Line; CN= Cognitively Normal; Obj-SCD= Objectively Defined Subtle Cognitive Decline; PD-MCI= Parkinson’s Disease Mild Cognitive Impairment; PDD= Parkinson’s Disease Dementia
Demographic and clinical characteristics are displayed in Table 1. At baseline, there were no significant group differences in mean age, education, or ADL (p > 0.05). The CN group had a larger representation of females relative to the other groups. The PD-MCI group demonstrated more severe motor symptoms on the UPDRS-III, relative to the CN group. The Obj-SCD group did not significantly differ from either the CN or PD-MCI group in terms of motor severity at baseline. The Obj-SCD significantly differed from both the CN and PD-MCI on all process scores. This is expected because impairment on process scores is required for classification of Obj-SCD, but is not required for CN or PD-MCI.
Table 1.
Group Baseline Characteristics
| CN (n=372) | Obj-SCD (n=40) | PD-MCI (n=71) | F/KW | p | |
|---|---|---|---|---|---|
| Mean Age (SD) | 61.0 (10) | 60.1 (10) | 61.8 (8) | 0.19 | 0.824 |
| Mean Education (SD) | 15.7 (3) | 15.3 (3) | 14.9 (4) | 2.28 | 0.104 |
| % Male | 62% | 78% | 79% | 10.69a,b | 0.005 |
| % Caucasian | 96% | 98% | 90% | 5.71 | 0.058 |
| Mean UPDRS Motor (SD) | 19.5 (9) | 19.5 (10) | 23.0 (9) | 4.50a | 0.012 |
| Mean ADL (SD) | 93.4 (6) | 94.8 (5) | 92.6 (6) | 1.69 | 0.185 |
| Mean MOCA (SD) | 27.5 (2) | 26.4 (2) | 25.9 (3) | 17.28a,b | <0.001 |
| Mean HVLT Immediate Recall (SD) | 0.06 (.78) | −0.43 (1) | −1.1 (.77) | 70.22a,b,c | <0.001 |
| Mean HVLT Delay Recall (SD) | −0.02 (.71) | −0.2 (1.5) | −1.1(1) | 43.42a,c | <0.001 |
| Mean JOLO (SD) | 0.16 (.86) | 0.28 (.97) | −0.3 (1) | 7.54a,c | .001 |
| Mean LNS (SD) | 0.10 (.79) | 0.17 (.96) | −0.72 (.97) | 29.96a,c | <0.001 |
| Mean Animal Fluency (SD) | −0.04 (.85) | −0.05 (.96) | −0.73 (.94) | 19.12a,c | <0.001 |
| Mean SDMT (SD) | −0.04 (.77) | −0.34 (.85) | −0.79 (.97) | 26.57a,b,c | <0.001 |
| Mean IIV (SD) | .87 (.27) | 1.39 (.31) | 1.25 (.41) | 92.69a,b,c | <0.001 |
| Mean HVLT Learning Slope (SD) | 3.5 (1.4) | 2.2 (2) | 3.2 (1) | 15.20b,c | <0.001 |
| Mean HVLT FP (SD) | 1.2 (2) | 3.6 (4.6) | 2.9 (3) | 29.47a,b | <0.001 |
Standard deviations are listed in parentheses. Normative z-scores are presented for cognitive tests. KW = Kruskal Wallis H-Test; CI = Cognitively Normal; Obj-SCD = Objectively Defined Subtle Cognitive Decline; PD-MCI= Parkinson’s Disease Mild Cognitive Impairment; UPDRS Motor= Unified Parkinson’s Disease Rating Scale- part III; HVLT = Hopkins Verbal Learning Test; JOLO = Judgement of Line Orientation; SDMT = Symbol Digit Modalities Test; MOCA = Montreal Cognitive Assessment; ADL = Schwab & England Activities of Daily Living Scale; IIV = intra-individual variability; FP = False Positives;
CN group significantly different from PD-MCI;
CN group significantly different from Obj-SCD;
Obj-SCD group significantly different from PD-MCI
Obj-SCD and Risk of Future PD-MCI and PDD
Aim 1 examined if individuals classified as Obj-SCD at baseline were at greater risk for developing PD-MCI or PDD, relative to CN individuals (Table 2). Relative to the CN group, individuals classified as Obj-SCD at baseline were at greater risk for developing PD-MCI or PDD within 5 years (Figure 2). The risk of developing PD-MCI or PDD was also associated with older age, and occasion (i.e., rates of PD-MCI and PDD increased as the study progressed).
Table 2.
Baseline Obj-SCD Predicts Future PD-MCI and PDD
| Odds Ratio | 95% Cl | P | |
|---|---|---|---|
| Gender | 1.098 | 0.870 to 1.386 | 0.432 |
| Age | 1.315 | 1.034 to 1.672 | 0.025 |
| Education | 0.801 | 0.602 to 1.065 | 0.126 |
| Occasion | 1.691 | 1.387 to 2.063 | <0.001 |
| UPDRS | 1.215 | 0.938 to 1.574 | 0.140 |
| Obj-SCD | 2.413 | 1.215 to 4.792 | 0.012 |
Significant p values depicted in bold. UPDRS = Unified Parkinson’s Disease Rating Scale; Obj-SCD = Objectively-defined Subtle Cognitive Decline; Gender was coded as: 0 = male, 1 = female
Fig. 2.
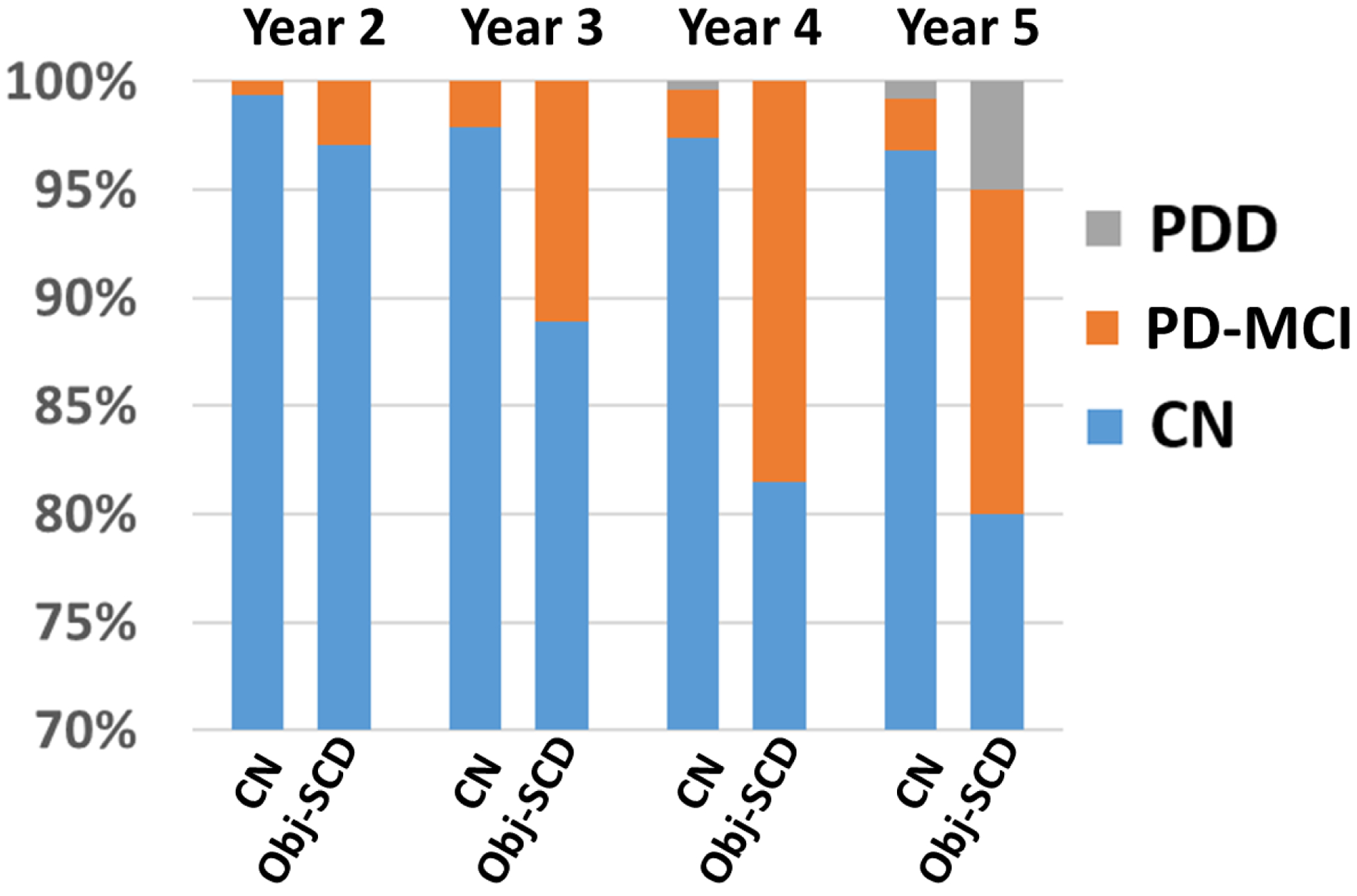
Baseline Obj-SCD and Risk of Future PD-MCI and PDD. CN= Cognitively Normal; Obj-SCD= Objectively Defined Subtle Cognitive Decline; PD-MCI= Parkinson’s Disease Mild Cognitive Impairment; PDD = Parkinson’s Disease Dementia
Cognitive Classifications, Cognitive Decline, and Functional Decline
Aim 2 examined the relationship between cognitive classification (CN, Obj-SCD, PD-MCI) and performance on an independent measure of global cognitive functioning (MoCA; Table 3). Findings revealed a significant Group X Occasion interaction effect, meaning that a diagnosis of a more severe stage of cognitive impairment (CN<Obj-SCD<MCI) was associated with a more rapid decline in cognition/MoCA scores (Figure 3). Worse global cognitive functioning was also associated with the main effect of group, less education, older age, male gender and more severe motor symptoms (all p values ≤ 0.001).
Table 3.
MLM: Cognitive Classifications Predicts Global Cognition
| Global Cognition | ||
|---|---|---|
| B | p | |
| Gender | 0.19 | 0.001 |
| Age | −0.27 | <0.001 |
| Education | 0.15 | <0.001 |
| Occasion | 0.02 | 0.276 |
| UPDRS | −0.11 | <0.001 |
| Group | −0.25 | <0.001 |
| Occasion X Group | −0.11 | <0.001 |
| Model Fit Indices | ||
| *Δ −2LL | 391 | <0.001 |
| *Δ AIC | 369 | <0.001 |
| *Δ BIC | 307 | <0.001 |
| Between-Person Pseudo r2 | 0.559 | |
| Within-Person Pseudo r2 | 0.215 | |
Significant p values depicted in bold. Group represented by ordinal rank (Cognitively Normal < Objectively Defined Subtle Cognitive Decline < Mild Cognitive Impairment); B = standardized beta weights; UPDRS = Unified Parkinson’s Disease Rating Scale; LL = Log Likelihood; AIC = Akaike Information Criterion; BIC = Bayesian Information Criterion. Gender was coded as: 0 = male, 1 = female. [Change in model indices relative to a null model with no predictors
Fig. 3.
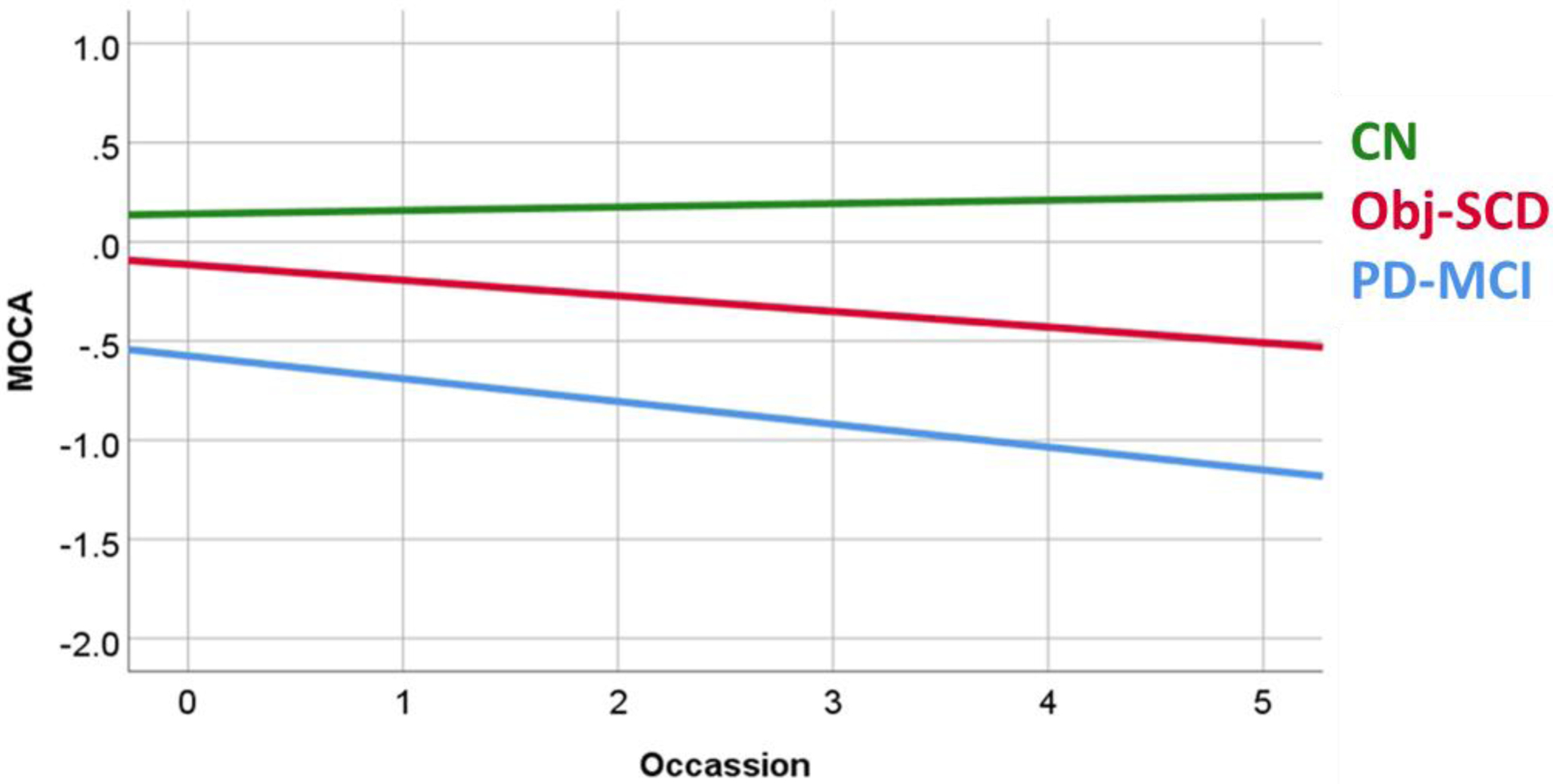
Group Differences in MoCA Scores. MoCA scores represented in a z-score metric. MoCA= Montreal Cognitive Assessment; CN= Cognitively Normal; Obj-SCD= Objectively Defined Subtle Cognitive Decline; PD-MCI= Parkinson’s Disease Mild Cognitive Impairment
An additional analysis examined the relationship between cognitive classification and difficulties with ADL. There was a significant Group X Occasion interaction effect (Table 4). A more severe stage of cognitive impairment was associated with more rapid declines in ADL (Figure 4). Greater difficulties with ADL was also associated with the main effects of group (CN<Obj-SCD<MCI), occasion (difficulties with ADL worsened over time), and more severe motor symptoms (all p values ≤ 0.003).
Table 4.
MLM: Cognitive Classifications Predict ADL
| ADL | ||
|---|---|---|
| B | p | |
| Gender | 0.05 | 0.292 |
| Age | 0.002 | 0.935 |
| Education | 0.01 | 0.631 |
| Occasion | −0.16 | <0.001 |
| UPDRS | −0.33 | <0.001 |
| Group | −0.07 | 0.002 |
| Occasion X Group | −0.06 | 0.003 |
| Model Fit Indices | ||
| *Δ −2LL | 959 | <0.001 |
| *Δ AIC | 937 | <0.001 |
| *Δ BIC | 874 | <0.001 |
| Between-Person Pseudo r2 | 0.499 | |
| Within-Person Pseudo r2 | 0.452 | |
Significant p values depicted in bold. Group represented by ordinal rank (Cognitively Normal < Objectively Defined Subtle Cognitive Decline < Mild Cognitive Impairment); B = standardized beta weights; UPDRS = Unified Parkinson’s Disease Rating Scale; LL = Log Likelihood; AIC = Akaike Information Criterion; BIC = Bayesian Information Criterion. Gender was coded as: 0 = male, 1 = female. [Change in model indices relative to a null model with no predictors
Fig. 4.
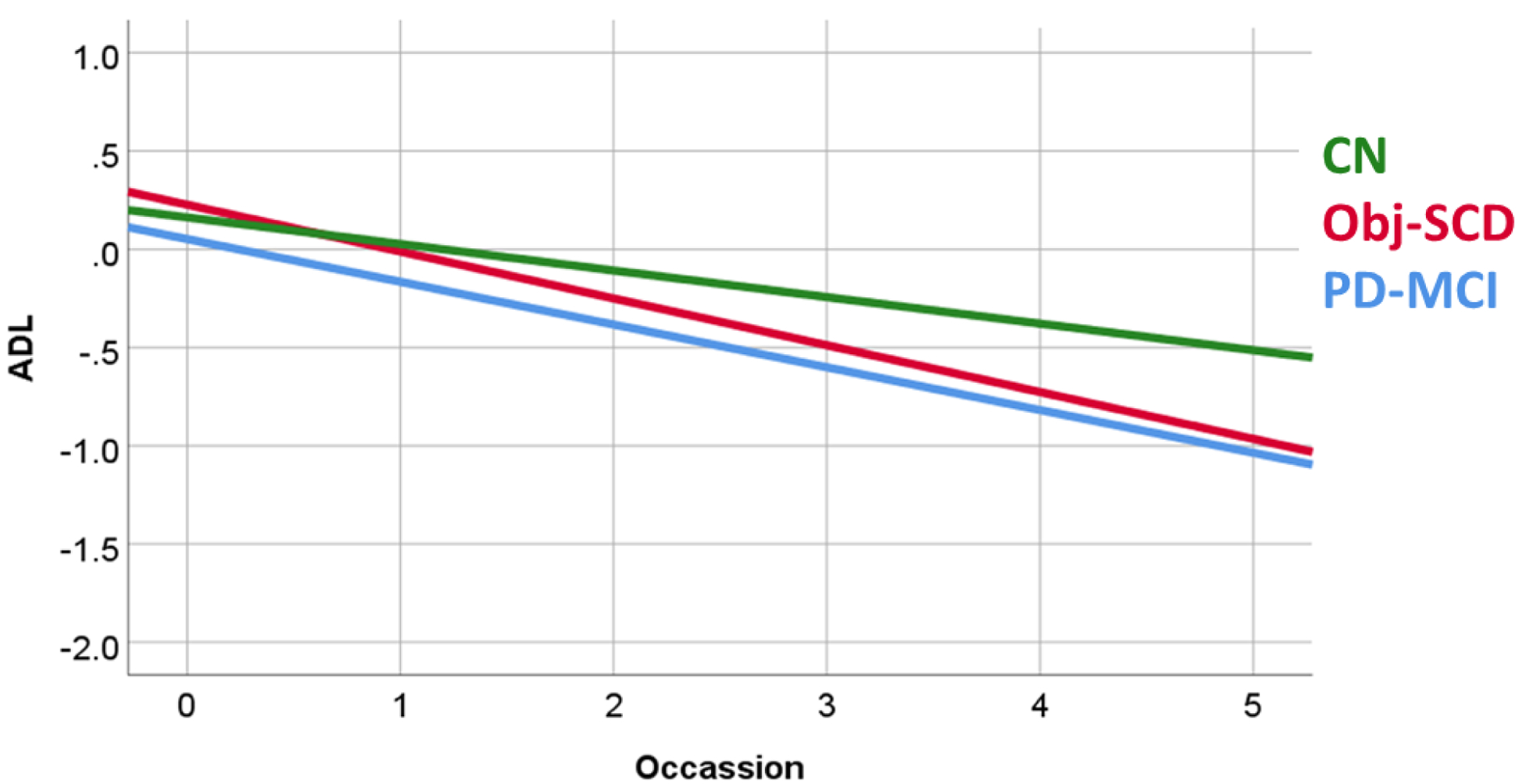
Group Differences in ADL Functioning. ADL scores represented in a z-score metric. ADL= Activities of Daily Living; CN= Cognitively Normal; Obj-SCD= Objectively Defined Subtle Cognitive Decline; PD-MCI= Parkinson’s Disease Mild Cognitive Impairment
Exploratory Aim: Subjective Cognitive Complaint (SCC) and Future Cognitive Decline
An exploratory aim examined if SCC was associated increased risk for future PD-MCI or PDD among CN participants. This aim was viewed as exploratory due to the fact that a standardized questionnaire accessing SCC was not administered as part of the PPMI study. Therefore, this exploratory analysis relied on a single self-reported item to assess SCC (see Supplemental Table 1 for a description of SCC classification and analyses). Analyses were similar to Aim 1, with the exception that SCC replaced Obj-SCD. Results revealed that SCC at baseline was not associated with increased risk for future PD-MCI or PDD (Supplemental Table 2).
DISCUSSION
The current study provides support for the utilization of Obj-SCD to detect cognitive decline in PD. Alternative neuropsychological metrics can provide objective criteria to identify individuals that are not detected by traditional PD-MCI criteria, but are at risk for future cognitive decline. Specifically, findings revealed that, relative to the CN group, Obj-SCD was a risk factor for transition to a more severe stage of cognitive impairment (PD-MCI or PDD). Additionally, Obj-SCD represented an intermediate level of cognitive and functional impairment relative to CN and PD-MCI groups.
This is the first study to examine Obj-SCD in the PD population. However, our findings are consistent with research among individuals with AD. Thomas and colleagues showed that in terms of progression to MCI, the Obj-SCD group progressed significantly faster than the cognitively intact participants [17]. Additionally, the Obj-SCD group represented an intermediate group (CN<Obj-SCD<MCI) in terms of amyloid and tau accumulation and was predictive of faster amyloid accumulation and entorhinal cortex thinning over time [19].
A unique aspect of the current study, and the previous studies by Thomas et al. [17,19], is the utilization of neuropsychological process scores to classify Obj-SCD. Past studies have taken alternative approaches to identify Obj-SCD. Zanchi et al. [27] investigated early structural brain changes among older adults who were cognitively intact but demonstrated evidence of a declining trajectory of cognitive functioning; relative to cognitively intact individuals with a stable trajectory of cognitive functioning. Specifically, they classified individuals as Obj-SCD if there was evidence of a 0.5 SD decline in cognitive functioning (between baseline and the following year) and the participant continued to perform in the intact range (e.g., a participant decline from 1 SD above the mean to 0.5 SD above the mean). Findings revealed greater hippocampal and amygdala atrophy among the Obj-SCD group relative to the stable cognitively intact group. Similar to our study, Zanchi et al. [27] provides evidence that individuals at risk for neurologic compromise can be identified prior to a diagnosis of MCI. However, the Zanchi et al.[27] study required multiple neuropsychological evaluations to detect Obj-SCD (i.e., a baseline and a follow-up assessment). A unique aspect of our proposed Obj-SCD classification utilizing neuropsychological process scores is that Obj-SCD can be classified after a single assessment, and therefore may be considered when multiple assessments are not available.
An alternative approach to identifying individuals at risk for cognitive decline prior to a diagnosis of PD-MCI has focused on the patient’s subjective complaints. Among cognitively intact individuals with PD, those with SCC are 8.4 times more likely to develop PD-MCI within 2 years relative to PD patients without SCC [13]. Another study showed that PD participants with SCC were more likely to develop PDD when compared to PD participants without SCC, but were less likely to develop PDD relative to PD-MCI participants [12]. However, findings should be interpreted with caution for two reasons. First, both studies were relatively limited in terms of the sample size of participants with PD (n < 50). Second, SCC may have a stronger relationship with depression than cognitive test performance [16]. The potential confound of depression raises concerns for the utility of SCC to predict cognitive impairment in PD. Not only are there higher rates of depression in PD relative to non-PD controls, but depression is an independent risk factor for future PD-MCI [28]. In addition to depression, anosognosia, which is reported in 16%−36% of PD participants with cognitive impairments, may also limit the utility of SCC to identify individuals with cognitive impairments [14]. In the current study, a single item assessing SCC did not significantly predict future PD-MCI or PDD. A lack of a relationship between SCC and future cognitive impairment highlights the importance of developing objective criteria for identifying a “pre-MCI stage.”
Limitations to the current study include the sample consisting entirely of newly diagnosed PD patients. Although this provides a unique opportunity to examine markers of early cognitive decline, findings may not generalize to the entire PD population. Futures studies are needed to investigate the utility of Obj-SCD in more advanced stages of PD when cognitive impairment is more common (e.g. 10 years after diagnosis). The current study was a secondary data analysis and restricted in terms of the number of neuropsychological tests administered. The current study was unable to classify PD-MCI with the recommended two tests per cognitive domains [6]. The current study utilized tests that are sometimes subsumed under the umbrella term “executive functioning” (i.e., working memory, verbal fluency), but future studies may benefit from additional measures of executive functioning. Obj-SCD criteria were determined based on the performance of three scores (IIV, learning slope, and recognition of false positives). Although the number of scores used to determine Obj-SCD is consistent with past studies [17,19], future studies utilizing a greater number of scores to determine Obj-SCD will likely result in deferring rates of Obj-SCD. Additionally, the study’s measure of ADL functioning relied on a clinician-rated measure, which may be heavily influenced by motor symptoms. We attempted to minimize the potential confound of motor symptoms by controlling for UPDRS-III scores in all analyses. However, future studies may benefit from an objective performance based functional measures of cognitively demanding tasks. Similarly, SCC classification relied on a single self/informant report item. Future studies may benefit by utilizing standard questionnaires assessing SCC and assessing if a hybrid classification of both process scores and subjective complaints adds value in identifying future cognitive decline. Future studies may additionally benefit by investigating the effect of medications and depression on Obj-SCD; including if medications or depression alter the rate that patients transition from Obj-SCD to PD-MCI or PDD.
Results from the current study provide initial evidence that Obj-SCD criteria utilizing alternative neuropsychological metrics may identify individuals at risk for cognitive decline. Furthermore, objective criteria may have advantages over criteria relying on subjective complaints. This is the first study to extend the Obj-SCD criteria to a new population beyond AD. This suggests these criteria may provide a flexible framework for identifying those at risk for future cognitive and functional decline, despite inclusion of different populations and process scores. Future studies are needed to support the clinical utilization of Obj-SCD and the conceptualization of Obj-SCD as a “pre-PD-MCI” stage and. Although Obj-SCD may identify at-risk individuals who are not detected by traditional PD-MCI criteria, future validation studies are needed to 1) examine if Obj-SCD represents a “pre-PD-MCI” stage in terms of biomarkers of cognitive impairment, and 2) examine if the utilization of Obj-SCD criteria leads to positive treatment/clinical outcomes. Ultimately, the clinical implications are that PD patients with no apparent cognitive impairment, but showing subtle cognitive decline on neuropsychological tests may be identified and potentially treated prior to the development of frank cognitive impairment.
Supplementary Material
Acknowledgements
Joseph Bunch was supported by NIH T34GM083883
Kelsey Thomas was support by the U.S. Department of Veterans Affairs Clinical Sciences Research and Development Service (Career Development Award-2 1IK2CX001865) and the Alzheimer’s Association (AARF-17-528918)
Data used in the preparation of this article were obtained from the Parkinson’s Progression Markers Initiative (PPMI) database (www.ppmi-info.org/data). For up-to-date information on the study, visit www.ppmi-info.org.
PPMI - a public-private partnership - is funded by the Michael J. Fox Foundation for Parkinson’s Research and funding partners, including AbbVie, Avid Radiopharmaceuticals, Biogen Idec, BioLegend, Bristol-Meyers Squibb, GE Healthcare, Genentech, GlaxoSmithKline, Eli Lilly and Company, Lundbeck, Merck, Meso Scale Discovery, Pfizer Inc., Piramal Imaging, Roche group, Sanofi-Genzyme, Servier, Takeda, TEVA, and UCB.
We would like to thank the CSUSB Institute for Child Development and Family Relations, and the Faculty Center for Excellence for supporting the publication of this paper with funded writing time.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflicts of interest/Competing interests: none
Ethics approval: The study was approved by the institutional review board at each site.
Consent to participate: Participants provided informed consent prior to all study activities.
Availability of data and material: https://www.ppmi-info.org/
References
- [1].Papagno C, Trojano L (2018) Cognitive and behavioral disorders in Parkinson’s disease: an update. I: cognitive impairments. Neurological Sciences 39(2):215–223. 10.1007/s10072-017-3154-8 [DOI] [PubMed] [Google Scholar]
- [2].Aarsland D, Bronnick K, Williams-Gray C, Weintraub D, Marder K, Kulisevsky J, Santangelo G (2010) Mild cognitive impairment in Parkinson disease: a multicenter pooled analysis. Neurology 75(12):1062–1069. 10.1212/WNL.0b013e3181f39d0e [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wen MC, Chan LL, Tan LC, & Tan EK (2017) Mild cognitive impairment in Parkinson’s disease: a distinct clinical entity?. Translational neurodegeneration 6(1): 24 10.1186/s40035-017-0094-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hely MA, Reid WG, Adena MA, Halliday GM, & Morris JG (2008) The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Movement disorders 23(6): 837–844. 10.1002/mds.21956 [DOI] [PubMed] [Google Scholar]
- [5].Fernandez HH, Crucian GP, Okun MS, Price CC, & Bowers D (2005) Mild cognitive impairment in Parkinson’s disease: The challenge and the promise. Neuropsychiatric Disease and Treatment 1(1): 37–50. 10.2147/nedt.1.1.37.52295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Litvan I, Aarsland D, Adler CH, Goldman JG, Kulisevsky J, Mollenhauer B, & Weintraub D (2011) MDS Task Force on mild cognitive impairment in Parkinson’s disease: critical review of PD-MCI. Movement disorders 26(10): 1814–1824. 10.1002/mds.23823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Roheger M, Kalbe E, & Liepelt-Scarfone I (2018) Progression of cognitive decline in Parkinson’s disease. Journal of Parkinson’s disease 8(2): 183–193. 10.3233/JPD-181306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bäckström D, Granåsen G, Domellöf ME, Linder J, Mo SJ, Riklund K, & Forsgren L (2018) Early predictors of mortality in parkinsonism and Parkinson disease: A population-based study. Neurology 91(22): e2045–e2056. 10.1212/WNL.0000000000006576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Jones JD, Mangal P, Lafo J, Okun MS, & Bowers D (2016) Mood differences among Parkinson’s disease patients with mild cognitive impairment. The Journal of neuropsychiatry and clinical neurosciences 28(3): 211–216. https://doi/org/10.1016/j.parkreldis.2017.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jones JD, Kuhn TP, & Szymkowicz SM (2018) Reverters from PD-MCI to cognitively intact are at risk for future cognitive impairment: Analysis of the PPMI cohort. Parkinsonism & related disorders 47: 3–7. 10.1016/j.parkreldis.2017.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Goldman JG, Holden SK, Litvan I, McKeith I, Stebbins GT, & Taylor JP (2018) Evolution of diagnostic criteria and assessments for Parkinson’s disease mild cognitive impairment. Movement Disorders 33(4): 503–510. 10.1002/mds.27323 [DOI] [PubMed] [Google Scholar]
- [12].Galtier I, Nieto A, Lorenzo JN, & Barroso J (2019) Subjective cognitive decline and progression to dementia in Parkinson’s disease: a long-term follow-up study. Journal of neurology 266(3): 745–754. 10.1007/s00415-019-09197-0 [DOI] [PubMed] [Google Scholar]
- [13].Hong JY, Sunwoo MK, Chung SJ, Ham JH, Lee JE, Sohn YH & Lee PH (2014) Subjective cognitive decline predicts future deterioration in cognitively normal patients with Parkinson’s disease. Neurobiology of aging 35(7): 1739–1743. 10.1016/j.neurobiolaging.2013.11.017 [DOI] [PubMed] [Google Scholar]
- [14].Orfei MD, Assogna F, Pellicano C, Pontieri FE, Caltagirone C, Pierantozzi M, & Spalletta G (2018) Anosognosia for cognitive and behavioral symptoms in Parkinson’s disease with mild dementia and mild cognitive impairment: Frequency and neuropsychological/neuropsychiatric correlates. Parkinsonism & related disorders 54: 62–67. 10.1016/j.parkreldis.2018.04.015 [DOI] [PubMed] [Google Scholar]
- [15].Mitchell AJ (2008) The clinical significance of subjective memory complaints in the diagnosis of mild cognitive impairment and dementia: a meta-analysis. International Journal of Geriatric Psychiatry: A journal of the psychiatry of late life and allied sciences 23(11): 1191–1202. 10.1002/gps.2053 [DOI] [PubMed] [Google Scholar]
- [16].Edmonds EC, Delano-Wood L, Galasko DR, Salmon DP, & Bondi MW (2015) Subtle cognitive decline and biomarker staging in preclinical Alzheimer’s disease. Journal of Alzheimer’s disease 47(1): 231–242. 10.3233/JAD-150128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Thomas KR, Edmonds EC, Eppig J, Salmon DP, Bondi MW, & Alzheimer’s Disease Neuroimaging Initiative (2018) Using neuropsychological process scores to identify subtle cognitive decline and predict progression to mild cognitive impairment. Journal of Alzheimer’s Disease 64(1): 195–204. 10.3233/JAD-180229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Thomas KR, Eppig J, Edmonds EC, Jacobs DM, Libon DJ, Au R, & Bondi MW (2018) Word-list intrusion errors predict progression to mild cognitive impairment. Neuropsychology 32(2): 235 10.1037/neu0000413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Thomas KR, Bangen KJ, Weigand AJ, Edmonds EC, Wong CG, Cooper S, & Alzheimer’s Disease Neuroimaging Initiative (2020) Objective subtle cognitive difficulties predict future amyloid accumulation and neurodegeneration. Neurology 94(4): e397–e406. 10.1212/WNL.0000000000008838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jones JD, Burroughs M, Apodaca M, & Bunch J (2019) Greater intraindividual variability in neuropsychological performance predicts cognitive impairment in de novo Parkinson’s disease. Neuropsychology. https://doi/org/10.1037/neu0000577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Brønnick K, Alves G, Aarsland D, Tysnes O, & Larsen J (2011) Verbal Memory in Drug-Naive, Newly Diagnosed Parkinson’s Disease. The Retrieval Deficit Hypothesis Revisited. Neuropsychology 25(1): 114–124. 10.1037/a0020857 [DOI] [PubMed] [Google Scholar]
- [22].Higginson C, Wheelock V, Carroll K, & Sigvardt K (2005) Recognition Memory in Parkinson’s Disease With and Without Dementia: Evidence Inconsistent with the Retrieval Deficit Hypothesis. Journal of Clinical and Experimental Neuropsychology 27(4): 516–528. 10.1080/13803390490515469 [DOI] [PubMed] [Google Scholar]
- [23].Weintraub D, Doshi J, Koka D, Davatzikos C, Siderowf AD, Duda JE & Clark CM (2011) Neurodegeneration across stages of cognitive decline in Parkinson disease. Archives of neurology 68(12): 1562–1568. 10.1001/archneurol.2011.725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Anderson AE, Jones JD, Thaler NS, Kuhn TP, Singer EJ, & Hinkin CH (2018) Intraindividual variability in neuropsychological performance predicts cognitive decline and death in HIV. Neuropsychology 32(8): 966 10.1037/neu0000482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bangen KJ, Weigand AJ, Thomas KR, Delano-Wood L, Clark LR, Eppig J, & Bondi MW (2019) Cognitive dispersion is a sensitive marker for early neurodegenerative changes and functional decline in nondemented older adults. Neuropsychology 33(5): 599 https://doi.org/0.1037/neu0000532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wyman-Chick KA, Martin PK, Weintraub D, Sperling SA, Erickson LO, Manning CA, & Barrett MJ (2018) Selection of Normative Group Affects Rates of Mild Cognitive Impairment in Parkinson’s Disease. Movement Disorders 33(5): 839–843. 10.1002/mds.27335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zanchi D, Giannakopoulos P, Borgwardt S, Rodriguez C, & Haller S (2017) Hippocampal and amygdala gray matter loss in elderly controls with subtle cognitive decline. Frontiers in aging neuroscience 9: 50 10.3389/fnagi.2017.00050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Jones JD, Kurniadi NE, Kuhn TP, Szymkowicz SM, Bunch J, & Rahmani E (2019) Depressive symptoms precede cognitive impairment in de novo Parkinson’s disease patients: Analysis of the PPMI cohort. Neuropsychology. 10.1037/neu0000583 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


