Abstract
Background and Aims:
Although highly effective, 10 to 20% of achalasia patients treated with laparoscopic Heller myotomy (LHM) or per-oral endoscopic myotomy (POEM) remain symptomatic. In evaluating such patients, we have observed a pattern of failure associated with a pseudodiverticulum, or blown out myotomy (BOM), in the distal esophagus. We aimed to assess risk factors and patient reported outcomes associated with a BOM.
Methods:
We reviewed our manometry database for achalasia patients previously treated with LHM or POEM. Included patients had a post-treatment esophagram within 1 year of their follow-up manometry. A BOM was defined radiographically as a wide-mouthed outpouching (>50% increase in esophageal diameter) in the area of the myotomy.
Results:
129 treated achalasia patients were included of whom 23 (17.8%) had a BOM. Comparing patients with a BOM to those without, post-treatment Eckardt scores were significantly greater (5 vs. 2, p=0.002), type III achalasia was more common (39.1% vs. 14.2%, p=0.005), and LHM was more common than POEM (73.9% vs. 26.1%, p=0.013), respectively. The integrated relaxation pressure (IRP) was also significantly greater in the BOM group (15.0 mmHg vs. 11.0 mmHg, p=0.025).
Conclusions:
BOM is a common complication after myotomy for achalasia not seen after pneumatic dilation. Pretreatment type III achalasia, LHM as opposed to POEM, and a greater post-treatment IRP were risk factors for developing a BOM. We speculate that esophageal wall strain in the area weakened by myotomy, whether from residual spastic contractility or continued esophageal outflow obstruction, may be the underlying mechanism of BOM development.
Keywords: achalasia, myotomy, esophageal motor disorder, manometry
Introduction
Achalasia is a major motor disorder characterized by incomplete lower esophageal sphincter (LES) relaxation and absent peristalsis. Although the exact etiology is unclear, the disease is primarily related to a loss or alteration of normal enteric neuronal function as an autoimmune process.1 Unfortunately, no therapy reverses the ganglionitis and all current definitive therapies instead focus on disrupting the LES via dilation or myotomy to improve emptying dynamics through the esophagogastric junction (EGJ).
The current treatment modalities for disrupting the LES are extremely effective, however, failure of treatment or recurrence of symptoms can occur in 10 to 20% of patients undergoing myotomy and 10 to 30% of patients undergoing pneumatic dilation.2–4 The presumed mechanisms of failure are incomplete myotomy or ineffective disruption of the LES.5, 6 However, we have encountered many patients with significant symptoms and bolus retention despite a complete myotomy as assessed by high-resolution manometry (HRM) and functional lumen imaging probe (FLIP).7–9 This presentation underscores the essential role the esophageal body in promoting normal emptying. Severely impaired or uncoordinated contractile patterns, extreme esophageal dilatation, diverticulum formation, sink-trapping, and severe tortuosity are some of the esophageal body abnormalities that can lead to persistent esophageal retention despite a complete myotomy.8
Another anatomical abnormality that presents after myotomy is a pseudodiverticulum at the myotomy site, or a blown out myotomy (BOM) evident as a focal increase in luminal diameter in the distal esophagus on barium esophagram. We and others have observed this pattern in a number of achalasia patients with recurrent symptoms after myotomy.8, 10, 11 These patients typically have an adequate myotomy defined on HRM or do not respond to further LES-targeted therapy making them a very difficult patient population. The aims of the current study were to assess the prevalence of BOM in achalasia patients presenting to our institution, determine the impact of a BOM on treatment outcome and describe the factors associated with the development of a BOM.
Methods
Subjects
Patients were retrospectively identified from the Northwestern Esophageal Center Achalasia Natural History Study. Patients included in the study were 18 to 85 years old and previously treated for achalasia with either per oral endoscopic myotomy (POEM), laparoscopic Heller myotomy (LHM), or pneumatic dilation at Northwestern Memorial Hospital or at an outside institution. As part of our Achalasia Natural History Study, patients undergo routine testing at one year post-treatment and every three years after that unless symptoms develop sooner. Unfortunately, some patients fail to follow-up and as such, BOM patients in this series represent a collection of patients as opposed to a true consecutive case series. Patients included in this study underwent a standardized HRM protocol between January 2015 and June 2017 at Northwestern Memorial Hospital and had an esophagram within one year of the HRM without any intervening achalasia treatment. Twenty four patients were evaluated after pneumatic dilation and none of them had a BOM. Hence, the study focused on patients who underwent POEM or LHM. Demographic information (age, sex, BMI), initial achalasia subtype, treatment-specific information (procedure type, myotomy length, location, prior treatment, post-treatment HRM, follow-up timing) and outcome data (Eckardt score (ES), timed barium esophagram (TBE) column height) were collected for patients treated with POEM or LHM when available. The study protocol was approved by the Northwestern University Institutional Review Board. A waiver of informed consent was obtained for this retrospective analysis.
High-resolution manometry
After a minimum 6-hour fast, HRM studies were completed using a 4.2-mm outer diameter solid-state assembly with 36 circumferential pressure sensors at 1-cm intervals (Medtronic Inc, Shoreview, MN). The HRM assembly was placed transnasally and positioned to record from the hypopharynx to the stomach with approximately three intragastric pressure sensors. Residual motility, median supine integrated relaxation pressure (IRP), and mean distal contractile integral (DCI) were recorded for each patient. Patients were identified as having residual motility if their post-myotomy DCI was >100 mmHg•s•cm. Initial achalasia subtype was determined for each patient based on review of the preoperative HRM when available. Patients were categorized as pretreatment achalasia subtype “unknown” when their preoperative manometry was not available for review.
Esophagram: BOM definition
Patient esophagrams were reviewed to assess for a BOM, defined as a distal wide-mouthed (>2 cm) diverticulum in the area of the prior myotomy with a greater than 50% increase in esophageal diameter (Figure 1). When available, timed barium esophagram column heights were recorded as previously described12.
Figure 1.
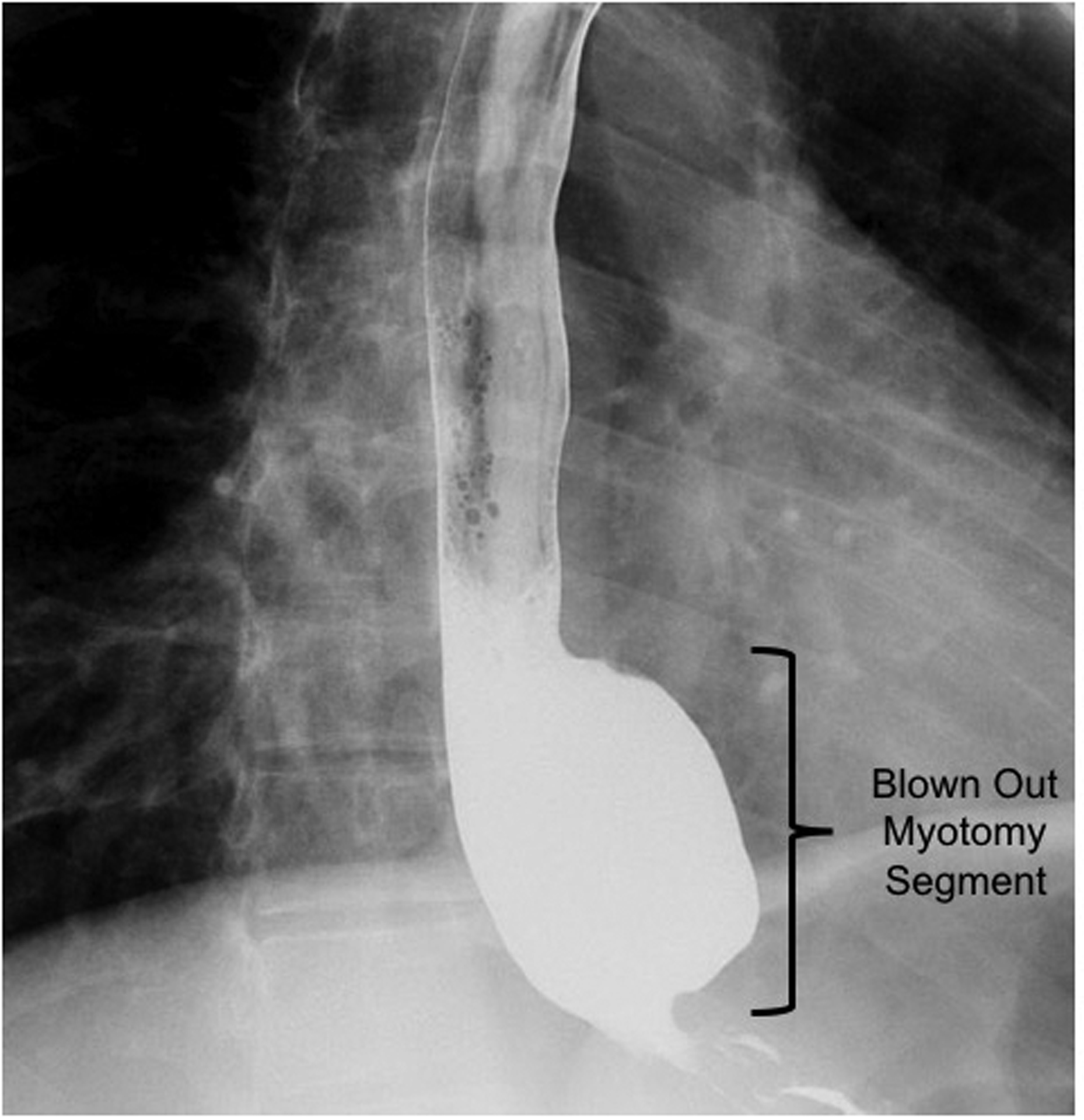
Blown out myotomy (BOM): wide-mouthed distal esophageal pseudodiverticulum in the area of prior myotomy.
Clinical Outcome
The ES is 4-item self-report scale measuring dysphagia, regurgitation, chest pain and weight loss.13 Each item is scored from 0 to 3 and summed yielding a maximum value of 12. Patient scores were collected at the time of post-treatment HRM. The PROMIS Global-10 questionnaire is a 10-item instrument representing degradations in multiple domains of health-related quality of life: overall physical health, mental health, social health, pain, fatigue, and overall perceived quality of life. The scale yields a Global Physical Health (GPH) score and a Global Mental Health (GMH) score.14
Statistical analysis
Data were analyzed using SPSS v25 (IBM, Armonk, NY). Comparisons using Pearson Chi-square analysis, Fisher’s Exact Test, t tests, or Mann-Whitney U tests were made among the groups depending on the data type and distribution. Analyses tested for a 0.05 level of statistical significance.
Results
Study Subjects
One-hundred and thirty-nine post-treatment achalasia patients were evaluated with HRM between January 2015 and July 2017 (POEM or LHM). One-hundred and twenty-nine (92.8%) were also evaluated with an esophagram and were included in this study. Of those identified, 23 (17.8%) were found to have a BOM on esophagram. There was no statistically significant difference in age, sex or BMI between patients with a BOM and those without (Table 1). Pre-treatment achalasia subtypes were different between the two groups (p=0.004) (Table 1). Type II achalasia was more common in patients without a BOM (42.5% vs. 17.4%, p=0.025) whereas, type III achalasia was more common in patients with a BOM (39.1% vs. 14.2%, p=0.005). There was no statistical difference in type I achalasia or achalasia of unknown subtype between patients with and without a BOM.
Table 1.
Patient demographics and pre-treatment achalasia subtypes. Values are median (interquartile range) or % (N) as indicated.
| Total N=129 | No BOM N=106 | BOM N=23 | p-value | |
|---|---|---|---|---|
| Age (yrs) | 51.0 (37.0 – 64.0) | 51.0 (37.5 – 64.0) | 49.0 (35.0 – 66.0) | 0.676 |
| Sex (Female) | 55.8% (72) | 54.7% (58) | 60.9% (14) | 0.59 |
| BMI (kg/m2) | 26.5 (22.9 – 30.7) | 26.7 (23.3 – 30.9) | 25.2 (22.9 – 30.5) | 0.47 |
| Achalasia Subtype | ||||
| Type 1 | 24.8% (32) | 27.4% (29) | 13.0% (3) | 0.004 |
| Type 2 | 38.0% (49) | 42.5% (45) | 17.4% (4) | |
| Type 3 | 18.6% (24) | 14.2% (15) | 39.1% (9) | |
Outcomes
The time to follow-up post-treatment was significantly longer for patients with a BOM than those without, median [IQR], 52.1 [18.4 – 134.2] months vs. 13.0 [8.7 – 38.2] months (p=0.002), respectively. However, the range of follow-up intervals was wider for patients without a BOM (1.2 – 306.2 months) compared to patients with a BOM (6.5 – 196.8 months). Twenty-four patients without a BOM had at least 4 years between treatment and follow-up assessment. Altough there were no significant difference in column heights measured at 1 or 5 minutes during timed barium esophagrams between patients with a BOM and those without (Table 2), post-treatment ESs for patients with a BOM were greater than those without a BOM, median [IQR], 5.0 [2.5 – 7.5] vs. 2.0 [1.0 – 4.3] (p=0.002). The proportion of patients with clinical failure, an ES of >3, was also greater in patients with a BOM 56.5% (13) compared to those without a BOM 29.2% (31) (p=0.009). Overall, 37% of patients included in the study had an ES >3. A breakdown of the Eckardt score into its components revealed significantly higher dysphagia scores, regurgitation scores and chest pain scores in patients with a BOM compared to those without (Supplemental Table 1). However, there was no difference in weight loss scores. Quality of life scores also revealed worse outcomes for patients with a BOM. Fifty-three patients without a BOM and 18 patients with a BOM completed the PROMIS Global-10 questionnaire. 37.7% (n=20) of those without a BOM and 61.1% (n=11) (p=0.084) of those with a BOM had a physical quality of life score lower than the national average. Furthermore, only 18.9% (n=10) of those without a BOM whereas 50% (n=9) (p=0.001) of those with a BOM had a mental quality of life score lower than the national average.
Table 2.
Clinical Outcomes. Values are median (interquartile range) or % (proportion) as indicated.
| Total | No BOM | BOM | p-value | |
|---|---|---|---|---|
|
Follow-up timing (months) |
14.9 (9.0 – 54.2) | 13.0 (8.7 – 38.2) | 52.1 (18.4 – 134.2) | 0.002 |
| Eckardt Score | 3 (1 – 5) | 2 (1 – 4.3) | 5 (2.5 – 7.5) | 0.002 |
| Eckardt Score >3 | 37.0% (44/119) | 29.2% (31/98) | 56.5% (13/21) | 0.47 |
| TBE column Height | ||||
| 1 min (cm) | 6.2 (1.2 – 10.3) | 6.4 (1.5 – 10.0) | 4.2 (0.0 – 12.3) | 0.953 |
Given that most outside hospital procedure reports were not available and the endoscopic and surgical approaches were not standardized, we separated patients based on treatment site to estimate the prevalence of a BOM as the mechanism of failure for patients with an ES >3. This analysis revealed that 5 of 27 (18.5%) patients treated at Northwestern and 8 of 17 (47.1%) treated elsewhere with an ES >3 had evidence of a BOM on imaging. In an attempt to more accurately assess the incidence of BOM development, we used data from patients treated at Northwestern during a single year (2015). This year was used as it had the most complete dataset in terms of follow-up. Seventy-four patients were treated with either POEM (n = 55) or LHM (n= 19) at Northwestern in 2015. Of patients treated that year who came back for follow-up, 4 (5.4%) developed a BOM, 3 (15.7%) after LHM and 1 (1.8%) after POEM.
Patients identified to have a BOM were recommended for a wide array of subsequent therapies which included repeat myotomy with POEM or LHM, fundoplication take down, dilation, diverticulectomy, esophagectomy, medical therapy (hyoscyamine), or no further therapy. The majority of patients 9 of 23 (39.1%) were recommended not to undergo further therapy. Unfortunately, ES were only available for 3 patients following therapy for BOM. One patient had improvement in the ES from 5 to 3 following a repeat POEM, one had worsening of the ES from 3 to 4 after a trial of hyoscyamine, and one had worsening of the ES from 8 to 9 following repeat POEM and subsequent esophagectomy.
Treatment
There was a significiant difference in BOM prevalence in patients who underwent LHM compared to POEM (p=0.013) as 17 of 65 LHM patients (26.2%) had a BOM compared to only 6 of 64 POEM patients (9.4%) (Table 3). The majority of patients treated with POEM were treated with an anterior approach, 95.2% (59). There was no statistically significant difference between anterior vs. posterior approach when comparing patients with and without a BOM. Of patients treated with LHM, Dor fundoplasty was the most common accompanying anti-reflux surgery, 28 (43.1%); 3 (4.6%) had a Nissen fundoplication (outside hospitals) and 22 (33.8%) had a Toupet fundoplication. There was no statistically significant difference in wrap type between patient who developed a BOM and those that did not. When available, myotomy length was recorded. The median (interquartile range [IQR]) esophageal myotomy length was 6 [6 – 6] and the median [IQR] gastric myotomy length was 3 [3 – 3] with no statistically significant difference in myotomy length between patients with a BOM compared to those without. Forty-seven (36.4%) patients underwent multiple/prior treatments for achalasia with either botulinum toxin, pneumatic dilation, prior POEM, or prior LHM. When comparing patients with a BOM to those without, multiple/prior treatment and the type of multiple/prior treatments performed, were not statistically different (Table 3). 20.2% (26) of the total number of patients identified (129) where treated at an outside hospital. These patients were all referred for persistent or recurrent symptoms and a disproportionate number of these patients had a BOM (p=0.012) (Table 3).
Table 3.
Patient treatment characteristics. Values are median (interquartile range) or % (proportion) as indicated.
| Total | No BOM | BOM | p-value | |
|---|---|---|---|---|
| Procedure | ||||
| LHM | 50.4% (65/129) | 73.8% (48/65) | 26.2% (17/65) | 0.013 |
| POEM | 49.6% (64/129) | 90.6% (58/64) | 9.4% (6/64) | |
| Fundoplication | ||||
| Nissen | 4.6% (3/65) | 33.3% (1/3) | 66.7% (2/3) | 0.119 |
| Toupet | 33.8% (22/65) | 86.4% (19/22) | 13.6% (3/22) | |
| Dor | 43.1% (28/65) | 75.0% (21/28) | 25.0% (7/28) | |
| Unknown | 18.5% (12/65) | 58.3% (7/12) | 41.7% (5/12) | |
| Myotomy Length | ||||
| Esophageal (cm) | 6 (6 – 6) | 6 (6 – 6) | 6 (5.8 – 8.5) | 0.218 |
| Gastric (cm) | 3 (3 – 3) | 3 (3 – 3) | 3.0 (2.0 – 3.3) | 0.517 |
| Total (cm) | 9 (9 – 10) | 9 (9 – 10) | 9 (8 – 12.3) | 0.766 |
| Multiple/Prior Achalasia Therapies | ||||
| Total | 36.4% (47/129) | 80.9% (38/47) | 19.1% (9/47) | 0.767 |
| PD | 21.7% (28/129) | 82.1% (23/28) | 17.9% (5/28) | 0.738 |
| POEM | 1.6% (2/129) | 50.0% (1/2) | 50.0% (1/2) | |
| LHM | 6.2% (8/129) | 75.0% (6/8) | 25.0% (2/8) | |
| >2 Prior Treatments | 3.9% (5/129) | 80.0% (4/5) | 20.0% (1/5) | |
| Location | ||||
| NMH | 79.8% (103/129) | 86.4% (89/103) | 13.6% (14/103) | 0.012 |
Interestingly, logistic regression analysis to assess for factors which could predict the development of a BOM with inputs including age, sex, BMI, initial achalasia subtype, post-treatment IRP, residual esophageal motility, location of procedure (OSH vs. Northwestern) and procedure type (LHM vs. POEM) revealed LHM compared to POEM as the only statistically significant predictor with an odds ratio of 3.778 and a 95% confidence interval of 1.046 – 13.639 (p=0.042).
Manometry
The median [IQR] IRP for the entire cohort was 12.0 [8.0 – 18.0] mmHg. Forty-one (31.8%) had a post-treatment IRP of >15 mmHg (the upper limit of normal). There was a trend toward a greater proportion of patients with a BOM compared to those without a BOM having a post-treatment IRP of >15 mmHg, 11 (47.8%) vs. 30 (28.4%) (p=0.068). The median [IQR] IRP was significantly greater in patients with a BOM 15.0 [11.0 – 22.0] mmHg compared to patients without a BOM 11.0 [7.8 – 17.0] (p=0.025). However, the resting EGJ pressure was not significantly different between these groups (data not shown). There was a trend towards patients with residual contractility (DCI >100 mmHg•s•cm) compared to those without residual contractility being more likely to develop a BOM 79.2% vs. 65.2% (p=0.149), respectively. Figures 2 demonstrates a patient that developed a BOM with evidence of residual contractility after myotomy on manometry.
Figure 2.
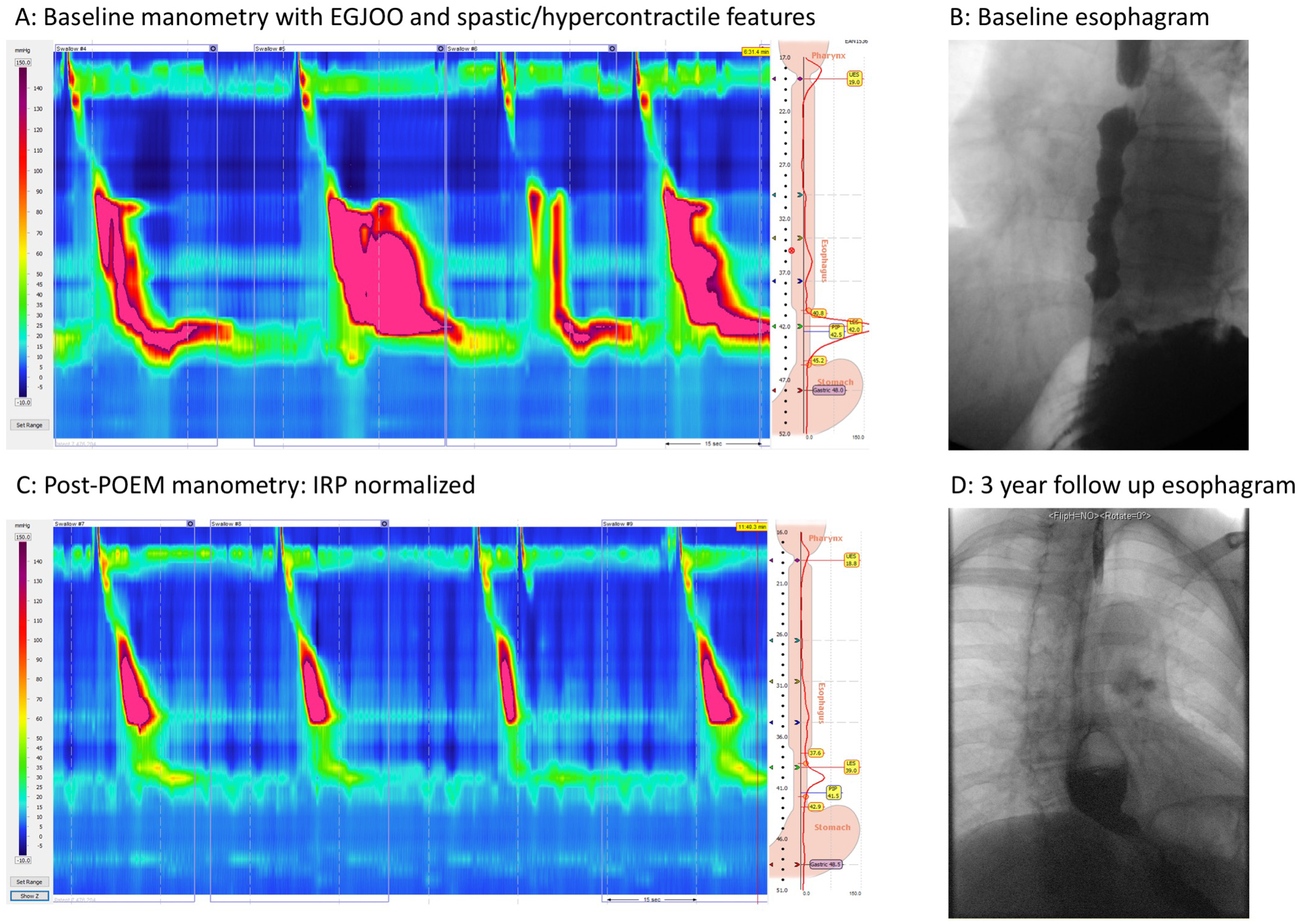
Patient presented with dysphagia and chest pain and was referred for manometry. Manometry was consistent with Type III achalasia (EGJOO and some component of jackhammer and short distal latency values) (Panel A). The esophagram was consistent with obstruction at the EGJ and there were spastic features and a normal caliber esophagus (Panel B). The patient underwent POEM with a standard 9cm myotomy and presented for follow-up evaluation with manometry which revealed normalized IRP and some residual contraction (Panel C). Approximately 3 years later she presented with new symptoms of hiccups and worsening dysphagia and chest pain and underwent an esophagram (Panel D). The esophagram revealed a large pouch along the myotomy length that failed to empty consistent with a blown out myotomy.
Discussion
This study assessed the prevalence and clinical impact of a BOM after myotomy in a large series of achalasia patients presenting at a tertiary referral center. Our findings suggest that the BOM pattern is associated with treatment failure as defined by post-treatment ES. We also found that this pattern was more common in patients with type III achalasia, after LHM as opposed to POEM, and in patients with a greater post-treatment IRP. The overall rate of BOM as a mechanism of failure in our post-myotomy achalasia patient population (with follow-up manometry and esophagram) was approximately 18% with almost half of the patients referred from outside centers for recurrent symptoms after myotomy having a BOM. Although the overall rate of clinical failure in this dataset was well above the 10 to 20% reported in the literature (37% overall and 65% of those referred from other centers) suggesting an enriched population, these findings demonstrate that a BOM may be an important mechanism of treatment failure in achalasia. Case reports have described comparable presentations that are similar to the patients described in this series.10, 11
Despite excellent outcomes with POEM and LHM, a significant number of patients have recurrent symptoms during long-term follow-up and our findings suggest that development of a BOM is an important mechanism of failure. LHM and POEM treat achalasia by reducing EGJ outflow obstruction thereby facilitating esophageal emptying with minimal pressurization. Diverticulum formation occurs in achalasia and other esophageal disorders due to the effect of pressure stress on the esophageal wall which leads to increased strain and deformation. When the deformation converts from elastic to plastic, a fixed diverticulum forms which no longer recoils or contributes to luminal pressurization (Figure 3). Although the stress/strain effects of bolus retention and intrabolus pressure are the likely stimuli for BOM formation and esophageal dilatation characteristic of achalasia, the underlying mechanisms leading to tissue remodeling are unclear. That said early failure may be more likely related to an acute complication such as an incomplete myotomy or tight wrap whereas late development of a BOM may be more likely due to the natural history of achalasia and a tight hiatal canal with persistent residual contraction over time leading to intrabolus pressurization and diverticulum formation.
Figure 3.
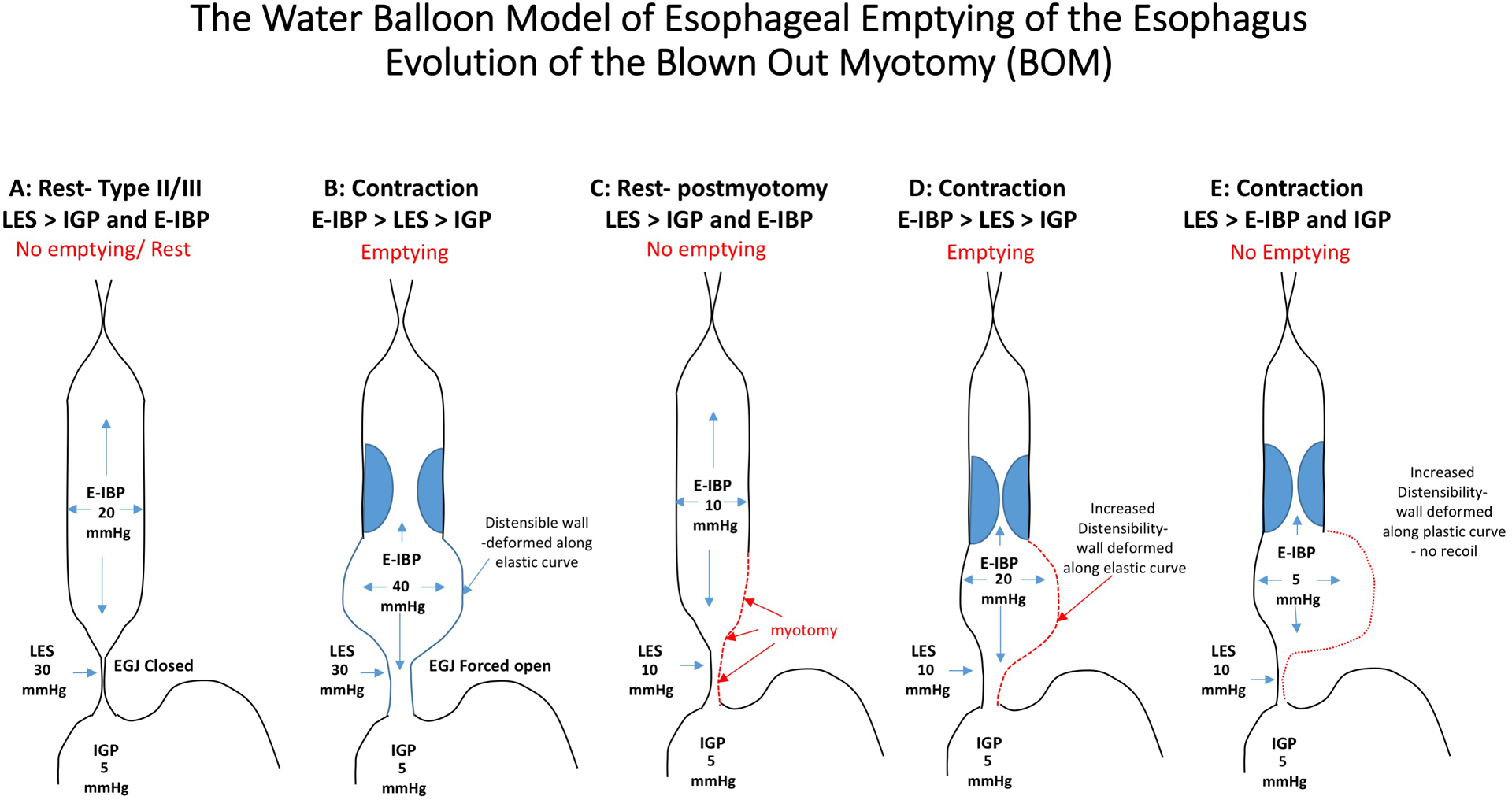
Panels A and B: Patients with type II and III achalasia generate high esophageal intrabolus pressure (E-IBP) through non-propagating contractions that reduce the lumen volume and this generates a high pressure in the esophagus (panesophageal pressurization or compartmentalized). The esophageal wall is distensible and elastic and the wall can accommodate the pressure increase by passively distending (Panel B) and recoiling back to its normal state (Panel A). This can generate enough pressure to open the poorly relaxing lower esophageal pressure (LES) and overcome the intra-gastric pressure (IGP). After a myotomy (Panels C-E), the pressure generated in the esophagus is lower because the myotomy reduces the outflow obstruction and the LES can open wider at lower E-IBP. However, there is still some resistance to flow related to the hiatus opening, anti-reflux surgery or an incomplete myotomy and the E-IBP will preferentially distend the area of the esophageal body where the myotomy was performed (Panel D). Continual stretch of this segment may eventually lead to remodeling in the are of the previous myotomy and the tissue may loose its elastic properties and the wall will deform and become more plastic (Panel E). This segment loses the ability to recoil and generate high E-IBP despite contractions and emptying is impeded by the inverted pressure gradient and the inability to generate a sufficient intrabolus opening pressure at esophagogastric junction. (Note: values depicted are to aid in conceptual understanding only)
Our findings suggest that retained contractions with residual restriction in EGJ opening likely generates the wall stress triggering BOM development. The myotomy is a point of weakness in the esophagus most vulnerable to this strain. Theoretically, that intra-esophageal pressure could be reduced by eliminating any residual outflow obstruction or esophageal contractility by performing a full thickness myotomy the entire length of the esophagus for all patients (Figure 3). However, this may further impede esophageal emptying and could lead to worse outcomes overall. An alternative approach would be to minimize the vulnerability to BOM formation by limiting the myotomy length in achalasia types I and II, restricting it to only the circular muscle layer of the esophageal wall, or limiting it only to the LES itself. The benefit of a limited myotomy is evident by the fact that we have not observed BOM formation in any patient who underwent pneumatic dilation. Based on these findings, it may be reasonable to reserve a tailored (longer) myotomy for type III achalasia in which case it should span the length of the spastic contractions and restrict the myotomy to the LES in patients with non-spastic achalasia. Furthermore, these data may suggest that LHM be avoided in patients with type III achalasia given the increased risk for continued outflow obstruction in the setting of a spastic proximal segment. The non-occluding contractions often observed in type II achalasia can also generate esophageal pressurization; however, this is typically beneficial with respect to emptying. The trade-off of reducing BOM formation at the expense of a potentially higher rate of incomplete myotomy seems appropriate being as an incomplete myotomy is more amenable to treatment than a BOM. However, proving this would require a large controlled trial given the 10–15% effect size of BOM development.
This study is limited by its descriptive nature and the fact that it was not prospective. Thus, a true incidence rate for a BOM could not be calculated. Of patients with treatment failure assessed during the study period, we did find approximately 18% had a BOM on imaging in the Northwestern treated cohort. This was compared to almost 50% of patients treated elsewhere. The disparity in these numbers may be due to endoscopic or surgical technique but may also represent referral bias. A complete dataset of all patients, regardless of symptoms, would provide more confidence in our assessment of incidence and risk factors. Another limitation was the arbitrary cut-off of a 50% increase in esophageal diameter in the area of the mytomy as the definition of a BOM. This was chosen for its simplicity after consultation with our radiologist colleagues. Future studies can address the optimal cutoff, but the 50% increase definition provides a reasonable starting point. Nonetheless, our findings are supported by biologic plausibility and principles of fluid mechanics (Figure 3). Although we did not see a major difference in terms of barium column height between patients with and without a BOM, the BOM seems to impede emptying at a low intrabolus pressure by increasing the volume of retention within the BOM site itself. Additionally, solid food may reside within these pockets as there is no mechanism to expel it across the EGJ. The fact that this complication can be seen in the context of a well done myotomy supports the contention that modifications in myotomy technique based on patient phenotype should be considered.
In summary, we have described a mechanism of achalasia treatment failure that is unique to myotomy. The BOM represents an important category of treatment failure as we suspect it is poorly responsive to further treatment targeting the LES leaving open only more invasive treatment options. We hypothesize that the occurrence of a BOM can be minimized by a myotomy tailored to the individual patient’s physiology. A future trial assessing a large cohort of achalasia patients with a precision myotomy approach would help define the optimal compensatory treatment strategy for this incurable disease.
Supplementary Material
Grant Support:
This work was supported by NIH grant 1P01DK117824-01
Abbreviations:
- LHM
laparoscopic Heller myotomy
- POEM
per-oral endoscopic myotomy
- BOM
blown out myotomy
- IRP
integrated relaxation pressure
- LES
lower esophageal sphincter
- EGJ
esophagogastric junction
- FLIP
functional luminal imaging probe
- HRM
high-resolution manometry
- ES
Eckardt score
- TBE
timed barium esophagram
- DCI
distal contractile integral
Footnotes
Disclosures:
John E. Pandolfino: Medtronic (Consultant, Grant, Speaking), Sandhill Scientific (Consulting, Speaking), Crospon (Stock Options), Takeda (Speaking), Ethicon (Consulting, Speaking); and Dustin A. Carlson: Medtronic (Consultant, Speaking). The remaining authors disclose no conflicts.
References
- 1.Pandolfino JE, Gawron AJ. Achalasia: A systematic review. JAMA 2015;313:1841–1852. [DOI] [PubMed] [Google Scholar]
- 2.Zaninotto G, Leusink A, Markar SR. Management of achalasia in 2019. Curr Opin Gastroenterol 2019. [DOI] [PubMed] [Google Scholar]
- 3.van Hoeij FB, Ponds FA, Werner Y, et al. Management of recurrent symptoms after per-oral endoscopic myotomy in achalasia. Gastrointestinal Endoscopy 2017. [DOI] [PubMed] [Google Scholar]
- 4.Richter JE. Update on the management of achalasia: balloons, surgery and drugs. Expert Rev Gastroenterol Hepatol 2008;2:435–45. [DOI] [PubMed] [Google Scholar]
- 5.Zaninotto G, Costantini M, Portale G, et al. Etiology, diagnosis, and treatment of failures after laparoscopic Heller myotomy for achalasia. Ann Surg 2002;235:186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ellis FH Jr., Failure after esophagomyotomy for esophageal motor disorders. Causes, prevention, and management. Chest Surg Clin N Am 1997;7:477–87; discussion 488. [PubMed] [Google Scholar]
- 7.Pandolfino JE, de Ruigh A, Nicodeme F, et al. Distensibility of the esophagogastric junction assessed with the functional lumen imaging probe (FLIP) in achalasia patients. Neurogastroenterol Motil 2013;25:496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jain AS, Carlson DA, Triggs J, et al. Esophagogastric Junction Distensibility on Functional Lumen Imaging Probe Topography Predicts Treatment Response in Achalasia-Anatomy Matters! Am J Gastroenterol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carlson DA, Lin Z, Kahrilas PJ, et al. High-Resolution Impedance Manometry Metrics of the Esophagogastric Junction for the Assessment of Treatment Response in Achalasia. Am J Gastroenterol 2016;111:1702–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Badillo R, Francis D, DeVault K. Formation of large esophageal diverticulum after peroral endoscopic myotomy. Gastrointest Endosc 2015;82:962; discussion 963. [DOI] [PubMed] [Google Scholar]
- 11.Sato H, Takahashi K, Takeuchi M, et al. Epiphrenic diverticulum of the esophagus after peroral endoscopic myotomy. Endoscopy 2015;47 Suppl 1 UCTN:E509–10. [DOI] [PubMed] [Google Scholar]
- 12.Triggs JR, Carlson DA, Beveridge C, et al. Upright Integrated Relaxation Pressure Facilitates Characterization of Esophagogastric Junction Outflow Obstruction. Clin Gastroenterol Hepatol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eckardt VF, Aignherr C, Bernhard G. Predictors of outcome in patients with achalasia treated by pneumatic dilation. Gastroenterology 1992;103:1732–8. [DOI] [PubMed] [Google Scholar]
- 14.Cella D, Riley W, Stone A, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol 2010;63:1179–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


