Abstract
Background.
The aim of this study was to evaluate outcomes in patients with peritoneal metastasis of colorectal cancer (pmCRC) who underwent cytoreductive surgery and intraperitoneal chemotherapy (CRS/IPC) in relation to the location of the primary tumor. Regional therapy including cytoreductive surgery and intraperitoneal chemotherapy has been associated with improved survival in patients with pmCRC. Location of the primary tumor has been shown to be prognostic in patients with metastasis.
Methods.
A retrospective review was performed for all patients who underwent complete cytoreduction and intraperitoneal chemotherapy from 2010 to 2017, examining patient and tumor characteristics, overall and recurrence-free survival, recurrence patterns, and tumor mutational profiles.
Results.
Ninety-three patients were included in the study: 49 (53%) with a right-sided and 44 (47%) with a left-sided primary tumor. Patients with a right-sided tumor had significantly shorter recurrence-free survival (median, 6.3 months [95% CI, 4.7–8.1] vs 12.3 months [95% CI, 3.6–21.7]; P = 0.02) and overall survival (median, 36.6 months [95% CI, 26.4–46.9] vs 83.3 months [95% CI 44.2–122.4]; P = 0.03). BRAF and KRAS mutations were more frequent in right-sided tumors, and APC and TP53 mutations were more frequent in left-sided tumors, which were more chromosomally instable. BRAF mutations were associated with early recurrence.
Conclusions.
Tumor sidedness is a predictor of oncological outcomes after CRS/IPC. Tumor sidedness and molecular characteristics should be considered when counseling patients regarding expected outcomes and when selecting or stratifying pmCRC patients for clinical trials of regional therapy.
INTRODUCTION
More than 20% of patients with colorectal cancer (CRC) will have metastatic disease, with worse outcomes seen in patients with peritoneal metastasis (pmCRC).1 Better understanding of tumor biology and response to treatment is key in personalizing therapy and in prognostication.
In the past few years, location of the primary tumor has emerged as an important factor and major biological determinant of treatment response and survival in patients with CRC. Initially, this importance surfaced when large databases and published clinical trials were reexplored to uncover significant differences between right- and left-sided tumors. In patients with metastasis or advanced locoregional disease, differences were noted both in responses to systemic therapy and in responses to targeted therapy.2–6 These differences were not observed in early, stage I, or stage II disease.7,8
Right- and left-sided tumors are derived from distinct anatomical and embryological origins (midgut and hindgut, respectively). Clinically, right-sided tumors at presentation are associated with older age, female sex, and advanced disease stage. Metastasis patterns also tend to differ, with right-sided tumors metastasizing to the peritoneal cavity more often than left-sided tumors, which metastasize more often to the liver.9
The differences between right- and left-sided colon cancers are apparent not only in clinical presentation and response to therapy. Differences in the spectrum of mutations, oncogenic alterations, and the pathways activated have also been described. Right-sided tumors tend to have more KRAS, BRAF, PIK3CA, PTEN, and SMAD4 mutations, while APC and TP53 mutations are seen more commonly in left-sided tumors.10–14
Previous studies showing an association between primary tumor sidedness and oncological outcomes following CRS/IPC for pmCRC, while undeniably important, had a few shortcomings.15 They were multi-institutional and lacked a uniform treatment protocol, they did not account for lead time bias, and most importantly, they lacked genetic and molecular information for the tumors.
In this study, we evaluated the association of primary tumor sidedness with recurrence and survival in patients with pmCRC who had complete cytoreduction surgery and intraperitoneal chemotherapy (CRS/IPC). We also investigated the genetic variations found in these tumors and potential predictors of recurrence and response.
METHODS
Study Design and Patients
A prospectively maintained database at Memorial Sloan Kettering Cancer Center was queried to identify patients who underwent CRS/IPC for management of pmCRC from 2010 to 2017. CRS/IPC patients with other pathologies, such as ovarian, appendiceal, mesothelioma, and small intestinal adenocarcinoma, were excluded from the study, as were patients who had incomplete cytoreduction or palliative surgery. All patients received preoperative chemotherapy; FOLFOX for chemotherapy naïve patients and FOLFIRI for previously treated patients. Typically, CRS was not attempted in the setting of disease progression based on symptoms, serum tumor makers, or imaging. CRS was performed to no gross residual disease and IPC consisted of either intra-operative hyperthermic intraperitoneal chemotherapy (HIPEC) with 40mg of Mitomycin C over 100 minutes or early post-operative intraperitoneal chemotherapy (EPIC) with 1000mg/m2 FUDR once daily for three days. The study was approved by the institutional review board of Memorial Sloan Kettering, and a waiver of Health Insurance Portability and Accountability Act authorization was obtained. Primary tumors in the cecum, ascending colon, hepatic flexure, or transverse colon were categorized as right-sided tumors. Primary tumors in the splenic flexure, descending colon, and sigmoid were categorized as left-sided tumors.
Clinical Data
Clinic notes, surgical notes, pathology reports, and imaging studies were reviewed to verify patient and tumor demographics and clinicopathological characteristics. Data collected included age at the time of CRS/IPC, sex, T and N stage, site of primary tumor, site of initial recurrence after colectomy, peritoneal carcinoma index (PCI), completeness of cytoreduction (CC), date of primary colonic tumor diagnosis, date of pmCRC diagnosis, date of CRS/IPC, date of recurrence after CRS/IPC, and date of and status at last follow-up. PCI was determined as described by Jacquet and Sugarbaker,16 and when not recorded, it was estimated based on surgical notes, with >15 corresponding to high burden and <15 corresponding to lower burden. Patients were selected for CRS if the PCI was estimated to be <20; however, select patients with slightly higher PCIs were treated based on other prognostic indicators. Resections were performed to CC 0 in all patients. Overall survival was analyzed from three separate time points, the time of primary tumor diagnosis, time of detection of pmCRC, and time of CRS/IPC to the time of death from any cause or censoring at last follow-up. This was done to account for lead time bias and other possible confounding therapeutic interventions. Recurrence-free survival was calculated from the time of CRS/IPC to the time of first recurrence (regardless of site) and to the time of peritoneal recurrence. If a recurrence occurred within 12 months following CRS/IPC it was categorized as an early recurrence. If pmCRC was diagnosed within 6 months of the primary disease, it was categorized as a synchronous presentation. The patients underwent a uniform surveillance protocol that included CT scans and carcinoembryonic antigen (CEA) measurement every 3–6 months.
Genomic Data
Genomic DNA from each patient’s tumor tissue and normal blood underwent targeted sequencing via MSK-IMPACT, a hybridization capture-based next-generation sequencing assay.17 Genomic alterations were filtered for oncogenic variants using OncoKB, a precision oncology knowledge base that identifies clinically actionable variants across cancer types for over 600 genes.18 Genes were consolidated into curated pathway templates, which were then used for enrichment analysis.19 Fisher’s exact test was used to compare frequencies of oncogenic alterations at both the gene level and the pathway level. The fraction of the genome altered was derived from copy number segmentation data. The Benjamini-Hochberg method was used to correct for multiple hypotheses.
Statistics
Continuous variables were reported as median and range. Categorical variables were reported as frequency and percentage. Differences between groups were assessed using Fisher’s exact test or the chi-square test. For continuous variables, the T-test and Wilcoxon rank-sum test were used for parametric and nonparametric data, respectively. Survival statistics were estimated using the Kaplan-Meier method and compared using the log-rank test. A Cox proportional hazards model was used to evaluate the association between individual factors and survival. Variables found to have a significant association with survival were included in a multivariable model to identify independent predictors. P values < 0.05 were considered significant. All analyses were performed with SPSS software version 25.
RESULTS
Patient and Tumor Characteristics
Ninety-three patients met the inclusion criteria: 49 (53%) with a right-sided primary tumor and 44 (47%) with a left-sided primary tumor. The characteristics of the two group of patients are listed in Table 1. Median age at the time of CRS/IPC was 61 years (range, 37–84) for patients with right-sided primaries and 54 years (range, 31–76) for patients with left-sided primaries (P < 0.001). The proportion of women did not differ significantly between the two groups: 29 (59%) vs 24 (55%) for right- and left-sided tumors, respectively (P = 0.65). Median follow up overall was 28 months; equal for left and right-sided primaries. All patients received systemic therapy prior to CRS/IPC and no patient received immunotherapy. The two groups did not differ in the frequency of synchronous peritoneal disease at presentation: 17 (35%) vs 16 (36%). Median PCI was 10 (range, 0–23) for right-sided tumors and 7 for left-sided tumors (range, 1–20) (P = 0.14). The two groups did not differ in the frequency of extraperitoneal oligometastatic disease in the liver 10 (20%) vs 12 (27%) or in the lung 1 (2%) vs 2 (4.5%) for right-sided and left-sided tumors respectively. In patients that had right-sided primary tumors, 39 patients were intended to receive EPIC and 10 patients received HIPEC. In patients with left-sided primaries, 34 were intended to receive EPIC and 10 patients received HIPEC. Three patients did not receive treatment due to complications from surgery; 2 patients with right-sided primaries and one patient with a left-sided primary. T and N stages in the two groups were comparable.
Table 1.
Patient and tumor characteristics
| Characteristics | Right colon n=49 | Left colon n=44 | P | ||
|---|---|---|---|---|---|
| Age, Median (range) | 61 | (37–84) | 54 | (31–76) | <0.001 |
| Sex N(%) | 0.65 | ||||
| Female | 29 | 59% | 24 | 55% | |
| Male | 20 | 41% | 20 | 45% | |
| Presentation N(%) | 0.8 | ||||
| Synchronous | 17 | 35% | 16 | 36% | |
| Metachronous | 32 | 65% | 28 | 64% | |
| Site of first recurrence N(%) | 0.5 | ||||
| Peritoneum | 38 | 78% | 30 | 68% | |
| Liver | 6 | 12% | 10 | 23% | |
| Lung | 0 | 0% | 1 | 2% | |
| Lung + peritoneum | 1 | 2% | 1 | 2% | |
| Liver + Peritoneum | 4 | 8% | 2 | 5% | |
| PCI, Median (range) | 10 | (0–23) | 7 | (1–20) | 0.14 |
| T stage N{%) | 0.54 | ||||
| 1 | 0 | 0% | 1 | 1% | |
| 2 | 0 | 0% | 1 | 1% | |
| 3 | 24 | 49% | 20 | 47% | |
| 4 | 25 | 51% | 22 | 51% | |
| N stage* N (%) | 0.31 | ||||
| 0 | 12 | 25% | 14 | 33% | |
| 1 | 14 | 29% | 16 | 37% | |
| 2 | 22 | 46% | 13 | 30% | |
PCI, peritoneal carcinoma index
One patient in each group was missing N stage information
Recurrence-Free Survival Median recurrence-free interval for any recurrence following CRS/IPC was 6.3 months for right-sided primaries (95% CI, 4.7–8.1) and 12.3 months (95% CI, 3.6–21.7) for left-sided primaries (log-rank P = 0.02; Figure 1A). In univariable analysis, right-sidedness (hazard ratio [HR], 1.7; 95% CI, 1.08–2.77), PCI < 15 (HR, 0.53; 95% CI, 0.27–0.84) and negative lymph nodes (HR, 0.53; 95% CI, 0.30–0.91) were associated with recurrence-free survival. In multivariable analysis, right-sided tumors (HR, 1.74; 95% CI, 1.08–2.81; P = 0.04) and negative lymph nodes (HR, 0.57; 95% CI, 0.33–0.99; P = 0.04) were identified as independent predictors of recurrence (Table 2). Peritoneal recurrence-free survival was also shorter on average for right-sided tumors (median, 8.2 months; 95% CI, 6.3–10) than for left-sided tumors (median, 14.7 months; 95% CI, 9.8–19.6) (P = 0.01; Figure 1B). In patients with right sided primary tumors, there were 35 early recurrences (≤ 12 months from surgery), and 14 patients without an early recurrence. In patients with left sided primary tumors, there were 22 early recurrences and 22 patients without an early recurrence.
Figure 1. Recurrence-Free Survival.
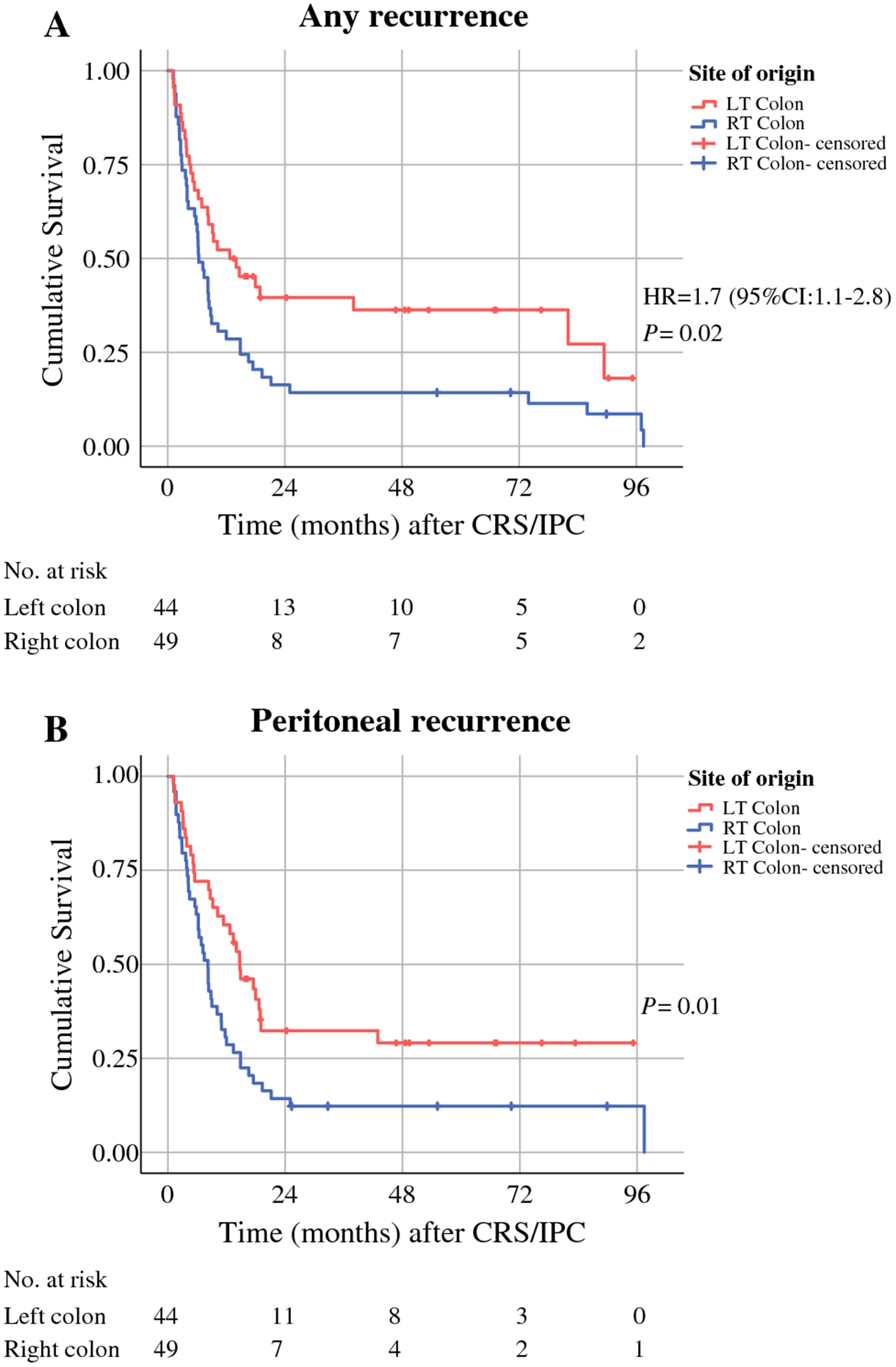
LT, left; RT, right; CRS/IPC, cytoreductive surgery with intraperitoneal chemotherapy.
Table 2.
Predictors of Recurrence-Free and Overall Survival after Cytoreductive Surgery and Intraperitoneal Chemotherapy
| Recurrence-Free Survival | Overall Survival | |||||||
|---|---|---|---|---|---|---|---|---|
| Factor | Univariable | Multivariable | Univariable | Multivariable | ||||
| HR (95% Cl) | P | HR (95% Cl) | P | HR (95% Cl) | P | HR (95% Cl) | P | |
| Age < 57 y | 0.81 (0.51–1.28) | .36 | 0.85 (0.45–1.63) | .63 | ||||
| Female sex | 0.85 (0.53–1.35) | .48 | 0.73 (0.38–1.42) | .36 | ||||
| Synchronous presentation | 0.85 (0.52–1.38) | .20 | 1.55 (0.80–3.01) | .20 | ||||
| Right sidedness | 1.73 (1.08–2.77) | .02 | 1.74 (1.08–2.81) | .04 | 2.09 (1.07–4.13) | .032 | 2.2 (1.12–4.44) | .002 |
| PCI < 15 | 0.53 (0.27–1.04) | .08 | 0.68 (0.34–1.36) | .28 | 0.36 (0.16–0.84) | .017 | 0.45 (0.19–1.05) | .06 |
| Lymph nodes negative | 0.53 (0.30–0.91) | .02 | 0.57 (0.33–0.99) | .04 | 0.14 (0.04–0.46) | .001 | 0.15 (0.05–0.49) | .022 |
PCI, peritoneal carcinoma index.
Overall Survival
Median overall survival after CRS/IPC was 36.6 months for patients with right-sided tumors (95% CI, 26.4–46.9), compared with 83.3 months for patients with left-sided tumors (95% CI, 44.2–122.4) (P = 0.03) (Figure 2A). To account for lead time bias and other possible confounding therapeutic interventions, including systemic chemotherapy, we analyzed overall survival from the diagnosis of the primary colonic tumor and from the diagnosis of peritoneal metastasis. Both time intervals showed significant differences in overall survival between patients with right-sided tumors and patients with left-sided tumors (Figure 2B and Figure 2C). Median overall survival from primary diagnosis in patients with right-sided tumors was 67 months (95% CI, 55.9–78.1), compared with 110 months (95% CI, 83–136) for patient with left-sided tumors (P = 0.04). Median overall survival from the diagnosis of peritoneal disease was 45.8 months (95% CI, 32–59.7) and 91 months (95% CI, 4–139) for patients with right-sided and left-sided tumors, respectively (P = 0.04). Univariable analysis showed that overall survival was associated with tumor right-sidedness (HR, 2.09; 95% CI, 1.07–4.13; P = 0.03), PCI < 15 (HR, 0.36; 95% CI, 0.16–0.84; P = 0.017), and negative lymph nodes (HR, 0.14, 95% CI, 0.04–0.46; P = 0.001) (Table 2 and Supplementary Figure 1). Controlling for confounders, tumor right-sidedness (HR, 2.2; 95% CI, 1.12–4.44; P = 0.002) and negative lymph nodes (HR, 0.15; 95% CI, 0.05–0.49; (P = 0.02) were identified in multivariable analysis as independent predictors of overall survival (Table 2).
Figure 2. Overall Survival.
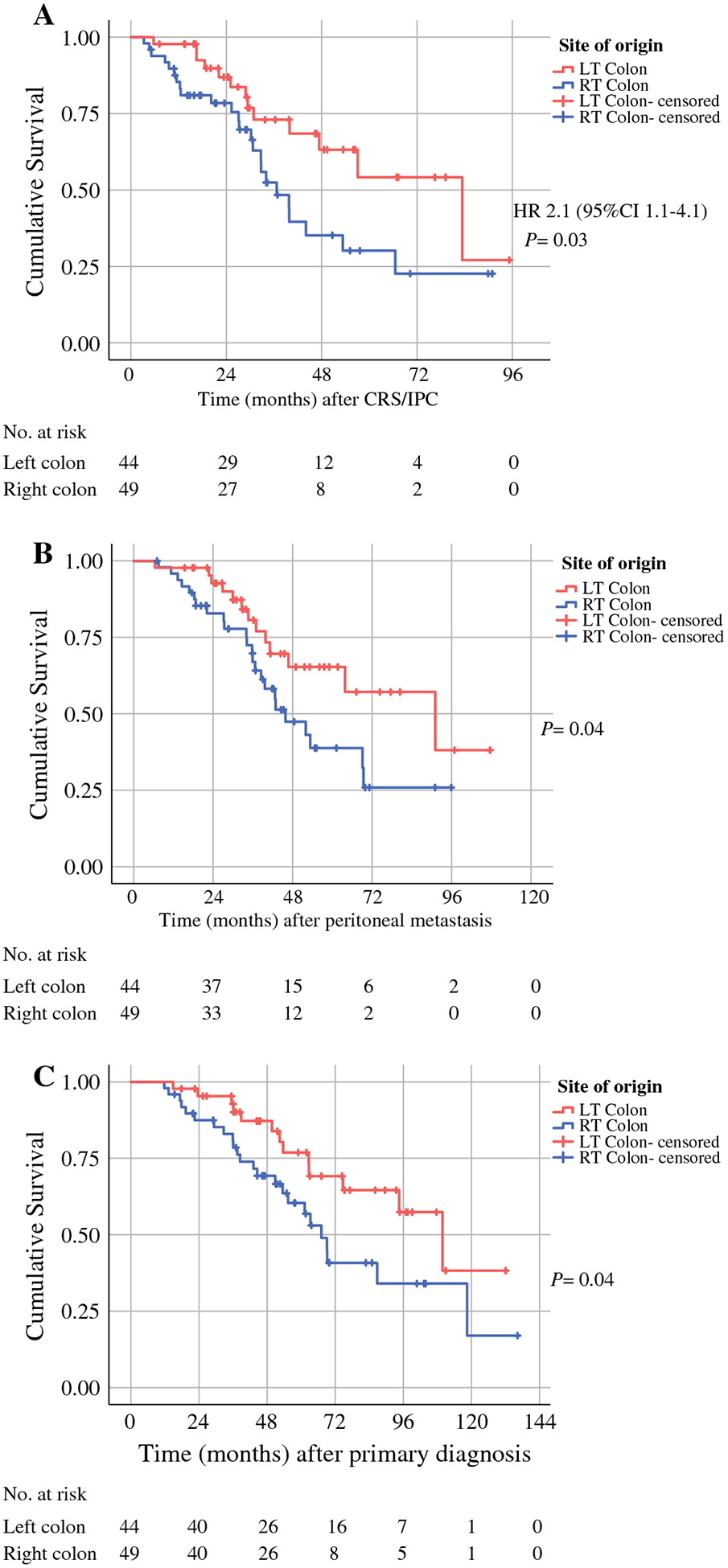
Survival was measured from cytoreductive surgery with intraperitoneal chemotherapy (CRS/IPC) (A), diagnosis of peritoneal metastasis (B), and diagnosis of the primary tumor (C). LT, left; RT, right.
Genomics
Of the 93 patients included in this study, 60 had samples that underwent sequencing by the MSK-IMPACT assay. Four of the 60 patients were excluded due to microsatellite instability or low sample purity. For the 56 evaluable patients, sequencing was performed on the primary tumor in 22 patients and on the peritoneal metastasis in 34 patients. After filtering for oncogenic driver variants18, the most frequently altered genes were APC (73%), TP53 (71%), KRAS (48%), SMAD4 (25%), PIK3CA (21%), FLT3 (13%), SOX9 (9%), PTEN (7%), MYC (7%), and BRAF (7%). TP53 and APC were more frequently altered in left-sided tumors (n = 30) than in right-sided tumors (n = 26): TP53, 83% vs 58% (P = 0.04; Q= 1); APC, 83% vs 62% (P = 0.08; Q= 1). KRAS and BRAF were more frequently altered in right-sided tumors than in left-sided tumors: KRAS, 58% vs 40% (P = 0.29; Q= 1); BRAF, 12% vs 3% (P = 0.33; Q = 1). At the pathway level, left-sided tumors were enriched in WNT, TP53, and cell cycle pathway alterations (87%, 80%, and 13%, respectively) compared with right-sided tumors (65%, 62%, and 0%, respectively): WNT pathway, P = 0.11, Q= 0.42; TP53 pathway, P = 0.08, Q= 0.42; cell cycle pathway, P = 0.12,Q = 0.42 (Figure 3A and Figure 3C). Left-sided tumors had a higher median fraction of genome altered (0.20 vs 0.10; P = 0.03) (Figure 3B). Patients were further stratified on the basis of when they had a recurrence, with early recurrence defined as ≤12 months after surgery and late recurrence defined as >12 months after surgery. BRAF alterations were found in 12% of the patients with an earlier recurrence and in none of the patients with a late recurrence (P = 0.14, Q= 1), whereas patients with a late recurrence tended to have more APC alterations (83% vs 67%; P = 0.23, Q= 1) (Figure 3A).
Figure 3.
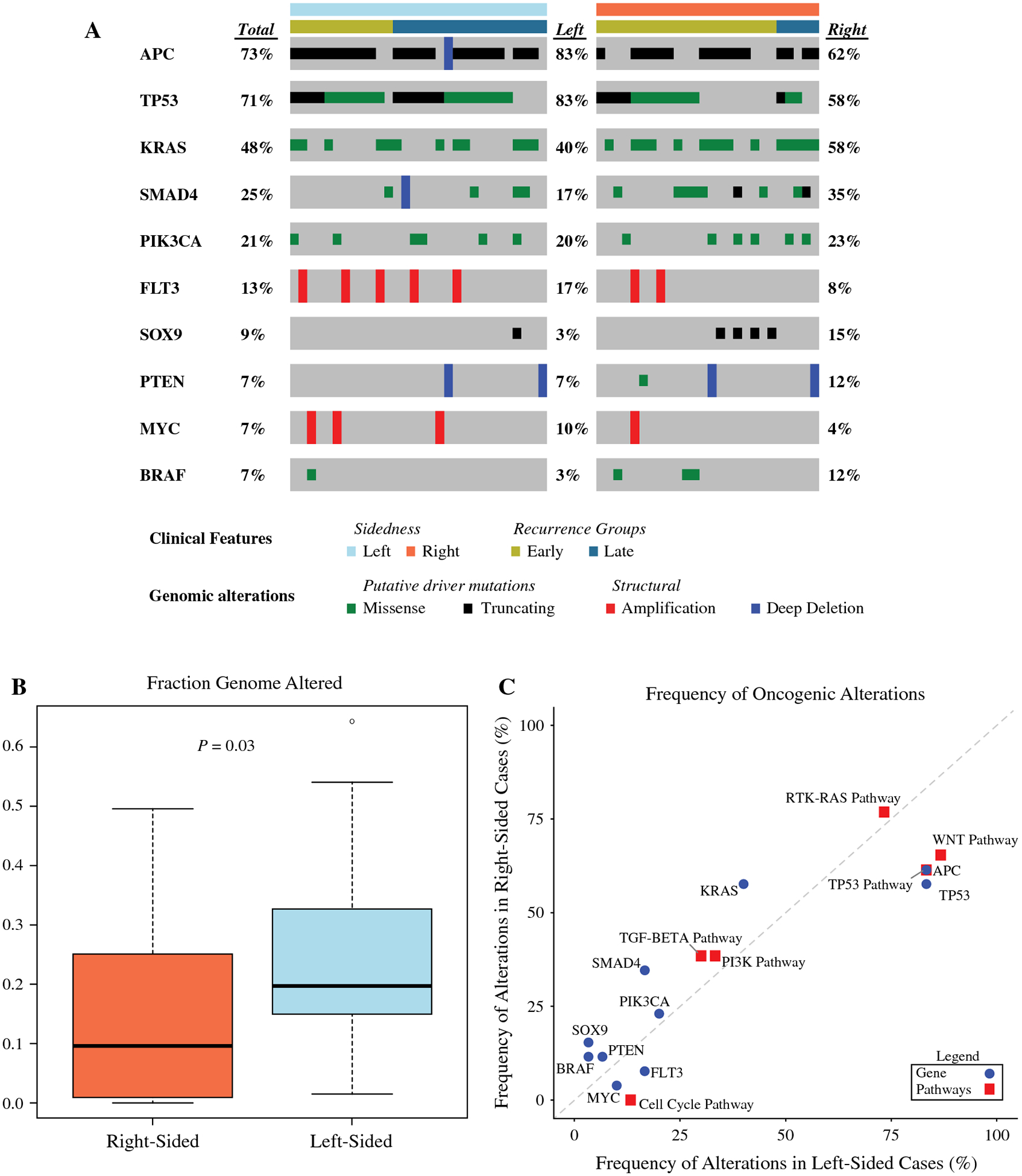
Genomic Comparison of Right-Colon Tumors and Left-Colon Tumors
DISCUSSION
Our study has identified significant differences in genomics, recurrence, and survival between patients with right-colon tumors and patients with left-colon tumors who underwent complete CRS/IPC for pmCRC. Despite comparable preoperative and operative interventions, right-sided tumors were associated with earlier recurrences (median, 6 vs 12 months) and shorter overall survival (median, 36 vs 83 months). BRAF and KRAS mutations were more frequent in right-sided tumors, whereas APC and TP53 mutations were more frequent in left-sided tumors. Chromosomal instability was higher among left-sided tumors. This finding may explain, in part, the better outcomes in patients with left-sided tumors and should be considered, along with PCI, additional sites of disease, and patient performance status, when evaluating patients for peritoneal therapy. Though the outcomes of patients with right-sided primary tumors were superior than that of many reported historical controls, median progression-free survival of 6 months suggests a limited, if any, benefit of regional therapy for at least half of these patients.
The poor prognosis of patients with pmCRC makes it imperative to tailor treatment to specific subgroups.20 An unpublished report from the PRODIGE 7 trial, presented at the 2018 Annual Meeting of the American Society of Clinical Oncology, indicated that CRS/IPC does not offer a survival benefit over CRS with systemic chemotherapy. Nevertheless, earlier trials that demonstrated better outcomes of regional therapy for pmCRC compared with systemic chemotherapy alone suggest a role for selective use of regional therapy.21–24 Our study has identified tumor sidedness as an easily evaluable clinical characteristic that can be used to refine and optimize regional therapy for pmCRC.
The evolving literature on CRS has identified several factors important in decision-making and patient selection for treatment of pmCRC. For instance, patients with high PCI tend to do worse than patients with lower PCI. Likewise, lymph node metastasis, perhaps reflecting hematogenous metastatic disease rather than regional disease involving only the peritoneal cavity, is also associated with shorter survival in patients who undergo regional therapy.25,26 Our findings are consistent with those reports. On univariable analysis, PCI, lymph node involvement, and tumor sidedness were significantly associated with recurrence and survival, while only lymph node involvement and tumor sidedness were identified as independent predictors on multivariable analysis. It appears that biology has a greater effect than tumor burden.
It appears that in patients with unresectable metastatic disease, tumor sidedness is an important prognostic factor of response to both systemic therapy and targeted therapy.4,27–32 Likewise, in patients with resectable metastatic disease, the site of the primary tumor has been identified as a predictor of survival: left tumor sidedness has been reported to be associated with better overall survival (but not disease-free survival) following liver resection for metastatic colon cancer.33,34
Similarly to our main findings, tumor sidedness and lymph node status were independent predictors of recurrence-free and overall survival in patients who had CRS for pmCRC with or without IPC in two recent studies.15 Kelly et al. demonstrated better RFS and OS in left sided versus right sided primaries (16 months vs. 14 months and 69 months vs. 36 months, respectively, n=115). Although these differences were not significant on univariable analysis, sidedness was an independent risk factor for both OS and RFS on multivariable analysis15. Kotha et al. also demonstrated better outcomes in left sided primaries (RFS of 13 months vs. 11.5 months and OS of 45 months vs. 30 months, n=336)15. Only OS was significant on univariable analysis, but sidedness was an independent risk factor for both endpoints on multivariable analysis.
There are some differences between the reports by Kelly et al., Kotha et al., and our study.15 In our study, sidedness was a risk factor for RFS and OS on both univariable and multivariable analysis. Transverse colon tumors were excluded in previous studies because of difficulty determining the exact localization. Those studies were performed at multiple institutions without a uniform treatment protocol. Some of the patients did not receive systemic chemotherapy before CRS or IPC following CRS, whereas all patients in our cohort received both types of chemotherapy. Median recurrence-free survival was shorter in our cohort, but median overall survival was longer, perhaps because of differences in patient selection and treatment algorithms. The previous studies described additional independent risk factors for recurrence-free or overall survival that we did not find (PCI, female sex) or did not investigate (poor differentiation, IPC). Our study also differed from those studies in that we accounted for lead time bias and analyzed the association between tumor sidedness and the molecular and genetic characteristics of the tumors.
We believe that the tumor sidedness-associated differences observed in this study are multifactorial. Response to systemic chemotherapy may play a major role; however, response to IPC should also be considered. The PRODIGE 7 study35 demonstrated longer than expected patient survival following CRS, raising the question of whether IPC of any kind has an added benefit for pmCRC. Subset analysis from PRODIGE 7 may shed light on the relevance of tumor sidedness for predicting response to IPC. Our study was conducted before initial reports from PRODIGE 7. At that time, we routinely used IPC in patients undergoing CRS for pmCRC. Our study does not attempt to address the value of IPC, but rather describes a consistent group of patients undergoing a standardized treatment and surveillance protocol for pmCRC. The colon portion of the ICARUS trial (NCT01815359), a prospective clinical trial randomizing patients to either EPIC or HIPEC following a CC 0 resection, has been completed. Further data regarding the role of IPC for pmCRC may be elucidated by the trial’s anticipated results.
The excellent outcomes for the highly selected group of patients in our study, especially for patients with left-sided primary tumors (median overall survival, 83 months), are among the best outcomes ever reported for CRS and are comparable to outcomes for resections of liver metastases.33
In this study, we explored the molecular features and genetic mutations that may govern the differences in outcomes for right and left sided pmCRC. We further stratified outcomes based on recurrence patterns to early and late recurrences to identify possible predictors in 60% (54/93) of our patient cohort. There was an enrichment of BRAF and KRAS mutations in the right-sided tumors, and left-sided tumors had significantly more APC and TP53 mutations, consistent with published literature for mCRC. We observed more chromosomal instability among left-sided tumors corresponding to the increased TP53 mutations. These findings could, in part, explain the better responses to chemotherapy noted in left-sided tumors and should be considered when evaluating patients for peritoneal therapy. Although sequencing was performed either on the primary tumor or the metastatic lesion, the genomics of these tissues have been found to be highly concordant14.
This study has several limitations. The retrospective analysis of a highly select group of patients and uncontrolled, associated selection bias may have had a confounding effect on the observed differences in outcomes. Patients were treated under uniform protocol at a single center, which may hinder generalizability. Multivariable analysis did not include genomic information, as data was available for only 60% of patients and inclusion of these data would be underpowered. Future such studies are planned as our pool of patients continues to expand.
Despite the limitations of selection bias herein, it is important to note that patient selection remains critical for this aggressive and potentially morbid surgery. The large majority of patients treated at our institution receive induction systemic chemotherapy before selection for CRS/IPC, and typically, patients whose disease progresses despite systemic chemotherapy are not considered candidates for regional therapy. This multilayered selection process, which probably helps avoid futile interventions, may be an important factor in the excellent outcomes achieved.
The finding that tumor sidedness is a major predictor of recurrence and survival in patients with pmCRC treated with CRS/IPC opens new opportunities for improving treatment. Tumor sidedness is a powerful factor in anticipating prognosis when planning CRS for pmCRC. Surgical oncologists may consider being more inclusive in selecting patients with left sided primary tumors. Based on these data, we believe that clinical trials should stratify for right and left-sided primary tumors as sidedness appears to be a surrogate for tumor biology and, possibly, response to treatment.
Supplementary Material
Supplemental Figure 1- Overall survival.
In relation to synchronous/metachronous pmCRC diagnosis (A), in relation to PCI (B). CRS/IPC, cytoreductive surgery with intraperitoneal chemotherapy; PCI, peritoneal cancer index.
SYNOPSIS.
In patients with peritoneal metastases of colorectal origin, location of the primary tumor was an independent predictor of survival. Worse outcomes were observed in patients with right-sided tumors. The genetic alterations were different in left-sided and right-sided tumors.
ACKNOWLEDGEMENTS
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748. Jonathan B. Yuval was supported in part by the NCI grant T32 CA009501. We thank Arthur Gelmis, BS, Department of Surgery, Memorial Sloan Kettering Cancer Center, for editing the manuscript.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest Disclosures: Dr. Yaeger has received research funding from Array BioPharma, Genentech, GlaxoSmithKline, and Novartis and has served as an advisory board member for GlaxoSmithKline. Dr. Garcia-Aguilar has received fees from Medtronic, Johnson & Johnson, and Intuitive Surgical. Dr. Cercek is an employee/paid consultant for Bayer and Proteus and reports receiving commercial research grants from Seattle Genetics. Dr. Nash has received meal reimbursement from Intuitive Surgical.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. January 2019;69(1):7–34. [DOI] [PubMed] [Google Scholar]
- 2.Boeckx N, Koukakis R, Op de Beeck K, et al. Primary tumor sidedness has an impact on prognosis and treatment outcome in metastatic colorectal cancer: results from two randomized first-line panitumumab studies. Ann Oncol. Aug 1 2017;28(8):1862–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ciombor KK, Goldberg RM. Primary Tumor Sidedness as Prognostic and Predictive Biomarker in Metastatic Colorectal Cancer: Further Validation of a Potentially Practice-Changing Variable. JAMA Oncol. February 1 2017;3(2):165–166. [DOI] [PubMed] [Google Scholar]
- 4.Modest DP, Stintzing S, von Weikersthal LF, et al. Exploring the effect of primary tumor sidedness on therapeutic efficacy across treatment lines in patients with metastatic colorectal cancer: analysis of FIRE-3 (AIOKRK0306). Oncotarget. December 1 2017;8(62):105749–105760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sunakawa Y, Ichikawa W, Tsuji A, et al. Prognostic Impact of Primary Tumor Location on Clinical Outcomes of Metastatic Colorectal Cancer Treated With Cetuximab Plus Oxaliplatin-Based Chemotherapy: A Subgroup Analysis of the JACCRO CC-05/06 Trials. Clin Colorectal Cancer. September 2017;16(3):e171–e180. [DOI] [PubMed] [Google Scholar]
- 6.Tejpar S, Stintzing S, Ciardiello F, et al. Prognostic and Predictive Relevance of Primary Tumor Location in Patients With RAS Wild-Type Metastatic Colorectal Cancer: Retrospective Analyses of the CRYSTAL and FIRE-3 Trials. JAMA Oncol. February 1 2017;3(2):194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiss JM, Pfau PR, O’Connor ES, et al. Mortality by stage for right- versus left- sided colon cancer: analysis of surveillance, epidemiology, and end results--Medicare data. J Clin Oncol. November 20 2011;29(33):4401–4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ha GW, Kim JH, Lee MR. Oncologic Effects of Primary Tumor-Sidedness on Patients with Stages 1–3 Colon Cancer: A Meta-Analysis. Ann Surg Oncol. May 2019;26(5):1366–1375. [DOI] [PubMed] [Google Scholar]
- 9.Benedix F, Kube R, Meyer F, et al. Comparison of 17,641 patients with right- and left-sided colon cancer: differences in epidemiology, perioperative course, histology, and survival. Dis Colon Rectum. January 2010;53(1):57–64. [DOI] [PubMed] [Google Scholar]
- 10.Yamauchi M, Morikawa T, Kuchiba A, et al. Assessment of colorectal cancer molecular features along bowel subsites challenges the conception of distinct dichotomy of proximal versus distal colorectum. Gut. Jun 2012;61(6):847–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Missiaglia E, Jacobs B, D’Ario G, et al. Distal and proximal colon cancers differ in terms of molecular, pathological, and clinical features. Ann Oncol. October 2014;25(10):1995–2001. [DOI] [PubMed] [Google Scholar]
- 12.Lee GH, Malietzis G, Askari A, Bernardo D, Al-Hassi HO, Clark SK. Is right-sided colon cancer different to left-sided colorectal cancer? - a systematic review. Eur J Surg Oncol. March 2015;41(3):300–308. [DOI] [PubMed] [Google Scholar]
- 13.Sinicrope FA, Mahoney MR, Yoon HH, et al. Analysis of Molecular Markers by Anatomic Tumor Site in Stage III Colon Carcinomas from Adjuvant Chemotherapy Trial NCCTG N0147 (Alliance). Clin Cancer Res. December 1 2015;21(23):5294–5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yaeger R, Chatila WK, Lipsyc MD, et al. Clinical Sequencing Defines the Genomic Landscape of Metastatic Colorectal Cancer. Cancer Cell. January 8 2018;33(1):125–136 e123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kotha NV, Baumgartner JM, Veerapong J, et al. Primary Tumor Sidedness is Predictive of Survival in Colon Cancer Patients Treated with Cytoreductive Surgery With or Without Hyperthermic Intraperitoneal Chemotherapy: A US HIPEC Collaborative Study. Ann Surg Oncol. July 2019;26(7):2234–2240. [DOI] [PubMed] [Google Scholar]
- 16.Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res. 1996;82:359–374. [DOI] [PubMed] [Google Scholar]
- 17.Cheng DT, Mitchell TN, Zehir A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A Hybridization Capture-Based Next-Generation Sequencing Clinical Assay for Solid Tumor Molecular Oncology. J Mol Diagn. May 2015;17(3):251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chakravarty D, Gao J, Phillips SM, et al. OncoKB: A Precision Oncology Knowledge Base. JCO Precis Oncol. July 2017;2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanchez-Vega F, Mina M, Armenia J, et al. Oncogenic Signaling Pathways in The Cancer Genome Atlas. Cell. April 5 2018;173(2):321–337 e310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franko J, Shi Q, Meyers JP, et al. Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: an analysis of individual patient data from prospective randomised trials from the Analysis and Research in Cancers of the Digestive System (ARCAD) database. Lancet Oncol. December 2016;17(12):1709–1719. [DOI] [PubMed] [Google Scholar]
- 21.Cashin PH, Mahteme H, Spang N, et al. Cytoreductive surgery and intraperitoneal chemotherapy versus systemic chemotherapy for colorectal peritoneal metastases: A randomised trial. Eur J Cancer. January 2016;53:155–162. [DOI] [PubMed] [Google Scholar]
- 22.Franko J, Ibrahim Z, Gusani NJ, Holtzman MP, Bartlett DL, Zeh HJ 3rd. Cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion versus systemic chemotherapy alone for colorectal peritoneal carcinomatosis. Cancer. August 15 2010;116(16):3756–3762. [DOI] [PubMed] [Google Scholar]
- 23.Elias D, Gilly F, Boutitie F, et al. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: retrospective analysis of 523 patients from a multicentric French study. J Clin Oncol. January 1 2010;28(1):63–68. [DOI] [PubMed] [Google Scholar]
- 24.Verwaal VJ, Bruin S, Boot H, van Slooten G, van Tinteren H. 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol. September 2008;15(9):2426–2432. [DOI] [PubMed] [Google Scholar]
- 25.Glehen O, Kwiatkowski F, Sugarbaker PH, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol. August 15 2004;22(16):3284–3292. [DOI] [PubMed] [Google Scholar]
- 26.da Silva RG, Sugarbaker PH. Analysis of prognostic factors in seventy patients having a complete cytoreduction plus perioperative intraperitoneal chemotherapy for carcinomatosis from colorectal cancer. J Am Coll Surg. December 2006;203(6):878–886. [DOI] [PubMed] [Google Scholar]
- 27.Wong HL, Lee B, Field K, et al. Impact of Primary Tumor Site on Bevacizumab Efficacy in Metastatic Colorectal Cancer. Clin Colorectal Cancer. June 2016;15(2):e9–e15. [DOI] [PubMed] [Google Scholar]
- 28.Chen KH, Shao YY, Chen HM, et al. Primary tumor site is a useful predictor of cetuximab efficacy in the third-line or salvage treatment of KRAS wild-type (exon 2 non-mutant) metastatic colorectal cancer: a nationwide cohort study. BMC Cancer. May 24 2016;16:327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arnold D, Lueza B, Douillard JY, et al. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann Oncol. August 1 2017;28(8):1713–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aljehani MA, Morgan JW, Guthrie LA, et al. Association of Primary Tumor Site With Mortality in Patients Receiving Bevacizumab and Cetuximab for Metastatic Colorectal Cancer. JAMA Surg. January 1 2018;153(1):60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cremolini C, Antoniotti C, Lonardi S, et al. Primary Tumor Sidedness and Benefit from FOLFOXIRI plus Bevacizumab as Initial Therapy for Metastatic Colorectal Cancer. Ann Oncol April 20 2018. [DOI] [PubMed] [Google Scholar]
- 32.Shida D, Tanabe T, Boku N, et al. Prognostic Value of Primary Tumor Sidedness for Unresectable Stage IV Colorectal Cancer: A Retrospective Study. Ann Surg Oncol. May 2019;26(5):1358–1365. [DOI] [PubMed] [Google Scholar]
- 33.Creasy JM, Sadot E, Koerkamp BG, et al. The Impact of Primary Tumor Location on Long-Term Survival in Patients Undergoing Hepatic Resection for Metastatic Colon Cancer. Ann Surg Oncol. February 2018;25(2):431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dupre A, Malik HZ, Jones RP, Diaz-Nieto R, Fenwick SW, Poston GJ. Influence of the primary tumour location in patients undergoing surgery for colorectal liver metastases. Eur J Surg Oncol. January 2018;44(1):80–86. [DOI] [PubMed] [Google Scholar]
- 35.Quenet F, Elias D, Roca L, et al. A UNICANCER phase III trial of hyperthermic intra-peritoneal chemotherapy (HIPEC) for colorectal peritoneal carcinomatosis (PC): PRODIGE 7. Journal of Clinical Oncology. 2018;36(18_suppl):LBA3503–LBA3503. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1- Overall survival.
In relation to synchronous/metachronous pmCRC diagnosis (A), in relation to PCI (B). CRS/IPC, cytoreductive surgery with intraperitoneal chemotherapy; PCI, peritoneal cancer index.


