Abstract
Adversities during juvenility increase the risk for stress-related disorders, such as post-traumatic stress disorder (PTSD) and alcohol use disorder. However, stress can also induce coping mechanisms beneficial for later stressful experiences. We reported previously that mice selectively bred for high alcohol preference (HAP) exposed to stress during adolescence (but not during adulthood) showed enhanced fear-conditioned responses in adulthood, as measured by fear-potentiated startle (FPS). However, HAP mice also showed enhanced responding to safety cues predicting the absence of foot shocks in adulthood. Here, we pursue these findings in HAP mice by investigating in further detail how juvenile stress impacts the acquisition of safety and fear learning. HAP mice were subjected to three days of juvenile stress (postnatal days 25, 27, 28) and discriminative safety/fear conditioning in adulthood. FPS was used to assess safety versus fear cue discrimination, fear learning, and fear inhibition by the safety cue. Both stressed and unstressed HAP mice were able to discriminate between both cues as well as learn the fear cue-shock association. Interestingly, it was only the previously stressed mice that were able to inhibit their fear response when the fear cue was co-presented with the safety cue, thus demonstrating safety learning. We also report an incidental finding of alopecia in the juvenile stress groups, a phenotype seen in stress-related disorders. These results in HAP mice may be relevant to understanding the influence of juvenile trauma for individual risk and resilience toward developing PTSD and how individuals might benefit from safety cues in behavioral psychotherapy.
Keywords: fear conditioning, fear-potentiated startle, juvenile stress, PTSD, resilience, safety learning
1. Introduction
Stress, particularly when experienced during sensitive developmental periods like juvenility, when the brain and stress response system are still developing, (e.g., [1]), is a known risk factor for a variety of disorders, including trauma and stressor-related disorders, like posttraumatic stress disorder (PTSD) [2], and alcohol use disorder (AUD) [3].
In humans, PTSD is characterized by unconditioned and conditioned symptom clusters. Hypervigilance resides among the unconditioned symptoms [4] and can manifest as an increased startle response [5]. Conditioned disturbances include memory disturbances, such as exaggerated fear responses to threat-associated stimuli [6], fear generalization to stimuli not predictive of threat [7, 8], and interestingly, an inability to inhibit the fear response in the presence of safety cues, i.e. stimuli that explicitly signal the non-occurrence of a threat [9, 10]. PTSD-like behavioral disturbances of both clusters can be modeled in rodents. For example, exposing rats to soiled cat litter at postnatal day (PND) 28 increases unconditioned acoustic startle responses in adulthood [11]. In mice, a three-day unpredictable stress regimen during juvenility induces fear generalization to the conditioning chamber after auditory cued fear conditioning in adulthood [12], and foot shock presentation during that time delays the ability to inhibit a fear response in the presence of a safety cue in adult rats [13].
Evidence indicates that common genetic factors increase the risk for developing PTSD and other commonly co-occurring psychiatric disorders, in particular, AUD [14, 15]. Exposure to environmental stress, particularly during development, interacts with genetic risk factors for these disorders [16]. Studies in people with PTSD and AUD, and in animal models, suggest that altered biological responses to stress may increase vulnerability toward stress-related pathological outcomes [17–19].
Mouse lines selectively bred for high or low alcohol preference (HAP/LAP lines) represent a useful animal model for identifying genetically correlated traits associated with selection for high or low alcohol drinking behavior and for exploring their underlying mechanisms. These lines show differences in emotional reactivity [20], affect-related behaviors [21], impulsivity [22], sensitization to alcohol’s locomotor-stimulant effects [23], and conditioned place preference [24] and conditioned taste aversion to alcohol [25]. Work in our laboratory indicates that these lines represent a good model for genetic vulnerability factors that may contribute to the development of co-morbid AUD and PTSD in humans (for a review see [26]). Previous studies have shown that HAP mice are vulnerable to stress-induced behaviors that mimic PTSD-related phenotypes in humans. HAP mice show greater sensitivity to anxiety-related behavior [27], conditioned fear-related behavior, assessed via fear-potentiated startle (FPS) [28], stress-related alcohol drinking in response to repeated fear-conditioning [29], and altered function of the hypothalamic-pituitary-adrenal (HPA) axis in response to fear-conditioning and testing [30] compared to their low-alcohol preferring (LAP) counterparts. HAP mice also show greater alcohol drinking behavior in adulthood when stressed during either adolescence [31] or adulthood [32], compared to no stress groups of the same mouse line. Finally, HAP, but not LAP, mice show increased FPS in adulthood after juvenile stress exposure compared to non-stressed mice of the same line [33].
In Barrenha and Chester (2007) we noted that, in addition to their pre-existing genetic vulnerability toward PTSD-like behavior, HAP but not LAP mice in the unpaired control groups showed evidence of learning the unpaired cues predicted the absence of foot shock (i.e. fear inhibition or “safety learning”) [28]. Thus, HAP mice show increased juvenile stress-induced conditioned fear behavior, but may also be better able to learn to inhibit fear-responses under certain experimental conditions. Similarly, it is interesting to note that in the literature it has been reported that electric foot shock presentation before fear conditioning in rats enhanced the fear response in one study [34], but it also enhanced fear inhibition, when presented before safety conditioning, in another study [35].
Excitation and inhibition of fear are both mechanisms relevant to the study of PTSD. Thus, building on the aforementioned findings in HAP mice, including their sensitivity to juvenile stress, the purpose of this study was to examine the effects of juvenile stress on subsequent learning to inhibit conditioned fear-related behavior in this mouse line. We presented male and female HAP mice with three days of stress during juvenility, followed by a discriminative safety/fear (S/F) conditioning paradigm in adulthood. Safety learning was assessed by the summation test and retardation of fear acquisition. To pass the summation test, the learned safety cue, when presented in compound with the learned fear cue, should reduce fear levels that are normally elicited by the learned fear cue alone. Furthermore, if the safety cue is acting as a conditioned inhibitor then it should also be slower to acquire fear properties when later paired with foot shocks than an initially neutral cue that is paired with foot shocks (i.e. retardation of fear acquisition). Despite the greater clinical relevance of the summation test, demonstrating a safety cue passes both tests offers more certainty that the safety cue indeed acquired safety valence, since the absence of a fear response to the safety cue does not allow differentiation between the possibilities of the safety cue having acquired safety valence or no valence at all [36, reviewed in 37]. We hypothesized juvenile stress would diminish the ability to learn safety.
2. Materials and Methods
2.1. Subjects
Alcohol-naive male and female mice from the third replicate of the selectively bred high alcohol preferring mouse line (generation 59) (HAP3, Indianapolis, IN) were used. Mice were bred at Purdue University and weaned at post-natal day (PND) 21 (birth = PND 1). Mice were group-housed (2–4/cage) on aspen bedding and had ad libitum access to water and food throughout the experiment. All experiments took place under white light in the light phase of a 12h light/dark cycle (lights on at 7am) and were approved by the Purdue University Animal Care and Use Committee.
2.2. Juvenile stress procedure
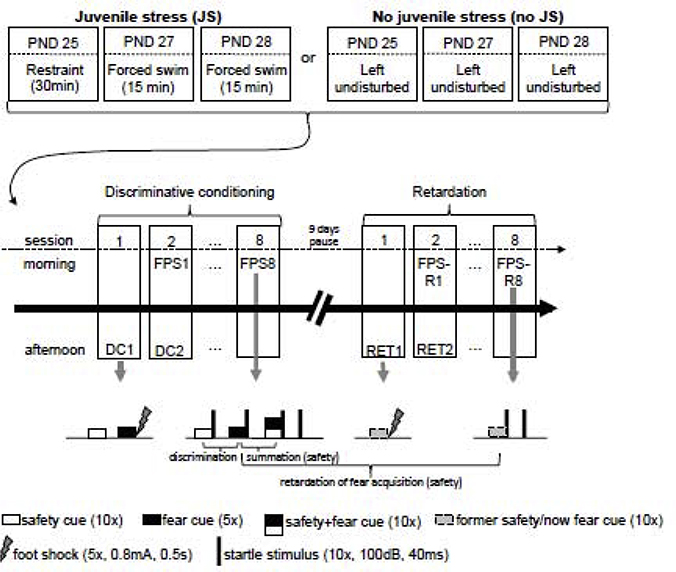
Mice were randomly assigned to ‘juvenile stress’ or ‘no juvenile stress’ groups. Juvenile stress (JS, N: m=15, f=15) comprised three days of stress presentation, adapted from [38]. Our stress procedure was short in duration, high in intensity and uncontrollable. It thus resembles human traumata, like natural catastrophes. On PND 25, mice were restrained for 30 minutes in a 20 mm wide and 102 mm long plastic enclosure. An opening in the front allowed them to breathe freely. Mice were left undisturbed on PND 26 and subjected to 15 minutes of forced swimming on PND 27 and 28. The water temperature was 24+/−2 °C and the water level was 10 cm to prevent mice from reaching the bottom of the beaker. No juvenile stress (no JS, N: m=14, f=16) mice were left undisturbed until behavioral tests started in adulthood. Mice within a cage were assigned to the same group to minimize disruption to the cage. Juvenile stress and adult behavioral testing were carried out in different rooms to avoid contextual reminder effects (Figure 1).
Figure 1:
Experimental outline. HAP3 mice were either presented with a three-day juvenile stress regimen (JS) or left undisturbed until behavioral testing started in adulthood (no JS). The conditioning paradigm consisted of a discriminative conditioning phase and a retardation of fear acquisition phase, each lasting 8 days with a 9-day break in between them. During conditioning (1/day, in the afternoon) mice were presented with 5 fear cue-foot shock pairings and 10 unreinforced safety cues. Fear potentiated startle (FPS) was assessed daily in the morning of these 8 days. FPS test sessions contained random presentations of 10 noise alone trials, 10 fear and 10 safety cues, as well as 10 compound safety+fear cues, each followed by the startle noise. During retardation conditioning the former safety cue was now paired with a footshock (5/session, 8 sessions on 8 consecutive days) and during FPS-retardation 10 former safety/now fear cues were followed by the startle noise. A tone (10kHz, 100dB, 20s) and a light (6W, 20s) served as the conditioned stimuli and were counter-balanced across groups and sexes (i.e. for half of each group the light was the safety cue, the tone was the fear cue, and vice versa for the other half). PND: postnatal day, FPS: fear potentiated startle, DC: discriminative conditioning, RET: retardation conditioning, FPS-R: fear potentiated startle-retardation.
2.3. Adult discriminative safety/fear conditioning
2.3.1. Apparatus
Discriminative conditioning and FPS testing took place in 8 dark, sound attenuated, acoustic startle chambers (Hamilton-Kinder Startle Monitor System, San Diego, CA). Each chamber contained a single weight-sensitive platform with a plexiglass mouse restraint holder (4 × 8.5 × 15 cm) on top. Peak forces of startle responses were recorded in Newton. For shock delivery during the conditioning sessions a metal grid floor was inserted underneath the enclosure. A fan provided 71–75 dB background noise. After every session the set up was cleaned with 70% ethanol.
2.3.2. General procedure
In adulthood (approximately PND 56), mice were exposed to a discriminative safety/fear conditioning procedure, consisting of 8 conditioning sessions on 8 consecutive days, carried out during the second half of the 12 h light cycle. Fear potentiated startle (FPS) responses were measured during the first half of the 12 h light cycle of each training day (adapted from [39]). Care was taken to keep a 1 hour gap from the light/dark switch and to space FPS testing and conditioning far enough apart to not interfere with the reconsolidation window. Body weight was monitored daily before each FPS session. Before the first conditioning session we noticed and recorded bald spots (alopecia), a phenotype that had not been observed during the juvenile stress period. If mice presented with a healthy coat and intact whiskers or with only small bald spots around the snout and/or curtailed whiskers, they were assigned to the “no alopecia (“no alop”) group. If mice presented with pronounced alopecia around the snout and absence of whiskers, they were assigned to the “alop” group. Alopecia (alop) grouping was based on Kalueff et al. (2006) [40].
2.3.3. Discriminative Conditioning 1–8 (DC 1–8)
After a 5 minute acclimatization period, 5 fear cues (20s) co-terminating with a foot shock (0.8mA, 0.5s) and 10 unreinforced safety cues were presented randomly with a variable inter-trial interval of 25–60s. In the first conditioning session, trials were preceded by the presentation of 10 startle noises alone (100dB white noise, 40ms, ITI: 20s) to habituate mice. For half of each group a tone (10kHz, 100dB, 20s) served as the fear cue and a light (6W, 20s) as the safety cue and vice versa for the other half.
2.3.4. Fear-potentiated startle 1–8 (FPS 1–8)
FPS sessions comprised the random presentation of 4 trial conditions (10 each, variable ITI: 25–60s) after 5 minutes of acclimatization: startle noise alone (100dB white noise, 40ms), safety cue+startle noise, fear cue+startle noise, compound safety cue/fear cue+startle noise to assess the safety cue’s ability to reduce the startle response elicited by the fear cue (summation test).
2.3.5. Retardation of fear acquisition 1–8 (RET 1–8)
Learning of a cue-shock association is expected to be impaired, if this cue has formerly been associated with safety, a phenomenon called retardation of fear acquisition [36]. Eight retardation training sessions were carried out in the afternoons (second half of the 12h light cycle) of 8 consecutive days, 9 days after the last DC session. After 5 minutes of acclimatization, mice were presented with 5 former safety cues (20s, variable ITI: 25–60s) that now co-terminated with a foot shock (0.8mA, 0.5s).
2.3.6. Fear potentiated startle-retardation 1–8 (FPS-R 1–8)
Similarly to the FPS (retrieval) sessions, FPS-R sessions took place in the first half of the 12h light cycle on retardation training days. They comprised the random presentation of the startle noise alone and the former safety/now fear cue+startle noise (10 each, variable ITI: 25–60 s), after 5 minutes acclimatization.
2.4. Statistical analyses
Per session, trials were averaged for each mouse and each trial type. % FPS was calculated using the formula [(startle amplitude on cue+noise trials – startle amplitude on noise-alone trials]/startle amplitude on noise-alone trials) × 100]. Data were analyzed using four-way analysis of variance (ANOVA) followed by lower-order ANOVAs and LSD post hoc tests or t-tests, where appropriate. For discriminative conditioning, the between-subject factors were juvenile stress group (JS, no JS) and sex (m, f) and the within-subject factors were trial-type [fear cue (F), safety cue (S), safety+fear cue (SF)] and testing sessions (FPS1, FPS8). For retardation of fear acquisition the between-subject factors were juvenile stress group (JS, no JS) and sex (m, f) and the within-subject factors were retardation [original fear cue (FPS), former safety/now fear cue (FPS-R)] and testing sessions (1, 8).
Body weight and startle amplitude were analyzed using a three-way ANOVA containing juvenile stress group (JS, no JS) and sex (m, f) as between subject factors and session (FPS1, FPS8) as the within subject factor. To compare the distributions of mice with and without alopecia in JS and no JS groups a Chi-square test was performed on the absolute number of mice. Alpha was set to p<0.05 and data are presented as mean+/−SEM.
3. Results
3.1. Summation test
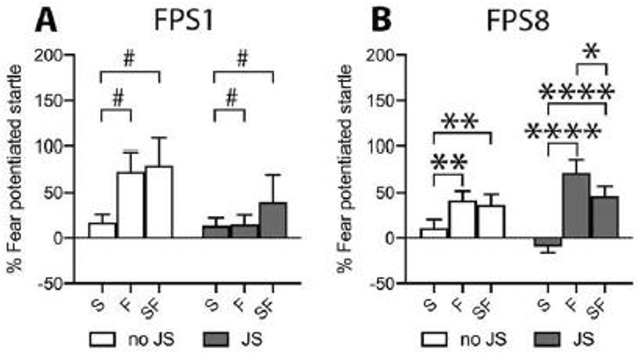
If the safety cue gained inhibitory properties through conditioning, it should dampen the fear response when co-presented with the fear cue; i.e. the inhibition elicited by the safety cue should summate with the excitation elicited by the fear cue [36]. A four-way ANOVA (stress, sex, session, trial type) revealed a significant main effect for trial type (Greenhouse-Geisser: F(1.562, 87.492)=15.966, p<0.0001) and a session x trial type x stress interaction (Greenhouse-Geisser: F(1.637, 91.693)=6.054, p=0.006). Since neither a significant sex difference, nor a significant interaction containing sex as a factor arose, males and females were collapsed for post hoc analyses. To examine the source of the 3-way interaction, 2-way ANOVAs (trial type x stress) for FPS1 and FPS8 were conducted. For FPS1, the ANOVA revealed a significant trial type effect (Greenhouse-Geisser: F(1.372, 79.554)=4.797, p=0.021) due to greater % FPS to the fear cue and the safety+fear cue compared to the safety cue (S vs F: p=0.01, S vs SF: p=0.02). Although this effect appeared to be mainly carried by the no JS group, a trial type x stress interaction was not significant (Greenhouse-Geisser: F(1.372, 79.554)=1.841, p=0.176) (Figure 2.A).
Figure 2:
Startle response after the first (FPS1) and last (FPS8) discriminative conditioning session. In FPS1 mice showed an increased startle response to the fear and the safety+fear cue, compared to the safety cue alone, indicating cue discrimination. Despite a non-significant stress x trial type interaction, this effect was carried by the no JS group (A). In FPS8, both the no JS and the JS group displayed higher FPS to the fear and the safety+fear cue compared to the safety cue alone. Moreover, in JS mice the FPS response to the safety+fear cue was significantly reduced compared to the fear cue, demonstrating successful fear inhibition and thus safety learning (B). Sex differences were not observed, therefore males and females were collapsed for graphical presentation. FPS: fear potentiated startle, JS: juvenile stress, no JS: no juvenile stress, S: safety cue, F: fear cue, SF: safety+fear cue; *p<0.05, **p<0.01, ****p<0.0001 between cues as indicated within the JS and the no JS group. #p<0.05 F and SF vs S collapsed over stress groups after insignificant stress x trial type interaction.
For FPS8, the ANOVA revealed a significant trial type effect (Greenhouse-Geisser: F(1.869, 108.380)=33.226, p<0.0001) and a significant trial type x stress interaction (Greenhouse-Geisser: F(1.869, 108.380)=6.501, p=0.003). Follow up analyses within the no JS group revealed the safety cue was significantly lower than the other two trial types (main effect of trial type: Greenhouse-Geisser: F(1.913, 55.468)=7.511, p=0.002; S vs F: p=0.002, S vs SF: p=0.002, F vs SF: p=0.577), indicating a lack of conditioned inhibition but intact safety/fear discrimination. Follow up analyses within the JS group revealed a significantly higher FPS-response to fear and safety+fear cues compared to the safety cue alone (main effect of trial type: Greenhouse-Geisser: F(1.841, 53.385)=26.654, p<0.0001; S vs F: p<0.0001, S vs SF: p<0.0001), indicating successful fear learning and safety/fear cue discrimination over the course of conditioning sessions (Figure 2.B). However, unlike the no JS group, their FPS-response to the compound safety+fear cue was also significantly lower than to the fear cue (F vs SF: p=0.038), demonstrating conditioned inhibition by the safety cue and thus safety learning (Figure 2.B).
3.2. Retardation of fear acquisition test
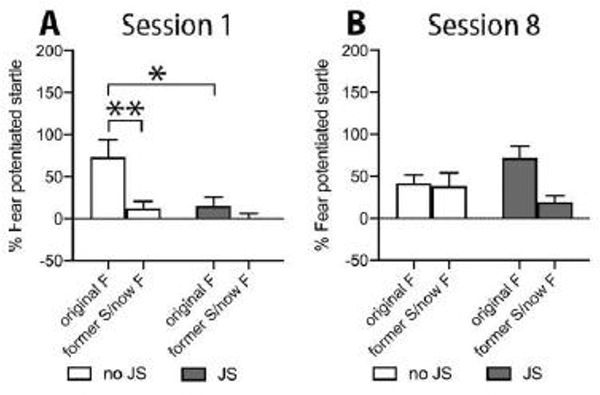
If the safety cue gained conditioned inhibitory properties during discriminative conditioning, re-conditioning this cue to a foot shock to instead become a fear cue should be less successful than a cue without any prior conditioning, i.e. the learning performance is expected to be retarded [36]. The fear potentiated startle response to the original fear cue obtained from discriminative safety/fear conditioning (FPS) and the former safety/now fear cue obtained from FPS-R sessions was compared between sexes and stress groups (Figure 3).
Figure 3:
Startle response after the first and the last conditioning session to the original fear cue (FPS1, FPS8) and the former safety/now fear cue (FPS-R1, FPS-R8). In session 1 (FPS1 and FPS-R1) the response to the original fear cue in no JS mice differed significantly from their response to the former safety/now fear cue and from the original fear cue response of the JS group (A). Session 8 revealed a significant main effect for retardation that appeared to be carried by the JS group. Since no sex differences were observed, males and females were collapsed for graphical presentation. JS: juvenile stress, no JS: no juvenile stress, F: fear cue, S: safety cue. *p<0.05, **p<0.01 as indicated.
A four-way ANOVA (stress x sex x session x retardation) revealed a significant main effect for retardation (Greenhouse-Geisser: F(1.000, 56.000)=14.471, p<0.0001) and a significant session x stress interaction (Greenhouse-Geisser: F(1.000, 56.000)=4.874, p=0.031), as well as a significant session x retardation x stress interaction (Greenhouse-Geisser: F(1.000, 56.000)=7.680, p=0.008). To examine the source of the 3-way interaction, 2-way ANOVAs (retardation x stress) for session 1 and session 8 were conducted. For session 1, the ANOVA indicated a significant main effect for retardation (Greenhouse-Geisser: F(1.000, 58.000)=10.926, p=0.002) and stress (F(1, 58)=6.439, p=0.014), as well as a significant retardation x stress interaction (Greenhouse-Geisser: F(1.000, 58.000)=4.031, p=0.049). Pairwise t-test comparisons (Bonferroni corrected) indicated a significant difference between the FPS-response of JS mice to the original fear cue and their response to the former safety/now fear cue (Bonferroni corrected paired t-test: t(29)=3.310, p=0.004). Moreover, FPS responses to the original fear cue differed significantly between JS and no JS mice (Bonferroni corrected unpaired t-test of the original fear cue: JS vs no JS: t(43.470)=2.483, p=0.034). Together, this indicates an initial, transient retardation of fear acquisition in the no JS group but not in the JS group during session 1 (Figure 3.A).
For session 8 two-way retardation x stress ANOVA revealed a significant main effect of retardation (Greenhouse-Geisser: F(1.000, 58.000)=4.877, p=0.031) and a retardation x stress interaction close to significance (F(1.000, 58.000)=3.777, p=0.057), due to the FPS response of the JS group to the former safety/now fear cue being retarded by 26% to that of the original fear cue (mean+/−SEM: 71.81+/−14.43 vs 19.18+/−7.99). In contrast, the FPS responses of no JS mice were not different from each other (mean+/−SEM: 41.62+/−10.14 vs 38.26+/−15.89 for original fear and former safety/now fear cue, respectively) (Figure 3.B).
3.3. Body weight, unconditioned startle, and alopecia
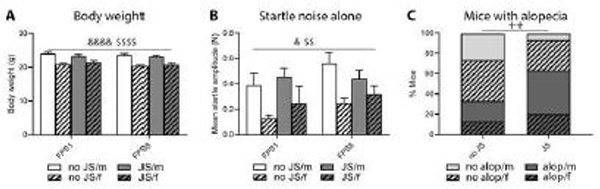
A three-way ANOVA (stress x sex x session) for body weight revealed a main effect for session (Greenhouse-Geisser: F(1.000, 56.000)=22.410, p<0.0001) and sex (F(1, 56)=51.417, p<0.0001). All groups reduced their body weight over the course of discriminative conditioning in adulthood, regardless of juvenile stress experience, and females weighed less than males (Figure 4.A).
Figure 4:
Body weight, unconditioned startle, and alopecia in the beginning (FPS1) and the end (FPS8) of adult discriminative S/F conditioning. Body weight reduced significantly over the course of adult conditioning regardless of juvenile stress and sex. Females weighed less than males throughout (A). The startle response to unsignalled startle noises alone increased uniformly in all groups over the course of adult conditioning and females showed a lower startle amplitude than males throughout sessions (B). The percentage of mice presenting with alopecia increased significantly after juvenile stress (C). JS: juvenile stress, no JS: no juvenile stress, m: males, f: females, alop: alopecia, no alop: no alopecia; &p<0.05, &&&&p<0.0001 session effect, $$p<0.01, $$$$p<0.0001 sex effect, ++p<0.01 distribution effect.
A three-way ANOVA (stress x sex x session) for the startle noise alone trials disclosed a main effect for session (Greenhouse-Geisser: F(1.000, 56.000)=7.003, p=0.011) and sex (F(1, 56)=10.721, p=0.002). Unconditioned startle responses increased in all groups from the first to the last session, and females presented lower startle responses compared to males (Figure 4.B).
As revealed by a Chi-square-test, the number of mice with alopecia (alop) was significantly higher in the JS group compared to the no JS group (Chi-square (3)=13.469, p=0.004) (Figure 4.C).
4. Discussion
The purpose of the present study was to assess fear- and safety-related learning mechanisms in HAP mice and the effect of juvenile stress on this behavior. Prior work had demonstrated that adult HAP mice displayed sensitivity to safety cues [28], and that HAP mice exposed to stress during adolescence (but not during adulthood) developed increased sensitivity to fear cues following fear conditioning in adulthood [33]. Based on these findings, we investigated here the consequences of juvenile stress on the acquisition of safety and fear learning in adult HAP mice using a discriminative classical conditioning task. Since PTSD patients are impaired in safety learning [9, 10] and childhood adversity has been linked to an increased risk for developing PTSD [41], our study bears translational relevance for understanding the human disorder. Contrary to our hypothesis that stress would impair safety/fear cue discrimination and safety learning, this study revealed that juvenile stress facilitated safety learning, as evidenced by the summation test and tendentially by the retardation of acquired fear test. In addition, we report that juvenile stress may have stimulated coping mechanisms in the form of barbering that manifested in alopecia.
Studies investigating the effects of stress on later learned behaviors utilize a variety of stress paradigms, which may differ in the types and intensity of stressors, leading to potentially varying results on learning. In our study, we used a juvenile stressor of restraint and forced swimming that was entirely unrelated to the foot shock used in the later learning paradigm. This potentially yielded contradictory results to Breit et al. (2016) where the same stressor was used throughout their study (electric foot shocks as the juvenile stressor and as the unconditioned stimulus in the fear conditioning procedure) [33], and thus the stressor may have served as a reminder of the juvenile trauma. This difference could explain why the adolescent stressors in Breit et al. (2016) enhanced FPS [33], whereas here we observed safety learning and lower FPS to the fear cue on the first session during safety training, and on the first session during retardation conditioning, in the JS group compared to the no JS group. A propensity to learn about safety cues in the HAP mice was suggested by results in Barrenha and Chester (2007), in which HAP and LAP mice were presented with temporally distant foot shocks and light cues [28]. Such explicitly unpaired protocols have been shown to assess safety learning, since the cue becomes a predictor of the non-occurrence of foot shocks through their temporal dissociation [42, 43]. In this paradigm, HAP, but not LAP, mice showed a lower startle response upon cue presentation compared to unsignalled noise presentation [28]. This suggests that, when HAP mice are offered cues to reduce fear, they are capable of doing so, although we did not observe safety learning in unstressed mice with our safety/fear conditioning protocol.
Another potential explanation for the contradictory results to Breit et al. (2016) could be that adult fear-conditioning parameters used in the Breit et al. (2016) were different (1 conditioning session with 40 0.8 mA foot shocks) from that used here (8 conditioning sessions with 5 0.8 mA foot shocks per session). The massed presentation of 40 shocks may have emulated a PTSD-inducing trauma better than our spaced presentation of the same number of shocks across 8 days. Moreover, our conditioning paradigm was discriminative in nature: in addition to a foot shock-associated fear cue, a safety cue associated with the absence of a foot shock was presented, while in the explicitly unpaired paradigm only one cue was presented. Non-discriminative and discriminative fear paradigms may require different learning strategies, which could impact how fear and safety cues are detected and learned. Finally, mice in the Breit et al. study (2016) received different duration and type of stress protocol (foot shock) and were older (mid-late adolescence) compared to the current study.
We observed safety learning only in mice previously exposed to juvenile stress. Stress-enhanced safety learning was also observed by Pollak et al (2008), who presented chronic mild stress before the explicitly unpaired protocol [42]. However, safety learning performance appears to be sensitive to the particular quality and timing of the stressor. A single session of restraint stress had no effect in the same explicitly unpaired protocol [35]. In rats subjected to discriminative safety learning, only four mild (0.4 mA) foot shocks presented in adolescence were enough to impair safety learning [13], while stronger foot shocks (15 foot shocks, 1 mA) presented one day prior to training in adulthood had no effect [44]. In contrast to our findings, in these studies the unstressed control groups did learn safety. Of note, the studies investigating discriminative learning, like we did here, utilized rats, whereas the mouse studies all utilized non-discriminative paradigms. Rats generally perform better in more cognitively demanding tasks [45] and may have an advantage in discriminative safety/fear tasks compared to mice. In fact, very few mouse studies have investigated fear inhibition in discriminative learning tasks [46, 47]. Moreover, the rats in the discriminative paradigm cited above were trained on a reward cue-reward association in addition to safety/fear discrimination. Safety and reward processing overlaps anatomically [48] and functionally [13], and stimulating the reward system simultaneously with safety/fear conditioning could thus support safety learning performance in otherwise naive animals.
Stress resulting in improved performance when later confronted with challenges [49–52] has been reported previously, and is believed to trigger coping mechanisms from which the individual can benefit. These stressors, however, are often social in nature and presented in the pre-weaning period. They include frequent interruptions of maternal care, using either the limited nesting paradigm [51], or brief episodes of maternal separation [49]. Santarelli et al. (2014, 2017) showed that mice reared and kept under disadvantageous conditions (limited nesting, single housing/chronic social defeat) performed similarly well in anxiety and sociability tests as compared to those reared and kept under advantageous conditions (early handling, group housing/housing with ovariectomized females). In contrast, mice, in which rearing and maintenance conditions did not match (i.e. advantageous rearing but disadvantageous maintenance conditions and vice versa) performed worse [50, 52].
In the present study we used an adapted three-day stress protocol; similar stress protocols induce PTSD-like disturbances [12, 38, 53–55]. When followed by fear conditioning in adulthood, they can induce memory generalization to the background context in an auditory cued fear conditioning task [12], and an exaggerated fear response to the fear cue [55], but these protocols did not offer cues to down-regulate fear like we did here. Despite this important difference, other differences in experimental variables and the genetic makeup of HAP mice may have resulted in improved (rather than the hypothesized disrupted) safety learning. Other studies have highlighted the impact of the interaction between genes and environment on mediating stress resilience. For example, three days of variable stress in juvenility induced PTSD-like memory disturbances in wild type mice, but not in heterozygous knockout mice for glutamic acid decarboxylase (GAD)65 [12], the enzyme that synthesizes GABA for synaptic use [56]. This resilience-like effect developed only after juvenile stress experience, since heterozygous GAD65 knockout mice did not differ from their wild type littermates when undisturbed, as assessed by anxiety-related behaviors [57], [58]. Interestingly, GAD65 knockout mice on the 129N2 background also show increased alcohol consumption and reduced alcohol withdrawal severity [59]. HAP mice show these same phenotypes (high alcohol consumption and low alcohol withdrawal) due to selective breeding [60]. One could therefore speculate that the stress effects on safety learning observed in HAP mice could be related to alterations in the GABAergic system, brought about by selective breeding. The observed stress effects on safety learning here could also be influenced by stress-interactions with monoaminergic systems. We reported that male and female HAP3 mice (same line used in the current study) have higher dopamine, the dopamine metabolite 3,4-dihydroxyphenylacetic acid, and dopamine turnover in the nucleus accumbens compared to LAP3 mice [61]. In addition, we recently reported that genetic differences in endocannabinoid system function may underlie the line differences in fear conditioning between HAP1 and LAP1 mice [62]. The endocannabinoid system is a promising mechanism for future study because of its reciprocal relationship with stress-axis function and role in regulating stress-related behaviors [63]. Indeed, the corticosterone response to foot shock stress differs between adult HAP2 and LAP2 mice [30], although no differences in the corticosterone response were found between adolescent HAP2 and LAP2 mice after 1 or 10 days of foot shock stress [33]. Future studies will include neurochemical assessments in the HAP/LAP mouse lines to further assess stress-related biological mechanisms that may influence safety learning and risk/resilience toward developing PTSD-like behavior.
Sex differences in stress susceptibility exist. For example, the incidence of PTSD is twice as high in women than in men [64], and female rodents present with greater contextual fear generalization [65] and impaired conditioned inhibition to a safety cue [66] compared to males. In the present study, sex differences in discriminative safety and fear learning were not found in either JS or no JS groups. This is in contrast to Foilb et al. (2018) and Day et al. (2015), who showed that females discriminated better between a fear cue and a non-reinforced cue early on in training [67, 68], but discrimination dissipated with continued training [67]. With respect to fear inhibition, rats and mice of both sexes are able to learn safety in explicitly unpaired paradigms [35, 69]. However, in the Kreutzmann et al. (2020) study, if foot shock stress, but not restraint stress, preceded safety conditioning in adulthood, males but not females showed improved safety learning [35]. In rats, fear inhibition assessed by summation has been shown to be impaired in females in a paradigm that simultaneously assessed reward consumption, which was increased in females early in training, possibly influencing safety learning performance [66]. Consistent with our findings, Foilb et al. (2018), who employed a similar training schedule of morning training and afternoon recall sessions, did not observe sex differences in the summation test [68]. Similarly, female HAP mice did not differ from males in the paired or explicitly unpaired training schedule in Barrenha and Chester (2007) [28]. These complex interactions between sex, stress and learning paradigms highlight the general importance of assessing both females and males in safety learning procedures (for a detailed review on this topic see Krueger and Sangha in this special issue).
Finally, stress protocols similar to ours have been previously shown to induce unconditioned disturbances, like reduced body weight [70] and increased anxiety-related behavior [12, 53]. We did not observe comparable disturbances, since JS and no JS groups did not differ from each other in weight at the beginning of adult conditioning. All groups uniformly reduced their body weight from the first to the last FPS-session during discriminative conditioning. Likewise, both JS and no JS groups increased their startle response to unsignalled noise-alone trials, indicating increased anxiety-related behavior over the course of adult conditioning. We did, however, find an increased number of mice that presented with alopecia in the JS group (see Figure 4) that did, however, not influence summation and retardation (see supplement). Since this was an incidental finding, data has to be interpreted with caution. A possible reason for alopecia is barbering, classically defined as plucking hair from the coat and/or whiskers from cage mates or oneself [40]. Barbering has been characterized as a stress-related behavior [40] but has also been associated with social behavior [71] and social dominance and/or aggression [40], both of which could be perturbed by stress. Since alopecia was mainly observed around the snout, it is possible that it resulted from excessive self-barbering and/or hetero-barbering from littermates, although hetero-grooming appears more likely due to its higher prevalence in mice [72]. While the etiology of barbering behavior is insufficiently understood, genetic background is a likely contributor. Kalueff et al., (2006) reported that inbred mouse strains expressed barbering behavior in a context- and strain-specific manner and that strains showed differential sensitivity to stress-induced barbering [40]. Barbering also serves as a model for Trichotillomania [72], or hair pulling disorder, an impulse control disorder in humans [73], for which juvenile stress is a risk factor [74, 75]. Interestingly, HAP mice are more impulsive than LAP mice [22]. Thus, studies assessing (stress-induced) barbering in both HAP and LAP mice are warranted to further test the idea that the alopecia observed in the current study is a stress-related phenotype associated with genetic selection for alcohol preference.
In conclusion, we found that juvenile stress facilitated safety learning in male and female high alcohol preferring mice, but also increased the incidence of alopecia, highlighting the many different facets of stress consequences and the importance of assessing a variety of stress-related behavioral outcomes. Our observations were surprising, since these mice previously showed features characteristic for PTSD and AUD, including increased FPS and alcohol drinking after exposure to a different stress paradigms [29, 31, 33]. These results in mice may be relevant to understanding the influence of juvenile trauma for individual risk and resilience toward developing PTSD. As well, these results are translationally relevant to understanding mechanisms of stress coping and resilience, namely, the potential of safety cues to help cope with aversive experiences that lead to PTSD and how individuals might benefit from implementing safety cues in behavioral psychotherapy.
7. Acknowledgements
We thank Dr. Nicholas J. Grahame for providing the HAP3 line breeders. We also thank Signe Hobaugh and Yolanda Jonker for excellent animal care.
5. Funding
IM was supported by the Alexander von Humboldt foundation. SS was supported by NIMH R01MH110425. HAP3 line breeding was funded by the National Institute on Alcohol Abuse and Alcoholism, grant number AA015512 to Dr. Richard Bell. None of the funding sources contributed to this study in any way other than providing financial support.
Footnotes
6. Declaration of interest
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Romeo RD, Kaplowitz ET, Ho A, and Franco D, “The influence of puberty on stress reactivity and forebrain glucocorticoid receptor levels in inbred and outbred strains of male and female mice,” Psychoneuroendocrinology, vol. 38, no. 4, pp. 592–596, 2013, doi: 10.1016/j.psyneuen.2012.07.019. [DOI] [PubMed] [Google Scholar]
- [2].Heim C and Nemeroff CB, “The Role of Childhood Trauma in the Neurobiology of Mood and Anxiety Disorders : Preclinical and Clinical Studies,” Biol. Psychiatry, vol. 49, no. 12, pp. 1023–1039, 2001, doi: 10.1016/S0006-3223(01)01157-X. [DOI] [PubMed] [Google Scholar]
- [3].Lee RS, Oswald LM, and Wand GS, “Early life stress as a predictor of co-occurring alcohol use disorder and post-traumatic stress disorder,” Alcohol Res. Curr. Rev, vol. 39, no. 2, pp. 147–159, 2018. [PMC free article] [PubMed] [Google Scholar]
- [4].Bisson JI, Cosgrove S, Lewis C, and Roberts NP, “Post-traumatic stress disorder,” BMJ, vol. 351, no. November, pp. 1–7, 2015, doi: 10.1136/bmj.h6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Shalev AY, Peri T, Orr SP, Bonne O, and Pitman RK, “Auditory startle responses in help-seeking trauma survivors,” Psychiatry Res, vol. 69, no. 1, pp. 1–7, 1997, doi: 10.1016/S0165-1781(96)03001-6. [DOI] [PubMed] [Google Scholar]
- [6].Fani N et al. , “Attention bias toward threat is associated with exaggerated fear expression and impaired extinction in PTSD,” Psychol. Med, vol. 42, no. 3, pp. 533–543, 2012, doi: 10.1017/S0033291711001565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lissek S, Kaczkurkin AN, Rabin S, Geraci M, Pine DS, and Grillon C, “Generalized anxiety disorder is associated with overgeneralization of classically conditioned fear,” Biol. Psychiatry, vol. 75, no. 11, pp. 909–915, 2014, doi: 10.1016/j.biopsych.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Thome J et al. , “Generalisation of fear in PTSD related to prolonged childhood maltreatment: An experimental study,” Psychol. Med, vol. 48, no. 13, pp. 2223–2234, 2018, doi: 10.1017/S0033291717003713. [DOI] [PubMed] [Google Scholar]
- [9].Jovanovic T et al. , “IMPAIRED FEAR INHIBITION IS A BIOMARKER OF PTSD BUT NOT DEPRESSION,” Depress. Anxiety, vol. 27, no. 3, pp. 244–251, 2010, doi: 10.1002/da.20663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jovanovic T, Kazama A, Bachevalier J, and Davis M, “Impaired safety signal learning may be a biomarker of PTSD,” Neuropharmacology, vol. 62, no. 2, pp. 695–704, 2012, doi: 10.1016/j.neuropharm.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bazak N et al. , “Pre-pubertal stress exposure affects adult behavioral response in association with changes in circulating corticosterone and brain-derived neurotrophic factor,” Psychoneuroendocrinology, vol. 34, no. 6, pp. 844–858, 2009, doi: 10.1016/j.psyneuen.2008.12.018. [DOI] [PubMed] [Google Scholar]
- [12].Müller I, Obata K, Richter-Levin G, and Stork O, “GAD65 haplodeficiency conveys resilience in animal models of stress-induced psychopathology.,” Front. Behav. Neurosci, vol. 8, no. August, p. 265, 2014, doi: 10.3389/fnbeh.2014.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Müller I, Brinkman AL, Sowinski EM, and Sangha S, “Adolescent conditioning affects rate of adult fear, safety and reward learning during discriminative conditioning,” Sci. Rep, vol. 8, no. 1, pp. 1–14, 2018, doi: 10.1038/s41598-018-35678-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sartor CE et al. , “Common genetic and environmental contributions to post- traumatic stress disorder and alcohol dependence in young women,” Psychol. Med, vol. 41, no. 7, pp. 1497–1505, 2011, doi: 10.1017/S0033291710002072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sheerin CM et al. , “Shared molecular genetic risk of alcohol dependence and posttraumatic stress disorder (PTSD).,” Psychology of Addictive Behaviors, vol. 34, no. 5, pp. 613–619, 2020, doi: 10.1037/adb0000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ryan J, Chaudieu I, Ancelin ML, and Saffery R, “Biological underpinnings of trauma and post-traumatic stress disorder: Focusing on genetics and epigenetics,” Epigenomics, vol. 8, no. 11, pp. 1553–1569, 2016, doi: 10.2217/epi-2016-0083. [DOI] [PubMed] [Google Scholar]
- [17].de Kloet CS, Vermetten E, Geuze E, Kavelaars A, Heijnen CJ, and Westenberg HGM, “Assessment of HPA-axis function in posttraumatic stress disorder: Pharmacological and non-pharmacological challenge tests, a review,” J. Psychiatr. Res, vol. 40, no. 6, pp. 550–567, 2006, doi: 10.1016/j.jpsychires.2005.08.002. [DOI] [PubMed] [Google Scholar]
- [18].Clarke T-K et al. , “HPA-axis activity in alcoholism: examples for a gene-environment interaction.,” Addict. Biol, vol. 13, no. 1, pp. 1–14, March 2008, doi: 10.1111/j.1369-1600.2007.00084.x. [DOI] [PubMed] [Google Scholar]
- [19].Suh J and Ressler KJ, “Alcohol Research: Current Reviews - Co-Occurring Alcohol Use Disorder and Post-Traumatic Stress Disorder,” Alcohol Res. Curr. Rev, vol. 39, no. 2, pp. 131–145, 2018. [PMC free article] [PubMed] [Google Scholar]
- [20].Matson LM and Grahame NJ, “Emotional Reactivity to Incentive Downshift as a Correlated Response to Selection of High and Low Alcohol Preferring Mice and an Influencing Factor on Ethanol Intake,” Alcohol, vol. 49, no. 7, pp. 657–664, 2015, doi: 10.1016/j.alcohol.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Can A, Grahame NJ, and Gould TD, “Affect-related behaviors in mice selectively bred for high and low voluntary alcohol consumption,” Behav. Genet, vol. 42, no. 2, pp. 313–322, 2012, doi: 10.1007/s10519-011-9505-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Oberlin BG and Grahame NJ, “High-Alcohol Preferring Mice Are More Impulsive Than Low-Alcohol Preferring Mice as Measured in the Delay Discounting Task,” Alcohol Clin Exp Res, vol. 33, no. 7, pp. 1294–1303, 2009, doi: 10.1111/j.1530-0277.2009.00955.x.High-Alcohol [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Grahame NJ, Rodd-Henricks K, Li TK, and Lumeng L, “Ethanol locomotor sensitization, but not tolerance correlates with selection for alcohol preference in high- and low-alcohol preferring mice,” Psychopharmacology (Berl), vol. 151, no. 2–3, pp. 252–260, 2000, doi: 10.1007/s002130000388. [DOI] [PubMed] [Google Scholar]
- [24].Grahame NJ, Chester JA, Rodd-Henricks K, Li TK, Lumeng L, and Grahame NJ, “Alcohol place preference conditioning in high- and low-alcohol preferring selected lines of mice,” Pharmacol. Biochem. Behav, vol. 68, no. 4, pp. 805–814, 2001, doi: 10.1016/S0091-3057(01)00476-2. [DOI] [PubMed] [Google Scholar]
- [25].Chester JA, Lumeng L, Li T-K, and Grahame NJ, “High- and low-alcohol-preferring mice show differences in conditioned taste aversion to alcohol.,” Alcohol. Clin. Exp. Res, vol. 27, no. 1, pp. 12–18, 2003, doi: 10.1097/01.ALC.0000046340.06154.9F. [DOI] [PubMed] [Google Scholar]
- [26].Chester JA, “9 - A Genetic Mouse Model for Comorbid Alcohol Use Disorder and Posttraumatic Stress Disorder,” in Neurobiology of Abnormal Emotion and Motivated Behaviors, Sangha S and Foti D, Eds. Academic Press, 2018, pp. 164–180, 10.1016/B978-0-12-813693-5.00009-5 [DOI] [Google Scholar]
- [27].Chester JA and Barrenha GD, “Acoustic startle at baseline and during acute alcohol withdrawal in replicate mouse lines selectively bred for high or low alcohol preference,” Alcohol. Clin. Exp. Res, vol. 31, no. 10, pp. 1633–1644, 2007, doi: 10.1111/j.1530-0277.2007.00462.x. [DOI] [PubMed] [Google Scholar]
- [28].Barrenha GD and Chester JA, “Genetic correlation between innate alcohol preference and fear-potentiated startle in selected mouse lines,” Alcohol. Clin. Exp. Res, vol. 31, no. 7, pp. 1081–1088, 2007, doi: 10.1111/j.1530-0277.2007.00396.x. [DOI] [PubMed] [Google Scholar]
- [29].Chester JA and Weera MM, “Genetic correlation between alcohol preference and conditioned fear: Exploring a functional relationship,” Alcohol, vol. 58, pp. 127–137, 2017, doi: 10.1016/j.alcohol.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Chester JA, Kirchhoff AM, and Barrenha GD, “Relation between corticosterone and fear-related behavior in mice selectively bred for high or low alcohol preference,” Addict. Biol, vol. 19, no. 4, pp. 663–675, 2014, doi: 10.1111/adb.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chester JA, Barrenha GD, Hughes ML, and Keuneke KJ, “Age- and sex-dependent effects of footshock stress on subsequent alcohol drinking and acoustic startle behavior in mice selectively bred for high-alcohol preference,” Alcohol. Clin. Exp. Res, vol. 32, no. 10, pp. 1782–1794, 2008, doi: 10.1111/j.1530-0277.2008.00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Chester JA, de Paula Barrenha G, DeMaria A, and Finegan A, “Different effects of stress on alcohol drinking behaviour in male and female mice selectively bred for high alcohol preference,” Alcohol Alcohol, vol. 41, no. 1, pp. 44–53, 2006, doi: 10.1093/alcalc/agh242. [DOI] [PubMed] [Google Scholar]
- [33].Breit KR and Chester JA, “Effects of Chronic Stress on Alcohol Reward- and Anxiety-Related Behavior in High- and Low-Alcohol Preferring Mice,” Alcohol. Clin. Exp. Res, vol. 40, no. 3, pp. 482–490, 2016, doi: 10.1111/acer.12992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Iwasaki S, Sakaguchi T, and Ikegaya Y, “Brief fear preexposure facilitates subsequent fear conditioning,” Neurosci. Res, vol. 95, pp. 66–73, 2015, doi: 10.1016/j.neures.2015.02.001. [DOI] [PubMed] [Google Scholar]
- [35].Kreutzmann JC et al. , “Neuropeptide-S-receptor deficiency affects sex-specific modulation of safety learning by pre-exposure to electric stimuli,” Genes, Brain Behav, vol. 19, no. 3, pp. 1–10, 2020, doi: 10.1111/gbb.12621. [DOI] [PubMed] [Google Scholar]
- [36].Rescorla RA, “Conditioned inhibition of fear resulting from negative CS-US contingencies,” J. Comp. Physiol. Psychol, vol. 67, no. 4, pp. 504–509, 1969, doi: 10.1037/h0027313. [DOI] [PubMed] [Google Scholar]
- [37].Sangha S, Diehl MM, Bergstrom HC, and Drew MR, “Know safety, no fear,” Neurosci. Biobehav. Rev, vol. 108, no. November 2019, pp. 218–230, 2020, doi: 10.1016/j.neubiorev.2019.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Tsoory M, Guterman A, and Richter-Levin G, “Exposure to stressors during juvenility disrupts development-related alterations in the PSA-NCAM to NCAM expression ratio: Potential relevance for mood and anxiety disorders,” Neuropsychopharmacology, vol. 33, no. 2, pp. 378–393, 2008, doi: 10.1038/sj.npp.1301397. [DOI] [PubMed] [Google Scholar]
- [39].Foilb AR, Flyer-adams JG, Maier SF, and Christianson JP, “Posterior insular cortex is necessary for conditioned inhibition of fear,” Neurobiol. Learn. Mem, vol. 134, pp. 317–327, 2016, doi: 10.1016/j.nlm.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kalueff AV, Minasyan A, Keisala T, Shah ZH, and Tuohimaa P, “Hair barbering in mice: Implications for neurobehavioural research,” Behav. Processes, vol. 71, no. 1, pp. 8–15, 2006, doi: 10.1016/j.beproc.2005.09.004. [DOI] [PubMed] [Google Scholar]
- [41].Jaffee SR, “Child Maltreatment and Risk for Psychopathology in Childhood and Adulthood,” Annu. Rev. Clin. Psychol, vol. 13, no. 1, pp. 525–551, 2017, doi: 10.1146/annurev-clinpsy-032816-045005. [DOI] [PubMed] [Google Scholar]
- [42].Pollak DD, Monje FJ, Zuckerman L, Denny CA, Drew MR, and Kandel ER, “An Animal Model of a Behavioral Intervention for Depression,” Neuron, vol. 60, no. 1, pp. 149–161, 2008, doi: 10.1016/j.neuron.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Pollak DD, Monje FJ, and Lubec G, “The learned safety paradigm as a mouse model for neuropsychiatric research,” Nat. Protoc, vol. 5, no. 5, pp. 954–964, 2010, doi: 10.1038/nprot.2010.64. [DOI] [PubMed] [Google Scholar]
- [44].Woon EP, Seibert TA, Urbanczyk PJ, Ng KH, and Sangha S, “Differential effects of prior stress on conditioned inhibition of fear and fear extinction,” Behav. Brain Res, vol. 381, no. November 2019, 2020, doi: 10.1016/j.bbr.2019.112414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ellenbroek B and Youn J, “Rodent models in neuroscience research: Is it a rat race?,” DMM Dis. Model. Mech, vol. 9, no. 10, pp. 1079–1087, 2016, doi: 10.1242/dmm.026120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Vouimba RM, Garcia R, Baudry M, and Thompson RF, “Potentiation of conditioned freezing following dorsomedial prefrontal cortex lesions does not interfere with fear reduction in mice,” Behav. Neurosci, vol. 114, no. 4, pp. 720–724, 2000, doi: 10.1037/0735-7044.114.4.720. [DOI] [PubMed] [Google Scholar]
- [47].Meyer HC et al. , “Ventral hippocampus interacts with prelimbic cortex during inhibition of threat response via learned safety in both mice and humans,” Proc. Natl. Acad. Sci, vol. 116, no. 52, pp. 26970–26979, December 2019, doi: 10.1073/PNAS.1910481116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Sangha S, Chadick JZ, and Janak PH, “Safety Encoding in the Basal Amygdala,” J. Neurosci, vol. 33, no. 9, pp. 3744–3751, 2013, doi: 10.1523/JNEUROSCI.3302-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Mcintosh J, Anisman H, and Merali Z, “Short- and long-periods of neonatal maternal separation differentially affect anxiety and feeding in adult rats : gender-dependent effects,” Brain Res Dev Brain Res, vol. 113, no. 1–2, pp. 97–106, 1999, doi: 10.1016/s0165-3806(99)00005-x. [DOI] [PubMed] [Google Scholar]
- [50].Santarelli S et al. , “Evidence supporting the match/mismatch hypothesis of psychiatric disorders,” Eur. Neuropsychopharmacol, vol. 24, no. 6, pp. 907–918, 2014, doi: 10.1016/j.euroneuro.2014.02.002. [DOI] [PubMed] [Google Scholar]
- [51].Hsiao YM, Tsai TC, Lin YT, Chen CC, Huang CC, and Sen Hsu K, “Early life stress dampens stress responsiveness in adolescence: Evaluation of neuroendocrine reactivity and coping behavior,” Psychoneuroendocrinology, vol. 67, pp. 86–99, 2016, doi: 10.1016/j.psyneuen.2016.02.004. [DOI] [PubMed] [Google Scholar]
- [52].Santarelli S et al. , “An adverse early life environment can enhance stress resilience in adulthood,” Psychoneuroendocrinology, vol. 78, pp. 213–221, 2017, doi: 10.1016/j.psyneuen.2017.01.021. [DOI] [PubMed] [Google Scholar]
- [53].Ilin Y and Richter-levin G, “Enriched Environment Experience Overcomes Learning Deficits and Depressive-Like Behavior Induced by Juvenile Stress,” PLoS One, vol. 4, no. 1, p. e4329, 2009, doi: 10.1371/journal.pone.0004329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Jacobson-Pick S and Richter-Levin G, “Differential impact of juvenile stress and corticosterone in juvenility and in adulthood, in male and female rats,” Behav. Brain Res, vol. 214, no. 2, pp. 268–276, 2010, doi: 10.1016/j.bbr.2010.05.036. [DOI] [PubMed] [Google Scholar]
- [55].Yee N, Schwarting RKW, Fuchs E, and Wöhr M, “Juvenile stress potentiates aversive 22-kHz ultrasonic vocalizations and freezing during auditory fear conditioning in adult male rats,” Stress, vol. 15, no. 5, pp. 533–544, 2012, doi: 10.3109/10253890.2011.646348. [DOI] [PubMed] [Google Scholar]
- [56].Kaufman DL, Houser CR, and Tobin AJ, “Two Forms of the γ-Aminobutyric Acid Synthetic Enzyme Glutamate Decarboxylase Have Distinct Intraneuronal Distributions and Cofactor Interactions,” J. Neurochem, vol. 56, no. 2, pp. 720–723, 1991, doi: 10.1111/j.1471-4159.1991.tb08211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Stork O et al. , “Postnatal development of a GABA deficit and disturbance of neural functions in mice lacking GAD65,” Brain Res, vol. 865, no. 1, pp. 45–58, 2000, doi: 10.1016/S0006-8993(00)02206-X. [DOI] [PubMed] [Google Scholar]
- [58].Bergado-Acosta JR, Sangha S, Narayanan RT, Obata K, Pape HC, and Stork O, “Critical role of the 65-kDa isoform of glutamic acid decarboxylase in consolidation and generalization of Pavlovian fear memory,” Learn. Mem, vol. 15, no. 3, pp. 163–171, 2008, doi: 10.1101/lm.705408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Blednov YA, Walker DL, Iyer SV, Homanics G, and Harris AR, “Mice lacking Gad2 show altered behavioral effects of ethanol, flurazepam and gabaxadol,” Addict. Biol, vol. 15, no. 1, pp. 45–61, 2010, doi: 10.1111/j.1369-1600.2009.00186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Barrenha GD and Chester JA, “Effects of Cross-Fostering on Alcohol Preference and Correlated Responses to Selection in High- and Low-Alcohol-Preferring Mice,” Alcohol. Clin. Exp. Res, vol. 36, no. 12, pp. 2065–2073, 2012, doi: 10.1111/j.1530-0277.2012.01839.x. [DOI] [PubMed] [Google Scholar]
- [61].Weera MM, Agim ZS, Cannon JR, and Chester JA, “Genetic correlations between nicotine reinforcement-related behaviors and propensity toward high or low alcohol preference in two replicate mouse lines,” Genes, Brain Behav, vol. 18, no. 3, pp. 1–10, 2019, doi: 10.1111/gbb.12515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Kirchhoff AM, Barker EL, and Chester JA, “Endocannabinoids and fear-related behavior in mice selectively bred for high or low alcohol preference,” Brain Sci, vol. 9, no. 10, 2019, doi: 10.3390/brainsci9100254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Akirav I, “Cannabinoids and glucocorticoids modulate emotional memory after stress,” Neurosci. Biobehav. Rev, vol. 37, no. 10, pp. 2554–2563, 2013, doi: 10.1016/j.neubiorev.2013.08.002. [DOI] [PubMed] [Google Scholar]
- [64].Kessler RC, Sonnega A, Bromet E, Hughes M, and Nelson CB, “Posttraumatic Stress Disorder in the National Comorbidity Survey,” Arch Gen Psyciatry, vol. 52, no. 12, pp. 1048–60, 1995, doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- [65].Keiser AA, Turnbull LM, Darian MA, Feldman DE, Song I, and Tronson NC, “Sex Differences in Context Fear Generalization and Recruitment of Hippocampus and Amygdala during Retrieval,” Neuropsychopharmacology, vol. 42, no. 2, pp. 397–407, 2017, doi: 10.1038/npp.2016.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Greiner EM, Müller I, Norris MR, Ng KH, and Sangha S, “Sex differences in fear regulation and reward-seeking behaviors in a fear-safety-reward discrimination task,” Behav. Brain Res, vol. 368, no. April, 2019, doi: 10.1016/j.bbr.2019.111903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Day HLL, Reed MM, and Stevenson CW, “Sex differences in discriminating between cues predicting threat and safety,” Neurobiol. Learn. Mem, vol. 133, pp. 196–203, 2016, doi: 10.1016/j.nlm.2016.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Foilb AR, Bals J, Sarlitto MC, and Christianson JP, “Sex differences in fear discrimination do not manifest as differences in conditioned inhibition,” Learn. Mem, vol. 25, no. 1, pp. 49–53, 2018, doi: 10.1101/lm.045500.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Kreutzmann JC, Jovanovic T, and Fendt M, “Infralimbic cortex activity is required for the expression but not the acquisition of conditioned safety,” Psychopharmacology (Berl), vol. 237, no. 7, pp. 2161–2172, 2020, doi: 10.1007/s00213-020-05527-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Brydges NM, Wood ER, Holmes MC, and Hall J, “Prepubertal stress and hippocampal function: Sex-specific effects,” Hippocampus, vol. 24, no. 6, pp. 684–692, 2014, doi: 10.1002/hipo.22259. [DOI] [PubMed] [Google Scholar]
- [71].Garner JP, Dufour B, Gregg LE, Weisker SM, and Mench JA, “Social and husbandry factors affecting the prevalence and severity of barbering (‘whisker trimming’) by laboratory mice,” Appl. Anim. Behav. Sci, vol. 89, no. 3–4, pp. 263–282, 2004, doi: 10.1016/j.applanim.2004.07.004. [DOI] [Google Scholar]
- [72].Garner JP, Weisker SM, Dufour B, and Mench JA, “Barbering (Fur and Whisker Trimming) by Laboratory Mice as a Model of Human Trichotillomania and Obsessive-Compulsive Spectrum Disorders,” Comp. Med, vol. 54, no. 2, pp. 216–224, 2004. [PubMed] [Google Scholar]
- [73].“https://icd.codes/icd10cm/F633,” online available on 2020–08-28
- [74].Lochner C et al. , “Childhood trauma in obsessive-compulsive disorder, trichotillomania, and controls,” Depress. Anxiety, vol. 15, no. 2, pp. 66–68, 2002, doi: 10.1002/da.10028. [DOI] [PubMed] [Google Scholar]
- [75].Özten E, Hizli Sayar G, Kağan G, Işik S, Karamustafalioğlu O, and Eryilmaz G, “The relationship of psychological trauma with trichotillomania and skin picking,” Neuropsychiatr. Dis. Treat, vol. 11, p. 1203, May 2015, doi: 10.2147/NDT.S79554. [DOI] [PMC free article] [PubMed] [Google Scholar]