Abstract
Introduction
The pediatric heart transplant community uses weight-based donor to recipient size matching almost exclusively, despite no evidence to validate weight as a reliable surrogate of cardiac size. Donor size mismatch is the second most common reason for refusal of donor hearts in current practice (~30% of all refusals). While case-by-case segmentation of total cardiac volume (TCV) by computed tomography (CT) for direct virtual transplantation is an attractive option, it remains limited by the unavailability of donor chest CT. We sought to establish a predictive model for donor TCV based on anthropomorphic and chest x-ray cardiac measures.
Methods
Banked imaging studies from 141 subjects with normal CT chest angiograms were obtained and segmented using 3D modeling to derive TCV. CXR data was available for 62 of those subjects. Three predictive models of TCV were fit via multiple linear regression using the variables: (A) weight only; (B) weight, height, sex and age; (C) weight, height, sex, age, and 1-view AP CXR maximal horizontal cardiac width.
Results
Model C provided the most accurate prediction of TCV (optimism corrected R2=0.99, testing set R2 = 0.98, mean absolute percent error MAPE= 8.6%) and outperformed Model A (optimism corrected R2=0.94, testing set R2=0.94, MAPE = 16.1%) and Model B (optimism corrected R2=0.97, testing set R2=0.97, MAPE = 11.1%).
Conclusions
TCV can be predicted accurately using readily available anthropometrics and a 1-view CXR from donor candidates. This simple and scalable method of TCV estimation may provide a reliable and consistent method to improve donor size matching.
Introduction
Children listed for heart transplantation face the highest waiting list mortality in all transplant medicine with an annual mortality rate of approximately 17%1. Overall, 58% of pediatric donor hearts were refused for pediatric transplantation from 2005 to 2014 and over 30% of potential donor organs are refused due to a perceived size mismatch by the candidate’s care team2. Thirty four percent of all donor hearts are discarded and 20% of donor hearts without marginal criteria are discarded3. Though efforts to increase organ donation continue, attention has shifted to optimizing the organ allocation process and ensuring use of all viable organs4,5.
Currently, donor-recipient body weight (DRBW) ratio is the primary measure used for donor-recipient size-matching in pediatric heart transplantation. Although body weight has been used as a surrogate measure for cardiac size, the precise relationship between body size and heart size has not been clearly defined in the pediatric population. Therefore, its use as the sole anatomic-based measurement to match donors to recipients for pediatric heart transplant may result in both unsuitable matches and missed transplant opportunities5–7. Unnecessarily refusing and passing organs on to the next recipient counteracts the intentions of priority-based organ allocation. Furthermore, DRBW-based size matching has not consistently been shown to correlate with outcomes, which provides incentive to look for additional size matching paradigms8,9. Previous studies have demonstrated the feasibility of expanding the donor upper limit of size match by direct visual confirmation of donor-to-recipient organ size match using cross-sectional imaging, often referred to as “virtual transplantation” 8–10. While a direct virtual transplant is a more sophisticated approach to donor-recipient organ matching than DRBW, this is logistically challenging due to the limited availability of cross-sectional imaging for most donors and the time required for segmentation and planning.
We propose an alternative method where the recipient TCV is directly measured from a recent cross-sectional imaging study, and then compared to the predicted TCV of the donor to assess for donor:recipient mismatch due to oversizing. The predicted TCV would be derived from available clinical data using a predefined mathematical model. Using readily available anthropometric data, we sought to develop a predictive model for donor TCV. We produced several models for the prediction of TCV based on weight, height, sex, age, and CXR maximal horizontal cardiac width. We hypothesized that the inclusion of height, sex, age, and 1-view CXR maximal horizontal cardiac width would enhance the accuracy of predictive models when compared to a weight-based model.
Methods
Data Source
This study was approved by Cincinnati Children’s Hospital Institutional Review Board prior to study initiation. A retrospective review of Cincinnati Children’s Hospital Picture Archiving and Communication Systems (PACS) database was performed to identify pediatric and young adult patients (age 0–30 years) with normal cardiac anatomy on clinically indicated chest computed tomography angiography (CTA). Subjects with incomplete capture of cardiac structures or any clinically identified cardiac abnormality, including nonspecific chamber dilation, were excluded. Additional exclusions included subjects with pulmonary embolism, chronic anemia, large airway malformations, parenchymal lung disease, large intrathoracic mass, genetic syndrome, and body mass index > 55. Demographic data was collected via chart review including date of birth, sex, ethnicity, race, weight, and height. Body surface and body mass index were derived from patient height and weight11.
CT Segmentation for Total Cardiac Volume
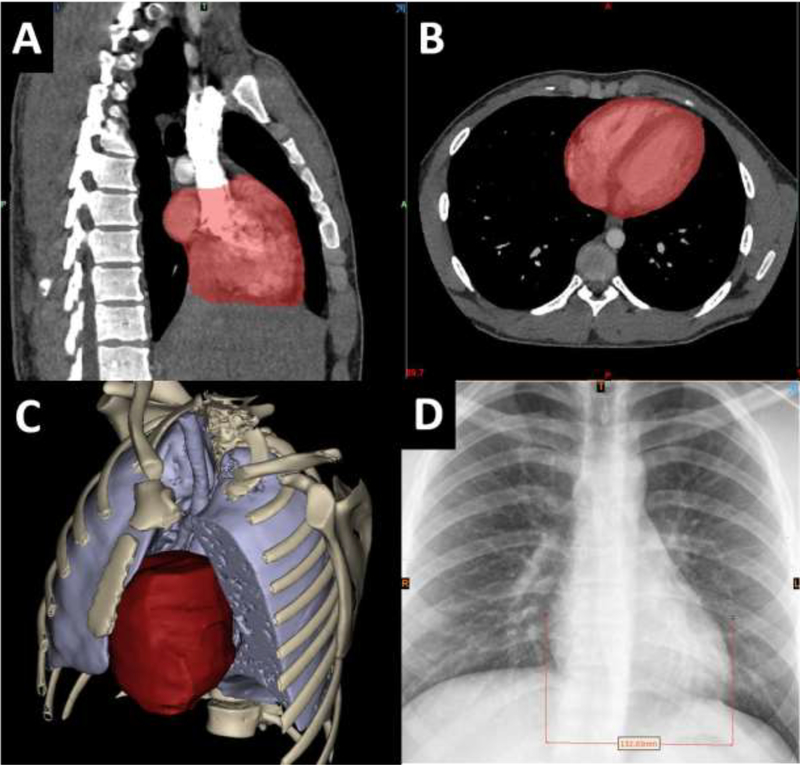
CT data was imported into Mimics 3D medical modeling software (Materialise Inc., Belgium) and semi-automatic segmentation of the chest structures was performed, as previously described8,10. The TCV segmentation was defined as the myocardial mass and internal heart chamber volume bounded at the approximate levels of surgical anastomosis for a bicaval orthotopic heart transplantation. Each TCV measurement included the border of the myocardial mass up to the junction of the superior vena cava (SVC) and inferior vena cava (IVC) to the right atrium junction of the pulmonary veins to the left atrium, and the great arteries to the level of the aortic and pulmonary roots (Figure 1). The primary variable of interest was TCV as this is the expected major determinant for heart-size matching success in bicaval orthotopic heart transplantation. When available, the most recent CXR (anteroposterior projection, 1-view) was reviewed and cardiac width was measured as the distance between the left and right heart borders as described previously12,13 (Figure 1–D). A 1-view anteroposterior projection CXR was used because this type of projection is routinely performed and available in critically ill transplant donor patients.
Figure 1.
An example 3D reconstruction showing A) sagittal view of segmentation mask showing SVC and IVC cutoff B) axial View of segmentation mask C) example 3d Reconstruction of heart, lungs, bones and D) example CXR heart diameter measurement.
Model Construction
Variables with a strong correlation with TCV were selected for model inclusion. These predictors included weight (kg), height (cm), age (years), and maximal horizontal cardiac width (cm). Sex was included in prediction models as a binary variable. Data on maximal horizontal cardiac width was available for 62 subjects. The statistical programming language R was used for all modeling and statistical analysis. Multiple imputation as implemented by the Hmisc package function aregImpute (version 4.2.0)14 was used to impute maximal horizontal cardiac width for those with unobserved values. All model predictors and TCV were included in the imputation model. A total of n=50 imputed datasets were generated using flexible parametric additive regression with three knots provided for each continuous variable. Ordinary least squares regression as implemented by the rms package function ols (version 5.1.3.1)14 was used to predict TCV after transformation to the natural log scale. Log transformation was performed to account for allometric growth patterns through the pediatric age range15,16.
Three primary models were developed. Model A used weight as the sole predictor and served as the base model to assess the performance of the current size matching process. Model B incorporated weight, height, sex, and age to assess the performance of additional anthropometric measures and sex on model predictions. Model C was similar to Model B, but with the addition of CXR 1-view maximal horizontal cardiac width and included imputed values whereas, no imputed values were used to develop Models A or B. An additive model with restricted cubic spline terms with knots placed at the 5th, 35th, 65th, and 95th percentiles to capture potential non-linear associations was first fit to gauge feature importance based on degree of freedom adjusted chi-square tests for each term. Age provided the smallest contribution to model fit and was therefore modeled as a linear term to reduce the model degrees of freedom. Four knots were retained for all other continuous predictors.
Analysis of Model Accuracy
Split-sample validation and internal resampling were used to assess model performance. A random selection of n=100 subjects were chosen to serve in the development set and the remaining n=41 subjects were held out to serve as the test set for split-sample validation. Internal resampling was conducted using 1000 bootstrap samples to obtain optimism corrected measures of model performance for the training set and full set of n=141 participants. The optimism corrected R2, and mean absolute percent error (MAPE) are reported for the development set and for models containing all 141 subjects. The model R2 and MAPE are reported for the full test set. Validation after multiple imputation was performed by averaging the values obtained for each multiply imputed dataset. Optimism correct estimates of model performance were obtained using the rms package function validate.
Inter-observer Variability
The primary observer (NAS) and an experienced imaging cardiologist (RAM) performed blinded intraobserver and interobserver repeat measurements of TCV on 10% of the subjects. Reliability of observations was assessed using intraclass correlation coefficient (ICC).
Results
Subject characteristics are summarized in Table 1. A 1-view CXR was available for 62 patients. There were no statistically significant differences in age, height, and weight between patients with or without a CXR. Male subjects (55%; median [IQR] = 9.8 [14.8] years) tended to be younger than female (15.0 [8.3] years) subjects on average (Wilcoxon rank-sum p-value = 0.02). The most common indications for CTA were evaluation for pulmonary embolus, trauma, and evaluation of anatomic abnormality such as a vascular ring or airway abnormality.
Table 1:
Patient characteristics of 141 subjects.
| Median | IQR | Range | |
|---|---|---|---|
| Weight (kg) | 47.5 | 14.6 – 71.7 | 2.2 – 149 |
| Height (cm) | 150 | 95 – 167 | 40 – 185 |
| Age (years) | 13.5 | 3.1 – 17.2 | 0.1 – 24.8 |
| BSA (m2) | 1.47 | 0.6 – 1.8 | 0.15 – 2.60 |
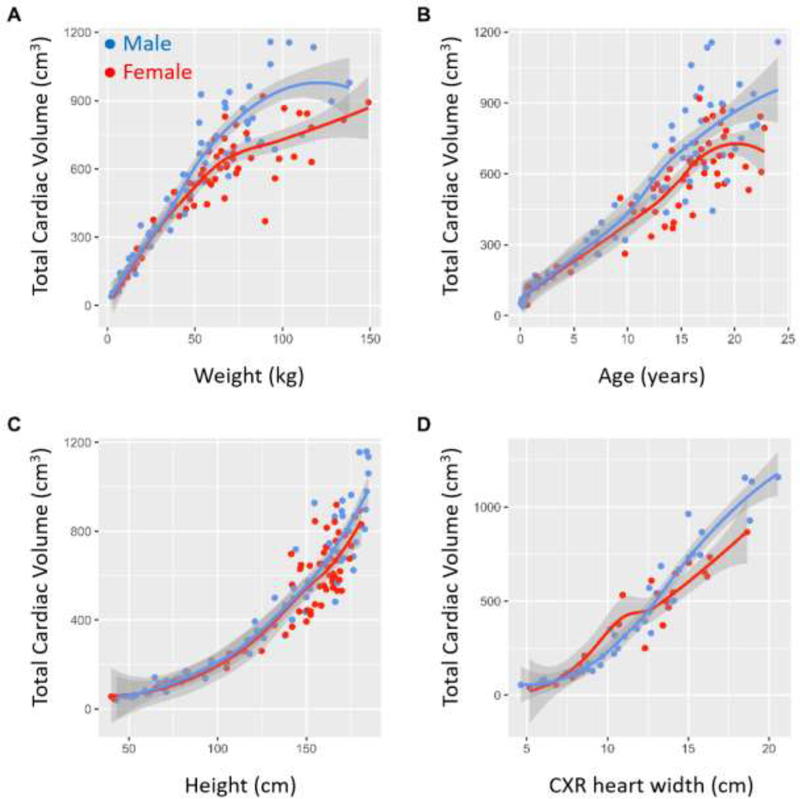
The univariate relationships between TCV and weight, height, age, and CXR heart width are shown in Figure 2. TCV is positively correlated with weight in a nonlinear manner with marked dispersion at higher weights. TCV is positively correlated with age but with high variability. TCV has an exponential relationship with height.
Figure 2.
Univariate relationship between predictor variables and TCV. Blue dots represent male patients and red dots represent female patients.
Model Performance
The parameters for model performance on a logarithmic scale for Models A, B, and C using split-and re-sampling technique are summarized in Table 2. There is incremental improvement in model performance with the inclusion of additional variables. Model C provides an accurate prediction of TCV (optimism corrected R2 = 0.98, validation set R2 = 0.99, mean absolute percent error MAPE = 1.11%) and performed better than Model A (optimism corrected R2 = 0.95, validation set R2 = 0.94, MAPE = 3.17%) and Model B (optimism corrected R2 = 0.97, validation set R2 = 0.97, MAPE = 2.19%) [See Supplemental Table 1]. Model A is shown to have greater error at lower and upper weight range as shown in the calibration plots when compared to Models B and C (Figure 3).
Table 2:
Model characteristics and performance (logarithmic scale) using split sampling
| Model A | Model B | Model C | |
|---|---|---|---|
| Model terms | Weight | Weight, Height, Age, Sex | Weight, Height, Age, Sex, CXR Heart Diameter |
| Training set | |||
| Sample size (n) | 100 | 100 | 100 |
| Optimism corrected R2 | 0.95 | 0.97 | 0.98 |
| MAPE | 2.76 | 1.91 | 1.49 |
| Testing set | |||
| Sample size (n) | 41 | 41 | 41 |
| R2 | 0.94 | 0.97 | 0.99 |
| MAPE | 3.17 | 2.19 | 1.11 |
Figure 3.
Testing Set calibration plots of models A, B, and C show improvement in model performance and the least amount of scatter in Model C.
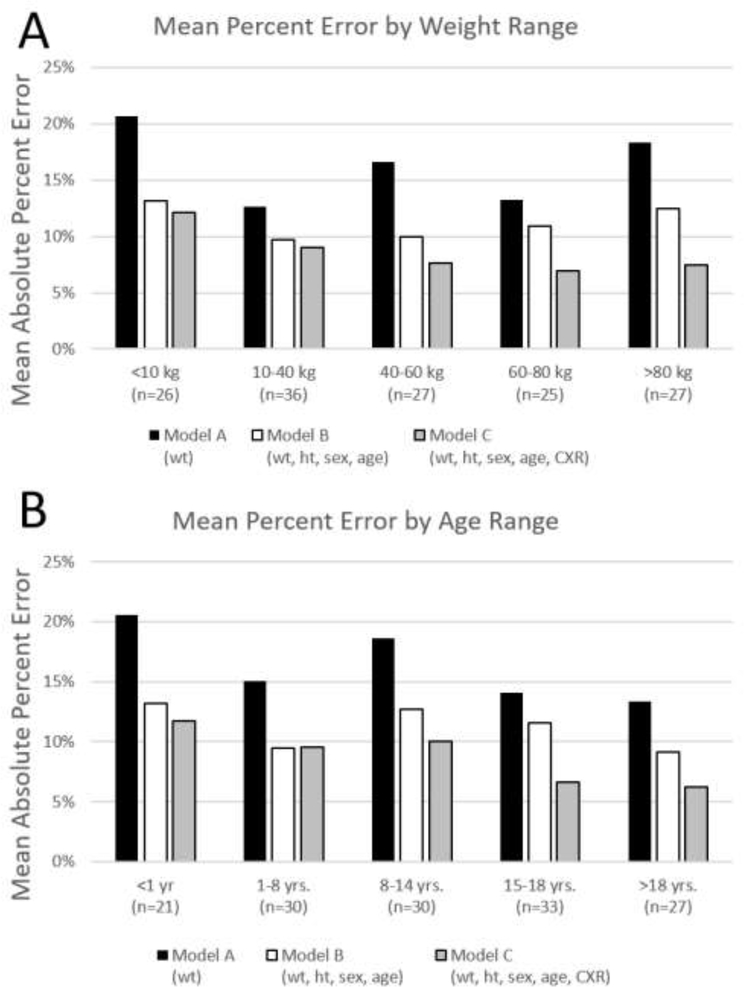
The MAPE calculated after transforming the observed and predicted values back to the original TCV scale were 16.1% for Model A and 11.1% for Model B. The MAPE on the original scale is lowest for Model C at 8.6%. Model C has the lowest error across all age ranges (Figure 4A) and weight ranges (Figure 4B). Model C is more accurate at higher age and weight ranges. In general, there is higher error in TCV prediction for all models at lower age and weight ranges.
Figure 4.
Model Mean Absolute Percent Error subcategorized by A) weight range and B) age range.
Reliability
For a randomly selected 10% of study subjects, reliability analysis was performed. The intraobserver ICC was 0.99 and the interobserver ICC was 0.99.
Discussion
The allometric relationship between body growth and heart growth is the basis for the weight-based criteria commonly used in pediatric cardiac transplantation, but this approach is confounded by uncertainty17–19. We now have robust imaging datasets that can directly define the relationships of body size to heart size. Consequently, the size match evaluation for heart transplant can and should be refocused on an evidence-based approach using such data. This study establishes an accurate method for estimating donor TCV using available clinical data and a multiple linear regression model. Though there is a complex relationship between indices of body size and TCV across the pediatric age range, we have shown that heart size can be accurately predicted by utilizing readily available patient specific measures. More importantly, this study describes the non-linear relationship of weight to allograft size and estimation methods using just weight which is key if a program, like ours, is still using weight for listing.
Most heart donors do not have cross-sectional imaging available for direct comparison of TCV, so we have devised a novel method for estimation of TCV to enable a size matching process for the clinical scenario where the recipient TCV is known. The TCV predictive models can be used by heart transplant centers to first determine the maximum weight limit for listing in UNOS and then to perform a rapid size match when a donor becomes available. At our institution, we recently started to select the maximum weight threshold by measuring the recipient TCV and comparing to the normative data for TCV. When a potential donor heart becomes available, a rapid size match assessment can be performed by comparing the directly measured recipient TCV to the predicted donor TCV provided using a predictive model.
Oversizing of donor hearts may lead to several immediate post-operative complications including pulmonary venous compression, bronchial compression, and post-operative open chest18. The uncertainty associated with weight-based size matching may cause donor centers to avoid borderline hearts; however, research focused on this hypothesis is needed. As shown in the case example below, a targeted approach to defining the upper limit of size matching can mitigate this uncertainty and increase the available donor pool for specific patients.
Case Example
Patient A is a 16 year old male (weight 64kg, height 177cm, BSA 1.79m2) with dilated cardiomyopathy with severe LV dysfunction status post Heartmate III VAD placement. He was listed for heart transplantation with an initial donor weight range of 60–80kg, corresponding to an upper limit of DRWR of 130%, consistent with the actual matched DRWR across the United States2. Using the process described above, the total cardiac volume from a CT scan was calculated to be 1164 cm3. After comparing this TCV to the normal patient donor pool, the upper limit of the weight range was increased to 100kg. After 5 days at an increased listing weight, the patient was offered a suitable donor heart from a male donor (22 years old, weight 91.5 kg, height 182 cm, BSA 2.13m2). The donor’s predicted total cardiac volume using Model B was 978 cm3 (~84% of recipient TCV). There were no intraoperative complications related to oversizing. He was extubated on post-operative day 1, transferred from the intensive care unit on post-operative day 8, and discharged on post-operative day 12.
We include heart width from 1-view CXR into this analysis because the majority of CXRs in the ICU are acquired as a single anteroposterior view, and heart width can be easily and repeatably measured12,13. The inclusion of CXR into the model provides an incremental improvement to model accuracy. To confirm that data imputation did not cause any bias toward model accuracy, the model analysis was repeated using only the 62 patients with CXR available. Bootstrap resampling was used rather than split sample validation for this method given the small sample size. The results are similar in that Model C (optimism corrected R2 = 0.98, MAPE = 1.41%) outperforming Model B (optimism corrected R2 = 0.97, MAPE = 2.17%) and Model A (optimism corrected R2 =0.95, MAPE=3.01) as summarized in Table 3.
Table 3.
Model characteristics and performance (log scale) for subject with CXR available
| Model A | Model B | Model C | |
|---|---|---|---|
| Model terms | Weight | Weight, Height, Age, Sex | Weight, Height, Age, Sex, CXR Heart Diameter |
| Sample size (n) | 62 | 62 | 62 |
| Optimism corrected R2 | 0.95 | 0.97 | 0.98 |
| MAPE | 3.01 | 2.17 | 1.41 |
Model C has the lowest error of all models across nearly all weight and age ranges, as shown in Figure 4. The consistently low error suggests that this model performs well across all potential donor sizes. Model C differs from Model B only in the addition of CXR as a predictor variable and shows decreased error for older patients and those with higher weight. This suggests that the variability seen in TCV at higher weight is mitigated by the use of CXR as an additional predictor variable.
There was a relative increase in error for all models at lower age and weight. This is likely due to the difficulty in creating a universal model for a wide spectrum of subjects from infant to young adult. In smaller subjects, even small degrees of variance in TCV prediction resulted in a larger percent error. In future studies, this error may be improved by creating a separate TCV prediction model for infants.
We anticipate that predictive modeling for the estimation of TCV will have significant value for the heart transplantation community related to size matching and maximizing donor usage. DRWR-based size matching is not consistently associated with outcomes, so there is potential with volume-based size matching to improve short and long-term outcomes of heart transplantation4. Gong et al. examined the effects of “undersizing” donor hearts in adult heart transplantation by using a validated anthropomorphic-based model for predicted heart mass (PHM) and found that the PHM was a better predictor of primary graft dysfunction when compared to size match based on total body weight17. Similarly, Kransdorf et al. demonstrated increased 1-year mortality for patients with undersized heart transplants based on PHM; undersizing based on weight, height, BSA, or BMI ratio had no effect on survival. Though PHM is shown to predict outcomes related to undersizing, it is not shown to predict outcomes related to oversizing of heart transplants20.
This tool for predicted TCV can primarily be used to examine both short-term morbidity related to allograft oversizing (delayed chest closure, bronchial compression, prolonged length of stay, etc.) as well as mortality. TCV carries potential advantage over PHM in assessing for oversizing by encompassing the entirety of the graft volume rather than just the ventricular mass. Similarly, TCV-based size matching may have advantages over DRWR-based matching, as was noted in a recent retrospective review of pediatric heart transplantations in UNOS from 1989 to 2019 where TCV-based size matching ratio predicted survival and DRWR-based matching did not21.
Though the focus of this manuscript is size mismatch, we recognize that donor:recipient matching for heart transplantation is a multifaceted system including donor organ quality, travel time, recipient stability, and immunological compatibilities. We believe that TCV provides a new additional measure to strengthen size match and will help mitigate the uncertainty of size matching though incorporation of multiple variables of donor size into a single measure.
This preliminary study of TCV for size matching must be validated in actual donor-recipient size matches before gaining widespread acceptance and use. Future studies will assess the reliability of TCV-based size matching in predicting adverse events related to oversizing in a retrospectively attained cohort of heart transplant recipients.
Limitations
This study is limited by being a single center retrospective study and potential bias from a convenience sample of patients. For widespread adoption of a cardiac volume-based size matching process, the model proposed in this study would need to be reproduced in a larger patient cohort. Additionally, control of operator error will be necessary to have comparable TCV values across institutions.
Measurement of TCV was found to be highly reliable within and between observers, which is likely attributable to the semi-automated nature of the 3D reconstruction protocol. Manual segmentation was required only for identifying the area of surgical anastomosis and several tissue interfaces. Despite this, there are minor differences between observers. Image segmentation is predominantly based on difference in contrast resolution between adjacent structures. Myocardium, liver, thymus, diaphragm and skeletal muscle have similar Hounsfield units, so segmentation of adjacent areas of these anatomic structures can lead to minor discrepancies amongst observers. Additionally, there is minor variation in the identification of surgical anastomosis cutoff location between observers.
Conclusion
TCV can be accurately predicted from readily available anthropometrics and CXR heart width. This simple method of TCV estimation can provide a reliable and consistent method to assess donor TCV. In future studies, TCV-based size matching can be used to set more accurate donor size criteria and assess the correlation of TCV with patient outcomes.
Supplementary Material
Acknowledgements and Disclosures
Dr. Zafar, Dr. Morales, and Dr. Moore’s time on this project was supported by NIH grant 1R01HL147957-01. Dr. Lorts reports grants from Berlin, grants and personal fees from Abbott, grants and personal fees from Medtronic, personal fees from Syncardia, personal fees from Abiomed, all outside the submitted work. Dr. Morales reports consultant fee from Cormatrix, Inc., personal fees from Syncardia, Inc., personal fees and consultant fee from Abbott Medical Inc., personal fees and consultant fee from Xeltis, Inc., consultant fee from Berlin Heart, Inc., all outside the submitted work. All other authors have no pertinent disclosures to report.
Abbreviations:
- CT
Computed tomography
- DICOM
Digital Imaging and Communications in Medicine
- TCV
Total cardiac volume
- UNOS
United Network for Organ Sharing
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Nicholas A. Szugye, The Heart Institute, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Division of Biostatistics and Epidemiology, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Department of Pediatrics, University of Cincinnati College of Medicine, Cincinnati, OH.
Farhan Zafar, The Heart Institute, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Division of Biostatistics and Epidemiology, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Department of Pediatrics, University of Cincinnati College of Medicine, Cincinnati, OH.
Nicholas J. Ollberding, The Heart Institute, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Division of Biostatistics and Epidemiology, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Department of Pediatrics, University of Cincinnati College of Medicine, Cincinnati, OH.
Chet Villa, The Heart Institute, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Division of Biostatistics and Epidemiology, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Department of Pediatrics, University of Cincinnati College of Medicine, Cincinnati, OH.
Angela Lorts, The Heart Institute, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Division of Biostatistics and Epidemiology, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Department of Pediatrics, University of Cincinnati College of Medicine, Cincinnati, OH.
Michael D. Taylor, The Heart Institute, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Division of Biostatistics and Epidemiology, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Department of Pediatrics, University of Cincinnati College of Medicine, Cincinnati, OH.
David L.S. Morales, The Heart Institute, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Division of Biostatistics and Epidemiology, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Department of Pediatrics, University of Cincinnati College of Medicine, Cincinnati, OH.
Ryan A. Moore, The Heart Institute, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Division of Biostatistics and Epidemiology, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Department of Pediatrics, University of Cincinnati College of Medicine, Cincinnati, OH.
References
- 1.Zafar F, Castleberry C, Khan MS, et al. Pediatric heart transplant waiting list mortality in the era of ventricular assist devices. J Heart Lung Transplant. 2015;34(1):82–88. [DOI] [PubMed] [Google Scholar]
- 2.Riggs KW, Giannini CM, Szugye N, et al. Time for evidence-based, standardized donor size matching for pediatric heart transplantation. J Thorac Cardiovasc Surg. 2019;158(6):1652–1660 e1654. [DOI] [PubMed] [Google Scholar]
- 3.Morrison AK, Gowda C, Tumin D, et al. Pediatric marginal donor hearts: Trends in US national use, 2005–2014. Pediatr Transplant. 2018;22(5):e13216. [DOI] [PubMed] [Google Scholar]
- 4.Rizwan R, Zafar F, Bryant R 3rd, et al. The Number of Refusals for Donor Organ Quality Does Not Impact Heart Transplant Outcomes in Children. Ann Thorac Surg. 2018;105(4):1223–1230. [DOI] [PubMed] [Google Scholar]
- 5.Zuckerman WA, Richmond ME, Singh RK, Chen JM, Addonizio LJ. Use of height and a novel echocardiographic measurement to improve size-matching for pediatric heart transplantation. J Heart Lung Transplant. 2012;31(8):896–902. [DOI] [PubMed] [Google Scholar]
- 6.Patel ND, Weiss ES, Nwakanma LU, et al. Impact of donor-to-recipient weight ratio on survival after heart transplantation: analysis of the United Network for Organ Sharing Database. Circulation. 2008;118(14 Suppl):S83–88. [DOI] [PubMed] [Google Scholar]
- 7.Delmo Walter EM, Huebler M, Schubert S, et al. Influence of size disparity of transplanted hearts on cardiac growth in infants and children. J Thorac Cardiovasc Surg. 2012;143(1):168–177. [DOI] [PubMed] [Google Scholar]
- 8.Szugye NA, Lorts A, Zafar F, Taylor M, Morales DLS, Moore RA. Can virtual heart transplantation via 3-dimensional imaging increase the maximum acceptable donor size? J Heart Lung Transplant. 2019;38(3):331–333. [DOI] [PubMed] [Google Scholar]
- 9.Plasencia JD, Kamarianakis Y, Ryan JR, et al. Alternative methods for virtual heart transplant-Size matching for pediatric heart transplantation with and without donor medical images available. Pediatr Transplant. 2018;22(8):e13290. [DOI] [PubMed] [Google Scholar]
- 10.Shugh SB, Szugye NA, Zafar F, et al. Expanding the donor pool for congenital heart disease transplant candidates by implementing 3D imaging-derived total cardiac volumes. Pediatr Transplant. 2020;24(1):e13639. [DOI] [PubMed] [Google Scholar]
- 11.Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition. 1989;5(5):303–311; discussion 312–303. [PubMed] [Google Scholar]
- 12.Chon SB, Oh WS, Cho JH, Kim SS, Lee SJ. Calculation of the cardiothoracic ratio from portable anteroposterior chest radiography. J Korean Med Sci. 2011;26(11):1446–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sahin H, Chowdhry DN, Olsen A, Nemer O, Wahl L. Is there any diagnostic value of anteroposterior chest radiography in predicting cardiac chamber enlargement? Int J Cardiovasc Imaging. 2019;35(1):195–206. [DOI] [PubMed] [Google Scholar]
- 14.Team R. R: A language and environment for statistical computing. R Foundation for Statistical Computing; https://www.r-project.org/ Published 2018 Accessed September 15, 2020, 2020. [Google Scholar]
- 15.Li JK. Cardiovascular Allometry: Analysis, Methodology, and Clinical Applications. Adv Exp Med Biol. 2018;1065:207–224. [DOI] [PubMed] [Google Scholar]
- 16.Thompson DAW. On Growth and Form.: Cambridge [Eng.]University press; 1917. [Google Scholar]
- 17.Gong TA, Joseph SM, Lima B, et al. Donor predicted heart mass as predictor of primary graft dysfunction. J Heart Lung Transplant. 2018;37(7):826–835. [DOI] [PubMed] [Google Scholar]
- 18.Razzouk AJ, Johnston JK, Larsen RL, Chinnock RE, Fitts JA, Bailey LL. Effect of oversizing cardiac allografts on survival in pediatric patients with congenital heart disease. J Heart Lung Transpl. 2005;24(2):195–199. [DOI] [PubMed] [Google Scholar]
- 19.Tremblay-Gravel M, Khush K. Predicted Heart Mass for Donor Organ Allocation: Getting Closer to the Target. Circ Heart Fail. 2019;12(12):e006634. [DOI] [PubMed] [Google Scholar]
- 20.Kransdorf E, Kittleson M, Patel J, et al. Predicted Heart Mass Is the Optimal Metric for Size Matching in Heart Transplantation. J Heart Lung Transpl. 2017;36(4):S113–S113. [DOI] [PubMed] [Google Scholar]
- 21.Dani A, Szugye NA, Thangappan K, et al. Total Cardiac Volume Ratio, Unlike Donor-recipient Weight Ratio, Correlates with Pediatric Heart Transplant Survival. Paper presented at: ASAIO Virtual On Demand 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.