Abstract
Background.
The preference for immediate rewards and high sensation seeking are both potent risk factors for alcohol use disorder (AUD), but how they interact during intoxication is poorly understood. To model decision-making linked to AUD risk, we tested heavy drinkers for impulsive choice (delay discounting with alcohol:money or money:money) and behavioral sensation seeking using a novel odor choice task. Laboratory tasks measured actual behavior with real contingencies. Our goals were to determine, in heavy drinkers, 1) alcohol’s effects on delay discounting, and 2) how AUD risk factors relate to delay discounting, and 3) how delay discounting with alcohol choices compares with strictly monetary choices.
Methods.
Thirty-five heavy drinkers (≥2 binges per month; age = 22.8 ±2.2; 20 male; 5.8 ±2.3 drinks/drinking day) performed cross-commodity discounting (CCD) of immediate alcohol vs. delayed money, a monetary delay discounting (DD), and behavioral sensation seeking tasks. CCD and DD were performed while sober and during controlled alcohol infusion targeting 0.08 g/dL. The behavioral sensation seeking task presented binary choices of odorants varying in intensity and novelty, and the risk of exposure to a malodorant.
Results.
CCD and DD behaviors were highly correlated across conditions, mean r = .64. Alcohol increased delayed reward preference in DD, p = .001, but did not alter mean CCD, p > .16. However, alcohol-induced changes in CCD correlated with behavioral sensation seeking, such that higher sensation seekers’ immediate alcohol preference increased when intoxicated, p = .042; self-reported sensation seeking was uncorrelated, ps > .08. Behavioral sensation seeking also correlated with “want” alcohol following a priming dose targeting 0.035 g/dL, p = .021. CCD and DD did not correlate with self-reported drinking problems or other personality risk traits.
Conclusions.
Alcohol increased impulsive alcohol choice in high sensation seekers, suggesting an interaction that may underlie impaired control of drinking, at least in a subset of heavy drinkers—consistent with models highlighting high novelty/sensation seeking AUD subtypes. Discounting behavior overall appears to be a generalized process, and relatively stable across methods, repeated testing, and intoxication. These findings further support the utility of behavioral tasks in uncovering key behavioral phenotypes in AUD.
Keywords: alcoholism, addiction, ethanol, novelty-seeking, loss of control
Introduction
Reward impatience is a putative endophenotype for alcohol and substance use disorders (Bickel et al., 2014, MacKillop, 2013), across drug type (Amlung et al., 2017), which is readily quantified as the devaluation of delayed rewards (Ainslie, 1975). Prior findings with monetary delay discounting (DD) suggest that steep discounting corresponds with addiction risk for alcohol and other drugs (Amlung et al., 2017, MacKillop et al., 2011). While possessing trait-like qualities (high test-retest reliability (Ohmura et al., 2006, Kirby, 2009) and between-commodity correlation (Odum, 2011)), discounting behavior can be manipulated with various interventions (see Koffarnus et al., 2013, Rung and Madden, 2018 for reviews). Single-commodity discounting paradigms reveal that consumable rewards (drug and non-drug) are consistently discounted more steeply than money (Odum, 2011), suggesting that commodity type interacts with temporal discounting processes (‘domain effect’; Baker et al., 2003). Discounting paradigms can also quantify reward impatience by posing choices between commodity types, mirroring many real-world decisions concerning rewards. This type of decision-making, cross-commodity discounting (CCD), is more sensitive than DD to drug deprivation (Mitchell, 2004), severity of use (Moody et al., 2017), and in predicting abstinence (Yoon et al., 2009). Arguably, CCD better models real-world decisions with mixed commodities, such as the decision to forgo another drink in favor of spending that money on a loved one. Thus, CCD may exceed DD in providing enhanced insight to pathological drug taking by better modeling naturalistic choices. Discounting tasks quantify the inhibition of immediate reward drive in service of delayed rewards expected in the future. Traits related to reward drive itself, such as novelty or sensation seeking, are also key addiction phenotypes, and widely shown to differentiate alcohol use disorder (AUD) subtypes (Cloninger et al., 1988, Ooteman et al., 2006, Mann et al., 2018).
Sensation seeking correlates with maladaptive behaviors such as drug and alcohol use, drunk driving, risky sexual behavior, criminality, and addiction (Arnett, 1994, Donohew et al., 2000, Zuckerman and Neeb, 1980, Arnett, 1990). Self-reported sensation seeking (risk-taking in search of intense, novel, and varied experiences) predicts later alcohol and drug use/abuse in longitudinal studies of children and adolescents (Cloninger et al., 1988, Pedersen, 1991), and corresponds with early-onset AUD, (Dom et al., 2006). Often characterized as an element of self-reported impulsivity, self-reported sensation seeking is an independent facet (Whiteside and Lynam, 2001) that shows a unique developmental profile (Steinberg et al., 2008), and may explain unique variance (Mitchell, 1999) in identifying risk severity in AUD (Dom et al., 2006). However, the oft-reported association between self-reported sensation seeking and alcohol and drug use may be partially confounded by social desirability response bias’s opposite effects on the two variables—that is, simultaneously over-reporting positively-perceived adventurous and risky activities (Toma and Carlson, 2015, Lupton and Tulloch, 2002), and under-reporting negatively-perceived alcohol and illicit drug use (Northcote and Livingston, 2011, Magura and Kang, 1996). Unreliable self-report can be exacerbated by inventories with unusual or novel items (Fan et al., 2006), which are typical in assessments of self-reported sensation seeking. Moreover, behavioral risk-taking explains additional variance in risky behavior compared to self-reported impulsivity or sensation-seeking alone (Lejuez et al., 2007).
Little prior work has utilized behavioral measures of sensation seeking, thus its relationship to addiction risk is largely unstudied. To address this gap, we recently developed and validated a laboratory measure of behavioral sensation seeking, and demonstrated that high behavioral sensation seeking corresponds with illicit drug taking (Oberlin et al., 2019). The relationship between behavioral sensation seeking and decision-making during intoxication remains unknown, but previous research using self-report measures suggests that, when compared to intoxicated low sensation seeking drinkers, high sensation seeking drinkers exposed to alcohol report increased alcohol reward and impaired baseline inhibitory control (Fillmore et al., 2009). We postulate that behavioral sensation seeking, as distinct from self-reported sensation seeking, may reveal important new interactions with discounting and intoxication.
Most discounting studies test non-intoxicated subjects, and fewer still test heavy drinkers or those with AUD. Those designs may fail to capture the reality of AUD, however, as heavy drinkers arguably make many relevant intertemporal choices after consuming their first drink, such as re-negotiating self-imposed drinking limits, and purchasing additional alcohol to continue drinking. Intoxicated decision-making can be assessed in the laboratory, but it requires precise alcohol delivery to control individual differences in brain exposure (e.g., physiologically-based pharmacokinetically modeled alcohol infusion; O’Connor et al., 1998). Defying conventional wisdom, alcohol’s acute effect on delay discounting is yet unclear, as studies find increased discounting (Reynolds et al., 2006, Reed et al., 2012), no effect (Richards et al., 1999, Bidwell et al., 2013, Dougherty et al., 2008, Rose and Grunsell, 2008, Bernhardt et al., 2019), or trends of decreased discounting (Ortner et al., 2003) in monetary DD tasks. In light of these varied findings, a task presenting choices for immediate alcohol against other rewards (CCD)—might better model alcohol’s effects on decision-making.
As we are unaware of published work in heavy drinkers performing alcohol-money cross-commodity discounting with actual alcohol rewards, we tested CCD to model alcohol decision-making in heavy drinkers, and compared this behavior with a well-established discounting paradigm (single-commodity monetary DD). We also compared behavioral sensation seeking to CCD and DD within subjects; while both sober and intoxicated. Our hypotheses were that: H1) Intoxication increases impulsivity in CCD, H2) CCD behavior correlates with AUD risk factors including behavioral sensation seeking, and H3) CCD correlates with monetary DD behavior.
Materials and Methods
Subjects
Forty-three healthy non-treatment seeking heavy drinkers, aged 21–45, and English-speaking, were recruited from community advertisements, provided informed consent prior to study procedures, and paid in cash for participating after study completion. Only the n=35 meeting criteria for analyses are included in this report. All procedures were approved by the Indiana University Institutional Review Board. Recent drinking and alcohol-related problems were determined at the in-person interview with the 35-day Timeline Followback self-report (TLFB; Sobell et al., 1986), the Alcohol Use Disorders Identification Test (AUDIT; Saunders et al., 1993), and the Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA; Bucholz et al., 1994). Heavy drinking was defined as a minimum of 2 heavy drinking days (≥5 or 4 drinks for males or females, respectively; Gunzerath et al., 2004) in a 4-week period, with 97% of the analyzed sample reporting ≥1 heavy drinking day per week, and 83% of the sample with AUDIT scores ≥8. Subjects also provided demographic information during the interview, including monthly income (from any source) and disposable income. On the study day, subjects reported to the Indiana University Clinical Research Center and completed personality measures, performed discounting tasks (monetary DD and cross-commodity), and underwent intravenous alcohol infusion. All tasks and inventories were presented on a laptop computer using Eprime 2.0 (Psychology Software Tools, Inc, Sharpsburg, PA). Subjects were instructed to abstain from alcohol or other drugs for 48 hours prior to the study day, and were excluded for current intoxication or recent illicit drug use—verified by breath alcohol concentration (BrAC) and urine screen (positive marijuana screens were accepted in the absence of visual and/or behavioral indicators of intoxication). Other exclusions included: history of smell or taste disorders, positive urine pregnancy screen, positive urine toxicology screen for illicit substances (except marijuana), current use of any psychotropic medication, history or presence of organic brain disease, current psychiatric disorders (including substance use disorder), or major medical disorders limiting behavioral performance. Daily tobacco users were provided a nicotine patch during the study to mitigate nicotine withdrawal. Four subjects were excluded from data analyses due to zero discounting (complete delay preference), one for complete discounting (complete immediate preference), and three for non-systematic discounting (Johnson and Bickel, 2008) in the cross-commodity task; the analysis sample (n=35) is described in Table 1.
Table 1.
Subject Characteristics (n=35)
| Mean (SD) | Range | n(%) | |
|---|---|---|---|
| Male | 20 (57) | ||
| Caucasian | 29 (83)h | ||
| Nicotinea | 4 (11) | ||
| Family history positiveb | 13 (37) | ||
| Age | 22.8 (2.2) | 21–28 | |
| Education (years) | 15.0 (1.0) | 13–17 | |
| Drinks per weekc | 15.7 (6.1) | 5–36 | |
| Drinks per drinking dayc | 5.8 (2.3) | 2.7–12.6 | |
| Heavy drinking days per weekc,d | 1.6 (0.6) | 0.6–3.2 | |
| Age regular drinkinge | 18.5 (1.7) | 15–21 | |
| AUDITf | 11.7 (4.4) | 5–22 | |
| DSM-IV criteriae,g | 2.1 (1.3) | 0–5 |
Daily use.
At least one first-degree relative with probable alcohol or substance use disorder; one subject’s FH unknown.
From the Alcohol Timeline Followback Interview (TLFB).
≥4 or 5 drinks per day for female or male, respectively.
From the Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA).
Alcohol Use Disorders Identification Test.
Diagnostic and Statistical Manual of Mental Disorders, Alcohol Use Disorder endorsements.
Others; ns=4 African-American, 2 mixed-race.
Procedures
Subjects performed CCD tasks and single-commodity monetary DD while sober, then later under controlled alcohol infusion in a single group pre- post design. We also assessed behavioral sensation seeking via the aroma choice task (ACT; Oberlin et al., 2019) and other personality traits (Figure 1) during sober periods.
Figure 1. Study Day Timeline.
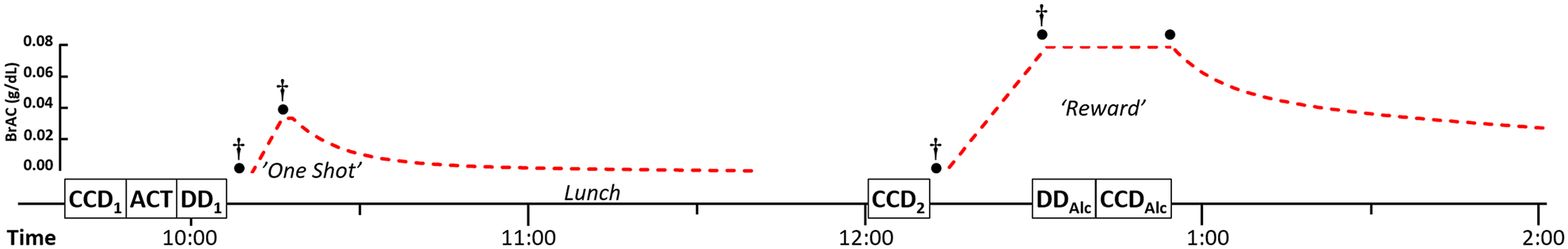
Subjects performed behavioral tasks (cross commodity discounting, CCD; the aroma choice task, ACT; and monetary delay discounting, DD), received ‘One Shot’ for reference intoxication, performed CCD again while sober (CCD2), and then completed DDAlc and CCDAlc maintained at a controlled level of intoxication (‘Reward’; BrAC of 0.08 g/dL). CCD1 and CCD2 differed only in the definition of “one drink”. Targeted BrAC (left y-axis labels) in time is shown as a dashed (red) line—reaching the dismissal level (0.02 g/dL) at ~5:00PM. Subjective ratings of intoxication were collected before infusions and at the BrAC peaks (daggers), with BrAC readings taken at the same times, plus at the end of the clamp (filled circles). Personality inventories were counterbalanced and administered during sober periods interspersed with behavioral tasks (not shown).
Subject instructions.
Prior to performing the first CCD, subjects were told, “Today you will be making choices about alcohol delivered immediately or money delivered in the future. When you are asked about alcohol as ‘one shot’, it is what you think of as ‘one drink’”. Instructions for the second and third CCD tasks—after the ‘one shot’ alcohol infusion (priming dose)—differed slightly; “When you are asked about alcohol as ‘one shot’, it is the same amount of alcohol as you got earlier”. Before each task, subjects were reminded, “It’s very important that you carefully consider each choice, and make every choice exactly as you want it, because some percentage of your choices will be selected at random by the computer and given according to your actual choices. That is, immediate choices will be paid today, and delayed money will be mailed after the specified delay”. Subjects were also instructed to make each choice in isolation, that is, “by itself, without considering other choices already made”. Although the precise nature of the contingency was intentionally obfuscated, the instructions were that any choice made in CCD or DD could potentially be reinforced (random computer choice), supporting the belief that all discounting tasks were incentivized. Importantly, subjects were reminded that “regardless of your choices, you may not leave before 5:00 PM”, to discourage the strategy of avoiding alcohol choices to hasten release. The ordering of CCD and DD and the three personality measures were pseudorandomized between-subjects, in the session prior to the ‘one shot’ alcohol infusion. The subject’s choice behavior could not shorten the time required for any task completion. After all CCD and DD procedures were completed, subjects were asked, “how much do you typically pay for a single drink?” to assess subjective alcohol price points.
Cross-commodity discounting (CCD, ~6 minutes).
The CCD task presented choices between an immediate ‘one shot’ of alcohol (intravenous alcohol infusion, described below) and delayed money, with the first and second CCD tasks differing only in the instructions regarding alcohol—this was to assess differences between the decision-making concerning a hypothetical ‘one drink’ and an actual ‘one drink’. We employed an adjusting-delay procedure to preserve an intuitive unit dose of intoxication for the immediate reward; to wit, ‘one shot’, rather than fractions of ‘one shot’, as would be required by adjusting (immediate) amount tasks (Green et al., 2007). The procedure converged on the relative preference for immediate alcohol, across amounts, using a staircase method (e.g., Du et al., 2002). The immediate option was always ‘one shot’. Delayed money amounts of $2, $4, $8, and $16 were used with a starting delay of 30 days, with amounts designed to bracket typical drink values. Choices for immediate alcohol decreased the next trial’s delay, while choices for delayed money increased delays. For example, a trial is presented as, “Which would you prefer: A “shot” of alcohol now OR $16 after 1 month”. For subsequent trials, the delay adjusts up by doubling—or down by halving—based on the previous choice for that amount. If the subject chose the delayed option in the current example, the options for the next trial offering $16 would be “A “shot” of alcohol now OR $16 after 2 months”. Twenty-four choice trials were presented; 6 adjusting trials for each of the 4 amounts. Four additional control trials assessed attentive responding. If subjects chose all delay options for a given amount, the final adjusted delay was estimated at 1.5 times the adjusted delay, given the uncertainty about the true adjusted delay in these cases. The instructions regarding alcohol delivery and payment were designed to convince subjects that the alcohol delivered was contingent on their decision-making (although with the precise amount obfuscated).
Single-commodity monetary delay discounting (DD, ~10 minutes).
The monetary discounting task quantified immediate money preference with an adjusting-amount procedure (Rachlin et al., 1991). Choices between smaller immediate vs. larger delayed monetary rewards adjusted the next trial’s immediate amount down for immediate choices and up for delayed choices, allowing the procedure to converge on the subjective preference for immediate money across delays (Du et al., 2002). Amounts of $20 and $200 were delayed by 2 days, 1 week, 1 month, 6 months, 1 year, and 5 years, with titrated immediate amounts. For each delay, the immediate amounts were initially half the delayed, and adjusted up or down on subsequent trials. Sixty choice trials were presented; 5 trials for each of the 6 delays, duplicated for the two amounts. Four additional control trials assessed attentive responding. Amount/delay combinations and the presentation side for each trial were pseudorandomized for CCD and DD tasks.
Aroma choice task (ACT, ~12 minutes).
The ACT is a validated behavioral test of sensation seeking that measures relative preference of an intense, novel, varied, risky option versus a mild, safe, ‘boring’ option, with odorants delivered in real-time. Subjects are instructed, “For the next 12 minutes, you will make choices about some smells. The choice labeled ‘Standard’ will likely be mild and pleasant. The choice labeled ‘Varied’ will likely be stronger and pleasant, but there is a chance that it will be unpleasant. Upon making a choice, please inhale deeply through your nose to receive the aroma.” Choice ratio, the proportion of ‘varied’ choices in a total of 40 binary choice trials, yields a single behavioral index reflecting behavioral sensation seeking (designed after self-reported sensation seeking trait descriptions (Arnett, 1994, Zuckerman et al., 1978)). The original ACT was developed with an air-dilution olfactometer (Oberlin et al., 2019), with the present version using experimenter-operated sniff bottles to deliver odorants (see Supplemental Data for convergent validity of the sniff bottle method demonstrated in a separate sample as compared to the air dilution olfactometer ACT).
Alcohol administration.
Brain exposure to alcohol was standardized between subjects by using a physiologically-based pharmacokinetic model of alcohol distribution and elimination (O’Connor et al., 1998). This integrated hardware/software package, the Computerized Alcohol Infusion System, incorporates subjects’ age, sex, height, and weight to generate model parameters infusion profiles to produce near-identical BrAC trajectories (Plawecki et al., 2007). The ‘one shot’ infusion (see Figure 1) provided subjects the experience of a discrete unit of intoxication for reference in CCD decision-making, and to familiarize them with the intravenous alcohol experience. Subjects were instructed that the ‘reward’ infusion was the actual alcohol they chose in the earlier CCD sessions (it was actually a fixed alcohol exposure, but this narrative was required to preserve the fiction of contingency). The ‘one shot’ infusion targeted a BrAC of 0.035 g/dL in 6 minutes, with the later, ‘reward’ infusion targeting a BrAC of 0.08 g/dL in a 20-min ramp. After the ‘one shot’, BrAC was allowed to drop and verified as 0.00 g/dL before proceeding. Before and during infusion, BrAC was measured and subjects rated their alcohol perceptions upon reaching the targeted peaks. For the ‘one shot’, the infusion terminated at the peak; for the ‘reward’ infusion, the pumps maintained (clamped) the BrAC at 0.08 g/dL; at which time both CCD and DD were presented, with the BrAC held constant until the tasks were completed.
Subjective alcohol ratings.
Subjects rated alcohol-related effects in a six-item questionnaire with a six-marker scale, at baseline and at the peak BrAC. The items “Right now, I feel as if I’ve had this many drinks” ranged from 0–5, and “I WANT a drink right now” ranged from “Strongly disagree” to “Strongly agree”. “I LIKE how I’m feeling right now”, “How INTOXICATED do I feel right now?”, “How ANXIOUS do I feel right now?”, “Do I feel NUMBNESS or TINGLING in any part of my body?”, and “How HIGH do I feel right now?” were anchored by “Not at All” to “Most Ever”.
Personality.
Personality assessments were collected at BrAC of 0 g/dL between tasks. The Arnett Inventory of Sensation Seeking (AISS; Arnett, 1994), an abbreviated Zimbardo Time Perspective Inventory1 (sZTPI; Zimbardo and Boyd, 1999), and the short-form UPPS-P Impulsive Behavior Scale (sUPPS-P; Cyders et al., 2014) were presented in pseudorandomized order between subjects.
Analyses and Statistics.
Power analysis for H1, based on the effect size from the alcohol-induced increase in discounting (experiential discounting task; Reynolds et al., 2006), suggests that n=35 subjects were required at 80% power. SPSS 24 (IBM Corp., Armonk, NY) was used for analyses; alpha was set to .05; in-text means are presented ± standard deviation.
Discounting behavior.
Area under the curve (AUC) quantified CCD and DD tasks, obviating theoretical assumptions about the form of the discounting curve (Myerson et al., 2001). Skewness in AUC data was minimized by cube-root normalization (mean AUC skewness for CCD and DD, 1.60 ± 0.84; cube-root AUC, 0.58 ± 0.30). Differences between CCD1 and CCD2 (both at BrAC = 0, but differing in instructions, and separated by the ‘one shot’ infusion), and the effects of alcohol (‘reward’) on CCD and DD were tested with paired t-tests (off vs. on alcohol). Effects of magnitude (discounting of $20 versus discounting of $200) and alcohol on DD were tested with a 2 (amount) × 2 (alcohol condition) repeated-measures ANOVA on transformed AUC. Similarity of discounting behavior between CCD and DD was assessed with Pearson correlation, and corrected for multiple comparisons with the D/AP procedure (Sankoh et al., 1997). All CCD and DD outcomes were assessed for correlation with income measures (natural-log normalized) to detect possible relationships with financial state.
Alcohol and discounting.
Alcohol’s effects on CCD and DD was calculated by subtracting the discounting behavior ON alcohol from behavior OFF alcohol (CCD2 – CCDAlc for CCD, and DD1$20 – DDAlc$20 and DD1$200 – DDAlc$200 for the two different magnitudes in DD).
Aroma choice task and alcohol effects.
ACT behavior was assessed for normality by a Shapiro-Wilk’s test. ACT choice ratio, the degree of preference for an intense, novel, and risky experience, was calculated as the number of ‘Varied’ choices divided by the total number of trials, i.e. 40. Continuous associations of behavioral sensation seeking (ACT choice ratio) with alcohol’s effect on CCD and DD was tested with Pearson correlation. To directly test if high and low sensation seekers differed in alcohol’s effect on behavior, subjects showing a significant preference (defined by ACT choice ratios outside the 95% confidence interval of indifference; 50%), were compared by group (t-test) on alcohol’s effect on CCD.
Self-reported risk factors, alcohol, and discounting.
Correlations were performed between self-reported risk factors and alcohol’s effects on CCD and DD. To identify orthogonal components and reduce dimensionality in self-report, principal components analysis reduced 17 self-reported drinking and personality scores2 to 5 components with eigenvalues > 1.0, (Table S1). These components were named according to their factor loadings, and assessed for correlation with discounting while sober (CCD1, CCD2, DD1$20, DD1$200) and intoxicated (CCDAlc, DDAlc$20, DDAlc$200). For completeness, we also report these correlations with the individual variables, corrected for multiple comparisons (D/AP procedure) in Table S2. Alcohol’s positively-valenced effects (“WANT”, “LIKE”, “HIGH”) were correlated with ACT choice ratio, corrected for multiple comparisons (D/AP).
Intravenous alcohol.
Computerized Alcohol Infusion System performance was tested by comparing measured and target BrAC exposures of 0.035 or 0.080 g/dL (one-sample t-test) for ‘one shot’ and ‘reward’ peaks, respectively, and for the maintenance of 0.080 g/dL at the end of the reward infusion.
Subjective alcohol effects.
The mean subjective effects of alcohol were evaluated with paired t-tests against the pre-infusion baseline assessment on “WANT”, “LIKE”, “INTOXICATED”, “ANXIOUS”, “NUMBNESS”, and “HIGH” ratings.
Alcohol and response times.
To evaluate whether alcohol affected response time (RT; the time from choice presentation to response), or if that related to effects on DD decision-making, RT was tested for equality (OFF versus ON alcohol, paired t), and assessed for correlations with AUC.
Alcohol price point.
Alcohol price points are reported to affirm that they fell within the monetary range used in CCD. These were derived from n=26, as some data were missing from the early subjects, prior to item inclusion in the study. Price points were also assessed for correlation with income measures.
Sex and excluded subjects.
To examine potential sex-related differences in drinking and behavioral performance, independent t-tests were performed on drinking-related measures. Subjects excluded for nonsystematic discounting were examined for differences from valid subjects by Chi-Square or t-test on characteristics listed in Table 1.
Results
Cross-Commodity Discounting (CCD).
CCD1 did not differ from CCD2, (p > .57), meaning that neither the ‘one shot’ morning infusion nor the differing instructions regarding “one shot” changed CCD. CCD behavior is illustrated in Figure 2A.
Figure 2. Discounting and intoxication.
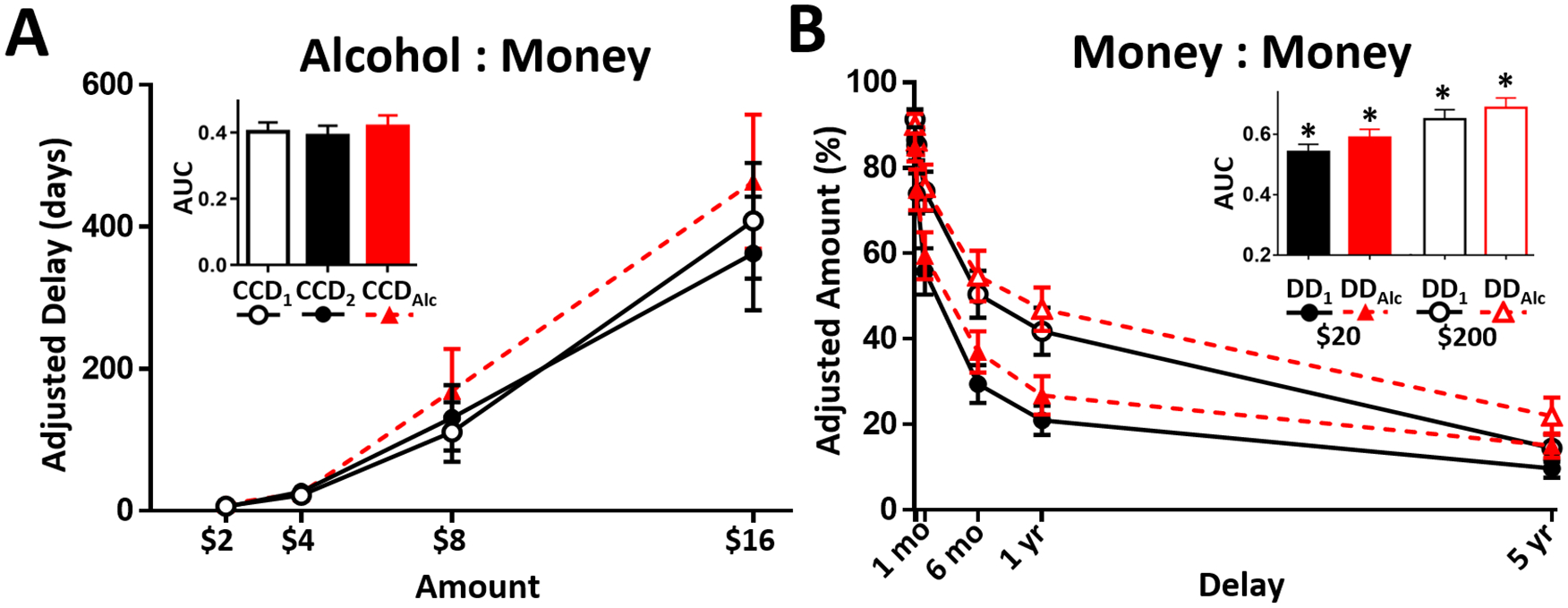
(A) Mean Alcohol:Money discounting differences were not detected between the two sober CCD tests, or CCD under alcohol (triangles/dashed lines; fixed BrAC of 0.08 g/dL); inset, area under the curve (AUC). (B) Both alcohol and larger money amounts decreased impulsive choice in single-commodity Money:Money discounting ($20 and $200 delayed amounts are filled or open, respectively; 2 day and 1 week delays omitted from x-axis labels); inset, AUC; *ps ≤ .001, main effects. Means are depicted ± SEM.
Single commodity monetary delay discounting (DD).
$200 increased delayed reward preference relative to $20; main effect of magnitude F(1,34) = 35.84, p < .001, and no interaction with alcohol, p = .61; Figure 2B. Neither CCD nor DD outcomes correlated with income: monthly income (ps > .19) or disposable income (ps > .38).
Alcohol’s effects on discounting.
Alcohol intoxication did not alter CCD, p > .16 (Figure 2A, red line/bar), but did increase preference for the delayed reward in DD; main effect of alcohol condition, F(1,34) = 13.55, p = .001 (Figure 2B, red lines/bars).
Sensation seeking, alcohol effects, and discounting.
ACT scores (behavioral sensation seeking) were normally distributed (p = .19) with mean choice ratio of 57.9% ± 26.0. Behavioral sensation seeking correlated with alcohol’s effect on CCD, with alcohol-induced increases in immediate alcohol choice correlating with higher behavioral sensation seeking, r(33) = .35, p = .042 (Spearman’s rho = .34, p = .044); Figure 3A. High sensation seekers (n=18) showed more alcohol-induced increases in immediate alcohol choice than low sensation seekers (n=8), t(24) = 3.07, p = .005. ACT choice ratios did not correlate with alcohol’s effects on DD, ps > .71, nor did ACT correlate with CCD or DD, per se; ps > .074. The self-reported sensation seeking PCA component was uncorrelated with alcohol effects, ps > .087.
Figure 3. Behavioral sensation seeking and alcohol effects.
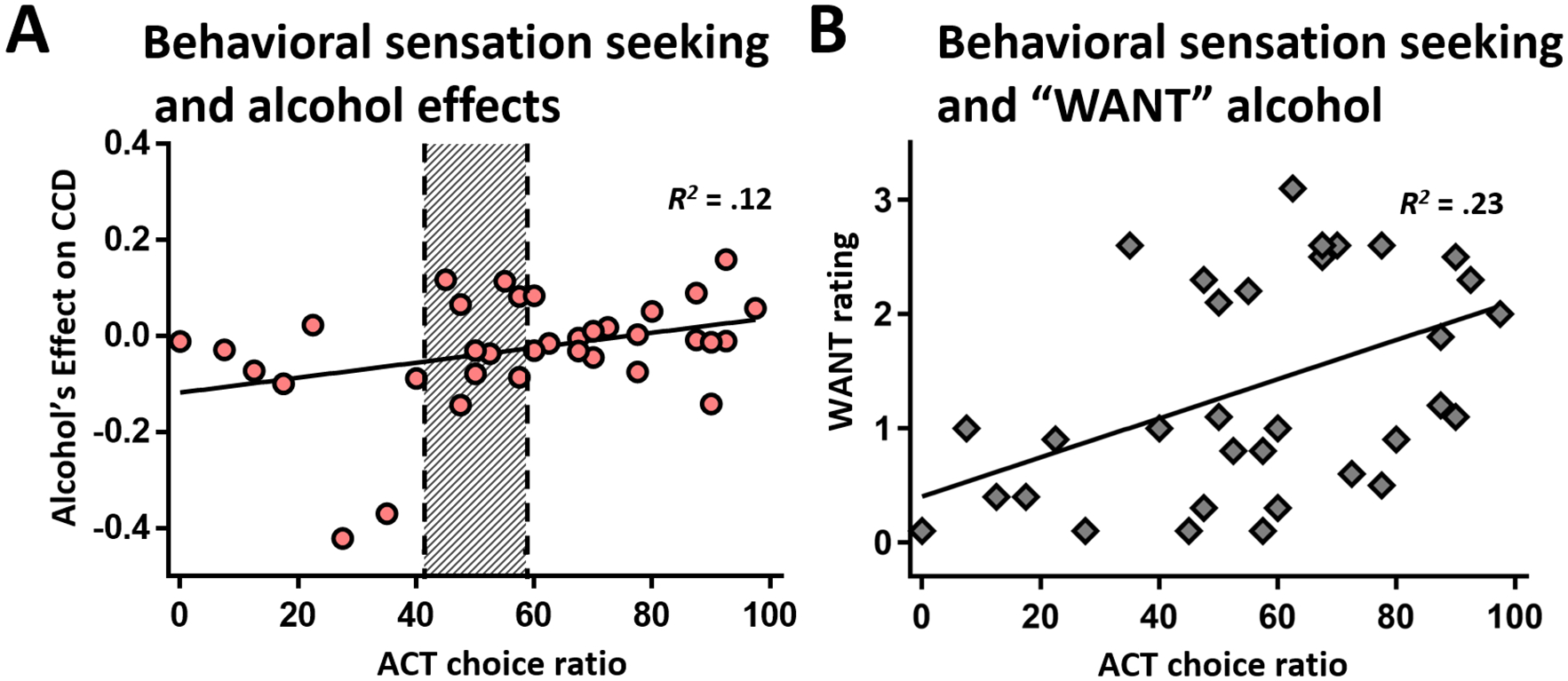
(A) ACT choice ratio correlated with alcohol’s effect on CCD (y-axis; CCD2 – CCDAlc), i.e., increased preference for immediate alcohol, p = .042. Lack of behavioral sensation seeking preference is indicated with shading (50% choice ratio ± 95% CI). (B) WANT alcohol at the peak of the ‘one shot’ infusion correlated with sensation seeking behavior, p = .022.
Discounting and self-report.
CCD and DD (off or on alcohol) did not correlate with any of the 5 PCA components (detailed in Table S1) representing self-reported drinking behaviors/problems, self-reported sensation seeking, or sUPPS-P impulsivity, psuncorrected > .055.
Discounting correlations by task type.
CCD and DD behaviors were strongly correlated, with the mean coefficient equal to .64 ± 0.15, psadj < .03; Table 2.
Table 2.
Discounting Task Correlations
| Measure | 1. | 2. | 3. | 4. | 5. | 6. |
|---|---|---|---|---|---|---|
| 1. CCD1 | — | |||||
| 2. CCD2 | .78*** | — | ||||
| 3. CCDAlc | .64*** | .81*** | — | |||
| 4. DD1$20 | .51** | .74*** | .56** | — | ||
| 5. DD1$200 | .46** | .57*** | .49** | .82*** | — | |
| 6. DDAlc$20 | .56** | .61*** | .50** | .86*** | .84*** | — |
| 7. DDAlc$200 | .41* | .51** | .53** | .69*** | .88*** | .77*** |
padj < .001,
padj ≤ .01,
padj < .05
Behavioral sensation seeking and intoxication ratings.
Subjects’ ratings of “WANT” a drink at the peak of ‘one shot’ correlated with behavioral sensation seeking (ACT), r(33) = .46, padj = .021 (Figure 3B), but other positive effects were not significant, psadj > .37.
Alcohol administration and perception.
Computerized Alcohol Infusion System performed well, slightly exceeding the targeted 0.035 g/dL peak for ‘one shot’ (mean 0.039 ± 0.004 g/dL), t(34) = 5.68, p < .001, and matching the targeted 0.080 g/dL at the peak of the ‘reward’ (0.081 ± 0.004 g/dL) and at the end of the clamp (0.080 ± 0.009 g/dL), ps > .17. The ‘one shot’ infusion significantly altered all subjective ratings, ts(34) > 2.48, ps < 0.019, except “I LIKE how I’m feeling right now”, p = .68. Exploratory correlational analyses revealed significant results between “HIGH” and “INTOXICATION” with “LIKE”, rs(33) > .39, ps < .019, but other measures were nonsignificant (ps > .19). The ‘reward’ infusion significantly altered all ratings, decreasing “ANXIOUS” and increasing all others; ts(34) > 2.64, ps < .013. The largest subjective alcohol effects are illustrated in Figure 4.
Figure 4. Alcohol Ratings.
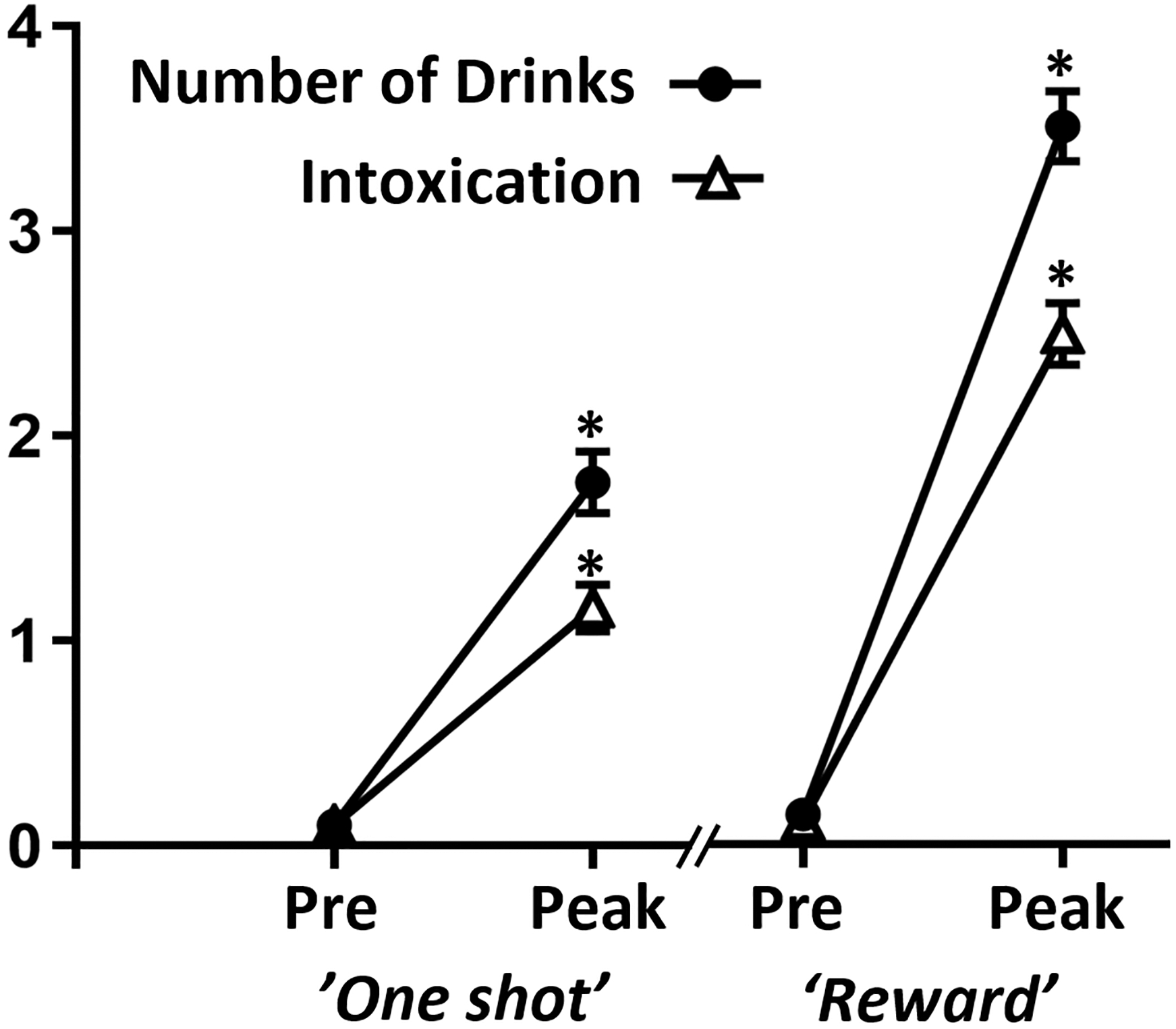
The ‘One Shot’ and ‘Reward’ intravenous alcohol infusions increased the perceived number of drinks (closed circles) and level of intoxication (open triangles); *ps < .001 relative to pre-infusion ratings (Pre), means are depicted ± SEM.
DD and RT.
RT correlated with AUC off alcohol, r(33) = .37, p = .031, but not on alcohol, p = .38; RT did not differ with intoxication, p = .09.
Alcohol price point.
Amount “typically paid for a drink” ranged from $0.40 to $6.50, median $4.00 (IQR = $3.00-$5.00); note that $0.40 (2.6 SD from mean) represented a participant who purchased inexpensive beer in bulk, but did not produce an extreme value in CCD or DD measures. Price point was uncorrelated with monthly income (p = .77) or disposable income (p = .82).
Sex and excluded subjects.
Subjects did not differ by sex on drinks per week (p = .99), drinks per drinking day (p = .82), age of commencing regular drinking (p = .53), AUDIT (p = .71), or DSM-IV criteria counts (p = .11), but differed by number of heavy drinking days per week, t(33) = 2.36, p = .024; means = 1.43 ± 0.44 and 1.87 ± 0.65 for males and females, respectively. Sex effects were not detected in CCD, DD, or ACT behavior, ps > .11. Subjects excluded for discounting performance (detailed in Methods) reported lower drinks per week than valid subjects (means 10.5 ± 3.9 and 15.7 ± 6.1, respectively; t(41) = 2.29, p = .027), but did not differ on any other characteristics, ps > .22.
Discussion
Beyond bypassing the limitations of self-report, behavioral tasks excel in detecting behavioral shifts in real-time, such as drug effects on decision-making. Using a task designed to model impulsive alcohol choice, we demonstrated that the alcohol:money discounting task detects intoxication-induced increases in alcohol preference as a function of behavioral sensation seeking. We did not find this association with single commodity discounting or self-reported sensation seeking. Interestingly, alcohol’s effects on mean discounting were task-specific; intoxication decreased impulsivity in single commodity monetary discounting, but had no net effect on CCD. Hypothesis H1 was not supported, as no mean overall effect of alcohol on CCD was detected. Hypothesis H2 was partially supported, as alcohol’s effect on CCD behavior correlated with behavioral sensation seeking, but not other self-reported AUD risk factors. Hypothesis H3 was supported, with discounting highly correlated across task type, and while both sober and intoxicated.
The CCD task models a canonical AUD-related behavior; preferring immediate alcohol over other types of delayed rewards. While alcohol and substance use disorders and behaviors are robustly associated with steep monetary discounting (Amlung et al., 2017), intoxication-induced increased drive for further intoxication potentially reflects a unique trait widely observed in AUD: impaired control. AUD has long been characterized by impaired control of alcohol drinking (Jellinek, 1960), and is represented in two DSM-5 substance use disorder diagnostic criteria; i.e. substance taken in larger amounts than intended, and unsuccessful efforts to control use (APA, 2013). Impaired control, also called “loss of control” or “priming effect”, predicts alcohol-related problems (Heather et al., 1993, Leeman et al., 2009), and correlates with self-reported impulsivity (Leeman et al., 2012). How impaired control relates to sensation seeking is less studied, but with self-report, high sensation seekers receiving alcohol reported greater alcohol-related reward and are more disinhibited, compared to low sensation seekers (Fillmore et al., 2009)—resembling the current behavioral sensation seeking findings. In contrast, we failed to detect associations between self-reported sensation seeking and alcohol effects. The positive correlation between behavioral sensation seeking and “WANT” alcohol further supports the association between sensation seeking and AUD risk, suggesting the possibility that increased alcohol reward in high sensation seekers shifts the balance of preference toward alcohol when intoxicated. While we did not explicitly test impaired control, converging evidence suggests that high sensation seeking may specifically exacerbate AUD/SUD risk via drug-induced impulsive choice mechanisms, one of which may be heightened drug reward sensitivity.
Similarly, the equivalence between CCD1 and CCD2 suggests that alcohol:money discounting was not affected by the instructions of the subject-defined “one drink” (versus the experimenter-administered ‘one shot’), or the alcohol exposure itself (as a priming dose before CCD2). Both results are consistent with decision-making governed by imagined reward—that is, a neural representation of value. While the phenomena of “subject-defined versus experimenter-defined” and “hypothetical versus real” are not identical, they both rely on imagined reward for decision-making. Considerable prior discounting work shows that hypothetical discounting does not differ from discounting of actual rewards (e.g., Johnson and Bickel, 2002, Madden et al., 2003), further supporting the primacy of imagined reward.
Although discounting shows consistent response patterns with different commodities in single commodity tasks (Odum, 2011), differing methods of measuring discounting may reveal potentially important differences. For instance, here, the relationship of sensation seeking to drug effects on discounting is not detected with traditional single commodity monetary discounting. Further, alcohol effects differed between DD and CCD—likely due to the inclusion of alcohol reward in the CCD decision process. The relationship of response time to discounting (longer RTs correspond with more delayed reward choice when sober), appears to be disrupted by alcohol; that is, subjects’ deliberation time only meaningfully indexes behavior, and presumably cognitive engagement, while sober. Finally, the lack of correlation between self-reported risk traits and discounting behavior, particularly self-reported sensation seeking with alcohol’s effect on CCD, argues for wider adoption of behavioral tasks, and is unsurprising given disparate findings between laboratory tasks and self-report (Cyders and Coskunpinar, 2011). We agree with others (Ebstein, 2006) that elucidating behavioral phenotypes in future studies will require carefully-designed laboratory tasks, and we propose that these show considerable promise in linking behavior to brain function.
While alcohol is widely believed to increase impulsivity, including impulsive choice, the evidence for alcohol increasing impulsive choice is equivocal (Richards et al., 1999, Bidwell et al., 2013, Dougherty et al., 2008, Rose and Grunsell, 2008, Bernhardt et al., 2019), with only one study (Reed et al., 2012) reporting this finding with amounts over $13. Reed and colleagues specifically tested family history negative women, making their sample difficult to directly compare with the current sample, which contained male and female drinkers of varying family history. Our results align more closely with Ortner et al. (2003), who found a trend-level decrease in alcohol-induced discounting (in males), and importantly, a correlation between BrAC and reduced discounting. While unintuitive, the idea that alcohol could decrease discounting is consistent with at least two plausible explanations: 1) reinforcer substitution (Green and Freed, 1993), that is, the satisfaction of a present reward state decreases the drive for other immediate reinforcers, and 2) behavioral choice theory (Rachlin, 1989, Vuchinich and Tucker, 1998), i.e., DD studies testing alcohol effects are generally conducted in laboratories or hospital settings, which typically provide minimal (immediate) alternate reinforcer opportunity, therefore biasing choice toward the delayed. Of note, alcohol did not decrease discounting in CCD—when alcohol was the immediate reinforcer—suggesting that the effect is commodity-specific (or potentially due to variation between primary and secondary reinforcers). Related, the specific effects of alcohol expectancies are difficult to isolate—even with a placebo control (heavy drinkers readily identify alcohol’s presence)—however prior work suggests that temporal impulsivity is not greatly affected by alcohol expectancies (Caswell et al., 2013). While we believe that our measurement of discounting largely reflects the pharmacological effect of alcohol on real-world decision-making, we cannot ignore the role of the proximal environment and alcohol-related expectancies in potentially modifying those choices during laboratory testing.
Some limitations warrant consideration. Our single-group design maximized power, but at the cost of a between-subjects placebo control. The A-B design used in this report does not control for effects of repeated testing on discounting, although the present CCD results suggest that discounting is generally stable with repeated testing, consistent with prior work (e.g., Lagorio and Madden, 2005). The results reported here should be regarded as preliminary, as a larger better-powered replication sample, with a balanced design, would be required for greater confidence in results. Payment in discounting studies often generates concerns germane to immediate reinforcement, due to uncontrolled interactions with financial need and expectancies. We mitigated this issue by specifying that delayed payment would be mailed after the chosen delay. Here, one possible strategy for a heavy drinker could be to choose all delayed money, knowing that the study participation payment received in the evening could be quickly converted into alcohol. Although the CCD task evidenced discounting—demonstrating valuation of immediate alcohol and sensitivity to delay—we speculate that such a strategy could interfere with measurement of unconstrained preference in some individuals. We actively discouraged this strategy by instructing subjects that they must remain in the facility until 5PM, regardless of BrAC, which enforced the divergent options of waiting while drunk or waiting while sober—instantiating our central question. The lack of correlations between discounting (CCD, DD) and drinking metrics might be unexpected (for meta-analysis; Amlung et al., 2017), however it is not unusual (Dennhardt and Murphy, 2011, MacKillop et al., 2007, Stojek et al., 2014). Previously-reported correlations between discounting and other metrics are frequently pooled across high- and low-drinking groups that may differ widely in a range of behaviors. While potentially reflecting mean differences rather than continuous associations (Aggarwal and Ranganathan, 2016), they often represent a large range over which to detect associations; thus the lack of a low drinking control group here may have reduced our ability to detect these relationships, if present.
Using a novel behavioral sensation seeking task with real-time consequences, together with discounting tasks employing actual reinforcers and controlled alcohol exposures, we uncovered a behavioral pattern that could potentially inform the impaired control phenotype. To fully understand this phenomenon, further work will be required to establish causal direction, the role of alcohol-related expectations, and parse subjective reward from choice behavior.
Supplementary Material
ACKNOWLEDGEMENTS
The IARC’s Computer-Assisted Alcohol Infusion System (oconnor1@iu.edu) managed the infusions employed in this research. We also thank Dr. Melissa Cyders for equipment and consulting, and Drs. Stephen Warrenburg and Aleksey Dumer of International Flavors and Fragrances for generously providing odorants. We are grateful to Victor Vitvitskiy for Computerized Alcohol Infusion System related programming support, Rachel Baum for technical assistance, Yitong Iris Shen for assistance in manuscript preparation, and Drs. Marta Karas and Jaroslaw Harezlak for statistical advice.
FUNDING AND DISCLOSURE
The authors report no conflicts of interest related to this work. Effort on this manuscript was supported by the National Institute on Alcohol Abuse and Alcoholism, grants R00 AA023296 (BGO), and P60 AA07611 (DAK, MHP) through the Indiana Alcohol Research Center (IARC). Clinical support was funded in part through the Indiana Clinical and Translational Sciences Institute Clinical Research Center, UL1 TR001108, NIH, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award (PI: Anantha Shekhar). The funders had no role in the conduct of the study, manuscript preparation, or the decision to submit for publication. The views expressed in this manuscript are those of the authors and do not necessarily reflect the position or policy of the funders.
Footnotes
Five items each with the highest factor loadings from Future Orientation and Present Hedonistic subscales comprised a shortened 10-item version. Items: Future (10, 13, 40, 45, 51); Present (8, 17, 23, 26, 42). #17 replaced #31 in Present Hedonistic to minimize redundancy.
Heavy drinking days per week, grams of alcohol per liter body water per week, grams of alcohol per liter of body water per drinking day, DSM-IV item counts from the SSAGA interview, age of first drink, age of regular drinking, age of first intoxication, AUDIT score, ZTPI present hedonistic, ZTPI future orientation, AISS novelty, AISS intensity, sUPPS-P negative urgency, sUPPS-P lack of perseverance, sUPPS-P lack of premeditation, sUPPS-P sensation seeking, sUPPS-P positive urgency.
Reynolds et al. (2006) detected alcohol-induced increases in discounting, but only with small probabilistic amounts ($0.30), and failed to detect alcohol effects on discounting with a DD task utilizing $10 amounts.
REFERENCES
- AGGARWAL R & RANGANATHAN P 2016. Common pitfalls in statistical analysis: The use of correlation techniques. Perspect Clin Res, 7, 187–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AINSLIE G 1975. Specious reward: a behavioral theory of impulsiveness and impulse control. Psychol Bull, 82, 463–96. [DOI] [PubMed] [Google Scholar]
- AMLUNG M, VEDELAGO L, ACKER J, BALODIS I & MACKILLOP J 2017. Steep delay discounting and addictive behavior: a meta-analysis of continuous associations. Addiction, 112, 51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APA 2013. Diagnostic and statistical manual of mental disorders (5th ed.), Arlington, VA, American Psychiatric Publishing. [Google Scholar]
- ARNETT J 1990. Drunk driving, sensation seeking, and egocentrism among adolescents. Personality and Individual Differences, 11, 541–546. [Google Scholar]
- ARNETT J 1994. Sensation seeking: A new conceptualization and a new scale. Person. individ. Diff, 16, 289–296. [Google Scholar]
- BAKER F, JOHNSON MW & BICKEL WK 2003. Delay discounting in current and never-before cigarette smokers: similarities and differences across commodity, sign, and magnitude. J Abnorm Psychol, 112, 382–92. [DOI] [PubMed] [Google Scholar]
- BERNHARDT N, OBST E, NEBE S, POOSEH S, WURST FM, WEINMANN W, SMOLKA MN & ZIMMERMANN US 2019. Acute alcohol effects on impulsive choice in adolescents. Journal of psychopharmacology, 0269881118822063. [DOI] [PubMed] [Google Scholar]
- BICKEL WK, KOFFARNUS MN, MOODY L & WILSON AG 2014. The behavioral- and neuro-economic process of temporal discounting: A candidate behavioral marker of addiction. Neuropharmacology, 76 Pt B, 518–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIDWELL LC, MACKILLOP J, MURPHY JG, GRENGA A, SWIFT RM & MCGEARY JE 2013. Biphasic effects of alcohol on delay and probability discounting. Exp Clin Psychopharmacol, 21, 214–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUCHOLZ KK, CADORET R, CLONINGER CR, DINWIDDIE SH, HESSELBROCK VM, NURNBERGER JI JR., REICH T, SCHMIDT I & SCHUCKIT MA 1994. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol, 55, 149–58. [DOI] [PubMed] [Google Scholar]
- CASWELL AJ, MORGAN MJ & DUKA T 2013. Acute alcohol effects on subtypes of impulsivity and the role of alcohol-outcome expectancies. Psychopharmacology (Berl), 229, 21–30. [DOI] [PubMed] [Google Scholar]
- CLONINGER CR, SIGVARDSSON S & BOHMAN M 1988. Childhood personality predicts alcohol abuse in young adults. Alcohol Clin Exp Res, 12, 494–505. [DOI] [PubMed] [Google Scholar]
- CYDERS MA & COSKUNPINAR A 2011. Measurement of constructs using self-report and behavioral lab tasks: Is there overlap in nomothetic span and construct representation for impulsivity? Clinical psychology review, 31, 965–982. [DOI] [PubMed] [Google Scholar]
- CYDERS MA, LITTLEFIELD AK, COFFEY S & KARYADI KA 2014. Examination of a short English version of the UPPS-P Impulsive Behavior Scale. Addict Behav, 39, 1372–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DENNHARDT AA & MURPHY JG 2011. Associations between depression, distress tolerance, delay discounting, and alcohol-related problems in European American and African American college students. Psychol Addict Behav, 25, 595–604. [DOI] [PubMed] [Google Scholar]
- DOM G, HULSTIJN W & SABBE B 2006. Differences in impulsivity and sensation seeking between early- and late-onset alcoholics. Addict Behav, 31, 298–308. [DOI] [PubMed] [Google Scholar]
- DONOHEW L, ZIMMERMAN R, CUPP PS, NOVAK S, COLON S & ABELL R 2000. Sensation seeking, impulsive decision-making, and risky sex: Implications for risk-taking and design of interventions. Personality and individual differences, 28, 1079–1091. [Google Scholar]
- DOUGHERTY DM, MARSH-RICHARD DM, HATZIS ES, NOUVION SO & MATHIAS CW 2008. A test of alcohol dose effects on multiple behavioral measures of impulsivity. Drug Alcohol Depend, 96, 111–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DU W, GREEN L & MYERSON J 2002. Cross-cultural comparisons of discounting delayed and probabilistic rewards. The Psychological Record, 52, 479–92. [Google Scholar]
- EBSTEIN RP 2006. The molecular genetic architecture of human personality: beyond self-report questionnaires. Mol Psychiatry, 11, 427–45. [DOI] [PubMed] [Google Scholar]
- FAN X, MILLER BC, PARK K-E, WINWARD BW, CHRISTENSEN M, GROTEVANT HD & TAI RH 2006. An exploratory study about inaccuracy and invalidity in adolescent self-report surveys. Field Methods, 18, 223–244. [Google Scholar]
- FILLMORE MT, OSTLING EW, MARTIN CA & KELLY TH 2009. Acute effects of alcohol on inhibitory control and information processing in high and low sensation-seekers. Drug Alcohol Depend, 100, 91–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN L & FREED DE 1993. The substitutability of reinforcers. J Exp Anal Behav, 60, 141–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN L, MYERSON J, SHAH AK, ESTLE SJ & HOLT DD 2007. Do adjusting-amount and adjusting-delay procedures produce equivalent estimates of subjective value in pigeons? J Exp Anal Behav, 87, 337–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUNZERATH L, FADEN V, ZAKHARI S & WARREN K 2004. National Institute on Alcohol Abuse and Alcoholism report on moderate drinking. Alcohol Clin Exp Res, 28, 829–47. [DOI] [PubMed] [Google Scholar]
- HEATHER N, TEBBUTT JS, MATTICK RP & ZAMIR R 1993. Development of a scale for measuring impaired control over alcohol consumption: a preliminary report. Journal of studies on alcohol, 54, 700–709. [DOI] [PubMed] [Google Scholar]
- JELLINEK EM 1960. The disease concept of alcoholism, New Haven, CT, College and University Press. [Google Scholar]
- JOHNSON MW & BICKEL WK 2002. Within-subject comparison of real and hypothetical money rewards in delay discounting. J Exp Anal Behav, 77, 129–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON MW & BICKEL WK 2008. An algorithm for identifying nonsystematic delay-discounting data. Exp Clin Psychopharmacol, 16, 264–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIRBY KN 2009. One-year temporal stability of delay-discount rates. Psychon Bull Rev, 16, 457–62. [DOI] [PubMed] [Google Scholar]
- KOFFARNUS MN, JARMOLOWICZ DP, MUELLER ET & BICKEL WK 2013. Changing delay discounting in the light of the competing neurobehavioral decision systems theory: a review. J Exp Anal Behav, 99, 32–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAGORIO CH & MADDEN GJ 2005. Delay discounting of real and hypothetical rewards III: steady-state assessments, forced-choice trials, and all real rewards. Behav Processes, 69, 173–87. [DOI] [PubMed] [Google Scholar]
- LEEMAN RF, PATOCK-PECKHAM JA & POTENZA MN 2012. Impaired control over alcohol use: An under-addressed risk factor for problem drinking in young adults? Experimental clinical psychopharmacology, 20, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEEMAN RF, TOLL BA, TAYLOR LA & VOLPICELLI JR 2009. Alcohol-induced disinhibition expectancies and impaired control as prospective predictors of problem drinking in undergraduates. Psychology of Addictive Behaviors, 23, 553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEJUEZ CW, AKLIN W, DAUGHTERS S, ZVOLENSKY M, KAHLER C & GWADZ M 2007. Reliability and validity of the youth version of the Balloon Analogue Risk Task (BART–Y) in the assessment of risk-taking behavior among inner-city adolescents. Journal of Clinical Child and Adolescent Psychology, 36, 106–111. [DOI] [PubMed] [Google Scholar]
- LUPTON D & TULLOCH J 2002. ‘Life would be pretty dull without risk’: voluntary risk-taking and its pleasures. Health, risk & society, 4, 113–124. [Google Scholar]
- MACKILLOP J 2013. Integrating behavioral economics and behavioral genetics: delayed reward discounting as an endophenotype for addictive disorders. J Exp Anal Behav, 99, 14–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKILLOP J, AMLUNG MT, FEW LR, RAY LA, SWEET LH & MUNAFO MR 2011. Delayed reward discounting and addictive behavior: a meta-analysis. Psychopharmacology (Berl), 216, 305–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKILLOP J, MATTSON RE, ANDERSON MACKILLOP EJ, CASTELDA BA & DONOVICK PJ 2007. Multidimensional assessment of impulsivity in undergraduate hazardous drinkers and controls. J Stud Alcohol Drugs, 68, 785–8. [DOI] [PubMed] [Google Scholar]
- MADDEN GJ, BEGOTKA AM, RAIFF BR & KASTERN LL 2003. Delay discounting of real and hypothetical rewards. Exp Clin Psychopharmacol, 11, 139–45. [DOI] [PubMed] [Google Scholar]
- MAGURA S & KANG SY 1996. Validity of self-reported drug use in high risk populations: a meta-analytical review. Subst Use Misuse, 31, 1131–53. [DOI] [PubMed] [Google Scholar]
- MANN K, ROOS CR, HOFFMANN S, NAKOVICS H, LEMENAGER T, HEINZ A & WITKIEWITZ K 2018. Precision Medicine in Alcohol Dependence: A Controlled Trial Testing Pharmacotherapy Response Among Reward and Relief Drinking Phenotypes. Neuropsychopharmacology, 43, 891–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITCHELL SH 1999. Measures of impulsivity in cigarette smokers and non-smokers. Psychopharmacology (Berl), 146, 455–64. [DOI] [PubMed] [Google Scholar]
- MITCHELL SH 2004. Effects of short-term nicotine deprivation on decision-making: delay, uncertainty and effort discounting. Nicotine Tob Res, 6, 819–28. [DOI] [PubMed] [Google Scholar]
- MOODY LN, TEGGE AN & BICKEL WK 2017. Cross-commodity delay discounting of alcohol and money in alcohol users. Psychol Rec, 67, 285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MYERSON J, GREEN L & WARUSAWITHARANA M 2001. Area under the curve as a measure of discounting. J Exp Anal Behav, 76, 235–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NORTHCOTE J & LIVINGSTON M 2011. Accuracy of self-reported drinking: observational verification of ‘last occasion’ drink estimates of young adults. Alcohol Alcohol, 46, 709–13. [DOI] [PubMed] [Google Scholar]
- O’CONNOR S, MORZORATI S, CHRISTIAN J & LI TK 1998. Clamping breath alcohol concentration reduces experimental variance: application to the study of acute tolerance to alcohol and alcohol elimination rate. Alcohol Clin Exp Res, 22, 202–10. [PubMed] [Google Scholar]
- OBERLIN BG, RAMER NE, BATES SM, SHEN YI, MYSLINSKI JS, KAREKEN DA & CYDERS MA 2019. Quantifying Behavioral Sensation Seeking with the Aroma Choice Task. Assessment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ODUM AL 2011. Delay discounting: trait variable? Behavioural processes, 87, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OHMURA Y, TAKAHASHI T, KITAMURA N & WEHR P 2006. Three-month stability of delay and probability discounting measures. Exp Clin Psychopharmacol, 14, 318–28. [DOI] [PubMed] [Google Scholar]
- OOTEMAN W, KOETER M, VERHEUL R, SCHIPPERS G & VAN DEN BRINK W 2006. Development and validation of the Amsterdam Motives for Drinking Scale (AMDS): an attempt to distinguish relief and reward drinkers. Alcohol Alcohol, 41, 284–92. [DOI] [PubMed] [Google Scholar]
- ORTNER CN, MACDONALD TK & OLMSTEAD MC 2003. Alcohol intoxication reduces impulsivity in the delay-discounting paradigm. Alcohol Alcohol, 38, 151–6. [DOI] [PubMed] [Google Scholar]
- PEDERSEN W 1991. Mental health, sensation seeking and drug use patterns: a longitudinal study. Br J Addict, 86, 195–204. [DOI] [PubMed] [Google Scholar]
- PLAWECKI MH, DECARLO R, RAMCHANDANI VA & O’CONNOR S 2007. Improved Transformation of Morphometric Measurements for a Priori Parameter Estimation in a Physiologically-Based Pharmacokinetic Model of Ethanol. Biomed Signal Process Control, 2, 97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RACHLIN H 1989. Judgment, decision, and choice: A cognitive/behavioral synthesis, New York, WH Freeman & Co. [Google Scholar]
- RACHLIN H, RAINERI A & CROSS D 1991. Subjective probability and delay. J Exp Anal Behav, 55, 233–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REED SC, LEVIN FR & EVANS SM 2012. Alcohol increases impulsivity and abuse liability in heavy drinking women. Exp Clin Psychopharmacol, 20, 454–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS B, RICHARDS JB & DE WIT H 2006. Acute-alcohol effects on the Experiential Discounting Task (EDT) and a question-based measure of delay discounting. Pharmacol Biochem Behav, 83, 194–202. [DOI] [PubMed] [Google Scholar]
- RICHARDS JB, ZHANG L, MITCHELL SH & DE WIT H 1999. Delay or probability discounting in a model of impulsive behavior: effect of alcohol. J Exp Anal Behav, 71, 121–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSE AK & GRUNSELL L 2008. The subjective, rather than the disinhibiting, effects of alcohol are related to binge drinking. Alcohol Clin Exp Res, 32, 1096–104. [DOI] [PubMed] [Google Scholar]
- RUNG JM & MADDEN GJ 2018. Experimental reductions of delay discounting and impulsive choice: A systematic review and meta-analysis. J Exp Psychol Gen, 147, 1349–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANKOH AJ, HUQUE MF & DUBEY SD 1997. Some comments on frequently used multiple endpoint adjustment methods in clinical trials. Stat Med, 16, 2529–42. [DOI] [PubMed] [Google Scholar]
- SAUNDERS JB, AASLAND OG, BABOR TF, DE LA FUENTE JR & GRANT M 1993. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption--II. Addiction, 88, 791–804. [DOI] [PubMed] [Google Scholar]
- SOBELL MB, SOBELL LC, KLAJNER F, PAVAN D & BASIAN E 1986. The reliability of a timeline method for assessing normal drinker college students’ recent drinking history: utility for alcohol research. Addict Behav, 11, 149–61. [DOI] [PubMed] [Google Scholar]
- STEINBERG L, ALBERT D, CAUFFMAN E, BANICH M, GRAHAM S & WOOLARD J 2008. Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: evidence for a dual systems model. Dev Psychol, 44, 1764–78. [DOI] [PubMed] [Google Scholar]
- STOJEK MM, FISCHER S, MURPHY CM & MACKILLOP J 2014. The role of impulsivity traits and delayed reward discounting in dysregulated eating and drinking among heavy drinkers. Appetite, 80, 81–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOMA CL & CARLSON CL 2015. How do Facebook users believe they come across in their profiles?: A meta-perception approach to investigating Facebook self-presentation. Communication Research Reports, 32, 93–101. [Google Scholar]
- VUCHINICH RE & TUCKER JA 1998. Choice, behavioral economics, and addictive behavior patterns In: MILLER WR & HEATHER N (eds.) Treating Addictive Behaviors. New York: Plenum Press. [Google Scholar]
- WHITESIDE SP & LYNAM DR 2001. The five factor model and impulsivity: using a structural model of personality to understand impulsivity. Person. individ. Diff, 30, 669–689. [Google Scholar]
- YOON JH, HIGGINS ST, BRADSTREET MP, BADGER GJ & THOMAS CS 2009. Changes in the relative reinforcing effects of cigarette smoking as a function of initial abstinence. Psychopharmacology (Berl), 205, 305–18. [DOI] [PubMed] [Google Scholar]
- ZIMBARDO PG & BOYD JN 1999. Putting time perspective: a valid, reliable individual-differences metric. Journal of Personality and Social Psychology, 77, 1271–88. [Google Scholar]
- ZUCKERMAN M, EYSENCK S & EYSENCK HJ 1978. Sensation seeking in England and America: cross-cultural, age, and sex comparisons. J Consult Clin Psychol, 46, 139–49. [DOI] [PubMed] [Google Scholar]
- ZUCKERMAN M & NEEB M 1980. Demographic influences in sensation seeking and expressions of sensation seeking in religion, smoking and driving habits. Personality and Individual Differences, 1, 197–206. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


