Abstract
Purpose:
Outcome differences driven by variation in Blacks’ biologic response to treatment may contribute to persistent racial disparities in asthma morbidity and mortality. This review assessed systematic variation in β2 agonist treatment outcomes among Blacks compared to other groups.
Methods:
We conducted a systematic review of studies reporting differential response to β2 agonists among Blacks, including studies identifying pharmacogenetic variants.
Results:
Of 3158 papers, 20 compared safety or efficacy of β2 agonists among Blacks as compared with other subgroups. Six papers evaluating efficacy of short-acting β2 agonists (SABA) found similar or improved results among Blacks compared with other groups, while one small study found reduced response to SABA therapy among Blacks. Reports of safety and efficacy of long-acting β2 agonists (LABA) indicated similar results among Blacks in four papers, while four reports found reduced safety among Blacks, as compared with other groups. Four papers assessed genomic variation and relative treatment response in Blacks, with two finding significant effects of the p.Arg16Gly variant in ADRB2 on β2 agonist response and one finding significant gene-gene IL6/IL6R interaction effects on albuterol response.
Conclusions:
Evidence suggests the potential for differences in β2 agonist outcomes among Blacks compared with other groups. This literature, however, remains small and significantly underpowered for substantive conclusions. There are notable opportunities for adequately-powered investigations exploring safety and efficacy of β2 agonists among Blacks, including pharmacogenomic modifiers of response.
Keywords: asthma, racial disparities, treatment response, safety, beta-2 adrenergic agonists
1. INTRODUCTION
Nearly 4 million Blacks in America with asthma suffer striking and ongoing health and healthcare disparities, including increased risk of emergency department visits and hospital admissions, as well as significantly increased morbidity and mortality.1 These issues affect Blacks across the lifespan, with children, adolescents, and adults experiencing adverse health effects due to asthma.2 While complex health care, patient, family, and environmental factors can contribute to poorer outcomes, disparities driven by variation in Blacks’ biologic response to treatment are not yet well understood. Systematic differences in response to asthma therapeutics may be an important key factor underlying some of the observed disparities in health outcomes.3,4
Disparate safety and efficacy signals across subgroups of interest can potentially be derived from existing studies that assess variation between different groups by race and ethnicity. Clinical outcomes of randomized controlled trials and cohort studies, for example, may demonstrate altered safety or efficacy profiles among subgroups, while pharmacogenomics research may explicitly examine the independent impact of genomic variation, such as single nucleotide polymorphisms (SNPs). Observed differences may significantly contribute to ongoing health disparities among Blacks with asthma, particularly when related to decreased therapeutic efficacy or increased risk of side effects.
Thus, the published literature, as a body of existing data, represents an important opportunity for illuminating treatment safety and efficacy patterns in the literature related to differences in race and ethnicity. Such patterns may further inform clinical practice and future research related to the care of individuals of different racial and ethnic backgrounds.
To begin to explore potential biologic factors that may affect treatment response among Blacks with asthma, including differences in safety and efficacy, we applied a systematic literature review approach to the short- and long-acting β2 adrenergic agonists, two classes of therapeutics used for treatment of this disease.5,6 The intent of this analysis was two-fold: to enable hypothesis formation to develop deeper understanding of the biologic explanations underpinning some of these results, and to inform selection of therapeutic courses when feasible and appropriate.
2. MATERIALS AND METHODS
2.1. Information Sources and Eligibility Criteria
We used data aggregated by IMS Institute for Healthcare Informatics to identify the most prescribed brand name and generic drugs in the US,7 prioritizing for this review those with FDA-approved indications for treatment of conditions with known health disparities among Blacks. We further refined and prioritized within these lists by searching for information on known racial/ethnic variations in drug response in sources such as package inserts and other drug information sources (e.g., AHFS Clinical Drug Information8), complemented by clinician input. This analysis reports the literature describing relative safety and efficacy of β2 adrenergic agonists among Blacks as compared with other races or ethnicities (note: another component of the larger review project employed these methods to the literature analyzing effects of atypical anti-psychotics among Blacks as compared with other groups, published in 20209).
We searched the MEDLINE database via PubMed from January 2000 to November 2016 using a combination of controlled vocabulary and key terms related to the drug classes, race, and genetic or drug response variation (e.g., treatment, genotype, polymorphism, race, Black, African American, albuterol, see Appendix). We selected the year 2000, when the first draft of the human genome was completed, as a starting date given that majority of evidence related to our pharmacogenomics objective would be published after this time.10 To ensure that our analysis incorporated the most current literature, we completed a final manual search of citations in PubMed from November 2016 through August 2019. We also explored the utility of searching EMBASE; comparison of results indicated this resource was duplicative of the literature retrieved using PubMed so we elected not to include EMBASE in our approach.
To facilitate review of the literature retrieved by our systematic search, we developed inclusion criteria in consultation with team experts and informed by our preliminary review of response variability information (Table 1). For this review, we sought both studies of differential response to drugs of interest and studies identifying genetic polymorphisms related to drug response variability or modification. We included: 1) empirical studies of any design and pooled trial analyses, systematic reviews, and meta-analyses addressing variation in effectiveness or safety of β2 adrenergic agonists (including both short-acting and long-acting agents) among Black adults or children compared with another racial/ethnic group and 2) original research studies evaluating genetic variations that may affect response to β2 adrenergic agonists in Blacks compared with other racial or ethnic groups. We also limited to English language studies. Four reviewers (KW, SK, NS, RJ) independently screened titles/abstracts and the full text of studies using predetermined criteria, with disagreements between reviewers resolved through discussion to reach consensus.
Table 1.
Inclusion criteria for the review
| Category | Criteria |
|---|---|
| Population | Black adults or children (including study-defined, self-reported or defined in-study via genetic ancestry approaches (e.g., genotyping ancestry informative markers, using the program STRUCTURE)) receiving pharmaceutical agent(s) of interest, short or long acting β2 agonists. |
| Publication languages | English only |
| Admissible evidence (study design and other criteria) |
Admissible designs Analyses of racial/ethnic response variation: systematic reviews, meta-analyses, database or registry studies, comparative studies (randomized controlled trials (RCTs), prospective or retrospective cohort studies, case series) Clinical trials with racial/ethnic subgroup analyses: RCTs, prospective or retrospective cohort studies, case series |
|
Other criteria Original research studies providing sufficient detail regarding methods and results to enable use and aggregation of the data and results | |
| Studies must have relevant population Studies must address one or more of the following: -Analyses of therapeutic response by race/ethnicity -Analyses of safety by race/ethnicity Studies must include comparison with at least one other race/ethnicity group Relevant outcomes must be able to be extracted from data in the papers Study published January 2000 – July 2019* |
Legend: This table describes the detailed criteria the team employed to select literature for inclusion in this review. The year 2000 was selected as a start date based on the rationale that pharmacogenomics analyses were likely to be rare before this date.
2.2. Data Extraction and Analysis
We extracted key study population characteristics, study design, treatment and follow-up details, and outcome data on constructs of interest (race/ethnicity, allelic variations, efficacy measures, safety data) from eligible studies. Given the seriousness of outcomes data comprising signals related to asthma exacerbation, treatment failure, hospitalization, respiratory related death, or life-threatening experience, we categorized studies with primary focus on these data as safety evaluations.
To accommodate heterogeneous terminology used within the literature to refer to subgroups by race and ethnicity, we use the term Black throughout this analysis to refer to any subgroup defined within studies as Black, African, African American, or of African ancestry, and use the term White to refer to subgroups defined within studies as White, Caucasian, European American, or of European ancestry. Other less frequently compared groups in this literature are described using the terminology used in the original studies (e.g., Mexican, Puerto Rican, Latino, Asian).
We synthesized studies qualitatively and report descriptive statistics in summary tables. We did not formally assess the methodologic quality of studies or risk of bias. To assess relative population frequency of variants with significant allelic or genotypic effects on efficacy and/or safety, we extracted data describing minor allele frequency (MAF) among the subgroups from gnomAD.11
3. RESULTS & DISCUSSION
3.1. Article selection and overview
Of the 3158 papers retrieved by our searches, we identified 20 papers describing use of β2 agonists for this review (Figure 1). Whites were the group most commonly compared to Blacks in this literature, with only a few studies analyzing a Hispanic or Latin American comparison group. Most analyses used participant self-reported race and ethnicity or did not provide operational definitions for these categories; only one analysis incorporated genetic ancestry markers.12
Figure 1. Disposition of studies identified for this review.
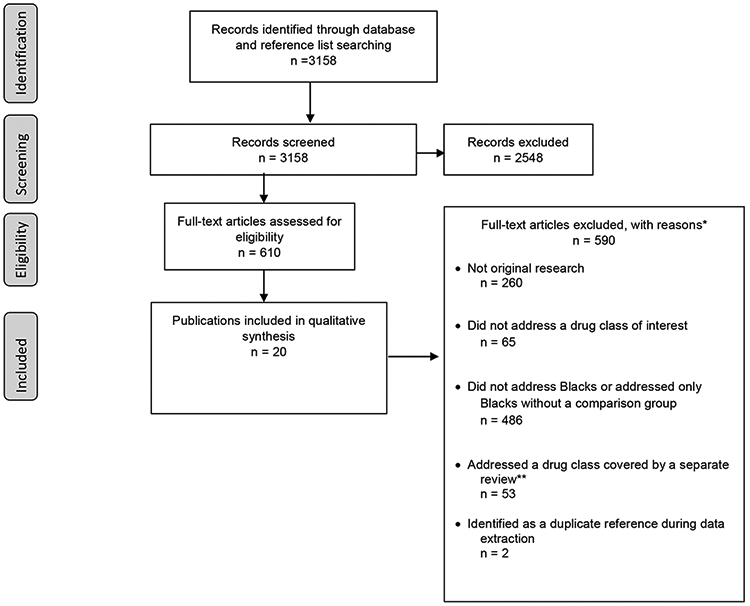
*Numbers do not tally as studies could be excluded for multiple reasons.
Abbreviations: n = Number.
** This review was part of a larger project including quasi-systematic reviews on two other drug classes, atypical antipsychotics9 and statins.
Legend: This figure illustrates the review process, including the disposition of all articles during each step of the process as related to the systematic application of inclusion criteria.
3.2. Studies reporting clinical data, without a pharmacogenetics component
Sixteen papers compared efficacy and/or safety outcomes associated with short-acting β2 agonist (SABA) or long-acting β2 agonist (LABA) therapy in Blacks as compared with other subgroups, including clinical data only, without analysis of genomic variation (Table 2). Eight of these papers did not report an operational definition for race and ethnicity;13-20 five papers indicated self-report of race and ethnicity;21-25 and three papers defined race by participant report that included both biologic parents, and all four biologic grandparents.26-28
Table 2:
β2 agonist studies reporting only clinical effectiveness or harms (no genetic findings)
| Author, Year Country |
Study Type Age group(s) Race/ethnicity (N) |
Operational definition for Black subgroup |
Condition(s)/ Drug(s) Studied |
Findings | Outcome type and relative directionality of effect among Blacks |
|---|---|---|---|---|---|
| SABA Efficacy | |||||
| McGarry 201526 USA, Puerto Rico SAGE II and GALA II studies |
Cross-sectional Children and adolescents Black (864) Mexican (687) Puerto Rican (974) Mixed/other Latino (435) |
Self-report; participant and all parents and grandparents self-identified as Black or African American | Asthma SABA (Albuterol) |
Blacks were less likely to be unresponsive to bronchodilator (BD) (positive BD response defined as ≥12% increase and a ≥200 mL increase in FEV1) as compared with Mexican Americans (OR:0.71, 95%CI: 0.50 to 0.99, p=0.04) | ↑ Efficacy |
| Wechsler 201122 USA Asthma Clinical Research Network |
Secondary analysis of data from 10 trials Adolescents and adults Black (233) White (795) |
Self-report | Asthma SABA (Agent not specified) |
No statistically significant difference observed in response between Blacks and Whites during short-acting β agonist therapy. | = Efficacy |
| Naqvi 2008;27 Naqvi 200728 USA, Mexico, Puerto Rico GALA and SAGE studies |
Cross-sectional Children, adolescents, and adults 199/200 Black (163/216) Mexican (106/294) Puerto Rican (246/365) |
Self-report; participant and all parents and grandparents self-identified as Black or African American | Asthma SABA (Albuterol) |
Puerto Ricans had lower bronchodilator responsiveness (% change in FEV1) as compared with Mexicans (p<0.001) and Blacks (p<0.001); the same signals were detected in the subset of asthmatics with FEV1 80% of predicted. Puerto Ricans of all ages and Black children with moderate-to-severe asthma demonstrated the lowest bronchodilator responsiveness overall. ICS use was associated with augmented bronchodilator responsiveness in Mexican Americans and Puerto Ricans (both p<0.05), but not in Blacks; these signals remained after excluding those who used long-acting β2 agonists |
↑ Efficacy (compared with Puerto Ricans) ↓ Efficacy (among Blacks with moderate-to-severe asthma) |
| Hardie 200720 | Cross-sectional Adults Black (16) White (16) |
NR | Asthma SABA (albuterol) |
FEV1 (in liters) before and after bronchodilator administration in Blacks and Whites, respectively, was 1.94±0.39 and 2.13±0.70 at baseline, 2.66±0.49 and 3.06±0.86 at 180 mcg albuterol, 2.88±0.48 and 3.37±0.91 at 360 mcg, and 2.42±1.29 and 3.47±0.95 at 540 mcg. The differences between Blacks and Whites in responsiveness were significant at 360 mcg (p<0.05) and 540 mcg (p<0.01). Visual Analog Scale and Borg score results for breathing discomfort did not differ significantly by group at all doses of albuterol. While words used by participants to describe asthma symptoms at 540 mcg of albuterol indicated a reduction in symptoms among Whites, 80% of Black participants used words indicating persistent upper airway symptoms. |
↓ Efficacy |
| El-Ekiaby 200623 | Cross-sectional Adults Blacks (155) Whites (140) |
Self-report | Asthma SABA (albuterol) |
There were no significant differences between Blacks and Whites in response to standard doses of albuterol, with mean peak expiratory flow rates increasing significantly in both groups. Further subgroup comparisons did not find any significant differences between Blacks and Whites with mild, severe, or life-threatening pretreatment obstruction. | = Efficacy |
| Lima 200013 USA |
Cross sectional Adults Black (15) White (18) |
NR | Asthma SABA (Albuterol) |
No difference in albuterol-induced change in FEV1 or percent predicted FEV1 between Blacks and Whites | = Efficacy |
| LABA Efficacy | |||||
| Murphy 201221 USA |
Secondary analysis of data from 4 RCTs Adolescents/Adults (12 years or older) Black (356) White (392) Other, including Hispanic (352) |
Self-report | Asthma LABA (Formoterol + Budesonide) |
Asthma event rates were significantly lower for participants receiving budesonide+formoterol as compared with participants treated with budesonide alone, regardless of race/ethnicity. | = Efficacy |
| Lemanske 201014 USA |
Randomized crossover trial Children and adolescents Black (45) White (67) Hispanic (39) Other (12) |
NR | Asthma LABA (Salmeterol) |
Race/ethnicity significantly associated with patterns of differential response to treatment (based on comparison of the need for treatment with oral prednisone for acute asthma exacerbation, number of asthma-control days, and FEV1) (p=0.005) Hispanic and non-Hispanic White participants most likely to have best response to LABA step-up, least likely to have best response to ICS step-up Black patients equally likely to have a best response to LABA or ICS step up therapy and less likely to have a best response to LTRA step-up. |
= Efficacy |
| LABA Safety | |||||
| Weinstein 201924 | RCT Adolescents and adults Black (705) Multiracial (968) Asian (550) American Indian or Alaska Native (440) Native Hawaiian or other Pacific Islander (9) Unknown (1) White (9056) |
Self-report Patients who selected Black plus another group were analyzed as part of a Black subgroup in the safety analysis (total revised N 1116) |
Asthma LABA (formoterol + mometasone furoate vs. mometasone furoate alone) |
Asthma exacerbations were more frequent among Black patients compared with non-Blacks in both treatment groups. Fewer asthma exacerbations occurred in Black patients in the mometasone furoate + formoterol group as compared with Blacks in the mometasone furoate only group (17.0% vs. 21.2%). Among non-Blacks, these frequencies were 11.5% in the combined treatment group and 12.5% in the mometasone-only group. Incidence of serious asthma outcomes (composite end point including asthma-related hospitalizations, intubations, and deaths) among Blacks were 1.2% in the combined group and 0.4% in the mometasone only group; among non-Blacks the frequency of hospitalization was 0.6% in both the combined treatment group and the mometasone only group. Adherence was lower in the Black subgroup; Black participants had a greater rate of treatment discontinuation as compared with non-Black |
= Safety (asthma exacerbations) ↓ Safety (composite of serious asthma outcomes) |
| Peters 201625 | RCT Adolescents and adults Black (401) White (4003) Asian (907) Other (536) |
Self-report | Asthma LABA (budesonide + formoterol vs. budesonide only) |
Risk of asthma exacerbation hazard ratios (95% CI, p) for comparison of the combined treatment group to the budesonide only group was 0.851 (0.608-1.192, p=0.348) among Blacks, 0.827 (0.720-0.950, p=0.007) among Whites, 0.776 (0.565-1.067, p=0.119) among Asians, and 1.026 (0.662-1.591, p=0.908) among participants of other race/ethnicity. | = Safety |
| Stempel 201616 AUSTRI trial Multiple countries |
RCT Adolescents and adults Black (1726) White (8783) Other (1170) |
NR | Asthma LABA (salmeterol+fl uticasone vs. fluticasone alone) |
No significant difference in rate of asthma-related hospitalization by race; investigators note that the trial was not powered to detect noninferiority in race/ethnicity subgroups. | = Safety |
| Stempel 201615 VESTRI trial Multiple countries |
RCT Children Black (1050) White (4030) Other (1128) |
NR | Asthma LABA (salmeterol+fl uticasone vs. fluticasone alone) |
Serious asthma-related event rates were less than 2% of the defined populations in all race subgroups. Time to event analysis of the first severe asthma exacerbation was not statistically significant between the fluticasone alone vs. salmeterol+fluticasone groups in the overall cohort or in the Black subgroup. Investigators note that the safety analyses were not powered to detect noninferiority in subgroups. |
= Safety |
| Wechsler 201122 USA Asthma Clinical Research Network |
Secondary analysis of data from 10 trials Adolescents and adults Black (233) White (795) |
Self-report | Asthma LABA, SABA (Specific agents not specified) |
Increased risk of treatment failures (asthma exacerbation, worsening lung function, increased use of asthma medication, or physician clinical judgment) in Blacks compared to Whites during treatment with long acting β agonists (LABA) (OR: 2.36, 95% CI 1.38-4.02, p=0.002), with or without other medications There was also an increased risk of treatment failure among Blacks as compared with Whites in the subgroup of patients receiving LABA plus a leukotriene receptor antagonist (OR 5.81 95% CI 2.11-16.02, p=0.0007). |
↓ Efficacy |
| Price 201017 Multinational UK/Sweden |
Pooled analysis of 41 trials Children and adolescents Black (369) White (8,947) Asian (1,043) Other or unknown (1,490) |
NR | Asthma LABA (Formoterol) |
No difference in hospital admission rates between formoterol treated and non-LABA-treated patients when analyzed by race/ethnicity. In general, Black participants tended to have higher hospital admission rates than Whites in both the formoterol (1.57% vs 0.65%) and the non-LABA treated (2.63% vs. 0.67%) groups. There was a decreased risk in asthma-related adverse events in the formoterol group (RR 0.67, 95% CI 0.46 to 0.98) and no effect of formoterol by gender or race. |
= Safety |
| Nelson 200618 USA SMART study Note: study terminated after interim analysis due to findings in Blacks and difficulties in enrollment Note: A Cochrane review by Walters et al. 200719 evaluated safety of LABA therapy in children; the subgroup analysis by race and ethnicity in this systematic review was limited to the SMART study, with slightly altered analytic approach. |
RCT Adolescents and adults Black (4,685) White (18,642) Hispanic (1,995) Asian (322) Other (454) |
NR | Asthma LABA (Salmeterol) |
Significant differences between salmeterol and placebo groups among Blacks for a combined outcome of respiratory related death or life threatening experience (20 vs. 5; RR: 4.1; 95% CI: 1.54 to 10.9), a combined outcome of asthma-related death or life threatening experience (19 vs. 4; RR: 4.92; 95% CI: 1.68 to 14.45), and combined all-cause death or life-threatening experience (24 vs 11, RR 2.17, 95% CI 1.06-4.41); no similar increases in risk observed among Whites. The subset of Blacks not using ICS at baseline experienced increased risk of combined respiratory-related death or life-threatening experience (RR 5.61, 95%CI 1.25-25.26) and of combined asthma-related death or life-threatening experience (RR 10.45 (1.34-81.58) with use of salmeterol as compared with placebo, as compared with those using ICS at baseline; no similar increases in risk observed among Whites. As calculated separately in the Cochrane review, Blacks were at particular risk for the respiratory related death and life-threatening asthma event (i.e. intubation, mechanical ventilation) as compared with Whites (RR: 3.9 (95% CI: 1.29 to 11.84). There was no significant difference between the two groups in asthma related deaths after LABA. |
↓ Safety |
Key: ↑ Efficacy=increased efficacy; ↑ Safety=increased safety; ↓ Efficacy=decreased efficacy; ↓ Safety=decreased safety; = Efficacy=equal efficacy; = Safety=equal safety; CI= confidence interval; ICS= inhaled corticosteroids; SABA = short acting β2 agonist; LABA= long-acting β2-agonist; LTRA= leukotriene receptor antagonists; OR=odds ratio; RCT=randomized controlled trial; relative risk; CI confidence interval; SAGE Study of African Americans, Asthma, Genes, and Environments; GALA Genes-environments and Admixture in Latino Americans; SMART Salmeterol Multicenter Asthma Research Trial
Legend: This table summarizes data from included studies evaluating safety and effectiveness of beta-agonists in Blacks as compared with other groups, including directionality of effects and focusing on the subset of studies that did not include any genetic data.
3.2.1. SABA Outcomes
3.2.1.1. SABA Efficacy:
Seven papers assessed relative response to treatment with a short-acting β2 agonist (albuterol) among Blacks as compared with one or more other subgroups.13,22,26-28 One paper focused on children and adolescents,26 one included both adolescents and adults,22 three were restricted to adults,13,20,23 and two papers included data from all three age groups.27,28
Three papers reported an improved response to albuterol among Blacks as compared with other groups.26-28 One paper, pooling cross-sectional data from the Study of African Americans, Asthma, Genes, and Environments (SAGE II) and Genes-environments and Admixture in Latino Americans (GALA II) observational studies of children and adolescents with asthma, found that Blacks were more likely to respond to a SABA bronchodilator (response defined as ≥ 12% increase and ≥200 mL increase in FEV1) as compared with Mexican Americans.26 Two cross-sectional analyses from the first SAGE and GALA observational studies assessed response to albuterol in children and adults with asthma,27,28 finding that Puerto Ricans had lower response as compared with both Mexicans and Blacks in both the overall group and in the subset of participants with FEV1 less than 80% of predicted (all p <0.001). In this same study, Puerto Ricans of all ages and the subset of Black children with moderate-to-severe asthma had the lowest response to albuterol. However, with regard to utility of concomitant therapy, inhaled corticosteroid use was associated with augmented response to albuterol in Mexican Americans and Puerto Ricans (both p<0.05) but not among Blacks; this signal remained after exclusion of those also using a LABA.
One small cross-sectional study found significantly reduced responsiveness to albuterol among Blacks as compared with Whites at 360 mcg (p<0.05) and 540 mcg (p<0.01); no significant difference was observed at 180 mcg.20 Qualitative analysis of patient-reported asthma experience at 540 mcg indicated persistent upper airway symptoms among 80% of Black participants, while White participants’ data suggested reduction in symptoms.20
The remaining three papers found no difference between Blacks and Whites in their response to a SABA, including a pooled analysis of 10 Asthma Clinical Research Network trials,22 a medium-sized cross sectional study,23 and a small cross sectional study.13
3.2.1.2. SABA Safety:
Our search did not identify any papers that analyzed differences in SABA-related safety issues among Blacks as compared with other groups.
3.2.2. LABA Outcomes
3.2.2.1. LABA Efficacy:
Two papers explored efficacy of LABA therapy in Blacks as compared with other groups.14,21 One of these analyses focused on children and adolescents14 and one included data from adolescents and adults.21
These two analyses found similar LABA efficacy among Blacks as compared with other racial and ethnic groups. In a secondary analysis of pediatric and adult data from four RCTs, asthma event rates were significantly lower for participants receiving budesonide plus formoterol as compared with participants treated with budesonide alone, regardless of race/ethnicity.21 In a randomized crossover trial, Hispanic and non-Hispanic White participants were most likely to have best response to LABA step-up and least likely to have best response to inhaled corticosteroids (ICS) step-up, while Blacks were equally likely to have a best response to LABA or ICS step up therapy and less likely to have a best response to leukotriene receptor antagonist (LTRA) step-up.14
3.2.2.2. LABA Safety:
Eight papers analyzed the relative safety of LABA therapy in racial and ethnic groups, including Blacks;15-19,22,24,25 two of these papers18,19 used the same dataset for analysis of differences by race and ethnicity and are discussed here together to avoid redundancy. Six of these analyses focused on adolescents and adults16,18,19,22,24,25 one included children and adolescents,17 and one focused on children.15
Two RCTs, a Cochrane review, and a pooled analysis of 10 RCTs, found serious increased risk associated with LABA therapy among Blacks compared with other groups; these analyses focused on adolescents and adults.18,19,22,24
In a recently published RCT, the incidence of a serious asthma outcome (composite of asthma-related hospitalizations, intubations, and deaths) was similar between the two randomized groups was equivalent among non-Blacks (0.6% in both groups), the incidence was greater among Blacks receiving the LABA formoterol plus the ICS mometasone furoate as compared with those receiving mometasone furoate alone (1.2% vs 0.4%).24 In this study, the overall rate of asthma exacerbations was similar between subgroups by intervention.
Another RCT, the Salmeterol Multicenter Asthma Research Trial (SMART), was terminated after interim analysis due to findings of serious increased risks among Blacks using salmeterol, as well as difficulties in enrollment.18 This interim analysis indicated significant differences between salmeterol and placebo groups among Blacks for a combined outcome of respiratory-related death or life-threatening experience (20 vs. 5, RR: 4.1, 95% CI 1.54 to 10.9), a combined outcome of asthma-related death or life-threatening experience (19 vs. 4, RR 4.92, 95% CI 1.68 to 14.45), and a combined all-cause death or life-threatening experience (24 vs. 11, RR 2.17, 95% CI 1.06-4.41). No similar increases in risk associated with salmeterol compared with placebo were observed among Whites.
Also reported in the SMART trial,18 the subset of Blacks not using ICS at baseline experienced increased risk of combined respiratory-related death/life-threatening experience (RR 5.61, 95% CI 1.25-25.26) and combined asthma-related death/life-threatening experience (RR 10.45, 95% CI 1.34-81.58) with use of salmeterol as compared with placebo, compared with those using ICS at baseline. No similar increases in risk were observed among Whites. As calculated separately in the related Cochrane review,19 Blacks were at particular risk for respiratory-related death and life-threatening asthma events (i.e., intubation, mechanical ventilation) compared with Whites (RR: 3.9, 95% CI 1.29 to 11.84). There was no significant difference in asthma-related deaths after LABA therapy between the two groups found in the Cochrane review.
A report analyzing pooled data from 10 RCTs in the Asthma Clinical Research Network22 revealed increased risk of treatment failures in Blacks compared to Whites during treatment with LABAs (OR 2.36, 95% CI 1.38-4.02, p=0.002), with or without concomitant use of other medications. There was also an increased risk of treatment failure among Blacks as compared with Whites in the subgroup of patients receiving LABA plus an LTRA (OR 5.81, 95% CI 2.11-16.02, p=0.0007).
Three RCTs and one pooled analysis of RCT data did not find a significant difference in safety among subgroups by race and ethnicity.15-17,25 Two RCTs comparing fluticasone alone to fluticasone plus salmeterol in asthma, one including children15 and one including adolescents and adults,16 found no difference in serious asthma events in the overall study cohort or among Black participants. The third RCT found similar risk of asthma exacerbation among Black patients receiving formoterol plus budesonide as compared with those receiving budesonide only (0.851, 95% CI 0.608-1.192, p=NS); interestingly, the risk of asthma exacerbation was significantly lower among Whites receiving the combined LABA + ICS as compared with those receiving ICS only (0.8278, 95% CI 0.720-0.950, p=0.007). Of note, none of these three RCTs were powered to detect noninferiority among subgroups.
A pooled analysis of 41 RCTs exploring use of formoterol in children and adolescents with asthma found no differential effect of race or ethnicity on hospital admission rates or asthma-related adverse events between formoterol-treated and non-LABA-treated patients.17 Blacks tended to have higher hospital admission rates than Whites in both the formoterol (1.57% vs 0.65%) and the non-LABA treated (2.63% vs. 0.67%) groups.
3.3. β2 Adrenergic agonists: Genetic variants and response.
In addition to these subgroup effects by race and ethnicity, we identified four papers exploring effects of interaction between genomic variation and race on response to β2 adrenergic agonists in asthma, including one meta-analysis,29 one cross-sectional study,12 one secondary analysis of randomized controlled trial (RCT) data,30 and one genome-wide association study (GWAS)31 (Table 3). One paper analyzed race by both self-report category (including biologic parents and grandparents) and ancestry informative markers,12 one defined race by participant report that included both biologic parents and all four biologic grandparents,31 and the other two papers did not provide operational definitions for race and ethnicity categories.29,30 These papers each focused on different age groups, including children and adolescents;29,31 children, adolescents and adults; and adults.30
Table 3:
β2 adrenergic agonist studies: genetic associations
| Author, Year Country |
Study Type Age Group(s) Race/Ethnicity (n) |
Operational definition for Black subgroup |
Conditions(s) Drug(s) Studied |
PGx Marker | Findings | Outcome type and relative directionality of effect among Blacks |
|---|---|---|---|---|---|---|
| Mak 201831 USA, Puerto Rico SAGE II and GALA II studies (discovery cohort); GALA, SAGE, HPR, SAPPHIRE, AND CHOP studies (replication cohorts) |
Cross-sectional Children and adolescents (discovery cohort); children, adolescents, and adults (replication cohort) Discovery cohort: Black (475) Puerto Rican (483) Mexican (483) Replication cohorts: Black (1443) Puerto Rican (522) Mexican (202() |
Self-report; participant and all parents and grandparents self-identified as Black or African American (discovery cohort) | Asthma SABA (Albuterol) |
Whole genome sequencing | No significant associations identified in association with albuterol response in subgroup-specific analyses by race/ethnicity. Trans-ethnic meta-analysis identified 10 unique loci (represented by 27 SNPs) significantly associated with albuterol response. |
= Efficacy |
| Finkelstein 200929 USA |
Meta-analysis Children and adolescents Black (125) White (639) Hispanic (84) Other/not defined (112) |
NR | Asthma SABA (drug not specified) |
c.46A>G, p.Arg16Gly and/or c.79C>G, p. Gln27Glu mutations in the β2 adrenergic receptor (ADRB2); position 16 (arg/Arg, Arg/Gly, or Gly/Gly) and/or 27 alleles (Glu/Glu, Glu/Gln, Gln/Gln) | Beneficial effect of Arg/Arg at position 16 on response to treatment with a SABA was most pronounced in Black children treated with SABA (OR=3.54; 95% CI [1.37, 9.13]), as compared with other ethnicities. Arg allele somewhat more prevalent among Blacks as compared with Whites (Table 4). | ↑ Efficacy |
| Corvol 200912 USA, Mexico, Puerto Rico GALA and SAGE studies |
Cross-sectional Children, adolescents and adults Black (267) Mexican (301) Puerto Rican (399) |
Self-report (participant, both biological parents, and all four biological parents must report same ethnic background); 104 ancestry informative markers imputed into STRUCTURE 2.1 program for assessment of individual genetic ancestry estimates | Asthma SABA (Albuterol) |
Four interleukin 6 (IL6) SNPs and one IL6R SNP | No single SNP consistently associated with response to albuterol (as measured by change in FEV1) in all 3 populations. The C allele of IL6 promoter region variant c.−572C>G (rs1800796) associated with lower drug response (change in FEV1 12%) among Mexicans (p=0.02); no significant effect among Puerto Ricans or Blacks. The C allele is also 3 times more prevalent among Latinos vs. Blacks (Table 4). The A allele of c.486T>A, p.Asp162Glu (rs13306435) associated with reduced risk of lower drug response among Mexicans (p=0.002); allele frequency too low in the other two populations to allow testing. The A allele is 35 times more common in Latinos as compared with Blacks. No significant effect of remaining IL6 SNPs or the IL6R SNP in any of the populations. IL6 and IL6R gene-gene interaction was found to be associated with higher drug response to albuterol among Latinos, but lower drug response among Blacks. |
↓ Efficacy |
| Wechsler 200930 USA LARGE study |
RCT Adults Black (17) White (58) Asian (3) Hispanic (9) |
NR | Asthma LABA (Salmeterol + ICS + ipratropium bromide as needed) |
ADRB2 (β2 Adrenergic Receptor) c.46A>G, p.Arg16Gly genotype | Genotype specific outcome effects observed in the Black subgroup not observed in the overall study group. Blacks with genotype Gly/Gly treated with LABA + ICS had significant improvement in AM and PM PEF (29 L/min, p=0.013 and 45 L/min, p=0.0005) compared to placebo + ICS and when treated with LABA only had improved PEF measured at clinic visits during spirometry compared to placebo (39 L/min, p=0.0016) Blacks with genotype Arg/Arg treated with LABA + ICS showed a lack of improvement in AM and PM PEF (−12 L/min, p=0.57 and −2.2 L/min, p=0.92) compared to placebo + ICS and when treated with LABA only showed lack of improved PEF measured at clinic visits during spirometry compared to placebo (−4.8 L/min, p=0.73) As noted above, Arg allele is somewhat more common among Blacks vs. Gly allele as compared with Whites. |
↑ Efficacy |
Key: ↑ Efficacy=increased efficacy; ↑ Safety=increased safety; ↓ Efficacy=decreased efficacy; ↓ Safety=decreased safety; = Efficacy=equal efficacy; = Safety=equal safety; CI confidence interval; ICS inhaled corticosteroid; SABA short-acting β2 agonist; LABA long-acting β2 agonist; OR odds ratio; FEV forced expiratory volume; PEF peak expiratory flow; AM morning; PM afternoon/evening: ICS inhaled corticosteroid; SAGE Study of African Americans, Asthma, Genes, and Environments; GALA Genes-environments and Admixture in Latino Americans; HPR Hartford/Puerto Rico; CHOP Children’s Hospital of Philadelphia; SAPPHIRE Study of Asthma Phenotypes and Pharmacogenomic Interactions by Race-ethnicity
Legend: This table summarizes data from included studies evaluating safety and effectiveness of beta-agonists in Blacks as compared with other groups, including directionality of effects reported in the literature focusing on the subset of studies that included analysis of genetic data.
Three analyses focused on SABA therapy (albuterol)12,29,31 and one on LABA therapy (salmeterol). Two of these papers assessed effects of the c.46A>G, p.Arg16Gly mutation in the β2 adrenergic receptor (ADRB2). A meta-analysis by Finkelstein et al.,29 which also found no significant effect of the p.Gln27Glu ADRB2 variant on treatment outcomes, observed a significant effect of c.46A>G, p.Arg16Gly genotype.
This analysis indicated a beneficial effect of Arg/Arg was most pronounced among Black children treated with a SABA (OR 3.54, 95% CI 1.37 – 9.13) as compared with other races and ethnicities. However, the opposite effect of the c.46A>G, p.Arg16Gly genotype was detected in a secondary analysis of RCT data in LABA-treated adults from the LARGE clinical trial.22 This RCT data indicated that Black Arg/Arg adults treated with LABA plus ICS showed a lack of improvement in AM and PM peak expiratory flow compared with Blacks treated with placebo plus ICS. The same lack of improvement was seen for those in the Black Arg/Arg subgroup treated with LABA only as compared with placebo. However, Black Gly/Gly participants treated with a LABA plus inhaled corticosteroids had significant improvement in AM and PM peak expiratory flow as compared with those treated with placebo plus ICS; the same trend was seen for Blacks with LABA only as compared with placebo. These effects were not observed in the overall study group or the White subset of participants in this RCT. Of note, the Arg allele is also somewhat more prevalent among Blacks as compared with Whites (Table 4).
Table 4:
Comparison of SNP minor allele frequency among populations for significant β2 agonist response variants
| First author, year |
Gene | SNP | MAF, Black* | MAF, White** | MAF, Latino | Summary of pharmacogenomic effects noted in Table 3 |
|---|---|---|---|---|---|---|
| Finkelstein, 200929; Wechsler 200930 | ADRB2 | c.46A>G, p.Arg16Gly (rs1042713) | 0.51 | 0.63 | 0.57 | Arg/Arg genotype associated with more pronounced response to SABA treatment in Blacks as compared with other ethnicities.29 Gly/Gly genotype associated with improvements in PEF in Blacks treated with LABA+ICS vs placebo+ICS; effect not observed in overall group and no subgroup effect for Blacks carrying Arg/Arg genotype.22 |
| Corvol, 200912 | IL6, promoter region | rs1800796 c.−572C>G | .09 | .04 | .27 | The C allele associated with lower response to SABA among Mexicans; no effect among Puerto Ricans or Blacks. |
| Corvol, 200912 | IL6R | c.486T>A, p.Asp162Glu (rs13306435) | .004 | .009 | .14 | The A allele associated with reduced risk of lower drug response among Mexicans; allele frequency too low to analyze among other populations. |
Note: MAF minor allele frequency; PEF peak expiratory flow; ICS inhaled corticosteroids; SABA short acting beta agonist; LABA long acting beta agonist; MAF data extracted from gnomAD browser11
prevalence from the gnomAD African subset
prevalence from the European (non-Finnish) gnomAD subset.
Legend: This table provides a snapshot of the relative population prevalence of single nucleotide polymorphisms associated with variability in treatment effects (safety or effectiveness) among Blacks as compared with other groups.
A cross sectional analysis of pooled data from the SAGE and GALA studies assessed the effects of SNPs in interleukin-6 (IL6) and the IL6 receptor (IL6R) on response to albuterol among children and adults with asthma.12 The C allele of IL6 promoter region variant c.−572G>C (rs1800796), known to affect IL6 gene expression and serum levels,32-35 was associated with lower drug response (change in FEV1 < 12%) among Mexicans (p=0.02); no significant effect of this variant was found among Puerto Ricans or Blacks. The C allele is also more prevalent among Latinos as compared with Blacks (Table 4). The A allele of c.486T>A, p.Asp162Glu (rs13306435), a variant affecting IL6 binding to gp130,36,37 was associated with reduced risk of lower treatment response among Mexicans but allele frequency was too low to analyze data from other subgroups. Finally, an IL6 and IL6R gene-gene interaction was associated with increased drug response to albuterol among Latinos, but lower drug response among Blacks.
Finally, the GWAS study employed data from the SAGE II and GALA II studies and found ten unique loci, including 27 SNPs, associated with albuterol response in the overall population; however, none of these associations were significant in subgroup analyses by race and ethnicity and none replicated in five additional independent populations, including a meta-analysis of those samples.31
3.4. Key findings
Although the issue of troubling and persistent health disparities among several minority populations is well-established, this review demonstrates both the use of existing evidence to detect effect signals, as well as the complexities involved in synthesizing these data. Several key issues such as small sample sizes, limited subgroup analyses, and likely heterogeneous populations limit the amount of synthesis possible. While mixed signals regarding differential effects among Blacks are difficult to align in a consistent direction given the limited data, this review underscores that important differences may exist and that clinicians should be aware in treatment planning and especially monitoring for safety issues.
While the literature is relatively small, data regarding the efficacy of SABA therapy in Blacks seems somewhat encouraging, though a cross sectional study indicating reduced responsiveness to albuterol among Blacks as compared with Whites suggests that further evaluations may be warranted, and informative. Further, any divergent safety signals associated with SABA therapy among Blacks appear to be underexplored at this time.
However, an increased risk of severe adverse outcomes among Blacks using the LABA salmeterol is concordant with the current FDA-mandated black box warning for this agent when used alone. The FDA recently removed the black box warning about increased risk of asthma-related death from products combining a LABA with a glucocorticoid based on four trials evaluating these outcomes.38 Of note, these trials were underpowered to detect noninferiority in racial subgroups.
Of interest for future research, while SABAs or LABAs may represent as only one component of a stepwise approach to management of asthma that incorporates multiple agents for more complex disease,39 evaluations of differences in outcomes by race and ethnicity for combination therapeutic approaches are also relatively sparse in the current literature.
Despite the small number of studies exploring pharmacogenomic influences on safety and efficacy of the β2 agonists, variation in minor allele frequencies (MAF) between populations by race and ethnicity is striking, suggesting that underlying genetic variation may be contributing significantly to variation in observed clinical outcomes during clinical treatment. Consideration of variation in MAF should be explicitly incorporated into further study of these agents’ safety and efficacy in diverse groups to promote understanding of how variants may help inform optimization and personalization of treatment planning.
Further, while the current work did not systematically explore the literature related to the efficacy and safety of the β2 agonists in those of Hispanic ethnicity, we uncovered initial data indicating disparate signals among Mexicans and Puerto Ricans. These incidental findings suggest the potential for significant value in future research devoted to systematic assessment of differences in asthma treatment outcomes in individuals from Hispanic and Latino subgroups.
3.5. Limitations
This review is subject to several limitations. Studies yielding data regarding possible racial disparities in clinical effects of β2 agonists, as well as genetic modifiers, are affected by relatively low sample sizes, especially within subgroups, including race and ethnicity as well as age groups, suggesting that this literature’s power to detect the full spectrum of predictors of efficacy and safety among Blacks is limited. Our approach did not include risk of bias scoring; future work may further assess this area to better estimate potential effects of bias on results as the relevant literature grows. Heterogeneity of studies also precluded quantitative analysis of results beyond the pooled analyses included in this review. Importantly, while differing presentations of asthma by patient age have important clinical implications, the relatively small size and heterogeneity of this literature precludes any meaningful differentiation between efficacy or safety among children, adolescents, and adults with asthma. Further, many studies did not include operational definitions for race and ethnicity categories, thus limiting assessment of heterogeneity related to this methodology.
We also limited our analysis to studies including both Blacks and another comparison group by race/ethnicity to allow for an assessment of relative risk. Though beyond the scope of our current review approach, there is an additional segment of the evidence that may provide additional insights for future hypothesis generation study design, and selection of pharmacogenetic markers to inform clinical practice - studies of SABA or LABA therapy that included only Black patients.40-44
4. IMPLICATIONS
Both types of β2 agonists considered here are key components of the National Heart, Lung and Blood Institute’s guidance for the treatment of asthma.39 Given the persistent disparities in health outcomes among Blacks affected by asthma,1 efforts to increase our understanding of systematic differences in treatment response among Blacks are essential. Further well-powered research investigations are called for, which should prioritize exploration of relative safety, efficacy, and potential pharmacogenomic modifiers of response to β2 agonists among Blacks. Background differences in allele frequency and effects by race and ethnicity must also be incorporated into future studies. In addition to making progress toward achieving our national goal of reducing asthma disparities among Blacks,45 this strengthening of our collective evidence base would enable the formulation of more tailored and effective asthma treatment plans.
Supplementary Material
Acknowledgments
Funding: This project described was supported by grant U54MD010722 from the National Institute on Minority Health and Health Disparities (USA) and the National Human Genome Research Institute (USA) and by CTSA award No. UL1 TR002243 from the National Center for Advancing Translational Sciences (USA). Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Institute on Minority Health and Health Disparities, the National Human Genome Research Institute, the National Center for Advancing Translational Sciences, or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest
The authors declare no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.CDC - Asthma - Most Recent Asthma Data. https://www.cdc.gov/asthma/most_recent_data.htm. Published July 14, 2017. Accessed January 8, 2018.
- 2.Loftus PA, Wise SK. Epidemiology of asthma. Curr Opin Otolaryngol Head Neck Surg. 2016;24(3):245–249. doi: 10.1097/MOO.0000000000000262 [DOI] [PubMed] [Google Scholar]
- 3.Cazzola M, Rogliani P, Sanduzzi A, Matera MG. Influence of ethnicity on response to asthma drugs. Expert Opin Drug Metab Toxicol. 2015;11(7):1089–1097. doi: 10.1517/17425255.2015.1047341 [DOI] [PubMed] [Google Scholar]
- 4.Brown RW, Cappelletti CS. Reaching beyond disparity: safely improving asthma control in the at-risk African-American population. J Natl Med Assoc. 2013;105(2):138–149. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell SJ, Bilderback AL, Okelo SO. Racial Disparities in Asthma Morbidity Among Pediatric Patients Seeking Asthma Specialist Care. Acad Pediatr. 2016;16(1):64–67. doi: 10.1016/j.acap.2015.06.010 [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease C, Prevention. Asthma Prevalence, Health Care Use and Mortality: United States, 2003-05. http://www.cdc.gov/nchs/data/hestat/asthma03-05/asthma03-05.htmfiles/11182/asthma03-05.html. Published March 8, 2016.
- 7.Medicines Use and Spending in the U.S. - A Review of 2015 and Outlook to 2020. IMS Health; http://www.imshealth.com/sites/en/thought-leadership/ims-institute/reports/medicines-use-and-spending-in-the-us-a-review-of-2015-and-outlook-to-2020. Published November 7, 2016. Accessed April 7, 2017. [Google Scholar]
- 8.American Society of Health-System Pharmacists. AHFS Drug Information (Online). Bethesda, MD: American Society of Health-System Pharmacists; 2016. http://proxy.library.vanderbilt.edu/login?url=https://online.statref.com/Document.aspx?grpalias=Vanderbilt&FxId=1. [Google Scholar]
- 9.Jerome RN, Pulley JM, Sathe NA, et al. Exploring Biologic Predictors Response Disparities to Atypical Antipsychotics among Blacks: A Quasi-Systematic Review. Ethn Dis. 2020;30(Suppl 1):229–240. doi: 10.18865/ed.30.S1.229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pirmohamed M. Pharmacogenetics and pharmacogenomics. Br J Clin Pharmacol. 2001;52(4):345–347. doi: 10.1046/j.0306-5251.2001.01498.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lek M, Karczewski KJ, Minikel EV, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536(7616):285–291. doi: 10.1038/nature19057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corvol H, De Giacomo A, Eng C, et al. Genetic ancestry modifies pharmacogenetic gene–gene interaction for asthma. Pharmacogenet Genomics. 2009;19(7):489–496. doi: 10.1097/FPC.0b013e32832c440e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lima JJ, Mohamed MH, Self TH, Eberle LV, Johnson JA. Importance of beta(2)adrenergic receptor genotype, gender and race on albuterol-evoked bronchodilation in asthmatics. Pulm Pharmacol Ther. 2000;13(3):127–134. doi: 10.1006/pupt.2000.0239 [DOI] [PubMed] [Google Scholar]
- 14.Lemanske RF Jr, Mauger DT, Sorkness CA, et al. Step-up therapy for children with uncontrolled asthma receiving inhaled corticosteroids. N Engl J Med. 2010;362(11):975–985. doi: 10.1056/NEJMoa1001278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stempel DA, Szefler SJ, Pedersen S, et al. Safety of Adding Salmeterol to Fluticasone Propionate in Children with Asthma. N Engl J Med. 2016;375(9):840–849. doi: 10.1056/NEJMoa1606356 [DOI] [PubMed] [Google Scholar]
- 16.Stempel DA, Raphiou IH, Kral KM, et al. Serious Asthma Events with Fluticasone plus Salmeterol versus Fluticasone Alone. N Engl J Med. 2016;374(19):1822–1830. doi: 10.1056/NEJMoa1511049 [DOI] [PubMed] [Google Scholar]
- 17.Price JF, Radner F, Lenney W, Lindberg B. Safety of formoterol in children and adolescents: experience from asthma clinical trials. Arch Child. 2010;95(12):1047–1053. doi: 10.1136/adc.2010.183814 [DOI] [PubMed] [Google Scholar]
- 18.Nelson HS, Weiss ST, Bleecker ER, Yancey SW, Dorinsky PM. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest. 2006;129(1):15–26. doi: 10.1378/chest.129.1.15 [DOI] [PubMed] [Google Scholar]
- 19.Walters EH, Gibson PG, Lasserson TJ, Walters JA. Long-acting beta2-agonists for chronic asthma in adults and children where background therapy contains varied or no inhaled corticosteroid. Cochrane Database Syst Rev. 2007;(1):Cd001385. doi: 10.1002/14651858.CD001385.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hardie GE, Brown JK, Gold WM. Adrenergic responsiveness: FEV1 and symptom differences in Whites and African Americans with mild asthma. J Asthma. 2007;44(8):621–628. doi: 10.1080/02770900701540481 [DOI] [PubMed] [Google Scholar]
- 21.Murphy KR, Uryniak T, Martin UJ, Zangrilli J. The effect of budesonide/formoterol pressurized metered-dose inhaler on predefined criteria for worsening asthma in four different patient populations with asthma. Drugs R D. 2012;12(1):9–14. doi: 10.2165/11630600-000000000-00000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wechsler ME, Castro M, Lehman E, et al. Impact of Race on Asthma Treatment Failures in the Asthma Clinical Research Network. Am J Respir Crit Care Med. 2011;184(11):1247–1253. doi: 10.1164/rccm.201103-0514OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El-Ekiaby A, Brianas L, Skowronski ME, et al. Impact of race on the severity of acute episodes of asthma and adrenergic responsiveness. Am J Respir Crit Care Med. 2006;174(5):508–513. doi: 10.1164/rccm.200603-431OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weinstein CLJ, Ryan N, Shekar T, et al. Serious asthma events with mometasone furoate plus formoterol compared with mometasone furoate. J Allergy Clin Immunol. 2019;143(4):1395–1402. doi: 10.1016/j.jaci.2018.10.065 [DOI] [PubMed] [Google Scholar]
- 25.Peters SP, Bleecker ER, Canonica GW, et al. Serious Asthma Events with Budesonide plus Formoterol vs. Budesonide Alone. N Engl J Med. 2016;375(9):850–860. doi: 10.1056/NEJMoa1511190 [DOI] [PubMed] [Google Scholar]
- 26.McGarry ME, Castellanos E, Thakur N, et al. Obesity and bronchodilator response in black and Hispanic children and adolescents with asthma. Chest. 2015;147(6):1591–1598. doi: 10.1378/chest.14-2689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naqvi M, Tcheurekdjian H, DeBoard JA, et al. Inhaled corticosteroids and augmented bronchodilator responsiveness in Latino and African American asthmatic patients. Ann Allergy Asthma Immunol Off Publ Am Coll Allergy Asthma Immunol. 2008;100(6):551–557. doi: 10.1016/S1081-1206(10)60055-5 [DOI] [PubMed] [Google Scholar]
- 28.Naqvi M, Thyne S, Choudhry S, et al. Ethnic-specific differences in bronchodilator responsiveness among African Americans, Puerto Ricans, and Mexicans with asthma. J Asthma Off J Assoc Care Asthma. 2007;44(8):639–648. doi: 10.1080/02770900701554441 [DOI] [PubMed] [Google Scholar]
- 29.Finkelstein Y, Bournissen FG, Hutson JR, Shannon M. Polymorphism of the ADRB2 gene and response to inhaled beta- agonists in children with asthma: a meta-analysis. J Asthma. 2009;46(9):900–905. doi: 10.3109/02770900903199961 [DOI] [PubMed] [Google Scholar]
- 30.Wechsler ME, Kunselman SJ, Chinchilli VM, et al. Effect of β2-adrenergic receptor polymorphism on response to longacting β2 agonist in asthma (LARGE trial): a genotype-stratified, randomised, placebo-controlled, crossover trial. Lancet. 2009;374(9703):1754–1764. doi: 10.1016/S0140-6736(09)61492-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mak AC, White MJ, Eckalbar WL, et al. Whole Genome Sequencing of Pharmacogenetic Drug Response in Racially Diverse Children with Asthma. Am J Respir Crit Care Med. March 2018. doi: 10.1164/rccm.201712-2529OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fang M, Huang Y, Zhang Y, Ning Z, Zhu L, Li X. Interleukin-6 -572C/G polymorphism is associated with serum interleukin-6 levels and risk of idiopathic pulmonary arterial hypertension. J Am Soc Hypertens JASH. 2017;11(3):171–177. doi: 10.1016/j.jash.2017.01.011 [DOI] [PubMed] [Google Scholar]
- 33.Brull DJ, Montgomery HE, Sanders J, et al. Interleukin-6 gene -174g>c and -572g>c promoter polymorphisms are strong predictors of plasma interleukin-6 levels after coronary artery bypass surgery. Arterioscler Thromb Vasc Biol. 2001;21(9):1458–1463. [DOI] [PubMed] [Google Scholar]
- 34.Fishman D, Faulds G, Jeffery R, et al. The effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis. J Clin Invest. 1998;102(7):1369–1376. doi: 10.1172/JCI2629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Terry CF, Loukaci V, Green FR. Cooperative influence of genetic polymorphisms on interleukin 6 transcriptional regulation. J Biol Chem. 2000;275(24):18138–18144. doi: 10.1074/jbc.M000379200 [DOI] [PubMed] [Google Scholar]
- 36.Liu Y, Berthier-Schaad Y, Fallin MD, et al. IL-6 haplotypes, inflammation, and risk for cardiovascular disease in a multiethnic dialysis cohort. J Am Soc Nephrol JASN. 2006;17(3):863–870. doi: 10.1681/ASN.2005050465 [DOI] [PubMed] [Google Scholar]
- 37.Noponen-Hietala N, Virtanen I, Karttunen R, et al. Genetic variations in IL6 associate with intervertebral disc disease characterized by sciatica. Pain. 2005;114(1-2):186–194. doi: 10.1016/j.pain.2004.12.015 [DOI] [PubMed] [Google Scholar]
- 38.Research C for DE and. Drug Safety and Availability - FDA Drug Safety Communication: FDA review finds no significant increase in risk of serious asthma outcomes with long-acting beta agonists (LABAs) used in combination with inhaled corticosteroids (ICS). https://www.fda.gov/Drugs/DrugSafety/ucm589587.htm. Accessed January 9, 2018.
- 39.Asthma NAE and PP Third Expert Panel on the Diagnosis and Management of. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. National Heart, Lung, and Blood Institute; (US: ); 2007. [Google Scholar]
- 40.Elbahlawan L, Binaei S, Christensen ML, Zhang Q, Quasney MW, Dahmer MK. Beta2-adrenergic receptor polymorphisms in African American children with status asthmaticus. Pediatr Crit Care Med. 2006;7(1):15–18. [DOI] [PubMed] [Google Scholar]
- 41.Moore PE, Ryckman KK, Williams SM, Patel N, Summar ML, Sheller JR. Genetic variants of GSNOR and ADRB2 influence response to albuterol in African-American children with severe asthma. Pediatr Pulmonol. 2009;44(7):649–654. doi: 10.1002/ppul.21033 [DOI] [PubMed] [Google Scholar]
- 42.Spector SL, Martin UJ, Uryniak T, O’Brien CD. Budesonide/formoterol pressurized metered-dose inhaler versus budesonide: a randomized controlled trial in black patients with asthma. J Asthma. 2012;49(1):70–77. doi: 10.3109/02770903.2011.633788 [DOI] [PubMed] [Google Scholar]
- 43.Brown RW, O’Brien CD, Martin UJ, Uryniak T, Lampl KL. Long-term safety and asthma control measures with a budesonide/formoterol pressurized metered-dose inhaler in African American asthmatic patients: a randomized controlled trial. J Allergy Clin Immunol. 2012;130(2):362–7.e9. doi: 10.1016/j.jaci.2012.03.028 [DOI] [PubMed] [Google Scholar]
- 44.Wechsler ME, Yawn BP, Fuhlbrigge AL, et al. Anticholinergic vs Long-Acting β-Agonist in Combination With Inhaled Corticosteroids in Black Adults With Asthma: The BELT Randomized Clinical Trial. JAMA. 2015;314(16):1720–1730. doi: 10.1001/jama.2015.13277 [DOI] [PubMed] [Google Scholar]
- 45.Reducing Asthma Disparities. http://www.nhlbi.nih.gov/health-pro/resources/lung/naci/discover/disparities.htmfiles/11177/disparities.html. Accessed March 8, 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


