Abstract
GATA4 is a transcription factor that regulates osteoblast differentiation. However, GATA4 is expressed at a higher level in mesenchymal stem cells (MSCs) than in osteoblasts. Therefore, the role of GATA4 in limb bud mesenchyme differentiation was investigated in mice by knocking out Gata4 using Cre-recombinase controlled by the Prx1 promoter (herein called Gata4 Prx-cKO mice). μCT analysis of the Gata4 Prx-cKO mice showed a decrease in trabecular bone properties compared with wildtype (Gata4fl/fl) littermates. Gata4 Prx-cKO mice have fewer MSCs as measured by CFU-F assays, mesenchymal progenitor cells (MPC2) (flow cytometry of Sca1+/CD45−/CD34−/CD44hi) and nestin immunofluorescence. Gata4 Prx-cKO bone marrow-derived MSCs have a significant reduction in WNT ligands, including WNT10B, and WNT signalosome components compared to control cells. Chromatin immunoprecipitation demonstrates that GATA4 is recruited to enhancers near Wnt3a, Wnt10b, Fzd6 and Dkk1. GATA4 also directly represses YAP in wildtype cells, and the absence of Gata4 leads to increased YAP expression. Together, we show that the decrease in MSCs is due to loss of Gata4 and a WNT10B-dependent positive autoregulatory loop. This leads to a concurrent increase of YAP and less activated β-catenin. These results explain the decreased trabecular bone in Gata4 Prx-cKO mice. We suggest that WNT signalosome activity in MSCs requires Gata4 and Wnt10b expression for lineage specification.
Keywords: GATA4, osteoblast, mesenchymal stem cell, WNT signaling, WNT10B
1. Introduction
GATA4 is a zinc-finger transcription factor that regulates osteoblast differentiation genes [1, 2]. In vivo and in vitro, reduction of Gata4 correlates with reduced Runx2 gene expression, along with reduced osteoblast mineralization. Chromatin immunoprecipitation (ChIP) demonstrated that Runx2 is a direct target of GATA4, that GATA4 is recruited to the two Runx2 promoters and an enhancer region. Furthermore, when Gata4 is knocked down, the chromatin at the Runx2 region is not open, as detected by DNase assays and ChIP with antibodies to the open chromatin marks H3K4me2 (histone 3 lysine 4 dimethylation) and H3K27ac (histone 3 lysine 27 acetylation) and the closed chromatin mark H3K27me3 (histone 3 lysine 27 trimethylation) [3].
We have also shown that GATA4 represses RANKL gene expression in osteoblasts. GATA4 is recruited to seven enhancers near RANKL. When Gata4 is knocked down, the chromatin at the RANKL region is opened, as detected by a reduction in H3K27me3 and an increase in H3K4me2 in the RANKL locus. In vitro, TRAP staining of cells from bone marrow cultures (containing both osteoblasts and osteoclasts) from Gata4 knockout cells shows that the increased levels of RANKL are sufficient for osteoclast formation [4]. These data demonstrate in vivo and in vitro that GATA4 is a key regulator of bone; GATA4 regulates the master intrinsic regulator of osteoblastogenesis and master exogenous regulator of osteoclastogenesis (RUNX2 and RANKL, respectively).
Osteoblasts differentiate from neural crest progenitors or mesenchymal stem cells (MSCs)[5]. MSCs can also differentiate into adipocytes, myocytes, chondrocytes and fibroblasts [6]. Markers of MSCs vary, depending on the species, source of cells and other experimental factors. For example, murine bone marrow-derived cells can be defined by two distinct mesenchymal progenitor cell populations (MPC1 and 2), with MPC2 (Sca1+, CD34−, CD45−, CD44+/hi), populations having the greater capacity of differentiating into osteoblasts, adipocytes and chondrocytes [7] compared to MPC1 (Sca1+, CD34−, CD45−, CD44−).
WNTs are known to control many types of stem cells [8], including MSCs. One of the key WNTs in bone is WNT10B [9]. WNT10B is required for maintenance of adult mouse bone density and WNT10B is a key regulator of mesenchymal progenitor fate because the number of bone marrow-derived mesenchymal progenitors is reduced in Wnt10b−/− mice [10].
Because Gata4 is expressed higher in MSCs and early in osteoblastogenesis than in differentiated osteoblasts [1], we sought to determine the role of GATA4 in MSCs using conditional knockout mice for Gata4 in limb bud mesenchyme using the Prx-1 promoter driving Cre-recombinase. Herein, we provide strong mechanistic evidence that MSCs require both Gata4 and Wnt10b expression to provide a positive autoregulatory loop, leading to differentiation into trabecular bone.
2. Materials and Methods
Mice
Animal experiments were approved by the Institutional Animal Care and Use Committee at the University of Tennessee Health Science Center. Animals were maintained in a specific pathogen free environment at 20–26°C with a relative humidity of 30–70% and a 12 h light/dark cycle. Commercial rodent chow (LM-485, Teklad, Madison, WI) and drinking water were available ad libitum. GATA4fl/fl mice were purchased from Jackson Laboratory (Gata4tm1.1Sad/J, JAX stock #008194). The floxed homozygous mice (GATA4fl/fl) were backcrossed for 10 generations to FVB background and then to C57BL/6 for 10 generations. The C57BL/6 GATA4fl/fl mice were crossed to B6.Cg-Tg(Prrx1-cre)1Cjt/J (JAX stock #005584) to produce GATA4fl/fl/Cre+ (Prx-cKO) and GATA4fl/fl/Cre− littermate control mice (referred to as WT mice in the text and figures).
GATA4-Flag-biotin mice (Flag-bio, Gt(ROSA)26Sortm1(birA)Mejr Gata4tm3.1Wtp/J)[11] were obtained from Jackson Labs (stock #018121).
WNT10B knockout mice (WNT10BKO, WNT10B−/−) were previously described [12].
Antibodies
The following antibodies were used: GATA4 (Santa Cruz Biotechnology, Clone G-4), Anti-dimethyl-Histone H3 (Lys4) Antibody (H3K4me2, Millipore cat. # 07–030), YAP (Cell Signaling, Cat. #4912), nestin (Aves Labs, Inc., Cat. #: NES), WNT10B (LSbio, Cat. # LS-C108739), FZD6 (Thermo Scientific Cat. #PA587953), activated β-catenin (ABC) (Cell Signaling, Cat. # 8814), LRP6 (Cell Signaling, Cat. #3395), pLRP6 (Cell Signaling, cat. # 2568), β-catenin (Santa Cruz Biotechnology, Cat. # sc-7199), AXIN2 (Abcam, Cat#ab32197), α-tubulin (Santa Cruz Biotechnology, Cat. #sc-8035), β-actin (Cell Signaling, Cat. #3700).
Micro-Computed Tomography (μCT)
μCT was performed as described [3, 4]. Tibiae, femora and vertebrae from 14-week-old mice were stored in PBS and then scanned using a Scanco μCT 40 (Brüttisellen, Switzerland) set at 55kVp/109μA. The entire femur and tibia were scanned in the sample holder with 12.3-mm diameter at medium resolution. These tubes were filled with PBS and the top of the tube was covered with Parafilm (American National Can, Chicago, IL, USA) to prevent dehydration. A scout view of each bone was taken and the sample height was adjusted to ensure the bone was within the field of view. The tibiae and femora images were obtained at 6 μm resolution. The integration time and Gaussian filter used for these samples was 300 ms and 1, respectively. Solid three-dimensional models were reconstructed from these images automatically after completion of each cone-beam image stack with the built-in software. The trabecular parameters were calculated on 200 slices of trabecular bone from a region just below the growth plate as described in [13] with a threshold of 250 and quoted using American Society of Bone and Mineral Research nomenclature. μCT was also used to measure the cortical properties of each diaphysis on 100 slices of cortical bone from region closest to the center of the shaft of the bone. The L5 vertebrae were isolated using the μCT X-ray scout view and scanned with an 8 μm voxel size. A section of the vertebral body measuring 0.5 mm immediately cranial to the caudal end plate was selected for analysis [14].
RNA and qPCR
Total RNA was isolated using TRIzol (Invitrogen, Carlsbad, CA, USA) and cDNA was prepared using ThermoScientific™ Maxima™ First Strand cDNA Synthesis Kit for RT-qPCR according to manufactures guideline and then quantified using TaqMan® Universal Master Mix II (Applied Biosystems; Foster City, CA, USA) or SYBR Green (ThermoFisher Scientific). The qPCR cycling conditions for TaqMan were initial denaturation at 50°C for 2 minutes; 95°C for 10 minutes, followed by 40 cycles of 95 °C for 15 seconds and 60 °C for 1 minute. The SYBR Green conditions used were 95 °C for 10 minutes; followed by 40 cycles of 95 °C for 15 seconds and 60 °C for 1 minute. Gata4 was quantified by TaqMan using primers from ThermoScientific (Assay Mm00484689_m1 and normalized to β-actin (Mm00607939_s1)). The oligonucleotide specific primers for SYBR Green assays used are listed in Supplemental Table 1. For analysis of the data, the values were normalized to β-actin values.
CFU-F, CFU-OB and CFU-Adipocytes
Bone marrow cells were isolated from tibias and femurs of 3–4 month old mice. Cells were flushed with MEM media, supplemented with 1% L-glutamine, and 1% penicillin-streptomycin and nucleated cells were counted with a hemocytometer. Total bone marrow was incubated for 5 days in mesenchymal stem cell media (MesenCult Basal Media, Stem-Cell Technologies Inc.).
For CFU-Fibroblasts (CFU-F) cells were cultured in MesenCult. Undifferentiated cells were fixed and stained with Giemsa (Ricca Chemical Company) and colonies were counted for CFU-F.
For differentiation into osteoblasts, cells were switched to osteogenic induction media (alpha MEM, supplemented with 1% L-glutamine, 1% penicillin-streptomycin, 50 μg/mL L-ascorbic acid 2-phosphate and 5 mM β-glycerophosphate) and the media was changed after every three days for two weeks. Osteoblasts were then fixed in 50% ethanol for 15 minutes at 4°C, followed by a 30-minute incubation with 1% Alizarin Red S (wt/vol with 0.1% ammonium hydroxide). The stain was then washed with distilled water, dried and photographed. The amount of mineral content was measured by eluting the Alizarin Red stain with 10% cetylpyridinium chloride and the optical density was measured at OD 570 nm.
For differentiation into adipocytes, cells were switched to adipogenic induction medium (alpha MEM, supplemented with 1% L-glutamine, 1% penicillin-streptomycin and 1 μM of the PPARγ agonist GW1929 [Tocris Bioscience]). Adipocyte differentiation was analyzed by ORO staining. Lipids were stained with 60% ORO solution for 10 minutes at room temperature. After washing and air-drying the ORO was eluted with 100% isopropanol and quantified by optical density measurement at 500 nm with a spectrophotometer.
Flow Cytometry
Bone marrow-derived MSCs were cultured as described [15]. The cells were stained with anti-CD34-Alexa 647 (clone RAM34), CD45-PE-Cyanine 7 (clone 30-F111), Sca1-PerCP-Cyanine5.5 (Clone D7), and CD44-eFluor450 (clone IM7) (all four stains from eBioscience). The cells were analyzed on a Propel/Bio-Rad Yeti/ZE5 Flow Cytometer. All flow cytometry data were generated in the Flow Cytometry and Cell Sorting core (FCCS) at The University of Tennessee Health Science Center with the assistance of Dr. Tony Marion.
Immunofluorescence
Slides were incubated in blocking buffer (1X TBST; 3%BSA; 1% normal goat or donkey serum; 0.2% Sodium Azide; 1% Triton X-100), followed by primary antibodies (in blocking buffer) overnight. Secondary antibodies conjugated to Alexa 488 were used to detect the primary antibody. Cells were counterstained with 6-diamidino-2-phenylindole (DAPI) in mounting medium (Vector Laboratories, Inc.) to identify nuclei.
For nestin staining, slides were incubated in BlokHen (Aves Labs, Inc). Chicken anti-nestin was diluted in BlokHen overnight. Fluorescein-labeled goat anti-chicken IgY (1:500 dilution, Aves Labs Cat. #F-1004) was used to detect Nestin. Cells were counterstained with 6-diamidino-2-phenylindole (DAPI) in mounting medium (Vector Laboratories, Inc.) to identify nuclei.
Calvaria
Mouse calvarial osteoblasts were isolated from 2-day-old CD1 or Flag-bio mice by sequential collagenase digestion [16]. Experiments were performed in α-MEM media with 10% fetal bovine serum (Omega Scientific, Tarzana, CA, USA). The cells were incubated for 40 minutes in α-MEM-1.0 mg/mL collagenase P-1.25% trypsin at 37°C. The cells were then washed in α-MEM and then incubated in α-MEM-1.0 mg/mL collagenase P-1.25% trypsin for 1 hour at 37°C. Collagenase digestion was stopped by addition of complete α-MEM media containing 10% FBS. The cells from second digest were obtained and allowed to proliferate in α-MEM media containing 10% FBS.
shGATA4
Lentivirus shC (a short hairpin that does not recognize any mammalian DNA) and shGATA4 were purchased from Sigma Aldrich (St. Louis, MO, USA). Two different shRNA from The RNAi Consortium (TRC) in pLKO vector were used to knockdown mouse Gata4 (TRCN0000095215: CCGGCCCAATCTCGATATGTTTGATCTCGAGATCAAACATATCGAGATTGGGTTTTT G and TRCN0000095217: CCGGCATCTCCTGTCACTCAGACATCTCGAGATGTCTGAGTGACAGGAGATGTTTT TG). The calvarial cells were plated in six-well plates at a seeding density of 2×105 cells per well. After 24 hours, the cells were infected with lentivirus at an MOI of 50 in α-MEM with 8 μg/mL polybrene per well. The plates were centrifuged at 1400 x g at 30°C for 45 minutes and left undisturbed for 24 hours after which the cells were washed with PBS and mineralization media was added. The knockdown of Gata4 was confirmed by qPCR.
Chromatin Immunoprecipitation (ChIP)
For chromatin immunoprecipitation, the calvarial cells were plated at a seeding density of 2×105 cells and left undisturbed for 24 hours prior to silencing using lentivirus directed towards shC or shGATA4 as mentioned above. After 24 hours of silencing the cells were washed with PBS and complete α-MEM media was added to each well and left for additional 3 days after which ChIP was performed using truChIP™ Ultra Low Cell Chromatin Shearing Kit (Covaris, Inc., Woburn, Massachusetts, USA) (see supporting information). qPCR using SYBR Green Mastermix (Life Technologies) was used to amplify the immunoprecipitated DNA.
GATA4-Flag-biotin mice (Gt(ROSA)26Sortm1(birA)Mejr Gata4tm3.1Wtp/J) [11] were obtained from Jackson Labs. The calvarial cells from 2-day old GATA4-Flag-biotin pups were isolated as described above for wildtype calvaria and grown under appropriate cell culture conditions in αMEM media with 10% fetal bovine serum in 15-cm dishes. Once confluent, the cells were fixed with 37% formaldehyde for 10 minutes, the excess formaldehyde was quenched with glycine for an additional 5 minutes as per the Magna ChIP™ A/G chromatin immunoprecipitation kit protocol (EMD Millipore, Massachusetts, USA). Wildtype CD1 mice were used as a control under identical conditions. The chromatin was lysed according to Millipore protocol and sonicated using a Biorupter sonicator (Diagenode, Denville, NJ) and incubated for 1 hour at 4°C with protein A beads to preclear the sheared chromatin followed by overnight incubation at 4°C with streptavidin beads (ThermoFisher Scientific). After the incubation, the samples were sequentially washed with cold SDS wash buffer (2% SDS), high salt buffer (50 mM HEPES pH 7.5, 500 mM NaCl, 1 mM disodium EDTA, 0.1% (w/v) sodium deoxycholate, 1% (v/v) Triton X-100), LiCl buffer (10 mM Tris-Cl pH 8.1, 250 mM LiCl, 1 mM disodium EDTA, 0.5% (v/v) Nonidet P-40 (NP-40), 0.5% (w/v) sodium deoxycholate), TE buffer (10 mM Tris-Cl, pH 7.5, 1 mM disodium EDTA) and Low Stringency IP Wash Buffer (EMD Millipore, Massachusetts, USA) [17]. To reverse the cross-links the IP beads and the input were resuspended in Millipore elution buffer and placed on a heat block for 2 hours at 65°C and an additional 15 minutes at 95°C, after which the DNA was validated by qPCR. Each ChIP was performed in biological triplicates. qPCR was performed as described above with primers listed in Supplemental Table 1 and normalized to input and an intergenic region on chromosome 2 that tested negative in multiple ChIP assays, including GATA4, (Intergenic sequence 7 (IS7)) primers).
Recombinant WNT proteins
Recombinant WNT10B (rWNT10B) and rWNT3A were purchased from R&D Systems. Cells were treated with 100 ng/mL rWNT10B or 40 ng/mL rWNT3A for 30 minutes.
Immunoblots
Protein extracts were prepared by homogenizing cells on ice in EBC buffer containing protease inhibitors. Protein concentrations were measured using the Bradford method. Immunoblots were performed with standard protocols using the antibodies listed above.
Statistical analysis
All experiments represent both biological and experimental triplicates. Unless otherwise stated, error bars represent mean ± 1 standard deviation. Statistical analyses, including student’s t-test, were performed using GraphPad Prism® (version 8) software.
3. Results
GATA4 knockout in limb bud mesenchyme leads to decreased trabecular bone
GATA4 regulates genes involved in early osteoblastogenesis, including RUNX2 and TGFβ [1, 3], supporting the hypothesis that GATA4 is necessary in mesenchymal stem cells (MSCs) and/or early in osteoblast differentiation. To test this hypothesis in vivo, we knocked-out Gata4 in limb bud mesenchyme using Cre-recombinase controlled by the Prx1 promoter. We observed the expected Mendelian ratio of knockout mice that were born (42/168, 25%) based on the breeding of Gata4fl/fl female mice and Gata4fl/+Prx-Cre+ male mice) and the mice had no gross abnormalities. At 14 weeks of age, the bones of these mice, here on called Gata4 Prx-cKO, were analyzed by μCT and representative images are shown (Figure 1 A–B). The percentage bone volume [bone volume (BV)/ tissue volume (TV)] was significantly reduced in femurs of female Gata4 Prx-cKO mice compared with wildtype (WT) littermates (Figure 1C). (All WT mice used are Gata4fl/flCre−.) In addition, there was a significant decrease in the trabecular thickness, while no change was seen in trabecular number and trabecular spacing (Figure 1D–F). Furthermore, no significant changes in cortical bone thickness nor porosity were detected between Gata4 Prx-cKO mice and their littermate controls (Figure 1 G–H). These results were consistent with our previously published conditional Gata4 knockout mice using either the Col1a1- (osteoblasts) or Bglap- (mature osteoblasts) driven Cre recombinase [3, 4]. Together, these mouse models confirm that GATA4 regulates trabecular bone properties.
Figure 1: Trabecular bone volume is decreased in Gata4 Prx-cKO mice.
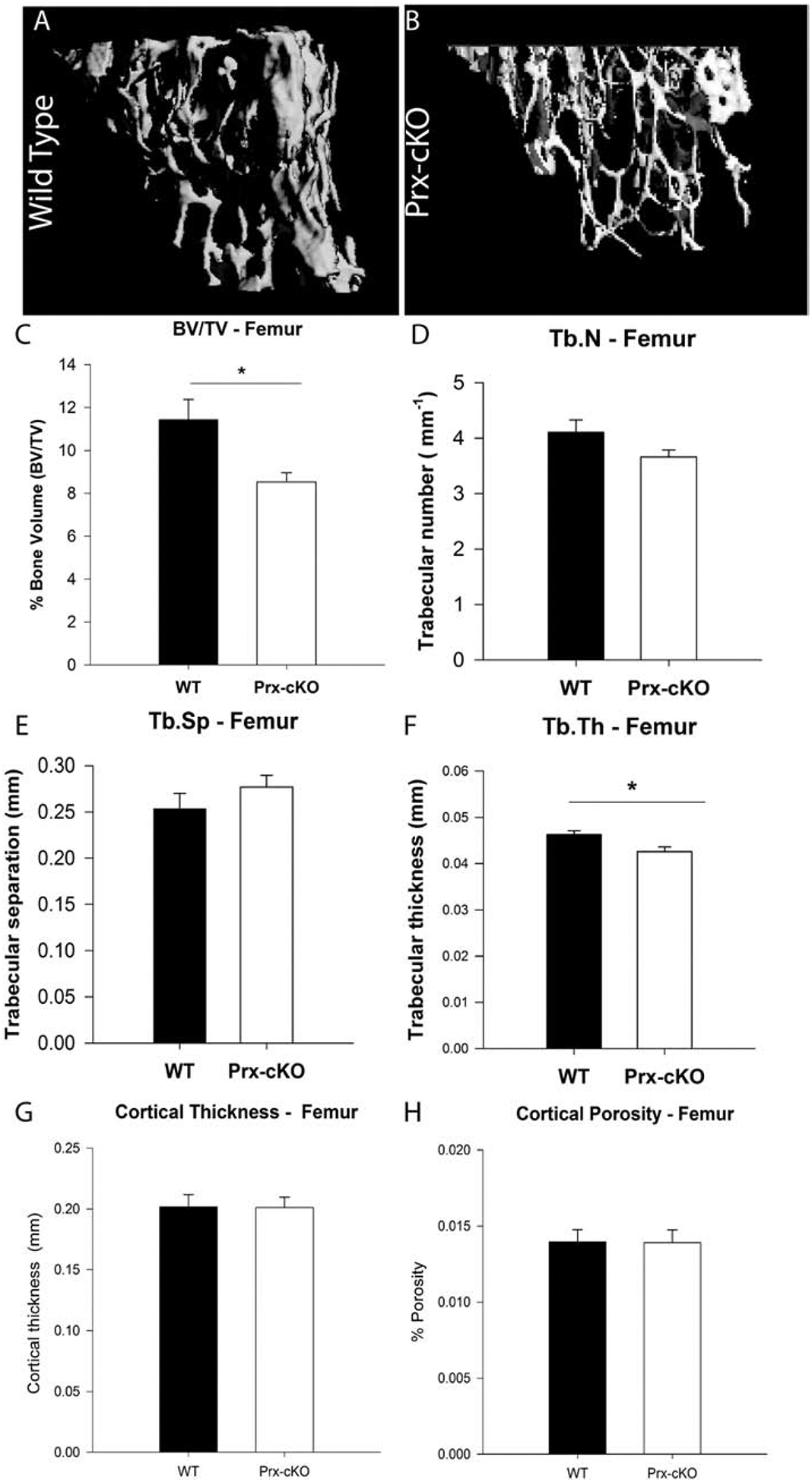
(A, B) μCT reconstruction of trabecular bone in the femur of 14-week-old female mice. (C) Mean percentage bone volume (BV/TV), (D) trabecular number (Tb.N), (E) trabecular separation (Tb.Sp), (F) trabecular thickness (Tb.Th), (G) cortical thickness and (H) cortical porosity of WT (Gata4FL/FL) and Prx-cKO mice. Black bars indicate wildtype (WT) mice; white bars indicate GATA4 Prx-cKO mice. Data are mean ± standard deviation from 12 WT and 12 cKO mice. Student’s t-test: *P < 0.05 compared with WT.
GATA4 is necessary for mesenchymal stem cell abundance and lineage specification
To test whether GATA4 plays a role in the regulation of MSC differentiation towards the osteoblast and adipocyte lineages, bone marrow cells were isolated from 3–4 month old WT and Gata4 Prx-cKO femurs and tibia. First, MSCs were differentiated ex vivo for 14 days in osteoblast mineralization media (containing ascorbic acid and β-glycerophosphate). The Gata4 expression level and mineralization were quantified at the end of the experiment. The Gata4 expression was not detected in cKO mice compared to WT mice (Figure 2A), along with reduced Alizarin Red staining and a significant reduction in mineral content (Figure 2 B–C). Furthermore, these cells had a reduction in the expression of Runx2, the “master” transcription factor for osteoblasts (Figure 2D) and osteocalcin (OCN, Bglap), a differentiated osteoblast marker (Figure 2E).
Figure 2: GATA4 regulates both osteogenesis and adipogenesis in vitro.
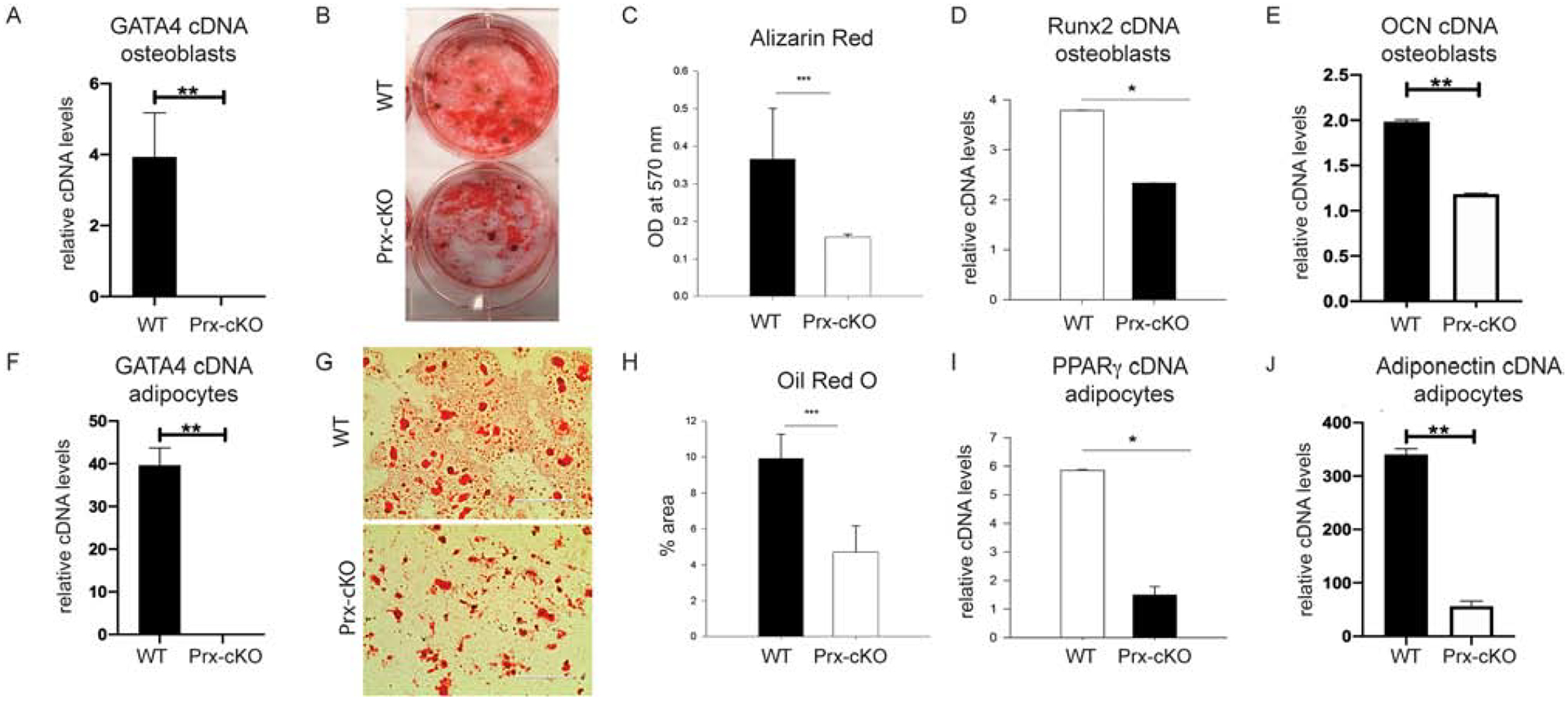
Bone marrow MSCs from WT and Gata4 Prx-cKO were differentiated in osteogenic media for two weeks. (A) RNA was obtained and qPCR was performed for Gata4 and normalized to actin mRNA. (B) Cells were fixed and stained for mineralization using Alizarin Red. (C) Alizarin Red from part B was eluted and the mineral content measured at an OD of 570 nm. (D) qPCR was performed for Runx2 and (E) osteocalcin (OCN, BGLAP). (F) Bone marrow MSCs were also differentiated for two weeks in adipogenic media and qPCR was performed for Gata4 normalized to actin mRNA. (G) Adipocytes were stained using Oil Red O and (H) the percentage of fat droplets were calculated using ImageJ. (I) PPARγ and (J) Adiponectin expression was evaluated in the WT and Prx-cKO adipocytes. Student’s t test; * P < 0.05; *** P < 0.001 compared with wild-type. OD = optical density.
Bone marrow cells were also differentiated to adipocytes using GW1929, a PPARγ agonist. After two weeks of differentiation, adipocytes were analyzed for the level of Gata4 (Figure 2F) and stained with Oil Red O (Figure 2G). Gata4 mRNA was not detected in the adipocytes from cKO mice. Quantification of Oil Red O staining showed a two-fold reduction in adipocytes (Figure 2H). Furthermore, these cells had a six-fold reduction in the expression of PPARγ (Pparg), the “master” transcription factor for adipocytes (Figure 2I), and adiponectin, a differentiated adipocyte marker (Figure 2J). Gata4 Prx-cKO bone marrow cells also had reduced expression of MyoD, the “master” transcription factor for myocytes, but not Sox9 (chondrocytes) or vimentin (a fibroblast marker) (Supplemental Figure 1). These results suggest that GATA4 is necessary for lineage specification from MSCs.
Next, the role of GATA4 was analyzed in undifferentiated MSCs by several different assays. First, the colony forming units-fibroblast (CFU-F) assay was performed using the mouse MesenCult Proliferation Kit™ (Stem Cell Technologies) for the expansion and enumeration of mouse MSCs. As expected, Gata4 was confirmed to be knocked out in these cells by qPCR (Figure 3A). In parallel cultures, colonies were stained with Giemsa (Figure 3B) and counted for the numbers CFU-F’s present (Figure 3C). Gata4 Prx-cKO bone marrow gave rise to over three-fold fewer CFU-F colonies than those from control littermates.
Figure 3: GATA4 regulates the number of mesenchymal stem cells.
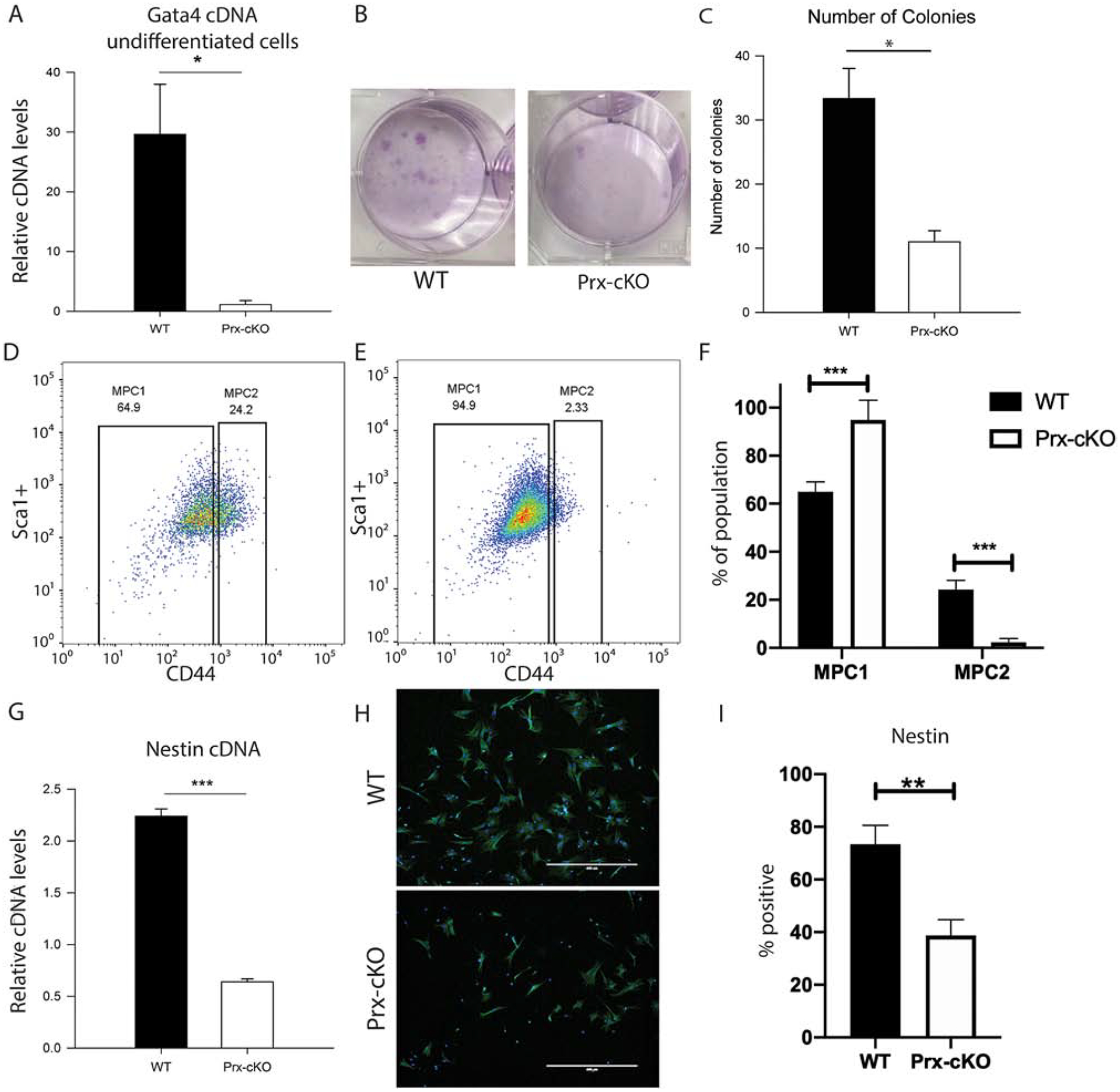
(A-C) Bone marrow cells from WT and Gata4 Prx-cKO mice were cultured in MesenCult media. (A) Gata4 mRNA expression levels in undifferentiated cells from WT and Gata4 Prx-cKO mice. (B) Cells were stained with Giemsa and (C) the number of Giemsa colonies in multiple wells was quantified using ImageJ. (D and E) MSCs were analyzed for the expression of Sca1+/CD45−/CD34−/CD44 markers using flow cytometry. (F) The number of Sca1+/CD45−/CD34−/CD44lo (MPC1) cells and Sca1+/CD45−/CD34−/CD44hi (MPC2) from D and E are graphed. (G) Nestin expression was measured by qPCR from cells grown as in parts A-C. (H) Immunofluorescence of nestin in WT and Gata4 Prx-cKO mice (I) Percentage of nestin positive cells in three replicates as in part H. Student’s t test; * P < 0.05; *** P < 0.001 compared with wild-type.
To further define the MSC population, we cultured MSCs according to Soleimani and Nadri [15], which enriches for MSCs in vitro by sequential media changes over a ~3 week period. After 3 weeks we harvested the enriched MSCs and conducted FACS analysis for the percentage of Sca1+/CD34−/CD45−/CD44+ cells [7] in both the WT and Gata4 Prx-cKO mice (Figure 3D–F). The enriched MSCs can be enumerated into both mesenchymal progenitor cell population 1 and 2 (MPC1 and MPC2) and we show that the MPC2 (Sca1+, CD34−, CD45−, CD44+/hi) are statistically significantly decreased in the Gata4 Prx-cKO mice. The MPC2 cells have a much greater capacity than the MPC1 (Sca1+, CD34−, CD45−, CD44−) cells to differentiate into osteoblasts and adipocytes [7]. These results are congruent with our previous data that showed fewer osteoblasts and adipocytes in the Gata4 Prx-cKO mice (Figure 2). We show our FACS scheme for enumerating the enriched MSC populations in Supplemental Figure 2.
Nestin-positive cells have been shown to contain all the bone-marrow colony-forming-unit fibroblastic activity and have the capacity of multilineage differentiation towards mesenchymal lineages and can self-renew in serial transplantation [18]. Thus, nestin-positive cells are representative of multipotent MSCs. qPCR for nestin mRNA showed a four-fold reduction in cells from Gata4 Prx-cKO mice (Figure 3G). These cells were also stained with an antibody to nestin and WT cells exhibited a 2-fold higher percentage of nestin-positive cells (Figure 3H and I).
In summary, by each of these three methods (CFU-F, flow cytometry and nestin expression), there are more MSCs (i.e. MPC2 cells) in WT mice compared to those without GATA4. We provide strong evidence that GATA4 expression is necessary for controlling the number of bone marrow-derived MSC cells.
GATA4 directly regulates WNT ligand and signalosome components
In search of a mechanism to explain the reduction in MSCs, we hypothesized that WNT signaling would be down-regulated, due to the role of WNT signaling in stem cells and that WNT10B-KO mice have fewer bone marrow-derived MSCs [8, 10]. Bone marrow cells from WT and Gata4 Prx-cKO mice were cultured in mouse MesenCult media, and in the absence of Gata4, the WNT ligands Wnt1, Wnt3a, Wnt5a and Wnt10b were down-regulated (Figure 4A). Wnt7b and Wnt11 were increased in Gata4 Prx-cKO cells, suggesting that these Wnt ligands are suppressed by GATA4 in undifferentiated MSCs. There was no change in mRNA expression for Wnt2, Wnt2b, Wnt3, Wnt4, Wnt5b, Wnt7a, Wnt9a, Wnt10a or Wnt16. Wnt8b and Wnt9b were not detected.
Figure 4: GATA4 directly regulates WNT signaling.
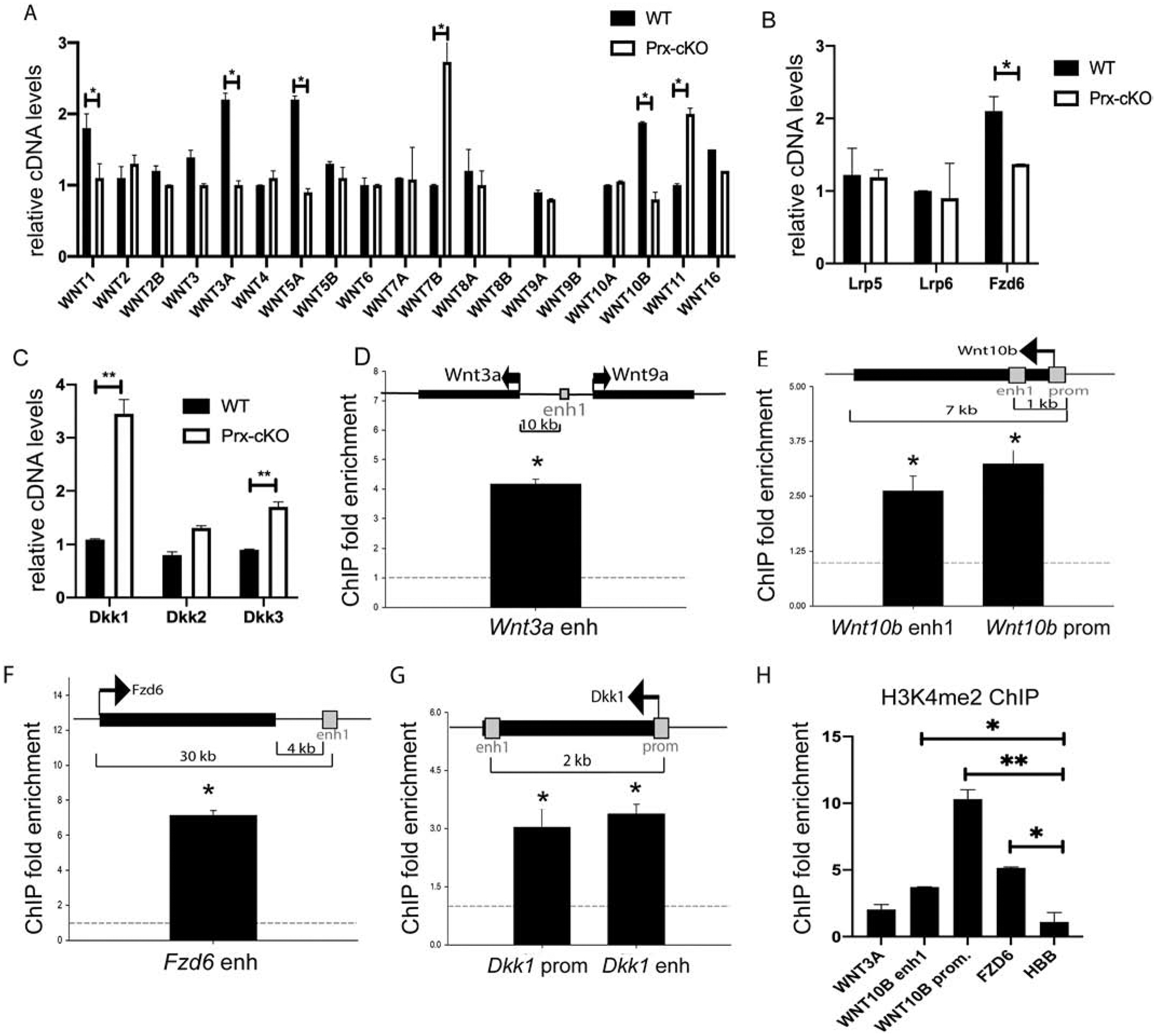
Bone marrow cells from WT and Gata4 Prx-cKO mice were cultured in MesenCult media. RNA was collected and qPCR was performed to the indicated (A) Wnt genes, (B) Wnt Co-receptors, and (C) Wnt signaling inhibitors. (D-G) Chromatin immunoprecipitation was performed at the indicated enhancers or promoters. Streptavidin-coated beads were used to precipitate Flag-biotin-GATA4. qPCR was performed with primers to the indicated regions (gray boxes). Each PCR was normalized to input and represented as fold enrichment over a negative genomic locus (dotted line). Insets show a schematic of the genomic region. Black bars indicate genes with arrows indicating the start and direction of transcription. Gray boxes indicate enhancer or promoter regions. (H) ChIP with antibodies to H3K4me2 was performed and qPCR was performed with primers to the indicated regions. Student’s t test; * P < 0.05; ** P < 0.01, *** P < 0.001 compared with wild-type (A-C) or negative genomic locus (D-H).
To determine if WNT signalosome components [19] were also altered, we analyzed for the expression of co-receptors, and inhibitors of Wnt signaling. mRNA levels of the lipoprotein receptor proteins, Lrp5 and Lrp6, that signal through a β-catenin dependent pathway and are often referred to as the canonical WNT signaling receptors [20], were unchanged (Figure 4B). Next, we analyzed for expression of Frizzled (FZD) family members that are co-activators with LRPs and directly interact with WNT-ligands. Fzd6 was decreased in Gata4 Prx-cKO MSC cells (Figure 4B). There were no other Fzd genes that were statistically decreased by more than 1.5 fold in Gata4 Prx-cKO cells (Supplemental Figure 3A). However, Fzd5 was increased by more than two-fold. In contrast, the WNT signaling secretable antagonist family members Dkk1 and Dkk3, but not Dkk2, were upregulated in these same cells (Figure 4C). These same WNT-ligands (Wnt1, −3a and −10b) and Fzd6 genes were also down-regulated in calvarial osteoblasts transduced with a lentiviral short hairpin shRNA to GATA4 (Supplemental Figure 3). Together, these data demonstrate that GATA4 regulates WNT ligands and its signalosome components.
We have previously analyzed GATA4 binding sites on DNA by genome-wide ChIP-sequencing, and discovered its functional role regulating both master regulators of osteogenesis (i.e. RUNX2) and osteoclastogenesis (i.e. RANKL) [3, 4]. Therefore, we searched for GATA4 binding sites near WNT ligands and WNT signalosome genes to determine if GATA4 directly binds to their cis-regulatory regions (at a p value <0.001). Genome-wide, GATA4 is enriched in introns (52.1% of binding sites), at distal intergenic regions (30.1% of binding sites) and less commonly, at the promoter of genes (7% of binding sites). GATA4 was found to bind at an enhancer near Wnt3a, at the promoter and an intronic enhancer for Wnt10b, at an enhancer near Fzd6, and the promoter and an intronic enhancer for Dkk1 (Figure 4D–G). Interestingly, the enhancer near Wnt3a is closer to Wnt9a, but Wnt9a mRNA is not influenced by the absence of Gata4 expression (Figure 4A). Based on our ChIP-seq cutoffs, we did not find GATA4 binding sites near Wnt1, Wnt5A, Lrp5, Lrp6, Dkk2 or Dkk3. ChIP-qPCR was performed to verify the ChIP-sequencing. The fold enrichment was determined by comparing the immunoprecipitated qPCR signal to input DNA and dividing by values of a negative control region (Figure 4D–G). Indeed, GATA4 is recruited to multiple promoters and enhancers regulating WNT signaling and WNT signalosome components. To demonstrate that these regions are active enhancers or promoters, ChIP with an antibody to histone 3 lysine 4 dimethylation (H3K4me2) was performed. The GATA4 sites near WNT10B and FZD6 are open, active enhancers (Figure 4H). The site near WNT3A was not statistically enriched for H3K4me2 and the qPCR values for Wnt3a cDNA indicated a low abundance of mRNA, suggesting a minor role, if any, for WNT3A in this system.
Based on the fact that Wnt10b knockout mice have fewer bone marrow-derived MSCs, similar to that of the Gata4 Prx-cKO, we wanted to confirm the loss of Wnt10b and Fzd6 expression in Gata4 Prx-cKO mice (Figure 5). Bone marrow cells were grown in MesenCult media to expand MSCs, and immunofluorescence was performed with antibodies to WNT10B and FZD6. Not all cells are positive for WNT10B and FZD6, but there is an almost complete loss of WNT10B and a greater than 50% reduction of FZD6 in Gata4 Prx-cKO cells (Figure 5A). To determine if downstream from the Wnt co-receptors has been affected by the loss of WNT10B and FZD6 expression, we stained for non-phosphorylated β-catenin that is often referred to as transcriptionally active β-catenin (ABC) [21] and demonstrated by immunofluorescence that there is a significant reduction of ABC in the Gata4 Prx-cKO cells (Figure 5A).
Figure 5: WNT signaling is attenuated in GATA4 Prx-cKO mice.
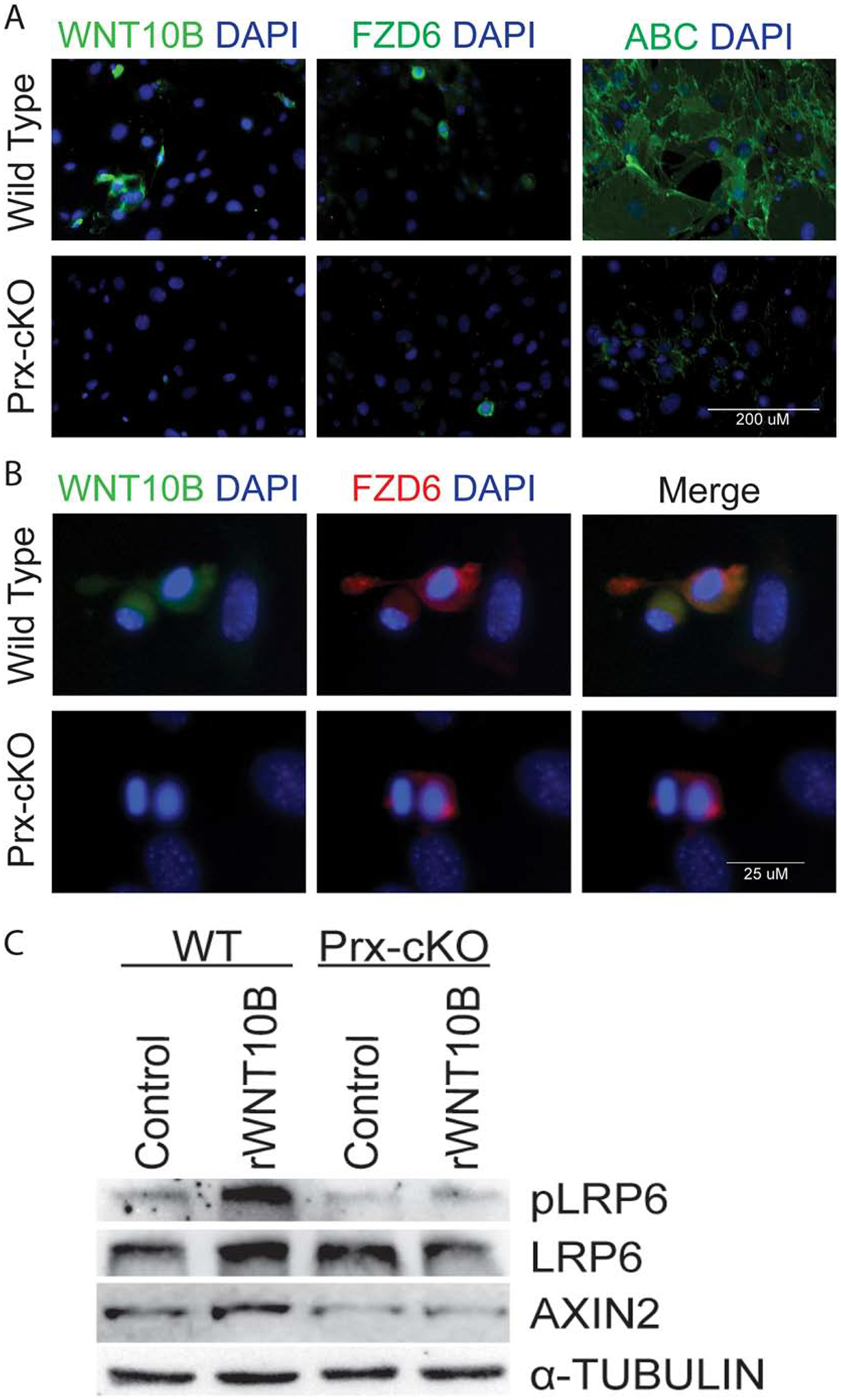
(A) Bone marrow cells from WT and Gata4 Prx-cKO mice were cultured in MesenCult media and then fixed and stained with antibodies to WNT10B (green), FZD (green) or activated β-catenin (green). Nuclei were identified with DAPI (blue). (B) Bone marrow cells from WT and Gata4 Prx-cKO mice were cultured in MesenCult media and then fixed and stained with antibodies to WNT10B (green) or FZD6 (red). Nuclei were identified with DAPI (blue). (C) Bone marrow cells from WT and Gata4 Prx-cKO mice were cultured in MesenCult media. Cells were treated with 100 ng/mL rWNT10B for 30 minutes and then lysed for protein. Immunoblots were performed with antibodies to pLRP6, LRP6, AXIN2 and α-tubulin.
WNT-Frizzled (FZD) interactions are mediated through the FZD’s cysteine-rich domain (CRD) [22]. Which of the 19 secreted Wnt ligands will physically interact with one or more of the 10 Frizzled receptors is still not well defined. The WNT10B ligand has been predicted to have an affinity for Fzd2/3/4/5/7/8/9 [23]. Which FZD receptor(s) that physically interacts with WNT10B in MSCs has not been described. Based on the above results, we performed immunofluorescence for the co-localization of WNT10B and FZD6. In wildtype cells, WNT10B and FZD6 are found in the same cells (Figure 5B). In Gata4 Prx-cKO cells, as there is a decrease in WNT10B, we don’t see co-localization of WNT10B and FZD6.
To further demonstrate loss of WNT signaling in the absence of Gata4, antibodies for total LRP6 and active, phosphorylated LRP6 (at Ser1490) were used in immunoblot assays with protein from wildtype and Gata4 Prx-cKO MSCs (Figure 5C). The basal expression levels for both are unchanged. The phosphorylation of Ser1490 on LRP6 only occurs when a functional complete signalosome has been activated at the level of the receptor and signal transduction has been initiated. When we exposed MSCs to recombinant WNT10B (rWNT10B) at 100 ng/mL for 30 minutes, rWNT10B was able to induce LRP6 phosphorylation at Ser1490, whereas it could not in Gata4 Prx-cKO cells. To determine if the addition of rWNT10B and increase in the LRP6 phosphorylation mediated β-catenin dependent transcriptional activation of canonical WNT-direct target(s) we immunoblotted for AXIN2 protein expression, using parallel cultures 24 hours after addition of rWNT10B. The results demonstrate an increase in AXIN2 protein expression after the exposure of rWNT10B in the WT cells, but not in Gata4 Prx-cKO cells. We further confirm that recombinant WNT3A (rWNT3a at 40 ng/mL for 30 minutes) is also able to induce phosphorylation of LRP6 only in wildtype cells (Supplemental Figure 4). These results are consistent with aberrant WNT signalosome component(s), (i.e. lower expression of FZD6 protein).
Taken together, in the absence of Gata4 expression the protein-protein interaction between WNT10B and FDZ6 is lost. More importantly, our data strongly suggest that in MSCs, WNT10B signals through a FZD6-LRP6 signalosome pathway to facilitate translocation of ABC into the nucleus to target its downstream WNT-direct target gene Axin2 (amongst others).
GATA4 and WNT10B are in an autoregulatory positive feedback loop in bone marrow-derived MSCs.
The Wnt10b knockout (WNT10BKO) mouse, in which WNT10B exons 2–5 have been globally deleted, has been shown to have a similar bone phenotype as Gata4 Prx-cKO mice, in that they both have trabecular bone loss [10]. Furthermore, we showed that Wnt10b deficiency results in an age-dependent progressive reduction of MSCs, as measured by CFU-F. To confirm this, we isolated bone marrow-derived MSCs from 3-month-old, aged-matched wildtype and WNT10BKO mice and performed CFU-F assays. Colonies were stained with Giemsa (Figure 6A) and counted (Figure 6B). WNT10BKO bone marrow gave rise to a statistically significant reduction of over two-fold fewer colonies than wildtype mice. To expand these results, we enumerated the number of Sca1+/CD45−/CD34−/CD44+ cells by flow cytometry, as was done for the Gata4 Prx-cKO mice (Figure 3). WNT10BKO mice have an approximately 4-fold lower number of MSCs when compared to WT mice (Figure 6C). Therefore, by CFU-F and flow cytometry, WNT10BKO mice have fewer MSCs, similar to what is observed for Gata4 Prx-cKO mice.
Figure 6: WNT10B regulates GATA4 expression.
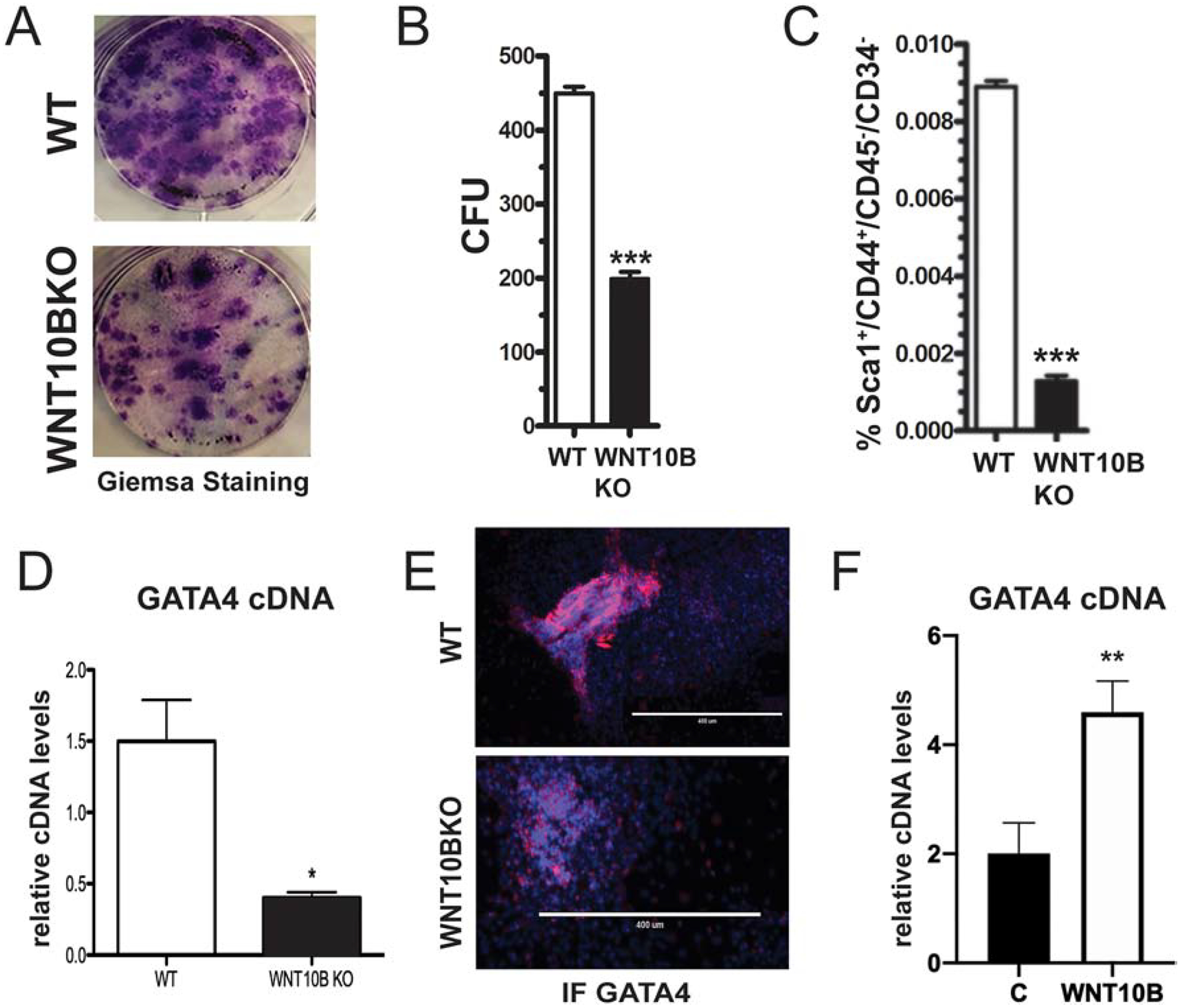
(A) Bone marrow cells from WT and WNT10BKO mice were cultured in MesenCult media. Cells were stained with Giemsa and (B) the number of Giemsa colonies (CFU) was quantified using ImageJ. (C) MSCs were analyzed for the expression of Sca1+/CD45−/CD34−/CD44+ markers using flow cytometry. (D) Bone marrow cells from WT and WNT10BKO mice were cultured in MesenCult media. Gata4 mRNA expression levels were analyzed by qPCR in undifferentiated cells from WT and WNT10BKO mice. (E) Bone marrow cells from WT and WNT10BKO mice were cultured in MesenCult media. Cells were fixed and stained with an antibody to GATA4 (pink). Nuclei were identified with DAPI (blue). (F) WT osteoblasts were untreated or treated with 100 ng/mL rWNT10B for 24 hours and then lysed for RNA. GATA4 mRNA was detected by qPCR.
Based on the above, we hypothesized that WNT10B expression could regulate the expression of Gata4 in MSCs. Therefore, we investigated the relationship between GATA4 and WNT10B expression in MSC cells. To this end, parallel cultures of bone marrow-derived MSC cells from Figure 6A–B were analyzed for the mRNA and protein levels of GATA4. WNT10BKO mice had a 3-fold lower level of Gata4 mRNA (Figure 6D). GATA4 protein, visualized by immunofluorescence, was also confirmed to be lower in the colonies obtained from WNT10BKO mice (Figure 6E). Finally, the addition of rWNT10B led to an over 2-fold increase in Gata4 mRNA (Figure 6F). Taken together, the loss of Gata4 expression leads to the loss of Wnt10b expression and that loss of Wnt10b expression leads to diminished expression of Gata4.
YAP is increased in GATA4 Prx-cKO MSCs
As key factors in MSCs and WNT signaling, we sought to determine YAP and TAZ levels in Gata4 Prx-cKO mice. YAP and TAZ are transcriptional regulators of the Hippo pathway and key regulators of MSCs [24]. YAP has been shown to associate with and repress RUNX2 activity [25]. YAP and TAZ also have been shown to be part of the β-catenin destruction complex, attenuating the steady-state levels of ABC [26].
Either knockdown of Gata4 in calvaria or bone marrow-derived MSCs from Gata4 Prx-cKO mice showed enhanced levels of YAP, but not TAZ, mRNA (Figure 7 and Supplemental Figure 3D). ChIP-sequencing identified two GATA4 binding sites in Yap1 introns (so-called Yap1 enhancer 1 and Yap1 enhancer 2, Figure 7B) and ChIP-qPCR confirmed the presence of GATA4 at these Yap1 enhancers, indicating that Yap1 is a direct target of GATA4. YAP protein was also elevated in Gata4 Prx-cKO bone marrow-derived MSCs compared to WT cells, as seen by both immunoblotting and immunofluorescence (Figure 7C and 7E). Like what is observed in Gata4 Prx-cKO bone marrow-derived MSCs, WNT10B KO cells also have increased YAP protein levels (Figure 7D). Interestingly, GATA4 represses Yap1 expression by an unknown mechanism. Together, these data described that another mechanism for the decreased number of MSCs is increased YAP, in addition to decreased WNT signaling. Not only is there less WNT ligand and lack of receptor activation at the membrane, but WNT signaling is affected by increased YAP levels and decreased activation of β-catenin. Both mechanisms are in congruence with fewer bone marrow-derived MSCs from the Gata4 Prx-cKO mice.
Figure 7: GATA4 directly represses YAP expression in MSCs.
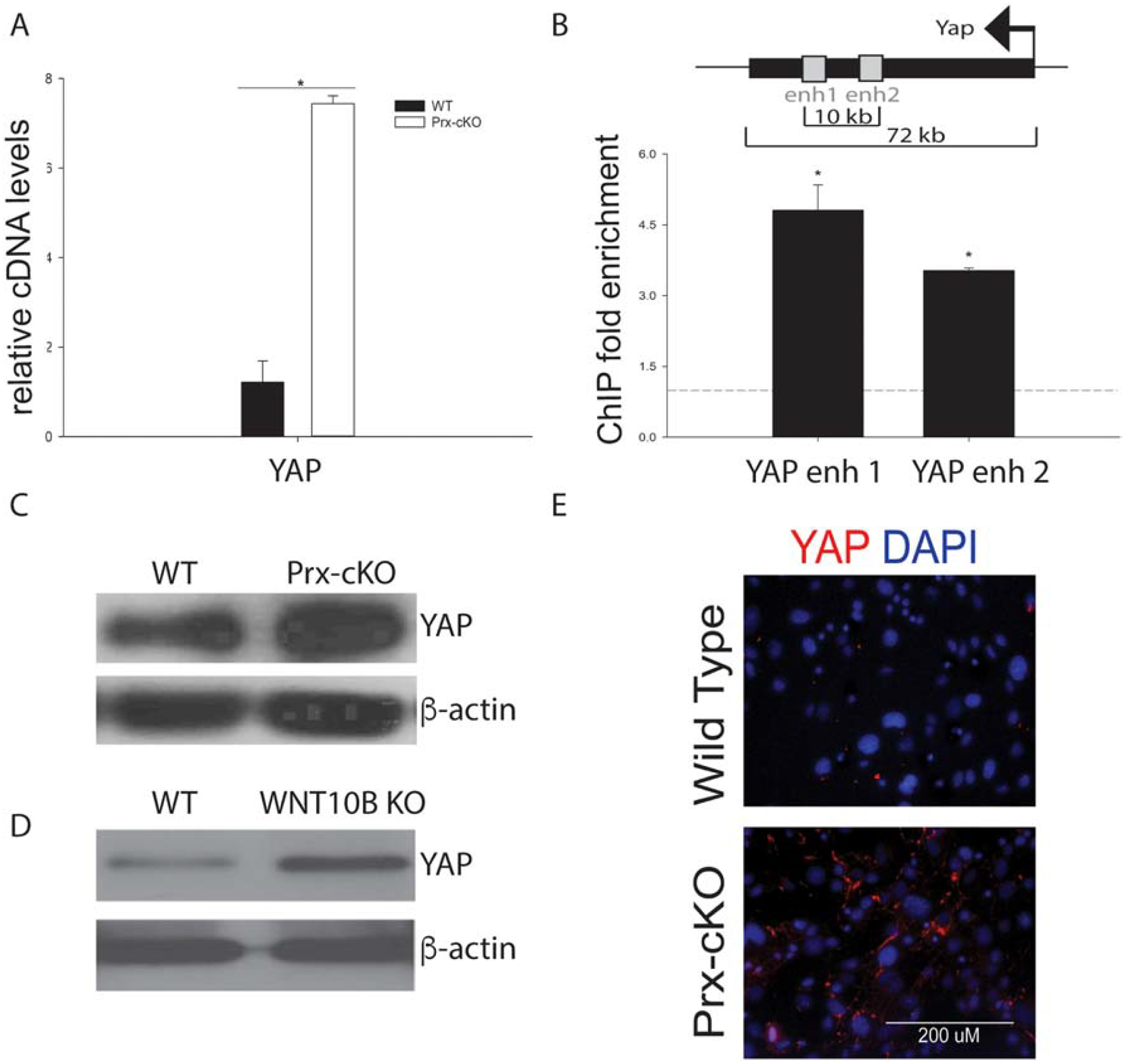
(A) Bone marrow cells from WT and Gata4 Prx-cKO mice were cultured in MesenCult media. YAP cDNA expression levels were analyzed by qPCR in undifferentiated cells from WT and Gata4 Prx-cKO. (B) Chromatin immunoprecipitation was performed at the indicated enhancers or promoters. Streptavidin-coated beads were used to precipitate Flag-biotin-GATA4. qPCR was performed with primers to the indicated regions (gray boxes). Each PCR was normalized to input and represented as fold enrichment over a negative genomic locus (dotted line). Inset shows a schematic of the genomic region. The black bar indicates the YAP gene with the arrow indicating the start and direction of transcription. Gray boxes indicate intronic enhancer regions. Student’s t test; * P < 0.05; compared with a negative genomic locus. (C) Immunoblot for YAP and beta-actin from WT and Prx-cKO bone marrow. (D) Immunoblot for YAP and beta-actin from WT and WNT10B KO bone marrow. (E) Bone marrow cells from WT and Gata4 Prx-cKO mice were cultured in MesenCult media. Cells were fixed and stained with an antibody to YAP (red). Nuclei were identified with DAPI (blue).
4. Discussion
It is estimated that ~300 million people are diagnosed with osteoporosis worldwide and the clinical need for new agents is high because current bone anabolic options have limitations. Mesenchymal stem cells (MSCs) have great therapeutic potential due to their capacity to self-renew and differentiate into a range of mesenchymal tissues including cartilage, muscle, adipose and bone. Here we show for the first time that GATA4 regulates MSCs via WNT signaling. GATA4 directly regulates the expression of Wnt ligands, Wnt receptors and Wnt inhibitors. Consequently, LRP6 is not activated and nor is subsequent downstream signaling (β-catenin) in primary MSCs.
WNTs are known to control many types of stem cells [8], and loss of coordinated WNT signaling would explain a loss of MSCs observed in Gata4 Prx-cKO mice. There are 19 mammalian WNTs, and many of them have been shown to be expressed in MSCs. Boland et al., showed that MSCs from adult human trabecular bone expressed Wnt1, Wnt3, Wnt5a, Wnt9a, Wnt10b, Wnt11 and Wnt13 [27]. WNT3A exposure affected MSC proliferation and caused a reversible suppression of osteogenic differentiation. Etheridge, et al., showed that human MSCs from bone marrow donors had active WNT signalosome components (i.e. LRP/DKKs) and expression of only Wnt2, Wnt4, Wnt5a, Wnt11 and Wnt16 [28]. The authors suggested that autocrine WNT signaling regulates mesenchymal linage specifications. We provide evidence that we can observe expression of each of these WNTs, and that five of these (including Wnt10b) are regulated by GATA4.
The consequences of decreased WNT signaling is decreased bone mineral density. Human mutations in LRP5 and sclerostin (SOST) highlight the importance of WNT signaling in bone, as mutations cause osteoporosis-pseudoglioma syndrome (OPPG) and van Buchem disease, respectively [29]. Furthermore, inhibition of sclerostin by Evenity (romosozumab) is now in clinical use for the treatment of osteoporosis. In mice, knockout of LRP5, LRP6 and β-catenin leads to defects in bone formation [30–32]. Wnt10b knockout mice and Wnt5a heterozygous mice have decreased trabecular bone [10, 33]. In comparison, Wnt16 knockout mice had thinner cortical bones [34]. Wnt9a knockout mice display skeletal abnormalities [35], due to defects in the joints. We fail to see a change in Wnt9a or Wnt16 in the Gata4 Prx-cKO mice, showing the specificity of GATA4 and WNT10B to trabecular bone.
Mechanistically, WNTs regulate transcription of key bone differentiation factors, such as RUNX2, osteocalcin and RANKL. Interestingly, we have shown that GATA4 also regulates these same factors [3, 4]. β-catenin and TCF1 have been shown to bind to the RUNX2 P1 promoter [36], as does GATA4 [3]. β-catenin and GATA4 also bind to the RANKL promoter and repress its transcription [4, 37]. Therefore, GATA4 and WNT signaling combinatorially regulate bone formation and osteoclastogenesis.
Very little is known about what regulates Wnt10b expression. We have identified that GATA4 binds to the Wnt10b promoter and increases its expression. Gata4 and Wnt10b have similar expression patterns in osteoblast differentiation from MSCs (high in MSCs and decreasing over differentiation). PATCH software [38] predicts nine GATA4 binding sites in the Wnt10b promoter. Furthermore, our ChIP-seq data reveal binding of GATA4 in a ~500 bp window around the promoter of Wnt10b. We also provide strong genetic evidence that WNT10BKO MSCs have both decreased GATA4 mRNA and protein expression and when WNT10B is added, GATA4 expression increases. These results argue for a positive feedback loop between Wnt10b and Gata4 expression in MSCs. The coordinated loss of expression of Gata4 and Wnt10b is timed for lineage specification and throughout osteoblast differentiation but differ during lineage specification for adipocyte differentiation. Disruption of WNT10B signaling promotes adipogenesis [39] and WNT10B is known as a regulator for the suppression of adipocytes, as it represses master fat transcriptional factors, such as PPARγ. In the MSC lineage specification for adipocytes, WNT10B ligand production must be turned off and WNT5B production must be on in preadipocytes that drive PPARγ expression [40]. Gata4 Prx-cKO MSCs produce significantly fewer adipocytes, contrasting the WNT10BKO phenotype. Therefore, WNT10B and GATA4 diverge at a critical stage in the MSC linage specification for adipocytes. Many knockout mice that have decreased bone mineral density have increased fat in the bone marrow, such as knockout of estrogen receptor alpha (ERα) and IGFBP2 [41, 42], but the GATA4 knockout mice have less fat AND less bone. Together, this data may delineate the hierarchy of factors in mesenchymal stem cell differentiation.
In summary, knockout of Gata4 early in osteoblastogenesis leads to decreased bone formation in vivo. Mechanistically, we have shown that GATA4 directly regulates multiple components of the WNT signalosome, including WNT10B, which in turn regulates MSC number.
Supplementary Material
HIGHLIGHTS.
Knockout of GATA4 in limb bud mesenchyme leads to reduced trabecular bone
There are fewer mesenchymal stem cells in GATA4 knockout mice
GATA4 regulates multiple components of the WNT signaling pathway
GATA4 and WNT10B are in an autoregulatory positive feedback loop
5. Acknowledgments
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number AR-064354 to SAK. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement: The authors declare that they have no conflict of interest.
6. References
- 1.Guemes M, Garcia AJ, Rigueur D, Runke S, Wang W, Zhao G, Mayorga VH, Atti E, Tetradis S, Peault B, Lyons K, Miranda-Carboni GA, and Krum SA, GATA4 is essential for bone mineralization via ERalpha and TGFbeta/BMP pathways. J Bone Miner Res, 2014. 29(12): p. 2676–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miranda-Carboni GA, Guemes M, Bailey S, Anaya E, Corselli M, Peault B, and Krum SA, GATA4 regulates estrogen receptor-alpha-mediated osteoblast transcription. Mol Endocrinol, 2011. 25(7): p. 1126–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khalid AB, Slayden AV, Kumpati J, Perry CD, Lillo MA, Arroyo SR, Miranda-Carboni G, and Krum SA, GATA4 directly regulates Runx2 expression and osteoblast differentiation JBMR Plus, 2018. 2(2): p. 81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khalid AB, Slayden AV, Kumpati J, Perry CD, Berryhill SB, Crawford JA, Fatima I, Morselli M, Pellegrini M, Miranda-Carboni GA, and Krum SA, GATA4 represses RANKL in osteoblasts via multiple long-range enhancers to regulate osteoclast differentiation. Bone, 2018. 116: p. 78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sera SR and Zur Nieden NI, microRNA Regulation of Skeletal Development. Curr Osteoporos Rep, 2017. 15(4): p. 353–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kemp KC, Hows J, and Donaldson C, Bone marrow-derived mesenchymal stem cells. Leuk Lymphoma, 2005. 46(11): p. 1531–44. [DOI] [PubMed] [Google Scholar]
- 7.Hachisuka H, Mochizuki Y, Yasunaga Y, Natsu K, Sharman P, Shinomiya R, and Ochi M, Flow cytometric discrimination of mesenchymal progenitor cells from bone marrow-adherent cell populations using CD34/44/45(−) and Sca-1(+) markers. J Orthop Sci, 2007. 12(2): p. 161–9. [DOI] [PubMed] [Google Scholar]
- 8.Nusse R, Wnt signaling and stem cell control. Cell Res, 2008. 18(5): p. 523–7. [DOI] [PubMed] [Google Scholar]
- 9.Wend P, Wend K, Krum SA, and Miranda-Carboni GA, The role of WNT10B in physiology and disease. Acta Physiol (Oxf), 2012. 204(1): p. 34–51. [DOI] [PubMed] [Google Scholar]
- 10.Stevens JR, Miranda-Carboni GA, Singer MA, Brugger SM, Lyons KM, and Lane TF, Wnt10b deficiency results in age-dependent loss of bone mass and progressive reduction of mesenchymal progenitor cells. J Bone Miner Res, 2010. 25(10): p. 2138–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He A, Shen X, Ma Q, Cao J, von Gise A, Zhou P, Wang G, Marquez VE, Orkin SH, and Pu WT, PRC2 directly methylates GATA4 and represses its transcriptional activity. Genes Dev, 2012. 26(1): p. 37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wend P, Runke S, Wend K, Anchondo B, Yesayan M, Jardon M, Hardie N, Loddenkemper C, Ulasov I, Lesniak MS, Wolsky R, Bentolila LA, Grant SG, Elashoff D, Lehr S, Latimer JJ, Bose S, Sattar H, Krum SA, and Miranda-Carboni GA, WNT10B/-catenin signalling induces HMGA2 and proliferation in metastatic triple-negative breast cancer. Embo Molecular Medicine, 2013. 5(2): p. 264–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khalid AB, Goodyear SR, Ross RA, and Aspden RM, Mechanical and material properties of cortical and trabecular bone from cannabinoid receptor-1-null (Cnr1(−/−)) mice. Med Eng Phys, 2016. 38(10): p. 1044–54. [DOI] [PubMed] [Google Scholar]
- 14.Willey JS, Livingston EW, Robbins ME, Bourland JD, Tirado-Lee L, Smith-Sielicki H, and Bateman TA, Risedronate prevents early radiation-induced osteoporosis in mice at multiple skeletal locations. Bone, 2010. 46(1): p. 101–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soleimani M and Nadri S, A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat Protoc, 2009. 4(1): p. 102–6. [DOI] [PubMed] [Google Scholar]
- 16.Krum SA, Miranda-Carboni GA, Lupien M, Eeckhoute J, Carroll JS, and Brown M, Unique ER{alpha} cistromes control cell type-specific gene regulation. Mol Endocrinol, 2008. 22(11): p. 3121–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He A and Pu WT, Genome-wide location analysis by pull down of in vivo biotinylated transcription factors. Curr Protoc Mol Biol, 2010. Chapter 21: p. Unit 21 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma’ayan A, Enikolopov GN, and Frenette PS, Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature, 2010. 466(7308): p. 829–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGough IJ and Vincent JP, APC Moonlights to Prevent Wnt Signalosome Assembly. Dev Cell, 2018. 44(5): p. 535–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiese KE, Nusse R, and van Amerongen R, Wnt signalling: conquering complexity. Development, 2018. 145(12). [DOI] [PubMed] [Google Scholar]
- 21.Maher MT, Mo R, Flozak AS, Peled ON, and Gottardi CJ, Beta-catenin phosphorylated at serine 45 is spatially uncoupled from beta-catenin phosphorylated in the GSK3 domain: implications for signaling. PLoS One, 2010. 5(4): p. e10184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeBruine ZJ, Ke J, Harikumar KG, Gu X, Borowsky P, Williams BO, Xu W, Miller LJ, Xu HE, and Melcher K, Wnt5a promotes Frizzled-4 signalosome assembly by stabilizing cysteine-rich domain dimerization. Genes Dev, 2017. 31(9): p. 916–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agostino M, Pohl SO, and Dharmarajan A, Structure-based prediction of Wnt binding affinities for Frizzled-type cysteine-rich domains. J Biol Chem, 2017. 292(27): p. 11218–11229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hong JH, Hwang ES, McManus MT, Amsterdam A, Tian Y, Kalmukova R, Mueller E, Benjamin T, Spiegelman BM, Sharp PA, Hopkins N, and Yaffe MB, TAZ, a transcriptional modulator of mesenchymal stem cell differentiation. Science, 2005. 309(5737): p. 1074–8. [DOI] [PubMed] [Google Scholar]
- 25.Zaidi SK, Sullivan AJ, Medina R, Ito Y, van Wijnen AJ, Stein JL, Lian JB, and Stein GS, Tyrosine phosphorylation controls Runx2-mediated subnuclear targeting of YAP to repress transcription. EMBO J, 2004. 23(4): p. 790–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Azzolin L, Panciera T, Soligo S, Enzo E, Bicciato S, Dupont S, Bresolin S, Frasson C, Basso G, Guzzardo V, Fassina A, Cordenonsi M, and Piccolo S, YAP/TAZ incorporation in the beta-catenin destruction complex orchestrates the Wnt response. Cell, 2014. 158(1): p. 157–70. [DOI] [PubMed] [Google Scholar]
- 27.Boland GM, Perkins G, Hall DJ, and Tuan RS, Wnt 3a promotes proliferation and suppresses osteogenic differentiation of adult human mesenchymal stem cells. J Cell Biochem, 2004. 93(6): p. 1210–30. [DOI] [PubMed] [Google Scholar]
- 28.Etheridge SL, Spencer GJ, Heath DJ, and Genever PG, Expression profiling and functional analysis of wnt signaling mechanisms in mesenchymal stem cells. Stem Cells, 2004. 22(5): p. 849–60. [DOI] [PubMed] [Google Scholar]
- 29.Lara-Castillo N and Johnson ML, LRP receptor family member associated bone disease. Rev Endocr Metab Disord, 2015. 16(2): p. 141–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kato M, Patel MS, Levasseur R, Lobov I, Chang BH, Glass DA 2nd, Hartmann C, Li L, Hwang TH, Brayton CF, Lang RA, Karsenty G, and Chan L, Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J Cell Biol, 2002. 157(2): p. 303–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riddle RC, Diegel CR, Leslie JM, Van Koevering KK, Faugere MC, Clemens TL, and Williams BO, Lrp5 and Lrp6 exert overlapping functions in osteoblasts during postnatal bone acquisition. PLoS One, 2013. 8(5): p. e63323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glass DA 2nd, Bialek P, Ahn JD, Starbuck M, Patel MS, Clevers H, Taketo MM, Long F, McMahon AP, Lang RA, and Karsenty G, Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell, 2005. 8(5): p. 751–64. [DOI] [PubMed] [Google Scholar]
- 33.Maeda K, Kobayashi Y, Udagawa N, Uehara S, Ishihara A, Mizoguchi T, Kikuchi Y, Takada I, Kato S, Kani S, Nishita M, Marumo K, Martin TJ, Minami Y, and Takahashi N, Wnt5a-Ror2 signaling between osteoblast-lineage cells and osteoclast precursors enhances osteoclastogenesis. Nat Med, 2012. 18(3): p. 405–12. [DOI] [PubMed] [Google Scholar]
- 34.Zheng HF, Tobias JH, Duncan E, Evans DM, Eriksson J, Paternoster L, Yerges-Armstrong LM, Lehtimaki T, Bergstrom U, Kahonen M, Leo PJ, Raitakari O, Laaksonen M, Nicholson GC, Viikari J, Ladouceur M, Lyytikainen LP, Medina-Gomez C, Rivadeneira F, Prince RL, Sievanen H, Leslie WD, Mellstrom D, Eisman JA, Moverare-Skrtic S, Goltzman D, Hanley DA, Jones G, St Pourcain B, Xiao Y, Timpson NJ, Smith GD, Reid IR, Ring SM, Sambrook PN, Karlsson M, Dennison EM, Kemp JP, Danoy P, Sayers A, Wilson SG, Nethander M, McCloskey E, Vandenput L, Eastell R, Liu J, Spector T, Mitchell BD, Streeten EA, Brommage R, Pettersson-Kymmer U, Brown MA, Ohlsson C, Richards JB, and Lorentzon M, WNT16 influences bone mineral density, cortical bone thickness, bone strength, and osteoporotic fracture risk. PLoS Genet, 2012. 8(7): p. e1002745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spater D, Hill TP, O’Sullivan RJ, Gruber M, Conner DA, and Hartmann C, Wnt9a signaling is required for joint integrity and regulation of Ihh during chondrogenesis. Development, 2006. 133(15): p. 3039–49. [DOI] [PubMed] [Google Scholar]
- 36.Gaur T, Lengner CJ, Hovhannisyan H, Bhat RA, Bodine PV, Komm BS, Javed A, van Wijnen AJ, Stein JL, Stein GS, and Lian JB, Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J Biol Chem, 2005. 280(39): p. 33132–40. [DOI] [PubMed] [Google Scholar]
- 37.Spencer GJ, Utting JC, Etheridge SL, Arnett TR, and Genever PG, Wnt signalling in osteoblasts regulates expression of the receptor activator of NFkappaB ligand and inhibits osteoclastogenesis in vitro. J Cell Sci, 2006. 119(Pt 7): p. 1283–96. [DOI] [PubMed] [Google Scholar]
- 38.Matys V, Kel-Margoulis OV, Fricke E, Liebich I, Land S, Barre-Dirrie A, Reuter I, Chekmenev D, Krull M, Hornischer K, Voss N, Stegmaier P, Lewicki-Potapov B, Saxel H, Kel AE, and Wingender E, TRANSFAC and its module TRANSCompel: transcriptional gene regulation in eukaryotes. Nucleic Acids Res, 2006. 34(Database issue): p. D108–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bennett CN, Ross SE, Longo KA, Bajnok L, Hemati N, Johnson KW, Harrison SD, and MacDougald OA, Regulation of Wnt signaling during adipogenesis. J Biol Chem, 2002. 277(34): p. 30998–1004. [DOI] [PubMed] [Google Scholar]
- 40.Kang S, Bennett CN, Gerin I, Rapp LA, Hankenson KD, and Macdougald OA, Wnt signaling stimulates osteoblastogenesis of mesenchymal precursors by suppressing CCAAT/enhancer-binding protein alpha and peroxisome proliferator-activated receptor gamma. J Biol Chem, 2007. 282(19): p. 14515–24. [DOI] [PubMed] [Google Scholar]
- 41.Wend K, Wend P, Drew BG, Hevener AL, Miranda-Carboni GA, and Krum SA, ERalpha regulates lipid metabolism in bone through ATGL and perilipin. J Cell Biochem, 2013. 114(6): p. 1306–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DeMambro VE, Clemmons DR, Horton LG, Bouxsein ML, Wood TL, Beamer WG, Canalis E, and Rosen CJ, Gender-specific changes in bone turnover and skeletal architecture in igfbp-2-null mice. Endocrinology, 2008. 149(5): p. 2051–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


