Abstract
Objectives:
To characterize monocyte subsets and activation in persons living with HIV (PLWH) with tuberculosis coinfection.
Design:
Cross-sectional study within a cohort of PLWH and HIV-uninfected participants at the Joint Clinical Research Centre in Kampala, Uganda.
Methods:
Participants were ≥45 years old with at least one cardiovascular risk factor. PLWH had an HIV viral load ≤1,000 copies/mL on stable antiretroviral therapy prior to cohort entry. QuantiFERON®-TB testing was performed to define latent tuberculosis infection (LTBI). Prior active TB was defined by self-report and verified by medical records. Blood was stained with monocyte subset markers (CD14, CD16), CD62p, CD69, CX3CR1, HLA-DR, and tissue factor, and examined with flow cytometry.
Results:
125 participants (83 PLWH and 42 without HIV) were included. Median CD4 count was 582 cells/uL in PLWH. PLWH had a higher frequency of total monocytes (4.3% vs 3.2%; p<0.001) and inflammatory monocyte subset (15.5% vs 11.7%; p=0.016) compared to HIV-uninfected individuals. No differences in the frequency of monocyte subsets were observed by TB status. Among PLWH, prior active TB was associated with increased frequency of total monocytes compared to LTBI (5.1% vs 3.7%; p=0.013). HLA-DR density on monocytes was 3-fold higher in PLWH with LTBI or prior TB compared to PLWH without LTBI (p=0.002). In multivariate analysis, a higher monocyte HLA-DR density remained associated with LTBI or prior TB in PLWH (log-MFI; b=1.17; p<0.001).
Conclusions:
Our findings indicate enhanced monocyte activation in PLWH with LTBI or prior active TB, which may contribute to the pathogenesis of non-communicable diseases in HIV.
Keywords: monocyte, immune activation, inflammation, HIV, tuberculosis
INTRODUCTION
Persons living with HIV (PLWH) are at increased risk of developing a number of non-communicable diseases (NCDs) compared to the general population [1, 2]. Persistent immune activation contributes to the pathogenesis of NCDs in PLWH, even among individuals on stable antiretroviral therapy (ART) [3]. Tuberculosis (TB), either on its latent or active form, has been associated with increased T cell activation in PLWH [4]. However, whether HIV/TB coinfection is associated with altered monocyte activation phenotypes has not been assessed.
Perturbations in circulating monocytes have been reported in inflammatory and infectious conditions including HIV [5, 6]. Three monocyte subsets are defined based on CD14 and CD16 expression [7, 8]. Classical monocytes (CD14+CD16-) and to a greater extent inflammatory monocytes (CD14+CD16+) produce pro-inflammatory cytokines upon microbial stimulation [9]. Patrolling monocytes (CD14dimCD16+) survey the endothelium and can recognize viral components [10, 11]. In this study, we performed immunophenotyping of fresh blood monocytes among PLWH from a high TB-prevalence area, to characterize circulating monocyte subsets and expression of activation markers (HLA-DR, CD69), coagulation-related markers (CD62p, tissue factor), and chemokine receptors (CX3CR1). We hypothesized that there would be altered monocyte activation phenotypes in PLWH with TB coinfection.
METHODS
Study design and population
Between April 2017 and January 2019, we conducted a nested cross-sectional study within an ongoing cardiovascular risk cohort study of HIV-infected and -uninfected individuals followed at the Joint Clinical Research Centre (JCRC) in Kampala, Uganda. At cohort entry, PLWH were on stable ART for at least 6 months, and had at least one HIV-1 viral load <1,000 copies/mL within the 6 months prior to cohort entry. All participants (PLWH and control) were ≥45 years old and had at least one of the following cardiovascular disease risk factors at entry: hypertension, low high-density lipoprotein cholesterol (HDL; <40mg/dL for men or <50mg/dL for women), diabetes mellitus, smoking, or family history of heart disease. Persons with uncontrolled inflammatory conditions, active chemotherapy or immunosuppression, pregnancy, or advanced chronic kidney disease (glomerular filtration rate <30 mL/min/1.73m2) were excluded. For this nested cross-sectional study, participants were administered a questionnaire on signs and symptoms of TB disease based on World Health Organization criteria, and provided blood for QuantiFERON®-TB Gold In-Tube (QFT) testing during their Year 2 research visit. Participants also provided clinical information and blood for fasting lipid profile and monocyte phenotyping by flow cytometry.
Definitions of TB infection status
We defined three TB infection categories based on results from the TB questionnaire and QFT test. Latent TB infection (LTBI) was defined by a positive QFT result and absence of TB symptoms. Prior TB disease was defined by self-reported history of active TB, and was verified by review of medical records. Persons without evidence of TB infection had a negative QFT, no TB symptoms, and no prior history of active TB.
Flow cytometry assays
Fresh blood samples were processed the same day of collection for flow cytometry analysis at the JCRC Immunology Laboratory. Briefly, 300μL of whole blood was lysed with 1x BD lysing buffer for 10 minutes at 40°C. After two washes, cells were stained using antibodies for 30 minutes at 4°C in FACS buffer. The flow cytometry panel included the following antibodies/conjugated fluorochromes: CD14/Pacific blue, CD16/PE, CD62p/PECy-5, CD69/PECy-7, CX3CR1/APC, HLA-DR/APCCy-7, and tissue factor/FITC. We used isotype-matched conjugated antibodies as controls. After staining, cells were washed and fixed with 1% paraformaldehyde. Data was acquired using a MACSQuant® flow cytometer and analyzed in FlowJo v10. Flow cytometry data was analyzed blinded to HIV and TB status.
Data collection and statistical analysis
Study data were collected and managed using REDCap [12, 13]. Data were analyzed in Stata v12 (College Station, TX). We used Mann-Whitney and Kruskal-Wallis tests for group comparisons of numeric and flow cytometry data. We used chi-square for comparisons of categorical data. For adjusted analyses, we used multivariable linear regression modeling of log-transformed flow cytometry variables of interest. Age, sex, diabetes mellitus, hyperlipidemia, and factors with a p<0.05 in univariable analyses were included in multivariable regression models. P values <0.05 were considered statistically significant.
RESULTS
We included 125 individuals with flow cytometry data and defined HIV and TB status in this analysis. Of these, 83 (66%) were PLWH and 42 (34%) were HIV-uninfected individuals (Supplementary Table 1). There were no significant differences between PLWH and HIV-uninfected individuals in terms of age (median, 55 vs. 61.5 years; p=0.063), male sex at birth (36% vs. 31% p=0.564), hypertension (88% vs. 83%, p=0.477), or family history of heart disease (13% vs. 12%; p=0.831). There was a lower proportion of diabetes mellitus (25% vs. 43%; p=0.045) and hyperlipidemia (8% vs. 24%; p=0.018) in PLWH compared to HIV-uninfected individuals. Among PLWH, the median HIV viral load was 20 copies/mL (interquartile range, 20 – 20), with only 2 (2%) persons with recent HIV viral load >1,000 copies/mL. The median CD4+ count was 582 cells/uL (interquartile range, 414 – 764). PLWH had a higher frequency of total monocytes (4.3% vs 3.2%; p<0.001) and inflammatory monocyte subset (15.5% vs. 11.7%; p=0.016) compared to HIV-uninfected individuals (Supplementary Figure 1). In multivariable regression, a higher frequency of inflammatory monocytes remained associated with HIV infection (b=0.37; p=0.019), after adjusting for TB status, age, sex, hyperlipidemia, and diabetes mellitus. Among the cellular markers measured on total monocytes, we found that HIV infection was associated with a higher frequency of CX3CR1 expression (b=1.79; p=0.002), after adjusting for TB status, age, sex, hyperlipidemia, and diabetes mellitus. This association remained significant within the classical and inflammatory monocyte subsets (b=2.06, p=0.004; b=1.85, p=0.004; respectively), and a trend was observed within patrolling monocytes (b=1.09; p=0.110). No significant differences were observed in monocyte expression of HLA-DR, CD69, CD62p, or tissue factor by HIV status (data not shown; all p>0.05).
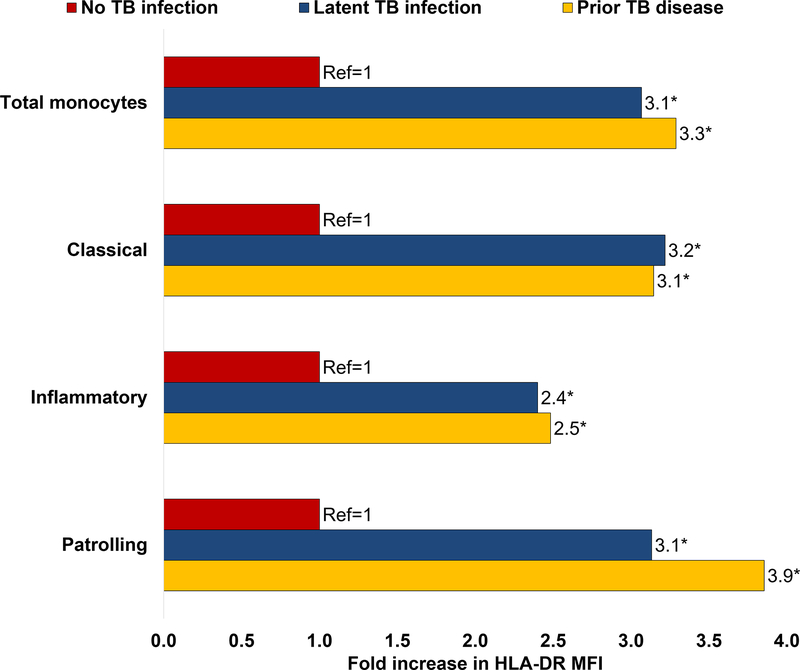
Among PLWH (see Table 1), there were no significant differences between individuals with LTBI, prior TB disease, and no TB coinfection in terms of age (median, 54 vs. 58 vs. 56; p=0.191), male sex at birth (33% vs. 48% vs. 30%; p=0.327), hypertension (89% vs. 88% vs. 88%; p=0.989), diabetes mellitus (11% vs. 36% vs. 25%; p=0.180), hyperlipidemia (17% vs. 8% vs. 5%; p=0.333), or family history of heart disease (22% vs. 12% vs. 10%; p=0.436). Compared to PLWH without TB coinfection, interferon-gamma levels in the QFT nil tube were higher in PLWH with LTBI (0.09 vs. 0.13; p=0.004) and PLWH with prior TB (0.09 vs. 0.12; p=0.04; Table 1). PLWH with prior TB showed a higher frequency of total monocytes compared to PLWH with LTBI (5.1% vs. 3.7%; p=0.013), but not when compared to PLWH without TB coinfection (5.1% vs. 4.5%; p=0.322; Table 1). There were no significant differences in the frequency of monocyte subsets between PLWH with prior active TB, LTBI, or no TB coinfection (data not shown). HLA-DR density in total and all monocyte subsets was higher in PLWH with LTBI or prior TB compared to PLWH without TB coinfection (see Figure 1; Supplementary Table 2). In multivariable regression, a higher density of HLA-DR on monocytes remained associated with LTBI or prior TB (log-MFI; b=1.17; p<0.001), after adjusting for age, sex, hyperlipidemia, and diabetes mellitus. No significant differences were observed in the expression of CD69, CD62p, CX3CR1, or tissue factor by TB status among PLWH (Supplementary Table 3).
Table 1.
Characteristics of HIV-infected participants by TB status (n=83)
| Characteristic | No TB infection (n=40) | LTBI (n=18) | Prior active TB (n=25) | p value |
|---|---|---|---|---|
| Age in years, median (IQR) | 56 (53 – 62) | 54 (51 – 59) | 58 (55 – 63) | 0.192 |
| Male sex at birth, n (%) | 12 (30) | 6 (33) | 12 (48) | 0.327 |
| Diabetes mellitus, n (%) | 10 (25) | 2 (11) | 9 (36) | 0.180 |
| Cardiovascular disease, n (%) | 1 (3) | 0 | 2 (8) | 0.333 |
| Hypertension, n (%) | 35 (88) | 16 (89) | 22 (88) | 0.989 |
| Hyperlipidemia, n (%) | 2 (5) | 3 (17) | 2 (8) | 0.333 |
| Chronic kidney disease, n (%) | 1 (3) | 0 | 1 (4) | 0.700 |
| Family history of heart disease, n (%) | 4 (22) | 3 (12) | 4 (10) | 0.436 |
| Most recent HIV viral load, median (IQR) | 20 (20 – 20) | 20 (20 – 20) | 20 (20 – 20) | 0.429 |
| Most recent CD4, median (IQR) | 572 (437 – 746) | 633 (385 – 899) | 589 (400 – 753) | 0.922 |
| Nadir CD4 count, median (IQR) | 162 (67 – 287) | 222 (85 – 287) | 149 (57 – 330) | 0.701 |
| Total cholesterol, median (IQR) | 214 (177 – 242) | 203 (167 – 220) | 206 (172 – 226) | 0.447 |
| Low density lipoprotein (LDL), median (IQR) | 130 (108 – 165) | 125 (92 – 138) | 131 (107 – 153) | 0.310 |
| High density lipoprotein (HDL), median (IQR) | 58 (45 – 74) | 52 (43 – 66) | 53 (43 – 66) | 0.691 |
| Non-HDL cholesterol, median (IQR) | 155 (122 – 184) | 142 (125 – 165) | 154 (125 – 167) | 0.499 |
| Creatinine, median (IQR) | 0.8 (0.7 – 0.9) | 0.7 (0.7 – 0.9) | 0.8 (0.7 – 0.9) | 0.783 |
| Total monocytes, median (IQR) | 4.5 (3.4 – 3.9) | 3.7 (3.1 – 4.2) | 5.1 (3.8 – 5.6) | 0.020* |
| Interferon-γ in QFT nil, IU/mL, median (IQR) | 0.09 (0.08 – 0.14) | 0.13 (0.11 – 0.33) | 0.12 (0.1 – 0.17) | 0.008** |
No TB infection vs. LTBI group, p=0.322; No TB infection vs. prior TB group, p=0.013.
No TB infection vs. LTBI group, p=0.004; No TB infection vs. prior TB group, p=0.04.
Figure 1.
Fold changes in HLA-DR MFI in total monocytes and monocytes subsets by TB status in PLWH (reference group no TB infection). The asterisk sign (*) indicates significant differences compared to the reference group (p<0.05)
Among HIV-uninfected individuals, no participants with prior TB disease were identified. There were no significant differences between individuals with LTBI and those without TB infection in terms of age, male sex, hypertension, diabetes mellitus, hyperlipidemia, or family history of heart disease (Supplementary Table 4). There were no differences in the proportion of total monocytes, monocyte subsets, or HLA-DR expression by TB infection status (Supplementary Table 5).
DISCUSSION
We found that both LTBI and prior TB disease were associated with increased HLA-DR expression in circulating monocytes of PLWH from a TB-endemic area, indicating increased monocyte activation in the setting of HIV/TB coinfection. To our knowledge, this is the first study to characterize monocyte activation phenotypes in PLWH with defined TB coinfection status. Consistent with other reports [5, 14, 15], we also found that HIV infection was associated with increased frequency of inflammatory monocytes and enhanced expression of the chemokine receptor CX3CR1 involved in cell adhesion and migration. Our findings indicate distinct perturbations in monocyte phenotypes in HIV and HIV/TB coinfection status.
HLA-DR is a cell surface receptor used for antigen presentation to CD4+ T cells [16]. Increased HLA-DR expression in monocytes usually indicates activation [17]. Individuals with primary and untreated HIV infection exhibit increased HLA-DR expression in monocytes [5, 18], and ART has shown to decrease monocyte HLA-DR levels [5]. In our study, we found no differences in monocyte HLA-DR expression between PLWH on stable ART compared to HIV-uninfected controls. However, among PLWH, HLA-DR expression was significantly higher in those with LTBI coinfection or prior TB disease, compared to PLWH without TB coinfection.
Our findings support that PLWH with TB coinfection, or at least a subset of them, have increased levels of immune activation. In South Africa, PLWH with active TB or LTBI coinfection showed enhanced activation of CD4+ and CD8+ T cells, compared to PLWH without TB coinfection [4]. It is possible that increased T cell activation in HIV/TB coinfection could augment the release of pro-inflammatory mediators such as interferon-gamma, which in turn may induce monocyte activation and enhanced HLA-DR surface expression [19]. Supporting this idea, we found that PLWH with TB coinfection had higher levels of unstimulated interferon-gamma compared to PLWH without TB coinfection, consistent with prior reports in HIV-uninfected individuals [20, 21]. Alternatively, TB infection may contribute to monocyte priming via epigenetic reprogramming, as has been observed with BCG mycobacteria [22]. Additional studies of immune activation in HIV/TB coinfection are needed, as augmented immune activation may contribute to the pathogenesis of NCDs in PLWH by increasing pro-inflammatory mediators, altering lipid metabolism, and accelerating immunosenescence which may favor the occurrence of NCDs at earlier ages [3, 23, 24]. HIV/TB coinfection has been associated with increased non-AIDS mortality [25], and thus persistent immune activation provides a plausible mechanistic link between TB and non-AIDS related events in PLWH.
This study had limitations. We may not have had enough power to detect differences due to sample size limitations. However, to our knowledge, this is the largest study conducted to date on monocyte activation phenotypes in PLWH with a defined TB status. Our HIV-infected population consisted of older adults on stable ART, and thus our results are not generalizable to other groups of PLWH. However, since most study participants had achieved sustained HIV virologic suppression, we were able to study the relationship between TB status and immune activation without confounding from uncontrolled HIV infection. We did not measure soluble markers of monocyte activation nor perform stimulation assays to assess the monocytes’ ability to produce pro-inflammatory cytokines. Future studies should consider these assays to gain further insight on monocyte activation in HIV/TB coinfection.
In conclusion, LTBI and prior active TB were associated with increased HLA-DR expression on all monocyte subsets in PLWH. These results indicate increased monocyte activation in the setting of HIV and TB coinfection, which may contribute to the pathogenesis of NCDs in PLWH.
Supplementary Material
ACKNOWLEDGEMENTS
We thank all of the study participants who made this study possible.
M.A.H. and C.T.L. designed the study. S.M.J. and M.A.H. analyzed flow cytometry data with input from D.A.Z. and C.T.L. All authors interpreted data. M.A.H. drafted the report. All authors provided input into the report and approved the final version of the report.
FUNDING
This work was supported by the National Center for Advancing Translational Science [grant number KL2 TR001426 to M.A.H.], the National Heart, Lung, and Blood Institute [grant number K23 HL123341 to C.T.L.], and the National Institute of Allergy and Infectious Diseases [grant numbers UM1AI069501 to C.J.F., UM1 AI068636 to M.A.H.] at the National Institutes of Health. M.A.H. also received support from a Faculty Pilot Award from the Department of Internal Medicine at the University of Cincinnati College of Medicine. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or the institutions with which the authors are affiliated. The funding source had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
POTENTIAL CONFLICTS OF INTEREST
C. J. F. reports grants from Gilead Sciences Inc, grants from ViiV Healthcare, grants from Janssen, grants from Amgen, grants from Merck, grants from Cytodyn, and personal fees from Clinical Care Options, outside the submitted work. All other authors report no potential conflicts.
REFERENCES
- 1.Patel P, Rose CE, Collins PY, Nuche-Berenguer B, Sahasrabuddhe VV, Peprah E, et al. Noncommunicable diseases among HIV-infected persons in low-income and middle-income countries: a systematic review and meta-analysis. AIDS 2018; 32 Suppl 1:S5–S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong C, Gange SJ, Moore RD, Justice AC, Buchacz K, Abraham AG, et al. Multimorbidity Among Persons Living with Human Immunodeficiency Virus in the United States. Clin Infect Dis 2018; 66(8):1230–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med 2011; 62:141–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sullivan ZA, Wong EB, Ndung’u T, Kasprowicz VO, Bishai WR. Latent and Active Tuberculosis Infection Increase Immune Activation in Individuals Co-Infected with HIV. EBioMedicine 2015; 2(4):334–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCausland MR, Juchnowski SM, Zidar DA, Kuritzkes DR, Andrade A, Sieg SF, et al. Altered Monocyte Phenotype in HIV-1 Infection Tends to Normalize with Integrase-Inhibitor-Based Antiretroviral Therapy. PLoS One 2015; 10(10):e0139474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Funderburg NT, Zidar DA, Shive C, Lioi A, Mudd J, Musselwhite LW, et al. Shared monocyte subset phenotypes in HIV-1 infection and in uninfected subjects with acute coronary syndrome. Blood 2012; 120(23):4599–4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, et al. Nomenclature of monocytes and dendritic cells in blood. Blood 2010; 116(16):e74–80. [DOI] [PubMed] [Google Scholar]
- 8.Thomas GD, Hamers AAJ, Nakao C, Marcovecchio P, Taylor AM, McSkimming C, et al. Human Blood Monocyte Subsets: A New Gating Strategy Defined Using Cell Surface Markers Identified by Mass Cytometry. Arterioscler Thromb Vasc Biol 2017; 37(8):1548–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ziegler-Heitbrock L The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J Leukoc Biol 2007; 81(3):584–592. [DOI] [PubMed] [Google Scholar]
- 10.Narasimhan PB, Marcovecchio P, Hamers AAJ, Hedrick CC. Nonclassical Monocytes in Health and Disease. Annu Rev Immunol 2019; 37:439–456. [DOI] [PubMed] [Google Scholar]
- 11.Cros J, Cagnard N, Woollard K, Patey N, Zhang SY, Senechal B, et al. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity 2010; 33(3):375–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform 2019; 95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo N, Chen Y, Su B, Yang X, Zhang Q, Song T, et al. Alterations of CCR2 and CX3CR1 on Three Monocyte Subsets During HIV-1/Treponema pallidum Coinfection. Front Med (Lausanne) 2020; 7:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krishnan S, Wilson EM, Sheikh V, Rupert A, Mendoza D, Yang J, et al. Evidence for innate immune system activation in HIV type 1-infected elite controllers. J Infect Dis 2014; 209(6):931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDevitt HO. Regulation of the immune response by the major histocompatibility system. N Engl J Med 1980; 303(26):1514–1517. [DOI] [PubMed] [Google Scholar]
- 17.McLeish KR, Wellhausen SR, Dean WL. Biochemical basis of HLA-DR and CR3 modulation on human peripheral blood monocytes by lipopolysaccharide. Cell Immunol 1987; 108(1):242–248. [DOI] [PubMed] [Google Scholar]
- 18.Gascon RL, Narvaez AB, Zhang R, Kahn JO, Hecht FM, Herndier BG, et al. Increased HLA-DR expression on peripheral blood monocytes in subsets of subjects with primary HIV infection is associated with elevated CD4 T-cell apoptosis and CD4 T-cell depletion. J Acquir Immune Defic Syndr 2002; 30(2):146–153. [DOI] [PubMed] [Google Scholar]
- 19.Nemeth J, Olson GS, Rothchild AC, Jahn AN, Mai D, Duffy FJ, et al. Contained Mycobacterium tuberculosis infection induces concomitant and heterologous protection. PLoS Pathog 2020; 16(7):e1008655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huaman MA, Deepe J, George S., Fichtenbaum CJ. Elevated Circulating Concentrations of Interferon-Gamma in Latent Tuberculosis Infection. Pathogens and Immunity 2016; 1(2):291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huaman MA, Henson D, Rondan PL, Ticona E, Miranda G, Kryscio RJ, et al. Latent tuberculosis infection is associated with increased unstimulated levels of interferon-gamma in Lima, Peru. PLoS One 2018; 13(9):e0202191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kleinnijenhuis J, Quintin J, Preijers F, Joosten LA, Ifrim DC, Saeed S, et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci U S A 2012; 109(43):17537–17542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Titanji B, Gavegnano C, Hsue P, Schinazi R, Marconi VC. Targeting Inflammation to Reduce Atherosclerotic Cardiovascular Risk in People With HIV Infection. J Am Heart Assoc 2020; 9(3):e014873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guaraldi G, Orlando G, Zona S, Menozzi M, Carli F, Garlassi E, et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis 2011; 53(11):1120–1126. [DOI] [PubMed] [Google Scholar]
- 25.Pettit AC, Giganti MJ, Ingle SM, May MT, Shepherd BE, Gill MJ, et al. Increased non-AIDS mortality among persons with AIDS-defining events after antiretroviral therapy initiation. J Int AIDS Soc 2018; 21(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.