Abstract
Background and aims
Cannabis, tobacco and alcohol use are prevalent among youth in the United States and may be risk factors for opioid use. The current study aimed at investigating associations between developmental trajectories of cannabis, tobacco and alcohol use in adolescence and opioid use in young adulthood in an urban cohort over the span of 12 years.
Design
Cohort study of adolescents originally recruited for a randomized prevention trial with yearly assessments into young adulthood.
Setting
Nine urban elementary schools in Baltimore, MD in the United States.
Participants
Participants (n = 583, 86.8% African American, 54.7% male) were originally recruited as first grade students.
Measurements
Cannabis, tobacco and alcohol use were assessed annually from ages 14–18 years and opioid use from ages 19–26. Socio-demographics were assessed at age 6. Intervention status was also randomly assigned at age 6. Gender, race, free/reduced-priced lunch and intervention status were included as covariates in individual and sequential growth models.
Findings
There were significant positive associations between the cannabis use intercept at age 14 and the opioid use intercept at age 19 (beta = 1.43; P = 0.028), the tobacco use intercept at age 14 and the opioid use intercept at age 19 (beta = 0.82; P = 0.042). Specifically, more frequent use of cannabis or tobacco at age 14 was associated with more frequent use of opioids at age 19.
Conclusions
Cannabis and tobacco use in early adolescence may be risk factors for opioid use in young adulthood among African Americans living in urban areas.
Keywords: African American, cannabis, gateway, opioids, tobacco, urban
INTRODUCTION
The opioid epidemic in the United States has been associated with a dramatic increase in opioid use and overdoses [1]. While the initial wave of the epidemic was primarily driven by prescription opioids and was concentrated in rural areas, there was a shift around 2010 to heroin-related deaths and, more recently, deaths due to the synthetic opioid fentanyl, both of which are concentrated in urban areas [2,3]. Youth living in urban areas, particularly African Americans, may be at risk for opioid misuse, as heroin and injection drug use has been on the rise in many urban centers in the United States [4]. Indeed, among African Americans, there have been substantial increases in deaths associated with prescription and synthetic opioids from 2002 to 2015 [5]. The high prevalence of opioid use disorder among African Americans in the United States may be partially attributed to the fact that African Americans are more likely to transition to opioid dependence after initial opioid use [6]. The significant public health burden associated with opioid use has prompted a considerable interest among researchers and policymakers alike in determining risk factors for opioid use, as well opioid use trajectories among adolescents and young adults.
Recent studies have investigated the role of cannabis as a risk factor for opioid use, but the findings have been inconsistent. Cannabis use is prevalent among youth in the United States, with as many as 15.4% of 12–17-year-olds reporting life-time use. Moreover, cannabis use may be associated with use of other substances, including opioids, in this age group [7]. Epidemiological studies among adults suggest that cannabis use may increase the risk of developing prescription opioid misuse and opioid use disorder [8] as well as other subsequent substance use disorders [9]. Conversely, a recent study from Canada found that cannabis use among street-involved adolescents and young adults was associated with a slower time to injection drug use in general, with a non-significant association with injection opioid use [10]. Taken together, there is conflicting evidence regarding the potential association between cannabis and opioid use among young people. Moreover, existing longitudinal studies mainly rely on two time-points [8,9], and do not provide the level of sensitivity necessary to evaluate whether cannabis use during adolescence predicts trajectories of opioid use in young adulthood. The recent landmark policy shift throughout the United States related to the legalization of medical cannabis, and more recently legalization for recreational cannabis use in 10 states and Washington, DC, make it even more important to answer this research question.
There has been a long-standing debate and conflicting results on the question of whether or not cannabis use serves as a gateway to other drug use, including opioids [11,12]. However, people rarely only use single substances, and polysubstance use is common [13–15]. For example, opioid use may not just be associated with cannabis, but also with alcohol [16] and tobacco use [17,18]. Instead of a gateway from cannabis to opioid use, there may be a common liability for use of different substances among young people [19–21]. This suggests that longitudinal studies investigating the potential role of cannabis use on the development of opioid use trajectories also need to control for other substance use behaviors common in adolescents and young adults.
However, there may be a unique contribution of early cannabis use to the risk for later opioid use. For example, twin studies have shown that cannabis use before age 17 increased the odds for life-time opioid use and opioid use disorder [22], even after controlling for early use of alcohol, tobacco and a number of internalizing and externalizing disorders. A potential explanation for this unique contribution of early cannabis use to later substance use risk may be neurodevelopmental. For example, frequent cannabis use in adolescence may sensitize the developing brain to experience enhanced rewards from opioid administration later in life, which has been demonstrated in animal models [23,24]. However, some have argued that levels equivalent to the substantial administration of cannabinoids in animal models are rarely seen in human self-administration [22]. Other reasons for early cannabis use as a risk factor for later opioid use may be the exposure to black markets and subsequent access to other illegal drugs [22] or greater exposure to drug using peers in social networks [25]. Taken together, more research on the potential association between cannabis and opioid use among young people is needed.
To address gaps in the existing literature, this study used data from a longitudinal prevention study to examine associations between developmental trajectories of cannabis use in adolescence and opioid use in young adulthood, controlling for trajectories of adolescent tobacco and alcohol use. The current study is unique, given the span of annual assessments of substance use from early adolescence into young adulthood. An additional strength of the study is its sample of predominantly African American youth.
METHOD
Participants and procedures
The original study sample consisted of 799 participants who were originally recruited as first grade students in the fall of 1993 as part of a randomized controlled, universal preventive intervention trial in nine Baltimore, MD elementary schools. The goals of the intervention were to improve academic achievement, decrease disruptive and/or aggressive behaviors and promote positive outcomes in adulthood. Participants were assigned to one of three arms (1, classroom-centered intervention; 2, family–school partnership intervention; and 3, control group). Participants were followed from first grade to young adulthood (age ~26) and yearly assessments were conducted. The study was reviewed and approved by the institutional review board of the Johns Hopkins Bloomberg School of Public Health. Additional information about the sample and intervention content can be found elsewhere [26].
For the current study, we restricted the sample to 583 individuals who had complete data on the covariates (i.e. participant gender, free/reduced-priced lunch status, race, intervention status) and cannabis, tobacco and alcohol data at age 14. In terms of differences between the analytical sample (n = 583) and those not included in the analyses due to missing data (n = 216), there was a greater percentage of African Americans in the analytical sample (86.8%) compared to those with missing data (79.6%; , P = 0.015). No other differences were found between individuals in the analytical sample compared to those excluded from analyses.
Measures
Participant demographics
Demographic data including participant gender, race and free/reduced-priced lunch status were drawn from the baseline assessments conducted at age 6 in the fall of first grade and from school records. Participant demographics were coded as follows: (race: black = 0, white = 1; gender: female = 0, male = 1; free/reduced lunch status: no = 0, yes = 1).
Intervention status
Intervention status was a binary variable that reflected assignment to one of the two intervention arms versus assignment to the control group. Participants were coded as 1 if they received an intervention and 0 if they did not.
Frequency of substance use
Frequency of cannabis, tobacco, alcohol and opioid use in the past year was assessed annually using an audio computer-assisted interview to increase accurate reporting of sensitive behavior. Questions were adapted from the Monitoring the Future survey [27]. Opioid use included past-year frequency of heroin use and the misuse of narcotic prescription drugs (e.g. morphine, oxycodone, hydrocodone, hydromorphone, etc.). Cannabis, tobacco and alcohol use were assessed annually between the ages of 14 and 18, whereas opioid use was assessed annually between the ages of 19 and 26.
Participants reported on their frequency of use in the past year on a 0–7 Likert scale: [0 (no use), 1 (once), 2 (twice), 3 (three to four times), 4 (five to nine times), 5 (10–19 times), 6 (20–39 times) and 7 (40 or more times)]. Preliminary analyses examined the distribution of the cannabis, tobacco, alcohol and opioid use variables which revealed a positive skew of these variables. We created new variables to more clearly reflect the distribution of these data using a 0–2 coding schema for cannabis, tobacco and opioids [0 (no use), 1 (once to three to four times) and 2 (five to 40 or more times)]; and a 0–3 coding schema for alcohol [0 (no use), 1 (once), 2 (twice to 19 times) and 3 (20–40 or more times)] (see Table 2) [28].
Table 2.
Frequency of use and ns for substance use variables for the analytical sample (n = 583).
| Frequency of use |
|||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | n | |
| Cannabis use age 14 | 507 | 66 | 10 | – | 583 |
| Cannabis use age 15 | 487 | 28 | 36 | – | 551 |
| Cannabis use age 16 | 386 | 65 | 58 | – | 509 |
| Cannabis use age 17 | 360 | 57 | 67 | – | 484 |
| Cannabis use age 18 | 367 | 68 | 62 | – | 497 |
| Tobacco use age 14 | 476 | 92 | 15 | – | 583 |
| Tobacco use age 15 | 453 | 52 | 46 | – | 551 |
| Tobacco use age 16 | 383 | 71 | 55 | – | 509 |
| Tobacco use age 17 | 351 | 53 | 80 | – | 484 |
| Tobacco use age 18 | 383 | 31 | 83 | – | 497 |
| Alcohol use age 14 | 475 | 102 | 4 | 2 | 583 |
| Alcohol use age 15 | 429 | 97 | 22 | 12 | 551 |
| Alcohol use age 16 | 332 | 138 | 29 | 24 | 509 |
| Alcohol use age 17 | 290 | 132 | 50 | 29 | 484 |
| Alcohol use age 18 | 322 | 126 | 43 | 24 | 497 |
| Opioid use age 19 | 483 | 12 | 1 | – | 496 |
| Opioid use age 20 | 469 | 14 | 3 | – | 486 |
| Opioid use age 21 | 478 | 16 | 1 | – | 495 |
| Opioid use age 22 | 475 | 7 | 5 | – | 488 |
| Opioid use age 23 | 464 | 7 | 7 | – | 478 |
| Opioid use age 24 | 475 | 10 | 5 | – | 490 |
| Opioid use age 25 | 474 | 7 | 5 | – | 486 |
| Opioid use age 26 | 463 | 7 | 7 | – | 477 |
Statistical analyses
Separate, unconditional growth models were first estimated in a step-wise fashion for cannabis, tobacco, alcohol and opioid use, consistent with recommended procedures for growth modeling [29] using Mplus version 8.0 [30]. These analyses were conducted to identify the shape of the trajectory of substance use behavior and whether the intercept-only model best fit the data compared to other models (e.g. intercept and linear slope; intercept and quadratic slope). To determine the model that best fitted the data, we examined changes in the BIC consistent with recommendations for evaluating the fit for categorical nested models [31].
Separate growth models for cannabis, tobacco, alcohol and opioid use were then estimated using the maximum likelihood ratio estimator and a numerical integration algorithm [30], with each model controlling for participant gender, race, intervention status and free/reduced-priced lunch status. Participant gender was included as a covariate, given that a number of studies have indicated gender differences in the base rates of cannabis and opioid use [13,32]. Intervention status was also controlled for, as participation in the interventions has been linked to a reduced likelihood of using drugs [33] and has been shown to interact with individual-specific features to predict age of initiation for tobacco and cannabis use [34,35]. We also controlled for free/reduced-priced lunch status, as this variable is a proxy for family income [36,37] and has been robustly associated with psychological impairments and substance use problems among youth [38,39]. Race was also controlled for in analyses, given that racial differences in opioid use have been observed previously [40] and the significant difference in race among included and excluded participants reported above.
For the adolescent cannabis use model, the inclusion of the intercept, slope and quadratic term resulted in the lowest BIC (see Supporting information, Appendix A1). The lowest BIC was also observed for the alcohol and tobacco models upon inclusion of the intercept, slope and quadratic terms. Regarding the young adult opioid use model, a model with the intercept term only yielded the lowest BIC. As a consequence, the sequential process growth model was estimated, including the intercept, linear slope and quadratic slope terms for the cannabis, tobacco and alcohol models; and the intercept term for the opioid model.
The sequential process growth model simultaneously estimated relationships between cannabis, tobacco, alcohol and opioid intercepts and slopes [41]. Planned analyses involved examining whether cannabis, tobacco and alcohol use at age 14 (intercepts) and changes over time in use from ages 14 to 18 (slopes) predicted age 19 opioid use (intercept) and changes in opioid use from ages 19 to 26 (slope) (see Figure 1 for conceptual model). Again, the final sequential process growth models controlled for participant gender, race, intervention status and free/reduced-priced lunch status.
Figure 1.
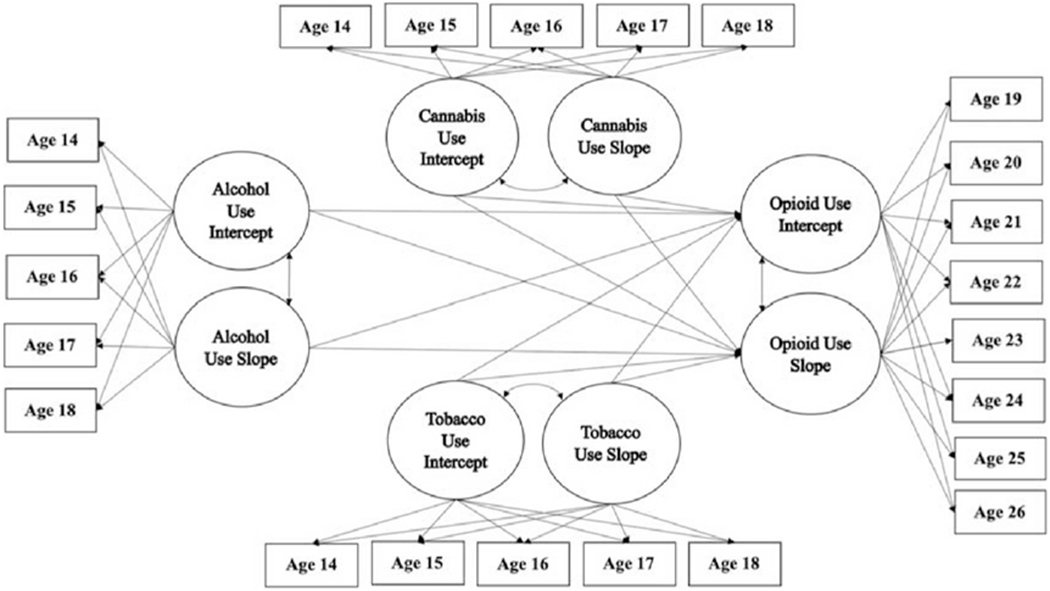
Parallel growth model of the proposed relationships between early cannabis use, alcohol, tobacco and opioid use in adulthood
Full information maximum likelihood (FIML) was used to address missing outcome data, which uses all available data to estimate model parameters but does not impute values [42]. This approach allows for inclusion of participants with missing data in model estimation and generates smaller errors in parameter estimates and standard errors relative to other missing data strategies (e.g. complete case-wise analysis or listwise deletion, complete case analyses or pairwise deletion), which may introduce bias [43,44].
RESULTS
The majority of our sample was male (54.7%) and African American (86.8%). Approximately 70% of the sample received free/reduced-priced lunch and two-thirds of the sample received an intervention (Table 1). The frequency of substance use endorsement can be found in Table 2. At age 14 years, 13.0, 18.4 and 18.1% of adolescents reported past-year cannabis, tobacco and alcohol use, respectively. At age 19 years, 26.2, 22.9 and 34.0% of adolescents reported past-year cannabis, tobacco and alcohol use, respectively. Endorsement of past-year opioid use was substantially lower and ranged from 2.6% of participants at age 19 to 2.9% of participants at age 26, with a peak of 3.5% at age 20.
Table 1.
Analytical sample characteristics (n = 583).
| Characteristic | n (%) |
|---|---|
| Gender | |
| Male | 319 (54.7%) |
| Female | 264 (45.3%) |
| Race | |
| White | 77 (13.2%) |
| African American | 506 (86.8%) |
| Free/reduced-priced lunch | |
| Yes | 411 (70.5%) |
| No | 172 (29.5%) |
| Intervention | |
| Yes | 388 (66.6%) |
| No | 195 (33.4%) |
Results of the sequential process growth model estimating cannabis, tobacco and alcohol use from ages 14 to 18 and opioid use from ages 19 to 26 are displayed in Table 3. There was a positive association between the adolescent cannabis use and young adult opioid use intercepts (b = 1.43, P = 0.028). In other words, more frequent use of cannabis at age 14 was associated with more frequent use of opioids at age 19. In addition, a positive association was also observed between the adolescent tobacco use and adult opioid use intercepts (b = 0.83, P = 0.042), such that more frequent use of tobacco at age 14 was associated with more frequent use of opioids at age 19.
Table 3.
Parameter estimates for parallel growth models involving cannabis, tobacco and alcohol use during adolescence (14–18) and opioid use in young adulthood (19–26) (n = 583).
| Beta (SE) | P | |
|---|---|---|
| Cannabis intercepta | – | – |
| Cannabis linear slope | 1.91 (1.21) | 0.114 |
| Cannabis quadratic slope | −0.29 (0.28) | 0.302 |
| Tobacco intercepta | – | – |
| Tobacco linear slope | 1.28 (0.71) | 0.073 |
| Tobacco quadratic slope | −0.26 (0.21) | 0.223 |
| Alcohol intercepta | – | – |
| Alcohol linear slope | 2.66 (0.73) | < 0.005 |
| Alcohol quadratic slope | −0.49 (0.16) | 0.002 |
| Opioid intercepta | – | – |
| Cannabis intercept → opioid intercept | 1.43 (0.65) | 0.028 |
| Cannabis linear slope → opioid intercept | 1.09 (0.65) | 0.093 |
| Cannabis quadratic slope → opioid intercept | −0.15 (0.16) | 0.368 |
| Tobacco intercept → opioid intercept | 0.82 (0.41) | 0.042 |
| Tobacco linear slope → opioid intercept | 0.21 (0.32) | 0.521 |
| Tobacco quadratic slope → opioid intercept | 0.15 (0.08) | 0.052 |
| Alcohol intercept → opioid intercept | 0.81 (0.44) | 0.068 |
| Alcohol linear slope → opioid intercept | 0.33 (0.40) | 0.402 |
| Alcohol quadratic slope → opioid intercept | −0.06 (0.09) | 0.472 |
Opioid use growth model adjusted for participant gender, race, intervention status, free/reduced-priced lunch status and age 14 tobacco and alcohol use.
In the parameterization of the growth models, the intercept growth factors are fixed at zero as the default. Parameters related to the research questions investigated are italicized.
With regard to covariates included in the sequential process growth model, participant race was negatively associated with the tobacco use intercept (b = −1.25, P = 0.018) and opioid use intercept (b = −1.11, P = 0.006). This means that white race was positively associated with more frequent use of tobacco at age 14 and opioids at age 19. Free/reduced-lunch status was positively associated with the cannabis use intercept (b = 1.54, P = 0.009), such that receiving free/reduced-lunch meals was positively related to using cannabis more frequently at age 14. Participant gender was positively associated with the intercept of adolescent opioid use (b = 0.67, P = 0.045), such that male participants endorsed more frequent use of opioids at age 19. No significant associations were observed between intervention status and cannabis, tobacco, alcohol or opioid use.
DISCUSSION
Opioid misuse remains a public health threat in the United States, and carries tremendous costs at both the individual and societal levels. The existing literature regarding the relationship between cannabis and opioids is mixed, with some studies indicating that cannabis use is a risk factor for opioid misuse [8], whereas other work has found no such association [10]. To further clarify the relationship between cannabis and opioid use, while controlling for simultaneous use of tobacco and alcohol, the current study investigated associations between trajectories of cannabis, tobacco and alcohol use in adolescence and opioid use in young adulthood. Building upon epidemiological work [8,9], we examined these relationships in a community sample of urban young people.
Using a sequential process growth model controlling for gender, race, free/reduced-priced lunch and intervention status, we found a significant association between the adolescent cannabis and tobacco use intercepts and the young adult opioid use intercept. Specifically, age 14 cannabis and tobacco use were associated with more opioid use at age 19. This finding is consistent with previous studies, which indicated that cannabis use increased the risk of developing prescription opioid misuse and opioid use disorder among adults [8]. Our results underline these findings, and show that cannabis use in adolescence can be a risk factor for opioid use. Moreover, our findings show this relationship in a predominantly low-income and African American cohort and thus expand the existing literature, which has predominantly investigated non-minority samples.
As our analyses controlled for tobacco and alcohol use trajectories, the increase in opioid use for adolescents reporting cannabis use at age 14 may signify that cannabis is a specific risk factor for opioid use above and beyond a common liability for substance use more generally [11,45]. As noted earlier, one potential explanation for early cannabis use as a distinct risk factor for later opioid use may be that exposure to black markets may facilitate subsequent access to other illegal drugs [22] or greater exposure to drug-using peers in social networks [25]. A second possible explanation is cross-substance sensitization of the adolescent brain, as found in animal models [23,24]. Cannabis exposure during adolescence, a sensitive period for brain development, may enhance the reinforcing effects of opioid use later in life, which may make early adolescent cannabis users more susceptible to the rewarding effects of opioids. A third explanation for the specific relationship between early cannabis use and later opioid use may be genetic contributions. For example, utilizing a twin design, a previous study found two genetic factors associated with substance use, one reflective of illicit (e.g. cannabis, cocaine) drug dependence and the other primarily related to licit (e.g. alcohol, caffeine) drug dependence [46]. Molecular genetics and candidate gene studies also support the notion of a shared liability between cannabis and opioid use. For example, some research has found significant—albeit small to moderate—genetic correlations between cannabis and opioid use [47], and other work has identified genetic variants that confer risk for both opioid and cannabis use disorders [48,49]. Thus, shared genetic liability, combined with neurobiological changes that result from using cannabis at an early age, may contribute to the positive association observed between cannabis and opioid use [50]. It should be noted, however, that a previous simulation study demonstrated that even controlling for other indicators of drug use propensity (e.g. tobacco and alcohol as conducted in the current analysis) cannot rule out an underlying common liability that may be responsible for both cannabis and opioid use or confirm the existence of a cannabis ‘gateway effect’ [21].
We also found significant associations between age 14 tobacco use and age 19 opioid use. These findings are consistent with existing studies that demonstrated a high likelihood of prescription opioid use among smokers [18]. Early nicotine exposure may sensitize the brain and enhance rewarding effects of opioid use [51]. Recent research has suggested that the substantial decline in cigarette smoking among US adolescents in the United States during the past 2 decades may have spillover effects and also reduce population-level opioid use [52]. Our results are in line with this conclusion, and suggest that a reduction in adolescent tobacco use may have an impact on opioid use in young adulthood.
Limitations
Findings of this study should be interpreted with a number of limitations in mind. First, the study sample was predominantly low-income and African American from one mid-Atlantic metropolitan area. While this sample is representative of students entering public schools in Baltimore in the 1990s, findings may not generalize to other samples or populations. Further, substance use behaviors were assessed using participant self-report without biochemical verification, and may thus be subject to bias. Moreover, relatively few participants reported opioid use during the course of the study. Due to relatively infrequent reported use, heroin and prescription opioid use were combined into a single opioid use outcome variable. It could be informative to disaggregate opioid use into heroin and prescription opioid use in future studies with larger samples. Our study followed-up participants throughout young adulthood, and future research is needed to investigate whether cannabis use during adolescence similarly confer risk for opioid use later in adulthood. Our analytical model used relatively few covariates and none of them were time-varying. Finally, the analysis was not pre-registered and results should be considered exploratory.
Conversely, this study also has several strengths. Most noteworthy is our large study sample of low-income, urban, primarily African American youth participating in a longitudinal study. While nationally representative studies can provide critical information on opioid use in the US population as a whole, they are less informative in understanding prevalence in subgroups, particularly low-income minority populations living in urban areas. The contribution of the current study is particularly timely, given that heroin and injection drug is on the rise in many urban centers in the United States [4] and recent increases in deaths associated with prescription and synthetic opioids among African Americans [5]. Moreover, this is one of the few studies that follows low-income African Americans from childhood to young adulthood and includes extensive measures of cannabis, tobacco, alcohol and opioid use over time.
Conclusions and future directions
In summary, our findings highlight the role of cannabis and tobacco use during adolescence as risk factors for opioid use in young adulthood. School prevention programs and services that address cannabis and tobacco use among urban African American adolescents are needed which, as a consequence, may attenuate opioid use in young adulthood. However, it should also be noted that only a small percentage of fewer than 4% of participants reported use of opioids in any given year, and the majority of adolescents using cannabis or tobacco never progressed to using opioids.
With regard to the effects of cannabis legalization for medical or recreational use [53], we can only speculate how those legislative changes may affect the association between cannabis and opioid use observed in the current study. For example, it is possible that legalization of cannabis will reduce adolescent exposure to the black market and subsequently also reduce their exposure to other drugs including opioids through these black-market channels. Conversely, an increase in adolescent cannabis use due to greater availability may put young people at risk for later opioid use, in line with arguments about potential cross-sensitization of the adolescent brain through early and repeated cannabis exposure.
In the context of increasing cannabis legalization in the United States, findings of the current study suggest that we need to carefully monitor not just the effects of these legal changes on cannabis use, but also potential unintended consequences with regards to the use of other substances, including opioids, by young people.
Supplementary Material
Table A1. Separate unconditional growth models parameter estimates involving cannabis, tobacco, and alcohol use during adolescence (age 14–18) and opioid use in young adulthood (age 19–26) (N = 583).
Acknowledgements
This study was funded by the National Institute on Drug Abuse (NIDA; R01 DA044184 02S1).
Footnotes
Declaration of interests
None.
Supporting Information
Additional supporting information may be found online in the Supporting Information section at the end of the article.
References
- 1.Skolnick P The opioid epidemic: crisis and solutions. Annu Rev Pharmacol Toxicol 2018; 58: 143–59. [DOI] [PubMed] [Google Scholar]
- 2.Jalal H, Buchanich JM, Roberts MS, Balmert LC, Zhang K, Burke DS Changing dynamics of the drug overdose epidemic in the United States from 1979 through 2016. Science 2018; 361: eaau1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kiang MV, Basu S, Chen J, Alexander MJ Assessment of changes in the geographical distribution of opioid-related mortality across the United States by opioid type, 1999–2016. JAMA Netw Open 2019; 2: e190040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brighthaupt S-C, Schneider KE, Johnson JK, Jones AA, Johnson RM Trends in adolescent heroin and injection drug use in nine urban centers in the U.S., 1999–2017. J Adolesc Health 2019; 65: 210–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kandel DB, Hu M-C, Griesler P, Wall M Increases from 2002 to 2015 in prescription opioid overdose deaths in combination with other substances. Drug Alcohol Depend 2017; 178: 501–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sartor CE, Kranzler HR, Gelernter J Rate of progression from first use to dependence on cocaine or opioids: a cross-substance examination of associated demographic, psychiatric, and childhood risk factors. Addict Behav 2014; 39: 473–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones CM, McCance-Katz EF Relationship between recency and frequency of youth cannabis use on other substance use. J Adolesc Health 2019; 64: 411–3. [DOI] [PubMed] [Google Scholar]
- 8.Olfson M, Wall MM, Liu S-M, Blanco C Cannabis use and risk of prescription opioid use disorder in the United States. Am J Psychiatry 2018; 175: 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blanco C, Hasin DS, Wall MM, Flórez-Salamanca L, Hoertel N, Wang S, et al. Cannabis use and risk of psychiatric disorders: prospective evidence from a US National Longitudinal Study. JAMA Psychiatry 2016; 73: 388–95. [DOI] [PubMed] [Google Scholar]
- 10.Reddon H, DeBeck K, Socias ME, Dong H, Wood E, Montaner J, et al. Cannabis use is associated with lower rates of initiation of injection drug use among street-involved youth: a longitudinal analysis. Drug Alcohol Rev 2018; 37: 421–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fergusson DM, Boden JM, Horwood LJ Cannabis use and other illicit drug use: testing the cannabis gateway hypothesis. Addiction 2006; 101: 556–69. [DOI] [PubMed] [Google Scholar]
- 12.Tarter RE, Vanyukov M, Kirisci L, Reynolds M, Clark DB Predictors of marijuana use in adolescents before and after licit drug use: examination of the gateway hypothesis. Am J Psychiatry 2006; 163: 2134–40. [DOI] [PubMed] [Google Scholar]
- 13.Banks DE, Rowe AT, Mpofu P, Zapolski TCB Trends in typologies of concurrent alcohol, marijuana, and cigarette use among US adolescents: an ecological examination by sex and race/ethnicity. Drug Alcohol Depend 2017; 179: 71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roche DJO, Bujarski S, Green R, Hartwell EE, Leventhal AM, Ray LA Alcohol, tobacco, and marijuana consumption is associated with increased odds of same-day substance co- and tri-use. Drug Alcohol Depend 2019; 200: 40–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montgomery L, Zapolski T, Banks DE, Floyd A Puff, puff, drink: the association between blunt and alcohol use among African American adolescents and young adults. Am J Orthopsychiatry 2019; 89: 609–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Novak SP, Peiper NC, Zarkin GA Nonmedical prescription pain reliever and alcohol consumption among cannabis users. Drug Alcohol Depend 2016; 159: 101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoon JH, Lane SD, Weaver MF Opioid analgesics and nicotine: more than blowing smoke. J Pain Palliat Care Pharmacother 2015; 29: 281–9. [DOI] [PubMed] [Google Scholar]
- 18.Zale EL, Dorfman ML, Hooten WM, Warner DO, Zvolensky MJ, Ditre JW Tobacco smoking, nicotine dependence, and patterns of prescription opioid misuse: results from a nationally representative sample. Nicotine TobRes 2015; 17: 1096–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mayet A, Legleye S, Beck F, Falissard B, Chau N The gateway hypothesis, common liability to addictions or the route of administration model a modelling process linking the three theories. Eur Addict Res 2016; 22: 107–17. [DOI] [PubMed] [Google Scholar]
- 20.Vanyukov MM, Tarter RE, Kirillova GP, Kirisci L, Reynolds MD, Kreek MJ, et al. Common liability to addiction and ‘gateway hypothesis’: theoretical, empirical and evolutionary perspective. Drug Alcohol Depend 2012; 123: S3–S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morral AR, McCaffrey DF, Paddock SM Reassessing the marijuana gateway effect. Addiction 2002; 97: 1493–504. [DOI] [PubMed] [Google Scholar]
- 22.Lynskey MT, Heath AC, Bucholz KK, Slutske WS, Madden PAF, Nelson EC, et al. Escalation of drug use in early-onset cannabis users vs co-twin controls. JAMA 2003; 289: 427–33. [DOI] [PubMed] [Google Scholar]
- 23.Cadoni C, Pisanu A, Solinas M, Acquas E, Di Chiara G Behavioural sensitization after repeated exposure to δ9-tetrahydrocannabinol and cross-sensitization with morphine. Psychopharmacology 2001; 158: 259–66. [DOI] [PubMed] [Google Scholar]
- 24.Ellgren M, Spano SM, Hurd YL Adolescent cannabis exposure alters opiate intake and opioid limbic neuronal populations in adult rats. Neuropsychopharmacology 2007; 32: 607–15. [DOI] [PubMed] [Google Scholar]
- 25.Galea S, Nandi A, Vlahov D The social epidemiology of substance use. Epidemiol Rev 2004; 26: 36–52. [DOI] [PubMed] [Google Scholar]
- 26.Ialongo NS, Werthamer L, Kellam SG, Brown CH, Wang S, Lin Y Proximal impact of two first-grade preventive interventions on the early risk behaviors for later substance abuse, depression, and antisocial behavior. Am J Community Psychol 1999; 27: 599–641. [DOI] [PubMed] [Google Scholar]
- 27.Bachman JG, Jonston LD, O’Malley PM Monitoring the Future: A Continuing Study of American Youth (12th-Grade Survey). Ann Arbor, MI: Inter-university Consortium for Political and Social Research; 1998. [Google Scholar]
- 28.Liu W, Lynne-Landsman SD, Petras H, Masyn K, Ialongo N The evaluation of two first-grade preventive interventions on childhood aggression and adolescent marijuana use: a latent transition longitudinal mixture model. Prev Sci 2013; 14: 206–17. [DOI] [PubMed] [Google Scholar]
- 29.Singer JD, Willett JB Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. New York, NY: Oxford University Press; 2003. [Google Scholar]
- 30.Muthén LK, Muthén BO Mplus User’s Guide. Muthén & Muthén: Los Angeles, CA; 2017. [Google Scholar]
- 31.Holtmann J, Koch T, Lochner K, Eid M A comparison of ML, WLSMV, and Bayesian methods for multilevel structural equation models in small samples: a simulation study. Multivar Behav Res 2016; 51: 661–80. [DOI] [PubMed] [Google Scholar]
- 32.Campesi I, Fois M, Franconi F Sex and gender aspects in anesthetics and pain medication. Handb Exp Pharmacol 2012. 265–78. [DOI] [PubMed] [Google Scholar]
- 33.Furr-Holden CDM, Ialongo NS, Anthony JC, Petras H, Kellam SG Developmentally inspired drug prevention: middle school outcomes in a school-based randomized prevention trial. Drug Alcohol Depend 2004; 73: 149–58. [DOI] [PubMed] [Google Scholar]
- 34.Musci RJ, Fairman B, Masyn KE, Uhl G, Maher B, Sisto DY, et al. Polygenic score × intervention moderation: an application of discrete-time survival analysis to model the timing of first marijuana use among urban youth. Prev Sci 2018; 19: 6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Musci RJ, Masyn KE, Uhl G, Maher B, Kellam SG, Ialongo NS Polygenic score × intervention moderation: an application of discrete-time survival analysis to modeling the timing of first tobacco use among urban youth. Dev Psychopathol 2015; 27: 111–22. [DOI] [PubMed] [Google Scholar]
- 36.Hobbs G, Vignoles A Is children’s free school meal ‘eligibility’ a good proxy for family income? Br Educ Res J 2010; 36: 673–90. [Google Scholar]
- 37.Huang J, Barnidge E Low-income Children’s participation in the National School Lunch Program and household food insufficiency. Soc Sci Med 2016; 150: 8–14. [DOI] [PubMed] [Google Scholar]
- 38.Goodman E The role of socioeconomic status gradients in explaining differences in US adolescents’ health. Am J Public Health 1999; 89: 1522–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hanson MD, Chen E Socioeconomic status and health behaviors in adolescence: a review of the literature. J Behav Med 2007; 30: 263–85. [DOI] [PubMed] [Google Scholar]
- 40.Martins SS, Santaella-Tenorio J, Marshall BDL, Maldonado A, Cerda M Racial/ethnic differences in trends in heroin use and heroin-related risk behaviors among non-medical prescription opioid users. Drug Alcohol Depend 2015; 151: 278–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim S-Y, Kim J-S Investigating stage-sequential growth mixture models with multiphase longitudinal data. Struct Equ Model Multidisc J 2012; 19: 293–319. [Google Scholar]
- 42.Enders CK A primer on maximum likelihood algorithms available for use with missing data. Struct Equ Modeling 2001; 8: 128–41. [Google Scholar]
- 43.Graham JW Missing data analysis: making it work in the real world. Annu Rev Psychol 2009; 60: 549–76. [DOI] [PubMed] [Google Scholar]
- 44.Little RJA, Rubin DB Statistical Analysis with Missing Data, 2nd edn Hoboken, NJ: Wiley-Interscience; 2002. [Google Scholar]
- 45.Woodcock EA, Lundahl LH, Stoltman JJK, Greenwald MK Progression to regular heroin use: examination of patterns, predictors, and consequences. Addict Behav 2015; 45: 287–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kendler KS, Myers J, Prescott CA Specificity of genetic and environmental risk factors for symptoms of cannabis, cocaine, alcohol, caffeine, and nicotine dependence. Arch Gen Psychiatry 2007; 64: 1313–20. [DOI] [PubMed] [Google Scholar]
- 47.Gillespie NA, Bates TC, Hickie IB, Medland SE, Verhulst B, Kirkpatrick RM, et al. Genetic and environmental risk factors in the non-medical use of over-the-counter or prescribed analgesics, and their relationship to major classes of licit and illicit substance use and misuse in a population-based sample of young adult twins. Addiction 2019; 114: 2229–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schwantes-An T-H, Zhang J, Chen L-S, Hartz SM, Culverhouse RC, Chen X, et al. Association of the OPRM1 variant rs1799971 (A118G) with non-specific liability to substance dependence in a collaborative de novo meta-analysis of European-ancestry cohorts. Behav Genet 2016; 46: 151–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang H, Wang F, Kranzler HR, Anton RF, Gelernter J Variation in regulator of G-protein signaling 17 gene (RGS17) is associated with multiple substance dependence diagnoses. Behav Brain Funct 2012; 8: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hurd YL, Michaelides M, Miller ML, Jutras-Aswad D Trajectory of adolescent cannabis use on addiction vulnerability. Neuropharmacology 2014; 76: 416–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vihavainen T, Piltonen M, Tuominen RK, Korpi ER, Ahtee L Morphine–nicotine interaction in conditioned place preference in mice after chronic nicotine exposure. Eur J Pharmacol 2008; 587: 169–74. [DOI] [PubMed] [Google Scholar]
- 52.Miech R, Keyes KM, O’Malley PM, Johnston LD The great decline in adolescent cigarette smoking since 2000: consequences for drug use among US adolescents. Tob Control 2020. 10.1136/tobaccocontrol-2019-055052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smart R, Pacula RL Early evidence of the impact of cannabis legalization on cannabis use, cannabis use disorder, and the use of other substances: findings from state policy evaluations. Am J Drug Alcohol Abuse 2019; 45: 644–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table A1. Separate unconditional growth models parameter estimates involving cannabis, tobacco, and alcohol use during adolescence (age 14–18) and opioid use in young adulthood (age 19–26) (N = 583).


