Abstract
Differences in overall cocaine intake can directly affect neuroadaptations, and this relationship can make it difficult to interpret neurobiological changes seen in drug-choice studies, since drug intake varies between subjects. Herein, a choice procedure that controls for cocaine intake was utilized to explore if neuronal activity, measured as cFos expression in the orbitofrontal cortex (OFC) and nucleus accumbens (NAc), was reflective of preference. Results demonstrated that cFos expression, in both the OFC and NAc, was independent of cocaine preference when cocaine intake was kept constant across individuals. However, when cocaine intake was systematically varied, the expression of cFos associated with cocaine preference was related to overall cocaine intake in the OFC, but not the NAc. Altogether, these results demonstrate that cocaine intake during choice can affect neurobiological outcome measures; thus, the neurobehavioral mechanisms underlying cocaine preference may be better isolated when controlling for cocaine frequency and intake. In all, some caution is warranted when interpreting results from choice studies evaluating the neurobehavioral mechanisms that underlie drug preference when drug frequency and intake is uncontrolled, and future research is needed to determine the role of drug frequency and intake on neurobiological measures associated with drug choice.
Keywords: Cocaine, choice, decision-making, immunohistochemistry, in situ hybridization
1. Introduction
Within the last decade there has been an increase in the number of studies investigating the decision-making processes underlying drug versus nondrug choice. Moreover, an ever-increasing number of these drug-choice studies have emerged in rodent models with the goal towards better understanding the neurobiological mechanisms that underlie drug preference (Banks and Negus, 2012). While existing studies into drug versus nondrug choice in rodent models have revealed many conditions in which nondrug alternatives can steer preference away from drugs of abuse (Beckmann et al., 2019; Thomsen et al., 2013), there is still much to be determined regarding the neurobiological mechanisms underlying these effects.
While not much is known regarding the neurobiology mediating drug preference, previous studies into substance use disorders provide some insight. Specifically, both drug reinforcers and nondrug reinforcers are shown to share overlapping neurobiological systems within the mesocorticolimbic circuit (Koob and Volkow, 2010; Robbins and Everitt, 1996). Both the nucleus accumbens (NAc) and orbitofrontal cortex (OFC), regions within the mesocorticolimbic pathway, are heavily implicated in reward valuation and the decision-making process (Gallagher et al., 1999; Salamone et al., 2007; Wallis, 2007). In vivo electrophysiological studies examining the NAc and OFC have also provided further insight into the neurobehavioral mechanisms associated with preference for drug and nondrug rewards. Electrophysiological recordings from neurons in the NAc have shown that there are non-overlapping neurons that will respond exclusively to cocaine or natural rewards (e.g., water) when presented separately (Carelli, 2002; Carelli et al., 2000). Likewise, electrophysiological recordings of neurons in the OFC also revealed that there are distinct populations of neurons that encode for qualitatively-different nondrug reinforcers (Padoa-Schioppa, 2013; Padoa-Schioppa and Assad, 2006). Moreover, studies measuring cFos expression have identified distinct neuronal populations, within the mesocorticolimbic circuit, that activate in response to drug and nondrug rewards (He et al., 2019; Xiu et al., 2014). Collectively, these studies suggest that there are neuronal populations within the mesocorticolimbic circuit that independently respond to specific reinforcers, and investigation into these independent neuronal populations may reveal insight into drug preference.
According to choice theory, preference is determined by differences in the relative dimensions of reinforcement for available alternatives (Davison and McCarthy, 1988). One dimension of reinforcement that has been consistently and repeatedly shown to drive drug preference is magnitude, or how much of a given reinforcer is available upon selection (Beckmann et al., 2019; Hutsell et al., 2015; Nader and Woolverton, 1990; Negus, 2003; Thomsen et al., 2013). Another reinforcer dimension known to determine drug preference is reinforcer frequency, or how often an organism comes into contact with available reinforcers (Anderson et al., 2002; Beckmann et al., 2019). Under most existing drug choice procedures, the relative number of drug-to-food reinforcers earned is subject-determined. Moreover, the relative measure for drug preference is the same relative measure of drug intake. Importantly, this covariation in drug choice and overall drug intake may significantly complicate the interpretation of neurobiological outcomes in drug-choice studies, since differential exposure to drugs of abuse, like cocaine, can directly influence neurobiological adaptations on its own (Hyman et al., 2006; Kalivas and O’Brien, 2008; Nestler, 2001).
Controlled reinforcer frequency schedules have been successfully utilized in a myriad of choice studies, including preclinical studies (c.f., Beckmann et al. 2019; Stubbs and Pliskoff 1969) and human clinical studies (e.g., Alsop et al., 2016; Pizzagalli et al., 2005) to help isolate preference and valuation processes that underlie behavioral disorders. Under the controlled reinforcer frequency choice procedures, differences in reinforcer experience and consequent intake are experimentally controlled, and the preference measures obtained are dissociable from reinforcer intake. The present study utilized a controlled reinforcer frequency choice procedure to control for the relative frequency of cocaine exposure across all subjects in order to determine the independent influence of cocaine intake and cocaine preference on neurobiological outcome measures associated with cocaine choice. Cocaine preference measures were correlated with cFos activity from the OFC and NAc to determine if the neurobiological adaptations observed were associated with cocaine preference. The immediate early gene cFos was chosen since its expression is indicative of neuronal activity (Cruz et al., 2015, 2013; Dragunow and Faull, 1989; He et al., 2019; Herrera and Robertson, 1996), and by using the known timeline of cFos expressed as mRNA or protein (Guzowski et al., 2001; Moratalla et al., 1993; Xue et al., 2017) different stimuli associated with conditions of cocaine and food preference can be used to induce cFos activity associated with each specific stimulus within a single subject (He et al., 2019; Xiu et al., 2014). If the neurobiological mechanisms driving cocaine preference are independent of overall cocaine intake, then under conditions where cocaine intake is held constant, a correlation between preference and cFos expression for cocaine should be observed. However, if differential cocaine intake influences the neurobiological changes then no correlation between preference measures and cFos activity should be observed when cocaine intake is held constant across individuals. Relatedly, if differential cocaine intake during drug vs. nondrug choice influences cFos expression then cocaine-related cFos expression should increase under conditions that systematically increase cocaine intake. Herein, we tested the above hypotheses over two independent, but related, experiments.
2. Methods
2.1. Subjects
Thirty-six adult male (data was collected prior to current NIH standards) Sprague-Dawley Rats (Harlan Inc.; Indianapolis, IN, USA), acquired at PND 60 and weighing approximately 250–275 g on arrival, were used. Rats were individually housed (12:12hr light:dark cycle) with ad libitum access to food and water in their home cage. All experimental protocols were conducted in accordance with the 2011, National Research Council: Guide for the Care and Use of Laboratory Animals (8th edition) and were approved by the Institutional Animal Care and Use Committee at the University of Kentucky.
2.2. Apparatus
Experiments were conducted in operant chambers (ENV-008CT, MED Associates, St. Albans, VT) enclosed within sound-attenuating compartments (ENV-018MD). For specifications see supplemental files.
2.3. Drugs
(−)-Cocaine hydrochloride, gifted from the National Institute on Drug Abuse (Bethesda, MD, USA), was mixed in sterile saline (0.9% NaCl).
2.4. Experiment 1: Neuronal activation when controlling for cocaine intake
Following a series of initial training procedures (see supplemental files), 12 rats were trained under a controlled reinforcer frequency schedule for cocaine vs. food choice, described previously (Beckmann et al., 2019). Briefly, the choice procedure consisted of 5 distinct blocks, where each block was signaled by its own accompanying tone pattern (Krägeloh and Davison, 2003; Pope et al., 2015). Each block consisted of a total of 6 trials, 3-cocaine and 3-food trials, where the dose of cocaine increased as a function of block (0, 0.032, 0.10, 0.32, and 1.0 mg/kg/infusion), while the food-option was always a single 45-mg palatable food pellet. In each block, both levers were extended during each trial but only one of the two reinforcers (i.e., cocaine or food) was randomly scheduled for delivery. Regardless of which lever the rat responded on, the reinforcer that was scheduled had to be earned to advance onto the next trial. Thus, responses for the scheduled reinforcer were recorded as forced responses, while responses for the unscheduled reinforcer were recorded as choice responses. Importantly, the randomization of reinforcer (i.e., cocaine vs. food) on each trial ensured that the availability for a given reinforcer was unpredictable. Upon reinforcer delivery, both levers would retract, and a corresponding cue-light would signal reinforcer delivery. All responses were scheduled on a fixed-ratio (FR) and required consecutive responding; a changeover in responding would reset the FR count. Rats were initially trained on a FR1 and were incrementally progressed up to an FR5. Rats were trained on the controlled reinforcer frequency choice procedure for 28 days. The resulting n-size was 10, and all attrition was due to catheter failure. Choice sessions lasted approximately 75 minutes.
2.5. Experiment 2: Neuronal activation under systematically varied cocaine:food frequency ratios
Following a series of initial training procedures, identical to Experiment 1, 24 rats were trained under the controlled reinforcer frequency choice procedure, where the relative ratio of cocaine:food reinforcers was equivalent (3:3) for 14 days. After 14 days, rats were matched for performance and placed in either a cocaine-replete or a cocaine-deplete condition of the controlled reinforcer frequency choice procedure. Note, the controlled reinforcer frequency choice procedure for cocaine-replete and -deplete conditions were identical to the initial controlled reinforcer frequency choice procedure, except for the relative number of cocaine to food trials available in each block (Beckmann et al., 2019). Briefly, under the cocaine-replete condition, the relative cocaine:food trial ratio per block was 5:1; under the cocaine-deplete condition, the relative cocaine:food trial ratio per block was 1:5. Rats trained under the systematically varied cocaine:food ratio conditions for 14 days. The resulting n-sizes were n=9 under cocaine-replete conditions, and n=8 under cocaine-deplete conditions. All attrition was due to catheter failure. Choice sessions lasted approximately 75 minutes for both ratio conditions.
2.6. Neuronal labeling for cocaine preference and food preference
By exposing rats to conditions where preference for cocaine and preference for food was observed (i.e., first and last block), the well-established timeline of cFos expression, as either mRNA or as protein, can be utilized to determine neuronal activity for more than one reinforcer (He et al., 2019; Xiu et al., 2014). cFos positive cells in the OFC and NAc were labeled using fluorescent immunohistochemistry (FIHC) to label cells activated by one reinforcer and fluorescent in situ hybridization (FISH) to label cells activated by the second reinforcer. Specifically, the reinforcer that was presented first expresses cFos protein, labeled via FIHC, and the reinforcer that was presented second expresses cFos mRNA, labeled via FISH. Importantly, the activation for cocaine and food preference conditions was counterbalanced. Thus, cFos expression identified by FIHC or FISH was indicative of activation in response to conditions where cocaine or food preference was seen, while overlap in FIHC and FISH labeling was indicative of cellular activation common to both reinforcers. Upon completion of controlled reinforcer frequency choice procedure training, for both Experiment 1 and 2, rats underwent two activation sessions, one for cocaine and one for food (counterbalanced across individuals), and then perfused. Activation sessions took approximately 15 minutes to complete for both reinforcers and all rats completed both activation sessions. The start of the first activation session occurred 135 minutes prior to perfusion, while the start of the second activation session occurred 30 minutes prior to perfusion; the time points used were based on previous studies examining cFos expression demonstrating that rising or peak levels of mRNA occurred around 30 to 60 minutes and rising or peak levels of protein occurred around 120 to 180 minutes (He et al., 2019; Hope et al., 1992; Kufahl et al., 2009; McClung et al., 2004; Moratalla et al., 1993; Morgan and Curran, 1991; Xiu et al., 2014; Xue et al., 2017). Brains were collected, sliced, and underwent FISH and FIHC (see Figure 1 for representative FISH/FIHC). See supplemental files for complete activation procedures, FISH/FIHC methods, and control conditions for FISH/FIHC labeling.
Figure 1.
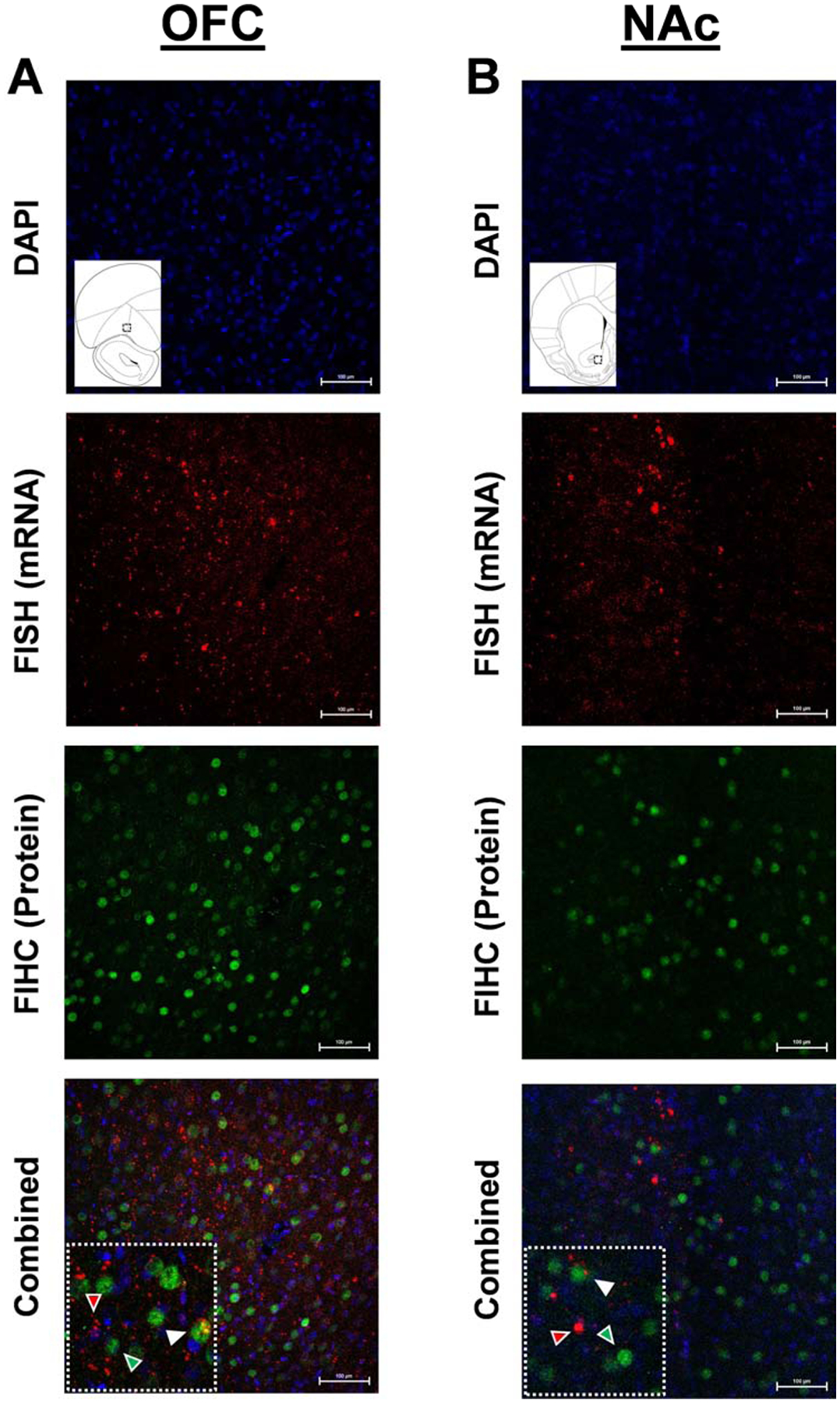
Representative FISH and FIHC cFos staining of the (A) OFC and (B) NAc, where FISH is staining is for food and FIHC staining is for cocaine. Black square within the inlet of the diagram of the region of interest represents the sample area. White arrows point to co-labeled FISH/FIHC/DAPI cells, red arrows point co-labeled with FISH/DAPI cells, and green arrows point to co-labeled FIHC/DAPI cells.
2.7. Analysis
2.7.1. Behavioral Analysis
Preference was expressed as percent choice for cocaine, calculated via the number of choice responses for cocaine divided by the total number of choice responses for cocaine and food. Importantly, this preference measure is comparable to more commonly-used drug preference measures (i.e., number of drug reinforcers earned over total reinforcers earned; Negus, 2003; Thomsen et al., 2013). For example, many previous preclinical studies utilizing controlled reinforcer frequency choice procedures have demonstrated that the preference measure is sensitive to the same factors that influence commonly-used preference measures (e.g., Llewellyn et al., 1976; Meisch and Gomez, 2013; Pope et al., 2015; Yates et al., 2019). Additionally, when preference was calculated using just the very-first response emitted on each trial (i.e., number of first reponses for cocaine over total number of trials) to mirror a binary scale (i.e., drug vs. nondrug) commonly-used in drug choice procedures (e.g., Negus, 2003; Thomsen et al., 2013), both preference measures (all choice responses vs. just first response) were practically identical (see supplemental Figure 3, r = 0.99; Beckmann et al. 2019). Finally, exploration into trial-by-trial response patterns both herein and in our previous publication (Beckmann et al., 2019) did not reveal any systematic pattern of responding suggestive of learning the random sequencing of scheduled reinforcers, supporting the unpredictability of the schedule. Altogether, the above indicate that preference as measured within the controlled reinforcer frequency choice procedures (i.e., responding on the unscheduled reinforcer) is reflective of reinforcer preference as it is in commonly-used binary measures of preference within procedures that do not control for reinforcer frequency.
Since cocaine and food are qualitatively different reinforcers, the following form of generalized matching (Beckmann et al., 2019) was applied:
| (1) |
where Bc represents behavior for cocaine, Bf represents behavior for food, and Mc represents the magnitude (i.e., dose) of cocaine. The free parameter sM represents the sensitivity to relative magnitude differences for cocaine:food reinforcement, while a represents the cocaine-food exchange rate, which can be conceptualized as a scaling constant such that a single 45-mg food pellet is scaled in unit dose of cocaine. Thus, Equation 1 was applied to experiments when the relative rate of reinforcement of cocaine and food was constant (3:3). For Experiment 1, best-fit model parameters (a and sM) were determined via nonlinear mixed-effects modeling (NLME; Pinheiro et al., 2006), with dose (continuous) as a within-subject factor, and subject as a random factor.
In Experiment 2 cocaine frequency and magnitude were systematically manipulated. Given that magnitude and frequency are independent dimensions of reinforcement (Davison and McCarthy, 1988) and varied across options in Experiment 2, the following form (Beckmann et al., 2019) of generalized matching was applied:
| (2) |
where Bc represents behavior for cocaine, Bf represents behavior for food, and Mc represents the magnitude (i.e., dose) of cocaine, Mf represents the magnitude of food, Rc represents the frequency of cocaine reinforcement, and Rf represents the frequency of food reinforcement. The free parameter sM represents the sensitivity to relative magnitude differences across cocaine-food alternatives, while sR represents the sensitivity to relative frequency differences across cocaine-food alternatives. For Experiment 2, a was set as 0.32, taken from baseline fits when the relative cocaine:food reinforcer frequency ratio was constant (3:3) using Equation 1, and best-fit model parameters for sM and sR were determined via NLME with dose (continuous) and frequency (continuous) as within-subject factors, and subject as a random factor.
2.7.2. Cellular Analysis
FISH/FIHC images from sample areas (3 sections) for each subject were obtained using a C2+ laser scanning confocal microscope (Nikon Instruments Inc, Melville, NY). Images were taken at 20× objective and in a single XY plane (1.2 mm × 1.2 mm; see Figure 1) with Z plane of 10 μm (z-stacks at 2 μm). OFC images were identified by the curvature of ventral and lateral subregions while NAc images were identified by the anterior commissure. Images were coded and counted in a blind fashion. Positive protein signals were identified as solid round- to oval-shaped with a diameter of 6 to 10 μm; positive mRNA signals were identified as round- to oval-shaped clusters (FISHji; Fontenete et al., 2016) forming an approximate diameter of 4 to 10 μm. Cells were automatically counted in ImageJ and verified manually (see supplement files for details). Importantly, cFos+ cells were identified by the co-labelling of DAPI; specifically, protein was identified by DAPI staining overlap and mRNA was identified by either DAPI staining overlap or by clustered staining surrounding a DAPI stain within 1 μm. Fluorescent signals from mRNA that did not form clusters of 4 to 10 um or were not overlapping or adjacent to the nucleus were not counted. Cell counts across each section were averaged for each subject.
Since cocaine and food activation occurred at different times that subsequently led to cFos expression for cocaine or food as either protein or mRNA (dependent on order of activation), and protein and mRNA expression for cFos are different forms of the same neuronal marker, cell counts (see supplemental Figure 2 for overall counts) were expressed as percent cocaine cFos+ cells. To be explicit, percent cocaine cFos+ cells were calculated as either the following:
| (3) |
or
| (4) |
Percent cocaine cFos+ cells for cocaine were analyzed using linear mixed-effects (LME) with brain regions (nominal) as a fixed factor and subject as a random factor for Experiment 1. Percent cocaine cFos+ cells for cocaine were also analyzed with LME with brain region (nominal) and cocaine:food ratio (continuous) as fixed factors and subject as a random factor for Experiment 2.
Correlations between parameter values from the matching equations and percent cocaine cFos+ cells were calculated using Pearson’s r. Specifically for Experiment 1, the cocaine-food exchange rate (a from Equation 1) was correlated with percent cocaine cFos+ cells and the sensitivity to relative magnitude differences was correlated with the number of overlapped cells. If neurobiological outcome measures for cocaine are reflective of cocaine preference, then a negative correlation between percent cFos+ cells and the cocaine-food exchange rate should be observed (e.g., high percent cocaine cFos+ cells are associated low values of cocaine-food exchange rate, a). Additionally, since cocaine and food reinforcers share some common neurobiological mechanisms (Koob and Volkow, 2010; Robbins and Everitt, 1996), and activity in overlapping cells could be indicative of some common encoding process(es) for given reinforcers (Kiani et al., 2014; Schultz, 2015), the number of cFos cells expressing both mRNA and protein was examined. Specifically, it was hypothesized that sensitivity to the effects of relative differences in cocaine and food reinforcement magnitude (sM from Equation 1) on choice would correlate with overlapping cell counts.
3. Results
Figure 2A illustrates percent choice for cocaine. NLME analysis revealed that the best-fitting cocaine-food exchange rate (a) was 0.36 and sensitivity to relative magnitude (sM) was 1.97 (for individual fits see supplemental Figure 4). Furthermore, there was no correlation between estimated whole-body cocaine levels and a (supplemental Figure 5), demonstrating that when using the controlled reinforcer frequency choice procedure preference can be dissociated from intake (Beckmann et al. 2019). Figure 2B illustrates percent cocaine cFos+ cells in the OFC and NAc for cocaine and food. LME analysis revealed no significant differences in percent cocaine cFos+ cells between the OFC and NAc. Further analysis revealed no correlations between individual a and individual percent cocaine cFos+ cells in the OFC (Figure 2C; Pearson’s r = 0.08, NS; p=0.8315) or NAc (Figure 2D; Pearson’s r = 0.24, NS; p=0.5110). Additionally, analysis revealed no correlations between sM and overlapped cells (supplemental Figure 6). In all, these data suggest that when the relative rate of reinforcement is kept constant, the relative percentage of cells activated by cocaine is not correlated with preference.
Figure 2.
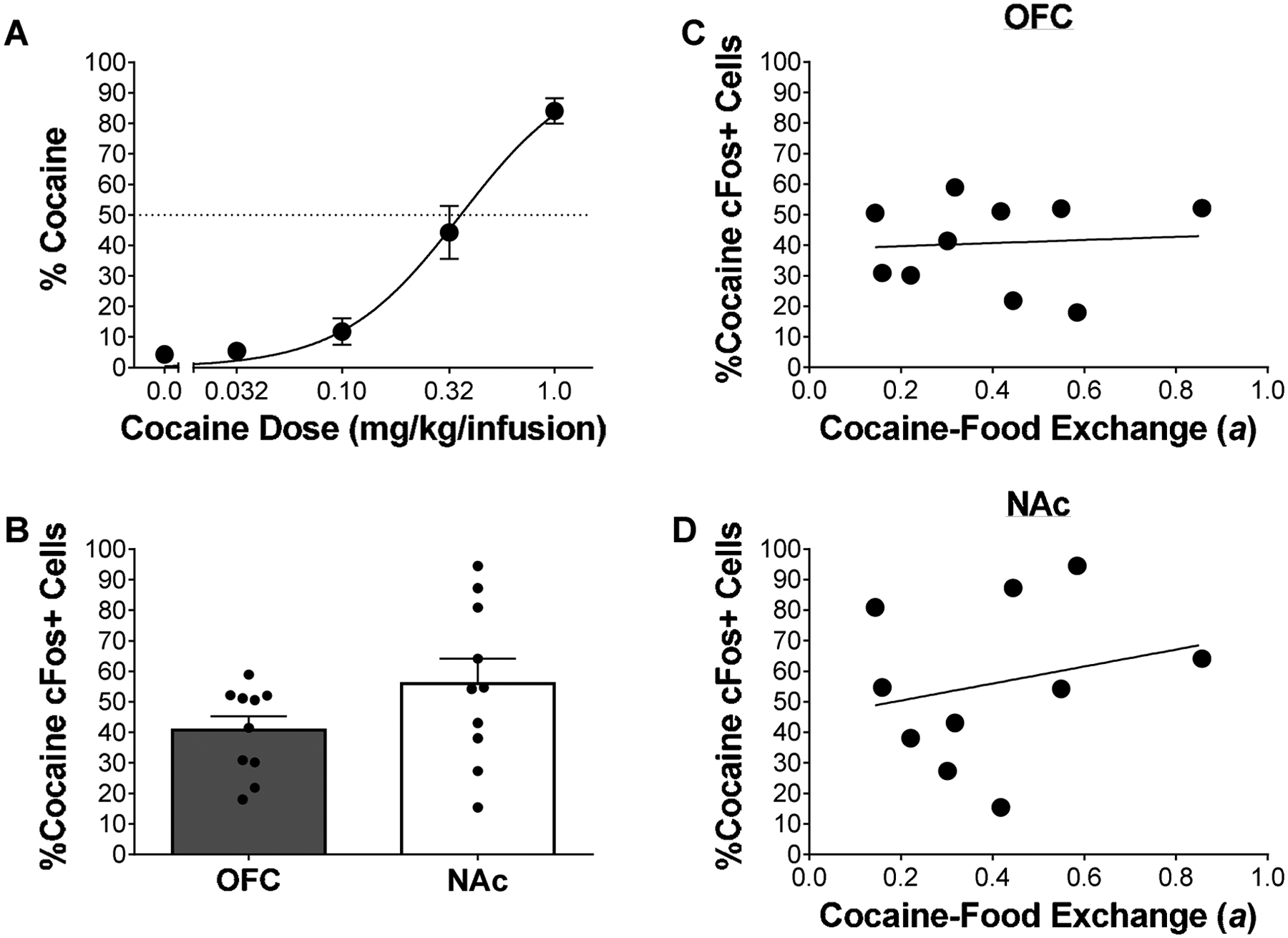
Cocaine versus food choice under the controlled reinforcer frequency choice procedure and neurobiological correlates (n=10). (A) Mean (±SEM) percent choice for cocaine. Line is best fit from NLME analysis. (B) Mean (±SEM) percent cocaine cFos+ cells, calculated via cocaine cFos+ cells divided by cocaine and food cFos+ cells. Correlations between individual cocaine-food exchange (a) rates and percent cocaine cFos+ cells in the (C) OFC and (D) NAc.
Figure 3A illustrates percent choice for cocaine prior to frequency manipulation and percent choice for cocaine under the different frequency ratio manipulations. NLME analysis revealed there were no significant differences between groups at baseline (supplemental Figure 7); also, analysis revealed that the cocaine-exchange rate (a) was 0.32 at baseline. To determine the effects of frequency manipulations on cocaine preference, NLME analysis revealed a significant effect of sensitivity to relative magnitude (sM = 2.11) [F(1,67)=142.20, p<0.0001] and a significant effect of sensitivity to relative frequency (sR = 1.32) [F(1,67)=26.83, p<0.0001], altogether indicating that the relative difference in magnitude for cocaine and food reinforcement, and frequency of reinforcement independently affected cocaine choice. Figure 3B illustrates averaged percent cocaine cFos+ cells in the OFC and NAc for cocaine and food following ratio manipulation. LME analysis revealed a main effect of cocaine:food ratio [F(1,15)=5.08, p=0.0396] in the OFC, indicating that the percent cocaine cFos+ cells in the cocaine-replete group was greater than the cocaine-deplete group. LME analysis revealed no significant differences in percent cocaine cFos+ cells in the NAc. Altogether, these results indicate that magnitude and frequency of reinforcement independently determine cocaine preference. Importantly, these results also demonstrate that greater overall intake of cocaine, via increased rate of reinforcement, increases the relative number of cocaine cells activated by cocaine.
Figure 3.
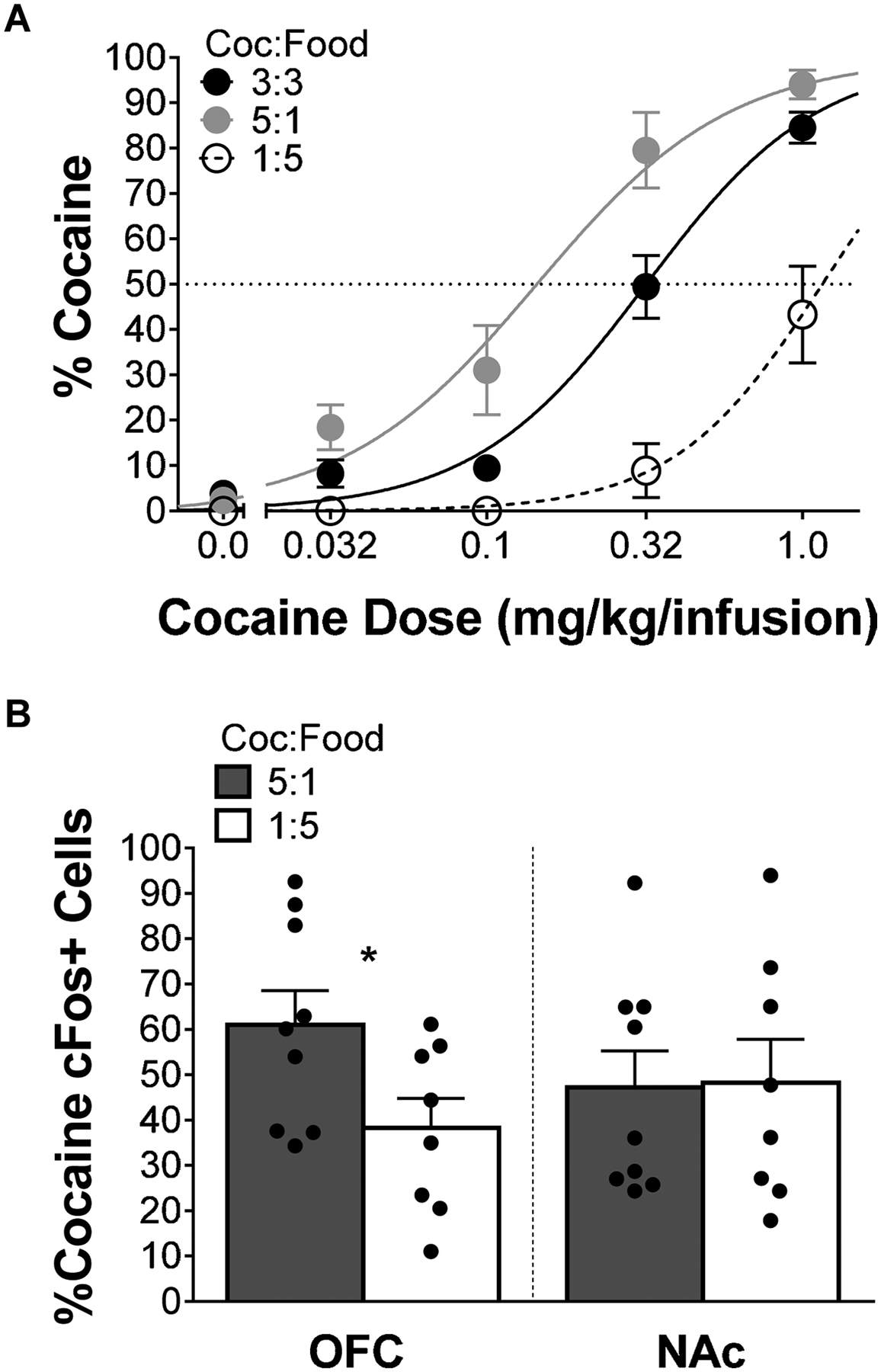
(A) Mean (±SEM) percent cocaine choice under relative reinforcer frequency manipulations. Lines are best fits from NLME analysis; 3:3 data is reflective of baseline preference for both frequency conditions. (B) Mean (±SEM) percent cocaine cFos+ cells OFC and NAc for both frequency manipulations. * indicates p<0.05. Note: 5:1 reflects cocaine-replete (n=9) and 1:5 reflects cocaine-deplete (n=8) frequency conditions.
4. Discussion
The experiments herein utilized a controlled reinforcer frequency choice procedure to explore the neurobehavioral mechanisms associated with cocaine preference. Consistent with previous reports (Beckmann et al., 2019), we obtained cocaine preference that was independent of cocaine intake (supplemental Figure 5), and cocaine preference was systematically determined by the relative differences in reinforcer dimensions across alternatives (Beckmann et al., 2019; Thomsen et al., 2013). More important to the present purpose, use of the controlled reinforcer frequency choice procedure removed individual differences in overall cocaine exposure and intake, controlling for its known effects on neuroadaptations (Freeman et al., 2002; Gao et al., 2017; Mantsch et al., 2004). When the cocaine-food exchange rate (a) was correlated with percent cocaine cFos+ cells, results revealed no correlation in either the OFC or the NAc. Importantly, these findings were found under conditions where the relative intake of cocaine was kept constant across all subjects. Subsequently, to determine if cocaine intake in choice procedures can affect neurobiological measures, the relative ratio of cocaine to food trials was systematically manipulated. Results from the frequency manipulation demonstrated that greater overall cocaine intake produced a greater number of cocaine-induced cFos+ cells in the OFC, but not the NAc, suggesting that cocaine exposure itself affects the relative expression of cFos+ cells for cocaine in the OFC, independent of preference. Altogether, these results are reflective of the known principle that drug intake itself directly affects neuroadaptations (Hyman et al., 2006; Kalivas and O’Brien, 2008; Nestler, 2001).
Also consistent with previous research, when cFos+ cells were labeled and counted, a pattern of independent populations of cells activated by cocaine and food was observed (Carelli, 2002; Carelli et al., 2000; Padoa-Schioppa, 2013; Padoa-Schioppa and Assad, 2006; Xiu et al., 2014). Furthermore, similar to previous studies examining cFos expression following cocaine self-administration, results (see supplemental files) herein demonstrated that there were generally more cFos+ cells in the OFC than the NAc (Thiel et al., 2010) in both Experiments 1 and 2. Given that there were differences in cFos+ counts in the brain regions examined herein, it possible that these brain regions (i.e., OFC vs. NAc) may have different timelines for cFos induction. However, given that the activation and degradation of cFos have similar patterns in different brain regions (Hope et al., 1992; Morgan et al., 1987; Xue et al., 2017), it is unlikely that the differences in counts are due to time course used; previous studies examining different brain regions have shown no differences in cFos expression for a given time course (e.g., Cifani et al., 2012). Despite the different levels of cFos expression in the OFC and NAc, the neurobiological measure herein focused on the relative difference in cocaine vs. food activated cells within a region. Additionally, when we examined the relationship between the number of cells expressing cFos as both protein and mRNA (i.e., cells activated by both cocaine and food) and individual sensitivities to the relative differences in cocaine and food magnitude, we found no correlation in either the OFC or NAc (supplement Figure 6); this result suggests that the process of mapping the effects of magnitude differences between available reinforcers on preference between them is independent of the degree of common neuronal activity induced by each available reinforcer.
In choice procedures that do not control for reinforcer frequency, choosing a specific option repeatedly results in a situation where the relative reward experienced (and consequent relative reward intake) across options becomes disproportionate (Johnstone and Alsop, 1999; McCarthy and Davison, 1984). This disproportionality itself may potentially affect neurobiological measures. For example, in a previous study where cocaine preference was examined under a choice schedule that did not control for reinforcer frequency, electrophysiological recordings suggested that cocaine preference was mediated by the activation of a large population of neurons in the OFC that specifically responded to cocaine (Guillem and Ahmed, 2018); furthermore, a significant correlation between cocaine preference and the number of cocaine encoding neurons was observed. While informative, these results are subject to the possibility that differences in relative reinforcement experience and consequent intake across subjects may underlie the reported correlation. Specifically, if one considers the covariation between the measure for drug preference (e.g., number of drug reinforcers earned over total reinforcers earned) and the measure for relative reinforcer intake (e.g., number of drug reinforcers earned over total reinforcers earned), the measures are identical and consequently interchangeable. Thus, the relationships between cocaine preference and neuronal activity, cocaine reinforcer frequency and neuronal activity, and cocaine intake and neuronal activity will all produce identical correlations. Therefore, the possible mediational role of any neurobiological measure in reinforcer preference cannot be dissociated from the effects of reinforcer frequency and consequent intake under such conditions. Furthermore, given that most studies investigating the neurobiological mechanisms of decision-making using nondrug reinforcers (e.g., Padoa-Schioppa and Assad, 2006; Simon et al., 2011; Stopper et al., 2014; Sugam et al., 2012; Zeeb et al., 2009) rely on choice procedures that do not control for reinforcer frequency, the results of such studies are also, at least in principal, subject to the confound highlighted above. Relatedly, research into probabilistic nondrug outcomes has demonstrated that neuronal signaling is strongly related to the likelihood of reinforcement (Joshua et al., 2009; Morris et al., 2006, 2004). Importantly, likelihood of reinforcement determines experienced reinforcer frequency and consequent reinforcer intake. As well, more likely/frequent reinforcement can lead to increased neuronal activity in response to the reinforcer and the cues that predict it (Joshua et al., 2009). Thus, increased experienced reinforcer frequency itself can result in increased neuronal activity in response to nondrug reinforcers, independent of preference.
Some potential drawbacks to the current study should be noted. For example, while the results herein demonstrate the effect that cocaine intake has on the neuroadaptations seen in the OFC during cocaine choice, these experiments were conducted in male rats only. Interestingly, previous research into cocaine versus food choice has demonstrated no sex differences in cocaine choice (Thomsen et al., 2013); however, use of a controlled reinforcer frequency drug choice procedure has yet to be conducted in female rats. In short, future research examining sex differences under a controlled reinforcer frequency schedule is warranted. Another consideration is that most studies investigating the neurobiological basis of decision-making processes have been completed using in vivo recording techniques and have identified potentially different choice-associated signals, such as option presentation, choice selection, and valuation of reinforcers (e.g., Padoa-Schioppa, 2013; Padoa-Schioppa and Assad, 2006). The activation and immunohistochemical methods used herein potentially capture all the aforementioned signals (and possibly more), making it difficult to differentiate which cFos+ cells are involved in a given potential process and to what degree. However, despite vast differences in methodological techniques, both cellular imaging studies examining cFos, a marker highly associated with neuronal activity (Cruz et al., 2015; Dragunow and Faull, 1989), and in vivo electrophysiological studies, which records neuronal activity, have identified similar brain regions involved in substance use disorder (see Cruz et al., 2015). Although this study used FISH and FIHC methods (Xiu et al., 2014; Zhang et al., 2018) to visualize the neural representations of distinct reinforcer preferences, the use of different techniques such as RNAscope combined with IHC or transgenic lines (Bobadilla et al., 2020; Cruz et al., 2015) may provide improved identification of the neural ensembles that underlie preference for a given reinforcer. Future work utilizing RNAscope will be especially useful since it is specifically designed to reduce background fluorescence that is commonly present in traditional FISH assays (Wang et al., 2012). In the current study, there was a modest amount of background RNA signal which is likely due to the specific procedure chosen (FISH vs RNAscope). However, it is possible that RNA antisense probes were non-specific. To err on the side of caution, only signals that clustered in close proximity to the nucleus were counted as Fos+ to minimize the risk of probe non-specificity. Additionally, future studies can utilize Fos-lacZ rats (Koya et al., 2009) to provide mechanistic insight into the role that these neurons have in the decision-making process. Lastly, it should be noted that the lack of a correlation in Experiment 1 could be due to a number of possible alternatives, and if the hypotheses tested herein were only predicated on the presence/absence of a correlation, this particular issue would be compounded. However, the results from Experiment 2 are consistent with and corroborate the lack of correlation in Experiment 1, and the two experiments collectively suggest that differential cocaine intake is largely responsible for changes in cFos activity. That said, future research utilizing additional neural methods should be coupled with choice procedures that control for differential reinforcer intake to replicate the current results and work toward a better understanding of the neurobehavioral mechanisms that govern drug preference.
5. Conclusion
Overall, the primary advantage of using a controlled reinforcer frequency schedule is its ability to eliminate any positive feedback loop between reinforcer preference, reinforcer experience, and consequent intake, helping to control for any effects of differential experienced reinforcer frequency/intake on neurobiological measures associated with reinforcer preference, as discussed above. While a single study is far from definitive, the present results suggest that differential cocaine reinforcer experience can itself produce systematic changes in neurobiological measures associated with preference. Given that the overwhelming majority of choice studies to date (both drug and nondrug) confound experienced reinforcer frequency/intake with reinforcer preference, future research into the neurobiological mechanisms that govern choice should systematically investigate the role of this factor in reinforcer preference to, at least, eliminate the confound as a possible important contributor to neurobiological results in choice studies.
Supplementary Material
Highlights.
Cocaine preference and intake were dissociable during choice.
Reinforcer frequency and magnitude independently determined cocaine preference.
Differential cocaine intake, not preference, influenced neurobiological outcome.
Acknowledgements
We would like to thank Josh N Lavy and Beckmann lab members for their technical support. Cocaine used in these experiments was generously gifted by the NIDA Drug Supply Program.
Funding and Disclosure
This work was supported by the National Institute on Drug Abuse grant DA016176 to JJC, DA033373 and DA045023 to JSB, and a NARSAD Young Investigator Award to RSH. All authors have no conflicts of interests to report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Alsop B, Furukawa E, Sowerby P, Jensen S, Moffat C, Tripp G, 2016. Behavioral sensitivity to changing reinforcement contingencies in attention-deficit hyperactivity disorder. J. Child Psychol. Psychiatry 57, 947–956. [DOI] [PubMed] [Google Scholar]
- Anderson KG, Velkey AJ, Woolverton WL, 2002. The generalized matching law as a predictor of choice between cocaine and food in rhesus monkeys. Psychopharmacology (Berl) 163, 319–326. [DOI] [PubMed] [Google Scholar]
- Banks ML, Negus SS, 2012. Preclinical determinants of drug choice under concurrent schedules of drug self-administration. Adv. Pharmacol. Sci 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann JS, Chow JJJ, Hutsell BA, 2019. Cocaine-associated decision-making: Toward isolating preference. Neuropharmacology 153, 142–152. 10.1016/j.neuropharm.2019.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobadilla A-C, Dereschewitz E, Vaccaro L, Heinsbroek JA, Scofield MD, Kalivas PW, 2020. Cocaine and sucrose rewards recruit different seeking ensembles in the nucleus accumbens core. Mol. Psychiatry 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli RM, 2002. Nucleus accumbens cell firing during goal-directed behaviors for cocaine vs. ‘natural’reinforcement. Physiol. Behav 76, 379–387. [DOI] [PubMed] [Google Scholar]
- Carelli RM, Ijames SG, Crumling AJ, 2000. Evidence that separate neural circuits in the nucleus accumbens encode cocaine versus “natural”(water and food) reward. J. Neurosci 20, 4255–4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cifani C, Koya E, Navarre BM, Calu DJ, Baumann MH, Marchant NJ, Liu Q-R, Khuc T, Pickel J, Lupica CR, 2012. Medial prefrontal cortex neuronal activation and synaptic alterations after stress-induced reinstatement of palatable food seeking: a study using c-fos-GFP transgenic female rats. J. Neurosci 32, 8480–8490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz FC, Koya E, Guez-Barber DH, Bossert JM, Lupica CR, Shaham Y, Hope BT, 2013. New technologies for examining the role of neuronal ensembles in drug addiction and fear. Nat. Rev. Neurosci 14, 743–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz FC, Rubio FJ, Hope BT, 2015. Using c-fos to study neuronal ensembles in corticostriatal circuitry of addiction. Brain Res 1628, 157–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison M, McCarthy D, 1988. The matching law: A research view
- Dragunow M, Faull R, 1989. The use of c-fos as a metabolic marker in neuronal pathway tracing. J. Neurosci. Methods 29, 261–265. [DOI] [PubMed] [Google Scholar]
- Fontenete S, Carvalho D, Lourenço A, Guimarães N, Madureira P, Figueiredo C, Azevedo NF, 2016. FISHji: New ImageJ macros for the quantification of fluorescence in epifluorescence images. Biochem. Eng. J 112, 61–69. [Google Scholar]
- Freeman WM, Brebner K, Lynch WJ, Patel KM, Robertson DJ, Roberts DCS, Vrana KE, 2002. Changes in rat frontal cortex gene expression following chronic cocaine. Mol. brain Res 104, 11–20. [DOI] [PubMed] [Google Scholar]
- Gallagher M, McMahan RW, Schoenbaum G, 1999. Orbitofrontal cortex and representation of incentive value in associative learning. J. Neurosci 19, 6610–6614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao P, Limpens JHW, Spijker S, Vanderschuren LJMJ, Voorn P, 2017. Stable immediate early gene expression patterns in medial prefrontal cortex and striatum after long-term cocaine self-administration. Addict. Biol 22, 354–368. [DOI] [PubMed] [Google Scholar]
- Guillem K, Ahmed SH, 2018. Preference for cocaine is represented in the orbitofrontal cortex by an increased proportion of cocaine use-coding neurons. Cereb. Cortex 28, 819–832. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Setlow B, Wagner EK, McGaugh JL, 2001. Experience-dependent gene expression in the rat hippocampus after spatial learning: a comparison of the immediate-early genesArc, c-fos, and zif268. J. Neurosci 21, 5089–5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q, Wang J, Hu H, 2019. Illuminating the activated brain: Emerging activity-dependent tools to capture and control functional neural circuits. Neurosci. Bull 35, 369–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera DG, Robertson HA, 1996. Activation of c-fos in the brain. Prog. Neurobiol 50, 83–107. [DOI] [PubMed] [Google Scholar]
- Hope B, Kosofsky B, Hyman SE, Nestler EJ, 1992. Regulation of immediate early gene expression and AP-1 binding in the rat nucleus accumbens by chronic cocaine. Proc. Natl. Acad. Sci 89, 5764–5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutsell BA, Negus SS, Banks ML, 2015. A generalized matching law analysis of cocaine vs. food choice in rhesus monkeys: Effects of candidate ‘agonist-based’medications on sensitivity to reinforcement. Drug Alcohol Depend 146, 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ, 2006. NEURAL MECHANISMS OF ADDICTION: The Role of Reward-Related Learning and Memory. Annu. Rev. Neurosci 29, 565–598. 10.1146/annurev.neuro.29.051605.113009 [DOI] [PubMed] [Google Scholar]
- Johnstone V, Alsop B, 1999. Stimulus presentation ratios and the outcomes for correct responses in signal-detection procedures. J. Exp. Anal. Behav 72, 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshua M, Adler A, Rosin B, Vaadia E, Bergman H, 2009. Encoding of probabilistic rewarding and aversive events by pallidal and nigral neurons. J. Neurophysiol 101, 758–772. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, O’Brien C, 2008. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology 33, 166–180. [DOI] [PubMed] [Google Scholar]
- Kiani R, Cueva CJ, Reppas JB, Newsome WT, 2014. Dynamics of neural population responses in prefrontal cortex indicate changes of mind on single trials. Curr. Biol 24, 1542–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND, 2010. Neurocircuitry of addiction. Neuropsychopharmacology 35, 217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koya E, Golden SA, Harvey BK, Guez-Barber DH, Berkow A, Simmons DE, Bossert JM, Nair SG, Uejima JL, Marin MT, 2009. Targeted disruption of cocaine-activated nucleus accumbens neurons prevents context-specific sensitization. Nat. Neurosci 12, 1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krägeloh CU, Davison M, 2003. Concurrent-schedule performance in transition: Changeover delays and signaled reinforcer ratios. J. Exp. Anal. Behav 79, 87–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufahl PR, Zavala AR, Singh A, Thiel KJ, Dickey ED, Joyce JN, Neisewander JL, 2009. c-Fos expression associated with reinstatement of cocaine-seeking behavior by response-contingent conditioned cues. Synapse 63, 823–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewellyn ME, Iglauer C, Woods JH, 1976. Relative Reinforcer Magnitude Under A Nonindependent Concurrent Schedule Of Cocaine Reinforcement In Rhesus Monkeys 1. J. Exp. Anal. Behav 25, 81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Yuferov V, Mathieu-Kia A-M, Ho A, Kreek MJ, 2004. Effects of extended access to high versus low cocaine doses on self-administration, cocaine-induced reinstatement and brain mRNA levels in rats. Psychopharmacology (Berl) 175, 26–36. [DOI] [PubMed] [Google Scholar]
- McCarthy D, Davison M, 1984. Isobias and alloiobias functions in animal psychophysics. J. Exp. Psychol. Anim. Behav. Process 10, 390. [PubMed] [Google Scholar]
- McClung CA, Ulery PG, Perrotti LI, Zachariou V, Berton O, Nestler EJ, 2004. ΔFosB: a molecular switch for long-term adaptation in the brain. Mol. Brain Res 132, 146–154. [DOI] [PubMed] [Google Scholar]
- Meisch RA, Gomez TH, 2013. Drug self-administration studies: a novel reinforcement schedule enhances choice. Behav. Pharmacol 24, 155–163. [DOI] [PubMed] [Google Scholar]
- Moratalla R, Vickers EA, Robertson HA, Cochran BH, Graybiel AM, 1993. Coordinate expression of c-fos and jun B is induced in the rat striatum by cocaine. J. Neurosci 13, 423–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JI, Cohen DR, Hempstead JL, Curran TOM, 1987. Mapping patterns of c-fos expression in the central nervous system after seizure. Science (80-. ) 237, 192–197. [DOI] [PubMed] [Google Scholar]
- Morgan JI, Curran T, 1991. Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annu. Rev. Neurosci 14, 421–451. [DOI] [PubMed] [Google Scholar]
- Morris G, Arkadir D, Nevet A, Vaadia E, Bergman H, 2004. Coincident but distinct messages of midbrain dopamine and striatal tonically active neurons. Neuron 43, 133–143. [DOI] [PubMed] [Google Scholar]
- Morris G, Nevet A, Arkadir D, Vaadia E, Bergman H, 2006. Midbrain dopamine neurons encode decisions for future action. Nat. Neurosci 9, 1057–1063. [DOI] [PubMed] [Google Scholar]
- Nader MA, Woolverton WL, 1990. Cocaine vs. food choice in rhesus monkeys: effects of increasing the response cost for cocaine, in: Problems of Drug Dependence 1990 Proceeding of the 52nd Annual Scientific Meeting p. 621. [PubMed] [Google Scholar]
- Negus SS, 2003. Rapid assessment of choice between cocaine and food in rhesus monkeys: effects of environmental manipulations and treatment with d-amphetamine and flupenthixol. Neuropsychopharmacology 28, 919–931. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, 2001. Molecular basis of long-term plasticity underlying addiction. Nat. Rev. Neurosci 2, 119–128. [DOI] [PubMed] [Google Scholar]
- Padoa-Schioppa C, 2013. Neuronal origins of choice variability in economic decisions. Neuron 80, 1322–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padoa-Schioppa C, Assad JA, 2006. Neurons in the orbitofrontal cortex encode economic value. Nature 441, 223–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D, 2006. nlme: linear and nonlinear mixed effects models, R package version 3, 1–76. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Pizzagalli DA, Jahn AL, O’Shea JP, 2005. Toward an objective characterization of an anhedonic phenotype: a signal-detection approach. Biol. Psychiatry 57, 319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope DA, Newland MC, Hutsell BA, 2015. Delay-specific stimuli and genotype interact to determine temporal discounting in a rapid-acquisition procedure. J. Exp. Anal. Behav 103, 450–471. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ, 1996. Neurobehavioural mechanisms of reward and motivation. Curr. Opin. Neurobiol 6, 228–236. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar A, Mingote SM, 2007. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology (Berl) 191, 461–482. [DOI] [PubMed] [Google Scholar]
- Schultz W, 2015. Neuronal reward and decision signals: from theories to data. Physiol. Rev 95, 853–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NW, Montgomery KS, Beas BS, Mitchell MR, LaSarge CL, Mendez IA, Bañuelos C, Vokes CM, Taylor AB, Haberman RP, 2011. Dopaminergic modulation of risky decision-making. J. Neurosci 31, 17460–17470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopper CM, Maric TL, Montes DR, Wiedman CR, Floresco SB, 2014. Overriding phasic dopamine signals redirects action selection during risk/reward decision making. Neuron 84, 177–189. [DOI] [PubMed] [Google Scholar]
- Stubbs DA, Pliskoff SS, 1969. CONCURRENT RESPONDING WITH FIXED RELATIVE RATE OF REINFORCEMENT 1. J. Exp. Anal. Behav 12, 887–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugam JA, Day JJ, Wightman RM, Carelli RM, 2012. Phasic nucleus accumbens dopamine encodes risk-based decision-making behavior. Biol. Psychiatry 71, 199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel KJ, Pentkowski NS, Peartree NA, Painter MR, Neisewander JL, 2010. Environmental living conditions introduced during forced abstinence alter cocaine-seeking behavior and Fos protein expression. Neuroscience 171, 1187–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Barrett AC, Negus SS, Caine SB, 2013. Cocaine versus food choice procedure in rats: environmental manipulations and effects of amphetamine. J. Exp. Anal. Behav 99, 211–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis JD, 2007. Orbitofrontal cortex and its contribution to decision-making. Annu. Rev. Neurosci 30, 31–56. [DOI] [PubMed] [Google Scholar]
- Wang F, Flanagan J, Su N, Wang L-C, Bui S, Nielson A, Wu X, Vo H-T, Ma X-J, Luo Y, 2012. RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J. Mol. diagnostics 14, 22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiu J, Zhang Q, Zhou Tao, Zhou Ting-ting, Chen Y, Hu H, 2014. Visualizing an emotional valence map in the limbic forebrain by TAI-FISH. Nat. Neurosci 17, 1552. [DOI] [PubMed] [Google Scholar]
- Xue Y-X, Chen Y-Y, Zhang L-B, Zhang L-Q, Huang G-D, Sun S-C, Deng J-H, Luo Y-X, Bao Y-P, Wu P, 2017. Selective inhibition of amygdala neuronal ensembles encoding nicotine-associated memories inhibits nicotine preference and relapse. Biol. Psychiatry 82, 781–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates JR, Prior NA, Chitwood MR, Day HA, Heidel JR, Hopkins SE, Muncie BT, Paradella-Bradley TA, Sestito AP, Vecchiola AN, 2019. Using a dependent schedule to measure risky choice in male rats: Effects of d-amphetamine, methylphenidate, and methamphetamine. Exp. Clin. Psychopharmacol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeb FD, Robbins TW, Winstanley CA, 2009. Serotonergic and dopaminergic modulation of gambling behavior as assessed using a novel rat gambling task. Neuropsychopharmacology 34, 2329–2343. [DOI] [PubMed] [Google Scholar]
- Zhang Q, He Q, Wang J, Fu C, Hu H, 2018. Use of TAI-FISH to visualize neural ensembles activated by multiple stimuli. Nat. Protoc 13, 118. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


