Abstract
The renin-angiotensin system (RAS) plays a critical role in the regulation of blood pressure. Inappropriate activation of the RAS, particularly stimulation of the ACE-Ang II-AT1 receptor axis is a key factor in hypertension and AT1R antagonists (ARBs) are first line therapies in the treatment of cardiovascular disease (CVD). Accumulating evidence suggests that the Ang II-AT1R axis may stimulate both innate and adaptive immune systems. Indeed, recent studies suggest that Ang II stimulates inflammatory events in an AT1R-independent manner by binding the MD2 accessory protein of the TLR4 complex in renal NRK-52E cells. Direct Ang II stimulation of the TLR4 complex is clinically relevant as ARBs increase circulating Ang II levels. Thus, the current study further investigated Ang II stimulation of the TLR4 pathway to release of the pro-inflammatory cytokine CCL2 under identical conditions to the TLR4 ligands LPS and palmitate in the NRK-52E cells. Although LPS (1 ng/mL) and palmitate (100 μM) stimulated CCL2 release 20-fold, Ang II (0.1–10 μM) failed to induce CCL2 release. Both the LPS and palmitate CCL2 responses were abolished by the TLR4 inhibitor Tak242 and significantly reduced by the MD2 inhibitor L48H37. Ang II (1 μM) had no additive effects on LPS (1 ng/mL) or palmitate (100 μM), and the ARB candesartan failed to attenuate CCL2 release to either agent alone. Ang II also failed to induce the release of the putative TLR4 ligand HMBG1. These studies failed to confirm that Ang II directly stimulates the MD2-TLR4 complex to induce cytokine release in NRK-52E cells.
1. INTRODUCTION
The renin-angiotensin system (RAS) is a classic circulating system that plays a critical role in the regulation of blood pressure and fluid homeostasis [3; 6; 12; 13; 46]. Inappropriate activation of the RAS, particularly the stimulation of the ACE-Ang II-AT1 receptor (AT1R) axis is likely a key factor in hypertension and the progression of other cardiovascular pathologies [3; 12; 13; 40]. Indeed, therapeutic approaches to block the RAS by AT1R antagonists (ARBs) and ACE inhibitors are well accepted therapeutic regimens in the treatment of cardiovascular disease (CVD) including hypertension, heart failure and diabetic nephropathy [12]. The functional tone of the RAS is mediated by the generation of the octapeptide Ang II which serves as the principal ligand for the AT1R, although alternative or non-classical pathways including the AT2R and the ACE2-Ang-(1–7)-MasR may play an antagonistic role [6; 12; 19]. Activation of the ACE-Ang II-AT1R axis leads to a complex array of cellular signaling pathways in various tissues that may, in part, encompass an increase in sympathetic tone, attenuation of the baroreflex pathway, stimulation of oxidative stress, reduced nitric oxide, release of aldosterone, vasopressin and endothelin, elevated intracellular calcium, as well as the stimulation of various sodium transporters within the kidney that may all contribute to a sustained increase in blood pressure and end organ damage [6; 12; 13; 46].
Mounting evidence suggests that the deleterious effects of the ACE-Ang II-AT1R axis on CVD may also reflect the stimulation of both innate and adaptive immune systems [3; 10; 14; 16; 17; 22; 28; 31; 32; 40; 43; 49; 50]. Blockade or knockdown of the toll-like 4 receptor (TLR4), an integral sensing and signaling component of the innate system, attenuates Ang II-dependent hypertension, as well as both cardiac and renal injury [9; 10; 14; 16; 43; 49]. In regards to the innate immune system, Nair et al [30] proposed that Ang II stimulates the AT1R to release the high-mobility group protein 1 (HMBG1), a ligand for the TLR4 to evoke inflammatory events [20; 37]. Knockdown of HMBG1 or a HMBG1 antibody attenuated the Ang II-AT1R stimulation of pro-inflammatory cytokines in the rat proximal tubule NRK-52E cell line [30]. In contrast, Liang and colleagues [14; 49] proposed that Ang II stimulates the innate system by directly activating TLR4 through an AT1R-independent mechanism. These investigators demonstrated that Ang II binds the accessory protein myeloid differentiator protein 2 (MD2) to induce association of the adaptor protein MyD88 with TLR4 and stimulate cytokine release in cardiac H9c2 and renal NRK-52E cells [14; 49]. The possibility for direct stimulation of the MD2-TLR4 complex by Ang II is clinically important as ARBs increase Ang II levels due to the disinhibition of renin release [6; 12; 46]. Thus, ARB treatment may have the unattended consequence of stimulating a TLR4-dependent inflammatory pathway that reflects elevated Ang II. Indeed, this mechanism could potentially underlie the less than optimal effects of ARBs in the treatment of CVD as proposed by Ferrario and Mullick [12]. Given this critically important interaction of Ang II with MD2-TLR4, the current study further investigated the ability of Ang II to induce the TLR4-dependent stimulation of the pro-inflammatory cytokine CCL2 (MCP-1) in the renal NRK-52E cells, particularly in comparison to the established TLR4 ligands lipopolysaccharide (LPS) and the saturated fatty acid palmitate [21; 33; 34; 35; 39; 42; 44; 47; 49]. In addition, we investigated the ability of Ang II to induce the release of the TLR4 ligand HMBG1 and augment TLR4 expression as an alternative mechanism for Ang II stimulation of the innate system in the renal cells
2. METHODS
2.1. Cell Culture
Normal kidney proximal tubule cells (NRK-52E) were obtained at passage 15 from American Tissue Type Culture (ATTC, Arlington VA). Cells were maintained at 37°C in plastic 75cm2 flasks in Dulbecco’s modified Eagle’s medium (DMEM) containing 5% calf serum (CS), 4.5 gm/L glucose, 2 mM L-Glutamine and 1.5 gm/L bicarbonate in the absence of the antibiotics penicillin and streptomycin as recommended by the ATTC [5]. Cell were passed into 24-well plates or 100 mm dishes. After 48 hours (h), the cell media was replaced with serum-free DMEM for 48 h prior to treatment; the serum free media was replaced daily. NRK-52E cells of passage 18 to 30 were used in this study.
2.2. Cell Treatments
NRK-52E cells, incubated in serum-free media for 48 h, were replenished with fresh media and treated with LPS (1 ng/mL, E. Coli 0111:B4, Sigma, St. Louis, MI, USA), palmitate (100 μM) or Ang II (0.1, 1.0 and 10.0 μM, Bachem Torrance CA USA) for 24 h. Cells were pre-treated with Tak 242 (10 μM, Cayman Chemical Ann Arbor, MI USA), L48H37 (100 μM, Sigma), or candesartan (10 μM, Selleckchem Houston, TX, USA) prior to the addition of LPS or palmitate. All treatments were expressed as the final concentration of each agent in the cell media. These agents were stored in stock solutions at −80°C and on the day of the experiment, a new vial was warmed to 37°C and diluted in warm DMEM media prior to addition to the cells.
Preparation of the saturated palmitate was based on the procedure of Spector [45]. The use of plastic ware was avoided whenever possible in the preparation of the palmitate-albumin complex. Fatty acid free bovine serum albumin (BSA, 2.835 g, Sigma #A6003) was dissolved in 50 mL of 150 mM NaCl in Millipore Milli-Q water at 37°C. To the BSA solution, 40 mL of palmitate (Sigma. #P9767) initially dissolved in 150 mM NaCl at 70°C was added in 5 mL aliquots and continuously stirred for 60 mins at 37°C until clear. The final volume was adjusted to 100 mL of 150mM NaCl and the pH adjusted to 7.4. The solution was passed through a 0.2 μmicron filter and stored in sterile glass vials at −20°C.
2.3. Cytokine and HMBG1 Assays
Media was collected from the NRK-52E cells and stored at −20°C until assessed for cytokine and HMBG1 content. Cytokines (CCL2, IL-6, TNFα) were quantified in the cell media from 24-well plates using a SimpleStep rat CCL2, IL-6 and TNFα ELISAs (Abcam, Cambridge MA USA). The cell media samples were thawed on ice, diluted in the appropriate ELISA assay buffer and directly added to the 96-well plate. The stated sensitivities of the ELISAs were CCL2 (5.7 pg/mL), IL-6 ( ) and TNFα. HMBG1 was also quantified in cell media using a rat HMBG1 ELISA kit (MB765190, MyBiosource, San Diego CA USA). Samples were also thawed on ice, diluted in the HMBG1 assay buffer and then added to the 96-well plate. The stated sensitivity of the HMGB1 ELISA was 18.0 pg/mL Sample dilutions for both assays were adjusted so as not to exceed the upper limits of the standard curves to assure the accurate measurement of both proteins under basal, inhibitory and stimulatory conditions.
2.4. Cell Fractionation
Nuclear and cytosolic fractions from the NRK-52E cells were prepared by a modification of the method described by Nabbi and Riabowol [29] in which the non-ionic detergent Triton X-100 was substituted for NP-40. Cells in 100 mm dishes were washed in ice cold PBS, scraped into 2 mL microcentrifuge tubes with cold PBS and pelleted at 5,000 g for 3 min at 4°C. For the isolation of nuclei, the buffer was removed and the cell pellets homogenized in 1 ml cold PBS/1% Triton X-100 with a 1 ml pipet (3 strokes) followed by a 1 mL syringe with a 27 gauge needle (5 strokes). The cell homogenates were centrifuge at 5,000 g for 10 min at 4°C. The resultant supernatant (“cytosol”) and pellet (“nuclei”) were stored at −20°C for HMBG1 expression by immunoblot. To examine the effect of serum on HMBG1 distribution, cells were maintained for 72 hr in the media with 0.1, 0.5, 1.0, 2.5 or 5.0% CS. For total cell homogenates, the buffer was removed, the cells washed in cold PBS and the cell pellets stored at −20°C for TLR4 expression by immunoblot.
2.5. Immunoblot
The cellular expression of HMBG1 and TLR4 was determined by immunoblotting as previously described [5]. In brief, cell pellets were prepared in 0.1 mL PBS containing orthovanadate (1 mM), sodium fluoride (50 mM), EDTA (5 mM), PMSF (0.1 mM) and a Sigma Protease Inhibitor cocktail (10 μL/mL; P8340) and immediately sonicated for 3 pulses at 3 seconds at an amplitude of 30% (Ultrasonic processor, Thermo-Fisher Scientific, Waltham MA USA). Protein was quantified using a BioRad Protein kit (BioRad, Hercules CA USA). The cell homogenate was diluted with an equal volume of Laemmli buffer and boiled for 5 minutes. Samples of 10 micrograms (μg) for TLR4 or 25 μg (nuclei, cytosol) were loaded onto a BioRad TGX Stain free 10% gel and proteins were separated electrophoretically at 120 volts for 70 min followed by transfer to a PVDF membrane using a BioRad Trans-Blot TurboTM transfer system. Membranes were blocked with 5% milk for 60 min at room temperature and probed overnight at 4°C with primary antibodies for TLR4 (1:2000; sc-293072, Santa-Cruz Biotechnology, Dallas TX, USA), HMBG1 (1:1000; #6893 Cell Signaling, Danvers MA USA) and nucleoporin 98 (1:1000; #2598, Cell Signaling). The following day, membranes were incubated for 60 min in an anti-rabbit secondary antibody (1:5000, NA934, GE Healthcare, Boston MA) in 5% milk at RT followed by a 5 min incubation with Femto (1:5 dilution, Thermo Scientific); reactive bands were visualized with the BioRad ChemiDoc system. HMBG1 or TLR4 were expressed as the ratio of each protein to the total protein in each lane of the blots.
2.6. Statistical analysis
All measurements are expressed as mean ± standard error (SEM). Group differences were analyzed by Students’-test (two-tailed) or one-way analysis of variance (ANOVA) with Tukey’s multiple comparisons analysis. Statistical analyses were performed and figures constructed with GraphPad Prism 8.1 (GraphPad Software, San Diego, California, USA). A probability value of P<0.05 was set for the minimum value of statistical significance.
3. RESULTS
3.1. CCL2 Release
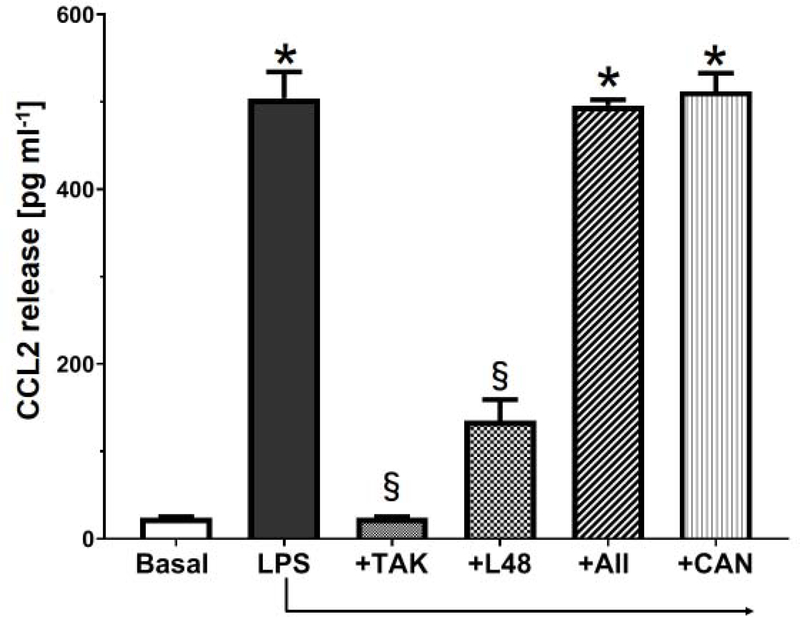
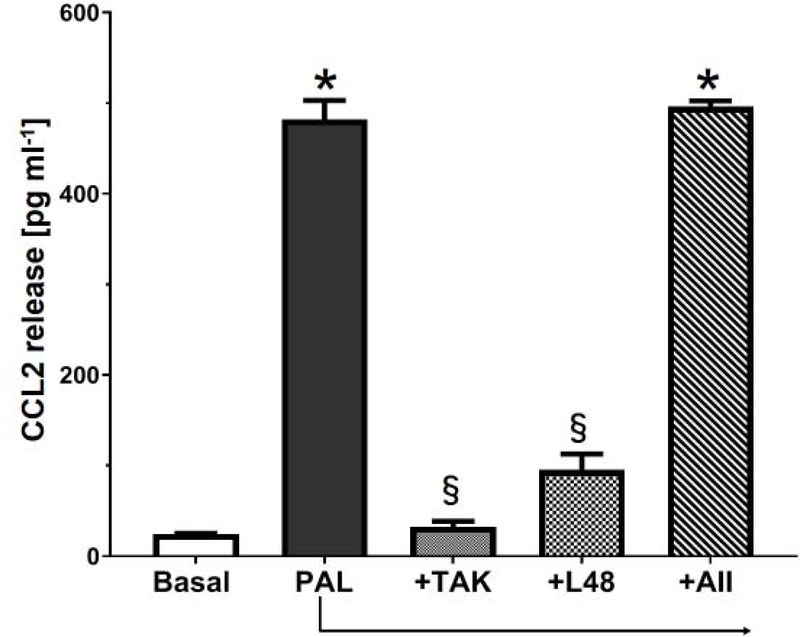
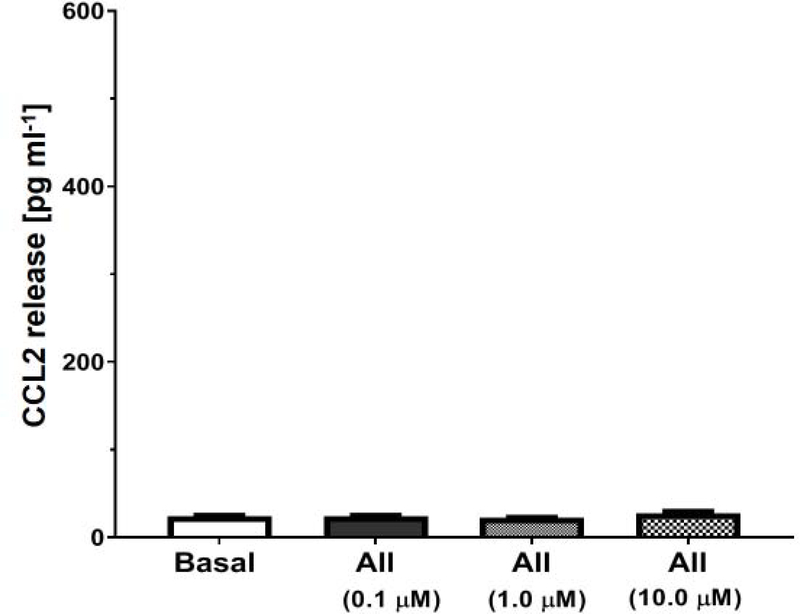
The potential pathway in the NRK-52E cells whereby Ang II, LPS or palmitate bind the MD2-TLR4 complex to induce cytokine release is depicted in Figure 1A. We utilized the curcumin analog L48H37 (Ki of 11 μM) to block the accessory protein MD2 and Tak 242, (IC50 of 10 nM) to block the association of TLR4 with adaptor proteins (MyD88, TRAM) that would inhibit downstream signaling [25; 48]. Initial studies determined the extent that the TLR4 ligands LPS and palmitate stimulated the release of CCL2 in the NRK-52E cells. The 24 h treatment with LPS (1 ng/ml) or palmitate (100 μM) elicited a robust increase in CCL2 release by approximately 20-fold (Figures 2 and 3, respectively). Pre-treatment of the cells with Tak 242 (10 μM) abolished both the LPS and palmitate-induced release of CCL2 (Figures 2 and 3, respectively). The MD2 inhibitor L48H37 (100 μM) also significantly reduced the release of CCL2 to both LPS and palmitate (Figures 2 and 3, respectively). These results strongly imply that the MD2-TLR4 pathway mediates the stimulatory effects of LPS and palmitate to induce CCL2 release and confirm previous reports in the NRK-52E cells and other cell lines [18; 21; 24; 26; 33;36; 44; 49]. In contrast, 24 h treatment with Ang II at concentrations from 0.1 to 10 μM failed to stimulate CCL2 release (Figure 4). Addition of Ang II (1 μM) to LPS (1 ng/ml) or palmitate (100 μM) also failed to exacerbate the stimulatory effects of LPS or palmitate (Figures 2 and 3, respectively). Pretreatment of the cells with the AT1R antagonist candesartan (10 μM) did not attenuate the release of CCL2 by LPS (Figure 2). Finally, LPS (1 ng/mL) increased IL-6 content approximately 5-fold [12 ± 2 vs. 63 ± 5 pg/mL, N=3; p<0.05] that was blocked by Tak 242 [11 ± 3 pg/mL, P<0.05 vs. LPS] while Ang II (1 μM) failed to stimulate IL-6 [12 ± 2 vs. 15 ± 3 pg/mL, N=3]; however, both LPS and Ang II failed to stimulate TNF-α levels in the NRK-52E cells (data not shown).
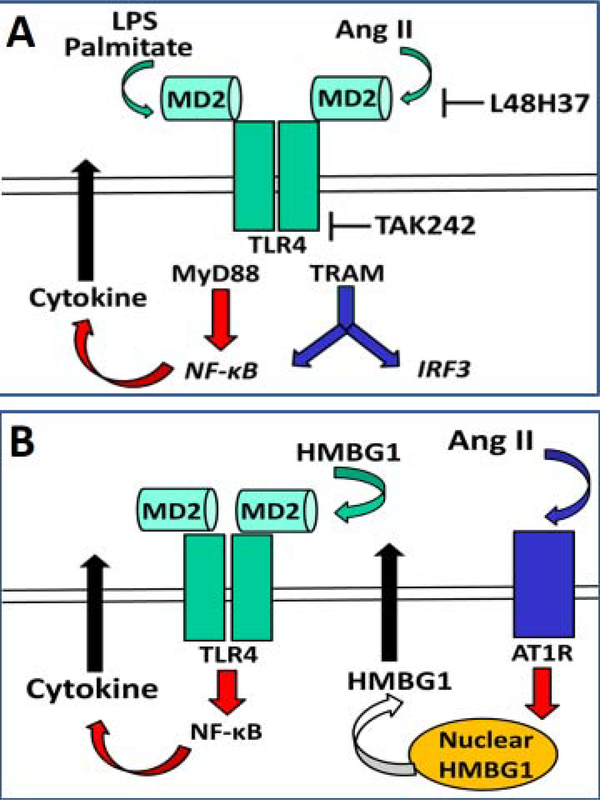
Figure 1: Potential pathways for Ang II stimulation of MD2-TLR4 innate pathway in NRK-52E cells.
Panel A: Ang II binds to the accessory protein MD2 to stimulate the TLR4 complex and subsequently NFkB to increase the release of pro-inflammatory cytokines. LPS and palmitate also bind to MD2 to stimulate the TLR4 pathway. L48H37 blocks binding to MD2 and Tak242 blocks TLR4 binding to accessory proteins MyD88 and TRAM. Panel B: Ang II binds the AT1 receptor (AT1R) to induce the nuclear translocation of HMBG1 to the cytosol and subsequent extracellular release. Extracellular HMBG1 activates the TLR4 complex by binding to the MD2 accessory protein to induce the release of pro-inflammatory cytokines.
Figure 2: LPS stimulates CCL2 release in NRK-52E cells.
LPS (1 ng/mL) stimulates CCL2 release over a 24 h period. CL2 release to LPS was abolished by Tak242 (+TAK, 10 μM) and attenuated by L48H37 (+L48, 100 μM). Addition of Ang II (+AII, 1 μM) or candesartan (+CAN, 5 μM) to LPS (1 ng/mL) did not change CCL2 levels as compared to LPS treatment alone. Data are means ± SEM; N=4 determinations from 4 separate cell passages. *P<0.01 vs. basal; §P<0.05 vs. LPS.
Figure 3: Palmitate stimulates CCL2 release in NRK-52E cells.
Palmitate (PAL, 100 μM) stimulates CCL2 release over a 24 h period. CCL2 release to PAL was abolished by Tak 242 (+TAK, 10 μM) and attenuated by L48H37 (+L48, 100 μM). Addition of Ang II (+AII, 1 μM) to PAL (+100 μM) did not change CCL2 levels as compared to PAL treatment alone. Data are means ± SEM; N=4 determinations from 4 separate cell passages. *P<0.01 vs. basal; §P<0.05 vs. PALM.
Figure 4: Ang II fails to stimulate CCL2 release in NRK-52E cells.
Ang II (AII) at concentrations of 0.1, 1.0 and 10 μM did not stimulate CCL2 release over a 24 h period. Data are means ± SEM; N=4 determinations from 4 separate cell passages.
3.2. HMBG1 Release
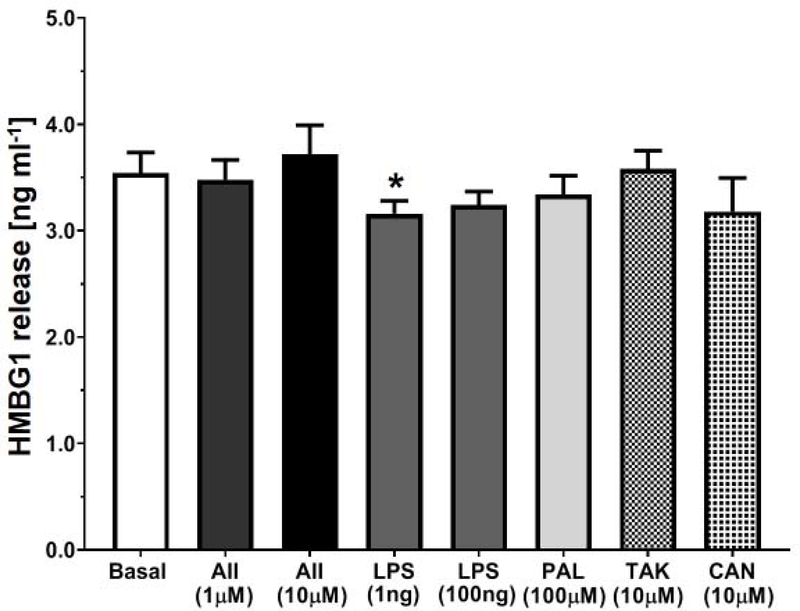
A putative activator of the TLR4 signaling cascade that belongs to the family of danger-associated molecular patterns molecules (DAMPs) is HMBG1 [20; 37]. As shown in Figure 1B, Ang II may bind the AT1R to induce the extracellular release of HMBG1 that subsequently stimulates the MD2-TLR4 complex [30]. The HMBG1 protein exclusively localizes to the cell nucleus, but following cell damage or stress, the protein undergoes extracellular release and may bind TLR4 to provoke an inflammatory response [4; 20; 37]. Nair and colleagues [30] reported that Ang II stimulated the release of HMBG1 to activate TLR4 and induce cytokine release in the NRK-52E cells. Although Ang II failed to stimulate CCL2 in our study, we determined whether Ang II or the TLR4 agonists LPS and palmitate induced the release of HMBG1 in the NRK-52E cells. As shown in Figure 6, basal levels of HMBG1 in the cell media assessed by the HMBG1 ELISA were essentially identical to that in the Nair study at ~3 ng/mL [30]. However, treatment with Ang II (1 and 10 μM), LPS (1 and 100 ng/ml) or palmitate (100 μM) failed to stimulate HMBG1 release (Figure 5). Indeed, HMBG1 release was significantly lower with the 1 ng/mL dose of LPS as compared to basal conditions (Figure 5) Pretreatment with the TLR4 inhibitor Tak 242 (10 μM) or the AT1R antagonist candesartan (10 μM) did not attenuate the basal levels of HMBG1 in the NRK-52E cells (Figure 5). Finally, LPS (1 ng/mL) increased IL-6 content approximately 3-fold [56 ± 6 vs.148 ± 13 pg/mL, N=3; p<0.05 vs. Control] which was blocked by Tak242 [53 ± 5 pg/mL, P<0.05 vs. LPS]; however Ang II (1 μM) failed to stimulate IL-6 [61 ± 10 pg/mL, P>0.05 vs. Control, N=3] and both LPS and Ang II failed to stimulate TNF-α levels in the NRK-52E cells (data not shown).
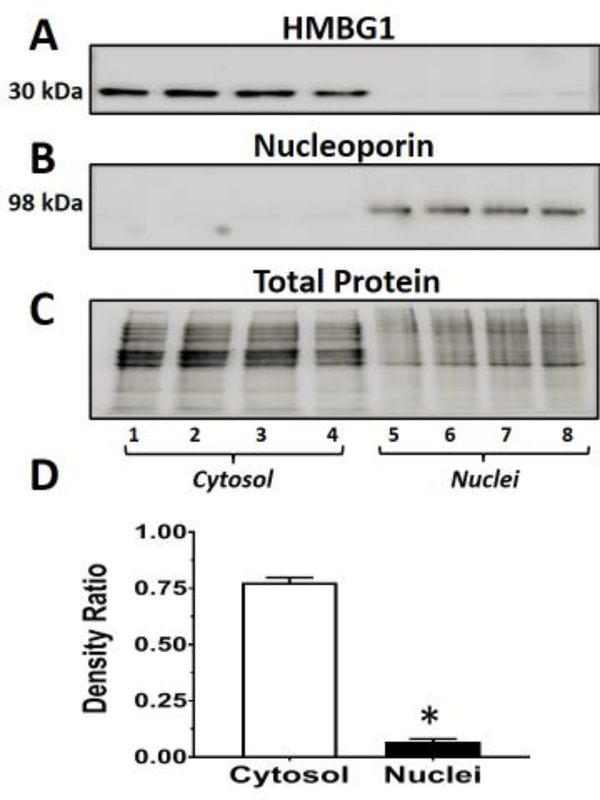
Figure 6: Nuclear and cytosolic expression of HMBG1 in the NRK-52E cells.
Nuclear and cytosolic fractions were prepared from control and palmitate-stimulated NRK-52E cells following a 48 hr period in serum-free conditions. The immunoblot was probed with an HMBG1 antibody (A), and the gel stripped and re-probed with a nucleoporin 98 antibody (B). The total protein for each lane is shown in C. The density ratios of HMBG1 to total protein of the combined cytosolic and nuclear fractions are shown in D (mean ± SEM; N=4; *P<0.5). Lanes 1–4 are cytosolic fractions from vehicle and palmitate at 50, 100 and 200 μM, respectively. Lanes 5–8 are the corresponding nuclear fractions from vehicle and palmitate at 50,100 and 200 μM, respectively. The immunoblot data are from a single experiment.
Figure 5: Ang II, LPS or palmitate fail to increase HMBG1 release in NRK-52E cells.
Effects of treatment with Ang II (AII, 1 and 10 μM), LPS (1 and 100 ng/mL), palmitate (PAL, 100 μM), Tak 242 (TAK, 10 μM), or candesartan (CAN, 10 μM) on extracellular levels of HMBG1 over a 24 hr period in NRK-52E cells. Data are means ± SEM; N=4 determinations from 4 separate cell passages. *P<0.05 vs. basal.
3.3. HMBG1 Localization
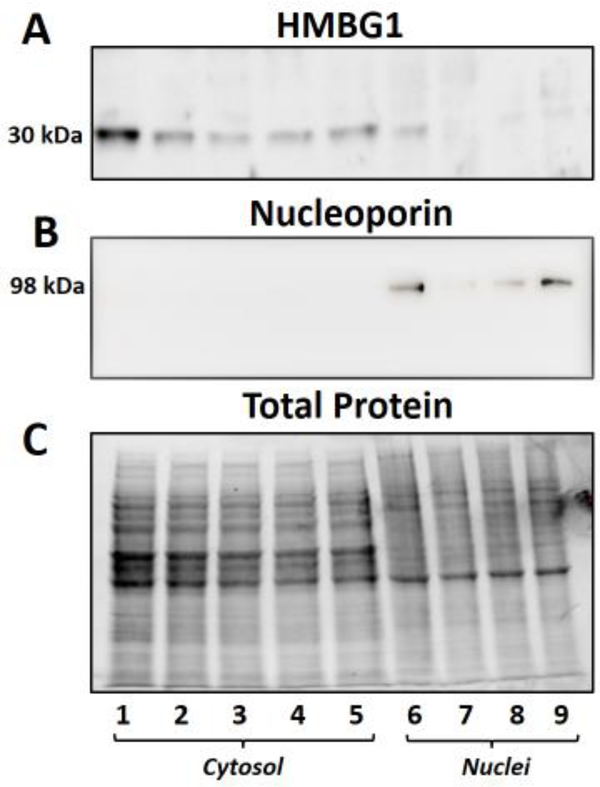
We then determined the intracellular distribution of HMBG1 in the cytosolic and nuclear fractions of the NRK-52E cells. As shown in Figure 6A, both vehicle (lanes 1 and 5) and palmitate-treated cells (lanes 2–4 and 6–8; 50, 100 and 200 μM, respectively) show prominent expression of the HMBG1 (30 kDa band) in the cytosolic fractions (lanes 1–4) versus the corresponding nuclear fractions (lanes 5–8). The blot was re-probed with the nuclear marker nucleoporin 98 to confirm nuclei (lanes 5–8) (Figure 6B). The total protein for each lane of the gel is shown in Figure 6C. We combined the cytosolic and nuclear data from this experiment to demonstrate a greater expression HMBG1 in the cytosolic fractions (Figure 6D). Given the prominent expression of HMBG1 in the cytosolic fraction, we addressed whether the cell culture conditions, particularly the lack of serum influenced the cytosolic localization of the protein. As shown in Figure 7, we observed comparable expression of HMBG1 in the cytosolic and nuclear fractions irrespective of the different concentrations of serum from 0.1 to 5% fetal bovine serum. Note that the subcellular data from Figures 6 and 7 are from single experiments in the NRK-52E cells.
Figure 7: Effect of serum on the nuclear and cytosolic expression of HMBG1 in the NRK-52E cells.
Nuclear and cytosolic fractions were prepared from NRK-52E cells maintained in different concentrations of calf serum (CS). The blot was probed with an HMBG1 antibody (A), the gel stripped and re-probed with a nucleoporin 98 antibody (B) for nuclei. The total protein for each lane is shown in C. Lanes 1–5 are cytosolic fractions from cells maintained in 5, 2.5, 1.0., 0.5 or 0.1% CS, respectively. Lanes 6–9 are the corresponding nuclear fractions from cells in 5, 2.5, 1 or 0.5% CS, respectively. The immunoblot data are from a single experiment.
3.4. TLR4 Expression
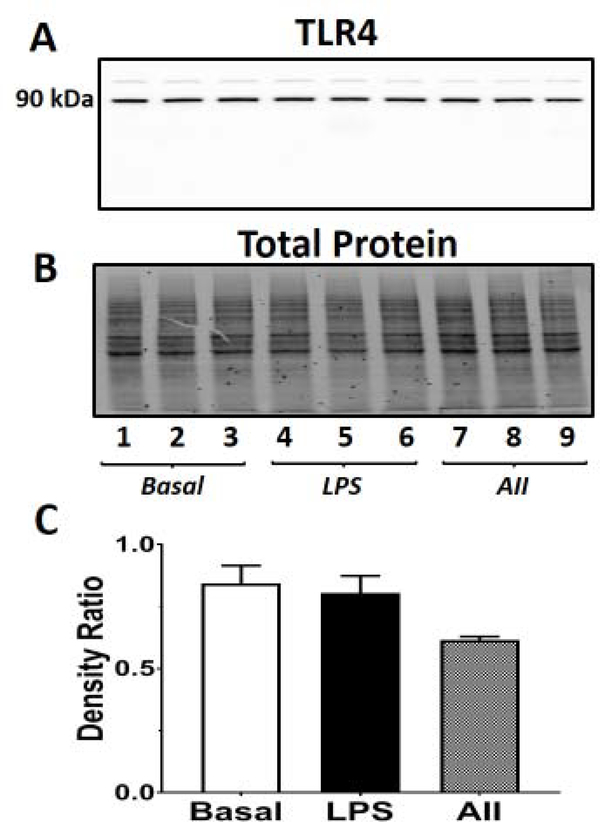
Finally, we determined the effects of Ang II (1 μM) and LPS (1 ng/mL) on the expression of TLR4 in the NRK-52E cells. An increase in TLR4 expression in the presence of extracellular levels of HMBG1 may potentially evoke TLR4 signaling (Figure 1B). As shown in the full length immunoblot, the major band corresponded to the molecular size of TLR4 at 90 kDa which confirms TLR4 expression, as well as both Tak 242 and L48H37 sensitivity in the NRK-52E cells (Figure 8A). The total protein for each lane is shown in Figure 8B. Comparison of the density ratios of the 90 kDa TLR4 band to the total protein of each lane revealed that neither LPS nor Ang II increased TLR4 expression in the NRK-52E cells (Figure 8C).
Figure 8: Ang II or LPS fail to increase TLR4 protein expression in the NRK-52E cells.
Effects of Ang II (1 μM) or LPS (1 ng/mL) on TLR4 expression over a 24 h period in NRK-52E cells. The total cell homogenate was prepared and the immunoblot probed with a TLR4 antibody that revealed a major band at 90 kDa (A). The total protein for each lane is shown in B. Ratio of the densities of the TLR4 90 kDa band to the total protein (C). Lanes 1–3 are vehicle treatment, lanes 4–6 are the corresponding LPS treatment and lanes 6–9 are the corresponding Ang II treatment. Data are means ± SEM; N=3 determinations from 3 separate cell passages.
4. Discussion
The present study assessed the ability of Ang II to stimulate the MD2-TLR4 pathway in the release of the pro-inflammatory cytokine CCL2 in rat proximal tubule NRK-52E cells. The NRK-52E cells are considered a cell model of the proximal tubule epithelium that express a complete RAS and exhibit pro-inflammatory and pro-fibrotic pathways that may parallel tubulointerstial injury in the kidney [1; 2; 5; 7; 38; 40; 41; 49]. Although we demonstrated a robust stimulation of CCL2 by the TLR4 ligands LPS and palmitate, Ang II over a dose range of 0.1 to 10 μM failed to stimulate CCL2 release in the NRK-52E cells. The combination of Ang II with LPS or palmitate failed to augment the release of CCL2 above that of LPS or palmitate alone. Treatment with the selective AT1R antagonist candesartan also failed to blunt the cytokine response to LPS and palmitate. Moreover, LPS stimulated IL-6 that was blocked by Tak 242, but Ang II failed to stimulate this cytokine. Finally, we assessed the role of HMBG1 in the cytokine response in the NRK-52E cells. The extracellular release of HMBG1 was not stimulated by LPS, palmitate or Ang II suggesting that HMBG1 is not involved in the LPS or palmitate dependent CCL2 response in these cells.
The Ang II-AT1R axis of the RAS plays an integral role in blood pressure regulation and dysregulation of this axis likely contributes to hypertension and other cardiovascular pathologies [3; 6; 12; 46]. Indeed, the blockade of the RAS by ACE inhibitors to attenuate Ang II formation or AT1R antagonists to block Ang II binding are important therapeutic regimens in the treatment of CVD [12; 46]. Accumulating experimental evidence suggests an important relationship between an activated Ang II-AT1R axis of the RAS and inflammatory pathways [3; 11; 17: 22; 31]. Blockade of TLR4 with Tak 242 or a TLR4 antibody attenuates the hypertensive response, vascular dysfunction and cardiac hypertrophy to Ang II [9; 10; 16; 26]. Moreover, TLR4 and MD2 knockout mice exhibit an attenuated response regarding cardiac and renal injury to Ang II that was independent of a reduction in blood pressure [14; 43; 49]. However, the mechanisms involved in the Ang II-dependent stimulation of TLR4 remain equivocal. Xu et al [49] reported that Ang II directly stimulates the TLR4 inflammatory pathway by binding to the TLR4 accessory protein MD2 and activating the TLR4-MyD88 pathway in renal NRK-52E cells (Figure 1A). Both Tak 242 and the MD2 inhibitor L6H21 abolished the Ang II-dependent release of pro-inflammatory cytokines; however, the AT1R antagonist valsartan failed to block the Ang II response suggesting the AT1R-independent stimulation of TLR4 [49]. The clinical relevance of these results reflects that ARBs increase the circulating levels of Ang II which may provoke the stimulation of the TLR4 inflammatory pathway [6; 12; 46].
The present study, however, could not confirm that Ang II directly stimulates the MD2-TLR4 pathway in the NRK-52E cells. Treatment with Ang II over a dose range of 0.1 to 10 μM failed to increase CCL2 release while the TLR4 ligands LPS and palmitate induced a robust CCL2 response. The stimulation of CCL2 to both LPS and palmitate was abolished by the selective TLR4 inhibitor Tak 242 and significantly reduced by the MD2 inhibitor L48H37. The combination of Ang II with either LPS or palmitate also failed to augment cytokine release above that of either agent alone suggesting that Ang II exhibits no additive effects. Moreover, treatment with candesartan failed to attenuate the response to LPS or palmitate and it is unlikely that either agent stimulates cellular levels of Ang II that contribute to a TLR4-dependent response. Xu et al [49] reported a binding constant (KD) of 200 μM for the association of Ang II and MD2 using an ex vivo protein interaction assay. As the highest dose of Ang II in our study was 10 μM, it’s possible that we did not achieve a sufficient Ang II concentration to stimulate the MD2-TLR4 complex. However, the Xu study [49] utilized 1 μM Ang II to stimulate cytokine release and TLR4-MyD88 association in the NRK-52E cells. At this time, we cannot explain the discrepancy between the KD value for Ang II-MD2 binding and the 200-fold lower dose of Ang II that stimulated cytokine release [49]. We note that an Ang II concentration of 200 μM for MD2 binding is unlikely to be achieved under in vivo conditions as circulating Ang II levels typically range from 10 to 100 pM [6].
Nair et al. [30] also reported that Ang II (1 μM) stimulated cytokine release in the NRK-52E cells; however, the Ang II response was abolished by the AT1R antagonist losartan. These investigators proposed that the Ang II-AT1R binding induced the downstream release of HMBG1 to activate the TLR4 complex [30] (Figure 1B). Ang II had a small (~20%) effect on HMBG1 release, but a more pronounced impact to increase TLR4 protein expression 3–4 fold [30]. Treatment with an antibody against HMBG1 or TLR4 siRNA reduced the Ang II response [30]. HMBG1 typically localizes to the nucleus to regulate chromatin organization and transcription, but the protein is released into the cytosol and extracellular compartment following cell stress or injury [4; 20; 37]. Indeed, extracellular HMBG1 may function as an endogenous ligand for TLR4 in injured tissues [20; 37]. We detected comparable extracellular levels of HMBG1 (~3 ng/mL or 100 pM) to those in the Nair study [30], but Ang II failed to stimulate the HMBG1 release or augment TLR4 protein expression in the NRK-52E cells. He et al [15] recently reported a KD of 0.6 μM for the binding of HMBG1 to MD2. The extracellular HMBG1 concentration of 100 pM in the NRK-52E cells is 6,000-fold below its KD value to bind MD2-TLR4 and potentially stimulate cytokine release. Further characterization of the NRK-52E cells under basal conditions or those treated with palmitate revealed HMBG1 expression predominantly in the cytosolic fraction of the cells. The cellular distribution of HMBG1 appeared similar in NRK-52E cells that were maintained in different amounts of serum. These preliminary data suggest that the incubation conditions (presence or absence of serum) did not influence the intracellular distribution of HMBG1. Additional studies are required to define the mechanisms that influence the subcellular localization and regulation of HMBG1 release in the NRK-52E cells, as well as reconcile the extracellular HMBG1 concentration to its KD value for MD2-TLR4 necessary to evoke cytokine release.
In conclusion, the present studies do not support the tenet that Ang II binds to the MD2-TLR4 complex to stimulate cytokine release under conditions that demonstrate a robust stimulatory effect of the TLR4 ligands LPS and palmitate. Moreover, we cannot confirm that TLR agonists LPS and palmitate or Ang II stimulates the release of HMBG1 or increase TLR4 expression in the renal epithelial cells that could potentially contribute to an inflammatory response. We acknowledge that the current study was performed solely in the NRK-52E cell line and that these results may not reflect the inflammatory pathways within the kidney or other tissues. Although the NRK-52E cells are reported to express a complete RAS including AT1R, we did not determine the presence of functional AT1Rs per se in the present study. In addition, our studies primarily focused on the release of CCL2, a key cytokine in tissue inflammation and fibrosis and we did not perform a comprehensive assessment of other cytokines nor attempt to directly inhibit the extracellular levels of HMBG1. However, in lieu of the current data and previous studies that TLR4 knockout or Tak 242 treatment has beneficial cardiovascular outcomes, a more plausible mechanism for the in vivo actions of Ang II is that the peptide indirectly stimulates TLR4 through the release of DAMPs that induce the innate immune pathway. Indeed, Zhao and colleagues [50] recently showed that the inflammatory response to Ang II reflected the blood pressure-dependent release of the DAMP ATP to stimulate P2X7 receptors rather than the direct actions of Ang II. In this regard, further studies are required to establish the mechanisms for Ang II-dependent stimulation of inflammatory events involved in cardiovascular disease that are mediated by TLR4.
Highlights.
Angiotensin II does not stimulate the MD2-TLR4 pathway to induce release of the cytokine CCL2 (MCP-1) in the rat renal epithelial NRK-52E cells.
The TLR4 agonists LPS and palmitate markedly stimulated CCL2 release that was abolished by the TLR4 inhibitor Tak 242 and reduced by the MD2 inhibitor L48H37.
Angiotensin II failed to stimulate the release of the TLR4 ligand HMBG1 to concentrations required to bind MD2-TLR or increase expression of TLR4 in the NRK-52E cells.
Subcellular fractionation revealed that HMBG1 was predominantly localized to the cytosolic fraction of the NRK-5E cells.
ACKNOWLEDGEMENTS
Support for this study was provided by National Institutes of Health grants HD-084227, HL-146818, HL146818-S1 and American Heart Association grants GRNT20480121 and TPA34170522, the Groskert Heart Fund, the Wake Forest Venture Fund and the Farley-Hudson Foundation (Jacksonville, NC).
Footnotes
CONFLICT OF INTEREST
The authors declare that there are no competing financial interests in the work described.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Alzayadneh EM, Chappell MC. Angiotensin-(1–7) abolishes AGE-induced cellular hypertrophy and myofibroblast transformation via inhibition of ERK 1/2. Cell Signal 26 (2014) 3027–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Alzayadneh EM, Chappell MC Nuclear expression of renin-angiotensin system components in NRK-52E renal epithelial cells. J Renin Angiotensin Aldosterone Syst. 16 (2015) 1135–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Biancardi VC, Bomfim GF, Reis WL, Al-Gassimi S, Nunes KP. The interplay between angiotensin II, TLR4 and hypertension. Pharmacol. Res. 120 (2017) 88–96. [DOI] [PubMed] [Google Scholar]
- [4].Bianchi ME, Agresti A. HMG proteins: dynamic players in gene regulation and differentiation. Current Opinion in Genetics & Development. 15 (2005) 496–506. [DOI] [PubMed] [Google Scholar]
- [5].Chappell MC, Pirro NT, Melo AC, Tallant EA, Gallagher PE. The microbiome product Urolithin A abrogates the TGFβ-EGFR-PAI1 pathway in NRK-52E renal cells. J Cell Signaling 5 (2020) 1–9. [Google Scholar]
- [6].Chappell MC. Biochemical assessment of the renin-angiotensin system: the good, bad and absolute? Am J Physiol Heart Circ 15 (2016) H137–H152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chou CH, Chuang LY, Lu CY, Goh JY. Interaction between TGFβ and ACE2-Ang-(1–7)-Mas pathway in high glucose-cultured NRK-52E cells. Mol Cell Endocrinol. 2013; 366:21–30. [DOI] [PubMed] [Google Scholar]
- [8].Culberg KB, Larsen JO, Perdersen SB, Richelsen B. Effects of LPS and dietary free fatty acids on MCP-1 in 3T3-L1 adipocytes and macrophages. Nutr Diabetes 24 (2014) e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dange RB, Agarwal D, Masson GS, Vila J, Wilson B, Nair A, Francis J. Central blockade of TLR4 improves cardiac function and attenuates myocardial inflammation in Angiotensin II-induced hypertension. Cardiovascular Research 103 (2014) 17–27. [DOI] [PubMed] [Google Scholar]
- [10].Batista De, Palacious R, Martin A, Hernanz R, Medici CT, Silva MA, Rosi EM, Aguado A, Vassallo DV, Saaices M, Alonso MJ. Toll-like receptor 4 upregulation by angiotensin II contributes to hypertension and vascular dysfunction through reactive oxygen species production. PLoS One. 9 (2014) e104020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Feng Q, Liu D, Lu Y, Liu Z. The interplay of renin-angiotensin system and Toll-Like Receptor 4 in the inflammation of diabetic nephropathy. J Immunol Res 2020 (2020) 1- [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ferrario CM, Mullick AE. Renin angiotensin aldosterone inhibition in the treatment of cardiovascular disease. Pharmacological Research 125 (2017) 57–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Forrester SJ, Booz GW, Sigmund CD, Coffman TM, Kawai T, Rizzo V, Scalia R, Eguchi S. Angiotensin II signal transduction: an update on mechanisms of physiology and pathophysiology. Physiol Rev 98 (2018) 1627–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Han J, Zou C, Mei L, Zhang Y, Aian Y, You S, Liang G. MD2 mediates Ang II-induced cardiac inflammation and remodeling via directly binding to Ang II and activating TLR4/NF-κB signaling pathway. Basic Res Cardiol 112 (2017) 1–15. [DOI] [PubMed] [Google Scholar]
- [15].He M, Bianchi ME, Coleman TR, Tracey KJ, Al-Abed Y Exploring the biological functional mechanism of the HMGB1/TLR4/MD-2 complex by surface plasmon resonance. Molecular Medicine 24 (2018) 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hernanz R, Martínez-Revelles S, Palacios R, Martín A, Cachofeiro V, Aguado A, García-Redondo L L, Barrús MT, de Batista PR, Briones AM, Salaices M, Alonso MJ. Toll-like receptor 4 contributes to vascular remodeling and endothelial dysfunction in Angiotensin II-induced hypertension. Br J Pharmacol. 172 (2015) 3159–3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jaén RI, Val-Blasco A, Prieto P, Gil-Fernández M, Smani T, López-Sendón JL, Delgado C, Boscá L, Fernández-Velasco M. Innate immune receptors, key actors in cardiovascular diseases. JACC Basic Transl Sci. 5 (2020) 735–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jiang P, Xu J, Zheng S, Huang J, Xiang Q, Fu X, Wang T. 17beta-estradiol downregulates lipopolysaccharide-induced MCP-1 production and cell migration in vascular smooth muscle cells. J Mol Endocrinol 45 (2010) 87–97. [DOI] [PubMed] [Google Scholar]
- [19].Kaschina E, Namsolleck P, Unger T. AT2 receptors in cardiovascular and renal diseases. Pharmacological Research 125 (2017) 39–47. [DOI] [PubMed] [Google Scholar]
- [20].Klune JR, Dhupar R, Cardinal J, Billiar TR, Tsung A. HMGB1: endogenous danger signaling. Molecular Medicine. 14 (2008) 476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Korbecki J, Bajdak-Rusinkek K. The effect of palmitic acid on inflammatory responses in macrophages. Inflamm Res 68 (2019) 915–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Laffer CL, Elijovich F. Inflammation and therapy for hypertension. Curr Hypertens Rep. 12 (2010) 233–242. [DOI] [PubMed] [Google Scholar]
- [23].Lee JY, Ye J, Gao Z, Youn HS, Lee WH, Zhao L, Sizemore N, Hwang DH. Reciprocal modulation of Toll-like receptor-4 signaling pathways involving MyD88 and phosphatidylinositol 3-kinase/AKT by saturated and polyunsaturated fatty acids. J Biol Chem. 278 (2003) 37041–37051. [DOI] [PubMed] [Google Scholar]
- [24].Luo Y, Wu MY, Deng BQ, Huang J, Hwang SH, Li MY, Zhou CY, Zhang QY, Yu HB, Zhao DK, Zhang G, Qin L, Peng A, Hammock BD, Liu JY. Inhibition of soluble epoxide hydrolase attenuates a high-fat diet-mediated renal injury by activating PAX2 and AMPK. Proc Natl Acad Sci U S A. 116 (2019) 5154–5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Matsunaga N, Tsuchimori N, Matsumoto T, Ii M. TAK-242 (resatorvid), a small-molecule inhibitor of Toll-like receptor (TLR) 4 signaling, binds selectively to TLR4 and interferes with interactions between TLR4 and its adaptor molecules. Mol. Pharmacol. 79 (2011) 34–41. [DOI] [PubMed] [Google Scholar]
- [26].Matsuda S. Umemoto S, Yoshimura K, Itoh S, Murata T, Fukai T, Matsuzaki M. Angiotensin II activates MCP-1 and induces cardiac hypertrophy and dysfunction via toll-like receptor 4. J. Arterioscler. Thromb 22 (2015) 834–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].McKernan K, Varghese M, Patel R, Singer K. Role of TLR4 in the induction of inflammatory changes in adipocytes and macrophages. Adipocyte 9 (2020) 212–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Moghimpour Bijani F, Vallejo JG, Rezaei N. Toll-like receptor signaling pathways in cardiovascular diseases: challenges and opportunities. Int Rev Immunol. 31 (2012) 379–395. [DOI] [PubMed] [Google Scholar]
- [29].Nabbi A, Riabowol K. Rapid isolation of nuclei from cells in vitro. Cold Spring Harbor Protocols 2015 (2015) 769–772. [DOI] [PubMed] [Google Scholar]
- [30].Nair AR, Ebenezer PJ, Saini Y, Francis J. Angiotensin II-induced hypertensive renal inflammation is mediated through HMBG1-TLR4 signaling in rat tubule-epithelial cells. Exp Cell Res 35 (2015) 238–247. [DOI] [PubMed] [Google Scholar]
- [31].E Norlander A, Madhur MS, Harrison DG. The immunology of hypertension. J. Exp. Med. 215 (2018) 21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Nunes KP, de Oliveira AA, Mowry FE, Biancardi VC. Targeting toll-like receptor 4 signaling pathways: can therapeutics pay the toll for hypertension? Br. J. Pharmacol. 176 (2019) 1864–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Oberbach A, Schlichting N, Blüher M, Kovacs P, Till H, Stolzenburg JU, Neuhaus J.. Palmitate induced IL-6 and MCP-1 expression in human bladder smooth muscle cells provides a link between diabetes and urinary tract infections. PLoS One. 28 (2010) e10882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Oblak A, Jerala R. The molecular mechanism of species-specific recognition of lipopolysaccharides by the MD-2/TLR4 receptor complex. Mol Immunol. 63 (2015) 134–142. [DOI] [PubMed] [Google Scholar]
- [35].Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO. The structural basis of lipopolysaccharide recognition by the TLR4–MD-2 complex. Nature 458 (2009) 1191–1195. [DOI] [PubMed] [Google Scholar]
- [36].Park CY, Heo JN, Suk K, Lee WH. Sodium azide suppresses LPS-induced expression MCP-1 through regulating IκBζ and STAT1 activities in macrophages. Cell Immunol. 2017. 315:64–70 [DOI] [PubMed] [Google Scholar]
- [37].Park JS, Gamboni-Robertson F, He Q, Svetkauskaite D, Kim JY, Strassheim D, Sohn JW, Yamada S, Maruyama I, Banerjee A, Ishizaka A, Abraham E. High mobility group box 1 protein interacts with multiple toll-like receptors. Am J Physiology. Cell Physiology 290 (2006) C917–C24. [DOI] [PubMed] [Google Scholar]
- [38].Qi R, Yang C. Renal tubular epithelial cells: the neglected mediator of tubulointerstial fibrosis after injury. Cell Death Disease 9 (2018) 11–26-1137.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Rocha DM, Caldas AP, Oliveira LL, Bressan J, Hermsdorff HH. Saturated fatty acids trigger TLR4-mediated inflammatory response. Atherosclerosis.244 (2016) 211–215. [DOI] [PubMed] [Google Scholar]
- [40].Rodriguez-Iturbe B, Pons H, Johnson RJ RJ. Role of the immune system in hypertension. Physiol Rev. 97 (2017) 1127–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Schnaper HW. The tubulointerstial pathophysiology of progressive kidney disease. Adv Chronic Kidney Dis.24 (2017) 107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Shimazu R, Akashi S, Ogata H, Nagai Y, Fukudome K, Miyake K, Kimoto M. MD-2, a molecule that confers lipopolysaccharide responsiveness on toll-like receptor 4. J. Exp. Med. 189 (1999), 1777–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Singh MV, Cicha MZ, Nunez S, Meyerholz DK, Chapleau MW, Abboud FM Angiotensin II-induced hypertension and cardiac hypertrophy are differentially mediated by TLR3- and TLR4-dependent pathways. Am J Physiol Heart Circ Physiol 316 (2019) H1027–H1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Soumura M, Kume S, Isshiki K, Takeda N, Araki S, Tanaka Y, Sugimoto T, Chin-Kanasak M, Nishio Y, Haneda M, Koya D, Kashiwagi A, Maegawa H, Uzu T. Oleate and eicosapentaenoic acid attenuate palmitate-induced inflammation and apoptosis in renal proximal tubular cell. Biochem Biophys Res Commun. 402 (2010) 265–271. [DOI] [PubMed] [Google Scholar]
- [45].Spector AA, Structure and lipid binding properties of serum albumin. Methods Enzymology 128 (1986) 320–339. [DOI] [PubMed] [Google Scholar]
- [46].Te RL, van Esch JH, Roks AJ, van den Meiracker AH, Danser AH. Hypertension: renin-angiotensin-aldosterone system alterations. Circ Res 116 (2015) 960–975. [DOI] [PubMed] [Google Scholar]
- [47].Wang Y, Qian Y, Fang Q, Zhong P, Li W, Wang L, Liang G Saturated palmitic acid induces myocardial inflammatory injuries through direct binding to TLR4 accessory protein MD2. Nature Comm 8 (2017) 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Wang Y, Shan X, Dai Y, Jiang L, Chen G, Zhang Y, Wang Z, Dong L, Wu J, Guo G, Liang G. Curcumin analog L48H37 prevents lipopolysaccharide-induced TLR4 signaling pathway activation and sepsis via targeting MD2. J Pharmacol Exp Ther. 353 (2015) 539–50. [DOI] [PubMed] [Google Scholar]
- [49].Xu Z, Li W, Han J, Zou C, Huang W, Yu W, Xhan X, Lum H, Li X, Liang G. Angiotensin II induces kidney inflammatory injury and fibrosis through binding to myeloid differentiation protein-2 (MD2). Sci Reports 21 (2017) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Zhao TV, Li Y, Ku X, Xia S, Shi P, Li L, Chen Z,, Yin C, Eriguchi M, Chen Y, Bernstein EA, Giani JF, Bernstein EE, Shen XZ. ATP release drives heightened immune responses associated with hypertension. Sci Immunol 4 (2019) 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]