Abstract
Objective
To evaluate whether the fetal linear growth effects of maternal nutrition supplementation would be maintained through 6 months postnatal age.
Study design
The Women First trial was a multicountry, individually randomized clinical trial that compared the impact of maternal nutrition supplementation-initiated preconception (Arm 1) vs at ~11 weeks of gestation (Arm 2), vs no supplement (Arm 3); the intervention was discontinued at delivery. Trial sites were Democratic Republic of Congo, Guatemala, India, and Pakistan. Analysis includes 2421 infants born to 2408 randomized women. Primary outcome was the trajectory of length-for-age z scores (LAZ) by arm, based on assessments at birth and 1, 3, and 6 months. We fitted longitudinal models on growth from birth to 6 months using generalized estimating equations; maternal intervention effects were evaluated, adjusting for site and baseline maternal covariates.
Results
Linear growth for Arms 1 and 2 was statistically greater than for Arm 3 in 3 of the 4 countries, with average pairwise mean differences in LAZ of 0.25 (95% CI 0.15–0.35; P < .001) and 0.19 (95% CI 0.09–0.28; P < .001), respectively. Compared with Arm 3, average overall adjusted relative risks (95% CI) for stunting (LAZ <–2) were lower for Arms 1 and 2: 0.76 (0.66–0.87; P < .001) and 0.77 (0.67–0.88; P < .001), respectively.
Conclusions
Improved linear growth in early infancy observed for the 2 intervention arms supports the critical importance of maternal nutrition before conception and in the early phase of gestation.
Trial registration
Reduction of childhood stunting is a global health priority.1–3 Despite recent progress toward this goal, nearly 150 million young children are stunted. The first several months of postnatal growth, which is normally very rapid, reflect both intrauterine and postnatal exposures, including nutritional adequacy. Population studies and observational cohorts from low- and middle-income settings indicate that linear growth faltering is often observed very early in the postnatal period,4–7 strongly suggesting a deprived intrauterine environment. To date, however, interventions to improve maternal nutritional status during pregnancy have resulted in increased birth size but have had only modest impact on postnatal growth.8–10
Although a strong theoretical rationale exists for the importance of preconceptional maternal nutritional health to fetal and early postnatal growth, data are very limited.11–14 In the multicountry “Women First” trial, a comprehensive maternal nutrition intervention initiated either before conception or by the end of the first trimester, resulted in greater newborn length and weight as well as lower rates of stunting and small for gestational age compared with no nutritional intervention.15 The a priori trial hypothesis examined in this report was that the gains of the intervention would be maintained at least through 6 months’ postpartum among the offspring of mothers who received nutritional supplementation.16 Specifically, we compared the trajectory of growth from birth to 6 months by maternal intervention arm for linear growth, weight gain, and head circumference (HC) gain, and for rates of stunting and wasting. Secondary objectives were to characterize the infant feeding patterns during the follow-up period and to analyze the effects of the maternal intervention on postnatal morbidity and mortality.
Methods
This report describes growth data obtained at birth, and 1, 3, and 6 months postnatal age for the offspring of the participants in the Women First preconception nutrition trial (clinicaltrials.gov NCT01883193).15,16 The original study was a multisite individually randomized clinical trial in which nonpregnant women were randomly assigned to 1 of 3 arms: initiation at time of randomization a daily small quantity lipid-based nutrient supplement (SQ-LNS) with continuation for at least 3 months before conception through to delivery (Arm 1); initiation of the same supplement late in the first trimester of pregnancy and continued through to delivery (Arm 2); or receipt of no trial supplement (Arm 3). In addition, women in Arm 1 and Arm 2 (once on the primary supplement) who were underweight or had inadequate gestational weight gain were provided an extra protein-energy supplement.16 The SQ-LNS provided modest quantities of protein and energy (2.6 g protein and 118 kcal), polyunsaturated fats in a favorable balance, and 22 micronutrients in quantities appropriate for pregnancy. The protein-energy supplement was also lipid-based and provided 300 kcal and 11 g protein (~15% of energy) without additional supplemental micronutrients. The total duration of exposure to the intervention supplement was 76.6 weeks for women in Arm 1, and 25.4 weeks for women in Arm 2.15
The study was conducted in rural and small city settings in the Democratic Republic of Congo, Guatemala, India, and Pakistan. Details of these sites have been provided previously.16
Participants
The infants in the present report are the offspring of the participants of the Women First trial. Birth anthropometric measurements, including length, weight, and HC, were obtained by at least 7 days of postnatal life, and 98.2% were obtained within 48 hours of birth. Data were collected between February 2015 and May 2017.
Anthropometry
Length, weight, and HC measurements were obtained by assessment teams who had not been directly involved in administration of the study intervention or the biweekly home visits throughout the trial. The assessors were trained and certified according to standardized procedures; they were recertified every 3 months. The equipment included infant electronic balances accurate to 10 g (seca 334), non-stretch, plasticized measuring tape (seca 201) (seca North America, Chino, California) and infantometers accurate to 1 mm (neonatal stadiometer, Ellard Instrumentation, Ltd, Monroe, Washington). Z scores, which accounted for sex and age at the time of measurement, were calculated for length, weight, body mass index (BMI, kg/m2), and HC from the World Health Organization (WHO) Child Growth Standards.17
Infant Feeding
Breastfeeding and overall feeding history was obtained at all anthropometry visits. Assessment included use of pre-lacteals in first 3 days of life; use of any/type of liquids besides breast milk and any/type of non-breast milk foods at subsequent visits; and use of any commercial fortified liquids or foods. Exclusive breastfeeding was defined as no other liquids or foods.
Infant Morbidity
Infant hospitalization or illness for which the family sought medical care, including visits to traditional healers, was recorded by research assistants at biweekly home visits throughout the 6-month study period. Specific questions addressed episodes of respiratory illness (treatment, yes/no); diarrhea (defined as 3 or more loose or watery stools per day); and malaria (confirmed by health worker and/or blood test, and treatment, yes/no).16
Ethics
The project was approved by the Colorado Multiple institutional review board, University of Colorado, the local and/or national ethics committees for each of the 4 sites (registered with US Office of Human Research Protection and with Federal-wide Assurance in place), and the data coordinating center. Written informed consent was obtained from all participants. The study protocol is available online at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4000057/. Throughout the intervention phase of the trial, a data monitoring committee designated by the Eunice Kennedy Shriver National Institute of Child Health and Human Development monitored safety of the trial. Adverse events that were monitored included pregnancy outcomes, adverse neonatal events, hospitalizations, and allergic reactions.15,16
Data Management and Analysis
After excluding biologically implausible anthropometric measurements (length and HC) according to WHO guidelines,17 the longitudinal analysis included all live-born infants with birth length measurements obtained by 7 days of age who had at least 2 follow-up visits (1-, 3-, or 6-month). Weight-for-length z scores (WLZ) could not be obtained for infants with length <45.0 cm at any visit due to limitations in the WHO standards17 and were set to missing for that visit.
Maternal baseline characteristics and unadjusted growth outcomes for infants were summarized using mean and SD for continuous variables and percentages for categorical variables. Primary and secondary outcomes were analyzed using longitudinal generalized estimating equations for each outcome and accounting for the correlation of repeated measures over time as well as study cluster (site/location). The models also were adjusted for age at visit, parity, and maternal education at baseline, the latter because the rates were significantly different across the 3 intervention arms in our analysis sample. Interactions between arm and other covariates (site, sex, and nulliparous/parous) were evaluated, using a cut-off of P < .10. The generalized estimating equation models used linear, log binomial or robust Poisson formulations, as appropriate, depending on whether the outcomes were continuous or binary. An autoregressive order 1 covariance structure was used to account for correlated longitudinal data. A 2-sided P < .05 was considered significant. Statistical analyses were conducted using SAS (Version 9.4, Cary, North Carolina). Treatment effects for continuous outcomes are presented as model adjusted pairwise mean differences and those for binary outcomes are presented as adjusted relative risks, along with respective 95% CIs.
Results
This analysis is based on 2408 women with a live birth in the longitudinal analysis population. Enrollment and randomization took place between December 2013 and October 2014. Maternal characteristics are presented in Table I (available at www.jpeds.com); no differences among arms were evident except for Arm 1 having more women with no education (P = .018). The infants represented in the longitudinal analysis for the combined sites included 2421 infants, with 785, 827, and 809 in Arms 1, 2, and 3, respectively. There were 2395 singletons, and 13 twin pairs (7, 2, and 4 pairs in Arm 1, 2, and 3, respectively) (Figure 1; available at www.jpeds.com).
Table I.
Overall baseline maternal characteristics among women who had a live birth in the longitudinal analysis subset* by intervention arm
| Variables | Total | Arm 1 | Arm 2 | Arm 3 | P value† |
|---|---|---|---|---|---|
| Randomized, n | 7387 | 2465 | 2460 | 2462 | |
| Women who had a live birth in the 6-mo longitudinal analysis population, n* |
2408 | 778 | 825 | 805 | |
| Maternal age, n (%), y | 2408 | 778 | 825 | 805 | .19 |
| <20 | 495 (21) | 149 (19) | 185 (22) | 161 (20) | |
| ≥20 | 913 (79) | 629 (81) | 640 (78) | 644 (80) | |
| Maternal education, n (%) | 2408 | 778 | 825 | 805 | .018 |
| No formal schooling | 766 (32) | 270 (35) | 245 (30) | 251 (31) | |
| Primary | 908 (38) | 259 (33) | 322 (39) | 327 (41) | |
| Secondary + | 734 (31) | 249 (32) | 258 (31) | 227 (28) | |
| BMI, n, kg/m2 | 2407 | 778 | 824 | 805 | |
| Mean ± SD | 21.4 ± 4 | 21.4 ± 4 | 21.5 ± 4 | 21.5 ± 4 | .90 |
| BMI <18.5, n (%) | 556 (23) | 183 (24) | 194 (24) | 179 (22) | |
| Height, N, cm | 2407 | 778 | 824 | 805 | |
| Mean ± SD | 151.3 ± 7 | 151.6 ± 7 | 151.2 ± 7 | 151.2 ± 7 | .51 |
| Stunted, n (%)‡ | 949 (39) | 282 (36) | 340 (41) | 327 (41) | |
| Parity, n (%) | 2408 | 778 | 825 | 805 | .22 |
| 0 (nulliparous) | 485 (20) | 181 (23) | 159 (19) | 145 (18) | |
| 1 | 764 (32) | 238 (31) | 263 (32) | 263 (33) | |
| ≥2 | 1159 (48) | 359 (46) | 403 (49) | 397 (49) | |
| Tally of indicators of higher SES, n (%)§ | 2408 | 778 | 825 | 805 | .90 |
| None (0 present) | 321 (13) | 104 (13) | 108 (13) | 109 (14) | |
| 1–2 present | 680 (28) | 234 (30) | 229 (28) | 217 (27) | |
| 3–4 present | 973 (40) | 300 (39) | 340 (41) | 333 (41) | |
| 5–6 present | 434 (18) | 140 (18) | 148 (18) | 146 (18) |
BMI, body mass index; SES, socioeconomic status.
Woman had a live birth in the longitudinal analysis population; includes all live born infants with birth length measurements obtained by 7 days of age and had at least 2 follow-up measurements at 1 month, 3 months, or 6 months.
P values from χ2 tests and ANOVA analysis to assess for differences between characteristics by treatment arm.
Stunted defined as −2 SD for the height-for-age z scores among girls from the 2007 WHO cutoffs. This is 147.9 cm for 15-year-old girls, 148.9 cm for 16-year-old girls, 149.5 cm for 17-year-old girls, 149.8 cm for 18-year-old girls, and 150 cm for 19+ year-old girls.1,2
The SES tally provides the number of indicators available from the following list: electricity, improved water source, sanitation, synthetic flooring, improved cooking fuels, and household assets.
Figure 1.
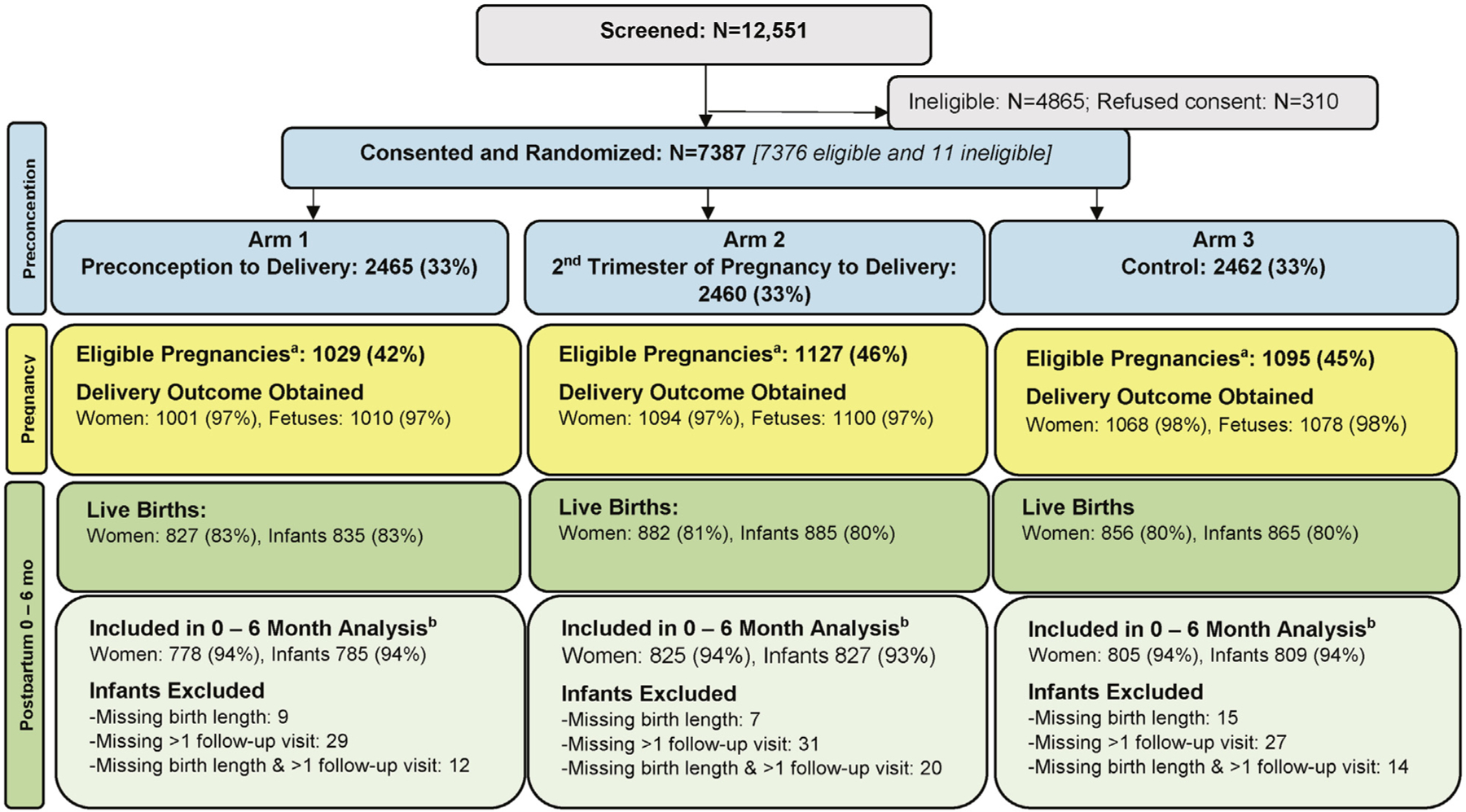
Consort diagram: screening, randomization, and longitudinal 0- to 6-month analyses population by intervention arm. a Excludes women who became pregnant <3 months into the study. The women who had eligible pregnancies may have had delivery data obtained or they may have exited the study before delivery. bAfter excluding extreme invalid measurements as determined by expert internal review, the 0- to 6-month longitudinal analysis subset includes all live-born infants with birth length measurements measured by 7 days of age and at least 2 follow-up visits (1-month, 3-month, or 6-month).
Anthropometric measurements at birth, 1, 3, and 6 months for the infants included in the longitudinal analysis are presented by arm in Table II. Mean unadjusted length-for-age z scores (LAZ) at birth were approximately −1.0 for both Arms 1 and 2, and −1.25 for Arm 3, similar to previously reported birth outcomes15 and indicated in Figure 2. The analysis for longitudinal LAZ and weight-for-age z score (WAZ) changes from birth to 6 months demonstrated no difference between Arms 1 and 2, but both were more positive compared with Arm 3 (Figure 2). For all arms, the mean LAZ scores were relatively stable from birth through 3 months but declined between 3 and 6 months (Figure 2).
Table II.
All sites unadjusted growth outcomes from birth to 6 months of age by intervention arm*
| Arm 1† |
Arm 2† |
Arm 3† |
Unadjusted pairwise arm comparisons and 95% CI‡ |
|||
|---|---|---|---|---|---|---|
| Variables | (N = 785) | (N = 827) | (N = 809) | Arm 1 vs 3 | Arm 2 vs 3 | Arm 1 vs 2 |
| Length, cm | ||||||
| Birth (<7 d) | 47.7 ± 2.2 (783) | 47.6 ± 2.0 (826) | 47.3 ± 2.1 (806) | 0.4 (0.2–0.6) | 0.3 (0.1–0.6) | 0.0 (−0.2 to 0.3) |
| 1 mo | 52.0 ± 2.4 (775) | 52.0 ± 2.3 (820) | 51.6 ± 2.4 (807) | 0.36 (0.1–0.6) | 0.4 (0.2–0.6) | −0.0 (−0.3 to 0.2) |
| 3 mo | 58.4 ± 2.5 (779) | 58.2 ± 2.5 (822) | 57.8 ± 2.6 (802) | 0.55 (0.3–0.8) | 0.4 (0.1–0.6) | 0.2 (−0.0 to 0.5) |
| 6 mo | 63.9 ± 2.6 (768) | 63.6 ± 2.7 (813) | 63.4 ± 2.6 (794) | 0.46 (0.2–0.7) | 0.2 (−0.0 to 0.5) | 0.2 (−0.0 to 0.5) |
| Weight, g | ||||||
| Birth (<7 d) | 2818 ± 434 (783) | 2812 ± 414 (826) | 2756 ± 415 (806) | 62.5 (20.7–104.3) | 56.7 (16.5–97.0) | 5.8 (−35.7 to 47.2) |
| 1 mo | 3738 ± 642 (777) | 3756 ± 617 (820) | 3703 ± 642 (807) | 35.2 (−28.1 to 98.5) | 52.5 (−8.7 to 113.7) | −17.3 (−79.1 to 44.5) |
| 3 mo | 5437 ± 894 (780) | 5401 ± 874 (822) | 5334 ± 896 (806) | 102.2 (14.1–190.4) | 66.8 (−19.2 to 152.8) | 35.4 (−51.2 to 122.0) |
| 6 mo | 6755 ± 1054 (770) | 6729 ± 1032 (816) | 86684 ± 1016 (795) | 70.4 (−32.3 to 173.0) | 44.7 (−55.4 to 144.9) | 25.6 (−77.2 to 128.4) |
| BMI, kg/m2 | ||||||
| Birth (<7 d) | 12.4 ± 1.3 (780) | 12.4 ± 1.2 (823) | 12.3 ± 1.2 (804) | 0.1 (−0.0 to 0.2) | 0.1 (−0.0 to 0.2) | −0.0 (−0.1 to 0.1) |
| 1 mo | 13.8 ± 1.6 (773) | 13.8 ± 1.6 (817) | 13.8 ± 1.6 (802) | −0.1 (−0.2 to 0.1) | −0.0 (−0.2 to 0.2) | −0.1 (−0.2 to 0.1) |
| 3 mo | 15.9 ± 1.9 (778) | 15.9 ± 1.9 (818) | 15.9 ± 1.9 (794) | −0.1 (−0.3 to 0.1) | −0.0 (−0.2 to 0.2) | −0.0 (−0.2 to 0.2) |
| 6 mo | 16.5 ± 1.9 (766) | 16.6 ± 1.8 (811) | 16.6 ± 1.8 (792) | −0.1 (−0.2 to 0.1) | −0.0 (−0.2 to 0.2) | −0.0 (−0.2 to 0.1) |
| Weight/length ratio, kg/m | ||||||
| Birth (<7 d) | 6.0 ± 0.7 (701) | 6.0 ± 0.6 (746) | 6.0 ± 0.6 (699) | 0.1 (−0.0 to 0.1) | 0.1 (−0.0 to 0.1) | 0.0 (−0.1 to 0.1) |
| 1 mo | 7.2 ± 1.0 (766) | 7.2 ± 1.0 (809) | 7.2 ± 1.0 (795) | 0.0 (−0.1 to 0.1) | 0.1 (−0.1 to 0.1) | −0.0 (−0.1 to 0.1) |
| 3 mo | 9.3 ± 1.3 (777) | 9.3 ± 1.3 (818) | 9.2 ± 1.3 (799) | 0.1 (−0.1 to 0.2) | 0.1 (−0.1 to 0.2) | 0.0 (−0.1 to 0.1) |
| 6 mo | 10.6 ± 1.4 (766) | 10.5 ± 1.3 (813) | 10.5 ± 1.3 (792) | 0.0 (−0.1 to 0.2) | 0.0 (−0.1 to 0.2) | 0.0 (−0.1 to 0.2) |
| HC, cm | ||||||
| Birth (<7 d) | 33.3 ± 1.5 (779) | 33.3 ± 1.4 (823) | 33.2 ± 1.5 (803) | 0.1 (−0.1 to 0.2) | 0.1 (−0.1 to 0.2) | −0.0 (−0.1 to 0.1) |
| 1 mo | 36.0 ± 1.5 (772) | 36.0 ± 1.4 (817) | 36.0 ± 1.5 (803) | 0.0 (−0.1 to 0.2) | 0.1 (−0.1 to 0.2) | −0.1 (−0.2 to 0.1) |
| 3 mo | 38.9 ± 1.5 (776) | 38.9 ± 1.4 (820) | 38.9 ± 1.5 (805) | 0.0 (−0.1 to 0.2) | −0.0 (−0.2 to 0.1) | 0.0 (−0.1 to 0.2) |
| 6 mo | 41.5 ± 1.6 (764) | 41.5 ± 1.5 (814) | 41.5 ± 1.5 (792) | −0.0 (−0.2 to 0.1) | −0.0 (−0.2 to 0.1) | 0.0 (−0.1 to 0.2) |
After we excluded extreme invalid measurements as determined by expert manual review, the longitudinal analysis subset includes all live-born infants with birth length measurements measured by 7 days of age who had at least 2 follow-up visits (1-month, 3-months, or 6-months) with length measurements.
Values presented as mean ± SD (n).
Unadjusted pairwise mean differences and 95% confidence limits were obtained from ± test assuming equal variance across arms.
Figure 2.
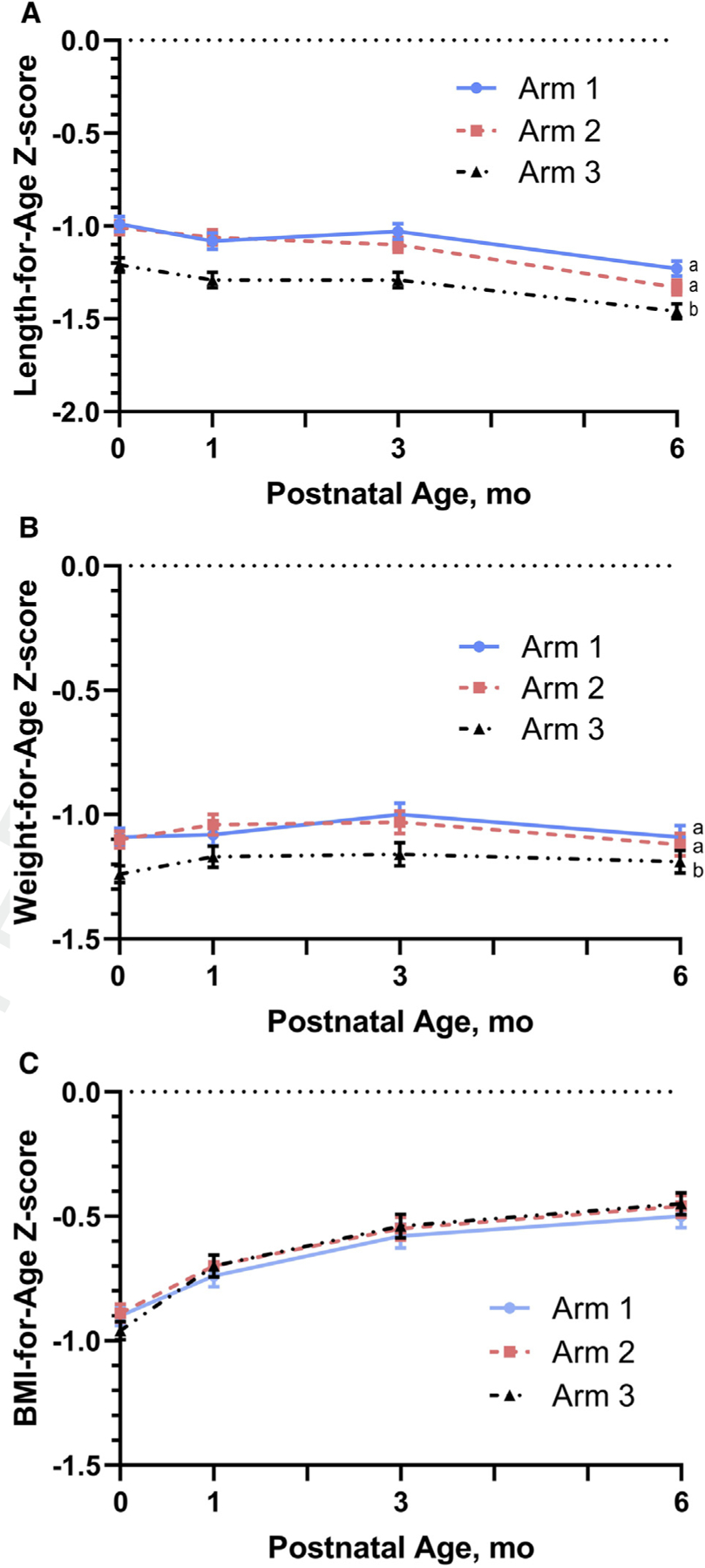
Longitudinal data for A, LAZ, B, WAZ; and C, BMI-for-age z score from birth to 6 months of age by intervention arm. Data are presented as mean ± SEM. Maternal participants in Arm 1 (N = 785 infants) started lipid-based nutrition supplements ≥3 months before conception; Arm 2 (N = 827 infants) started the same supplement at ~11 weeks of gestation; and Arm 3 (control, N = 809 infants) received no trial supplements. Different subscripts denote significant differences between arms when adjusted for site, cluster, maternal education, parity, and (for LAZ) site-by-arm interaction (P < .05).
The adjusted pairwise mean differences for length over the 6-month study period were 0.49 cm (95% CI 0.28–0.70, P < .001) and 0.34 cm (95% CI 0.13–0.54, P = .001) for Arms 1 and 2, respectively, compared with Arm 3 (Table III). The adjusted longitudinal analysis showed that the interaction between arm and site was close to our cut-off for significance for LAZ (P = .07) and was, therefore, included in the final model. Adjusted mean differences for Arm 1 vs Arm 3 for LAZ were statistically different, ranging from 0.21 to 0.44, for all sites but Guatemala (Table III). Mean differences for Arm 2 vs Arm 3 were smaller and statistically significant only for Democratic Republic of the Congo and Guatemala (Table III). No other interactions for combined sites were significant and were excluded from all other final models for all other outcome measures.
Table III.
Treatment effects for 0–6 month continuous and categorical anthropometric outcomes*† among the longitudinal analysis subset‡
| Variables§ |
Arm 1 vs Arm 3 |
Arm 2 vs Arm 3 |
Arm 1 vs Arm 2 |
|||
|---|---|---|---|---|---|---|
| Continuous variables | Adjusted mean difference (95% CI)* | P value* | Adjusted mean difference (95% CI)* | P value* | Adjusted mean difference (95% CI)* | P value* |
| Length, cm | 0.50 (0.30–0.71) | <.001 | 0.33 (0.12–0.53) | .002 | 0.18 (−0.03 to 0.38) | .09 |
| LAZ¶* | ||||||
| DRC | 0.44 (0.24–0.64) | <.001 | 0.27 (0.09–0.46) | .004 | 0.17 (−0.02 to 0.36) | .08 |
| Guatemala | 0.06 (−0.11 to 0.23) | .51 | 0.18 (0.01–0.34) | .03 | −0.12 (−0.30 to 0.05) | .17 |
| India | 0.21 (0.01–0.40) | .04 | 0.15 (−0.05 to 0.35) | .15 | 0.06 (−0.03 to 0.15) | .57 |
| Pakistan | 0.33 (0.12–0.55) | .002 | 0.13 (−0.08 to 0.34) | .22 | 0.2 (0.00–0.40) | .05 |
| Weight, g | 104.6 (45.4–163.8) | <.001 | 61.5 (4.0–119.1) | .04 | 43.1 (−15.1 to 101.2) | .15 |
| WAZ | 0.19 (0.09–0.28) | <.001 | 0.13 (0.04–0.22) | .005 | 0.06 (−0.15 to 0.26) | .20 |
| BMI, kg/m2 | 0.08 (−0.03 to 0.19) | .17 | 0.04 (−0.06 to 0.15) | .44 | 0.03 (−0.07 to 0.14) | .53 |
| BMI-for-age z score | 0.06 (−0.02 to 0.14) | .15 | 0.04 (−0.04 to 0.12) | .33 | 0.02 (−0.06 to 0.10) | .60 |
| Weight/length ratio | 0.12 (0.04–0.20) | .003 | 0.07 (−0.01 to 0.14) | .10 | 0.06 (−0.02 to 0.14) | .15 |
| WLZ | −0.01 (−0.09 to 0.07) | .77 | −0.03 (−0.10 to 0.05) | .48 | 0.02 (−0.06 to 0.09) | .69 |
| HC, cm | 0.08 (−0.04 to 0.20) | .21 | 0.05 (−0.07 to 0.16) | .41 | 0.03 (−0.09 to 0.15) | .63 |
| HC-for-age z score | 0.07 (−0.02 to 0.17) | .12 | 0.06 (−0.03 to 0.15) | .20 | 0.02 (−0.08 to 0.11) | .72 |
| Categorical variables | Relative risk (95% CI)† | P value† | Relative risk (95% CI)† | P value** | Relative risk (95% CI)† | P value† |
| LAZ <–2 | 0.76 (0.66–0.87) | <.001 | 0.77 (0.67–0.88) | <.001 | 0.98 (0.84–1.14) | .82 |
| WAZ <–2** | 0.83 (0.72–0.95) | .008 | 0.84 (0.73–0.97) | .02 | 0.98 (0.84–1.13) | .78 |
| BMI-for-age z score <–2 | 0.89 (0.75–1.04) | .13 | 0.92 (0.78–1.07) | .27 | 0.97 (0.82–1.13) | .68 |
| WLZ <–2†† | 1.00 (0.82–1.23) | .98 | 1.12 (0.92–1.37) | .26 | 0.89 (0.73–1.09) | .27 |
| HCAZ <−2 | 0.99 (0.83–1.17) | .88 | 0.92 (0.78–1.09) | .34 | 1.07 (0.90–1.27) | .42 |
DRC, Democratic Republic of the Congo.
Adjusted mean differences, 95% CI, and P values comparing the means of continuous growth outcomes for the pairwise comparisons of the intervention arms were obtained from general linear models with generalized estimating equations for the outcome of interest, adjusted for country, cluster nested within country, maternal education level (α = 0.10), primiparous/multiparous birth indicator, interaction between intervention x primiparous/multiparous birth indicator (α = 0.10), chronological age at the visit, interaction between intervention × country (α = 0.10), sex and its interaction with intervention (α = 0.10) while modeling the within infant correlations across visits with an auto-regressive order 1 (ie, AR[1]). As these are exploratory analyses, no correction for multiple comparisons were made.
Unless otherwise specified, relative risks, 95% CI, and P values comparing proportions of categorical outcomes for the pairwise comparisons of the intervention arms are obtained from log-binomial generalized linear models with generalized estimating equations to estimate parameters while adjusting for country, cluster nested within country, maternal education level (α = 0.10), primiparous/multiparous birth indicator, interaction between intervention × primiparous/multiparous birth indicator (α = 0.10), chronological age at the visit, interaction between intervention × country (α = 0.10), sex and its interaction with intervention (α = 0.10) while modeling the within infant correlations across visits with an auto-regressive order 1 (ie, AR[1]). The robust Poisson generalized linear model was fitted when the log-binomial model failed to converge. If this model also failed to converge, a model which did not account for the clustering of the data was used. As these are exploratory analyses, no correction for multiple comparisons were made.
After we excluded extreme invalid measurements as determined by expert internal manual review, the longitudinal analysis subset includes all live-born infants with birth length measurements measured by 7 days of age and at least 2 follow-up visits (1-, 3-, or 6-month[s]) completed.
All LAZ, WAZ, WLZ, BMI-for-age z score, and HC-for-age z scores were calculated using the expanded tables of the WHO Child Growth Standards.1 LAZ, WAZ, BMI-for-age z score, WLZ, and HC-for-age z score were within the biologically plausible range according to WHO standards (—6≤ LAZ or WAZ ≤6, 5≤ BMI-for-age z score or WLZ or HC-for-age z score ≤5). If an infant was found to have a biologically implausible LAZ or WAZ at a visit, all growth outcomes at the visit were set to missing. If an infant was found to have a biologically implausible BMI-for-age z score, WLZ, or HC-for-age z score at a visit, only the corresponding measurement and z score at the visit were set to missing. WLZ could not be obtained for infants with a length of less than 45.0 cm at any visit due to limitations in the WHO standards and were set to missing for that visit.1
In the longitudinal analysis of LAZ, the intervention × country interaction was significant at α = 0.10 (P = .07) and thus was controlled for in the model.
For the binary growth outcomes WAZ <–2, the log-binomial generalized linear model failed to converge, and thus the specified robust Poisson model† was fitted.
Due to clusters with no infants having a WLZ <–2 the specified log-binomial generalized linear model† did not converge, and therefore, the specified log-binomial generalized linear model† was fitted without adjusting for clusters.
Unadjusted means of WAZ at birth were also slightly lower than −1.0 for Arms 1 and 2, and as for LAZ, the longitudinal mean WAZ for both arms were consistently less negative than that of Arm 3 over the 6-month period (Figure 2). In contrast to the LAZ, however, the WAZ did not decline appreciably over the birth to 6 months period (Figure 2). The adjusted mean differences in WAZ for both Arm 1 and Arm 2 compared with control Arm 3 were smaller than for LAZ, although both differences were statistically significant (P < .001 and .005, respectively) (Table III). No differences by arm at birth or in the longitudinal analyses were observed among arms for BMI-for-age z score, WLZ, or HC-for-age z score (Table III).
No growth differences were observed between sexes, and models adjusted for maternal characteristics, including parity and education, demonstrated similar findings as the unadjusted models. Longitudinal analyses further adjusted for parity (primiparous vs multiparous) did not substantially change the comparisons of mean differences for continuous variables or the adjusted relative risks for the binary outcomes (data not shown).
Stunting rates across the 6-month period were similar for Arms 1 and 2 and were significantly lower than those for Arm 3 (Figure 3). The adjusted mean probability for stunting changed only slightly between birth and 3 months for all arms, but rates increased for all arms between 3 and 6 months (Figure 3). The adjusted relative risks for stunting over the study period were 0.76 (95% CI 0.66–0.87) and 0.77 (95% CI 0.67–0.88) for Arm 1 vs Arm 3 (P < .001) and for Arm 2 vs Arm 3 (P < .001), respectively (Table III). At 6 months of age, the percentages of stunted infants were 22.9%, 25.2%, and 30.7% for Arms 1, 2, and 3, respectively (Figure 3). In contrast to stunting, no statistical differences in the longitudinal risk of wasting (BMI-for-age z score <–2 and WLZ <–2) or of low head circumference (HC-for-age z score <–2) were observed for any comparison among arms; risk of underweight (WAZ <–2) was reduced for both Arms 1 and 2 compared with Arm 3 (Table III).
Figure 3.
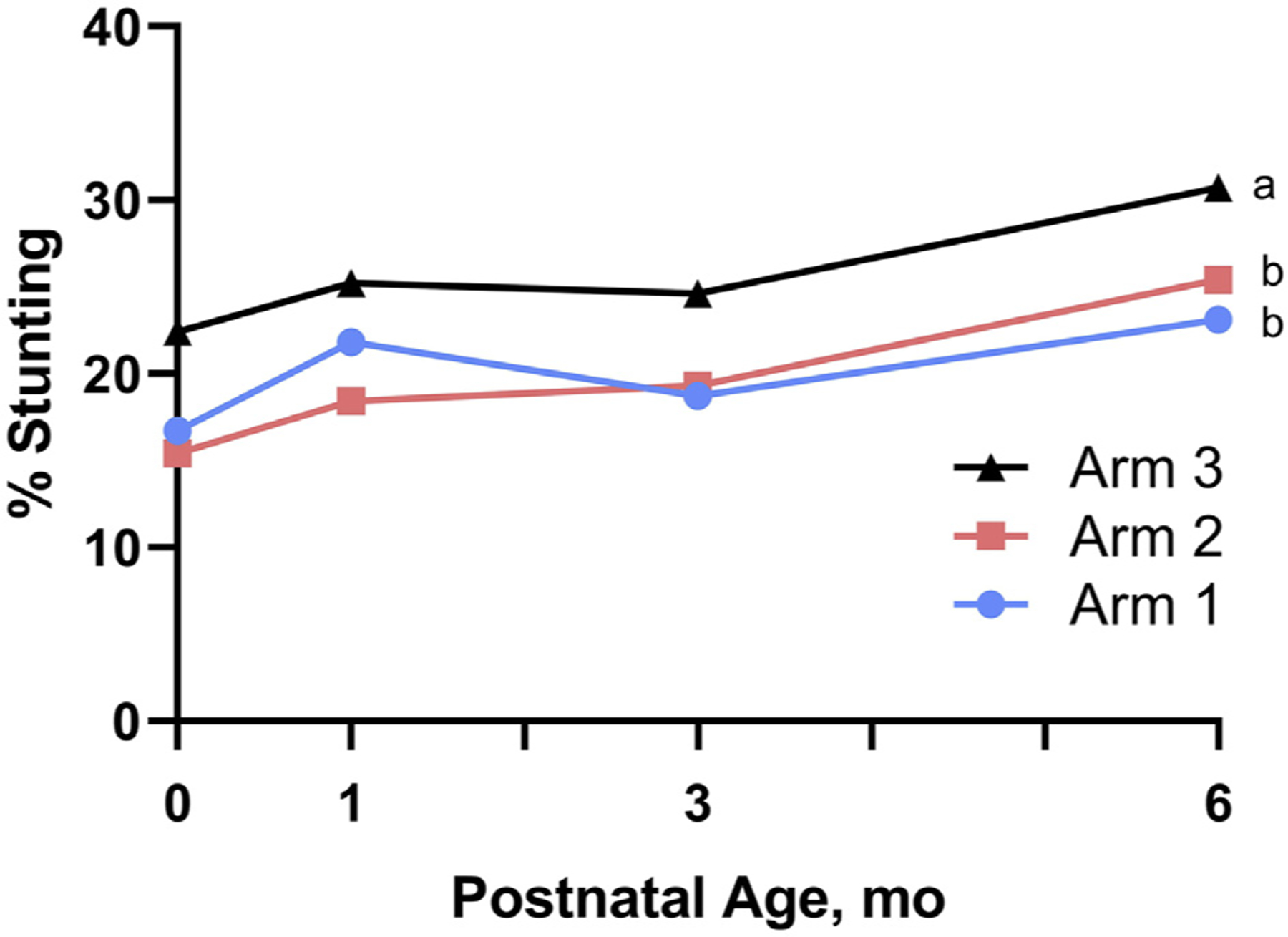
Progression of stunting from birth to 6 months of age by intervention arm. Data represent unadjusted means. Maternal participants in Arm 1 (N = 785 infants) started lipid-based nutrition supplements ≥3 months before conception; Arm 2 (N = 827 infants) started same supplement at ~11 weeks of gestation; and Arm 3 (control, N = 809 infants) received no trial supplements. Different superscripts denote significant differences between arms.
Maternal reports of infant feeding practices indicated essentially universal breastfeeding, with rates of >99% for women in all arms throughout the study period. Pre-lacteal feeds in the first 3 days after birth were reported by 20%, 17%, and 16% of women in Arms 1, 2, and 3, respectively. The most commonly reported pre-lacteal fluids were traditional medicines (10%), infant formula (2.1%), and plain water (1.7%), with no differences by arm. Exclusive breastfeeding at 14 days was reported by 80%−84% of women in the three intervention arms; at 1 and 3 months, the figures were lower at 72%−74% and 69%−71%, respectively. The dominant non-breast milk liquid was traditional medicine (20%), followed by other milk (4–5%), and plain water (2%−8%). By 6 months, most mothers (58%) reported offering non-breast milk liquids, most commonly plain water (39%), traditional medicine (18%), and thin porridge (15%). At 3 months, less than 5% of mothers reported use of non-liquid complementary foods, whereas by 6 months, most reported offering some type of complementary foods, with cereals (non-commercial), fruits and vegetables, and some type of fat being the foods most commonly reported. No differences were evident among arms at any time point or for any liquid or food, with the differences between arms typically less than 3%.
Reported illnesses for all livebirth singletons did not differ by arm, but the number of hospitalizations was higher for Arm 1 (5%) compared with Arm 3 (2.8%) (relative risk 1.80, 95% CI 1.09–2.97, P = .02) (Table IV; available at www.jpeds.com). No statistical differences were observed among arms for respiratory, diarrheal, or malaria illnesses (Table IV). With respect to mortality, Arm 2 had a greater rate of neonatal deaths (<28 days) compared with Arm 3, with neonatal mortality rates (NMR) 41.9 per 1000 vs 23.3 per 1000, respectively (RR 1.79; 95% CI 1.08–2.97; P = .02); rate for Arm 1 vs Arm 3 was intermediate at 37.2/1000 (RR 1.56; 95% CI 0.87–2.81; P = .14). For all arms, most (75%−84%) of the neonatal deaths occurred during the first week of life. Primary causes of death were “infection/trouble breathing” (44%), “prematurity or low birth weight” (13%), and “birth asphyxia” (8%). Inspection of the by-site data indicated only Pakistan had an NMR greater for Arm 2 vs Arm 3: 66.4 per 1000 vs 23.4 per 1000 (RR 2.84; 95% CI 1.05–7.68; P = .04). Infant deaths over the entire 6-month study period did not differ by arm (Table IV).
Table IV.
All-sites morbidity and mortality by intervention arm among all live-birth singletons*
| Intervention Arm |
Arm 1 vs Arm 3† |
Arm 2 vs Arm 3† |
Arm 1 vs Arm 2† |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcomes | Arm 1 (N = 807)* | Arm 2 (N = 869) | Arm 3 (N = 839) | Relative risk (95% CI)* | P Value | Relative risk (95% CI)* | P Value | Relative risk (95% CI)* | P Value |
| Illness/health problem, n/N (%) | 319/787 (40.5) | 309/841 (36.7) | 286/824 (34.7) | 1.11 (0.99–1.24) | .07 | 1.05 (0.95–1.15) | .37 | 1.06 (0.96–1.17) | .23 |
| No illnesses: 0, n/N (%) | 468/787 (59.5) | 532/841 (63.3) | 538/824 (65.3) | ||||||
| Number of illnesses: 1, n/N (%) | 158/787 (20.1) | 159/841 (18.9) | 155/824 (18.8) | ||||||
| Number of illnesses: 2, n/N (%) | 83/787 (10.5) | 87/841 (10.3) | 80/824 (9.7) | ||||||
| Number of illnesses: ≥3, n/N (%) | 78/787 (9.9) | 63/841 (7.5) | 51/824 (6.2) | ||||||
| Hospitalization, n/N (%) | 39/787 (5.0) | 30/839 (3.6) | 23/824 (2.8) | 1.80 (1.09–2.97) | .02 | 1.30 (0.77–2.21) | .33 | 1.38 (0.87–2.19) | .17 |
| Respiratory illness, n/N (%) | 141/786 (17.9) | 151/836 (18.1) | 138/822 (16.8) | 1.09 (0.93–1.28) | .31 | 1.08 (0.90–1.29) | .43 | 1.01 (0.85–1.20) | .89 |
| Diarrhea, n/N (%) | 50/786 (6.4) | 36/836 (4.3) | 42/822 (5.1) | 1.19 (0.85–1.67) | .32 | 0.83 (0.53–1.32) | .44 | 1.42 (0.97–2.09) | .07 |
| Malaria, n/N (%) | 21/786 (2.7) | 18/836 (2.2) | 14/822 (1.7) | 1.50 (0.77–2.90) | .23 | 1.25 (0.63–2.48) | .52 | 1.20 (0.65–2.21) | .57 |
| Mortality | |||||||||
| Neonatal mortality (<28 d), n/N (rate/1000)‡ | 31/833 (37.2) | 37/883 (41.9) | 20/859 (23.3) | 1.56 (0.87–2.81) | .14 | 1.79 (1.08–2.97) | .03 | 0.87 (0.58–1.32) | .52 |
| Infant mortality (<6 mo), n/N (rate/1000) | 29/799 (36.3) | 39/863 (45.2) | 29/829 (35.0) | 0.99 (0.59–1.66) | .96 | 1.27 (0.87–1.87) | .22 | 0.78 (0.54–1.12) | .18 |
Since this was collected at the family level (vs by infant), data for live birth twins are unidentifiable and therefore excluded. The data presented in this table are for all singleton live births or twin births resulting in only 1 live birth.
Relative risks, 95% CIs, and P values comparing proportions for the pairwise comparisons of the intervention arms are obtained from generalized linear models with generalized estimating equations adjusting for country while controlling for cluster correlations with an independent working correlation structure and a robust sandwich estimator (empirical estimates). This specified model did not converge for hospitalization and malaria, and thus, a log-binomial model without adjust for cluster correlations was fitted. As these are exploratory analyses, no correction for multiple comparisons have been made.
The denominators for neonatal mortality rate at 28 days are all live births.
Discussion
The key finding in this analysis was the persistence through the first 6 months of postnatal life of the benefits for fetal growth that resulted from comprehensive maternal nutritional supplementation initiated before conception or at the end of the first trimester. Despite no postnatal intervention, both length and weight, and the respective z scores, were significantly greater, and stunting rates were lower at 6 months for infants of mothers randomized to the intervention arms compared with those of the women in the control group. The multisite study was undertaken in diverse, low=resource settings, all with rates of young child stunting ≥40%.1 LAZ scores declined and stunting rates increased for all 3 groups, especially between 3 and 6 months, but stunting rates remained 20%−30% greater at 6 months for the infants of mothers in the control arm compared with those of women who received the intervention.
Our findings contrast with the limited literature on growth patterns in early infancy after maternal nutrition supplementation during pregnancy and lactation. Two studies of pre- and postnatal maternal micronutrient supplementation with SQ-LNS that were conducted in Bangladesh and Malawi reported no difference in length velocity or LAZ by group for the birth to 6 months’ postnatal period.9,10 Our findings also contrast with those of a randomized trial in Burkina Faso that compared effects of LNS vs multiple micronutrient supplements initiated in the second trimester and continued until parturition. In that study, although birth length was significantly greater in infants of mothers who received LNS, the postnatal growth was actually slower in the LNS group, and by 6 months the growth trajectories had converged, and no difference in LAZ at 6 months was observed.8 The differing results among these studies and our findings for early growth may reflect differences in study populations, baseline maternal nutritional status, and/or in study design, eg, the early timing and/or the comprehensive nature of the nutrition intervention, which included both the SQ-LNS and a lipid-based balanced protein and energy supplement without additional micronutrients.
The relatively steady linear growth between birth and 3 months also differs from multiple reports of substantial linear growth faltering in the early weeks of postnatal life.4,5,9,10,18
Cross-sectional data from national anthropometric measurements from 54 countries demonstrated an early and progressive decline in LAZ, particularly in Sub-Saharan Africa and South Asia.4 In a longitudinal observational study in the same area in Guatemala, the sharpest decline in LAZ occurred between birth and 3 months, with more gradual decline thereafter through 6 months.5 The large multicountry longitudinal birth cohort study, MAL-ED (Etiology, Risk Factors and Interactions of Enteric Infections and Malnutrition and the Consequences for Child Health and Development), which linked growth, enteric infections, and nutritional intake, reported considerable variability in the onset of stunting. In that study, sites in Africa and South America demonstrated increases in the proportion of stunted infants by approximately 3 months, but in the other sites increases occurred primarily after 6 months.18 In MAL-ED, asymptomatic enteropathogen detection, not diarrhea, was associated with slower linear growth as early as the first 2 months of postnatal life.19 Enteropathogen carriage was not tracked in our study and may have differed among the Women First trial sites. Indicators of socioeconomic status and water and sanitation ranged widely among sites20 but did not differ among the intervention arms for those women with birth outcomes.15 The significant interaction between intervention arm and site that we observed for LAZ supports this heterogeneity of linear growth faltering, but the significant positive effects of the intervention in three of the four sites for the preconception arm also supports generalizability of our findings.
Lack of exclusive breastfeeding has been associated with poor growth and infectious morbidity including, especially, diarrhea.21 Although rates of exclusive breastfeeding steadily declined, the rates were comparable with or exceeded those of the observational MAL-ED birth cohort study, which found no association of breastfeeding (full or partial) and linear growth in the first 6 months.19 In addition, 2 trials of maternal LNS supplementation for 6 months postpartum reported no benefit on linear growth.9,10 The randomized design of the intervention trial, the nearly parallel LAZ trajectories for all three arms, and the similarity of reported feeding practices suggest that prenatal factors were more potent than postnatal diet in influencing growth during this observation period. In addition, maternal nulliparity and baseline anemia were identified as critical effect modifiers in the Women First trial such that the women with these characteristics had newborns with substantially greater birth length and weight, especially for those in the preconception group.22 Although continued follow-up of these offspring of the Women First trial will be essential to determine the durability of these growth effects as other environmental factors, including dietary, water, and hygiene factors become progressively influential,19 our findings emphasize the importance of maternal nutrition for this critical period of post-natal growth and development.
The unexpected findings of greater hospitalizations for the infants of mothers in the preconception arm (Arm 1) compared with the controls, and of greater NMR for the infants of women in the prenatal arm (Arm 2) compared with the controls are difficult to explain, especially in light of the more favorable growth for infants of mothers in both of the intervention arms. The outstanding observation for the Pakistan site was actually the very low and somewhat-implausible NMR for the control arm, which was less than half that reported from the same communities in a multicountry maternal neonatal health registry.23,24 The NMR for Arms 1 and 2 in the Pakistan site were in line with the registry reports, suggesting that the Arm 3 finding was due to factors unrelated to the intervention. No differences among arms were observed for rates of either miscarriages (<20 weeks of gestation) or stillbirths (≥20 weeks of gestation), thus arguing against intervention group-based differences in potential vulnerability of the newborns.15 Moreover, a recent review that compared maternal and infant outcomes for prenatal SQ-LNS vs iron-folate supplements found no difference in neonatal deaths.25 We also found no differences among the intervention arms or among the sites in exclusive breastfeeding, which has consistently been found to be strongly protective against morbidity and mortality, especially related to diarrhea and other infectious diseases.26–28 Thus, in the absence of a plausible biological etiology and unless our findings are replicated in studies with larger sample sizes and more intense monitoring, we conclude that the greater NMR for the infants of the women in the prenatal intervention arm was due to chance and should not discourage ongoing interest in the use of SQ-LNS as a potentially effective nutrition-specific intervention.
The strengths of this study include the randomized assignment to the nutrition intervention arm before conception for all women; the relatively large sample size; and the high rate of follow-up for the postnatal measurements, with approximately 95% of live-born infants in the intervention phase of the trial having at least 2 measurements for the current analysis. We also acknowledge limitations, including the relatively low intensity of surveillance for morbidity and mortality outcomes, which was not sufficient to provide more insight for underlying explanation(s) for the observed findings.
In summary, in these diverse low-resource settings, both linear and ponderal growth from birth through 6 months were significantly greater and stunting rates were lower for infants whose mothers received nutrition supplementation in the preconception period or late in the first trimester compared with infants whose mothers received only the standard of care in their setting. These improved growth patterns impacted infants at the crucial time of most rapid postnatal growth and were observed despite no postnatal intervention to either the mothers or infants. Our results add to the current understanding of growth in the first half of the first 1000 days and support the critical and possibly enduring importance of optimized maternal nutrition before conception and the early phase of gestation.
Supplementary Material
Acknowledgments
Supported by the Bill & Melinda Gates Foundation, Seattle, WA (OPP1055867), and the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the Office of Dietary Supplements (U10 HD 076474 [to N.K. and K.H.] and UG1 HD 076474 [to N.K.]).
Glossary
- BMI
Body mass index
- HC
Head circumference
- LAZ
Length-for-age z score
- MAL-ED
Etiology, Risk Factors and Interactions of Enteric Infections and Malnutrition and the Consequences for Child Health and Development
- NMR
Neonatal mortality rate
- SQ-LNS
Small quantity-lipid nutrient supplement
- WAZ
Weight-for-age z score
- WHO
World Health Organization
- WLZ
Weight-for-length z score
Appendix
List of Women First Preconception Maternal Nutrition Study Group members.
Justin Gado; Kinshasha School of Public Health, Kinshasha, Democratic Republic of the Congo.
Manjunath S. Somannavar and Veena Herekar; KLE Academy of Higher Education and Research’s Jawaharlal Nehru Medical College, Belagavi, India.
Omrana Pasha and Umber Khan; Aga Khan University, Karachi, Pakistan.
Elizabeth M. McClure; RTI International, Durham, NC.
Marion Koso-Thomas; Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health, Bethesda, MD.Data ProfileConsort Checklist
Footnotes
List of Women First Preconception Maternal Nutrition Study Group members available at www.jpeds.com
The authors declare no conflicts of interest.
Portions of this study were presented at the annual meeting of the American Society for Nutrition (“Nutrition 2019”), June << >>, 2019, Baltimore, MD.
Data Statement
Data sharing statement available at www.jpeds.com.
References
- 1.World Bank. World Bank Annual Report 2016. Washington, DC: World Bank; 2016. [Google Scholar]
- 2.World Health Organization. Global nutrition targets 2025: policy brief series (WHO/NMH/NH/14.2). Geneva: World Health Organization; 2014. [Google Scholar]
- 3.United Nations Children’s Fund, World Health Organization, World Bank. Joint Child Malnutrition Estimates. Global Database on Child Growth and Malnutrition; 2015. ed2019. [Google Scholar]
- 4.Victora CG, de Onis M, Hallal PC, Blossner M, Shrimpton R. Worldwide timing of growth faltering: revisiting implications for interventions. Pediatrics 2010;125:e473–80. [DOI] [PubMed] [Google Scholar]
- 5.Berngard SC, Berngard JB, Krebs NF, Garces A, Miller LV, Westcott J, et al. Newborn length predicts early infant linear growth retardation and disproportionately high weight gain in a low-income population. Early Hum Dev 2013;89:967–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Solomons NW, Vossenaar M, Chomat AM, Doak CM, Koski KG, Scott ME. Stunting at birth: recognition of early-life linear growth failure in the western highlands of Guatemala. Public Health Nutr 2015;18: 1737–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mal-Ed Network Investigators. Childhood stunting in relation to the pre- and postnatal environment during the first 2 years of life: the MAL-ED longitudinal birth cohort study. PLoS Med 2017;14:e1002408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lanou H, Huybregts L, Roberfroid D, Nikiema L, Kouanda S, Van Camp J, et al. Prenatal nutrient supplementation and postnatal growth in a developing nation: an RCT. Pediatrics 2014;133:e1001–8. [DOI] [PubMed] [Google Scholar]
- 9.Dewey KG, Mridha MK, Matias SL, Arnold CD, Cummins JR, Khan MS, et al. Lipid-based nutrient supplementation in the first 1000 d improves child growth in Bangladesh: a cluster-randomized effectiveness trial. Am J Clin Nutr 2017;105:944–57. [DOI] [PubMed] [Google Scholar]
- 10.Ashorn P, Alho L, Ashorn U, Cheung YB, Dewey KG, Gondwe A, et al. Supplementation of maternal diets during pregnancy and for 6 months postpartum and infant diets thereafter with small-quantity lipid-based nutrient supplements does not promote child growth by 18 months of age in rural Malawi: a randomized controlled trial. J Nutr 2015;145: 1345–53. [DOI] [PubMed] [Google Scholar]
- 11.Fleming TP, Watkins AJ, Velazquez MA, Mathers JC, Prentice AM, Stephenson J, et al. Origins of lifetime health around the time of conception: causes and consequences. Lancet 2018;391:1842–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.King JC. A summary of pathways or mechanisms linking preconception maternal nutrition with birth outcomes. J Nutr 2016;146:1437S–44S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramakrishnan U, Nguyen PH, Gonzalez-Casanova I, Pham H, Hao W, Nguyen H, et al. Neither preconceptional weekly multiple micronutrient nor iron-folic acid supplements affect birth size and gestational age compared with a folic acid supplement alone in rural Vietnamese women: a randomized controlled trial. J Nutr 2016;146:1445S–14452S. [DOI] [PubMed] [Google Scholar]
- 14.Potdar RD, Sahariah SA, Gandhi M, Kehoe SH, Brown N, Sane H, et al. Improving women’s diet quality preconceptionally and during gestation: effects on birth weight and prevalence of low birth weight—a randomized controlled efficacy trial in India (Mumbai Maternal Nutrition Project). Am J Clin Nutr 2014;100:1257–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hambidge KM, Westcott JE, Garces A, Figueroa L, Goudar SS, Dhaded SM, et al. A multicountry randomized controlled trial of comprehensive maternal nutrition supplementation initiated before conception: the Women First trial. Am J Clin Nutr 2019;109:457–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hambidge KM, Krebs NF, Westcott JE, Garces A, Goudar SS, Kodkany BS, et al. Preconception maternal nutrition: a multi-site randomized controlled trial. BMC Pregnancy Childbirth 2014;14:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization. The WHO Child Growth Standards: Length/height-for-age, weight-for-age, weight-for-height and body mass index-for-age Methods and development. Geneva: WHO; 2006. [Google Scholar]
- 18.Lang D, Mal-Ed Network Investigators. Opportunities to assess factors contributing to the development of the intestinal microbiota in infants living in developing countries. Microb Ecol Health Dis 2015;26:28316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mal-Ed Network Investigators. Relationship between growth and illness, enteropathogens and dietary intakes in the first 2 years of life: findings from the MAL-ED birth cohort study. BMJ Glob Health 2017;2:e000370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hambidge KM, Krebs NF, Garces A, Westcott JE, Figueroa L, Goudar SS, et al. Anthropometric indices for non-pregnant women of childbearing age differ widely among four low-middle income populations. BMC Public Health 2017;18:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, et al. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 2008;371:243–60. [DOI] [PubMed] [Google Scholar]
- 22.Hambidge KM, Bann CM, McClure EM, Westcott JE, Garces A, Figueroa L, et al. Maternal characteristics affect fetal growth response in the Women First Preconception Nutrition Trial. Nutrients 2019;11: 2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bose CL, Bauserman M, Goldenberg RL, Goudar SS, McClure EM, Pasha O, et al. The Global Network Maternal Newborn Health Registry: a multi-national, community-based registry of pregnancy outcomes. Reprod Health 2015;12(suppl 2):S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pasha O, Saleem S, Ali S, Goudar SS, Garces A, Esamai F, et al. Maternal and newborn outcomes in Pakistan compared to other low and middle income countries in the Global Network’s Maternal Newborn Health Registry: an active, community-based, pregnancy surveillance mechanism. Reprod Health 2015;12(suppl 2):S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Das JK, Hoodbhoy Z, Salam RA, Bhutta AZ, Valenzuela-Rubio NG, Weise Prinzo Z, et al. Lipid-based nutrient supplements for maternal, birth, and infant developmental outcomes. Cochrane Database Syst Rev 2018;8:CD012610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lamberti LM, Fischer Walker CL, Noiman A, Victora C, Black RE. Breastfeeding and the risk for diarrhea morbidity and mortality. BMC Public Health 2011;11(suppl 3):S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khan J, Vesel L, Bahl R, Martines JC. Timing of breastfeeding initiation and exclusivity of breastfeeding during the first month of life: effects on neonatal mortality and morbidity—a systematic review and meta-analysis. Matern Child Health J 2015;19:468–79. [DOI] [PubMed] [Google Scholar]
- 28.Sankar MJ, Sinha B, Chowdhury R, Bhandari N, Taneja S, Martines J, et al. Optimal breastfeeding practices and infant and child mortality: a systematic review and meta-analysis. Acta Paediatr 2015;104:3–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


