Abstract
Trimethylamine (TMA), formed by intestinal microbiota, and its Flavin-Monooxygenase 3 (FMO3) product Trimethylamine-N-Oxide (TMAO), are potential modulators of host cardiometabolic phenotypes. High circulating levels of TMAO are associated with increased risk for cardiovascular diseases. We hypothesized that TMA/TMAO could directly change the vascular tone. Perivascular adipose tissue (PVAT) helps to regulate vascular homeostasis and may also possess FMO3. Thoracic aorta with(+) or without(−) PVAT, also + or − the endothelium (E), of male Sprague Dawley rats were isolated for measurement of isometric tone in response to TMA/TMAO (1nM–0.5 M). Immunohistochemistry (IHC) studies were done to identify the presence of FMO3. TMA and TMAO elicited concentration-dependent arterial contraction. However, at a maximally achievable concentration (0.2 M), contraction stimulated by TMA was of a greater magnitude (141.5±16% of maximum phenylephrine contraction) than that elicited by TMAO (19.1±4.03%) with PVAT and endothelium intact. When PVAT was preserved, TMAO-induced contraction was extensively reduced the presence (19.1±4.03%) versus absence of E (147.2±20.5%), indicating that the endothelium plays a protective role against TMAO-induced contraction. FMO3 enzyme was present in aortic PVAT, but the FMO3 inhibitor methimazole did not affect contraction stimulated by TMA in aorta + PVAT. However, the L-type calcium channel blocker nifedipine reduced TMA-induced contraction by ~50% compared to the vehicle. Though a high concentration of these compounds was needed to achieve contraction, the findings that TMA-induced contraction was independent of PVAT and E and mediated by nifedipine-sensitive calcium channels suggest metabolite-induced contraction may be physiologically important.
Keywords: Trimethylamine (TMA), Trimethylamine N-oxide (TMAO), Perivascular adipose tissue (PVAT), Flavin-monooxygenase 3 (FMO3), aorta, vascular endothelium
Graphical abstract
1. Introduction
Cardiovascular diseases (CVD) are the leading cause of death and morbidity worldwide [1]. It is likely that the gut microbiota substantially contributes to the global epidemic of CVD [2–10] and cardiometabolic risk [11]. Clinical studies worldwide have identified trimethylamine N-oxide (TMAO), a molecule derived from gut microbiota products, to be independently associated with risk for CVD, predicting incident risks for myocardial infarction, stroke, and death [12–15]. In animal studies, TMAO administration promotes vascular inflammation [16,17] and atherosclerotic formations [12].
Trimethylamine (TMA) is an intermediate product derived from nutrients by the action of host gut microbiota [18,19] and is converted to TMAO mainly in the liver [20] by flavin-dependent monooxygenases. Dietary TMA containing compounds include choline, lecithin, and carnitine, which are found in meats and dairy products. Of the five functional flavin-dependent monooxygenases (FMOs 1-5) of humans, only FMO3, a key rate-limiting enzyme primarily expressed in the liver [21] but also prominently found in adipose tissue [22], effectively catalyzes the conversion of TMA to TMAO [23].
TMA and TMAO are found in high amounts in the systemic circulation, particularly in patients with CVD disease. In healthy patients, TMA plasma levels (0.5-1.2 μM) are lower than TMAO. In disease patients, variable results have been observed. TMA plasma concentration was reported as both decreased [24] or elevated [25] vs. controls.
TMAO plasma levels from healthy adults vary from 3.5 μM [26] to approximately 40 μM [25]. By contrast, patients with diseases, such as chronic kidney disease (CKD), present 40-fold elevated TMAO levels compared with control subjects [27,28]. TMAO, at a 3 mM concentration, acutely augments myocardial contractile force ex-vivo in both murine and human cardiac tissue [29], which is proposed to contribute to cardiac dysfunction during CKD. Rodents, the model used presently, are similar to humans in that they possess relatively similar levels of circulating TMA and TMAO to the human (TMA 2.5 μM and TMAO 10-15 μM in mice [12,26]). Three-month-old Sprague-Dawley rats were demonstrated to present 64.3 μM and 7.3 μM in plasma levels of TMA and TMAO, respectively [30].
Besides being associated with both prevalent atherosclerotic heart disease and adverse cardiac events [12–14;31–35], increased blood levels of TMAO is a risk factor for the development of hypertension and endothelial dysfunction in humans [36]. Aging is associated with increased plasma TMA in rats. There is a significant increase in TMA plasma levels in 18 months-old compared with 3-month-old rats [37]. TMA leads to degradation of the protein structure of lactate dehydrogenase and albumin, which is associated with deleterious actions on rats’ vascular smooth muscle and cardiac cells, also with increase arterial blood pressure [37,38]. No studies have investigated the direct vascular response to TMA or TMAO, nor whether these metabolites influence the function of PVAT and endothelium, key-players of vascular tone.
Dysfunctional PVAT or endothelium, such as observed in obesity or aging, are associated with abnormal changes in the vascular tone [39]. The existence of PVAT is critical to the maintenance of the vasculature in normal functional status [39,40], partially through paracrine factors. PVAT is also a site for metabolism [41]. Substances synthesized by PVAT, such as cytokines, are involved in atherosclerosis plaques development [42] and vascular alterations associated with hypertension, diabetes mellitus and obesity [43]. On the other hand, nitric oxide (NO) produced both by the endothelium cells and by PVAT promotes an anti-atherogenic effect via platelet aggregation inhibition and vascular smooth muscle relaxation and reduced mitogenesis/migration [44,45].
There are strong links between the TMA/FMO3/TMA0 pathway and adipose tissue function [22], as well as links between PVAT and the tone of vessels from different vascular beds [41,46,47]. The inflammatory mediators highly expressed in PVAT (mainly white-like ([43]), are similar to those associated with vascular inflammation promoted by increased systemic levels of TMAO [17, 40]. Considering common features connecting TMA/TMAO and PVAT, such as inflammatory processes, perhaps these gut microbiota metabolites are feasible factors to be considered in modifying vascular tone.
To our knowledge, few studies have examined the ability of TMA and TMAO to change vascular tone directly [29], let alone whether PVAT or the endothelium modifies a TMA- or TMAO-induced response. Thus, we hypothesize that TMA and TMAO are vasoactive compounds whose effects are modulated by FMO3 that is expressed in PVAT.
2. Materials and Methods
2.1. Chemicals.
All chemicals were purchased from Sigma-Aldrich (Saint Louis, MO USA). Phenylephrine (PE), acetylcholine (ACh), trimethylamine (TMA), trimethylamine-N-oxide (TMAO) and the FMO3 inhibitor methimazole (MTMZ) were solubilized and diluted in water. The L type calcium channel inhibitor nifedipine (NIF), was first diluted in DMSO (10 mM), then diluted in water to achieve a stock 100μM solution.
2.2. Animals and tissue preparation.
Male Sprague-Dawley rats with 10±1 week old (225-275 grams, Charles River, Indianapolis, IN USA) were used. All protocols were approved by the MSU Institutional Animal Care and Use Committee and follow the “Guide for the Care and Use of Laboratory Animals,” 8th edition (2011) and ARRIVE guidelines. Rats were anesthetized with sodium pentobarbital (60-80 mg/kg, IP). Deep anesthesia was verified by a lack of paw pinch and eye-blink reflexes. Death was assured by pneumothorax and exsanguination, and tissues were removed for one of the following protocols. Thoracic aorta was removed and placed in a Silastic® filled dish and kept under physiological salt solution [PSS in mM; NaCl 130; KCl 4.7; KH2PO4 1.18; MgSO4·7H2O 1.17; NaHCO3 14.8; dextrose 5.5; CaNa2EDTA 0.03, CaCl2 1.6 (pH 7.2)] during dissection.
Rat thoracic aorta rings (4 mm) were employed in vascular studies in different conditions: free of perivascular adipose tissue (− PVAT) or with adipose tissue intact (+ PVAT), in the presence (E +) or absence (E −) of the endothelium. To keep the aortic rings + PVAT, this adipose tissue was cleaned of blood clots while minimally handling the PVAT itself. For those protocols performed in the absence of endothelium (E −), the vascular endothelium was mechanically removed by gentle rubbing. In order to remove the E in vessel ring without damaging the PVAT, the aortic ring was held from the lumen in two stainless steel hooks and turned around itself from inside, always keeping it in the Petri dish containing PSS solution. The endothelium removal was completed when the ring was suspended in the organ bath chamber containing PSS before adding basal tension, in which the rings were loosely hung between the hook passing through their lumen, and the bubbles (95%O2/5%CO2 gas) gently turned the aortic rings around the two hooks during 30 seconds for one side and more 30 seconds for the other side. When this procedure was completed, before adding tension, the PSS solution was changed.
For the immunohistochemical studies, unmodified aortic rings were immediately formalin-fixed and processed by MSU Investigative Pathology Services to create paraffin-embedded sections.
2.3. Immunohistochemistry protocol
Slides with 8 micron thick sections of rat liver (positive control; Zyagen, San Diego CA USA) or dissected thoracic aorta were submerged twice in HistoChoice Clearing Agent (catalog no. H103; Amresco, Solon, OH, USA), four times in 100% isopropanol, and twice in dH2O, for 3 min each. Slides were submerged and microwaved in a 1% antigen unmasking solution (catalog no. H-330; Vector Laboratories, Burlingame, CA, USA) for antigen retrieval and rinsed in dH2O. Sections were incubated with the FMO3-specific antibody 1:100 (catalog no. ARP44434_P050; AVIVA Systems Biology, San Diego CA) or no primary antibody overnight at 4°C in a humidified chamber. After washes, slides were incubated with the appropriate secondary antibody, washed and developed using 3, 3-diaminobenzidine (catalog no. SK-4100; Vector) for 2 min. Hematoxylin QS (catalog no. H-3404; Vector) was added for 30 s as a counterstain. Coverslips were mounted using Vectamount (catalog no. H-5000; Vector). Images were photographed on a Nikon TE2000 inverted microscope using MMI Cellcut Software (MMI, Haslett, MI).
2.4. Vascular reactivity protocols
Four preparations (4 mm rings) made from each thoracic aorta were mounted individually in tissue baths (30mL) for isometric tension recordings using Grass FT03 transducers. Data were acquired using a PowerLab Data Acquisitions unit (ADInstruments, Colorado Springs, CO, USA). Baths contained warmed (37°C), oxygenated PSS (95% O2/5% CO2). After one hour equilibrating under optimum resting tension [4 grams], with washes every 15 min, tissues were tested with an initial concentration of 10 μM PE. Tissues were then washed repeatedly. The presence of an intact endothelial cell layer was confirmed by acetylcholine (ACh, 1 μM) inducing at least 90% relaxation in half-maximal PE-induced contraction (PE EC50) tissues. The absence of the endothelium was confirmed by less than a 5% relaxation to ACh after the contraction with PE EC50. Tissues were washed again until tone returned to baseline before starting the specific protocols. The four different experiment groups (+PVAT/E+; −PVAT/E+; +PVAT/E−; −PVAT/E−) were randomized to the four different tissue baths daily. At the end of each experiment, tissues were washed and stimulated with 10 μM PE to test their viability.
Before and after experiments, samples of the PSS were collected from the organ bath to measure the pH, which revealed to be stable.
2.4.1. Effect of TMA and TMAO on vascular contraction
Cumulative responses were performed to TMA or TMAO (1 nM – 0.5 M), added at baseline tone. Approximately 2-3 minutes were allowed between cumulative additions. When contractions were observed, the time necessary to achieve a contractile plateau was allowed before the next addition.
2.4.2. Effect of inhibitors on the vascular contraction induced by TMA
Tissues incubated with either the appropriate vehicle, 100 μM MTMZ, or 0.1 μM NIF for one hour without washing. Cumulative curves for TMA (1 nM – 0.5 M) were then generated.
2.5. Data processing and statistical analysis
For immunohistochemical images, all images taken were modified for brightness and contrast as a whole, never in part. For contractility, data are reported as means ± SEM for the number of animals indicated by N. Statistical analyses were performed with GraphPad Prism 7.0 (GraphPad Software, Inc., La Jolla, CA). Data for contraction studies are expressed as a percentage of the maximum contraction induced by 10 μM PE at the beginning of the respective protocol. Potency is defined as the −log EC50 [M] of agonist, and values were calculated using GraphPad Prism 7.0. Data were analyzed by unpaired Student t-test or Two-way ANOVA followed by the Bonferroni method for posthoc comparisons (equal variances validated by Bartlett’s test). P < 0.05 was considered statistically significant.
3. Results:
3.1. FMO3 protein is present in aortic PVAT
To determine if a metabolic pathway to convert TMA into TMAO was present in aortic PVAT, immunohistochemical sections of the aorta + PVAT were incubated with an antibody directed against FMO3. Hepatic tissue was used as a control since FMO3 is highly expressed in the liver. The antibody used produced a strong positive signal in liver sections (compare figures 1C and 1D). FMO3 protein was clearly expressed in PVAT, with an evident loss of signal in sequential sections of aorta + PVAT incubated without the primary antibody. FMO3 is also expressed in the smooth muscle and the endothelium layers (compare figures 1A and 1B). This knowledge was important for interpreting some of the following studies.
Figure 1.
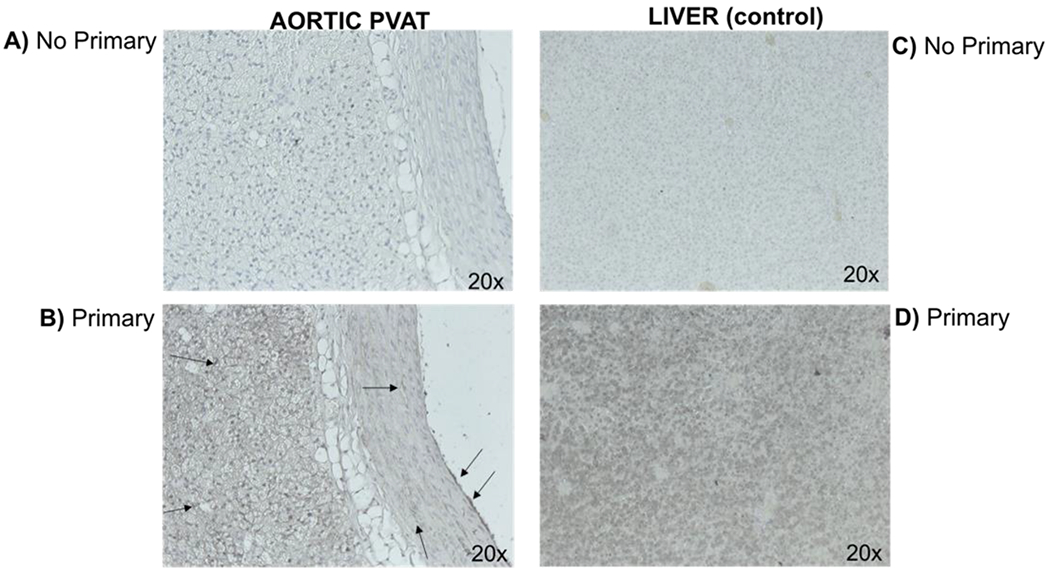
Immunohistochemical staining of Flavin-Mono-Oxidase-3 (FMO3) in the rat thoracic aorta (left) and rat liver (right) as a positive control for FMO3 expression. Section incubated without (panels A and C) and with primary FMO3-specific antibody (B and D). Images (magnification 20x) depict representative of a minimum of four separate animals. Adjustments in brightness and contrast were made to the whole panel of a photograph, not a portion. Arrows indicate the presence of staining for FMO3. Representative of five (5) separate animals.
3.2. Aorta vascular reactivity
3.2.1. Both metabolites induce arterial contraction, but TMA was more potent than TMAO.
TMA and TMAO induced contraction of all experimental groups in a cumulative fashion (figure 2A–D). The magnitude of the initial challenge of PE is reported in the figure legend, validating that the contractile potential of each of these four groups was similar. In all these experiments, potency could only be estimated because a maximum contraction was not always acquired; we could achieve no higher concentration of the metabolites in the tissue bath. Independent of E and/or PVAT, the potency of TMA was of a higher magnitude than the potency of TMAO. However, when the endothelium and PVAT were simultaneously intact (+PVAT/E+), not only potency but also the efficacy of the contraction stimulated by TMA was higher than by TMAO (figure 2A). In this same experimental group (+ PVAT / E+), TMAO-induced maximum contraction was lower than in any other group (figure 2A). Table 1 shares the values of TMA and TMAO’s efficacy (maximum effect), and estimated potency (−log EC50) for curves shared in figure 2.
Figure 2.
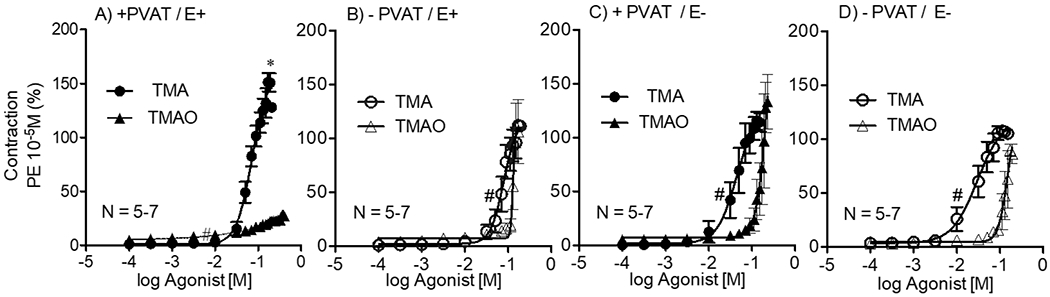
Contractile concentration-response curves to TMA and TMAO in the thoracic aortic rings + and − PVAT, and + and − the endothelium (A-D). Points represent means ± SEM obtained as a percentage of the maximum contraction induced by 10 μM PE for the number (N) of animals indicated in the graphs. *p<0.05 (unpaired Students’ t-test) denotes difference between the efficacy (maximum effect) stimulated by TMA vs TMAO for + PVAT; E+ variable. #p<0.05 (unpaired Students’ t-test) denotes the difference between the potencies of TMA vs. TMAO for all the variables. Values of Max effects and potencies (−Log EC50) are reported in table 1.
Magnitude of 10 μM PE-induced contraction: +PVAT/E+: 1414±117mg; −PVAT/E+: 1515±85mg; +PVAT/E− :1350±101mg; −PVAT/E−: 1870±112mg (p>0.05 by Two-way ANOVA).
Table 1.
Potency (−Log EC50 in [M]) and efficacy (Max Eff - % of initial maximum PE contraction) of TMA and TMAO in the thoracic aorta.
| +PVAT |
−PVAT |
|||||||
|---|---|---|---|---|---|---|---|---|
| TMA |
TMAO |
TMA |
TMAO |
|||||
| Max. Eff. (%) | −Log EC50 | Max. Eff.(%) | −Log EC50 | Max. Eff.(%) | −Log EC50 | Max. Eff. (%) | −Log EC50 | |
| E+ | 141.5±16.0 | 1.2±0.04 | 19.1±4.03 | 2.2±0.4 | 114.8±5.4 | 1.1±0.07 | 107.7±24.1 | 3.6±1.2 |
| E− | 133.0±13.4 | 1.3±0.1 | 147.2±20.5 | 0.8±0.06 | 110.8±4.5 | 1.6±0.1 | 103.7±5.6 | 0.9±0.04 |
Values are expressed as means ± SEM of the magnitude of 10 μM PE induced maximum contraction. Potency (−Log EC50 [M]) or efficacy (Max Eff - %) of contractions stimulated by each of the agents in aortic rings + PVAT and − PVAT were analyzed through Two-way ANOVA comparing the responses obtained in vessels E+ and E−. Symbols (*, #, f) denote significant difference (P<0.05). * indicates the difference between the efficacy (maximum effect) stimulated by TMAO E+ vs. TMAO E−. # indicates the difference between the potency (−Log EC50) stimulated by TMAO E+ vs. TMAO E−. f indicates the difference between the potency (−Log EC50) stimulated by TMA E+ vs TMA E−.
In the presence of PVAT (+PVAT), both potency and efficacy of TMAO-induced contraction were reduced in aortic rings with intact endothelium (E+) vs. without (E−). When PVAT was removed (−PVAT), there were no differences either between −logEC50 or Max contractile responses stimulated by TMAO in E+ vs. E− (table 1).
In aorta with PVAT (+PVAT), E+ vs. E− did not result in differences for either potency or efficacy parameters for TMA-stimulated contraction. Similarly, in rings without PVAT (−PVAT), the efficacy of TMA contraction was not affected by the presence or absence of endothelium (E+ vs. E−). Potency, however, was lower when the endothelium was removed (E−).
3.2.2. TMA-induced contraction is not dependent on FMO3
IHC studies revealed FMO3 in aortic PVAT, with FMO3, the enzyme that converts the more potent TMA into a less potent TMAO. We tested whether this occurred functionally in the PVAT, using aortic rings E+ and E−, by examining the ability of the FMO3 inhibitor methimazole (MTMZ) to the leftward shift the cumulative contractile-response curves to TMA; this would happen if TMA was actively metabolized by FMO3. In tissues with PVAT, FMO3 inhibition did not alter the contractile stimulated by TMA (figure 3). There were no statistical differences (P>0.05) between maximum contraction stimulated with TMA after incubation with MTMZ or with its vehicle in aortic rings + PVAT in both E + (Max. Effect +MTMZ= 167.9± 14.4%; Max. Effect + vehicle= 187.2 ± 29%; figure 3A) or E − (Max. Effect +MTMZ= 92.0 ± 12.6%; Max. Effect + vehicle= 104.2.0 ± 6.7%; figure 3B).
Figure 3.
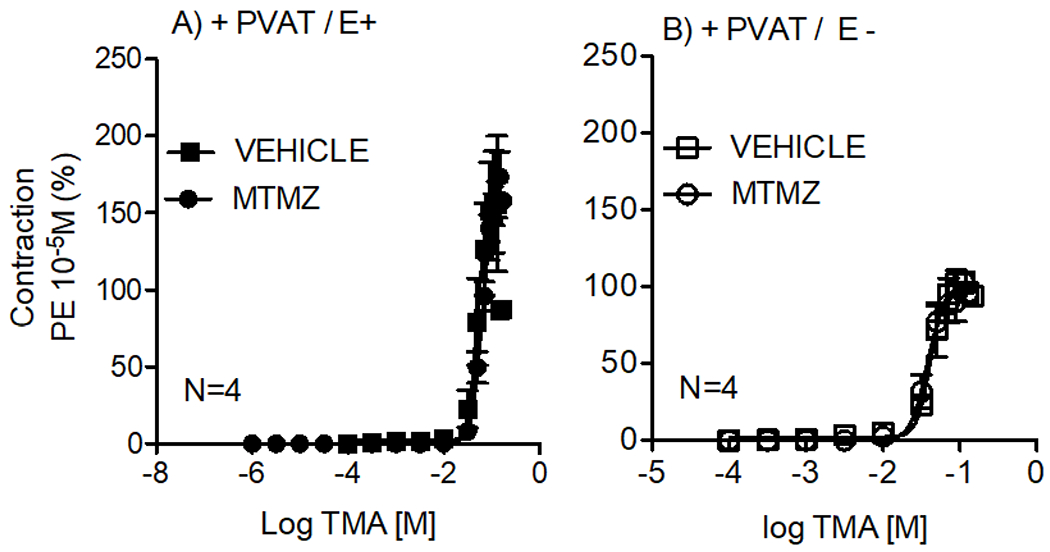
Contractile concentration-response curves to TMA in the thoracic aortic rings + PVAT / E + (A) and +PVAT / E− (B) after 1-hour incubation with vehicle or methimazole (MTMZ 100 μM). Points represent means ± SEM obtained as a percentage of the maximum contraction induced by 10 μM PE for the number (N) of animals indicated in the graphs.
3.2.3. TMA-induced contraction was partially dependent on the Ca2+ influx via L-type channels
Given that cytoplasmic Ca2+ is critical for smooth muscle contraction, we evaluated if Ca2+ influx was important for TMA-induced contraction. This experiment was done in both the presence and absence of PVAT. The L-type Ca2+ channel blocker nifedipine (NIF) reduced TMA-induced contraction in aorta E+ compared to the vehicle, independently of the presence of PVAT (figure 4). In aortic rings + PVAT incubated with vehicle, TMA caused 149.8 ± 18% of maximum PE-induced contraction; NIF reduced the maximum contraction by half [73 ± 9% (P<0.001; figure 4A)]. Similarly, TMA elicited 111.8 ± 9% of maximum contraction in vehicle-exposed rings −PVAT, while incubation with NIF reduced contraction to 63 ± 6.7% (P<0.001; figure 4B). Thus, in both experiments, NIF significantly reduced contraction caused by TMA.
Figure 4.
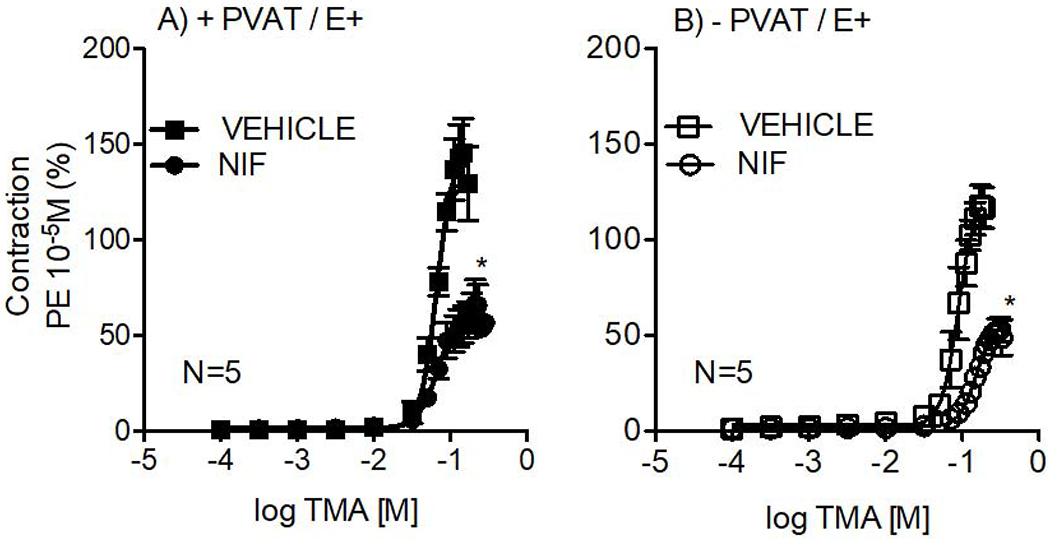
Contractile concentration-response curves to TMA in the thoracic aortic rings with intact endothelium (E+) in +PVAT (A) and − PVAT (B) after 1-hour incubation with vehicle or nifedipine (NIF 0.1 μM). Points represent means ± SEM obtained as a percentage of the maximum contraction induced by 10 μM PE for the number (N) of animals indicated in the graphs. *p<0.05, Two-way ANOVA.
4. Discussion:
The present study aimed to investigate the ability of the microbial metabolites TMA and TMAO to cause vascular contraction under the paracrine influence of PVAT and endothelium. We determined that TMA and TMAO do cause constriction, with significant PVAT and endothelium effects on TMAO-induced contraction. These findings are novel because they describe a hereto unknown functional interaction between PVAT and the TMA/FMO3/TMA0 pathway in aortic-contractility.
TMA and TMAO caused contraction in the aorta
Our present focus was to determine whether these metabolites caused contraction and perform initial studies into mechanisms of how contraction would occur. At high concentrations, both TMA and TMAO stimulate arterial contraction. Because elevated plasma levels of TMAO are observed with progression of cardiovascular diseases [12,24–26] these compounds may have a direct effect on increasing vascular tone. Oakley et al [29] published a study to which we can directly compare our results. The highest concentration of TMAO used was 300 μM in the isolated rat thoracic aorta [29]. Our findings are consistent with theirs in that, at this concentration, we did not observe direct contraction stimulated by TMAO.
PVAT opposes TMAO-induced contraction in the aorta with preserved endothelium
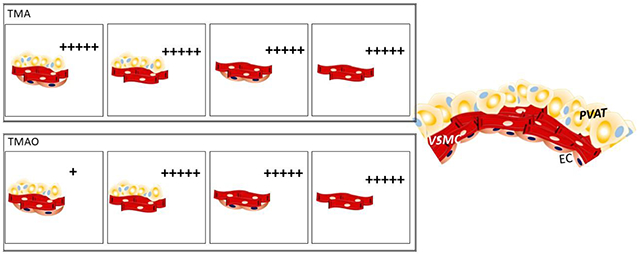
When PVAT was preserved, efficacy and potency of the TMAO-induced contraction were extensively reduced in the presence versus absence of endothelium, indicating that the endothelium plays a protective role against TMAO-induced contraction. Without PVAT, the presence or absence of endothelial cells did not affect either the potency or efficacy of contractions to TMAO (figure 5).
Figure 5:
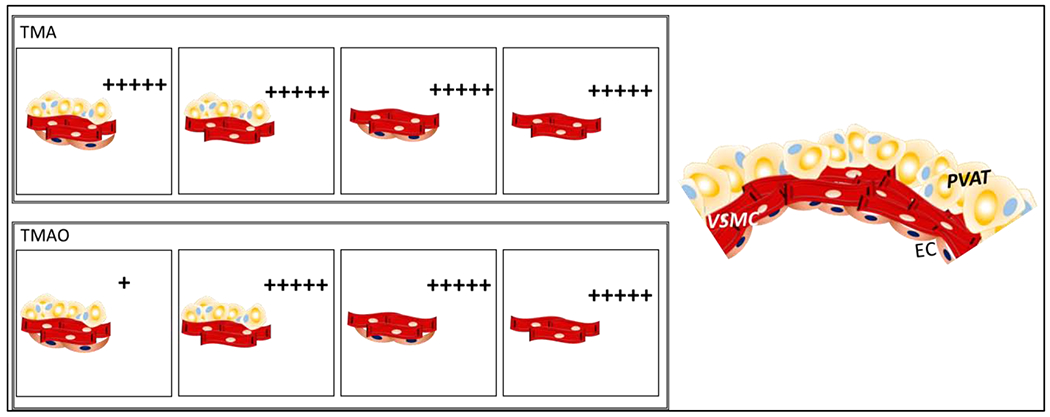
Graphical abstract of results and conclusions supported by the data in this manuscript. “+” indicates the intensity of the maximum contraction stimulated by TMA (upper panel) and TMAO (lower panel) under different experimental conditions. PVAT= perivascular adipose tissue. VSMC= vascular smooth muscle cells. EC= endothelial cells.
Soltis and Cassis [48] were the first to demonstrate that PVAT significantly reduced NE-induced contraction in rat aorta. Many subsequent studies confirmed that, in healthy animals, PVAT exerts a largely anti-contractile function [49,50] playing an essential role in regulating and modulating vascular function by releasing relaxant molecules, including nitric oxide [45, 51–55]. Our data support that PVAT exerts anti-contractile effects on TMAO-induced contractions, but only when the endothelium is present. Explanations for this could include: (1) TMAO enhances a cross-talk between endothelium and PVAT in a manner that relaxing factors released by both structural vascular layers are increased; (2) as a contractile agent, TMAO is not potent/efficacious enough to counteract the additive relaxant actions from endothelium plus PVAT.
These findings could be important, for example, in diseases in which the endothelium is damaged (such as atherosclerosis). The anti-contractile effect of PVAT to stimulation by TMAO would be diminished, leading to an increased vascular tone, consequently aggravating associated co-morbidities. By contrast, the anti-contractile action of PVAT in the presence of endothelium observed to TMAO-induced contractions did not occur when the agonist was its substrate, TMA.
PVAT exerts contractile actions to TMA-induced contraction in the aorta with preserved endothelium.
TMA caused more potent contractions than TMAO in all the four experimental scenarios: presence (+) or the absence (−) of PVAT or endothelium (E+ or E−). A significantly higher efficacy was obtained by stimulation with TMA than TMAO, but only when in the presence of +PVAT with intact E. We conclude PVAT is directly modulating the endothelium to affect TMA-induced contraction. The high contractions to TMA when the endothelium and PVAT are simultaneously preserved is intriguing (figure 5). TMA-induced contractions are due, at least in part, through direct mechanisms located in the vessel. Whether FMO3 is active or not, we demonstrated that independent of the endothelium, TMA-induced contraction was not reliant on local FMO3.
Besides its anti-contractile effects, PVAT, as a source of endothelium-derived contracting factors (EDCF), modifies contraction to other agonists through releasing NE and activation of alpha-adrenergic receptors [41, 56]. In this sense, we suggest there are synergic or additive mechanisms between PVAT and TMA. In addition, PVAT may enhance contraction to TMA, and endothelial relaxing factors cannot counteract it. It is interesting PVAT acts in opposite ways on TMAO- and TMA-induced vascular contraction, and that these different effects are dependent on the presence of endothelium. A reduced action of nitric oxide (NO) could explain this, since there is an alternative route involving TMA metabolization to dimethylamine (DMA), which leads to negative feedback in the NO production/releasing [57–59].
Because TMA caused a more potent and efficacious contraction than TMAO in normal vessels, we further investigated the mechanism of TMA-induced contraction.
Mechanism of TMA-induced contractions
Importantly, nifedipine reduced TMA contractions by 50% in rat aorta. These data indicate there is contractile machinery activated by TMA in the aortic ring + PVAT / E+, which is significantly dependent on Ca2+ influx through L-type channels. Increased intracellular Ca2+ concentration stimulated by TMA has been reported in neuron of snails [60]. TMA-induced intracellular Ca2+ mobilization from endoplasmic reticulum (ER) stores contributes towards cellular stress [61] associated with cardiometabolic dysfunctions such as obesity and diabetes [62]. Although the TMA-induced contraction was significantly reduced, it was not abolished by nifedipine 100nM. At this concentration, nifedipine maximally inhibits KCl-induced contraction in the isolated rat thoracic aorta [63]. In this sense, it is reasonable that other pathways for Ca2+ influx or even Ca2+ released from intracellular stores are involved in the TMA-induced contraction. The substantial contraction independent of voltage-operated calcium channels may be due to store and/or receptor-operated contraction mechanisms and are worthy of investigation.
TMA is a full agonist at human G-protein coupled receptor trace amine-associated receptor 5 (TAAR5) [64–67]; TAAR5 does not recognize TMAO [68]. TMA might mediate contraction by activating TAAR5 located in the aorta. Calling into play receptor activation is logical given that concentration-dependent contraction to TMA, sometimes saturable, was observed. However, while TAAR5 is highly expressed in the central nervous system as chemosensory receptors [69], its presence in the vasculature is not known. To date, Timberol® is the only available TAAR5 antagonist as a candidate for studying receptors involved in the olfactory perception of TMA. Timberol®, an amber-woody fragrance, is not validated for scientific protocols such as reactivity studies in isolated organs [70, 71]. There are two other compounds, which property as TAAR5 antagonists are under investigation, but similarly to Timberol®, they are not validated for use in experiments outside of the olfactory system [72]. As such, experimental approaches to identify receptors for TMA in vascular beds make it an attractive candidate molecule for investigating the role of the microbiota in regulating vascular tone in health and disease.
5. Limitations
First, we did not examine for the presence of microbiota (as a source of TMA) in aortic components, including PVAT. Is it possible that it is from here that TMA or TMAO could come from, or must they be systemic? Second, the concentrations of TMA/TMAO we used are high and may not be physiologically relevant unless considered in a local fashion in which high concentrations could be achieved. Third, FMO3 enzymatic activity in rat aorta and particularly in its PVAT, is needed to confirm TMA’s conversion to TMAO. Fourth, studies of the effects of TMA in other vascular beds and/or other species are necessary. Preliminary investigations showed that TMA stimulated contractions in the superior mesenteric artery (SMA) isolated from rats (results are shown as supplemental material). Fifth, the same concentrations of neither TMAO nor the FMO3 substrate TMA caused aortic relaxation when compared to that caused by time-course with vehicle (water) (supplemental material); this is also consistent with work by Oakley et al. [29]. To be more thorough in the investigations on the relaxation mediated by TMA and TMAO, a set concentration of TMA or TMAO could be tested for its ability to rightward shift contraction to a vascular agonist such as PE, one interpretation of which could be reducing vascular tone. However, the outcome of this experiment would be difficult to interpret without the ability to prove direct relaxation. It is also likely that the concentration of TMA and TMAO that would be necessary alone would also cause contraction, also making interpretation difficult. Finally, the involvement of TAAR5 in the TMA-induced contraction in the aorta remains to be investigated.
6. Conclusion
The microbial metabolites TMA and TMAO direct stimulate vascular contraction in the rat aorta. TMA was more potent than TMAO. The TMA-induced contraction was mediated by Ca2+ influx through voltage-gated L-type channels. PVAT exerted anti-contractile actions when TMAO was the agonist and contractile actions when TMA was the agonist. This different modulation by PVAT depends on the presence of endothelium, which plays a protective role against the increased contraction stimulated by TMAO, but not by TMA.
The present findings suggest that endogenous TMA and TMAO may contribute to cardiovascular diseases via direct and indirect actions on vascular smooth muscle. Our results emphasize the importance of understanding the relationship between TMA/TMAO and PVAT in modulating vascular tone in cardiometabolic diseases, particularly in those associated with impaired endothelium or phenotypic changes in PVAT as seen in hypertension, atherosclerosis, or inflammatory diseases.
Supplementary Material
7. Acknowledgments:
Supported by FIL70687 (NIH) to SWW and GDF and by the State of Sao Paulo Research Foundation to CBAR (Fundacao de Amparo a Pesquisa do Estado de Sao Paulo: Fapesp: 2015/25822-9).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
8. References:
- [1].Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, et al. (2016) Heart Disease and Stroke Statistics—2016 Update: A Report From the American Heart Association. Circulation. 133, e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- [2].Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. (2004) The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci. 101 (44): 15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. (2005) Obesity alters gut microbial ecology. Proc Natl Acad Sci. 102(31): 11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Turnbaugh PJ, Gordon JI (2009). The core gut microbiome, energy balance and obesity. J. Physiol 587, 4153–4158. doi: 10.1113/jphysiol.2009.174136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Koren O, Spor A, Felin J, Fåk F, Stombaugh J, Tremaroli V, Behre CJ, Knight R, Fagerberg B, Ley RE, Bäckhed F (2011) Human oral, gut, and plaque microbiota in patients with atherosclerosis, Proc Natl Acad Sci. 108(1):4592–4598. doi: 10.1073/pnas.1011383107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hyvärinen K, Mäntylä P, Buhlin K, Paju S, Nieminen MS, Sinisalo J, Pussinen PJ. (2012) A common periodontal pathogen has an adverse association with both acute and stable coronary artery disease. Atherosclerosis. 223(2):478–484 doi: 10.1016/j.atherosclerosis.2012.05.021. [DOI] [PubMed] [Google Scholar]
- [7].Karlsson FH, Fåk F, Nookaew I, Tremaroli V, Fagerberg B, Petranovic D, Bäckhed F, Nielsen J. (2012) Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat Commun. 3:1245. doi: 10.1038/ncomms2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cox LM, Yamanishi S, Sohn J, Alekseyenko AV, Leung JM, Cho I, Kim SG, Li H, Gao Z, Mahana D, et al. (2014) Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell. 158(4):705–721. doi: 10.1016/j.cell.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Fåk F, Tremaroli V, Bergström G, Bäckhed F. (2015) Oral microbiota in patients with atherosclerosis. Atherosclerosis. 243(2):573–578. doi: 10.1016/j.atherosclerosis.2015.10.097. [DOI] [PubMed] [Google Scholar]
- [10].Jie Z, Xia H, Zhong SL, Feng Q, Li S, Liang S, Zhong H, Liu Z, Gao Y, Zhao H, et al. (2017) The gut microbiome in atherosclerotic cardiovascular disease. Nat Commun. 8(1):845. doi: 10.1038/s41467-017-00900-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bu J, Wang Z (2018) Cross-Talk between Gut Microbiota and Heart via the Routes of Metabolite and Immunity Gastroenterology. Research and Practice. ID 6458094, 8 pages. doi: 10.1155/2018/6458094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, et al. (2011). Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 472(7341):57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, et al. (2013). Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 19(5):576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. (2013) Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 368(17):1575–8154. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wang Z, Zhao Y (2018) Gut microbiota derived metabolites in cardiovascular health and disease. Protein Cell. 9(5):416–431. doi: 10.1007/s13238-018-0549-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Singh GB, Zhang Y, Boini KM, Koka S. (2019) High Mobility Group Box 1 Mediates TMAO-Induced Endothelial Dysfunction. Int J Mol Sci. 20(14):3570. doi: 10.3390/ijms20143570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Seldin MM, Meng Y, Qi H, Zhu W, Wang Z, Hazen SL, Lusis AJ, Shih DM. (2016) Trimethylamine N-Oxide Promotes Vascular Inflammation Through Signaling of Mitogen-Activated Protein Kinase and Nuclear Factor-κB. J Am Heart Assoc.5(2):e002767. doi: 10.1161/JAHA.115.002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Seim H, Schulze J, and Strack E (1985) Catabolic pathways for high-dosed L(−)- or D(+)-carnitine in germ-free rats? Biol Chem Hoppe Seyler 366:1017–1021. [DOI] [PubMed] [Google Scholar]
- [19].al-Waiz M, Mikov M, Mitchell SC, Smith RL. (1992) The exogenous origin of trimethylamine in the mouse. Metabolism. 41(2):135–136. doi: 10.1016/0026-0495(92)90140-6. [DOI] [PubMed] [Google Scholar]
- [20].Higgins T, Chaykin S, Hammond KB, Humbert JR. (1972) Trimethylamine N-oxide synthesis: a human variant. Biochem Med. 6(4):392–396. doi: 10.1016/0006-2944(72)90025-7. [DOI] [PubMed] [Google Scholar]
- [21].Bennett BJ, de Aguiar Vallim TQ, Wang Z, Shih DM, Meng Y, Gregory J, Allayee H, Lee R, Graham M, et al. (2013). Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 17(1):49–60. doi: 10.1016/j.cmet.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Schugar RC, Shih DM, Warrier M, Helsley RN, Burrows A, Ferguson D, Brown AL, Gromovsky AD, Heine M, Chatterjee A et al. (2017) The TMAO-Producing Enzyme Flavin-Containing Monooxygenase 3 Regulates Obesity and the Beiging of White Adipose Tissue. Cell Rep. 2017. June 20; 19(12):2451–2461. doi: 10.1016/j.celrep.2017.05.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lang DH, Yeung CK, Peter RM, Ibarra C, Gasser R, Itagaki K, Philpot RM, Rettie AE (1998) Isoform specificity of trimethylamine N-oxygenation by human flavin-containing monooxygenase (FMO) and P450 enzymes: selective catalysis by FMO3. Biochem Pharmacol 56(8):1005–1012. doi: 10.1016/s0006-2952(98)00218-4. [DOI] [PubMed] [Google Scholar]
- [24].Boini KM, Hussain T, Li PL, Koka S (2017) Trimethylamine-N-oxide instigates NLRP3 inflammasome activation and endothelial dysfunction. Cell Physiol Biochem 44:152–162. 10.1159/000484623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bain MA, Faull R, Fornasini G, Milne RW, Evans AM (2006) Accumulation of trimethylamine and trimethylamine-N-oxide in end-stage renal disease patients undergoing haemodialysis. Nephrol Dial Transplant. 21 (5): 1300–1304. doi: 10.1093/ndt/gfk056 [DOI] [PubMed] [Google Scholar]
- [26].Wang Z, Levison BS, Hazen JE, Donahue L, Li XM, Hazen SL (2014) Measurement of trimethylamine-N-oxide by stable isotope dilution liquid chromatography tandem mass spectrometry. Anal Biochem. 455:35–40. doi: 10.1016/j.ab.2014.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mueller DM, Allenspach M, Othman A, Saely CH, Muendlein A, Vonbank A, Drexel H, von Eckardstein A (2015) Plasma levels of trimethylamine-N-oxide are confounded by impaired kidney function and poor metabolic control. Atherosclerosis. 243(2):638–44. doi: 10.1016/j.atherosclerosis.2015.10.091. [DOI] [PubMed] [Google Scholar]
- [28].Hai X, Landeras V, Dobre MA, DeOreo P, Meyer TW, Hostetter TH (2015) Mechanism of Prominent Trimethylamine Oxide (TMAO) Accumulation in Hemodialysis Patients. PLoS One.10(12):e0143731. doi: 10.1371/journal.pone.0143731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Oakley CI, Vallejo JA, Wang D, Gray MA, Tiede-Lewis LM, Shawgo T, Daon E, Zorn G 3rd, Stubbs JR, Wacker MJ (2020) Trimethylamine-N-oxide acutely increases cardiac muscle contractility. Am J Physiol Heart Circ Physiol. 318(5):H1272–H1282. doi: 10.1152/ajpheart.00507.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Jaworska K, Konop M, Hutsch T, Perlejewski K, Radkowski M, Grochowska M, Bielak-Zmijewska A, Mosieniak G, Sikora E, Ufnal M (2020) Trimethylamine But Not Trimethylamine Oxide Increases With Age in Rat Plasma and Affects Smooth Muscle Cells Viability. J Gerontol A Biol Sci Med Sci. 75(7):1276–1283. doi: 10.1093/gerona/glz181. [DOI] [PubMed] [Google Scholar]
- [31].Lever M, George PM, Slow S, Bellamy D, Young JM, Ho M, McEntyre CJ, Elmslie JL, Atkinson W, Molyneux SL, et al. (2014) Betaine and trimethylamine-N-oxide as predictors of cardiovascular outcomes show different patterns in diabetes mellitus: an observational study. PLoS One. 9:e114969. doi: 10.1371/journal.pone.0114969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Mente A, Chalcraft K, Ak H, Davis AD, Lonn E, Miller R, Potter MA, Yusuf S, Anand SS, McQueen MJ (2015) The Relationship Between Trimethylamine-N-Oxide and Prevalent Cardiovascular Disease in a Multiethnic Population Living in Canada. Clinical Research. 31(9): 1189–1194. 10.1016/j.cjca.2015.06.016. [DOI] [PubMed] [Google Scholar]
- [33].Tang WH, Hazen SL (2014) The contributory role of gut microbiota in cardiovascular disease. J Clin Invest.124(10):4204–4211. doi: 10.1172/JCI72331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Trøseid M, Andersen GO, Broch K, Hov JR (2020) The gut microbiome in coronary artery disease and heart failure: Current knowledge and future directions. EBioMedicine 52: 102649. doi: 10.1016/j.ebiom.2020.102649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wang Z, Tang WH, Buffa JA, Fu X, Britt EB, Koeth RA, Levison BS, Fan Y, Wu Y, Flazen SL (2014) Prognostic value of choline and betaine depends on intestinal microbiota-generated metabolite trimethylamine-N-oxide. 35(14):904–910. doi: 10.1093/eurheartj/ehu002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ge X, Zheng L, Zhuang R, Yu P, Xu Z, Liu G, Xi X, Zhou X, Fan H (2020) The gut microbial metabolite trimethylamine N-oxide and hypertension risk: a systematic review and dose-response meta-analysis. Adv Nutr 11:66–76. doi: 10.1093/advances/nmz064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Jaworska K, Bielinska K, Gawrys-Kopczynska M; Ufnal M. (2019) TMA (trimethylamine), but not its oxide TMAO(trimethylamine-oxide), exerts hemodynamic effects—Implications for interpretation of cardiovascular actions of gut microbiome. Cardiovasc. Res 115(14): 1948–1949, 10.1093/cvr/cvz231. [DOI] [PubMed] [Google Scholar]
- [38].Jaworska K, Hering D, Mosieniak G, Bielak-Zmijewska A, Pilz M, Konwerski M, Gasecka A, Kapłon-Cieślicka A, Filipiak K, Sikora E et al. (2019). TMA, A Forgotten Uremic Toxin, but Not TMAO, Is Involved in Cardiovascular Pathology. Toxins (Basel). 11 (9):490. doi: 10.3390/toxins11090490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Chang L, Garcia-Barrio MT, Chen YE. (2020) Perivascular Adipose Tissue Regulates Vascular Function by Targeting Vascular Smooth Muscle Cells. Arterioscler Thromb Vase Biol. 40(5): 1094–1109. doi: 10.1161/ATVBAHA.120.312464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ma G, Pan B, Chen Y, Guo C, Zhao M, Zheng L, Chen B (2017) Trimethylamine N-oxide in atherogenesis: impairing endothelial self-repair capacity and enhancing monocyte adhesion. Biosci Rep. 37(2):BSR20160244. doi: 10.1042/BSR20160244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ayala-Lopez N, Martini M, Jackson WF, Darios E, Burnett R, Seitz B, Fink GD, Watts SW (2014) Perivascular adipose tissue contains functional catecholamines. Pharmacol Res Perspect. 2(3):e00041. doi: 10.1002/prp2.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Tanaka K, Sata M (2018) Roles of Perivascular Adipose Tissue in the Pathogenesis of Atherosclerosis. Front Physiol. 9:3. doi: 10.3389/fphys.2018.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].van Dam AD, Boon MR, Berbée JFP, Rensen PCN, van Flarmelen V (2017) Targeting white, brown and perivascular adipose tissue in atherosclerosis development. Eur J Pharmacol;816:82–92. doi: 10.1016/j.ejphar.2017.03.051 [DOI] [PubMed] [Google Scholar]
- [44].Qi XY, Qu SL, Xiong WFI, Rom O, Chang L, Jiang ZS (2018) Perivascular adipose tissue (PVAT) in atherosclerosis: a double-edged sword. Cardiovasc Diabetol 17:134 doi: 10.1186/s12933-018-0777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Xia N, Florke S, Flabermeier A, Closs EI, Reifenberg G, Gericke A, Mikhed Y, Münzel T, Daiber A, Förstermann U et al. (2016). Uncoupling of Endothelial Nitric Oxide Synthase in Perivascular Adipose Tissue of Diet-Induced Obese Mice. Arterioscler Thromb Vase Biol. 36(1):78–85. doi: 10.1161/ATVBAHA.115.306263. [DOI] [PubMed] [Google Scholar]
- [46].Ayala-Lopez N, Thompson JM, Watts SW (2017) Perivascular adipose tissue’s impact on norepinephrine-induced contraction of mesenteric resistance arteries. Front Physiol. 8:37. doi: 10.3389/fphys.2017.00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Restini CBA, Ismail A, Kumar RK, Burnett R, Garver FI, Fink GD, Watts SW (2018) Renal perivascular adipose tissue: form and function. Vascul Pharmacol. 106:37–45. doi: 10.1016/j.vph.2018.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Soltis EE, Cassis LA (1991) Influence of perivascular adipose tissue on rat aortic smooth muscle responsiveness. Clin Exp Hypertens A. 13(2):277–296. doi: 10.3109/10641969109042063. [DOI] [PubMed] [Google Scholar]
- [49].Ozen G, Topal G, Gomez I, Ghorreshi A, Boukais K, Benyahia C, Kanyinda L, Longrois D, Teskin O, Uydes-Dogan BS, et al. (2013) Control of human vascular tone by prostanoids derived from perivascular adipose tissue. Prostaglandins Other Lipid Mediat. 107:13–17. doi: 10.1016/j.prostaglandins.2013.06.002 [DOI] [PubMed] [Google Scholar]
- [50].Lynch FM, Withers SB, Yao Z, Werner ME, Edwards G, Weston AH, Heagerty AM (2013) Perivascular adipose tissue-derived adiponectin activates BK(Ca) channels to induce anticontractile responses. Am J Physiol Heart Circ Physiol. 304(6):H786–95. doi: 10.1152/ajpheart.00697.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Fernaádez-Alfonso MS, Gil-Ortega M, Aranguez I, Souza D, Dreifaldt M, Somoza B, Dashwood MR (2017) Role of PVAT in coronary atherosclerosis and vein graft patency: friend or foe? Br J Pharmacol. 174(20):3561–3572. doi: 10.1111/bph.13734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].da Costa RM, da Silva JF, Alves JV, Dias TB, Rassi DM, Garcia LV, Lobato NS, Tostes RC (2018) Increased O-GIcNAcylation of Endothelial Nitric Oxide Synthase Compromises the Anti-contractile Properties of Perivascular Adipose Tissue in Metabolic Syndrome. Front Physiol. 9:341. doi: 10.3389/fphys.2018.00341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Dashwood MR, Dooley A, Shi-Wen X, Abraham DJ, Souza DS (2007) Does periadventitial fat-derived nitric oxide play a role in improved saphenous vein graft patency in patients undergoing coronary artery bypass surgery? J Vase Res. 44(3): 175–181. doi: 10.1159/000099833. [DOI] [PubMed] [Google Scholar]
- [54].Xia N, Li H. (2017) The role of perivascular adipose tissue in obesity-induced vascular dysfunction. Br J Pharmacol. 174(20):3425–3442. doi: 10.1111/bph.13650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Lee RM, Lu C, Su LY, Gao YJ (2009) Endothelium-dependent relaxation factor released by perivascular adipose tissue. J Hypertens. 27(4):782–790. doi: 10.1097/HJH.0b013e328324ed86 [DOI] [PubMed] [Google Scholar]
- [56].Kumar RK, Darios ES, Burnett R, Thompson JM, Watts SW (2019) Fenfluramine-induced PVAT-dependent contraction depends on norepinephrine and not serotonin. Pharmacol Res. 140:43–49. doi: 10.1016/j.phrs.2018.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Chhibber-Goel J, Gaur A, Singhal V, Parakh N, Bhargava B, Sharma A. (2016) The complex metabolism of trimethylamine in humans: endogenous and exogenous sources. Expert Rev Mol Med. 18:e8. doi: 10.1017/erm.2016.6. [DOI] [PubMed] [Google Scholar]
- [58].Janeiro MH, Ramirez MJ, Milagro FI, Martinez JA, Solas M. (2018) Implication of Trimethylamine N-Oxide (TMAO) in Disease: Potential Biomarker or New Therapeutic Target. Nutrients 10(10): 1398. doi: 10.3390/nu10101398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Fennema D, Phillips IR, Shephard EA (2016) Trimethylamine and Trimethylamine N-Oxide, a Flavin-Containing Monooxygenase 3 (FMO3)-Mediated Host-Microbiome Metabolic Axis Implicated in Health and Disease. Drug Metab Dispos. 44(11): 1839–1850. doi: 10.1124/dmd.116.070615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Willoughby D, Thomas R, and Schwiening C (2001) The effects of intracellular pH changes on resting cytosolic calcium in voltage-clamped snail neurones. J Physiol 530(3):405–416. doi: 10.1111/j.1469-7793.2001.0405k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Schönthal AH (2012) Endoplasmic reticulum stress: its role in disease and novel prospects for therapy. Scientifica (Cairo). 2012, 857516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Cnop M, Foufelle F, Velloso LA (2012) Endoplasmic reticulum stress, obesity and diabetes. Trends Mol Med. 18(1):59–68. doi: 10.1016/j.molmed.2011.07.010. [DOI] [PubMed] [Google Scholar]
- [63].Florian FA, Watts SW. (1998) Integration of Mitogen-Activated Protein Kinase Kinase Activation in Vascular 5-Hydroxytryptamine2A Receptor Signal Transduction. Journal of Pharmacology and Experimental Therapeutics 284 (1) 346–355. [PubMed] [Google Scholar]
- [64].Liberies SD, Buck LB (2006) A second class of chemosensory receptors in the olfactory epithelium. Nature 442, 645–650. doi: 10.1038/nature05066 [DOI] [PubMed] [Google Scholar]
- [65].Wallrabenstein I, Kuklan J, Weber L, Zborala S, Werner M, et al. (2013) Human Trace Amine-Associated Receptor TAAR5 Can Be Activated by Trimethylamine. PLoS ONE 8(2): e54950. doi: 10.1371/journal.pone.0054950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Zhang J, Pacifico R, Cawley D, Feinstein P, and Bozza T (2013) Ultrasensitive detection of amines by a trace amine-associated receptor. J Neurosci 33:3228–3239. doi: 10.1523/JNEUROSCI.4299-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Berry MD, Gainetdinov RR, Hoener MC, Shahid M (2017). Pharmacology of human trace amine-associated receptors: Therapeutic opportunities and challenger. Pharmacology and Therapeutic Reviews. 180: 161–180. 10.1016/j.pharmthera.2017.07.002 [DOI] [PubMed] [Google Scholar]
- [68].Li Q, Korzan WJ, Ferrero DM, Chang RB, Roy DS, Buchi M, Lemon JK, Kaur AW, Stowers L, Fendt M, Liberies SD (2013) Synchronous evolution of an odor biosynthesis pathway and behavioral response. Curr. Biol 23:11–20. doi: 10.1016/j.cub.2012.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Liberies SD (2015) Trace amine-associated receptors: ligands, neural circuits, and behaviors. Curr Opin Neurobiol. 34:1–7. doi: 10.1016/j.conb.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Wallrabenstein I, Singer M, Panten J, Hatt H, Gisselmann G (2015) Timberol® Inhibits TAAR5-Mediated Responses to Trimethylamine and Influences the Olfactory Threshold in Humans. PLoS ONE 10(12): e0144704. doi: 10.1371/journal.pone.0144704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Espinoza S, Sukhanov I, Efimova EV, Kozlova A, Antonova KA, llliano P, Leo D, Merkulyeva N, Kalinina D, Musienko P, et al. (2020). Trace Amine-Associated Receptor 5 Provides Olfactory Input Into Limbic Brain Areas and Modulates Emotional Behaviors and Serotonin Transmission. Front Mol Neurosci. 5; 13:18. doi: 10.3389/fnmol.2020.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Cichero E, Espinoza S, Tonelli M, Franchini S, Gerasimov AS, Sorbi C et al. (2016). Homology modelling-driven study leading to the discovery of the first mouse Trace Amine-Associated Receptor 5 (TAAR5) antagonists. Med.Chem.Commun 7:353–364. doil: 10.1039/c5md00490j [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


