Abstract
Introduction:
Smoking is associated with cardiac arrhythmia, stroke, heart failure, and sudden cardiac arrest, all of which may derive from increased sympathetic influence on cardiac conduction system and altered ventricular repolarization. However, knowledge of the effects of smoking on supraventricular conduction, and the role of the sympathetic nervous system in them, remains incomplete.
Methods:
Participants with intermediate-high cardiovascular disease risk were measured for urinary catecholamines and cotinine, and 12-lead electrocardiograms (ECGs) were measured for atrial and atrioventricular conduction times, including P duration, PR interval, and PR segment (lead II), which were analyzed for associations with cotinine by generalized linear models. Statistical mediation analyses were then used to test whether any significant associations between cotinine and atrioventricular conduction were mediated by catecholamines.
Results:
ECG endpoints and urinary metabolites were included from a total of 136 participants in sinus rhythm. Atrial and atrioventricular conduction did not significantly differ between smokers (n=53) and non-smokers (n=83). Unadjusted and model-adjusted linear regressions revealed cotinine significantly and inversely associated with PR interval and PR segment, but not P duration. Dopamine, norepinephrine, and epinephrine all inversely associated with PR interval, whereas only dopamine was also inversely associated with PR segment (p<0.05). Dopamine and norepinephrine (but not epinephrine) also associated positively with cotinine. Dopamine mediated the relationship between cotinine and PR interval, as well as the relationship between cotinine and PR segment.
Conclusion:
Smoking is associated with accelerated atrioventricular conduction and elevated urinary dopamine and norepinephrine. Smoking may accelerate atrioventricular nodal conduction via increased dopamine production.
Implications
The electrocardiographic markers of atrioventricular conduction (PR interval and PR segment) are particularly sensitive to adrenergic stimulation and predictive of all-cause and cardiovascular mortality. Our study findings suggest that cigarette smoke exposure accelerates atrioventricular conduction by augmenting dopamine. These observations identify a pathway by which smoking may increase risk for cardiovascular morbidity and mortality, and a potential for therapeutic target.
Keywords: cigarette, cotinine, electrocardiography, catecholamines, PR interval, PR segment, P wave, atrioventricular, smoking, dopamine
Introduction
Smoking is the leading preventable cause of cardiovascular diseases (CVD), including coronary heart disease, stroke and sudden death. There is also a strong dose-response relationship between cigarette smoking and risk for arrhythmia, irrespective of coronary artery disease 1. It remains unclear which individual tobacco smoke constituents and biological pathways mediate this increased risk; however, nicotine may drive some of the effects of smoking through sympathetic neural stimulation and systemic catecholamine release 2. Smoking compromises oxygen balance within the myocardium, especially in coronary artery disease patients, by increasing cardiac workload and coronary resistance 3. Such effects may occur secondary to adrenergic receptor activation following nicotine-induced catecholamine release. Indeed, both acute and chronic activation of the sympatho-adrenal system, expressed as increased levels of circulating catecholamines, promotes cardiovascular dysfunction and disease, including arrhythmia, hypertension, heart failure, myocardial infarction, and ischemic stroke 4.
Although the cardiovascular effects of smoking have been well studied, the advent of new nicotine delivery systems such as e-cigarettes and heat-not-burn products has increased the need to delineate the sympathomimetic effects of nicotine and elucidate the cardiovascular effects of nicotine-containing products. Assessment of cardiac electrophysiology can provide clear insight into cigarette smoking’s cardiovascular effects, which may derive partly from the sympathomimetic effects of nicotine. Previous work on cigarette-induced electrophysiological abnormalities have mainly focused on heart rate variability (HRV) 5 and ventricular repolarization 6–9, with little interest in ECG measures of atrial activity and atrioventricular (AV) node conduction such as PR interval and its subcomponent, PR segment. Yet, because the AV node is particularly responsive to autonomic modulation, sympathetic stimulation often manifests as changes in PR interval. Moreover, PR interval is predictive of adverse outcomes. Abnormally low and high PR interval are both associated with increased risk of atrial fibrillation 10 and cardiovascular mortality 11. Likewise, several studies suggest smokers have increased atrial fibrillation risk 12, which may also contribute to smoking-associated stroke. However, a mode of action for smoking-induced supraventricular arrhythmia remains unidentified. Thus, PR interval and segment provide salient indices of smoking-related cardiac risk and may bear key relationships with nicotine and catecholamines in smokers.
The current study was therefore designed to study the effect of nicotine and cigarette smoke exposure on PR interval and its components in a cohort of patients with intermediate-to-high CVD risk and to discern the role of sympatho-adrenal activity in these effects on atrioventricular conduction through analyzing urinary metabolites of nicotine and catecholamines (dopamine, norepinephrine, and epinephrine).
Methods
Participants and design
The study was approved by the Institutional Review Board at the University of Louisville. Individuals (>18 years of age) with intermediate to high CVD risk were recruited from the University of Louisville Hospital and affiliated clinic system between October 2009 and March 2011 as described previously 13. All accessible patients visiting the clinics during this time period were pre-screened through a review of medical records prior to recruitment in order to exclude individuals that did not meet the enrollment criteria. In addition, persons unwilling or unable to provide informed consent or with significant and/or severe comorbidities were excluded. Exclusion criteria included: significant chronic lung, liver, kidney, hematological, or neoplastic disease, chronic neurological or psychiatric illness, chronic infectious disease such as HIV or hepatitis, severe coagulopathies, drug/substance abuse, and chronic cachexia. Pregnant women, prisoners, and other vulnerable populations were also excluded from the study. Patients who met the enrollment criteria and gave written consent were consented and administered a questionnaire to provide demographic information and baseline characteristics. Medical records were reviewed for past medical history, vital signs and medication history. To reduce selection bias, all consecutive participants who were eligible for this study were recruited. For our analyses, only those patients with complete urinary biomarkers and ECG with normal sinus rhythm were included (Supplementary Figure 1).
Electrocardiogram (ECG) measurement protocol
Standard 12-lead ECGs with 2.5 seconds of each lead and 10 seconds of rhythm strip (lead II) from medical records were used for ECG analyses. The following ECG intervals were measured: P wave duration (from beginning to end of P) and PR interval from lead II (from beginning of P to beginning of Q) 11, QRS duration from lead V6 (from beginning of Q to end of S) 14, and QT interval from V5 (from beginning of Q to end of T) 15. The PR segment was calculated as the difference between PR interval and P wave duration. QT was also corrected using the Framingham formula. All intervals were measured with electronic calipers and adjusted to scale by reported automated measures of RR (or heart rate). Two trained analysts (CA and AI) independently and manually measured each ECG interval (except RR) from the first 3 sinus beats. HRV parameters were derived from digital caliper measurements of all RRs in the rhythm strip. When average of any ECG interval for a given patient differed between the two analysts by >10%, both investigators re-measured that ECG interval independently. If the parameter remained >10% different between analysts, the analysts reviewed the ECG together and reached consensus on the appropriate measure.
Urinary measurements
A spot urine sample was collected on the day of study enrollment. Urinary cotinine is a well-established metabolite for cigarette smoke exposure 16, and was measured by Ultra performance liquid chromatography - tandem mass spectrometer (UPLC-MS/MS) using D3-cotinine as an internal standard 17. For UPLC-MS/MS analysis of dopamine, norepinephrine, epinephrine, and their metabolites (metanephrine, normetanephrine, vanillylmandelic acid, 3-methoxytyramine, homovanillic acid), urine samples were thawed on ice, vortexed and diluted 1:50 with 0.2% formic acid containing isotopic labeled internal standards. 1 μL of the mixture was analyzed on an UPLC-MS/MS instrument (ACQUITY UPLC H-Class system and Xevo TQ-S micro triple quadrupole mass spectrometer, all from Waters Inc., MA). Separation was performed on an Acquity UPLC HSS PFP (150 mm × 2.1 mm, 1.8 μm) column (Waters Inc., MA) with a binary gradient comprised of 0.2% formic acid (Solvent A) and methanol (Solvent B). Three multiple reaction monitoring (MRM) transitions were set up for each sample: one for quantification, one for confirmation, and one for labeled internal standard. At least 12 data points were collected for each peak. Analytes were quantified using peak area ratio based on 8 point-standard curves run before and after the urine samples. The concentration values of analytes were normalized to creatinine level which was measured on a COBAS MIRA-plus analyzer (Roche, NJ) with Infinity Creatinine Reagent (Thermo Fisher Scientific, MA).
Statistical Analysis
Baseline subject characteristics were summarized by smoking status. Categorical characteristics, frequencies and percentages are reported along with Chi-square test p-values, which were used to compare distributions across study groups. In addition to visual inspection of histograms, Shapiro-Wilk tests were conducted for continuous characteristics to determine if the characteristics were approximately normally distributed. Mean and standard deviation are reported for continuous characteristics with a normal distribution, whereas median and interquartile range are reported for continuous characteristics with a skewed distribution, and the study groups were compared by the appropriate statistical test based on normality. P-values were derived from Student’s t-tests for study group comparisons of normally-distributed variables, whereas Mann-Whitney tests were used for variables lacking a normal distribution. The urinary metabolites (cotinine, dopamine, norepinephrine and epinephrine and their daughter metabolites) were log-transformed because their distribution was skewed. We tested the associations between ECG parameters and urinary metabolites by linear regression. ECG parameters were dichotomized by their median levels into high- and low-value groups. Baseline characteristics associated with ECG variables in bivariate analyses (with p<0.01) were used to build fully adjusted models using linear regression analyses. Smoking status was determined as reported by the participant (active smoker, former smoker or non-smoker) and by urinary cotinine levels of 50 μg/g 16. Finally, mediation was assessed by the bootstrapping technique and macro put forth by Preacher and Hayes 18. Statistical analyses were performed using Statistical Package for Social Sciences (SPSS) software (version 24, SPSS, Inc, Chicago, IL, USA).
Results
Baseline characteristics (Table 1)
Table 1:
Baseline characteristics
| All patients | Current smoker | |||
|---|---|---|---|---|
| N=136 | Yes (N=53, 39%) | No (N=83, 61%) | p value | |
| Age (years)* | 52, 10 | 53, 10 | 50, 9 | 0.05 |
| BMI (kg/m2)* | 33, 8 | 34, 8 | 31, 7 | 0.06 |
| SBP (mm Hg)* | 133, 23 | 132, 22 | 136, 24 | 0.15 |
| DBP (mm Hg)* | 81, 13 | 80, 11 | 81, 16 | 0.37 |
| Heart rate (beats/min)* | 73, 15 | 74, 15 | 73, 15 | 0.99 |
| Male gender | 72, 53% | 43, 52% | 30, 57% | 0.58 |
| Caucasian | 77, 57% | 46, 55% | 31, 59% | 0.73 |
| Hypertension | 118, 87% | 74, 89% | 45, 85% | 0.47 |
| Prior MI | 62, 46% | 40, 48% | 23, 43% | 0.58 |
| Diabetes | 43, 32% | 28, 34% | 15, 28% | 0.51 |
| Stroke | 23, 17% | 14, 17% | 10, 19% | 0.77 |
| Arrhythmia | 45, 33% | 28, 34% | 17, 32% | 0.84 |
| Beta blocker | 98, 73% | 65, 78% | 34, 64% | 0.07 |
| CCB | 32, 24% | 24, 29% | 8, 15% | 0.06 |
| ACEI or ARB | 89, 66% | 54, 65% | 35, 66% | 0.91 |
| Statin | 84, 62% | 55, 66% | 30, 57% | 0.26 |
| Aspirin | 81, 60% | 49, 59% | 33, 62% | 0.71 |
| Diuretics | 58, 43% | 38, 46% | 20, 38% | 0.35 |
Abbreviations: BMI - Body mass index, SBP- Systolic Blood Pressure, DBP – Diastolic Blood Pressure, MI – Myocardial Infarction, CCB – Calcium Channel Blocker, ACEI - Angiotensin-Converting Enzyme Inhibitor, ARB - Angiotensin II Receptor Blocker
For each variable counts and column percentage has been reported except for where indicated
where the mean and standard deviation (SD) are reported
A total of 136 participants were in normal sinus rhythm and had ECGs and urinary metabolites available. The participants were approximately evenly split by gender (male n=72, 53%), about half of all participants were Caucasians (n=77, 57%), and mean age was 52 years. Participants had a high prevalence of CVD risk factors; with a majority diagnosed with hypertension (n=119, 87%) and/or on beta blockers (n=98, 73%). Several of the participants were diagnosed with diabetes (n=43, 32%) or prior myocardial infarction (n=62, 46%), and/or were taking calcium channel blockers (n=32, 24%) or beta blockers (n=98, 73%). The relationship of baseline characteristics with dichotomized PR interval, P duration, and PR segment are shown in Supplementary Table 1. Mean age and proportion of females were significantly higher among individuals in the upper stratum for PR interval, whereas mean BMI, or proportion of participants who were female, hypertensive, or taking calcium channel, ACE, or angiotensin receptor inhibitors were higher among those with longer P duration. Conversely, only the number of participants on diuretics were higher among those with longer PR segment
Smoking status and catecholamine levels
Overall smoking prevalence was 61% (n=83) by self-report and 46% when defined by urinary cotinine >50 μg/g (Cohen’s κ=0.79, p<0.001 for agreement between these measures). Three participants self-reported as never smokers had urinary cotinine levels >50 μg/g and two who self-reported as active smokers had urinary cotinine levels of ≤50 μg/g (Supplementary Table 2). None of the cohort characteristics were significantly different between active smokers and the rest of patients (Table 1); although trends of higher age, BMI, and use of calcium channel and/or beta blockers was observed among smokers. All urinary metabolites (cotinine, dopamine, epinephrine and norepinephrine) were higher among current smokers vs never-smokers. None of the catecholamines were different between current smokers and ex-smokers (p>0.05); however, catecholamines were higher in the high-cotinine group relative to the low-cotinine group (Supplementary Table 3 A).
Supplementary table 4 shows that cotinine was significantly associated with dopamine and norepinephrine, but not with epinephrine. We also explored the possible effect of cotinine on catecholamine metabolism by the association of cotinine and the daughter metabolites and their ratios. Each catecholamine’s intermediate metabolite, and none of the final metabolites, had a significant positive association with cotinine. Among the ratio of intermediate/parent metabolites, cotinine associated positively with 3-methoxytyramine/Dopamine (an index of catechol-o-methyltransferase activity) and inversely with vanillyl mandelic acid/normetanephrine and vanillyl mandelic acid/metanephrine (both indices of monoamine oxidase activity).
Association of cotinine with P- and PR-parameters
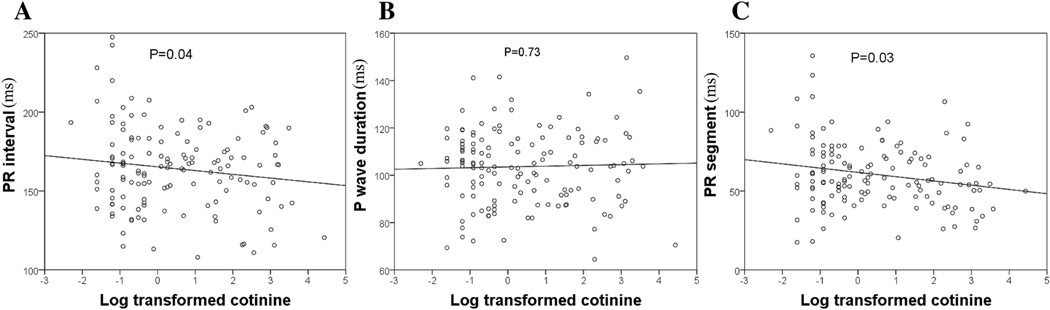
Smokers did not differ from non-smokers in proportion of participants within strata of long or short P duration, PR interval, or PR segment. Distribution of participants into high- or low-PR or P-duration parameters also did not differ by cotinine dichotomization (Supplementary Table 3 B). When analyzed as a continuous variable, cotinine was significantly higher among participants with short PR segment compared to those with long PR segment (p=0.03). PR interval also showed a similar trend (p=0.06), whereas cotinine did not differ between participants with long vs. short P duration (p=0.25) (Table 2). Among all participants, cotinine had a significant negative association with PR interval and PR segment (but not with P duration) in linear regressions, even after adjusting for age, gender and heart rate (Table 3). Figure 1 shows the scatterplot distributions of PR interval, P duration, and PR segment across log-transformed urinary cotinine levels normalized by creatinine. Cotinine was not significantly associated with other ECG parameters, including QRS duration, QT interval and corrected QT, in unadjusted and adjusted models (supplementary Table 5).
Table 2:
Comparison of creatinine-normalized urinary biomarkers (median [interquartile range]) among participants dichotomized into high and low atrial and atrioventricular conduction parameters.
| All patients | PR interval | P wave duration | PR segment | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ≤ 163.32 ms | > 163.33 ms | p value | ≤ 104.73 ms | > 104.74 ms | p value | ≤ 55.20 ms | > 55.21 ms | p value | ||
| Cotinine (μg/g) | 23.1 [1.6 – 794.1] | 30.6 [1.5 – 948.4] | 23.0 [1.8 – 647.7] | 0.45 | 238.3 [2.3 – 102.5] | 5.8 [1.4 – 627.1] | 0.08 | 130.7 [2.4 – 1070.1] | 9.5 [1.2 – 591.3] | 0.03 |
| Dopamine (μg/g) | 165.4 [127.6 – 214.1] | 179.8 [142.3 – 228.5] | 150.6 [119.7 – 189.5] | 0.01 | 180.1 [142.0 – 234.7] | 111.6 [180.3 – 168.8] | 0.01 | 168.8 [140.7 – 219.8] | 160.5 [119.7 – 204.9] | 0.07 |
| Epinephrine (μg/g) | 4.7 [2.4 – 7.9] | 5.6 [2.9 – 8.6] | 8.6 [4.1 – 7.5] | 0.23 | 5.9 [3.5 – 9.8] | 3.9 [1.7 – 6.4] | 0.01 | 5.2 [2.1 – 8.9] | 4.2 [2.5 – 7.5] | 0.75 |
| Norepinephrine (μg/g) | 37.2 [26.0 – 53.3] | 36.7 [26.9 – 52.6] | 38.0 [22.9 – 56.4] | 0.86 | 41.2 [30.7 – 54.5] | 31.9 [22.1 – 52.3] | 0.03 | 36.4 [28.0 – 51.7] | 37.5 [22.7 – 55.6] | 0.75 |
Table 3:
Estimated effects (β-coefficients) of an increase in cotinine on PR interval, P wave, and P segment, with corresponding P-values, from unadjusted and adjusted linear regressions. Urinary cotinine was log-transformed.
| PR interval | P duration | PR segment | ||||
|---|---|---|---|---|---|---|
| β | p value | β | p value | β | p value | |
| Unadjusted Cotinine | −2.38 | 0.04 | 0.33 | 0.73 | −2.70 | 0.03 |
| *Adjusted Cotinine | −2.67 | 0.05 | 0.87 | 0.32 | −2.10 | 0.04 |
model adjusted for age, body mass index, and gender (PR interval); BMI, SBP, DBP, gender,hypertension, CCB, and ACEI, and ARB (P duration); or BMI, beta-blocker, and diuretics (PR segment).
Figure 1:
Scatterplot and linear relationships of (A) PR interval, (B) P wave and (C) PR segment with log transformed urinary cotinine levels. P values represent unadjusted linear regression.
Catecholamines and PR interval
All three catecholamines were significantly elevated among participants with short P duration relative to those with long P duration (Table 2). Dopamine was also elevated among those with shorter PR-interval relative to those with longer PR-interval (p=0.01). Dopamine showed a trend of being higher among patients with short vs. those with long PR-segment. The adjusted linear regression showed all three catecholamines inversely associated with PR interval (p<0.05), none of the catecholamines associated with P duration, and only dopamine inversely associated with PR segment (table 4).
Table 4:
Association between catecholamine and atrial or atrioventricular conduction determined by unadjusted and adjusted linear regressions. Catecholamines were creatinine-normalized and log-transformed.
| PR interval | P wave | PR segment | |||||
|---|---|---|---|---|---|---|---|
| β | p value | β | p value | β | p value | ||
| Unadjusted | Dopamine | −17.13 | <0.01 | −8.45 | <0.01 | −8.68 | 0.04 |
| Epinephrine | −5.47 | 0.02 | −2.8 | 0.04 | −2.66 | 0.16 | |
| Norepinephrine | −7.31 | 0.11 | −4.79 | 0.08 | −2.52 | 0.49 | |
| *Adjusted | Dopamine | −12.3 | 0.02 | −3.56 | 0.28 | −8.51 | 0.04 |
| Epinephrine | −6.62 | <0.01 | −2.73 | 0.06 | −2.53 | 0.19 | |
| Norepinephrine | −10.16 | 0.02 | −3.39 | 0.18 | −2.69 | 0.46 | |
models adjust for age and gender (PR interval); body mass index, gender, hypertension, calcium channel blocker, ACE-inhibitor or angiotensin II receptor blocker (P duration); and diuretics (PR segment).
Mediation analyses
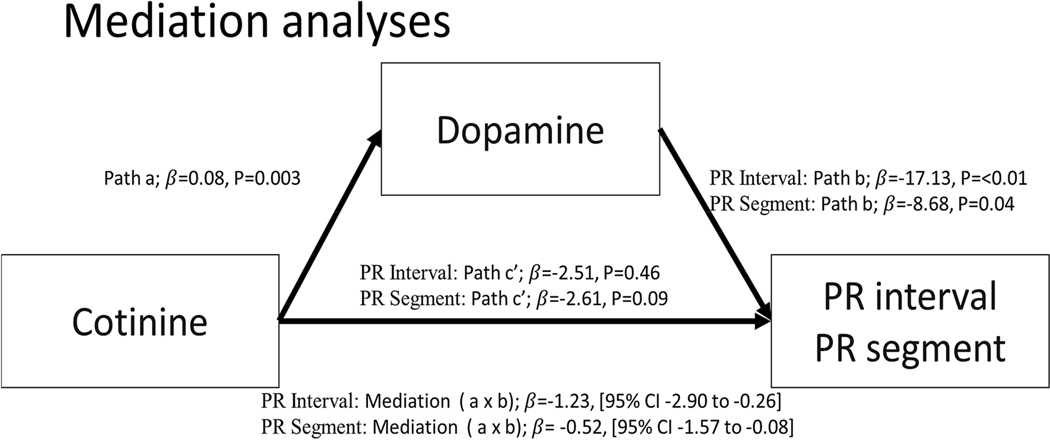
In linear regression analyses cotinine was inversely associated with PR segment and PR interval, and dopamine was the only catecholamine significantly associated with both PR segment and PR interval (inversely in both cases). We therefore conducted mediation analyses to determine whether dopamine mediated the association between cotinine and PR segment and also between cotinine and PR interval. Figure 2 shows the relationship between cotinine and dopamine (path a); the relationship between dopamine and PR interval/segment (path b); and the total effect of cotinine on PR interval/segment (path c). The total effect (c) is the sum of direct (c’) and indirect (ab) effects. The direct effect is the relationship between cotinine and PR interval/segment while controlling for mediators. The indirect effect represents the mediated effect. Mediation analysis showed that dopamine completely mediated the association of cotinine with PR interval (c’, p=0.46) and PR segment (c’, p=0.09). Specifically, the indirect effect (path a × b) of cotinine on PR interval through dopamine had a point estimate of −1.23 and an upper and lower 95% CI of −2.90 and −0.26; and the indirect effect (path a × b) of cotinine on PR segment through dopamine had a point estimate of −0.52 and an upper and lower 95% CI of −1.57 and −0.08 (Figure 2).
Figure 2:
Mediation analyses of cotinine, dopamine, and PR interval/segment.
Discussion
The effects of smoking on atrial and AV nodal conduction velocity, and their underlying mechanisms, are currently unknown. We thus measured PR interval, P wave duration, and PR segment from ECGs, in conjunction with measures of urinary catecholamines, nicotine, and nicotine metabolites, in a cohort of 136 participants with intermediate-high cardiovascular risk. We report three major findings from this study. First, cotinine was inversely associated with PR interval and PR segment, but not with P wave duration, in adjusted linear regression models. Second, urinary dopamine inversely correlated with PR interval and both of its components (PR segment and P duration), whereas epinephrine and norepinephrine associated with PR interval but not PR segment or P duration. Third, mediation analyses indicated that the cotinine-associated shortening of PR interval and PR segment, may, at least in part, be mediated by dopamine. Together, these findings suggest smoking may increase cardiovascular morbidity and mortality through nicotine-associated increases in catecholamine release and downstream modulation of AV conduction.
Nicotine and PR interval and its components
Previous investigations into the chronic effects of smoking on PR interval have produced mixed results. Some studies found short PR interval at baseline among chronic smokers vs non-smokers 19,20, while others found no difference 21,22. Similarly, a few studies showed increased P wave duration among smokers 23,24, while other studies did not show any significant difference 25,26 or found a trend towards decreasing P wave duration with smoking 19. There are several reasons for the wide variation in the results of prior studies; 1) small sample size, 2) insufficient consideration for the role of the components of PR interval, and 3) inadequate assessment of nicotine exposure and its potential impact. To the best of our knowledge, to date, this is the first investigation into the effects of nicotine exposure on PR interval and its components in humans. This is particularly important in light of recent findings in large cohort studies that short PR interval associates with increased risk for atrial fibrillation and cardiovascular mortality 10,11. Beyond overall cardiovascular mortality, smoking also associates with increased risk of atrial fibrillation through unknown mechanisms 12. Thus, by demonstrating that exposure to cigarette smoke (and perhaps nicotine specifically) accelerates atrioventricular conduction, our findings provide further insight into how smoking may confer cardiovascular risk, including risk for atrial fibrillation.
In the present study, cotinine inversely associated with PR interval and PR segment, indicating that increased cigarette smoke exposure accelerates atrioventricular conduction. PR interval is mainly influenced by the sum of atrial activity and atrioventricular nodal conduction. Atrial pathology usually results in prolonged (rather than shortened) PR interval, whereas shortened PR interval typically results from accelerated AV nodal conduction. Hence, it is plausible from our results that nicotine exposure expedites AV nodal conduction. Moreover, because P wave duration was not associated with cotinine, our findings suggest nicotine has limited effects on atrial conduction. Using our linear regression coefficient, we found that each 100 μg/g cotinine corresponded with a 12.3-ms decrease in PR interval and 9.7-ms decrease in PR segment. Despite that the observed PR interval and PR segment values among smokers may fall within clinically normal ranges, our findings that a 100-μg/g increase in cotinine corresponded with a 12.3-ms decrease in PR interval are within range of recent observations in a larger cohort (9,637 participants), for whom 10-ms declines in PR interval below 162 ms corresponded with significant increases in cardiovascular mortality and morbidity 27.
The parasympathetic branch of the ANS directly modulates AV nodal activity through vagal-mediated release of acetylcholine (ACh), which stimulates M2 muscarinic receptors and, in turn, G-protein coupled inward rectifying K+ channels (GIRK) that inhibit AV conduction via Gi 28. It is plausible that sympathetic dominance, via either acute nicotine-induced sympathetic activation or chronic smoking-induced enhancements in sympathetic tone, would diminish parasympathetic slowing of dromotropy and thereby enable PR interval shortening.
Cotinine and catecholamines
Cigarette smoking and nicotine exposure result in increased central and peripheral sympatho-adrenal activation. The activation of nicotinic acetylcholine receptors in the adrenal medulla leads to increased circulating catecholamines. We found urinary dopamine and norepinephrine (but not epinephrine) were significantly higher among smokers and those with higher cotinine, suggesting smokers have increased sympathetic neuronal activity (norepinephrine), but similar adrenal medullary hormone secretion (epinephrine). Interestingly, we also found that cotinine significantly associated with the intermediate metabolites of all three catecholamines, but not with the final metabolite, and there was a negative association between cotinine and the ratio of the final and intermediate metabolites of all three catecholamines. This positive association between cigarette smoking and synthesis of both dopamine and norepinephrine thus corresponded with a concomitant decrease in catabolism of the intermediate metabolites of all three catecholamines. These findings corroborate several other studies that determined cigarette smoking inhibits the activity of monoamine oxidase 29,30, an enzyme primarily responsible for catabolism of the intermediate metabolites of norepinephrine and epinephrine to their final metabolites., both atrial and AV conduction did not significantly differ between smokers and non-smokers
Nicotine may increase AV nodal conduction velocity through a number of mechanisms. Nicotine stimulates sympathetic neurotransmission via activation of nicotinic acetylcholine receptors localized on peripheral postganglionic sympathetic nerve endings and the adrenal medulla, which in turn causes catecholamine release. Catecholamines mediate positive chronotropic, inotropic, dromotropic, and bathmotropic effects (i.e., increased rate, force conductivity, and excitability). Furthermore, evidence from animal models and ex-vivo studies suggests nicotine may also directly cause endothelial cell injury 31, a pro-fibrotic state 32, and inhibition of cardiac A type potassium channels 33.
A novel component to our study, mediation analysis revealed that, among catecholamines, dopamine fully mediated the relationship between cotinine and shortened PR interval/segment. In accordance with our observations, the positive dromotropic effects of dopamine accelerate AV nodal conduction 34 while not affecting atrial depolarization time 35, and would thus be expected to shorten the PR interval/segment without affecting P wave duration.
Cotinine was associated with altered metabolism of the three catecholamines (ratio of metabolite/parent). The positive association of cotinine with 3-methoxytyramine/Dopamine ratio suggests smoking increases dopamine synthesis and/or systemic secretion and, in compensation, also increases catechol-O-methyltransferase (COMT) activity. Additionally, cotinine’s inverse association with Vanillylmandelic acid/Normetanephrine and Vanillylmandelic acid/Metanephrine suggest that smoking decreases monoamine oxidase (MAO) activity, consistent with observations of decreased MAO-B in the amygdalae of smokers and the antidepressant effects of nicotine 36
Catecholamines and PR interval
Interestingly, dopamine, and neither epinephrine nor norepinephrine, was associated with PR interval/segment. As all catecholamines are known to exert a dromotropic effect, plausible reasons for our findings include:
long-term smoking disproportionately affects circulating dopamine relative to norepinephrine and epinephrine 37, potentially via nicotine-mediated decline in dopamine uptake;
cigarette smoking and/or nicotine increase central neuronal release of dopamine, which, with nicotine-mediated increases in permeability of the blood-brain barrier 38, lead to increased circulating dopamine;
dopamine has less temporal variability than its metabolites, epinephrine and norepinephrine, making it a more stable marker of chronic sympathetic nervous system activation;
at low pathophysiological levels, dopamine has a higher affinity than epinephrine and norepinephrine for β1- and β2- adrenergic receptors, which may modulate dromotropy in the atrioventricular node 39.
Other ECG parameters
We did not observe any association of smoking status or cotinine with QRS, QT or QTc. Previous studies in this area have yielded mixed results, including QT prolongation 6–8, QT shortening 40, or no relationship 9 with smoking.
Limitations
While this is the first known investigation of the role of catecholamines in nicotine-mediated alterations in cardiac conduction, the sample size is limited and participants were drawn at random from the outpatient clinic setting with intermediate to high cardiovascular risk. Thus, we might have missed significant associations between cotinine and other parameters (e.g., P-wave duration) due to insufficient statistical power. Additionally, ECGs were obtained retrospectively from medical records and not on the day of enrollment. The median and interquartile range of number of days from study enrollment (urine sample collection) and ECG was 79 [20 – 320] days. We did not collect data on time of last cigarette/nicotine exposure and states that may affect catecholamines (stress, noise, discomfort, body position, consumption of food, caffeinated beverages and drugs). Nevertheless, we used urinary analytes (cotinine and catecholamines) that are established markers for chronic nicotine and sympatho-adrenal activation and are unlikely to exhibit large acute variation in the outpatient clinic setting from which this cohort was derived. We did not collect 24-hour urine to account for diurnal and intra-individual variation in catecholamines; however, summative analysis of 24-h catecholamine production may mask elevations due to dilution. Notably, in secondary analyses, we found a heart rate variability parameter (RMSSD: square root of the mean of squared differences of successive NN intervals), when dichotomized, tended to inversely associate with urinary norepinephrine and dopamine (r= −0.13, p=0.10 for both), suggesting concordance between measures of sympathetic activity in both ECG and subsequent urine samples. Although most participants were on beta blockers, prevalence did not significantly differ by cotinine strata, and PR and P wave durations did not differ by beta blocker use. This latter point accords with observations that beta blockers do not alter resting PR interval whereas they partially attenuate PR and RR interval shortening during exercise-induced sympatho-excitation 41. Additionally, we were unable to assess associations between other cigarette components or smoking habits (e.g., frequency) and cardiac electrophysiology. Other constituents within tobacco smoke (e.g., particulate matter and aldehydes) have been shown to alter autonomic balance and may thus plausibly alter catecholamine synthesis, secretion, and metabolism. Finally, smoking and nicotine can affect myocardial conduction velocity through induction of cardiac remodeling, oxidative stress, and/or ion channel dysfunction, which were not assessed in this study and may occur independent of sympathetic activation. Most of these are limitations inherent to any mediation analyses that are not complemented by manipulation of the putative mediator (dopamine). Nevertheless, sympathetic activation can induce all three of these pathogenic processes 4 and may thereby indirectly mediate atrioventricular conduction defects.
Conclusions
Collectively, our findings suggest that exposure to cigarette smoke accelerates atrioventricular conduction and that dopamine mediates these effects. More research is warranted to examine the specificity and selectivity of these effects and to delineate the direct contribution of nicotine. These observations identify a pathway by which smoking may increase risk for cardiovascular morbidity and mortality.
Supplementary Material
Supplementary figure 1: Baseline characteristics
Supplementary table 1: Baseline characteristics by high and low PR interval, P wave duration and PR segment
Supplementary table 2: Distribution of urinary cotinine levels by self-reported active smoking status among all participants.
Supplementary table 3: Comparison among participants stratified by self-reported smoking status or by cotinine level of (A) urinary cotinine and catecholamines (median [interquartile range], creatinine-normalized), and (B) high and low PR interval, P wave duration and PR segment
Supplementary table 4: Association between creatinine-normalized log transformed cotinine and parent catecholamines, their intermediate and final metabolites, and their metabolism, denoted by the ratios of intermediate:parent and final:intermediate metabolites.
Supplementary table 5: Associations of QRS duration, QT interval and corrected QT with urinary cotinine in unadjusted and adjusted linear regressions. Urinary cotinine was normalized by urine creatinine and log-transformed.
Acknowledgement:
We thank Karen Beatty, Jessica Nystoriak, and Dan Riggs for technical support and assistance in ECG procurement.
Funding: Research reported in this publication was supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Numbers R01HL147343, HL071739, N01-HC-95159, and N01-HC-95169, the AHA Tobacco Regulation and Addiction Center (A-TRAC), and FDA Center for Tobacco Products (CTP) (P50HL120163). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. C.A. was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Competing Interests:
The authors have declared that no competing interests exist.
Conflicts of interest: None (All authors)
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Goldenberg I, Moss AJ, McNitt S, et al. Cigarette smoking and the risk of supraventricular and ventricular tachyarrhythmias in high-risk cardiac patients with implantable cardioverter defibrillators. J Cardiovasc Electrophysiol. 2006;17(9):931–936. [DOI] [PubMed] [Google Scholar]
- 2.Haass M, Kubler W. Nicotine and sympathetic neurotransmission. Cardiovasc Drugs Ther. 1997;10(6):657–665. [DOI] [PubMed] [Google Scholar]
- 3.Klein LW, Ambrose J, Pichard A, Holt J, Gorlin R, Teichholz LE. Acute coronary hemodynamic response to cigarette smoking in patients with coronary artery disease. J Am Coll Cardiol. 1984;3(4):879–886. [DOI] [PubMed] [Google Scholar]
- 4.Carll AP, Farraj AK, Roberts AM. The Role of the Autonomic Nervous System in Cardiovascular Toxicity In: Campen MJ, ed. Comprehensive Toxicology. Vol Vol. 13 3rd ed Oxford: Elsevier, Ltd; 2018. [Google Scholar]
- 5.Dinas PC, Koutedakis Y, Flouris AD. Effects of active and passive tobacco cigarette smoking on heart rate variability. Int J Cardiol. 2013;163(2):109–115. [DOI] [PubMed] [Google Scholar]
- 6.Dilaveris P, Pantazis A, Gialafos E, Triposkiadis F, Gialafos J. The effects of cigarette smoking on the heterogeneity of ventricular repolarization. Am Heart J. 2001;142(5):833–837. [DOI] [PubMed] [Google Scholar]
- 7.Fauchier L, Maison-Blanche P, Forhan A, et al. Association between heart rate-corrected QT interval and coronary risk factors in 2,894 healthy subjects (the DESIR Study). Data from an Epidemiological Study on the Insulin Resistance syndrome. Am J Cardiol. 2000;86(5):557–559, A559. [DOI] [PubMed] [Google Scholar]
- 8.Ileri M, Yetkin E, Tandogan I, et al. Effect of habitual smoking on QT interval duration and dispersion. Am J Cardiol. 2001;88(3):322–325. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Post WS, Dalal D, Blasco-Colmenares E, Tomaselli GF, Guallar E. Coffee, alcohol, smoking, physical activity and QT interval duration: results from the Third National Health and Nutrition Examination Survey. PLoS One. 2011;6(2):e17584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nielsen JB, Pietersen A, Graff C, et al. Risk of atrial fibrillation as a function of the electrocardiographic PR interval: results from the Copenhagen ECG Study. Heart Rhythm. 2013;10(9):1249–1256. [DOI] [PubMed] [Google Scholar]
- 11.Soliman EZ, Cammarata M, Li Y. Explaining the inconsistent associations of PR interval with mortality: the role of P-duration contribution to the length of PR interval. Heart Rhythm. 2014;11(1):93–98. [DOI] [PubMed] [Google Scholar]
- 12.Watanabe I Smoking and risk of atrial fibrillation. J Cardiol. 2018;71(2):111–112. [DOI] [PubMed] [Google Scholar]
- 13.DeJarnett N, Conklin DJ, Riggs DW, et al. Acrolein exposure is associated with increased cardiovascular disease risk. J Am Heart Assoc. 2014;3(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldberger AL. Electrocardiography In: Longo DL KD FA, Hauser SL, Jameons JL, Loscalzo J, ed. Harrison’s Principles of Internal Medicine. 18 ed.: New York: McGraw-Hill; 2012. 2012. [Google Scholar]
- 15.Salles G, Xavier S, Sousa A, Hasslocher-Moreno A, Cardoso C. Prognostic value of QT interval parameters for mortality risk stratification in Chagas’ disease: results of a long-term follow-up study. Circulation. 2003;108(3):305–312. [DOI] [PubMed] [Google Scholar]
- 16.Benowitz NL, Hukkanen J, Jacob P 3rd. Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol. 2009(192):29–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Man CN, Gam LH, Ismail S, Lajis R, Awang R. Simple, rapid and sensitive assay method for simultaneous quantification of urinary nicotine and cotinine using gas chromatography-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;844(2):322–327. [DOI] [PubMed] [Google Scholar]
- 18.Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput. 2004;36(4):717–731. [DOI] [PubMed] [Google Scholar]
- 19.Sharma NK, Jaiswal KK, Meena SR, et al. ECG Changes in Young Healthy Smokers: A Simple and Cost-Effective Method to Assess Cardiovascular Risk According to Pack-Years of Smoking. J Assoc Physicians India. 2017;65(6):26–30. [PubMed] [Google Scholar]
- 20.Chatterjee S, Kumar S, Dey SK, Chatterjee P. Chronic effect of smoking on the electrocardiogram. Jpn Heart J. 1989;30(6):827–839. [DOI] [PubMed] [Google Scholar]
- 21.Sandhya Metta, Satyanarayana Uppala, Joyarani D. Study of ECG changes and left ventricular diastolic dysfunction as hemodynamic markers of myocardial stress in chronic smokers. International Journal of Research in Medical Sciences. 2015;3(3):588–592. [Google Scholar]
- 22.Srivastava Amit, Poonia Anuj, Shekhar Suman, Tewari RP A Comparative Study of Electrocardiographic Changes between Non smokers and Smokers International Journal of Computer Science Engineering & Technology. 2012;2(5):1231. [Google Scholar]
- 23.Yaniel Castro-Torresa RC-P, Castañeda-Carsarvilla Luis. Increased maximum p wave duration in smoking patients with ST-elevation acute myocardial infarction and its relationship with inflammatory markers. Cor et Vasa. 2017;59(3):e246–e250. [Google Scholar]
- 24.Baden L, Weiss ST, Thomas HE Jr., Sparrow D. Smoking status and the electrocardiogram: a cross-sectional and longitudinal study. Arch Environ Health. 1982;37(6):365–369. [DOI] [PubMed] [Google Scholar]
- 25.Venkatesh G, RM S. A Study of Electrocardiographic changes in smokers compared to normal human beings. Biomedcal Research. 2010;21(4):389–392. [Google Scholar]
- 26.Goette A, Lendeckel U, Kuchenbecker A, et al. Cigarette smoking induces atrial fibrosis in humans via nicotine. Heart. 2007;93(9):1056–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holmqvist F, Thomas KL, Broderick S, et al. Clinical outcome as a function of the PR-interval-there is virtue in moderation: data from the Duke Databank for cardiovascular disease. Europace. 2015;17(6):978–985. [DOI] [PubMed] [Google Scholar]
- 28.Harvey RD, Belevych AE. Muscarinic regulation of cardiac ion channels. Br J Pharmacol. 2003;139(6):1074–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fowler JS, Logan J, Wang GJ, Volkow ND. Monoamine oxidase and cigarette smoking. Neurotoxicology. 2003;24(1):75–82. [DOI] [PubMed] [Google Scholar]
- 30.Launay JM, Del Pino M, Chironi G, et al. Smoking induces long-lasting effects through a monoamine-oxidase epigenetic regulation. PLoS One. 2009;4(11):e7959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zimmerman M, McGeachie J. The effect of nicotine on aortic endothelium. A quantitative ultrastructural study. Atherosclerosis. 1987;63(1):33–41. [DOI] [PubMed] [Google Scholar]
- 32.Goette A. Nicotine, atrial fibrosis, and atrial fibrillation: do microRNAs help to clear the smoke? Cardiovasc Res. 2009;83(3):421–422. [DOI] [PubMed] [Google Scholar]
- 33.Wang H, Shi H, Zhang L, et al. Nicotine is a potent blocker of the cardiac A-type K(+) channels. Effects on cloned Kv4.3 channels and native transient outward current. Circulation. 2000;102(10):1165–1171. [DOI] [PubMed] [Google Scholar]
- 34.Bianchi C, Diaz R, Gonzales C, Beregovich J. Effects of dobutamine on atrioventricular conduction. Am Heart J. 1975;90(4):474–478. [DOI] [PubMed] [Google Scholar]
- 35.Tisdale JE, Patel R, Webb CR, Borzak S, Zarowitz BJ. Electrophysiologic and proarrhythmic effects of intravenous inotropic agents. Prog Cardiovasc Dis. 1995;38(2):167–180. [DOI] [PubMed] [Google Scholar]
- 36.Karolewicz B, Klimek V, Zhu H, et al. Effects of depression, cigarette smoking, and age on monoamine oxidase B in amygdaloid nuclei. Brain Res. 2005;1043(1–2):57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Snider SR, Kuchel O. Dopamine: an important neurohormone of the sympathoadrenal system. Significance of increased peripheral dopamine release for the human stress response and hypertension. Endocr Rev. 1983;4(3):291–309. [DOI] [PubMed] [Google Scholar]
- 38.Hawkins BT, Abbruscato TJ, Egleton RD, et al. Nicotine increases in vivo blood-brain barrier permeability and alters cerebral microvascular tight junction protein distribution. Brain Res. 2004;1027(1–2):48–58. [DOI] [PubMed] [Google Scholar]
- 39.Motomura S, Hashimoto K. Beta 2-adrenoceptor-mediated positive dromotropic effects on atrioventricular node of dogs. Am J Physiol. 1992;262(1 Pt 2):H123–129. [DOI] [PubMed] [Google Scholar]
- 40.Karjalainen J, Reunanen A, Ristola P, Viitasalo M. QT interval as a cardiac risk factor in a middle aged population. Heart. 1997;77(6):543–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luceri RM, Brownstein SL, Vardeman L, Goldstein S. PR interval behavior during exercise: implications for physiological pacemakers. Pacing Clin Electrophysiol. 1990;13(12 Pt 2):1719–1723. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure 1: Baseline characteristics
Supplementary table 1: Baseline characteristics by high and low PR interval, P wave duration and PR segment
Supplementary table 2: Distribution of urinary cotinine levels by self-reported active smoking status among all participants.
Supplementary table 3: Comparison among participants stratified by self-reported smoking status or by cotinine level of (A) urinary cotinine and catecholamines (median [interquartile range], creatinine-normalized), and (B) high and low PR interval, P wave duration and PR segment
Supplementary table 4: Association between creatinine-normalized log transformed cotinine and parent catecholamines, their intermediate and final metabolites, and their metabolism, denoted by the ratios of intermediate:parent and final:intermediate metabolites.
Supplementary table 5: Associations of QRS duration, QT interval and corrected QT with urinary cotinine in unadjusted and adjusted linear regressions. Urinary cotinine was normalized by urine creatinine and log-transformed.