Abstract
Background:
While deep brain stimulation has been successful in treating movement disorders, such as in Parkinson’s disease, its potential application in alleviating memory disorders is inconclusive.
Objective/Hypothesis:
We investigated the role of the location of the stimulating electrode on memory improvement and hypothesized that entorhinal white versus gray matter stimulation would have differential effects on memory.
Methods:
Intracranial electrical stimulation was applied to the entorhinal area of twenty-two participants with already implanted electrodes as they completed visual memory tasks.
Results:
We found that stimulation of right entorhinal white matter during learning had a beneficial effect on subsequent memory, while stimulation of adjacent gray matter or left-sided stimulation was ineffective. This finding was consistent across three different visually guided memory tasks.
Conclusion(s):
Our results highlight the importance of precise stimulation site on modulation of human hippocampal-dependent memory and suggest that stimulation of afferent input into the right hippocampus may be an especially promising target for enhancement of visual memory.
Keywords: Intracranial electrical stimulation, declarative memory, deep brain stimulation, white matter, entorhinal cortex, hippocampus
Introduction
The ability to remember new facts and experienced events depends on the hippocampus and associated structures in the medial temporal lobe (MTL), including entorhinal, perirhinal and parahippocampal cortices [1]. Much of our knowledge regarding the coordination of these areas to encode and retrieve declarative memories is based on work with animal models. Originally reported in the rabbit hippocampus [2], the persistent strengthening of hippocampal synapses through coordinated neuronal firing, or Hebbian plasticity, is generally accepted as the cellular basis of memory. Mimicking endogenous neuronal behavior in rodents, electrical stimulation of the afferent white matter input to the hippocampus from the entorhinal cortex (i.e., perforant path) can elicit long-term potentiation (LTP) and acetylcholine release. Both outcomes have been associated with enhanced memory [3–5], as well as increased hippocampal neurogenesis in the downstream dentate gyrus [6, 7]—a region thought responsible for producing a sparse representation of entorhinal input. Conversely, saturation or blockade of LTP results in impaired learning [8, 9]. Stimulation of the perforant pathway, therefore, may be a primary way to modulate hippocampal activity and, within certain constraints, improve hippocampal-dependent memory.
The potential clinical applications of these basic research concepts are vast; with preliminary animal and human studies showing some promise for the treatment of memory disorders such as Alzheimer’s disease (AD), traumatic brain injury (TBI) and Rett syndrome (RTT). There is evidence that stimulation of the fornix, another major white matter input/output pathway within the MTL, increases cerebral glucose metabolism and may slow cognitive decline in a subset of patients with mild AD [10] while stimulation of the entorhinal cortex rescues spatial memory in a mouse model of AD [11]. Stimulation of the fornix or septohippocampal circuit has improved spatial learning in TBI rodent models [12], though this remains to be translated to humans. In the realm of basic research with human participants, a few studies on the effect of electrical stimulation in the MTL region have arrived at contradictory results, with some reporting memory enhancement [13–17] and others reporting memory impairment [18–21]. This variability could stem from methodological differences, including the behavioral task parameters, the precise site of the stimulation electrode, and the spatiotemporal profile and amplitude of stimulation [22].
Because the invasive nature of data collection in these human studies limits the number of experiments that can be conducted, it is impossible to systematically explore the entire parameter space. Instead, we focus here on investigating whether the precise location of the stimulating electrode is critical for the effect of stimulation on subsequent memory and to determine the robustness and generalizability of this effect across different hippocampal-dependent memory tasks. We employed automated segmentation software and co-registration of high-resolution magnetic resonance imaging (MRI) and CT scans to identify the precise location of each stimulating electrode within the entorhinal area and assessed the memory effect of stimulation in each area for each memory task, as well as in the combined dataset. We sought to examine how the precise location of the stimulating electrode may contribute to the efficacy of stimulation for memory enhancement. In particular, we asked whether previously reported white matter (angular bundle) stimulation, as well as lateralization, effects [16] could be generalized to other memory tasks.
Materials and Methods
Experimental Design
The design of the study was a within-subjects experiment, comparing memory performance between a stimulated and non-stimulated condition. The research objective was to measure whether memory was better for items that received stimulation than those that did not, and whether this was affected by the location of the stimulating electrodes. Our a priori hypothesis prior to data collection was that the location of the stimulating electrode in entorhinal white matter vs. surrounding gray matter would provide differential effects (white/gray). Due to findings reported by Titiz and colleagues [16] indicating the importance of lateralization of the stimulating electrode (left/right), we chose to analyze white/gray * left/right.
Participants were patients with pharmacoresistant epilepsy who had been implanted with intracranial depth electrodes (see details below). Each participant completed at least one of the following memory tasks: person recognition, object recognition, or face-name associative memory (see Fig 1 and “Behavioral Tasks” below), and sometimes completed multiple sessions of a given task. Within each individual experimental session, a randomly selected half of the items were delivered with stimulation. The participants were told that stimulation would be applied but were blinded to which items received stimulation. The experimenters were able to observe stimulation artifact in real time, which was used to ensure stimulation was delivered appropriately.
Fig 1. Overview of the experimental protocol.
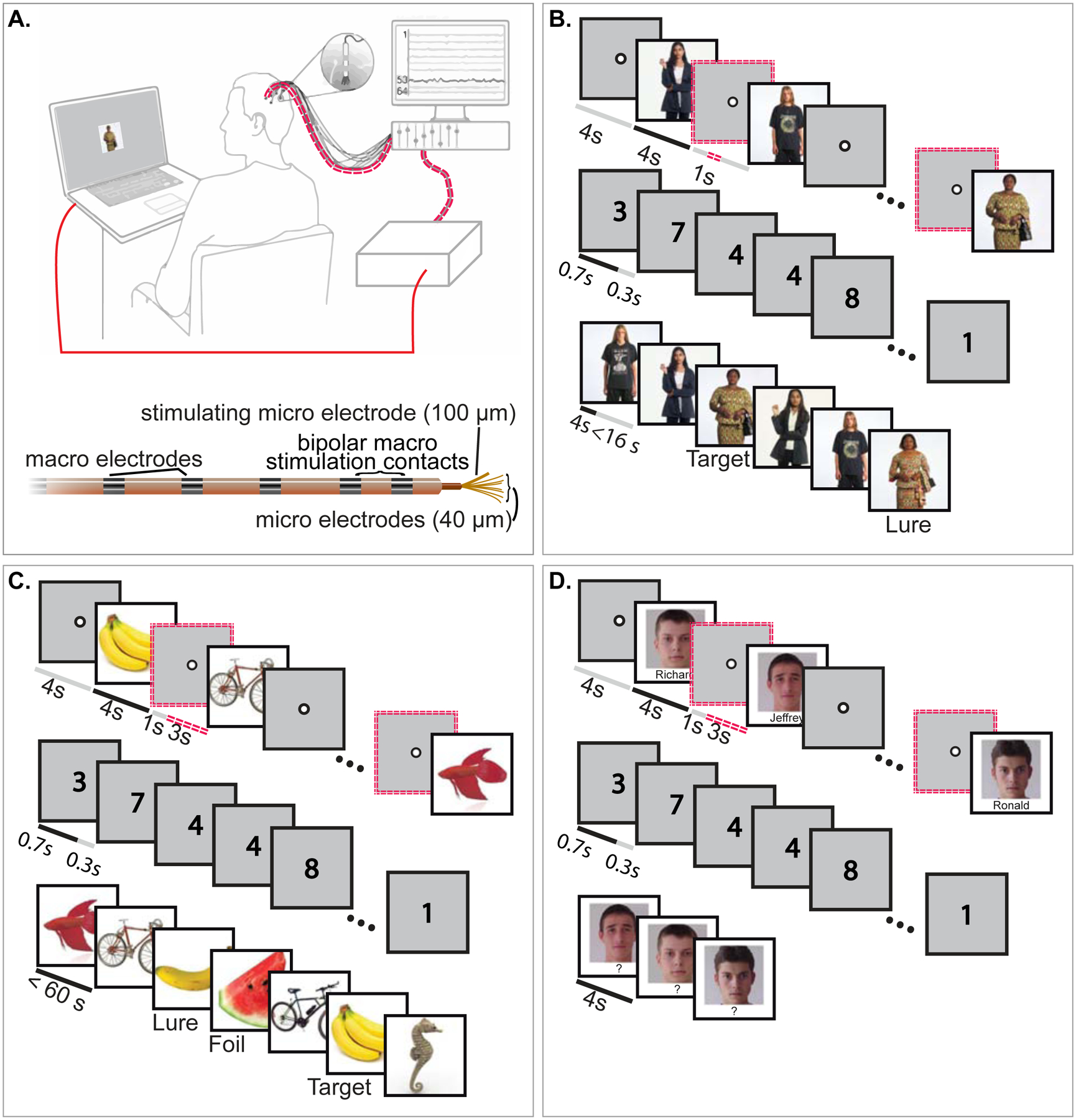
(A). A cartoon illustration of a participant completing a memory task. Red lines indicate the stimulation pathway: the laptop on which the participant conducts the task sends a signal to the stimulation box, which sends electrical pulses to a splitter, which then transmits the pulses to the prescribed electrode within the brain. Below this cartoon is a diagram of the depth electrode used for macro- and micro-stimulation (A). (B-D). Participants completed a person recognition (B), an object recognition (C), and/or a face-name associative memory task (D), each of which included an encoding, distractor, and retrieval stage. During encoding, a random half of the items were selected to receive stimulation during the prior fixation period, denoted as double, red, dotted lines. After encoding, participants were asked to do a distractor task, during which randomly selected single digits were rapidly presented, and participants were asked to indicate whether each number was odd or even. During retrieval in the person memory task, a shuffled set of previously seen photographs (“Targets”) and similar-looking photographs (“Lures”) were presented, and participants were asked to rate whether the images were “new” or “old” and then assess their confidence. During object memory retrieval, the original (“Target”), a very similar (“Lure”), or a dissimilar (“Foil”) image was shown, and participants were asked to rate whether the images were “new” or “old” and to assess their confidence. During face-name associative memory retrieval, participants were asked to recall the name associated with each image. Participants completed 6 blocks with the same stimuli to facilitate learning of the associations. Although the order of presentation varied for each repeated block, the same items received stimulation.
No explicit sample size computation was performed prior to beginning experiments and no specific rule was used for stopping data collection. However—due to the extreme scarcity of experimental participants— it is common in the field of invasive human stimulation/physiology to include a minimum of 6 participants. Our goal was to test the effect of stimulation across multiple stimulation conditions (white/right, white/left, gray/right, gray/left). We thus considered the critical sample variable to be the number of individual sessions collected within each condition, rather than the number of participants, and sought to include a minimum of 15 data points (experimental sessions) per condition when combined across tasks. We were not able to precisely balance the number of experimental sessions completed across the three tasks and four stimulation conditions, due to the complexity of factors that contribute to how many conditions and tasks each participant was able to complete (e.g. clinically-determined electrode locations and duration of hospital stay). We collected data from 22 participants, which yielded at least 19 individual experimental sessions within each condition when combined across tasks (see Figure 3). Criteria for data exclusion are described within “Participant Details”.
Fig. 3. Analysis of the change in behavioral performance as a function of stimulation location in tasks.
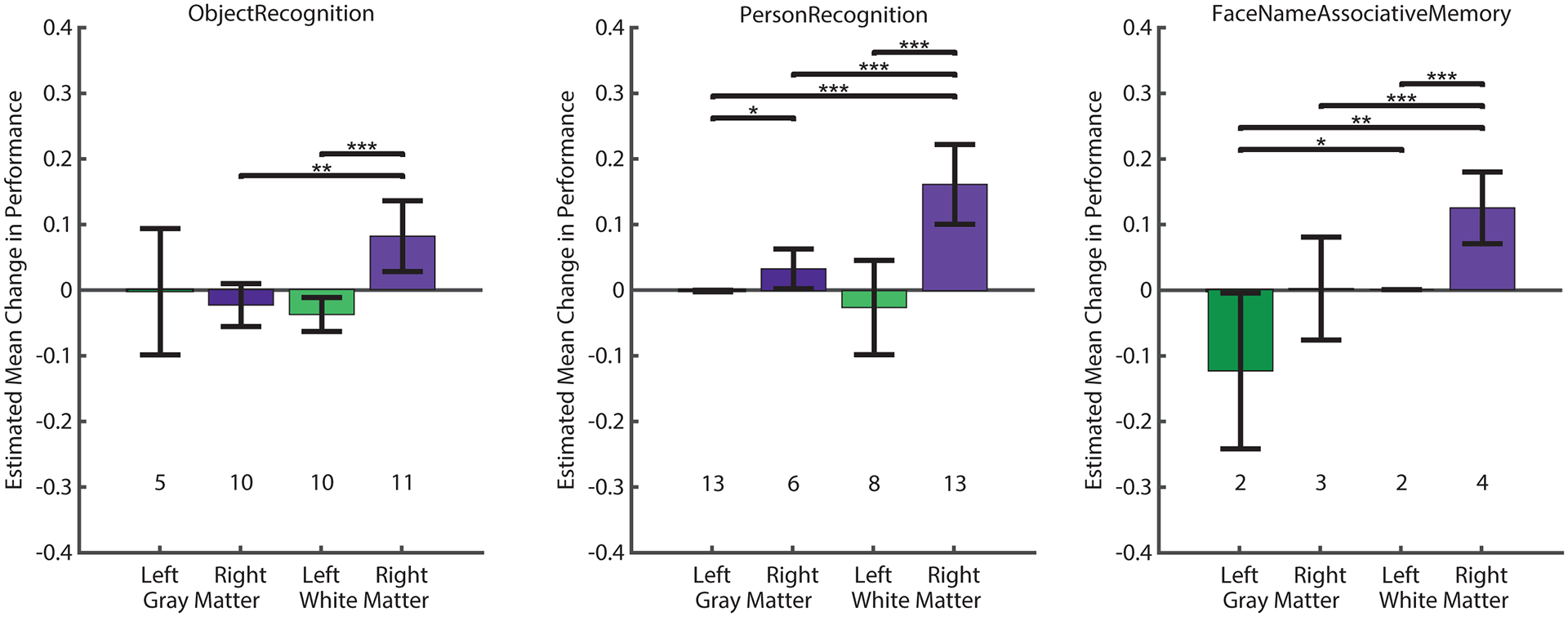
Generalized estimating equations (GEEs) were used to model the effect of gray versus white matter stimulation on the behavioral outcomes of individual memory tasks: Person Recognition (left), Object Recognition (middle), and Face Name Associative memory (right). For each task, estimated means and Wald 95% confidence intervals (error bars) of the change in performance are shown. Positive values indicate memory improvement and negative values indicate memory impairment in stimulated trials. The number of sessions used within each condition is noted. In all three behavioral tasks, stimulation of the right entorhinal white matter showed significantly positive outcome on memory performance, indicated by positive estimated means with confidence intervals that do not include zero. Pairwise comparisons of the different stimulation conditions are indicated within each task (* P < 0.05, ** P < 0.01, *** P < 0.001). Source data and code are included in the Supplementary Materials.
Because some participants performed the experiment multiple times, we used generalized estimating equations (GEEs) to analyze our data, as they model the effects of within-subject correlations without losing statistical power (as happens when the average for each participant is computed prior to performing a t-test; see “Statistical Analysis” below). Nonetheless, GEEs do not provide a traditional measure of effect size, which limited our ability to perform an a priori power analysis for our study.
Participant Details
The study participants were 22 patients (Table S1) with pharmacoresistant epilepsy who had been implanted with intracranial depth electrodes and stayed in the hospital for 7–34 days, during which time intracranial electroencephalographic (iEEG) activity was monitored to determine epileptogenic zones and guide possible surgical resection [23]. Pre-determined clinical criteria guided placement of the 9–14 Behnke-Fried electrodes (Adtech Medical, Racine WI), which were implanted stereotactically with the aid of digital subtraction angiography or CT angiography (CTA) as well as magnetic resonance imaging (MRI) [23]. Each Behnke-Fried macro-micro depth electrode contained seven macro-electrode contacts (1.5 mm in diameter), which were spaced 1.5 mm apart along the shaft and a Behnke-Fried inner platinum-iridium micro-wire bundle (California Fine Wire, Grover Beach, CA). The micro-stimulation electrode contact extended past the tip of the macro-electrode by 3 mm, 2 mm shorter than the tip of the other micro-wires (Fig. 1A). All surgeries were performed by one surgeon (IF). Neuropsychological test scores were determined for each participant, including tests of memory and executive function (Table S1). All research was carried out at the UCLA Ronald Reagan Medical Center. Before participating in the study, all participants provided written informed consent on a study protocol approved by the UCLA Institutional Review Board.
A subset of data from these participants was previously published elsewhere [16]. However, the current study is unique in combining multiple new data sets with previously explored data to investigate a novel research question concerning the consistency of the effect of white vs. gray matter stimulation and left vs. right-sided stimulation.
Three participants were excluded from analysis. One participant was excluded because of psychological issues that arose during the hospital stay (this participant was also listed as an excluded patient in the study by Titiz and colleagues [16]); one participant was removed because the MRI voxel resolution was too low and the proximity of the stimulating electrode to the white/gray matter boundary prevented confident assignment of the electrode location to a specific group; one participant was excluded because they only participated in face-name association and completed fewer than 6 blocks on the task.
Stimulation Parameters
A board-certified neurologist was present for each stimulation session to monitor the clinical iEEG recordings for after-discharges and ensure patient safety. Before experimental sessions, each participant was given a short series of test stimulation pulses while a neurologist monitored the clinical iEEG recording for after-discharges. Unaware of the exact timing of stimulation onset, participants were asked to report any unusual feelings or sensations. They were also instructed to report any usual feelings or sensations during the experimental session. Participants failed to consciously notice any effects of stimulation and were unaware of which trials within each behavioral paradigm were stimulated. Stimulation of epileptogenic areas was avoided when possible, no sessions were administered within two hours following a seizure, and only one seizure was noted in one participant during the two-hour period following a session. No seizures were elicited as a result of stimulation. For each paradigm, the stimulation preceded each stimulus by 3 s and was applied for a duration of 1–3 s, depending on the task (Supplementary Materials and Methods).
Macro-stimulation
A CereStim R96 Macro-stimulator (BlackRock Microsystems) was used to deliver electrical stimulation to the Behnke-Fried depth electrode bipolar macro-contacts. Charge-balanced and current-regulated biphasic rectangular pulses were set below the current amplitude that elicited an after-discharge, which was identified through pretesting with a neurologist, and ranged from 0.4 to 6.0 mA. Remaining stimulation parameters were identical to those used previously [24]. Briefly, bipolar macro-electrodes were spaced 1.5 mm apart (surface area, 0.059 cm2; Fig. 2, red circles) and electrical stimulation was delivered at 50 Hz and with a 300-μsec pulse width. Stimulation ranged between 2 and 30 μC of charge per square centimeter per phase, and electrode impedance was measured using the clinical Neurofax EEG-1200A system (Nihon Koden corporation) and ranged from 0.3 to 17.0 kΩ.
Fig 2. Examples of co-registration and automated segmentation methods for electrode localization.
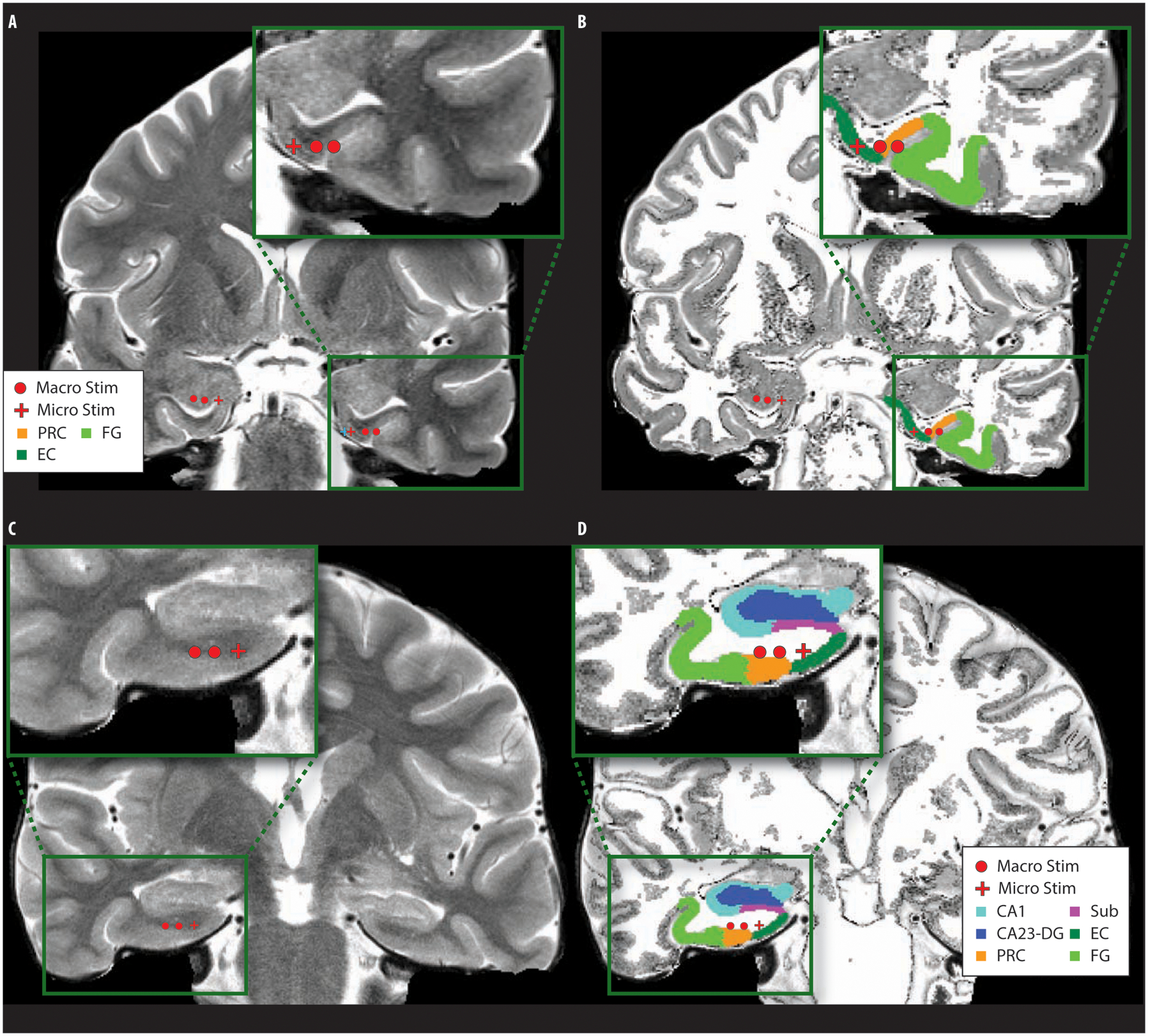
Example participant electrode locations (macro-electrodes and micro-electrodes) overlaid onto the original high-resolution (A, C) and segmented MRI (B, D) (Example co-registration of CT and MRI are shown in Fig. S1; localizations for each participant are available in Fig. S2). Macro-stimulation was delivered to the two macro-electrode contacts and micro-stimulation to the 100-μm micro-electrode. Example automatic segmentations of MTL subregions are shown with delineated hippocampal (CA1, CA3-DG, subiculum) and cortical areas (entorhinal, perirhinal, and fusiform). Top is an example micro- and macro-electrode placement within nearby gray matter regions; bottom is an example bipolar macro-electrode and micro-electrode placement within the entorhinal white matter region. (Macro Stim: bipolar macro-electrode contacts, 1.5mm apart, surface area 59 mm2, Micro Stim: 100μm micro-electrode, Sub: subiculum, EC: entorhinal cortex, PRC: perirhinal cortex, FG: fusiform gyrus).
Micro-stimulation
A Blackrock R96 stimulator (BlackRock Microsystems, Salt Lake City, Utah) was used to deliver monopolar current regulated micro-stimulation, directed through a 100-μm Platinum-Iridium micro-wire (Fig. 2, red crosshair) with insulation removed from 1 mm around the tip. Micro-stimulation parameters were identical to our previous study showing improved memory with micro-stimulation [16]. Briefly, 150 mA cathodic-first, biphasic theta-burst micro-stimulation was applied for 1-second at 100 Hz with a pulse width of 200 μs and an inter-pulse interval of 100 μs. A theta-burst stimulation protocol was used (i.e., 4 pulses at 100 Hz, occurring every 200 msec) [16]. This stimulation protocol resulted in a charge delivery of 30 nC per phase and a charge density of 9.32 μC/cm2. Impedance was measured prior to each stimulation session using an electrode impedance meter (Bak Electronics, Inc.) combined with a switch box composed of a single pole multiple throw wafer switch to manually check the impedances. Impedance values were determined to be less than 80 kΩ in each session (mean ± SD: 27.64 ± 17.47 kΩ).
Electrode Localization
Methods for determining the location of the stimulating electrodes were as described previously [16]. Briefly, a high-resolution post-operative computed tomography (CT) scan was co-registered to a pre-operative whole brain magnetic resonance imaging (MRI) and high-resolution MRI (Fig. S1). MTL regions were delineated using the Automatic Segmentation of Hippocampal Subfields (ASHS [25]) software using boundaries determined from MRI visible landmarks that correlate with underlying cellular histology (Fig. 2, S2). Macro- and micro-electrode contacts were identified and outlined on the post-operative CT scan and overlaid with the results from automated segmentation. If the more distal of the two stimulating macro-electrodes fell within the white matter region, it was classified as “white.” The co-registered CT electrode locations and high-resolution MRIs of example participants are shown in Fig. S2. Table S3 includes additional information—both the localization result for each electrode contact as well as the corresponding clinical label. See also Electrode Localization and Brain Imaging Parameters in Supplementary Materials and Methods.
Behavioral Tasks
Participants completed at least one of the following three behavioral tasks, designed to probe hippocampal-dependent declarative memory: Person Recognition, Object Recognition, and Face-Name Associative Memory (Fig. 1). All tasks were presented on a laptop running Mathworks’ Matlab with Psychtoolbox extensions [26]. To coordinate the tasks and stimulator onset, a stimulation pulse was sent via USB from the experimental laptop to a USB-to-TTL converter box. This in turn sent a TTL pulse to the stimulator, triggering a predetermined stimulation protocol. Tasks and the order in which they were presented to the patients were decided with respect to the given research priority and patient factors at the time of testing. When possible, the tasks were administered multiple times to each patient. All tasks were designed to be hippocampal-dependent with the introduction of a cognitively engaging distractor task between encoding and retrieval phases. The tasks shared the same basic structure: each task began with a learning (encoding) period, followed by a 30 s distraction task, and then a test (retrieval) period. The Face-Name Associative memory task was repeated 6 times with the same stimuli to increase learning; the same stimuli received stimulation in each encoding block, but the order of presentation and cued recall was randomized between blocks. For each task, the number of stimuli to be learned was pre-determined based on neuropsychological testing and/or pre-testing sessions, in order to prevent a ceiling or floor effect. Electrical stimulation was provided prior to the onset of stimulus presentation during half of all encoding trials in a randomized fashion for each participant using a within-subject design. The distractor task was a 30 s odd/even task in which numbers were presented quickly, for 600–750 ms with a jittered 250–400 ms delay between them, and participants were instructed to classify them as ‘odd’ or ‘even’ by using one of two key presses. This distractor task was used between encoding and retrieval phases for each memory task. The specifics of each behavioral task are detailed in the supplementary methods and Fig.1.
Statistical Analysis
The effect of location (site within the angular bundle and hemisphere) and size of stimulating electrode on memory performance was investigated using generalized estimating equations (GEE). GEE is class of Generalized Linear Models capable of modeling data with potential (unknown) correlations between the outcomes, thus making it suitable for analyzing within-subject repeated measure designs [16, 27]. For both the All Data analysis and the individual task analysis, the behavioral performance measure for each individual session was calculated for stimulated items and non-stimulated items separately. The difference between these two values, a metric describing memory modulation (positive values: enhancement; negative values: impairment) was modeled with the stimulation site (white/gray matter), the stimulation hemisphere (left/right) specified as model effects, as well as the interaction between site and hemisphere. Additionally, electrode size (macro/micro) was included as a term in an additional model for the All Data analysis. Although we report uncorrected P-values for the “All Data” and “All Data Alternate Model” (Table 1), Bonferroni correction of these values does not change their significance level. Participant ID was defined as the repeated subject variable, and session number*task identity was included as a within-subjects variables to account for the non-independence of repeated sessions by the same participant. The memory modulation score was specified as a linear scale response, with identity link function, and a working correlation matrix with exchangeable structure was used. Means and confidence intervals reported are the estimated means and 95% Wald Confidence Intervals generated by this model. Additionally, the model computed the statistics associated with the pairwise comparisons of all site-hemisphere combinations.
Table 1. The effects of stimulation site, hemisphere, and electrode size on subsequent memory using GEE models.
(A) Generalized estimating equations (GEEs) were used to model behavioral performance for each individual session (difference in fraction of recalled trials in stimulated vs non-stimulated condition) as a function of the stimulating electrode site (white matter vs. adjacent gray matter), stimulation hemisphere (left vs. right), and the interaction term between the two. For a detailed description of the model, see Statistical Analysis section (Methods). Our data, collected from 22 participants across three behavioral paradigms, included 87 experimental sessions, with the following number of each type: Nwhite = 48, Ngray = 39; Nright = 47, Nleft = 40. We report the P-value, the coefficient for each factor in the model and its standard error (shown as B ± Std. Error), and the Wald Chi-Square test statistic (shown as Chi-Square). (B) Because some stimulation sessions were delivered with macro stimulation (Nmacro = 8, Nmicro = 79), we compared the original model to one that included a term for the impact of electrode size. Note that the macro vs micro term was not significant. Because we computed two models on the All Data set, multiple comparisons corrections should be applied to the P values. Bonferroni corrected P-values are shown in parentheses. Source data and code are available in Supplementary Materials.
| (A) | Gray vs White | Left vs Right | White × Right | ||
|---|---|---|---|---|---|
| Person Recognition | P | 0.0002 | 0.00009 | 0.002 | |
| B ± std. Error | −0.13 ± 0.03 | −0.19 ± 0.05 | 0.15 ± 0.05 | ||
| Chi-Square | 13.81 | 15.3 | 9.58 | ||
| Object Recognition | P | 0.005 | 0.0001 | 0.000002 | |
| B ± std. Error | −0.10 ± 0.04 | −0.12 ± 0.03 | 0.14 ± 0.03 | ||
| Chi-Square | 7.92 | 14.77 | 22.16 | ||
| Face Name Association | P | 0 | 0.000007 | 0.99 | |
| B ± std. Error | −0.12 ± 0.01 | −0.12 ± 0.03 | −0.001 ± 0.073 | ||
| Chi-Square | 100.91 | 20.04 | 0 | ||
| All Data | P | 0.0017 (0.0034) | 0.00039 (0.00079) | 0.0014 (0.0029) | |
| B ± std. Error | −0.13 ±0.04 | −0.14 ± 0.04 | 0.13 ± 0.04 | ||
| Chi-Square | 9.84 | 12.57 | 10.17 | ||
| (B) | Gray vs White | Left vs Right | White × Right | Macro vs Micro | |
| All Data (Alternate Model) | P | 0.0021 (0.0043) | 0.00027 (0.00055) | 0.00052 (0.0010) | 0.167 |
| B ± std. Error | −0.12 ± 0.04 | −0.13 ± 0.04 | 0.11 ± 0.03 | −0.05 ± 0.04 | |
| Chi-Square | 9.41 | 13.24 | 12.03 | 1.91 | |
GEEs were calculated using SPSS (IBM). Data and source code for conducting the analysis are included in the supplementary material as Data File S1 and Source Code S1.
Results
Study Design and Participants
We collected data from twenty-two participants with intracranial depth electrodes implanted for clinical epilepsy evaluation. Demographics and neuropsychological test scores are shown in Table S1. Amongst the 22 participants in the study, a total of 30 electrode sites were used to deliver electrical stimulation. Based on the results of an automated electrode localization procedure, which combined co-registration of high-resolution MRI and CT scans with automated hippocampal segmentation software (see Electrode Localization in Materials and Methods), 15 electrode locations were determined to be in white matter (5 in left hemisphere) and 15 in gray matter (6 in left hemisphere) (Fig. 2, S1, S2; Tables S2, S3).
Each participant completed at least one of the three behavioral tasks: person recognition, object recognition, and face-name associative memory. For all memory tasks in this study, stimulation was provided during the learning phase for half of the trials in a within-subjects design. Moreover, these tasks shared a similar structure in that they consisted of three phases (encoding, distraction, and recall), and involved visual demands in the recognition of persons, faces, or objects. The face-name associative memory task differed from the others in that participants repeated the same encoding-distractor-recall sequence six times for each set of stimuli, as participants often required multiple repetitions to learn the associations. To measure the overall effect of stimulation on learning, we restricted our analysis to the final block. For a detailed description of each task, see Fig. 1 and the Behavioral Tasks section of Methods. A subset of the data from the person recognition task were published previously [16].
Location Specific Effects of Stimulation in Each Task
Within each behavioral paradigm, we sought to understand the effects of hemisphere and site of stimulation (whether the electrode was located within the angular bundle or in adjacent gray matter). To test the influence of each of these factors on stimulation’s effect on memory, we used generalized estimating equations (GEE) to exploit the within-subject repeated-measure design of the tasks (see Statistical Analysis section, as well as [16], for justification of this approach). For each experimental session, the fraction of correct trials was computed for stimulated versus non-stimulated trials, and the difference between the two was modeled as a function of white vs gray matter, left vs right hemisphere, and the interaction between these.
In each task, we found significant main effects of both stimulation site and hemisphere (object recognition; site: P = 0.005, hemisphere: P < 0.001; person recognition; site: P < 0.001, hemisphere P < 0.001, face name associative memory: site: P < 0.001; hemisphere: P < 0.001). In the object and person recognition tasks, there was also a significant effect of the interaction between site and hemisphere (object recognition: P < 0.001; person recognition: P = 0.002), whereas in the face-name associative memory task, the interaction was not significant (P = 0.990) (Fig. 3). In all three tasks, stimulating in right white matter had a significantly positive effect on memory performance (Fig. 3; see Table 1 for model outputs). In addition, the stimulation-driven performance boost for right-sided white matter stimulation was significantly greater than any other location combination (except in object recognition, the left-gray/white-right difference was not significant), as demonstrated by the pair-wise comparisons of the stimulation conditions (Fig. 3).
Location Specific Effects of Stimulation in a Combined Dataset
We next combined data from the three different hippocampal-dependent behavioral tasks. By sampling from a diverse dataset this approach has the advantage of potentially averaging out unreliable effects (such as those arising from task demands) while amplifying more consistent ones, as well as increasing statistical power. Aligned with our results from the individual paradigms, we found significant effects of stimulation site (P = 0.002), hemisphere (P < 0.001), and their interaction (P = 0.001) on subsequent memory performance. Here, too, stimulation of the right white matter yielded significant memory enhancement (Estimated Mean = 11.45%; CI = 6.00–16.94%)—and different from all other combinations of stimulation location (P < 0.01 for all comparisons; Fig. 4).
Fig. 4. Combined task analysis demonstrated that stimulation of the right entorhinal white matter consistently improved memory.
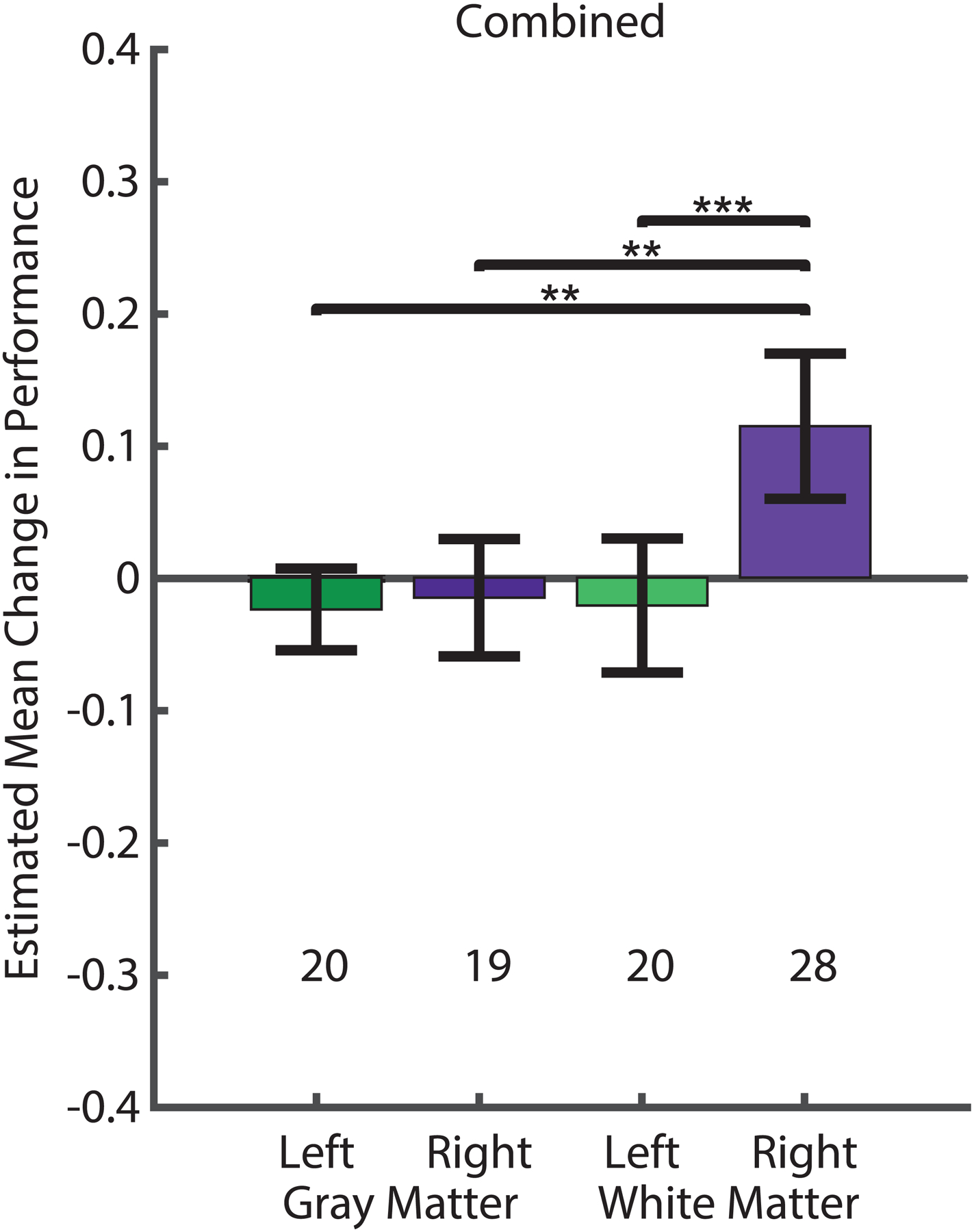
Generalized estimating equations (GEEs) were used to model the change in memory performance driven by stimulation. Estimated mean change in fraction recalled for stimulated vs non-stimulated trials (colored bars; error bars denote Wald 95% confidence intervals) was positive if performance on stimulated trials was better than on non-stimulated trials. The location of the stimulating electrode in white or gray matter and left or right hemisphere was examined; the number of sessions in each condition is noted.
Across all trials of all behavioral tasks, stimulation of right white matter was the only condition that led to performance differences with a positive mean and a confidence interval that did not include zero. Furthermore, stimulation-related memory enhancement in the right white matter was significantly different from all other condition (pair-wise comparisons of right white matter stimulation with: left white matter, P= 0.0003; right gray matter, P = 0.002; left gray matter, P = 0.003; see Methods). Source data and code are included in the Supplementary Materials.
Unlike the study by Titiz and colleagues [16], the current dataset includes not only micro-stimulation but also studies in which stimulation was delivered through macro-electrodes (6 bipolar electrode pairs); we thus also considered the type of stimulation delivered. It should be noted that stimulation amplitudes and charge densities were often higher in macro-stimulation (charge density: 2–30 μC/cm2; amplitude: 0.4–6 mA) compared to micro-stimulation (charge density: 9.32 μC/cm2; amplitude: 150 μA), so these may each contribute to any possible differences observed between micro- and macro-stimulation. We introduced micro- versus macro- stimulation as an additional factor in our GEE model. While stimulation location in white vs gray matter (P = 0.002), stimulation hemisphere (P < 0.001) and their interaction (P = 0.001) were still significant factors in predicting stimulation-related memory performance, we did not observe an effect for electrode size (P = 0.167; Table 1). However, the spatial extent of stimulation (be it in the form of the size of the stimulating electrode or the amplitude of the electrical current used) warrants investigations in future studies.
Taken together, these results indicate that the location of the stimulation electrode is a robust predictor of the effect of stimulation on subsequent memory. Within and across all three tasks, stimulation in the white matter of the right entorhinal area consistently improved visuospatial memory while other stimulation locations had null or impairment effects. The persistence, and replication, of this observation across three tasks lends strength to the tenet that targeting stimulation to the entorhinal white matter is critical in modulating human memory.
Discussion
Our findings suggest that deep brain stimulation (DBS) applied to the entorhinal area can result in modulation of human memory. Overall, we found that stimulation of the angular bundle in the right hemisphere during encoding was the most effective for visuospatial memory enhancement. These results are in line with recent results from clinical DBS studies aimed at treating essential tremor and depression, which emphasize the importance of accurate electrode placement for maximal therapeutic efficacy [28]. A recent review on the targeting of neuronal circuits [29] also stresses the principle of afferent-tract targeting, noting that regardless of the specific intervention—whether intracranial stimulation [30] or optogenetic control [31] — targeting white matter is crucial for effective treatment of these conditions [32]. Because we desired to affect memory, we targeted our stimulation to the entorhinal area, whose white matter includes the afferent input to the hippocampus, the chief organ of declarative memory. Consistent with the principle of afferent tract-targeting, stimulation was more effective at enhancing memory when the stimulating electrode was positioned in the white matter.
Though the specific mechanisms contributing to the beneficial memory effect of our stimulation protocols remain unclear, previous rodent studies have shown that stimulation of the perforant pathway can aid potentiating neural mechanisms of learning and memory [3–5]. Recent imaging studies in humans confirmed that perforant pathway fibers are quite densely bundled within an area similar to our localized entorhinal white matter electrodes, from which they divide and disperse to various hippocampal subregions [33, 34]. By focusing stimulation on this region of the angular bundle, the perforant pathway in humans may be best targeted, thereby allowing for increased specificity of modulation. Further downstream, this could possibly result in long term potentiation or the depolarization of hippocampal neurons closer to their threshold potential, leading to more action potentials and successful memory formation. Conversely, adjacent gray matter stimulation may have a neutral or disruptive effect on encoding, either affecting fewer perforant fibers or introducing an overwhelming amount of noise to regions thought responsible for containing the sparsely-encoded memory trace [1].
Earlier studies of intracranial MTL stimulation in humans have yielded mixed results regarding the efficacy of short-term electrical stimulation for memory enhancement [reviewed in 22]. A few studies involving electrical stimulation of the fornix white matter, the chief efferent pathway of the hippocampus, suggested memory enhancement [13, 14], though these should be considered with caution, due to the small sample size, divergent results (i.e. Miller et al.[14] demonstrated memory improvement in only one of the two presented tasks), and inter-study variability of electrode placement along the fornix—both anterior and posterior [35]. We have previously found enhanced memory by stimulation of the entorhinal region during learning [15, 16]. Other studies targeting either the hippocampus directly or other MTL gray matter areas showed either null [15] or disruptive [18, 19, 21, 36] effects on memory. Thus, the present results aim to help to clarify this literature by specifically examining stimulation site across multiple visuospatial memory tasks.
Together, the findings that afferent tract targeting is critical in clinical DBS treatment for non-mnemonic conditions, perforant path stimulation aids memory and LTP in rodents, and white matter stimulation in the MTL has shown positive memory effects in human patients led us to hypothesize that the precise localization of stimulating electrode to white or gray matter in the entorhinal area might be a critical factor driving the success or failure of stimulation to enhance memory across a wide variety of tasks. We therefore considered the effect of stimulating electrode location across 30 entorhinal stimulating electrodes in 22 participants who completed 87 sessions among three hippocampal-dependent visuospatial memory tasks. There is some evidence that the hemisphere of stimulation may also contribute to efficacy [16, 18], so we evaluated the effects of both stimulation hemisphere and stimulation site in white or gray matter.
Across the entire dataset, we found that stimulation was uniquely effective for memory enhancement when it was delivered in the white matter of the right entorhinal area. This confirmed our hypothesis that targeting the afferent input to the hippocampus was important. Another finding that emerged was the strong influence of laterality, with stimulation of the right hemisphere producing the only consistent benefits.
In fact, there is prior evidence on lateralized involvement of the hippocampus in delayed recognition memory. Coleshill and colleagues found that, on a delayed recognition memory task, right-sided hippocampal stimulation interfered with recognition memory for faces but not for words, while left-sided hippocampal stimulation interfered with recognition memory for words but not faces [18]. Thus, the present findings showing lateralized enhancing effects of entorhinal white matter stimulation may be due in part to the tasks used. All three tasks had a strong visual component. Two tasks were explicitly visual recognition tasks, while success on face-name association requires the ability to recognize a face, though also to associate a name to it. It is possible, therefore, that the right white matter stimulation enhanced visual recognition in this task in the same manner as it did for the others. We anticipate that for memory tasks that depend on the processing of non-spatial verbal material, stimulation applied in the left hemisphere may also provide modulatory effects on memory [37], consistent with related findings in prior studies [38].
Although there remains some debate, familiarity-based recognition memory has been proposed to be mediated by different neuronal processes than recollection [39], including recognition of unfamiliar faces [40]. In particular, it has been suggested that familiarity-based recognition memory may be supported by the perirhinal cortex in a manner complementary to hippocampal support of recollection. In this case, it may seem surprising that stimulation of the input to the hippocampus was effective. In our recognition tasks, however, we considered an item to be remembered only if the item was recognized and the corresponding close lure was correctly rejected, which required a degree of memory specificity and recollection that likely relied on hippocampal processes [33].
This is the largest-scale analysis of entorhinal stimulation that we are aware of. Nonetheless, we recognize that there are limitations of our current study and its conclusions. Although we had the same number of stimulating electrodes in the white and gray matter (15 in each), the distribution within micro- and macro-stimulating electrodes was not symmetric—a slightly higher number of micro-stimulating electrodes were in the white (15) compared to gray (9) matter—potentially introducing sampling bias. It is also worth mentioning that the absence of a statistical effect of macro- vs micro- stimulation is likely due to an underpowered statistical test, given that macro-stimulation was limited to gray matter in this study. Further, given the difficulty in acquiring data within monitored epilepsy patients with implanted electrodes, not all participants in the current study were able to complete all three of the memory tasks. Finally, it is likely that a combination of other variables that we did not specifically measure or test for, contribute to the overall efficacy of stimulation. For instance, other spatial factors (e.g., the proximity of stimulation to the perforant pathway [41], or size and spacing of the stimulating electrode), temporal factors (e.g., timing of stimulation with respect to native brain rhythms [42], or ongoing brain “state” at the time of stimulation [43]), or stimulation waveform parameters (amplitude, frequency, pulse width, pulse duration, etc.) may play a role [37]. As such, a model-driven stimulation protocol, with spatiotemporal patterns that are tailored to each person, may be required to fully address the complexity of stimulation’s effects on memory [17].
We acknowledge that DBS is a complex intervention, where a large number of methodological differences can lead to opposing results [22, 35]. In our data, we held certain factors constant while allowing others to vary. Within our particular sub-region of parameter space, we found that stimulation of the entorhinal white matter was advantageous. We hope that this could provide insight for designing future studies and evaluating differences among published results. For example, in a recently published study, Jacobs and colleagues [20] reported that electrical stimulation of the hippocampal and entorhinal regions impaired both spatial memory and verbal free recall. It is important to note, however, that across both tasks in that study, only 6 participants were reported to have received white matter stimulation in the entorhinal area, and of these, only two appear to have been stimulated on the right side. That dataset, thus, under-represents the stimulating conditions where we found the most promise for memory enhancement. Together with other methodological differences, these points could help explain why Jacobs and colleagues found primarily impairment.
There are several factors that remain to be explored. It is possible that left-sided stimulation could improve memory on more verbally-based memory tasks and that our findings of the benefits of right-sided stimulation here are highly specific to visuospatial memory tasks. Since we do not include a non-visual, verbally-based memory task in the current study, laterality effects of stimulation on various types of memory will require direct exploration in future studies. Further, since processing of spatial and non-spatial information are thought to rely on different MTL cortical subregions and hemispheres [44, 45], characterization of the precise effects of stimulation at different locations within the MTL during spatial vs. non-spatial and visual vs. verbal memory tasks will be an important focus for future large-sample studies. There are significant challenges associated with acquiring data within monitored epilepsy patients with implanted electrodes. Given the clinically determined nature of intracranial electrode placements, within-subject designs for all comparisons are rarely feasible. Further, not all participants can complete multiple behavioral tasks due to clinical reasons and the limited time during their hospital stay. Thus, meta-analyses across datasets or future multi-institutional efforts may be better suited to studying stimulation effects on specific memory functions, and perhaps across multiple sensory modalities (e.g., auditory versus visual). Additionally, with DBS-enabled neural implants becoming more ubiquitous, it may be possible to probe the effect of stimulation on multiple behavioral tasks at different times within the same participant [37].
Yet another question for future studies is whether stimulation is more effectively applied bilaterally than unilaterally. Although the present study confirms our previous findings that unilateral stimulation may be sufficient to modulate memory [16, 24], the efficacy of unilateral vs. bilateral stimulation of the entorhinal region has yet to be tested directly. Further, optimization of the precise spatiotemporal pattern of stimulation [17] and other stimulation parameters may provide a more personalized approach and even strengthen the effects of entorhinal white matter stimulation on memory [22, 37]. Finally, in the present study we delineated entorhinal white from gray matter, but the combination of high-resolution MRI with diffusion tensor imaging (DTI) methods could allow for more fine-grained insight into the effects of electrode positioning relative to the perforant pathway or other white matter tracts across participants.
Conclusions
Altogether, our findings suggest that deep brain electrical stimulation offers a unique opportunity to improve learning and memory performance in humans, which may have clinical relevance to the development of therapeutic treatments for debilitating memory disorders. Although considerable research is still needed to identify the methods and parameters that will be the most effective, our results indicate that stimulation targeted specifically to entorhinal white matter of the right hemisphere during learning hold considerable promise for memory enhancement.
Supplementary Material
Deep brain stimulation (DBS) can improve human memory.
DBS of memory is most effective when applied in the entorhinal white matter.
DBS consistently improved memory across three different visually guided tasks.
Acknowledgments
General: We thank Tony Fields, Kirk Shattuck, Eric Behnke, Michael Jenkins, and Antonio Campos for technical assistance; Alec Gasperian, J.R. Miller, Yasmine Sherafat, Deena Pourshaban, Marianna Holliday, Samantha Briones, Nancy Guerrero, Patricia Walshaw, Sonja Hiller, and Brooke Salaz for general assistance; the IDRE statistical consulting group at UCLA, in particular Christine Wells and Andy Lin, for providing insightful discussions regarding statistical analysis methods; the participants for their participation.
Funding: Supported by grants from the National Institute of Neurological Disorders and Stroke (NS103802, NS084017, NS033221, NS108930 and NS058280), DARPA Restoring Active Memory program (Agreement number: N66001-14-2-4029), NSF 1756473, and the A.P. Giannini Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none
Data and materials availability: Data and source code are available in the supplementary materials.
References
- [1].Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci 2004;27:279–306. 10.1146/annurev.neuro.27.070203.144130 [DOI] [PubMed] [Google Scholar]
- [2].Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol 1973;232(2):331–56. 10.1113/jphysiol.1973.sp010273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Feuerstein T, Seeger W. Modulation of acetylcholine release in human cortical slices: possible implications for Alzheimer’s disease. Pharmacol Ther 1997;74(3):333–47. 10.1016/S0163-7258(97)00006-5 [DOI] [PubMed] [Google Scholar]
- [4].Pastalkova E, Serrano P, Pinkhasova D, Wallace E, Fenton AA, Sacktor TC. Storage of spatial information by the maintenance mechanism of LTP. Science 2006;313(5790):1141–4. 10.1126/science.1128657 [DOI] [PubMed] [Google Scholar]
- [5].Roy DS, Arons A, Mitchell TI, Pignatelli M, Ryan TJ, Tonegawa S. Memory retrieval by activating engram cells in mouse models of early Alzheimer’s disease. Nature 2016;531(7595):508–12. 10.1038/nature17172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Stone SS, Teixeira CM, Devito LM, Zaslavsky K, Josselyn SA, Lozano AM, et al. Stimulation of entorhinal cortex promotes adult neurogenesis and facilitates spatial memory. J Neurosci 2011;31(38):13469–84. 10.1523/jneurosci.3100-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Toda H, Hamani C, Fawcett AP, Hutchison WD, Lozano AM. The regulation of adult rodent hippocampal neurogenesis by deep brain stimulation. J Neurosurg 2008;108(1):132–8. 10.3171/JNS/2008/108/01/0132 [DOI] [PubMed] [Google Scholar]
- [8].Morris R, Anderson E, Lynch Ga, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP 5. Nature 1986;319(6056):774–6. 10.1038/319774a0 [DOI] [PubMed] [Google Scholar]
- [9].Moser EI, Krobert KA, Moser M-B, Morris RG. Impaired spatial learning after saturation of long-term potentiation. Science 1998;281(5385):2038–42. 10.1126/science.281.5385.2038 [DOI] [PubMed] [Google Scholar]
- [10].Laxton AW, Tang-Wai DF, McAndrews MP, Zumsteg D, Wennberg R, Keren R, et al. A phase I trial of deep brain stimulation of memory circuits in Alzheimer’s disease. Ann Neurol 2010;68(4):521–34. 10.1002/ana.22089 [DOI] [PubMed] [Google Scholar]
- [11].Mann A, Gondard E, Tampellini D, Milsted JAT, Marillac D, Hamani C, et al. Chronic deep brain stimulation in an Alzheimer’s disease mouse model enhances memory and reduces pathological hallmarks. Brain Stimul 2018;11(2):435–44. 10.1016/j.brs.2017.11.012 [DOI] [PubMed] [Google Scholar]
- [12].Sweet JA, Eakin KC, Munyon CN, Miller JP. Improved learning and memory with theta-burst stimulation of the fornix in rat model of traumatic brain injury. Hippocampus 2014;24(12):1592–600. 10.1002/hipo.22338 [DOI] [PubMed] [Google Scholar]
- [13].Hamani C, McAndrews MP, Cohn M, Oh M, Zumsteg D, Shapiro CM, et al. Memory enhancement induced by hypothalamic/fornix deep brain stimulation. Ann Neurol 2008;63(1):119–23. 10.1002/ana.21295 [DOI] [PubMed] [Google Scholar]
- [14].Miller JP, Sweet JA, Bailey CM, Munyon CN, Luders HO, Fastenau PS. Visual-spatial memory may be enhanced with theta burst deep brain stimulation of the fornix: a preliminary investigation with four cases. Brain 2015;138(7):1833–42. 10.1093/brain/awv095 [DOI] [PubMed] [Google Scholar]
- [15].Suthana N, Fried I. Percepts to recollections: insights from single neuron recordings in the human brain. Trends Cogn Sci 2012;16(8):427–36. 10.1016/j.tics.2012.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Titiz AS, Hill MRH, Mankin EA, Aghajan ZM, Eliashiv D, Tchemodanov N, et al. Theta-burst microstimulation in the human entorhinal area improves memory specificity. Elife 2017;6 10.7554/eLife.29515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hampson RE, Song D, Robinson BS, Fetterhoff D, Dakos AS, Roeder BM, et al. Developing a hippocampal neural prosthetic to facilitate human memory encoding and recall. J Neural Eng 2018;15(3):036014 10.1088/1741-2552/aaaed7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Coleshill SG, Binnie CD, Morris RG, Alarcon G, van Emde Boas W, Velis DN, et al. Material-specific recognition memory deficits elicited by unilateral hippocampal electrical stimulation. J Neurosci 2004;24(7):1612–6. 10.1523/jneurosci.4352-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Halgren E, Wilson CL, Stapleton JM. Human medial temporal-lobe stimulation disrupts both formation and retrieval of recent memories. Brain Cogn 1985;4(3):287–95. 10.1016/0278-2626(85)90022-3 [DOI] [PubMed] [Google Scholar]
- [20].Jacobs J, Miller J, Lee SA, Coffey T, Watrous AJ, Sperling MR, et al. Direct Electrical Stimulation of the Human Entorhinal Region and Hippocampus Impairs Memory. Neuron 2016;92(5):983–90. 10.1016/j.neuron.2016.10.062 [DOI] [PubMed] [Google Scholar]
- [21].Lacruz ME, Valentin A, Seoane JJ, Morris RG, Selway RP, Alarcon G. Single pulse electrical stimulation of the hippocampus is sufficient to impair human episodic memory. Neuroscience 2010;170(2):623–32. 10.1016/j.neuroscience.2010.06.042 [DOI] [PubMed] [Google Scholar]
- [22].Suthana N, Aghajan ZM, Mankin EA, Lin A. Reporting Guidelines and Issues to Consider for Using Intracranial Brain Stimulation in Studies of Human Declarative Memory. Front Neurosci 2018;12(905). 10.3389/fnins.2018.00905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Fried I, Wilson C, Zhang J, Behnke E, Watson V. Implantation of depth electrodes for EEG recording Stereotactic surgery and radiosurgery Madison, WI: Medical Physics Publishing; 1993:149–58. [Google Scholar]
- [24].Suthana N, Haneef Z, Stern J, Mukamel R, Behnke E, Knowlton B, et al. Memory enhancement and deep-brain stimulation of the entorhinal area. N Engl J Med 2012;366(6):502–10. 10.1056/NEJMoa1107212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Yushkevich PA, Wang H, Pluta J, Das SR, Craige C, Avants BB, et al. Nearly automatic segmentation of hippocampal subfields in in vivo focal T2-weighted MRI. Neuroimage 2010;53(4):1208–24. 10.1016/j.neuroimage.2010.06.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Brainard DH. The Psychophysics Toolbox. Spat Vis 1997;10(4):433–6. 10.1163/156856897X00357 [DOI] [PubMed] [Google Scholar]
- [27].Hubbard AE, Ahern J, Fleischer NL, Van der Laan M, Lippman SA, Jewell N, et al. To GEE or not to GEE: comparing population average and mixed models for estimating the associations between neighborhood risk factors and health. Epidemiology 2010;21(4):467–74. 10.1097/EDE.0b013e3181caeb90 [DOI] [PubMed] [Google Scholar]
- [28].Riva-Posse P, Choi KS, Holtzheimer PE, McIntyre CC, Gross RE, Chaturvedi A, et al. Defining critical white matter pathways mediating successful subcallosal cingulate deep brain stimulation for treatment-resistant depression. Biol Psychiatry 2014;76(12):963–9. 10.1016/j.biopsych.2014.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Rajasethupathy P, Ferenczi E, Deisseroth K. Targeting Neural Circuits. Cell 2016;165(3):524–34. 10.1016/j.cell.2016.03.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Whitmer D, De Solages C, Hill BC, Yu H, Henderson JM, Bronte-Stewart H. High frequency deep brain stimulation attenuates subthalamic and cortical rhythms in Parkinson’s disease. Front Hum Neurosci 2012;6:155 10.3389/fnhum.2012.00155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Optical deconstruction of parkinsonian neural circuitry. Science 2009;324(5925):354–9. 10.1126/science.1167093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci 2007;10(9):1116–24. 10.1038/nn1944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Yassa MA, Muftuler LT, Stark CE. Ultrahigh-resolution microstructural diffusion tensor imaging reveals perforant path degradation in aged humans in vivo. Proceedings of the national academy of sciences 2010;107(28):12687–91. 10.1073/pnas.1002113107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zeineh MM, Palomero-Gallagher N, Axer M, Grabetael D, Goubran M, Wree A, et al. Direct Visualization and Mapping of the Spatial Course of Fiber Tracts at Microscopic Resolution in the Human Hippocampus. Cereb Cortex 2016. 10.1093/cercor/bhw010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Fried I Brain stimulation and memory. Brain 2015;138(Pt 7):1766–7. 10.1093/brain/awv121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Merkow MB, Burke JF, Ramayya AG, Sharan AD, Sperling MR, Kahana MJ. Stimulation of the human medial temporal lobe between learning and recall selectively enhances forgetting. Brain Stimul 2017;10(3):645–50. 10.1016/j.brs.2016.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Mankin EA, Fried I. Modulation of Human Memory by Deep Brain Stimulation of the Entorhinal-Hippocampal Circuitry. Neuron 2020;106(2):218–35. 10.1016/j.neuron.2020.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kucewicz MT, Berry BM, Miller LR, Khadjevand F, Ezzyat Y, Stein JM, et al. Evidence for verbal memory enhancement with electrical brain stimulation in the lateral temporal cortex. Brain 2018. 10.1093/brain/awx373 [DOI] [PubMed] [Google Scholar]
- [39].Davachi L, Mitchell JP, Wagner AD. Multiple routes to memory: distinct medial temporal lobe processes build item and source memories. Proc Natl Acad Sci U S A 2003;100(4):2157–62. 10.1073/pnas.0337195100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Bird CM, Burgess N. The hippocampus supports recognition memory for familiar words but not unfamiliar faces. Curr Biol 2008;18(24):1932–6. 10.1016/j.cub.2008.10.046 [DOI] [PubMed] [Google Scholar]
- [41].Wishard TJ, Aghajan ZM, Mankin EA, Villaroman D, Kuhn T, Fried I, et al. Perforant pathway structural properties predict declarative memory effects of deep brain stimulation. Rome, Italy: Organization of Human Brainmapping; 2019. [Google Scholar]
- [42].Siegle JH, Wilson MA. Enhancement of encoding and retrieval functions through theta phase-specific manipulation of hippocampus. eLife 2014;3:e03061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ezzyat Y, Wanda PA, Levy DF, Kadel A, Aka A, Pedisich I, et al. Closed-loop stimulation of temporal cortex rescues functional networks and improves memory. Nature Communications 2018;9(1):365 10.1038/s41467-017-02753-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Frisk V, Milner B. The relationship of working memory to the immediate recall of stories following unilateral temporal or frontal lobectomy. Neuropsychologia 1990;28(2):121–35. 10.1016/0028-3932(90)90095-6 [DOI] [PubMed] [Google Scholar]
- [45].Smith ML, Milner B. Right hippocampal impairment in the recall of spatial location: Encoding deficit or rapid forgetting? Neuropsychologia 1989;27(1):71–81. 10.1016/0028-3932(89)90091-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


