Abstract
Aims:
Heart transplantation is a life-saving therapy for end-stage organ failure. Organ deterioration during transportation limits storage to 4 hours, limiting hearts available. Approaches ameliorating organ damage could increase the number of hearts acceptable for transplantation. Prior studies show that adipose-derived stem/stromal cell secretome (ASC-S) rescues tissues from post-ischemic damage in vivo. The study tested whether ASC-S preserved the function of mouse hearts and human iPS-derived cardiomyocytes (iCM) exposed to organ transportation and transplantation conditions.
Methods and Results:
Hearts were subjected to cold University of Wisconsin (UW) cardioplegic solution ± ASC-S for 6 hours followed by analysis using the Langendorff technique. In parallel, the effects of ASC-S on the recovery of iCM from UW solution was examined when provided either during or after cold cardioplegia. Exposure of hearts and iCM to UW deteriorated contractile activity and caused cell apoptosis, worsening in iCM as a function of exposure time; these were ameliorated by augmenting with ASC-S. Silencing of SOD3 and catalase expression prior to secretome generation compromised the ASC-S cardiomyocyte-protective effects.
Conclusion:
A novel in vitro iCM model was developed to complement a rodent heart model in assessing efficacy of approaches to improve cardiac preservation. ASC-S displays strong cardioprotective activity on iCM either with or following cold cardioplegia. This effect is associated with ASC-S mediated cellular clearance of ROS. The effect of ASC-S on the temporal recovery of iCM function supports the possibility of lengthening heart storage by augmenting cardioplegic transport solution with ASC-S, expanding the pool of hearts for transplantation.
Graphical Abstract
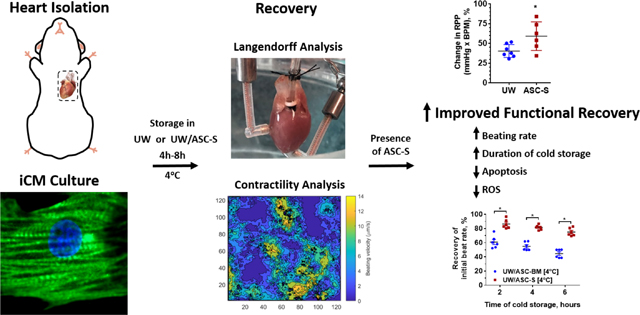
The damaging effects of UW solution are ameliorated by the inclusion of ASC-S. Mouse hearts stored in UW supplemented with ASC-S demonstrated increased survivability ex vivo. This same phenomenon was observed in hiPSC derived cardiomyocytes in vitro with cells demonstrating increased survival and functionality when treated with ASC-S either during or immediately following UW incubation.
INTRODUCTION
Heart transplantation is a critical life-saving therapy for patients with end-stage cardiovascular disease. However, use of this curative treatment is limited by a severe shortage in donor organ supply.1, 2 While there are approximately 50,000 patients with severe heart failure that are candidates for cardiac transplantation, only around 3000 cardiac transplants are conducted yearly in the USA. Due to a progressively aging population, the number of patients on the waiting list for heart transplantation is constantly growing, while the supply of acceptable donor hearts has not markedly increased, resulting in an approximate 20% yearly mortality among the patients waiting for heart transplantation.1–3 In addition, data prepared by the Association of Organ Procurement Organizations indicates that in the USA, up to 70% of hearts available by consent of organ donors are rejected for transplantation yearly due to stringent limitations of acceptance criteria;4 and approximately one half of these organs are discarded due to anticipated time of cold transportation from donor to recipient. Overall, there is an urgent need to develop effective approaches to increase the pool of donor hearts and their actual acceptance for transplantation.
Maintenance of viability during storage/preservation of the heart is particularly challenging, due to its high metabolic requirements and its consequently limited tolerance to pre-transplant ischemic damage. During organ procurement, cardiac arrest is immediately induced by introduction of cold cardioplegia solution following aortic clamping in order to limit cardiac energy expenditure. However, cardiac metabolic processes continue, so that cardioplegia exposure is accompanied by a spectrum of cellular alterations which progress over time and predispose the heart to injury in conjunction with implantation, adversely affecting cardiac functional recovery.5, 6
The ischemic time of the donor heart is recognized as a significant negative contributor to outcomes of transplantation and to increases in prevalence of both acute and delayed graft dysfunction. While short ischemic periods during cold storage are reasonably well-tolerated, functional deterioration occurs with increasing frequency when the cold ischemia extends beyond 4 hours and is particularly severe when storage time is greater than 6 hours.7, 8 Mitigation of injury during and following organ storage/transportation will improve donor organ preservation, translating into an increase in acceptable transport time, which will in turn expand the geographic area within which patients in need may be served.
Mesenchymal stem cell-based therapies have emerged as promising approaches to mitigate ischemic conditions in tissues and organs. Adipose tissue-derived stem/stromal cells (ASCs), a subtype of mesenchymal stem cells that are readily available from fat via minimally invasive liposuction, are recognized as a particularly attractive cell type for both autologous and allogeneic therapies. We initially described,9 and other laboratories have also reported10 that ASCs secretome (ASC-S) contains paracrine factors that mediate the therapeutic effects of ASCs in a wide range of ischemic disease models, including critical limb ischemia, myocardial infarction and brain ischemia.11–15 The ASC-S target multiple physiological pathways involved in tissue rescue/repair, including inhibition of cell apoptosis and inflammation, stimulation of angiogenesis and endogenous progenitor cell cycling.13 The recognition that mesenchymal stem cells (MSCs), including ASCs, provide their therapeutic effects predominantly through secreted bioactive factors, rather than through donor cell integration into damaged tissue, has prompted multiple studies involving administration of concentrated MSC or ASC secretome.16–22 Indeed, the administration of MSC secretome during and following ischemia/reperfusion in vivo has been found to ameliorate infarction size as well as preserve myocardial function in rats as well as pigs.23, 24
While multiple prior studies have explored cell-based therapies to effect tissue rescue from ischemia in an intact organism, little work thus far has been done to evaluate their ability to ameliorate ischemic damage of isolated tissues and organs destined for transplant. With respect to human heart preservation in particular, while animal models may have limited predictive value, experimental use of intact healthy human hearts is impractical due to the unfulfilled need for this organ for transplantation procedures, whereas human mature cardiomyocytes are difficult to isolate and maintain in culture. To overcome these challenges, human induced pluripotent stem cell (hiPSC)-derived cardiomyocytes (iCM) have been employed for preclinical evaluation of therapeutic approaches.25, 26 Here we used iCM-derived contracting syncytial sheets to extend findings in ex vivo rodent heart function, both the responses of human cardiomyocytes to conditions mimicking current clinical protocols for cardiac transportation including exposure to cold cardioplegic solution, and to explore the efficacy and mechanisms of protective effects of human ASC-S.
METHODS
An Extended Methods can be found in the Supplemental Materials.
1. Ethics statement
Animal studies were approved by the Institutional Animal Care and Use Committee at Indiana University School of Medicine. The animal procedures conformed to the NIH Guide for the Care and Use of Laboratory Animals. Deidentified human hearts from donors that were deemed unsuitable for transplantation and donated to research were collected through the Indiana Donor Network under a standard protocol for receiving tissue from deceased individuals. All human tissue collection conformed to the Declaration of Helsinki.
2. Ex vivo perfusion of isolated beating mouse hearts (Langendorff)
Hearts were isolated from adult male C57BL/6 mice as previously described.27 Immediately afterwards, 1 ml of cold University of Wisconsin (UW) cardioplegic solution (Bridge to Life, USA) alone or with ASC-S was infused into the coronary circulation, after which the arrested hearts were stored in UW solution ± ASC-S at 4°C for 6 hours. After storage, spontaneous contraction was re-established. Heart rate and left ventricular developed pressure (LVDP) were recorded and the rate-pressure product values were used as an index of cardiac function.
3. hiPSC culture and differentiation into iCMs
To produce iCM, hiPSCs (Harvard Stem Cell Institute, USA), were seeded on Geltrex-coated (Thermo Fisher Scientific, USA) tissue culture plastic and expanded. At 80% confluence hiPSCs were detached and re-seeded into 12-well tissue culture plates until cell monolayers reached 95% confluence. iCM were then generated by temporal activation and deactivation of the Wnt signaling pathway following a previously established protocol.28 Briefly, hiPSCs were incubated in basal differentiation media (BDM(−): RPMI Medium 1640 (Life Technologies, USA), 2% B27 without insulin (Invitrogen, USA), 3.4 × 10−4 % β-mercaptoethanol (Promega, USA), 100 U/ml penicillin, 100 μg/ml streptomycin) supplemented with 12.5 μM Wnt activator CHIR99021 (CHIR, Stemgent, USA) for 24 hours, followed by 2-day incubation in BDM(−) alone. On day 4, cells were exposed to BDM(−) supplemented with 5 μM Wnt inhibitor IWP-4 (Stemgent, USA) with media exchange to BDM(−) on day 6. On day 9, medium was exchanged to BDM(−) with B27 supplement and insulin (Invitrogen) (BDM(+)). iCMs were reseeded into fibronectin-coated 24-well tissue culture plates. To define the proportion of fully differentiated iCM after completion of differentiation protocol, cells were harvested and analyzed using a FACSAria II analyzer (BD Biosciences).
4. Human heart collection and processing
Hearts deemed unsuitable for transplant were flushed with cardioplegic solution to remove the blood, placed on ice, and brought to the University of Notre Dame. Samples of left ventricle tissue were used for analysis of RNA expression or immunohistochemistry (IHC).
5. ASC conditioned media (ASC-S) generation
Human ASCs were isolated from human subcutaneous adipose tissue, as previously described,9 and cryo-banked. To generate ASC-S, cells were plated at 5×103 cells/cm2 and incubated in EGM-2mv (Lonza, Walkersville, MD) for 3 days. After reaching 5.5×104 cells/cm2 (~90% confluence), the ASC monolayers were incubated under 0.2 ml DMEM/F12 media per cm2 (CellGro, Manassas, VA) twice for 48 hours. Media from both incubations were combined, concentrated using Amicon Ultra-15 Centrifugal Filter Units with 3kDa NMWL in such a way that 1 ml of concentrated ASC-S was conditioned by 8×106 cells for 48h, and stored at −80°C. In most experiments, ASC-S was diluted tenfold in UW or culture media. ASC-S preparations were characterized based on protein concentration (509 ± 126 μg/ml; Mean±SEM) and expression of selected growth factors and cytokines using Human Angiogenesis Array & Growth Factor 17-plex Array (Eve Technology; HDAGP17) and standard ELISA kits from RnD Systems for VEGF, HGF and SDF-1 (Supplemental Figure 1). To test the contribution of selected antioxidant enzymes to ASC effects, cell monolayers were transfected with either superoxide dismutase 1 (SOD1), superoxide dismutase 3 (SOD3), or catalase silencing RNA or with scrambled RNA (all siRNA reagents were from Invitrogen, USA). One day after transfection, the media on the cells was exchanged to DMEM/F12 to produce ASC-S using the same protocol as above.
6. In vitro iCM functional analysis
After physiologically relevant beating of reseeded iCM recommenced, the baseline beat rate was recorded. Subsequently, media on iCM was replaced with UW solution alone or supplemented with various preparations of ASC-S. iCM were incubated in UW ± ASC-S for 2–8 hours either at 4°C or 37°C. After the UW exposure was completed, UW was replaced with either iCM complete culture media or with RPMI alone or supplemented with ASC-S. Time-lapse videos were taken intermittently for 24 hours after UW treatment/cold storage was completed.
7. Beating velocity assay
A previously developed block-matching algorithm25 was applied to analyze contractility of iCM. This was done for all frames of the time-lapse video to create a time series of iCM beating velocity vectors. The peak velocity for each vector determined over time was then averaged with that of all other vectors within each beating cluster to yield a single value representing each such active syncytium.
8. Reactive oxygen species accumulation assay
Accumulation of intracellular reactive oxygen species (ROS) was assessed in iCM that were exposed to UW solution alone or with ASC-S. The level of superoxide or hydroxyl radicals in the cells was determined at specified timepoints with ROS and H2O2 Assay kits (eEnzyme, USA). Following incubation at 37°C, three randomly selected fields of view were imaged for each sample the averaged intensity of the fluorescent signal was used to define the representative ROS activity at each timepoint.
9. RNA and protein expression analysis
Total RNA was isolated using RNAeasy Plus Mini Kit (Qiagen, Germany) from confluent beating iCM and human left ventricle tissue. cDNA was synthesized and expression of selected genes was evaluated. Gene expression was normalized to the level of the housekeeping gene GAPDH (for iCM analysis, RT-qPCR materials were from Biorad, USA). All primers are provided in Supplementary Table 1.
To evaluate the efficiency of silencing of antioxidant enzyme expression, total RNA was isolated from ASC at day 5 post-transfection followed by cDNA synthesis. Expression of selected genes was evaluated utilizing RT-qPCR (for ASC analysis, RT-qPCR materials were TaqMan predesigned primer/probe assays from ThermoFisher, USA) and normalized to the level of the housekeeping gene β-actin. Efficiency of silencing was also confirmed on the protein level. Cell lysates were fractionated on SDS–polyacrylamide and transferred onto nitrocellulose membranes. Membranes were incubated with SOD2, SOD3, catalase and beta-actin IgG (Cell Signaling) followed by incubation with IRDye® 800CW anti-mouse or IRDye® 680RD anti-rabbit IgG, and imaging on Odyssey CLx Imaging system (all from Li-Cor).
10. Immunohistochemistry
Standard immunohistochemistry staining was performed. Samples were fixed with paraformaldehyde, permeabilized, blocked, and then stained with primary antibody overnight at 4°C. The following day samples were stained with secondary antibody for 6 hours at 4°C, followed by DAPI staining for 10 minutes at room temperature. Samples were evaluated using a Nikon Confocal Ni-E Upright Research Microscope or Zeiss Axio Observer.Z1 fluorescence microscope equipped with a Hamamatsu digital camera. Acquired images were processed using Nikon NIS-Elements software (for the confocal microscope) or Zeiss Zen software (for the fluorescent microscope).
To determine the percent of caspase-3 positive cells, three randomly selected fields of view were imaged and the number of caspase-3+ nuclei was determined and normalized to the total number of nuclei in each image.
11. Statistical Analysis
Data are presented as an average ± standard error of the mean. A one-way ANOVA with Tukey’s post hoc test was performed for all experiments. All p values are two-sided and statistical significance was defined as p < 0.05 and sample size (n) ≥ 3 for all experiments.
12. Data Availability Statement
The data from this study is available from the corresponding authors upon reasonable request.
RESULTS
1. Mouse hearts are functionally compromise following prolonged cold UW exposure, which was ameliorated by ASC-S
Exposure of explanted mouse hearts to UW solution resulted in immediate cardiac arrest; following storage for 6 hours at 4°C and flushing with oxygenated Krebs-Henseleit buffer, hearts began spontaneous contraction and gradually recovered function. Hearts stored in UW for 6 hours exhibited significantly compromised left ventricular function after 60 minutes of re-animation as demonstrated by reduction of rate-pressure product (RPP) to approximately 40% of control hearts evaluated immediately after explantation. However, hearts exposed to UW admixed with human ASC-S showed significant improvement in RPP to about 60% of baseline function (Figure 1A).
Figure 1. Damaging effects of UW solution on cardiomyocyte survival and function in vitro and ex vivo are ameliorated by ASC-S.
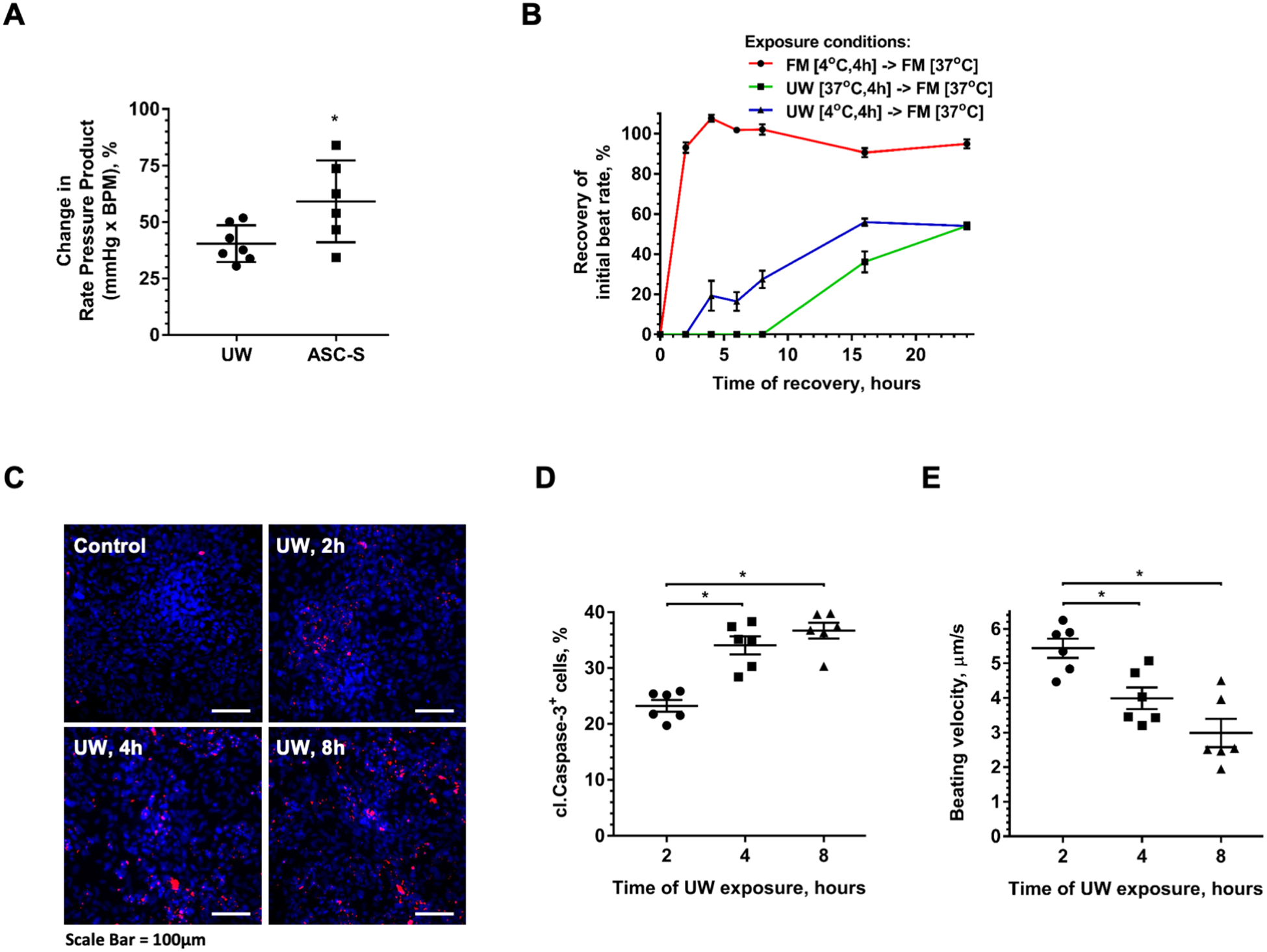
(A) Functional analysis of mouse hearts on Langendorff apparatus. Hearts were extracted from mice and either immediately placed on the Langendorff apparatus for data collection, or initially stored in UW solution alone or with ASC-S at 4°C for 6 hours. (B) Dynamics of beating rate recovery of iCM after exposure to either standard iCM culture media (full media, FM) at 4°C, or to UW at 4°C, or to UW at physiological temperature (37°C) for 4 hours, followed by incubation in FM at 37°C for 24 hours. Time “0” represents the moment when UW was exchanged to FM. (C, D) Representative images (C) and quantitative analysis (D) of cleaved caspase-3 (red) expression in iCM after cell exposure to UW for 2–8 hours at 4°C, followed by 24-hour recovery in FM under standard culture conditions. Nuclei were revealed with DAPI (blue). (E) Beating velocity of iCM exposed to UW for 2–8 hours at 4°C followed by recovery in FM for 24 hours. Statistics: (A) student t-test; (B, D, E): one-way ANOVA with Tukey’s post hoc test; n=6 for each graph; *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001. Scale Bar = 100μm.
2. iCM display phenotypic similarities with human myocardium
The degree of hiPSCs differentiation to iCM was evaluated by immunostaining cell monolayers for vimentin, as a progenitor cell marker, and for cardiac troponin, as a cardiomyocyte marker.29 By day 35 of differentiation, monolayers exhibited a limited number of vimentin-positive cells and were enriched with cells expressing striated cardiac troponin (Supplemental Figure 2A). Additionally, translocation of connexin-43 from nuclei to cell membrane was observed, which is typical in human myocardium (Supplemental Figure 2A). These findings, along with spontaneous rhythmic contraction of cell sheets suggest hiPSC differentiation into iCM with establishment of a syncytium. Analysis of differentiated iCM monolayers by flow cytometry revealed that by day 35 more than 95% of cells were cardiac troponin positive, confirming a high degree of homogeneity of iCM cultures (Supplemental Figure 2B). Strengthening our findings, quantitative PCR analysis that revealed prominent expression levels of canonical cardiomyocyte transcripts, such as TNNT, MHC6, and NKX2.5 in iCM as well as human left ventricle tissue, used as a positive control (Supplemental Figure 2C).
3. iCM mimics human myocardial response to UW exposure
To explore the behavior of human iCM following cold temperature and cardioplegia exposure, iCM monolayers with established contractile activity were exposed to either standard iCM incubation media (full medium, FM) at cold temperature (4°C), or to UW solution at either cold (4°C) or physiological (37°C) temperatures for 4 hours. iCM viability and contractility (rate and displacement) were then evaluated as a function of time during recovery in FM under standard incubation conditions. Exposure of iCM to FM at 4°C resulted in gradual cessation of contraction within minutes, while subsequent return to physiological temperature restored the syncytial beating rate (SBR) within several minutes to pre-cold stress levels (Figure 1B). Introduction of UW solution to iCM at both 4°C and 37°C caused immediate cessation of calcium transients (Supplemental Video 1) and contractile activity (Supplemental Video 2), emulating the behavior seen with intact human hearts. iCM exposure to UW at 37°C for 4 hours followed by media exchange to iCM culture media to induce cell recovery, was associated with re-initiation of rhythmic contractile activity in the iCM syncytium following a delay period of several hours where no contraction was observed (Figure 1B). The SBR gradually increased yet remained significantly slower than the basal rate even after 24 hours of recovery. Interestingly, iCM that were incubated in UW at 4°C and returned to iCM culture media re-initiated contractile activity after a significantly shorter delay than those exposed to UW at 37°C; and manifested an asymptotic approach to a SBR closer to that observed for cells that were exposed to cold iCM culture media. Specifically, the SBR of iCM reached 20% of the control culture SBR within 4 hours, whereas the cells incubated in UW at 37°C did not begin beating until after 16 hours of incubation in control media (Figure 1B).
Measurement of cell apoptosis based on anti-active (cleaved) caspase-3 staining in iCM as a function of exposure time to UW at 4°C revealed numerous cells (23%) initiating apoptosis already after 2 hours of exposure, which increased with prolonged exposure to UW (Figure 1C, D). A similar frequency of nuclei positive for cleaved caspase-3 was found in the samples of human myocardium prepared from hearts which were rejected for transplantation for reasons unrelated to cardiac physiology and were exposed to cold UW solution for similar time periods as iCM (Supplemental Figure 3).
Analysis of iCM beating velocity after exposure to cold UW solution for 2–8 hours, followed by recovery in standard media for 24 hours, revealed a negative correlation between cardioplegia exposure and recovery of beating velocity (Figure 1E).
4. ASC-S preserves iCM function during UW exposure
To test whether admixture of ASC-S into UW would improve survival and recovery of iCM function following cold storage regimens, iCM were exposed to UW supplemented with either DMEM/F12, as a control (ASC basal media, UW/ASC-BM), or with 10% ASC-S (UW/ASC-S) at 4°C for 4–8 hours. Cells exposed to UW/ASC-BM for 4 hours recovered SBR to 60% of baseline by 24 hours after UW washout (Figure 2A). Exposure of iCM to UW/ASC-BM for longer times further decreased iCM’s ability to recover SBR: by 24 hours after UW washout, SBR reached 55.0±2.4% and 44.7±2.5% of the initial values following 6 and 8 hours of UW incubation respectively (Figure 2A). The addition of ASC-S to UW significantly improved SBR recovery (Figure 2A) for all durations of iCM exposure to cold cardioplegia (86.4±2.6%, 81.6±1.4%, and 75.1±2.3% following 4, 6, 8 hours of exposure correspondingly). In addition, ASC-S promoted a significant decrease (~30%) in the lag period needed for iCM to re-initiate contractile activity, regardless of duration of cardioplegia exposure (Figure 2B). Use of 10% ASC-S was considered to be optimal, since it was similar to 20% ASC-S, but superior to 5% ASC-S effects (Supplemental Figure 4).
Figure 2. ASC-S protects iCM during UW exposure.
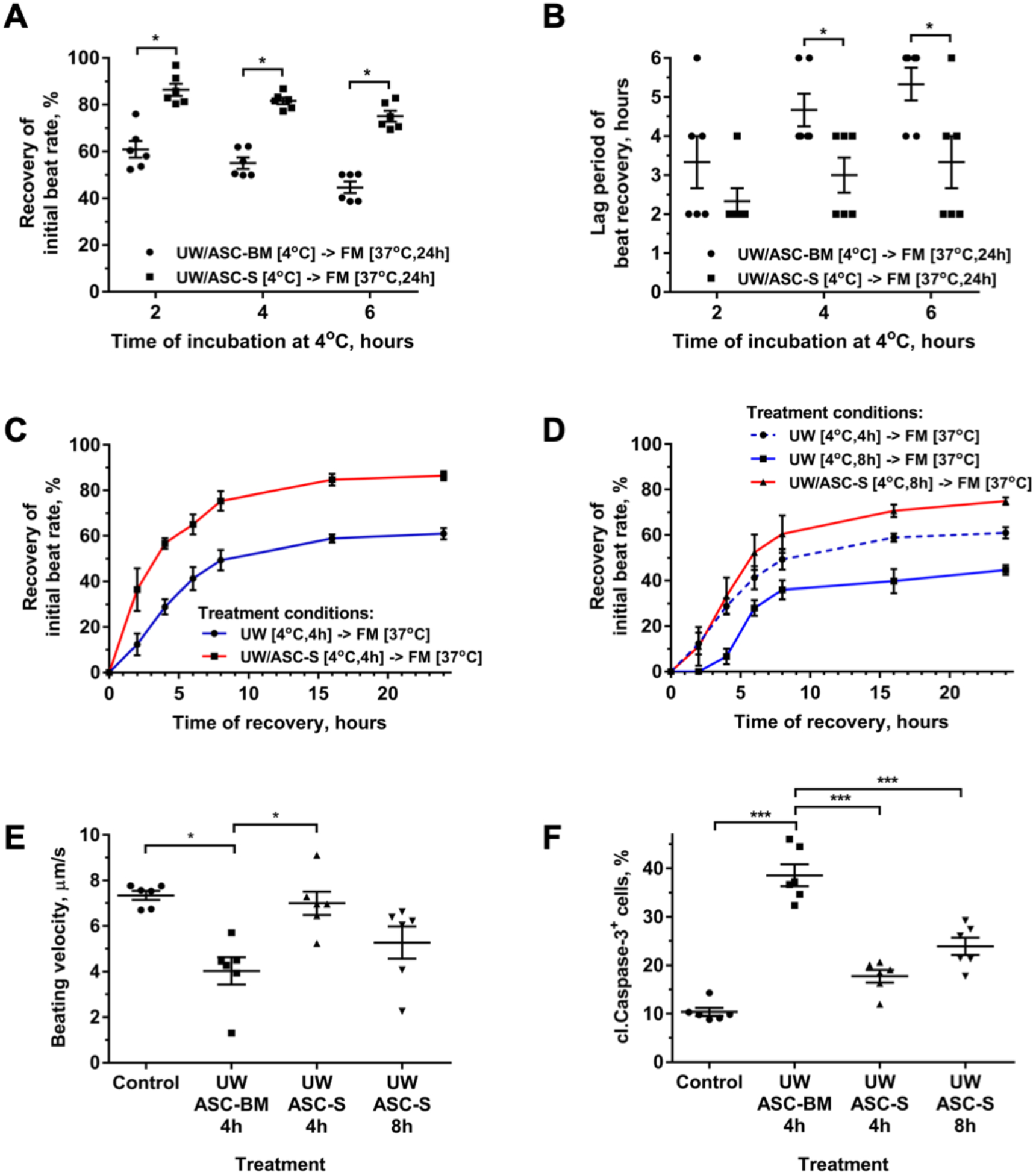
(A) Recovery of beating rate of iCM incubated in either UW/ASC-BM or UW/ASC-S at 4°C for 4–8 hours followed by recovery in standard iCM culture media (FM) at 37°C for 24 hours. (B) Analysis of the minimal period required for iCM to re-establish stable beating rate after exposure to UW/ASC-BM or to UW/ASC-S at 4°C for 4–8 hours. iCM after exposure to UW/treatment were incubated in FM at 37°C and the incidence of iCM contraction was checked hourly. (C, D) Dynamics of beating rate recovery in iCM incubated in FM after cell exposure to either UW/ASC-BM (C: for 4 hours; D: 4 and 8 hours) or to UW/ASC-S (C: for 4 hours; D: 8 hours) at 4°C. (E, F) Beating velocity of iCM (E) and prevalence of cleaved caspase-3+ iCM (F) after cell exposure to UW/ASC-BM for 4 hours or to UW/ASC-S for 4 and 8 hours at 4°C, followed by cell recovery in FM for 24 hours. Time “0” represents the timepoint at which UW solution ± supplement was exchanged to FM. iCM incubated in FM throughout the experiment represent the positive control. Statistics: (A-F): one-way ANOVA with Tukey’s post hoc test; n=6 for each graph; *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001.
To evaluate in more detail the dynamics of iCM functional recovery after 4-hour exposure to cold UW, iCM contraction was evaluated repeatedly over a 24-hour period following media exchange from cold UW to FM and cell incubation at physiological temperature: at each timepoint the presence of ASC-S in UW significantly mitigated the detrimental effect of UW, resulting in 24-hour SBR recovery to 86.4±2.6% and 61.0.0±3.5% respectively (Figure 2C). To test whether augmentation of UW with ASC-S would permit extension of the duration of cardioplegia exposure to UW while maintaining functional activity, iCM were exposed to cold cardioplegia alone or with ASC-S for 8 hours. iCM exposed to UW/ASC-S for 8 hours exhibited recovery profiles superior or at least equivalent to those shown by cells exposed to UW alone for 4 hours for each measured parameter: iCM SBR (Figure 2D), iCM contraction velocity (Figure 2E), and iCM viability (Figure 2F). No additional iCM recovery was observed after 24 hours in either group (Supplemental Figure 5).
5. ASC-S promotes iCM recovery post UW treatment
Based on the observation of improved functional recovery of iCM exposed to ASC-S occurring within only 2 hours of UW washout, we explored whether ASC-S would confer a protective effect when provided to iCM after, rather than during, UW exposure. iCM were incubated in cold UW for 4 hours, after which UW was replaced with iCM basal (RPMI) media alone or supplemented with ASC-S, and monitored for 24 hours. As shown in Figure 3A, iCM exposed to RPMI media augmented with ASC-S manifested markedly enhanced SBR recovery, compared to iCM recovering in RPMI alone (62.1±2.6% vs 34.3±2.2%), confirming rapid onset of ASC-S activity. Furthermore, this treatment decreased the level of cell apoptosis by 65%, as revealed by iCM cleaved caspase-3 expression at the 24-hour recovery timepoint (Figure 3B); and restored the beating velocity of iCM to the level found in cells incubated in control media during the entire experiment (Figure 3C). When ASC-S was provided to the cells both during UW exposure and after UW washout, the recovery curves for SBR and velocity were indistinguishable from those observed for iCM treated with ASC-S solely during UW exposure or recovery phases (Figure 3D, E).
Figure 3. ASC-S promotes iCM recovery post-UW treatment.
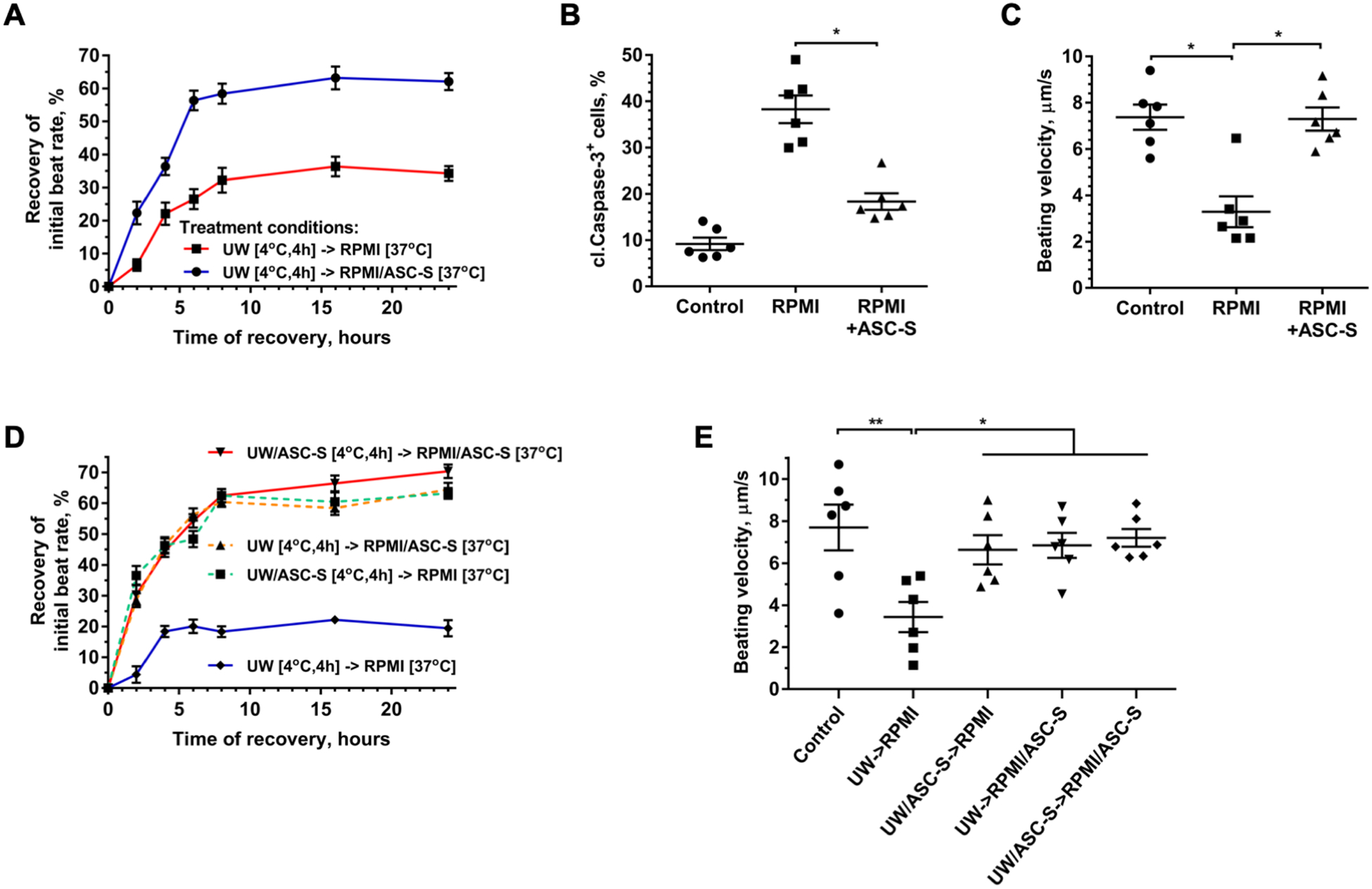
(A) Dynamics of beating rate recovery in iCM after exposure to UW at 4°C for 4 hours, followed by incubation in RPMI media alone or supplemented with ASC-S at 37°C for 24 hours. Time “0” represents the moment when UW solution was exchanged to RPMI ± supplements. (B, C) Prevalence of cleaved caspase-3+ iCM (B) and beating velocity of iCM (C) evaluated after cells were exposed to UW for 4 hours, followed by incubation in RPMI ± ASC-S for 24 hours. (D) Dynamics of beating rate recovery in iCM treated with ASC-BM or ASC-S either only during UW incubation, only during the recovery phase, or during both incubations. (E) Beating velocity of iCM evaluated after cells were exposed to UW/supplements (ASC-BM or ASC-S) at 4°C for 4 hours, followed by incubation in RPMI ± supplements (ASC-BM or ASC-S) for 24 hours. Statistics: (A-E): one-way ANOVA with Tukey’s post hoc test was performed: n=6 for each graph; *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001.
6. ASC-S cardio-protective effects are mediated by the antioxidant enzymes, SOD3 and catalase
Based on the observation that exposure of iCM to ASC-S both during and after cold storage in UW solution markedly dampened the damaging effects of UW with a rapid time course (Figure 4 A–C), we reasoned that the underlying mechanisms were largely independent of transcriptional or translational modulations. We thus tested whether the effect of ASC-S on iCM might be due to enzymatic activities, specifically antioxidant enzymes, which are secreted by ASC. To explore this concept, the expression of SOD1 (mitochondrial isoform), SOD3 (extracellular isoform), and catalase were silenced in ASC, either individually or in combination, by transfecting cells with corresponding siRNA constructs. RT-qPCR analysis confirmed a >85% decrease in mRNA expression for each intended target when tested at day 5 post-transfection (Supplemental Figure 6A). Using western blotting we also confirmed silencing of catalase at the protein level, while no changes in expression of SOD2, used as a positive control, were observed. Attempts to detect SOD3 protein both in control and transfected cells were unsuccessful despite testing several target-specific antibodies. This may be attributed to expression/secretion of SOD3 by ASC at levels below the detection range of the tested antibodies (Supplemental Figure 6B). iCM were then exposed to UW supplemented with either ASC-BM or ASC-S produced by either control ASC (ASCC), or ASC transfected with silencing RNA. Analysis revealed that iCM recovered rapidly from UW exposure when exposed to either ASCC-S or ASCSOD1-S, whereas the potencies of ASC-S from cells with knockdown of SOD3 (ASCSOD3-S) or catalase (ASCCat-S) expression were significantly compromised compared to iCM SBR recovery. Data recorded at the 8-hour time-point of the recovery phase showed that iCM exposed to ASCC-S demonstrated 76.5±0.8% recovery of the baseline SBR, whereas the SBR of iCM treated with ASCSOD3-S and ASCCat-S, were respectively 64.4±1.6% and 60.3±1.1% of baseline (Figure 4A). Conversely, ASCSOD1-S did not attenuate SBR recovery by comparison with ASCC-S, demonstrating that the extracellular superoxide dismutase isoform, SOD3, but not SOD1, was necessary for the activity of ASC-S (Figure 4A). ASCSOD3-S and ASCCat-S also displayed limited ability to recover beating velocity or to rescue iCM from apoptosis (Figures 4B, C). To exclude the possibility that silencing of SOD3 and catalase altered the expression of other factors, and thus could be indirectly responsible for loss of ASC-S effects, we evaluated the expression of several factors, known to be responsible for ASC therapeutic effects in many models. Expression of vascular endothelial growth factor, hepatocyte growth factor, and stromal cell-derived factor-1 were found to be unaffected in modified cells (Supplemental Figure 6C).
Figure 4. SOD3 and catalase are key mediators of ASC-S cardio-protective effects.
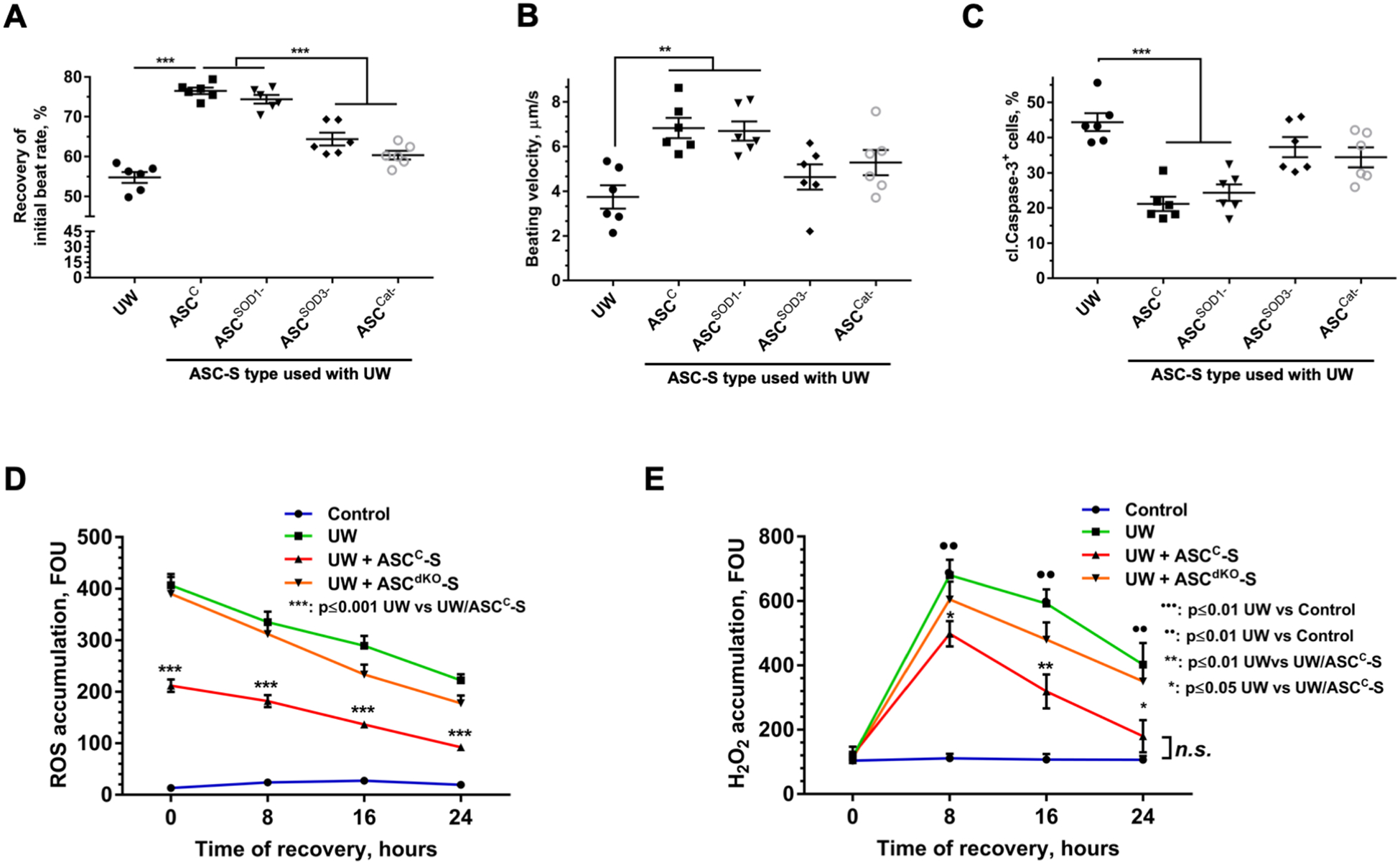
(A) Recovery of iCM beating rate after cell exposure to UW with ASC-S generated by either control ASC (ASCC), or ASC transfected with silencing RNA directed to SOD1 (ASCSOD1-), SOD3 (ASCSOD3-), catalase (ASCCat-), or SOD3 and catalase in combination (ASCdKO). Cells exposed to UW ± ASC-S at 4°C for 4 hours, were allowed to recover in standard iCM culture media for 8 hours prior to data collection. (B, C) Analysis of iCM beating velocity (B) and prevalence of cleaved caspase-3+ iCM (C). Cells exposed to UW ± ASC-S at 4°C for 4 hours were allowed to recover in standard iCM culture media (FM) for 24 hours prior to data collection and staining. (D, E) Dynamics of ROS (D) and H2O2 (E) accumulation in the cytoplasm of iCM exposed to UW alone or supplemented with either ASCC-S or ASCdKO-S (secretome produced by ASC silenced for SOD3 and catalase in combination) at 4°C for 4 hours, followed by recovery in FM. Time “0” represents the moment when UW solution was exchanged to FM. Statistics: (A-E): one-way ANOVA with Tukey’s post hoc test; n=6 for each graph; *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001.
Based on the apparent key role for catalase and SOD3 in the activity of ASC-S, we hypothesized that exposure of iCM to UW is associated with cellular accumulation of reactive oxygen species, which, in turn, leads to progressive cardiomyocyte dysfunction as the time of exposure to cardioplegic conditions increases. To test this hypothesis, accumulation of overall ROS and H2O2 were assayed in iCM at various time-points during recovery following exposure to UW alone or with several preparations of ASC-S. The exposure of iCM to cold UW was associated with a very prominent accumulation of ROS, with its highest level manifested at the end of UW exposure. iCM exposed to UW with ASCC-S demonstrated a twofold decrease in ROS levels, while ASCdKO-S was ineffective in accelerating the clearance of iCM ROS. The dynamics of H2O2 concentration were distinct from those of ROS: at the time of UW washout (t=0), the level of H2O2 in all treatment groups was similarly low, with a subsequent increase in H2O2 seen during recovery in control media. As with ROS measurements, iCM incubated in UW with ASCC-S showed lower levels of H2O2 at each time-point in compared to the UW group; while incubation with ASCdKO-S treatment produced little effect. Taken together, these data confirm the substantial action of ASC-S to accelerate clearance of ROS via SOD3- and catalase-dependent mechanisms (Figure 4D, E).
DISCUSSION
Cardioplegia solutions are commonly used clinically to arrest heart contraction and decelerate the deterioration of cardiac function during the period of cold ischemia encountered during organ transport prior to transplantation. The permissible duration of cold storage of the heart in cardioplegic solution has been limited practically by the empiric observation that cold ischemic periods extending beyond 4 hours progressively compromise recovery of heart function and worsen prognosis following transplantation. This limitation in acceptable duration of heart exposure to cold cardioplegia defines the maximal distance between the donor and the recipient, and thus may limit the access of the patients in need to life-saving therapy. Amelioration of the heart damage during storage/transportation may help to overcome this issue. Our group and others have demonstrated that ASC markedly reduce the loss of cardiac tissue and function when provided soon after onset of myocardial infarction.13, 30–32 We have shown in multiple pre-clinical models that ASC therapeutic effects are mediated by paracrine mechanisms,11–15, 33 via production of a myriad of factors involved in anti-apoptotic, pro-angiogenic, matrix remodeling, and immunomodulatory pathways. These in vivo findings demonstrate that ASC-S preserves heart function after acute ischemic episodes and prompted us to explore whether provision of ASC-S during the ischemic period that accompanies storage/transportation of the hearts from donor to recipient would ameliorate the associated damage to donor hearts and accordingly extend the permissible time of heart transportation.
We initially evaluated the therapeutic potential of ASC-S with the commonly used Langendorff model of whole rodent heart function ex vivo, and observed that augmentation of cardioplegia with ASC-S limited the decline in heart function during the storage process (Figure 1A). However, many non-human models of myocardial physiology have limitations with respect to assessing the therapeutic effects of human cell-based therapies, given the occurrence of ligand-receptor pairs with sufficient non-homology to impair binding and transduction interactions. Meanwhile, the use of non-diseased adult human cardiac tissue or explants for experimentation is impractical due to the need for donor hearts. In this context, iCM have recently emerged as a superior model system in which to test and define the cardio-protective effects of various therapeutic interventions.25, 34, 35 Accordingly, in this study, encouraged by the results from the ex vivo murine model, we developed a novel pre-transplant transport model employing human iCM in an environment simulating the clinical context of hypothermic organ movement from donor to recipient. Under these conditions, we explored functional and molecular endpoints to define the effects of human ASC-S on human iCM, uncovering for the first time that key therapeutic effects of ASC-S are based on the delivery of specific enzymatic activities to accelerate the disposal of ROS and H2O2.
The observation that human iCM exposed to cold UW exhibit delayed and diminished contractile activity following washout of UW, with impairment proportional to the time of UW exposure, parallels well with clinical observations of the effects of post-cardioplegic ischemia/reperfusion compromise of cardiac function in transplant recipients. While short periods of cold storage are well-tolerated, recipient survival decreases with increased ischemic time during donor heart transport, especially for storage times beyond 4–6 hours.7, 8
The primary readouts used in this study were the time-dependent return of cardiomyocyte contractile rate and function. Comparison of iCM recovery following immersion in UW transport solution at 4°C or 37°C revealed that iCM deterioration during UW exposure was accelerated at physiological temperature, presumably due to an increased metabolic rate of the cells during warm UW exposure (Figure 1B), supporting the accepted concept that preservation of the isolated heart is enhanced at lower temperatures. Remarkably, storage of the cells in cold standard culture media did not result in significant functional deterioration, whereas iCM stored at 4°C in UW show significantly reduced functional recovery (Figure 1B). These findings, along with the observations of increased levels of cleaved caspase-3 (Figure 1C and 1D, Supplemental Figure 3) and decreased beating velocity (Figure 1E) as a function of UW exposure time, prompts the notion that UW may cause toxic effect in cardiomyocytes. Surprisingly, despite the wide use of cardioplegic solutions for organ preservations, there are no studies that address, in depth, the toxicity of cardioplegic solutions on cardiomyocytes. Several in vivo and ex vivo studies on whole organs have shown that cardioplegic solutions have detrimental effects on cardiac tissue, especially during long-term storage.36–41 This area of research has been forgotten or ignored for several decades and few advances have been made with respect to the composition of cardioplegic solutions. This supports the emerging concept that new improvements in transport media technology are a critical need for improved heart preservation.42
During organ procurement, cardiac arrest and rapid cooling are introduced promptly after aortic clamping to reduce the rate of cardiac metabolism. However, this treatment also causes rapid metabolic remodeling toward anaerobic processes, resulting in the generation and accumulation of protons, lactate, hypoxanthine and reactive oxygen species, as well as a rapid alteration of the cardiomyocyte proteome so as to diminish anti-oxidant activity.43 Upon organ re-warming and implantation, the accumulated metabolites continue to generate reactive oxygen species, including both free radicals and H2O2, which then contribute to cell damage and overall loss of organ function. Although iCM ex vivo do not undergo physiologic ischemia/reperfusion, the replacement of culture media with cold (or warm) UW is clearly sufficient to result in marked ROS and H2O2 accumulation (Figure 4D, E).
The beneficial effects of anti-oxidants on preservation of isolated organs have been known for more than two decades. Gharagozloo et. al.44 have shown that perfusion of sheep hearts with cardioplegic solution augmented with SOD and catalase proteins resulted in a significant improvement in post-cardioplegic left and right ventricular compliance, myocardial function and oxygen consumption, reduction in tissue edema, and preservation of normal LV end diastolic pressure. Similarly, beneficial effects of free radical scavengers have been shown for kidney transplants.45, 46
Our study demonstrates for the first time that iCM recovery following exposure to conditions mimicking clinical organ transport for transplantation is markedly enhanced by the admixture of concentrated ASC-S. We have previously shown that ASC-S can improve survival of endothelial cells,9 neurons,16, 18 epithelial cells,47 and pancreatic islets.48 Our observations that the protective effects of ASC-S are markedly diminished when the expression of either SOD3 or catalase is selectively repressed suggests that the catalytic disposal of ROS plays a central role ASC-S effect. These novel findings highlight, in addition to the previously recognized activities of ASC secretome to promote angiogenic, anti-apoptotic, and immunomodulatory pathways, a critical anti-oxidant function of ASC secretome which occurs by direct provision of catalytic protein activity. In concert with our finding, provision of MSC secretome was previously demonstrated to limit oxidative modification of myocardial protein after ischemic injury in vivo; and secretome derived from rat MSC was recently shown to improve the function of rat hearts with compromised systolic as well as diastolic function due to increased age following heterotopic transplantation.49, 50 Moreover, this effect was found to be associated with enhanced anti-oxidant gene expression in the secretome-treated grafts. Our findings also prompt the notion that selected pre-treatments of ASC or MSC to upregulate expression of catalase and SODs, as has been done with melatonin16, 46, 49, 50 may enhance the potency of their secretome to provide organ protection.
We recognize that other proteins as well as miRNAs secreted by ASC likely play complementary roles in heart preservation,12, 21, 51–53 and also that paracrine effects on non-myocytes likely contribute to rescuing post-ischemic cardiac function by affecting myocardial endothelial cells and fibroblasts, as well as by modulating the inflammatory milieu. Indeed, our prior studies have demonstrated that ASC-S contains factors that support endothelial cell preservation and function,9, 21 and thus could also preserve vascular integrity during cold UW exposure. Interestingly, recent analysis strongly suggests that the quantity of miRNA present in MSC exosomes is insufficient to account for their activity, whereas the quantity of multiple enzymes present in MSC exosomes can indeed explain metabolic activity in particular.54 Follow-up studies are needed to compare the effects of ASC-S and purified anti-oxidant enzymes on endothelial cell preservation in cold cardioplegic solution when cultured alone and in combination with iCM in myocardium-on-a chip constructs28 to further uncover the full cardio-protective potential of these therapeutic agents. In addition, multiple studies have shown that HSP70 and HSP90 play a key role in recovery of cardiomyocytes after ischemia/reperfusion injuries,55–57 and overexpression of HSP70 further improves organ recovery. Since it is well documented that ASC-S is enriched with exosomes,58 which have a high level of HSP70,59 it is reasonable to hypothesize that ASC-S may also provide complementary therapeutic effects by direct upregulation of intracellular HSPs through exosome-mediated transfer.59 However, based on the relatively rapid response dynamic (minutes to hours) of iCMs we observed to ASC-S, we believe an enzymatic action, rather than cellular pathway modulation which could require longer timescales (several hours to days), is more plausible.
The exact mechanisms by which ASC-S functions to so rapidly provide the enzymes capable of clearing ROS from iCM require further clarification. It has been recognized that ASC-S is enriched with exosomes and extracellular vesicles that carry bioactive molecules, including enzymes, and that these vehicles efficiently deliver their cargo to the cell cytoplasm by fusing with the cell membrane.60, 61 We speculate that while purified proteins may provide effects through removal of ROS from the extracellular media, ASC-S may facilitate access to both extra-cellular and intra-cellular ROS, and thus may confer more efficient protective activity.
Interestingly, similarly robust protective effects on the iCM were noted when cells were exposed to ASC-S during the period of cold UW exposure, during the subsequent period of recovery in control media at physiological temperature, or when the cells were exposed to ASC-S for the duration of the entire experiment (Figure 2D). While current methods may not be sensitive enough to detect small differences in treatment regimens, the observed findings also suggest that presentation of exogenous ROS scavengers to the cells at any point of the preservation period may be sufficient to eliminate generated ROS during exposures on the timescale of several hours. This finding has direct implications for translation, since it suggests that exposure of the heart to standard transport solution admixed with ASC-S may provide significant augmentation of cardiac transplant preservation without the need for additional treatment of the recipient. These findings also support the complementary notion of treatment to improve functional recovery even in situations when ASC-S is not available at the time of organ procurement, by exposing the organ to ASC-S prior to implantation in order to improve procedure outcomes. The recovery of iCM in media supplemented with ASC-S significantly improved both iCM survival and the recovery of function (Figure 3) even when provided to the cells at the time of cardioplegia washout; this is also consistent with prior studies in which MSC secretome was effective to limit regional myocardial infarction whether provided immediately or with modest delay after the time of reperfusion.23, 24
While the conditions of this study most closely simulate those of the “brain-death” organ recovery scenario with an absence of a warm ischemia period, we postulate that the use of ASC-S could be extended to protection or even recovery of organs obtained from delayed circulatory death (DCD) patients and to preservation of the heart during heart surgeries. Finally, recent promising results suggesting the utility of perfusion at physiological temperatures to extend the transport of heart and other organs62, 63 may be complemented by the addition of ASC-S to the circulation of such systems.
Conclusion
This study strongly suggests that heart preservation in a standard cardioplegic solution can be significantly improved. Augmentation of cardioplegic solution with ASC secretome substantially improves preservation of both murine heart and human iCM function and permits extension of the permissible duration of iCM cold storage. iCM storage in cold UW solution led to a marked increase in intracellular ROS accumulation, which could be efficiently cleared by the activities of anti-oxidant enzymes, catalase and SOD3, present in ASC secretome. We speculate that incorporation of fractionated, concentrated ASC-S into clinical practice by admixture with cardioplegic solutions at the site of organ donation may expand the usable donor pool by as much as 50–100%, addressing a critical medical need for patients with advanced heart failure.
Supplementary Material
Funding:
This work was supported by Veterans Administration Merit Review [1I01BX003888-01] to KLM, PZ, DT]; University of Notre Dame Naughton Fellowship to [BE], American Heart Association [17IRG33460277] to [KLM, DT]; and National Institutes of Health [R01 HL141909-01A1] to [PZ, KLM].
Footnotes
Conflict of interest: K.M. declared patent holder for the secretome approach to transplant, Consultant and stock ownership of Theratome Bio. All of the other authors declared no potential conflicts of interest.
References
- 1.Singhal AK, Abrams JD, Mohara J, Hasz RD, Nathan HM, Fisher CA, Furukawa S, Goldman BI. Potential suitability for transplantation of hearts from human non-heart-beating donors: data review from the Gift of Life Donor Program. J Heart Lung Transplant 2005;24:1657–1664. [DOI] [PubMed] [Google Scholar]
- 2.Taylor DO, Stehlik J, Edwards LB, Aurora P, Christie JD, Dobbels F, Kirk R, Kucheryavaya AY, Rahmel AO, Hertz MI. Registry of the International Society for Heart and Lung Transplantation: Twenty-sixth Official Adult Heart Transplant Report-2009. J Heart Lung Transplant 2009;28:1007–1022. [DOI] [PubMed] [Google Scholar]
- 3.Iyer A, Gao L, Doyle A, Rao P, Jayewardene D, Wan B, Kumarasinghe G, Jabbour A, Hicks M, Jansz PC, Feneley MP, Harvey RP, Graham RM, Dhital KK, Macdonald PS. Increasing the tolerance of DCD hearts to warm ischemia by pharmacological postconditioning. Am J Transplant 2014;14:1744–1752. [DOI] [PubMed] [Google Scholar]
- 4.http://www.organdonor.gov/statistics-stories/statistics.html ODSODI.
- 5.Crisostomo PR, Wang Y, Markel TA, Wang M, Lahm T, Meldrum DR. Human mesenchymal stem cells stimulated by TNF-alpha, LPS, or hypoxia produce growth factors by an NF kappa B-but not JNK-dependent mechanism. Am J Physiol Cell Physiol 2008;294:C675–682. [DOI] [PubMed] [Google Scholar]
- 6.Wang M, Zhang W, Crisostomo P, Markel T, Meldrum KK, Fu XY, Meldrum DR. STAT3 mediates bone marrow mesenchymal stem cell VEGF production. J Mol Cell Cardiol 2007;42:1009–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson MR, Meyer KH, Haft J, Kinder D, Webber SA, Dyke DB. Heart transplantation in the United States, 1999–2008. Am J Transplant 2010;10:1035–1046. [DOI] [PubMed] [Google Scholar]
- 8.Young JB, Hauptman PJ, Naftel DC, Ewald G, Aaronson K, Dec GW, Taylor DO, Higgins R, Platt L, Kirklin J. Determinants of early graft failure following cardiac transplantation, a 10-year, multi-institutional, multivariable analysis. J Heart Lung Transplant 2001;20:212. [DOI] [PubMed] [Google Scholar]
- 9.Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV, March KL. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 2004;109:1292–1298. [DOI] [PubMed] [Google Scholar]
- 10.Wang M, Crisostomo PR, Herring C, Meldrum KK, Meldrum DR. Human progenitor cells from bone marrow or adipose tissue produce VEGF, HGF, and IGF-I in response to TNF by a p38 MAPK-dependent mechanism. Am J Physiol Regul Integr Comp Physiol 2006;291:R880–884. [DOI] [PubMed] [Google Scholar]
- 11.Bhang SH, Lee S, Shin JY, Lee TJ, Jang HK, Kim BS. Efficacious and clinically relevant conditioned medium of human adipose-derived stem cells for therapeutic angiogenesis. Mol Ther 2014;22:862–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai L, Johnstone BH, Cook TG, Liang Z, Traktuev D, Cornetta K, Ingram DA, Rosen ED, March KL. Suppression of hepatocyte growth factor production impairs the ability of adipose-derived stem cells to promote ischemic tissue revascularization. Stem Cells 2007;25:3234–3243. [DOI] [PubMed] [Google Scholar]
- 13.Cai L, Johnstone BH, Cook TG, Tan J, Fishbein MC, Chen PS, March KL. IFATS collection: Human adipose tissue-derived stem cells induce angiogenesis and nerve sprouting following myocardial infarction, in conjunction with potent preservation of cardiac function. Stem Cells 2009;27:230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev 2008;88:581–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei X, Du Z, Zhao L, Feng D, Wei G, He Y, Tan J, Lee WH, Hampel H, Dodel R, Johnstone BH, March KL, Farlow MR, Du Y. IFATS collection: The conditioned media of adipose stromal cells protect against hypoxia-ischemia-induced brain damage in neonatal rats. Stem Cells 2009;27:478–488. [DOI] [PubMed] [Google Scholar]
- 16.Wei X, Zhao L, Zhong J, Gu H, Feng D, Johnstone BH, March KL, Farlow MR, Du Y. Adipose stromal cells-secreted neuroprotective media against neuronal apoptosis. Neurosci Lett 2009;462:76–79. [DOI] [PubMed] [Google Scholar]
- 17.Fontanilla CV, Gu H, Liu Q, Zhu TZ, Zhou C, Johnstone BH, March KL, Pascuzzi RM, Farlow MR, Du Y. Adipose-derived Stem Cell Conditioned Media Extends Survival time of a mouse model of Amyotrophic Lateral Sclerosis. Sci Rep 2015;5:16953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walker CL, Meadows RM, Merfeld-Clauss S, Du Y, March KL, Jones KJ. Adipose-derived stem cell conditioned medium impacts asymptomatic peripheral neuromuscular denervation in the mutant superoxide dismutase (G93A) transgenic mouse model of amyotrophic lateral sclerosis. Restor Neurol Neurosci 2018;36:621–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie J, Broxmeyer HE, Feng D, Schweitzer KS, Yi R, Cook TG, Chitteti BR, Barwinska D, Traktuev DO, Van Demark MJ, Justice MJ, Ou X, Srour EF, Prockop DJ, Petrache I, March KL. Human adipose-derived stem cells ameliorate cigarette smoke-induced murine myelosuppression via secretion of TSG-6. Stem Cells 2015;33:468–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu H, Cook T, Poirier C, Merfeld-Clauss S, Petrache I, March KL, Bogatcheva NV. Pulmonary Retention of Adipose Stromal Cells Following Intravenous Delivery Is Markedly Altered in the Presence of ARDS. Cell Transplant 2016;25:1635–1643. [DOI] [PubMed] [Google Scholar]
- 21.Lu H, Poirier C, Cook T, Traktuev DO, Merfeld-Clauss S, Lease B, Petrache I, March KL, Bogatcheva NV. Conditioned media from adipose stromal cells limit lipopolysaccharide-induced lung injury, endothelial hyperpermeability and apoptosis. J Transl Med 2015;13:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prochazka V, Jurcikova J, Lassak O, Vitkova K, Pavliska L, Porubova L, Buszman PP, Krauze A, Fernandez C, Jaluvka F, Spackova I, Lochman I, Jana D, Merfeld-Clauss S, March KL, Traktuev DO, Johnstone BH. Therapeutic Potential of Adipose-Derived Therapeutic Factor Concentrate for Treating Critical Limb Ischemia. Cell Transplant 2016;25:1623–1633. [DOI] [PubMed] [Google Scholar]
- 23.Timmers L, Lim SK, Arslan F, Armstrong JS, Hoefer IE, Doevendans PA, Piek JJ, El Oakley RM, Choo A, Lee CN, Pasterkamp G, de Kleijn DP. Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem cell research 2007;1:129–137. [DOI] [PubMed] [Google Scholar]
- 24.Timmers L, Lim SK, Hoefer IE, Arslan F, Lai RC, van Oorschot AA, Goumans MJ, Strijder C, Sze SK, Choo A, Piek JJ, Doevendans PA, Pasterkamp G, de Kleijn DP. Human mesenchymal stem cell-conditioned medium improves cardiac function following myocardial infarction. Stem cell research 2011;6:206–214. [DOI] [PubMed] [Google Scholar]
- 25.Acun A, Nguyen TD, Zorlutuna P. In vitro aged, hiPSC-origin engineered heart tissue models with age-dependent functional deterioration to study myocardial infarction. Acta Biomaterialia 2019;94:372–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Machiraju P, Greenway SC. Current methods for the maturation of induced pluripotent stem cell-derived cardiomyocytes. World J Stem Cells 2019;11:33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang C, Gu H, Yu Q, Manukyan MC, Poynter JA, Wang M. Sca-1+ cardiac stem cells mediate acute cardioprotection via paracrine factor SDF-1 following myocardial ischemia/reperfusion. PLoS One 2011;6:e29246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ellis BW, Acun A, Can UI, Zorlutuna P. Human iPSC-derived myocardium-on-chip with capillary-like flow for personalized medicine. Biomicrofluidics 2017;11:024105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Can UI, Nagarajan N, Vural DC, Zorlutuna P. Muscle-Cell-Based “Living Diode”. Adv Biosyst 2017;1. [DOI] [PubMed] [Google Scholar]
- 30.Henry TD, Pepine CJ, Lambert CR, Traverse JH, Schatz R, Costa M, Povsic TJ, David Anderson R, Willerson JT, Kesten S, Perin EC. The Athena trials: Autologous adipose-derived regenerative cells for refractory chronic myocardial ischemia with left ventricular dysfunction. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions 2017;89:169–177. [DOI] [PubMed] [Google Scholar]
- 31.Houtgraaf JH, den Dekker WK, van Dalen BM, Springeling T, de Jong R, van Geuns RJ, Geleijnse ML, Fernandez-Aviles F, Zijlsta F, Serruys PW, Duckers HJ. First experience in humans using adipose tissue-derived regenerative cells in the treatment of patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol 2012;59:539–540. [DOI] [PubMed] [Google Scholar]
- 32.Perin EC, Sanz-Ruiz R, Sanchez PL, Lasso J, Perez-Cano R, Alonso-Farto JC, Perez-David E, Fernandez-Santos ME, Serruys PW, Duckers HJ, Kastrup J, Chamuleau S, Zheng Y, Silva GV, Willerson JT, Fernandez-Aviles F. Adipose-derived regenerative cells in patients with ischemic cardiomyopathy: The PRECISE Trial. Am Heart J 2014;168:88–95 e82. [DOI] [PubMed] [Google Scholar]
- 33.Roch AM, Maatman TK, Cook TG, Wu HH, Merfeld-Clauss S, Traktuev DO, March KL, Zyromski NJ. Therapeutic Use of Adipose-Derived Stromal Cells in a Murine Model of Acute Pancreatitis. J Gastrointest Surg 2020;24:67–75. [DOI] [PubMed] [Google Scholar]
- 34.Acun A, Zorlutuna P. Engineered myocardium model to study the roles of HIF-1alpha and HIF1A-AS1 in paracrine-only signaling under pathological level oxidative stress. Acta Biomater 2017;58:323–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Acun A, Zorlutuna P. CRISPR/Cas9 Edited Induced Pluripotent Stem Cell-Based Vascular Tissues to Model Aging and Disease-Dependent Impairment. Tissue Eng Part A 2019;25:759–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tortolani AJ, Powell SR, Misik V, Weglicki WB, Pogo GJ, Kramer JH. Detection of alkoxyl and carbon-centered free radicals in coronary sinus blood from patients undergoing elective cardioplegia. Free Radic Biol Med 1993;14:421–426. [DOI] [PubMed] [Google Scholar]
- 37.Yamazaki K, Miwa S, Ueda K, Tanaka S, Toyokuni S, Unimonh O, Nishimura K, Komeda M. Prevention of myocardial reperfusion injury by poly(ADP-ribose) synthetase inhibitor, 3-aminobenzamide, in cardioplegic solution: in vitro study of isolated rat heart model. Eur J Cardiothorac Surg 2004;26:270–275. [DOI] [PubMed] [Google Scholar]
- 38.Digerness SB, Vanini V, Wideman FE. In vitro comparison of oxygen availability from asanguinous and sanguinous cardioplegic media. Circulation 1981;64:II80–83. [PubMed] [Google Scholar]
- 39.Dobsak P, Siegelova J, Wolf JE, Rochette L, Eicher JC, Vasku J, Kuchtickova S, Horky M. Prevention of apoptosis by deferoxamine during 4 hours of cold cardioplegia and reperfusion: in vitro study of isolated working rat heart model. Pathophysiology 2002;9:27. [DOI] [PubMed] [Google Scholar]
- 40.Hegge JO, Southard JH, Haworth RA. Preservation of metabolic reserves and function after storage of myocytes in hypothermic UW solution. Am J Physiol Cell Physiol 2001;281:C758–772. [DOI] [PubMed] [Google Scholar]
- 41.Yamazaki K, Miwa S, Toyokuni S, Nemoto S, Oriyanhan W, Takaba K, Saji Y, Marui A, Nishina T, Ikeda T, Komeda M. Effect of edaravone, a novel free radical scavenger, supplemented to cardioplegia on myocardial function after cardioplegic arrest: in vitro study of isolated rat heart. Heart Vessels 2009;24:228–235. [DOI] [PubMed] [Google Scholar]
- 42.Giwa S, Lewis JK, Alvarez L, Langer R, Roth AE, Church GM, Markmann JF, Sachs DH, Chandraker A, Wertheim JA, Rothblatt M, Boyden ES, Eidbo E, Lee WPA, Pomahac B, Brandacher G, Weinstock DM, Elliott G, Nelson D, Acker JP, Uygun K, Schmalz B, Weegman BP, Tocchio A, Fahy GM, Storey KB, Rubinsky B, Bischof J, Elliott JAW, Woodruff TK, Morris GJ, Demirci U, Brockbank KGM, Woods EJ, Ben RN, Baust JG, Gao D, Fuller B, Rabin Y, Kravitz DC, Taylor MJ, Toner M. The promise of organ and tissue preservation to transform medicine. Nat Biotechnol 2017;35:530–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li X, Arslan F, Ren Y, Adav SS, Poh KK, Sorokin V, Lee CN, de Kleijn D, Lim SK, Sze SK. Metabolic adaptation to a disruption in oxygen supply during myocardial ischemia and reperfusion is underpinned by temporal and quantitative changes in the cardiac proteome. J Proteome Res 2012;11:2331–2346. [DOI] [PubMed] [Google Scholar]
- 44.Gharagozloo F, Melendez FJ, Hein RA, Shemin RJ, DiSesa VJ, Cohn LH. The effect of superoxide dismutase and catalase on the extended preservation of the ex vivo heart for transplantation. J Thorac Cardiovasc Surg 1988;95:1008–1013. [PubMed] [Google Scholar]
- 45.Land W, Schneeberger H, Schleibner S, Illner WD, Abendroth D, Rutili G, Arfors KE, Messmer K. The beneficial effect of human recombinant superoxide dismutase on acute and chronic rejection events in recipients of cadaveric renal transplants. Transplantation 1994;57:211–217. [DOI] [PubMed] [Google Scholar]
- 46.Nakagawa K, Koo DD, Davies DR, Gray DW, McLaren AJ, Welsh KI, Morris PJ, Fuggle SV. Lecithinized superoxide dismutase reduces cold ischemia-induced chronic allograft dysfunction. Kidney Int 2002;61:1160–1169. [DOI] [PubMed] [Google Scholar]
- 47.Dhong ES, Hwang NH, Kim DW, Rajashekhar G, Johnstone BH, March KL. Morphologic changes in photodamaged organotypic human skin culture after treatment of autologous adipose-derived stromal cells. J Craniofac Surg 2012;23:805–811. [DOI] [PubMed] [Google Scholar]
- 48.Kono TM, Sims EK, Moss DR, Yamamoto W, Ahn G, Diamond J, Tong X, Day KH, Territo PR, Hanenberg H, Traktuev DO, March KL, Evans-Molina C. Human adipose-derived stromal/stem cells protect against STZ-induced hyperglycemia: analysis of hASC-derived paracrine effectors. Stem Cells 2014;32:1831–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arslan F, Lai RC, Smeets MB, Akeroyd L, Choo A, Aguor EN, Timmers L, van Rijen HV, Doevendans PA, Pasterkamp G, Lim SK, de Kleijn DP. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem cell research 2013;10:301–312. [DOI] [PubMed] [Google Scholar]
- 50.Korkmaz-Icoz S, Li SL, Huttner R, Ruppert M, Radovits T, Loganathan S, Sayour AA, Brlecic P, Lasitschka F, Karck M, Szabo G. Hypothermic perfusion of donor heart with a preservation solution supplemented by mesenchymal stem cells. J Heart Lung Transpl 2019;38:315–326. [DOI] [PubMed] [Google Scholar]
- 51.Dehaini H, Awada H, El-Yazbi A, Zouein FA, Issa K, Eid AA, Ibrahim M, Badran A, Baydoun E, Pintus G, Eid AH. MicroRNAs as Potential Pharmaco-targets in Ischemia-Reperfusion Injury Compounded by Diabetes. Cells 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moghaddam AS, Afshari JT, Esmaeili SA, Saburi E, Joneidi Z, Momtazi-Borojeni AA. Cardioprotective microRNAs: Lessons from stem cell-derived exosomal microRNAs to treat cardiovascular disease. Atherosclerosis 2019;285:1–9. [DOI] [PubMed] [Google Scholar]
- 53.Qiu G, Zheng G, Ge M, Wang J, Huang R, Shu Q, Xu J. Mesenchymal stem cell-derived extracellular vesicles affect disease outcomes via transfer of microRNAs. Stem Cell Res Ther 2018;9:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Toh WS, Lai RC, Zhang B, Lim SK. MSC exosome works through a protein-based mechanism of action. Biochem Soc Trans 2018;46:843–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Song YJ, Zhong CB, Wang XB. Heat shock protein 70: A promising therapeutic target for myocardial ischemia-reperfusion injury. J Cell Physiol 2019;234:1190–1207. [DOI] [PubMed] [Google Scholar]
- 56.Peng W, Zhang Y, Zheng M, Cheng H, Zhu W, Cao CM, Xiao RP. Cardioprotection by CaMKII-deltaB is mediated by phosphorylation of heat shock factor 1 and subsequent expression of inducible heat shock protein 70. Circ Res 2010;106:102–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang C, Liu X, Miao J, Wang S, Wu L, Yan D, Li J, Guo W, Wu X, Shen A. Heat shock protein 70 protects cardiomyocytes through suppressing SUMOylation and nucleus translocation of phosphorylated eukaryotic elongation factor 2 during myocardial ischemia and reperfusion. Apoptosis 2017;22:608–625. [DOI] [PubMed] [Google Scholar]
- 58.Baglio SR, Rooijers K, Koppers-Lalic D, Verweij FJ, Perez Lanzon M, Zini N, Naaijkens B, Perut F, Niessen HW, Baldini N, Pegtel DM. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res Ther 2015;6:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vicencio JM, Yellon DM, Sivaraman V, Das D, Boi-Doku C, Arjun S, Zheng Y, Riquelme JA, Kearney J, Sharma V, Multhoff G, Hall AR, Davidson SM. Plasma exosomes protect the myocardium from ischemia-reperfusion injury. J Am Coll Cardiol 2015;65:1525–1536. [DOI] [PubMed] [Google Scholar]
- 60.Li SP, Lin ZX, Jiang XY, Yu XY. Exosomal cargo-loading and synthetic exosome-mimics as potential therapeutic tools. Acta Pharmacol Sin 2018;39:542–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Dongen HM, Masoumi N, Witwer KW, Pegtel DM. Extracellular Vesicles Exploit Viral Entry Routes for Cargo Delivery. Microbiol Mol Biol Rev 2016;80:369–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ardehali A, Esmailian F, Deng M, Soltesz E, Hsich E, Naka Y, Mancini D, Camacho M, Zucker M, Leprince P, Padera R, Kobashigawa J, investigators PIt. Ex-vivo perfusion of donor hearts for human heart transplantation (PROCEED II): a prospective, open-label, multicentre, randomised non-inferiority trial. Lancet 2015;385:2577–2584. [DOI] [PubMed] [Google Scholar]
- 63.Warnecke G, Van Raemdonck D, Smith MA, Massard G, Kukreja J, Rea F, Loor G, De Robertis F, Nagendran J, Dhital KK, Moradiellos Diez FJ, Knosalla C, Bermudez CA, Tsui S, McCurry K, Wang IW, Deuse T, Leseche G, Thomas P, Tudorache I, Kuhn C, Avsar M, Wiegmann B, Sommer W, Neyrinck A, Schiavon M, Calabrese F, Santelmo N, Olland A, Falcoz PE, Simon AR, Varela A, Madsen JC, Hertz M, Haverich A, Ardehali A. Normothermic ex-vivo preservation with the portable Organ Care System Lung device for bilateral lung transplantation (INSPIRE): a randomised, open-label, non-inferiority, phase 3 study. Lancet Respir Med 2018;6:357–367. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data from this study is available from the corresponding authors upon reasonable request.


