Abstract
Background:
Eicosapentaenoic acid (EPA) supplementation is an effective treatment option in major depressive disorder (MDD) associated with chronic low-grade inflammation. EPA is the precursor of specialized pro-resolving lipid mediators (SPMs) termed resolvins (Rv), that serve important roles in the resolution of inflammation. The objective of this study was to assess the effects of different doses of EPA on plasma concentrations of EPA metabolites and SPMs in MDD patients.
Methods:
In a 2-site study, 61 MDD patients with body mass index >25 kg/m2 and serum high-sensitivity C-reactive protein ≥3 μg/mL were enrolled in a 12-week randomized trial comparing EPA 1, 2, and 4 g/d to placebo. Plasma EPA (mol%) and SPMs (pg/mL) were measured in 42 study completers at baseline and at the end of treatment by liquid chromatography/mass spectrometry.
Results:
Plasma EPA and SPM concentrations did not change in the placebo group during 12 weeks of treatment. Plasma EPA and EPA-derived metabolites increased significantly and dose-dependently in all EPA supplementation arms. The increase in 18-hydroxyeicosapentaenoic acid (18-HEPE), the precursor of RvE1–3, was significantly greater with the 4 g/d EPA dose than the other doses from week 4 to 12. RvE1 was undetected in all treatment groups, while RvE2 was detected in half of the subjects both at baseline and after treatment, with dose-dependent increases. RvE3 was detected only after supplementation, dose-dependently. A significant reduction in plasma arachidonic acid (AA), relative to baseline, was observed in all EPA arms. This was in contrast with an increase in AA-derived SPM lipoxin B4 (LXB4) in the 4 g/d arm.
Conclusions:
Our results show a robust effect of EPA 4 g/d supplementation in increasing plasma EPA and 18-HEPE levels, associated with improved conversion to RvE2–3, and LXB4 levels.
Keywords: omega-3 fatty acids, eicosapentaenoic acid (EPA), pro-resolving lipid mediators (SPM), major depressive disorder (MDD)
1. Introduction
Chronic inflammation, a characteristic feature of cardiometabolic diseases such as obesity, metabolic syndrome, diabetes and cardiovascular disease (1,2), has emerged as a significant contributor to the development of psychiatric disorders, including major depressive disorder (MDD) (3,4). Inflammation is a central element of the innate immune response against injury or pathogens. Under normal conditions, inflammation is a self-limiting process, followed by a resolution phase during which activated inflammatory cells are cleared and tissue repair is initiated (5). When the resolution phase is impaired, chronic inflammation and tissue injury ensues (5,6). Resolution of inflammation is a highly regulated process, mediated in part by lipid molecules called specialized pro-resolving lipid mediators (SPMs), that are derived endogenously from omega-3 (n-3) fatty acids such as eicosapentaenoic acid (EPA, 20:5 n-3) and docosahexaenoic acid (DHA, 22:6 n-3) (7–9). Possibly due to the well-documented anti-inflammatory effects of EPA (10), some randomized clinical intervention trials of EPA supplementation in MDD patients have shown significant reductions in depressive symptoms (11–13). Recently, an expert panel from the International Society for Nutritional Psychiatry Research (ISNPR) supported clinical use of n-3 fatty acids in MDD, particularly as augmentation therapy (14), and a meta-analysis demonstrated the clinical benefits of EPA, but not DHA, supplementation in MDD (15).
EPA is the precursor of a variety of bioactive lipid molecules, many with anti-inflammatory effects at concentrations 100 to 1000 times lower than EPA. Among the lipid metabolites derived from EPA are the 3-series prostaglandins (PGs) and thromboxanes (TXs) and 5-series leukotrienes (LTs). These eicosanoids are known to be less inflammatory than the 2-series PGs and TXs and 4-series LTs derived from arachidonic acid (AA, 20:4 n-6) (16). In addition, EPA is metabolized to E-series resolvins (RvE), a major class of SPMs (17,18). RvE1, RvE2 and RvE3, are synthesized from EPA via an intermediate precursor, 18-hydroxyeicosapentaenoic acid (18-HEPE) (9). In animal models of acute inflammation, such as murine peritonitis, synthesis of RvEs increases dramatically in tissues following injury (18) and leads to a host of biological effects, including reduction in neutrophil infiltration and inhibition of macrophage cytokine expression and NLRP3 inflammasome activation (19).
EPA can be further desaturated and elongated to docosapentaenoic acid (DPA, 22:5, n-3) and then to docosahexaenoic acid (DHA, 22:6 n-3) (20,21). These n-3 fatty acids also serve as precursors of other SPMs, including DPA-derived D-series resolvins (RvDn-3 DPA) and maresins (MaR1n-3 DPA) and DHA-derived RvD1–6, protectin D1 (PD1) and maresin 1 (MaR1) (18,22). Little is known about the time- and dose-effects of EPA supplementation on the production of EPA-derived lipid mediators and SPMs in humans with chronic inflammation or MDD. Therefore, the objective of this double-blind randomized placebo-controlled study was to characterize the time course of the changes in plasma concentrations of lipid metabolites originating from the n-3 fatty acids EPA and DHA and the n-6 fatty acid AA in overweight/obese adults with both MDD and concomitant inflammation given oral fish-oil derived EPA enriched supplements containing 1, 2, or 4 g/day EPA versus placebo over a 12-week period of intervention.
1. Subjects and Methods
1.1. Subjects and study design.
Sixty-one overweight or obese adult participants (body mass index, BMI, ≥ 25 kg/m2) with MDD (Inventory of Depressive Symptomatology – 30 items, IDS-C30, ≥ 25) and chronic inflammation (high-sensitivity C reactive protein, hs-CRP, ≥ 3 μg/mL) were enrolled in a 12-week randomized, placebo-controlled, double-blind, parallel-group study assessing the effect of three different EPA supplementation doses on clinical symptoms of depression and biochemical outcomes. Subjects were recruited at two sites, Emory University (Atlanta GA) and Massachusetts General Hospital (Boston, MA). The study protocol was approved by the IRB at both institutions IRB and is registered on ClinicalTrials.gov (NCT02553915). Subjects with a high dietary intake of n-3 fatty acids, as assessed by dietary history using Food Processor (23) or taking fish oil supplements were excluded from the study. EPA was supplemented at doses of 1 g/d, 2 g/d or4 g/d with each capsule containing approximately 823 mg n-3 fatty acids in triglyceride form, with an EPA:DHA ratio of 3.9:1 (about 590 mg of EPA and 152 mg DHA). Matched placebo capsules contained soybean oil (approximately 51% linoleic acid, 25% oleic acid and 6% α-linolenic acid, but no EPA or DHA). Patients were randomized equally to one of four treatment arms: 1) EPA 1 g/day; 2) EPA 2 g/day; 3) EPA 4 g/day; or 4) placebo. In each arm of the study, participants were instructed to take eight capsules each day, four in the morning and four in the evening, with the corresponding meals. All subjects provided informed consent. A total of 42 subjects completed the study and provided plasma for the assessment of fatty acids and/or lipid metabolites (Table 1).
Table 1.
Baseline characteristics of study participants.
| Placebo (n=10) | EPA 1 g/d (n=11) | EPA 2 g/d (n=11) | EPA 4 g/d (n=10) | |
|---|---|---|---|---|
| Gender, M/F | 2/8 | 3/8 | 3/8 | 4/6 |
| Age, y1 | 52±13 | 43±17 | 47±15 | 46±14 |
| BMI, kg/m2 2 | 38.8 (10.7) | 33.2 (5.2) | 34.3 (13.0) | 35.0 (10.7) |
| hs-CRP, μg/mL2 | 6.3 (11.0) | 6.6 (8.8) | 6.4 (3.0) | 3.9 (1.1) |
| IDS-C301 | 37.8±8.2 | 37.7±6.0 | 33.7±7.7 | 34.8±8.5 |
mean±SD;
median (interquartile range: 75th – 25th percentile)
BMI: body mass index; hs-CRP: high sensitivity C reactive protein; IDS-C30: Inventory of depressive symptomatology – 30 items
Subjects were instructed to avoid taking any non-steroidal anti-inflammatory drugs for at least 24 hours before blood draws. Blood for the measurement of plasma fatty acids, lipid mediators, and SPMs was collected in EDTA plasma tubes (Becton Dickinson) after a 12-hour fast at baseline (week 0) and at weeks 4, 8 and 12. Plasma was obtained after centrifugation at 1000 × g for 25 min at 4 °C and then immediately stored at −80 °C until further analyses.
1.2. Fatty acid analysis
Plasma samples were hydrolyzed and then the fatty acid concentrations, representing the fatty acids contained in all plasma lipid families (triglycerides, phospholipids, cholesteryl esters) were assessed by ultrahigh-performance liquid chromatography (UPLC, as previously described (24), coupled with a QTRAP5500 mass analyzer. Chromatography was performed on Targa C8 (2×10 mm, 5μ) columns. The concentration of individual fatty acids was validated against standard curves generated for each fatty acid. Fatty acid concentrations were then converted to molar percent of total plasma fatty acids (mol%).
1.3. Lipid mediators and SPM analysis
The plasma concentrations of lipid mediators, defined here as the intermediate lipid molecules derived from the enzymatic conversion of EPA, DHA and AA and with biological activity, and of SPMs were assessed by liquid chromatography-mass spectrometry (LC-MS) as previously described (9,25). All samples were batch-analyzed at the end of the study. Briefly, 100 μl of plasma was spiked with 5 ng each of PGE1-d4, RvD2-d5, LTB4-d4, and 15S-hydoxyeicosatetraenoic acid-d8 (15S-HETE-d8) as internal standards for analyte recovery and quantitation, followed by extraction for fatty acyl lipid metabolites with C18 extraction columns. Samples were then subjected to LC-MS analysis. Data were collected with Analyst 1.6 software and quantitated using MultiQuant software (AB Sciex). All quantified lipid mediators were identified by comparison with authenticated standards (Cayman Chemicals, Ann Arbor. MI).
1.4. Statistical analyses
Statistical analyses were conducted in R (version 3.5.2) with RStudio IDE (www.rstudio.com). Due to their skewed distributions, plasma levels of fatty acids, lipid mediators and SPMs are presented as median and interquartile range (75 percentile – 25 percentile). For the time effect, the significance of the change in values at weeks 4, 8, and 12 from baseline (week 0) within each arm was determined by Wilcoxon Signed Rank tests. The dose effect was assessed by comparing changes from week 0 at each time point among the four different treatment arms using the Wilcoxon Mann-Whitney tests with Benjamini-Hochberg adjustment for multiple comparisons. Associations between fatty acid and lipid mediator concentrations were assessed by Spearman’s rank-order correlation tests. A p value <0.05 was considered statistically significant.
Results
1.5. Plasma fatty acids
Plasma EPA (mol%) did not change in the placebo arm (Table 2 and Figure 1). Relative to week 0, plasma EPA significantly increased in all EPA supplementation arms at week 4 and this increase remained stable until week 12. At week 12, relative to week 0, plasma EPA increased by 4.4 fold in the 1 g/day arm, 5.1 fold in the 2 g/d arm, and 11.3 fold in the 4 g/d arm. During supplementation, plasma EPA was significantly greater than placebo in all treatment arms. At 12 weeks, the 4 g/d dose group reached plasma EPA levels greater than those at 1 and 2 g/d, while levels at 1 g/d and 2 g/d were similar.
Table 2.
Proportion of fatty acids (mol%) in plasma, by treatment arm.1
| Placebo (n=8) | EPA 1 g/d (n=12) | EPA 2g/d (n=11) | EPA 4 g/d (n=10) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wk0 | Wk4 | Wk8 | Wk12 | Wk0 | Wk4 | Wk8 | Wk12 | Wk0 | Wk4 | Wk8 | Wk12 | Wk0 | Wk4 | Wk8 | Wk12 | |
| SFA | ||||||||||||||||
| 12:0 | 0.75 (1.16) | 0.62 (1.13) | 0.60 (0.40) | 0.73 (0.37) | 0.68 (1.27) | 0.68 (0.83) | 0.60 (0.62) | 0.50 (0.10) | 0.54 (1.19) | 0.54 (0.49) | 0.53 (0.18) | 0.59 (0.62) | 0.77 (1.25) | 0.54 (0.46) | 0.55 (0.55) | 0.55 (0.45) |
| 14:0 | 2.99 (1.03) | 2.83 (2.03) | 3.01 (1.29) | 3.17 (1.05) | 2.08 (1.13) | 2.66 (1.53) | 2.75 (1.71) | 2.31 (1.54) | 2.39 (1.28) | 2.19 (0.63) | 1.90 (0.53) | 1.81 (0.76) | 3.40 (1.67) | 3.40 (1.56) | 3.08 (0.97) | 3.00 (1.33) |
| 15:0 | 1.23 (1.09) | 1.07 (1.10) | 1.47 (1.90) | 1.76 (0.78) | 1.16 (0.81) | 1.47 (1.58) | 1.68 (1.33) | 1.32 (0.76) | 1.11 (0.72) | 1.10 (0.22) | 0.96 (0.46) | 0.98 (0.93) | 1.77 (0.67) | 2.16 (1.16) | 1.61 (0.70) | 1.39 (0.88) |
| 16:0 | 16.3 (1.6) | 15.5 (3.1) | 17.7 (3.0) | 16.1 (3.2) | 16.9 (2.1) | 18.3 (3.0) | 17.6 (3.1) | 17.0 (2.6) | 17.3 (3.2) | 19.4 (1.2) | 16.7 (3.3) | 18.3 (1.9) | 16.9 (6.1) | 19.0 (5.4) | 17.7 (3.3) | 17.0 (1.0) |
| 17:0 | 0.94 (0.80) | 1.05 (0.71) | 1.18 (1.06) | 1.13 (0.63) | 1.03 (0.65) | 1.19 (0.80) | 1.23 (0.68) | 1.12 (0.48) | 1.11 (0.47) | 0.98 (0.73) | 0.98 (0.23) | 0.97 (0.51) | 1.26 (1.38) | 1.26 (1.52) | 1.25 (1.05) | 1.22 (1.00) |
| 18:0 | 11.6 (0.8) | 11.7 (3.1) | 12.7 (1.4) | 12.2 (1.6) | 13.1 (5.3) | 12.0 (1.8) | 13.2 (5.3) | 11.9 (4.3) | 13.2 (5.6) | 11.8 (2.9) | 12.2 (3.1) | 11.9 (3.6) | 12.8 (3.7) | 11.4 (2.0) | 11.4 (2.2) | 11.8 (2.9) |
| 20:0 | 0.19 (0.11) | 0.19 (0.09) | 0.27 (0.16) | 0.22 (0.08) | 0.25 (0.19) | 0.21 (0.16) | 0.31 (0.21) | 0.22 (0.26) | 0.28 (0.16) | 0.23 (0.27) | 0.23 (0.10) | 0.21 (0.16) | 0.29 (0.28) | 0.21 (0.25) | 0.22 (0.21) | 0.25 (0.29) |
| 22:0 | 0.08 (0.06) | 0.07 (0.06) | 0.10 (0.10) | 0.08 (0.03) | 0.10 (0.13) | 0.09 (0.11) | 0.13 (0.12) | 0.09 (0.13) | 0.10 (0.09) | 0.08 (0.14) | 0.09 (0.04) | 0.11 (0.13) | 0.12 (0.16) | 0.10 (0.16) | 0.13 (0.12) | 0.10 (0.12) |
| 24:0 | 0.19 (0.10) | 0.12 (0.16) | 0.23 (0.09) | 0.17 (0.10) | 0.22 (0.33) | 0.20 (0.24) | 0.23 (0.29) | 0.19 (0.21) | 0.27 (0.24) | 0.14 (0.19) | 0.17 (0.08) | 0.20 (0.36) | 0.24 (0.32) | 0.21 (0.26) | 0.15 (0.22) | 0.23 (0.16) |
| 26:0 | 0.05 (0.02) | 0.03 (0.06) | 0.06 (0.03) | 0.04 (0.01) | 0.05 (0.08) | 0.06 (0.07) | 0.06 (0.10) | 0.04 (0.05) | 0.07 (0.08) | 0.03 (0.03) | 0.04 (0.02) | 0.04 (0.08) | 0.08 (0.08) | 0.07 (0.07) | 0.03 (0.08) | 0.06 (0.06) |
| MUFA | ||||||||||||||||
| 16:1 n-7 | 3.07 (1.14) | 3.11 (1.60) | 2.64 (1.08) | 2.35 (0.96) | 2.62 (1.20) | 2.07 (1.17) | 2.16 (1.48) | 1.90 (0.99) | 1.80 (0.62) | 1.81 (0.90) | 1.42 (0.83) | 1.73 (1.06) | 2.38 (1.20) | 1.61 (1.59) | 1.58 (0.36) | 1.53 (0.89) |
| 17:1 n-7 | 0.26 (0.06) | 0.25 (0.12) | 0.28 (0.19) | 0.25 (0.12) | 0.24 (0.10) | 0.25 (0.10) | 0.22 (0.15) | 0.24 (0.08) | 0.23 (0.09) | 0.20 (0.06) | 0.17 (0.05) | 0.21 (0.17) | 0.28 (0.13) | 0.25 (0.13) | 0.22 (0.06) | 0.24 (0.11) |
| 18:1 n-9 | 12.1 (1.0) | 12.3 (2.4) | 12.1 (0.6) | 12.1 (1.0) | 12.1 (1.8) | 10.7 (2.3) | 10.8 (1.0) | 11.3 (2.8) | 11.4 (3.2) | 11.2 (3.2) | 11.3 (3.6) | 11.0 (1.9) | 12.5 (2.2) | 10.7 (1.7) | 11.3 (0.8) | 11.2 (2.5) |
| 20:1 n-9 | 0.12 (0.04) | 0.15 (0.06) | 0.12 (0.04) | 0.16 (0.07) | 0.12 (0.07) | 0.10 (0.07) | 0.14 (0.07) | 0.11 (0.07) | 0.13 (0.08) | 0.08 (0.10) | 0.11 (0.04) | 0.12 (0.07) | 0.15 (0.08) | 0.10 (0.04) | 0.10 (0.06) | 0.09 (0.05) |
| PUFA | ||||||||||||||||
| 20:3 n-9 | 0.60 (0.05) | 0.62 (0.20) | 0.56 (0.14) | 0.63 (0.24) | 0.60 (0.31) | 0.47 (0.25) | 0.38 (0.17) | 0.42 (0.10) | 0.65 (0.29) | 0.45 (0.19) | 0.47 (0.21) | 0.41 (0.18) | 0.63 (0.31) | 0.30 (0.14) | 0.35 (0.19) | 0.30 (0.29) |
| 18:2 n-6 | 23.1 (4.7) | 22.6 (5.6) | 22.8 (2.4) | 21.5 (5.2) | 24.1 (7.0) | 22.2 (4.0) | 21.0 (3.6) | 21.8 (6.9) | 23.6 (6.7) | 20.6 (3.8) | 22.8 (3.7) | 21.9 (7.0) | 21.5 (7.4) | 16.8 (4.7) | 17.3 (3.0) | 19.9 (6.3) |
| 18:3 n-6 + n-3 | 0.49 (0.09) | 0.55 (0.10) | 0.43 (0.08) | 0.44 (0.15) | 0.31 (0.08) | 0.37 (0.18) | 0.30 (0.12) | 0.31 (0.21) | 0.37 (0.24) | 0.29 (0.14) | 0.25 (0.08) | 0.26 (0.18) | 0.31 (0.20) | 0.21 (0.16) | 0.20 (0.19) | 0.32 (0.13) |
| 20:2 n-6 | 0.27 (0.07) | 0.28 (0.09) | 0.24 (0.04) | 0.30 (0.08) | 0.28 (0.09) | 0.23 (0.08) | 0.26 (0.11) | 0.27 (0.06) | 0.33 (0.09) | 0.24* (0.14) | 0.26 (0.12) | 0.24 (0.08) | 0.28 (0.14) | 0.18* (0.05) | 0.19 (0.10) | 0.22 (0.09) |
| 20:3 n-6 | 1.92 (0.29) | 1.75 (0.34) | 1.67 (0.40) | 1.78 (0.24) | 1.84 (0.63) | 1.51 (0.29) | 1.53 (0.46) | 1.68 (0.36) | 1.73 (0.44) | 1.65 (0.57) | 1.57 (0.24) | 1.39 (0.50) | 1.76 (0.81) | 1.26 (0.64) | 1.37 (0.20) | 1.33 (0.45) |
| 20:4 n-6 | 11.2 (1.6) | 10.1 (2.0) | 10.0 (2.0) | 10.9 (1.7) | 11.4 (4.2) | 9.2 (2.2) | 9.1 (2.8) | 10.1 (1.8) | 9.4 (3.8) | 8.2 (3.3) | 9.4 (3.5) | 9.0 (3.9) | 10.9 (5.2) | 7.7 (2.9) | 8.1 (2.5) | 8.8 (3.3) |
| 22:4 n-6 | 0.19 (0.05) | 0.20 (0.11) | 0.15 (0.10) | 0.20 (0.08) | 0.17 (0.11) | 0.10 (0.07) | 0.12 (0.06) | 0.11 (0.07) | 0.13 (0.06) | 0.06 (0.07) | 0.07 (0.04) | 0.07 (0.09) | 0.15 (0.10) | 0.04 (0.05) | 0.06 (0.07) | 0.08 (0.10) |
| 22:5 n-6 | 0.23 (0.13) | 0.23 (0.10) | 0.19 (0.13) | 0. 22 (0.06) | 0.21 (0.08) | 0.15* (0.11) | 0.13 (0.21) | 0.18 (0.08) | 0.19 (0.07) | 0.15 (0.14) | 0.17 (0.14) | 0.18 (0.08) | 0.20 (0.12) | 0.17 (0.10) | 0.18 (0.09) | 0.24†¶ (0.06) |
| 18:4 n-3 | 0.08 (0.07) | 0.07 (0.06) | 0.06 (0.03) | 0.07 (0.04) | 0.02 (0.02) | 0.04 (0.05) | 0.05* (0.04) | 0.04 (0.03) | 0.02 (0.06) | 0.06 (0.05) | 0.04 (0.05) | 0.04 (0.06) | 0.02 (0.03) | 0.07 (0.11) | 0.11 (0.06) | 0.10 (0.06) |
| 20:5 n-3 | 0.55 (0.31) | 0.51 (0.14) | 0.48 (0.11) | 0.51 (0.20) | 0.43 (0.27) | 1.39* (1.34) | 1.24* (0.94) | 1.89* (1.21) | 0.48 (0.40) | 2.77*† (2.03) | 2.30*† (2.26) | 2.43* (1.87) | 0.32 (0.19) | 4.14*¶ (2.21) | 4.14*† (1.52) | 3.62*†¶ (3.28) |
| 22:5 n-3 | 0.44 (0.19) | 0.43 (0.20) | 0.39 (0.14) | 0.42 (0.23) | 0.37 (0.15) | 0.44 (0.36) | 0.46* (0.42) | 0.60 (0.40) | 0.36 (0.20) | 0.76* (0.29) | 0.86*† (0.22) | 0.75* (0.50) | 0.31 (0.25) | 0.77* (0.21) | 0.82* (0.37) | 0.82* (0.54) |
| 22:6 n-3 | 8.5 (3.6) | 7.1 (2.3) | 7.2 (2.6) | 8.6 (1.8) | 7.9 (2.4) | 9.7 (2.3) | 10.0 (3.4) | 10.7* (2.4) | 9.4 (4.8) | 12.7*† (5.1) | 11.8* (3.0) | 10.4 (4.6) | 6.0 (3.1) | 11.5*† (5.1) | 12.8*† (6.3) | 11.8*†¶ (6.4) |
median (interquartile range).
SFA, saturated fatty acids; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids
Bold numbers indicate statistical significance, relative to week 0, within each arm as determined by Wilcoxon Signed Rank tests (unadjusted p<0.05).
Within each time-point (week 0, 4, 8 and 12), the difference among arms was assessed by pairwise Wilcoxon Mann-Whitney tests with the Benjamini-Horchberg adjustment for multiple comparisons;
P<0.05 versus placebo;
P<0.05 versus 1 g/d;
P<0.05, versus 2 g/d.
Figure 1.
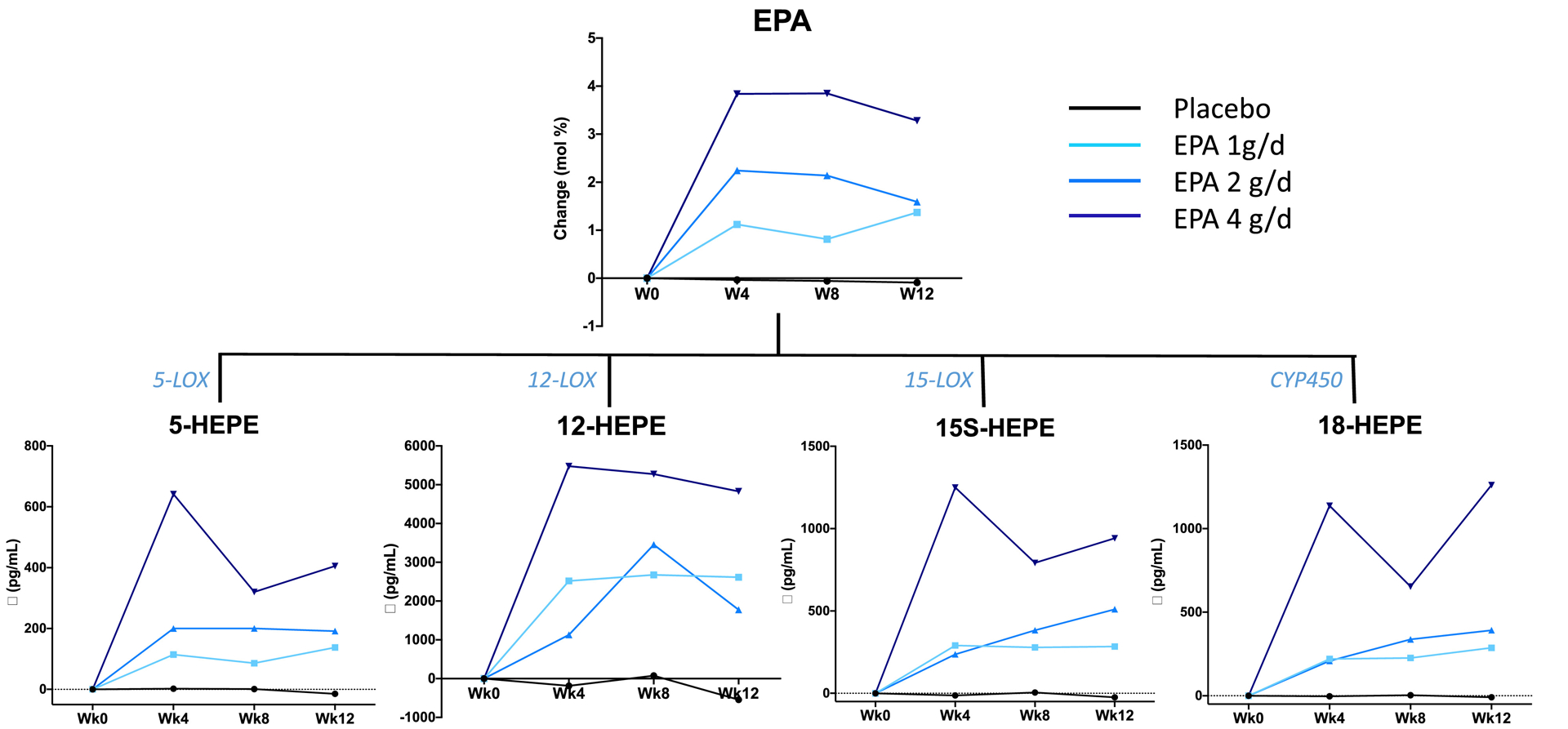
Median changes in plasma EPA and EPA-derived lipid mediators at weeks 4, 8 and 12, relative to week 0, by treatment arm. The enzymes involved in the synthesis of the lipid mediators are indicated.
DPA can be generated by elongation of EPA and the supplementation capsules contained approximately 150 mg DHA. Therefore these n-3 fatty acids were assessed in plasma. DPA and DHA levels were unaffected in the placebo group. Relative to week 0, DPA significantly increased in all EPA treatment arms at week 4 and remained significantly elevated up to week 12 (Table 2). At week 12, DPA was significantly higher in the 2 g/d and 4 g/d arms than placebo. DHA also increased in all supplementation arms relative to week 0. Relative to placebo, at 12 weeks DHA levels were significantly higher in the 4 g/d arm than in the other arms.
The increase in the proportion of n-3 fatty acids during EPA supplementation occurred concomitantly with a decrease in other fatty acids in plasma, especially AA, which was significantly reduced in all EPA treatment arms at weeks 4 and 8, relative to week 0 (Table 2). Only the 2 g/d EPA group showed a significant reduction in AA at 12 weeks. However, there was no dose-dependent effect of EPA supplementation on AA. No change in AA was observed in the placebo arm.
1.6. Plasma lipid mediators and SPM
The n-3 and n-6 fatty acid-derived lipid metabolites that were detected in all or the majority (> 80%) of plasma samples at all time-points are shown in Table 3. No change in EPA-, DHA- and AA-derived metabolites was observed in the placebo arm. A significant increase in plasma levels of EPA lipid mediators occurred in all EPA supplementation arms, starting from week 4 and remained relatively stable up to week 12 (Table 3 and Figure 1). The effect of the 4 g/d dose on EPA-derived lipid mediators at 12 weeks was significantly greater than in all other treatment arms (Table 4); the increase in these lipid metabolites did not significantly differ between the 1 g/d and 2 g/d arms. In addition, when all the treatment arms were pooled, we found that at week 12 plasma concentrations of 18-HEPE, 15-HEPE, 12-HEPE and 5-HEPE were significantly associated with plasma abundance of EPA (Rho=0.75, 0.69, 0.64 and 0.71, respectively, all p<0.001) (Figure 2). Of those, 18-HEPE showed a somewhat stronger association. Similar association were also detected at week 4 (Rho=0.66, 0.69, 0.55 and 0.79, respectively, p<0.001) and week 8 (Rho=0.59, 0.70, 0.59 and 0.73, respectively, p<0.001).
Table 3.
Plasma concentrations of lipid mediators (pg/mL, unless otherwise noted) derived from EPA, DHA and AA, by treatment arm.1
| Placebo (n=10) | EPA 1 g/d (n=11) | EPA 2g/d (n=11) | EPA 4 g/d (n=10) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wk0 | Wk42 | Wk8 | Wk12 | Wk0 | Wk4 | Wk8 | Wk12 | Wk0 | Wk4 | Wk8 | Wk12 | Wk0 | Wk4 | Wk82 | Wk12 | |
| EPA | ||||||||||||||||
| 5-HEPE | 84 (35) | 57 (7) | 62 (35) | 61 (21) | 48 (28) | 172 (111) | 158 (80) | 208 (214) | 53 (25) | 244 (152) | 251 (178) | 255 (329) | 67 (60) | 721 (444) | 438 (369) | 506 (218) |
| 11-HEPE | 58 (29) | 51 (22) | 38 (32) | 37 (26) | 42 (53) | 266 (259) | 317 (222) | 223 (281) | 42 (37) | 275 (450) | 247 (458) | 433 (359) | 49 (36) | 987 (588) | 643 (318) | 779 (565) |
| 12-HEPE3 | 1.2 (1.3) | 1.0 (0.5) | 1.7 (2.0) | 1.0 (2.3) | 0.5 (2.2) | 3.0 (6.7) | 4.8 (7.4) | 3.1 (4.6) | 1.8 (3.2) | 4.0 (4.6) | 4.5 (5.6) | 3.2 (6.9) | 0.7 (0.4) | 8.1 (13.2) | 6.3 (4.8) | 7.7 (12.6) |
| 15-HEPE | 108 (61) | 91 (72) | 108 (27) | 95 (112) | 83 (48) | 331 (217) | 339 (407) | 366 (353) | 105 (117) | 386 (529) | 521 (447) | 539 (705) | 79 (55) | 1340 (1102) | 875 (445) | 1024 (687) |
| 18-HEPE | 62 (30) | 57 (26) | 57 (34) | 52 (22) | 47 (21) | 289 (350) | 285 (332) | 359 (218) | 50 (48) | 274 (230) | 377 (474) | 431 (457) | 49 (25) | 1251 (1003) | 690 (1318) | 1354 (1675) |
| DHA | ||||||||||||||||
| 4-HDHA | 71 (33) | 59 (34) | 67 (38) | 61 (32) | 61 (37) | 88 (53) | 86 (40) | 91 (84) | 53 (35) | 145 (62) | 112 (65) | 121 (57) | 60 (90) | 198 (232) | 161 (92) | 193 (92) |
| 7-HDHA | 22 (12) | 24 (17) | 21 (14) | 19 (8) | 19 (8) | 31 (17) | 36 (20) | 41 (39) | 16 (20) | 49 (29) | 46 (21) | 43 (31) | 27 (17) | 109 (103) | 73 (20) | 84 (45) |
| 13-HDHA | 143 (288) | 102 (100) | 122 (100) | 152 (138) | 190 (285) | 240 (575) | 415 (474) | 288 (316) | 141 (171) | 368 (362) | 222 (307) | 253 (344) | 104 (145) | 513 (512) | 316 (211) | 372 (547) |
| 14-HDHA2 | 2.9 (2.1) | 2.1 (2.1) | 2.6 (2.7) | 2.2 (4.2) | 1.6 (3.6) | 3.4 (4.8) | 3.8 (5.2) | 3.0 (4.3) | 2.9 (5.3) | 2.3 (3.1) | 4.4 (3.8) | 2.5 (3.7) | 1.7 (1.2) | 3.2 (5.3) | 2.9 (2.5) | 4.2 (4.8) |
| 17-HDHA | 27 (30) | 28 (8) | 27 (15) | 35 (28) | 28 (18) | 44 (37) | 62 (62) | 59 (26) | 32 (41) | 60 (62) | 64 (42) | 63 (79) | 32 (12) | 93 (91) | 57 (38) | 88 (58) |
| RvD14 | 103 (65) | 71 (53) | 81 (49) | 110 (108) | 74 (37) | 73 (83) | 97 (99) | 70 (188) | 91 (135) | 76 (82) | 75 (325) | 156 (131) | 78 (110) | 75 (97) | 108 (144) | 125 (247) |
| AA | ||||||||||||||||
| 5-HETE | 548 (560) | 603 (362) | 474 (246) | 400 (146) | 438 (244) | 354 (125) | 310 (191) | 359 (211) | 332 (178) | 311 (161) | 238 (224) | 319 (114) | 677 (1089) | 501 (278) | 397 (231) | 356 (178) |
| 11-HETE3 | 1.9 (1.8) | 1.0 (0.5) | 1.1 (1.1) | 1.1 (1.4) | 1.3 (2.9) | 1.7 (2.4) | 2.6 (3.4) | 1.3 (0.9) | 1.1 (1.1) | 0.8 (1.1) | 0.8 (1.5) | 0.9 (1.1) | 1.6 (2.7) | 1.2 (1.8) | 0.9 (0.6) | 1.1 (1.2) |
| 12-HETE3 | 13.2 (6.7) | 7.7 (14.7) | 10.3 (10.4) | 9.7 (14.9) | 5.9 (23.7) | 9.8 (13.9) | 11.9 (18.2) | 6.2 (16.3) | 10.6 (18.4) | 4.5 (3.4) | 7.6 (10.2) | 8.8 (8.7) | 10.6 (6.7) | 5.3 (7.2) | 4.2 (6.4) | 7.6 (11.9) |
| 15-HETE3 | 1.4 (0.4) | 1.1 (0.4) | 1.1 (0.5) | 1.3 (0.6) | 0.9 (1.2) | 1.2 (0.8) | 1.5 (1.3) | 1.2 (0.8) | 1.3 (1.2) | 0.8 (0.5) | 1.0 (1.1) | 1.0 (1.0) | 1.4 (1.0) | 0.8 (0.9) | 0.8 (0.3) | 1.1 (0.5) |
| PGD24 | 243 (269) | 185 (208) | 121 (234) | 200 (232) | 271 (241) | 323 (354) | 428 (497) | 172 (123) | 219 (189) | 102 (218) | 203 (314) | 132 (63) | 138 (200) | 145 (318) | 150 (94) | 305 (227) |
| PGE23,4 | 0.8 (1.9) | 0.9 (0.9) | 0.5 (2.2) | 0.9 (1.6) | 1.0 (1.8) | 1.3 (1.8) | 1.8 (3.0) | 0.9 (0.5) | 0.5 (1.0) | 0.6 (0.9) | 0.4 (1.3) | 0.6 (0.3) | 1.0 (2.9) | 0.8 (1.9) | 0.6 (1.3) | 0.8 (1.6) |
| TXB23 | 1.9 (2.8) | 1.8 (2.6) | 1.3 (3.7) | 2.5 (3.8) | 2.1 (5.8) | 3.0 (3.8) | 2.9 (6.1) | 1.8 (2.6) | 1.0 (3.7) | 0.8 (1.1) | 1.2 (2.3) | 1.2 (1.5) | 2.2 (2.6) | 1.2 (2.9) | 1.5 (1.0) | 3.1 (3.7) |
median (interquartile range);.
data in n=9;
ng/mL;
detected in >80% of plasma samples
Bold numbers indicate statistical significance, relative to week 0, within each arm as determined by Wilcoxon Signed Rank tests (unadjusted p<0.05).
Table 4.
Changes in plasma EPA (mol %) and EPA-derived lipid mediators (pg/mL) at week 4, 8 and 12, relative to week 0, by treatment arm.
| Week 4 | Week 8 | Week 12 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | EPA 1 g/d | EPA 2 g/d | EPA 4 g/d | Placebo | EPA 1 g/d | EPA 2 g/d | EPA 4 g/d | Placebo | EPA 1 g/d | EPA 2 g/d | EPA 4 g/d | |
| 8 | 12 | 11 | 10 | 8 | 12 | 11 | 9 | 8 | 12 | 11 | 10 | |
| n (SPM) | 9 | 11 | 11 | 10 | 10 | 11 | 11 | 9 | 10 | 11 | 11 | 10 |
| EPA | −0.03 (0.2) | 1.1 (1.1)* | 2.2 (1.7)* | 3.8 (2.0)*†¶ | −0.06 (0.1) | 0.8 (1.1)* | 2.1 (2.2)*† | 3.9 (1.4)*† | −0.1 (0.2) | 1.4 (1.3)* | 1.6 (1.6)*† | 3.3 (2.8)*†¶ |
| 5_HEPE | 2 (44) | 114 (98)* | 200 (146)* | 642(469)*†¶ | 1 (46) | 86 (65)* | 200 (166)* | 320 (443)*†¶ | −15 (45) | 138 (165)* | 191 (321)* | 405 (380)*†¶ |
| 11_HEPE | −7 (34) | 247 (226)* | 170 (417)* | 908 (610)*†¶ | −19 (25) | 223 (165)* | 202 (482)* | 575 (327)*†¶ | −26 (17) | 156 (193)* | 377 (360)* | 710 (579)*†¶ |
| 12_HEPE2 | −0.2 (1.1) | 2.5 (4.2)* | 1.1 (3.3) | 5.5 (9.6)* | 0.08 (0.8) | 2.7 (6.3)* | 3.5 (5.7)* | 5.3 (3.9)* | −0.5 (1.0) | 2.6 (3.1)* | 1.8 (6.7)* | 4.8 (8.2)* |
| 15_HEPE | −13 (40) | 290 (193)* | 236 (466)* | 1250 (796)*†¶ | 5 (51) | 278 (284)* | 382 (520)* | 792 (294)* | −25 (57) | 284 (305)* | 510 (771)* | 942 (694)*†¶ |
| 18 HEPE | −3 (23) | 220 (345)* | 208 (308)* | 1137 (992)*†¶ | 3 (15) | 226 (357)* | 337 (526)* | 653 (1258)*†¶ | −9 (25) | 287 (223)* | 392 (477)* | 1261 (1629)*†¶ |
| DHA | −0.1 (2.5) | 1.0 (1.5)* | 3.5 (3.1) | 4.7 (3.2)*†¶ | −0.2 (2.7) | 1.8 (1.5) | 3.4 (3.2)* | 6.6 (2.8)*† | −0.3 (1.6) | 2.4 (1.6) | 1.2 (4.0)* | 6.5 (3.2)*† |
| 4_HDHA | −18 (40) | 27 (27) | 73 (59) | 126 (263) | −4 (49) | 25 (48) | 35 (104) | 73 (87) | −16 (49) | 35 (57) | 50 (54) | 88 (131) |
| 7_HDHA | 2 (11) | 20 (11)* | 22 (24)* | 92 (127)* | −2 (10) | 10 (21) | 24 (23) | 41 (33) | −3 (9) | 16 (34)* | 21 (31)* | 47 (78)* |
| 13_HDHA | −64 (277) | 33 (176) | 190 (239)* | 438 (336)*†¶ | −18 (109) | 61 (318) | 180 (416) | 172 (188) | −54 (111) | 45 (183) | 53 (258)* | 303 (316)* |
| 14_HDHA2 | −1.1 (3.5) | 0.9 (1.2) | 0.6 (4.7) | 1.4 (3.0) | 0.4 (3.2) | 0.7 (1.7) | 0.9 (5.4) | 1.2 (1.8) | 0.2 (3.3) | 1.5 (1.7) | 1.3 (0.6) | 1.8 (3.5) |
| 17_HDHA | 2 (16) | 13 (13)* | 8 (41) | 41 (44)* | −4 (23) | 16 (29) | 25 (49) | 26 (14) | 0 (31) | 25 (30) | 28 (48) | 42 (48) |
| RvD1 | −17 (44) | 12 (84) | 7 (89) | −14 (46) | −24 (32) | 17 (89) | −3 (168) | 27 (165) | −12 (126) | 21 (82) | 84 (166) | 8 (242) |
| AA | −0.2 (2.7) | −1.2 (3.6) | −1.4 (2.0) | −2.5 (1.7) | −0.8 (2.3) | −1.6 (2.4) | −1.1 (2.3) | −2.2 (3.0) | −0.2 (0.7) | −1.1 (1.5) | −1.5 (2.2) | −1.8 (2.6) |
| 5_HETE | −143 (439) | −60 (314) | −1 (95) | −59 (476) | −26 (238) | −110 (323) | −67 (228) | −356 (1039) | −134 (555) | −84 (347) | −45 (156) | −187 (1260) |
| 11_HET E2 | −0.6 (1.6) | −0.1 (0.9) | −0.2 (1.0) | −0.1 (0.9) | −0.3 (0.9) | −0.2 (1.0) | 0.1 (1.2) | −1.0 (2.4) | −0.3 (1.2) | −0.4 (1.2) | −0.2 (0.9) | −0.2 (1.7) |
| 12_HETE2 | −1.9 (8.9) | −0.3 (8.1) | 0.7 (16.9) | −3.6 (8.3) | −1.2 (7.2) | 1.2 (7.0) | −0.7 (10.8) | −5.9 (7.6) | 0.7 (10.6) | −0.3 (8.6) | 0.2 (14.2) | −1.43 (10.9) |
| 15_HETE2 | −0.2 (0.9) | −0.07 (0.4) | 0.08 (0.6) | −0.2 (0.6) | −0.2 (1.0) | −0.02 (0.4) | −0.2 (0.7) | −0.8 (0.7) | −0.2 (1.2) | 0.01 (0.7) | 0.1 (0.8) | −0.4 (0.9) |
| PGD2 | −13 (179) | −20 (238) | −30 (95) | −4 (270) | −32 (80) | −35 (354) | −17 (254) | −10 (184) | 21 (106) | −15 (100) | −123 (133) | 198 (319) |
| PGE22 | −0.8 (0.8) | −0.2 (1.1) | −0.1 (0.6) | −0.3 (0.9) | 0.07 (0.4) | −0.3 (1.1) | −0.09 (1.1) | −0.7 (2.3) | 0.1 (1.0) | −0.4 (0.9) | −0.1 (0.7) | −0.3 (1.6) |
| TXB22 | −0.3 (1.4) | −0.4 (3.5) | −0.02 (3.0) | −0.4 (1.6) | 0.05 (1.5) | −0.2 (3.8) | −0.02 (1.7) | −1.96 (2.0) | 0.7 (1.4) | −0.5 (4.6) | −0.2 (2.8) | 0.1 (3.4) |
| LTB4 | −22 (19) | −4 (31) | −8 (5) | 3 (165) | −6 (14) | −22 (13) | −8 (15) | −63 (134) | −19 (588) | −8 (20) | −4 (23) | −2 (136) |
Data presented as median (interquartile range);
pg/mL
Within each time-point, the difference among arms was assessed by pairwise Wilcoxon Mann-Whitney tests with the Benjamini-Horchberg adjustment for multiple comparisons;
P<0.05 versus placebo;
P<0.05 versus 1 g/d;
P<0.05, versus 2 g/d.
Figure 2.

Associations of plasma EPA (mol%) with the plasma concentration of EPA-derived lipid mediators at the end of treatment (week 12).
Modest increases in DHA-derived metabolites were observed during EPA supplementation, without a clear time and dose effect (Table 3 and Figure 3). Specifically, 17-HDHA, the precursors of RvDs, was significantly elevated at week 12 relative to week 0 in all treatment arms. Significant increases in 14-HDHA, the precursor of Mar1, were detected in the 1 g/d and 4 g/d arms at 12 weeks. The other DHA-derived lipid mediators, including 4-HDHA, 7-HDHA and 13-HDHA, were significantly increased at week 12 relative to week 0 in the 2 g/d and 4 g/d arms. The modest effect of supplementation on DHA-derived lipid mediators is also evident in Table 4, where a significant increase in 17-HDHA levels compared to placebo was observed at week 4 in the 1 and 4 g/d arms, but not at week 8 and 12. 14-HDHA changes were not significantly different from placebo in all EPA arms.
Figure 3.

Median changes in plasma DHA and DHA-derived lipid mediators at weeks 4, 8 and 12, relative to week 0, by treatment arm. The enzymes involved in the synthesis of the lipid mediators are indicated.
There was a limited effect of EPA supplementation on AA-derived lipid mediators, with significant reductions in 5-HETE, 12-HETE and 15-HETE concentrations observed only in the 4 g/d arm at week 8 (Table 3 and Figure 4). Moreover, there was no significant change in AA-derived intermediate mediators in the EPA treatments versus placebo (Table 4).
Figure 4.
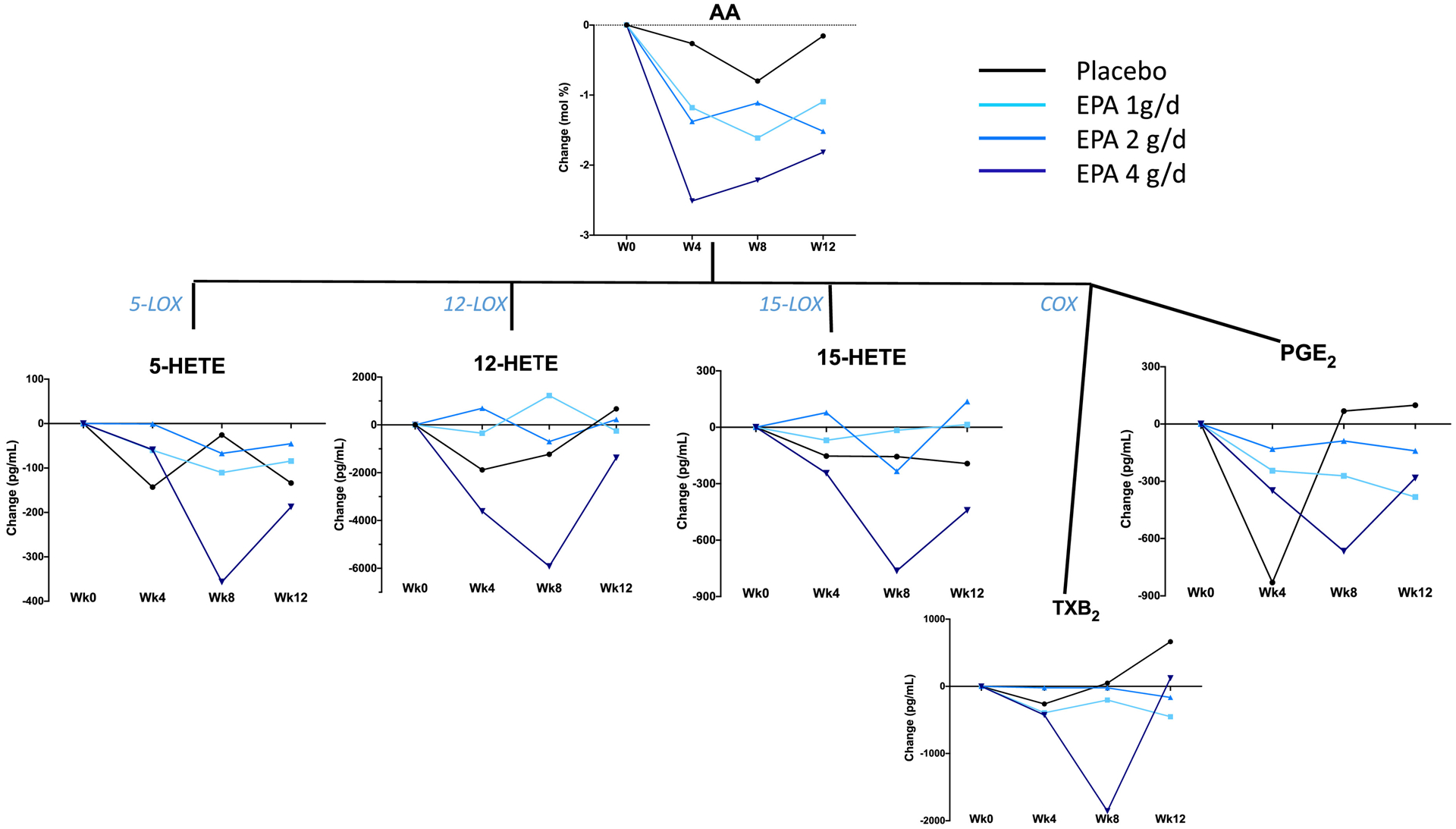
Median changes in plasma AA and AA-derived lipid mediators and eicosanoids at weeks 4, 8 and 12, relative to week 0, by treatment arm. The enzymes involved in the synthesis of the lipid mediators are indicated.
Despite the profound increase in the precursor molecule 18-HEPE, plasma levels of EPA-derived SPMs remained undetectable in some of the subjects following the EPA supplementation. Of note, however, RvE2 showed a dose-dependent increase in plasma concentrations following supplementation (Figure 5). In addition, there was a clear dose-dependent increase in the number of subjects with detectable RvE3 and, furthermore, a trend for a time-dependent effect within the 4 g/d group with progressively higher levels of RvE3 over the course of the supplementation period (Figure 5). RvD1 levels were not affected by EPA supplementation (Table 3). Of the other DHA-derived SPMs, RvD5, PD1 and Mar1 were detected in approximately 50% of plasma samples without clear time or treatment dose effect (Figure 5). Unexpectedly, lipoxin B4 (LXB4), an AA-derived SPM with pro-resolving actions, was rarely detected at week 0 in all treatment arms, but became detectable, dose-dependently, across the 12 weeks of EPA supplementation (Figure 5).
Figure 5.

Plasma levels of SPMs derived from EPA (Panel A), DHA (panel B), and AA (Panel C) in the placebo and the EPA treatment arms. SPMs were variably detected, usually in less than 80% of plasma samples. Only subjects with detectable levels of each SPM are shown in the figure. Medians are based on detectable levels only.
2. Discussion
The discovery of SPMs derived from EPA and DHA has opened a new area of research in inflammation, specifically the resolution phase of inflammation and the mechanisms by which these SPMs function (5,8,9,18). The novel aspect of our study is the assessment of the time- and dose-dependent effects of EPA supplementation on plasma levels of lipid mediators and SPMs in overweight/obese MDD subjects with chronic inflammation. Using an EPA supplement with an EPA:DHA ratio of 3.9:1, we found that all three doses, 1 g/d, 2 g/d and 4 g/d, were effective in increasing both plasma EPA and EPA-derived lipid mediators in a dose-dependent fashion, relative to placebo, with most of the increases already occurring by week 4, the first on-treatment time-point.
In a recent study conducted in healthy volunteers receiving different doses of a marine oil containing EPA, DHA, AA, and several SPM precursors, it was shown that plasma lipid mediators and SPMs increased progressively to peak at 2–3 hr post-administration and returned to baseline 24 hrs after administration (26). In our study, most of the changes in fatty acids and lipid mediators were observed at week 4 and maintained until week 12. Differently from our study, another study reported a linear increase in EPA and lipid mediators following fish oil supplementation over a period of 12 months (27). However, in that study, EPA was assessed only in plasma phosphatidylcholine, not in total plasma lipid pool. Unlike intermediate lipid mediators, EPA-derived SPMs, and RvE3 in particular, increased time- and dose-dependently during the 4 g/d dose EPA supplementation. This suggests that a prolonged EPA supplementation may be required to achieve sustained elevations in plasma SPMs. RvE3, the most recently discovered RvE synthesized from 18-HEPE via the 15-lipoxygenase (15-LOX) pathway (28), has been shown to reduce airway inflammation in an animal model of asthma, leading to improved airway hyper-responsiveness (29). Recently, RvE3 was also shown to reduce symptoms of depression caused by LPS administration in mice (30). Considering that the plasma levels of the RvE3 precursor 18-HEPE had reached a plateau by week 4, the progressive increase in the number of subjects with detectable plasma RvE3 as well as its plasma concentrations over time may be explained by increased synthesis or reduced degradation. A similar, though less pronounced, trend was also observed for RvE2. Taken together, these observations suggest that at least 8 to 12 weeks may be necessary to obtain clinical benefits from EPA supplementation through enhanced RvE-driven resolution of inflammation.
It should be pointed out that the study participants at baseline had chronic low-grade inflammation as per the inclusion criterion of hs-CRP, ≥ 3 μg/mL. It has been suggested that plasma levels of SPMs derived from n-3 fatty acids are lower in subjects with chronic inflammation, relative to healthy subjects, possibly due an impaired ability to synthesize pro-resolving mediators (31). The 4 g/d dose in our study shows that increased substrate availability may overcome defects in EPA metabolism in subjects with chronic inflammation. In a recent large randomized placebo-controlled clinical trial conducted in patients with established cardiovascular disease or increased risk of cardiovascular disease and on a statin, treatment with 4 g/d EPA significantly reduced the risk of cardiac events relative to placebo (Hazard ratio= 0.75) (32). This suggests that EPA doses higher than previously administered may be needed to reduce inflammation or activate the resolution of inflammation.
In our study, plasma levels of DPA also increased following EPA supplementation. The changes in DPA were, however, modest relative to those observed for EPA. There is evidence that DPA can be generated by elongation of EPA (20,21), therefore it is likely that the increase in plasma DPA observed in the EPA arms is due to EPA elongation. The recently discovered DPA-derived SPMs RvD5n-3 DPA and MaR1n-3 DPA were also detected in plasma in the present study, but only in a limited number of subjects, with no apparent effect of EPA treatment (data not presented).
DHA was present in the supplementation capsules, corresponding to approximately 300 mg, 600 mg, and 1,200 mg DHA in the 1 g/d, 2 g/d and 4 g/d EPA supplementation arms, respectively. Plasma levels of DHA increased only modestly but in a dose-dependent manner, with the 4g/d arm reaching DHA levels above all other arms at week 12. Plasma 14-HDHA and 17-HDHA, the precursors of DHA-derived SPMs, increased variably among the treatment arms and we did not observe significant time- or dose-dependent changes in the DHA-derived SPMs RvD1, RvD5, PD1 and MaR1. In a study conducted in healthy volunteers receiving n-3 fatty acid supplementation equivalent to 1, 2 or 4 fish portions/week for 12 weeks, it was reported that 17-HDHA plasma levels increased dose-dependently (27). Although the 4 g/d dose in our study corresponds to approximately the 4 portions of fatty fish/week in the previous study, we had a smaller number of subjects and observed a greater inter-individual variation in plasma lipid mediators, which potentially explains the lack of significant effects of our supplementation on DHA-derived mediators. In a short-term supplementation trial of 1g EPA and 672 mg DHA, a large increase in EPA-derived lipid mediators but modest increase in DHA-derived lipid mediators were observed (33). Our observations, and those from the latter study, may support preferential use of EPA as substrate by lipoxygenases and CYP450, compared to DHA (34).
The increase in plasma EPA following EPA supplementation occurred at the expense of AA, a finding consistent with the literature (35). However, the reduction in plasma AA resulted only in modest changes in AA-derived metabolites: 5-HETE was significantly reduced only at week 8 in the 1 g/d and 4 g/d doses whereas 12-HETE and 15-HETE were significantly reduced at week 8 only in the 4 g/d dose. Some, but not all, previous n-3 fatty acid supplementation studies have also failed to show significant changes in AA metabolites (30,35,36). A novel observation in our study was the dose-dependent increase in the plasma levels of LXB4, an AA-derived SPM, in the context of a reduction in plasma levels of its precursor, 15-HETE. LXB4 inhibits inflammation and promotes resolution by counteracting the pro-inflammatory effects of leukotrienes and prostaglandins and stimulating the removal of apoptotic neutrophils by macrophages, respectively (37,38). LXB4 also mediates inhibition of cytokine secretion from stimulated T cells (39). In healthy subjects who received n-3 fatty acid supplementation after LPS challenge, LBX4 was one of several SPMs that increased (9). Of note, ours is the first report of an elevation in LXB4 concentrations after EPA supplementation without LPS challenge, in subjects with chronic inflammation. Since 12/15-lipoxygenases mediate the synthesis of LXB4 from AA (37), the metabolic pathway(s) responsible for the increase in LXB4 in the context of the concurrent reduction in AA and other AA-derived 12- and 15-LOX metabolites during the 4 g/d EPA dose merits further investigation. As for RvE3, the increase in LXB4 is under the regulation of 15-LOX activity, and may be due to preferential activation of this pathway, higher substrate preference of LOX for their precursors, or reduced degradation following EPA supplementation. The changes in PGE2 and TxB4, the AA-derived eicosanoids with pro-inflammatory activities, remained rather small during the EPA supplementation, in agreement with the variable and often non-significant changes in AA levels.
Despite the noticeable dose-dependent increases in EPA and EPA-derived lipid mediators during the EPA supplementation, we observed a large inter-individual variability in fatty acid, lipid mediator and SPM levels. This may be due in part to variations in genes coding for enzymes involved in lipid metabolism and eicosanoid production (40,41). There are several other limitations of this current study worth noting. Although we did perform a three-day food diary to assess diet prior to entry into the study, we asked subjects to maintain their usual diet thorough the study but did not monitor dietary intake at other time points in the study. A second limitation is that the sample size for each group is rather small; however, the consistent and dose-dependent increase in EPA and SPMs levels suggests the validity of these initial results. We acknowledge the need for the replication and extension of these findings in larger studies. In conclusion, the present study provides evidence of a dose effect of EPA supplementation on EPA-derived plasma levels of lipid mediators and SPMs in obese/overweight adults with MDD and chronic inflammation, with the 4 g/d dose eliciting the stronger increase in these metabolites. In addition, our data indicate more sustained increases in SPMs over time, especially at the highest dose of EPA.
Highlights.
EPA supplementation dose-dependently increases EPA-derived lipid mediators
A daily dose of 4 g EPA is associated with increases in EPA-derived resolvin E2 and E3
A daily dose of 4 g EPA is associated with increases in AA-derived lipoxin B4
Acknowledgments:
We would like to thank Dr. Wendy Weber for her contribution to the design of the study and oversight.
Funding: This work was supported by the National Institutes of Health grant UG3 AT008857.
Abbreviations:
- AA
arachidonic acid
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- HDHA
hydroxydocosahexaenoic acid
- HEPE
hydroxyeicosapentaenoic acid
- HETE
hydroxyeicosatetraenoic acid
- LT
leukotriene
- MDD
major depressive disorder
- PG
prostaglandin
- SPM
specialized pro-resolving lipid mediator
- Rv
resolvin
- TXs
thromboxane
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: Lamon-Fava S., So J, Rapaport MH, Kinkaid B, Shettler P and Maddipati KR report no conflict of interest. Mischoulon D has received research support from Nordic Naturals, consulted for Pharmavite LLC and Gnosis USA, Inc. Ziegler, TR is receiving research funding from Takeda. Dunlop B has received research support from Acadia, Compass, Aptinyx, Sage, and Takeda, and has served as a consultant to Greenwich Biosciences, Myriad Neuroscience, Otsuka, and Sophren Therapeutics. Felger J has received consulting fees from Otsuka. Fava M has consulted for Axsome, Benckiser Pharmaceuticals, Inc., BioClinica, Inc, Biogen, BioHaven, Cambridge Science Corporation, Cerecor, Gate Neurosciences, Inc., GenOmind, LLC, Gentelon, LLC, Happify, Johnson & Johnson, Lundbeck Inc., Marinus Pharmaceuticals, Methylation Sciences, Inc., Millennium Pharmaceutics, Inc. Minerva Neurosciences, Neuralstem, NeuroRX Inc., Novartis, Otsuka, Pfizer, Premiere Research International, Relmada Therapeutics Inc., Reckitt, Shenox Pharmaceuticals, Stanley Medical Research Institute (SMRI), Taisho, Takeda, Vistagen, National Institute of Drug Abuse (NIDA); National Institutes of Health (NIH), National Institute of Mental Health (NIMH), and PCORI.
Clinicatrial.gov registration NCT02553915.
References
- 1.Ross R Atherosclerosis--an inflammatory disease. The New England journal of medicine. 1999;340:115–26. [DOI] [PubMed] [Google Scholar]
- 2.Libby P, Okamoto Y, Rocha VZ and Folco E. Inflammation in atherosclerosis: transition from theory to practice. Circulation journal: official journal of the Japanese Circulation Society. 2010;74:213–20. [DOI] [PubMed] [Google Scholar]
- 3.Morris AA, Zhao L, Ahmed Y, Stoyanova N, De Staercke C, Hooper WC, Gibbons G, Din-Dzietham R, Quyyumi A and Vaccarino V. Association between depression and inflammation--differences by race and sex: the META-Health study. Psychosom Med. 2011;73:462–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kohler CA, Freitas TH, Maes M, de Andrade NQ, Liu CS, Fernandes BS, Stubbs B, Solmi M, Veronese N, Herrmann N, Raison CL, Miller BJ, Lanctot KL and Carvalho AF. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr Scand. 2017;135:373–387. [DOI] [PubMed] [Google Scholar]
- 5.Serhan CN and Savill J. Resolution of inflammation: the beginning programs the end. Nature immunology. 2005;6:1191–7. [DOI] [PubMed] [Google Scholar]
- 6.Merched AJ, Ko K, Gotlinger KH, Serhan CN and Chan L. Atherosclerosis: evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2008;22:3595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ariel A and Serhan CN. Resolvins and protectins in the termination program of acute inflammation. Trends in immunology. 2007;28:176–83. [DOI] [PubMed] [Google Scholar]
- 8.Buckley CD, Gilroy DW and Serhan CN. Proresolving lipid mediators and mechanisms in the resolution of acute inflammation. Immunity. 2014;40:315–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Norris PC, Skulas-Ray AC, Riley I, Richter CK, Kris-Etherton PM, Jensen GL, Serhan CN and Maddipati KR. Identification of specialized pro-resolving mediator clusters from healthy adults after intravenous low-dose endotoxin and omega-3 supplementation: a methodological validation. Scientific reports. 2018;8:18050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calder PC. Omega-3 fatty acids and inflammatory processes: from molecules to man. Biochem Soc Trans. 2017;45:1105–1115. [DOI] [PubMed] [Google Scholar]
- 11.Rapaport MH, Nierenberg AA, Schettler PJ, Kinkead B, Cardoos A, Walker R and Mischoulon D. Inflammation as a predictive biomarker for response to omega-3 fatty acids in major depressive disorder: a proof-of-concept study. Mol Psychiatry. 2016;21:71–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jahangard L, Sadeghi A, Ahmadpanah M, Holsboer-Trachsler E, Sadeghi Bahmani D, Haghighi M, Brand S. Influence of adjuvant omega-3-polyunsaturated fatty acids on depression, sleep, and emotion regulation among outpatients with major depressive disorders - Results from a double-blind, randomized and placebo-controlled clinical trial. J Psychiatr Res. 2018;107:48–56. [DOI] [PubMed] [Google Scholar]
- 13.Ginty AT, Conklin SM. Short-term supplementation of acute long-chain omega-3 polyunsaturated fatty acids may alter depression status and decrease symptomology among young adults with depression: A preliminary randomized and placebo controlled trial. Psychiatry Res. 2015;229:485–9 [DOI] [PubMed] [Google Scholar]
- 14.Guu TW, Mischoulon D, Sarris J, Hibbeln J, McNamara RK, Guu TW, Hamazaki K, Freeman MP et al. International Sociaty for Nutritional Psychiatry Research Practice Guidelines for omega-3 fatty acids in the treatment of Major Depressive Disorder. Psychoter Psychosom 2019:88:263–73., [DOI] [PubMed] [Google Scholar]
- 15.Liao Y, Xie B, Zhang H, He Q, Guo L, Subramaniapillai M, Fan B, Lu C and McLntyer RS. Efficacy of omega-3 PUFAs in depression: A meta-analysis. Transl Psychiatry. 2019;9:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bagga D, Wang L, Farias-Eisner R, Glaspy JA and Reddy ST. Differential effects of prostaglandin derived from omega-6 and omega-3 polyunsaturated fatty acids on COX-2 expression and IL-6 secretion. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:1751–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N and Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. The Journal of experimental medicine. 2000;192:1197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oh SF, Dona M, Fredman G, Krishnamoorthy S, Irimia D, Serhan CN. Resolvin E2 formation and impact in inflammation resolution. J Immunol. 2012;188:4527–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopategi A, Flores-Costa R, Rius B, Lopez-Vicario C, Alcaraz-Quiles J, Titos E and Claria J. Frontline Science: Specialized proresolving lipid mediators inhibit the priming and activation of the macrophage NLRP3 inflammasome. J Leukoc Biol. 2019;105:25–36. [DOI] [PubMed] [Google Scholar]
- 20.Goyens PL, Spilker ME, Zock PL, Katan MB and Mensink RP. Compartmental modeling to quantify alpha-linolenic acid conversion after longer term intake of multiple tracer boluses. Journal of lipid research. 2005;46:1474–83. [DOI] [PubMed] [Google Scholar]
- 21.Guo XF, Tong WF, Ruan Y, Sinclair AJ and Li D. Different metabolism of EPA, DPA and DHA in humans: A double-blind cross-over study. Prostaglandins, leukotrienes, and essential fatty acids. 2019:102033. [DOI] [PubMed] [Google Scholar]
- 22.Gobbetti T, Dalli J, Colas RA, Federici Canova D, Aursnes M, Bonnet D, Alric L, Vergnolle N, Deraison C, Hansen TV, Serhan CN and Perretti M. Protectin D1n-3 DPA and resolvin D5n-3 DPA are effectors of intestinal protection. Proceedings of the National Academy of Sciences of the United States of America. 2017;114:3963–3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Block G, Wakimoto P, Jensen C, Mandel S, Green RR. Validation of a food frequency questionnaire for Hispanics. Prev Chronic Dis. 2006. July;3:A77. [PMC free article] [PubMed] [Google Scholar]
- 24.Hellmuth C, Weber M, Koletzko B and Peissner W. Nonesterified fatty acid determination for functional lipidomics: comprehensive ultrahigh performance liquid chromatography-tandem mass spectrometry quantitation, qualification, and parameter prediction. Analytical chemistry. 2012;84:1483–90. [DOI] [PubMed] [Google Scholar]
- 25.Maddipati KR, Romero R, Chaiworapongsa T, Zhou SL, Xu Z, Tarca AL, Kusanovic JP, Munoz H and Honn KV. Eicosanomic profiling reveals dominance of the epoxygenase pathway in human amniotic fluid at term in spontaneous labor. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2014;28:4835–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Souza PR, Marques RM, Gomez EA, Colas RA, De Matteis R, Zak A, Patel M, Collier DJ and Dalli J. Enriched Marine Oil Supplements Increase Peripheral Blood Specialized Pro-Resolving Mediators Concentrations and Reprogram Host Immune Responses: A Randomized Double-Blind Placebo-Controlled Study. Circulation research. 2020;126:75–90. [DOI] [PubMed] [Google Scholar]
- 27.Ostermann AI, West AL, Schoenfeld K, Browning LM, Walker CG, Jebb SA, Calder PC and Schebb NH. Plasma oxylipins respond in a linear dose-response manner with increased intake of EPA and DHA: results from a randomized controlled trial in healthy humans. The American journal of clinical nutrition. 2019;109:1251–1263. [DOI] [PubMed] [Google Scholar]
- 28.Isobe Y, Arita M, Matsueda S, Iwamoto R, Fujihara T, Nakanishi H, Taguchi R, Masuda K, Sasaki K, Urabe D, Inoue M and Arai H. Identification and structure determination of novel anti-inflammatory mediator resolvin E3, 17,18-dihydroxyeicosapentaenoic acid. The Journal of biological chemistry. 2012;287:10525–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sato M, Aoki-Saito H, Fukuda H, Ikeda H, Koga Y, Yatomi M, Tsurumaki H, Maeno T, Saito T, Nakakura T, Mori T, Yanagawa M, Abe M, Sako Y, Dobashi K, Ishizuka T, Yamada M, Shuto S, Hisada T. Resolvin E3 attenuates allergic airway inflammation via the interleukin-23-interleukin-17A pathway. FASEB J. 2019;11:12750–12759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deyama S, Shimoda K, Ikeda H, Fukuda H, Shuto S and Minami M. Resolvin E3 attenuates lipopolysaccharide-induced depression-like behavior in mice. J Pharmacol Sci. 2018;138:86–88. [DOI] [PubMed] [Google Scholar]
- 31.Kooij G, Derada Troletti C, Leuti A, Norris PC, Riley I, Albanese M, Ruggieri S, Libreros S, van der Pol SMA, van Het Hof B, Schell Y, Guerrera G, Buttari F, Mercuri NB, Centonze D, Gasperini C, Battistini L, de Vries HE, Serhan CN, Chiurchiù V. Specialized pro-resolving lipid mediators are differentially altered in peripheral blood of patients with multiple sclerosis and attenuate monocyte and blood-brain barrier dysfunction. Haematologica. 2019;. pii: haematol.2019.219519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT Jr., Juliano RA, Jiao L, Granowitz C, Tardif JC, Ballantyne CM and Investigators R-I. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. The New England journal of medicine. 2019;380:11–22. [DOI] [PubMed] [Google Scholar]
- 33.Schuchardt JP, Schneider I, Willenberg I, Yang J, Hammock BD, Hahn A and Schebb NH. Increase of EPA-derived hydroxy, epoxy and dihydroxy fatty acid levels in human plasma after a single dose of long-chain omega-3 PUFA. Prostaglandins & other lipid mediators. 2014;109–111:23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fischer R, Konkel A, Mehling H, Blossey K, Gapelyuk A, Wessel N, von Schacky C, Dechend R, Muller DN, Rothe M, Luft FC, Weylandt K and Schunck WH. Dietary omega-3 fatty acids modulate the eicosanoid profile in man primarily via the CYP-epoxygenase pathway. Journal of lipid research. 2014;55:1150–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shearer CG, Harris WS, Pedersen TL, Newman JW. Detection of pmega-3 oxylipins in human plasma and response to treatment withomega-3 acid ethyl esters. J Lipid Res 2010;51:2074–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schebb NH, Ostermann AI, Yang J, Hammock BD, Hahn A, Schuchardt JP. Comparison of the effects of long-chain omega-3 fatty acid supplementation on plasma levels of free and esterified oxylipins. Prostaglandins Other Lipid Mediat. 2014;113–115:21–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Serhan CN. Lipoxins and aspirin-triggered 15-epi-lipoxins are the first lipid mediators of endogenous anti-inflammation and resolution. Prostaglandins, leukotrienes, and essential fatty acids. 2005;73:141–62. [DOI] [PubMed] [Google Scholar]
- 38.Parkinson JF. Lipoxin and synthetic lipoxin analogs: an overview of anti-inflammatory functions and new concepts in immunomodulation. Inflamm Allergy Drug Targets. 2006;5:91–106. [DOI] [PubMed] [Google Scholar]
- 39.Ariel A, Chiang N, Arita M, Petasis NA and Serhan CN. Aspirin-triggered lipoxin A4 and B4 analogs block extracellular signal-regulated kinase-dependent TNF-alpha secretion from human T cells. Journal of immunology. 2003;170:6266–72. [DOI] [PubMed] [Google Scholar]
- 40.Stephensen CB, Armstrong P, Newman JW, Pedersen TL, Legault J, Schuster GU, Kelley D, Vikman S, Hartiala J, Nassir R, Seldin MF and Allayee H. ALOX5 gene variants affect eicosanoid production and response to fish oil supplementation. Journal of lipid research. 2011;52:991–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berthelot CC, Kamita SG, Sacchi R, Yang J, Nording ML, Georgi K, Hegedus Karbowski C, German JB, Weiss RH, Hogg RJ, Hammock BD and Zivkovic AM. Changes in PTGS1 and LOX12 Gene Expression in Peripheral Blood Mononuclear Cells Are Associated with Changes in Arachidonic Acid, Oxylipins, and Oxylipin/Fatty Acid Ratios in Response to Omega-3 Fatty Acid Supplementation. PloS one. 2015;10:e0144996. [DOI] [PMC free article] [PubMed] [Google Scholar]


