Abstract
BACKGROUND:
Despite evidence suggesting detrimental effects of perioperative hyperoxia, hyperoxygenation remains commonplace in cardiac surgery. Hyperoxygenation may increase oxidative damage and neuronal injury leading to potential differences in postoperative neurocognition. Therefore, we tested the primary hypothesis that intraoperative normoxia, as compared to hyperoxia, reduces postoperative cognitive dysfunction in older patients having cardiac surgery.
METHODS:
We conducted a randomized double-blind trial in patients aged 65 years or older having coronary artery bypass graft (CABG) surgery with cardiopulmonary bypass (CPB). A total of 100 patients were randomized to one of two intraoperative oxygen delivery strategies. Normoxic patients (n=50) received a minimum fraction of inspired oxygen (FiO2) of 0.35 to maintain a PaO2 above 70 mmHg before and after CPB and between 100 and 150mmHg during CPB. Hyperoxic patients (n=50) received an FiO2 of 1.0 throughout surgery, irrespective of PaO2 levels. The primary outcome was neurocognitive function measured on postoperative day two using the Telephonic Montreal Cognitive Assessment. Secondary outcomes included neurocognitive function at one, three and six months, as well as postoperative delirium, mortality, and durations of mechanical ventilation, intensive care unit, and hospital stay.
RESULTS:
The median age was 71 years (interquartile range, 68–75) and the median baseline neurocognitive score was 17 (16–19). The median intraoperative PaO2 was 309 (285–352) in the hyperoxia group and 153 (133–168) mmHg in the normoxia group (p<0.001). The median Telephonic Montreal Cognitive Assessment score on postoperative day two was 18 (16–20) in the hyperoxia group and 18 (14–20) in the normoxia group (p=0.42). Neurocognitive function at one, three, and six months, as well as secondary outcomes, were not statistically different between groups.
CONCLUSIONS:
In this randomized controlled trial, intraoperative normoxia did not reduce postoperative cognitive dysfunction when compared to intraoperative hyperoxia in older patients having cardiac surgery. Although the optimal intraoperative oxygenation strategy remains uncertain, our results indicate that intraoperative hyperoxia does not worsen postoperative cognition after cardiac surgery.
INTRODUCTION
Each year over one million patients undergo cardiac surgery utilizing cardiopulmonary bypass (CPB)1 globally, with approximately 500,000 of those operations performed in the United States.2 Morbid neurological sequelae of cardiac surgery, including delayed neurocognitive recovery and postoperative neurocognitive disorder3 are common, with reported incidences of up to 80% at hospital discharge and 20–40% after six months.4 The more vulnerable older surgical patient increasingly comprises a larger subset of the cardiac surgical population and is at a substantially higher risk for developing long-term cognitive decline, reduced level of overall cognitive function, lower quality of life, and increased mortality.4–9
Oxygen, the most widely administered therapy in modern hospitals, has classically been administered liberally to avoid hypoxemia and maintain tissue oxygenation.10 However, the excessive use of oxygen leading to hyperoxia has recently been shown to be potentially injurious, especially in the context of ischemia-reperfusion injury.11–15 Cardiac surgery with CPB is associated with a profound exposure to ischemia-reperfusion, and patients in this high-risk setting are frequently treated with higher concentrations of oxygen to guard against myocardial and cerebral hypoxia.16,17 Given the burden of disease from postoperative neurocognitive disorders and the possible link between hyperoxia and poorer outcomes in cardiac surgery, a prospective investigation of regulated intraoperative normoxia to ameliorate postoperative neurocognitive disorders following cardiac surgery is warranted.18 Investigation into such a potentially simple cost-effective intervention could impact hundreds of thousands of cardiac surgery patients a year.
Therefore, we conducted a clinical trial with the objective of determining the effect of intraoperative normoxia versus hyperoxia on postoperative neurocognition. Our hypothesis was that titration of intraoperative oxygenation to achieve normoxia, as compared to standard practice hyperoxia, reduces postoperative cognitive dysfunction in older patients having cardiac surgery on postoperative day two. Secondarily we tested the hypotheses that intraoperative normoxia reduces the incidence, severity and duration of delirium, length of stay and time to extubation, and neurocognitive function at longitudinal follow-up to six months, when compared to intraoperative hyperoxia.
MATERIALS AND METHODS
Study Design
This parallel group randomized controlled trial enrolled patients at Beth Israel Deaconess Medical Center in Boston Massachusetts between July 2015 and July 2017. Institutional Review Board approval was obtained on February 2, 2015 from the Committee on Clinical Investigations and all patients provided written informed consent. Full details of the study protocol have been previously published.19 The trial was registered with ClinicalTrials.gov (NCT02591589, https://clinicaltrials.gov/ct2/show/NCT02591589, Principal Investigator: Shahzad Shaefi, Registration Date: October 29, 2015). The study protocol is available on ClinicalTrials.gov. The trial was designed to assess the effect of two intraoperative oxygen titration strategies, namely hyperoxia and normoxia, with postoperative neurocognitive function among older patients having cardiac surgery.
Study Participants
Patients 65 years or older having elective or urgent coronary artery bypass graft (CABG) surgery requiring CPB were eligible for trial inclusion. Patients who were undergoing emergent CABG, procedures requiring single lung ventilation, off-pump CABG, or patients with signs of cardiogenic shock, as dictated by preoperative inotropic, intra-aortic balloon counterpulsation, or mechanical circulatory support, were excluded. Non-English speaking patients were excluded because the neurocognitive assessments could not be administered in languages other than English.
Randomization and Masking
Patients were randomized using a permuted block randomization schedule with block sizes of four, in which patients were randomly assigned in a 1:1 allocation to the normoxia or hyperoxia arm. Randomization assignments were allocated using sealed sequentially numbered opaque envelopes. The randomization schema was generated by a unblinded statistician and concealed from study investigators. At the time of randomization the anesthesiologist would open the next sequentially numbered envelope to obtain the randomization assignment. Study team members assessing neurocognitive function and postoperative clinical outcomes were blinded to patient arm, as were patients. Surgeons, anesthesiologists and perfusionists involved in providing clinical care intraoperatively were not blinded due to the need for protocol adherence.
Study Procedures
Study ventilator settings were applied after induction of general anesthesia and successful endotracheal intubation and continued throughout surgery. In the normoxia group, the fraction of inspired oxygen (FiO2) was set at a minimum of 0.35 to maintain an arterial partial pressure of oxygen (PaOO2) above 70 mmHg or oxygen saturations (SpO2) greater than or equal to 92%. If required, the FiO2 was titrated up to prevent hypoxemia (SpO2 < 92%) during the pre- and post-bypass periods. During CPB, a blended air/oxygen mixture was titrated to arterial blood gas analysis in order to maintain the PaO2 between 100 and 150 mmHg. In the hyperoxic arm, the FiO2 was set at 1.0 throughout the intraoperative period including CPB. Both groups received an anesthetic regimen at the discretion of the treating provider. Mechanical ventilation was based on the institutional standard of care, with a tidal volume of 6 to 8 ml/kg (employing ideal body weight) and positive end expiratory pressure of 5 cmH20.
Outcomes
The primary outcome was postoperative cognition, as assessed by the Telephonic Montreal Cognitive Assessment score on postoperative day two. Cognitive function was measured preoperatively as baseline and subsequently postoperatively daily until discharge unless the participant was in the intensive care unit (ICU) and non-verbal. The telephonic assessment is an adaptation of the Montreal Cognitive Assessment, a validated screening instrument that is highly sensitive for detecting mild cognitive impairment.20–23 The Telephonic Montreal Cognitive Assessment is evaluated on a 22-point scale, with lower scores indicating worse cognitive status. The assessment comprises an aggregate score of individual assessments of attention and concentration, executive functions, memory, language, conceptual thinking, calculations, and orientation. The items do not require writing or visual cues to complete, and therefore can be easily adapted to either in-person or telephone administration. In addition to the primary outcome, neurocognitive scores at one, three and six months were evaluated via telephone interviews as secondary outcomes. Patients were contacted by telephone for one, three, and six-month assessments. Research staff attempted to reach patients until their call window expired (before or after seven days for the one month follow up call and before or after fourteen days for the three and six month follow up) or after ten attempts. The rationale for using the abbreviated Telephonic Montreal Cognitive Assessment was to facilitate the use of a consistent scale continuously throughout the study from the inpatient, in person assessments to over the phone assessments at one, three and six months.
Additional secondary outcomes included the incidence and severity of postoperative delirium, time to extubation, days of mechanical ventilation, length of ICU and hospital stay, and mortality at thirty days and six months. Postoperative delirium was assessed each postoperative day until discharge with the Confusion Assessment Method or Confusion Assessment Method-ICU for non-verbal (intubated) patients. As with the Telephonic Montreal Cognitive Assessment, delirium assessments were administered by study staff members who were blinded to group assignment. Delirium severity was scored using the long Confusion Assessment Method Severity score, which assigns points from 0 to 19, with worsening delirium characterized by higher scores.24 The worst Confusion Assessment Method Severity score for their hospital stay was analyzed. Time to extubation was reported as the number of hours from when patients were initially intubated for surgery to when they were last extubated. Hospital length of stay was defined as the number of days spent in the hospital after surgery and ICU length of stay was defined as the number of days spent in the ICU prior to transfer to the general inpatient cardiac surgery ward.
Adverse Events
Because the patient population under study is by definition critically ill, we collected data on serious adverse events and unexpected non-serious adverse events that were possibly related to the study. Patients were assessed daily while in the hospital for a maximum of three days postoperatively. Additional adverse outcome data was collected from the Society of Thoracic Surgeons database including sternal wound infection, renal failure, myocardial infarction, reoperation, and stroke. This trial did not include any interim analyses to stop for safety, efficacy, or futility, thus the trial was not stopped early, nor was there a dedicated data safety monitoring board. Adverse event monitoring was performed by members of the study team and reviewed by the Principal Investigator at regular intervals.
Sample Size Calculation
The minimal clinically important difference on the Mini-Mental State Examination in postoperative change following cardiac surgery has been shown to be two points, with postoperative day two reported as the in-hospital postoperative nadir time point.25 As there is no reported minimal clinically important difference for the Telephonic Montreal Cognitive Assessment, we utilized two points as our minimal clinically important difference based on prior validated crosswalk methods.26,27 Using a two-sided alpha of 0.05 and 80% power, we determined that a sample size of 74 patients was needed in order to detect a mean difference in Telephonic Montreal Cognitive Assessment scores of two points (standard deviation of 3) between the hyperoxia and normoxia groups. To allow for potential longer term attrition from loss to follow up or withdrawal, a total of 100 participants were enrolled and underwent surgery.
Statistical Analysis
Descriptive statistics of the data are presented as means ± standard deviation, medians (interquartile range), or counts and proportions depending on variable type and distribution. Normality of continuous data was assessed with the Shapiro-Wilk test. Differences in continuous variables were assessed using independent sample t-tests or Wilcoxon Rank-Sum tests as appropriate. Differences in categorical data were assessed with a chi-square or Fisher’s Exact test. Our primary outcome, the Telephonic Montreal Cognitive Assessment score on postoperative day two, was assessed with a non-parametric Wilcoxon Rank-Sum test. The Hodges-Lehmann Estimation of Shift is reported as the location shift and associated 95% confidence interval (CI). Differences in neurocognition at the follow up time periods was also assessed between groups with the use of a Wilcoxon-Rank-Sum test. In a post-hoc analysis the incidence of delirium was assessed among all participants, and the time to delirium and delirium severity was reported among only those who developed delirium. Full details of the statistical analysis plan were previously published in the protocol paper.19 SAS 9.4 (SAS Institute Inc. Cary, NC) was utilized for analysis. For all analyses two-sided p-values < 0.05 were considered statistically significant.
RESULTS
Study Population
A total of 492 patients were screened, of whom 343 met eligibility criteria. A total of 100 patients were randomized, received the study intervention, and analyzed (Figure 1). Overall the majority of participants were male (84%), white (95%), had a median age of 71 (interquartile range 68, 75) years and a median baseline Telephonic Montreal Cognitive Assessment score of 17 (16, 19). All patients underwent isolated CABG only. No significant differences were found with regard to baseline demographics, medical comorbidities, or surgical characteristics between the groups (Table 1). Baseline functional status was not statistically different between groups, with the majority of participants living independently and had a high school degree or greater (eTable 1).
Figure 1. Patients Screening and Enrollment in the Trial.
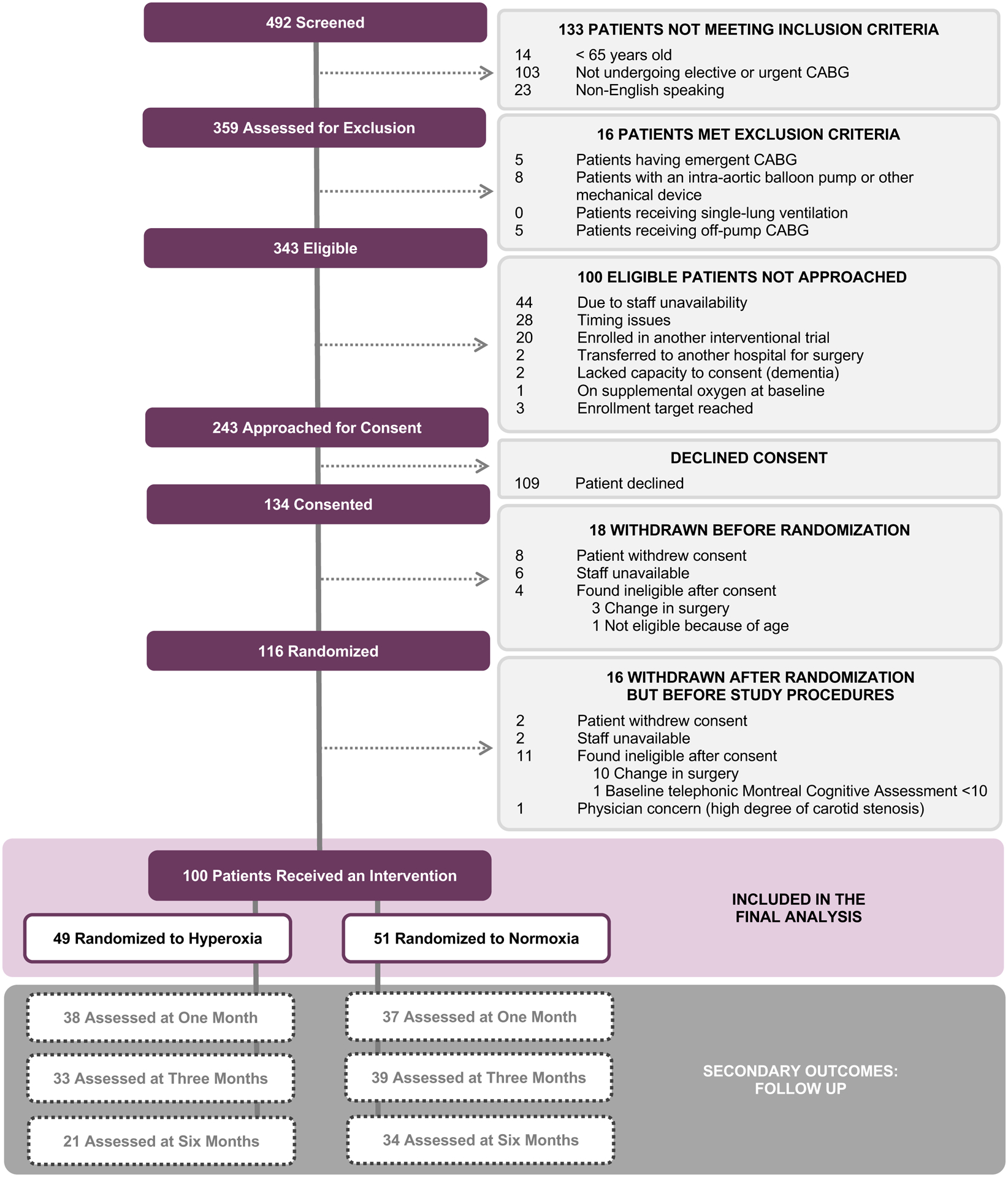
Depicted above is the study flow diagram, including data on patients that were screened, eligible, enrolled and excluded. Abbreviations: CABG = coronary artery bypass grafting.
Table 1.
Baseline Characteristics of the Study Cohort
| Hyperoxia N = 49 |
Normoxia N = 51 |
P-Value | |
|---|---|---|---|
| Demographics | |||
| Age, years | 71 (67, 75) | 70 (68, 75) | 0.88 |
| Male Sex | 43 (87.76) | 41 (80.39) | 0.32 |
| Weight, kilograms | 86.7 ± 13.7 | 87.9 ± 18.0 | 0.70 |
| Height, centimeters | 175 (170, 178) | 173 (168, 180) | 0.81 |
| Body Mass Index, kilograms/meter2 | 29.3 (26.6, 31.8) | 29.0 (24.4, 32.0) | 0.91 |
| Race | 0.74 | ||
| White | 46 (93.88) | 49 (96.08) | |
| Black / African American | 1 (2.04) | 1 (1.96) | |
| Asian | 0 (0) | 1 (1.96) | |
| Unknown / Not Specified | 1 (2.04) | 0 (0) | |
| Other | 1 (2.04) | 0 (0) | |
| Hispanic or Latino | 1 (2.04) | 0 (0) | 0.49 |
| Surgical Characteristics | |||
| Previous Cardiac Surgery | 1 (2.04) | 0 (0) | 0.49 |
| Previous Carotid Endarterectomy | 4 (8.16) | 2 (3.92) | 0.43 |
| Previous Percutaneous Coronary Intervention | 11 (22.45) | 19 (37.25) | 0.11 |
| Other Previous Vascular Surgery | 2 (4.08) | 2 (3.92) | 0.97 |
| Preoperative Medications | |||
| Aspirin (Within Five Days) | 42 (85.71) | 42 (82.35) | 0.65 |
| Clopidogrel/Plavix (Within Seven Days) | 5 (10.20) | 5 (9.80) | 0.95 |
| Medical Characteristics | |||
| Charlson Comorbidity Index | 4 (3, 5) | 4 (3, 5) | 0.41 |
| Peripheral Vascular Disease | 5 (10.20) | 5 (9.80) | 0.95 |
| Connective Tissue Disease | 3 (6.12) | 5 (9.80) | 0.72 |
| Ulcer Disease | 1 (2.04) | 4 (7.84) | 0.36 |
| Mild liver Disease | 1 (2.04) | 2 (3.92) | 0.58 |
| Diabetes (Without Complications) | 17 (34.69) | 20 (39.22) | 0.64 |
| Diabetes (With End Organ Damage) | 7 (14.29) | 6 (11.76) | 0.71 |
| Moderate or Severe Renal Disease | 6 (12.24) | 6 (11.76) | 0.94 |
| Solid tumor (Non-metastatic) | 9 (18.37) | 7 (13.73) | 0.53 |
| Leukemia | 2 (4.08) | 0 (0) | 0.24 |
| Lymphoma / Multiple Myeloma | 1 (2.04) | 1 (1.96) | 0.98 |
| Moderate or Severe Liver Disease | 2 (4.08) | 1 (1.96) | 0.61 |
| Acquired Immune Deficiency Syndrome | 1 (2.04) | 0 (0) | 0.49 |
| Depression | 9 (18.37) | 6 (11.76) | 0.36 |
| Chronic Pain | 5 (10.20) | 5 (9.80) | 0.95 |
| None | 6 (12.24) | 11 (21.57) | 0.21 |
| Baseline telephonic-Montreal Cognitive Assessment Score | 17 (15, 19) | 17 (16, 19) | 0.96 |
Values are presented as mean ± standard deviation, median (quartile 1, quartile 3), or n (%) depending on type and distribution.
Oxygen Administration and Protocol Adherence
Protocol adherence was enforced immediately after endotracheal intubation. Reliable oxygenation separation between groups was achieved with a median intraoperative PaO2 of 309 (285, 352) in the hyperoxia group and 153 (133, 168) mmHg in the normoxia group (p<0.0001). Median intraoperative oxygen saturations were 99.2% (98.6%, 99.6%) in the hyperoxic group and 96.7% (95.7%, 97.8%) in the normoxic group (p < 0.0001; Figure 2). Other surgical characteristics are depicted in Table 2. Observed intraoperative CPB-related characteristics were not statistically different between the hyperoxia and normoxia groups regarding cross clamp (69 [54, 80] vs 69 [57, 78]; p = 0.89) and total CPB minutes (83 [66, 97] vs 81 [68, 91]; p = 0.67).
Figure 2. Protocol Adherence throughout Intraoperative Period.
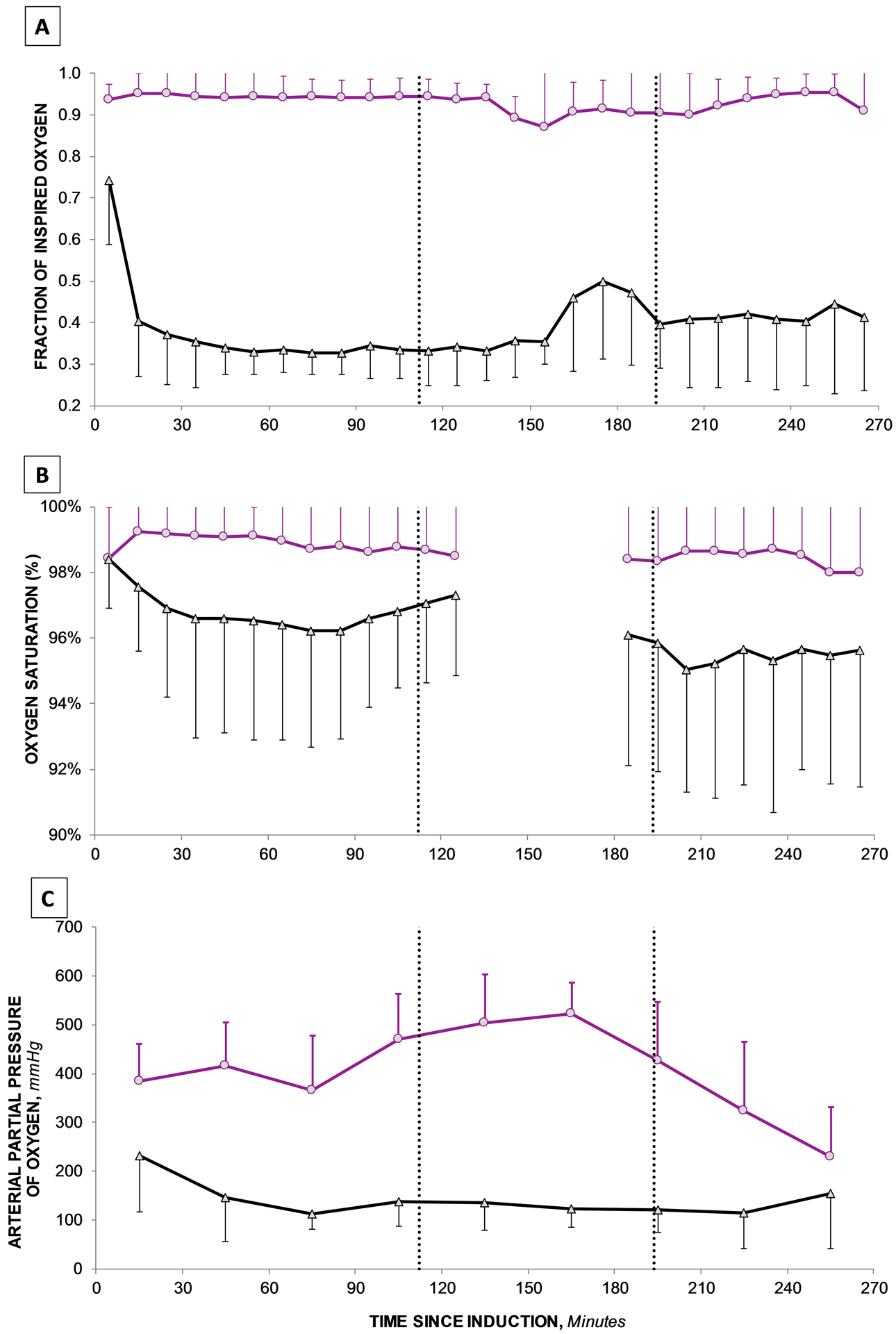
Components of trial adherence including fraction of inspired oxygen administered (FiO2), oxygen saturation (SpO2) and partial pressure of oxygen (PaO2) are depicted for both the normoxia (black triangles) and hyperoxia (purple circles) groups.
Table 2.
Operative Characteristics
| Hyperoxia N = 49 |
Normoxia N = 51 |
P-Value | |
|---|---|---|---|
| Operative Characteristics | |||
| Urgent Procedure | 12 (24.49) | 18 (35.29) | 0.24 |
| Surgical Cross Clamp Time (minutes) | 67(54, 80) | 69 (57, 78) | 0.89 |
| Total CPB Time (minutes) | 83 (66, 97) | 81 (68, 91) | 0.67 |
| Left Ventricular Ejection Fraction (%) | 55 (53, 55) | 55 (46, 55) | 0.95 |
| Intraoperative Arterial Partial Pressure of Oxygena | |||
| Throughout Surgery | 309 (285, 352) | 153 (133, 168) | <0.0001 |
| Before Bypass | 395 (333, 436) | 163 (121, 210) | <0.0001 |
| During Bypass | 527 (485, 557) | 127 (109, 147) | <0.0001 |
| After Bypass | 194 (136, 223) | 156 (135, 180) | 0.01 |
| Fraction of Inspired Oxygena | |||
| Throughout Surgery | 94 (92, 98) | 38 (35, 41) | <0.0001 |
| Before Bypass | 95 (93, 98) | 38 (34, 42) | <0.0001 |
| During Bypass | 86 (79, 92) | 42 (34, 61) | <0.0001 |
| After Bypass | 94 (92, 98) | 37 (34, 43) | <0.0001 |
| Oxygen Saturationa | |||
| Throughout Surgery | 99.2 (98.6, 99.6) | 96.7 (95.7, 97.8) | <0.0001 |
| Before Bypass | 99.2 (98.7, 99.6) | 97.5 (96.0, 98.4) | <0.0001 |
| After Bypass | 99.4 (98.2, 99.8) | 96.2 (94.7, 97.5) | <0.0001 |
Values are presented as median (quartile 1, quartile 3), or n (%) depending on variable type. Abbreviations: CPB = cardiopulmonary bypass;
For each patient the average of all of their values was calculated for each of the periods of surgery individually. Then, the median off all of these values among patients in the hyperoxia and normoxia groups are presented.
Primary Outcome
There was no significant difference in median Telephonic Montreal Cognitive Assessment score on postoperative day two between the hyperoxia and normoxia groups (18 [16, 20] vs. 18 [14, 20]; p=0.42). On postoperative day the between group difference between normoxia and hyperoxia was −1 (95% CI: −2, 1). It should be noted that the primary outcome could not be assessed in 10 (10%) of patients due to prolonged intubation, patient refusal or withdrawal from the study. The trajectory of neurocognition between groups is portrayed between groups in Figure 3. Although a slight increase was observed in both arms over time, this is likely attributable to the known and expected learning effects associated with repeated tests. When reported stratified by sex, the overall Telephonic Montreal Cognitive Assessment score on postoperative day two among males was 18 (14, 20) and 18 (16, 19) among females. There were no significant differences between groups in neurocognitive scores at one, three or six months. These results, especially those at the longer follow up, should be interpreted with extreme caution, as only 55% of patients could be contacted at the six month time period (eTable 2).
Figure 3. Cognitive Trajectories of Study Participants.
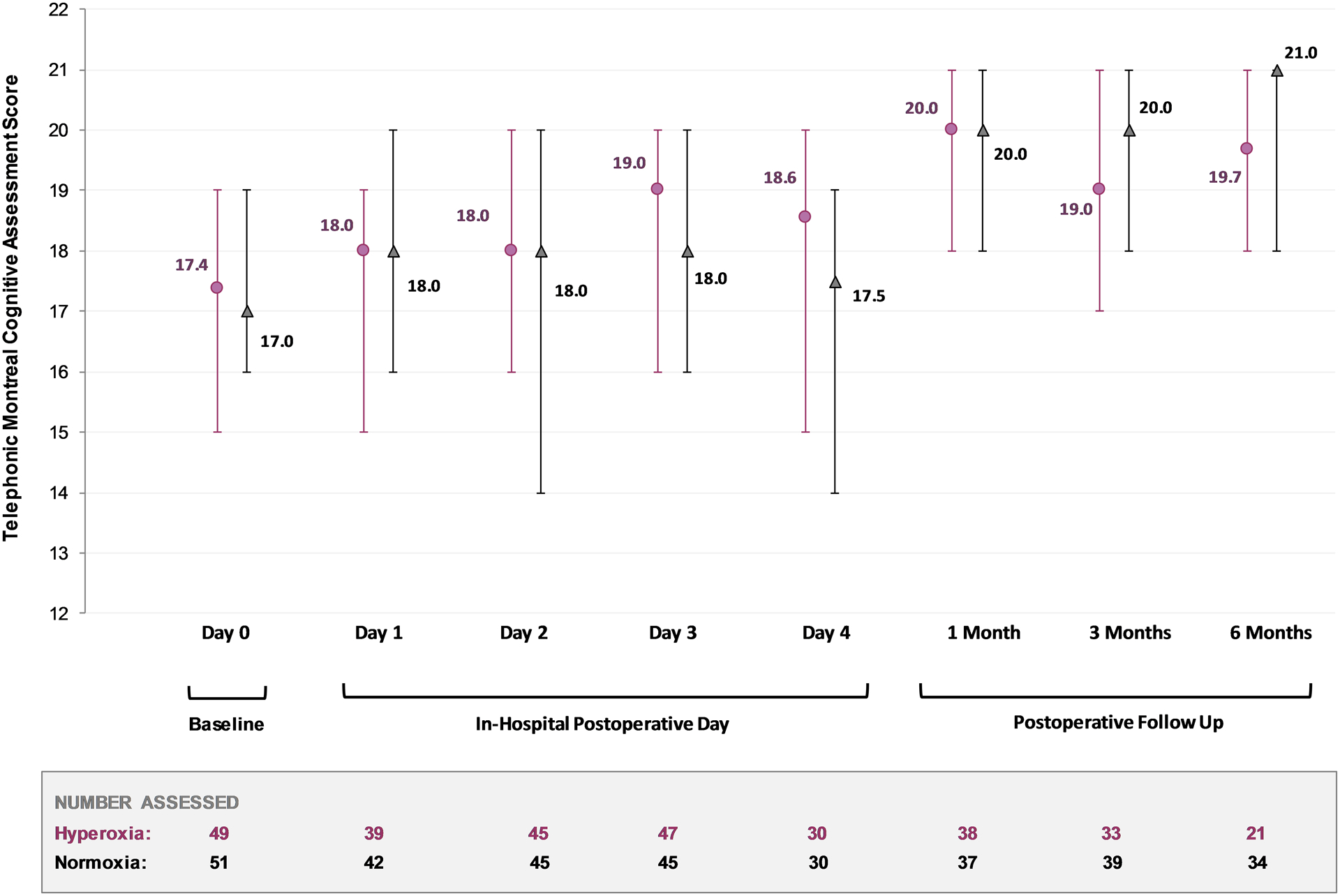
Neurocognitive scores for the study period are presented over time. Values are reported for the hyperoxia (purple circles) and normoxia (black triangles) randomization groups individually, with medians presented and their associated interquartile range (error bars).
Other Secondary Outcomes
The incidence of postoperative delirium in the hyperoxia group was 30.6%, compared to 31.4% in the normoxia group (p=0.93; eFigure 1). Clinical outcomes of study patients are described in Table 3. No statistically significant difference was observed between those randomized to the hyperoxia or normoxia groups in regards to hospital days (8 [5, 11] vs 7 [5, 10] days, respectively; p = 0.70) or ICU (2 [1, 3] vs 1 [1, 3] days; p = 0.34) length of stay. No adverse events were determined to be possibly or probably related to the study intervention. The incidence of adverse events including mortality, stroke, pneumonia, acute kidney injury, reoperation and atrial fibrillation were not statistically significantly different between groups.
Table 3.
Study Outcomes
| Hyperoxia N = 49 |
Normoxia N = 51 |
P-Value | |
|---|---|---|---|
| Primary Outcome | |||
| Postoperative Day 2 telephonic Montreal Cognitive Assessment | 18 (16, 20) | 18 (14, 20) | 0.42 |
| Change from Baselinea | −1.0 (−2.9, 1.0) | 0.0 (−2.0, 2.0) | 0.12 |
| Secondary Neurocognitive Outcomes | |||
| Neurocognition | |||
| One Month telephonic Montreal Cognitive Assessment | 20 (18, 21) | 20 (18, 21) | 0.60 |
| Days After Surgery | 31.0 ± 4.5 | 30.5 ± 4.0 | 0.58 |
| Number Assessed | 38 | 37 | |
| Three Months telephonic Montreal Cognitive Assessment | 19 (17, 21) | 20 (18, 21) | 0.62 |
| Days After Surgery | 91.8 ± 10.6 | 91.6 ± 7.4 | 0.92 |
| Number Assessed | 33 | 39 | |
| Six Months telephonic Montreal Cognitive Assessment | 20 (18, 21) | 21 (18, 21) | 0.34 |
| Days After Surgery | 181.9 ± 7.7 | 179.7 ± 9.3 | 0.38 |
| Number Assessed | 21 | 34 | |
| Delirium | 15 (30.61) | 16 (31.37) | 0.93 |
| Delirium Severity (Worst) | 11 (8, 13) | 8 (7, 11) | 0.23 |
| Time to Delirium | 1 (1, 2) | 2 (1, 3) | 0.17 |
| Time Characteristics | |||
| Hospital Length of Stay, days | 8 (5, 11) | 7 (5, 10) | 0.70 |
| ICU Length of Stay, days | 2 (1, 3) | 1 (1, 3) | 0.34 |
| Hours of initial intubation | 4.8 (3.7, 8.7) | 5.5 (3.7, 8.7) | 0.76 |
| Adverse Clinical Outcomes | |||
| Mortality | |||
| In-Hospital | 0 (0) | 0 (0) | --- |
| 30 Day | 0 (0) | 1 (1.96) | 0.32 |
| Six Monthb | 0 (0) | 1 (2.56) | 0.37 |
| Stroke | 0 (0) | 0 (0) | --- |
| Pneumonia | 3 (6.12) | 1 (1.96) | 0.36 |
| Renal Failure | 0 (0) | 1 (1.96) | 0.32 |
| Reoperation (Bleeding) | 0 (0) | 1 (1.96) | 0.32 |
| Atrial Fibrillation | 14 (28.57) | 16 (31.37) | 0.83 |
Values are presented as mean ± standard deviation, median (quartile 1, quartile 3), or n (%) depending on type or distribution. Abbreviations: POD = Postoperative Day.
The change from baseline is calculated as baseline telephonic Montreal Cognitive Assessment – telephonic Montreal Cognitive Assessment on postoperative day two.
Six month mortality status was not available for all patients. Mortality status could be confirmed for 31 hyperoxia and 39 normoxia patients. The patient who died within 30 days is the same patient denoted as dead at six months.
In a post-hoc analysis of patients who developed delirium, there was no statistically significant difference in time to delirium (1 [1, 2] vs 2 [1, 3] days; p = 0.17; eFigure 1) or delirium severity (as assessed by Confusion Assessment Method Severity score; 11 [8, 13] vs 8 [7, 11]; p = 0.23) in the hyperoxia group as compared with the normoxia group.
DISCUSSION
This randomized clinical trial assessed the effect of normoxic versus hyperoxic intraoperative oxygen conditions on postoperative cognition, measured using Telephonic Montreal Cognitive Assessment scores in an older population of patients having CABG. While statistically and clinically significant differences in protocol-defined oxygen titration between groups was achieved, no significant difference was observed in postoperative cognition or delirium at any time point between groups. Additionally, oxygen titration strategy did not impact time to extubation, length of ICU and hospital stay, or patient mortality.
The results of this study do not provide clarity on the optimal oxygenation strategy for patients having cardiac surgery with CPB with regards to neurocognition and delirium. Potential reasons hyperoxia may be harmful in patients having cardiac surgery utilizing CPB are due to cardiovascular dysfunction28,29, enhancement of ischemia-reperfusion injury30,31 and direct injury from reactive oxygen species32–35. However, we did not find evidence of such harm in our study, at least as manifested as cognitive dysfunction. In interpreting these results, it is entirely plausible that the arterial oxygen content is a minor variable in the response to ischemia-reperfusion injury, dwarfed by that from the systemic inflammatory response to CPB. Other studies both in cardiac surgery16,17,36 and allied specialties have not demonstrated statistically significant results regarding the detrimental effects of hyperoxia, albeit via varied outcome measurements.12,37–39 It must be borne in mind, this was a study of moderate compared with severe hyperoxia. More contemporary studies are attempting to examine differences in tight normoxic and mild hyperoxic conditions assuming that any oxygen titration benefit may be gleaned in this more physiological window.40–43 Other cardiac surgical studies have employed continuous arterial blood gas analysis to give ability for closer real time normoxic titration of non-pulsatile PaO2.16
In the broader context of identifying the ideal perioperative oxygenation strategy, there is currently substantial debate. There is not a consensus definition of hyperoxia, leading to difficulties in advocating for a specific titration strategy as well as significant heterogeneity in both clinical trials and clinical practice. Currently, both the World Health Organization and the Centers for Disease Control and Prevention recommend high concentrations of perioperative oxygen, largely based on a subgroup analysis of a single trial of high FiO2 to reduce surgical site infection.44,45 These recommendations have elicited criticism due to the inconclusive nature of the evidence and ignore evidence of harm from hyperoxia.46–48 It is likely that lower oxygenation targets can still provide adequate tissue oxygenation in the perioperative period, even for high risk patients. In fact, a series of recent larger studies have demonstrated that lower oxygen targets could be applied safely during CPB without detrimental cardiac and renal outcomes.16,17,36 Our findings are in congruence with a large retrospective study of 1018 patients having cardiac surgery with CPB which failed to demonstrate a relationship between arterial hyperoxia and neurocognitive function six weeks after surgery.18 Our study did not find any significant differences in adverse outcomes with the use of a lower oxygenation target, although interpretation of these results in support of the safety of a lower oxygenation target must be made with caution. Although evidence suggests that liberal oxygen supplementation and hyperoxia may lead to neurotoxicity in the context of ischemia-reperfusion injury, ours and other studies aiming to prevent such complications by simply reducing the amount of oxygen administered have yet to show consistent benefit, suggesting additional factors may be at play. It should be noted however that further focus upon delirium severity in this population may be warranted. Higher delirium severity perhaps confers a higher risk of long term cognitive decline.49
This study has several limitations, some of all of which may have contributed to these findings. Our intervention was over a broad period of oxygenation focus rather than specifically at a critical time point such as myocardial reperfusion. The inclusion of only CABG patients with relatively brief bypass times and thus at lower risk for ischemia-reperfusion injury than patients having more extensive surgery may have limited our exposure to injury and thus baseline risk. Interestingly, the patients in this study were relatively low-risk, with few baseline risk characteristics or postoperative events that might put them at risk for postoperative decline. This could potentially contribute to the lack of differences we observed. Our intervention was based on FiO2 rather than PaO2 targets, therefore it is possible that different exposure definitions could have resulted in changes. However, this is unlikely given the large separation we observed between groups. Additionally, the Telephonic Montreal Cognitive Assessment is a relatively new tool to evaluate neurocognitive function in this population. Previous studies have shown that the Telephonic Montreal Cognitive Assessment is able to reliably identify mild cognitive impairment and has been employed in surgical populations.23,50,51 In our study we identified relatively low variability, which could suggest that either our patients were very homogenous, or that the instrument is perhaps not sensitive enough to detect small cognitive differences. Although it does address memory, attention, language, abstraction, recall, and several other important components of neurocognitive function, we are unable to comment on visuospatial or executive cognitive domains. We did not evaluate the individual domains because of our sample size, however this would be an interesting avenue for future research in this patient population. Furthermore, scores may improve with repeat testing during short intervals. These issues could be mitigated by a wider testing battery with multiple individual tests, non-surgical comparator groups and/or factor analysis in future studies. It should also be noted that our conclusions for longer term follow up should be interpreted with extreme caution, due in large part because of loss to follow up. That is, only 55% of patients could be contacted at the six-month mark. Despite our attempts to contact participants, it is possible that this missing data biased our interpretation of longer-term neurocognition. Although this only occurred for a few patients in our study, the impact of prolonged intubation or delirium may also make it hard to interpret values for our primary outcome. Further limiting our study, the sample size for this trial was based on expected cardiac surgical cognitive changes seen postoperatively using the Mini-Mental State Examination25, and subsequent extrapolation to our primary endpoint using a validated crosswalk between the Mini-Mental State Examination and Telephonic Montreal Cognitive Assessment26, however the effect size we observed is not as pronounced as anticipated. Additionally, assumptions were made using a parametric distribution, however we found that neurocognitive scores were not normally distributed. Because non-parametric analyses require larger sample sizes, we are therefore potentially underpowered to detect a difference if one truly exists. Despite these potential limitations, we were able to show no difference in short-term postoperative cognition among older cardiac surgical patients undergoing differential titration of intraoperative oxygen therapy.
In conclusion, this trial demonstrated that the titration of intraoperative oxygenation resulted in no significant differences in postoperative cognition after cardiac surgery. These results suggest that a varied intraoperative oxygen strategy may be safely employed without impairing postoperative neurocognitive function.
Supplementary Material
6. Acknowledgments:
Protocol development, execution and adherence was supported by the Center for Anesthesia Research Excellence within the Beth Israel Deaconess Medical Center Department of Anesthesia, Critical Care and Pain Medicine.
10. Sources of Funding and Support
Funding for this study was provided by the Foundation for Anesthesia Education and Research in the form of a Mentored Training Research Grant. Funds have been allotted from this organization to support Principal Investigator time and effort. Additional funds have been awarded by the Beth Israel Deaconess Medical Center Chief Academic Officer Pilot Award for supporting study staff salary, statistical support, and regulatory compliance consulting.
Footnotes
Conflicts of Interest
Dr. Shaefi received speaking honorarium for University of North Carolina Visiting Professorship lecture. Ms. Mueller receives statistical consulting fees from The University of Chicago. Dr. O’ Gara receives consulting fees from Sedana Medical (Danderyd, Sweden). Dr. Bagchi receives consulting fees from Lungpacer Medical Inc (British Columbia, Canada). Ms. Banner-Goodspeed received salary support from several NIH and DoD grants, unrelated to this project. Dr. Subramaniam receives grant support from Mallinckrodt Pharmaceuticals (Staines-upon-Thames, United Kingdom) and Edward Lifesciences (Irvine CA). The other authors declare no competing interests.
Clinical Trial Number and Registry URL
The trial was registered with ClinicalTrials.gov; Identifier NCT02591589; Principal Investigator: Shahzad Shaefi; Registration Date: October 29, 2015 https://clinicaltrials.gov/ct2/show/NCT02591589
Prior Presentations
Shankar P, Mueller A, Marcantonio E, Subramaniam B, O’Gara B, Shaefi S (October 20, 2019) Intraoperative Oxygen Concentration and Neurological Outcome in Cardiac Surgery – A Randomized Controlled Trial – Poster and Oral Presentation presented at Anesthesiology Annual Meeting 2019; Orlando FL, USA
REFERENCES
- 1.Punjabi PP, Taylor KM: The science and practice of cardiopulmonary bypass: From cross circulation to ECMO and SIRS. Glob Cardiol Sci Pract 2013; 2013: 249–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Statistics CfDCNCfH: National Hospital Discharge Survey: 2010 table, Procedures by selected patient characteristics – Number by procedure category and age, 2010
- 3.Evered L, Silbert B, Knopman DS, Scott DA, DeKosky ST, Rasmussen LS, Oh ES, Crosby G, Berger M, Eckenhoff RG, Nomenclature Consensus Working G: Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery-2018. Br J Anaesth 2018; 121: 1005–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newman MF, Kirchner JL, Phillips-Bute B, Gaver V, Grocott H, Jones RH, Mark DB, Reves JG, Blumenthal JA, Neurological Outcome Research G, the Cardiothoracic Anesthesiology Research Endeavors I: Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N Engl J Med 2001; 344: 395–402 [DOI] [PubMed] [Google Scholar]
- 5.Phillips-Bute B, Mathew JP, Blumenthal JA, Grocott HP, Laskowitz DT, Jones RH, Mark DB, Newman MF: Association of neurocognitive function and quality of life 1 year after coronary artery bypass graft (CABG) surgery. Psychosom Med 2006; 68: 369–75 [DOI] [PubMed] [Google Scholar]
- 6.Newman MF, Grocott HP, Mathew JP, White WD, Landolfo K, Reves JG, Laskowitz DT, Mark DB, Blumenthal JA, Neurologic Outcome Research G, the Cardiothoracic Anesthesia Research Endeavors Investigators of the Duke Heart C: Report of the substudy assessing the impact of neurocognitive function on quality of life 5 years after cardiac surgery. Stroke 2001; 32: 2874–81 [DOI] [PubMed] [Google Scholar]
- 7.Berger M, Terrando N, Smith SK, Browndyke JN, Newman MF, Mathew JP: Neurocognitive Function after Cardiac Surgery: From Phenotypes to Mechanisms. Anesthesiology 2018; 129: 829–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tully PJ, Baune BT, Baker RA: Cognitive impairment before and six months after cardiac surgery increase mortality risk at median 11 year follow-up: a cohort study. Int J Cardiol 2013; 168: 2796–802 [DOI] [PubMed] [Google Scholar]
- 9.Fontes MT, Swift RC, Phillips-Bute B, Podgoreanu MV, Stafford-Smith M, Newman MF, Mathew JP: Predictors of cognitive recovery after cardiac surgery. Anesth Analg 2013; 116: 435–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Driscoll BR, Howard LS, Bucknall C, Welham SA, Davison AG: British Thoracic Society emergency oxygen audits. Thorax 2011; 66: 734–5 [DOI] [PubMed] [Google Scholar]
- 11.Baigrie RJ, Lamont PM, Kwiatkowski D, Dallman MJ, Morris PJ: Systemic cytokine response after major surgery. Br J Surg 1992; 79: 757–60 [DOI] [PubMed] [Google Scholar]
- 12.Girardis M, Busani S, Damiani E, Donati A, Rinaldi L, Marudi A, Morelli A, Antonelli M, Singer M: Effect of Conservative vs Conventional Oxygen Therapy on Mortality Among Patients in an Intensive Care Unit: The Oxygen-ICU Randomized Clinical Trial. Jama 2016; 316: 1583–1589 [DOI] [PubMed] [Google Scholar]
- 13.Stub D, Smith K, Bernard S, Nehme Z, Stephenson M, Bray JE, Cameron P, Barger B, Ellims AH, Taylor AJ, Meredith IT, Kaye DM, Investigators A: Air Versus Oxygen in ST-Segment-Elevation Myocardial Infarction. Circulation 2015; 131: 2143–50 [DOI] [PubMed] [Google Scholar]
- 14.Kilgannon JH, Jones AE, Shapiro NI, Angelos MG, Milcarek B, Hunter K, Parrillo JE, Trzeciak S, Emergency Medicine Shock Research Network I: Association between arterial hyperoxia following resuscitation from cardiac arrest and in-hospital mortality. JAMA 2010; 303: 2165–71 [DOI] [PubMed] [Google Scholar]
- 15.Rincon F, Kang J, Maltenfort M, Vibbert M, Urtecho J, Athar MK, Jallo J, Pineda CC, Tzeng D, McBride W, Bell R: Association between hyperoxia and mortality after stroke: a multicenter cohort study. Crit Care Med 2014; 42: 387–96 [DOI] [PubMed] [Google Scholar]
- 16.McGuinness SP, Parke RL, Drummond K, Willcox T, Bailey M, Kruger C, Baker M, Cowdrey KA, Gilder E, McCarthy L, Painter T: A Multicenter, Randomized, Controlled Phase IIb Trial of Avoidance of Hyperoxemia during Cardiopulmonary Bypass. Anesthesiology 2016; 125: 465–73 [DOI] [PubMed] [Google Scholar]
- 17.Smit B, Smulders YM, de Waard MC, Boer C, Vonk AB, Veerhoek D, Kamminga S, de Grooth HJ, Garcia-Vallejo JJ, Musters RJ, Girbes AR, Oudemans-van Straaten HM, Spoelstra-de Man AM: Moderate hyperoxic versus near-physiological oxygen targets during and after coronary artery bypass surgery: a randomised controlled trial. Crit Care 2016; 20: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fontes MT, McDonagh DL, Phillips-Bute B, Welsby IJ, Podgoreanu MV, Fontes ML, Stafford-Smith M, Newman MF, Mathew JP, Neurologic Outcome Research Group of the Duke Heart C: Arterial hyperoxia during cardiopulmonary bypass and postoperative cognitive dysfunction. J Cardiothorac Vasc Anesth 2014; 28: 462–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaefi S, Marcantonio ER, Mueller A, Banner-Goodspeed V, Robson SC, Spear K, Otterbein LE, O’Gara BP, Talmor DS, Subramaniam B: Intraoperative oxygen concentration and neurocognition after cardiac surgery: study protocol for a randomized controlled trial. Trials 2017; 18: 600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horton DK, Hynan LS, Lacritz LH, Rossetti HC, Weiner MF, Cullum CM: An Abbreviated Montreal Cognitive Assessment (MoCA) for Dementia Screening. Clin Neuropsychol 2015; 29: 413–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong A, Nyenhuis D, Black SE, Law LS, Lo ES, Kwan PW, Au L, Chan AY, Wong LK, Nasreddine Z, Mok V: Montreal Cognitive Assessment 5-minute protocol is a brief, valid, reliable, and feasible cognitive screen for telephone administration. Stroke 2015; 46: 1059–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H, Zietemann V, Kopczak A, Muller C, Wollenweber FA, Dichgans M: The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment Validation of the Telephone Interview of Cognitive Status and Telephone Montreal Cognitive Assessment Against Detailed Cognitive Testing and Clinical Diagnosis of Mild Cognitive Impairment After Stroke. J Am Geriatr Soc 2005; 53: 695–9 [DOI] [PubMed] [Google Scholar]
- 23.Austin CA, O’Gorman T, Stern E, Emmett D, Stürmer T, Carson S, Busby-Whitehead J: Association Between Postoperative Delirium and Long-term Cognitive Function After Major Nonemergent Surgery. JAMA Surg 2019; 154: 328–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inouye SK, Kosar CM, Tommet D, Schmitt EM, Puelle MR, Saczynski JS, Marcantonio ER, Jones RN: The CAM-S: development and validation of a new scoring system for delirium severity in 2 cohorts. Ann Intern Med 2014; 160: 526–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saczynski JS, Marcantonio ER, Quach L, Fong TG, Gross A, Inouye SK, Jones RN: Cognitive trajectories after postoperative delirium. N Engl J Med 2012; 367: 30–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saczynski JS, Inouye SK, Guess J, Jones RN, Fong TG, Nemeth E, Hodara A, Ngo L, Marcantonio ER: The Montreal Cognitive Assessment: Creating a Crosswalk with the Mini-Mental State Examination. J Am Geriatr Soc 2015; 63: 2370–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roalf DR, Moore TM, Mechanic-Hamilton D, Wolk DA, Arnold SE, Weintraub DA, Moberg PJ: Bridging cognitive screening tests in neurologic disorders: A crosswalk between the short Montreal Cognitive Assessment and Mini-Mental State Examination. Alzheimers Dement 2017; 13: 947–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spoelstra-de Man AM, Smit B, Oudemans-van Straaten HM, Smulders YM: Cardiovascular effects of hyperoxia during and after cardiac surgery. Anaesthesia 2015 [DOI] [PubMed] [Google Scholar]
- 29.Farquhar H, Weatherall M, Wijesinghe M, Perrin K, Ranchord A, Simmonds M, Beasley R: Systematic review of studies of the effect of hyperoxia on coronary blood flow. Am Heart J 2009; 158: 371–7 [DOI] [PubMed] [Google Scholar]
- 30.Peng YW, Mohammed A, Deatrick KB, Major T, Cheng D, Charpie I, Charpie JR: Differential Effects of Normoxic and Hyperoxic Reperfusion on Global Myocardial Ischemia-Reperfusion Injury. Semin Thorac Cardiovasc Surg 2019; 31: 188–198 [DOI] [PubMed] [Google Scholar]
- 31.Brueckl C, Kaestle S, Kerem A, Habazettl H, Krombach F, Kuppe H, Kuebler WM: Hyperoxia-induced reactive oxygen species formation in pulmonary capillary endothelial cells in situ. Am J Respir Cell Mol Biol 2006; 34: 453–63 [DOI] [PubMed] [Google Scholar]
- 32.Topcu AC, Bolukcu A, Ozeren K, Kavasoglu T, Kayacioglu I: Normoxic management of cardiopulmonary bypass reduces myocardial oxidative stress in adult patients undergoing coronary artery bypass graft surgery. Perfusion 2020: 267659120946733. [DOI] [PubMed] [Google Scholar]
- 33.Peng YW, Major T, Mohammed A, Deatrick KB, Charpie JR: Normoxic re-oxygenation ameliorates end-organ injury after cardiopulmonary bypass. J Cardiothorac Surg 2020; 15: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lopez MG, Hughes CG, DeMatteo A, O’Neal JB, McNeil JB, Shotwell MS, Morse J, Petracek MR, Shah AS, Brown NJ, Billings FTt: Intraoperative Oxidative Damage and Delirium after Cardiac Surgery. Anesthesiology 2020; 132: 551–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lopez MG, Pandharipande P, Morse J, Shotwell MS, Milne GL, Pretorius M, Shaw AD, Roberts LJ 2nd, Billings FTt: Intraoperative cerebral oxygenation, oxidative injury, and delirium following cardiac surgery. Free Radic Biol Med 2017; 103: 192–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abou-Arab O, Huette P, Martineau L, Beauvalot C, Beyls C, Josse E, Touati G, Bouchot O, Bouhemad B, Diouf M, Lorne E, Guinot PG: Hyperoxia during cardiopulmonary bypass does not decrease cardiovascular complications following cardiac surgery: the CARDIOX randomized clinical trial. Intensive Care Med 2019; 45: 1413–1421 [DOI] [PubMed] [Google Scholar]
- 37.Hofmann R, James SK, Jernberg T, Lindahl B, Erlinge D, Witt N, Arefalk G, Frick M, Alfredsson J, Nilsson L, Ravn-Fischer A, Omerovic E, Kellerth T, Sparv D, Ekelund U, Linder R, Ekstrom M, Lauermann J, Haaga U, Pernow J, Ostlund O, Herlitz J, Svensson L: Oxygen Therapy in Suspected Acute Myocardial Infarction. N Engl J Med 2017 [Google Scholar]
- 38.Mackle D, Bellomo R, Bailey M, Beasley R, Deane A, Eastwood G, Finfer S, Freebairn R, King V, Linke N, Litton E, McArthur C, McGuinness S, Panwar R, Young P: Conservative Oxygen Therapy during Mechanical Ventilation in the ICU. N Engl J Med 2019 [DOI] [PubMed] [Google Scholar]
- 39.Roffe C, Nevatte T, Sim J, Bishop J, Ives N, Ferdinand P, Gray R: Effect of Routine Low-Dose Oxygen Supplementation on Death and Disability in Adults With Acute Stroke: The Stroke Oxygen Study Randomized Clinical Trial. Jama 2017; 318: 1125–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Young P, Mackle D, Bellomo R, Bailey M, Beasley R, Deane A, Eastwood G, Finfer S, Freebairn R, King V, Linke N, Litton E, McArthur C, McGuinness S, Panwar R: Conservative oxygen therapy for mechanically ventilated adults with sepsis: a post hoc analysis of data from the intensive care unit randomized trial comparing two approaches to oxygen therapy (ICU-ROX). Intensive Care Med 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schjorring OL, Perner A, Wetterslev J, Lange T, Keus F, Laake JH, Okkonen M, Siegemund M, Morgan M, Thormar KM, Rasmussen BS: Handling Oxygenation Targets in the Intensive Care Unit (HOT-ICU)-Protocol for a randomised clinical trial comparing a lower vs a higher oxygenation target in adults with acute hypoxaemic respiratory failure. Acta Anaesthesiol Scand 2019; 63: 956–965 [DOI] [PubMed] [Google Scholar]
- 42.Barrot L, Asfar P, Mauny F, Winiszewski H, Montini F, Badie J, Quenot JP, Pili-Floury S, Bouhemad B, Louis G, Souweine B, Collange O, Pottecher J, Levy B, Puyraveau M, Vettoretti L, Constantin JM, Capellier G: Liberal or Conservative Oxygen Therapy for Acute Respiratory Distress Syndrome. N Engl J Med 2020; 382: 999–1008 [DOI] [PubMed] [Google Scholar]
- 43.Lopez MG, Pretorius M, Shotwell MS, Deegan R, Eagle SS, Bennett JM, Sileshi B, Liang Y, Gelfand BJ, Kingeter AJ, Siegrist KK, Lombard FW, Richburg TM, Fornero DA, Shaw AD, Hernandez A, Billings FTt: The Risk of Oxygen during Cardiac Surgery (ROCS) trial: study protocol for a randomized clinical trial. Trials 2017; 18: 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berrios-Torres SI, Umscheid CA, Bratzler DW, Leas B, Stone EC, Kelz RR, Reinke CE, Morgan S, Solomkin JS, Mazuski JE, Dellinger EP, Itani KMF, Berbari EF, Segreti J, Parvizi J, Blanchard J, Allen G, Kluytmans J, Donlan R, Schecter WP: Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection, 2017. JAMA Surg 2017; 152: 784–791 [DOI] [PubMed] [Google Scholar]
- 45.Allegranzi B, Zayed B, Bischoff P, Kubilay NZ, de Jonge S, de Vries F, Gomes SM, Gans S, Wallert ED, Wu X, Abbas M, Boermeester MA, Dellinger EP, Egger M, Gastmeier P, Guirao X, Ren J, Pittet D, Solomkin JS: New WHO recommendations on intraoperative and postoperative measures for surgical site infection prevention: an evidence-based global perspective. Lancet Infect Dis 2016; 16: e288–e303 [DOI] [PubMed] [Google Scholar]
- 46.Meyhoff CS: Perioperative hyperoxia: why guidelines, research and clinical practice collide. Br J Anaesth 2019; 122: 289–291 [DOI] [PubMed] [Google Scholar]
- 47.Mattishent K, Thavarajah M, Sinha A, Peel A, Egger M, Solomkin J, de Jonge S, Latif A, Berenholtz S, Allegranzi B, Loke YK: Safety of 80% vs 30–35% fraction of inspired oxygen in patients undergoing surgery: a systematic review and meta-analysis. Br J Anaesth 2019; 122: 311–324 [DOI] [PubMed] [Google Scholar]
- 48.de Jonge S, Egger M, Latif A, Loke YK, Berenholtz S, Boermeester M, Allegranzi B, Solomkin J: Effectiveness of 80% vs 30–35% fraction of inspired oxygen in patients undergoing surgery: an updated systematic review and meta-analysis. Br J Anaesth 2019; 122: 325–334 [DOI] [PubMed] [Google Scholar]
- 49.Vasunilashorn SM, Fong TG, Albuquerque A, Marcantonio ER, Schmitt EM, Tommet D, Gou Y, Travison TG, Jones RN, Inouye SK: Delirium Severity Post-Surgery and its Relationship with Long-Term Cognitive Decline in a Cohort of Patients without Dementia. J Alzheimers Dis 2018; 61: 347–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shelton KT, Qu J, Bilotta F, Brown EN, Cudemus G, D’Alessandro DA, Deng H, DiBiasio A, Gitlin JA, Hahm EY, Hobbs LE, Houle TT, Ibala R, Loggia ML, Pavone KJ, Shaefi S, Tolis G, Westover MB, Akeju O: Minimizing ICU Neurological Dysfunction with Dexmedetomidine-induced Sleep (MINDDS): protocol for a randomised, double-blind, parallel-arm, placebo-controlled trial. BMJ Open 2018; 8: e020316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zietemann V, Kopczak A, Müller C, Wollenweber FA, Dichgans M: Validation of the Telephone Interview of Cognitive Status and Telephone Montreal Cognitive Assessment Against Detailed Cognitive Testing and Clinical Diagnosis of Mild Cognitive Impairment After Stroke. Stroke 2017; 48: 2952–2957 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


