Abstract
Dopamine signaling mediates the formation of some types of social relationships, including reproductive pair bonds in the socially monogamous prairie vole (Microtus ochrogaster). In addition to these pair bonds with mates, prairie voles demonstrate selective preferences for familiar same-sex peers. The dependence of peer relationships on dopamine signaling has not been tested, and the mechanisms supporting these relationships may differ from those underlying pair bonds. We examined the effects of pharmacological manipulations of dopamine signaling on peer partner preference and socially conditioned place preference in female prairie voles. Haloperidol blockade of dopamine receptors at multiple doses did not alter selective preferences for familiar same-sex partners, suggesting that dopamine neurotransmission is not necessary for the formation of prairie vole peer relationships, unlike mate relationships. Dopamine receptor agonist apomorphine facilitated peer partner preferences under conditions normally insufficient for partner preference formation; however, in the absence of successful blockade, it is difficult to distinguish between a role for dopamine in partner preference formation and the generally rewarding properties of a dopamine agonist. Prairie voles also exhibited socially conditioned place preferences for new but not long-term same-sex peers, and these preferences were not blocked by haloperidol. These results suggest that prairie vole peer relationships are less dependent on dopamine signaling than pair bonds, while still being rewarding. The data support distinct roles of dopamine and motivation in prairie vole peer relationships relative to mate relationships, suggesting that reproductive bonds are mediated differently from non-reproductive ones.
Keywords: prairie vole, reward, dopamine, social behavior, affiliation, socially conditioned place preference, partner preference
INTRODUCTION
Dopamine signaling plays an important role in promoting and maintaining social relationships in social animals, such as those between parent and offspring and those between mates (reviewed in Curtis et al., 2006; O’Connell and Hofmann, 2011; Trezza et al., 2011; Numan and Young, 2016). Relationships between same-sex conspecifics are fundamental to the social systems of many group living species, but the mechanisms mediating these peer relationships are poorly understood relative to those mediating reproductive relationships. We investigated the role of dopamine signaling in peer relationships in female prairie voles (Microtus ochrogaster), a species that has been largely studied for its reproductive pair bonds, which are dopamine-dependent. Using pharmacological manipulations of dopamine signaling, we sought to determine whether the mechanisms supporting peer relationships in prairie voles are more like pair bonds with mates in prairie voles (thus species-specific) or more like selective peer relationships in meadow voles (relationship type-specific).
Prairie voles are socially monogamous rodents that have been extensively studied for their ability to form pair bonds with mates (reviewed in Gobrogge and Wang, 2016; Carter, 2017; Walum and Young, 2018). These pair bonds are selective, stable, and motivated (Williams et al., 1992; Aragona et al., 2003; Bosch et al., 2009; Beery et al., 2019; Goodwin et al., 2019). Prairie voles also exhibit selective, stable preferences for familiar same-sex peers (DeVries et al., 1997; Beery et al., 2018; Lee et al., 2019), but these peer relationships have been understudied compared to prairie vole pair bonds. Much of the research on peer relationships in prairie voles has focused on the effects of isolation from same-sex cage-mates (Grippo et al., 2008; Lieberwirth et al., 2012), as well as social buffering by same-sex cage-mates (Smith and Wang, 2014; Burkett et al., 2016). Relationships between peers are a critical component of animal social systems, including human societies, and the mechanisms underlying the formation and maintenance of these relationships merit further investigation. Prairie voles provide an excellent opportunity to assess the mechanisms mediating social behavior across different relationship types. It is possible that non-reproductive relationships between peers differ from reproductive relationships including pair bonding and biparental care, which are highly motivated and reinforced by conserved reward pathways.
The role of reward in the contexts of sexual behavior and appetitive behaviors like drug addiction is well established (reviewed in Young et al., 2011; Beloate and Coolen, 2017). Social reward specifically has been well studied in the maternal behavior and social play of rodents, especially rats and mice (Trezza et al., 2011). Prairie voles also exhibit behavioral reward for mates: they show socially conditioned place preferences for mates but not long-term same-sex cage-mates (Goodwin et al., 2019), and will lever-press at high rates for access to their mates, with sex differences in the extent of pressing for peers (Beery et al., 2019).
Several studies have focused on the mesocorticolimbic dopaminergic pathway as an important factor mediating pair bonds in prairie voles. The rewarding properties of a mate appear to be mediated in part by dopamine signaling, both for bond formation and maintenance. In prairie voles, dopamine D2-type receptor activation, and interaction with oxytocin receptors, is necessary for pair bond formation (Wang et al., 1999; Liu and Wang, 2003). Whereas D2-like activation in the rostral shell of the nucleus accumbens promotes pair bond formation, D1-like activation inhibits it (Gingrich et al., 2000; Aragona et al., 2003, 2006; Liu and Wang, 2003). D1-type receptors are instead important for pair bond maintenance (Aragona et al., 2006), and interact with κ-opioid receptors to promote pair bond maintenance (Resendez et al., 2016). Similarly, D1-type receptors mediate pair bond maintenance in the socially monogamous titi monkey (Rothwell et al., 2019).
The meadow vole (Microtus pennsylvanicus) is a closely related species that does not form pair bonds with mates, and is often studied as a comparison to prairie voles (Beery, 2019). While meadow voles do not form pair bonds with mates in the wild, they exhibit group living in winter months (Beery, 2018), and show selective peer preferences as in prairie voles, but unlike rats and mice (Beery et al., 2018; Schweinfurth et al., 2017). Importantly, comparative work in prairie and meadow voles suggests that the necessary role of dopamine signaling may be specific to pair bonding with mates. Despite distinct mating systems, both prairie and meadow voles show increases in extracellular dopamine in the striatum after mating (Curtis et al. 2003). Prairie voles treated with dopamine receptor antagonist also exhibit no change in mating bouts relative to control (Wang et al., 1999). In contrast to prairie vole pair bonds, dopamine receptor blockade does not prevent same-sex peer partner preference formation in meadow voles (Beery et al. 2010). Meadow voles also do not exhibit socially conditioned place preferences for peers (Goodwin et al., 2019). Meadow voles provide insight into how the roles of reward and dopamine signaling may differ across species and across mating systems.
Differing involvement of dopamine receptors in prairie vole mate preferences and meadow vole peer preferences gives rise to two competing hypotheses about prairie vole peer relationships: (a) Prairie vole peer relationships depend on dopamine signaling (similar to prairie vole pair bonds), and thus the role of dopamine signaling in the formation of selective social relationships is more similar within than across species, regardless of relationship type. OR (b) Prairie vole peer relationships do not depend on dopamine signaling (similar to meadow vole peer relationships), and thus the role of dopamine signaling in the formation of social preferences is specific to the relationship type (peer vs. mate).
We used pharmacological manipulations to evaluate the necessity and sufficiency of dopamine signaling for the formation of peer partner preferences in female prairie voles, as determined by partner preference tests. Our goal was to assess the extent to which prior work on prairie vole pair bonds generalizes to prairie vole peer relationships. We also assessed the ability of female prairie voles to form socially conditioned place preferences for peer partners, in order to assess social reward associated with a partner, and to determine whether these preferences are affected by changes in dopamine signaling. Prairie voles provide an excellent opportunity to investigate the mechanisms underlying bond formation in a comparative context (across species and across partner type). This research will allow us to determine whether, in voles, reliance on dopamine signaling is reserved for reproductive relationships.
MATERIALS AND METHODS
Animal subjects
Prairie voles were bred locally at Smith College. Female prairie voles were group weaned at 21 ± 1 days, then separated to pair-housing with either a same-sex sibling or an age-matched same-sex non-sibling (cross-litter pairing) within one week. Voles were maintained on a long day (LD) light cycle (14h light; 03:00 to 17:00 EST). Subjects were housed in clear plastic cages (45x25x15cm) with aspen bedding (Harlan TekLab), nesting material (Lab Supply Enviro-dri and a nestlet), and a PVC hiding tube. Food (Labdiet Mouse Chow 5015 supplemented with Labdiet Rabbit Chow 5326) and water were available ad libitum, with every-other-day supplementation with fresh produce (apple or carrot). Rooms were maintained at ~20°C.
All procedures adhered to federal and institutional guidelines and were approved by the Institutional Animal Care and Use Committee.
Experimental timeline
Prior to performing experiments 1–3, sufficient and insufficient pairing times were determined for both same-sex and opposite-sex pairings. Voles were paired with new partners for 3, 6, or 24 hr before testing in a partner preference test. Under control (non-treated) conditions, 6 hr of pairing were insufficient for peer partner preference formation (figure S1), and were used as the insufficient duration in experiment 2.
For experiments 1–3, one week prior to pairing with a new same-sex partner, female prairie voles were separated from their cage-mates and solo-housed until re-pairing. One week of separation was chosen because female prairie voles display more fighting upon re-pairing with a new same-sex partner after shorter separation times (24–48 hr) compared to longer ones (1 wk) (personal observation). Only female prairie voles were tested because this study focused on formation of new peer relationships, and a prior study revealed males could not be safely re-paired with a same-sex partner in adulthood (Lee et al., 2019).
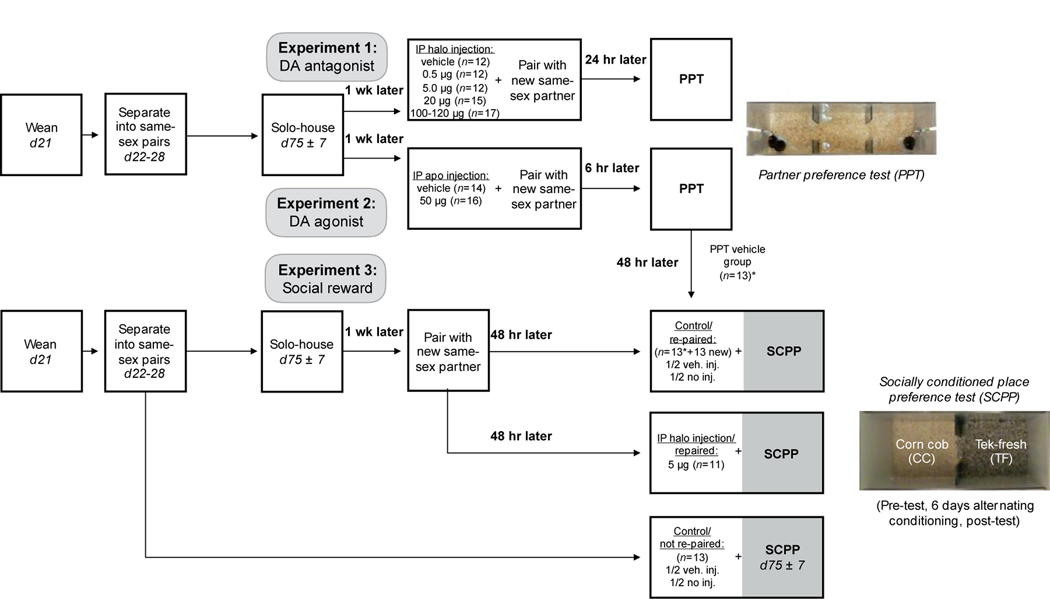
Experiment 1 (n=12–17/group): Voles were given one intraperitoneal injection of one of 4 doses of dopamine antagonist haloperidol or vehicle (control) immediately prior to 24 hr cohabitation with a new same-sex partner, followed by a partner preference test. The highest haloperidol dose has a slightly larger sample size (n=17) because it consists of two partial groups. Experiment 2 (n=14–16/group): Voles were given one intraperitoneal injection of dopamine agonist apomorphine or vehicle immediately prior to 6 hr cohabitation with a new same-sex partner, followed by a partner preference test. Voles from the vehicle group then underwent socially conditioned place preference testing. Experiment 3 (n=11–13/group): Voles were either re-paired or maintained with their partner since weaning, then went through the socially conditioned place preference paradigm with no injection, a vehicle injection, or one 5 μg injection of haloperidol 30 minutes prior to the post-test. Because there was no effect of vehicle injection, injected and non-injected subjects were pooled in the re-paired and not re-paired groups. Subjects in the re-paired group were pooled with an initial set of animals tested at the conclusion of Experiment 2, for a total n=26.
Pharmacological manipulations
The non-selective dopamine receptor antagonist haloperidol (Sigma-Aldrich, St. Louis, MO) was dissolved in a solution of 0.3% tartaric acid (Sigma-Aldrich) in ultra-pure water. Prairie voles received approximately 0.5 μg (0.0125 mg/kg), 5.0 μg (0.125 mg/kg), 20 μg (0.5 mg/kg), or a higher dose (100–120 μg/2.5–3.0 mg/kg) of haloperidol. Doses were scaled by body weight, and doses in μg are listed for a sample 40 g vole. The first two doses successfully blocked partner preference formation in a study of prairie vole mates (Wang et al., 1999); the last dose was added after initial testing with lower doses suggested no effect of haloperidol (as high as 100 μg of haloperidol has been used in prairie voles) (Lonstein, 2002).
The non-selective dopamine receptor agonist apomorphine (Sigma-Aldrich) was dissolved in 0.9% saline. Voles received approximately 50 μg (1.25 mg/kg) of apomorphine, which successfully induced partner preference formation in a study of prairie vole mates (Wang et al., 1999). Voles received one intraperitoneal injection of drug or vehicle (200 μl/40 g body weight) immediately prior to pairing with a new same-sex partner (followed by partner preference test), or 30 minutes prior to a socially conditioned place preference post-test.
Peer partner preference test
Peer partner preference testing was conducted as a classic partner preference test (Williams et al. 1992), but with all female subjects. The testing apparatus consisted of three equal-sized plastic compartments arranged linearly (75x20x30cm), as previously described (e.g. Ahern et al., 2009; Anacker et al., 2016a; Beery et al., 2018; Lee et al., 2019). The cage-mate of the focal prairie vole (the partner) was tethered at one end of the apparatus, and an age-matched, unrelated, same-sex novel vole (the stranger) was tethered at the other end. Strangers were pair-housed from weaning and used no more than 3 times each. The focal vole was placed in the center chamber and allowed to move freely for the duration of the 180-minute test. Tests were video recorded, and trained observers used a custom scoring script (Intervole Timer1.6.pl, AKB) to quantify the amount of time focal voles spent huddling (side-by-side or one on top of the other), duration in each chamber, and number of times the focal vole crossed between chambers. Scorers were unaware of subject treatment and position of the partner/stranger.
Socially conditioned place preference
Socially conditioned place preference testing was conducted in a rectangular plastic apparatus consisting of two equal-sized compartments arranged linearly (50x20x30cm). Both individuals in each pair were tested. Two novel beddings, corn cob (Bed-o’Cobs 1/8”, ScottPharma, Marlborough, MA) and TEK-Fresh were introduced to the prairie voles in a 30 min pre-test (d1 of testing). Voles were placed in the center of the two-chamber apparatus and allowed to roam freely for the duration of the test. 100 g of TEK-Fresh evenly covered one chamber and 200 g of corn cob the other. Different amounts of corn cob and TEK-Fresh bedding were used to keep floor coverage by bedding relatively consistent, as corn cob is denser than TEK-Fresh. After testing, voles were returned to their normal cages. On d2, they were moved as a pair into an opaque white cage with 300 g of corn cob, followed by solo-housing in 200 g of TEK-Fresh on d3. This continued for a total of 6 days of alternating social/corn cob housing and isolate/TEK-Fresh housing. On d8, voles underwent a 30 min post-test. A prior study in the lab using this paradigm found that voles prefer TEK-Fresh over corn cob bedding (Goodwin et al., 2019); thus, the corn cob was paired with the social stimulus (CS+) while the preferred TEK-Fresh was associated with isolation (CS−) to promote counter-conditioning.
Tests were video recorded, and trained observers used a custom scoring script to quantify the amount of time prairie voles spent on each bedding type. Scorers were unaware of subject groups.
Statistical analyses
Group differences in partner huddling, stranger huddling, and preference score (partner/total huddling, e.g. Beery and Zucker, 2010; Anacker et al., 2016b; Harbert et al., 2020) were assessed by one-way ANOVA for multiple groups, and by Welch’s t-tests assuming unequal variances for two groups. Peer partner preference within groups was defined as significantly more time huddling with the partner than the stranger, and Welch’s t-tests were also used for these comparisons. Within-group comparisons in SCPP pre-test vs. post-test were assessed by paired t-tests. Cohen’s d was calculated with an online calculator (University of Colorado Colorado Springs, Lee A. Becker) to determine effect size.
Statistical analyses were performed in JMP 12 (SAS) and Prism 7 (GraphPad Software) unless otherwise noted, all tests were two-tailed, and results were deemed significant at p < 0.05.
RESULTS
Cohabitation times and partner preference formation
Cohabitation durations that were sufficient or insufficient for peer partner preference formation were first assessed in the absence of manipulations including injections. In a previous study, we determined that female prairie voles exhibited highly significant preferences for huddling with a peer partner over a stranger after 24 hr of cohabitation with the new same-sex partner (Lee et al., 2019). After 6 hr of cohabitation with a new same-sex partner, female prairie voles did not exhibit significant peer partner preferences (figure S1a).
In contrast, in opposite-sex pairings, female prairie voles exhibited a significant partner preference for the familiar male after 6 hr, but not 3 hr, of cohabitation with a mate, regardless of mating status (t=5.78, p<0.0001, df=19, d=5.78; figure S1b).
Dopamine signaling is not necessary for peer partner preference
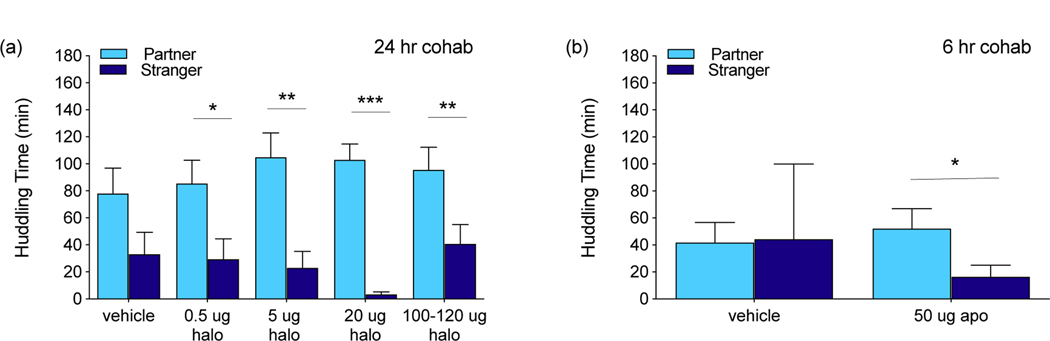
There were no differences in partner huddling across groups, or in stranger huddling across groups (experiment 2, figure 2a, one-way ANOVAs). There was also no significant difference in preference score (partner/total huddling) between groups of voles treated with different doses of haloperidol. Within groups, peer partner preferences were significant in the 0.5 μg (t=2.45, p=0.023, df=21.53, d=1.06), 5 μg (t=3.77, p=0.0013, df=19.24, d=1.72), 20 μg (t=8.39, p<0.0001, df=14.67, d=4.38), and 100–120 μg (t=2.95, p=0.0062, df=30, d=1.72) haloperidol groups. Partner vs. stranger huddling approached significance in the control group (t=1.82, p=0.083, df=21.52, d=1.08). There were also no differences in activity across groups.
Figure 2.
Peer partner preference tests. (a) Haloperidol does not block peer partner preference formation at any dose after 24 hr cohabitation, a normally sufficient cohabitation time. (b) Apomorphine induces peer partner preference formation after 6 hr cohabitation, a normally insufficient cohabitation time. * p < 0.05, ** p < 0.01, *** p < 0.005.
Dopamine signaling may be sufficient to promote peer partner preference
Administration of apomorphine was sufficient to induce significant partner preferences after 6 hr of peer cohabitation in experiment 3 (t=2.091, p=0.047, df=24.14, d=0.85), whereas the control group did not exhibit peer partner preferences after 6 hr (figure 2b). There was no significant difference in partner huddling or preference score between vehicle-treated and apomorphine-treated prairie voles, and stranger huddling decrease was not significant (t=1.63, p=0.12, df=21.07, d= 0.71).
Socially conditioned place preference
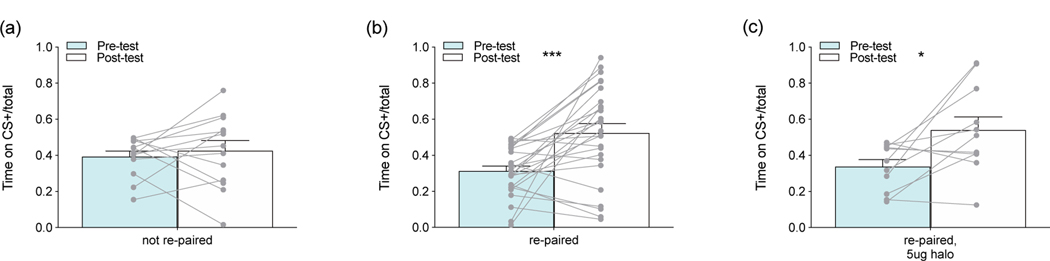
Consistent with our previous findings (Goodwin et al., 2019), voles co-housed since weaning with a same-sex partner in experiment 4 did not exhibit socially conditioned place preferences for their partners, defined as a significant difference between pre-test and post-test in the proportion of time spent on the conditioned stimulus over the total testing time (CS+/total) (figure 3a). However, prairie voles re-paired with new peer partners in adulthood exhibited significant socially conditioned place preferences (t=4.077, p=0.0004, df=25, d=1.009) (figure 3b). This conditioned preference was not blocked by treatment with 5 μg haloperidol (t=2.72, p=0.022, df=10, d=1.050) (figure 3c).
Figure 3.
Socially conditioned place preference tests. (a) Consistent with our prior findings, prairie voles housed with their peer partners since weaning (not re-paired, ½ injected with vehicle control) did not exhibit significant socially conditioned place preference. (b) Prairie voles re-paired with new same-sex partners in adulthood (½ injected with vehicle control) did exhibit significant socially conditioned place preference. (c) Haloperidol administration (re-paired, 5ug halo) at pairing did not block socially conditioned place preference. * p < 0.05, *** p < 0.005.
DISCUSSION
Investigation of prairie vole peer relationships allows for the comparative assessment of mechanisms underlying bond formation across relationship type (mate vs. peer), as well as across species (prairie vs. meadow). Blockade of dopamine receptors did not prevent the formation of peer partner preferences after a cohabitation time that was sufficient in controls, suggesting that dopamine signaling is not necessary for the formation of prairie vole peer relationships. Distinct from prairie vole mate relationships, neither prairie vole nor meadow vole peer partnerships rely on dopamine signaling; thus, the necessary role of dopamine signaling appears specific to reproductive pair bonds rather than to all prairie vole relationships. This finding is consistent with work on behavioral reward in prairie voles indicating that mates are more rewarding than peers in multiple tests of motivation (Goodwin et al., 2019; Beery et al., 2019).
Pharmacologically unmanipulated prairie voles housed with a novel peer in adulthood exhibited socially conditioned place preferences for beddings associated with these partners, suggesting that peer exposure can be reinforcing. Haloperidol did not interfere with the expression of socially conditioned place preferences. The focus of the current study was on formation of peer relationships, which requires assessment of newly established pairs. However, we tested a group of voles co-housed with their partners since weaning alongside voles re-paired in adulthood and found that unlike new peer relationships, long-term peer relationships were not reinforcing. Similarly, Goodwin et al. (2019) tested long-term partnerships and found that prairie voles did not exhibit socially conditioned place preferences for chambers containing a bedding associated with their long-term peer partners. This difference between the reinforcing properties of old vs. new partners suggests that there is an effect of novelty on peer social reward. All voles re-paired in adulthood were separated for one week prior to re-pairing to mitigate aggression, so it is also possible that this period of isolation prior to formation of new pairs increases social motivation. Prairie voles isolated from their same-sex partners for 4 weeks exhibit anxiety- and depressive-like behaviors (Grippo et al., 2008) that may be consistent with increased social motivation after a shorter isolation time.
Although pharmacological blockade of dopamine signaling did not prevent formation of new peer partner preferences, injection of a dopamine receptor agonist facilitated the formation of peer partner preferences after a normally insufficient cohabitation time. This suggests that dopamine signaling may be sufficient to promote prairie vole peer relationships, as it is in mate partnerships. While the sufficiency—but not necessity—of dopamine signaling may at first seem incongruent, this finding is not surprising in light of numerous studies on drugs of abuse that employ dopamine receptor agonists to condition and reinforce artificial behaviors. For example, male rats treated with the D2-type receptor agonist quinpirole can be conditioned to exhibit same-sex social preferences in 20 min preference tests (Triana-Del Rio et al., 2011), and will even prefer familiar male partners over receptive females (Cibrian-Llanderal et al., 2012). Rats, like mice and many other rodents studied in the lab, are gregarious and do not normally display preferences for familiar peers (Schweinfurth et al., 2017). Such studies highlight the generally reinforcing properties of dopamine receptor agonism, and thus we must exercise caution in extrapolating between sufficiency and normal biological function. Although dopamine signaling may not play an essential role in the endogenous processes that occur during formation of new peer relationships, it may be co-opted to induce many behaviors, including ones that do not naturally occur.
Our results provide evidence that dopamine does not play a necessary role in prairie vole peer relationships, but also that prairie voles find peers rewarding. It is possible, then, that a different reward system mediates the formation of these peer relationships. Studies on sucrose reward in rats (reviewed in Berridge and Robinson, 1998) and mice (Cannon and Palmiter, 2003) show that animals deficient in dopamine display normal sucrose preference. There is also evidence that dopamine antagonism or depletion of dopamine in the nucleus accumbens does not inhibit other rewarding behaviors, such as approach and choice of estrous females and copulation (reviewed in Salamone and Correa, 2002). There can be reward without dopamine, and dopamine likely only mediates specific aspects of reward (reviewed in Berridge and Robinson, 2003; Salamone, 2007; Fujita et al., 2019). Other systems that may regulate reward and selective aggression in prairie vole peer relationships include opioid, oxytocin, and other signaling pathways found to be important in prairie vole pair bonds.
Relationships between peers are an important aspect of the social system of prairie voles and other social animals, including humans. This study adds to a growing body of evidence that selective non-reproductive relationships should be studied in their own right, as they are likely mediated by different mechanisms than reproductive relationships. While dopamine signaling is necessary for prairie vole pair bonds, pharmacological data from the present study suggest that prairie vole peer relationships are not dopamine-dependent. However, prairie vole peer relationships are still reinforced; behavioral data suggests that they are intermediate between prairie vole mate relationships and meadow vole peer relationships, with prairie vole peers providing less reinforcement than mates. This is unlike meadow voles, who conditioned away from social bedding in the same paradigm (Goodwin et al., 2019). It is not surprising that reproductive relationships are highly reinforced and mediated by conserved reward mechanisms, and that this may not necessarily be the case for non-reproductive relationships. Thus, study of non-reproductive relationships is essential to fully understanding the mechanisms that underlie social behavior as a whole.
Supplementary Material
Supplementary Figure 1. Partner preference tests in female prairie voles. (a) 6 hr cohabitation is not sufficient for partner preference formation with peers (n=5). (b) 3 hr cohabitation is not sufficient for partner preference formation with mates (n=10). 6 hr cohabitation, with or without mating, is sufficient for partner preference formation with mates (n=13). *** p < 0.005.
Figure 1.
Experimental design: In experiments 1 & 2, female prairie voles underwent injections of haloperidol, apomorphine, or vehicle prior to pairing with a new same-sex partner, followed by partner preference testing (PPT). The vehicle group in experiment 2 subsequently underwent socially conditioned place preference (SCPP) testing; in experiment 3, a new cohort of voles was also re-paired prior to SCPP, and thus the two groups were pooled (indicated with an asterisk) for SCPP analysis. Voles were re-paired with a new same-sex partner prior to SCPP (½ no injection, ½ vehicle injection), re-paired with a new-same sex partner and given a dose of haloperidol, or were not re-paired (½ no injection, ½ vehicle injection).
Highlights:
Prairie voles form same-sex partner preferences for familiar peers
Females showed socially conditioned place preferences (SCPP) for new peer partners
Dopamine receptor agonist facilitated peer partner preference formation
Dopamine receptor antagonist did not prevent formation of peer partner preferences
Dopamine receptor antagonist did not block SCPP for peer partners
ACKNOWLEDGEMENTS
We are grateful to Kate Shambaugh, Sarah Lopez, Jessie Marsh, Maddie Lerner, Karina Lieb, Asia DeRosby, and Paige Salters for their help with behavioral testing and scoring. We would also like to thank the staff of the Smith College Animal Care Facility for animal care and colony maintenance.
FUNDING SOURCES
This research was supported by the National Institute of Mental Health of the National Institutes of Health under Award Number R15MH113085.
Footnotes
DECLARATION OF INTEREST
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ahern TH, Modi ME, Burkett JP, Young LJ. 2009. Evaluation of two automated metrics for analyzing partner preference tests. J Neurosci Methods 182:180–188. 10.1016/j.jneumeth.2009.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker AMJ, Christensen JD, LaFlamme EM, Grunberg DM, Beery AK. 2016a. Septal oxytocin administration impairs peer affiliation via V1a receptors in female meadow voles. Psychoneuroendocrinology 68:156–162. 10.1016/j.psyneuen.2016.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker AMJ, Reitz KM, Goodwin NL, Beery AK. 2016b. Stress impairs new but not established relationships in seasonally social voles. Horm Behav 79:52–57. 10.1016/j.yhbeh.2016.01.004 [DOI] [PubMed] [Google Scholar]
- Aragona BJ, Liu Y, Curtis JT, Stephan FK, Wang Z. 2003. A critical role for nucleus accumbens dopamine in partner-preference formation in male prairie voles. J Neurosci 23:3483–3490. 10.1523/JNEUROSCI.23-08-03483.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona BJ, Liu Y, Yu YJ, Curtis JT, Detwiler JM, Insel TR, Wang Z. 2006. Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nat Neurosci 9:133–139. 10.1038/nn1613 [DOI] [PubMed] [Google Scholar]
- Beery AK. 2019. Frank Beach award winner: Neuroendocrinology of group living. Horm Behav. 10.1016/j.yhbeh.2018.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK, Chen J, Lopez S, Lee NS. 2019. Social selectivity and social reward in prairie voles. Program No 06923 2019 Neurosci Meet Plan Chic IL Soc Neurosci 2019 Online. [Google Scholar]
- Beery AK, Christensen JD, Lee NS, Blandino KL. 2018. Specificity in sociality: mice and prairie voles exhibit different patterns of peer affiliation. Front Behav Neurosci 12:50 10.3389/fnbeh.2018.00050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK, Zucker I. 2010. Oxytocin and same-sex social behavior in female meadow voles. Neuroscience 169:665–673. 10.1016/j.neuroscience.2010.05.023 [DOI] [PubMed] [Google Scholar]
- Beloate LN, Coolen LM. 2017. Influences of social reward experience on behavioral responses to drugs of abuse: Review of shared and divergent neural plasticity mechanisms for sexual reward and drugs of abuse. Neurosci Biobehav Rev 83:356–372. 10.1016/j.neubiorev.2017.10.024 [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. 1998. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Rev 28:309–369. 10.1016/S0165-0173(98)00019-8 [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. 2003. Parsing reward. Trends Neurosci 26:507–513. 10.1016/S0166-2236(03)00233-9 [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Nair HP, Ahern TH, Neumann ID, Young LJ. 2009. The CRF system mediates increased passive stress-coping behavior following the loss of a bonded partner in a monogamous rodent. Neuropsychopharmacol 34:1406–1415. 10.1038/npp.2008.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkett JP, Andari E, Johnson ZV, Curry DC, Waal FBM de, Young LJ. 2016. Oxytocin-dependent consolation behavior in rodents. Science 351:375–378. 10.1126/science.aac4785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon CM, Palmiter RD. 2003. Reward without dopamine. J Neurosci 23:10827–10831. 10.1523/JNEUROSCI.23-34-10827.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS. 2017. The oxytocin–vasopressin pathway in the context of love and fear. Front Endocrino 8: 356 10.3389/fendo.2017.00356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cibrian-Llanderal T, Rosas-Aguilar V, Triana-Del Rio R, Perez CA, Manzo J, Garcia LI, Coria-Avila GA. 2012. Enhaced D2-type receptor activity facilitates the development of conditioned same-sex partner preference in male rats. Pharmacol Biochem Behav 102:177–183. 102:177–183 10.1016/j.pbb.2012.04.007 [DOI] [PubMed] [Google Scholar]
- Curtis JT, Liu Y, Aragona BJ, Wang Z. 2006. Dopamine and monogamy. Brain Res 1126:76–90. 1126:76–90. 10.1016/j.brainres.2006.07.126 [DOI] [PubMed] [Google Scholar]
- Curtis JT, Stowe JR, Wang Z. 2003. Differential effects of intraspecific interactions on the striatal dopamine system in social and non-social voles. Neuroscience. 118(4):1165–73. 10.1016/S0306-4522(03)00032-0 [DOI] [PubMed] [Google Scholar]
- DeVries AC, Johnson CL, Carter CS. 1997. Familiarity and gender influence social preferences in prairie voles (Microtus ochrogaster). Can J Zool 75:295–301. 10.1139/z97-037 [DOI] [Google Scholar]
- Fujita M, Ide S, Ikeda K. 2019. Opioid and nondopamine reward circuitry and state-dependent mechanisms. Ann N Y Acad Sci 1451:29–41. 10.1111/nyas.13605 [DOI] [PubMed] [Google Scholar]
- Gingrich B, Liu Y, Cascio C, Wang Z, Insel TR. 2000. Dopamine D2 receptors in the nucleus accumbens are important for social attachment in female prairie voles (Microtus ochrogaster). Behav Neurosci 114:173–183. 10.1037/0735-7044.114.1.173 [DOI] [PubMed] [Google Scholar]
- Gobrogge K, Wang Z. 2016. The ties that bond: neurochemistry of attachment in voles. Curr Opin Neurobiol 38:80–88. 10.1016/j.conb.2016.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin NL, Lopez SA, Lee NS, Beery AK. 2019. Comparative role of reward in long-term peer and mate relationships in voles. Horm Behav 111:70–77. 10.1016/j.yhbeh.2018.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Wu KD, Hassan I, Carter CS. 2008. Social isolation in prairie voles induces behaviors relevant to negative affect: toward the development of a rodent model focused on co-occurring depression and anxiety. Depress Anxiety 25:E17–E26. 10.1002/da.20375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbert KJ, Pellegrini M, Gordon KM, Donaldson ZR. 2020. How prior pair-bonding experience affects future bonding behavior in monogamous prairie voles. Horm Behav:104847. 10.1101/2020.06.05.135160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee NS, Goodwin NL, Freitas KE, Beery AK. 2019. Affiliation, aggression, and selectivity of peer relationships in meadow and prairie voles. Front Behav Neurosci 13:52 10.3389/fnbeh.2019.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberwirth C, Liu Y, Jia X, Wang Z. 2012. Social isolation impairs adult neurogenesis in the limbic system and alters behaviors in female prairie voles. Horm Behav 62:357–366. 10.1016/j.yhbeh.2012.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wang ZX. 2003. Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience 121:537–544. 10.1016/s0306-4522(03)00555-4 [DOI] [PubMed] [Google Scholar]
- Lonstein JS. 2002. Effects of dopamine receptor antagonism with haloperidol on nurturing behavior in the biparental prairie vole. Pharmacol Biochem Behav 74:11–19. 10.1016/S0091-3057(02)00952-8 [DOI] [PubMed] [Google Scholar]
- Numan M, Young LJ. 2016. Neural mechanisms of mother–infant bonding and pair bonding: Similarities, differences, and broader implications. Horm Behav 77:98–112. 10.1016/j.yhbeh.2015.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell LA, Hofmann HA. 2011. The vertebrate mesolimbic reward system and social behavior network: A comparative synthesis. J Comp Neurol 519:3599–3639. 10.1002/cne.22735 [DOI] [PubMed] [Google Scholar]
- Resendez SL, Keyes PC, Day JJ, Hambro C, Austin CJ, Maina FK, Eidson LN, PorterStransky KA, Nevárez N, McLean JW, others. 2016. Dopamine and opioid systems interact within the nucleus accumbens to maintain monogamous pair bonds. ELife 5:e15325. 10.7554/eLife.15325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell ES, Mendoza SP, Ragen BJ, Bales KL. 2019. Dopamine D1-like receptors regulate agonistic components of pair bond maintenance behaviors in male titi monkeys (Callicebus cupreus). Psychoneuroendocrinology 106:259–67. 10.1016/j.psyneuen.2019.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD. 2007. Functions of mesolimbic dopamine: changing concepts and shifting paradigms. Psychopharmacology (Berl) 191:389–389. 10.1007/s00213-006-0623-9 [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M. 2002. Motivational views of reinforcement: implications for understanding the behavioral functions of nucleus accumbens dopamine. Behav Brain Res 137:3–25. 10.1016/S0166-4328(02)00282-6 [DOI] [PubMed] [Google Scholar]
- Schweinfurth MK, Neuenschwander J, Engqvist L, Schneeberger K, Rentsch AK, Gygax M, Taborsky M. 2017. Do female Norway rats form social bonds? Behav Ecol Sociobiol 71:98 10.1007/s00265-017-2324-2 [DOI] [Google Scholar]
- Smith AS, Wang Z. 2014. Hypothalamic oxytocin mediates social buffering of the stress response. Biol Psychiatry 76:281–288. 10.1016/j.biopsych.2013.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V, Campolongo P, Vanderschuren LJMJ. 2011. Evaluating the rewarding nature of social interactions in laboratory animals. Dev Cogn Neurosci 1:444–458. 10.1016/j.dcn.2011.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triana-Del Rio R, Montero-Domínguez F, Cibrian-Llanderal T, Tecamachaltzi-Silvaran MB, Garcia LI, Manzo J, Hernandez ME, Coria-Avila GA. 2011. Same-sex cohabitation under the effects of quinpirole induces a conditioned socio-sexual partner preference in males, but not in female rats. Pharmacol Biochem Behav 99:604–613. 10.1016/j.pbb.2011.06.006 [DOI] [PubMed] [Google Scholar]
- Walum H, Young LJ. 2018. The neural mechanisms and circuitry of the pair bond. Nat Rev Neurosci:1 10.1038/s41583-018-0072-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Yu G, Cascio C, Liu Y, Gingrich B, Insel TR. 1999. Dopamine D2 receptor-mediated regulation of partner preferences in female prairie voles (Microtus ochrogaster): a mechanism for pair bonding? Behav Neurosci 113:602 10.1037//0735-7044.113.3.602 [DOI] [PubMed] [Google Scholar]
- Williams JR, Catania KC, Carter CS. 1992. Development of partner preferences in female prairie voles (Microtus ochrogaster): The role of social and sexual experience. Horm Behav 26:339–349. 10.1016/0018-506X(92)90004-F [DOI] [PubMed] [Google Scholar]
- Young KA, Gobrogge KL, Liu Y, Wang Z. 2011. The neurobiology of pair bonding: Insights from a socially monogamous rodent. Front Neuroendocrinol 32:53–69. 10.1016/j.yfrne.2010.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Partner preference tests in female prairie voles. (a) 6 hr cohabitation is not sufficient for partner preference formation with peers (n=5). (b) 3 hr cohabitation is not sufficient for partner preference formation with mates (n=10). 6 hr cohabitation, with or without mating, is sufficient for partner preference formation with mates (n=13). *** p < 0.005.