Abstract
Alterations in white matter integrity have been demonstrated in a number of psychiatric disorders involving emotional disruptions. One such pathway – the uncinate fasciculus – connects the orbitofrontal cortex (OFC) to the medial temporal lobes (MTL) and has been associated with early life adversity, maltreatment, anxiety, and depression. While it is purported to play a role in episodic memory and discrimination, its exact function remains poorly understood. We have previously described the role of the amygdala and dentate (DG)/CA3 fields of the hippocampus in the mnemonic discrimination of emotional experiences (i.e. emotional pattern separation). However, how this computation may be modulated by connectivity with the orbitofrontal cortex remains unknown. Here we asked if the uncinate fasciculus plays a role in influencing MTL subregional activity during emotional pattern separation. By combining diffusion imaging with high-resolution fMRI, we found that reduced integrity of the UF is related to elevated BOLD fMRI activation of the DG/CA3 subregions of the hippocampus during emotional lure discrimination. We additionally report that higher levels of DG/CA3 activity are associated with poorer memory performance, suggesting that greater activation in this network (possibly driven by CA3 recurrent collaterals) is associated with memory errors. Based on this work we suggest that the UF is one pathway that may allow the OFC to exert control on this network and improve discrimination of emotional experiences, although further work is necessary to fully evaluate this possibility. This work provides novel insight into the role of prefrontal interactions with the MTL, particularly in the context of emotional memory.
Keywords: Uncinate fasciculus, pattern separation, hippocampus, memory, emotion
Introduction
Episodic memory – memory for facts and events – involves contributions from the medial temporal lobe (MTL), prefrontal cortex (PFC), and their interactions (Eichenbaum, 2017; Preston & Eichenbaum, 2013). While a considerable imaging literature in humans has focused on the hippocampus, relatively few studies have examined the role of white matter pathways connecting the MTL to the PFC in supporting episodic memory computations.
One such anatomical connection, the uncinate fasciculus (UF), is a major white matter bundle connecting the MTL to the orbitofrontal cortex (OFC) in primates. This particular connection forms an “anterior pathway” by which the MTL can communicate with the PFC. Although often described as innervating the hippocampus, human dissection and axonal tracing studies in primates indicate that this bundle more accurately innervates the basolateral amygdala (BLA) and the entorhinal cortices of the MTL and projects to a number of prefrontal regions including the OFC (Figure 1; Ebeling and Von Cramon, 1992; Von der Hide et al., 2013; Thiebaut de Schotten et al., 2012).
Figure 1.

Circuitry of orbitofrontal connectivity with the medial temporal lobe subfields via the uncinate fasciculus. The uncinate innervates both the lateral amygdala and cortices of the MTL. The cortices of the MTL feed into the hippocampal subfields of DG, CA3, and CA1 via multiple pathways one of which includes the perforant path. The perforant pathway feeds into the DG/CA3 subfields where the neuronal computation known as pattern separation is thought to occur. In this manner, the uncinate fasciculus is a possible direct route of inhibitory information to these regions.
In humans, abnormalities of the UF have been implicated in psychiatric disorders characterized by emotional dysregulation such as major depression and anxiety, as well as in response to early life stress and maltreatment (Taylor et al., 2007; Zhang et al., 2012; Eluvathingal et al., 2006; Ho et al., 2017; Hanson et al., 2015).
While the exact functional role of the UF remains unclear, it is thought to be involved in episodic memory, language, and socio-emotional processing (Von Der Heide et al., 2013). The UF was recently hypothesized to play a role in adjudicating among competing episodic memory representations at retrieval (Alm et al., 2016). Competition among memory traces during retrieval could be mediated, at least in part, by pattern separation. This process, as first suggested by Marr (Marr, 1971), is defined as the neural computation used to orthogonalize overlapping similar stimuli, a process that particularly involves the dentate gyrus and CA3 regions of the hippocampus (Yassa & Stark, 2011; Leal and Yassa 2018). Functional MRI studies have noted pattern separation related activity in the DG/CA3 region during incidental encoding of similar lure items (Bakker et al. 2008; Lacy et al. 2011) as well as explicit performance of mnemonic discrimination tasks (Yassa et al. 2010a, 2010b; Reagh et al. 2018). These tasks require participants to determine during retrieval if items highly similar to the previously encoded items are “new” or “old”, which requires suppression of interference from competing memory representations. In the context of emotional memories, we have previously shown this process extends to also include the amygdala (Leal et al. 2014b; Zheng et al. 2019). Given this prior work, we reasoned that tasks assessing pattern separation would be particularly well-suited to understanding the impact of the UF connection on emotional episodic memory discrimination. However, its role in this process has never before been investigated.
Using multimodal neuroimaging, we tested the hypothesis that the UF may play a role in regulating mnemonic discrimination specifically for emotional items by modulating BOLD fMRI activity in the DG/CA3 region, which, would predict emotional discrimination performance. To assess the structure of the UF, we implemented a new method to anatomically track the pathway using non-tensor-based deterministic tractography and utilized a model-free diffusion metric (normalized quantitative anisotropy – nQA) designed to capture the degree of diffusion among primary fiber orientations accounting for partial volume effects inherent in the tensor model (Yeh et al., 2013). To assess the functional role of the DG/CA3 region of the hippocampus, we used high-resolution (1.5mm isotropic) BOLD fMRI capable of resolving hippocampal subfields, while participants performed the emotional discrimination (i.e. pattern separation) task (Figure 2). The outcome measure was the “lure discrimination index”, a measure of how well participants are able to discriminate among similar emotional and neutral scenes, corrected for response bias.
Figure 2.

Schematic of the emotional pattern separation task. The left side of the image shows the incidental encoding phase of the task where participants are asked to rate images as “negative”, “neutral”, or “positive”. On the right side of the image is the memory portion of the task. This phase, known as the Old/New Recognition phase shows participants several variants of previously seen images (lures), as well as repeated images (repeats), and never before seen images (foils). Subjects are asked to make “old” or “new” judgements during the trial and response times are recorded.
Materials and Methods
Participants:
A total sample of 27 participants (15 female) were recruited from Johns Hopkins University and received monetary compensation for their participation. Informed consent was given by all participants and all procedures approved by the Johns Hopkins University Institutional Review Board. Subjects were administered a neuropsychological battery which is summarized in Table 1. All participants were screened against major medical or psychiatric morbidities, substance abuse history, and additional criteria of MRI contraindications like metal in the body. Results of the fMRI analysis of this dataset were previously published in Leal et al. 2014b with the exception of a single subject who did not complete the DWI scan.
Table 1.
Participant Demographics and Neuropsychological Results
| N = 27 (15 female) | Mean (± SD) |
|---|---|
| Age | 21 ± 3.38 |
| Beck Depression Inventory-II | 10.55 ± 11.48 |
| RAVLT Immediate Recall | 12.59 ± 1.97 |
| RAVLT Delayed Recall | 11.63 ± 3.44 |
| Digit Span Forward | 11.96 ± 1.85 |
| Digit Span Backward | 8.29 ± 2.03 |
| Mini Mental State Exam | 29.22 ± 0.97 |
| Trails Making Test A | 19.58 ± 5.31 |
| Trails Making Test B | 45.59 ± 11.91 |
Imaging Data Collection:
All data were collected on a 3 Tesla Philips scanner. We collected an ultrahigh-resolution structural MPRAGE scan that we developed for accurate delineation of hippocampal subfields and high-resolution diffeomorphic alignment (0.55 mm isotropic resolution; 273 sagittal slices, field of view = 240 x 240 mm, flip angle = 9, TR/TE = 13/5.9 ms, matrix size = 448 x 448, inversion pulse TI = 1110 ms). SENSE parallel imaging was used in two directions (2 x 1.5). The SAR (<10%) and PNS (<75%) were within required limits based on the scanner-calculated values. Task-activated functional data were collected and processed in the same manner as Leal et al., 2014b. Briefly, these data were collected with a high-speed EPI single-shot pulse sequence (1.5mm isotropic, 19 oblique axial slices parallel to the principal axis of the hippocampus, field of view = 96 x 96 mm, flip angle= 70, SENSE parallel reduction factor = 2, TR/TE = 1500/30 ms, matrix size = 64 x 64. Diffusion weighted imaging were collected with an echo planar imaging sequence. The data were 3 mm isotropic, TR/TE = 6800/67 ms, 65 slices, 32 non-collinear directions, one B0, b-value = 700 s/mm2. Diffusion MR data were collected during the same scan session as the fMRI scanning.
Emotional Pattern Separation Task:
The emotional pattern separation task consists of an incidental encoding phase where subjects are instructed to rate the valence of each image from positive, negative, or neutral based on a priori ratings outlined in Leal et al., 2014a. Subjects were later tested during the “Old/New Recognition” portion of the task. In this portion, foils (new images), targets (same images), and high and low similarity lures were presented. Subjects were asked to make “Old” or “New” recognition judgments (Figure 2). In this case, correctly identifying that a lure item was “New” is termed as a Correct Rejection (CR) and falsely claiming that a lure item is “Old” would be termed a False Alarm (FA). We assessed behavioral discrimination on this task by quantifying a lure discrimination index (LDI) which measures performance on the task accounting for response bias: P(“New”|Lure) – P(“New”|Target) or lure correct rejections minus target misses. This was calculated for high and low similarity lures across the three valence types. This is a measure we have frequently employed in the past as it accounts for response bias (e.g. Leal et al. 2014; Reagh et al. 2018). LDI of highly similar lure items was chosen in this case because of the UFs’ purported role in adjudicating between similar items during retrieval.
Functional MRI Image Analysis
Task data were analyzed using the open source package Analysis of Functional Neuroimages (AFNI) (Cox, 1996) and are previously described in detail in Leal et al. (2014b). Briefly, images were corrected for slice timing and subject motion censoring motion of 3° of rotation or 2mm translocation in any direction relative to prior acquisition. Upon processing for motion we then registered our functional images to the structural (MPRAGE) using AFNI/s 3dAllineate program. We use Advanced Normalization Tools (Avants et al., 2011) which implements a robust diffeomorphic algorithm to warp structural scans to a common template based on the entire sample. The same transformations were then applied to the functional data. Finally, behavioral vectors were created based on trial type orthogonalizing trials unique in emotion, similarity, and behavioral decision. These vectors there then used in a deconvolution approach based on multiple linear regression and the resulting fit coefficients (betas) estimated activity versus novel foils as an implicit baseline for a given time points and trial type in a voxel was calculated. We used the sum of the fit coefficients over an expected hemodynamic response of 3–12 seconds after trial onset as the model’s estimate of response to each trial type as described in Leal et al., 2014b. Finally, fit coefficients were extracted using a region of interest approach for the DG/CA3 and CA1 subfields of the hippocampus according to Leal et al., 2014b.
Diffusion Weighted Imaging Analysis:
Diffusion data were processed for eddy correction using FSLs eddy_correct and analyzed using DSI-Studio (http://dsi-studio.labsolver.org). Individual subject data were then processed using DSI-Studios Q-Spin Diffeomorphic Reconstruction (QSDR) method which calculates the orientational distribution of the density of diffusing water in MNI space (Yeh & Tseng, 2011). All subjects met the criterion of fitting to the template space by obtaining an R-squared value of greater than 0.63 as suggested by the software developers. QSDR was chosen as the reconstruction method in order to overcome several limitations of the tensor model. First, QSDR provides reconstruction in a template space allowing the creating of standardized “regions-of-avoidance” to filter out known false-projecting fibers. Second, QSDR allowed for the computation of orientation distribution functions rather than tensor-based computation of diffusion signals. This is important because the orientation distribution functions calculated with this method are presumed to resolve partial volume fractions, model crossing-fibers, and are thought to result in more accurate deterministic tractography (Yeh et al., 2013; Maier-Hein et al., 2017). Our tractography protocol included a customized region-of-avoidance by merging three intersecting planes (one axial, one coronal, one sagittal) with a sphere over the striatum and nucleus accumbens complex (Figure 3). The use of this region allowed us to filter out false projections along the inferior longitudinal fasciculi and projections extending medially toward the striatum and anterior cingulate cortex. We included bilateral uncinate fasciculus regions from the Johns Hopkins White Matter Atlas available through DSI-Studio as “seed regions” and Brodmann’s areas 11 and 47 as “end regions”. The tractography settings were: seed count = 500,000, threshold index = QA, FA threshold = 0.0, seed plan = “0”, initial direction=”0”, interpolation= “0”, step size = “0”, turning angle = “55”, smoothing = 0.6, min length = 0, max length = 150. The tractography protocol was repeated for the left and right hemisphere separately by using the left or right uncinate seed region in each iteration. In order to quantify the integrity of the resulting streamlines we used the model-free measure of normalized quantitative anisotropy (nQA) to quantify the degree of diffusion along principle orientations on an ODF. This measure is thought to more accurately represents the degree of diffusion among principal axonal directions within a single voxel rather than fractional anisotropy (FA), which is a singular tensor-based measure that does not account for multiple orientations. The nQA measure also accounts for the isotropic component of diffusion and, similar to FA, is normalized on a scale from 0–1 and reflects greater diffusivity among primary fiber orientations (Yeh et al., 2013; Yeh et al., 2010). Together, these methods allowed for consistent modeling of the UF in a standardized and automated way with no manual intervention.
Figure 3.

In-vivo dissection of the uncinate fasciculus derived from model-free QSDR and deterministic tractography in the template space. (a) The “region-of-avoidance” is depicted in yellow, uncinate fasciculus “seed region” depicted in purple, and “end” region in blue containing merged Brodmanns areas 11 and 47. (b) The resulting UF tractography for the left hemisphere. The tractography shows branching in the prefrontal cortex region as anatomical studies suggest. We also provide tractography results for 6 random subjects viewed from a sagittal perspective in the template space. Here we show the consistent nature of the custom protocol in modeling of this complex pathway. In each of the subjects the U-shaped fanning bundle is clearly seen with individual difference in the amount of branching in the prefrontal cortex.
Statistical Analysis:
All statistical analyses were conducted using Prism GraphPad 7 and R-studio. We conducted all correlational analysis in Prism 7 computing two-tailed Pearson correlation coefficients with 95% confidence intervals. Unlike our previous work, we did not choose an arbitrary cutoff to be used for a binary classification of “healthy” and “depressed” as we did not recruit a clinically assessed sample. Instead, multiple linear regressions were used to assess the influence of Beck Depression Inventory-II (on a continuous basis) on relationships between behavior, fMRI, and diffusion measures and were conducted in R-studio. Bootstrap analysis were conducted in R-studio using the Boot package with 1000 repetitions. 95% confidence intervals are reported. Statistical values were considered significant at an alpha level of 0.05. Since we only tested targeted hypotheses and used a priori planned comparisons, correction for multiple comparisons was not necessary (Rothman, 1990; Saville, 1990).
Results
UF Integrity Predicts DG/CA3 Activity During Emotional Lure Discrimination
To test the hypothesis that UF structural integrity is associated with MTL subregional activation profiles involved in discrimination of highly similar lures, we used normalized quantitative anisotropy (nQA) to quantify the UF and the average of beta coefficients of signal in the DG/CA3 region during correct discrimination as our variables of interest. Here we separately assessed the magnitude of this relationship (between UF integrity and DG/CA3 activity) in highly similar negative, neutral, and positive correct discrimination trials within each hemisphere. We investigated each hemisphere separately as recent investigations modeling the uncinate fasciculus using diffusion spectrum imaging have reported hemispheric differences (Leng et al., 2016). We focused on highly-similar lures as prior work suggested that DG/CA3 signals are particularly sensitive to this more challenging condition (Leal et al. 2014b). We found a significant negative correlation between left UF nQA and left DG/CA3 activity for highly similar negative (r = −0.41, P = 0.035) and positive (r = −0.44, P = 0.022) but not neutral (r = 0.12, P = 0.55) CRs (Figure 4a–c). To rule out the possibility of individual points having a large contribution to the aforementioned effects we conducted bootstrap analysis with 1000 simulations. The 95% confidence interval for the Pearson correlation coefficient bootstrap effect of the relationship between left UF nQA and left DG/CA3 activity for negative CRs ranged from - to −0.12 (SE = 0.14). The 95% confidence interval for the Pearson correlation coefficient bootstrap effect of the relationship between left UF nQA and left DG/CA3 activity for positive CRs ranged from −0.82 to −0.11 (SE = 0.18). As a result of these analyses, we then tested if the slope of the relationship between UF integrity and DG/CA3 activity during “emotional” CRs was significantly different the relationship between UF integrity and DG/CA3 activity during neutral lure discrimination. We found that the slope of the relationship between UF integrity and DG/CA3 activity during negative correct rejections was significantly different than the slope of the relationship between UF integrity and DG/CA3 activity during neutral lure CRs (Z = 2.057, P = 0.040). Similarly, we found that the slope of the relationship between UF integrity and DG/CA3 activity during positive correct rejections was significantly different than the slope of the relationship between UF integrity and DG/CA3 activity during neutral lure CRs (Z = 2.22, P = 0.027). These data suggest that lower integrity of the left UF is associated with higher levels of activity in the left DG/CA3 during correct emotional but not neutral discrimination, suggesting a possible role in limiting the activity of this subregion during emotional conditions.
Figure 4.
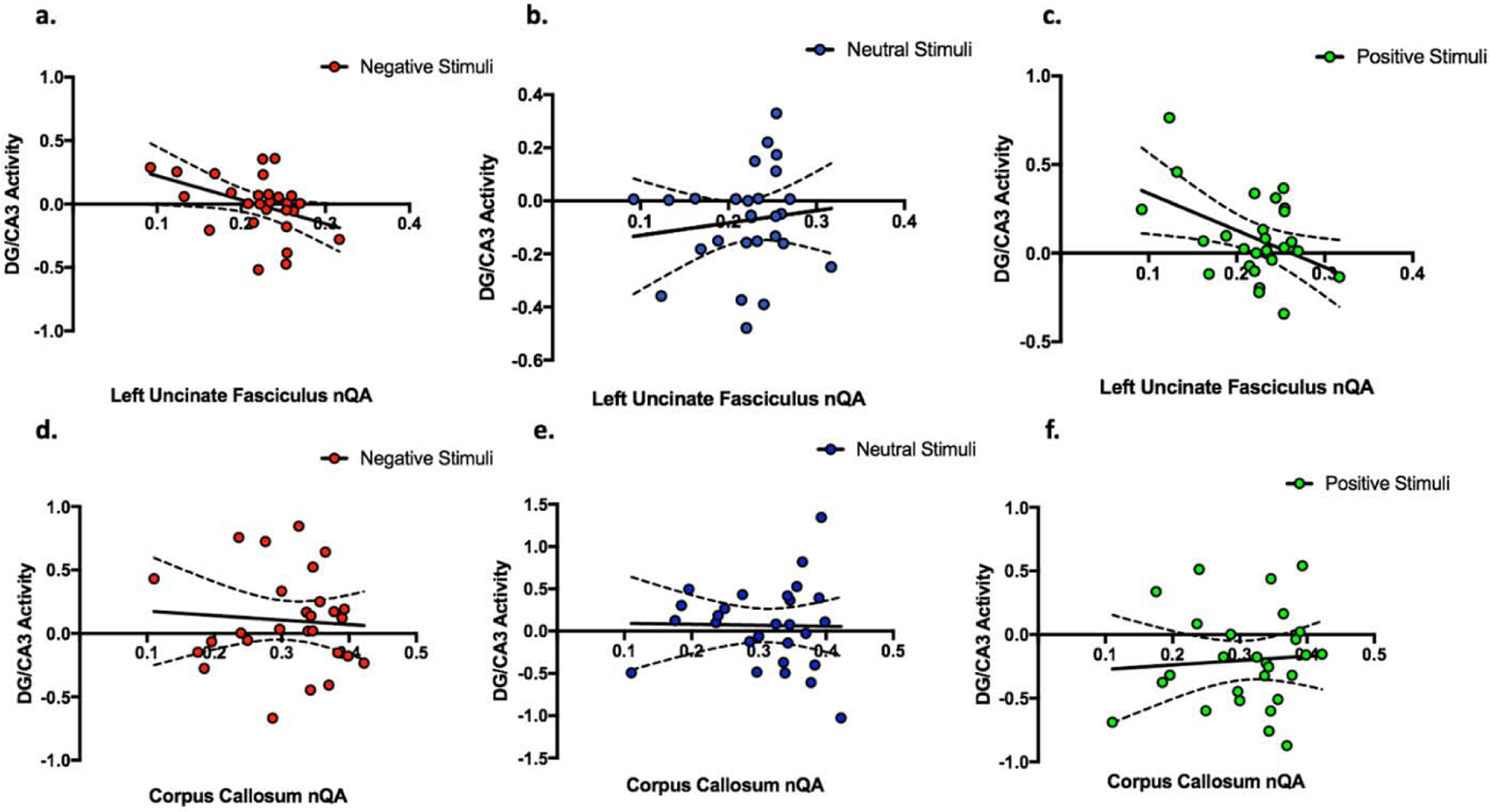
Relation between structural integrity (nQA) predicting BOLD activation of DG/CA3 during correct lure discrimination trials. The top row (a-c) shows left uncinate fasciculus nQA predicting left DG/CA3 BOLD activity during highly similar lure correct rejections. Specifically, we show that greater left uncinate fasciculus nQA predicts decreased DG/CA3 BOLD response during the correct discrimination of (a) negative (r = −0.41, P = 0.035) and (c) positive (r = −0.44, P = 0.022) highly similar lure items but not (b) neutral (r = 0.12, P = 0.55). (d–f) Shows the corpus callosum as our negative control. We show there is no relationship between corpus callosum nQA and average left and right DG/CA3 activity during the correct discrimination of (d) negative, (e) neutral, and (f) positive highly similar lure items.
As the UF has been previously implicated in psychiatric disorders characterized by emotional disturbances, we asked if this effect could be related to symptoms of depression. Using the Beck Depression Inventory II (BDI-II) we quantified depressive symptoms in all participants and included the overall score in a regression model (BDI-II and UF nQA predicting DG/CA3 activity). We found that the relationship between left UF nQA and left DG/CA3 activity for highly similar negative lure correct discrimination trials remained significant (B = −1.8, P = 0.048) after accounting for BDI-II scores (B = −0.0029, P = 0.46). Similarly, the relationship between left UF nQA and left DG/CA3 activity for highly similarly positive items remained significant (B = −1.94, P 0.032) after accounting for BDI-II scores (B = −0.0045, P = 0.24).
In order to assess the specificity of our findings, we conducted several additional control analyses. First, we asked whether this relationship between activation and white matter is specific to the UF or if it’s a general feature of white matter connectivity. We tested the relationship between general connectivity and DG/CA3 activity using the corpus callosum as a control pathway. Specifically, we tested if corpus callosum nQA predicted averaged DG/CA3 response during high similarity negative, positive and neutral lures. We found that none of the relationships were statistically significant (Figure 4d–f). Second, we asked whether the relationship between UF nQA and DG/CA3 activity generalizes to other hippocampal subfields. We tested whether UF integrity was associated with CA1 activity and found left UF nQA did not predict with the responses of the left CA1 subfield during highly similar lure correct rejection trials of any valence (Supplementary Table 1a). Additionally, these effects seem specific to the left hemisphere. Right UF integrity did not predict right DG/CA3 nor CA1 activity during highly similar lure discrimination for any valence type (Supplementary Table 1b). Finally, to test the specificity of UF nQA predicting DG/CA3 activity during highly similar emotional lure discrimination, we asked whether UF nQA also predicted DG/CA3 activity during low similarity lure correct rejections. Left UF nQA did not predict DG/CA3 activity for negative (r = 0.084, P = 0.68), neutral (r = 0.21, P = 0.28), or positive (r = 0.15, P = 0.45) low similarity correct rejections.
UF Integrity, DG/CA3 Functional Activity, and Lure Discrimination Performance
Given the relationship between UF integrity and DG/CA3 activity during correct rejections of emotional stimuli, we asked whether left UF integrity was directly associated with lure discrimination performance. We found that UF integrity did not directly predict lure discrimination performance. We then asked whether the relationship between DG/CA3 subfield activity during discrimination of highly similar lure was associated with overall performance on those trial types. Past work in our group and others has suggested that higher levels of activity in the DG/CA3 region is associated with overgeneralization errors. We found a significant negative correlation between left DG/CA3 activity during correct discrimination of negative stimuli and the lure discrimination index on the same trials (r = −0.42, P = 0.029, Figure 5). We once again assessed whether depressive symptoms impacted this relationship by including BDI-II score as a regressor and found that that the relationship between left DG/CA3 activity and negative LDI remained significant (B = −0.31, P = 0.039) after accounting for depressive symptoms (B = 0.00089, P = 0.76).
Figure 5.

Relationship between DG/CA3 BOLD response during highly similar lure discrimination and lure discrimination behavior. Here left DG/CA3 activity during high similarity CRs is inversely related with lure discrimination for (a) negative (r = −0.42, p-value = 0.029), but not (b) neutral or (c) positive stimuli.
Discussion
In this study, we tested the hypothesis that the integrity of the uncinate fasciculus is associated with medial temporal lobe dynamics during an emotional pattern separation task. This stems from the observation that prefrontal cortex modulation of MTL signaling is implicated in memory processing (Jones & Wilson, 2005; Kim et al., 2011; Brincat & Miller, 2015; Kesteren et al., 2010), however, the exact pathways by which this modulation occurs in humans have remained elusive. Studies in rodents have been hindered by the major species differences in PFC anatomy as well as the absence of certain anatomical connections that are present in primates, thus this study offers novel insight into human MTL-PFC interactions.
Accounting for depressive symptoms, we found that in the left hemisphere, lower uncinate fasciculus integrity predicted greater activation of the DG/CA3 subfield of the hippocampus during emotional (positive and negative but not neutral) highly similar correct discrimination trials. To our knowledge, this is the first demonstration that differences in uncinate fasciculus integrity may be associated with alterations of functional activity in the DG/CA3 subfields of the hippocampus during discrimination of similar information.
In addition to providing a link between uncinate structure and DG/CA3 activity, we found that greater left DG/CA3 activity during highly similar negative correct rejections was related to poorer memory performance for highly similar negative items (i.e. higher rate of false alarms). Although at first this result seems surprising, given what is known about the role of DG/CA3 in pattern separation, a finding of increased activation in DG/CA3 linked to poor performance has been previously reported in older age (Yassa et al., 2011; Sinha et al., 2018). An interesting possibility is that the increased activation is related to CA3’s recurrent collateral network which is thought to play a role in pattern completion (or overgeneralization in the case of a discrimination task). While the noted activity occurred during the correct trials, pattern completion is assumed to occur also during lure discrimination (i.e. “recall to reject”). Here, much like the data in older adults, higher levels of DG/CA3 activity during correct lure discrimination is linked to poorer overall memory performance at the individual subject level. Overall, this suggests that greater activation in this subfield is associated with worse cognitive outcomes. That said, other work has shown that a within-subject increase in DG/CA3 activity as a function of mild aerobic exercise is associated with enhanced discrimination (Suwabe et al. 2018), however this finding also was associated with increased functional connectivity with regions involved in high precision recollection of memories (e.g. retrosplenial cortex, angular gyrus). Given this evidence, it is possible that there is an inverted U-shaped dose-response relationship where activity in DG/CA3 needs to be held in balance, with too much or too little activity being associated with worse outcomes. This particular topic should be subject to further investigation.
Our analysis of lure discrimination performance provides evidence that while the structural integrity of the UF is not related to lure discrimination, it is possible that the impact of this MTL-PFC bundle on memory is directed by its impact on DG/CA3 function. Further research is needed in order to test this hypothesis appropriately. The lack of correlation between uncinate integrity and discrimination performance is consistent with other results from Bennet et al., (2015), who found no link between uncinate integrity and object discrimination performance. Instead, the authors found a relationship between the fornix and performance, a relationship that was consistent across young and older adults. The possibility that the uncinate’s impact on behavior is mediated by functional signals in the DG/CA3 is consistent with a top-down modulatory control from the PFC to the MTL during discrimination.
Although we tested a focused hypothesis about the uncinate fasciculus and checked its specificity against a control pathway (the corpus callosum), one possible limitation is that other pathways might influence these emotional memory processes. In general, the relative contribution of the uncinate fasciculus, cingulum, and fornix bundles in innervating their respective targets within the medial temporal lobe are poorly understood in humans. The 3-dimensional structures of these pathways have not been extensively resolved in humans or non-human primates. Along these lines, it is possible that information flow is segregated according to information content and valence. Signaling involving emotional items could be reliant on OFC integration and thus be communicated via the uncinate fasciculus, whereas signaling involving non-emotional items could be reliant on mPFC (or other PFC region) integration and thus be communicated via the cingulum or fornix. This may be too simplified, as it is not currently known whether these pathways innervate overlapping subdivisions of the PFC, and it is unclear what the functional consequences of these dissociations may be. Another possible limitation is the relatively small sample size collected in this investigation. While we provide additional statistical analysis to demonstrate these results are not sensitive to outliers, it is still unclear how these results would generalize in larger clinically-assessed population.
One issue is the difficulty of even modern tractography algorithms and data acquisition schemes to accurately and non-invasively model even the largest white matter bundles with sufficient accuracy in humans (Maier-Hein et al., 2017). Tractography of the uncinate fasciculus is a particularly challenging endeavor due to its branching and turning pattern and the possibility for false continuation medially toward the striatum and posteriorly along the inferior fronto-occipital fasciculus and inferior longitudinal fasciculus. In this manner, the use of more sophisticated tractography algorithms that might account for multiple fiber orientations within each voxel might aid in the delineation of this morphologically complex fiber. To address these issues we used normalized quantitative anisotropy, a measure that resolves multiple fiber orientations within a voxel and appropriately accounts for for the isotropic component of diffusion, which addresses partial volume confounds. We combined this approach with anatomical regions of avoidance consisting of three intersecting planes and a sphere over the striatal area to ultimately generate a highly reproducible scheme for tractography of the uncinate fasciculus while avoiding common false continuations with respect to anatomy.
In this study, we have shown that decreased UF integrity is associated with greater activity of DG/CA3 during emotional lure discrimination and that greater DG/CA3 activity is associated with poor performance on an emotional pattern separation task. As a result of this finding, we hypothesize that these results reflect a possible scenario in which the UF mediates important top-down control from the OFC to the MTL which is critical for processing of emotional memories, but that the relationship between UF integrity and behavior is mediated by functional activation of the hippocampal network.
Conclusions
Together our results suggest that UF integrity may be a marker for emotional and cognitive health, which is consistent with prior work implicating the UF in emotional dysregulation conditions including major depressive disorder and anxiety (Zhang et al., 2012), as well as work linking UF integrity with early life stress and maltreatment (Eluvathingal et al., 2000). Future studies are needed to determine whether these effects manifest beyond emotional discrimination tasks or if there are alterations in the relationship between UF and functional signals across the lifespan.
Supplementary Material
Highlights.
There is little focus on influence of white matter in pattern separation processes
Used emotional mnemonic discrimination task and multimodal MR Imaging
Uncinate integrity predicts emotional pattern separation-related DG/CA3 activity
Greater DG/CA3 activity during negative CRs related to poorer memory performance
Acknowledgements:
We would like to acknowledge Ms. Jessica Noche, Ms. Amanda Chun, and Ms. Elizabeth Murray for their help with data collection and Dr. Zachariah Reagh and Mr. Jared Roberts (posthumously) for their assistance with analysis of the functional MRI data. This work was supported by NIMH R01 MH102392 (PI: Yassa).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests:
None
References:
- Alm KH, Rolheiser T, & Olson IR (2016). Inter-Individual Variation in Fronto-Temporal Connectivity Predicts the Ability to Learn Different Types of Associations. Neuroimage, 132, 213–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MC, Bunce JG, & Barbas H (2016). Neurobiology of Learning and Memory Prefrontal – hippocampal pathways underlying inhibitory control over memory. Neurobiology of Learning and Memory, 134, 145–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MC, Ochsner KN, Kuhl B, Cooper J, Robertson E, Gabrieli SW, Gabrieli JDE (2004). Neural Systems Underlying the Suppression of Unwanted Memories, Science, 303, 232–236. [DOI] [PubMed] [Google Scholar]
- Avants BB, Tustison NJ, Song G, Cook PA, Klein A, & Gee JC (2011). A reproducible evaluation of ANTs similarity metric performance in brain image registration. NeuroImage, 54, 2033–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker A, Kirwan CB, Miller M Stark CE (2008). Pattern separation in the human hippocampal CA3 and Dentate gyrus. Science 219: 1640–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett IJ, Huffman DJ, & Stark CEL (2015). Limbic Tract Integrity Contributes to Pattern Separation Performance Across the Lifespan, Cerebral Cortex 25, 2988–2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit RG, & Anderson MC (2012). Opposing Mechanisms Support the Voluntary Forgetting of Unwanted Memories. Neuron, 76(2), 450–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brincat SL, & Miller EK (2015). Frequency-specific hippocampal-prefrontal interactions during associative learning. Nat. Neurosci 18, 576–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW (1996) AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29:162–173. [DOI] [PubMed] [Google Scholar]
- Depue BE, Curran T, Banich MT (2007). Prefrontal Regions Orchestrate via a Two-Phase Process, Science 317, 215–220. [DOI] [PubMed] [Google Scholar]
- Ebeling U, & Cramon D. v. (1992). Topography of the uncinate fascicle and adjacent temporal fiber tracts. Acta Neurochirurgica, 115(3–4), 143–148. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H (2017). Prefrontal–hippocampal interactions in episodic memory. Nature Reviews Neuroscience, 18, 547. [DOI] [PubMed] [Google Scholar]
- Eluvathingal TJ Chugani HT, Behen ME, Juhász C, Muzik O, Maqbool M, Chugani DC, Makki M (2006). Abnormal Brain Connectivity in Children After Early Severe Socioemotional Deprivation: A Diffusion Tensor Imaging Study. Pediatrics, 117, 2093–2100. [DOI] [PubMed] [Google Scholar]
- Hanson JL, Knodt AR, Brigidi BD, Hariri AR (2015). Lower structural integrity of the uncinate fasciculus is associated with a history of child maltreatment and future psychological vulnerability to stress. Development and Psychopathology, 27, 1611–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho TC, King LS, Leong JK, Colich NL, Humphreys KL, Ordaz SJ, & Gotlib IH (2017). Effects of Sensitivity to Life Stress on Uncinate Fasciculus Segments in Early Adolescence. Social Cognitive and Affective Neuroscience, 12, 1460–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MW, & Wilson MA (2005). Theta rhythms coordinate hippocampal–prefrontal interactions in a spatial memory task. PLoS Biol 3: 402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DK (2008). Studying connections in the living human brain with diffusion MRI, Cortex, 44, 936–952. [DOI] [PubMed] [Google Scholar]
- Van Kesteren MT, Fernández G, Norris DG, Hermans EJ (2010). Persistent schema-dependent hippocampal-neocortical connectivity during memory encoding and postencoding rest in humans. Proc Natl AcadSci 107(16):7550–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Delcasso S, Lee I (2011). Neural correlates of object-in-place learning in hippocampus and prefrontal cortex. J. Neurosci 31, 16991–17006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy JW, Yassa MA, Stark SM Muftuler LT, Stark CE (2010) Distinct pattern separation related transfer functions in human CA3/dentate and CA1 revealed using high-resolution fMRI and variable mnemonic similarity. Learn mem 18: 15–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal SL, Tighe SK, Yassa MA (2014a). Asymmetric effects of emotion on mnemonic interference. Neurobiol Learn Mem, 111:41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal SL, & Yassa MA (2018). Integrating new findings and examining clinical applications of pattern separation. Nature Neuroscience, 21(2), 163–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal SL, Tighe SK, Jones CK, Yassa MA (2014b). Pattern separation of emotional information in hippocampal dentate and CA3. Hippocampus, 24(9), 1146–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutgeb JK Leutgeb S, Moser MB, Moser EI (2007) Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science, 315, 961–966. [DOI] [PubMed] [Google Scholar]
- Maier-Hein KH (2017). The challenge of mapping the human connectome based on diffusion tractography. Nature Communications, 8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek R, Sun Y, Sah P (2018). Neural circuits for a top-down control of fear and extinction. Psychopharmacology, 236, 313–320. [DOI] [PubMed] [Google Scholar]
- Marr D (1971) Simple memory: a theory for archicortex. Philos. Trans. R. Soc. Lond. B: Biol. Sci, 262, 23–81. [DOI] [PubMed] [Google Scholar]
- Olson IR, Heide RJ Von Der, Alm KH, & Vyas G (2015). Development of the uncinate fasciculus: Implications for theory and developmental disorders. Developmental Cognitive Neuroscience, 14, 50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston AR, & Eichenbaum H (2013). Interplay of hippocampus and prefrontal cortex in memory. Current biology, 23(17), R764–R773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reagh ZM, Noche JA, Tustison NJ, Delisle D, Murray EA, & Yassa MA (2018). Functional Imbalance of Anterolateral Entorhinal Cortex and Hippocampal Dentate/CA3 Underlies Age-Related Object Pattern Separation Deficits. Neuron, 97(5), 1187–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman KJ (1990). No adjustments are needed for multiple comparisons. Epidemiology, 1: 43–46. [PubMed] [Google Scholar]
- Saville DJ (1990) Multiple Comparison Procedures: The Practical Solution. The American Statistician, 44:174–180 [Google Scholar]
- Sinha N, Berg CN, Tustison NJ, Shaw A, Hill D, Yassa MA, & Gluck MA (2018). Neurobiology of Aging APOE ε 4 status in healthy older African Americans is associated with deficits in pattern separation and hippocampal hyperactivation. Neurobiology of Aging, 69, 221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Quirk GJ (2010) Prefrontal control of fear: more than just extinction.(Current Opinion in Neurobiology 20: 231–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwabe K, Byun K, Hyodo K, Reagh ZM, & Roberts JM (2018). Rapid stimulation of human dentate gyrus function with acute mild exercise, PNAS 115(41). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WD, MacFall JR, Gerig G, & Krishnan RR (2007). Structural integrity of the uncinate fasciculus in geriatric depression: Relationship with age of onset. Neuropsychiatric Disease and Treatment, 3(5), 669–674. [PMC free article] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Dell’Acqua F, Valabregue R, Catani M, 2012. Monkey to human comparative anatomy of the frontal lobe association tracts. Cortex 48 (1), 82–96. [DOI] [PubMed] [Google Scholar]
- Von Der Heide RJ, Skipper LM, Klobusicky E, & Olson IR (2013). Dissecting the uncinate fasciculus: Disorders, controversies and a hypothesis. Brain, 136(6), 1692–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, & Olson IR (2018). The Original Social Network: White Matter and Social Cognition. Trends in Cognitive Sciences, 22(6), 504–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, & Stark CEL (2011). Pattern separation in the hippocampus. Trends in Neurosciences, 34(10), 515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Lacy JW, Stark SM, Albert MS, Gallagher M, & Stark CEL (2011). Pattern Separation Deficits Associated With Increased Hippocampal CA3 and Dentate Gyrus Activity in Nondemented Older Adults, Hippocampus 979, 968–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Stark SM, Bakker A, Albert MS, Gallagher M, & Stark CE (2010). High-resolution structural and functional MRI of hippocampal CA3 and dentate gyrus in patients with amnestic Mild Cognitive Impairment. NeuroImage, 51(3), 1242–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh FC, & Tseng WYI (2011). NTU-90: A high angular resolution brain atlas constructed by q-space diffeomorphic reconstruction. NeuroImage, 58(1), 91–99. [DOI] [PubMed] [Google Scholar]
- Yeh FC, Verstynen TD, Wang Y, Fernández-Miranda JC, & Tseng WYI (2013). Deterministic diffusion fiber tracking improved by quantitative anisotropy. PLoS ONE, 8(11), 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A, Leow A, Ajilore O, Lamar M, Yang S, Joseph J, Medina J, Zhan L, Kumar A (2012). Quantitative tract-specific measures of uncinate and cingulum in major depression using diffusion tensor imaging. Neuropsychopharmacology, 37(4), 959–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Stevenson RF, Mander BA, Mnatsakanyan L, Hsu F, Vadera S, Lin, Knight RT, Yassa MA, Lin JJ (2019). Multiplexing of Theta and Alpha Rhythms in the Amygdala-Hippocampal Circuit Supports Pattern Separation of Emotional Information. Neuron, 102(4), 887–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


