Abstract
Background:
Male marijuana use has increased steadily over the last decade, but its effect on risk of spontaneous abortion to our knowledge has not been studied.
Methods:
We analyzed data from Pregnancy Study Online (PRESTO), a North American prospective cohort study of pregnancy planners (2013-2019). During the preconception period, male and female participants completed baseline questionnaires on demographics, medical history, and behavioral factors, including marijuana use. Female participants identified pregnancy losses on bimonthly follow-up questionnaires and questionnaires completed in early and late pregnancy. We categorized frequency of male marijuana use in the 2 months before baseline as: none, <1 time/week, or ≥1 time/week. We estimated the association between preconception male marijuana use and spontaneous abortion, adjusting for male and female confounders.
Results:
Among 1,535 couples who conceived during follow-up, 9% of men reported preconceptional marijuana use <1 time/week and 8% ≥1 time/week. Nineteen percent of pregnancies ended in spontaneous abortion. Compared with no use, adjusted hazard ratios (HR) for male marijuana use were 1.1 (95% CI: 0.64-1.7) for <1 time/week and 2.0 (95% CI: 1.2-3.1) for ≥1 time/week. The association for ≥1 time/week persisted after restricting to couples where the female partner did not use marijuana (HR=2.0, 95% CI: 1.1-3.3), and was stronger for losses at <8 weeks’ gestation (HR=2.5, 95% CI: 1.4-4.3) and among males aged ≥35 years (HR=4.1, 95% CI: 1.54-11).
Conclusions:
Couples with male partners who used marijuana ≥1 time/week during preconception had greater risk of spontaneous abortion than couples with males who did not use marijuana.
Keywords: Cannabis, Marijuana, Spontaneous Abortion, Pregnancy, Preconception, Cohort study
Introduction
The prevalence of marijuana use in North America is one of the highest worldwide. Data from the 2016 National Survey on Drug Use and Health indicate 24 million Americans (9%) used marijuana in the past month.1 Marijuana use has increased over the last decade in most age groups,1 and is greater for men than women (17% vs. 10% in past year).2 Marijuana policy continues to change at a rapid pace, and recreational marijuana is now legal in 11 states, the District of Columbia, and Canada. Despite increasing legalization and prevalence, few studies have investigated the influence of marijuana use on the male reproductive system.
Tetrahydrocannabinol, an exogenous cannabinoid, is the primary psychoactive component of marijuana.3 Exogenous cannabinoids bind to cannabinoid receptors, which are present in human and animal testicular tissue and in spermatozoa.4-8 Male exposure to exogenous cannabinoids may disrupt the endocannabinoid system, adversely affecting semen quality and the integrity of sperm DNA.
There is limited research on male marijuana use and reproductive outcomes. Some human and animal studies show chronic or frequent marijuana exposure is associated with lower sperm concentration and motility, abnormal sperm morphology, sperm DNA fragmentation, and lower concentrations of testosterone and luteinizing hormone,8-11 while other studies show associations with improved semen parameters12 and higher testosterone concentrations.10,13 Although sperm with DNA damage are capable of fertilizing oocytes,14,15 poor semen quality and sperm DNA fragmentation have been associated with spontaneous abortion in some studies.15-18 More than 50% of first-trimester spontaneous abortions are attributed to chromosomal abnormalities19 and the male gamete contributes half the genome to the human embryo. In a prior publication, we reported little association between male marijuana use and fecundability.20 To our knowledge, no study has assessed the association between male marijuana use and pregnancy loss. Herein, we investigated the hypothesis that preconceptional male marijuana use increases risk of spontaneous abortion.
Methods
Pregnancy Study Online (PRESTO) is an ongoing prospective cohort study of couples trying to conceive, described previously.21 Eligible participants are women aged 21-45 years who reside in the United States or Canada, are trying to conceive without fertility treatments, and have male partners aged ≥21 years. PRESTO was approved by the Boston Medical Center Institutional Review Board. All participants provided informed consent.
Data Collection
All data collection is conducted online. Eligible women complete a baseline questionnaire and are then prompted to invite their male partners to complete an optional baseline questionnaire. Baseline questionnaires collect data on demographics, medical history, and lifestyle. Women complete follow-up questionnaires every 8 weeks for up to 12 months or until a pregnancy is reported; those who report a pregnancy are invited to complete two additional questionnaires in early (<12 weeks’ gestation) and late pregnancy (~32 weeks’ gestation).
Analytic Sample
Between June 2013-July 2019, 5,486 women reported a pregnancy during follow-up and were thus at risk for spontaneous abortion. We excluded couples without male marijuana data, including 2,361 women who did not invite their male partners and 1,590 women whose male partners did not agree to participate. The final study population included 1,535 couples.
Exposure Assessment
On the baseline questionnaire, men were asked: “Have you used marijuana during the last two months?” Those who responded “yes” were then asked, “How often have you used marijuana?” with response options of “every day”, “4–6 times/week”, “1–3 times/week”, and “less than 1 time/week”. We categorized marijuana use as follows: non-use, use <1 time/week, and use ≥1 time/week.
Outcome Assessment
On each follow-up questionnaire, women reported the date of the first day of their last menstrual period (LMP) and whether they were currently pregnant (“yes,” “no,” “don’t know”). Women who responded, “yes” or “don’t know” were asked, “Since the last questionnaire, have you had a miscarriage (including chemical pregnancy)?” Women who reported being currently pregnant were directed to the early pregnancy questionnaire where they reported on any intervening pregnancy losses since their last follow-up, their due date, and their date of first positive pregnancy test. Pregnancy losses occurring after the early pregnancy questionnaire were identified on the late pregnancy questionnaire. Women who reported a loss on any questionnaire were asked how many weeks the pregnancy lasted and on what date the pregnancy ended.
We attempted to find women lost to follow-up by email or phone. If a woman no longer wanted to participate, we asked her to provide information on her pregnancy status, including whether she experienced a pregnancy loss, the date of loss, and the number of weeks’ gestation at loss. We identified the absence of pregnancy losses by linking participant data to birth registries in select states (CA, FL, MA, MI, OH, PA, TX); if we identified a live birth in the registry with a date of birth corresponding to an LMP date during the study period, we assumed there was no pregnancy loss. Women who reported a therapeutic abortion or ectopic pregnancy were censored at the gestational week of those outcomes.
We estimated gestational weeks at pregnancy loss based on reported completed weeks the pregnancy lasted. If missing, we calculated gestational weeks at loss using the pregnancy end date, pregnancy due date, and LMP date. We calculated gestational weeks at loss (in completed weeks) as follows: (pregnancy end date-(pregnancy due date-280))/7. For women with missing pregnancy due dates, we calculated gestational weeks at loss as follows: (pregnancy end date-last menstrual period date)/7.
Data Analysis
We used an Anderson–Gill data structure22 with one observation per gestational week. We estimated the crude probability of spontaneous abortion by male marijuana frequency using life-table methods and Cox proportional hazards regression models to estimate hazard ratios (HRs) and 95% confidence intervals (CI) for the association between preconceptional male marijuana use and spontaneous abortion. The time-scale was gestational weeks, beginning at the gestational week of pregnancy detection (when available) or 4 weeks (when date of pregnancy detection was unavailable), until spontaneous abortion or censoring (20 weeks’ gestation).
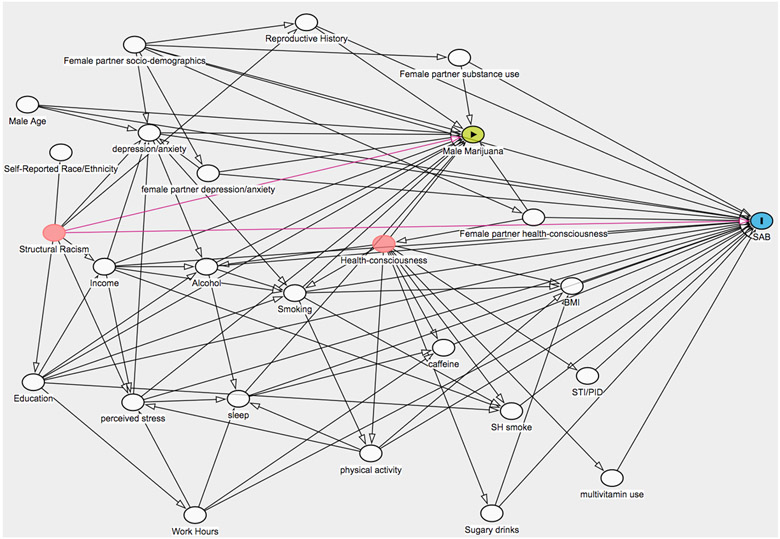
We created a directed acyclic graph to identify male and female confounders (eFigure 1). We fit two multivariable-adjusted models. Adjusting for reproductive history variables may result in over-adjustment bias if marijuana use remained relatively constant over time and was a risk factor for prior and future pregnancy loss.23,24 Therefore, the first model controlled for male and female confounders of male marijuana use and spontaneous abortion, excluding reproductive history. These variables included male and female: age (years), education (≤12, 13-15, ≥16 years), race/ethnicity (White, Black, Asian, mixed race, non-Hispanic other, Hispanic), alcohol (drinks/week), smoking status (current, past, never), current environmental tobacco exposure (yes vs. no), caffeine intake (<100, 100-199, 200-299, ≥300 mg/day), sugar-sweetened beverage intake (0, 1, 2-6, ≥7 drinks/week), BMI (<25, 25-29, 30-34, ≥35 kg/m2), physical activity (<10, 10-19, 20-39, ≥40 metabolic equivalent of task (MET)-hours/week), multivitamin or prenatal vitamin use (yes vs. no), hours of work/week (unemployed, <30, 30-39, 40-49, ≥50), history of sexually transmitted infections (yes vs. no), depression/anxiety diagnosis; male only: household income/1,000 USD (<50,000, 50,000-99,999, 100,000-149,999, >150,000), sleep duration (<7, 7-8, ≥9 hours/day), perceived stress scale score (continuous); and female only: marijuana use frequency (none, <1/month, ≥1/month). The second model controlled for all variables in model 1 plus female-reported time-to-pregnancy of the index pregnancy (cycles), female history of spontaneous abortion (yes vs. no), female parity (parous vs. nulliparous), and male history of impregnating a female partner (yes vs. no). To assess for multicollinearity, we examined a correlation matrix and the variance inflation factors of our confounders. We determined there was no multicollinearity or strong correlations between covariates, as all correlation coefficients were <0.80 (max=0.62 for female and male age), and variance inflation factors were <10 (max=2.1 for female age).
eFigure 1.
Directed Acyclic Graph of male and female confounders, male marijuana exposure, and spontaneous abortion.
Male participation in PRESTO was optional. As only 28% of female participants had male partner data on marijuana use, we evaluated potential for selection bias by weighting participants by the inverse probability of male participation in a sensitivity analysis.25 We constructed stabilized weights by fitting logistic regression models among the full sample before excluding male non-participants (n=5,486). To estimate the denominator of the weights, we used an indicator for male participation as the dependent variable, and predictors of male participation and spontaneous abortion as independent variables. Predictors of male participation and spontaneous abortion included, for males and females, age, education, household income, and cigarette smoking status; and for females only, race/ethnicity, history of sexually transmitted diseases, alcohol use, marijuana use, multivitamin use, anxiety and depression diagnosis, perceived stress scale score, history of spontaneous abortion, cycles of attempt time at study entry, hours of work per week, and parity. The numerator of the weights was estimated as the probability of male participation.
Conditioning on couples who conceive may also induce selection bias if preconception marijuana use affects conception, and unmeasured confounders affect both conception and subsequent spontaneous abortion.26 Using the full sample of couples who either did or did not conceive (n=10,253), we calculated additional stabilized weights representing the inverse probability of pregnancy using the same approach described above. Our weight models included the same covariates as those included in the models for male participation, with the addition of female BMI, use of methods to improve chances of pregnancy, and use of a hormonal method of last contraception. We then multiplied these weights by the inverse probability of male participation weights for a final set of weights. Accounting for selection bias due to pregnancy complicates the causal question, as the effect of male marijuana use on spontaneous abortion is only relevant to those who become pregnant and are at risk of the outcome.26,27 Therefore, we present estimates both accounting for, and not accounting for, selection bias due to pregnancy.
We conducted secondary analyses restricting to female non-users of marijuana at baseline and females with no history of spontaneous abortion. We stratified analyses by pregnancy attempt time at study entry (<3 vs. ≥3 cycles) and male age at baseline (<35 vs. ≥35 years) because longer attempt times may indicate underlying fertility problems and older age is a risk factor for poorer semen quality. Last, we stratified analyses by timing of spontaneous abortion (<8 vs. ≥8 weeks’ gestation) because a greater proportion of earlier pregnancy losses are due to chromosomal abnormalities and risk factors for early vs. late loss may differ.28 For example, if male marijuana use affects spontaneous abortion via sperm DNA fragmentation, we would expect to observe a stronger association between male marijuana use and earlier loss. To evaluate potential for recall bias, we excluded 110 men who completed their baseline questionnaires after the reported positive pregnancy test date. As we did not collect time-varying marijuana measures, we additionally restricted to couples who completed male baseline questionnaires within 3 months of their positive pregnancy test date to ensure marijuana exposure occurred closer to conception (i.e., reduce potential for exposure misclassification). Finally, we calculated an E-value to quantify the minimum strength of association an unmeasured confounder would need to have with both marijuana exposure and spontaneous abortion, conditional on measured covariates, to completely explain the observed association for our primary analysis.29 The E-value was calculated for the point estimate and lower confidence interval value assuming outcome prevalence >15% with the following equation:
Missing data ranged from <1% (age) to 39% (male secondhand cigarette smoke exposure, which was added to the questionnaire in July 2015). We assumed data to be missing at random conditional on measured covariates and used multiple imputation to impute missing data on exposure, covariates, and gestational weeks at loss.
Results
Among 1,535 couples who conceived, 83% reported no male marijuana use in the 2 months before baseline, 9% reported marijuana <1 time/week, and 8% reported marijuana ≥1 time/week. Among men who used marijuana ≥1 time/week, 51% reported using every day, 23% used 4-6 times/week, and 27% used 1-3 times/week. Men who used marijuana ≥1 time/week were more likely to have lower income and education, a diagnosis of anxiety or depression, smoke cigarettes, and have female partners who smoked cigarettes, used marijuana, or had a history of spontaneous abortion (Table 1).
Table 1.
Baseline characteristicsa of 1,535 men according to male preconceptional marijuana use, PRESTO (2013-2019).
| Male marijuana use in the 2 months before baseline |
|||
|---|---|---|---|
| Characteristic | None | <1 time/week |
≥1 time/week |
| Number of couples (n) | 1,267 | 140 | 128 |
| Male age at baseline (mean years) | 32 | 32 | 32 |
| Female partner age at baseline (mean years) | 30 | 30 | 29 |
| Non-Hispanic White (%) | 88 | 89 | 86 |
| Household income <$50,000/year (%) | 14 | 7 | 26 |
| Less than college degree (%) | 27 | 28 | 49 |
| Body mass index, kg/m2 (mean) | 28 | 27 | 27 |
| Metabolic equivalent of task-hours/week of physical activity (mean) | 33 | 35 | 31 |
| Alcohol, drinks/week (mean) | 6 | 11 | 8 |
| Caffeine 300+ mg/day (%) | 14 | 16 | 17 |
| Sugar-sweetened soda intake, drinks/week (mean) | 2 | 2 | 3 |
| Daily multivitamin use (%) | 37 | 35 | 25 |
| Average sleep duration <7 hours/night (%) | 34 | 26 | 37 |
| Work ≥50 hours/week (%) | 28 | 24 | 23 |
| Perceived stress scale score (mean) | 14 | 15 | 17 |
| Ever diagnosed with anxiety (%) | 7 | 7 | 14 |
| Ever diagnosed with depression (%) | 9 | 12 | 20 |
| Ever pregnant/impregnated someone (%) | 47 | 39 | 57 |
| History of sexually transmitted infections (%) | 4 | 7 | 7 |
| Current environmental tobacco smoke exposure (%) | 9 | 11 | 20 |
| Current regular smoker (%) | 4 | 8 | 24 |
| Current occasional smoker (%) | 4 | 9 | 5 |
| Past smoker (%) | 16 | 28 | 31 |
| Female partner current regular smoker at baseline (%) | 2 | 6 | 11 |
| Female partner current marijuana user at baseline (%) | 3 | 34 | 46 |
| Prior any pregnancy loss (female partner) (%) | 29 | 32 | 39 |
| History of spontaneous abortion (female partner) (%) | 23 | 18 | 28 |
| History of therapeutic abortion (female partner) (%) | 7 | 14 | 15 |
All male characteristics except for age are standardized to male baseline age of cohort, and female characteristics except for age standardized to female baseline age of cohort.
When we compared characteristics reported by females whose male partners did vs. did not participate in PRESTO, male non-participants had lower educational attainment (≤high school: 9% vs. 13%), were more likely to smoke cigarettes (10% vs. 16%), and were more likely to have female partners who smoked cigarettes (4% vs. 7%), used marijuana (9% vs. 13%), or had a history of spontaneous abortion (23% vs. 26%) than male participants.
Over follow-up, 292 (19%) couples reported a spontaneous abortion (Table 2). Median gestational weeks at loss was 6 (interquartile range: 5-9). Spontaneous abortions were reported among 19% of couples with no male marijuana use, 16% with use <1 time/week, and 31% with use ≥1 time/week. In adjusted models, male marijuana use ≥1 time/week during preconception was associated with 2.0 times the risk of spontaneous abortion compared with no use (95% CI: 1.2-3.1). Results persisted after adjustment for reproductive history (HR: 2.0, 95% CI: 1.3-3.2), and after restricting to female non-users of marijuana (HR: 2.0, 95% CI: 1.1-3.4) and females with no history of spontaneous abortion (HR: 2.2, 95% CI: 1.2-4.0). Male marijuana use <1 time/week showed little association with spontaneous abortion in all models.
Table 2.
Male baseline marijuana use frequency and spontaneous abortion among 1,535 PRESTO couples (2013-2019)
| Marijuana use in the 2 months before baseline |
SABs | Gestational- Weeks of observation |
Median Gestational Weeks at Loss (IQR) |
Crude HR (95% CI) |
Adjusted HR (95% CI)a |
Adjusted HR (95% CI)b |
|---|---|---|---|---|---|---|
| Full sample (n=1,535) | ||||||
| None | 234 | 16,269 | 6 (5-9) | REF | REF | REF |
| <1 time/week | 22 | 1,819 | 5 (5-7) | 0.85 (0.55-1.3) | 1.1 (0.64-1.7) | 1.1 (0.65-1.7) |
| ≥1 time/week | 36 | 1,381 | 6 (5-9) | 1.7 (1.2-2.4) | 2.0 (1.2-3.1) | 2.0 (1.3-3.2) |
| Inverse probability of male participation weighted sample (n=1,535)c | ||||||
| None | 234 | 16,269 | 6 (5-9) | REF | REF | REF |
| <1 time/week | 22 | 1,819 | 5 (5-7) | 0.87 (0.57-1.3) | 1.0 (0.63-1.6) | 1.0 (0.64-1.6) |
| ≥1 time/week | 36 | 1,381 | 6 (5-9) | 1.7 (1.3-2.3) | 1.8 (1.2-2.8) | 1.9 (1.3-2.9) |
| Inverse probability of male participation and pregnancy weighted sample (n=1,535)d | ||||||
| None | 234 | 16,269 | 6 (5-9) | REF | REF | REF |
| <1 time/week | 22 | 1,819 | 5 (5-7) | 0.73 (0.47-1.1) | 0.87 (0.53-1.4) | 0.88 (0.53-1.5) |
| ≥1 time/week | 36 | 1,381 | 6 (5-9) | 1.5 (1.1-1.9) | 1.9 (1.3-2.9) | 2.0 (1.4-3.1) |
| Restricted to couples with no female marijuana use at baseline (n=1,394) | ||||||
| None | 228 | 15,824 | 6 (5-9) | REF | REF | REF |
| <1 time/week | 15 | 1,209 | 5 (5-7) | 0.88 (0.53-1.5) | 1.1 (0.62-1.9) | 1.1 (0.62-1.9) |
| ≥1 time/week | 18 | 792 | 6 (5-8) | 1.5 (0.94-2.5) | 2.0 (1.1-3.4) | 2.0 (1.1-3.4) |
| Restricted to couples with no female history of spontaneous abortion (n=1,078)e | ||||||
| None | 152 | 11920 | 6 (5-9) | REF | REF | REF |
| <1 time/week | 13 | 1254 | 6 (5-7) | 0.80 (0.46-1.4) | 0.84 (0.45-1.6) | 0.82 (0.44-1.6) |
| ≥1 time/week | 20 | 835 | 6 (5-9) | 1.7 (1.1-2.8) | 2.2 (1.2-4.0) | 2.1 (1.1-3.8) |
Gestational-weeks of observation is the number of weeks from detected pregnancy (i.e. entry into cohort) until SAB or censoring. SAB: spontaneous abortion; HR: Hazard Ratio; CI: Confidence Interval;
Adjusted for male and female: age, education, race/ethnicity, alcohol frequency, smoking status, environmental tobacco exposure, caffeine intake, sugar-sweetened beverage intake, BMI, weekly exercise, multivitamin use, hours work per week, history of STIs, depression/anxiety; male only: household income, hours of sleep per night, perceived stress scale; and female only: marijuana use frequency.
Additionally adjusted for reproductive history: female parity and history of spontaneous abortion, male ever impregnated someone, and total time to pregnancy (cycles).
Weighted by the inverse probability of male participation.
Weighted by the inverse probability of male participation and pregnancy. Weights are the product of the male participation and pregnancy weights.
No models adjust for history of spontaneous abortion.
When using inverse probability of selection weights for male participation, stabilized weights had a mean of 1.0 (range 0.6–3.0). Variables most predictive of male participation included greater female and male education, lack of male cigarette smoking, lack of female marijuana use, absence of diagnosed depression, and female Asian or Black race (vs. White race). Weighted estimates accounting for male participation were slightly attenuated for male marijuana use ≥1 time/week (HR: 1.8, 95% CI: 1.2-2.8). When using inverse probability of selection weights for pregnancy, stabilized weights had a mean of 1.0 (range 0.5-19.4). Variables most predictive of pregnancy included shorter cycles of attempt time at study entry, greater female and male education, and lower BMI. Additionally adjusting for selection bias due to conditioning on pregnancy did not substantially alter results (weighted HRs for <1 and ≥1 time/week: 0.87, 95% CI: 0.53-1.4 and 1.9, 95% CI: 1.3-2.9) (Table 2).
In secondary analyses (Tables 3-5), the association between male marijuana use ≥1 time/week and spontaneous abortion persisted among couples with pregnancy attempt times of <3 cycles (HR: 2.1, 95% CI: 1.1-3.9), and was stronger among couples with attempt times ≥3 cycles (HR: 3.4, 95% CI: 1.5-7.7) (Table 3). The association for ≥1 time/week was slightly stronger for pregnancy losses at <8 weeks’ gestation (HR: 2.5, 95% CI: 1.4-4.3), but was attenuated for later pregnancy loss (HR: 1.3, 95% CI: 0.56-3.1) (Table 4). Results were substantially stronger but more imprecise among men ≥35 years (HR: 4.1, 95% CI: 1.5-11.0) (Table 5). Excluding men who completed their baseline questionnaires after the reported pregnancy test date did not appreciably change results (<1 and ≥1 time/week: HR=0.98, 95% CI: 0.59-1.6 and HR=2.0, 95% CI: 1.2-3.2, respectively). Restricting to men who completed their baseline questionnaires within 3 months of pregnancy detection also did not appreciably change results (<1 and ≥1 time/week: HR=1.1, 95% CI: 0.54-2.2 and HR=2.7, 95% CI: 1.4-5.2). The adjusted HR for ≥1 time/week of 2.0 in our primary full sample analysis corresponds to an E-value of 2.6; the lower confidence interval value of 1.2 corresponds to an E-value of 1.6.
Table 3.
Male baseline marijuana use frequency and spontaneous abortion among 1,535 PRESTO couples, stratified by attempt time at study entry (2013-2019)
| Marijuana use in the 2 months before baseline |
SABs | Gestational- Weeks of observation |
Median Gestational Weeks at Loss (IQR) |
Crude HR (95% CI) |
Adjusted HR (95% CI)a |
Adjusted HR (95% CI)b |
|---|---|---|---|---|---|---|
| Attempt Time <3 cycles (n=1,045 couples) | ||||||
| None | 146 | 11,280 | 6 (5-9) | REF | REF | REF |
| <1 time/week | 18 | 1,547 | 5 (5-7) | 1.1 (0.69-1.8) | 1.5 (0.87-2.7) | 1.5 (0.86-2.7) |
| ≥1 time/week | 20 | 1,050 | 5 (5-8) | 1.7 (1.1-2.8) | 2.1 (1.1-3.9) | 2.0 (1.1-3.9) |
| Attempt Time ≥3 cycles (n=490 couples) | ||||||
| None | 88 | 4,989 | 6 (5-9) | REF | REF | REF |
| <1 time/week | 4 | 560 | 5 (5-7) | 0.41 (0.15-1.1) | 0.43 (0.14-1.4) | 0.40 (0.13-1.3) |
| ≥1 time/week | 16 | 567 | 6.5 (5-9.5) | 1.6 (0.93-2.7) | 3.4 (1.5-7.7) | 3.7 (1.6-8.3) |
Gestational-weeks is the length of time pregnant until SAB or censoring. Gestational-weeks of observation is the number of weeks from detected pregnancy (i.e. entry into cohort) until SAB or censoring. SAB: spontaneous abortion; HR: Hazard Ratio; CI: Confidence Interval.
Adjusted for male and female: age, education, race/ethnicity, alcohol frequency, smoking status, environmental tobacco exposure, caffeine intake, sugar-sweetened beverage intake, BMI, weekly exercise, multivitamin use, hours work per week, history of STIs, depression/anxiety; male only: household income, hours of sleep per night, perceived stress scale; and female only: marijuana use frequency.
Additionally adjusted for reproductive history: female parity and history of spontaneous abortion, and male ever impregnated someone.
Table 5.
Male baseline marijuana use frequency and SAB among 1,535 PRESTO couples, stratified by male age at study entry (2013-2019)
| Marijuana use in 2 months before baseline |
SABs | Gestational- Weeks of observation |
Median Gestational Weeks at Loss (IQR) |
Crude HR (95% CI) |
Adjusted HR (95% CI)a |
Adjusted HR (95% CI)b |
|---|---|---|---|---|---|---|
| Male age <35 years (n=1,191 couples) | ||||||
| None | 180 | 12,726 | 6 (5-9) | REF | REF | REF |
| <1 time/week | 13 | 1,420.5 | 5 (5-6) | 0.65 (0.37-1.2) | 0.85 (0.45-1.6) | 0.84 (0.45-1.6) |
| ≥1 times/week | 24 | 986 | 6 (5-8.5) | 1.6 (1.0-2.4) | 1.9 (1.1-3.5) | 1.9 (1.1-3.4) |
| Male age ≥35 years (n=344 couples) | ||||||
| None | 54 | 3,543 | 7 (5-10) | REF | REF | REF |
| <1 time/week | 9 | 398 | 5 (5-8) | 1.5 (0.76-3.1) | 2.3 (0.85-6.3) | 2.7 (1.0-7.3) |
| ≥1 times/week | 12 | 395 | 5.5 (4.5-9.5) | 2.0 (1.1-3.7) | 4.1 (1.5-11) | 4.3 (1.6-11) |
Gestational-weeks is the length of time pregnant until SAB or censoring. Gestational-weeks of observation is the number of weeks from detected pregnancy (i.e. entry into cohort) until SAB or censoring. HR: Hazard Ratio; CI: Confidence Interval.
Adjusted for male and female: education, race/ethnicity, alcohol frequency, smoking status, environmental tobacco exposure, caffeine intake, sugar-sweetened beverage intake, BMI, weekly exercise, multivitamin use, hours work per week, history of STIs, depression/anxiety; male only: household income, hours of sleep per night, perceived stress scale; and female only: age, marijuana use frequency.
Additionally adjusted for reproductive history: female parity and history of spontaneous abortion, male ever impregnated someone, and total time to pregnancy (cycles).
Table 4.
Male baseline marijuana use frequency and spontaneous abortion among 1,535 PRESTO couples, stratified by timing of pregnancy loss (2013-2019)
| Marijuana use in the 2 months before baseline |
SABs | Gestational- Weeks of observation |
Median Gestational Weeks at Loss (IQR) |
Crude HR (95% CI) |
Adjusted HR (95% CI)a |
Adjusted HR (95% CI)b |
|---|---|---|---|---|---|---|
| Earlier loss (<8 weeks)c (n=1,533) | ||||||
| None | 154 | 4,726 | 5 (5-6) | REF | REF | REF |
| <1 time/week | 17 | 525.5 | 5 (5-5) | 1.0 (0.61-1.7) | 1.5 (0.86-2.6) | 1.5 (0.84-2.6) |
| ≥1 time/week | 24 | 456 | 5 (5-6) | 1.7 (1.1-2.6) | 2.5 (1.4-4.3) | 2.3 (1.3-4.0) |
| Later loss (≥8 weeks)d (n=1,113) | ||||||
| None | 80 | 11,543 | 10 (9-11) | REF | REF | REF |
| <1 time/week | 5 | 1,293 | 9 (9-11) | 0.56 (0.23-1.4) | 0.38 (0.13-1.1) | 0.42 (0.15-1.2) |
| ≥1 time/week | 12 | 925 | 10 (9-11.5) | 1.8 (1.0-3.4) | 1.3 (0.56-3.1) | 1.3 (0.54-3.1) |
Gestational-weeks is the length of time pregnant until SAB or censoring. Gestational-weeks of observation is the number of weeks from detected pregnancy (i.e. entry into cohort) until SAB or censoring. SAB: spontaneous abortion; HR: Hazard Ratio; CI: Confidence Interval.
Adjusted for male and female: age, education, race/ethnicity, alcohol frequency, smoking status, environmental tobacco exposure, caffeine intake, sugar-sweetened beverage intake, BMI, weekly exercise, multivitamin use, hours work per week, history of STIs, depression/anxiety; male only: household income, hours of sleep per night, perceived stress scale; and female only: marijuana use frequency.
Additionally adjusted for reproductive history: female parity and history of spontaneous abortion, male ever impregnated someone, and total time to pregnancy (cycles).
Includes all pregnant women. Gestational weeks of observation is the length of time until SAB or censoring at 8 weeks.
Includes women who are still pregnant after 8 weeks. Gestational weeks of observation is the length of time from week 8 until SAB or censoring at 20 weeks.
Discussion
In this prospective cohort study, male preconceptional marijuana use ≥1 times/week was associated with an increased risk of spontaneous abortion compared with no male marijuana use. The association was stronger among those with early pregnancy losses and among men aged ≥35 years. The association was materially unchanged after accounting for several potential confounders and two sources of selection bias. There was no meaningful increased risk for men who reported using marijuana <1 time/week.
Most research to date has focused on female risk factors for spontaneous abortion, but male factors may also be important. Some studies have explored the association between male preconception caffeine consumption, alcohol use, and cigarette smoking and spontaneous abortion.30 Male caffeine consumption was associated with increased risk of pregnancy loss in a preconception cohort study31 and lower probability of live birth in an infertility treatment study,32 while other studies have found no association.33 Studies of male alcohol intake are similarly inconsistent: in couples undergoing fertility treatment, male alcohol use was associated with both a lower34 and higher probability of live birth,32 whereas another study reported no association.33 In three studies of couples from the general population, preconception male alcohol use had no association with spontaneous abortion,31,35,36 and yet male alcohol use was associated with 2-3 times the risk of spontaneous abortion in a prospective Danish study.37 Male cigarette smoking has known deleterious effects on semen quality;38 some studies find no association of preconception male smoking with pregnancy outcomes,30,31 while others suggest an increased risk.39
Marijuana use has been associated with DNA fragmentation, which may lead to increased risk of spontaneous abortion.15 This mechanism is supported by data showing advanced paternal age is associated with increased risk of spontaneous abortion,40 as older men have greater sperm DNA fragmentation and other lower semen quality parameters.41 Research on paternal age and spontaneous abortion similarly show an association with earlier but not later pregnancy loss,40 consistent with DNA-related mechanisms: a greater proportion of earlier versus later pregnancy losses is attributed to chromosomal abnormalities.32 The association between male marijuana use was substantially stronger in our cohort among men ≥35 years. Prior studies indicate paternal age is a risk factor for pregnancy loss, thus it is reasonable to hypothesize older men may be more vulnerable to an effect of marijuana on spontaneous abortion.40
In a prior study based on the same cohort,20 we found little association between male marijuana use and fecundability. Though additional studies are needed, the results of our prior study coupled with the current study suggest that male marijuana use may have little effect on conception, but might contribute to adverse outcomes post-conception. It has been demonstrated that sperm with DNA damage are capable of fertilization while subsequently leading to early pregnancy loss.14,15
If male participation is associated with both male marijuana use and underlying risks of spontaneous abortion, either directly or through other factors, this may induce selection bias. We compared female-reported characteristics of men who participated with those who did not, and found men who participated tended to have higher socioeconomic status, were less likely to smoke cigarettes and have partners who used marijuana or had a history of spontaneous abortion. It is plausible that men who participated were less likely to use marijuana than men who did not, but given the prospective design, it is unlikely that future pregnancy outcomes influenced male participation. Indeed, the prevalence of spontaneous abortion in PRESTO did not differ appreciably between couples with (18%) and without (19%) male participation. In sensitivity analyses, we attempted to control for selection bias by weighting participants by the inverse probability of male participation; we found little difference in associations.
It is possible that conditioning on pregnancy might bias estimates of the association between preconception exposures and pregnancy outcomes.26,42 We explored this source of selection bias by additionally weighting participants by the inverse probability of pregnancy, but we found little difference in results. One reason for the similarity in results could be the lack of association between male marijuana use and fecundability in this cohort.20 There is a debate in the literature about the validity of conditioning on pregnancy;26 some say that it creates a selection bias that should be removed,42,43 whereas others believe that it is a selection factor inherent to human reproduction that we should not attempt to remove.27 On one hand, simulations and causal diagrams demonstrate that bias can be induced through reproductive selection processes.26,42 On the other hand, accounting for selection bias due to pregnancy through inverse probability weighting results in a causal question that is difficult to interpret: the effect of male marijuana use on spontaneous abortion if everyone in the preconception cohort became pregnant.26,27 The question is unrealistic because there will always be those who do not conceive, and the association is only relevant to the subpopulation at risk of the outcome.
Because marijuana use was self-reported and we did not collect time-varying marijuana measures, exposure misclassification is likely. In some cases reported exposure may be up to 12 months before conception. However, when restricting to men who completed baseline within 3 months of pregnancy detection, and thus ensuring a more etiologically relevant time-window for exposure, results were slightly stronger. We also did not collect information on dose or mode of use (e.g. ingestion, vaping, smoking). Other considerations for exposure misclassification include variation in North American marijuana policies, and the rise in popularity of cannabidiol (CBD) products. Men living in states without legalization of marijuana may underreport marijuana use.44,45 In our data, there was lower prevalence of marijuana use ≥1 time/week among those living in states with no marijuana legalization (5%) compared with those living in places with at least medical marijuana legalization (9%). It is unknown whether this represents a true difference in prevalence, or whether men who lived in states with no legalization are underreporting. In addition, some participants who use CBD-only products may report this as using marijuana, while others may report no marijuana use. CBD is an exogenous cannabinoid present in marijuana, and some animal model studies indicate CBD exposure might adversely affect reproductive outcomes.46,47 However, it is unknown how much exposure to isolated CBD differs from exposure to marijuana overall. Given the prospective design, any exposure misclassification is likely non-differential and expected to bias estimates in the highest category towards the null.48
Further, there may be misclassification of spontaneous abortion. Pre-implantation losses will not be captured in this study. And very early post-implantation losses often go undetected if home pregnancy testing is not used early in gestation (e.g., several days before a missed period). More than 95% of PRESTO women reported using home pregnancy tests and the median time at first pregnancy testing was 4 weeks across both exposure groups, indicating that most women test early. Because timing of early pregnancy identification was unrelated to male marijuana use, this is unlikely a large source of bias. The prevalence of spontaneous abortion in PRESTO (19%) agrees with estimates from a nationally representative population.49
Reverse causation is possible if men experiencing reproductive issues used marijuana under claims that cannabis products, particularly CBD, improve reproductive health. However, our estimates persisted after controlling for reproductive history and after stratifying by pregnancy attempt time at study entry, thereby allaying this concern. Finally, our E-value sensitivity analysis suggests that an unmeasured confounder would need to be associated with 1.6 times the risk of both marijuana exposure and spontaneous abortion to explain the observed association completely. We controlled for several female and male covariates, though we cannot rule out potential for residual or unmeasured confounding (e.g., from adverse childhood experiences, stressful life events, illicit drug use, or early life exposure to endocrine-disrupting chemicals).
In summary, frequent male preconceptional marijuana use was associated with an increased risk of spontaneous abortion in this prospective cohort study of North American couples. The association appeared to be driven by early pregnancy losses and was stronger among older men. The extent to which the association is explained by adverse effects on semen parameters (e.g., DNA fragmentation) is unclear.
Acknowledgments:
We thank the PRESTO participants for their contributions to this study. We additionally thank Mr. Michael Bairos for his help developing the web-based infrastructure of the PRESTO study. Finally, we thank Ms. Alina Chaiyasarikul for her work in research coordination.
Source of funding: This work was supported by the Eunice Kennedy Shriver National Institute for Child Health and Human Development (grants R01-HD086742, R21-HD072326, and R03-HD090315).
Footnotes
Conflicts of interest: None declared
Data availability:
Data analysis code is available upon request. The data are not available for replication because the Institutional Review Board does not permit the sharing of study data to other investigators, as the participants did not provide consent for data sharing.
References
- 1.Substance Abuse and Mental Health Services Administration. (2017). Key substance use and mental health indicators in the United States: Results from the 2016 National Survey on Drug Use and Health (HHS Publication No. SMA 17-5044, NSDUH Series H-52). Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration; Retrieved from https://www.samhsa.gov/data/ [Google Scholar]
- 2.Carliner H, Mauro PM, Brown QL, et al. The widening gender gap in marijuana use prevalence in the U.S. during a period of economic change, 2002-2014. Drug Alcohol Depend. 2017;170:51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaoni Y, Mechoulam R. Isolation, Structure, and Partial Synthesis of an Active Constituent of Hashish. J Am Chem Soc. 1964;86(8):1646–1647. [Google Scholar]
- 4.Rossato M, Ion Popa F, Ferigo M, Clari G, Foresta C. Human sperm express cannabinoid receptor Cb1, the activation of which inhibits motility, acrosome reaction, and mitochondrial function. J Clin Endocrinol Metab. 2005;90(2):984–991. [DOI] [PubMed] [Google Scholar]
- 5.Gye MC, Kang HH, Kang HJ. Expression of cannabinoid receptor 1 in mouse testes. Arch Androl. 2005;51(3):247–255. [DOI] [PubMed] [Google Scholar]
- 6.Bovolin P, Cottone E, Pomatto V, et al. Endocannabinoids are involved in male vertebrate reproduction: Regulatory mechanisms at central and gonadal level. Front Endocrinol (Lausanne). 2014;5(54):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grimaldi P, Di Giacomo D, Geremia R. The endocannabinoid system and spermatogenesis. Front Endocrinol (Lausanne). 2013;4:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Payne KS, Mazur DJ, Hotaling JM, Pastuszak AW. Cannabis and Male Fertility: A Systematic Review. J Urol. 2019;202(4):674–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphy SK, Itchon-Ramos N, Visco Z, et al. Cannabinoid exposure and altered DNA methylation in rat and human sperm. Epigenetics. 2018;13(12):1208–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gundersen TD, Jørgensen N, Andersson A-M, et al. Association Between Use of Marijuana and Male Reproductive Hormones and Semen Quality: A Study Among 1,215 Healthy Young Men. Am J Epidemiol. 2015;182(6):473–481. [DOI] [PubMed] [Google Scholar]
- 11.Vescovi P, Pedrazzoni M, Michelini M, Maninetti L, Bernardelli F, Passeri M. Chronic effects of marihuana smoking on luteinizing hormone, follicle-stimulating hormone and prolactin levels in human males. Drug Alcohol Depend. 1992;30(1):59–63. [DOI] [PubMed] [Google Scholar]
- 12.Nassan FL, Arvizu M, Mínguez-Alarcón L, et al. Marijuana smoking and markers of testicular function among men from a fertility centre. Hum Reprod. 2019;34(4):715–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thistle JE, Graubard BI, Braunlin M, et al. Marijuana use and serum testosterone concentrations among U.S. males. Andrology. 2017;5(4):732–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gandini L, Lombardo F, Paoli D, et al. Full-term pregnancies achieved with ICSI despite high levels of sperm chromatin damage. Hum Reprod. 2004;19(6):1409–1417. [DOI] [PubMed] [Google Scholar]
- 15.Robinson L, Gallos ID, Conner SJ, et al. The effect of sperm DNA fragmentation on miscarriage rates: a systematic review and meta-analysis. Hum Reprod. 2012;27(10):2908–2917. [DOI] [PubMed] [Google Scholar]
- 16.Eisenberg ML, Sapra KJ, Kim SD, Chen Z, Buck Louis GM. Semen quality and pregnancy loss in a contemporary cohort of couples recruited before conception: data from the Longitudinal Investigation of Fertility and the Environment (LIFE) Study. Fertil Steril. 2017;108(4):613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramasamy R, Scovell JM, Kovac JR, Cook PJ, Lamb DJ, Lipshultz LI. Fluorescence in situ hybridization detects increased sperm aneuploidy in men with recurrent pregnancy loss. Fertil Steril. 2015;103(4):906–909.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jayasena CN, Radia UK, Figueiredo M, et al. Reduced Testicular Steroidogenesis and Increased Semen Oxidative Stress in Male Partners as Novel Markers of Recurrent Miscarriage. Clin Chem. 2019;65(1):161–169. [DOI] [PubMed] [Google Scholar]
- 19.Jurkovic D, Overton C, Bender-Atik R. Diagnosis and management of first trimester miscarriage Davor Jurkovic consultant gynaecologist. BMJ. 2013. doi: 10.1136/bmj.f3676 [DOI] [PubMed] [Google Scholar]
- 20.Wise LA, Wesselink AK, Hatch EE, et al. Marijuana use and fecundability in a North American preconception cohort study. J Epidemiol Community Health. 2018;72(3):208–215. [DOI] [PubMed] [Google Scholar]
- 21.Wise LA, Rothman KJ, Mikkelsen EM, et al. Design and Conduct of an Internet-Based Preconception Cohort Study in North America: Pregnancy Study Online. Paediatr Perinat Epidemiol. 2015;29(4):360–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Therneau TM, Grambsch PM. Modeling Survival Data : Extending the Cox Model.; 2000. [Google Scholar]
- 23.Howards PP, Schisterman EF, Poole C, Kaufman JS, Weinberg CR. “Toward a clearer definition of confounding” revisited with directed acyclic graphs. Am J Epidemiol. 2012;176(6):506–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weinberg CR. Toward a clearer definition of confounding. Am J Epidemiol. 1993;137(1):1–8. [DOI] [PubMed] [Google Scholar]
- 25.Robins JM, Finkelstein DM. Correcting for noncompliance and dependent censoring in an AIDS clinical trial with inverse probability of censoring weighted (IPCW) log-rank tests. Biometrics. 2000;56(3):779–788. [DOI] [PubMed] [Google Scholar]
- 26.Snowden JM, Bovbjerg ML, Dissanayake M, Basso O. The Curse of the Perinatal Epidemiologist: Inferring Causation Amidst Selection. Curr Epidemiol Reports. 2018;5(4):379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Werler MM, Parker SE. Letters to the Editor Bias from conditioning on live-births in pregnancy cohorts: an illustration based on neurodevelopment in children after prenatal exposure to organic pollutants (Liew et al. 2015). Int J Epidemiol. 2015:1079–1080. [DOI] [PubMed] [Google Scholar]
- 28.Agenor A, Bhattacharya S. Infertility and miscarriage: Common pathways in manifestation and management. Women’s Heal. 2015;11(4):527–541. [DOI] [PubMed] [Google Scholar]
- 29.Van Der Weele TJ, Ding P. Sensitivity analysis in observational research: Introducing the E-Value. Ann Intern Med. 2017;167(4):268–274. [DOI] [PubMed] [Google Scholar]
- 30.Mínguez-Alarcón L, Chavarro JE, Gaskins AJ. Caffeine, alcohol, smoking, and reproductive outcomes among couples undergoing assisted reproductive technology treatments. Fertil Steril. 2018;110(4):587–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buck Louis GM, Sapra KJ, Schisterman EF, et al. Lifestyle and pregnancy loss in a contemporary cohort of women recruited before conception: The LIFE Study. Fertil Steril. 2016;106(1):180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karmon AE, Toth TL, Chiu Y-H, et al. Male caffeine and alcohol intake in relation to semen parameters and in vitro fertilization outcomes among fertility patients. Andrology. 2017;5(2):354–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Braga DP, Halpern G, Figueira Rde C, Setti AS, Iaconelli AJ, Borges EJ. Food intake and social habits in male patients and its relationship to intracytoplasmic sperm injection outcomes. Fertil Steril. 2012;97(1):53–59. [DOI] [PubMed] [Google Scholar]
- 34.Klonoff-Cohen HS, Natarajan L, Chen RV. A prospective study of the effects of female and male marijuana use on in vitro fertilization (IVF) and gamete intrafallopian transfer (GIFT) outcomes. Am J Obstet Gynecol. 2006;194(2):369–376. [DOI] [PubMed] [Google Scholar]
- 35.Windham GC, Fenster L, Swan SH. Moderate Maternal and Paternal Alcohol Consumption and the Risk of Spontaneous Abortion. Epidemiology. 1992;3(4):364–370. [DOI] [PubMed] [Google Scholar]
- 36.Parazzini F, Bocciolone L, La Vecchia C, Negi E, Fedele L. Maternal and paternal moderate daily alcohol consumption and unexplained miscarriages. BJOG An Int J Obstet Gynaecol. 1990;97(7):618–622. [DOI] [PubMed] [Google Scholar]
- 37.Henriksen TB, Hjollund NH, Jensen TK, et al. Alcohol Consumption at the Time of Conception and Spontaneous Abortion. Am J Epidemiol. 2004;160(7):661–667. [DOI] [PubMed] [Google Scholar]
- 38.Sharma R, Harlev A, Agarwal A, Esteves SC. Cigarette Smoking and Semen Quality: A New Meta-analysis Examining the Effect of the 2010 World Health Organization Laboratory Methods for the Examination of Human Semen. Eur Urol. 2016;70(4):635–645. [DOI] [PubMed] [Google Scholar]
- 39.Venners SA, Wang X, Chen C, et al. Paternal Smoking and Pregnancy Loss: A Prospective Study Using a Biomarker of Pregnancy. Am J Epidemiol. 2004;159(10):993–1001. [DOI] [PubMed] [Google Scholar]
- 40.Brandt JS, Cruz Ithier MA, Rosen T, Ashkinadze E. Advanced paternal age, infertility, and reproductive risks: A review of the literature. Prenat Diagn. 2019;39(2):81–87. [DOI] [PubMed] [Google Scholar]
- 41.Johnson SL, Dunleavy J, Gemmell NJ, Nakagawa S. Consistent age-dependent declines in human semen quality: a systematic review and meta-analysis. Ageing Res Rev. 2015;19:22–33. [DOI] [PubMed] [Google Scholar]
- 42.Liew Z, Olsen J, Cui X, Ritz B, Arah OA. Bias from conditioning on live birth in pregnancy cohorts: an illustration based on neurodevelopment in children after prenatal exposure to organic pollutants. Int J Epidemiol. 2015:345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raz R, Kioumourtzoglou MA, Weisskopf MG. Live-Birth Bias and Observed Associations between Air Pollution and Autism. Am J Epidemiol. 2018;187(11):2292–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kerr WC, Ye Y, Subbaraman MS, Williams E, Greenfield TK. Changes in marijuana use across the 2012 washington state recreational legalization: Is retrospective assessment of use before legalization more accurate? J Stud Alcohol Drugs. 2018;79(3):495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuhns JB, Miller RN. Exploring the Impact of Medical Marijuana Laws on the Validity of Self-Reported Marijuana Use Among Juvenile Arrestees Over Time. Crim Justice Policy Rev. 2012;23(1):40–66. [Google Scholar]
- 46.Carvalho RK, Souza MR, Santos ML, et al. Chronic cannabidiol exposure promotes functional impairment in sexual behavior and fertility of male mice. Reprod Toxicol. 2018;81:34–40. [DOI] [PubMed] [Google Scholar]
- 47.Chang MC, Schuel H. Reduction of the fertilizing capacity of sea urchin sperm by cannabinoids derived from marihuana. II. Ultrastructural changes associated with inhibition of the acrosome reaction. Mol Reprod Dev. 1991;29(1):60–71. [DOI] [PubMed] [Google Scholar]
- 48.Dosemeci M, Wacholder S, Lubin JH. Does nondifferential misclassification of exposure always bias a true effect toward the null value? Am J Epidemiol. 1990;132(4):746–748. http://www.ncbi.nlm.nih.gov/pubmed/2403115. Accessed April 18, 2019. [DOI] [PubMed] [Google Scholar]
- 49.Rossen LM, Ahrens KA, Branum AM. Trends in Risk of Pregnancy Loss Among US Women, 1990-2011. Paediatr Perinat Epidemiol. 2018;32(1):19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data analysis code is available upon request. The data are not available for replication because the Institutional Review Board does not permit the sharing of study data to other investigators, as the participants did not provide consent for data sharing.