Abstract
Purpose:
To evaluate the safety and feasibility of combining exercise (EX) and resveratrol to treat older adults with physical function limitations.
Methods:
Three-arm, two-site pilot randomized, controlled trial (RCT) for community-dwelling adults (N = 60), 71.8 ± 6.3 years of age with functional limitations. Participants were randomized to receive either 12 weeks of (1) EX + placebo [EX0], (2) EX + 500 mg/day resveratrol [EX500], or (3) EX + 1,000 mg/day resveratrol [EX1000]. EX consisted of two sessions a week for 12 weeks of center-based walking and whole-body resistance training. Safety was assessed through adverse events and feasibility through exercise session and supplement (placebo, or resveratrol) protocol adherence. Outcome measures included a battery of indices of physical function as well as skeletal muscle mitchondrial function. Data were adjusted for age and gender using the Intent-To-Treat approach.
Results:
Adverse event frequency and type were similar between groups (n=8 EX0, n=12 EX500, and n=7 EX1000). Overall, 85% of participants met the supplement adherence via pill counts while 82% met the exercise session adherence. Adjusted within group mean differences (95% confidence interval) from week 0 to 12 for gait speed ranged from −0.04 (EX0: −0.1, 0.03) m/s to 0.04 (EX1000: −0.02, 0.11) and the six-minute walk test mean differences were 9.45 (EX0: −9.02, 27.7), 22.9 (EX500: 4.18, 41.6), and 33.1 (EX1000: 13.8, 52.4) meters. Unadjusted mean differences for citrate synthase were −0.80 (EX0: −15.45, 13.84), −1.38 (EX500: −12.16, 9.39), and 7.75 (EX1000: −4.68, 20.18) mU/mg. COX activity mean within group changes ranged from −0.05 (EX0) to 0.06 (EX500) k/sec/mg. Additional outcomes are detailed in the text.
Conclusion:
The pilot RCT indicated that combined EX + resveratrol was safe and feasible for older adults with functional limitations and may improve skeletal muscle mitochondrial function and mobility-related indices of physical function. A larger trial appears warranted and is needed to formally test these hypotheses.
Keywords: aging, clinical trial, exercise, functional status, mitochondria
Introduction
Maintaining physical function during the aging process is critical to ward off disability and extend independence. [1–3] However, despite the well-established relationship of decreased physical function with subsequent adverse health outcomes, few therapeutic strategies exist that are shown to improve these outcomes among older adults. To date, interventions incorporating physical exercise have demonstrated the most consistent results for improving physical function among older adults. [4] Still, despite the general benefits of exercise, physiologic responses to exercise can be quite variable [5 6] – thus adjuvant strategies may be useful to enhance the efficacy of exercise.
One potential such adjuvant is the nutritional supplement resveratrol, a compound commonly found in red grapes. Resveratrol is a natural polyphenol with purported anti-oxidant, anti-inflammatory, and metabolic benefits. [7–11] Preclinical studies have suggested greater potential benefits in combining exercise with resveratrol supplementation compared to exercise alone, [12–14] but existing data from humans are less supportive. [15–17] Still, the literature in humans remains sparse and novel interventions are needed to enhance the efficacy of exercise among older adults with physical limitations. Accordingly, the current pilot RCT expands on the limited literature to evaluate dose-dependent effects of resveratrol combined with physical exercise for older adults with functional limitations with an emphasis on safety and feasibility. As outlined previously [18], we designed a pilot, randomized controlled trial (RCT) to begin to test the hypothesis that resveratrol may potentiate the functional benefits of exercise among older adults at least partly by enhancing beneficial adaptations in skeletal muscle mitochondrial function in response to chronic exercise.
The objective of this pilot trial was to collect data on the safety, feasibility, and potential efficacy of resveratrol supplementation combined with exercise training on indices of physical function and skeletal muscle mitochondrial function among older adults with functional limitations. The present manuscript provides findings from the pilot study designed to support or refute the need for a larger-scale trial necessary to fully test our central hypothesis. Notably, due to additional resources and tissue availability, were able to expand our initial battery of mitochondrial measures (mitochondrial DNA copy number, citrate synthase, cytochrome C oxidase) to include a more comprehensive evaluation of skeletal muscle mitochondrial function including indices of mitochondrial damage, fusion, fission, transcriptional regulation, as well as vascular inflammation and oxidative stress. These data provide important biologic insight, in addition to the clinical measures, to aid in evaluation of the potential need for a future, larger-scale trial in this area.
Material and Methods
2.1. Study Overview
This three-arm pilot RCT was designed to evaluate the safety and efficacy of two resveratrol doses (500 mg/d and 1,000 mg/d) combined with structured exercise training. 1000 mg/d dose was selected based on a previous 12 week resveratrol dosing pilot study that demonstrated its safety for older adults [19] while 500 mg/d was deemed sufficient for improving mitochondrial function [20] as outlined in the trial design [18].
Study participants were older adults with functional limitations, and both resveratrol protocols were compared to a placebo. The study design was described in detail previously [18] with the addition that the study was completed across two clinical data collection sites (Gainesville, FL and Birmingham, AL). This study was carried out in accordance with the Declaration of Helsinki. The protocol was approved by the University of Florida and the University of Alabama at Birmingham Institutional Review Boards. Prior to enrollment, all participants provided written informed consent. The study is registered at www.clinicaltrials.gov (NCT02523274).
Analyses were performed by biostatisticians who remained masked to the intervention assigned until the analyses were completed, providing a triple-masked design including study staff and participants. Moreover, the study intervention and study assessments were conducted by separate staff and in separate physical locations to prevent bias.
2.2. Participants
Older adults ≥ 65 years old, (N = 60) were recruited from the Gainesville, FL and Birmingham, AL (USA) areas from 2016 – 2018. Recruitment involved direct residential mailings and print advertisements. Eligibility criteria included (1) age ≥ 65 years; (2) < 150 minutes/ week of moderate physical activity on the Community Health Activities Model Program for Senior (CHAMPS) questionnaire; [21] (3) > 290 s indicating functional limitations during the long-distance corridor test; [1] (4) willingness to be randomized to either treatment group; (5) willingness to participate in all study procedures.
Exclusion criteria included (1) a failure to provide consent, (2) regular consumption of a resveratrol supplement; (3) current involvement in a supervised rehabilitation program; (4) absolute contraindication(s) to exercise training according to the American College of Sports Medicine [22] (5) Pain classification > Grade 3 on a Graded Chronic Pain Scale (84); (6) significant cognitive impairment, [23] or a Mini-Mental State Examination (MMSE) score < 24; (7) severe cardiac, neurologic, rheumatologic, orthopedic, or other conditions which prohibited safe participation, or (8) simultaneous participant in another intervention trial. For participation in the muscle biopsy, additional exclusion criteria included (1) use of prescription anti-platelet medications (not including aspirin); (2) prescription anti-coagulant use (e.g. warfarin); (3) conditions which would reduce healing; or (4) known allergy to lidocaine.
2.3. Randomization
Published recommendations for pilot trial design indicate 15–20 participants per randomized arm could indicate feasibility for a full-scale RCT. This sample size was also appropriate to determine the estimation of mean changes (e.g. 95% confidence intervals) for each treatment group. [24 25] Thus, sample size for the pilot was based on published literature for the conduct of pilot studies.
Participants that met the inclusion and exclusion criteria were asked to return for a baseline assessment and subsequent randomization. Upon randomization, participants were randomized 1:1:1 to three study treatments. To preserve masking, treatment allocation was concealed to all directly involved (investigators, study staff, biostatisticians, and participants) until the end of the trial.
2.4. Supplement Intervention
Participants that met the study inclusion and exclusion criteria were randomized to consume either a placebo, 500 mg/day, or 1,000 mg/day of resveratrol provided by Reserveage Organics (Gainesville, FL, USA) via visually identical capsules. Delivery was provided twice a day with instructions to consume one capsule 15–30 minutes before breakfast and another 15–30 minutes before dinner in which the 500 mg/day resveratrol group received one placebo capsule and one 500 mg/day resveratrol capsule. Participants were instructed to bring back all unused doses at each assessment visit allowing for study staff to conduct pill counts for adherence purposes.
2.5. Exercise Intervention
All groups performed a twice a week for 12 weeks, center-based, supervised walking and whole-body resistance exercise training program. Participants began with a brief warm-up followed by 30-minutes of walking encouraged at moderate-intensity for 20 minutes and moderate to vigorous intensity walking for the last 10 minutes. If walking was not well-tolerated, participants pedaled on a recumbent, stationary bicycle (Life Fitness, Schiller Park, IL, USA). After aerobic training, participants transitioned to whole-body strength training, and ended with flexibility exercises and balance training at intensities between rating of perceived exertion (RPE) moderate (5–6) to vigorous (7–8). [26] Leg press, leg extension, chest press, and seated row were performed on day one of the week while leg curl, calf flexion, arm curl, and triceps extensions were conducted on day two of training guided to elicit a 6–7 on the Borg CR10 RPE (0–10 scale). [26] All resistance exercises were performed using standard isotonic resistance training equipment (Life Fitness, Schiller Park, IL, USA). Exercise session data including walk distance, rating of perceived exertion, and heart rate (beats per minute). Participants at the second site, The University of Alabama at Birmingham (N = 23), one repetition maximum (1 RM) strength was assessed at weeks 0 and 12 for leg press, leg extension, and chest press.
2.6. Safety, Recruitment, and Adherence
Safety was closely monitored throughout the duration of the study. Participants were encouraged to notify study staff immediately if any adverse events occurred that was potentially related to the study intervention. Moreover, study staff monitored all adverse events at each study visit and exercise intervention visit. In addition, clinical safety blood tests (e.g., lipid panel, comprehensive metabolic panel) were used to monitor hematologic and metabolic abnormalities in response to the interventions and were performed at weeks 0, 6, and 12. Blood pressure was also assessed using an automatic blood pressure monitor (Microlife®, Omron, Kyoto, Japan) at assessment visits (weeks 0, 6, and 12) and immediately prior to the start of each exercise training session.
Participant recruitment was performed via community advertising in form of both print and digital media advertising. Adherence to exercise was calculated as scheduled session attendance while supplement adherence (placebo and resveratrol) conducted via pill counting.
2.7. Assessments
Study outcomes of interest included changes in gait speed, walking endurance, mean composite knee extensor peak torque, validated physical function test battery, habitual physical activity, and circulating indices of cardiovascular risk assessed at weeks 0 and 12 as described previously. [18]
Functional Assessments
Gait speed was assessed via a 4 m usual-paced walk test at week 0 and 12. This simple and cost-effective physical performance tool assesses functional status. [27 28] Walking endurance was measured through the fast-paced six-minute walk test on an indoor walk course as described previously. [29 30] Mean composite knee extensor peak torque was assessed across three angular velocities (60, 90, 120°/s) as unilateral isokinetic strength of the knee extensors in the dominant limb via a dynamometer (Biodex Medical Systems, New York, NY, USA) as previously published. [31 32] Participants also completed the validated short physical performance battery (SPPB), which involves balance tests, the 4 meter usual-paced walk, and a repeated chair stand task. [33]
The Late Life Function and Disability Instrument (LLFDI) was used to report self-assessed physical function [34 35] through a 16-item questionnaire that assesses both function and disability. Specifically the function component evaluates an individual’s ability to perform actions or activities whereas the disability assessed the individual’s performance of socially defined tasks as described previously. [36] Raw scores were transformed and presented as the total scaled for each component.
Three-day diet records were conducted in which participants were asked to carefully record all foods, drinks as well as the time and portion sizes for two weekdays and one weekend day within a seven-day period. The diet record was analyzed using a food processor program package (ESHA, Salem, OR, USA) as published previously. [37 38]
Habitual physical activity was assessed through a hip worn, solid-state triaxial accelerometer (Actigraph wGT9X-BT, Pensacola, FL, USA) with an adjustable strap for seven consecutive days to monitor potential changes in physical activity in the community setting [39 40]. Participants were encouraged to record any time during the seven days in which the accelerometer was not worn in a provided log. Data were sampled for 1 min epochs for each day to estimate minutes of activity. [41].
Biospecimen Collection
At weeks 0 and 12, venous blood was collected according to standard clinical procedures after approximately eight hours of fasting. Blood samples were utilized for clinical safety parameters, including a comprehensive metabolic panel, as well as study assays. Skeletal muscle samples (0, 12 weeks) were also provided by a subset of participants (n = 22; EX0 = 7, EX500 = 7, EX1000 = 8). Post-exercise muscle biopsy collection occurred at least 48 hours following the last exercise session, with biopsies occurring 2–5 days post-training pending participant availability.
Percutaneous skeletal muscle samples (200–225 mg) were obtained from the vastus lateralis skeletal muscle under local anesthesia. Muscle biopsies were collected by a trained, licensed study clinician approximately midway between the patella and iliac crest in the vastus lateralis utilizing a 5-mm Bergstrom biopsy needle with suction as previously published. [42–45] After collection, skeletal muscle samples were immediately snap frozen in liquid nitrogen and stored at −80°C prior to analysis.
Skeletal Muscle Mitochondrial Function
Mitochondrial outcome measures included skeletal muscle expression of peroxisome proliferator-activated receptor gamma receptor gamma coactivator 1-alpha (PGC-1α), mitochondrial DNA (mtDNA) copy number, mitochondrial DNA damage, and mitochondrial complex enzyme activity assays for citrate synthase and cytochrome C oxidase (COX) activity. [18]
Mitochondrial DNA Content
As described previously, [46–54] total DNA was isolated using the QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) and quantified using the Quant-iT PicoGreen dsDNA Assay Kit (Invitrogen, Carlsbad, CA). A 236 bp (short), highly-conserved region of the mitochondrial genome was amplified from 15ng of total DNA, in a GeneAmp PCR system 2400 using a quantitative polymerase chain reaction (QPCR), GoTaq DNA Polymerase (Promega), 32P radiolabeled ATP (Perkin-Elmer) and primers 1066F/N1007R. 20μL of each “short” QPCR product was resolved (vertical electrophoresis) on 8–12% polyacrylamide gels at 90 volts for 2h. Dried gels were exposed to phosphor screens for 12h and quantified with ImageQuant (Molecular Dynamics, Sunnyvale, CA, USA). Quantified bands were normalized to mitochondrial DNA (mtDNA) copy number (content) reported as a percentage of a positive control.
Mitochondrial DNA Damage
The entire mitochondrial genome (long) was then amplified by QPCR from 15ng of total DNA, using AccuPrime Taq DNA Polymerase (Invitrogen), 32P radiolabeled ATP (Perkin-Elmer, Waltham, MA, USA) and primers 1065F/1066R. [46–54] 25μL of each “long” QPCR product was resolved (vertical electrophoresis) on 1.2% agarose gels at 90 volts for 3h, dried and exposed to phosphor screens for 12h, before being quantified with ImageQuant (Molecular Dynamics, Sunnyvale, CA USA). Quantified band densities for each “long” were expressed as a ratio relative to the “short”, with each participants’ post-intervention values (Week 12) expressed relative to the pre-intervention (Week 0) values. The mtDNA lesion frequency for each sample was then calculated using the Poisson equation [49], –ln(Week 12/Week 0).
Mitochondrial Volume
Citrate synthase, a surrogate index of mitochondrial volume, [55 56] was measured using a coupled reaction with oxaloacetate, acetyl-CoA, and 5, 5-dithiobis-(2,4-nitrobenzoic acid). [57 58] COX activity was measured at 550nm. [59] For biochemical assays of muscle proteins involved in signaling and transcription, skeletal muscle samples were lysed, measured for concentration using bicinchoninic acid (BCA) protein assay and bovine serum albumin (BSA) standards.
Skeletal Muscle Mitochondrial Protein Abundance
Capillary nano-immunoassays were performed using WES™ [60] (ProteinSimple Inc., San Jose, CA, USA); according to the manufacturer’s using the 12–230 kDA Master Kit Separation Module [61] (ProteinSimple Inc., San Jose, CA, USA; SM 004–600). Prior to analysis, skeletal muscle samples were lysed, measured for concentration using bicinchoninic acid (BCA) protein assay and bovine serum albumin (BSA) standards. Sodium dodecyl sulfate (SDS), dithiothreitol (DTT), and fluorescent labeled molecular weight marker were added into the capillary columns. [62] The capillary was probed with target specific antibodies and conjugated secondary antibodies for the following proteins (abbreviation: company, catalog number): P38-mitogen activated protein kinase α (P38α: R&D Systems, AF8691); mitochondrial dynamin like GTPase Opa1 (OPA1: Novus, NB110–55290), Mitochondrial transcription factor A (TFAM: Novus, NBP1–71648); Mitofusin-2 (MFN2: ABCam, AB56889); Mitochondrial fission 1 protein (Fis1: PTGLAB, 10956–1-ap), PGC-1α (ABCam, AB54481), Nuclear respiratory factor 1 (NRF1: PTGLAB, 12482–1-AP); Receptor-interacting protein 140 (RIP140: Sigma-Aldrich, MABS1917); Sirtuin (Sirt1: Cell Signaling, 9475). Capillary plates were analyzed using the Compass Software (ProteinSimple Inc., San Jose, CA, USA) normalized to total protein. The chemiluminescent light generation was measured by a charge coupled device to analyze the protein size and peak area. The WES™ analysis has good reproducibility through its automated operation, assisting with protocol standardization. [61 63]
Vascular Inflammation & Oxidative Stress
Serum sample preparation for ELISA kits involved the collection of whole blood samples (3.5mL) in serum separator (SST) vacutainer blood collection tubes (BD Medical, Franklin Lakes, NJ, USA). Samples were positioned upright for 30–60 min at ambient temperate and then centrifuged for 20 min (3,380 x g). The serum aliquot was stored at −80°C until the final analyses.
Whole blood (4.0mL) was collected in ethylenediaminetetraacetic acid (EDTA) vacutainer blood collection tubes (BD Medical, Franklin Lakes, NJ, USA). Plasma was isolated by centrifugation at ambient temperature (3,380 x g, 10 min). Supernatants were collected and a secondary spin, at ambient temperature (10,000 x g, 10 min) was conducted. The final aliquot was stored at −80°C until the final analyses. Circulating markers of vascular inflammation and oxidative stress were analyzed through commercially available ELISAs kits for the following targets: interleukin-6 (IL-6, D6050), vascular cellular adhesion molecule 1 (VCAM-1, DVC00), E-selectin (DSLE00), myeloperoxidase (MPO, DMYE00B) (R&D Systems, Minneapolis, MN, USA), and oxidized low density lipoprotein (OxLDL) (Mercodia, Uppsala, Sweden). The BioTek Gen5 30.4 software was utilized with the BioTek 800 TS microplate reader (BioTek Instruments, Inc., Winooski, VT, USA) to analyze ELISA findings. The intra-assay coefficient of variation for ELISA kits were the following: IL-6 (9.67%), VCAM-1 (6.8%), E-selectin (3.71%), MPO (5.38), and OxLDL (7.96%).
As an additional indicator of potential oxidative stress, plasma concentrations of nitrate and nitrite were also evaluated. For plasma nitrate and nitrite level assessment, [64] plasma was thawed on ice and then ice-cold methanol (1:1 volume) added and the sample vortex mixed for 10 seconds. The mixture was then centrifuged at 10,000 x g for 10 min and supernatant collected and measured by High Performance Liquid Chromatography (HPLC) coupled to the Griess reaction using the ENO-30 N (EiCom, Japan). [65] Data collection, calibration, and analysis were conducted by Eicom Envision software (Eicom, Corp., Kyoto, Japan). Nitrite and nitrate concentrations were determined using calibration curves generated daily with sodium nitrite and sodium nitrate standards. Detecting nitrite and nitrate, major metabolites of nitric oxide can be used to determine nitric oxide levels. 75% of participants provided week 0 and 12 samples that were viable and analyzed for nitrite, nitrate, and total protein concentrations. Protein was measured using the Biorad assay.
2.8. Statistical Analysis
The primary analysis was an Intent-To-Treat approach. Descriptive analyses were conducted overall and stratified by study site. Summary values, i.e. mean (standard deviation) and frequency (percentage), were computed for all analysis variables. Linear mixed models were applied for each outcome, accounting for heterogeneity between study participants and allowing incorporation of data from participants who missed a visit. All model assumptions were assessed visually and by conducting the Kolmogorov-Smirnov test upon model residuals. A group-by-time interaction was used to estimate intervention-based changes from baseline, and additional adjustment was included for exercise group, and assessment visit (weeks 0, 6, and 12). Additional adjustments included age (continuous) and gender (categorical). As this study was not powered to detect differences in outcomes (i.e. p-value < 0.05), estimated mean differences are presented with 95% confidence intervals or standard error. This presentation style is consistent with published guidelines for reporting findings from pilot studies. [66 67] Biospecimen results are reported as unadjusted modeled values. Unadjusted clinical data as well data for an efficacy analysis [among participants who maintained ≥ 75% for both exercise attendance and pill adherence] are provided in supplementary materials. All statistical analyses were performed using R version ≥3.6.0, using a number of open-source software packages. [68–73]
Results
3.1. Enrollment and Retention
Of those who attended the in-person screening visit, 60/145 = 41.38% of participants met the necessary in-person screening inclusion and exclusion criteria (Figure A.1). Screen fail rates were 55% at the University of Florida (59/108) and 38% at the University of Alabama at Birmingham (14/37). Of those who failed to meet inclusion/exclusion criteria, most participants (n = 22) either walked faster than 290 seconds on the long corridor walk test, [21 33 74] or performed more than < 150 minutes of moderate physical activity as assessed by the CHAMPS Questionnaire (n = 20). Other screen fails included: uncontrolled high blood pressure (>160 mm Hg systolic or >90 mm Hg diastolic; n = 9), pain classification >3 on the Chronic Pain Scale (n = 4), and peripheral vascular disease and MMSE <24/30 (n = 3), while the remaining criteria accounted for eleven other screen fails.
A total of 60 participants were recruited and randomized with n = 20 allocated to exercise + placebo (EX0), n = 20 to EX + 500 mg/day resveratrol (EX500), and n = 20 to EX + 1,000 mg/day resveratrol (EX1000). Participant baseline demographic characteristics were generally similar between groups (Table A.1), with some modest variability expected given the relatively small sample size in a pilot. A total of n = 4 participants withdrew from the study, and n = 1 was a lost to follow-up. In the Intent-To-Treat analysis, N = 60 participants were included (Figure A.1).
As indicated in Table A.1, participants commonly used prescription and supplementary medications among the groups included anti-hypertensive, glucose-lowering, statins, and nonsteroidal anti-inflammatory (NSAID) drugs. Although there are no known interactions reported for anti-hypertensive, glucose-lowering, and statin medications, potential interactions of NSAIDs and resveratrol has been documented, [75 76] which may warrant further investigation in future studies.
3.2. Safety and Adherence
A review of the safety was conducted through assessment of adverse events during the pilot RCT. In total, 27 related, or possibly related individual adverse event reports occurred. Overall, adverse events were reported with similar rates among groups (n = 8 for EX0, n = 12 for EX500, and n = 7 for EX1000). The most common adverse events were gastrointestinal issues (n= 9). Of those participants, two were in the EX1000 mg/day resveratrol group, and five were in the EX500 mg/day resveratrol group. Other common adverse events were musculoskeletal events (n = 6), and dizziness (n = 2). Two serious adverse events were reported which were unrelated to the trial. Changes in clinical safety laboratory values are shown in Supplementary information Table B.1.
Overall adherence for exercise was 82% (EX0: 84 ± 21%, EX500: 79 ± 24%, and EX1000: 81 ± 20%). Overall supplement (placebo, 500 mg/day, or 1,000 mg/day of resveratrol) adherence was 85% (EX0: 85 ± 20%. EX500: 86 ± 13%, and EX1000: 84 ± 18%).
For the efficacy analysis, exercise session adherence was set at ≥ 75% [77] for all 24 exercise sessions. 73% of all participants across groups met this threshold (individual groups EX0: 75%, EX500: 75%, and EX1000: 70%). The efficacy analysis for supplement adherence was also set at ≥ 75% in which 70% of all participants met the threshold (individual groups EX0: 70%, EX500: 65%, and EX1000: 75%). With the exercise session and supplemental adherence efficacy threshold combined, 60% of all participants met both guidelines (EX0: 60% (12/20), EX500: 60% (12/20), and EX1000: 60% (12/20)).
3.3. Exercise Data
Participant walking distance during exercise sessions are displayed in Figure A.2. Changes in walking distance from session 1 to session 24 are presented for all groups including: EX500 (342 m) and EX1000 (449 m) compared to EX0 (245 m).
3.4. Physical Function
Relative changes (from week 0 to week 12) in measures of physical function are shown in Figure A.3. Mean changes in gait speed ranged from −0.04 (EX0; 95% CI: −0.1, 0.03) m/s to 0.04 (EX1000: −0.02, 0.11) m/s.
The six minute walk distance had similar estimated week 0 mean ranges (412.16 – 413.47) m, yet different patterns of within group distances covered were evident ranging from 9.45 (EX0: −9.02, 27.7) m to 33.1 (EX1000: 13.8, 52.4) m.
SPPB week 0 means ranged from 10.33 – 10.41 (out of 12 total) with similar within group results after 12 weeks ranging from 0.13 (EX1000 −0.42, 0.69) total SPPB to 0.38 (EX0: −0.17, 0.93) total SPPB.
Mean changes in quadricep strength ranged from −3.24 (EX0: −9.7, 3.23) Nm to 10.5 (EX500: 4.28, 16.7) Nm.
All groups had similar LLFDI limitation scaled total for week 0 with estimated means (ranging 73.60 – 73.81) scaled total score, and 12 week within group patterns from 3.36 (EX1000: −1.5, 8.22) score to 5.72 (EX500: 0.93, 10.5) scaled total.
The LLFDI frequency total had similar estimated week 0 means (ranging from 52.18 to 52.33) with week 12 within group changes ranging from 1.41 (EX0: −1.52, 4.35) total scaled to 2.59 (EX1000: −0.44, 5.62) total scaled.
Overall, results from the efficacy analysis appears similar to the intent to treat primary analysis (Supplementary information Figure B.1). The 4 m usual paced gait speed for EX1000 had a positive change of 0.08 (EX1000: −0.01, 0.17) m/sec while EX0 and EX500 had relative negative changes in gait speed. Walking endurance for both EX + resveratrol groups was 30.3 (EX500: 6.17, 54.3) m, and 36.4 (EX1000:12.3, 60.5) m. Mean changes in quadricep strength ranged from 8.72 (EX500: 1.44, 16) Nm to 10.6 (EX1000: 2.9, 18.4) Nm, while the LLFDI limitation total for EX500 was 7.43 (0.08, 14.8) scaled units and the LLFDI functional total was 2.17 (0.1, 4.24) scaled units.
The unadjusted indices of physical function are displayed in Supplementary information Figure B.2. Within group mean changes for endurance walking ranged from 8.96 (EX0: −13.04, 31) m to 32.93 (EX1000: 9.9, 56) m.
3.5. Supportive Outcomes
For 1RM data, leg press within group changes ranged from 21.4 (EX0: −7.88, 50.7) lbs to 54.3 (EX500: 22.9, 85.6) lbs. Chest press had smaller relative within group changes including 2.65 (EX500: −7.45, 12.7) lbs to 5.75 (EX0: −3.68, 15.2) lbs. Knee extension within group changes ranged from 2.3 (EX0: −17.3, 41.9) lbs to 27.7 (−3.73, 59.1) lbs. Changes in diet, outside physical activity, and supportive outcomes related to cardiometabolic health are shown in Supplementary information Table B.2.
3.6. Skeletal Muscle Mitochondrial Volume and Damage
Skeletal muscle mitochondrial volume and damage are displayed in Figure A.4. Citrate synthase displayed relevant within group mean changes ranging from −1.38 (EX500: −12.16, 9.39) mU/mg to 7.75 (EX1000: −4.68, 20.18) mU/mg. COX activity showed similar within group changes including −0.05 (EX0: −0.22, 0.11) k/sec/mg to 0.06 (EX500: −0.06, 0.19) k/sec/mg.
Lesion frequency, had negative relative differences, less within group damage, for both EX and resveratrol groups including −0.08 (EX500: −0.34, 0.18), and −0.03 (EX1000: 0.11, −0.23). Mitochondrial copy number was normalized to mitochondrial DNA ranging from −3.1 × 105 (EX500: −1.4 × 107, 1.4 107) arb. units to 5.3 × 106 (EX1000: −1.1 × 107, 2.1 × 107) arb. units.
3.7. Skeletal Muscle Mitochondrial Protein Abundance
Skeletal muscle mitochondrial relative protein abundance is shown in Figure A.5. TFAM ranged from 0.23 (EX1000: −0.51, 0.98) peak area to 0.61 (EX500: −0.16, 1.38) peak area. For NRF1, we observed similar with group changes (ranging from .10 to 0.15) with 0.15 (EX500: −0.1, 0.39) peak area. MFN2 had relative positive changes for all groups ranging from 0.5 (EX500: 0.06, 0.93) peak area to 0.12 (EX1000: −0.31, 0.54) peak area. Opa1 had a relative positive changes for resveratrol and exercise groups including 0.17 (EX1000: −3.41, 3.75) peak area to 2.42 (EX500: −1.52, 6.35) peak area. FIS1 had positive within group changes including 0.22 (EX0: −0.36, 0.58) to .99 (EX500: −0.004, 2.01) peak area. RIP140 had relative changes included −0.01 (EX0: −0.02, 0) to 0.01 (0, 0.02) peak area for both EX + resveratrol groups. SIRT1 relative changes ranged from 0 (EX1000: −0.1, 0.09) peak area to 0.05 (EX0: −0.05, 0.16) peak area. PGC-1α had relative positive changes for all groups including 0.12 (EX0: −0.41, 0.65) to 0.37 (EX500: −0.12, 0.87) peak area. P38α relative changes ranged from −0.03 (EX1000: −0.28, 0.22) peak area to 0.19 (EX500: −0.07, 0.45) peak area.
3.8. Inflammation and Oxidative Stress Biomarkers
Inflammatory markers VCAM-1, E-Selectin, and IL-6 are shown in Figure A.6. VCAM-1 had negative within group changes for all groups ranging from −257 (EX1000: −374, −140) ng/ml to −139 (EX0: −267, −11) ng/ml. E-selectin within group changes included −32 (EX0: −43, −21) ng/ml to −22 (EX500: −32, −12) ng/ml. All groups had a relative increase in IL-6 ranging from 0.83 (EX500: 0.15, 1.52) pg/ml to 1.69 (EX0: 0.69, 2.31) pg/ml. MPO in Figure A.6 ranged from −35 (EX500: −57, −14) ng/ml to 36 (EX1000: 14, 58) ng/ml. OxLDL had relative within group decreases including −4.5 × 104 (EX0: −5.6 × 104, −3.5 × 104) mU/L to 3.2 × 104 (EX500: −4.2 × 104, −2.1 × 104) mU/L, in Figure A.6.
Plasma nitrite (NO2-) is presented relative to total plasma protein ranging from −0.005 (EX500: −0.002, 0.0009) μM to 0.003 (EX0: 0.001, 0.004) μM. Plasma nitrate (NO3-), is also presented as relative to total protein at week 0 varied for all groups, within group means including −0.07 (EX1000: −.092, −0.044) μM to 0.019 (EX0: −0.009, 0.047) μM in Figure A.6.
Discussion
The three-arm, pilot RCT collected critical data on safety and feasibility for combining chronic exercise with two doses of orally-consumed resveratrol. The purpose was to refine and finalize elements critical to conducting a future, fully-powered RCT to definitively test whether resveratrol combined with chronic exercise improves functional limitations among older adults. Our results suggest that a fully powered RCT is feasible and safe.
Overall, there was a relatively low number of related, or possibly related adverse events during the pilot RCT. Although gastrointestinal issues were the most reported (n = 9), only two of those participants received the 1,000 mg/day resveratrol. Regarding the feasibility of a fully-powered RCT, components of recruitment were considered, [78] and the recruitment target was achieved for the trial. Moreover, the trial provided useful information regarding the relative recruitment yield at two potential field sites for a future trial.
Adherence to intervention protocols are essential in evaluating the pilot RCT. Adherence was determined for exercise sessions and pill supplement (placebo, 500 mg/day or 1,000 mg/day of resveratrol). The intent to treat adherence for exercise session was 82% and supplement pill adherence was 85%.
Another key consideration of the pilot RCT was assessing dose response of resveratrol (500 mg/d vs. 1,000 mg/d). As a pilot RCT, our results should not be over interpreted and are presented as mean ± 95% confidence intervals as reported previously. [37 38] However, the observed 33.1 m improvement in the six min walk test for the EX1000 group is a clinically meaningful difference [79] offering potential support for combined exercise and 1,000 mg/d resveratrol supplementation. In addition, Figure A.2 describes the average total distance walked during exercise sessions for all groups. Regardless of the intervention randomization, all groups average walking distance increased from week 0 to week 12. Notably, the EX1000 group had the largest increase with 449 m. Mitochondrial function measures yielded some interesting results. Aligned with Hart et al. [13] findings in pre-clinical models, exercise combined with resveratrol may have enhanced aerobic capacity through skeletal muscle mitochondrial function. In support, citrate synthase, a common marker of mitochondrial volume, increased in the EX1000 group. Moreover, COX enzyme activity that modulates oxygen uptake increased for both EX and resveratrol groups while EX0 had a relative decrease in COX enzymatic activity. Although, normalized mtDNA content increased for the EX1000 group, EX0 also had similar values at week 12.
While all groups had similar results, the relative increase in PGC-1α in the EX500 group had a higher expression at week 12 compared to the relative change in the EX1000 group. As previous research indicates, PGC-1α may be reduced in sedentary individuals and may increase with exercise. [80]. Although speculative, it is possible that (1) the 12 week time frame in between skeletal muscle biopsies were too far apart to denote considerable protein abundance changes, and/or (2) the time between the last exercise session and the week 12 muscle biopsy may have been too far apart.
With skeletal muscle and blood samples available, protein abundance in skeletal muscle mitochondrial was assessed. TFAM and NRF1 had relative increases in all groups, with visually steeper curves for EX500. RIP 140, a nuclear coregulator involved in metabolism observed relative within group positive changes for both EX + resveratrol groups, aligned with pre-clinical models adaptation to endurance exercise [81]. MFN2, a marker of mitochondrial outer membrane fusion, had relative positive changes for all groups, with EX500 group relative change of 0.5 (0.06, 0.93) peak area. Mitochondrial fission marker, FIS1, had similar results. Opa1, a marker for inner membrane fusion observed a relative increase in the EX500 group, and relative decreases in the EX0 group. However, SIRT1, downstream from PCG-1α signaling had a relative positive changes for EX0 and EX500 groups. In comparison, P38α signaling had relative negative changes for EX0 and EX1000 which may indicate a reduction in the proinflammatory pathway and inhibition of reactive oxidative species, although EX500 had a relative positive change.
Moreover, the down regulation of VCAM-1 may have been influenced by the anti-inflammatory and anti-oxidant properties in resveratrol. [82] Our results showed that EX1000 had a relative decrease in nitrite was dose-dependent. Nitrite, an intermediate of nitric oxide, may have an integral role in mitochondrial function [83]. Specifically, there are multiple potential mechanisms that may have influenced the nitrite levels. The most likely mechanisms involve endothelial nitric oxide synthase (eNOS), renal function, and oral nitrate reductase (e.g. diet). A future trial design should consider assessing the role of eNOS in skeletal muscle to determine specific mechanistic changes.
In evaluating any future trial, mention of a few additional considerations is warranted. Although our three groups were randomized, some variation in the baseline characteristics should be acknowledged. The EX1000 group had 18/20, 90% females randomized compared to 13/20, 65% in EX500, and 14/20, 70% in the EX0 group. The EX1000 group also had a relatively younger population compared to the EX0 group (70.3 vs 73.3 years old). Moreover, resting, seated systolic blood pressure ranged in the exercise and resveratrol groups, (EX1000: 133 ± 9.6 mm Hg) to (EX500: 145 ± 15.1 mm Hg). In Supplementary information Table B.2, there were no within group changes in systolic blood pressure, but there was a notable diastolic blood pressure within group change with a baseline of 82.4 mm Hg and a −6.68 (EX500: −10.8, −2.56) mm Hg change. Participants’ diabetic history varied from 3/20, 15% in the EX1000 to 8/20, 42% in the EX500 group. Upon further review of the clinical safety outcomes described in Supplementary Table B.1, there were no within group changes total cholesterol as previously documented. [84] Collectively, the preliminary data gathered on physical function, biochemistry, as well as variations in participant characteristics should be acknowledged when proceeding with a future, fully powered trial.
Conclusions
Aging of the worldwide population will have a massive burden of clinical and economic costs associated with age-related physical function. Moreover, declines in physical function occur with age and disablement. Research studies have demonstrated that exercise is a needed, yet insufficient, component of interventions to prevent age-related physical function. Therefore, it is critical to identify adjuvant therapies designed to optimize the efficacy of exercise which could ultimately have a positive impact on reducing functional limitations. Our pilot RCT has concluded that combined EX + resveratrol can be a safe and feasible for older adults with functional limitations. Moreover, EX + 1,000 mg/d resveratrol may have benefits for physical function and mitochondrial function. Ultimately, a fully powered trial is necessary to understand the effects of exercise and resveratrol combined for older adults with functional limitations.
Supplementary Material
Highlights.
Combined exercise + resveratrol can be safe and feasible for older adults with functional limitations.
Combined exercise + resveratrol may have benefits for both physical function and mitochondrial function in older persons.
A fully powered trial is necessary to understand the efficacy of exercise and resveratrol combined for older adults with functional limitations.
Acknowledgments
We would like to thank the participants who volunteered their time. In addition, we would like to thank the University of Alabama at Birmingham Diabetes Research Center Bioanalytical Redox Biology Core who performed the skeletal muscle mitochondrial complex analyses.
Funding
Research was supported by the National Institute on Aging (R21AG049974, P30AG028740, P30AG050886), National Center for Medical Rehabilitation Research (1P2CHD086851 and T32HD071866), American Heart Association (Postdoctoral Fellowship 20POST34990005), and the UAB Center for Exercise Medicine.
Abbreviations
- RCT
randomized, controlled trial
- EX0
exercise + placebo
- EX500
exercise + 500 mg/d resveratrol
- EX1000
exercise + 1,000 mg/d resveratrol
- COX
cytochrome c oxidase
- CHAMPS
community healthy activities model program for seniors
- NYHA
New York Heart Association
- MMSE
mini-mental examination score
- RPE
rating of perceived exertion
- 1RM
1 repetition maximum
- LLFDI
late life function and disability instrument
- SPPB
short physical performance battery
- mtDNA
mitochondrial DNA
- qPCR
quantitative PCR
- ELISA
enzyme-linked immunosorbent assay
- SST
serum separator tube
- EDTA
ethylenediaminetetraacetic acid
- BCA
bicinchoninic acid
- BCA
bovine serum albumin
- SDS
sodium dodecyl sulfate
- DTT
dithiothreitol
- P38α
P38-mitogen activated protein kinase α
- OPA1
mitochondrial dynamin like GTPase Opa1
- TFAM
mitochondrial transcription factor A
- MFN2
mitofusin-2
- Fis1
mitochondrial fission 1
- NRF1
nuclear respiratory factor 1
- RIP140
receptor-interacting protein 140
- SIRT1
sirtuin
- VCAM-1
vascular cellular adhesion molecule 1
- MPO
myeloperoxidase
- OXLDL
oxidized low density lipoprotein
- HPLC
high performance liquid chromatography
- CI
confidence intervals
- eNOS
endothelial nitric oxide synthase
Table A.1.
Participant baseline characteristics according to the randomization group.
| EX0 (n = 20) | EX500 (n = 20) | EX1000 (n = 20) | |
|---|---|---|---|
| Age, years | 73.3 ± 7.8 | 72 ± 5.1 | 70.3 ± 5.8 |
| Sex, Female | 14 (70) | 13 (65) | 18 (90) |
| Race, White | 14 (70) | 13 (65) | 16 (80) |
| Ethnicity, Hispanic | 1 (5) | 1 (5) | 1 (5) |
| Site, UF | 13 (65) | 14 (70) | 10 (50) |
| Education – professional/graduate degree | 5 (25) | 8 (40) | 10 (50) |
| Height, cm | 163.0 ± 9.5 | 166.1 ± 8.3 | 162.4 ± 6.6 |
| Weight, kg | 83.4 ± 19.8 | 86.1 ± 24.5 | 84.4 ± 19.3 |
| Body Mass Index, kg/m2 | 31.1 ± 5.5 | 31.3 ± 7.6 | 32 ± 6.9 |
| Waist Circumference, cm | 98.4 ± 26.1 | 105 ± 20.3 | 103 ± 17.1 |
| Systolic blood pressure, mm Hg | 140 ± 13.7 | 145 ± 15.1 | 133 ± 9.6 |
| Diastolic blood pressure, mm Hg | 79.3 ± 11.8 | 82.4 ± 9.5 | 78.2 ± 8.2 |
| MMSE, points | 28.2 ± 1.4 | 27.4 ± 2 | 28.7 ± 1.5 |
| CHAMPS Questionnaire, physical activity moderate intensity, min | 53.0 ± 51.8 | 36.6 ± 45 | 30.2 ± 43.3 |
| Chronic Pain Scale, score | 1.2 ± 0.8 | 1.2 ± 0.8 | 0.9 ± 0.5 |
| 400 m walk gait speed, m/s | 1.2 ± 0.2 | 1.2 ± 0.1 | 1.2 ± 0.1 |
| Total number of prescription medications, n | 6 ± 3.4 | 5.3 ± 3.3 | 6.1 ± 4.3 |
| Total number of supplements, n | 3 ± 3.3 | 3.9 ± 2.9 | 2.3 ± 2.6 |
| History of hypertension, n | 15 (79) | 12 (60) | 11 (55) |
| History of diabetes, n | 6 (32) | 8 (42) | 3 (15) |
| History of lower-limb osteoarthritis, n | 2 (10) | 3 (15) | 2 (10) |
Notes: Values represent unadjusted participant baseline demographics and characteristics. Missing data have been excluded from all summary statistics and missing data is assumed to be missing at random. Data are presented as mean ± SD, or n (percentage).
Abbreviations: EX0 = exercise + placebo, EX500 = exercise + 500 mg/d resveratrol, EX1000 = exercise + 1,000 mg/d resveratrol, UF = University of Florida, MMSE = Mini-Mental State Examination, CHAMPS = Community Healthy Activities Model Program for Seniors, SPPB = Short Physical Performance Battery.
Figure A.1. Consolidated standards of reporting trials (CONSORT).
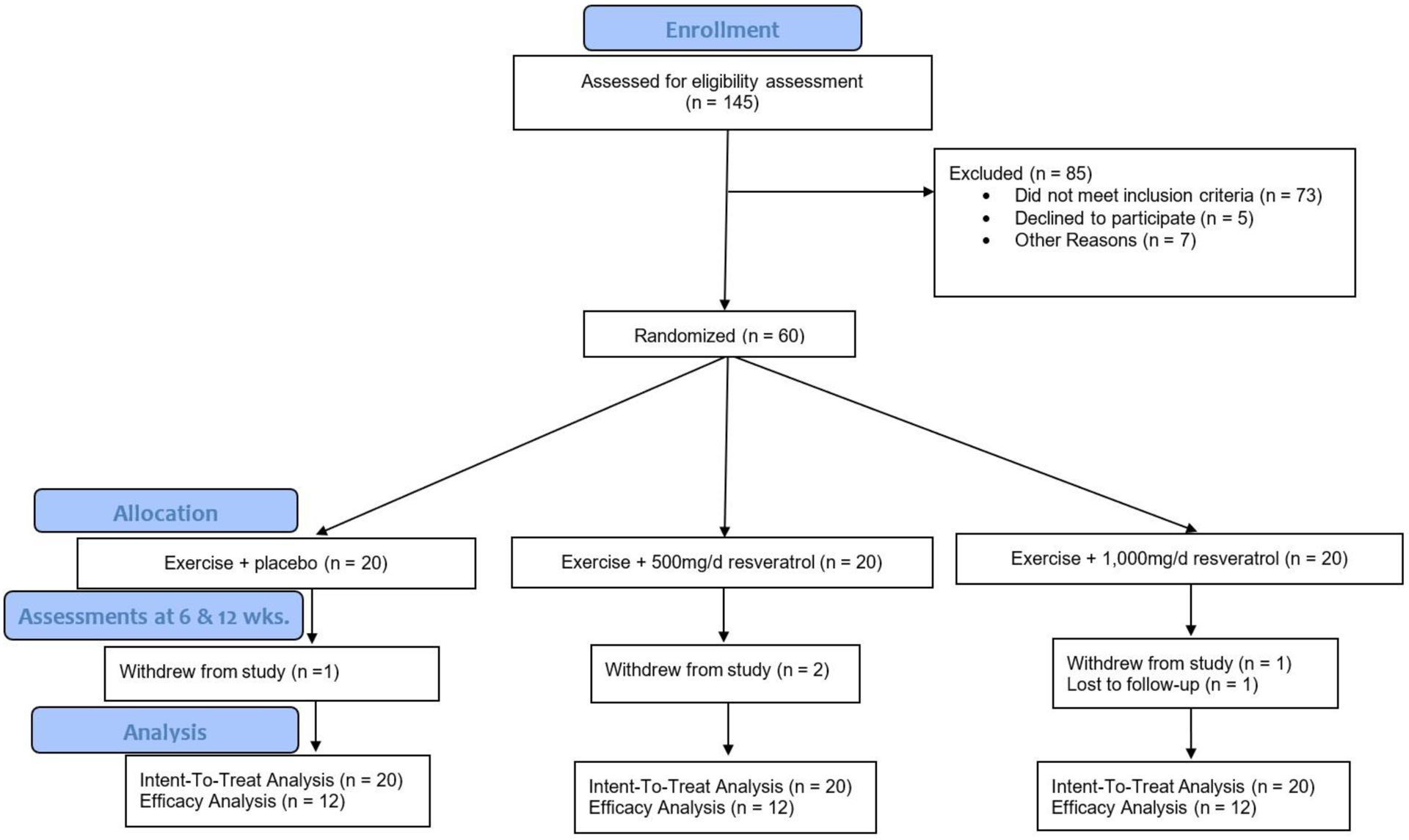
The overview of study trial includes the intent-to-treat and efficacy analyses. The efficacy analysis included participants who had ≥ 75% adherence for both the exercise session and pill counts.
Figure A.2. Walking distance per session.
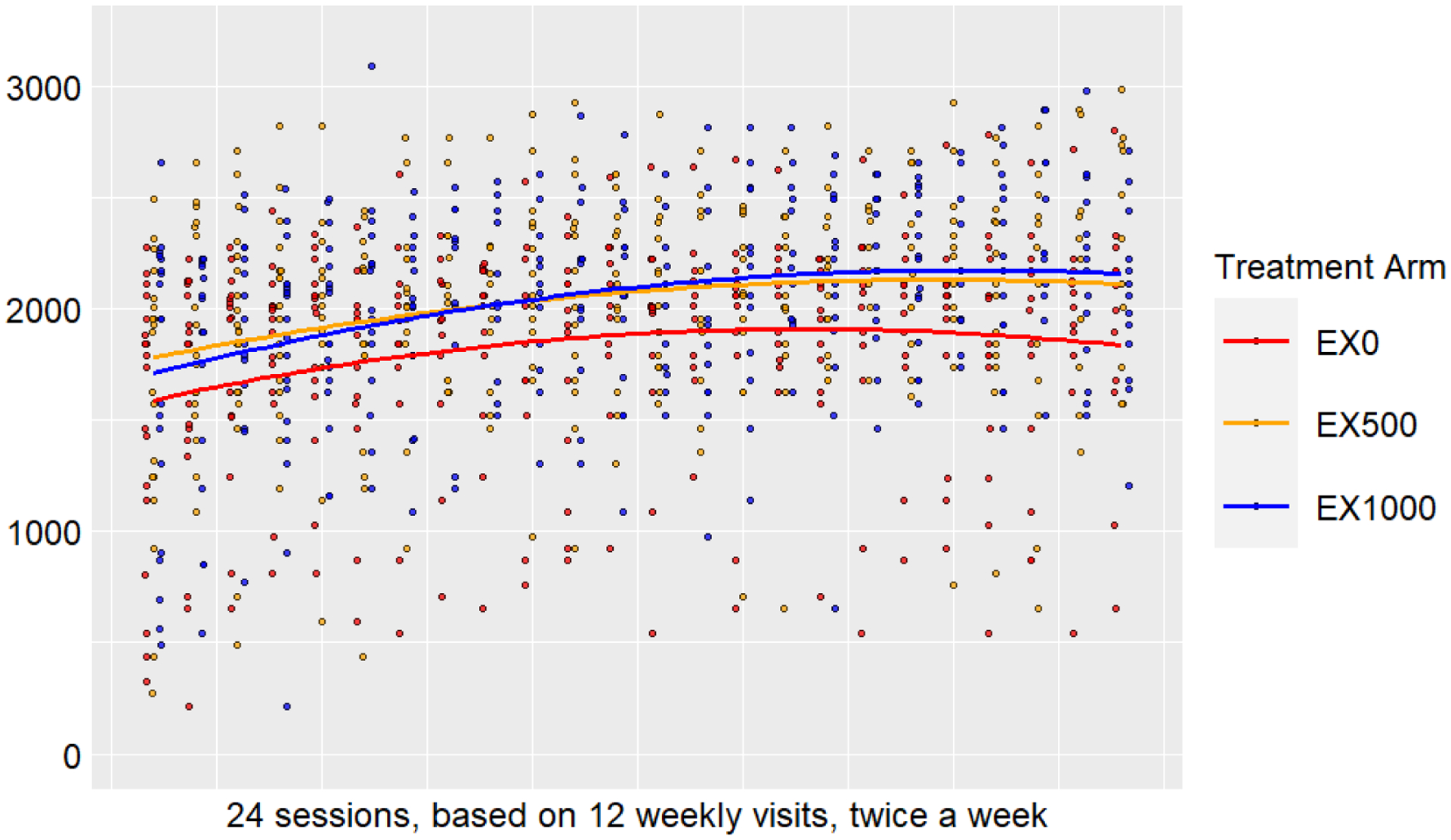
Data are reported as the unadjusted participant average session walking distance (m) over 24 sessions, based on 12 weekly visits, twice a week represented as a red, orange, or blue dot. The treatment group (EX0, EX500, or EX1000) overall average walking distance for each group is representative by the colored line.
Figure A.3. Indices of physical function.
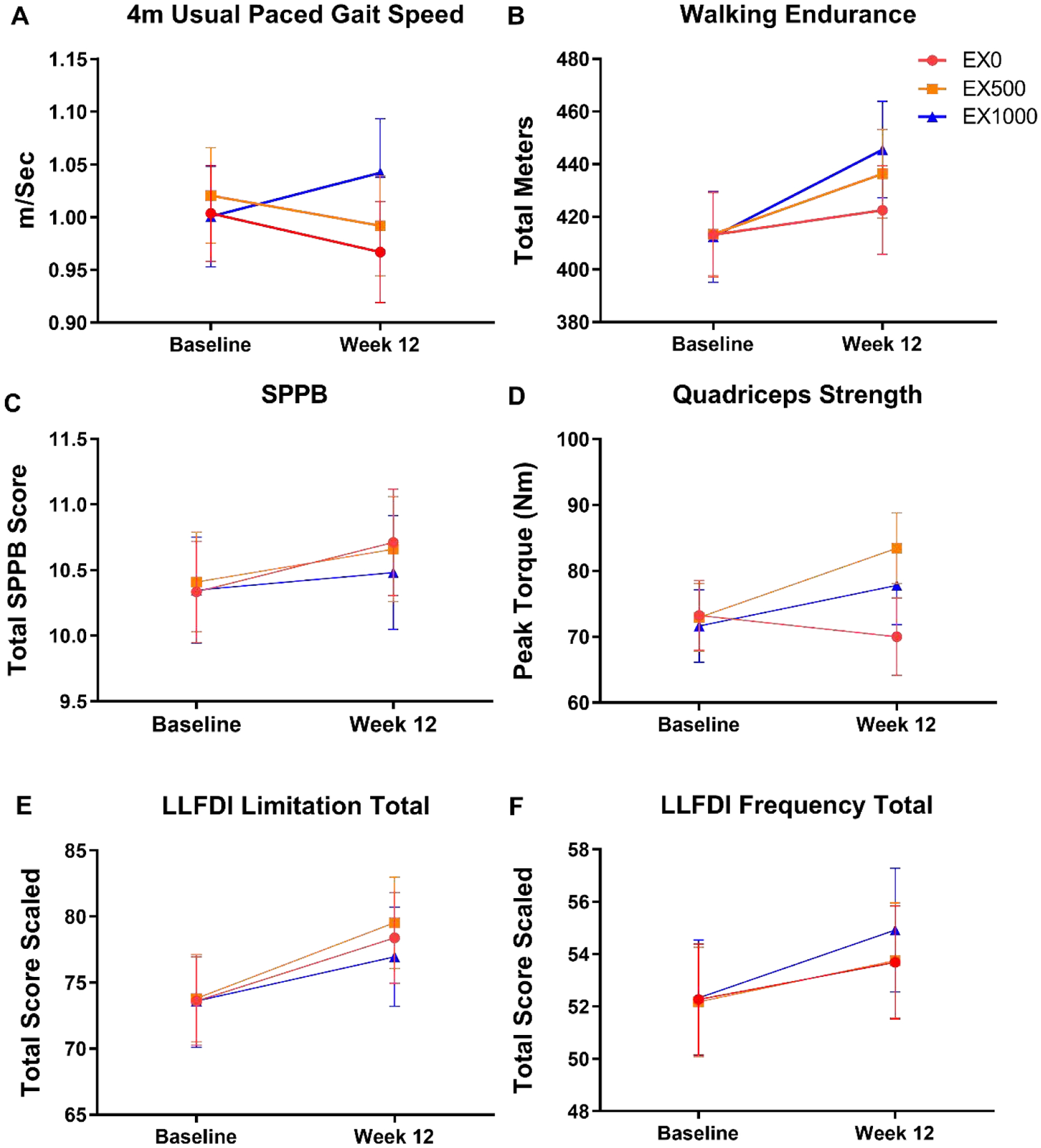
Data are presented as the intent-to-treat adjusted relative changes in (A) 4 meter usual gait speed, (B) walking endurance, (C) the Short Physical Performance Battery (SPPB), (D) quadriceps strength (Nm) across three angular velocities of movement (60, 90, 120°/s) is presented as the mean composite strength, (E) late life function and disability instrument limitation total, F) late life function and disability instrument frequency total in which a higher score indicates less limitation among older adults following 12-weeks of either EX0, EX500, or EX1000. Data indicate estimated marginal mean ± 95% confidence intervals.
Figure A.4. Skeletal muscle mitochondrial damage and biogenesis.
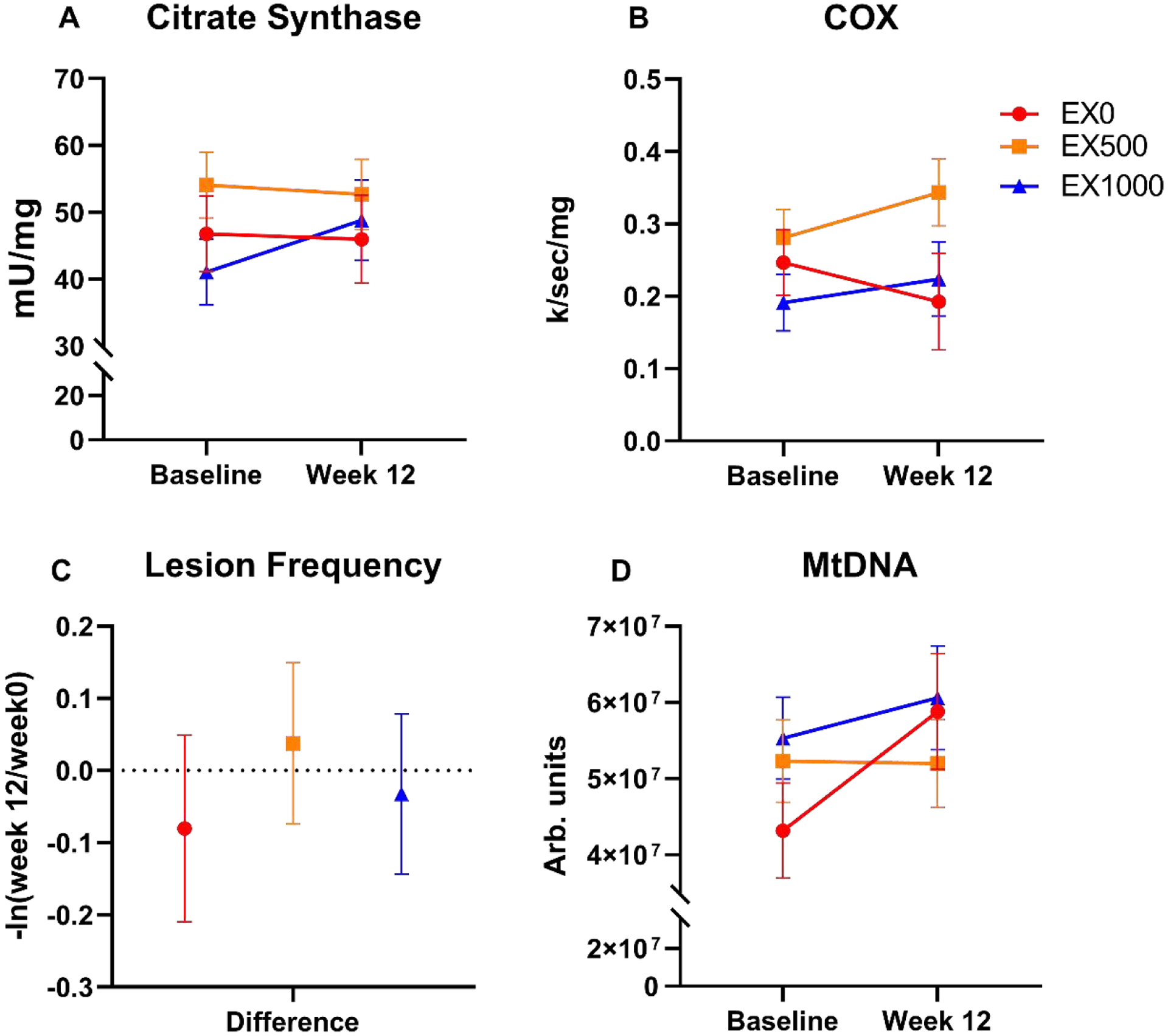
Presented as the unadjusted within group changes, 12-week changes for citrate synthase (A), cytochrome c oxidase (COX) activity (B), lesion frequency (C), and MtDNA (D). Data indicate unadjusted mean ± SE. Abbreviations: EX0 = exercise + placebo, EX500 = exercise + 500 mg/d resveratrol, EX1000 = exercise + 1,000 mg/d resveratrol.
Figure A.5. Skeletal muscle mitochondrial protein abundance.
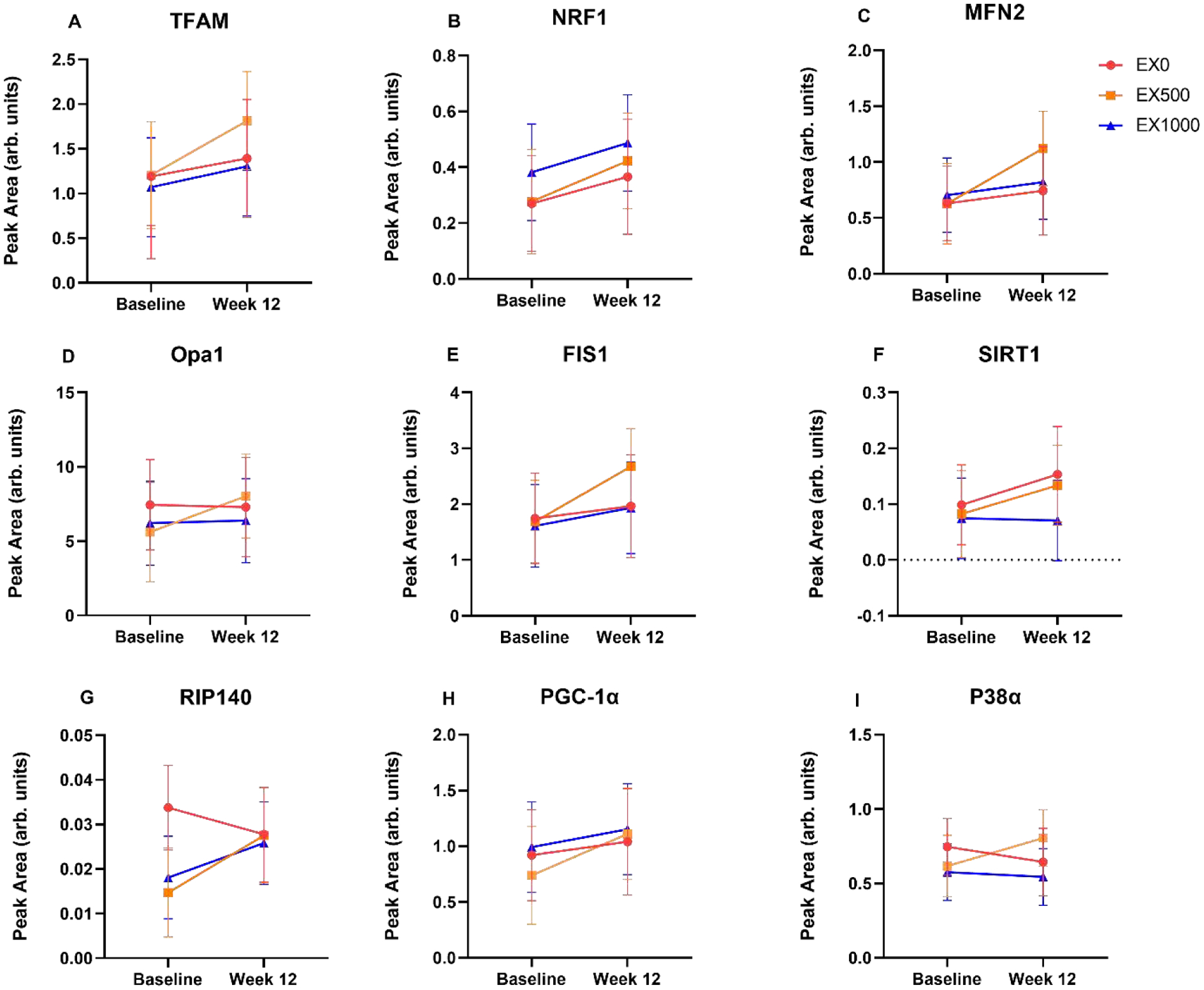
Presented as the unadjusted within group changes, 12-week changes. Data are reported as mean ± SE. Abbreviations: TFAM: Transcription factor A (A); NRF1: Nuclear respiratory factor 1 (B); MFN2: Mitofusin-2 (C); OPA1 mitochondrial dynamin like GTPase Opa1 (D); FIS1: Mitochondrial fission 1 (E); SIRT1: Sirtuin 1 (F); RIP140: Nuclear receptor-interacting protein 1 (G); PGC-1a: Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (H); P38a mitogen-activated protein kinase (I). Abbreviations: EX0 = exercise + placebo, EX500 = exercise + 500 mg/d resveratrol, EX1000 = exercise + 1,000 mg/d resveratrol.
Figure A.6. Systematic inflammation and oxidative stress measures.
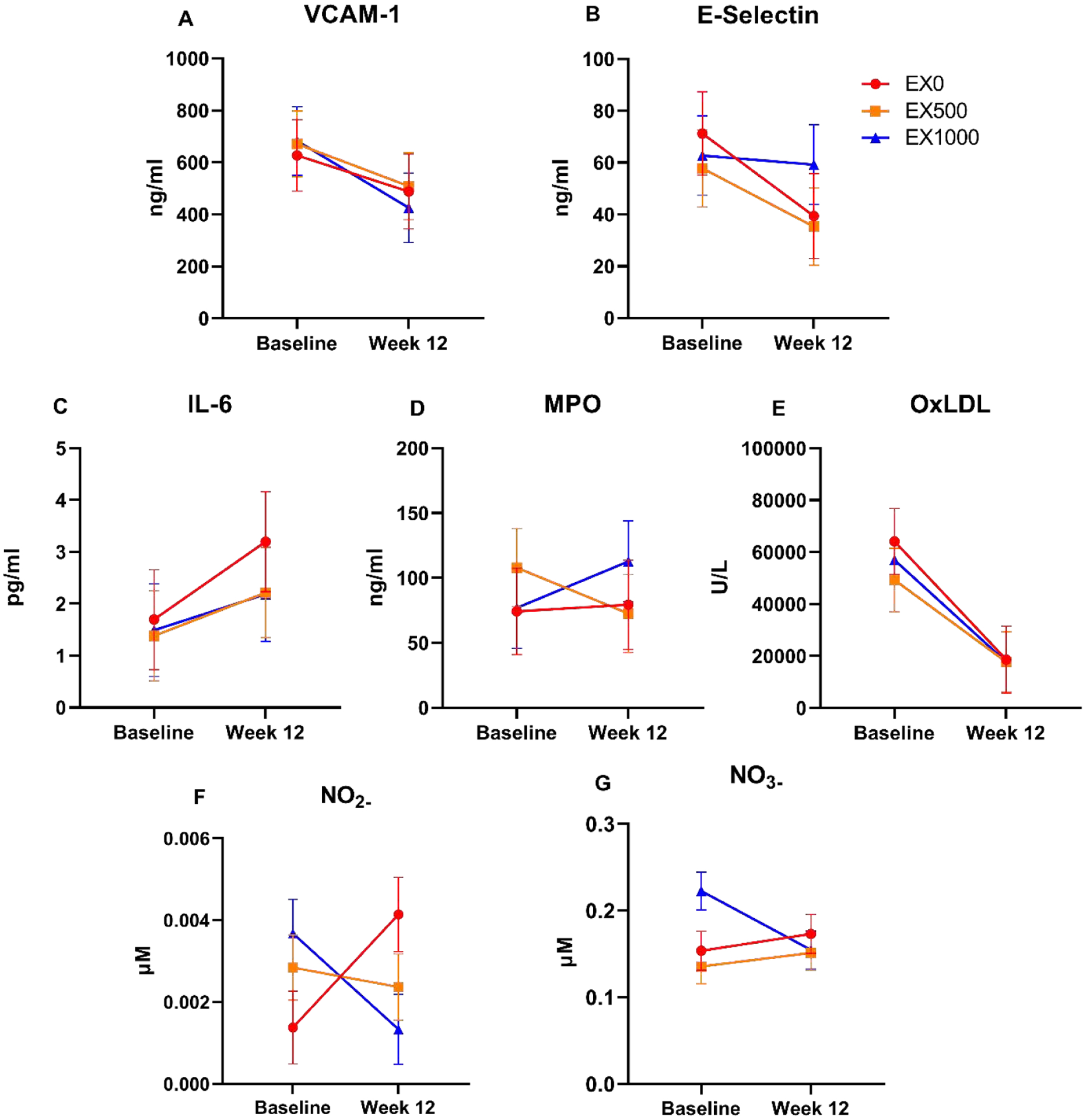
Circulating markers of vascular inflammation and oxidative stress including vascular cellular adhesion molecule 1 (VCAM-1, A), E-selectin (B), interleukin-6 (IL-6, C), myeloperoxidase (MPO, D), and oxidized low density lipoprotein (OxLDL, E). High Performance Liquid Chromatography (HPLC) with the ENO-30 NOx Analyzer (Eicom, Corp., Kyoto, Japan) was used to assess nitrite and nitrate values the Griess reaction measuring indirect colorimetric of nitric oxide in plasma for nitrite (NO2-, F), and nitrate (NO3-, G). Data are presented as week 12 relative to week 0, reported as mean ± SE. Abbreviations: EX0 = exercise + placebo, EX500 = exercise + 500 mg/d resveratrol, EX1000 = exercise + 1,000 mg/d resveratrol, No2- = nitrite, No3- = nitrate.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest
SDA is a consultant for Reserveage Organics (Gainesville, FL, USA) that provided that resveratrol and placebo pills.
References
- 1.Newman AB, Simonsick EM, Naydeck BL, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. Jama 2006;295(17):2018–26 doi: 10.1001/jama.295.17.2018. [DOI] [PubMed] [Google Scholar]
- 2.Shaw LJ, Olson MB, Kip K, et al. The value of estimated functional capacity in estimating outcome: results from the NHBLI-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study. Journal of the American College of Cardiology 2006;47(3 Suppl):S36–43 doi: 10.1016/j.jacc.2005.03.080. [DOI] [PubMed] [Google Scholar]
- 3.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. Jama 2011;305(1):50–8 doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manini TM, Pahor M. Physical activity and maintaining physical function in older adults. British journal of sports medicine 2009;43(1):28–31 doi: 10.1136/bjsm.2008.053736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buford TW, Anton SD, Clark DJ, Higgins TJ, Cooke MB. Optimizing the benefits of exercise on physical function in older adults. PM & R : the journal of injury, function, and rehabilitation 2014;6(6):528–43 doi: 10.1016/j.pmrj.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keysor JJ, Brembs A. Exercise: necessary but not sufficient for improving function and preventing disability? Current opinion in rheumatology 2011;23(2):211–8 doi: 10.1097/BOR.0b013e3283432c41. [DOI] [PubMed] [Google Scholar]
- 7.Custodero C, Mankowski RT, Lee SA, et al. Evidence-based nutritional and pharmacological interventions targeting chronic low-grade inflammation in middle-age and older adults: A systematic review and meta-analysis. Ageing Res Rev 2018;46:42–59 doi: 10.1016/j.arr.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kursvietiene L, Staneviciene I, Mongirdiene A, Bernatoniene J. Multiplicity of effects and health benefits of resveratrol. Medicina (Kaunas, Lithuania) 2016;52(3):148–55 doi: 10.1016/j.medici.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Li H, Xia N, Förstermann U. Cardiovascular effects and molecular targets of resveratrol. Nitric Oxide 2012;26(2):102–10 doi: 10.1016/j.niox.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 10.Poulsen MM, Fjeldborg K, Ornstrup MJ, Kjaer TN, Nohr MK, Pedersen SB. Resveratrol and inflammation: Challenges in translating pre-clinical findings to improved patient outcomes. Biochimica et biophysica acta 2015;1852(6):1124–36 doi: 10.1016/j.bbadis.2014.12.024. [DOI] [PubMed] [Google Scholar]
- 11.Wu J, Xia S, Kalionis B, Wan W, Sun T. The Role of Oxidative Stress and Inflammation in Cardiovascular Aging. BioMed Research International 2014;2014:615312 doi: 10.1155/2014/615312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dolinsky VW, Jones KE, Sidhu RS, et al. Improvements in skeletal muscle strength and cardiac function induced by resveratrol during exercise training contribute to enhanced exercise performance in rats. J Physiol 2012;590(11):2783–99 doi: 10.1113/jphysiol.2012.230490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hart N, Sarga L, Csende Z, et al. Resveratrol enhances exercise training responses in rats selectively bred for high running performance. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association 2013;61:53–9 doi: 10.1016/j.fct.2013.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodriguez-Bies E, Tung BT, Navas P, Lopez-Lluch G. Resveratrol primes the effects of physical activity in old mice. The British journal of nutrition 2016;116(6):979–88 doi: 10.1017/s0007114516002920. [DOI] [PubMed] [Google Scholar]
- 15.Gliemann L, Schmidt JF, Olesen J, et al. Resveratrol blunts the positive effects of exercise training on cardiovascular health in aged men. The Journal of physiology 2013;591(20):5047–59 doi: 10.1113/jphysiol.2013.258061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olesen J, Gliemann L, Bienso R, Schmidt J, Hellsten Y, Pilegaard H. Exercise training, but not resveratrol, improves metabolic and inflammatory status in skeletal muscle of aged men. The Journal of physiology 2014;592(8):1873–86 doi: 10.1113/jphysiol.2013.270256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scribbans TD, Ma JK, Edgett BA, et al. Resveratrol supplementation does not augment performance adaptations or fibre-type-specific responses to high-intensity interval training in humans. Appl Physiol Nutr Metab 2014;39(11):1305–13 doi: 10.1139/apnm-2014-0070. [DOI] [PubMed] [Google Scholar]
- 18.Layne AS, Krehbiel LM, Mankowski RT, et al. Resveratrol and exercise to treat functional limitations in late life: design of a randomized controlled trial. Contemporary clinical trials communications 2017;6:58–63 doi: 10.1016/j.conctc.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crandall JP, Oram V, Trandafirescu G, et al. Pilot study of resveratrol in older adults with impaired glucose tolerance. The journals of gerontology. Series A, Biological sciences and medical sciences 2012;67(12):1307–12 doi: 10.1093/gerona/glr235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Timmers S, Konings E, Bilet L, et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab 2011;14(5):612–22 doi: 10.1016/j.cmet.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Medicine and science in sports and exercise 2001;33(7):1126–41 [DOI] [PubMed] [Google Scholar]
- 22.American College of Sports M. ACSM's guidelines for exercise testing and prescription: Sixth edition Philadelphia: : Lippincott Williams & Wilkins, [2000] ©2000, 2000. [Google Scholar]
- 23.Hensel A, Angermeyer MC, Riedel-Heller SG. Measuring cognitive change in older adults: reliable change indices for the Mini-Mental State Examination. Journal of neurology, neurosurgery, and psychiatry 2007;78(12):1298–303 doi: 10.1136/jnnp.2006.109074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hertzog MA. Considerations in determining sample size for pilot studies. Res Nurs Health 2008;31(2):180–91 doi: 10.1002/nur.20247. [DOI] [PubMed] [Google Scholar]
- 25.Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies: recommendations for good practice. Journal of evaluation in clinical practice 2004;10(2):307–12 doi: 10.1111/j..2002.384.doc.x. [DOI] [PubMed] [Google Scholar]
- 26.Borg G. Borg’s preceived exertion and pain scales. Champaign, IL: Human Kinetics, 1998. [Google Scholar]
- 27.Afilalo J, Eisenberg MJ, Morin JF, et al. Gait speed as an incremental predictor of mortality and major morbidity in elderly patients undergoing cardiac surgery. Journal of the American College of Cardiology 2010;56(20):1668–76 doi: 10.1016/j.jacc.2010.06.039. [DOI] [PubMed] [Google Scholar]
- 28.Dumurgier J, Elbaz A, Ducimetiere P, Tavernier B, Alperovitch A, Tzourio C. Slow walking speed and cardiovascular death in well functioning older adults: prospective cohort study. BMJ (Clinical research ed.) 2009;339:b4460 doi: 10.1136/bmj.b4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buford TW, Manini TM, Hsu FC, et al. Angiotensin-converting enzyme inhibitor use by older adults is associated with greater functional responses to exercise. Journal of the American Geriatrics Society 2012;60(7):1244–52 doi: 10.1111/j.1532-5415.2012.04045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buford TW, Miller ME, Church TS, et al. Antihypertensive Use and the Effect of a Physical Activity Intervention in the Prevention of Major Mobility Disability Among Older Adults: The LIFE Study. The journals of gerontology. Series A, Biological sciences and medical sciences 2016;71(7):974–81 doi: 10.1093/gerona/glv222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buford TW, Cooke MB, Manini TM, Leeuwenburgh C, Willoughby DS. Effects of age and sedentary lifestyle on skeletal muscle NF-kappaB signaling in men. J Gerontol A Biol Sci Med Sci 2010;65(5):532–7 doi: 10.1093/gerona/glp196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buford TW, Cooke MB, Redd LL, Hudson GM, Shelmadine BD, Willoughby DS. Protease supplementation improves muscle function after eccentric exercise. Medicine and science in sports and exercise 2009;41(10):1908–14 doi: 10.1249/MSS.0b013e3181a518f0. [DOI] [PubMed] [Google Scholar]
- 33.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. Journal of gerontology 1994;49(2):M85–94 [DOI] [PubMed] [Google Scholar]
- 34.Haley SM, Jette AM, Coster WJ, et al. Late Life Function and Disability Instrument: II. Development and evaluation of the function component. The journals of gerontology. Series A, Biological sciences and medical sciences 2002;57(4):M217–22 [DOI] [PubMed] [Google Scholar]
- 35.Jette AM, Haley SM, Coster WJ, et al. Late life function and disability instrument: I. Development and evaluation of the disability component. The journals of gerontology. Series A, Biological sciences and medical sciences 2002;57(4):M209–16 [DOI] [PubMed] [Google Scholar]
- 36.Buford TW, Fillingim RB, Manini TM, Sibille KT, Vincent KR, Wu SS. Kaatsu training to enhance physical function of older adults with knee osteoarthritis: Design of a randomized controlled trial. Contemporary clinical trials 2015;43:217–22 doi: 10.1016/j.cct.2015.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baptista LC, Jaeger BC, Anton SD, et al. Multimodal Intervention to Improve Functional Status in Hypertensive Older Adults: A Pilot Randomized Controlled Trial. J Clin Med 2019;8(2) doi: 10.3390/jcm8020196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harper SA, Roberts LM, Layne AS, et al. Blood-Flow Restriction Resistance Exercise for Older Adults with Knee Osteoarthritis: A Pilot Randomized Clinical Trial. J Clin Med 2019;8(2) doi: 10.3390/jcm8020265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Migueles JH, Cadenas-Sanchez C, Ekelund U, et al. Accelerometer Data Collection and Processing Criteria to Assess Physical Activity and Other Outcomes: A Systematic Review and Practical Considerations. Sports medicine (Auckland, N.Z.) 2017;47(9):1821–45 doi: 10.1007/s40279-017-0716-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nawrocka A, Mynarski W, Cholewa J. Adherence to physical activity guidelines and functional fitness of elderly women, using objective measurement. Annals of agricultural and environmental medicine : AAEM 2017;24(4):632–35 doi: 10.5604/12321966.1231388. [DOI] [PubMed] [Google Scholar]
- 41.Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Medicine and science in sports and exercise 1998;30(5):777–81 doi: 10.1097/00005768-199805000-00021. [DOI] [PubMed] [Google Scholar]
- 42.Buford TW, Cooke MB, Shelmadine BD, Hudson GM, Redd LL, Willoughby DS. Differential gene expression of FoxO1, ID1, and ID3 between young and older men and associations with muscle mass and function. Aging Clin Exp Res 2011;23(3):170–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Larkin KA, Macneil RG, Dirain M, Sandesara B, Manini TM, Buford TW. Blood flow restriction enhances post-resistance exercise angiogenic gene expression. Medicine and science in sports and exercise 2012;44(11):2077–83 doi: 10.1249/MSS.0b013e3182625928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Layne AS, Larkin-Kaiser K, MacNeil RG, et al. Effects of blood flow restriction on biomarkers of myogenesis in response to resistance exercise. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme 2017;42(1):89–92 doi: 10.1139/apnm-2016-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harper SA, Baptista LC, Roberts LM, et al. Angiotensin Converting Enzyme Inhibitors Combined with Exercise for Hypertensive Seniors (The ACES Trial): Study Protocol of a Randomized Controlled Trial. Front Med (Lausanne) 2019;6:327 doi: 10.3389/fmed.2019.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ballinger SW. Mitochondrial dysfunction in cardiovascular disease. Free radical biology & medicine 2005;38(10):1278–95 doi: 10.1016/j.freeradbiomed.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 47.Ballinger SW, Patterson C, Knight-Lozano CA, et al. Mitochondrial integrity and function in atherogenesis. Circulation 2002;106(5):544–9 doi: 10.1161/01.cir.0000023921.93743.89. [DOI] [PubMed] [Google Scholar]
- 48.Ballinger SW, Patterson C, Yan CN, et al. Hydrogen peroxide- and peroxynitrite-induced mitochondrial DNA damage and dysfunction in vascular endothelial and smooth muscle cells. Circ Res 2000;86(9):960–6 doi: 10.1161/01.res.86.9.960. [DOI] [PubMed] [Google Scholar]
- 49.Ballinger SW, Van Houten B, Jin GF, Conklin CA, Godley BF. Hydrogen peroxide causes significant mitochondrial DNA damage in human RPE cells. Experimental eye research 1999;68(6):765–72 doi: 10.1006/exer.1998.0661. [DOI] [PubMed] [Google Scholar]
- 50.Fetterman JL, Holbrook M, Westbrook DG, et al. Mitochondrial DNA damage and vascular function in patients with diabetes mellitus and atherosclerotic cardiovascular disease. Cardiovascular diabetology 2016;15:53 doi: 10.1186/s12933-016-0372-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Knight-Lozano CA, Young CG, Burow DL, et al. Cigarette smoke exposure and hypercholesterolemia increase mitochondrial damage in cardiovascular tissues. Circulation 2002;105(7):849–54 doi: 10.1161/hc0702.103977. [DOI] [PubMed] [Google Scholar]
- 52.Krzywanski DM, Moellering DR, Westbrook DG, et al. Endothelial Cell Bioenergetics and Mitochondrial DNA Damage Differ in Humans Having African or West Eurasian Maternal Ancestry. Circulation. Cardiovascular genetics 2016;9(1):26–36 doi: 10.1161/circgenetics.115.001308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yakes FM, Van Houten B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc Natl Acad Sci U S A 1997;94(2):514–9 doi: 10.1073/pnas.94.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang Z, Knight CA, Mamerow MM, et al. Prenatal environmental tobacco smoke exposure promotes adult atherogenesis and mitochondrial damage in apolipoprotein E−/− mice fed a chow diet. Circulation 2004;110(24):3715–20 doi: 10.1161/01.Cir.0000149747.82157.01. [DOI] [PubMed] [Google Scholar]
- 55.Hoppeler H. Exercise-induced ultrastructural changes in skeletal muscle. Int J Sports Med 1986;7(4):187–204 doi: 10.1055/s-2008-1025758. [DOI] [PubMed] [Google Scholar]
- 56.Leek BT, Mudaliar SR, Henry R, Mathieu-Costello O, Richardson RS. Effect of acute exercise on citrate synthase activity in untrained and trained human skeletal muscle. American journal of physiology. Regulatory, integrative and comparative physiology 2001;280(2):R441–7 doi: 10.1152/ajpregu.2001.280.2.R441. [DOI] [PubMed] [Google Scholar]
- 57.Kelly NA, Ford MP, Standaert DG, et al. Novel, high-intensity exercise prescription improves muscle mass, mitochondrial function, and physical capacity in individuals with Parkinson’s disease. Journal of applied physiology (Bethesda, Md. : 1985) 2014;116(5):582–92 doi: 10.1152/japplphysiol.01277.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shepherd D, Garland PB. The kinetic properties of citrate synthase from rat liver mitochondria. The Biochemical journal 1969;114(3):597–610 doi: 10.1042/bj1140597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ragan CI WM, Darley-Usmar VM, Lowe PN. Mitochondria: A Practical Approach: Oxford, 187. [Google Scholar]
- 60.Nguyen UT, Bittova L, Muller MM, et al. Accelerated chromatin biochemistry using DNA-barcoded nucleosome libraries. Nature methods 2014;11(8):834–40 doi: 10.1038/nmeth.3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen JQ, Wakefield LM, Goldstein DJ. Capillary nano-immunoassays: advancing quantitative proteomics analysis, biomarker assessment, and molecular diagnostics. Journal of translational medicine 2015;13:182 doi: 10.1186/s12967-015-0537-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nelson GM, Guynn JM, Chorley BN. Procedure and Key Optimization Strategies for an Automated Capillary Electrophoretic-based Immunoassay Method. Journal of visualized experiments : JoVE 2017(127) doi: 10.3791/55911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harris V. Protein Detection by Simple Western Analysis. Methods in Molecular Biology 2015;1312:465–8 [DOI] [PubMed] [Google Scholar]
- 64.Bryan NS, Grisham MB. Methods to detect nitric oxide and its metabolites in biological samples. Free radical biology & medicine 2007;43(5):645–57 doi: 10.1016/j.freeradbiomed.2007.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lang JD Jr., Teng X, Chumley P, et al. Inhaled NO accelerates restoration of liver function in adults following orthotopic liver transplantation. The Journal of clinical investigation 2007;117(9):2583–91 doi: 10.1172/jci31892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eldridge SM, Chan CL, Campbell MJ, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. Pilot Feasibility Stud 2016;2:64 doi: 10.1186/s40814-016-0105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Horne E, Lancaster GA, Matson R, Cooper A, Ness A, Leary S. Pilot trials in physical activity journals: a review of reporting and editorial policy. Pilot and Feasibility Studies 2018;4(1):125 doi: 10.1186/s40814-018-0317-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Douglas Bates MM, Ben Bolker, Steve Walker. Fitting Linear Mixed-Effects Models Using Ime4. Journal of Statistical Software 2015;67(1):1–48 doi: doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- 69.Glue: Interpreted String Literals [program], 2019.
- 70.tibbleOne: Table One for ‘Latex’, ‘Word’, and ‘Html’ ‘R Markdown’ Documents. [program], 2019.
- 71.emmeans: Estimated Marginal Means, aka Least-Squares Means [program], 2019.
- 72.RStudio: Integrated Development for R. [program] Boston, MA, 2015. [Google Scholar]
- 73.Team RC. R: A language and environment for statistical computing. Secondary R: A language and environment for statistical computing; 2019. https://www.R-project.org/. [Google Scholar]
- 74.Chang M, Cohen-Mansfield J, Ferrucci L, et al. Incidence of loss of ability to walk 400 meters in a functionally limited older population. Journal of the American Geriatrics Society 2004;52(12):2094–8 doi: 10.1111/j.1532-5415.2004.52570.x. [DOI] [PubMed] [Google Scholar]
- 75.Hsu Y-J, Ho C-S, Lee M-C, Ho C-S, Huang C-C, Kan N-W. Protective Effects of Resveratrol Supplementation on Contusion Induced Muscle Injury. Int J Med Sci 2020;17(1):53–62 doi: 10.7150/ijms.35977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Salehi B, Mishra AP, Nigam M, et al. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines 2018;6(3):91 doi: 10.3390/biomedicines6030091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Martin KA, Sinden AR. Who will stay and who will go? A review of older adults’ adherence to randomized controlled trials of exercise. Journal of aging and physical activity 2001;9(2):91–114 [Google Scholar]
- 78.Rajadhyaksha V Conducting feasibilities in clinical trials: an investment to ensure a good study. Perspect Clin Res 2010;1(3):106–09 [PMC free article] [PubMed] [Google Scholar]
- 79.Bohannon RW, Crouch R. Minimal clinically important difference for change in 6-minute walk test distance of adults with pathology: a systematic review. Journal of evaluation in clinical practice 2017;23(2):377–81 doi: 10.1111/jep.12629. [DOI] [PubMed] [Google Scholar]
- 80.Ji LL, Kang C. Role of PGC-1alpha in sarcopenia: etiology and potential intervention - a mini-review. Gerontology 2015;61(2):139–48 doi: 10.1159/000365947. [DOI] [PubMed] [Google Scholar]
- 81.Wu H, Gallardo T, Olson EN, Williams RS, Shohet RV. Transcriptional analysis of mouse skeletal myofiber diversity and adaptation to endurance exercise. Journal of muscle research and cell motility 2003;24(8):587–92 doi: 10.1023/b:jure.0000009968.60331.86. [DOI] [PubMed] [Google Scholar]
- 82.Fry JL, Al Sayah L, Weisbrod RM, et al. Vascular Smooth Muscle Sirtuin-1 Protects Against Diet-Induced Aortic Stiffness. Hypertension 2016;68(3):775–84 doi: 10.1161/hypertensionaha.116.07622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Larsen FJ, Weitzberg E, Lundberg JO, Ekblom B. Effects of dietary nitrate on oxygen cost during exercise. Acta physiologica (Oxford, England) 2007;191(1):59–66 doi: 10.1111/j.1748-1716.2007.01713.x. [DOI] [PubMed] [Google Scholar]
- 84.Simental-Mendía LE, Guerrero-Romero F. Effect of resveratrol supplementation on lipid profile in subjects with dyslipidemia: A randomized double-blind, placebo-controlled trial. Nutrition 2019;58:7–10 doi: 10.1016/j.nut.2018.06.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


