Abstract
Context:
This systematic review aims to: (1) characterize strategies used to identify individuals at increased risk for hereditary breast and ovarian cancer syndrome and Lynch syndrome outside of oncology and clinical genetic settings, (2) describe the extent to which these strategies have extended the reach of genetic services to underserved target populations; and (3) summarize indicators of the potential scalability of these strategies.
Evidence acquisition:
Investigators searched PubMed, Embase, and PsycINFO for manuscripts published from October 2005 to August 2019. Eligible manuscripts were: published in English, described strategies to identify those at risk for hereditary breast and ovarian cancer syndrome or Lynch syndrome, implemented outside of an oncology or genetic specialty clinic, and included measures of cancer genetic services uptake. This study assessed strategies used to increase the reach of genetic risk screening and counseling services. Each study was evaluated using the 16-item quality assessment tool and results were reported according to PRISMA guidelines.
Evidence synthesis:
Of the 16 eligible studies, 11 were conducted in clinical settings and 5 in public health settings. Regardless of setting, most (63%, 10/16) used brief screening tools to identify people with a family history suggestive of hereditary breast and ovarian cancer syndrome or Lynch syndrome. When reported, genetic risk screening reach (range=11%–100%) and genetic counseling reach (range=11%–100%) varied widely across studies. Strategies implemented in public health settings appeared to be more successful (median counseling reach=65%) compared with those implemented in clinical settings (median counseling reach=26%). Most studies did not describe fundamental components relevant for broad scalability.
Conclusions:
Efforts to expand cancer genomic services are limited outside of traditional oncology and genetic clinics. This is a missed opportunity, as evidence thus far suggests these efforts can be successful in expanding reach of genetic services with the potential to reduce health inequities in access. This review highlights the need for accelerating research that applies evidence-based implementation strategies and frameworks along with process evaluation to understand barriers and facilitators to scalability of strategies with high reach.
CONTEXT
National and international guidelines (e.g., the U.S. Preventive Services Task Force, Evaluation of Genomic Applications in Practice and Prevention Working Group)1–3 and population health organizations (e.g., Healthy People 2020)4 all recommend that individuals at heightened risk for hereditary cancers receive genetic counseling, and as appropriate, genetic testing. Implementing these guidelines is of critical importance as mutation carriers and their blood relatives have the potential to receive life-saving prevention and treatment options.1,2 Much of these implementation efforts have focused on identifying carriers of genetic mutations associated with hereditary breast and ovarian cancer syndrome (HBOC) and Lynch syndrome (LS), as >1 million people in the U.S. are at increased risk for these conditions and related adverse health outcomes.5,6
Currently, efforts to identify carriers of genetic mutations are conducted predominantly in specialty cancer clinics (e.g., oncology, clinical genetic settings). However, the majority of mutation carriers and their relatives remain unidentified. For example, in the U.S., genetic counseling referral and genetic testing rates are approximately 24% to 52% of the breast cancer patient population and 15% to 48% in the ovarian cancer patient population.7–9 In addition, 28% to 70% of colon cancer patients who have LS remain unidentified as genetic screening has been limited to tumor testing for patients in specialty care settings who meet certain age or family history criteria.10–13 It has been suggested that expansion of genetic service reach will require that programs be extended beyond specialty care clinics.14 This is especially critical for subgroups that are more difficult to reach. Those who live in rural settings, racial ethnic minorities, and those with low education and income are unlikely to have access to genetic services.15–17
The scope of efforts that are being implemented outside of specialty care clinics is largely unknown, and the investigation of optimal ways to implement and expand the reach of cancer genetic services is limited.18,19 Implementation science frameworks (e.g., reach, effectiveness, adoption, implementation, maintenance [RE-AIM],20 Proctor’s implementation outcomes21) suggest processes and critical components to be considered in evaluating the likelihood that any “intervention” strategy will be scalable. These components include but are not limited to: strategy complexity, setting characteristics, organizational supports, and cost.22 Guided by the above considerations, the authors conducted a systematic review to: (1) describe strategies used to identify individuals at increased risk for HBOC and LS outside of oncology and clinical genetic settings, (2) describe the extent to which these strategies have extended the reach of genetic services to underserved target populations, and (3) summarize components suggested by implementation frameworks to support the potential scalability of these strategies.
EVIDENCE ACQUISITION
Eligibility Criteria
For the purposes of this review, a “strategy” is defined as an intervention or systematic effort that is designed to identify individuals at increased risk of carrying a mutation for HBOC or LS. Manuscripts were eligible for this review if they included: (1) strategies designed to identify individuals at risk for HBOC and LS (e.g., systematic implementation of family history assessment), (2) studies conducted outside of an oncology or genetic specialty clinic settings (e.g., conducted by a community organization), (3) studies that measured an outcome related to the uptake of cancer genetic services (e.g., complete genetic risk screening), and (4) studies published in English. The authors excluded studies in which cascade screening was the sole strategy used (e.g., mutation carrier engaged to identify family members), or quality improvement initiatives (e.g., establishing a new cancer genetic clinic). Studies not accessible in full text, conference and meeting abstracts, and non-research studies (e.g., commentaries, editorials, study protocols, literature reviews) were excluded.
Search Strategy
Three electronic databases (PubMed [National Library of Medicine], Embase [Elsevier], and PsycINFO [EBSCOhost]) were searched using the terms genetic counseling, genetic testing, genetic screening, population surveillance registry, referral and consultation, screening, or mass screening combined with terms related to HBOC and LS (Appendix Table 1). The search was restricted to peer-reviewed journal articles published from October 2005 to August 2019. This timeframe was chosen because it follows the 2005 release of the U.S. Preventive Services Task Force’s evidence-based HBOC screening recommendations when these genetic services outreach efforts were widely endorsed.23
Study Selection
A systematic review was performed in accordance with PRISMA guidelines24 and describe the process of study inclusion using a PRISMA flow diagram (Figure 1). The initial search executed on March 29, 2018 identified 18,455 publications and 15,548 of these were unique titles. Investigators conducted an updated search on August 9, 2019 and identified 2,271 additional unique manuscripts published between March 2018 and August 2019. Two independent coders (YG and CMM) piloted the eligibility criteria and exhibited good agreement. Four members of the research team (YG, CGA, JZ, CMM) reviewed 17,819 titles/abstracts and excluded 17,732 manuscripts from full-text review. The 4 reviewers evaluated 87 full-text manuscripts for eligibility and 16 studies met the inclusion criteria.
Figure 1. PRISMA flowchart of the process of study selection.
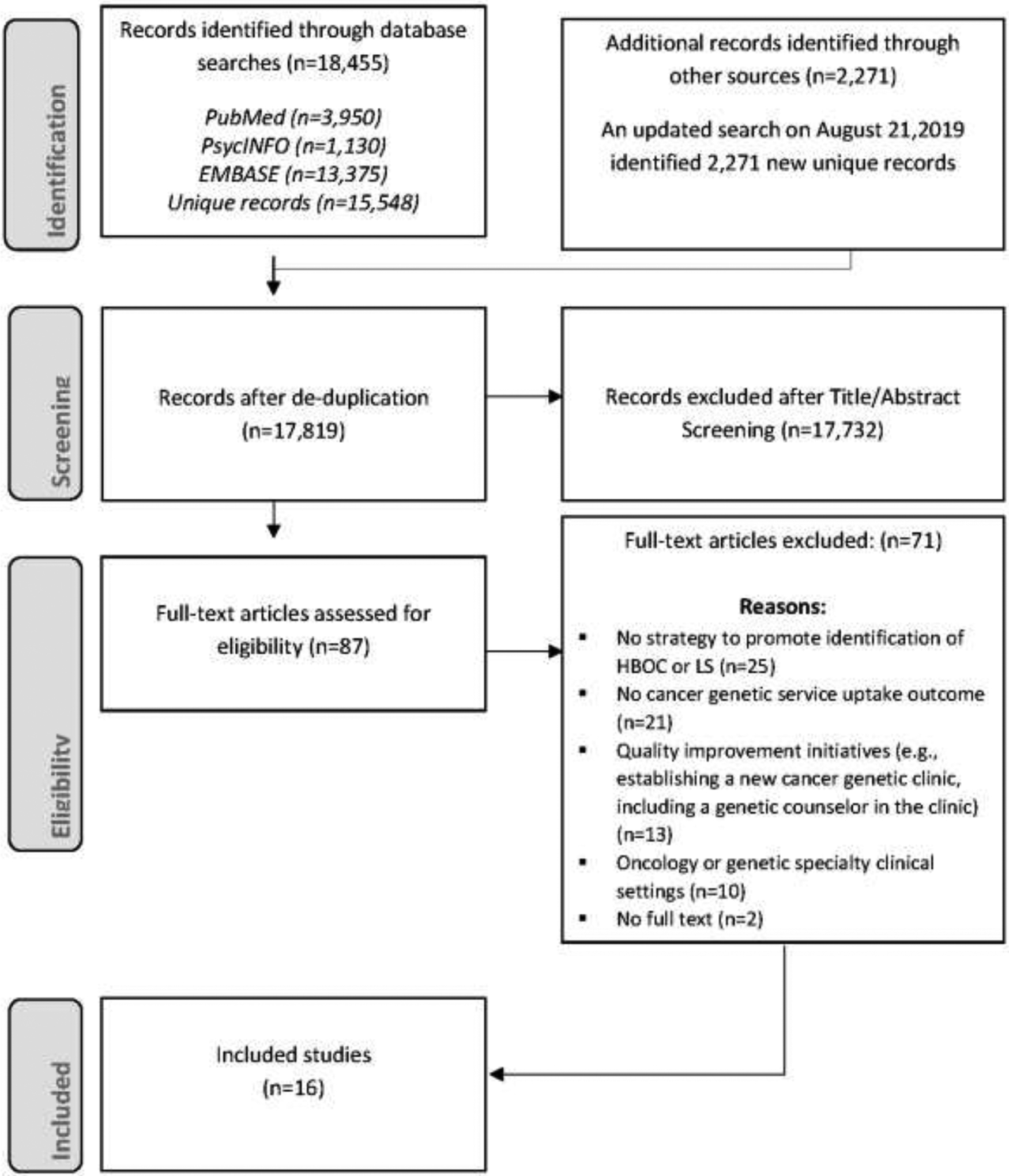
HBOC, hereditary breast and ovarian cancer syndrome; LS, Lynch syndrome.
Data Extraction
The population, intervention, comparator, outcomes, timeframe, and study design (PICOTS) framework25 to guide the general characteristics of included studies to be extracted including: purpose, country, cancer type, study design, study setting, target population, and outcome measures. For intervention studies, the authors coded and reported strategy components that were evaluated to improve uptake of genetic services; the usual care or control groups were not described.
Informed by implementation science frameworks,20,21 reach was characterized as “the absolute number, proportion and representativeness of individuals willing to participate in a given initiative.” For the purposes of this review, to describe the extent to which the strategies have been successful in extending the reach of genetic services, 2 reach variables were operationalized: (1) genetic risk screening reach, the number of individuals who completed genetic risk screening divided by the number of individuals who could have been screened, and (2) genetic counseling service reach, the number of individuals who completed genetic counseling divided by the number of individuals found to be eligible for genetic counseling.
The risk screening reach variable is a required initial step for extending genetic services reach, as individuals at high genetic risk must be identified first to be referred for genetic counseling. The genetic counseling service reach variable aligns with professional guidelines that genetic counseling be offered to all identified to be at heightened risk. Subsequent actions following genetic counseling (e.g., uptake of genetic testing) generally are not assessed in contexts outside of specialty clinical settings and are more fraught with complexity and nuance due to factors such as personal preferences.
Additionally, the authors reviewed details about how the strategy was implemented to gain insight into whether there was support for its potential scalability (Table 1). All studies were coded on whether they included any assessments that aligned with implementation framework indicators of sustainability (1=presence, 0=absent).
Table 1.
Indicators of the Potential Scalability of Strategies
| Domain/Code | Definition |
|---|---|
| Strategy implementation | |
| Complexity | Components of the strategy, time/number of steps required to complete the strategy |
| Setting | Geographic location, type of research setting |
| Organizational implementers | |
| People deliver the strategy | Description of people who deliver the strategy, their expertise and roles |
| Process factors | |
| Target population needs | Description of the target population, their needs and resources |
| User engagement | User engagement in the planning stage to gain feedback informing the strategy design |
| Process evaluation | Process evaluation to get feedback on strategy implementation process |
| Maintenance factors | |
| Resources | Training, education, or technical support dedicated for implementation |
| Costs | Start-up cost, cost of strategy delivery, or cost of maintenance |
Three members of the research team (YG, CGA, JZ) independently coded all eligible articles after coding 5 articles together for agreement. Any disagreement in the data collection process was resolved through discussion and consensus between the 2 reviewers and, if needed, with a third party (CMM).
Quality Assessment
This study used the 16-item quality assessment tool to assess the quality of each included study.26 Each study was rated on a scale of 0 to 3 for each criterion, with a higher score indicating greater methodological rigor. Scores on the quality assessment tool can range from 0 to 42 (qualitative and quantitative studies) or 48 (mixed methods studies). The overall rating, calculated as the total score divided by the total possible score, placed each study into categories of low- (<50%), medium- (50%–80%) or high- (>80%) quality evidence.26 The 3 reviewers coded 5 articles for agreement (YG, CGA, JZ) and 1 reviewer (YG) independently coded the remaining articles.
Data Analysis
The authors analyzed the data extracted from the included studies using simple frequency counts and a narrative approach to illustrate similarities and differences across strategies.27 They described general characteristics of included studies, participants, setting, and outcomes. Percentages were reported that reflected the extent of reach and counts of studies that included any implementation framework indicators of sustainability.
EVIDENCE SYNTHESIS
Study Design
Of the 16 included studies, 11 were single-arm designs (Appendix Table 1): 10 were cross-sectional28–37 and 1 was a pre–post design.38 Two studies were RCTs that compared different reach strategies,39,40 2 were non-RCTs,41,42 and 1 employed a mixed methods design.43 Ten studies focused on identifying individuals at risk for HBOC28,29,32,34,36,37,39,40,42 and 3 focused on LS.30,35,38 Another 3 studies evaluated reach strategies for several hereditary cancers simultaneously.31,33,43 The majority of studies (n=12) were conducted in the U.S.28,30,32–40,43; 4 were conducted in European countries, including Italy,29 Latvia,31 the Netherlands,42 and 1 in Israel.41
Implementation Setting
Most strategies were implemented in clinical settings (n=11, 69%),28,29,32,33,35–38,41–43 such as primary care practices (n=4),32,33,42,43 community mammography screening practices (n=4),28,29,36,37 community gastroenterology practices (n=2),35,38 and multiple clinics (n=1).41 Additional strategies were implemented within public health settings (n=5, 31%)30,31,34,39,40: collaborating with population-based cancer registries (n=2),30,34 national or local healthcare call centers (n=2),39,40 or another unspecified community setting (n=1).31
Target Population
Among studies conducted in clinical settings, 9 (56%) included patients only,28,29,32,33,35–38,41 and 2 (13%) solely targeted primary care physicians.42,43 In public health settings, 4 studies (25%) focused on the general public31,39–41 and 2 studies (13%) focused on patients identified from population-based cancer registries.30,34
Studies employed a variety of approaches. Participants were proactively recruited through postal invitations, telephone calls, and targeted advertisements,34,38,42,43 or opportunistically invited when they accessed a call-in service39–41 or at clinic appointments.28,29,32,33,35–37,41 Studies commonly reported inclusion and exclusion criteria (n=15, 94%),28–33,35–43 and characteristics of participants (n=13, 81%).28,30,32–41,43 However, representativeness of participants was often not computable, as few studies compared characteristics of those who participated with those who declined or were not engaged (n=6, 38%).30,32,33,37,40,43
In 4 studies, researchers partnered with local community healthcare practices to expand the reach of genetic risk assessment to minority and low-income populations. For instance, Wernke et al.36 administered family history-based screening among Black women with low SES who were underinsured and receiving care in a safety net hospital. Participants in McGuinness and colleagues’ study37 were predominantly Hispanic (77%) and were recruited from a low-income, multiethnic population in New York. Anderson et al.32 also focused on minority women (74% Black, 26% Hispanic) seen at 2 federally qualified health centers’ clinics in Chicago. Pasick and colleagues40 partnered with a statewide cancer screening call center that served low-income populations in San Francisco Bay Area counties to reach participants from diverse ethnic backgrounds (30% White, 9% Black, 16% Asian, 40% Hispanic, 5% other race).
Study Outcomes
Most studies were designed to evaluate the uptake of genetic risk assessment28,30–32,34,36–38,43 or genetic counseling as the primary outcomes.29,33,35,39–42 Few studies (n=3) included the primary outcome of completing genetic testing33,35,41 (Figure 2).
Figure 2.
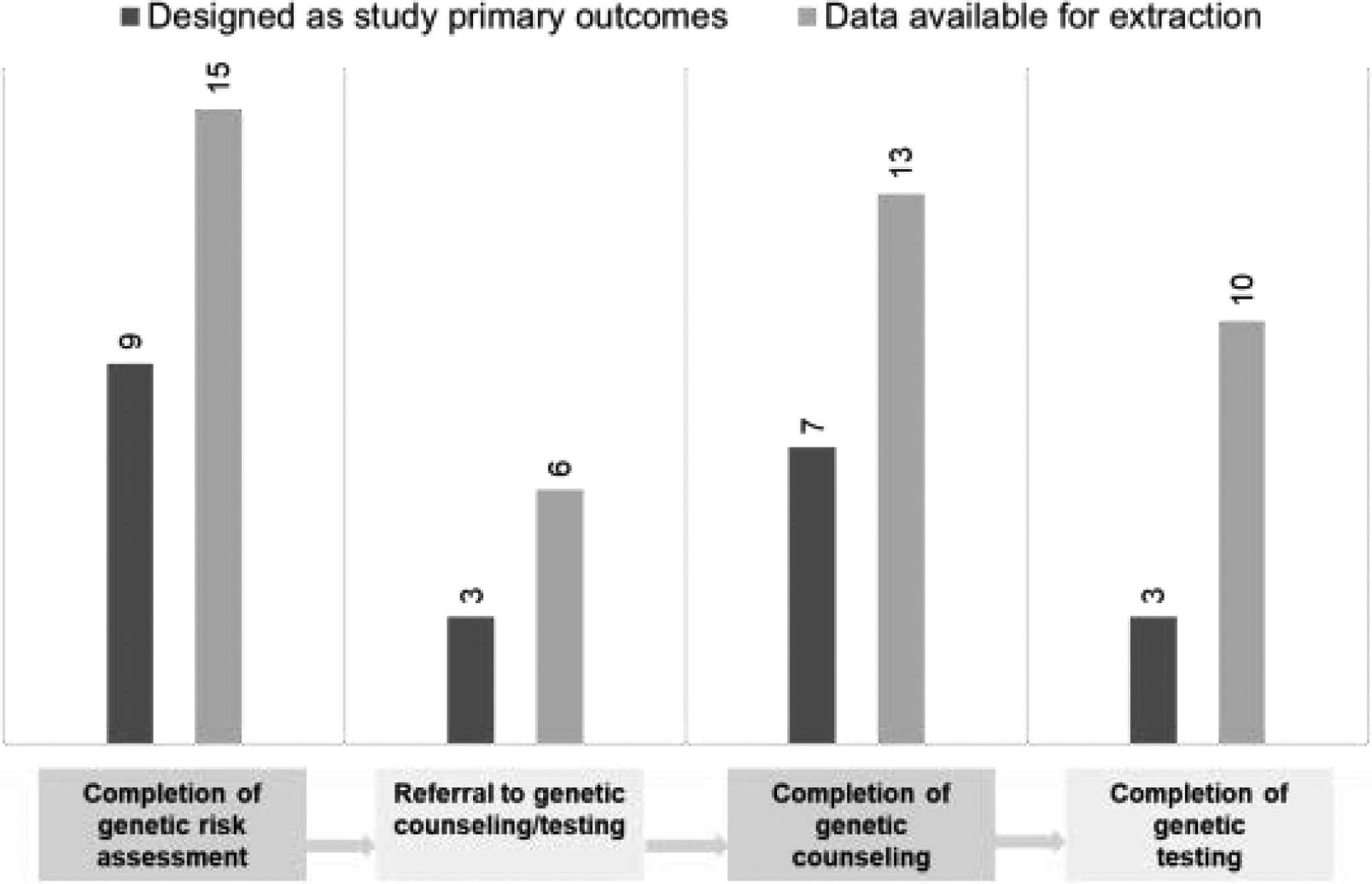
Number of studies reported cancer genetic service uptake outcomes.
Of studies reporting the number or proportion of individuals who completed cancer genetic services, 15 reported completion of genetic risk assessment for HBOC or LS (63%),28,30–43 6 reported referral to genetic counseling or testing (38%),28,36,38,40,42,43 13 reported completion of genetic counseling (81%),28–30,33–36,38–43 and 10 reported completion of genetic testing (63%).29–31,33,35,38,41–43
Reach
Genetic risk screening reach (i.e., number of individuals who completed genetic risk screening among individuals who could have been screened) was available in 13 studies (81%).30–40,42,43 It is noteworthy that the denominator for target populations varied widely across studies (mean=4,798, median=1,212), ranging from 30 (patients with a diagnosis of ovarian cancer)42 to 24,210 (general population).31 Genetic risk screening reach in clinical settings varied widely, ranging from 11% to 100% (median=57%). The 2 studies with 100% screening reach were conducted in clinical settings. Helsper et al.42 used medical records to identify all patients with an ovarian cancer diagnosis (N=30) in a primary care practice. Gunaratnam and colleagues38 implemented risk assessment among all patients (N=6,031) referred during the study period to open access colonoscopy at a community-based practice.
There was less variability in reach of public health strategies, ranging from 31% to 77% (median=57%). Genetic risk screening reach was highest (77%, 18,642/24,210) in a study that implemented family history screening among all adult residents in 4 towns in Latvia.31
Genetic counseling service reach (i.e., number of individuals who completed genetic counseling among individuals found to be eligible for genetic counseling) was reported in 10 studies (63%); 8 of these studies reported counseling uptake based on clinical validation28,33,36,38,40–43 and 2 studies used participants’ self-report.30,34 Strategies implemented in public health settings (median=65%, range=11%–66%) had generally higher reach compared with those implemented in clinical settings (median=26%, range=1%–100%).
Programs that achieved high service reach included the program of Pasick’s et al.,40 in which HBOC screening assessment was conducted among callers to a community-based cancer screening call center; free genetic counseling and testing was provided. This program achieved a 68% (30/44) counseling service reach. Niendorf and colleagues34 targeted individuals diagnosed with cancer enrolled in a population-based cancer registry to consider cancer genetic services (service reach=65%, 500/769). One clinical study41 achieved a 100% (1,771/1,771) service reach by implementing population-based streamlined BRCA genetic counseling and testing for Ashkenazi Jewish participants in multiple clinics (e.g., ambulatory clinics, mammogram screening clinics).
Indicators of the Potential Scalability of Strategies
Strategy implementation.
Strategies used were heterogeneous across studies and typically included multiple components (Appendix Table 1). The most commonly reported component was the use of family history-based risk assessment tools as part of the genetic risk screening process (n=10, 63%).28,29,31,32,34–37,39,40 In particular, 6 studies (38%) implemented family history screening tools in person, in primary care practice, or in community clinics.28,29,32,35–37 Three more studies (19%) conducted telephone family risk assessment through local healthca6re call centers39,40 or by reaching out to those identified via a state’s cancer registry.34 One study implemented a family history questionnaire at the population level in 4 Latvian towns.31
Other elements included developing educational materials about hereditary cancers, genetic risk assessment, genetic counseling, and testing (n=6, 38%),30,34,35,39,42,43 establishing new infrastructure supports (e.g., telemedicine, electronic medical record system; n=6, 38%),32,33,35,38,42,43 and providing free in-house genetic counseling or testing services (n=3, 19%).28,30,40 None of the studies specified details about demands of the screening supports (e.g., time to complete the family history screening or the educational supplements) that would be important for assessing scalability.
Organizational implementers.
Ten studies (63%) used existing personnel of the institution (e.g., clinicians, staff) to administer the strategy28–31,33–35,38–40; 6 of these studies involved non-genetic professionals who conducted genetic risk assessment (e.g., endoscopists, registry staff with no medical training; 37%),31,34,35,38–40 and 4 relied on a genetic counselor28,30,33 or medical geneticist29 to provide genetic counseling services. Less than half of the studies (n=6, 37%) mostly relied on research staff outside the institution to implement the strategy.32,36,37,41–43
Process factors.
The majority of studies described the needs and resources of the target population (n=10, 63%).28,30,33,35–37,39,40,42,43 A couple of studies described tailoring their strategy to target populations (e.g., translating the tool to different languages).28,40 Reported approaches to engage the intended target population included focus groups, usability testing, and surveys. However, user engagement in designing the strategy was infrequent (n=5, 31%).28,30,39,40,43 Most studies did not assess the quality of the implementation process. However, 2 studies conducted evaluations through surveys and interviews with staff clinicians to assess their attitudes and opinions regarding the implementation process.35,43
Maintenance factors.
Providing training or technical support for implementation was not commonly reported (n=5, 31%).28,34,38–40 Such informational support was mainly for individuals without genetic training (e.g., registry and clinic staff). None of the included studies reported numerical values for intervention development cost, or implementation cost indicators (i.e., capacity building, maintenance, formal cost analysis).
Quality Assessment
Based on the quality assessment tool criteria, 2 of the 16 studies were rated as high quality (13%),39,43 9 were medium quality (56%),30–38,40–42 and 5 (31%) were low quality28,29,36,37,42 (Appendix Table 2, Figure 1). The 2 high-quality studies included an RCT and a mixed methods design.39,43
DISCUSSION
Description of Strategies Implemented Outside of Specialty Clinical Settings
Evidence-based guidelines were established more than a decade ago to address how to broaden screening to identify individuals with HBOC or LS. However, little empirical work (0.1%, 16 of 17,819 publications) has been conducted to implement these guidelines outside of cancer specialty settings (e.g., urban cancer centers). The most common strategy used was family history-based risk assessment, which looks promising with respect to screening and service reach in resource-limited settings. Ten of 16 studies implemented brief screening tools to identify people with a family history suggestive of HBOC or LS. This approach was typically combined with other institutional-level strategies such as establishing supportive infrastructure, personnel education and training, and financial support. Strategy reach and potential for scalability may be most promising in settings with an existing population that offers ongoing cancer-related services (e.g., registries, healthcare call centers).
Reach of Cancer Genetic Services to Underserved Populations
With respect to increasing access among subgroups such as minorities and those living in rural settings, family history-based screening in these groups specifically showed some success in both clinical and public health settings. Family history screening for HBOC provided in settings that serve a large proportion of minorities have shown high reach potential for risk screening and genetic counseling.32,40 Though the research base is limited, these findings taken together support continued efforts to explore context-specific approaches for implementing family history-based screening to reach underserved populations and reduce disparities in access to cancer genetic services.
Indicators of the Potential Scalability of Strategies
It is noteworthy, however, that only 6 of the 16 studies reported the racial/ethnic status of the target population: 2 study populations consisted primarily of Whites,33,39 while 4 studies focused on low-SES areas or minority ethnic groups.32,36,37,40 Clearer characterization of the target population intended for expanded reach will be critically important going forward to inform strategy development and evaluation.
Strategies implemented in public health settings appeared to be most consistently successful in reaching the target population compared with those implemented in clinical settings. Studies reporting greatest service reach embedded risk assessment into existing infrastructures that had an established and delineated target population. For example, Pasick et al.40 implemented risk assessments for HBOC among callers to a community-based healthcare call center and provided free genetic counseling and testing. Niendorf and colleagues34 targeted individuals diagnosed with cancer enrolled in a population-based cancer registry to consider cancer genetic services. Given the relatively small number of studies, it is difficult to draw any firm conclusions. Yet, clearly, there is a need to continue to explore linking genetic risk identification and service access through public health infrastructures.
Descriptions of most studies did not include foundational components relevant to scalability. With regard Proctor’s implementation outcomes,21 only 5 studies28,30,39,40,43 reported using collaborative processes such as engaging the target population to guide their strategy design and few conducted process evaluations for acceptability. No study reported adaptations, maintenance plans, or monetary costs related to building new infrastructure or the workforce necessary to deliver the strategy within the clinical and public health settings. This lack of consideration of scalability potential is not specific to genetic services and continues to be a well-recognized gap in the field. Moving forward, assessment components to determine whether a strategy is scalable across multiple subgroups, settings, or time are needed.22,44
To the authors’ knowledge, this is the first review to systematically characterize efforts to broaden cancer genomic service reach outside of specialty clinical settings. Previous reviews on genomic medicine implementation have focused on screening in highly specialized clinical settings,18 or using a cascade testing approach where the mutation carrier was already identified in a family.19
Limitations
Although the reported results carry important implications for implementation research in precision public health, there were limitations to this systematic review. It only included studies published in English and in peer-reviewed literature. Many initiatives do not progress to published literature, particularly programs operated by state public health departments, so publication bias is likely to be present.
The results are based only on what was reported in the article and the research team did not correspond with authors to assess additional details of study design. There were generally few details provided regarding the strategy implementation experience, which limited the ability to identify clear patterns that distinguished studies with high or low reach. The lack of reporting should not be viewed as a quality issue of the study design, but rather highlights the need for future research to incorporate implementation science to understand barriers and facilitators and implementation strategies for genomic interventions that could inform the scale up of effective strategies to diverse populations and settings.
CONCLUSIONS
The pressing challenge for addressing heritable cancer syndromes is to expand the reach of screening and genetic services beyond traditional cancer specialty centers. These findings suggest that these efforts are still nascent. Extending the reach of genetic services is an ambitious goal that can only be achieved through collaborations across multiple disciplines. Future efforts need to be partnered with appropriate access to risk-reducing screening and treatment services for mutation carriers. In addition, emerging clinical practice is emphasizing use of multigene panels. This approach will undoubtedly introduce new challenges around the amount and complexity of outreach strategies.
That said, the findings suggest that implementing family history-based screening as a part of existing infrastructures that are already reaching well-delineated target populations has the potential to expand reach of genetic services related to hereditary cancers, especially for ethnic minorities and those living in low-resource settings. These results highlight the need for accelerating research that applies evidence-based implementation strategies and frameworks along with process evaluation to understand barriers and facilitators to scalability of strategies with high reach.
Supplementary Material
Table 2.
Summary of the Strategy Reach (N=16)
| Reference | Genetic risk screening reach (n=13) | Genetic counseling service reach (n=10) | ||||
|---|---|---|---|---|---|---|
| Number of individuals who completed genetic risk screening (Numerator) | Number of individuals who could have been screened (Denominator) | Screening reach (%) | Number of individuals who completed genetic counseling (Numerator) | Number of individuals found to be eligible for genetic counseling (Denominator) | Counseling reach (%) | |
| Clinical settings | ||||||
| General practice | ||||||
| Scheuner (2014) | 1,275 | 2,321 | 55% | 104 | 166 | 63% |
| Anderson (2015) | 237 | 448 | 53% | NA | NA | NA |
| Bradbury (2016) | 82 | 100 | 82% | 61 | 100 | 61% |
| Helsper (2018) | 30 | 30 | 100% | 5 | 19 | 26% |
| Community screening mammography practice | ||||||
| Lee (2005) | 7,316 | NA | NA | 74 | 280 | 26% |
| Seymour (2008) | NA | NA | NA | NA | 707 | NA |
| Wernke (2019) | 126 | 1,169 | 11% | 4 | 35 | 11% |
| McGuinness (2019) | 3,055 | 18,502 | 17% | NA | NA | NA |
| Community gastroenterology practice | ||||||
| Gunaratnam (2016) | 6,031 | 6,031 | 100% | 7 | 848 | 1% |
| Luba (2018) | 3,134 | 5,287 | 59% | 177 | NA | NA |
| Multiple clinics (e.g., mammography center, ambulatory clinics) | ||||||
| Lieberman (2017) | 1,771 | NA | NA | 1,771 | 1,771 | 100% |
| Public health settings | ||||||
| Healthcare call center | ||||||
| Miller (2005) | 279 | 492 | 57% | NA | NA | NA |
| Pasick (2016) | 709 | 1,212 | 58% | 30 | 44 | 68% |
| Population-based cancer registry | ||||||
| Lowery (2010) | 181 | 575 | 31% | 20 | 181 | 11% |
| Niendorf (2016) | 869 | 1,992 | 44% | 500 | 769 | 65% |
| Unclear community setting | ||||||
| Vanags (2010) | 18,642 | 24,210 | 77% | NA | NA | NA |
NA, not available.
ACKNOWLEDGMENTS
YG is funded by the Winship Cancer Institute of Emory University, through Winship Invest$ Winter 2020 Cycle. CGA is funded by the Cancer Epidemiology Education in Special Populations (CEESP) Program through the National Cancer Institute’s grant (R25 CA112383), and by the National Cancer Institute of the NIH under Award Number F99CA253576. CE is funded by the Cancer Prevention and Control Research Network through U48 DP006377. The CPCRN is part of the Prevention Research Centers Program at the Centers for Disease Control and Prevention. The findings and conclusions in this publication are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No financial disclosures were reported by the authors of this paper.
REFERENCES
- 1.Evaluation of Genomic Applications in Practice and Prevention Working Group. Recommendations from the EGAPP Working Group: genetic testing strategies in newly diagnosed individuals with colorectal cancer aimed at reducing morbidity and mortality from Lynch syndrome in relatives. Genet Med. 2009;11:35–41. 10.1097/gim.0b013e31818fa2ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nelson HD, Pappas M, Cantor A, Haney E, Holmes R. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2019;322(7):666–685. 10.1001/jama.2019.8430. [DOI] [PubMed] [Google Scholar]
- 3.Modell SM, Greendale K, Citrin T, Kardia SL. Expert and advocacy group consensus findings on the horizon of public health genetic testing. Healthcare (Basel). 2016;4(1):14 10.3390/healthcare4010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Healthy People 2020. Genomics. Office of Disease Prevention and Health Promotion, Centers for Disease Control and Prevention. https://www.healthypeople.gov/2020/topics-objectives/topic/genomics/objectives. Accessed September 22, 2020.
- 5.Petrucelli N, Daly MB, Pal T. BRCA1- and BRCA2-associated hereditary breast and ovarian cancer In: Adam MP, Ardinger HH, Pagon RA, eds. GeneReviews®[Internet]. Seattle, Washington: University of Washington, Seattle, 2016. https://www.ncbi.nlm.nih.gov/books/NBK1247/. Accessed September 22, 2020. [Google Scholar]
- 6.Kohlmann W, Gruber SB. Syndrome Lynch. In: Adam MP, Ardinger HH, Pagon RA, et al. , eds. GeneReviews®[Internet]. Seattle, Washington: University of Washington, Seattle, 2018. https://www.ncbi.nlm.nih.gov/books/NBK1211/. Accessed September 22, 2020. [Google Scholar]
- 7.Drescher CW, Beatty JD, Resta R, et al. The effect of referral for genetic counseling on genetic testing and surgical prevention in women at high risk for ovarian cancer: results from a randomized controlled trial. Cancer. 2016;122(22):3509–3518. 10.1002/cncr.30190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Powell CB, Littell R, Hoodfar E, Sinclair F, Pressman A. Does the diagnosis of breast or ovarian cancer trigger referral to genetic counseling? Int J Gynecol Cancer. 2013;23(3):431–436. 10.1097/igc.0b013e318280f2b4. [DOI] [PubMed] [Google Scholar]
- 9.Wright JD, Chen L, Tergas AI, et al. Underuse of BRCA testing in patients with breast and ovarian cancer. Am J Obstet Gynecol. 2016;214(6):761–763. 10.1016/j.ajog.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 10.Hampel H, Frankel WL, Martin E, et al. Feasibility of screening for Lynch syndrome among patients with colorectal cancer. J Clin Oncol. 2008;26(35):5783–5788. 10.1200/jco.2008.17.5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morrison J, Bronner M, Leach BH, Downs-Kelly E, Goldblum JR, Liu X. Lynch syndrome screening in newly diagnosed colorectal cancer in general pathology practice: from the revised Bethesda guidelines to a universal approach. Scand J Gastroenterol. 2011;46(11):1340–1348. 10.3109/00365521.2011.610003. [DOI] [PubMed] [Google Scholar]
- 12.Trano G, Sjursen W, Wasmuth HH, Hofsli E, Vatten LJ. Performance of clinical guidelines compared with molecular tumour screening methods in identifying possible Lynch syndrome among colorectal cancer patients: a Norwegian population-based study. Br J Cancer. 2010;102:482–488. 10.1038/sj.bjc.6605509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Lier MG, Leenen CHM, Wagner A, et al. Yield of routine molecular analyses in colorectal cancer patients <70 years to detect underlying Lynch syndrome. J Pathol. 2012;226(5):764–774. 10.1002/path.3963. [DOI] [PubMed] [Google Scholar]
- 14.Khoury MJ, Bowen MS, Clyne M, et al. From public health genomics to precision public health: a 20-year journey. Genet Med. 2018;20:574–582. 10.1038/gim.2017.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dheensa S, Lucassen A, Fenwick A. Limitations and pitfalls of using family letters to communicate genetic risk: a qualitative study with patients and healthcare professionals. J Genet Couns. 2018;27(3):689–701. 10.1007/s10897-017-0164-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pozzar RA, Berry DL. Patient-centered research priorities in ovarian cancer: a systematic review of potential determinants of guideline care. Gynecol Oncol. 2017;147(3):714–722. 10.1016/j.ygyno.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Williams CD, Bullard AJ, O’Leary M, Thomas R, Redding IV TS, Goldstein. Racial/ethnic disparities in BRCA counseling and testing: a narrative review. J Racial Ethn Health Disparities. 2019;6:570–583. 10.1007/s40615-018-00556-7. [DOI] [PubMed] [Google Scholar]
- 18.Roberts MC, Kennedy AE, Chambers DA, Khoury MJ. The current state of implementation science in genomic medicine: opportunities for improvement. Genet Med. 2017;19:858–863. 10.1038/gim.2016.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberts MC, Dotson WD, DeVore CS, et al. Delivery of cascade screening for hereditary conditions: a scoping review of the literature. Health Aff (Millwood). 2018;37(5):801–808. 10.1377/hlthaff.2017.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health. 1999;89(9):1322–1327. 10.2105/ajph.89.9.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Proctor E, Silmere H, Raghavan R, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health. 2011;38:65–76, (2011). 10.1007/s10488-010-0319-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Milat AJ, King L, Bauman AE, Redman S. The concept of scalability: increasing the scale and potential adoption of health promotion interventions into policy and practice. Health Promot Int. 2013;28(3):285–298. 10.1093/heapro/dar097. [DOI] [PubMed] [Google Scholar]
- 23.U.S. Preventive Services Task Force. Genetic risk assessment and BRCA mutation testing for breast and ovarian cancer susceptibility: recommendation statement. Ann Intern Med. 2005;143(5):355–361. 10.7326/0003-4819-143-5-200509060-00011. [DOI] [PubMed] [Google Scholar]
- 24.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65–W94. 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 26.Sirriyeh R, Lawton R, Gardner P, Armitage G. Reviewing studies with diverse designs: the development and evaluation of a new tool. J Eval Clin Pract. 2012;18(4):746–752. 10.1111/j.1365-2753.2011.01662.x. [DOI] [PubMed] [Google Scholar]
- 27.Greenhalgh T, Robert G, Macfarlane F, Bate P, Kyriakidou O, Peacock R. Storylines of research in diffusion of innovation: a meta-narrative approach to systematic review. Soc Sci Med. 2005;61(2):417–430. 10.1016/j.socscimed.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Lee R, Beattie M, Crawford B, et al. Recruitment, genetic counseling, and BRCA testing for underserved women at a public hospital. Genet Test. 2005;9(4):306–312. 10.1089/gte.2005.9.306. [DOI] [PubMed] [Google Scholar]
- 29.Seymour IJ, Casadei S, Zampiga V, et al. Results of a population-based screening for hereditary breast cancer in a region of North-Central Italy: contribution of BRCA1/2 germ-line mutations. Breast Cancer Res Treat. 2008;112:343–349. 10.1007/s10549-007-9846-7. [DOI] [PubMed] [Google Scholar]
- 30.Lowery JT, Axell L, Vu K, Rycroft R. A novel approach to increase awareness about hereditary colon cancer using a state cancer registry. Genet Med. 2010;12:721–725. 10.1097/gim.0b013e3181f1366a. [DOI] [PubMed] [Google Scholar]
- 31.Vanags A, Strumfa I, Gardovskis A, et al. Population screening for hereditary and familial cancer syndromes in Valka district of Latvia. Hered Cancer Clin Pract. 2010;8:8 10.1186/1897-4287-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson EE, Tejeda S, Childers K, Stolley MR, Warnecke RB, Hoskins KF. Breast cancer risk assessment among low-income women of color in primary care: a pilot study. J Oncol Pract. 2015;11(4):e460–e467. 10.1200/jop.2014.003558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bradbury A, Patrick-Miller L, Harris D, et al. Utilizing remote real-time videoconferencing to expand access to cancer genetic services in community practices: a multicenter feasibility study. J Med Internet Res. 2016;18(2):e23 10.2196/jmir.4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niendorf KB, Geller MA, Isaksson Vogel R, et al. A model for patient-direct screening and referral for familial cancer risk. Fam Cancer. 2016;15:707–716. 10.1007/s10689-016-9912-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luba DG, DiSario JA, Rock C, et al. Community practice implementation of a self-administered version of PREMM1,2,6 to assess risk for Lynch syndrome. Clin Gastroenterol Hepatol. 2018;16(1):49–58. 10.1016/j.cgh.2017.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wernke K, Bellcross C, Gabram S, Ali N, Stanislaw C. Impact of implementing BRST(TM) to screen for hereditary breast and ovarian cancer on risk perception and genetic counseling uptake among women in an academic safety net hospital. Clin Breast Cancer. 2019;19(4):e547–e555. 10.1016/j.clbc.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 37.McGuinness JE et al. Uptake of genetic testing for germline BRCA1/2 pathogenic variants in a predominantly Hispanic population. Cancer Genet. 2019;235‒236:72–76. 10.1016/j.cancergen.2019.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gunaratnam NT, Akce M, Al Natour R, et al. Screening for cancer genetic syndromes with a simple risk-assessment tool in a community-based open-access colonoscopy practice. Am J Gastroenterol. 2016;111(5):589–593. 10.1038/ajg.2016.84. [DOI] [PubMed] [Google Scholar]
- 39.Miller SM, Fleisher L, Roussi P, et al. Facilitating informed decision making about breast cancer risk and genetic counseling among women calling the NCI’s Cancer Information Service. J Health Commun. 2005;10(suppl 1):119–136. 10.1080/07366290500265335. [DOI] [PubMed] [Google Scholar]
- 40.Pasick RJ, Joseph G, Stewart S, et al. Effective referral of low-income women at risk for hereditary breast and ovarian cancer to genetic counseling: a randomized delayed intervention control trial. Am J Public Health. 2016;106(10):1842–1848. 10.2105/ajph.2016.303312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lieberman S, Tomer A, Ben-Chetrit A, et al. Population screening for BRCA1/BRCA2 founder mutations in Ashkenazi Jews: proactive recruitment compared with self-referral. Genet Med. 2017;19:754–762. 10.1038/gim.2016.182. [DOI] [PubMed] [Google Scholar]
- 42.Helsper CW, Van liet LM, Velthuizen ME, et al. Identifying patients with a history of ovarian cancer for referral for genetic counselling: non-randomised comparison of two case-finding strategies in primary care. Br J Gen Pract. 2018;68(676):e750–e756. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scheuner MT, Hamilton AB, Peredo J, et al. A cancer genetics toolkit improves access to genetic services through documentation and use of the family history by primary-care clinicians. Genet Med. 2014;16:60–69. 10.1038/gim.2013.75. [DOI] [PubMed] [Google Scholar]
- 44.Chambers DA, Feero WG, Khoury MJ. Convergence of implementation science, precision medicine, and the learning health care system: a new model for biomedical research. JAMA. 2016;315(18):1941–1942. 10.1001/jama.2016.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


