Abstract
Background:
We compared oncologic outcomes of patients who received neoadjuvant chemotherapy (CT) with those of patients who received neoadjuvant chemotherapy plus chemoradiation (CRT) for resectable gastric adenocarcinoma.
Methods:
We compared oncologic and survival outcomes of patients who received CT or CRT for gastric adenocarcinoma at our institution between July 1995 and July 2018. We analyzed propensity score–matched cohorts based on age, sex, race, tumor histologic characteristics, and clinical stage.
Results:
We identified 440 patients (mean age 61 ± 12 years, 62% male, 55% white); 345 (78%) received CRT and 95 (22%) received CT. The propensity score–matched cohorts included 65 patients who received CT and 65 who received CRT. The CRT group had similar frequencies of R1 resection margins to the CT group (7.7% vs 6.2%, p=0.75) but significantly higher frequency of pathologic complete response (27.7% vs 1.5%, p<0.001). The CRT group had lower pathologic stages (p=0.002). Median disease-free survival was 50.9 months (95% confidence interval [CI]: 4.7–97.2) in the CT group and 122.1 months (95% CI: 69.0–175.1) in the CRT group (p=0.07). Median overall survival was 70.7 months (95% CI: 23.9–117.5) in the CT group and 122.1 months (95% CI: 68.7–175.4) in the CRT group (p=0.21).
Conclusions:
Compared with CT, CRT for resectable gastric adenocarcinoma is associated with higher rates of pathologic complete response and subsequent lower final pathologic stage, but survival differences are not significant. Ongoing investigation is necessary to better determine the optimal neoadjuvant therapy and identify patients who receive optimal benefit from CRT.
Keywords: gastric adenocarcinoma, preoperative therapy, surgery, survival
INTRODUCTION
The treatment paradigm for resectable gastric adenocarcinoma (GA) has shifted over the past 15 years after randomized studies showed that various adjuvant therapies improve patient survival [1–4] with multimodality therapy is now the standard treatment for GA [5, 6]. The Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) trial showed that neoadjuvant therapy improved survival outcomes; as a result, the use of neoadjuvant chemotherapy (CT) in patients with T2+ GA in the United States increased from 34% in 2006 to 65% in recent years [7]. Most recently, results of the FLOT4 trial have provided increasing momentum for a perioperative chemotherapy approach to gastric cancer [8]. This paradigm shift has necessitated a change in the American Joint Committee on Cancer (AJCC) staging manual to include a post-neoadjuvant therapy pathologic classification system [9].
In addition to CT, survival benefits have also been found for neoadjuvant chemotherapy plus chemoradiotherapy (CRT) in resectable locally advanced gastroesophageal (GE) junctional cancer [10]. CRT has been successfully adapted to patients with GA in some settings; our institution frequently administers CRT prior to planned curative resection for GA because we have shown high rates of pathologic complete response (pathCR) and microscopically negative (R0) resection, as well as promising long-term survival outcomes [11]. Currently, the international phase III Trial Of Preoperative Therapy for Gastric and Esophagogastric Junction Adenocarcinoma (TOPGEAR) is comparing perioperative chemotherapy with perioperative chemotherapy plus CRT [12, 13]. Thus, the optimal neoadjuvant therapy regimen for resectable GA is not yet defined.
The purpose of the current study was to compare perioperative and long-term oncologic outcomes of patients who received CT with those of patients who received CRT for resectable GA.
METHODS
The University of Texas MD Anderson Cancer Center Institutional Review Board approved the current study. We reviewed our prospectively maintained database on patients with gastric cancer treated at MD Anderson from July 1, 1995, through July 1, 2018. Patient selection and variables collected were similar to those used in a previous study by our group [14–16]. The current study included patients with primary GA, including Siewert type III GE junctional tumors, who underwent potentially curative gastrectomy. The patient and tumor characteristics collected were age, sex, race/ethnicity, primary tumor location, cT category, cN status, final yp stage according to the 8th edition of the AJCC staging manual, and histologic features. The cN status was determined via endoscopic ultrasound (EUS). Treatment variables included use of CT or CRT. Rates of microscopically positive (R1) resection and pathCR, as well as final pathologic stage, were assessed.
CT and CRT techniques at our institution have been previously described [14–16]. CT regimens administered included doublet epirubicin+platinum regimen or 5-FU/capecitabine+platinum regimen, a triplet epirubicin+5-FU/capecitabine+platinum or 5-FU/capecitabine+taxol+platinum regimen, or other combination agents according to clinical indication and physician discretion. Agents and duration of CT was driven by the standard of care as determined from existing high-level evidence at the time of treatment [1, 2, 8], as well as from patient tolerance to therapy. The clinical treatment volume of the radiotherapy included the primary gastric tumor, a 3-cm mucosal margin, involved nodes, and at-risk nodal regions. Radiation oncologists identified gastric lymph node stations to aid in definition of the elective clinical treatment volume for three-dimensional conformal techniques, intensity-modulated radiotherapy, or volumetric-modulated arc radiotherapy. The standard regimen of CRT used for patients with gastric cancer was a radiation dose of 45-Gy administered concurrently with 5-fluorouracil, most commonly after induction chemotherapy with a 5-fluorouracil–based regimen [17]. Patients with distal tumors received similar CT; our phase II trials showing the benefit of CRT included patients with distal tumors [11]. Approximately 6–8 weeks after the completion of CRT, patients underwent restaging with computed tomography, positron emission tomography/computed tomography, or EUS, followed by resection. Patients who did not undergo surgery owing to progression of disease or comorbidities were excluded from the current study.
Our standard surveillance practice is follow-up assessment with imaging every 4–6 months. Owing to low rates of locoregional relapse following curative resection, with low yield and high costs associated with rigorous surveillance, we do not routinely perform surveillance endoscopic evaluation [18].
Statistical analysis.
Data are reported as mean ± standard deviation if normally distributed or median (interquartile range) if not. To test univariate associations, differences between treatment groups were compared using the Student t test for parametric data and the Mann-Whitney U test for nonparametric data. Categorical data were compared using Pearson chi-square; if cell counts were <5, the Fisher exact test was used.
Perioperative outcomes, including frequency of R1 resection margin, pathologic complete response (pathCR), final pathologic stage, and peri-operative complications, as well as hospital length of stay (LOS, in days) were compared between treatment groups using Pearson chi-square and Mann-Whitney U test. For long-term oncologic outcomes, Kaplan-Meier survival analyses were used to evaluate overall survival (OS) and disease-free survival (DFS) with treatment groups compared using the log-ranktest. To account for differences in baseline characteristics between the groups, we used propensity matching. Propensity scores were assigned to each patient using a logistic regression model for predicting whether a patient would receive CT using only pretreatment variables including patient and tumor characteristics. These variables included age, sex, race/ethnicity, histologic grade, presence of signet ring cells, linitis plastica, Lauren classification, as well as both cT and cN stage separately. Patients with synchronous malignancies, those with uncertainty of stable disease on preoperative staging, and those who did not receive complete planned neoadjuvant therapy were excluded from the propensity score–matched cohorts. A 1:1 fixed ratio of nearest neighbor matching was used to minimize bias without sacrificing power [19]. Comparisons between propensity score–matched cohorts were performed. Significance was assessed at p<0.05. Statistical analyses were performed using SPSS version 24 (IBM Corporation; Armonk, NY).
RESULTS
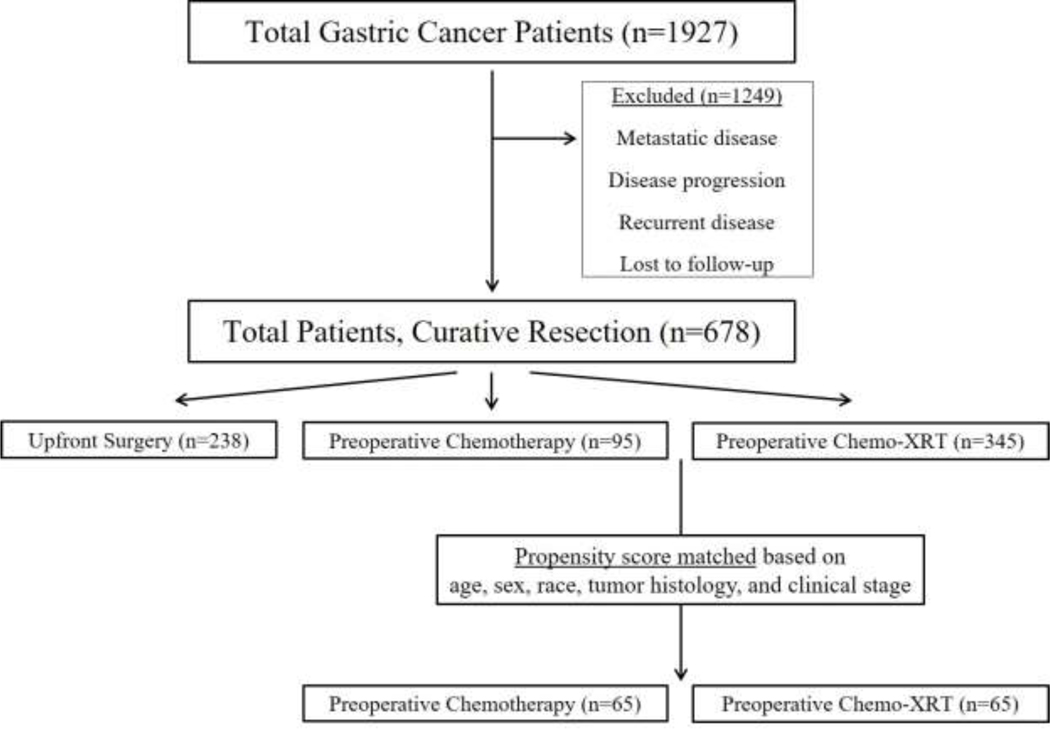
The patient selection process for inclusion in the analysis is seen in Figure 1. Of 1,927 patients in our institutional database, 678 underwent potentially curative gastrectomy, of which 440 received neoadjuvant therapy. These patients who underwent neoadjuvant therapy were age 61±12 years, 62% were male, and 55% were white. The median OS for the entire cohort was 70.7 months (95% confidence interval [CI]: 49.2–92.2 months) with a median follow-up time of 38.4 months.
Figure 1:
Patient selection process for our study.
Of the entire cohort, 345 (78%) received CRT and the remaining 95 (22%) received CT. There was high uniformity in radiation delivery among the CRT group; only 13 patients received less than 45 Gy owing to toxicities and/or other logistical reasons. Table 1 shows the demographic and clinical features of the 440 patients who received neoadjuvant therapy. Patients who received CRT, compared with those who received CT, more often presented with locally advanced cT4 lesions (13.1% vs 6.1%, p=0.07) and/or with cN2+ regional nodal disease (9.8% vs 2.5%, p=0.01) on EUS. Median DFS for CT and CRT was 40.8 (95% CI: 26.5–55.0) vs 67.8 (95% CI: 42.3–93.1) months (p=0.08), respectively. Median OS was 50.9 (95% CI: 23.1–78.7) vs 85.1 (95% CI: 63.0–107.2) months with a 5-year OS of 47.8% vs 55.1% (p=0.17), respectively.
Table 1:
Demographic and clinical characteristics of patients who underwent resection for gastric adenocarcinoma following neoadjuvant therapy (n=440).a
| CT (n=95) | CRT (n=345) | P Value | |
|---|---|---|---|
| Age, years | 62±13 | 61±12 | 0.234 |
| Male | 55.8% | 63.3% | 0.234 |
| Race | 0.123 | ||
| White | 54.7% | 54.8% | |
| Hispanic | 18.9% | 14.0% | |
| Black | 11.6% | 6.7% | |
| Other | 14.8% | 24.4% | |
| Histology | 0.675 | ||
| Well Differentiated | 1.1% | 0.3% | |
| Moderately Differentiated | 24.2% | 23.7% | |
| Poorly Differentiated | 70.5% | 68.4% | |
| Other | 4.2% | 7.6% | |
| Signet Ring Cells | 51.6% | 48.8% | 0.728 |
| Linitis Plastica | 10.9% | 6.0% | 0.109 |
| EUS T stage | 0.517 | ||
| cT1 | 2.4% | 0.6% | |
| cT2 | 18.5% | 13.1% | |
| cT3 | 70.4% | 71.6% | |
| cT4 | 6.1% | 13.1% | |
| cTx | 2.5% | 1.3% | |
| EUS N Stage | 0.204 | ||
| cN0 | 53.1% | 43.1% | |
| cN1 | 40.7% | 42.5% | |
| cN2 | 2.5% | 7.8% | |
| cN3 | 0.0% | 2.0% | |
| cNx | 3.7% | 4.6% | |
CT, neoadjuvant chemotherapy; CRT, neoadjuvant chemotherapy plus chemoradiotherapy; EUS: endoscopic ultrasound.
To control for differences between groups, we assigned propensity matching scores. As shown in Table 2, 65 patients who received CT were effectively matched to 65 patients who received CRT. Groups were similar by age, sex, race/ethnicity, histologic characteristics, and preoperative clinical stage. Patients who received CRT, compared with CT, had similar rates of R1 resection margins (7.7% vs 6.2%, p=0.75) yet significantly higher rates of pathCR (27.7% vs 1.5%, p<0.001). We found no differences in length of stay or any other major complications, including leak rates, intra-abdominal infections, or bleeding (all p>0.05; see Table 2).
Table 2:
Comparison of propensity score–matched patients who underwent resection for gastric adenocarcinoma following neoadjuvant therapy (n=130).a
| CT (n=65) | CRT (n=65) | P Value | |
|---|---|---|---|
| Age, years | 62±12 | 61±13 | 0.843 |
| Male | 58.5% | 53.8% | 0.362 |
| Race | 0.751 | ||
| White | 50.8% | 60.0% | |
| Hispanic | 21.5% | 15.4% | |
| Black | 12.3% | 13.8% | |
| Other | 15.4% | 10.8% | |
| Histology | 0.362 | ||
| Well Differentiated | 1.5% | 0.0% | |
| Moderately Differentiated | 24.6% | 23.1% | |
| Poorly Differentiated | 70.8% | 76.9% | |
| Other | 3.1% | 0.0% | |
| Signet Ring Cells | 53.8% | 43.1% | 0.146 |
| Linitis Plastica | 6.3% | 1.6% | 0.187 |
| EUS T stage | 0.543 | ||
| cT1 | 3.0% | 1.5% | |
| cT2 | 13.8% | 7.7% | |
| cT3 | 73.8% | 72.3% | |
| cT4 | 6.2% | 13.9% | |
| cTx | 3.1% | 3.1% | |
| EUS N Stage | 0.762 | ||
| cN0 | 52.3% | 46.2% | |
| cN1 | 41.5% | 47.7% | |
| cN2 | 3.1% | 1.5% | |
| cN3 | 0.0% | 1.5% | |
| cNx | 3.1% | 3.1% | |
| Procedure Performed | 0.155 | ||
| Total Gastrectomy | 41.5% | 58.5% | |
| Subtotal/Distal Gastrectomy | 53.9% | 38.5% | |
| Proximal Gastrectomy | 4.6% | 3.1% | |
| Lymphadenectomy, 15 or more | 84.6% | 81.5% | 0.408 |
| Mean LN Removed | 32±20 | 25±12 | 0.011 |
| Median LOS, days | 11(9–14) | 11(10–17) | 0.665 |
| Complications | |||
| Wound | 18.5% | 16.9% | 0.501 |
| Intraabdominal Infection | 9.2% | 12.3% | 0.389 |
| Leak | 1.5% | 7.7% | 0.104 |
| Respiratory | 7.7% | 15.4% | 0.136 |
| Renal | 1.5% | 3.1% | 0.501 |
| Cardiac | 4.6% | 6.2% | 0.499 |
| Anemia | 6.2% | 4.6% | 0.501 |
| Other | 18.5% | 10.8% | 0.161 |
| ypT Stage | <0.001 | ||
| ypT0 | 1.5% | 27.7% | |
| ypT1 | 23.0% | 16.9% | |
| ypT2 | 10.8% | 23.1% | |
| ypT3 | 36.9% | 23.1% | |
| ypT4 | 26.2% | 9.2% | |
| ypN Stage | 0.039 | ||
| ypN0 | 42.2% | 66.2% | |
| ypN1 | 26.6% | 18.5% | |
| ypN2 | 17.2% | 9.2% | |
| ypN3 | 14.1% | 6.2% | |
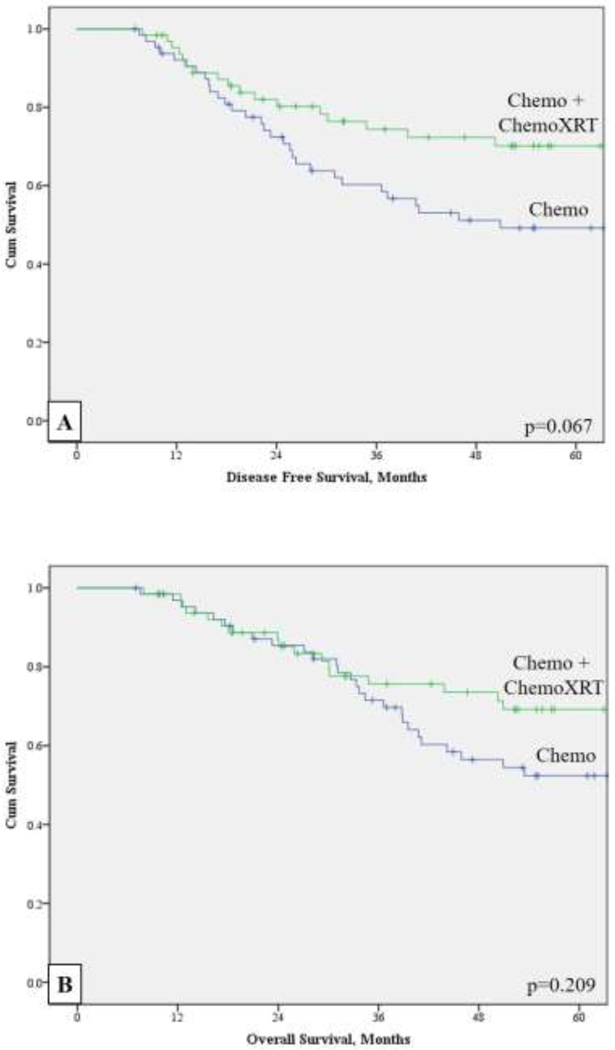
| DFS | 0.067 | ||
| Median DFS, mo(95% CI) | 51(5–97) | 122(69–175) | |
| 5 year DFS | 49.2% | 70.1% | |
| OS | 0.209 | ||
| Median OS, mo(95% CI) | 71(24–118) | 122(69–175) | |
| 5 year OS | 52.4% | 69.2% | |
CT, neoadjuvant chemotherapy; CRT, neoadjuvant chemotherapy plus chemoradiotherapy; EUS, endoscopic ultrasound; IQR, interquartile range; DFS, disease-free survival; CI, confidence interval; OS, overall survival.
Although the propensity score–matched cohorts had similar preoperative clinical staging, those who received CRT had significantly lower final pathologic stage (p=0.002). Figure 2 shows OS and DFS for the matched cohorts. Median DFS for CT and CRT was 50.9 (95% CI: 4.7–97.2) vs 122.1 (95% CI: 69.0–175.1) months (p=0.07), respectively. Median OS was 70.7 (95% CI: 23.9–117.5) vs 122.1 (95% CI: 68.7–175.4) months with a 5-year OS of 52.4% vs 69.2% (p=0.21), respectively.
Figure 2:
Kaplan-Maier analyses of the propensity score–matched cohorts (n=130) for (a) disease-free survival and (b) overall survival.
Among the propensity score–matched patients who received CT, reasons for exclusion from radiation included tumor-related variables (n=17, 26%), patient preference due to logistical/financial reasons (n=7, 11%), toxicity related to CT (n=5, 8%), and other/physician preference (n=36, 55%).
DISCUSSION
The results of our single-institution, retrospective analysis of neoadjuvant therapy for GA indicate that CRT is associated with higher rates of pathCR and subsequent lower overall final pathologic stage compared with CT. Absolute differences in DFS and OS did not reach significance. There were no differences in perioperative morbidity based on neoadjuvant treatment regimen.
CRT is a standard component of our institution’s treatment approach for patients being considered for curative resection. An important goal of CRT is reduction in primary tumor volume to facilitate resection and improve rates of R0 resection, as well as subsequently decrease local recurrence. Additional potential benefits include treatment effect on micrometastatic disease and decreased spread of tumor cells at the time of resection. Furthermore, patients with aggressive disease and the potential for early recurrence after an upfront resection may experience disease progression during neoadjuvant therapy instead, thus avoiding a potentially morbid operation with little survival benefit [20]. Although multiple studies have demonstrated the benefit of a multimodality treatment approach with perioperative therapy, up to 50% of patients undergoing gastrectomy never receive adjuvant therapy, presumably owing to poor tolerance, whereas neoadjuvant therapy is more reliably delivered and may be better tolerated [21]. Finally, pathologic evaluation of a tumor’s response to neoadjuvant therapy provides additional prognostic information and may aid in the selection of additional adjuvant therapies [22].
In support of our institutional practices, CRT has been shown to effectively downstage primary tumors and nodal disease in single-institution and phase II single-arm trials [11, 23–25]. In the absence of randomized comparative trials as of yet, in the current study, we compared outcomes of propensity score–matched patients who received CRT with those of patients who received CT. Although no survival benefit was seen with CRT, we did see higher rates of both pathCR and downstaging in the final pathologic stage in patients who received CRT compared with those who received CT. These factors may play an important role in decreasing local recurrence rates and influencing survival [11, 26, 27], thus supporting our continued use of CRT in patients with resectable GA. A recent analysis of the National Cancer Database (NCDB) showed similar results, with a pathCR rate of 16.1% in the CRT group, similar to the rate seen in the current study [27]. The ChemoRadiotherapy for Oesophageal cancer followed by Surgery Study (CROSS trial) also showed a promising 29% incidence of pathCR as well as an OS benefit of CRT for patients with GE junctional cancers [10]. Although that study included only patients with esophageal cancer, several phase II clinical trials have shown similarly high rates of pathCR in patients with GA [23–25].
Still, with regards to gastric cancer treatment specifically, a survival benefit of CRT over CT based on comparative analysis has yet to be demonstrated in a large randomized phase III trial. Survival differences between the two groups in the current study did not reach statistical significance, similar to the findings of a recent NCDB study that used propensity matching; that study did not find CRT to be independently predictive of survival [27]. However, as previously mentioned, the ongoing TOPGEAR trial is comparing perioperative chemotherapy with perioperative chemotherapy plus CRT to better define the best perioperative treatment regimen for gastric cancer [12, 13]. The ongoing CRITICS-II trial will compare preoperative chemotherapy alone, preoperative chemoradiotherapy alone, and preoperative chemotherapy plus chemoradiotherapy [28]. These trials together will provide valuable high-quality data to better determine the optimal neoadjuvant therapy for gastric cancer.
We also found no differences in postoperative complications in patients who received CRT compared with those who received CT. Although patients who received CRT had higher rates of anastomotic leak, this difference did not reach statistical significance, and our study was not specifically designed to assess this outcome. However, previous studies have shown no increases in anastomotic leak rates or intra-abdominal fluid collections after CRT [14]. Furthermore, an interim analysis of the TOPGEAR trial supported these findings, thus confirming the safety of CRT [13].
The current study had some limitations. Immeasurable differences between patients who received CT and those who received CRT are likely, and these unmeasured confounders could not be completely accounted for with multivariable statistical adjustment in the propensity matched analysis. Due to the retrospective nature of the study, the regimens were not standardized, and actual agents and duration employed varied within this cohort. Randomized trials with standardized protocols are thus needed to better determine the optimal preoperative regimen in this context. In addition, decisions between CT and CRT were dependent on various factors, including tumor-related variables, patient preference, financial or logistical factors for the patient, physician preference, and other reasons that could not be captured via retrospective data collection. More insight on why patients do not receive their recommended care, such as from financial or logistical barriers, are areas for additional investigation. Our institutional preference, based on the results of phase II trials and single-institution studies, is to routinely offer CRT [11, 23–25]. Although this approach is recommended by the current National Comprehensive Cancer Network guidelines, we acknowledge that this approach is unique to our institution and a limitation to the generalizability of our work. Thus, because we routinely offer CRT, the relatively low number of patients who received CT presents a size limitation of the current analysis, potentially contributing to type II error for survival comparisons. Given the limited population size, we were unable to assess stage-specific outcomes such as advanced nodal disease. Also, because only a small portion of patients received less than the planned 45-Gy dose of radiation for CRT, we were unable to compare outcomes by radiation dose or regimen. In addition, the radiotherapy techniques changed during the period studied. In most cases, three-dimensional conformal techniques were used before 2009 and intensity-modulated radiotherapy was used after 2009. The extent of the radiation field also varied but did not routinely include extra-regional lymph nodes, such as para-aortic lymph nodes or hepatoduodenal ligament lymph nodes, unless these nodes were enlarged, raising suspicion for metastasis. The radiation field and technique used for CRT may vary by institution in the United States, which may affect the external validity of our findings. The nature of the study fails to allow for an intention-to-treat analysis. As this prospective database was primarily built to obtain detailed data on those who underwent resection, attempting to compare progression or drop-out rates between groups in a retrospective manner would be unreliable due to limitations of unstandardized data reporting in the medical records. Although it is possible patients progressed during XRT, other studies have found that if patients progress during preoperative therapy, it is likely during induction chemotherapy, prior to XRT [23–25]. Additionally, the interim results of TOPGEAR, a randomized phase III trial comparing preoperative CT and preoperative CRT, showed the proportion of patients proceeding to surgery was 90% in the CT group and 85% in the CRT group [13]. Finally, our present study shows survival outcomes better than recently reported in larger multi-institutional trials. This may in part be due to our treatment approach but may also be a result of our limited sample size and single institutional data. Despite these limitations, the current study represents one the largest head-to-head comparisons of oncologic outcomes of patients who receive CT with those who receive CRT for resectable GA.
In conclusion, compared with CT alone, CRT is associated with higher rates of pathCR and subsequent lower overall final pathologic stage, yet differences in DFS and OS did not reach significance. Ongoing investigation is needed to better determine the optimal preoperative therapeutic regimen and identify specific subsets of patients who would benefit from CRT.
Synopsis:
The optimal neoadjuvant therapy regimen for resectable gastric adenocarcinoma is not defined. This retrospective, propensity score-matched analysis demonstrates higher rates of pathologic response, lower final pathologic stages, and possible survival advantages with neoadjuvant chemotherapy plus chemoradiotherapy when compared to neoadjuvant chemotherapy alone.
ACKNOWLEDGMENTS
This research was supported by the National Institutes of Health/National Cancer Institute under award number P30CA016672 and The No Stomach for Cancer Award for Gastric Cancer Research. The authors would like to acknowledge Erica Goodoff in Scientific Publications, Medical Research Library at MD Anderson Cancer Center for editing assistance.
AUTHOR CONTRIBUTIONS
CJA was directly responsible for all aspects of the study. He participated in the collection, analysis, and interpretation of data and drafting and revision of the manuscript, figures, and tables. ANB and GLS participated in the analysis and interpretation of data and drafting and revision of the manuscript, figures, and tables. PD, BDM, MB, JA, and PFM participated in the review and revision of the manuscript, figures, and tables. NI participated in the design, analysis, and revision of the manuscript, figures, and tables. BDB had overall responsibility for the study, including conception and experimental design, analysis and interpretation of data, drafting and revision of the manuscript, obtaining funding for the project, and supervision.
Portions of these data will be presented as an oral presentation at the Society of Surgical Oncology 73rd Annual Cancer Symposium, 2020, in Boston, MA.
Footnotes
Evidence level: III
Disclosures: The authors have no potential conflicts of interest to declare.
REFERENCES
- 1.Cunningham D, et al. , Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med, 2006. 355(1): p. 11–20. [DOI] [PubMed] [Google Scholar]
- 2.Ychou M, et al. , Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol, 2011. 29(13): p. 1715–21. [DOI] [PubMed] [Google Scholar]
- 3.Macdonald JS, et al. , Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med, 2001. 345(10): p. 725–30. [DOI] [PubMed] [Google Scholar]
- 4.Sakuramoto S, et al. , Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med, 2007. 357(18): p. 1810–20. [DOI] [PubMed] [Google Scholar]
- 5.Badgwell B, Multimodality Therapy of Localized Gastric Adenocarcinoma. J Natl Compr Canc Netw, 2016. 14(10): p. 1321–1327. [DOI] [PubMed] [Google Scholar]
- 6.Badgwell B, Das P, and Ajani J, Treatment of localized gastric and gastroesophageal adenocarcinoma: the role of accurate staging and preoperative therapy. J Hematol Oncol, 2017. 10(1): p. 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ikoma N, et al. , Racial disparities in preoperative chemotherapy use in gastric cancer patients in the United States: Analysis of the National Cancer Data Base, 2006–2014. Cancer, 2018. 124(5): p. 998–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Batran SE, et al. , Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet, 2019. 393(10184): p. 1948–1957. [DOI] [PubMed] [Google Scholar]
- 9.Amin MB, American Joint Committee on Cancer., and American Cancer Society., AJCC cancer staging manual. Eight edition / editor-in-chief, Amin Mahul B., MD, FCAP; editors, Edge Stephen B., MD, FACS and 16 others; Gress Donna M., RHIT, CTR - Technical editor; Meyer Laura R., CAPM - Managing editor. ed. 2017, Chicago IL: American Joint Committee on Cancer, Springer; xvii, 1024 pages. [Google Scholar]
- 10.Shapiro J, et al. , Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol, 2015. 16(9): p. 1090–1098. [DOI] [PubMed] [Google Scholar]
- 11.Ikoma N, et al. , Nodal Downstaging in Gastric Cancer Patients: Promising Survival if ypN0 is Achieved. Ann Surg Oncol, 2018. 25(7): p. 2012–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leong T, et al. , TOPGEAR: a randomised phase III trial of perioperative ECF chemotherapy versus preoperative chemoradiation plus perioperative ECF chemotherapy for resectable gastric cancer (an international, intergroup trial of the AGITG/TROG/EORTC/NCIC CTG). BMC Cancer, 2015. 15: p. 532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leong T, et al. , TOPGEAR: A Randomized, Phase III Trial of Perioperative ECF Chemotherapy with or Without Preoperative Chemoradiation for Resectable Gastric Cancer: Interim Results from an International, Intergroup Trial of the AGITG, TROG, EORTC and CCTG. Ann Surg Oncol, 2017. 24(8): p. 2252–2258. [DOI] [PubMed] [Google Scholar]
- 14.Ikoma N, et al. , Preoperative Chemoradiation Therapy Does Not Increase Risk of Anastomotic Leak in Patients With Gastric Cancer. Int J Radiat Oncol Biol Phys, 2017. 99(3): p. 660–666. [DOI] [PubMed] [Google Scholar]
- 15.Ikoma N, et al. , Evaluation of the American Joint Committee on Cancer 8th edition staging system for gastric cancer patients after preoperative therapy. Gastric Cancer, 2018. 21(1): p. 74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ikoma N, et al. , Patterns of Initial Recurrence in Gastric Adenocarcinoma in the Era of Preoperative Therapy. Ann Surg Oncol, 2017. 24(9): p. 2679–2687. [DOI] [PubMed] [Google Scholar]
- 17.Moertel CG, et al. , Combined 5-fluorouracil and supervoltage radiation therapy of locally unresectable gastrointestinal cancer. Lancet, 1969. 2(7626): p. 865–7. [DOI] [PubMed] [Google Scholar]
- 18.Elimova E, et al. , Patterns of relapse in patients with localized gastric adenocarcinoma who had surgery with or without adjunctive therapy: costs and effectiveness of surveillance. Oncotarget, 2017. 8(46): p. 81430–81440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Austin PC, Statistical criteria for selecting the optimal number of untreated subjects matched to each treated subject when using many-to-one matching on the propensity score. Am J Epidemiol, 2010. 172(9): p. 1092–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiong HQ, et al. , Chemoradiation for resectable gastric cancer. Lancet Oncol, 2003. 4(8): p. 498–505. [DOI] [PubMed] [Google Scholar]
- 21.Tormo Ferrero V, et al. , Evaluation of the toxicity of the combined treatment of chemoradiotherapy, according to the scheme of Macdonald, after radical surgery in patients diagnosed of gastric cancer. Clin Transl Oncol, 2006. 8(8): p. 611–5. [DOI] [PubMed] [Google Scholar]
- 22.Yao JC, et al. , Combined-modality therapy for gastric cancer. Semin Surg Oncol, 2003. 21(4): p. 223–7. [DOI] [PubMed] [Google Scholar]
- 23.Ajani JA, et al. , Paclitaxel-based chemoradiotherapy in localized gastric carcinoma: degree of pathologic response and not clinical parameters dictated patient outcome. J Clin Oncol, 2005. 23(6): p. 1237–44. [DOI] [PubMed] [Google Scholar]
- 24.Ajani JA, et al. , Multi-institutional trial of preoperative chemoradiotherapy in patients with potentially resectable gastric carcinoma. J Clin Oncol, 2004. 22(14): p. 2774–80. [DOI] [PubMed] [Google Scholar]
- 25.Ajani JA, et al. , Phase II trial of preoperative chemoradiation in patients with localized gastric adenocarcinoma (RTOG 9904): quality of combined modality therapy and pathologic response. J Clin Oncol, 2006. 24(24): p. 3953–8. [DOI] [PubMed] [Google Scholar]
- 26.Reed VK, et al. , Incidence, natural history, and patterns of locoregional recurrence in gastric cancer patients treated with preoperative chemoradiotherapy. Int J Radiat Oncol Biol Phys, 2008. 71(3): p. 741–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ikoma N, et al. , Preoperative chemoradiation therapy induces primary-tumor complete response more frequently than chemotherapy alone in gastric cancer: analyses of the National Cancer Database 2006–2014 using propensity score matching. Gastric Cancer, 2018. 21(6): p. 1004–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Slagter AE, et al. , CRITICS-II: a multicentre randomised phase II trial of neo-adjuvant chemotherapy followed by surgery versus neo-adjuvant chemotherapy and subsequent chemoradiotherapy followed by surgery versus neo-adjuvant chemoradiotherapy followed by surgery in resectable gastric cancer. BMC Cancer, 2018. 18(1): p. 877. [DOI] [PMC free article] [PubMed] [Google Scholar]