Abstract
Objective:
To synthesize investigations into the role of lipid-mediated recruitment and activation of ILC2s in AERD.
Data Sources:
A comprehensive literature review of reports pertaining to AERD cellular mechanisms, cytokine and lipid mediators in AERD, and ILC2 activation and recruitment was performed using PubMed and Google Scholar.
Study Selections:
Selections of studies were based on reports of lipid mediators in AERD, cytokine mediators in AERD, type two effector cells in AERD, platelets in AERD, AERD treatment, ILC2s in allergic airway disease, and ILC2 activation, inhibition, and trafficking.
Results:
The precise mechanisms of AERD pathogenesis are not well understood. Greater levels of proinflammatory lipid mediators and type two cytokines are found in tissues derived from AERD patients relative to controls. Following pathognomonic COX-1 inhibitor reactions, proinflammatory mediator concentrations (prostaglandin D2 and cysteinyl leukotrienes) are rapidly increased, as are ILC2 levels in the nasal mucosa. ILC2s, which potently generate type 2 cytokines in response to lipid mediator stimulation, may play a key role in AERD pathogenesis.
Conclusion:
While the literature suggests that lipid-mediated ILC2 activation may occur in AERD, there is a dearth of definitive evidence. Future investigations leveraging novel next-generation single cell sequencing approaches along with recently developed AERD murine models will better define lipid mediator-induced ILC2 trafficking in patients with AERD.
Introduction
Allergic airway disease is a major global health problem. As of 2018, the CDC has reported that over 24 million people in the US (7.7%) are living with asthma and greater than 28 million people (11.6%) are afflicted with chronic rhinosinusitis (CRS). 20% of CRS patients will develop nasal polyps (CRSwNP), noncancerous growths of the sinus cavities. A subset of asthmatics (9%) are diagnosed with aspirin-exacerbated respiratory disease (AERD). The prevalence of AERD in asthmatics increases from 9% to 30% in those with comorbid CRSwNP..1 AERD was previously known as Samter’s Triad (also aspirin-intolerant-asthma and non-steroidal anti-inflammatory drug exacerbated respiratory disease) as it was first described by Max Samter in 1968 and is characterized by three clinical hallmarks, which typically develop in sequence in the 3rd−4th decade of life: CRSwNP, moderate-to-severe asthma, and pathognomonic respiratory reactions to COX-1-inhibition. The underlying cause of AERD is unknown; however, it is clear that AERD is a type 2 immune-mediated respiratory disease, like asthma and CRS, but with a particularly marked proinflammatory lipid signature involving cysteinyl leukotrienes (CysLTs) and prostaglandin D2 (PGD2).
Innate lymphoid cells (ILCs) are recently discovered innate counterparts to adaptive helper T (Th) cells. The family of ILCs includes ILC1s, ILC2s, and ILC3s, which correspond to Th1, Th2, and Th17/22 cells, respectively.2 ILCs mirror Th cell expression of transcription factors and cytokines. For example, ILC2s and Th2 cells are defined by expression of the key transcriptional regulator GATA-3 and produce IL-4, IL-5, and IL-13. However, unlike Th cells, ILCs lack T cell receptors and antigen specificity. Instead, ILCs respond rapidly and robustly to the local cytokine (IL-33, IL-25, TSLP) and lipid mediators (CysLTs, PGD2). Largely residential to mucosal surfaces, ILCs are positioned as immune sentinels, and ILC2s are the primary ILC found in the airways. Various environmental stressors, including allergens, viruses, and parasites, initiate ILC2 responses. More recently, ILC2s were shown to be to be recruited to the airway in AERD patients. Here, we review the current understanding of mediators that regulate and recruit ILC2s and how ILC2s might contribute to AERD pathogenesis.
Lipid mediators in AERD that regulate ILC2s
Cysteinyl leukotrienes
Long before ILC2s were discovered and shown to be activated by CysLTs3–5, Ferreri et al. provided the first insight into the role of CysLTs in AERD pathogenesis.6 Following aspirin challenge, the levels of nasal leukotriene (LT) C4 were greater in aspirin intolerant patients compared to tolerant controls. LTC4 is a member of the cysteinyl leukotriene (CysLT) family of bioactive lipid molecules, which are products of the 5-lipoxygenase (5-LO) branch of arachidonic acid (AA) metabolism.7,2 5-LO converts AA to leukotriene A4 (LTA4), which is subsequently metabolized into leukotriene B4 (LTB4) or LTC4. The former is a terminal metabolite, whereas the latter rapidly converts to leukotriene D4 (LTD4), and finally leukotriene E4 (LTE4). CysLTs include LTC4, LTD4, and LTE4 and are mediators of bronchoconstriction, mucus hypersecretion, and eosinophilia. Since Ferreri’s initial discovery, a wealth of evidence supporting LT involvement in AERD has been reported. Baseline urinary levels of LTE4 are increased in AERD patients relative to healthy controls, and concentrations are further increased following aspirin challenge.8 While elevated levels of urinary LTE4 are also found in patients with asthma and CRSwNP, the LTE4 concentrations in urine from AERD patients are increased above those with asthma and CRSwNP. These observations were later extended to LTB4, though the concentrations reported were lower than those of LTE4.9 In support of these findings, 5-LO was found to be increased in AERD nasal mucosa, and LTC4-synthase shown to be increased in both the lungs and nasal mucosa.
Increased PGD2
In addition to 5-LO, AA is also metabolized by the two cyclo-oxygenase (COX) enzymes, COX-1 and COX-2. This process yields the prostaglandin (PG) family of lipid mediators that regulate inflammatory and anti-inflammatory responses in allergic airway disease. High levels of PGD2, a known bronchoconstrictor, are also associated with severe asthma.2,10,11 COX products are of particular interest as AERD is characterized by by COX-1 inhibitor reactions. Significantly higher concentrations of PGD2 are found in nasal polyps from aspirin intolerant patients compared to aspirin tolerant patients.12,13 Further, baseline urinary PGD2 metabolites (PGD2M) levels are greater in AERD patients compared to controls, and PGD2M concentrations negatively correlate with tolerance to aspirin desensitization.13 Importantly, human ILC2s are identified by expression of CRTH22, one of the receptors for PGD2, and therefore the reported effects of PGD2 on ILC2 recruitment and activation are particularly relevant to AERD (Figure 1).14,15
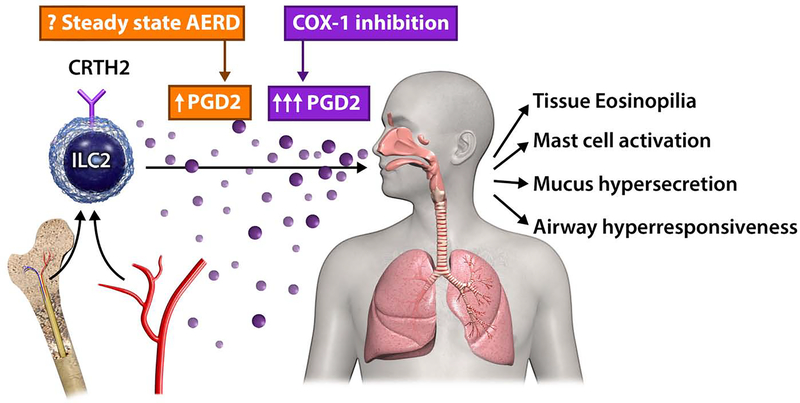
Figure 1. Recruitment of ILC2s to respiratory mucosa in AERD.
In patients with AERD challenged with COX-1 inhibitors, CRTH2+ ILC2s are reduced in the blood and accumulate in the nasal mucosa concomitantly with increased urinary prostaglandin D2 metabolites. This suggests that ILC2s that express CRTH2 (receptor for PGD2) may traffic into respiratory tissues from the bone marrow during COX-1 inhibition in AERD patients. Whether ILC2 recruitment occurs over time in the absence of COX-1 inhibitor challenge (‘steady state AERD’) is not known. Once in tissues, type 2 cytokine production by ILC2s and other cell types can promote tissue eosinophilia, mast cell activation, mucus hypersecretion, and airway hyperresponsiveness.
Reduced PGE2
Prostaglandin E2 (PGE2) is also formed by through COX processing of AA. Unlike PGD2, PGE2 plays a protective role in allergic airway disease by attenuating airway inflammation and supporting normal lung function.16 Studies have demonstrated that PGE2 reduces both 5-LO activity and allergen-induced IL-33 release may explain the former mechanism.17,18 Importantly, defects in PGE2 responses have been linked to AERD as expression of PGE2 is lower in nasal polyps from aspirin intolerant patients compared to controls.12,19,20 Additionally, the PGE2 receptor is downregulated in nasal and lung tissue derived from aspirin intolerant patients.21,22 Further, a recent report demonstrated that suppression of CysLT production by PGE2 was diminished in AERD patients thus highlighting complex interplay between these eicosanoids.20 In addition to activation by CysLTs and PGD2, human ILC2 responses are inhibited by PGE2.23 Together, these studies suggest that tissue ILC2s may be more pro-inflammatory in patients with AERD. Overall, AERD pathophysiology driven by high levels of pro-inflammatory mediators may be compounded by concomitant impairment of PGE2 signaling.
ILC2-activating cytokine mediators in AERD
Epithelial-cell derived cytokines
In response to injury, airway epithelial cells release proinflammatory “alarmin” cytokines IL-25, IL-33, and TSLP which activate many type 2 cytokine producing cell types including ILC2s.2 These mediators have been linked to asthma, CRS, nasal polyposis, and, more recently, AERD. While early investigation into AERD pathogenesis focused on bioactive lipid mediators, recent studies have elucidated the role of these epithelial-cell derived cytokines. Increased expression of IL-33 and TSLP were found in nasal polyps from AERD patients relative to those derived from aspirin tolerant individuals.24,25 Another study reported higher baseline plasma levels of IL-25 in AERD patients relative to aspirin tolerant controls.26 Importantly, alarmin induced type 2 cytokines drive proliferation of PGD2- and CysLT-producing eosinophils and mast cells. Taken together, these reports suggest a revised model of AERD pathogenesis wherein epithelial injury triggers alarmin release and subsequent PGD2 and CysLT production.
Pro-inflammatory type 1 and 2 cytokines
Epithelial cell derived cytokines drive immune cells (primarily ILC2s and Th2 cells) to produce the type 2 cytokines IL-4, IL-5, IL-13, and IL-9, which have been inextricably linked to allergic airway disease. Recent studies of nasal polyposis have demonstrated type 2 cytokine involvement in AERD. AERD polyps contain greater IL-4 transcripts relative to chronic hyperplastic eosinophilic sinusitis polyps, and surprisingly, higher levels of the type 1 cytokine IFN-γ from eosinophils.27 Levels of IL-5 are also greater in nasal polyps from allergic individuals with type 2 disease compared to non-allergic individuals, and nasal secretions from patients with CRSwNP contain more IL-5 than those from patients with CRSsNP.28–30 Further, IL-9, GATA-3, IL-5, and IL-13 expression is increased in tissue from patients with CRSwNP compared to CRSsNP and non-diseased controls.31,32 Overall, nasal polyp tissue from AERD patients contains a high type 2 cytokine signature consistent with the presence of ILC2s, though the type 1 cytokine IFN-γ may also contribute to pathogenesis.
Type 2 effector cells in AERD
Mast cells and eosinophils
IL-4, IL-5, IL-13, and IL-9 are known to drive accumulation of eosinophils and mast cells, which are primary sources of PGD2 and CysLTs and implicated in AERD. In the same study that first linked LTE4 to AERD, it was also demonstrated that nasal histamine levels were greater in aspirin intolerant patients compared to tolerant controls following aspirin challenge which suggested mast cell involvement in AERD.6 Consistent with this, another 1991 study reported that systemic mast cell activation occurred in a subset of aspirin intolerant patients after aspirin challenge.33 More recently, Cahill et al. provided evidence bolstering these early reports.34 AERD patients with high levels of mast cells exhibited increased symptom scores and reduced forced expiratory volume in one second (FEV1) following aspirin challenge. This subset of patients also had greater levels of the inflammatory mediators and metabolites, PGD2M and LTE4. Tissue eosinophil infiltration, a hallmark of type 2 allergic responses, are enriched in lung and nasal polyp tissue from aspirin intolerant compared to aspirin tolerant individuals and are also an important source of PGD2.35–37 Peripheral eosinophilia can also be found in AERD, but is reduced during aspirin challenge, indicating possible tissue migration from blood. Eosinophils derived from aspirin intolerant patients also produce more ECP, indicating an enhanced activation state.35 Thus, studies using human samples have revealed enhanced production of critical lipid mediators and accumulation and activation of mast cells and eosinophils in AERD patient tissue.
Platelets in AERD
Interestingly, platelets have also been implicated in AERD pathogenesis. Unlike mast cells and eosinophils, platelets do not possess the full machinery required to produce PGD2 and CysLTs. However, platelets express high levels LTC4-synthase and can form aggregates with 5-LO expressing white blood cells, a process that is required for pathogenesis in murine asthma models.38 Enriched levels of granulocyte-adherent platelets levels were detected in AERD polyp and blood samples and contributed significantly to LTC4 overproduction.38 Highlighting the importance of platelets in type 2 inflammation, platelet depletion in a murine asthma models significantly attenuated airway eosinophilia as well as ILC2 activation.39,40 More recently, platelets were also found to express functional IL-33 supporting a role for both lipid and alarmin contributions to inflammation in AERD.41
Activation and recruitment of ILC2s
Innate type 2 allergic airway inflammation
Elevated levels of epithelial cell derived cytokines and type 2 effector cells, coupled with an antigen-independent pathognomonic COX-1 inhibitor reactions, suggest that AERD pathogenesis may be orchestrated by an innate type 2 cytokine producing cell. ILC2s highly express the PGD2 receptor (CRTH2), are responsive to IL-33 and PGD2, and are potent producers of the type 2 cytokines IL-4, IL-5, IL-9, and IL-13.42–45 In mice, allergens induce release of ILC2-activating epithelial cell derived cytokines IL-33, IL-25, and TSLP (Figure 2). Upon stimulation, lung ILC2s rapidly drive eosinophilia, airway hyperresponsiveness, mucus hypersecretion, and mast cell accumulation independent of T and B cells. ILC2s are also enriched in nasal polyps from patients with the eosinophilic endotype, which is uniformly present in AERD.46,47 Further, the number of ILC2s is significantly greater in tissues from patients with allergic airway diseases compared to healthy controls and correlates with moresevere disease.48,49 ILC2s from allergic individuals also have greater cytokine producing and chemotactic potential compared to non-diseased controls.48,50
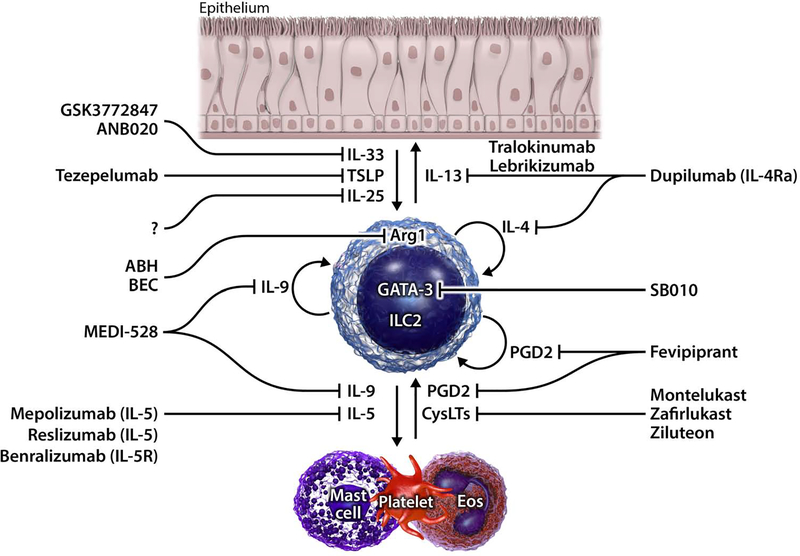
Figure 2. Proposed mechanisms and targets of ILC2-mediated inflammation in AERD.
In AERD, epithelial cytokines IL-33 and TSLP are increased in tissues and can stimulate ILC2s to produce IL-4, IL-5, IL-9, and IL-13. IL-13 promotes hyperresponsiveness and mucus production. IL-4 and IL-9 have autocrine effects on ILC2 activation in addition to IL-9 promoting mast cell accumulation. IL-5 stimulates eosinophil production and egress from bone marrow as well as contributing to recruitment and survival of eosinophils in tissues. In turn, eosinophils and mast cells produce PGD2 and CysLTs (that are elevated in AERD) which can activate ILC2s. PGD2 is also produced by ILC2s and has autocrine actions to promote cytokine production. ILC2s also highly express GATA-3 that is required for type 2 cytokine production. Approved and non-approved therapies that target various mediators in ILC2 driven inflammation are shown.
Eicosanoid regulation of ILC2s
In 2013, ILC2s were first shown to be activated by bioactive lipids.4,51 Our group demonstrated that murine ILC2s highly express CysLT1R and that CysLTs, particularly LTD4, potently induced ILC2 Th2 cytokine production and calcium flux.4 In a CysLT1R-dependent fashion, LTD4 elicited IL-5 and IL-13 production on par with IL-33, but also promoted IL-4 production unlike IL-33. These findings were later translated to human ILC2s, which also express CysLT1R.52 However, in humans LTE4, rather than LTD4, more potently promotes ILC2 survival and cytokine production. While original studies found ILC2 induction by CysLTs to be CysLT1R-dependent, a more recent study indicated that LTC4 activates ILC2s through a CysLT2R-dependent mechanism.53 The authors also reported that ILC2s were indirectly activated through a CysLT2R through LTC4 stimulation of IL-33 by alveolar type 2 cells. Overall, CysLTs which are highly expressed in AERD also potently activate ILC2s, suggesting a link with ILC2s in AERD pathogenesis.
Human ILC2s were first identified by their high expression of CRTH2, one of the receptors for PGD2.42 Three after the initial discovery that human CRTH2+ ILC2s, PGD2 was shown to induce migration and activation of ILC2s through CRTH2.54,55 Similar to LTs, PGD2 rapidly and potently induces IL-4, IL-5, and IL-13 production.4 In contrast, other members of the PG family can negatively regulate ILC2s. In a 2013 report, Barnig et al. demonstrated that Lipoxin A4 (LXA4) inhibits human peripheral blood ILC2 IL-13 production.51 While LXA4 concentrations are suppressed in some individuals with severe asthma, it remains unclear whether levels are further reduced in severe asthmatics with AERD. More recent studies have shown that PGI2 and PGE2 also suppress both human and mouse ILC2s, and PGE2.56–58, While PGE2 resistance has been described in AERD, whether this occurs specifically in ILC2s requires additional investigation. Importantly, two very recent studies showed that COX inhibition potentiates IL-33-induced murine ILC2 activation suggesting that the sum of prostaglandin effects are to inhibit ILC2 responses.17,59
Lipid enhancement of alarmin-induced ILC2 activation
In 2017, our group and others reported that lipid mediators could potentiate Il-33-induced activation of ILC2s suggesting non-redundant roles for lipids in ILC2 responses.60,61 Specifically, CysLTs enhanced IL-33-induced activation through an NFAT- and CysLT1R-depedent mechanism. These findings were later translated to human ILC2s as LTE4 synergized with epithelial derived cytokines (IL-33, IL-25, and TSLP) to further activate ILC2s.52 ILC2s achieved the highest level of activation when stimulated with the combination of PGD2, LTE4, and epithelial derived cytokines which are all implicatedin in AERD. IL-25 and IL-33 receptor transcript levels were also upregulated in the combined group, which may have contributed to the potentiation. In another study, LTC4 was found to both directly and indirectly activate ILC2s, the latter being through enhanced IL-33 release.53 A more recent report demonstrated that ILC2s may utilize lipid and cytokine synergistic activation to promote homeostasis.62 Interestingly, in this study ILC2s were shown to generate and respond to PGD2, which was necessary for activation by epithelial derived cytokines as CRTH2 inhibitors attenuated responses in isolated human ILC2s. Thus, lipids and cytokines important in AERD pathogenesis regulate ILC2 activation in multiple ways.
Tissue trafficking of ILC2s
In addition to the role AERD-related lipid and cytokine mediators play in ILC2 survival and activation, several of these mediators have also been shown to induce ILC2 migration in vitro. PGD2 induces ILC2 chemotaxis through CRTH2 in a dose-dependent fashion, and ILC2s from asthmatics and allergic individuals are more chemotactic both at baseline and after PGD2 treatment.4,54,55,63 IL-33 and IL-25 induce migration of human ILC2s, albeit more modestly than PGD2.54,63 CysLTs, particularly LTE4, promote ILC2 chemotaxis as well and LTE4 potentiates PGD2 induced migration, as it does for cytokine production.52 Other mediators have also recently been described to induce ILC2 migration. TGF-β increases the distance and speed of ILC2 travel and CCL8 was reported to drive ILC2 migration, in a dose- and CCR8-dependent fashion.63,64
While ILC2s are largely considered to be tissue resident cells that locally proliferate, the aforementioned chemotaxis studies have led researchers to investigate whether ILC2 migration plays a role in allergic airway disease pathogenesis. Initial studies investigating ILC2 migration in vivo demonstrated that intranasal PGD2 challenge induced peripheral ILC2 tafficking to the lungs in a CRTH2-dependent manner and revealed that CRTH2 expression was downregulated once ILC2s reached the lungs.65 More recently, systemic IL-33 treatment was also found to promote ILC2 migration to the lungs in a PGD2-dependent fashion.66
Evidence of ILC2 recruitment to the lung is not limited to artificial exogenous mediator-induced trafficking, but also includes migration invoked by clinically relevant allergens in animal models. Alternaria was shown to induce ILC2 progenitor egress from the bone marrow and ILC2 accumulation in the lung in an IL-33R-dependent fashion.67 Additionallly, our group demonstrated that inhibition of the β2 integrin adhesion receptor inhibited mouse ILC2 lung accumulation after Alternaria challenge without affecting ILC2 proliferation or apoptosis.68
More recently, Huang et al. published a seminal report detailing several novel aspects of ILC2 migration in vivo. The authors reported that ILC2s traffic through lymphatics and blood to distal tissues to participate in immune responses.69 Specifically, ILC2s were shown to traverse the gut to the lung. In vivo ILC2 migration was S1P-dependent as the S1P-antagonist FTY720 inhibited migration. This process may be relevant to AERD as gastrointestinal manifestations occur in a subset of patients. To support this idea, a study found that, compared to aspirin tolerant controls, AERD patients have greater levels of serum S1P, the levels of which positively correlate with the change in FEV1 following aspirin challenge.70 Thus, there are mulitple possible mechanisms by which ILC2 trafficking into tissues may occurr in AERD.
ILC2 accumulation in allergic rhinitis
Investigations into allergic airway diseases more broadly have provided insight into the potential role of ILC2s in AERD. At baseline, levels of blood ILC2s are greater in patients with allergic rhinitis (AR) compared to controls.48, 71 Moreover, ILC2s are further enriched in AR patient blood during allergy season.72,73 In 2014, we reported that nasal cat allergen challenge in sensitized patients elicited ILC2 expansion in PBMCs relative to control challenge.73 A more recent study with a similar design showed that nasal allergen challenge led to nasal tissue enrichment of ILC2s, the level of which positively correlated with symptom severity and markers of type 2 inflammation.74 Conversely, immunotherapy decreases ILC2 levels and symptom severity.72,75 While the kinetics of ILC2 mobilization to the nasal mucosa remain unclear, it is evident that ILC2s are recruited from distal tissues and accumulate at the site of environmental insult.
ILC2s in AERD
Since the discovery of ILC2s, numerous groups have found elevated levels of ILC2s in nasal polyps.42,46,76,77 On average, ILC2s are more enriched in nasal polyps compared to any other tissue. Until recently, no studies had specifically assessed changes in ILCs in AERD patients. In 2017, we reported that ILC2 recruitment occurs during AERD desensitization reactions.78 We assessed levels of ILC2s in blood and nasal mucosa samples from AERD patients before, during, and after COX-1 inhibitor challenges. ILC2s were significantly enriched in the mucosa and depleted in the blood during reactions compared to baseline, while control patient ILC2 levels were unaltered. Further, ILC2 levels positively correlated with urinary LTE4 and PGD2 concentrations as well as symptom severity. This study suggests that ILC2s traffic from the blood to the nose during aspirin challenge and may contribute to disease exacerbation.
Platelet aggregates and ILC2 activation
Nearly 20 years ago, mouse platelets were demonstrated to form aggregates with leukocytes.79 Without platelets, leukocyte migration into the lung after allergen exposure was severely attenuated. Recently, our group showed that platelet attachment to ILC2s occurs in mice.40 Depletion of platelets suppressed ILC2 cytokine production, indicating that platelets interact with ILC2s in order to support ILC2 homeostasis and function. Thus, platelets might promote ILC2 responses in the lung and nasal mucosa in AERD.
Pharmacological intervention of ILC2 pathways in AERD
Both conventional and potential novel treatments for AERD mirror those for asthma and CRSwNP. More traditional drugs include beta agonists and corticosteroids. In addition to their direct effects on airway physiology, beta agonists may also work through ILC2-intrinsic mechanisms (Figure 2). Human and mouse lung ILC2s were recently found to express the beta 2 adrenergic receptor. In a model of Alternaria-induced allergic airway inflammation, a beta agonist reduced ILC2 numbers and cytokine production.80 Corticoteroids are often the first line of therapy for patients with allergic airway diseases, yet some patients do not respond adequately.81 Importantly, a recent landmark study demonstrated that TSLP renders human ILC2s steroid resistant.82 Further, the level of TSLP positively correlated with the magnitude of steroid resistance in BAL ILC2s. Finally, the authors showed that steroid resistance could be reversed through MEK and STAT5 inhibition.
AERD therapy also includes the CysLT1R antagonists, montelukast and zafirlukast, and the 5-LO inhibitor, ziluteon. Montelukast also directly inhibits CysLT-induced ILC2 cytokine production.4,54 A recent study showed that a diet with a high ratio of omega-3 to omega-6 fatty acids reduced urinary LTE4 and PGD2M and improved overall symptom scores.83 ILC2 suppression could contribute to this effect as a 2015 study showed that ILC2 are inhibited by the omega-3 derivative, maresin-1.84 More recently, a clinical trial was conducted investigating whether prasugrel, an anti-platelet drug and potential ILC2-platelet aggregate inhibitor, would ameliorate aspirin induced reactions in AERD patients. While prasugrel lacked effectiveness in the total study cohort, subset analysis revealed complete abrogation of symptomology in patients with greater levels of platelet activation and less severe respiratory reactions to aspirin.85
Newly developed biologics and small molecules targeting various aspects of ILC2 biology have recently been shown to be efficacious in treating allergic airway disease. The anti-TSLP antibody tezepelumab reduces airway inflammation and limits exacerbations in asthmatics.86,87 Numerous CRTH2 antagonists were previously shown to improve lung function and reduce eosinophilia in asthmatics, though efficacy in recent trials have been disappointing.88–90 Dupilumab, an FDA-approved IL-4 receptor blocking antibody that inhibits both IL-13 as well as IL-4 signaling, was shown to be efficacious in asthmatics and patients with nasal polyposis.91 The anti-IL-5 antibody mepolizumab is an effective treatment for eosinophilic asthma and hypereosinophilic syndromes.92–95 IL-13 blockade with lebrikizumab or tralokinumab yielded limited improvements in lung function, but studies for efficacy are ongoing.96,97 Finally, a small clinical trial in asthmatics found that SB010, a novel DNA enzyme that inhibits GATA-3 prevented reductions in FEV1, as well as reduced plasma IL-5, sputum eosinophilia and tryptase levels.98
Several additional compounds targeting pro-ILC2 mediators and ILC2 receptors, enzymes, cytokines are currently being developed. Antibodies that block IL-25 signaling reduce airway inflammation, hyperresponsiveness, and polyposis in animal models.99–101 MEDI-528, a humanized IL-9 blocking antibody is effective in murine asthma models, however its effect in humans thus far is inconclusive and requires additional investigation.102 Mouse and human ILC2s require the metabolic enzyme arginase-1 (Arg1) to function, and removal of ILC Arg1 attenuates allergic airway inflammation in mice.103 Arginase inhibitors are effective in asthma models and are currently being trialed as cancer therapeutics.104 More recently, a CRTH2-depletion antibody was shown to eliminate ILC2s, eosinophils, and Th2 cells in a humanized CRTH2 mouse model105. Finally, IL-33 signaling blocking antibodies are currently in development for treatment of allergic disease.106
ILC2s in mouse models of AERD
Investigation into AERD pathogenesis has been limited to human samples for decades as the disease is difficult to model in mice. While murine asthma models have shed light on the pathogenesis of type 2 allergic airway inflammation in general, AERD is unique due to the pathognomonic COX-1 inhibitor sensitivity. In 2013, the first AERD mouse model was described.107 The authors identifed prostaglandin E-synthase deficient mice as being AERD-like. Aspirin administration to house dust mite challenged PGE-synthase deficient mice enhanced levels of mast cell activation, CysLT production, and airway hyperresponsiveness. In a later study, AERD-like mice were found to have elevated airway epithelium IL-33 levels and increased lung eosinophilia.24 More recently, ILC2s were reportedly enriched threefold in the lungs of AERD mice relative to WT mice, and that ILC2 depletion nearly abrogated the response to aspirin.53 Thus, some features of AERD can be modeled in mice, and such models could help decipher mechanisms of AERD pathogenesis, including the roles of ILC2s, and test newly developed pharmacological agents.
Conclusion
In summary, AERD is a complex disease though recent studies have provided great insight into the pathogenesis. Proinflammatory bioactive lipids and type two cytokines are particularly high in tissues from AERD patients and levels are further increased during pathognomonic reactions to COX-1 inhibition. These hallmarks position ILC2s, central drivers of innate type 2 airway inflammation in animal models, as potential orchestrators of AERD pathophysiology. This model is further supported by recent findings of rapid ILC2 recruitment to the airways following COX-1 inhibitor challenge. Future studies are needed to more fully characterize ILC2s in AERD patients longitudinally across tissues, at baseline, and after COX-1 inhibitor therapy. Additionally, studies investigating mechanisms of ILC2 recruitment and activation in newly described AERD mouse models are warranted. Such research may lead to the identification of novel therapeutic targets for not only AERD, but all allergic airway diseases that involve pathogeneic ILC2 responses.
Key Messages:
AERD patients have elevated levels of pro-inflammatory lipids and cytokines in nasal tissue at baseline, and levels increase further after COX-1 inhibitor challenge
Following COX-1 inhibitor challenge, ILC2s decrease in the blood and increase in the nasal mucosa
Lipid and cytokine mediators induce ILC2 migration in vivo
A recently described mouse model recapitulating features of AERD demonstrates pathognomonic COX-1 inhibitor reactions are ILC2-dependent
Novel therapeutics in the developmental pipeline for AERD and other allergic airway diseases target ILC2-mediated processes
Acknowledgments
T.A.D. is supported by NIH grants AI 114585 and AI 070535 as well as Veterans Affairs Merit Review Award BX005073.
K.J.C. is supported by NIH grant T32 DK007202
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.White AA, Stevenson DD. Aspirin-Exacerbated Respiratory Disease. The New England journal of medicine 2018;379:1060–70. [DOI] [PubMed] [Google Scholar]
- 2.Cavagnero K, Doherty TA. Cytokine and Lipid Mediator Regulation of Group 2 Innate Lymphoid Cells (ILC2s) in Human Allergic Airway Disease. J Cytokine Biol 2017;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lund SJ, Portillo A, Cavagnero K, et al. Leukotriene C4 Potentiates IL-33-Induced Group 2 Innate Lymphoid Cell Activation and Lung Inflammation. J Immunol 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doherty TA, Khorram N, Lund S, Mehta AK, Croft M, Broide DH. Lung type 2 innate lymphoid cells express cysteinyl leukotriene receptor 1, which regulates TH2 cytokine production. The Journal of allergy and clinical immunology 2013;132:205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salimi M, Stoger L, Liu W, et al. Cysteinyl leukotriene E4 activates human group 2 innate lymphoid cells and enhances the effect of prostaglandin D2 and epithelial cytokines. The Journal of allergy and clinical immunology 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferreri NR, Howland WC, Stevenson DD, Spiegelberg HL. Release of leukotrienes, prostaglandins, and histamine into nasal secretions of aspirin-sensitive asthmatics during reaction to aspirin. The American review of respiratory disease 1988;137:847–54. [DOI] [PubMed] [Google Scholar]
- 7.Weiss JW, Drazen JM, Coles N, et al. Bronchoconstrictor effects of leukotriene C in humans. Science (New York, NY 1982;216:196–8. [DOI] [PubMed] [Google Scholar]
- 8.Christie PE, Tagari P, Ford-Hutchinson AW, et al. Urinary leukotriene E4 concentrations increase after aspirin challenge in aspirin-sensitive asthmatic subjects. The American review of respiratory disease 1991;143:1025–9. [DOI] [PubMed] [Google Scholar]
- 9.Mita H, Higashi N, Taniguchi M, Higashi A, Akiyama K. Increase in urinary leukotriene B4 glucuronide concentration in patients with aspirin-intolerant asthma after intravenous aspirin challenge. Clin Exp Allergy 2004;34:1262–9. [DOI] [PubMed] [Google Scholar]
- 10.Fajt ML, Gelhaus SL, Freeman B, et al. Prostaglandin D2 pathway upregulation: relation to asthma severity, control, and TH2 inflammation. The Journal of allergy and clinical immunology 2013;131:1504–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnston SL, Freezer NJ, Ritter W, O’Toole S, Howarth PH. Prostaglandin D2-induced bronchoconstriction is mediated only in part by the thromboxane prostanoid receptor. Eur Respir J 1995;8:411–5. [DOI] [PubMed] [Google Scholar]
- 12.Yoshimura T, Yoshikawa M, Otori N, Haruna S, Moriyama H. Correlation between the prostaglandin D(2)/E(2) ratio in nasal polyps and the recalcitrant pathophysiology of chronic rhinosinusitis associated with bronchial asthma. Allergol Int 2008;57:429–36. [DOI] [PubMed] [Google Scholar]
- 13.Cahill KN, Bensko JC, Boyce JA, Laidlaw TM. Prostaglandin D(2): a dominant mediator of aspirin-exacerbated respiratory disease. The Journal of allergy and clinical immunology 2015;135:245–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang JE, Doherty TA, Baum R, Broide D. Prostaglandin D2 regulates human type 2 innate lymphoid cell chemotaxis. The Journal of allergy and clinical immunology 2014;133:899–901 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xue L, Salimi M, Panse I, et al. Prostaglandin D activates group 2 innate lymphoid cells through chemoattractant receptor-homologous molecule expressed on T2 cells. The Journal of allergy and clinical immunology 2014;133:1184–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pavord ID, Tattersfield AE. Bronchoprotective role for endogenous prostaglandin E2. Lancet 1995;345:436–8. [DOI] [PubMed] [Google Scholar]
- 17.Zhou W, Zhang J, Toki S, et al. COX Inhibition Increases Alternaria-Induced Pulmonary Group 2 Innate Lymphoid Cell Responses and IL-33 Release in Mice . J Immunol 2020;205:1157–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flamand N, Surette ME, Picard S, Bourgoin S, Borgeat P. Cyclic AMP-mediated inhibition of 5-lipoxygenase translocation and leukotriene biosynthesis in human neutrophils. Mol Pharmacol 2002;62:250–6. [DOI] [PubMed] [Google Scholar]
- 19.Roca-Ferrer J, Garcia-Garcia FJ, Pereda J, et al. Reduced expression of COXs and production of prostaglandin E(2) in patients with nasal polyps with or without aspirin-intolerant asthma. The Journal of allergy and clinical immunology 2011;128:66–72.e1. [DOI] [PubMed] [Google Scholar]
- 20.Laidlaw TM, Cutler AJ, Kidder MS, et al. Prostaglandin E2 resistance in granulocytes from patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol 2014;133:1692–701.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ying S, Meng Q, Scadding G, Parikh A, Corrigan CJ, Lee TH. Aspirin-sensitive rhinosinusitis is associated with reduced E-prostanoid 2 receptor expression on nasal mucosal inflammatory cells. The Journal of allergy and clinical immunology 2006;117:312–8. [DOI] [PubMed] [Google Scholar]
- 22.Corrigan CJ, Napoli RL, Meng Q, et al. Reduced expression of the prostaglandin E2 receptor E-prostanoid 2 on bronchial mucosal leukocytes in patients with aspirin-sensitive asthma. The Journal of allergy and clinical immunology 2012;129:1636–46. [DOI] [PubMed] [Google Scholar]
- 23.Maric JRA, Mazzurana L, Bjorklund AK, Acker AV, Rao A, Friberg D, Dahlen S, Heinemann A, Konya V, Mjosberg J. PGE suppresses human group 2 innate lymphoid cell function The Journal of allergy and clinical immunology 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu T, Kanaoka Y, Barrett NA, et al. Aspirin-Exacerbated Respiratory Disease Involves a Cysteinyl Leukotriene-Driven IL-33-Mediated Mast Cell Activation Pathway. J Immunol 2015;195:3537–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buchheit KM, Cahill KN, Katz HR, et al. Thymic stromal lymphopoietin controls prostaglandin D2 generation in patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol 2016;137:1566–76.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee JU, Chang HS, Lee HJ, et al. Association of interleukin-25 levels with development of aspirin induced respiratory diseases. Respiratory medicine 2017;123:71–8. [DOI] [PubMed] [Google Scholar]
- 27.Steinke JW, Liu L, Huyett P, Negri J, Payne SC, Borish L. Prominent role of IFN-γ in patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol 2013;132:856–65.e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamilos DL, Leung DY, Huston DP, Kamil A, Wood R, Hamid Q. GM-CSF, IL-5 and RANTES immunoreactivity and mRNA expression in chronic hyperplastic sinusitis with nasal polyposis (NP). Clin Exp Allergy 1998;28:1145–52. [DOI] [PubMed] [Google Scholar]
- 29.Bachert C, Wagenmann M, Hauser U, Rudack C. IL-5 synthesis is upregulated in human nasal polyp tissue. The Journal of allergy and clinical immunology 1997;99:837–42. [DOI] [PubMed] [Google Scholar]
- 30.Riechelmann H, Deutschle T, Rozsasi A, Keck T, Polzehl D, Bürner H. Nasal biomarker profiles in acute and chronic rhinosinusitis. Clin Exp Allergy 2005;35:1186–91. [DOI] [PubMed] [Google Scholar]
- 31.Van Bruaene N, Pérez-Novo CA, Basinski TM, et al. T-cell regulation in chronic paranasal sinus disease. The Journal of allergy and clinical immunology 2008;121:1435–41, 41.e1–3. [DOI] [PubMed] [Google Scholar]
- 32.Olcott CM, Han JK, Cunningham TD, Franzese CB. Interleukin-9 and interleukin-17C in chronic rhinosinusitis. Int Forum Allergy Rhinol 2016;6:841–7. [DOI] [PubMed] [Google Scholar]
- 33.Bosso JV, Schwartz LB, Stevenson DD. Tryptase and histamine release during aspirin-induced respiratory reactions. The Journal of allergy and clinical immunology 1991;88:830–7. [DOI] [PubMed] [Google Scholar]
- 34.Cahill KN, Murphy K, Singer J, Israel E, Boyce JA, Laidlaw TM. Plasma tryptase elevation during aspirin-induced reactions in aspirin-exacerbated respiratory disease. The Journal of allergy and clinical immunology 2019;143:799–803.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cowburn AS, Sladek K, Soja J, et al. Overexpression of leukotriene C4 synthase in bronchial biopsies from patients with aspirin-intolerant asthma. The Journal of clinical investigation 1998;101:834–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feng X, Ramsden MK, Negri J, et al. Eosinophil production of prostaglandin D2 in patients with aspirin-exacerbated respiratory disease. The Journal of allergy and clinical immunology 2016;138:1089–97 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Payne SC, Early SB, Huyett P, Han JK, Borish L, Steinke JW. Evidence for distinct histologic profile of nasal polyps with and without eosinophilia. The Laryngoscope 2011;121:2262–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laidlaw TM, Kidder MS, Bhattacharyya N, et al. Cysteinyl leukotriene overproduction in aspirin-exacerbated respiratory disease is driven by platelet-adherent leukocytes. Blood 2012;119:3790–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paruchuri S, Tashimo H, Feng C, et al. Leukotriene E4-induced pulmonary inflammation is mediated by the P2Y12 receptor. The Journal of experimental medicine 2009;206:2543–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karta MR, Cavagnero K, Miller M, et al. Platelets attach to lung ILC2 expressing PSGL-1 and influence ILC2 function. The Journal of allergy and clinical immunology 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takeda T, Unno H, Morita H, et al. Platelets constitutively express IL-33 protein and modulate eosinophilic airway inflammation. The Journal of allergy and clinical immunology 2016;138:1395–403.e6. [DOI] [PubMed] [Google Scholar]
- 42.Mjosberg JM, Trifari S, Crellin NK, et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol 2011;12:1055–62. [DOI] [PubMed] [Google Scholar]
- 43.Moro K, Yamada T, Tanabe M, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature 2010;463:540–4. [DOI] [PubMed] [Google Scholar]
- 44.Neill DR, Wong SH, Bellosi A, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 2010;464:1367–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Price AE, Liang HE, Sullivan BM, et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proceedings of the National Academy of Sciences of the United States of America 2010;107:11489–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walford HH, Lund SJ, Baum RE, et al. Increased ILC2s in the eosinophilic nasal polyp endotype are associated with corticosteroid responsiveness. Clinical immunology (Orlando, Fla 2014;155:126–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Poposki JA, Klingler AI, Tan BK, et al. Group 2 innate lymphoid cells are elevated and activated in chronic rhinosinusitis with nasal polyps. Immun Inflamm Dis 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bartemes KR, Kephart GM, Fox SJ, Kita H. Enhanced innate type 2 immune response in peripheral blood from patients with asthma. J Allergy Clin Immunol 2014;134:671–8.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith SG, Chen R, Kjarsgaard M, et al. Increased numbers of activated group 2 innate lymphoid cells in the airways of patients with severe asthma and persistent airway eosinophilia. J Allergy Clin Immunol 2016;137:75–86.e8. [DOI] [PubMed] [Google Scholar]
- 50.Chang JE, Doherty TA, Baum R, Broide D. Prostaglandin D2 regulates human type 2 innate lymphoid cell chemotaxis. J Allergy Clin Immunol 2014;133:899–901.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barnig C, Cernadas M, Dutile S, et al. Lipoxin A4 regulates natural killer cell and type 2 innate lymphoid cell activation in asthma. Sci Transl Med 2013;5:174ra26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salimi M, Stoger L, Liu W, et al. Cysteinyl leukotriene E4 activates human group 2 innate lymphoid cells and enhances the effect of prostaglandin D2 and epithelial cytokines. J Allergy Clin Immunol 2017;140:1090–100.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu T, Barrett NA, Kanaoka Y, et al. Type 2 Cysteinyl Leukotriene Receptors Drive IL-33-Dependent Type 2 Immunopathology and Aspirin Sensitivity. J Immunol 2018;200:915–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xue L, Salimi M, Panse I, et al. Prostaglandin D2 activates group 2 innate lymphoid cells through chemoattractant receptor-homologous molecule expressed on TH2 cells. The Journal of allergy and clinical immunology 2014;133:1184–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chang JE, Doherty TA, Baum R, Broide D. Prostaglandin D2 regulates human type 2 innate lymphoid cell chemotaxis. The Journal of allergy and clinical immunology 2013;S0091–6749(13)01464–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou W, Toki S, Zhang J, et al. Prostaglandin I2 Signaling and Inhibition of Group 2 Innate Lymphoid Cell Responses. American journal of respiratory and critical care medicine 2016;193:31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou Y, Wang W, Zhao C, et al. Prostaglandin E2 Inhibits Group 2 Innate Lymphoid Cell Activation and Allergic Airway Inflammation Through E-Prostanoid 4-Cyclic Adenosine Monophosphate Signaling. Front Immunol 2018;9:501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maric J, Ravindran A, Mazzurana L, et al. Prostaglandin E2 suppresses human group 2 innate lymphoid cell function. The Journal of allergy and clinical immunology 2018;141:1761–73 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maruyama N, Takai T, Kamijo S, et al. Cyclooxygenase inhibition in mice heightens adaptive- and innate-type responses against inhaled protease allergen and IL-33. Allergy 2019;74:2237–40. [DOI] [PubMed] [Google Scholar]
- 60.Lund SJ, Portillo A, Cavagnero K, et al. Leukotriene C4 Potentiates IL-33-Induced Group 2 Innate Lymphoid Cell Activation and Lung Inflammation. J Immunol 2017;199:1096–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.von Moltke J, O’Leary CE, Barrett NA, Kanaoka Y, Austen KF, Locksley RM. Leukotrienes provide an NFAT-dependent signal that synergizes with IL-33 to activate ILC2s. The Journal of experimental medicine 2017;214:27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maric J, Ravindran A, Mazzurana L, et al. Cytokine-induced endogenous production of prostaglandin D2 is essential for human group 2 innate lymphoid cell activation. The Journal of allergy and clinical immunology 2019;143:2202–14.e5. [DOI] [PubMed] [Google Scholar]
- 63.Puttur F, Denney L, Gregory LG, et al. Pulmonary environmental cues drive group 2 innate lymphoid cell dynamics in mice and humans. Sci Immunol 2019;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Denney L, Byrne AJ, Shea TJ, et al. Pulmonary Epithelial Cell-Derived Cytokine TGF-beta1 Is a Critical Cofactor for Enhanced Innate Lymphoid Cell Function. Immunity 2015;43:945–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wojno ED, Monticelli LA, Tran SV, et al. The prostaglandin D(2) receptor CRTH2 regulates accumulation of group 2 innate lymphoid cells in the inflamed lung. Mucosal immunology 2015;8:1313–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Oyesola OO, Duque C, Huang LC, et al. The Prostaglandin D2 Receptor CRTH2 Promotes IL-33-Induced ILC2 Accumulation in the Lung. J Immunol 2020;204:1001–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stier MT, Zhang J, Goleniewska K, et al. IL-33 promotes the egress of group 2 innate lymphoid cells from the bone marrow. The Journal of experimental medicine 2018;215:263–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Karta MR, Rosenthal PS, Beppu A, et al. beta2 integrins rather than beta1 integrins mediate Alternaria-induced group 2 innate lymphoid cell trafficking to the lung. The Journal of allergy and clinical immunology 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang Y, Mao K, Chen X, et al. S1P-dependent interorgan trafficking of group 2 innate lymphoid cells supports host defense. Science (New York, NY 2018;359:114–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Trinh HK, Kim SC, Cho K, et al. Exploration of the Sphingolipid Metabolite, Sphingosine-1-phosphate and Sphingosine, as Novel Biomarkers for Aspirin-exacerbated Respiratory Disease. Sci Rep 2016;6:36599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhong H, Fan XL, Yu QN, et al. Increased innate type 2 immune response in house dust mite-allergic patients with allergic rhinitis. Clinical immunology (Orlando, Fla 2017;183:293–9. [DOI] [PubMed] [Google Scholar]
- 72.Lao-Araya M, Steveling E, Scadding GW, Durham SR, Shamji MH. Seasonal increases in peripheral innate lymphoid type 2 cells are inhibited by subcutaneous grass pollen immunotherapy. The Journal of allergy and clinical immunology 2014;134:1193–5.e4. [DOI] [PubMed] [Google Scholar]
- 73.Doherty TA, Scott D, Walford HH, et al. Allergen challenge in allergic rhinitis rapidly induces increased peripheral blood type 2 innate lymphoid cells that express CD84. J Allergy Clin Immunol 2014;133:1203–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dhariwal J, Cameron A, Trujillo-Torralbo MB, et al. Mucosal Type 2 Innate Lymphoid Cells Are a Key Component of the Allergic Response to Aeroallergens. Am J Respir Crit Care Med 2017;195:1586–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fan D, Wang X, Wang M, et al. Allergen-Dependent Differences in ILC2s Frequencies in Patients With Allergic Rhinitis. Allergy Asthma Immunol Res 2016;8:216–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Miljkovic D, Bassiouni A, Cooksley C, et al. Association between group 2 innate lymphoid cells enrichment, nasal polyps and allergy in chronic rhinosinusitis. Allergy 2014;69:1154–61. [DOI] [PubMed] [Google Scholar]
- 77.Ho J, Bailey M, Zaunders J, et al. Group 2 innate lymphoid cells (ILC2s) are increased in chronic rhinosinusitis with nasal polyps or eosinophilia. Clin Exp Allergy 2015;45:394–403. [DOI] [PubMed] [Google Scholar]
- 78.Eastman JJ, Cavagnero KJ, Deconde AS, et al. Group 2 innate lymphoid cells are recruited to the nasal mucosa in patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol 2017;140:101–8.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pitchford SC, Yano H, Lever R, et al. Platelets are essential for leukocyte recruitment in allergic inflammation. The Journal of allergy and clinical immunology 2003;112:109–18. [DOI] [PubMed] [Google Scholar]
- 80.Moriyama S, Brestoff JR, Flamar AL, et al. β(2)-adrenergic receptor-mediated negative regulation of group 2 innate lymphoid cell responses. Science (New York, NY 2018;359:1056–61. [DOI] [PubMed] [Google Scholar]
- 81.Martin RJ, Szefler SJ, King TS, et al. The Predicting Response to Inhaled Corticosteroid Efficacy (PRICE) trial. The Journal of allergy and clinical immunology 2007;119:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu S, Verma M, Michalec L, et al. Steroid resistance of airway type 2 innate lymphoid cells from patients with severe asthma: The role of thymic stromal lymphopoietin. The Journal of allergy and clinical immunology 2018;141:257–68.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schneider TR, Johns CB, Palumbo ML, Murphy KC, Cahill KN, Laidlaw TM. Dietary Fatty Acid Modification for the Treatment of Aspirin-Exacerbated Respiratory Disease: A Prospective Pilot Trial. The journal of allergy and clinical immunology In practice 2018;6:825–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Krishnamoorthy N, Burkett PR, Dalli J, et al. Cutting edge: maresin-1 engages regulatory T cells to limit type 2 innate lymphoid cell activation and promote resolution of lung inflammation. J Immunol 2015;194:863–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Laidlaw TM, Cahill KN, Cardet JC, et al. A trial of type 12 purinergic (P2Y(12)) receptor inhibition with prasugrel identifies a potentially distinct endotype of patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol 2019;143:316–24.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Corren J, Parnes JR, Wang L, et al. Tezepelumab in Adults with Uncontrolled Asthma. The New England journal of medicine 2017;377:936–46. [DOI] [PubMed] [Google Scholar]
- 87.Gauvreau GM, O’Byrne PM, Boulet LP, et al. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. The New England journal of medicine 2014;370:2102–10. [DOI] [PubMed] [Google Scholar]
- 88.Kuna P, Bjermer L, Tornling G. Two Phase II randomized trials on the CRTh2 antagonist AZD1981 in adults with asthma. Drug Des Devel Ther 2016;10:2759–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hall IP, Fowler AV, Gupta A, et al. Efficacy of BI 671800, an oral CRTH2 antagonist, in poorly controlled asthma as sole controller and in the presence of inhaled corticosteroid treatment. Pulmonary pharmacology & therapeutics 2015;32:37–44. [DOI] [PubMed] [Google Scholar]
- 90.Barnes N, Pavord I, Chuchalin A, et al. A randomized, double-blind, placebo-controlled study of the CRTH2 antagonist OC000459 in moderate persistent asthma. Clin Exp Allergy 2012;42:38–48. [DOI] [PubMed] [Google Scholar]
- 91.Wenzel S, Castro M, Corren J, et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting β2 agonist: a randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet 2016;388:31–44. [DOI] [PubMed] [Google Scholar]
- 92.Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. The New England journal of medicine 2014;371:1198–207. [DOI] [PubMed] [Google Scholar]
- 93.Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet 2012;380:651–9. [DOI] [PubMed] [Google Scholar]
- 94.Busse WW, Ring J, Huss-Marp J, Kahn JE. A review of treatment with mepolizumab, an anti-IL-5 mAb, in hypereosinophilic syndromes and asthma. The Journal of allergy and clinical immunology 2010;125:803–13. [DOI] [PubMed] [Google Scholar]
- 95.Rothenberg ME, Klion AD, Roufosse FE, et al. Treatment of patients with the hypereosinophilic syndrome with mepolizumab. The New England journal of medicine 2008;358:1215–28. [DOI] [PubMed] [Google Scholar]
- 96.Hanania NA, Korenblat P, Chapman KR, et al. Efficacy and safety of lebrikizumab in patients with uncontrolled asthma (LAVOLTA I and LAVOLTA II): replicate, phase 3, randomised, double-blind, placebo-controlled trials. Lancet Respir Med 2016;4:781–96. [DOI] [PubMed] [Google Scholar]
- 97.Brightling CE, Chanez P, Leigh R, et al. Efficacy and safety of tralokinumab in patients with severe uncontrolled asthma: a randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Respir Med 2015;3:692–701. [DOI] [PubMed] [Google Scholar]
- 98.Krug N, Hohlfeld JM, Kirsten AM, et al. Allergen-induced asthmatic responses modified by a GATA3-specific DNAzyme. The New England journal of medicine 2015;372:1987–95. [DOI] [PubMed] [Google Scholar]
- 99.Ballantyne SJ, Barlow JL, Jolin HE, et al. Blocking IL-25 prevents airway hyperresponsiveness in allergic asthma. The Journal of allergy and clinical immunology 2007;120:1324–31. [DOI] [PubMed] [Google Scholar]
- 100.Beale J, Jayaraman A, Jackson DJ, et al. Rhinovirus-induced IL-25 in asthma exacerbation drives type 2 immunity and allergic pulmonary inflammation. Sci Transl Med 2014;6:256ra134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shin HW, Kim DK, Park MH, et al. IL-25 as a novel therapeutic target in nasal polyps of patients with chronic rhinosinusitis. The Journal of allergy and clinical immunology 2015;135:1476–85.e7. [DOI] [PubMed] [Google Scholar]
- 102.Antoniu SA. MEDI-528, an anti-IL-9 humanized antibody for the treatment of asthma. Curr Opin Mol Ther 2010;12:233–9. [PubMed] [Google Scholar]
- 103.Monticelli LA, Buck MD, Flamar AL, et al. Arginase 1 is an innate lymphoid-cell-intrinsic metabolic checkpoint controlling type 2 inflammation. Nature immunology 2016;17:656–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bratt JM, Zeki AA, Last JA, Kenyon NJ. Competitive metabolism of L-arginine: arginase as a therapeutic target in asthma. J Biomed Res 2011;25:299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Huang T, Hazen M, Shang Y, et al. Depletion of major pathogenic cells in asthma by targeting CRTh2. JCI Insight 2016;1:e86689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Scott IC, Houslay KF, Cohen ES. Prospects to translate the biology of IL-33 and ST2 during organ transplantation into therapeutics to treat graft-versus-host disease. Ann Transl Med 2016;4:500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu T, Laidlaw TM, Katz HR, Boyce JA. Prostaglandin E2 deficiency causes a phenotype of aspirin sensitivity that depends on platelets and cysteinyl leukotrienes. Proceedings of the National Academy of Sciences of the United States of America 2013;110:16987–92. [DOI] [PMC free article] [PubMed] [Google Scholar]