Summary
The way in which aberrant neural circuits contribute to epilepsy remains unclear. To elucidate this question, we dissected the circuit mechanisms underlying epileptogenesis using a mouse model of focal cortical malformation with spontaneous epileptiform discharges. We found that spontaneous spike-wave discharges and optogenetically induced hyperexcitable bursts in vivo were present in a cortical region distal to (>0.7 mm) freeze-lesion induced microgyrus, instead of near the microgyrus. ChR2-assisted circuit mapping revealed ectopic interlaminar excitatory input from infragranular layers to layers 2/3 pyramidal neurons as the key component of hyperexcitable circuitry. This hyperactivity disrupted the balance between excitation and inhibition and was more prominent in the cortical region distal to the microgyrus. Consistently, the inhibition from both parvalbumin-positive interneurons (PV) and somatostatin-positive interneurons (SOM) to pyramidal neurons were altered in a layer- and site-specific fashion. Finally, closed-loop optogenetic stimulation of SOM, but not PV, terminated spontaneous spike-wave discharges. Together, these results demonstrate the occurrence of highly site- and cell-type specific synaptic reorganization underlying epileptic cortical circuits and provide new insights into potential treatment strategies.
Keywords: Epilepsy, Channelrhodopsin-2, neocortex, interneuron, spike-wave discharge, microcircuit, cortical malformation
In Brief
Yang et al. report key features of the circuit rewiring that contributes to epiletogenesis in a mouse model of cortical malformation. The authors further demonstrate that spontaneous spike-wave discharges can be curtailed by selectively activating somatostatin interneurons in a small cortical region distal to the microgyrus.
Introduction
A significant portion of intractable epilepsy in patients is intimately associated with the malformation of cortical development (MCD)[1–4]. Polymicrogyria (PMG) is one among the most common forms of MCD in human epilepsy patients and often occurs as an isolated brain malformation [5–8]. A typical characteristic found in human PMG patients is a four-layered abnormal lamination surrounded by normal-appearing six-layered cortical tissue [9, 10]. Patients with PMG are highly prone to refractory epilepsy (60% to 85%), and surgical removal of the microgyrus area only generates positive outcomes for <50% of patients with intractable epilepsy, mainly due to PMG [7, 8, 11]. A more effective treatment requires a deeper understanding of how neural circuits become rewired into a highly epileptogenic neural network.
Rodent models of PMG by environmental or genetic manipulation capture some essential features of human patients [12, 13]. Dvorak et al. first induced experimental microgyri in postnatal day zero rats via transcranial freeze lesion (FL) [14, 15]. Subsequent studies in brain slices demonstrated that epileptiform activities are capable of propagating over a long distance and were initiated in cortical regions adjacent to the experimentally induced microgyrus, but not in the microgyrus itself [16, 17]. However, electrographic or behavioral seizures were not found to be present in FL treated rats [18–21], at least, without a provoking event [22]. We recently developed a mouse FL model of MCD and demonstrated continuous spontaneous spike-wave discharges in both anesthetized and freely behaving mice [23–25]. Noticeably, pathological high frequency oscillations, which have been used as a reliable biomarker for identifying seizure-generating zones [26, 27] in resection surgical treatment of refractory epilepsies [28], were elevated in the superficial cortical area ~500 μm to the microgyrus in this model [23]. These studies suggest that the mouse PMG by FL may serve as a valuable model to study the circuit mechanisms underlying epileptogenesis.
To tackle the question of how specific circuits become rewired to give rise to epiletogenesis, we used ChR2-assisted circuit mapping (CRACM) to identify aberrant synapses and assess their E/I balance [29]. We then combined optogenetic stimulations with linear microelectrode array recordings in vivo and found that the epileptogenesis site was about >0.7 mm distal to the microgyrus, which was confirmed by CRACM analysis. Strikingly, an unexpected aberrant L5B ➔ L2/3 input, located distal to the microgyrus, contributed significantly to the abnormal hyperexcitable circuit. Concurrently, two major cortical interneurons (INs), PV and SOM, underwent site-specific reorganization that were consistent with their contributions to the E/I imbalance. Closed-loop optogenetic stimulation of SOM, but not PV, terminated spontaneous spike-wave discharges. Together, these experiments indicate that balanced synaptic excitation and inhibition becomes interrupted in a site-, layer- and cell type-specific manner in the somatosensory cortex of FL-induced epileptic mice. We further hypothesize that these abnormal local circuits are the key to understanding epileptogenesis in this mouse model.
Results
Reversed layer-specific E/I balance in epileptic mice, primarily due to de novo inter-laminal excitatory inputs to L2/3 neurons
We first examined the spontaneous excitatory or inhibitory postsynaptic currents in FL mice (Figure S1A). These results showed that the neurons in FL mice exhibited a significant increase in EPSCs frequency (unpaired t test, P = 0.045, Figure S1A). These mice also tended to receive stronger EPSCs compared to the neurons in sham mice (unpaired t test, P = 0.18), suggesting the FL mice were in a hyperexcitable state as previously shown (Sun et al., 2016; Williams and Sun, 2019). The amplitude but not frequency of IPSCs increased significantly in the FL group (unpaired t test, P = 0.041, Figure S1A).
To uncover the prominent changes associated with epileptic circuits, we sought to examine the properties of canonical circuits within the barrel field of the somatosensory cortex (vS1), but not in the microgyrus, using the CRACM approach in brain slices [29–32]. We used CRACM methods to map local E/I ratios in both L2/3 and L5B pyramidal cells (PCs), the major cortical outputs to other brain regions [33–35]. Injected AAV-ChR2 virus transfected both excitatory and inhibitory neurons in vS1, typically covering multiple barrel columns (Figures 1A, Methods). This allows us to study the effects of local optogenetic activations of inputs to recorded PCs. Stimuli were delivered on a photo-stimulation grid (16 × 12, 75 μm spacing) covering a 1.44 mm2 area surrounding the recorded neuron (Figures 1A).
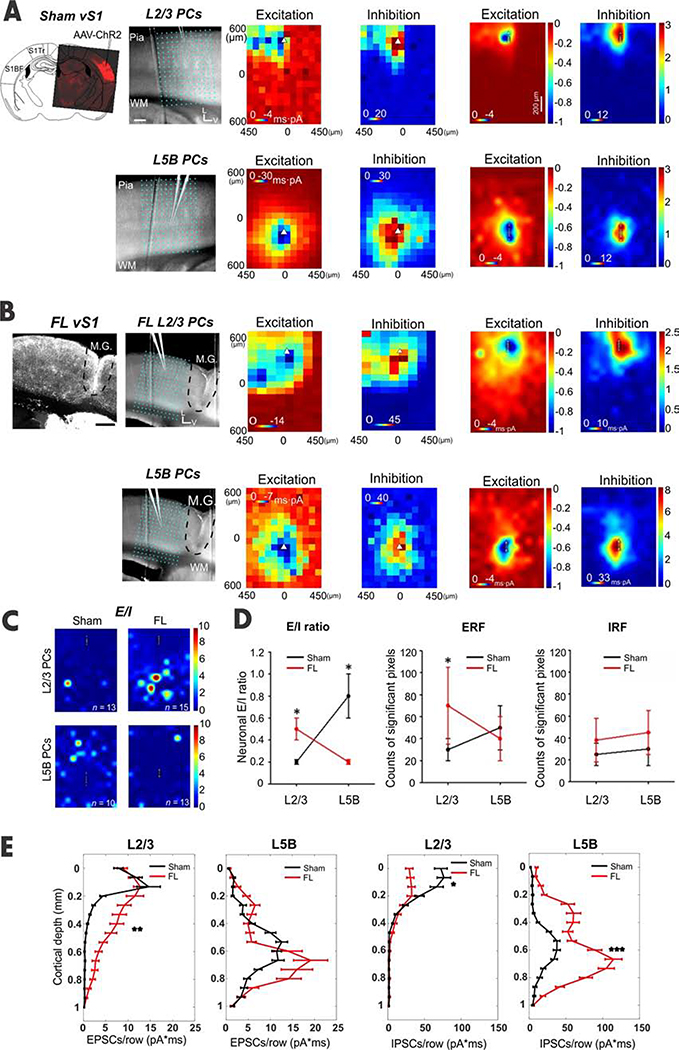
Figure 1. Aberrant synaptic inputs to superficial PCs in S1.
(A) CRACM of EPSCs and IPSCs in L2/3 and L5B PCs in sham mice. Top Left: confocal image of AAV-ChR2 virus expression pattern in vS1; Right: an overlay of a brain slice with a 16-by-12 photo-stimulation grid illustrates the photo-stimulation and recording sites for each group. Other panels: an example of CRACM maps of excitatory synaptic inputs (“Excitation”) and inhibitory synaptic inputs (“Inhibition”) in a L2/3 and L5B PC. Triangles indicate neuronal soma locations in this and in following panels and figures. Average excitation and inhibition maps to L2/3 and L5BPCs (neuron and mice numbers see C). Scale bar, 200 μm. (B) Similar to A-C but for FL treated mice. Note the excessive excitatory input to L2/3 PCs in FL mice (compare Excitation maps in A). (C) E/I heat maps obtained by calculating EPSC/IPSCS ratios in each of the 192 sites (16 X 12 pixels) in L2/3 (n=13 neurons for sham and n=15 for FL) and L5B PCs (n = 10 neurons for sham and n = 13 for FL) in sham (n=5 mice) vs. FL mice (n=5 mice). (D) E/I ratios were obtained by calculating the ratios of the summed EPSCs charges over summed IPSCs charges in heat maps. The E/I ratio in L2/3 PCs in the FL group was significantly larger than the ratio in the sham group (unpaired t-test, P=0.027); meanwhile, the E/I ratio in FL L5B PCs was significantly reduced compared to that in sham counterparts (unpaired t-test, P=0.012). Excitatory receptive field (ERF), obtained by counting significant EPSC pixels (>2X s.d.) in each group. The ERF in sham L2/3 PCs was significantly higher in the FL group than in their counterparts in the sham group (unpaired t-test, P=0.037); the ERF in L5B PCs was similar across groups (unpaired t-test, P = 0.83). Inhibitory receptive field (IRF), obtained by counting significant IPSC pixels in each group. No significant IRF change was found in both L2/3 and L5B PCs across groups (unpaired t-test, P=0.71 for L2/3 and P=0.63 for L5B). (E) Laminar distribution of average charges or strength of EPSCs and IPSCs for L2/3 and L5B PCs from sham (black) and FL (red) mice. For L2/3 PCs, the EPSCs charge was significantly increased in the FL group (unpaired t-test, P=0.0013). In contrast, L5B EPSCs charge in the FL group was similar to that seen in the sham group (unpaired t-test, P=0.134). However, for the IPSCs charge, the layer specific difference was roughly reversed between FL and sham groups (L5B, unpaired t-test, P=0.000; L2/3, P=0.044).
See also Figures S1 and S2.
Polysynaptic input to recorded neurons was typically maximized at the perisomatic region (Figure 1A–B). Excitatory or inhibitory postsynaptic currents (EPSC/IPSC) displayed shorter latency around perisomatic regions (Figure S1C–G). In pilot experiments, we applied TTX and 4-AP to eliminate spontaneous AP firing and polysynaptic activities. Under this condition, only monosynaptic inputs from ChR2-expressing neurons were evoked and recorded (Figures S1H and I) This was consistent with previous results [31]. We used an excitatory or inhibitory “receptive field” (ERF/IRF), which consisted of pixels with significant synaptic events (see Methods), to visualize the spatial patterns of PSC events. TTX eliminated ~70% of ERF of L2/3 PCs (paired t test, P=0.009, n = 5) and ~60% of ERF of L5B PCs (paired t test, P=0.04, n = 5). Unless otherwise stated, all CRACM studies were performed without TTX and 4-AP.
In the L2/3 PCs of sham mice, local optogenetically evoked excitatory and inhibitory responses were restricted mainly to supragranular layers (Figure A - an example neuron and averaged maps). The median E/I ratio across pixels (pixel E/I ratio) was 0.19 (n = 13 neurons, Figure S2A). Different stimuli intensities led to similar neuronal E/I ratios (summed E and I across pixels, see Methods, Figure S2A1), suggesting that E/I ratios are intrinsically regulated and balanced, as previously reported in a normal mouse cortex [29, 36, 37]. Simultaneously stimulating the majority, if not all of the synaptic inputs to the recorded neurons using whole-field LED illumination showed a similar result (Figure S2F). Importantly, in FL mice, extensive supragranular and ‘ectopic’ infragranular excitatory inputs to L2/3 PCs were revealed (compare the Excitation maps in Figures 1A vs B, S2E). As a result, numerous ‘hot pixels’ (i.e. higher E/I ratios) appeared within the E/I ratio map (Figure 1C, top right), mainly in the infragranular layers, which was a sharp contrast to results in the sham mice group (Figure 1C, top left). The neuronal E/I ratios were significantly elevated in L2/3 FL group (Figure 1D, see Legend for statistics); the ERF appeared larger in L2/3 FL group (Figure 1D). Consistent with the above findings, the layer-origin of the significant increases in optogenetically evoked EPSCs to L2/3 PCs spans from lower L2/3 all the way to L6 (Figure 1E). Also, the average median value of pixel E/I ratios of L2/3 PCs showed a significant increase from 0.19 in sham to 0.63 in FL (Figure S2C, P=0.0026). The IRF size did not differ between FL and sham groups for both L2/3 and L5B PCs (Figure 1D). These results suggest that the synaptic E/I balance in FL treated L2/3 PCs had been altered in favor of stronger excitation.
Surprisingly, FL L5B PCs received stronger inhibition compared to their sham counterparts (Figures 1). Despite slight increases in IRF (Figure 1D), the IPSC strength at L5B PCs was much stronger (Figure 1E), leading to significantly lower E/I ratios in the FL group (Figure 1C, Figure S2B, D, median 0.41 vs. 0.13, S2E). Like L2/3 PCs, sham L5B E/I ratios maintained a similar value under two different intensities (Figure S2B). However, neuronal E/I ratios decreased with higher laser intensity in FL (n = 9 neurons, Figure S2D), suggesting enhanced inhibition of L5B PCs in FL mice. Thus, the E/I ratios in FL L2/3 PCs were three fold higher than their sham counterparts, whereas the E/I ratios in FL L5B PCs were significantly lower than their sham counterparts (Figure 1D, Figures S2E). These results indicate that a drastic layer-dependent rewiring has occurred at the circuit level in the epileptic mouse cortex.
The hyperexcitable site was located in supragranular layers distal to the center of microgyrus
Previous in vitro studies using the FL rat model demonstrated hyperexcitability adjacent to the FL-induced microgyrus [21, 38]. However, it is unclear how cortical circuits are reorganized in mouse PMG models, and what their relationship to the hyperexcitable site is in vivo. Using in vivo microelectrode array recordings, we found that not only were extracellular waveforms altered in FL animals, but also there was a spatial change in both local field potential (LFP) and multi-unit activities (MUA) relative to the distance from the microgyrus (Figure 2). As such, FL data was further divided into regions either ‘distal’ (>0.7 mm) or ‘proximal’ (<0.7 mm) to the center of microgyrus as determined from histological analysis of post-mortem brain tissues (Figure S3B).
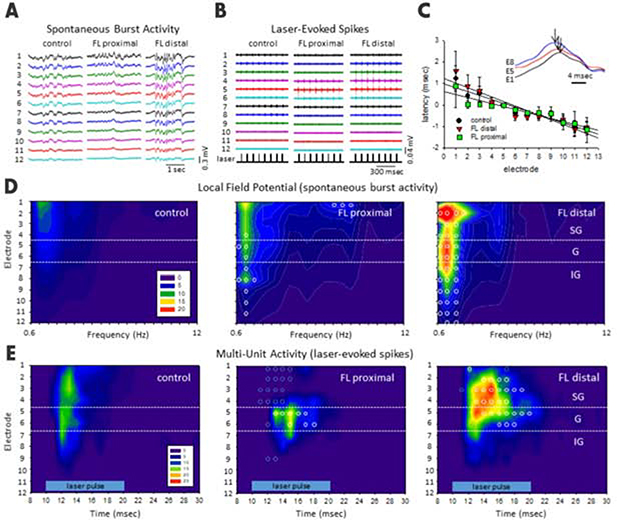
Figure 2. Site-specific hyperexcitability revealed by measuring spectral power of spontaneous burst activity and laser-evoked MUA in epileptic mice.
(A) Exemplar unfiltered LFP recordings showed much stronger burst activities in the distal region (>0.7 mm from the FL microgyrus) from S1BF of anesthetized FL mice compared to those in anesthetized sham-treated mice or proximal (<0.7 mm from the FL microgyrus) region of FL mice. (B) Exemplar laser-evoked MUA from control, FL-proximal and FL-distal groups. (C) Shift in latency of burst waveforms computed from the peak offset of the cross-correlation values between electrodes (see Methods, r2 = 0.913 (control), 0.874 (FL-distal), and 0.879 (FL-proximal)), inset shows the corresponding shift in waveform peaks across 3 electrodes (E1, E5, and E8, black arrows indicate peak deflection). (D) Composite heat maps of power spectral density (PSD) values from spontaneous burst activities in the three groups. (E) Composite heat maps of laser-evoked MUA counts across cortical lamina (horizontal blue bar, laser intensity level 8). D-E, Heat maps were constructed using the average values of spike counts from each experimental group; control (n=6 mice), FL-distal (n=4 mice) and FL-proximal (n=4 mice). Horizontal white dotted lines indicating the transitions between supragranular (SG), granular (G), and infragranular (IG) layers. Blue indicates lower spike counts while red indicates higher spike counts. Open circles indicate significant differences compared to control groups (P=0.04, Holm-Sidak post-hoc analysis). See also Figure S3.
To probe the hyperexcitability sites in vivo, we used two complementary approaches: anesthesia-induced and optogenetically-evoked burst discharges (see Methods). First, we measured the latency of optogenetically evoked bursts in vivo, as this can be accurately measured with regard to laser stimulation time (Methods). Under isoflurane anesthesia, extracellular waveforms of laser-evoked burst activity typically progressed from deeper to more superficial layers of the cortex (Figure 2C) as indicated by the temporal shift in waveform peaks of the recorded LFP signals across layers (Figure 2C, inset). An average delay of 2–3 ms was observed in the supragranular layers compared to the infragranular layer (Figure 2C). The rapid progression of the burst waveform from the deeper to more superficial cortical layers was also associated with a significant increase in total signal power (0–20 Hz) in the supragranular cortical layers only for the FL groups (P=0.035, Holm-Sidak post-hoc analysis). Next, we examined anesthesia-induced spontaneous bursts discharges, which we found previously to be significantly enhanced by FL (Figure 2A)[24]. Further analysis indicated significant differences in the spectral power and frequency spectrum of these abnormal burst-discharges associated with the FL-treatment (P=0.027, multivariate ANOVA) as displayed in the laminar heat maps in Figure 2D. In comparison, the strongest increases in spectral power were observed in the supragranular layers of the FL-distal group, particularly in the slow-wave (i.e. delta) frequency range (0–4 Hz). Lastly, because evoked MUA is the gold standard for accurate mapping of the origin of evoked bursts/waves, we examined the laminar and lateral location of optogenetically induced bursts and found that optogenetically-evoked MUA showed significant increases in spike count and also lasted much longer in the upper cortical layers of the FL-distal group (Figure 2E, P=0.031, multivariate ANOVA). Interestingly, a significant reduction in spike count was also observed across some FL-proximal regions, particularly in the supragranular layers (Figure 2E). Taken together, this in vivo data indicates that the distal region to the microgyrus is a locus of hyperexcitability and that epileptogenesis may originate from the infragranular layer.
Identifying the location and sources of hyperexcitable foci within the malformed cortex
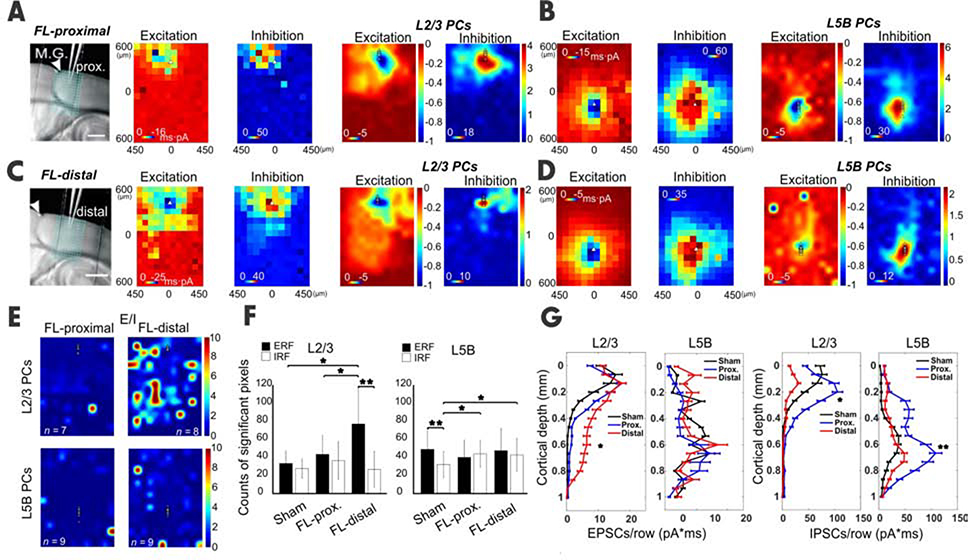
Next, we asked how the previously mentioned hyperexcitability aligns with cortical regions proximal or distal to microgyrus at the cellular and synaptic level. We further divided the FL data in Figure 1 into two groups: FL-proximal and FL-distal (Figures 3A–D, Figure S4A, B). Importantly, L2/3 PCs in the distal group displayed a significantly larger E/I ratio than both the proximal group (Figure S4C, E/I ratio = 1.1 vs 0.3) and the sham group (Figure 3E, E/I ratio = 1.1 vs 0.2), respectively. Comparisons of the neuronal E/I ratios between proximal vs. distal groups under two laser intensities support the findings mentioned earlier (Figure S4E). The ERF size in L2/3 PCs increased significantly compared to both the distal and sham groups (Figure 3F). The ERF and IRF sizes for L2/3 PCs were comparable in sham and proximal groups; however, ERF size significantly outnumbered IRF size in the distal group (Figure 3F). Consistently, the EPSC strength at distal L2/3 PCs was also significantly increased (Figure 3G); meanwhile IPSC strength in the distal group was sharply reduced when compared to IPSC strength in the proximal group (Figure 3G, Figure S4E). Importantly, the source of the ERF to L2/3 PCs came from the infragranular layers (Figure 3G). These results indicate that the hyperexcitability foci were mainly located at cortical regions distal (>0.7 mm) to the center of the microgyrus.
Figure 3. Site- and layer-specific synaptic inputs measured by CRACM.
(A-B) CRACM of local vS1 excitatory and inhibitory synaptic inputs to L2/3 or L5B PCs in a region proximal to the M.G. The layout is similar to Figure 1. (C-D) Same as above but for the FL-distal region. The excitatory input to distal L2/3 PCs was more extensive compared to its proximal counterpart, particularly in infragranular layers (compare Excitation maps in A,C, also F); meanwhile the inhibitory input to distal L2/3 PCs was much smaller compared to its proximal counterpart (compare Inhibition maps in A,C, also F). (E) E/I heat maps in L2/3 (n=7 neurons for FL-proximal and n = 9 for FL-distal, 4 mice) and L5B PCs (n=8 neurons for sham and n=9 for FL, 4 mice). (F) Comparison of ERF and IRF between groups, obtained by counting total significant pixels in A-D. Note the L2/3 ERF in FL-distal was significantly increased when compared to sham and FL-proximal (unpaired t-test, P < 0.05); While in L5B, the IRF of both FL-proximal and FL-distal increased significantly (unpaired t-test, P < 0.05). (G) Laminal distribution of charge of EPSCs and IPSCs for L2/3 and L5B PCs in sham (black) vs. FL-proximal (blue) and FL-distal (red) groups. G, L2/3 EPSCs strength in FL-distal group was significantly larger for infragranular layers compared to that in the FL-proximal group (P = 0.032). No difference was found in EPSCs charge across layers between groups. L2/3 IPSCs charge was significantly lower in FL-distal group compared to that in FL-proximal group (P<0.05), mainly in supragranular layers. L5B IPSCs strength was significantly lower in FL-distal group compared to that in FL-distal group (paired t-test, P=0.013), but mainly in infragranular layers. See also Figure S4.
In contrast, L5B PCs in both proximal and distal groups exhibited hypoexcitability (more obvious in the proximal region), with median E/I ratio values of 0.12 and 0.3 respectively, compared to the sham group (0.4, Figure S4D). The IRF increased significantly in L5B PCs of both proximal and distal groups compared to the sham group (Figure 3F). While the ERF size was larger than the IRFs’ size in the sham group, the ERF size was consistent with the IRFs’ size in both proximal and distal FL groups (Figure 3F). The combined results from L2/3 and L5B data highlight the location-, and layer-specific aberrant circuitry in FL cortices.
To further examine the contribution from specific layers or input sources to the general E/I balance, we used a layer- and interneuron cell type-specific strategy to study the aberrant circuitry associated with FL-treatment.
L4-derived synapses contribute to an imbalanced E/I ratio in aberrant circuitry
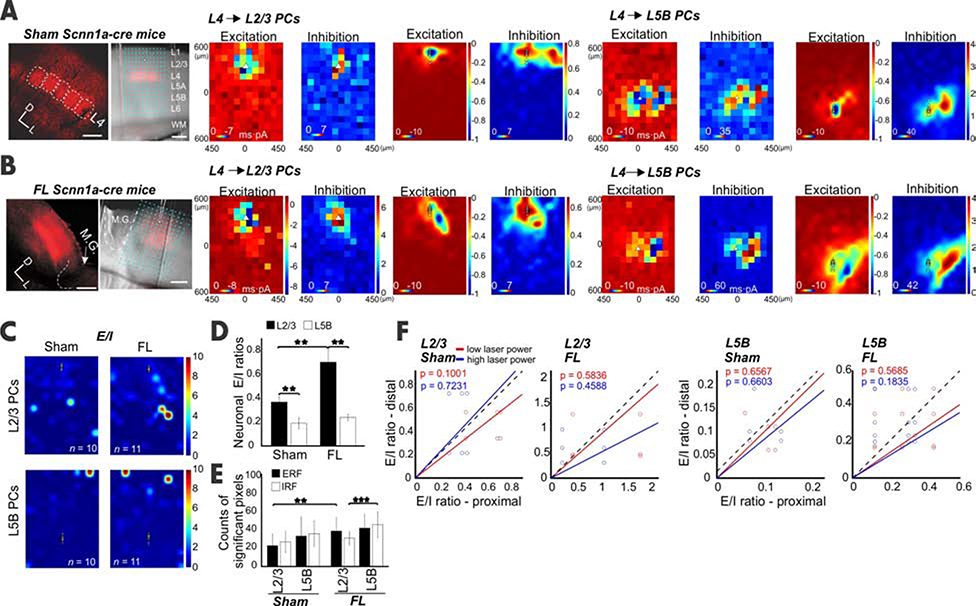
L4 spiny neurons carry bottom-up information from the thalamus to cortical L2/3 neurons [39–41] and L5B PCs [31]. Does abnormal hyperexcitability (for L2/3 neurons) and hypoexcitability (for L5B PCs) result from differential re-organization of L4 synaptic input? We addressed this question by examining L4-specific inputs to L2/3 and L5B PCs in sham and FL mice. L4-specific expressions of ChR2 were achieved via injection of flexed ChR2 in Scnn1a-cre mice (see Methods, Figures 4A, [42]). Sham L2/3 PCs showed a centralized pattern in excitation maps and a bimodal distribution in inhibition maps (Figure 4A), presumably due to lateral inhibition from a neighboring barrel column. This lateral inhibition was also found in L5B PCs in the sham group (Figure 4A). L2/3 (Figure 4A), but not L5B PCs (Figure 4A) in FL groups displayed increased E/I ratios compared to their counterparts in sham groups (Figure 4C Figures S5A–E, neuronal E/I ratio in L2/3: 0.43 vs 0.28; L5B: 0.21 vs 0.11). In addition, the lateral inhibition observed in sham PCs was interrupted in L2/3 but not L5B PCs (Figures 4A vs 4B) in the FL group. The ERF size increased significantly in FL L2/3 PCs compared to their sham counterparts (Figure 4E, P<0.01). A significant increase in IRF was also observed in FL L5B compared to FL L2/3 PCs, which was comparable in the sham groups between L2/3 and L5B PCs (Figure 4E, P<0.001). These results suggest that the L4 ➔ L2/3 inputs contribute to the reorganization of inter-laminal and inter-columnal circuits.
Figure 4. Input from the granular layer contributes to the E/I imbalance in epileptic mice.
(A) Left, confocal image showed the barrels in vS1 expressing ChR2 from a sham Scnn1a-cre mouse. Right, an exemplar recording from a L2/3 PC. Scale bar, 200 μm. CRACM of synaptic input from L4 to L2/3 PCs or to L5B PCs in sham mice. Note the scale difference in Inhibition maps between two layers. (B) Same as A but for FL mice. The pixel number of inputs to L5B PCs for both Excitation and Inhibition increased (F). (C) E/I heat maps for L2/3 (Sham, n=10, FL, n=11) and L5B PCs (Sham, n = 11, FL, n = 11). (D) The averaged E/I ratios across neurons in FL were significantly higher in L2/3 PCs (P=0.023) but not in L5B PCs. (E) The ERF size in FL was also significantly higher in L2/3 (P=0.007); in contrast, the IRF, instead of ERF, increased significantly in L5B (P=0.000), but not L2/3. (F) No significant E/I ratio differences were observed between proximal and distal locations from L4 to L2/3 or L5B PCs in both sham and FL groups. The relationship between E/I ratios in sham vs FL mice did not change under two different laser intensities. See also Figure S5.
To determine whether the circuit differences in proximal vs. distal regions were contributed by differential L4 inputs, we compared the proximal vs. distal neuronal E/I ratios in L4 ➔ L2/3 and L4 ➔ L5B PCs. The results showed that neuronal E/I ratios did not change significantly for either L4 ➔ L2/3 or L4 ➔ L5B PCs between proximal and distal groups (Figures 4D, F), suggesting the overabundant excitatory input to FL-distal L2/3 PCs observed in previous experiments (Figure 1) came from sources other than local L4, likely from intra- and/or inter-columnar L5B (Figure 2C) and local L2/3 PCs. Together, these data suggest that the L4 only partially contributed to hyperexcitability in L2/3 PCs. However, the hypoexcitability in L5B PCs remains unclear. Is it due to altered inhibition from a specific subtype of interneuron? Is it a compensatory effect of cortical circuitry in response to epileptogenesis?
Reorganization of inhibitory circuits in the FL cortex
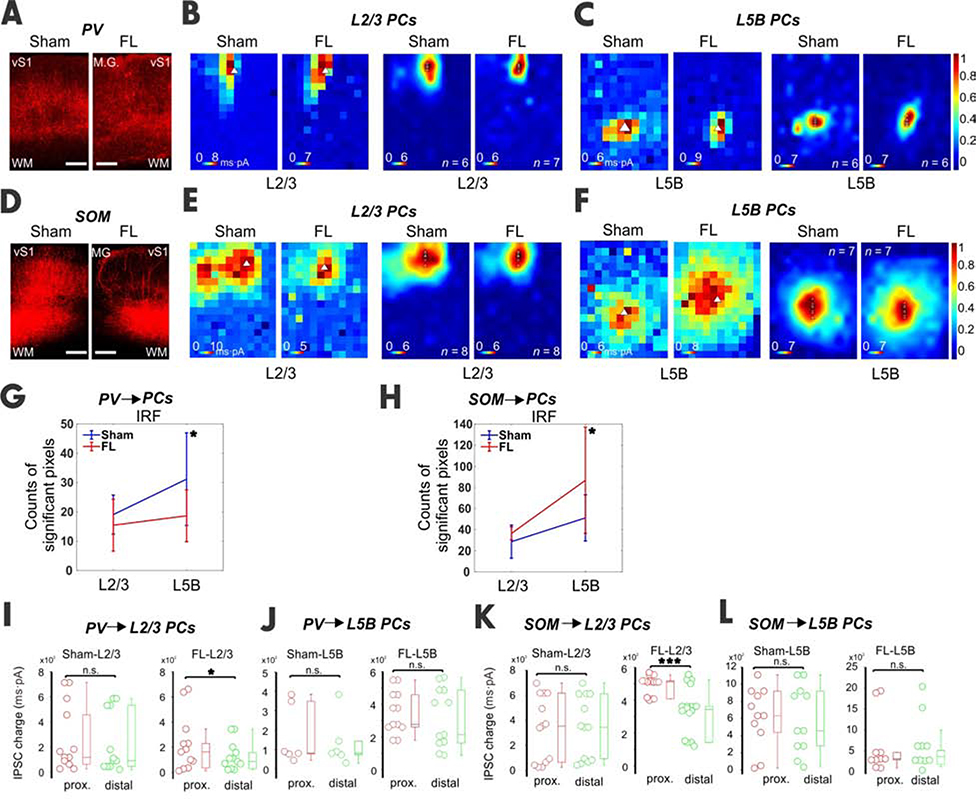
Next, we examined if inhibition from distinct interneuron subtypes was differentially altered in L2/3 and L5B of the FL cortices. PV and SOM INs are the primary subtypes that provide direct synaptic inhibition onto PCs [43] and were therefore the subjects of study. PVs mainly target the perisomatic region of pyramidal neurons [44], whereas SOMs innervate the distal dendritic areas [45]. We examined the inhibition from PVs or SOMs onto L2/3 and L5B PCs (see Methods). As expected, CRACM revealed a perisomatic inhibition pattern in L2/3 and L5B PCs in PV-cre mice (Figure 5B–C). The IRF size of L5B PCs dropped significantly (Figure 5G), presumably due to reduced inhibition from PV INs. In SOM-cre mice, CRACM demonstrated an apparent dendritic targeting pattern of SOM input to PCs in both layers as expected (Figure 5E–F). The IRF size of L5B PCs in the FL group increased dramatically, suggesting the formation of more dendritic inhibitory synapses by SOM in the FL mice (Figure 5H). To confirm the identities of PV and SOM interneurons, we performed whole-cell current clamp recordings and examined excitabilities of presumed PV and SOM cells (via their expression of tdtomato marker). Indeed, many parameters of the intrinsic and action potentials are significantly different (Table S1): supporting these were two distinct types of interneurons. This demonstrates that SOM, but not PV, contributes to the hypoexcitability in L5B PCs.
Figure 5. Inhibitory inputs from PVs and SOMs to PCs of vS1 in epileptic mice.
(A) Confocal images showed ChR2 expression in PVs for both sham and FL slices. (B) An example of and averaged heat maps showed the inhibitory input from PVs to L2/3 PCs. (C) An example of and averaged heat maps showing the inhibitory input from PVs to a L5B PC. (D-F) Similar to above but for SOMs. (G) The IRF in L5B was significantly reduced in the FL group (sham, n=6, 3 mice; FL, n=6, paired t test, DF=5, 3 mice P=0.04). (H) Similar to G but for SOMs. IRF in L5B was also significantly increased in the FL group (sham, n=7; FL, n=7, unpaired t test, DF=6, P=0.014). (I) In sham L2/3 PCs, no difference between proximal and distal groups was found in IPSC charge from PVs. In FL mice L2/3 PCs, IPSC charge from PV INs was reduced in the distal group (P<0.05). (J) In both the sham and FL L5B PCs, no difference between proximal and distal groups was found in IPSC charge from PVs. (K-L) Similar to I-J but for SOMs. Note the significant difference in IPSC charge between proximal and distal groups for FL L2/3 PCs (P=0.000). See also Table S1.
We also compared the absolute IPSC charge between proximal and distal regions for both sham and FL groups (Figure 5I–L). As expected, the IPSC charge of the sham PV and SOM ➔ L2/3 PCs did not differ between the two regions (Figure 5I, K, n = 12 pairs, DF=11). However, FL PV-L2/3 PCs showed a significantly lower IPSC charge in the FL-distal region compared to FL-proximal region (Figure 5I, n = 12 pairs; DF=11). SOM ➔ L2/3 PCs in FL-distal regions showed significantly lower IPSC charge than in FL-proximal regions (Figure 5K, n = 16 pairs, DF=15). The L5B IPSC charge of proximal vs. distal regions was not significantly different in PV and SOM INs (Figures 5J and L). These results suggest that the functions of both PV and SOM INs in L2/3 were compromised in the distal FL region, leading to the hyperexcitable states in L2/3 of this region. The hypoexcitable state in L5B may be caused by either enhanced SOM inhibitory innervation of the distal dendritic sites of L5B PCs, (which is presumably caused by axonal sprouting of SOM INS), or increased SOM neuron count due to the migration gradient by FL. If former is the case, then we predict that optogenetic manipulation of these SOM inputs will have a larger influence compared to PV cells’ influence on spike-wave discharges observed in this FL model previously [25].
Closed-loop optogenetic activation of remaining SOMs, but not PVs, distal to the microgyrus irreversibly abolished spontaneous SWDs in vivo
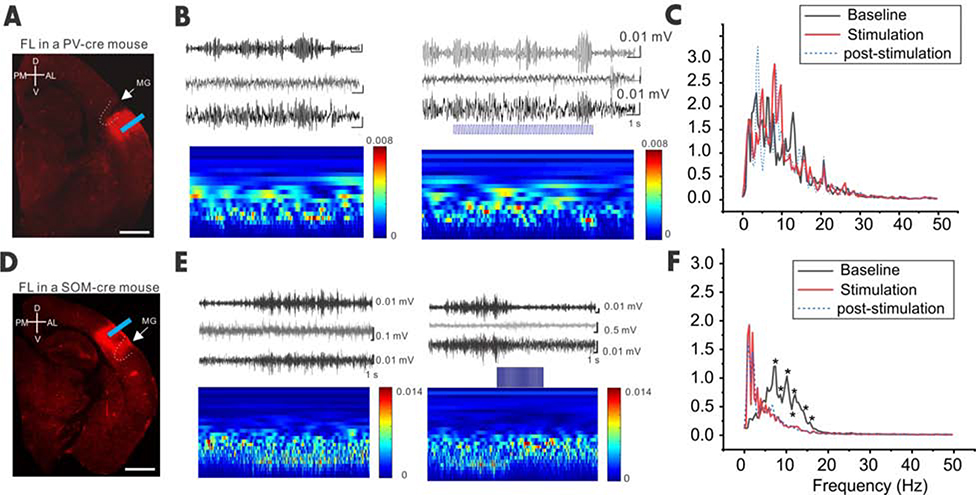
To further examine roles of SOM or PV INs during spontaneous spike-wave discharges, we applied closed-loop optogenetic stimulation in vivo on unanesthetized animals while they exhibited spontaneous spike-wave. Flexed ChR2 AAV was injected in FL-distal regions in PV- and SOM-cre mice, respectively, and a fiber-optic probe was implanted in the same regions (Figure 6A and 6D). After waiting for at least two weeks upon viral expressions, we performed EEG recordings and closed-loop optogenetic stimulations. Once SWD activities were detected (Figure 6B and 6E, based on the characteristic α (8–12 Hz) and θ (4–8 Hz) activity of the SWD [25]), we applied closed-loop optogenetic activation (5Hz) of PVs or SOMs in distal regions of the microgyrus. Our results showed that optogenetic activation of SOM, but not PV INs, irreversibly abolished the spontaneous SWD events (Figure 6C and 6F), suggesting that by enhancing remaining SOM INs, but not t PV INs, this eliminates the characteristic α (8–12 Hz) and θ (4–8 Hz) activities of the SWD in FL- treated mice.
Figure 6. Closed-loop optogenetic activation of SOM but not PVs terminated the spontaneous SWD in vivo.
(A) Confocal image showing ChR2 expression in a FL PV-cre mouse. (B) EEG traces (top) and time frequency plot (continuous wavelet transformation of the same EEG traces) show SWD before, during and after optogenetic stimulation (5Hz) in the perimicrogyri area. (C) Power spectrum of the baseline, optogenetic stimulation, and post-stimulation period (2 seconds in each period, n=8 mice, p=0.67595, Stim. vs. baseline, DF=127). (D-F) Same as A-C but for SOM-cre mice. Note that the power was significantly reduced at the frequency ranging from 7–15 Hz upon optical stimulation compared to baseline (n=5 mice, p=0.03078, Stim. vs. baseline as one group, DF= 127, * denotes specific frequencies for P values <0.05).
Discussion
Spontaneous spike-wave discharges are core features of our focal MCD model. We used in vivo and in vitro electrophysiology together with laser scanning photostimulation mapping to characterize how aberrant circuits lead to epileptogenesis. Our data suggests that excessive network excitation of supragranular PCs, originating at least in part from granular and infragranular layers, are the major driving forces of hyperexcitability in distal areas. In sharp contrast, the otherwise active L5B PCs experienced hypoexcitability in a FL cortex. Furthermore, we found that the chronic hyperexcitability foci are located at regions distal to the microgyrus, both in vivo and in vitro. Strikingly, despite the functional deficits of both PVs and SOMs in the infragranular layer, IRF size dropped for PV, but increased for SOM. Neither functional deficit nor RF change has been found for either subtype of interneuron in L2/3. Importantly, activation of SOMs at FL-distal regions abolished epileptic activities irreversibly, at least during the time period we tested.
Previous studies have probed the synaptic connectivity onto L2/3 and L5 PCs in a rat FL model [21]. One main finding by Brill and Huguenard was greater excitatory input to L5 PCs but not L2/3 PCs. Several possibilities could have lead to this discrepancy: 1) difference in species used between a mouse and a rat; 2) The Brill and Huguenard study used rats aged P16–22 while our study used adult mice (~2 months old), and excitatory axonal sprouting takes months to occur[46]; 3) Brill and Huguenard used a glutamate uncaging method to optically excite neuronal soma but not axon terminals. This mapping of monosynaptic input may have revealed the location of the synaptic input source but missed the location of the synaptic terminals. The CRACM method activates axon terminals reliably, even when the parent soma has not been activated enough to generate a spike [30, 31]. In FL mice, axon terminals from certain neurons (e.g. SOMs) may abnormally sprout, resulting in abnormal innervations that could potentially contribute to epileptogenesis. Further studies are needed to generate a comprehensive picture of abnormal circuit rewiring through a broad developmental period.
It has been well documented that synaptic excitation and inhibition in cortical neurons shows a proportionality, or balance, through the use of recurrent local synaptic connections to maintain normal brain functions [29, 37, 47–52]. L2/3 and L5B PCs play different roles in the horizontal propagation of recurrent-network activities, including epileptiform activities [53, 54]. In canonical cortical circuitry, L2/3 receives its prominent excitatory drive from L4 and subsequently sends a major output to L5B [40]. In a healthy barrel cortex, L2/3 PCs display a lower synaptic E/I ratio compared to L5B PCs within the same columns [29, 51, 55], consistent with their respective functions, e.g. the sparse coding strategy used by supragranular neurons for feature integration [56, 57] and active feedback to subcortical regions [34, 58]. L2/3 PCs also send their outputs to other ipsi- and contra-lateral cortical regions. Their hyperexcitability in the malformed cortex may facilitate abnormal intracortical propagation and generalize epileptiform activities. Intrinsically bursting L5B PCs have also been implicated in the generation of epileptiform activities due to their intrinsic properties, extensive horizontal intracortical axons, and feedback modulation of ascending sensory information [37, 59]. However, our results indicate that circuits formed by L5B PC neurons are hypoexcitable in FL vS1. This result is consistent with our in vivo extracellular data, which shows an absence of optogenetically induced epileptiform activities. We would like to point out that the hypoexcitability in L5B PCs does not mean that these cells were not involved in the induction of hyperexcitability in L2/3 PCs. On the contrary, L5B PCs may actually lead to excitation of L2/3 PCs if their connections to L2/3 are enhanced, which is indeed the case here (Figure. 1H and I). Thus, our results shown here lead to a new hypothesis that the interplay between infra- and supra-granular layers is necessary for epileptogenesis in the FL-induced PMG model. Our hypothesis is consistent with an idea put forth by prior studies [54, 60], regarding the propagation of self-sustained activities within a normal neocortex. Our data has provided support to a long-standing hypothesis that was put forward by several researchers that the normal long-range neocortical network is ‘hijacked’ by the epileptic brain to give rise to epileptiform activities[61–63].
In an epileptic brain, the E/I imbalance directly leads to epileptic discharges of the “epileptic neuron” [64]. However, the mechanisms contributing to epileptogenesis in malformed cortices are largely unknown. It is also unknown why seizures associated with cortical malformations are medically intractable [65, 66]. A focal epileptogenesis mechanism has been proposed in previous studies, characterized by an initiation of epileptiform activities in an adjacent 6-layered cortical area distal to the microgyrus [12, 16]. This hypothesis has yet to be tested in a mouse model exhibiting spontaneous seizures. Importantly, severing a microgyrus from an adjacent cortical region does not prevent the initiation of epileptiform activities [12, 16]. Human MCD is rarely a localized or even regional process, but instead an electrophysiologically, functionally, and ultimately clinically integrated neural network disorder. Our results demonstrate that aberrant ectopic inter-laminar network excitation of L2/3 neurons in distal paramicrogyral areas may be a critical constituent of epileptogenesis. This epileptic tendency could be further shaped by differential compensatory effects in subtypes of INs. This difference was not due to IN density change in the FL model [67, 68]. The hypoexcitability of L5B PCs could be explained partially by either axonal sprouting of SOM INs within the same layer [69], or increased SOM neuron count due to migration gradient by FL, indicating a selected disturbance of IN subtypes in this model. This aberrant elevated activity of SOM INs may counteract the excitatory drive from hyperexcitable L2/3 PCs, which may explain how closed-loop activation of SOMs alleviates epileptogenetic activities. Other untested possibilities include excessive excitatory drive in INS, and drive from intralaminar input or thalamocortical inputs [70]. GABAA receptor reduction and increased AMPA receptor density in the paramicrogyral area, as reported earlier [71], may also contribute to an imbalanced E/I ratio. Understanding the fine layer- and cell type-specific circuitry that gives rise to aberrant activities in the epileptic brain is an important step towards developing a revolutionized novel treatment.
In an earlier study [23], using a combination of intracranial EEG (iEGG) and linear electrode array recordings, we identified high-frequency ripple (HFR) oscillations in the cortical granular and supragranular layers and concluded that a hyperexcitable supragranular layer ‘near the malformed cortex’ may play a key role in SWD epileptogenesis. However, the source of inputs that triggers the HFR has yet to be identified. In current study, both enhanced network excitation (Figure 1H and I), as well as reduced SOM inhibition (Figure 5K) in this region strongly supports the idea that over-excitation of L2/3 may be the key circuit feature that causes SWD discharges. Indeed, our in vivo optogenetic data in Figure 6, where activation of SOM INS in this area irreversibly terminated SWD discharges, supports this interpretation. Taking our data from these two studies together, we suggest that normal thalamocortical sleep spindle waves during NREM sleep may drive the malformed cortical columnar circuits in a manner that generates SWDs. Thalamic spindles arrive in L5B, which in the malformed cortices provide extensive recurrent excitation to L2/3 (Figure 1B and E), which were matched with reduced network inhibitions. To fully test this hypothesis, the next logical step is to conduct in vivo simultaneous recordings in multiple cortex layers and the thalamus while the animal exhibits SWD seizures. This will generate insightful data. Our prediction is that there will be an enhanced coherence between the cortex and thalamus through a wide range of frequencies (delta-through HFRs), but there will not necessarily be an enhanced firing in the thalamus (as we have reported previously [23]), which may underlie the epileptogenesis of SWDs in this model.
STAR Methods
Unique link: https://star-methods.com/?rid=KRT5e9decf29da8b
Resource Availability
Lead Contact
Further information and requests for resources and reagents may be directed to and will be fulfilled by Lead Contact, Dr. Qian-Quan Sun, neuron@uwyo.edu.
Materials Availability
This study did not generate new unique reagents or materials.
Data and Code Availability
The published article includes all datasets generated or analyzed during this study. Detailed datasets and codes supporting the current study are available from the corresponding authors on request.
Experimental Model and Subject Details
All experiments were performed under protocols approved by the Institutional Animal Care and Use Committee of the University of Wyoming. Mice were housed in a vivarium maintained at 22–23 °C on a 12:12 h light-dark cycle. Food and water were available ad libitum. Mice of both sexes were used in this study. Unless otherwise specified, mice of a CD-1 background were used. Scnn1a-cre mice (Jackson lab) were used for L4-specific experiments; PV-Cre (Jackson lab) and Som-Cre (Jackson lab) mice were used for inhibitory CRACM experiments. All cre lines were of a C57BL/6J background. Both C57BL/6J and CD-1 mouse strains had been used for an earlier study of FL induced spontaneous spike-wave discharges and no significant differences were found between the two strains [25].
Method Details
Experimental microgyrus in mice
An experimental microgyrus was induced in postnatal day 0 (P0) mouse pups via the use of the transcranial freeze-lesion (FL) as previously described [14, 15, 25, 38, 72]. The pups were first briefly anesthetized by being surrounded with ice for ~2 minutes, the skull exposed, and a copper bar (1 mm tip diameter and pre-submerged in liquid nitrogen for ~10-min) was gently placed on the surface of the right barrel cortex region for ~2 s (from bregma: 2.2±0.2 mm lateral and 0±0.1 mm AP). Controls were sham-operated littermates, treated the same as the FL pups except for the use of another copper bar kept at room temperature (23 °C). The scalp was sutured with super glue. Treated mice were allowed to recover for ~30-min in a heated cage before being returned to the dam. Examination of slices for electrophysiological experiments at age of P46–180 or post hoc histological observation indicated 4-layered abnormal cortical structures which were reliably induced as previously reported [14, 15, 25, 38, 72] (Figure S2C). The microgyrus was primarily located around the central or lateral side of the barrel field. The recorded neurons were mostly located medial to the FL-induced microsulcus. Some neurons were located lateral to the microsulcus, which showed similar results to the medial neurons, and thus were included for analysis. The microgyrus with an abnormal 4-layered structure typically spanned a 400 ± 80 μm (tangential) region (Figure S2C). Cortical regions surrounding the microgyrus displayed a normal 6-layered structure as previously reported [14, 15, 25, 38, 72]. While most recorded neurons were located in the S1 barrel cortex, some neurons in distal regions fell into the S1 trunk region.
Stereotaxic viral injection
Virus injection was performed in sham or FL mice aged P14-P16. Mice were anesthetized with 3% isoflurane (vol/vol) and maintained with oxygenated 2% isoflurane mixture throughout the surgery procedure. AAV2.1.CAG.hChR2(H134R)-mCherry (University of Pennsylvania Vector Core) was injected in vS1 area of CD1 mice. AAV2/1.CAGGS.flex.ChR2.tdTomato.WPRE.SV40 (University of Pennsylvania Vector Core) was injected in vS1 of Scnn1a-cre mice, PV-cre mice, or SOM-cre mice. The viral vector (2 μl) was loaded into the tip (~10 μm in diameter) of a beveled glass micropipette (Drummond Scientific Co.). A custom stereotactic apparatus was used to deliver the viral vector to the cortex through a small hole drilled in the skull. For vS1 injection, the virus was injected at two depths: 400 μm and 800 μm; for L4 injection, 400 μm and 600 μm. For each depth, a volume of ~100 nl was injected within 1–2 minutes using a micromanipulator (MP-285-system, Sutter Instrument). The injection pipette was kept in place for 5 minutes per depth, after injection. Injected mice were warmed in a heated cage until full recovery from surgery before being returned to the dams. After weaning day, the pups were separated by gender until experiments took place. Electrophysiological experiments would not start until at least two weeks after viral injection.
Microelectrode arrays
Extracellular signals were recorded using linear microelectrode SmartProbe™ arrays (NeuroNexus, Ann Arbor, MI) from the vS1 of control and FL mice [23, 24]. The electrode array consisted of a single shank with 16 individual electrodes separated by 100 μm, with on-board electronics for digital conversion of the signals, and linked to a SmartBox™ control and data streaming system through SmartLink headstage (NeuroNexus). The array was positioned perpendicular to the pia. Each signal was filtered digitally (1–10000 Hz band-pass and 60 Hz notch filters) and recorded with a sample rate of 20 kHz using Smartbox 2.01 (NeuroNexus). Additional off-line digital filtering was used to define local field potentials (LFPs, 1–100 Hz) and multi-unit (MU) activity (800–5000 Hz).
In vivo Extracellular recordings
All extracellular recordings were obtained from anesthetized (0.5–1.0% isoflurane delivered in oxygen) and head-restrained mice (stereotaxic frame supplied with mouse and gas anesthesia adaptors, Stoelting, Wood Dale, IL) as previously described [24]. Normal animal body temperature was maintained during recordings with a circulating water bath (Haake, Thermo Electron, Newington, NH), infused custom heating pad and continuous rectal temperature probe (BK precision, Yorba Linda, CA). A scalp incision was made to expose the skull over the right vS1. The recording stage was performed in a custom-built Faraday cage on vibration isolation table (Vibraplane, Kinetic Systems, Boston, MA). Single or multiple burr holes (1 mm diameter) were made through the skull with a dental drill for stereotaxic insertion of linear microelectrode arrays and fiber optic probes (laser stimulation). Fiber optic probes were placed directly over the virus injection ports. The microelectrode arrays were positioned laterally within 1 mm of the fiber optic probe at an angle of 40 degrees from vertical for perpendicular insertion into the S1BF. A 3-axis micromanipulator (hydraulic, Narishige, Amityville, NY) was used to advance the array slowly into the brain and was then stationary for 20 minutes once set in place (~1650 μm depth). Electrode placement was visually guided under a dissecting microscope to verify that all electrodes had penetrated the surface of the brain. The abrupt change in the local field potential (LFP) between cortical and subcortical layers was used as a visual marker to verify electrode depth [23, 24]. The first 12 electrodes were subsequently analyzed for laser-evoked multi-unit (MU) activity. The dura was found to offer only minimal resistance to electrode insertion and was therefore left intact. Warm physiologically isotonic saline was administered to the craniotomy site during the recording period. The silver wire reference electrode was placed under the skin flap of the scalp incision.
Intracranial EEG (iEEG) recordings
Methods of iEEG surgery, recording and analysis are similar to the methods described in our previous publication [23, 25]. Briefly, polyimide-insulated stainless steel wires (125 μm, California Fine Wire) and connecting pins will be implanted into S1 (right side S1R), VPM and contralateral hippocampus. Upon animals fully recovering from the surgery, EEG recordings will be performed in a 24-hour cycle with simultaneous video behavior monitoring and automated infrared (IR)-activity tracking at a frequency of once per week. EEG signals will be amplified via a differential AC amplifier (Model 1700, A-M system), digitized at 1000Hz using Power 1401, and analyzed using the Spike-2® program.
Closed-loop optogenetic stimulation
We implanted a multi-mode fiber optic patch cable (Ø150μm, Thorlabs) via a 1.25mm OD multimode ceramic zirconia ferrule (Precision Fiber Products, Inc, Milpitas, CA 95035), which was glued together with the S1R electrode to form an optrode configuration and implanted in the distal site (~1–1.5mm) of the microgyrus location (Figure 6a, d). The multi-mode fiber optic patch cable was coupled to a blue laser (473nm, 100 mW, DPSS blue laser, www.lasercentury.com) via a SMA mating end. Laser pulses were delivered via a custom-made pulse generator at three frequencies (1, 5 and 10Hz). It was estimated that an area approximately 0.3mm deep and 0.3mm wide was illuminated upon activation of the fiber, based on tests using a similar fiber in brain slices in vitro [73]. During the online recording period, bandwidth filtered (8–15Hz) of the EEG trace is displayed for the detection of spike-wave discharges, which is defined based on our initial characterization [25]. Upon detection of spike-wave discharges, laser stimulation is delivered for a period of 5–10 seconds.
Histology after in vivo recording
Following all in vivo electrophysiology recordings, animals were anesthetized and transcardially perfused with 0.9% saline followed by 4% paraformaldehyde. Brains were extracted, immersed in 4% paraformaldehyde for 24 h, then transferred to 0.1 M phosphate buffer containing sequentially increasing levels of sucrose (10%, 20%, 30%, pH 7.4, 4°C) across 3 consecutive days. Brain tissue was then removed from the sucrose solution and cut into serial sections through the area of interest. The microelectrode was immersed with DiI (2 mg/ml in ethanol,). Several techniques were used to visualize the probe track in relation to the freeze lesion and S1 barrel field. Thick coronal sections (300 μm) were cut on a vibratome for direct visualization of the probe track (DiI) in relation to the freeze lesion. A cryostat was used to cut thinner coronal sections (50–80 μm) for staining with cytochrome oxidase (CO) to visualize the barrel cortex in relation to the freeze lesion. Coronal sections were incubated at room temperature in a solution of CO (0.5 mg/ml, Sigma), sucrose (0.4 mg/ml, Sigma), and DAB (0.625 mg/ml, Sigma) in 0.1 M phosphate buffer (pH 7.4) for 60–90 min until cortical barrels were visible under a dissecting microscope. All sections were then mounted on glass slides with a DAPI mounting medium (Vectashield, Vector Laboratories, Burlingame, CA) and coverslipped. Brain images were evaluated under a light/fluorescent microscope (Zeiss Axioskop 2, Ontario, CA) and digitally imaged using Axiovision software (ver. 4.6, Zeiss). Histological analysis of serial brain sections was used to construct cortical maps to mark the location of the recording sites in relation to the induced microgyric lesions.
Data and statistical analysis for in vivo extracellular recordings
NeuroExplorer (Nex Technologies, Madison, AL) was used for data analysis, visual inspection, and digital filtering of MU activity. Power spectral density (PSD) values were computed across a frequency range of 1–20 Hz with a single taper Hann FFT using a bin size of .0012 Hz and 50% overlap between bins to mitigate data loss at the spectral edge of each bin. Correlation analysis was used to compute the cross-correlation and latency shift between simultaneously recorded signals from a single linear microarray recording using standard correlograms across a −0.3 to +0.3 sec offset (bin size of 0.05 ms based on the A/D sampling rate)[23, 24]. Data files were exported to Spike2 (Cambridge Electronic Design Limited, Cambridge, England) for spike sorting. MU spikes were detected using template matching software triggered by negative threshold deflections greater than −0.02 mV (Spike2 software, see Figures 2D–2F) within a 50 ms window surrounding each laser pulse (10 ms pre-stimulus recording). Post-stimulus time histograms and raster plots of MU activity were computed from 32 laser-evoked responses at each recording site (NeuroExplorer) and spike count was averaged across each group (Sigmaplot). Following this analysis, raw data was exported to Microsoft Excel for tabulation of statistical averages and standard error values or Sigmaplot (ver. 11.0, SYSTAT software Inc., San Jose, CA) for graphical display and statistical analysis between groups. Group averages are presented mean ± S.E.M. A multifactorial analysis of variance (ANOVA) was used to evaluate the main effects and interactions when multiple independent variables were present, including spike count, electrode, laser intensity, time, experimental group (e.g. control vs. FL), followed by a Holm-Sidak post-hoc analysis to evaluate significant differences between individual groups. A P value of <0.05 was considered significant.
Slice preparation and electrophysiology
Mice aged P46–180 were decapitated under isoflurane anesthesia. The brain was quickly removed and placed into an ice cold cutting solution (in mM: 2.5 KCl, 1.25 NaH2PO4, 10.0 MgCl2, 0.5 CaCl2, 26.0 NaHCO3, 11.0 glucose and 234.0 sucrose). Coronal brain slices were cut (TPI, St. Louis, MO) into a 300-μm thickness. Slices were incubated in 35 °C oxygenated (95% O2 and 5% CO2) aCSF (in mM: 126.0 NaCl, 2.5 KCl, 1.25 NaH2PO4, 1.0 MgCl2, 2.0 CaCl2, 26.0 NaHCO3 and 10.0 glucose, 295 mOsm) for 1 h. The incubation chamber was then kept at room temperature for up to 5 h throughout experiments. All recordings were performed at room temperature in circulating aCSF (1ml/min). Only slices with prominent barrels were used. Whole-cell recordings were obtained using borosilicate glass pipettes with a filament (resistance 4–5 MΩ). Recording pipettes were filled with a Cs-based intracellular solution (in mM: 120 cesium gluconate, 10 phosphocreatine-Tris, 3 MgCl2, 0.07 CaCl2, 4 EGTA, 10 HEPES, 4 Na2-ATP, and 1 Na-GTP) with the pH adjusted to 7.35 with CsOH (289 mOsm). Neurobiotin was always included in the intracellular solution (0.5%, wt/vol, Vector Labs). Whole-cell recordings were obtained with a Multiclamp 700B amplifier (Molecular Devices) and a 1322A AD/DA converter. Data from CRACM experiments was collected using the Matlab-based software Ephus [74]. Cells were recorded at depths ranging from 50 to 110 μm within the brain slice. For CRACM experiments, EPSCs were recorded via a voltage-clamp with holding potential at −45 to −48 mV (L2/3 PCs) or −45 to −53 mV (L5B PCs) such that a fast (several ms) downward current was evoked when a laser beam was directed to recorded neuronal soma position. IPSCs were recorded in the same mode with holding potential at 0 mV. L2/3 and L5B PCs were verified by post hoc morphological confirmation of the existence of thick proximal dendrites.
Photostimulation and CRACM
The blue laser beam (Shanghai Laser and Optics Century Co., 473 nm) was carefully aligned with routing mirrors, Pockels cell (ConOptics) and a pair of galvanometer scanners (Cambridge Scanning, Inc.) to generate a relatively cylindrical beam through a 4x objective (0.10 NA, Olympus) at the specimen plane (~15 μm in diameter, full-width at half max) as reported [36, 75]. A portion of the laser beam was redirected to a photodiode (silicon detector, Edmund Optics) through a beam splitter (30:70 split, 450–650 nm range, Thorlabs). The detected voltage was coupled to laser intensity measurements using a handheld laser power meter (Edmund Optics) placed at the immediate back of the 4x objective. The majority of recorded neurons were measured under two different intensities. The laser power (0.1–1.6 mW, 1 ms duration) was adjusted so that the largest IPSCCRACM had peak values ranging between 80–150 pA at the lower intensity, while the values ranged between 200–400 pA at the higher intensity [30–32]. Slices with insufficient virus expression (weak fluorescence) were excluded from subsequent analysis. Each cell was mapped with a 16 × 12 photostimulation grid (distance between adjacent points, 75 μm) covering the majority, if not all of the polysynaptic inputs to the dendritic arbor of recorded neurons. Each map was averaged using simulation results that were repeated 3x. The photostimulation sequence was given in a pseudo-random manner to maximize the intervals between adjacent locations receiving the photostimulation [30–32].
LED field stimulation
In a subset of experiments, blue LED (LEDD1B, 488 nm, Thorlabs) field stimulation was used via a 4x objective to examine E/I ratio differences when compared to the CRACM method (Figure S2F). The light was pulsed for a duration of 2 ms. Light intensity (0.1–0.8 mW) was adjusted accordingly as mentioned above for a two-intensity stimulation. The interval between sweeps (20 repetitions) were 10 s. Holding potentials for the voltage clamp were identical to those used for CRACM experiments for the same recorded neurons.
Data analysis for slice mapping experiments
We performed data analysis using custom-written programs in MATLAB (MATLAB, MathWorks). Each trace of raw data represented a 400-ms time window of averaged recorded current under the voltage clamp: the first 100 ms was the baseline before photostimulation and laser delivery at exactly 100 ms, followed by the 300 ms window which showed the evoked EPSC/IPSC (i.e. Figure S1C). The area of the postsynaptic potential, defined as the integral of the recorded potential above the baseline between 0 and 200 ms after laser onset, was calculated. The pixel values of the CRACM map correspond to the average EPSC or IPSC charge calculated as previous. This time window essentially captured the monosynaptic and major components of polysynaptic activities of evoked EPSC/IPSC. EPSC/IPSC charge within this time window was used in calculating their corresponding strengths and ratios. For each pixel of a CRACM map (16×12=192 pixels in total), we calculated the charges for both EPSCs and IPSCs and converted them to heat maps (Figure S1C1). Within each averaged EPSC map and its corresponding IPSC map of each cell, the values of the individual pixels were normalized to the pixel value of maximum charge within each EPSC map (e.g. Figure 1B, two outside scale bars). Considering the variability of ChR2 expression across different animals, we further unified the EPSC map scales per class to the scale of sham-L2/3 experiments (e.g. Figure 1B, two inside scale bars) by dividing or multiplying by a factor such that the maximum charge of each class of experiment was the same. The scales of corresponding IPSC maps were also altered accordingly.
E/I ratio maps were derived by dividing the EPSC charge by IPSC charge (pixel E/I ratio, e.g. Figure 1E, pixels with only either EPSC or IPSC or neither were excluded). This analysis gave rise to a spatial distribution pattern of E/I ratios of one recorded neuron across its dendritic arbor or soma with its polysynaptic input. The ratio limit was arbitrarily set as 10 (E/I ratio>10 was forced to 10). The E/I ratio is calculated by dividing the sum of the individual pixel EPSC charges by the sum of individual pixel IPSC charges. This calculation generated one single value reflecting the overall excitability profile of recorded neurons. In addition to the E/I ratio, response size for each synaptic class was also calculated to determine the polysynaptic or monosynaptic (for PV or SOM INs only) “receptive field” (RF) of each recorded neuron. Only pixels with significant responses (>2x standard deviation from the baseline) were included in the analysis.
Quantification and statistical analysis
Group averages are presented mean ± S.E.M. and a P value of <0.05 is considered significant. Following our analysis, raw data was exported to Microsoft Excel for tabulation of statistical averages and standard error values or Sigmaplot (ver. 11.0, SYSTAT software Inc., San Jose, CA) for graphical display and statistical analysis between groups. A multifactorial analysis of variance (ANOVA) was used to evaluate the main effects and interactions when multiple independent variables were present, including spike count, electrode, laser intensity, time, and experimental group (e.g. control vs. FL), and this was followed by a Holm-Sidak post-hoc analysis to evaluate significant differences between individual groups. A paired t test was used for neuronal E/I ratio comparisons at the two intensities. Nonparametric Wilcoxon signed-rank tests were used for pixel size comparisons between paired groups as well as for E/I ratio comparisons. Multivariate ANOVA was used for spectrum power analysis. One-way ANOVA with Bonferroni correction was used for multiple comparison between groups. Statistical methods were not used to pre-determine sample size.
Supplementary Material
KEY RESOURCES TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and Virus Strains | ||
| AAV2.1.CAG.hChR2(H134R)-mCherry | University of Pennsylvania Vector Core | Addgene_20938MOD |
| AAV2/1.CAGGS.flex.ChR2.tdTomato.WPRE.SV40 | University of Pennsylvania Vector Core | Addgene_18917 |
| Biological Samples | ||
| Cytochrome c from equine heart | Millipore Sigma | C-2506 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Alexa Fluor 594 | Invitrogen | A10438 |
| 3,3′-Diaminobenzidine tetrahydrochloride | Millipore Sigma | D-5905 |
| VECTASHIELD PLUS Antifade Mounting Medium with DAPI | Vector Laboratoties | H-2000 |
| Experimental Models: Organisms/Strains | ||
| Scnn1a-cre transgenic mouse | Jackson Lab | 009613 |
| PV-cre transgenic mouse | Jackson Lab | 008069 |
| Som-cre mouse | Jackson Lab | 013044 |
| C57BL/6J wild-type mouse | Charles River Laboratory | 027 |
| Recombinant DNA | ||
| AAV2.1.CAG.hChR2(H134R)-mCherry | University of Pennsylvania Vector Core | Addgene_20938MOD |
| AAV2/1.CAGGS.flex.ChR2.tdTomato.WPRE.SV40 | University of Pennsylvania Vector Core | Addgene_18917 |
| Software and Algorithms | ||
| MATLAB v. 2007b & 2011a | MathWorks | RRID: SCR_001622 |
| Spike-2 | Cambridge Electronic Design | RRID: SCR_000903 |
| Neuroexplorer | Nex Technologies. | RRID:SCR_001818 |
Highlights.
Ectopic infragranular excitatory inputs cause hyperexcitability and epileptogenesis
Disrupted E/I balance is more prominent in distal malformed cortex
SOM Inhibition in the distal supragranular layers are weakened
Closed-loop optogenetic activation SOM interneurons stops seizure in vivo
Acknowledgments
Funding for this research is provided by the NINDS/NIH grant 5R01NS094550 and P20GM121310. We thank the Sun lab members for assistance with animal care and breeding, proof reading of the manuscript, and Chen Zhou for performing the EEG electrode implants and recordings and closed-loop optogenetic stimulations. We thank Dr. Bryan M. Hooks from the Department of Neurobiology, University of Pittsburgh School of Medicine for sharing CRACM analysis tools and Dr. Karel Svoboda, HHMI, for generous assistance with building the CRACM rig. We thank Dr. John Huguenard for the recommendation on laminar plots shown in Figure 1E and Figure 3G.
Footnotes
Declaration of Interests
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barkovich AJ, Guerrini R, Kuzniecky RI, Jackson GD, and Dobyns WB (2012). A developmental and genetic classification for malformations of cortical development: update 2012. Brain 135, 1348–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guerrini R, and Dobyns WB (2014). Malformations of cortical development: clinical features and genetic causes. Lancet Neurol 13, 710–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuzniecky RI (1994). Magnetic resonance imaging in developmental disorders of the cerebral cortex. Epilepsia 35 Suppl 6, S44–56. [DOI] [PubMed] [Google Scholar]
- 4.Pang T, Atefy R, and Sheen V (2008). Malformations of cortical development. Neurologist 14, 181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barkovich AJ, Kuzniecky RI, Jackson GD, Guerrini R, and Dobyns WB (2005). A developmental and genetic classification for malformations of cortical development. Neurology 65, 1873–1887. [DOI] [PubMed] [Google Scholar]
- 6.Jansen A, and Andermann E (2005). Genetics of the polymicrogyria syndromes. J Med Genet 42, 369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maillard L, and Ramantani G (2018). Epilepsy surgery for polymicrogyria: a challenge to be undertaken. Epileptic Disord 20, 319–338. [DOI] [PubMed] [Google Scholar]
- 8.Wang DD, Knox R, Rolston JD, Englot DJ, Barkovich AJ, Tihan T, Auguste KI, Knowlton RC, Cornes SB, and Chang EF (2016). Surgical management of medically refractory epilepsy in patients with polymicrogyria. Epilepsia 57, 151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuzniecky R, Andermann F, and Guerrini R (1993). Congenital bilateral perisylvian syndrome: study of 31 patients. The CBPS Multicenter Collaborative Study. Lancet 341, 608–612. [DOI] [PubMed] [Google Scholar]
- 10.McBride MC, and Kemper TL (1982). Pathogenesis of four-layered microgyric cortex in man. Acta Neuropathol 57, 93–98. [DOI] [PubMed] [Google Scholar]
- 11.Leventer RJ, Jansen A, Pilz DT, Stoodley N, Marini C, Dubeau F, Malone J, Mitchell LA, Mandelstam S, Scheffer IE, et al. (2010). Clinical and imaging heterogeneity of polymicrogyria: a study of 328 patients. Brain 133, 1415–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luhmann HJ (2016). Models of cortical malformation--Chemical and physical. J Neurosci Methods 260, 62–72. [DOI] [PubMed] [Google Scholar]
- 13.Wong M, and Roper SN (2016). Genetic animal models of malformations of cortical development and epilepsy. J Neurosci Methods 260, 73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dvorak K, and Feit J (1977). Migration of neuroblasts through partial necrosis of the cerebral cortex in newborn rats-contribution to the problems of morphological development and developmental period of cerebral microgyria. Histological and autoradiographical study. Acta Neuropathol 38, 203–212. [DOI] [PubMed] [Google Scholar]
- 15.Dvorak K, Feit J, and Jurankova Z (1978). Experimentally induced focal microgyria and status verrucosus deformis in rats--pathogenesis and interrelation. Histological and autoradiographical study. Acta Neuropathol 44, 121–129. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs KM, Gutnick MJ, and Prince DA (1996). Hyperexcitability in a model of cortical maldevelopment. Cereb Cortex 6, 514–523. [DOI] [PubMed] [Google Scholar]
- 17.Luhmann HJ, and Raabe K (1996). Characterization of neuronal migration disorders in neocortical structures: I. Expression of epileptiform activity in an animal model. Epilepsy Res 26, 67–74. [DOI] [PubMed] [Google Scholar]
- 18.Gibbs SA, Scantlebury MH, Awad P, Lema P, Essouma JB, Parent M, Descarries L, and Carmant L (2008). Hippocampal atrophy and abnormal brain development following a prolonged hyperthermic seizure in the immature rat with a focal neocortical lesion. Neurobiol Dis 32, 176–182. [DOI] [PubMed] [Google Scholar]
- 19.Kellinghaus C, Moddel G, Shigeto H, Ying Z, Jacobsson B, Gonzalez-Martinez J, Burrier C, Janigro D, and Najm IM (2007). Dissociation between in vitro and in vivo epileptogenicity in a rat model of cortical dysplasia. Epileptic Disord 9, 11–19. [DOI] [PubMed] [Google Scholar]
- 20.Scantlebury MH, Gibbs SA, Foadjo B, Lema P, Psarropoulou C, and Carmant L (2005). Febrile seizures in the predisposed brain: a new model of temporal lobe epilepsy. Ann Neurol 58, 41–49. [DOI] [PubMed] [Google Scholar]
- 21.Brill J, and Huguenard JR (2010). Enhanced infragranular and supragranular synaptic input onto layer 5 pyramidal neurons in a rat model of cortical dysplasia. Cereb Cortex 20, 2926–2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luhmann HJ, Kilb W, and Clusmann H (2014). Malformations of cortical development and neocortical focus. Int Rev Neurobiol 114, 35–61. [DOI] [PubMed] [Google Scholar]
- 23.Williams AJ, and Sun QQ (2019). Cortical Layer and Spectrotemporal Architecture of Epileptiform Activity in vivo in a Mouse Model of Focal Cortical Malformation. Front Neural Circuits 13, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams AJ, Zhou C, and Sun QQ (2016). Enhanced Burst-Suppression and Disruption of Local Field Potential Synchrony in a Mouse Model of Focal Cortical Dysplasia Exhibiting Spike-Wave Seizures. Front Neural Circuits 10, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun QQ, Zhou C, Yang W, and Petrus D (2016). Continuous spike-waves during slow-wave sleep in a mouse model of focal cortical dysplasia. Epilepsia 57, 1581–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Worrell GA, Parish L, Cranstoun SD, Jonas R, Baltuch G, and Litt B (2004). High-frequency oscillations and seizure generation in neocortical epilepsy. Brain 127, 1496–1506. [DOI] [PubMed] [Google Scholar]
- 27.Bragin A, Wilson CL, Almajano J, Mody I, and Engel J Jr. (2004). High-frequency oscillations after status epilepticus: epileptogenesis and seizure genesis. Epilepsia 45, 1017–1023. [DOI] [PubMed] [Google Scholar]
- 28.Haegelen C, Perucca P, Chatillon CE, Andrade-Valenca L, Zelmann R, Jacobs J, Collins DL, Dubeau F, Olivier A, and Gotman J (2013). High-frequency oscillations, extent of surgical resection, and surgical outcome in drug-resistant focal epilepsy. Epilepsia 54, 848–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang W, and Sun QQ (2018). Circuit-specific and neuronal subcellular-wide E-I balance in cortical pyramidal cells. Sci Rep 8, 3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petreanu L, Huber D, Sobczyk A, and Svoboda K (2007). Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections. Nat Neurosci 10, 663–668. [DOI] [PubMed] [Google Scholar]
- 31.Petreanu L, Mao T, Sternson SM, and Svoboda K (2009). The subcellular organization of neocortical excitatory connections. Nature 457, 1142–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mao T, Kusefoglu D, Hooks BM, Huber D, Petreanu L, and Svoboda K (2011). Long-range neuronal circuits underlying the interaction between sensory and motor cortex. Neuron 72, 111–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aronoff R, and Petersen CC (2006). Controlled and localized genetic manipulation in the brain. J Cell Mol Med 10, 333–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li N, Chen TW, Guo ZV, Gerfen CR, and Svoboda K (2015). A motor cortex circuit for motor planning and movement. Nature 519, 51–56. [DOI] [PubMed] [Google Scholar]
- 35.Harris KD, and Shepherd GM (2015). The neocortical circuit: themes and variations. Nat Neurosci 18, 170–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wehr M, and Zador AM (2003). Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature 426, 442–446. [DOI] [PubMed] [Google Scholar]
- 37.Xue M, Atallah BV, and Scanziani M (2014). Equalizing excitation-inhibition ratios across visual cortical neurons. Nature 511, 596–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacobs KM, Hwang BJ, and Prince DA (1999). Focal epileptogenesis in a rat model of polymicrogyria. J Neurophysiol 81, 159–173. [DOI] [PubMed] [Google Scholar]
- 39.Lubke J, Roth A, Feldmeyer D, and Sakmann B (2003). Morphometric analysis of the columnar innervation domain of neurons connecting layer 4 and layer 2/3 of juvenile rat barrel cortex. Cereb Cortex 13, 1051–1063. [DOI] [PubMed] [Google Scholar]
- 40.Thomson AM, and Bannister AP (2003). Interlaminar connections in the neocortex. Cereb Cortex 13, 5–14. [DOI] [PubMed] [Google Scholar]
- 41.Bureau I, von Saint Paul F, and Svoboda K (2006). Interdigitated paralemniscal and lemniscal pathways in the mouse barrel cortex. PLoS Biol 4, e382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Connor DH, Hires SA, Guo ZV, Li N, Yu J, Sun QQ, Huber D, and Svoboda K (2013). Neural coding during active somatosensation revealed using illusory touch. Nat Neurosci 16, 958–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rudy B, Fishell G, Lee S, and Hjerling-Leffler J (2011). Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Dev Neurobiol 71, 45–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Packer AM, and Yuste R (2011). Dense, unspecific connectivity of neocortical parvalbumin-positive interneurons: a canonical microcircuit for inhibition? J Neurosci 31, 13260–13271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Silberberg G, and Markram H (2007). Disynaptic inhibition between neocortical pyramidal cells mediated by Martinotti cells. Neuron 53, 735–746. [DOI] [PubMed] [Google Scholar]
- 46.Dudek FE, and Staley KJ (2012). The Time Course and Circuit Mechanisms of Acquired Epileptogenesis In Jasper’s Basic Mechanisms of the Epilepsies, 4th Edition, Noebels JL, Avoli M, Rogawski MA, Olsen RW and Delgado-Escueta AV, eds. (Bethesda (MD)). [PubMed] [Google Scholar]
- 47.Okun M, Steinmetz N, Cossell L, Iacaruso MF, Ko H, Bartho P, Moore T, Hofer SB, Mrsic-Flogel TD, Carandini M, et al. (2015). Diverse coupling of neurons to populations in sensory cortex. Nature 521, 511–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Isaacson JS, and Scanziani M (2011). How inhibition shapes cortical activity. Neuron 72, 231–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shu Y, Hasenstaub A, and McCormick DA (2003). Turning on and off recurrent balanced cortical activity. Nature 423, 288–293. [DOI] [PubMed] [Google Scholar]
- 50.Zhou M, Liang F, Xiong XR, Li L, Li H, Xiao Z, Tao HW, and Zhang LI (2014). Scaling down of balanced excitation and inhibition by active behavioral states in auditory cortex. Nat Neurosci 17, 841–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun QQ, and Zhang Z (2011). Whisker experience modulates long-term depression in neocortical gamma-aminobutyric acidergic interneurons in barrel cortex. J Neurosci Res 89, 73–85. [DOI] [PubMed] [Google Scholar]
- 52.Bridi MCD, Zong FJ, Min X, Luo N, Tran T, Qiu J, Severin D, Zhang XT, Wang G, Zhu ZJ, et al. (2019). Daily Oscillation of the Excitation-Inhibition Balance in Visual Cortical Circuits. Neuron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wester JC, and Contreras D (2013). Generating waves in corticothalamocortical networks. Neuron 77, 995–997. [DOI] [PubMed] [Google Scholar]
- 54.Telfeian AE, and Connors BW (1998). Layer-specific pathways for the horizontal propagation of epileptiform discharges in neocortex. Epilepsia 39, 700–708. [DOI] [PubMed] [Google Scholar]
- 55.Adesnik H, and Scanziani M (2010). Lateral competition for cortical space by layer-specific horizontal circuits. Nature 464, 1155–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harris KD, and Mrsic-Flogel TD (2013). Cortical connectivity and sensory coding. Nature 503, 51–58. [DOI] [PubMed] [Google Scholar]
- 57.Crochet S, Poulet JF, Kremer Y, and Petersen CC (2011). Synaptic mechanisms underlying sparse coding of active touch. Neuron 69, 1160–1175. [DOI] [PubMed] [Google Scholar]
- 58.Groh A, Meyer HS, Schmidt EF, Heintz N, Sakmann B, and Krieger P (2010). Cell-type specific properties of pyramidal neurons in neocortex underlying a layout that is modifiable depending on the cortical area. Cereb Cortex 20, 826–836. [DOI] [PubMed] [Google Scholar]
- 59.Vogels TP, Sprekeler H, Zenke F, Clopath C, and Gerstner W (2011). Inhibitory plasticity balances excitation and inhibition in sensory pathways and memory networks. Science 334, 1569–1573. [DOI] [PubMed] [Google Scholar]
- 60.Wester JC, and Contreras D (2012). Columnar interactions determine horizontal propagation of recurrent network activity in neocortex. J Neurosci 32, 5454–5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Beenhakker MP, and Huguenard JR (2009). Neurons that fire together also conspire together: is normal sleep circuitry hijacked to generate epilepsy? Neuron 62, 612–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McCormick DA, and Contreras D (2001). On the cellular and network bases of epileptic seizures. Annu Rev Physiol 63, 815–846. [DOI] [PubMed] [Google Scholar]
- 63.Steriade M (2006). Neuronal substrates of spike-wave seizures and hypsarrhythmia in corticothalamic systems. Adv Neurol 97, 149–154. [PubMed] [Google Scholar]
- 64.Prince DA, and Wilder BJ (1967). Control mechanisms in cortical epileptogenic foci. “Surround” inhibition. Arch Neurol 16, 194–202. [DOI] [PubMed] [Google Scholar]
- 65.Wong M (2008). Mechanisms of epileptogenesis in tuberous sclerosis complex and related malformations of cortical development with abnormal glioneuronal proliferation. Epilepsia 49, 8–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schwartzkroin PA, and Walsh CA (2000). Cortical malformations and epilepsy. Ment Retard Dev Disabil Res Rev 6, 268–280. [DOI] [PubMed] [Google Scholar]
- 67.Schwarz P, Stichel CC, and Luhmann HJ (2000). Characterization of neuronal migration disorders in neocortical structures: loss or preservation of inhibitory interneurons? Epilepsia 41, 781–787. [DOI] [PubMed] [Google Scholar]
- 68.Hablitz JJ, and DeFazio T (1998). Excitability changes in freeze-induced neocortical microgyria. Epilepsy Res 32, 75–82. [DOI] [PubMed] [Google Scholar]
- 69.Jiang X, Lupien-Meilleur A, Tazerart S, Lachance M, Samarova E, Araya R, Lacaille JC, and Rossignol E (2018). Remodeled cortical inhibition prevents motor seizures in generalized epilepsy. Ann Neurol 84, 436–451. [DOI] [PubMed] [Google Scholar]
- 70.Meeren HK, Veening JG, Moderscheim TA, Coenen AM, and van Luijtelaar G (2009). Thalamic lesions in a genetic rat model of absence epilepsy: dissociation between spike-wave discharges and sleep spindles. Exp Neurol 217, 25–37. [DOI] [PubMed] [Google Scholar]
- 71.Zilles K, Qu M, Schleicher A, and Luhmann HJ (1998). Characterization of neuronal migration disorders in neocortical structures: quantitative receptor autoradiography of ionotropic glutamate, GABA(A) and GABA(B) receptors. Eur J Neurosci 10, 3095–3106. [DOI] [PubMed] [Google Scholar]
- 72.Dvorak K., and Feit J. (1978). Testing of the course of neurogenesis and gliogenesis in the germinative zones of the CNS of embryonal and early postnatal rats by means of the gel reaction for the histochemical demonstration of the thiamine-pyrophosphatase. Histochemical and autoradiographical study. Acta Histochem 63, 89–104. [DOI] [PubMed] [Google Scholar]
- 73.Wang X, and Sun QQ (2012). Characterization of axo-axonic synapses in the piriform cortex of Mus musculus. J Comp Neurol 520, 832–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Suter BA, O’Connor T, Iyer V, Petreanu LT, Hooks BM, Kiritani T, Svoboda K, and Shepherd GM (2010). Ephus: multipurpose data acquisition software for neuroscience experiments. Front Neural Circuits 4, 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cruikshank SJ, Lewis TJ, and Connors BW (2007). Synaptic basis for intense thalamocortical activation of feedforward inhibitory cells in neocortex. Nat Neurosci 10, 462–468. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The published article includes all datasets generated or analyzed during this study. Detailed datasets and codes supporting the current study are available from the corresponding authors on request.