Abstract
Radiation therapy, along with surgery and chemotherapy, is one of the main treatments for cancer. While radiotherapy is highly effective in the treatment of localized tumors, its main limitation is its toxicity to normal tissue. Previous preclinical studies have reported that ultra-high dose-rate (FLASH) irradiation results in reduced toxicity to normal tissues while controlling tumor growth to a similar extent relative to conventional-dose-rate (CONV) irradiation. To our knowledge this is the first report of a dose-response study in mice comparing the effect of FLASH irradiation vs. CONV irradiation on skin toxicity. We found that FLASH irradiation results in both a lower incidence and lower severity of skin ulceration than CONV irradiation 8 weeks after single-fraction hemithoracic irradiation at high doses (30 and 40 Gy). Survival was also higher after FLASH hemithoracic irradiation (median survival >180 days at doses of 30 and 40 Gy) compared to CONV irradiation (median survival 100 and 52 days at 30 and 40 Gy, respectively). No ulceration was observed at doses 20 Gy or below in either FLASH or CONV. These results suggest a shifting of the dose-response curve for radiation-induced skin ulceration to the right for FLASH, compared to CONV irradiation, suggesting the potential for an enhanced therapeutic index for radiation therapy of cancer.
INTRODUCTION
The main limitation of radiation therapy in the treatment of cancer is the toxicity associated with radiation (1). A primary method currently used in the clinic aimed to avoid this collateral damage is to use image-guided conformal therapy to minimize undesired irradiation of normal tissues. However, intermediate dose spill to adjacent normal tissues is unavoidable, as is high-dose irradiation of normal tissue within the target volume (2–5). Recently, novel preclinical observations on the biological effects of ultra-rapid FLASH irradiation have been described (6). Favaudon et al. demonstrated that delivery of FLASH irradiation (variably defined by dose rates ≥40 Gy/s) results in less toxicity to lung tissue compared to conventional-dose-rate irradiation (≤0.03 Gy/s), as measured by the induction of fibrosis. Similar effects of reduced normal tissue toxicity associated with FLASH irradiation have been reported on mouse brain and intestine (7–9).
Skin is a tissue for which there is dose-limiting toxicity in clinical scenarios. Sparing of mini-pig skin by FLASH has been reported on a single animal whose back was irradiated in different spots with single-fraction electron beam doses of 28–34 Gy at FLASH and conventional dose rates (10). In the same work, it was reported that no late skin toxicity other than alopecia was observed in six cats with spontaneous nasal planum squamous cell carcinoma treated with FLASH in single fractions of 25–41 Gy. Here, we report the skin toxicity outcomes of a dose-response experiment in which cohorts of mice received single-fraction hemithoracic FLASH or conventional-dose-rate irradiation, using an electron beam from a clinical linear accelerator configured to deliver dose rates up to the FLASH range. We evaluated the end points of the severity of skin toxicity and overall survival.
MATERIALS AND METHODS
All procedures for use of animals and their care were approved by the Institutional Animal Care and Use Committee of Stanford University (Stanford, CA) in accordance with institutional and NIH guidelines.
Mouse Irradiation
Female C57BL/6 mice were purchased from Charles River Laboratories (Wilmington, MA) at age 7 weeks and allowed to acclimate for 1 week before being irradiated. Mice were anaesthetized with a combination of ketamine [100 mg/kg; intraperitoneal (IP)] and xylazine (10 mg/kg; IP). FLASH and CONV irradiations were performed using an electron beam irradiation setup as described elsewhere (8, 11). In brief, mice were immobilized in a custom 3D-printed stereotactic positioning frame that was registered to a 3D-printed shield comprising a polylactic acid (PLA) plastic shell containing layers of aluminum oxide powder and tungsten spheres. In this study, the shield had a 2 × 2 cm opening. The field borders were designed to encompass the right hemithorax, with positions determined from microCT images of mice in the stereotactic positioning frame. Transverse and depth dose profiles for both FLASH and CONV geometries were measured by Gafchromic™ EBT3 films (Ashland™ Inc., Bridgewater NJ) between layers of water-equivalent polystyrene. The beam parameters included electron energy of 16 MeV. Beam parameters for FLASH irradiation were: pulse repetition rate 90 Hz, dose per pulse 2.0 Gy, average dose rate 180 Gy/s, and instantaneous dose rate (in 5 μs pulse) 4.0 × 105 Gy/s. Beam parameters for CONV irradiation were: pulse repetition rate 72 Hz, dose per pulse 0.00104 Gy, average dose rate 0.0747 Gy/s, instantaneous dose rate (in 5 μs pulse) 207 Gy/s. Entrance dose for every individual mouse irradiation was recorded by Gafchromic EBT3 films placed inside the positioning frame, and the average entrance dose in the central 8.5 × 8.5 mm square of the field was recorded as the delivered dose. We administered doses of 10, 16, 20, 30 or 40 Gy FLASH or CONV in a single fraction to 5 mice in each dose cohort.
Skin Toxicity Scoring
An ordinal scale to score skin toxicity was developed using the following criteria: A score of 0 = normal; 1 = <50% depigmentation within the radiation field; 2 = ≥ 50% depigmentation within the radiation field; 3 = <50% alopecia (± depigmentation) within the radiation field; 4 = ≥50% alopecia (± depigmentation) within the radiation field; 5 = <50% ulceration (± alopecia and depigmentation) within the radiation field; and 6 = ≥50% ulceration (± alopecia and depigmentation) within the radiation field. At 8 weeks (or earlier if required by euthanasia criteria, i.e., on day 52 in these cases), all mice were scored by two independent observers blinded to the intervention. If scores deviated between observers, the average of the two scores was used as the final score. Inter-rater reliability by Cohen’s kappa coefficient was calculated to determine the agreement between the independent observers.
Mouse Monitoring and Euthanasia Criteria
All mice were monitored weekly for 8 weeks for development of skin toxicity within the radiation field. Mice noted to develop cutaneous ulcerations were monitored by Veterinary Service Center staff and received a single dose of buprenorphine SR (1 mg/kg; SQ) and daily topical Neosporin®. Mice whose cutaneous lesions progressed despite treatment were humanely euthanized via CO2 asphyxiation and cardiac exsanguination at, or prior to, the 8-week timepoint.
Histopathology
After euthanasia, skin within the radiation field was fixed in 10% neutral buffered formalin for at least 72 h. A single strip of skin was harvested through the radiation field parallel to the hair growth. Formalin-fixed skin samples were processed routinely, embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin (H&E; Histo-Tec Laboratory Inc., Hayward, CA).
Survival Analysis
Survival was monitored for up to six months postirradiation. Euthanasia in mice meeting skin toxicity euthanasia criteria was scored as a mortality event. Mice that were sacrificed for tissue harvesting for a concurrent study were censored at the time of sacrifice (at days 52 or 56). Kaplan-Meier survival analysis was performed.
Statistical Analysis
A Mann-Whitney t test was used to calculate significance between equal FLASH and CONV doses. A log-rank test was used to calculate significance for the survival curves. Inter-rater reliability was determined by calculating Cohen’s kappa.
RESULTS
FLASH Irradiation Results in Reduced Severe Skin Toxicity Compared to CONV Irradiation at High Doses
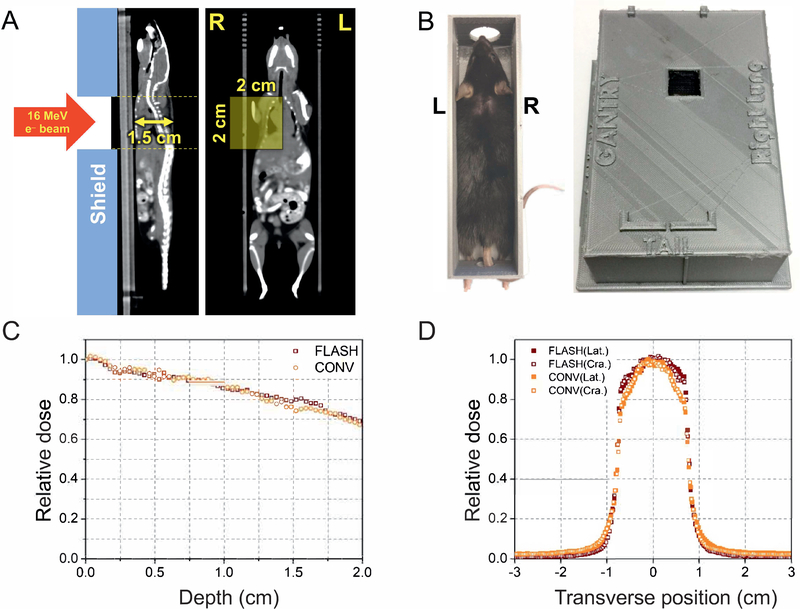
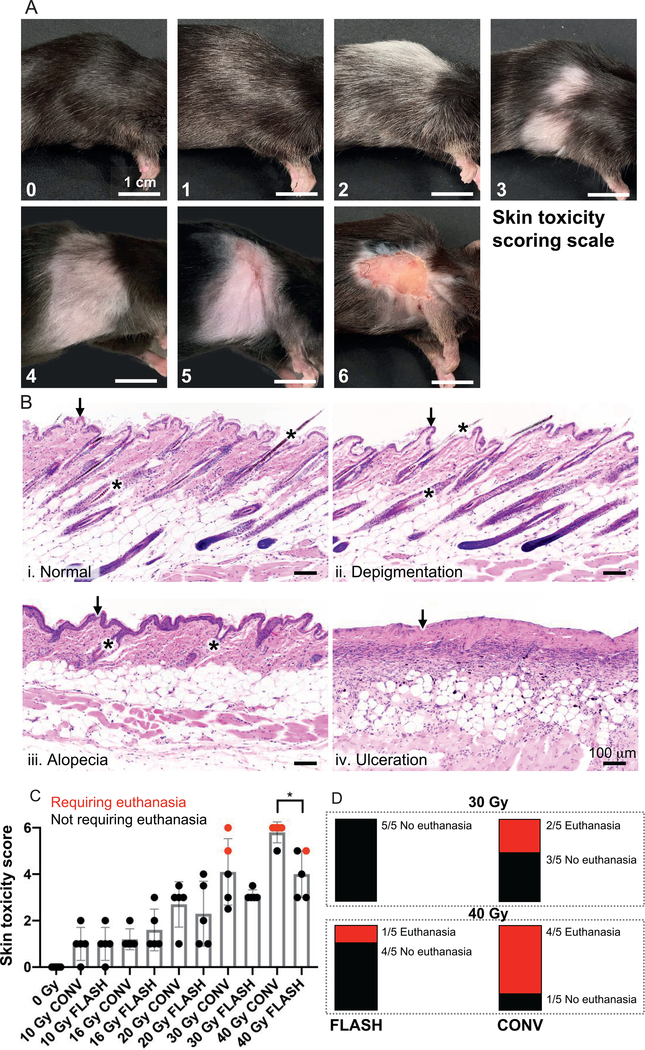
To compare the effects of FLASH vs. CONV irradiation, we tested different doses of each. First, we took CT scans of mice to outline the irradiation field and to verify correct positioning inside a stereotactic positioning frame (Fig. 1A). We then built a radiation shield with a 2 × 2 cm opening designed for right-sided hemithoracic irradiation (Fig. 1B). Eight-week-old, female C57BL/6 mice received 10, 16, 20, 30 or 40 Gy FLASH or CONV irradiation. Both FLASH and CONV setups produce equal and relatively homogeneous dose distributions throughout the irradiated volume (Fig. 1C and D). At 8 weeks postirradiation, all mice were assigned a skin toxicity score based on our scoring system (Fig. 2A). Histologic analysis demonstrated microscopic correlates of the gross findings of depigmentation, alopecia and ulceration (Fig. 2B) that were the basis of our skin toxicity score. Seven mice were euthanized on day 52 because of progressive ulceration, with 5 out of those 7 mice receiving a score of 6 while the remaining 2 mice received a score of 5. A high inter-rater reliability was determined based on a Cohen’s kappa coefficient of 0.91. Table 1 summarizes the number of mice per group for each skin toxicity score (0–6). Only mice that received 30 or 40 Gy CONV or 40 Gy FLASH irradiation developed skin ulcerations at approximately week 8 postirradiation. There was significantly decreased (P < 0.05) skin toxicity in the 40 Gy FLASH-irradiated group compared to the 40 Gy CONV-irradiated group (Fig. 2C). All mice in the 40 Gy CONV-irradiated group developed ulcerations, and 4 out of 5 mice had a score of 6 and were euthanized, with the remaining mouse having a score of 5 (Fig. 2C and D). In contrast, only 2 out of 5 mice in the 40 Gy FLASH-irradiated group developed ulcerations with a score of 5. Of these 2 mice, only 1 had to be euthanized (Fig. 2C and D), indicating a skin-sparing effect of FLASH irradiation. In the 30 Gy CONV-irradiated cohort, 1 mouse had a score of 6 and another had a score of 5; both had to be euthanized. By comparison, 4 out of 5 mice in the 30 Gy FLASH-irradiated cohort had a score of 3, one had a score of 3.5, and none had to be euthanized (Fig. 2C and D), again consistent with a skin-sparing effect of FLASH irradiation. At lower doses, mice did not develop ulcerations. While FLASH was associated with lower skin toxicity scores than CONV irradiation, on histology we observed no visible differences between FLASH and CONV irradiation in the character of the pathologic features (i.e., depigmentation, alopecia and ulceration) except in frequency or severity grossly.
FIG. 1.
Irradiation setup and dosimetry. Panel A: CT images (coronal and sagittal slices) showing mouse positioning within the stereotactic positioning frame. The yellow shaded rectangle indicates the irradiated region, designed for right hemithoracic irradiation. The thickness of the mice is about 1.5 cm. The direction of radiation (ventral to dorsal) is shown. Panel B: A mouse placed within our stereotactic immobilization frame (left) with the left and right sides indicated by L and R, respectively. The stereotactic frame registers to a fixed location on the radiation shield (right), producing a fixed relationship between the opening in the shield relative to the mouse anatomy, in this case producing a 2 × 2 cm hemithoracic irradiation field. Panels C and D: Depth and transverse (lateral and craniocaudal) dose profiles of FLASH vs. CONV dose-rate setups measured by film in a water-equivalent polystyrene phantom. Doses are relative to the maximum dose: at the center of the field for the transverse profiles recorded at the exit of the shield at the entrance surface of the stereotactic positioning frame; at the entrance surface of the mouse for the depth profile (after the buildup from the 1-mm PLA wall of the stereotactic positioning frame) recorded along the central axis of the field. Both FLASH and conventional setups produce equal and relatively homogeneous dose distributions throughout the irradiated volume.
FIG. 2.
FLASH irradiation results in reduced severe skin toxicity compared to CONV dose-rate irradiation at high doses. Panel A: Skin toxicity scoring scale. Lesions were graded on a 0–6 scale using the following criteria:← 0 = normal; 1 = <50% depigmentation within the radiation field; 2 = ≥50% depigmentation within the radiation field; 3 = <50% alopecia (± depigmentation) within the radiation field; 4 = ≥50% alopecia (± depigmentation) within the radiation field; 5 = <50% ulceration (± alopecia and depigmentation) within the radiation field; 6 = ≥50% ulceration (± alopecia and depigmentation) within the radiation field. Representative images are shown of the scores assigned. Inter-rater reliability score (Cohen’s kappa) between two observers blinded to the interventions was 0.91. Scale bar = 1 cm. Panel B: Representative histologic images (H&E stained) of (i) normal mouse skin, (ii) depigmentation, (iii) alopecia and (iv) ulceration. In the normal mouse skin, the epidermis is 1–2 cell layers thick (i, black arrow) and there are numerous, evenly-spaced anagen hair follicles (i, asterisks) containing pigmented hair shafts. Mice exhibiting depigmentation retain the 1–2 cell layer thick epidermis (ii, black arrow) and evenly-spaced anagen hair follicles, but have increased numbers of depigmented hair shafts (ii, asterisks). Mice exhibiting alopecia have fewer, unevenly-spaced catagen hair follicles (iii, asterisks) and epidermal hyperplasia (iii, black arrow). Mice with ulceration have complete loss of the epidermis (iv, black arrow) and all associated hair follicle structures. Histologically, we observed no visible differences between FLASH and CONV irradiation in the character of these pathologic features except in frequency or severity grossly. Scale bar = 100 μm. Panel C: Severity of skin toxicity per cohort at 8 weeks postirradiation. The 40 Gy FLASH-irradiated cohort had significantly lower scores than the 40 Gy CONV-irradiated cohort; n = 5 per cohort; red dots indicate animals that had to be euthanized for meeting skin toxicity criteria; bars represent mean ± SD. *P < 0.05. Panel D: Mice in high-dose irradiated cohorts that met euthanasia criteria by week 8 postirradiation. Only mice in the 30 and 40 Gy CONV irradiated, and 40 Gy FLASH irradiated cohorts had to be euthanized for meeting skin toxicity criteria. Each bar represents a fraction of the total in each cohort that either met or did not meet euthanasia criteria. These results indicate a skin-sparing effect of FLASH compared to CONV irradiation at high doses of 30–40 Gy in a single fraction. At doses up to 20 Gy, there was no ulceration in either FLASH or CONV irradiated animals at 8 weeks.
TABLE 1:
Number of Mice by Cohort per Skin Toxicity Score
| Radiation dose/modality | Skin toxicity score |
||||||
|---|---|---|---|---|---|---|---|
| 0 | 1 (1.5) | 2 (2.5) | 3 (3.5) | 4 (4.5) | 5 (5.5) | 6 | |
| 0 Gy | 5 | - | - | - | - | - | - |
| 10 Gy conventional | 1 | 3 | 1 | - | - | - | - |
| 10 Gy FLASH | 1 | 3 | 1 | - | - | - | - |
| 16 Gy conventional | - | 4 | 1 | - | - | - | - |
| 16 Gy FLASH | - | 3 | 1 | 1 | - | - | - |
| 20 Gy conventional | - | 1 | - | 3(1) | - | - | - |
| 20 Gy FLASH | - | 2 | 1 | - (1) | 1 | - | - |
| 30 Gy conventional | - | - | - (1) | 1 | 1 | 1 | 1 |
| 30 Gy FLASH | - | - | - | 4 (1) | - | - | - |
| 40 Gy conventional | - | - | - | - | - | 1 | 4 |
| 40 Gy FLASH | - | - | - | 2 | 1 | 2 | |
Note. 5 mice per cohort.
FLASH Irradiation Results in Reduced Mortality Compared to CONV Irradiation at High Doses
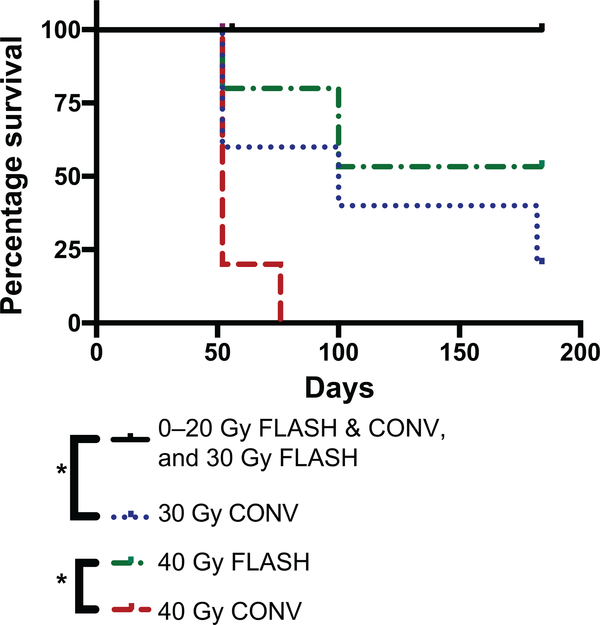
After observing that FLASH irradiation results in less severe skin toxicity than CONV irradiation at high doses, we sought to determine if there was a difference in long-term survival between the cohorts. Of note, 2 mice in each cohort were euthanized on days 52 or 56 for tissue harvesting for a concurrent study (except the 40 Gy and 30 Gy conventionally irradiated cohorts, which had 4 and 2 mice, respectively, euthanized due to skin toxicity euthanasia criteria) and were censored at that time. We found significantly longer survival of mice that received FLASH irradiation compared to CONV irradiation at high doses (Fig. 3). The 40 Gy CONV-irradiated cohort had a median survival of 52 days postirradiation (due to skin toxicity euthanasia criteria) and only 1 mouse survived to day 72 postirradiation (spontaneous death). By comparison, only 1 mouse in the 40 Gy FLASH cohort was euthanized due to skin toxicity euthanasia criteria on day 52 postirradiation (Figs. 2C and 3), 1 mouse survived to day 100 (spontaneous death), and two mice were still alive on day 184 postirradiation (Fig. 3). The 30 Gy CONV-irradiated cohort had a median survival of 100 days postirradiation, with 2 mice euthanized on day 52 (due to skin toxicity euthanasia criteria), 1 mouse surviving to day 100 postirradiation (spontaneous death), 1 mouse surviving to day 182 postirradiation (spontaneous death), and 1 mouse still alive on day 184 postirradiation. In contrast, all mice left in the 30 Gy FLASH cohort were still alive on day 184 postirradiation (Fig. 3). All the mice in the remaining cohorts were still alive on day 184 postirradiation (Fig. 3).
FIG. 3.
FLASH irradiation results in reduced mortality compared to CONV dose-rate irradiation at high doses. Kaplan-Meier survival curves of mice. Control: 0 Gy (n = 5); FLASH: 10, 16, 20, 30 and 40 Gy (n = 5 each); CONV: 10, 16, 20, 30 and 40 Gy (n = 5 each). Control (0 Gy), FLASH (10, 16, 20 and 30 Gy) and CONV (10, 16, 20 Gy) irradiation groups all had a 100% survival and are shown as a single curve. Censored subjects are shown as ticks. There was significantly longer survival with 30 Gy FLASH compared to 30 Gy CONV irradiation, and also significantly longer survival with 40 Gy FLASH irradiation compared to 40 Gy CONV irradiation; *log-rank P < 0.05.
DISCUSSION
Radiation-induced skin toxicity has historically been one of the dose-limiting toxicities of cancer radiation therapy. Skin sparing by the use of high-energy X rays and more conformal plans using beams from multiple directions have mitigated this issue, but it remains a clinical problem, particularly when treating target volumes that include superficial tissues, including the skin itself. FLASH irradiation has been shown preclinically to increase the radiobiological therapeutic index, primarily through decreased normal tissue toxicity, as seen in multiple organ systems including lung, brain and intestinal tract (6–9). With respect to skin sparing by FLASH, to date there has been one reported study comparing FLASH and CONV-dose-rate irradiation in a single mini-pig and the suggestion of a favorable therapeutic index in a single-arm dose-escalation trial of FLASH for nasal planum squamous cancer in six cats (10). In the first human case report of FLASH radiation therapy, a patient treated with 15 Gy FLASH (delivered in 90 ms) to a cutaneous lymphoma lesion had a complete response and no late skin toxicity, suggesting that the therapeutic index advantage of FLASH may translate to the clinical setting (12).
Here, we conducted a preclinical dose-response study of FLASH irradiation vs. CONV dose-rate hemithoracic irradiation in mice, covering the dose range of 10–40 Gy in a single fraction. The results of our study suggest that at high doses (30–40 Gy), compared to CONV irradiation, FLASH produced less toxicity on the two end points of severe skin toxicity (especially ulceration) and mortality. However, at smaller doses of 20 Gy or less, there was no severe toxicity associated with either FLASH or CONV irradiation. Further studies with larger cohorts are needed to establish if there is a difference between FLASH and CONV irradiation at lower doses.
The mechanism behind the reduced toxicity of FLASH irradiation on normal tissues has not been elucidated. One hypothesis is that the dose rate of FLASH is sufficiently high to cause radiochemical depletion of molecular oxygen before oxygen can be replenished by circulation in tissues in vivo, leading to an anoxic dose response and increased survival of stem cells (13–16). At the same time, normal tissues may be afforded a relative advantage over tumor cells because tumors have impaired ability to sequester labile iron that contributes to magnification through Fenton chemistry of the burst of damaging free radical chain reactions generated by FLASH irradiation relative to CONV irradiation (17). Further studies, to understand the mechanism behind FLASH sparing of normal tissue while maintaining tumor cytotoxicity, are needed to fully implement FLASH radiotherapy in the clinic as technologies to deliver conformal FLASH therapy to human patients are being developed (18–20).
In conclusion, we present proof-of-principle for a shift to the right of the radiation dose-response relationship for skin toxicity and mortality for FLASH irradiation compared to CONV dose-rate hemithoracic irradiation in mice. The difference in severe toxicity was evident at high doses (30–40 Gy), but lack of severe toxicity at lower doses (20 Gy or less) limits the assessment of differences at those doses. Our data, together with other studies, suggest that FLASH radiotherapy could increase the therapeutic index over current radiotherapy, potentially allowing higher, more curative doses to treat tumors while sparing normal tissue.
ACKNOWLEDGMENTS
This research was supported by funds from the National Cancer Institute (grant nos. R01CA233958 and R01CA197136-S1), the Tobacco-Related Disease Research Program of the University of California (grant no. T29IP0443), the American Lung Association (award no. LCD-565487), the National Institutes of Health (Training Grant no. CA09302), the Stanford University Department of Radiation Oncology, and SLAC National Accelerator Laboratory. We also gratefully acknowledge the generous support of philanthropic donors to the Department of Radiation Oncology. We thank Miguel Jimenez, James Clayton and Daniel Pawlak from Varian Medical Systems for their technical assistance on the project. KB, PGM, EEG and BWL have received research support from Varian Medical Systems. PGM and BWL are co-founders of TibaRay. BWL is a board member of TibaRay.
REFERENCES
- 1.De Ruysscher D, Niedermann G, Burnet NG, Siva S, Lee AWM, Hegi-Johnson F. Radiotherapy toxicity. Nat Rev Disease Primers 2019; 5:13. [DOI] [PubMed] [Google Scholar]
- 2.Shah JL, Loo BW Jr. Stereotactic Ablative Radiotherapy for early-stage lung cancer. Sem Rad Onc 2017; 27:218–28. [DOI] [PubMed] [Google Scholar]
- 3.Pfeffer MR. Radiotherapy for breast cancer: curing the cancer while protecting the heart. Isr Med Assoc J 2018; 20:582–3. [PubMed] [Google Scholar]
- 4.Ho JC, Phan J. Reirradiation of head and neck cancer using modern highly conformal techniques. Head Neck 2018; 40:2078–93. [DOI] [PubMed] [Google Scholar]
- 5.Hauer-Jensen M, Denham JW, Andreyev JN. Radiation enteropathy–Pathogenesis, treatment and prevention. Nat Rev Gastroenterol Hepatol 2014; 11:470–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Favaudon V, Caplier L, Monceau V, Pouzoulet F, Sayarath M, Fouillade C, et al. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci Trans Med 2014; 6:245ra93. [DOI] [PubMed] [Google Scholar]
- 7.Montay-Gruel P, Petersson K, Jaccard M, Boivin G, Germond JF, Petit B, et al. Irradiation in a flash: Unique sparing of memory in mice after whole brain irradiation with dose rates above 100 Gy/s. Radiother Oncol 2017; 124:365–9. [DOI] [PubMed] [Google Scholar]
- 8.Levy K, Natarajan S, Wang J, Chow S, Eggold J, Loo P, et al. FLASH irradiation enhances the therapeutic index of abdominal radiotherapy in mice. bioRxiv 2019.12.12.873414. [Google Scholar]
- 9.Diffenderfer ES, Verginadis II, Kim MM, Shoniyozov K, Velalopoulou A, Goia D, et al. Design, implementation, and in vivo validation of a novel proton FLASH radiation therapy system. Int J Rad Onc Biol Phys 2020; 106:440–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vozenin MC, De Fornel P, Petersson K, Favaudon V, Jaccard M, Germond JF, et al. The advantage of FLASH radiotherapy confirmed in mini-pig and cat-cancer patients. Clin Cancer Res 2019; 25:35–42. [DOI] [PubMed] [Google Scholar]
- 11.Schuler E, Trovati S, King G, Lartey F, Rafat M, Villegas M, et al. Experimental platform for ultra-high dose rate FLASH irradiation of small animals using a clinical linear accelerator. Int J Rad Onc Biol Phys 2017; 97:195–203. [DOI] [PubMed] [Google Scholar]
- 12.Bourhis J, Sozzi WJ, Jorge PG, Gaide O, Bailat C, Duclos F, et al. Treatment of a first patient with FLASH-radiotherapy. Radiother Oncol 2019; 139:18–22. [DOI] [PubMed] [Google Scholar]
- 13.Vozenin MC, Hendry JH, Limoli CL. Biological benefits of ultra-high dose rate FLASH radiotherapy: Sleeping beauty awoken. Clin Onc 2019; 31:407–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pratx G, Kapp DS. Ultra-high-dose-rate FLASH irradiation may spare hypoxic stem cell niches in normal tissues. Int J Rad Onc Biol Phys 2019; 105:190–2. [DOI] [PubMed] [Google Scholar]
- 15.Pratx G, Kapp DS. A computational model of radiolytic oxygen depletion during FLASH irradiation and its effect on the oxygen enhancement ratio. Phys Med Biol 2019; 64:185005. [DOI] [PubMed] [Google Scholar]
- 16.Petersson K, Adrian G, Butterworth K, McMahon SJ. A quantitative analysis of the role of oxygen tension in FLASH radiotherapy. Int J Rad Onc Biol Phys 2020; 107:539–47. [DOI] [PubMed] [Google Scholar]
- 17.Spitz DR, Buettner GR, Petronek MS, St-Aubin JJ, Flynn RT, Waldron TJ, et al. An integrated physico-chemical approach for explaining the differential impact of FLASH versus convensional dose rate irradiation on cancer and normal tissue responses. Radiother Oncol 2019; 139:23–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bourhis J, Montay-Gruel P, Jorge PG, Bailat C, Petit B, Ollivier J, et al. Clinical translation of FLASH radiotherapy: Why and how? Radiother Oncol 2019; 139:11–17. [DOI] [PubMed] [Google Scholar]
- 19.Colangelo NW, Azzam EI. The importance and clinical implications of FLASH ultra-high dose-rate studies for proton and heavy ion radiotherapy. Rad Res 2020; 193:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maxim PG, Tantawi SG, Loo BW Jr. PHASER: A platform for clinical translation of FLASH cancer radiotherapy. Radiother Oncol 2019; 139:28–33. [DOI] [PubMed] [Google Scholar]