Abstract
Given significant species-specific differences in liver functions, cultures of primary human hepatocytes (PHHs) are useful for assessing drug metabolism and to mitigate the risk of drug-induced hepatotoxicity in humans. While significant advances have been made to keep PHHs highly functional for 2–4 weeks in vitro, especially upon co-culture with both liver- and non-liver-derived non-parenchymal cells (NPCs), the functional lifespan of PHHs is 200–400 days in vivo. Therefore, it is desirable to determine culture conditions that can further prolong PHHs functions in vitro for modeling chronic drug exposure, disease pathogenesis, and to provide flexibility to the end-user for staggering drug incubations across multiple culture batches. Most PHH culture platforms utilize supraphysiologic levels of glucose and insulin and bovine-derived serum when including NPCs, which can alter PHH functions. Therefore, here we developed a culture medium containing physiologic levels of glucose (5 mM), insulin (500 pM), and human serum (10% v/v) and tested its effects on micropatterned co-cultures (MPCCs) in which PHHs are organized onto collagen domains of empirically optimized dimensions and surrounded by 3T3-J2 murine fibroblasts that express liver-like molecules and induce higher PHH functions than liver-derived NPCs. Our physiologically-inspired culture medium allowed better retention of PHH morphology, polarity, and functions (albumin and urea, cytochrome-P450 activities, and sensitivity to insulin-mediated inhibition of gluconeogenesis) for up to 10 weeks relative to the traditional medium. Finally, PHHs in the physiologic medium displayed clinically-relevant responses to prototypical drugs for hepatoxicity and CYP induction. Ultimately, our physiologic culture medium could find broader utility for the continued development of PHH-NPC co-cultures for drug development, investigating the effects of patient-derived sera on PHH functions and disease phenotypes, and for use in cell-based therapies.
Keywords: hepatotoxicity, CYP induction, human serum, insulin resistance
INTRODUCTION
Preclinical detection of drug-induced liver injury (DILI) is a critical part of drug development towards mitigating the risk to patients in clinical trials and the marketplace (Abboud and Kaplowitz 2007). Furthermore, there are considerable efforts to discover safe and efficacious drugs against global liver diseases such as hepatitis B viral (HBV) infection and non-alcoholic fatty liver disease (NAFLD) that currently have no permanent cures. While testing drug candidates in live animals serves an important role in drug development that is mandated by regulatory agencies, it has become clear over several high-profile drug failures that animal models cannot suffice on their own to mitigate the risk of DILI to humans due to significant differences in drug metabolism pathways of the liver (Khetani et al. 2013; Shih et al. 1999). Additionally, either the animal models for certain liver diseases (e.g. NAFLD) show significant differences in disease pathogenesis than their human counterparts or the available animal model is too expensive and/or restricted for routine use during drug development (e.g. chimpanzee for HBV infection) (Delire et al. 2015; Takahashi et al. 2012).
Given the above limitations of animals for drug testing, the use of in vitro human liver models throughout the drug development pipeline has steadily increased over the last few decades. While human liver models can be fabricated using transformed hepatic cell lines and hepatocyte-like cells derived from induced pluripotent stem cells, the lower drug metabolism capacities of these cell sources relative to the adult human liver make their use limited to the earlier stages of drug development (i.e. initial metabolism and toxicity screening) (Schwartz et al. 2014; Wilkening et al. 2003). In contrast, precision-cut liver slices retain the architectural and cellular complexity of the liver, they do not help address throughput needs during the early stages of drug development and display a rapid decline in drug metabolism capacities (Underhill and Khetani 2018). Therefore, primary human hepatocyte (PHH) cultures are widely considered to the ‘gold standard’ for generating in vitro models of the human liver for drug development and disease modeling.
Since conventional 2-dimensional (2D) confluent monolayers of PHHs on collagen-coated plastic are known to display a rapid decline in phenotypic functions, several culture techniques have been developed to prolong PHH functional lifetime in vitro to 2–4 weeks in vitro (Godoy et al. 2013). Typically, the highest and longest-lasting PHH functions are observed upon co-culture with both liver- and non-liver-derived non-parenchymal cells (NPCs) in both 2D and 3-dimensional (3D) culture formats (Underhill and Khetani 2018). However, hepatocyte lifespan in vivo is typically 200 to 400 days (Macdonald 1961; Magami et al. 2002), which suggests that further improvements can be made to PHH culture models to improve functional lifetime that can prove useful for elucidating the long-term effects of pharmaceuticals on normal PHHs and those subjected to disease-causing stimuli (e.g. HBV, over-nutrition). One such area of improvement is the type of serum and levels of hormones used in hepatocyte monocultures and hepatocyte-NPC co-cultures. PHH monocultures, as well as PHH-NPC co-cultures, are typically cultured with supraphysiologic levels of glucose and insulin, which can lead to excessive lipid accumulation (steatosis) and ensuing insulin resistance as well as alterations of drug metabolism activities in PHHs (Davidson et al. 2016; Davidson et al. 2015). Furthermore, PHH-NPC co-culture media generally contains serum derived from bovine sources, which does not entirely mimic the composition of human serum. Therefore, culture medium composition can be further improved for PHH cultures towards enabling more physiological outcomes; in particular, better insulin sensitivity in PHH cultures may help maintain higher metabolic activity for assessing drug metabolism and drug-induced hepatotoxicity, as well as prove useful for investigating the mechanisms by which over-nutritional stimuli (e.g. high glucose, fructose, and fatty acids) lead to the development of excessive steatosis and insulin resistance over time in PHHs.
Here, we hypothesized that a physiologically inspired culture medium, composed of human serum and physiologic levels of glucose and insulin, could maintain PHH functions for a longer duration in vitro. To test our hypothesis, we utilized well-established micropatterned co-cultures (MPCC) in which PHHs are organized onto collagen-coated circular islands of empirically optimized dimensions and subsequently surrounded by 3T3-J2 murine embryonic fibroblasts that express liver-like molecules to support hepatocytes from multiple species (Underhill and Khetani 2018). MPCCs were cultured for ~10 weeks either in the traditional culture medium previously reported (i.e. containing bovine serum and high levels of insulin) or the physiologically inspired culture medium developed here. PHH phenotype in both culture media formulations was assessed via morphological observations, secretions of urea and albumin in the supernatants, activities of cytochrome-P450 (CYP) enzymes, and bile canaliculi formation and retention. Finally, the utility of MPCCs cultured in the physiologic medium was assessed for the detection of drug-induced hepatotoxicity and drug-mediated CYP induction, two key applications of human liver models in the drug development pipeline.
METHODS
Cell culture
Cryopreserved primary human hepatocytes (PHHs) were obtained from BioIVT (Baltimore, MD) or Lonza, Inc. (Walkersville, MD). PHH lots included EJW (29 year old female Caucasian, BioIVT), HUM4055A (54 year old female Caucasian, Lonza), and HUM4011 (26 year old male Caucasian, Lonza). Micropatterned co-cultures (MPCCs) were created as previously described (Lin and Khetani 2017). Briefly, rat tail collagen-I (Corning Life Sciences, Tewksbury, MA) was lithographically patterned to create 500 μm diameter circular domains spaced 1200 μm apart, center-to-center. PHHs selectively attached to the collagen domains leaving ~25,000 attached PHHs on ~90 collagen-coated islands or ~3,500 PHHs on ~13 islands within each well of a 24-well or 96-well plate, respectively. 3T3-J2 murine embryonic fibroblasts, that were first growth-arrested by incubating with 2 μg/mL mitomycin-C (Sigma-Aldrich, St. Louis, MO) for 4 hours, were seeded the next day at 3:1 ratio with PHHs to create MPCCs (Fig. 1a). MPCCs were cultured in a medium composed of 5 mM D-glucose added (Fisher BioReagents, Pittsburgh, PA) to a glucose-free Dulbecco’s Modified Eagle’s Medium (DMEM) base (Corning Life Sciences); additional medium components included glucagon (2nM), dexamethasone (0.5μM), HEPES (15mM), L-glutamine (4mM), penicillin-streptomycin (1% v/v), transferrin (6.35 μg/ml), selenium (6.25 ng/ml), and various concentrations of insulin (500 pM, physiologic or 1 μM, supraphysiologic typically used in PHH culture medium). Finally, 10% bovine serum (Thermofisher, Waltham, MA) or 10% pooled human serum (Biocell, Rancho Dominguez, CA and Lonza) was added to the medium described above to generate either the ‘traditional’ or ‘physiologic’ culture medium formulations; culture medium was replaced on MPCCs every 2 days.
Figure 1. Schematic for MPCC fabrication and hepatocyte morphology over time.
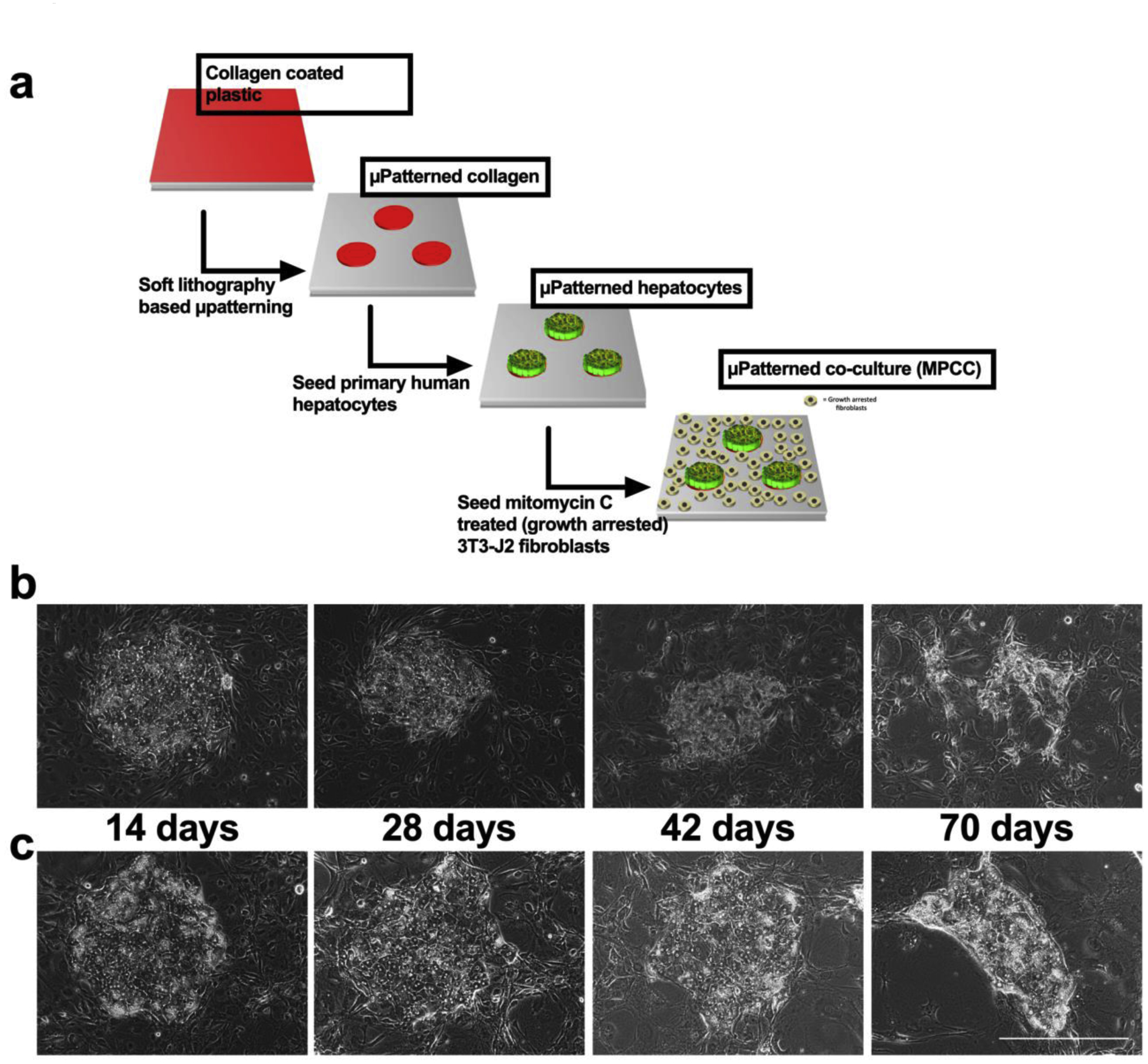
(a) MPCC fabrication schematic. Representative phase contrast images over time of PHH islands surrounded by fibroblasts in MPCCs cultured in the (b) traditional medium and (c) physiologic medium. Scale bar represents 400 μm.
Biochemical assays
Urea concentration in culture supernatants was measured using a colorimetric end-point assay utilizing diacetyl monoxime with acid and heat (Stanbio Labs, Boerne, TX) (Khetani and Bhatia 2008). Since human serum contains a significant amount of human albumin, we first washed MPCCs three times with 1x PBS and then incubated for 24 hours in a serum-free medium. De novo cell secreted albumin was then measured in this medium using an enzyme-linked immunosorbent assay with horseradish peroxidase detection and 3,3’,5,5’-tetramethylbenzidine (TMB, Rockland Immunochemicals, Boyertown, PA) as the substrate (Khetani and Bhatia 2008).
CYP3A4 and CYP2C9 activities were measured by incubating cultures with luciferin-IPA or luciferin-H substrates (Promega, Madison, WI) for 1 or 3 hours, respectively; the metabolite, luciferin, was quantified via luminescence detection. CYP1A2 and CYP2A6 activities were measured by incubating cultures with 5 μM 7-ethoxyresorufin or 50 μM coumarin (Sigma-Aldrich) for 1 hour, respectively; the supernatants were then incubated with a mixture of beta-glucuronidase and aryl-sulfatase solution (Roche, IN) to remove the glucuronide and sulfate groups from the resulting metabolites, respectively, as detailed previously (Khetani and Bhatia 2008). Finally, the deconjugated metabolites, resorufin and 7-hydroxycoumarin (7-HC), generated from 7-ethoxyresorufin and coumarin, respectively, were quantified via fluorescence detection (excitation/emission: 550/585 nm for resorufin, and 355/460 nm for 7-HC).
To assess insulin sensitivity/resistance, MPCCs were first cultured in hormone-free, serum-containing medium for 24 hours, and then washed 3 times with 1x PBS to remove residual glucose. Then, cultures were incubated in a glucose-free medium containing 4 mM L-glutamine, 1% penicillin/ streptomycin, 1.5% HEPES buffer, gluconeogenic substrates, 20 mM lactate (Sigma) and 2 mM pyruvate (Sigma), and +/− 10 nM insulin. Glucose levels in the culture supernatants were measured using the Amplex Red glucose/glucose-oxidase assay kit (Thermo-Fisher). Insulin resistance was calculated by dividing the insulin-stimulated glucose output by the basal (no insulin) level of glucose output.
Cell imaging
Culture morphology was monitored using an EVOS® FL microscope (Thermo-Fisher). Additionally, cultures were first washed 1X with serum-free culture medium and then incubated for 15 minutes with 2 μM of 5(6)-carboxy-2’,7’-dichlorofluorescein-diacetate (CDCFDA) that gets cleaved into 5(6)-carboxy-2’,7’-dichlorofluorescein (CDF) by esterases in hepatocytes and exported into the bile canaliculi by multidrug resistance protein-2 (Zamek-Gliszczynski et al. 2003). Cultures were then washed 3X with a serum-free culture medium and imaged using the GFP (green fluorescent protein) light cube to visualize the CDF stain. The area of excreted CDF was assessed by setting a threshold that included all fluorescent signal and subsequently quantifying the threshold area per PHH island. The number of individual bile canaliculi branches on thresholded images was also quantified across multiple PHH islands.
Drug studies
Drugs were purchased from Sigma-Aldrich or Cayman Chemicals (Ann Arbor, MI) and dissolved in 100% dimethylsulfoxide (DMSO, Corning). MPCC were treated with hepatotoxic (amiodarone, clozapine, diclofenac, piroxicam, troglitazone) or non-hepatotoxic drugs (aspirin, dexamethasone, miconazole, prednisone, rosiglitazone) dissolved in serum-free culture medium at 25× and 100× Cmax (Cmax: maximum drug concentration measured in human plasma) for each drug; cultures were treated with drugs twice over 5 days. Drug/Cmax (μM): amiodarone/0.806, aspirin/5.526, clozapine/0.951, dexamethasone/0.224, diclofenac/8.023, miconazole/0.024, piroxicam/5.135, prednisone/0.068, rosiglitazone/1.120, troglitazone/6.387 (Xu et al. 2008). Following drug exposure, urea secretion, previously shown to correlate highly with hepatotoxicity markers such as ATP (Kang et al. 2020; Khetani et al. 2013), was measured as above. DMSO concentration in the medium was kept at 0.1% (v/v), and a DMSO-only control culture was used to calculate relative changes in endpoints due to drug treatment.
For CYP induction, MPCCs were exposed to serum-free culture medium containing rifampin (25 μM), phenobarbital (1 mM), omeprazole (10 μM), or dimethylsulfoxide (DMSO) alone (0.1% v/v); cultures were treated with drugs for 4 days with fresh drug added to the culture medium at the 2-day medium exchange. Following drug exposure, CYP3A4, CYP2C9, and CYP1A2 activities were quantified as above.
Data analysis
All findings were confirmed in 3–4 wells per condition across different 2–3 PHH donors. Data processing was performed using Microsoft Excel. GraphPad Prism (La Jolla, CA) was used for displaying results. Mean and standard deviation are displayed for all data sets for each data point. Statistical significance was determined using Student’s t-test (two groups) or one-way ANOVA followed by Dunnett’s multiple comparison test (p< 0.05) for more than two groups.
RESULTS
Development of a physiologic culture medium formulation for PHHs
Traditional hepatocyte maintenance medium for MPCCs utilizes bovine serum and supraphysiologic amounts of glucose (25mM) and insulin (1 μM). Bovine serum could potentially have proteins (e.g. Fetuin-A) that inhibit hepatocyte insulin pathways (Mathews et al. 1997). Additionally, high amounts of glucose and insulin are major contributors to fatty liver disease and we have previously shown that MPCCs become steatotic and insulin resistant with such a medium formulation (Davidson et al. 2016). Accordingly, we formulated a medium with physiologic glucose (~5mM), human serum in place of bovine serum, and physiologic levels of insulin (500 pM). We have previously shown that glucose concentration can be reduced to physiologic levels in medium without adverse effects on MPCC performance (Davidson et al. 2016); thus, here we systematically assessed how changing insulin levels and serum type affect hepatocyte function and longevity.
We first characterized the effects of insulin concentration on MPCCs. MPCCs cultured in bovine serum (10% v/v) with 500 pM insulin showed a gradual decline in hepatocyte urea production and CYP3A4 activity when compared to MPCCs in bovine serum with 1 μM insulin (Supplemental Figure 1). On the other hand, MPCCs cultured in human serum (10% v/v) containing medium with 500 pM insulin maintained stable hepatocyte urea production and CYP3A4 activity for over 3 weeks in culture. These results suggest that human serum enables the relatively stable culture of hepatocytes in MPCCs at physiologic insulin concentrations, whereas MPCCs cultured in bovine serum decline in hepatocyte functions without supraphysiologic levels of insulin. Consequently, the remainder of the studies were carried out using the “traditional medium” containing 1 μM insulin and bovine serum or “physiologic medium” containing 500 pM insulin and human serum.
To further optimize the medium formulation, we cultured MPCCs in different concentrations of serum and assessed the effects of serum concentration on hepatocyte albumin production and urea synthesis. Within the range we tested, 5–10% serum-containing medium, we found no significant change in hepatocyte function with different amounts of human serum. Importantly, we found that 10% bovine serum was the optimal serum concentration for MPCC albumin production, with significant decreases in albumin production for MPCCs cultured in 7.5% and 5% bovine serum-containing media (Supplemental Figure 2). Therefore, subsequent studies were carried out using 10% human and 10% bovine serum. Since supporting fibroblasts in MPCCs could differentially proliferate in response to different serum types, we growth-arrested fibroblasts using mitomycin C before incorporation into MPCCs (Figure 1a); we have previously shown that similar to their proliferative counterparts, growth-arrested fibroblasts can also support PHH functions (Davidson and Khetani 2020).
Effects of physiologic culture medium on secretory and CYP functions of PHHs
PHH morphology and island integrity in MPCCs were diminished by 6 weeks in the traditional culture medium whereas these parameters were better retained for up to 10 weeks in the physiologic culture medium containing human serum and physiologic level of insulin (Figure 1b–c). At the functional level, CYP3A4 activity of MPCCs cultured in the traditional medium dropped to ~1% of week 1 levels after 10 weeks in culture, while CYP3A4 activity of MPCCs cultured in the physiologic medium was ~60% of week 1 levels after 10 weeks (Figure 2a). Similarly, CYP2A6 activity of MPCCs cultured in the traditional medium was undetectable after 8 weeks of culture, while CYP2A6 activity in MPCCs cultured in the physiologic medium was ~45% of week 1 levels after ~9 weeks of culture (Figure 2b). We also found higher levels (1.4- to 2.5-fold) of CYP1A2 and CYP2C9 activity in MPCCs after 3 weeks of culture in the physiologic culture medium as compared to the traditional medium (Supplemental Figure 3). Urea synthesis by MPCCs was also better maintained in physiologic medium at 57% and 23% of week 1 levels after 5 and 10 weeks in culture, respectively; in contrast, urea synthesis of MPCCs in the traditional medium was ~7% and 5% of week 1 levels by 5 weeks and 10 weeks in culture, respectively (Figure 2c). Finally, albumin production was ~3 fold higher after 3 weeks of culture in the physiologic medium relative to the traditional medium (Figure 2d).
Figure 2. Hepatic functions are better maintained over time in physiologic medium.
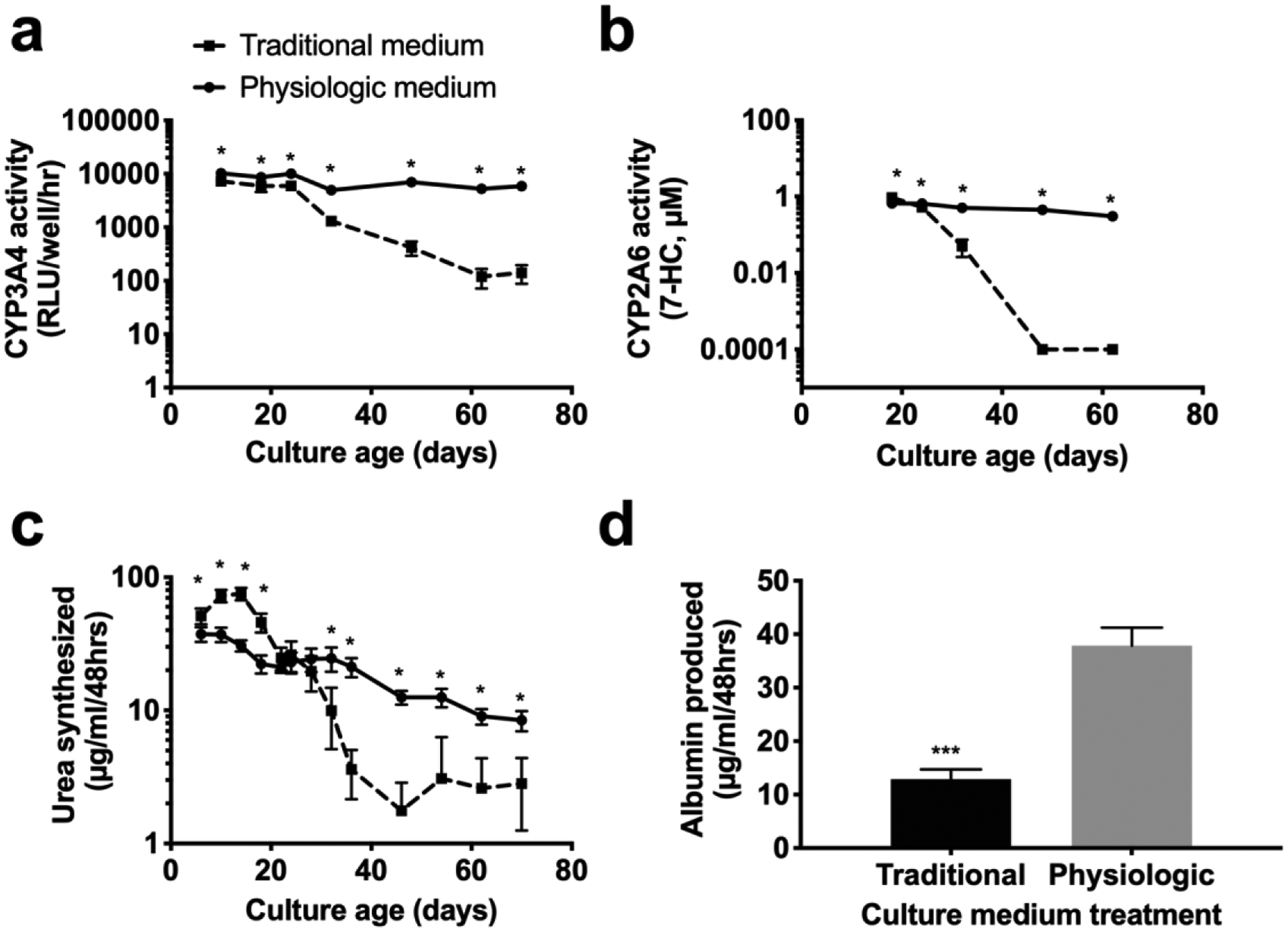
MPCCs were cultured in traditional or physiologic medium for 8–10 weeks and assessed for (a) CYP3A4 and (b) CYP2A6 enzyme activities. Secretory functions, including (c) urea synthesis and (d) albumin production were assessed in cultures treated with traditional or physiologic medium over 10 weeks and at 3 weeks of culture, respectively. Error bars represent SD. *,*** represent p<0.05, p<0.001 respectively.
Effects of physiologic culture medium on functional bile canaliculi of PHHs
Since hepatocyte islands maintained better integrity and size in the physiologic culture medium as compared to the traditional culture medium (Figure 1b–c), we hypothesized that hepatocyte islands would have greater levels of transporter function in the physiologic medium. To probe transporter function, we used a fluorescent dye, which is selectively transported through the hepato-specific multidrug resistant-like proteins 2 and 3 (MRP2, −3). At early time points, PHHs had significant transporters in both medium formulations (not shown), while after 4 weeks there was a significant loss of transporters in PHH islands cultured in the traditional medium as compared to PHH islands cultured in the physiologic medium (Figure 3).
Figure 3. Hepatic bile canaliculi are better retained over time in physiologic medium.
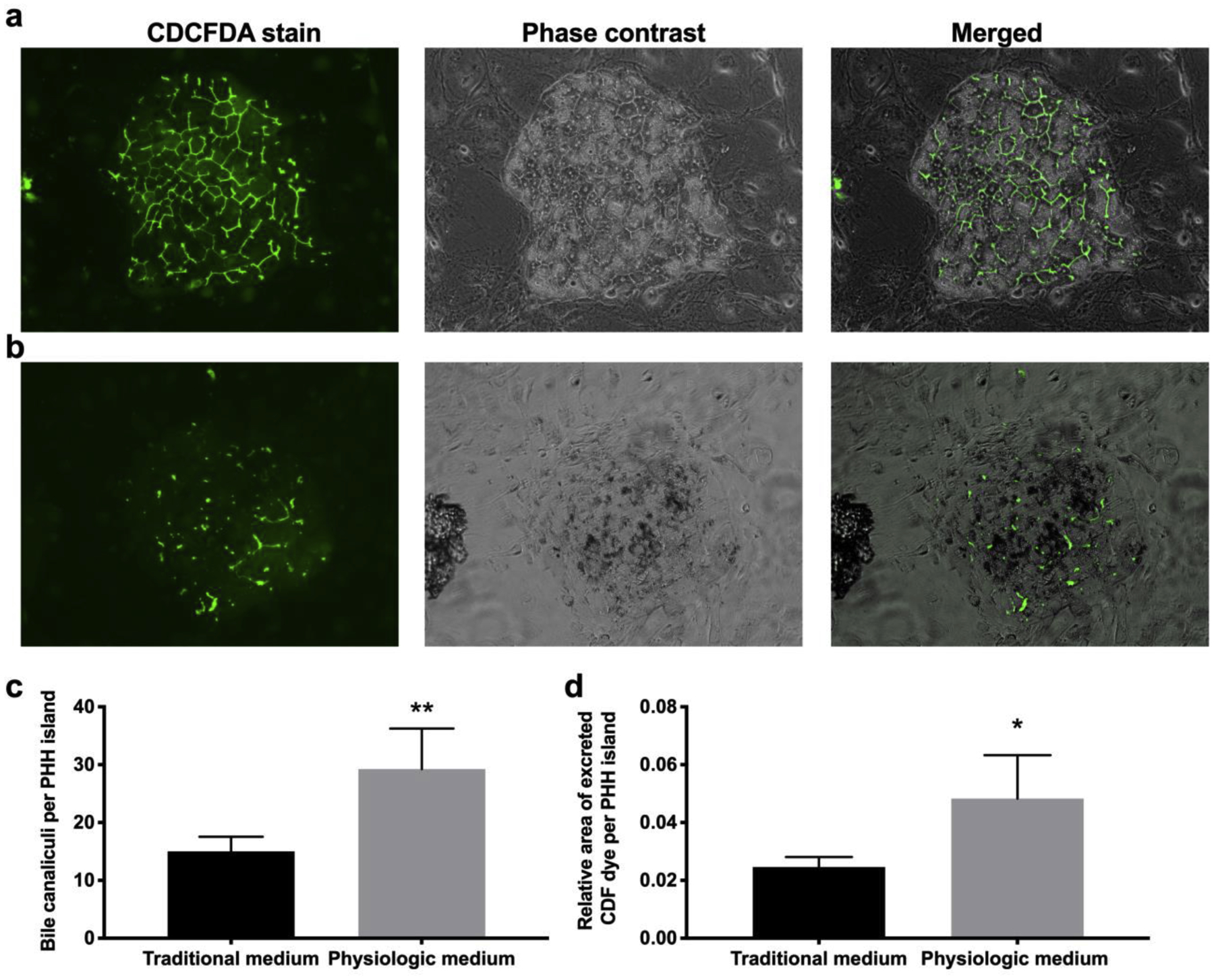
MPCCs were cultured for 4 weeks in (a) physiologic or (b) traditional culture medium and then assessed for bile canaliculi functionality, with the CDCFDA transporter dye, and corresponding phase contrast imaging. Scale bar represents 250 μm. (c) The number of bile canaliculi branches per PHH island (n=5) in MPCCs cultured for 4 weeks in the two media types. (d) The relative area of excreted CDF per PHH island (n=5) in MPCCs cultured for 4 weeks in the two media types. Error bars represent SD. *,** represent p<0.05 and p<0.01, respectively.
Effects of physiologic culture medium on insulin sensitivity of PHHs
One critical function of the liver is to maintain glucose levels in the blood by responding to hormones secreted from the pancreas. This function of liver cells is generally overlooked in in vitro liver models, although it is a key determinant of liver health. Additionally, bovine factors, such as fetuins and high amounts of insulin could contribute to insulin resistance in vitro (Cook et al. 2015; Mathews et al. 1997; Meex et al. 2015). Therefore, we assessed MPCC insulin sensitivity/resistance after treatment with the traditional or physiologic medium for 4 weeks. Insulin resistance was calculated by dividing the insulin-stimulated glucose output from cultures by the non-insulin-stimulated glucose output over 8 hours of glucose production, where completely insulin resistant cultures have a value of 1 and perfectly insulin-sensitive cultures have a value of 0 (Figure 4a). We found that PHHs in the traditional medium had an insulin resistance value of 0.5 after 2 weeks of culture and became completely insulin-resistant after 4 weeks of culture. In contrast, hepatocytes cultured in the physiologic medium were almost completely insulin-sensitive with an insulin resistance value of 0.025 after 2 weeks of culture and a value of 0.23 after 4 weeks of culture. Insulin resistance in MPCCs cultured in bovine serum was likely due to factors in bovine serum, since MPCCs cultured in physiologic insulin (500 pM) with bovine serum developed insulin resistance faster than cultures in the physiologic culture medium containing human serum (Supplemental Figure 4).
Figure 4. Hepatocyte insulin sensitivity is retained in physiologic medium.
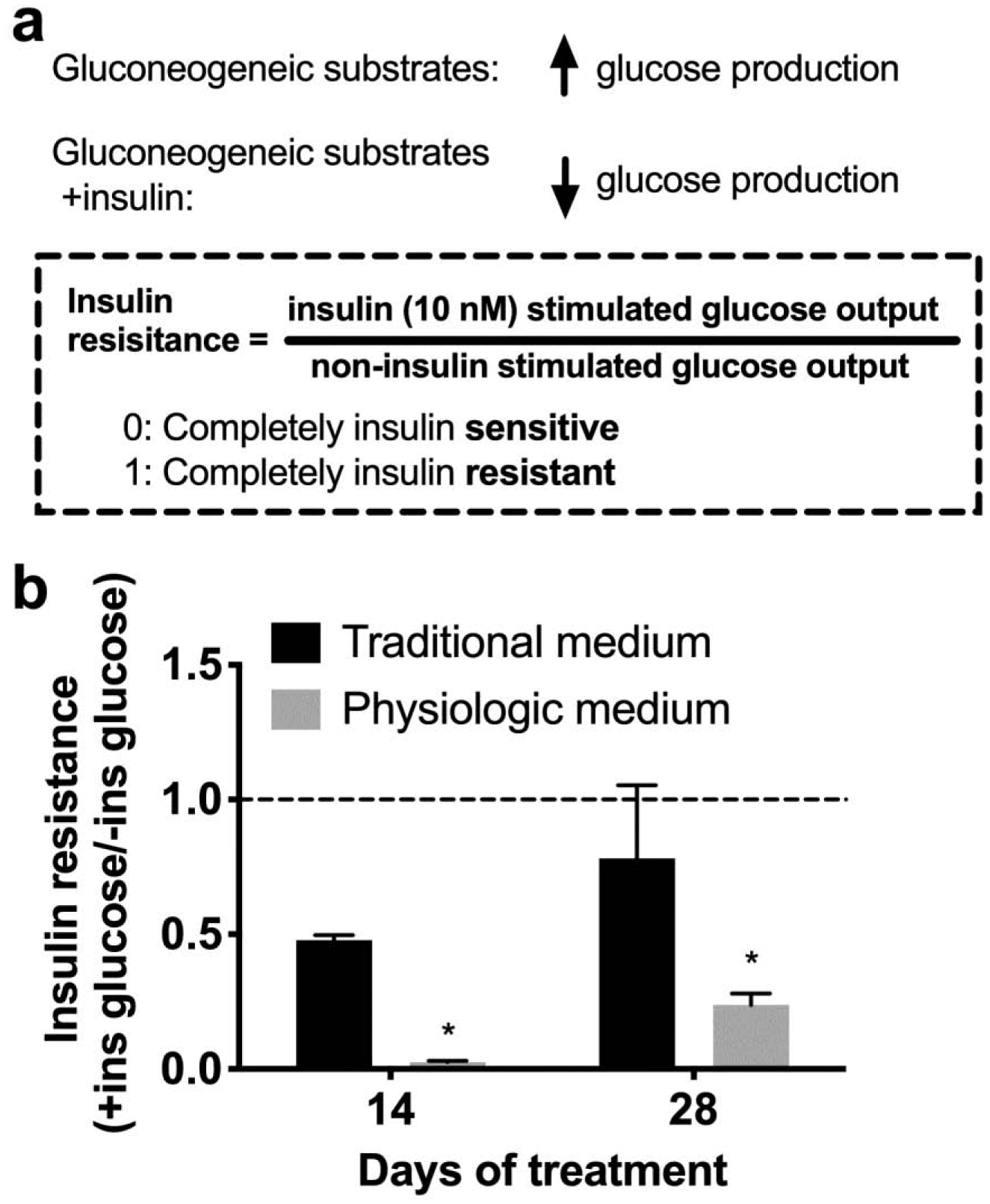
(a) Schematic describing how insulin resistance was calculated. (b) Insulin resistance after 2 and 4 weeks of culture in traditional and physiologic medium. Error bars represent SD. * represents p<0.05.
Effects of physiologic culture medium on drug-induced hepatotoxicity and CYP induction in PHHs
We have previously shown that MPCCs cultured for 2–3 weeks in the traditional medium can a) detect the toxicity of several hepatotoxic drugs while retaining specificity for non-hepatotoxic drugs and b) respond in a clinically-relevant way by upregulating CYP activity in the presence of specific inducer drugs. Since here we showed that MPCC functional lifetime can be improved using a physiologic culture medium containing human serum and physiologic levels of insulin, we sought to determine if similar outcomes for drug-induced hepatotoxicity and CYP induction could be obtained for practical use of the modified culture medium in the drug development pipeline. Therefore, we carried out a proof-of-concept drug screen with cultures after they were subjected to the physiologic medium for 10 days. Specifically, cultures were treated in a serum-free medium for a total of 5 days with 5 hepatotoxic drugs (amiodarone, clozapine, diclofenac, piroxicam, troglitazone) or 5 non-hepatotoxic drugs (aspirin, dexamethasone, miconazole, prednisone, rosiglitazone) at 25× and 100× Cmax (Cmax: maximum drug concentration measured in human plasma (Xu et al. 2008)) for each drug. Previously, 5–9 days of treatment with drugs at concentrations up to 100× Cmax in serum-free medium was found to increase the sensitivity for drug toxicity detection without an increase in the false positive rate over 1-day drug treatment in MPCCs (Khetani et al. 2013). The DMSO concentration in the culture medium was kept at 0.1% (v/v) for all drugs since DMSO at high levels can inhibit CYP3A4 activity (Easterbrook et al. 2001). A DMSO-only control culture was used to calculate relative changes in functional endpoints due to the drug treatment. Urea secretion, specific to PHHs, were measured after the 5-day drug exposure period since downregulation of urea was previously shown to correlate with toxicity endpoints such as ATP in MPCCs (Kang et al. 2020; Khetani et al. 2013). Compounds were categorized as toxic if urea synthesis dropped below 50% of the vehicle (DMSO) treated control (Khetani et al. 2013). After 2 treatments with drugs over 5 days, MPCCs cultured in the physiologic medium correctly identified all 5 hepatotoxic drugs as toxic (Figure 5a) and all 5 non-hepatotoxic drugs were detected as non-toxins (Figure 5b). These results suggest that hepatocytes cultured in physiologic medium retain the ability to correctly identify potential hepatotoxins and non-hepatotoxic compounds.
Figure 5. MPCCs maintained in physiologic medium are useful for drug-induced hepatotoxicity and CYP induction testing.
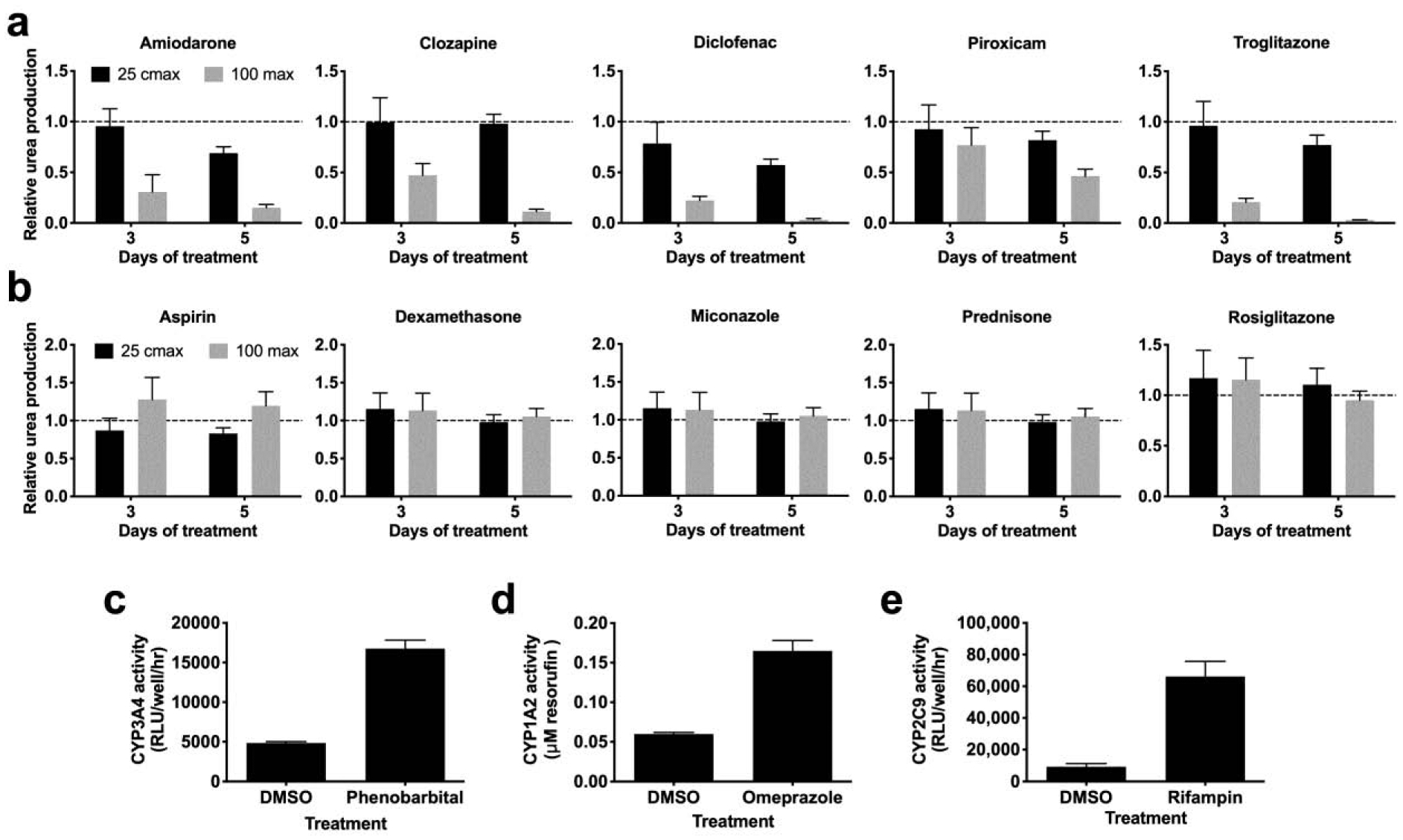
MPCCs were cultured in physiologic medium for 10 days and then treated with prototypical drugs. (a) Urea synthesis from MPCCs treated with (a) hepatotoxic or (b) non-hepatotoxic drugs for 5 days. Induction of (c) CYP3A4, (d) CYP1A2, and (e) CYP2C9 enzymes following treatment with indicated prototypical drugs or DMSO control (0.1% v/v) for 4 days. Error bars represent SD.
To assess whether MPCCs cultured in the physiologic medium can be used for drug-mediated CYP induction assessment (a measure of drug-drug interactions), MPCCs that were 10 days old were treated for a total of 4 days in serum-free medium with single concentration of either a CYP1A2 inducer (omeprazole), CYP3A4 inducer (phenobarbital), or a CYP2C9 inducer (rifampin), with fresh drug added every 2 days. Control cultures were treated with DMSO alone. The activities of the corresponding CYP enzymes were assessed as detailed in methods. MPCCs in physiologic medium showed a 3.5 (± 0.25) fold induction of CYP3A4 activity, 2.75 (± 0.24) fold induction of CYP1A2, and a 7.2 (± 1.8) fold induction of CYP2C9 activity relative to activities in the DMSO control (Figure 5c). These results suggest that MPCCs cultured in physiologic medium retain the ability to identify drug-mediated CYP induction and the potential for drug-drug interactions.
DISCUSSION
PHH functions can be significantly enhanced via several advanced culture techniques. However, most of these platforms utilize a culture medium containing supraphysiologic levels of glucose and insulin as well as serum from bovine sources, all of which can lead to non-physiologic outcomes in the cells (e.g. steatosis, insulin resistance, and alteration in metabolism) (Davidson et al. 2016). Here, we developed a culture medium containing physiologic levels of glucose/insulin and human serum. When tested on well-established MPCCs as a model human liver platform, our physiologic culture medium led to significantly better retention of PHH morphology and functions for up to 10 weeks as compared to the traditional medium with bovine serum. Finally, maintaining MPCCs in the physiologic medium did not compromise their ability to be utilized for drug-induced hepatotoxicity and CYP induction screening.
MPCCs cultured in the traditional medium were previously shown to improve PHH morphology and functions for ~3–4 weeks (Lin et al. 2016). Furthermore, 3T3-J2 fibroblasts in MPCCs induce the highest levels of PHH functions relative to liver-derived NPCs (Underhill and Khetani 2018). However, MPCCs become excessively steatotic over time due to the use of supraphysiologic levels of insulin and glucose in the traditional medium; furthermore, even in the absence of steatosis, MPCC functions decline after 3–4 weeks. In contrast, we showed here that in the presence of human serum, PHHs were better retained on the islands even after 10 weeks as compared to the traditional medium. We also observed better retention of functional hepatic bile canaliculi in the physiologic culture medium as compared to traditional medium. While reducing the percent of human serum down to 5% did not appreciably affect PHH functions as compared to 10% human serum, PHH functions were higher with 10% bovine serum as compared to lower concentrations of bovine serum. However, the levels of steatosis in PHHs were not considerably different across the traditional and physiologic media, potentially due to lipids in both sera types being taken up by the PHHs. Lastly, the growth-arrested supportive fibroblasts were able to maintain their confluency in both media formulations, suggesting that the media components are acting directly on PHHs to modulate their functions.
CYP2A6 and CYP3A4 activities were significantly better retained in the physiologic culture medium relative to the traditional medium (e.g. after 10 weeks, MPCCs retained ~45% and ~60% of week 1 CYP2A6 and CYP3A4 activities, respectively, in the physiologic medium, whereas CYP26 was undetectable and CYP3A4 was 1% of week 1 levels in the traditional medium). Similarly, CYP1A2 and CYP2C9 activities were 1.4- to 2.5-fold higher in the physiologic medium relative to the traditional medium. Additionally, secreted urea levels in the physiologic medium were 57% and 23% of week 1 levels after 5 and 10 weeks, respectively, whereas they were 7% and 5% of week 1 levels in the traditional medium. Finally, as with urea secretion, albumin secretion was higher (~3-fold) in the physiologic medium relative to the traditional medium.
The use of supraphysiologic insulin levels (~1 μM) is common with PHH culture since these cells spontaneously lose sensitivity to insulin in monocultures on collagen-coated plastic +/− Matrigel™ overlay (Davidson et al. 2015). While MPCCs cultured in the traditional medium are more sensitive to insulin than monocultures, even MPCCs become significantly insulin resistant after 2 weeks. Here, we reduced the insulin concentration to within the physiologic range (500 pM) in the traditional medium containing bovine serum but found that urea synthesis and CYP3A4 activity were reduced relative to high insulin. In contrast, the use of 500 pM insulin in the physiologic medium containing human serum did not affect CYP3A4 activity; however, urea secretion was downregulated by 1.3–1.5 fold relative to high insulin. However, the culture of MPCCs in the physiologic medium with 500 pM insulin led to significantly better insulin sensitivity over 4 weeks than the traditional medium, which became completely insulin resistant after 4 weeks. While reducing insulin in the traditional medium improved insulin sensitivity over 4 weeks relative to the high insulin, sensitivity was still not improved to the same level as the physiologic medium. Therefore, human serum in the culture medium improves insulin sensitivity of MPCCs, which can be useful for studying insulin signaling under physiologic and pathophysiologic scenarios (e.g. over-nutritional stimuli such as high glucose, fructose, and fatty acids).
PHH-NPC co-cultures (including PHH-3T3-J3 fibroblast co-cultures), in 2D or 3D formats, are more sensitive for the detection of drug-induced hepatoxicity with repeat drug exposure than PHH monocultures (Lin and Khetani 2016). Furthermore, PHH-NPC co-cultures can be typically used for drug-mediated CYP induction studies over the entire culture duration, thereby providing screening flexibility to the end-user. We have previously shown that MPCCs in the traditional medium can be used for both drug-induced hepatotoxicity and drug-mediated CYP induction studies over 2–3 weeks of culture (Khetani et al. 2013; Lin et al. 2016). Here, we determined whether culturing MPCCs in the physiologic medium has any impact on their utility for the above-mentioned applications. Thus, we incubated MPCCs over 5 days with five hepatotoxic and five non-hepatotoxic drugs at concentrations up to 100× Cmax, a threshold that takes into account interindividual variabilities in plasma drug concentrations, higher exposure of the liver to orally administered drugs through the portal vein, and changes in plasma drug concentrations due to drug-drug and drug-diet interactions; most importantly, the use of the 100× Cmax threshold increases the sensitivity for drug toxicity detection without increasing the false positives (Khetani et al. 2013; Xu et al. 2008). To assess adverse effects, we tracked urea secretions, which was previously shown to be more sensitive than intracellular ATP for detecting drug-induced hepatotoxicity in both human and animal-derived MPCCs (Kang et al. 2020; Khetani et al. 2013). Furthermore, in addition to allowing tracking of the same wells over time, urea is a hepatospecific endpoint as opposed to ATP which cannot be used to demarcate cell type-specific toxicity in co-cultures.
All 5 hepatotoxic drugs were found to be toxic in MPCCs cultured in the physiologic medium (i.e. >50% downregulation of urea relative to DMSO controls) whereas the 5 non-hepatotoxic drugs did not cause any appreciable loss of urea secretion. For drug-mediated CYP induction, MPCCs cultured in the physiologic medium appropriately responded to prototypical inducers (phenobarbital induced CYP3A4, rifampin induced CYP2C9, and omeprazole induced CYP1A2) as previously observed with MPCCs cultured in the traditional medium (Lin et al. 2016). Therefore, culturing MPCCs in the physiologic medium does not compromise their ability to be used for drug-induced hepatotoxicity and drug-mediated CYP induction studies. However, how the sensitivity and specificity for drug-induced hepatotoxicity studies as well as fold changes for drug-mediated CYP induction studies compare across the two media types remains to be elucidated. Furthermore, whether improved insulin signaling and thus functional lifetime of MPCCs in the physiologic medium can translate to more in vivo-like profiles for the study of non-alcoholic fatty liver disease than the traditional medium needs follow-up studies.
While we showed that a human serum-based physiologic medium better maintains PHH morphology/functions in MPCCs relative to the traditional medium, the specific components in each type of serum that cause such effects remain unclear. One possibility is fetuins in bovine serum that may cause decreased insulin signaling, which could lead to premature cell death since insulin signaling is necessary for cell survival (Brunet et al. 1999). Some clear differences between human and bovine serum are the ratios of high-density lipoprotein to low-density lipoprotein as well as the amounts and species of fatty acids (Haylett and Moore 2002). Lipids and cholesterol have numerous effects on hepatocyte metabolism and survival, which may explain the benefits of human serum observed here. Another clear difference between the sera types is the bile acid pool, which can be species-specific (de Aguiar Vallim et al. 2013). Bile acids have pleiotropic effects on hepatocytes via binding to nuclear receptors, which has been shown to affect hepatocyte pathways and aid in maintaining hepatic differentiation (Halilbasic et al. 2013; Sawitza et al. 2015).
While being significantly better than the traditional medium in the retention of PHH functions over time, the human serum was not sufficient to fully stabilize PHH functions over 10 weeks; in particular, we found that after about 8 weeks of culture, the PHH islands began to morph into non-circular shapes and there was the loss of some PHHs from the islands, which correlates with downregulation of functions even in the physiologic medium by 10 weeks. These trends suggest that additional soluble factors or media manipulations may be necessary to further stabilize PHH functions and improve longevity. For instance, we have developed an intermittent serum/hormone starvation protocol (Davidson and Khetani 2020) that could be used in conjunction with the human serum-based physiologic medium to further improve PHH functional lifetime. Additionally, while we have found that liver NPCs do not sustain PHH functions to the same levels as the 3T3-J2 fibroblasts (Underhill and Khetani 2018), their inclusion in the MPCCs alongside the fibroblasts under fluid perfusion in a microfluidic device may further improve PHH functions via better gas/nutrient/waste exchange than static formats. Furthermore, how the physiologic medium affects the functions of different types of liver NPCs, such as liver sinusoidal endothelial cells, Kupffer cells, and hepatic stellate cells, in the presence or absence of PHHs in MPCCs and other culture formats as compared to the traditional medium is pending investigation. Lastly, while the insulin level used here (500 pM) is within the physiologic range after ingestion of a meal (Feaver et al. 2016), our glucagon concentration is supraphysiologic (~50–100 pM versus 2000 pM) (Alford et al. 1977). Therefore, reducing glucagon as well to physiologic levels and studying its interactions with insulin signaling is pending further investigation to further tune our physiologic medium.
In conclusion, we developed a medium containing human serum and physiologic levels of insulin and glucose that significantly improved PHH morphological integrity, polarity, albumin and urea secretions, CYP activity, and insulin sensitivity in MPCCs without compromising the ability to conduct drug-induced hepatotoxicity and drug-mediated CYP induction studies as compared to the traditional medium with bovine serum. We anticipate that our physiologic culture medium can find broad utility for a) the continued development of PHH cultures and PHH-NPC co-cultures for drug development, b) investigating the effects of patient-derived sera on PHH functions and disease phenotypes, and c) use in cell-based therapies with xeno-free culture components.
Supplementary Material
Table 1.
Comparison of traditional and physiologic culture media compositions with in vivo values for humans.
| Culture medium component | Traditional Medium | Physiologic Medium | In vivo in humans |
|---|---|---|---|
| Insulin concentration | 1 μM | 500 pM | 1 – 800 pM |
| Glucose concentration | 5 mM | 5 mM | 3.9 – 6.1 mM |
| Serum source | Bovine | Human | Human |
Acknowledgements
The authors would like to thank Christine Lin and Brenton Ware for cell culture assistance.
Funding
Funding was provided by the National Institutes of Health (1R03EB019184-01 to SRK) and the National Science Foundation (CBET-1351909 to SRK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCES
- Abboud G and Kaplowitz N 2007. Drug-induced liver injury. Drug safety : an international journal of medical toxicology and drug experience 30, 277–294. [DOI] [PubMed] [Google Scholar]
- Alford FP, Bloodm SR and Nabarro JD 1977. Glucagon levels in normal and diabetic subjects: use of a specific immunoabsorbent for glucagon radioimmunoassay. Diabetologia 13, 1–6. [DOI] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J and Greenberg ME 1999. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96, 857–868. [DOI] [PubMed] [Google Scholar]
- Cook JR, Langlet F, Kido Y and Accili D 2015. Pathogenesis of selective insulin resistance in isolated hepatocytes. The Journal of biological chemistry 290, 13972–13980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson MD, Ballinger KR and Khetani SR 2016. Long-term exposure to abnormal glucose levels alters drug metabolism pathways and insulin sensitivity in primary human hepatocytes. Scientific Reports 6, 28178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson MD and Khetani SR 2020. Intermittent Starvation Extends the Functional Lifetime of Primary Human Hepatocyte Cultures. Toxicol Sci 174, 266–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson MD, Lehrer M and Khetani SR 2015. Hormone and drug-mediated modulation of glucose metabolism in a microscale model of the human liver. Tissue Engineering, Part C: Methods 21, 716–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Aguiar Vallim TQ, Tarling EJ and Edwards PA 2013. Pleiotropic roles of bile acids in metabolism. Cell metabolism 17, 657–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delire B, Stärkel P and Leclercq I 2015. Animal Models for Fibrotic Liver Diseases: What We Have, What We Need, and What Is under Development. J Clin Transl Hepatol, pp. 53–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easterbrook J, Lu C, Sakai Y and Li AP 2001. Effects of organic solvents on the activities of cytochrome P450 isoforms, UDP-dependent glucuronyl transferase, and phenol sulfotransferase in human hepatocytes. Drug Metabolism and Disposition 29, 141–144. [PubMed] [Google Scholar]
- Feaver RE, Cole BK, Lawson MJ, Hoang SA, Marukian S, Blackman BR, Figler RA, Sanyal AJ, Wamhoff BR and Dash A 2016. Development of an in vitro human liver system for interrogating nonalcoholic steatohepatitis. JCI Insight 1, e90954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godoy P, Hewitt NJ, Albrecht U, Andersen ME, Ansari N, Bhattacharya S, Bode JG, Bolleyn J, Borner C, Böttger J, Braeuning A, Budinsky RA, Burkhardt B, Cameron NR, Camussi G, Cho C-S, Choi Y-J, Craig Rowlands J, Dahmen U, Damm G, Dirsch O, Donato MT, Dong J, Dooley S, Drasdo D, Eakins R, Ferreira KS, Fonsato V, Fraczek J, Gebhardt R, Gibson A, Glanemann M, Goldring CEP, Gómez-Lechón MJ, Groothuis GMM, Gustavsson L, Guyot C, Hallifax D, Hammad S, Hayward A, Häussinger D, Hellerbrand C, Hewitt P, Hoehme S, Holzhütter H-G, Houston JB, Hrach J, Ito K, Jaeschke H, Keitel V, Kelm JM, Kevin Park B, Kordes C, Kullak-Ublick GA, LeCluyse EL, Lu P, Luebke-Wheeler J, Lutz A, Maltman DJ, Matz-Soja M, McMullen P, Merfort I, Messner S, Meyer C, Mwinyi J, Naisbitt DJ, Nussler AK, Olinga P, Pampaloni F, Pi J, Pluta L, Przyborski SA, Ramachandran A, Rogiers V, Rowe C, Schelcher C, Schmich K, Schwarz M, Singh B, Stelzer EHK, Stieger B, Stöber R, Sugiyama Y, Tetta C, Thasler WE, Vanhaecke T, Vinken M, Weiss TS, Widera A, Woods CG, Xu JJ, Yarborough KM and Hengstler JG 2013. Recent advances in 2D and 3D in vitro systems using primary hepatocytes, alternative hepatocyte sources and non-parenchymal liver cells and their use in investigating mechanisms of hepatotoxicity, cell signaling, and ADME. Archives of Toxicology 87, 1315–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halilbasic E, Claudel T and Trauner M 2013. Bile acid transporters and regulatory nuclear receptors in the liver and beyond. J Hepatol 58, 155–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haylett AK and Moore JV 2002. Comparative analysis of foetal calf and human low density lipoprotein: relevance for pharmacodynamics of photosensitizers. J Photochem Photobiol B 66, 171–178. [DOI] [PubMed] [Google Scholar]
- Kang W, Podtelezhnikov AA, Tanis KQ, Pacchione S, Su M, Bleicher KB, Wang Z, Laws GM, Griffiths TG, Kuhls MC, Chen Q, Knemeyer I, Marsh DJ, Mitra K, Lebron J and Sistare FD 2020. Development and Application of a Transcriptomic Signature of Bioactivation in an Advanced In Vitro Liver Model to Reduce Drug-induced Liver Injury Risk Early in the Pharmaceutical Pipeline. Toxicol Sci 177, 121–139. [DOI] [PubMed] [Google Scholar]
- Khetani SR and Bhatia SN 2008. Microscale culture of human liver cells for drug development. Nature Biotechnology 26, 120–126. [DOI] [PubMed] [Google Scholar]
- Khetani SR, Kanchagar C, Ukairo O, Krzyzewski S, Moore A, Shi J, Aoyama S, Aleo M and Will Y 2013. Use of micropatterned cocultures to detect compounds that cause drug-induced liver injury in humans. Toxicological Sciences 132, 107–117. [DOI] [PubMed] [Google Scholar]
- Lin C and Khetani SR 2016. Advances in engineered liver models for investigating drug-induced liver injury. BioMed Research International 2016, 1829148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C and Khetani SR 2017. Micropatterned co-cultures of human hepatocytes and stromal cells for the assessment of drug clearance and drug-drug interactions. Currrent Protocols in Toxicology 72, 14 17 11–14 17 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C, Shi J, Moore A and Khetani SR 2016. Prediction of drug clearance and drug-drug interactions in microscale cultures of human hepatocytes. Drug Metabolism and Disposition 44, 127–136. [DOI] [PubMed] [Google Scholar]
- Macdonald RA 1961. “Lifespan” of liver cells. Autoradio-graphic study using tritiated thymidine in normal, cirrhotic, and partially hepatectomized rats. Arch Intern Med 107, 335–343. [DOI] [PubMed] [Google Scholar]
- Magami Y, Azuma T, Inokuchi H, Kokuno S, Moriyasu F, Kawai K and Hattori T 2002. Cell proliferation and renewal of normal hepatocytes and bile duct cells in adult mouse liver. Liver 22, 419–425. [DOI] [PubMed] [Google Scholar]
- Mathews ST, Srinivas PR, Leon MA and Grunberger G 1997. Bovine fetuin is an inhibitor of insulin receptor tyrosine kinase. Life Sci 61, 1583–1592. [DOI] [PubMed] [Google Scholar]
- Meex RC, Hoy AJ, Morris A, Brown RD, Lo JC, Burke M, Goode RJ, Kingwell BA, Kraakman MJ, Febbraio MA, Greve JW, Rensen SS, Molloy MP, Lancaster GI, Bruce CR and Watt MJ 2015. Fetuin B Is a Secreted Hepatocyte Factor Linking Steatosis to Impaired Glucose Metabolism. Cell Metab 22, 1078–1089. [DOI] [PubMed] [Google Scholar]
- Sawitza I, Kordes C, Gotze S, Herebian D and Haussinger D 2015. Bile acids induce hepatic differentiation of mesenchymal stem cells. Sci Rep 5, 13320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz RE, Fleming HE, Khetani SR and Bhatia SN 2014. Pluripotent stem cell-derived hepatocyte-like cells. Biotechnology advances 32, 504–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih H, Pickwell GV, Guenette DK, Bilir B and Quattrochi LC 1999. Species differences in hepatocyte induction of CYP1A1 and CYP1A2 by omeprazole. Human and Experimental Toxicology 18, 95–105. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Soejima Y and Fukusato T 2012. Animal models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol 18, 2300–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underhill GH and Khetani SR 2018. Bioengineered Liver Models for Drug Testing and Cell Differentiation Studies. Cell Mol Gastroenterol Hepatol 5, 426–439 e421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkening S, Stahl F and Bader A 2003. Comparison of primary human hepatocytes and hepatoma cell line Hepg2 with regard to their biotransformation properties. Drug Metabolism and Disposition 31, 1035–1042. [DOI] [PubMed] [Google Scholar]
- Xu JJ, Henstock PV, Dunn MC, Smith AR, Chabot JR and de Graaf D 2008. Cellular imaging predictions of clinical drug-induced liver injury. Toxicological Sciences 105, 97–105. [DOI] [PubMed] [Google Scholar]
- Zamek-Gliszczynski MJ, Xiong H, Patel NJ, Turncliff RZ, Pollack GM and Brouwer KLR 2003. Pharmacokinetics of 5 (and 6)-carboxy-2’,7’-dichlorofluorescein and its diacetate promoiety in the liver. Journal of Pharmacology and Experimental Therapeutics 304, 801–809. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


