Abstract
Background
We describe the temporal pattern of COVID-19 admissions to a tertiary care children’s hospital in central New Jersey during the SARS-Cov-2 surge, covering the time period from March 29 to July 26, 2020.
Methods
Medical charts were reviewed for the date of admission, past medical history and demographic variables, presenting signs and symptoms, admitting laboratory values, diagnostic imaging, diagnosis, treatment modalities, and outcomes including length of stay and disease severity.
Results
Patients with symptomatic SARS-Cov-2 infection tended to present with pneumonia early during the study period, which coincided with the early surge in New Jersey cases. Approximately two weeks after the peak in reported SARS-Cov-2 cases in New Jersey we began to see fewer pneumonia cases and an increase in admissions for Multi-Inflammatory Syndrome in Children (MIS-C) as well as cases of acute appendicitis in association with a diagnosis of SARS-CoV-2 infection.
Conclusions
We present a novel association of acute appendicitis in children infected with SARS-CoV-2 and postulate that it may represent a post-infectious hyperinflammatory complication of SARS-CoV-2 infection occurring 2 weeks after the early manifestation of acute pneumonia disease in children.
Keywords: SARS-CoV-2, COVID-19, children, MIS-C, pneumonia, appendicitis
INTRODUCTION
On March 11, 2020, the World Health Organization declared the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) outbreak as a global pandemic.1,2 As of June 28, 2020, the United States (US) Centers for Disease Control and Prevention (CDC) reported 2,504,175 confirmed SAR-CoV-2 cases and 125,484 deaths in the US.3 Fortunately, children represent only a fraction of reported US cases, accounting for only 5.49 percent of confirmed SARS-CoV-2 cases in the June 28, 2020 CDC report.3
Children appear to be as likely as adults to be infected with SARS-CoV-2, but are more likely to remain asymptomatic or develop only mild upper respiratory tract symptoms—they may therefore be less likely to get tested and receive a confirmed diagnosis.4–6 A CDC report revealed that children were less likely than adults to experience fever, cough, or dyspnea (73% versus 95%), or to require hospitalization (5.7% versus 10%).4 Two epidemiologic studies of confirmed SARS-CoV-2 cases from China showed similar results.5,6 Dong and colleagues described 728 children with PCR-confirmed SARS-CoV-2 of which 12.9% were asymptomatic and 43.1% had only mild upper respiratory tract (URI) symptoms.5 In a separate study of 171 children with confirmed SARS-CoV-2 infection 15.8% were asymptomatic, 19.3% had signs and symptoms consistent with URI, and 64.9% had CT-confirmed pneumonia.6
As of October 10, 2020, 61,364 patients had been hospitalized in the US with confirmed SARS-CoV-2 infection, with children accounting for only 1.6 percent of hospitalizations.7 According to the CDC, children of all ages were less likely than adults to require hospitalization (estimated hospitalization rates of 5.7%–20% and 10–33%, respectively) or intensive care unit (ICU) admission (0.58%–2.0% and 1.4%–4.5%, respectively), but hospitalization rates were higher in children less than one year of age.4 In their pediatric cohort of 728 PCR-confirmed SARS-CoV-2 cases, Dong and colleagues reported a 5.2 percent incidence of severe respiratory infections with clinical progression to dyspnea and a 0.6 percent incidence of critical infections with clinical deterioration to acute respiratory distress syndrome (ARDS) and/or signs of other organ dysfunction.5
Although earlier reports stated that children younger than 5 years old were at greater risk of severe SARS-Cov-2 disease; in late April/early May 2020, the European Union and United States reported clusters of older children and adolescents presenting with shock, fever and hyperinflammation associated with SARS-CoV-2 infection. In May 2020, the Centers for Disease Control and Prevention (CDC) issued a national health advisory to report on cases meeting the criteria for Multisystem Inflammatory Syndrome in Children (MIS-C). This subset of children develop a dysregulated immune response with host tissue damage and hyperinflammation, resembling Kawasaki’s disease (KD), toxic shock syndrome or macrophage activating syndrome with the median age of onset being 8.3 years.8,9,14,15 In cases with MIS-C, the majority of children were hospitalized (80–88%) with most requiring intensive care management (80%) for multiorgan dysfunction.15,16 Gastrointestinal and cardiovascular organ systems were the two of the most commonly affected (92% and 80% respectively).15
Gastrointestinal (GI) symptoms, such as diarrhea and abdominal pain, are a known feature of SARS-CoV-2 infection and have been reported in adults and children with mild to severe SARS-CoV-2 infection.24,38 Other SARS-CoV-2 associated GI manifestations that have been reported are ileus and mesenteric adenopathy in adults and children with terminal ileitis presenting as atypical appendicitis.24,38 Individual case reports of pseudoappendicitis and acute surgical abdomen have been reported in the literature in pediatric and adult SARS-CoV-2-positive patients.36–39
New York and New Jersey (NJ) were early centers of SARS-CoV-2 infection and spread.4 The first known NJ case was reported on March 4, 2020, followed by an exponential increase in reported cases and hospitalizations.17 We report the clinical presentation and management of 48 children admitted to the Bristol Myers Squibb Children’s Hospital (BMSCH), a tertiary care facility affiliated with Robert Wood Johnson (RWJ) -Barnabas Health in New Brunswick, NJ, with confirmed SARS-CoV-2 infection between March 29 and July 26, 2020. We compare the characteristics of cases seen during the early period of the pandemic surge to those seen during the later period, focusing on children in the later period who presented with acute appendicitis.
METHODS
Study Methods
A retrospective analysis of pediatric patients with confirmed SARS-CoV-2 infection admitted between March 29, 2020 to July 26, 2020 was performed. During the pandemic, the age for BMSCH admission was increased to 25 years. Hospitalized patients were tested for SARS-Cov-2 based on suspicion of disease. Universal SARS-CoV-2 PCR screening of hospitalized patients via nasopharyngeal specimens was initiated on April 12, 2020, utilizing the Xpert® Xpress SARS-CoV-2 (Cepheid, Sunnyvale, CA), Simplexa® COVID-19 Direct Kit (DiaSorin Molecular LLC, Cypress, CA), or Cobas® 6800 SARS-CoV-2 test (Roche Diagnostics, Indianapolis, IN) based on availability. The SARS-CoV-2 IgG assay (Abbott Diagnostics, Lake Forest, IL) became available on April 20, 2020.
Data collection
Inclusion criteria included all hospitalized patients 25 years old or less with positive SARS-CoV-2 diagnostic testing by PCR and/or SARS-CoV-2 antibody testing. IRB approval was received, and patient information was de-identified. The electronic medical record was used to obtain pertinent information.
Demographic data included age, gender, race and ethnicity, underlying comorbidities, history of SARS-CoV-2 exposure, and admission weight. Weight percentiles were determined per CDC growth charts, and categorized as normal (5th to less than 85th percentile), overweight (85th to less than 95th percentile), or obese (greater than 95th percentile).18
Presenting signs and symptoms included number of days of fever prior to admission (PTA) and respiratory (cough, shortness of breath (SOB), rhinitis), gastrointestinal (GI; nausea, vomiting, diarrhea, abdominal pain), dermatologic (rash), or other (conjunctivitis, cervical lymphadenopathy, myalgias, dizziness, headache) signs and symptoms. Admitting laboratory values included serum white blood cell (WBC) count, absolute lymphocyte count (ALC), absolute neutrophil count (ANC), platelet (PLT) count, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase (LDH), D-dimer, fibrinogen, ferritin, prothrombin time (PT), partial thromboplastin time (PTT), and interleukin-6 (IL-6).
Clinical data included the days of fever following admission, radiologic imaging findings, the need for and duration of supplemental oxygen (O2) use, need for pediatric intensive care unit (PICU) admission, hospital length of stay (LOS), and medication regimens.
We also collected demographic and clinical data for all appendicitis cases by ICD-10 code admitted during the same timeframe which included demographic data, GI symptoms, presence of fevers and length of stay.
Disease severity classification
Disease severity was classified into 3 groups: (1) Mild—did not require supplemental oxygen, inotropes or PICU admission; (2) Moderate—required supplemental oxygen via nasal cannula or face mask, and may have required PICU admission, but did not need intubation or inotropic support; (3) Severe—required mechanical ventilation or inotropic support in the PICU.
Statistical Methods
Normally-distributed continuous demographic characteristics was presented as mean ± standard deviation (SD), and non-normally-distributed variables as median and interquartile range (IQR). Categorical variables were presented as frequency (percentage). Spearman Rank Order Correlation tests were used to investigate the potential relationships between demographic variables and presenting symptoms, disease severity, and hospital length of stay. A One Way Analysis of Variance (ANOVA; for normally-distributed variables) or Kruskal-Wallis One Way ANOVA on Ranks (for non-normally-distributed variables) was used to compare SARS-CoV-2 pneumonia, MIS-C, and appendicitis patients with respect to continuous admitting and outcomes variables. A Chi square test was used to compare categorical admitting and outcomes data, with adjusted residuals used for post hoc testing in the case of a significant P value. A P value less than 0.05 was considered significant for all analyses.
RESULTS
Forty-eight patients with confirmed SARS-CoV-2 infection were admitted between March 29 and July 26, 2020 (Figure 1). On review, we classified patients into 5 separate diagnostic categories: (1) Pneumonia—respiratory signs and symptoms and Chest radiograph (CXR) findings consistent with pneumonia; (2) MIS-C—met the CDC criteria for MIS-C;16 also classified as having KD if they met American Heart Association guidelines for classic or incomplete KD;19 (3) Appendicitis—signs, symptoms and radiographic imaging compatible with appendicitis; (4) Unconventional - signs and symptoms clinically compatible with SARS-CoV-2 but did not fit any of the other categories; (5) Asymptomatic - admitted for an unrelated diagnosis and SARS-CoV-2 positive on routine screening.
Figure 1.
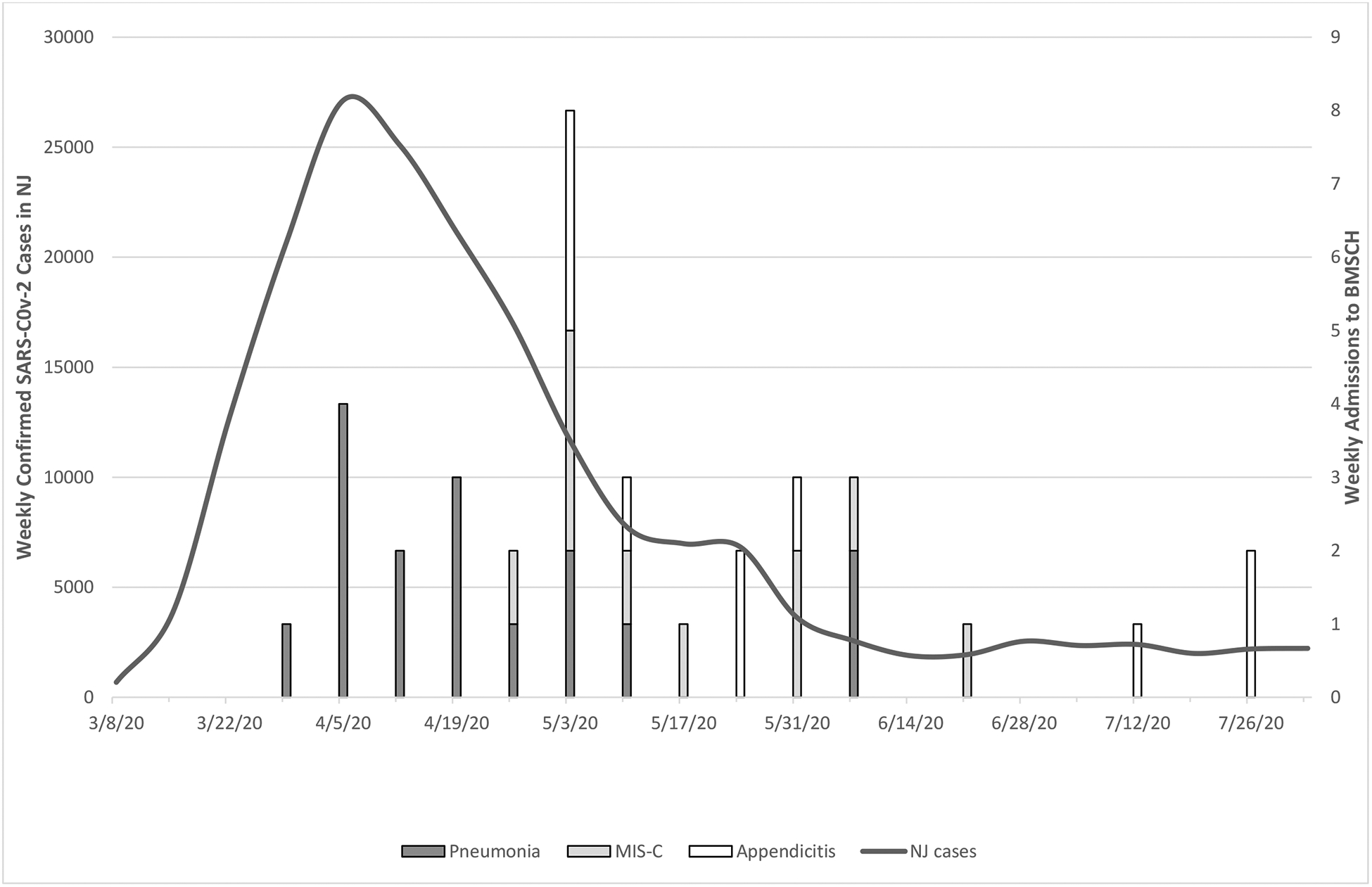
Timeline of SARS-CoV-2-positive admissions at BMSCH by diagnosis
https://njhealth.maps.arcgis.com/apps/opsdashboard/index.html#/81a17865cb1a44d
Seven patients were SARS-CoV-2 asymptomatic and were excluded from further analysis. Of the remaining 41 patients, 16 were diagnosed with SARS-CoV-2 pneumonia, 10 with appendicitis, 10 with MIS-C and 5 with unconventional symptoms. As Figure 1 shows, SARS-CoV-2 pneumonia was more frequent during the first 4–6 weeks (11 of 16 cases) while all 10 appendicitis cases and nine of ten MIS-C cases were seen more frequently during the final 12 weeks.
Demographic characteristics of all patients are given in Table 1. The median (IQR) age of all 41 patients was 9 (0.2, 17) years. The median (IQR) age was significantly greater in patients with pneumonia or appendicitis [15 (4.25, 18.5) and 12 (6, 13.5) years respectively] than in those with unconventional symptoms [0.275 (0.183, 1.575) years, P=0.003]. Overall, most patients were male (56.1%) and Hispanic or Latino (51.2%). When comparing the four cohorts by ethnicity, there were fewer non-Hispanic children with appendicitis than expected (9 Hispanic versus only one non-Hispanic patient; P=0.021). Although no other racial or ethnic differences were significant, non-Hispanic Black children predominated in the MIS-C group (6 non-Hispanic Black versus 3 Hispanic and 1 other). Fourteen patients (34.1%) had underlying comorbidities and 19 (46.3%) had exposure to a confirmed or suspected SARS-CoV-2 case, prior to admission (PTA). Patients diagnosed with appendicitis or MIS-C were significantly less likely than expected to have comorbidities (only one child in the appendicitis and MIS-C cohort; P=0.012). There was a non-significant trend toward a higher median weight percentile in MIS-C and pneumonia patients. There were no other differences in demographic variables. Patients with SARS-CoV-2 unconventional infection were excluded from further analysis.
Table 1.
Demographic characteristics
| Appendicitis (n=10) | MIS-C (n=10) | Pneumonia (n=16) | Unconventional (n=5) | P value | |
|---|---|---|---|---|---|
| Age (years) [Median (IQR)] | 12 (6, 13.5)c | 9 (5.75, 12.5) | 15 (4.25, 18.5)b | 0.3 (0.205, 1.5)b,c | 0.003a |
| Gender [No. (%)] | NSd | ||||
| M, 6 (60.0) | M, 7 (70.0) | M, 7 (43.75) | M, 3 (60.0) | ||
| Ethnicity [No. (%)] | −− | ||||
| O, 0 | O, 1 (10.0) | O, 1 (6.25) | O, 2 (40.0) | ||
| Ethnicity [No. (%)] | 0.021d | ||||
| non-H, 1 (10.0)e | non-H, 7 (70.0) | non-H, 9 (56.25) | non-H, 3 (60.0) | ||
| Median (IQR) weight percentile (%) | 75 (47.5, 91.25) | 90 (83, 99) | 87 (38.75, 99) | 79.5 (16.5, 99) | NSa |
| Comorbidities [No. (%)] | 0.012d | ||||
| No, 9 (90.0) | No, 9 (90.0) | No, 6 (37.5) | No, 2 (50.0) | ||
| Exposure to Confirmed case [No. (%)] | NSd | ||||
| No, 7 (70.0) | No, 6 (60.0) | No, 7 (43.75) | No, 2 (60.0) |
Asymptomatic COVID patients were excluded from this analysis; F, female; M, male; W, non-Hispanic White; B, non-Hispanic Black; H, Hispanic or Latino; O, Other;
Kruskal-Wallis One Way Analysis of Variance on Ranks for comparison of SARS-CoV-2 pneumonia, MIS-C, and appendicitis patients;
P<0.05 by Dunn’s All Pairwise Multiple Comparison Procedures Method,
Chi square test for comparison of SARS-CoV-2 pneumonia, MIS-C, and appendicitis patients;
P<0.05 adjusted residual post-hoc method; PTA, prior to hospital admission; NS, not significant
Clinical characteristics and outcomes are given in Table 2. Overall, among the 3 cohorts, there was a significant correlation between the days of fever PTA (P=<0.001) disease severity (P=0.008), and hospital LOS (P=0.049). Patients with appendicitis had significantly fewer days of fever PTA [0 (0, 1.25)] than did patients with MIS-C or pneumonia [5 (4, 6) and 5 (1.25, 7.75) days, respectively; P<0.001]. Appendicitis patients presented predominantly with nausea, vomiting and abdominal pain. Both MIS-C and pneumonia patients had longer febrile days both prior to admission and while in hospital. MIS-C patients were also noted to present with gastrointestinal features, similar to appendicitis patients, but reported a higher frequency of diarrheal symptoms at time of presentation. Patients with appendicitis had a significantly lower median (IQR) disease severity ranking than either pneumonia or MIS-C patients, and were significantly less likely than MIS-C patients to require ICU admission. Appendicitis patients also had a significantly shorter hospital LOS than did patients with pneumonia [3 (1, 8) versus 7.5 (6, 16.25) days, respectively; P=0.049]. One death occurred in a patient with SARS-CoV-2 pneumonia, but this patient was admitted with terminal acute myelocytic leukemia.
Table 2.
Comparison of clinical characteristics and outcomes by diagnosis
| Appendicitis (n=10) | MIS-C (n=10) | Pneumonia (n=16) | P value | |
|---|---|---|---|---|
| Days fever PTA [Median (IQR)] | 0 (0, 1.25)b,c | 5 (4, 6)b | 5 (1.25, 7.75)c | <0.001a |
| Days fever in hospital [Median (IQR)] | 0.5 (0, 2.5) | 1.5 (0, 2.75) | 1 (1, 3) | NSa |
| Disease severity [Median (IQR)] | 1 (1, 1)b,c | 2.5 (1, 3)b | 2 (1.25, 2)c | 0.008a |
| Hospital LOS [Median (IQR)] | 3 (1, 8)b | 9 (4.5, 12) | 7.5 (6, 16.25)b | 0.049a |
Kruskal-Wallis One Way Analysis of Variance on Ranks;
P<0.05 by Dunn’s All Pairwise Multiple Comparison Procedures method; PTA, prior to hospital admission; LOS, length of stay; NS, not significant
Admitting laboratory values are given in Table 3. Appendicitis and MIS-C patients had significantly higher WBC and ANC values than pneumonia patients. CRP values were significantly higher in MIS-C patients than in pneumonia patients. Appendicitis patients had significantly lower AST and LDH values than did patients with MIS-C or pneumonia, and significantly lower ALT values than patients with MIS-C. There was a trend toward a higher ALC and lower D-dimer value in patients with appendicitis but it should be noted that age-adjusted ALC values were low in most patients (56.25%, 25%, 60%, respectively of patients with SARS-CoV-2 pneumonia, appendicitis and MIS-C) and D-dimer values elevated in most patients (56.25%, 25%, and 60%, respectively, of patients with SARS-CoV-2 pneumonia, appendicitis and MIS-C).
Table 3.
Comparison of median (IQR) admitting inflammatory markers by clinical diagnosis
| Appendicitis (n=10) | MIS-C (n=10) | Pneumonia (n=16) | P value | |
|---|---|---|---|---|
| WBC (103 cells/mm3) | 12.75 (10.075, 16.5)b | 12.6 (8.9, 20.45)c | 5.15 (3.475, 11.125)b,c | 0.002a |
| ALC (103 cells/mm3) | 1.15 (0.785, 2.17) | 1.04 (0.54, 1.652) | 1.07(0.86, 1.6) | NSa |
| ANC (103 cells/mm3) | 11.19 (7.415, 15.16)b | 10.78 (4.99, 18.65)c | 3.5(2.15, 6.3)b,c | 0.002a |
| ESR (mm/hr) | 27 (18.5, 70) | 43 (31.25, 78.75) | 56(20, 84.75) | NSa |
| CRP (mg/dL) | 9.62 (4.278, 20.255) | 27.395 (10.668, 30.502)b | 6.17 (2.63, 8.565)b | 0.001a |
| AST (U/L) | 20 (14.25, 25)b,c | 57.5 (35.5, 68.75)b | 49.5 (27.75, 86)c | 0.004a |
| ALT (U/L) | 15 (9, 19)b | 45 (20.25, 71)b | 34 (12.75, 63.25) | 0.016a |
| LDH (U/L) | 184 (135, 210.5)b,c | 297 (259.75, 457.25)b | 288.5 (201.75, 605.5)c | 0.020a |
| D-dimer (ng FEU/mL) | 930 (318.75, 10162.75) | 2372 (1447, 4175) | 1082.5 (641.75, 1589) | NSa |
| Fibrinogen (mg/dL) | 571.5 (390.5, 733) | 690.5 (581, 848) | 714 (489, 795) | NSa |
| Ferritin (ng/mL) | 107 (78, 181)b | 664.5 (327.5, 1293.25)b | 378.5 (160, 1133.75) | 0.015a |
Kruskal-Wallis One Way Analysis of Variance on Ranks for comparison of SARS-CoV-2 pneumonia, MIS-C, and appendicitis patients;
P<0.05 by Dunn’s All Pairwise Multiple Comparison Procedures method; NS, not significant
Table 4 compares the demographic characteristics of our 10 SARS-CoV-2-positive appendicitis patients to 61 patients admitted to the BMSCH for acute appendicitis during the same time period who tested negative for SARS-CoV-2. When comparing patients with appendiceal rupture there were significantly fewer non-Hispanic or Latino patients than expected in the SARS-CoV-2 negative group (P=0.047).
Table 4.
Comparison of SARS-CoV-2 positive and negative appendicitis patients by demographics, rupture, and days of GI symptoms prior to admission
| Appendiceal rupture | No rupture | |||||
|---|---|---|---|---|---|---|
| SARS-CoV-2 negative (n=22) | P value | SARS-CoV-2 Negative (n=39) | P value | |||
| Age (years) Mean ± SD | 11.0 ± 3.67 | NSa | 12.1 ± 4.90 | NSa | ||
| Gender No. (%) | M, 15 (68.2) | NSb | M, 20 (51.3) | NSb | ||
| F, 7 (31.8) | F, 19 (48.7) | |||||
| Ethnicity No. (%) | H, 10 (45.5) | 0.047b | H, 17 (43.6) | NSb | ||
| Non-H, 12 (54.5) | Non-H, 22 (56.4) | |||||
| Days of GI symptoms PTA [Median (IQR)] | 2 (1, 3) | NSd | 1 (1, 2) | NSd | ||
Student’s t test;
Fisher’s exact test;
P<0.05 adjusted residual post-hoc method;
Mann-Whitney rank sum test; GI, gastrointestinal; PTA, prior to admission; M, Male; F, Female; H, Hispanic; non-H, non-Hispanic; SD, standard deviation; IQR, interquartile range
Clinical Course
SARS-Cov-2 Pneumonia.
Fifteen of the sixteen pneumonia patients were SARS-CoV-2 PCR-positive; one patient was SARS-CoV-2 antibody positive. Multilobar disease was the most common CXR finding, noted in 10 patients, with ground glass opacities in only 2 patients. Presenting clinical characteristics noted most often included fever, cough, and shortness of breath.
MIS-C.
Ten children met the CDC criteria for MIS-C. Three were SARS-CoV-2 PCR-positive and six were PCR-negative and SARS-CoV-2 antibody positive; one patient was both SARS-CoV-2 PCR and antibody positive.
Five of the 10 MIS-C patients met the criteria for KD and the most prominent symptoms noted with fever were maculopapular rash and conjunctivitis. Eight of the ten MIS-C patients had echocardiographic abnormalities suggestive of myocarditis and 2 of these patients had abnormal coronaries.
Unconventional SARS-CoV-2.
The 5 unconventional COVID −19 cases that were admitted were all children 2 years of age and under (ages 0.16, 2years) who presented with a variety of symptoms. Patient 1 was a 7-week-old white male admitted with fever and unilateral conjunctivitis with a normal CXR. He had 48 hours of fever and was discharged home on hospital day 3 with resolution of symptoms. Patient 2 was a 2-year-old Hispanic female with chronic medical conditions (holoprosencephaly, chronic lung disease, seizure disorder, failure to thrive and developmental delay) admitted with fever for 1 day and shortness of breath, requiring oxygen by nasal cannula. CXR showed peribronchial thickening, consistent with her chronic lung disease, and no infiltrates. Patient 3 was a 3-month-old ex-34-week gestation male admitted with vomiting and shortness of breath. CXR showed peribronchial thickening with no infiltrates. Patient 4 was a 1-year-old female admitted with vomiting and abdominal pain and no fever. She had a normal CXR and abdominal CT scan showed small bowel obstruction with no appendicitis. She had an exploratory laparotomy with excision of an adhesion in the distal ileum and was discharged on the 6th hospital day. Patient 5 was a 4-month-old Hispanic male admitted with fever for 1 day, vomiting with shortness of breath and cough. His CXR was normal and he was discharged within 48 hours of rehydration. Three of the five children had a household COVID-19 contact. All five children were SARS-CoV-2 PCR positive.
Appendicitis:
Five of the 10 children with a radiologic diagnosis of appendicitis had a ruptured appendix and only 1 of 8 had radiologic evidence of mesenteric adenopathy. Eight of the 10 patients underwent an appendectomy and the other 2 were medically managed.
The major presenting clinical findings were fever, nausea, vomiting, and abdominal pain. CXR was performed in six children and three had abnormal findings (unilateral or bilateral basilar opacity); however, none had respiratory symptoms. Three of the 7 appendicitis patients were overweight or obese.
When comparing SARS-CoV-2-positive and negative appendicitis patients with appendiceal rupture to those without, there was a trend in both groups toward a longer duration of GI symptoms prior to admission in children with appendiceal rupture (Table 5). The patients with non-ruptured appendicitis in both groups presented earlier to the hospital with shorter days of GI symptoms as compared to the cases with ruptured appendicitis and although not significant, it was noted that those with SARS-CoV-2 infection had a longer duration of symptoms prior to admission than those that were negative. None of the SARS-CoV-2 positive appendicitis patients received remdesivir, steroids or biologics during their hospital stay.
Table 5.
Comparison of rupture and non-rupture appendicitis in SARS-CoV-2 positive patients by clinical course
| Ruptured appendix (n=5) | Non-Ruptured appendix (n=5) | P value | |
|---|---|---|---|
| Days of GI symptoms PTA [Median (IQR)] | 4 (2.5, 5) | 1 (0.75, 2.5) | 0.032a |
| Days fever PTA [Median (IQR)] | 0 (0, 2) | 0 (0, 1) | NSa |
| Days fever in hospital [Median (IQR)] | 2 (0.5, 4.5) | 0 (0, 0.5) | NSa |
| Disease severity [Median (IQR)] | 1 (1, 1.5) | 1 (1, 1) | NSa |
| Hospital LOS [Median (IQR)] | 8 (4.25, 12.5) | 1 (1, 2) | 0.016a |
Mann-Whitney Rank Sum Test;
Fisher’s Exact test; PTA, prior to hospital admission; GI, gastrointestinal; LOS, length of stay; NS, not significant
DISCUSSION
We report our experience with pediatric cases of SARS-CoV-2 infection in children hospitalized at BMSCH. In total, we admitted 48 patients with a diagnosis of SARS-CoV-2 infection in the first 4 months of the peak epidemic in New Jersey. Pneumonia and MIS-C are well-known associations with pediatric SARS-CoV-2 disease; however, we also report a cluster of 10 SARS-CoV-2-positive children admitted with acute appendicitis during the New Jersey pandemic between March 29 and July 26, 2020.5,6,15 Appendicitis accounted for 20.8 percent of the children admitted during this time period who tested positive for SARS-CoV-2. The pattern of admissions (Figure 1) followed the overall trend of primarily seeing SARS-CoV-2 pneumonia cases early, particularly during the first six weeks, followed approximately four weeks later by admissions for MIS-C and acute appendicitis. Pneumonia is a well-known clinical manifestation of acute SARS-CoV-2 infection, which was reflected in our 16 patients with presenting signs, symptoms, laboratory variables, chest radiographs, and clinical course that has been previously reported.5,6
The clinical presentation, course, and management of our MIS-C patients was similar to previous reports concerning this condition in children.10–15 Six of the 10 children were overweight or obese, with a seventh child at the 84th weight percentile. Most of our MIS-C patients had cardiac injury with some level of carditis (8 out of 10), and presented with ventricular dysfunction and cardiogenic shock as has been noted in previous studies.10–15 Ethnic minorities accounted for the majority of patients.
MIS-C may represent a post-infectious hyperinflammatory complication of SARS-CoV-2 since it presents later in the timeline of the epidemic and MIS-C patients are often PCR negative but antibody positive suggesting a late manifestation. Of the 99 children with MIS-C reported by Dufort and colleagues, only 19 percent were PCR-positive and antibody-negative, while 33 percent were positive by both assays and 47 percent antibody-positive and PCR-negative, an observation that supports the concept of MIS-C being a post-infectious complication.14 Serologic testing for antibodies to SARS-CoV-2 was not available at our institution until April 20, 2020. Seven of the 10 appendicitis cases were PCR positive (the remaining two were antibody positive), while six of the 10 patients with MIS-C were antibody positive (the remainder 3 were PCR positive). One patient in each category was both PCR and antibody positive. Also, to note, antibody testing was not routinely done at our institution for the appendicitis and MIS-C cases if they were PCR positive, so we cannot comment on possible antibody positivity in addition to PCR positivity in these cases. It is possible that many of the PCR positive appendicitis cases may have also tested antibody positive given that they presented later on our epidemic curve.
In our cohort, all five young infants classified as having unconventional disease were managed on the general Pediatric floor, had mild to moderate symptoms and did not progress to severe disease as has previously been reported for this age group.4,5 Three of the five children presented with gastrointestinal symptoms, as has been observed in other studies.24,25 Interestingly, intussception has been described in infants with SARS-CoV-2 infection and we postulate that SARS-CoV-2 infection may have been an inciting factor for small bowel obstruction observed in our patient.
We admitted 10 SARS-CoV-2 positive patients and 61 SARS-CoV-2 negative patients with acute appendicitis between March 29 and July 26, 2020. Ninety percent of these children had no underlying comorbidities, and 50 percent were overweight or obese. Nine of the 10 SARS-CoV-2 positive patients were Hispanic or Latino. Previous studies have clearly established that Hispanic or Latino and non-Hispanic Black communities are being disproportionately impacted by the SARS-CoV-2 pandemic.14,15,20,21 Due to a variety of socioeconomic barriers including poor access to care, these patients are more likely to be hospitalized with SARS-CoV-2 and more likely to experience a severe disease course.
Although not statistically significant, the racial and ethnic distribution of the children in the two appendicitis cohorts was different. There was no difference in the race/ethnic makeup in the SARS-CoV-2 negative group, whereas 9 of the 10 patients in the SARS-CoV-2 positive group were Hispanic. This reiterates the fact that minority groups are disproportionately being affected with SARS-CoV-2.
Racial and ethnic minorities are also at a greater risk for appendiceal rupture in the US, due to poor access to healthcare.33,34 Ponsky and colleagues reported an increased rupture rate in minority children when compared to White children [adjusted OR (95%CI) ratio of appendiceal rupture in Black children of 1.13 (1.01–1.3) and Asian children of 1.66 (1.24–2.23)]. Smink and colleagues found a similar relationship [OR (95%CI) of appendiceal rupture was 1.23 (1.1–1.39) for Black and 1.20 (1.1–1.3) for Hispanic children, compared with White children].34 In contrast, our study, did not find a significant racial difference in patients with appendiceal rupture (data not shown).
When comparing SARS-CoV-2-positive and negative appendicitis patients some more intriguing observations emerged. In SARS-CoV-2-negative patients the overall rupture rate was 36.1 percent; in contrast, 50 percent of SARS-CoV-2-positive children experienced appendiceal rupture. Established risk factors for appendiceal rupture in children revolve around delays in diagnosis and the risk of perforation increases dramatically if left untreated beyond 48 hours. Age is therefore an important risk factor, as infants and many preschoolers are unable to verbalize their symptoms and accurately locate the region of pain.33,35 Accordingly, infants and children less than three years of age are more likely to be misdiagnosed early on and have a reported perforation rate of 82–92 percent.35 Ponsky and colleagues reported that children between 5–12 years of age were more likely than those 13–17 years of age, to present with ruptured appendix (OR and 95% CI was 1.41(1.3,1.53)33 In our study, we did not see an age-related difference for the ruptured appendicitis patients in both groups with the median (IQR) age of 12 years in the SARS-CoV-2 positive group and 11 years in the SARS-CoV-2 negative group. The overall rupture rate among the 61 SARS-CoV-2-negative patients (36.1%) correlates well with that reported by Ponsky and colleagues, however, a 50 percent rate was observed in our 10 SARS-CoV-2-positive patients. If we assume that a delay in obtaining healthcare due to fear of exposure to SARS-CoV-2 in hospitals was the reason for an increase in ruptured appendicitis cases, then the SARS-negative cohort should have been equally affected. Although our numbers are small, we postulate that an exaggerated inflammatory response may be at play among SARS-CoV-2 infected patients leading to rupture.
The cause of appendicitis is unknown, although obstruction of the lumen of the appendix secondary to an initiating factor such as appendicolith formation or mesenteric adenopathy is suspected.1 All three of our patients with rupture who were screened for appendicoliths were positive, compared to two of five patients without rupture. Mesenteric lymphadenopathy was noted in one of three with rupture and none of the patients without rupture.
Could acute appendicitis represent another post-infectious, hyperinflammatory complication of SARS-CoV-2, or rather does acute GI infection and inflammation trigger the development of appendicitis? ACE2 receptors are widely distributed in both smooth muscle and endothelial cells of the stomach, small intestine, and colon, as well as arterial and venous endothelial cells throughout the body.22 The exact cause of appendicitis remains poorly understood. Although family clusters and a seasonal pattern have been observed, consistent evidence of a viral trigger is lacking.26,27
Cai and colleagues reported on five SARS-CoV-2-positive children presenting with GI signs and symptoms, one with CT-confirmed acute appendicitis with rupture and localized peritonitis.25 Previous reports suggest that patients with SARS-CoV-2 infection can present with GI signs and symptoms suggestive of acute appendicitis, but subsequently ruled out by abdominal CT.23,24,36 In contrast to the reports of pseudo-appendicitis, our experience shows that appendicitis can in fact occur in SARS-CoV-2 infected pediatric patients.
In conclusion, we describe an experience with SARS-CoV-2 infection in children seen at a tertiary care hospital in central NJ who presented with different clinical manifestations during the SARS-CoV-2 surge in NJ. The temporal pattern of admissions suggest that pneumonia is likely to be seen early during an epidemic curve, followed by hyperinflammatory post-infectious manifestations such as MIS-C. To the best of our knowledge, this is the first report of a temporal association of SARS-CoV-2 infection with multiple cases of acute appendicitis in children. Although our numbers are small and more studies are needed to verify this association, clinicians should be made aware of the possibility that appendicitis can present as a post infectious manifestation of SARS-CoV-2 infection in children.
Disclosure of Funding:
“Research reported in this publication was supported (in part) by the New Jersey Alliance for Clinical and Translational Science and the National Center for Advancing Translational Sciences (NCATS), a component of the National Institute of Health (NIH) under award number UL1TR003017. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.”
REFERENCES
- 1.WHO Director-General’s opening remarks at the media briefing on COVID-19 – 11 March 2020, https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19-−−11-march-2020, accessed 5 May 2020.
- 2.Zimmermann P, Curtis N. Coronavirus Infections in Children Including COVID-19: An Overview of the Epidemiology, Clinical Features, Diagnosis, Treatment and Prevention Options in Children. Pediatr Infect Dis J 2020;39:355–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention Coronavirus Disease 2019 (COVID-19) Cases, Data, & Surveillance, https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html, accessed 19 October 2020.
- 4.Coronavirus Disease 2019 in Children — United States, February 12–April 2, 2020. MMWR April 10, 2020;69(14):422–426, US Department of Health and Human Services/Centers for Disease Control and Prevention, https://www.cdc.gov/mmwr/volumes/69/wr/mm6914e4.htm?s_cid=mm6914e4_w, accessed 17 May 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong Y, Mo X, Hu Y, et al. Epidemiological characteristics of 2143 pediatric patients with 2019 coronavirus disease in China. Pediatrics 2020; doi: 10.1542/peds.2020-0702 [DOI] [Google Scholar]
- 6.Lu X, Zhang L, Du H, et al. SARS-CoV-2 Infection in Children. New Engl J Med, March 18, 2020, DOI: 10.1056/NEJMc2005073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coronavirus Disease 2019 (COVID-19)-Associated Hospitalization Surveillance Network (COVID-NET), updated October 10, 2020, https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covid-net/purpose-methods.html, accessed 19 Oct 2020
- 8.Ye Q, Wang B, Mao J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. Journal of Infection 2020, 10.1016/j.jinf.2020.03.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tay MZ, Poh CM, Rénia L et al. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol (2020). 10.1038/s41577-020-0311-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whittaker E, Bamford A, Kenny J, et al. Clinical Characteristics of 58 Children With a Pediatric Inflammatory Multisystem Syndrome Temporally Associated With SARS-CoV-2. JAMA. Published online June 08, 2020. doi: 10.1001/jama.2020.10369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. www.thelancet.com Published online May 6, 2020. 10.1016/S0140-6736(20)31094-1 [DOI] [PMC free article] [PubMed]
- 12.Nathan N, Prevost B, Corvol H. Atypical presentation of COVID-19 in young infants www.thelancet.com Vol 395 May 9, 2020, 10.1016/S0140-6736(20)30979-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verdoni L, Mazza A, Gervasoni A, Martelli L, Ruggeri M, Ciuffreda M, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. www.thelancet.com Published online May 13, 2020. 10.1016/S0140-6736(20)31103-X [DOI] [PMC free article] [PubMed]
- 14.Dufort EM, Koumans EH, Chow EJ, Rosenthal EM, Muse A, Rowlands J, et al. Multisystem Inflammatory Syndrome in Children in New York State. New Engl J Med 2020; DOI: 10.1056/NEJMoa2021756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son MBF, et al. Multisystem Inflammatory Syndrome in U.S. Children and Adolescents. New Engl J Med 2020; DOI: 10.1056/NEJMoa2021680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with Coronavirus Disease 2019 (COVID-19), CDC Health Alert Network, May 20, 2020, https://emergency.cdc.gov/han/2020/han00432.asp, accessed July 13, 2020
- 17.New Jersey Department of Health COVID-19 Confirmed Case Summary, available at https://www.nj.gov/health/cd/documents/topics/NCOV/COVID_Confirmed_Case_Summary.pdf, accessed 18 May 2020.
- 18.Barlow SE and the Expert Committee. Expert Committee Recommendations Regarding the Prevention, Assessment, and Treatment of Child and Adolescent Overweight and Obesity: Summary Report. Pediatrics 2007;120(Suppl 4):S164–S192; DOI: 10.1542/peds.2007-2329C [DOI] [PubMed] [Google Scholar]
- 19.McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, Baker AL, Jackson MA, Takahashi M, Shah PBMD, Kobayashi T, Wu M-H, Saji TT, Pahl E. AHA Scientific Statement. Diagnosis, treatment, and long-term management of Kawasaki Disease: a scientific statement for health professionals from the American Heart Association. Circulation 2017;135(17):e927–e999, 10.1161/CIR.0000000000000484 [DOI] [PubMed] [Google Scholar]
- 20.Tai DBG, Shah A, Doubeni CA, Sia IG, Wieland ML. The Disproportionate Impact of COVID-19 on Racial and Ethnic Minorities in the United States [published online ahead of print, 2020 Jun 20]. Clin Infect Dis. 2020;ciaa815. doi: 10.1093/cid/ciaa815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Azar KMJ, Shen Z, Romanelli RJ, et al. Disparities In Outcomes Among COVID-19 Patients In A Large Health Care System In California. Health Aff (Millwood). 2020;39(7):1253–1262. doi: 10.1377/hlthaff.2020.00598 [DOI] [PubMed] [Google Scholar]
- 22.Hamming I, Timens W, Bulthuis MLC, Lely AT, Navis GJ, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 2004;203:631–637, DOI: 10.1002/path.1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pautrat K, Chergui N. SARS-CoV-2 infection may result in appendicular syndrome: chest CT scan before appendectomy. Journal of Visceral Surgery 2020, doi: 10.1016/j.jviscsurg.2020.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tullie L, Ford K, Bisharat M, Watson T, Thakkar H, Mullassery D, et al. Gastrointestinal features in children with COVID-19: an observation of varied presentation in eight children. Lancet Child Adolesc Health 2020; Published Online May 19, 2020, 10.1016/S2352-4642(20)30165-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cai X, Ma Y, Li S, Chen Y, Rong Z, Li W. Clinical Characteristics of 5 COVID-19 Cases With Non-respiratory Symptoms as the First Manifestation in Children. Frontiers in Pediatrics 2020;6(Article 258), doi: 10.3389/fped.2020.00258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alder AC, Fomby TB, Woodward WA, Haley RW, Sarosi G, Livingston EH. Association of viral infection and appendicitis. Arch Surg. 2010;145(1):63–71. doi: 10.1001/archsurg.2009.250 [DOI] [PubMed] [Google Scholar]
- 27.Richardsen I, Schöb DS, Ulmer TF, Steinau G, Neumann UP, Klink CD, Lambertz A. Etiology of Appendicitis in Children: The Role of Bacterial and Viral Pathogens. Journal of Investigative Surgery 2020;29(2):74–79, DOI: 10.3109/08941939.2015.1065300 [DOI] [PubMed] [Google Scholar]
- 28.Arnbjörnsson E, Bengmark S. Obstruction of the appendix lumen in relation to pathogenesis of acute appendicitis. Acta Chir Scand. 1983;149(8):789–791 [PubMed] [Google Scholar]
- 29.Gao Z, Xu Y, Sun C, Wang X, Guo Y, Qiu S, Ma K. A Systematic Review of Asymptomatic Infections with COVID-19 [published online ahead of print, 2020 May 15]. J Microbiol Immunol Infect 2020;10.1016/j.jmii.2020.05.001. doi: 10.1016/j.jmii.2020.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, Zhou W, Yang L, You R. Physiological and pathological regulation of ACE2, the SARS-CoV-2 receptor. Pharmacol Res 2020;157:104833. doi: 10.1016/j.phrs.2020.104833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen K, Bi J, Su Y, Chappell MC, Rose JC. Sex-Specific Changes in Renal Angiotensin-Converting Enzyme and Angiotensin-Converting Enzyme 2 Gene Expression and Enzyme Activity at Birth and Over the First Year of Life. Reprod Sci. 2016;23(2):200–210. doi: 10.1177/1933719115597760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pan Y, Yu X, Du X, Li Q, Li X, Qin T, Wang M, Jiang M, Li J, Li W, Zhang Q, Xu Z, Zhang L. Epidemiological and Clinical Characteristics of 26 Asymptomatic Severe Acute Respiratory Syndrome Coronavirus 2 Carriers. J Infect Dis. 2020;221(12):1940–1947. doi: 10.1093/infdis/jiaa205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ponsky TA, Huang ZJ, Kittle K, Eichelberger MR, Gilbert JC, Brody F, Newman KD. Hospital and patient level characteristics and the risk of appendiceal rupture and negative appendectomy in children. JAMA. 2004;292(16):1977–1982. [DOI] [PubMed] [Google Scholar]
- 34.Smink DS, Fishman SJ, Kleinman K, Finkelstein JA. Effects of race, insurance status, and hospital volume on perforated appendicitis in children. Pediatrics. 2005;115(4):920–925. [DOI] [PubMed] [Google Scholar]
- 35.Almaramhy H Acute appendicitis in young children less than 5 years: review article. Ital J Pediatr. 2017;43(15). Doi: 10.1186/s13052-017-0335-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abdalhadi A, Alkhatib M, Mismar AY, Awouda W, Albarqouni L. Can COVID-19 present like appendicitis? ID Cases. 2020;21:e00860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suwanwongse K, Shabarek N. Pseudoappendicitis in an adolescent with COVID-19. Cureus. 2020;12(7):e9394 Doi: 10.7759/cureus.9394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suresh Kumar VC, Mukherjee S, Harne PS, Subedi A, Ganapathy MK, Patthipati VS, Sapkota B. Novelty in the gut: a systematic review and meta-analysis of the gastrointestinal manifestations of COVID-19. BMJ Open Gastr. 2020. 7e000417 Doi: 10.1136/bmjgast-2020-000417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saeed U, Selevoll HB, Young VS, Sandbaek G, Glomsaker T, Mala T. COVID-19 may present with acute abdominal pain. Br J Surg. 2020;107:e186–e187. [DOI] [PMC free article] [PubMed] [Google Scholar]


