Abstract
Background and purpose:
It is unclear if sex differences explain some of the variability in the outcomes of stroke patients who undergo endovascular treatment (EVT). In this study we assess the effect of sex on radiological and functional outcomes in EVT-treated acute stroke patients and determine if differences in baseline perfusion status between men and women might account for differences in outcomes.
Methods:
We included patients from the CRISP (Computed tomographic perfusion to Predict Response to Recanalization in ischemic stroke) study, a prospective cohort study of acute stroke patients who underwent EVT up to 18 hours after last seen well. We designed ordinal regression and univariable and multivariable regression models to examine the association between sex and infarct growth, final infarct volume and 90-day mRS score.
Results:
We included 198 patients. At baseline, women had smaller perfusion lesions, more often had a target mismatch perfusion profile, and had better collateral perfusion. Women experienced less ischemic core growth (median 15 mL vs. 29 mL, p < 0.01) and had smaller final infarct volumes (median 26 mL vs. 50 mL, p < 0.01). Female sex was associated with a favorable shift on the modified Rankin Scale (adjusted cOR 1.79 [1.04 – 3.08; p = 0.04]) and lower odds of severe disability or death (adjusted OR 0.29 [0.10 – 0.81]; p = 0.02).
Conclusions:
The results suggest that women have better collaterals and, therefore, more often exhibit a favorable imaging profile on baseline imaging, experience less lesion growth, and have better clinical outcomes following endovascular therapy.
Keywords: Stroke, Thrombectomy, Magnetic Resonance Imaging, perfusion imaging, Women, Sex
Subject Terms: Cerebrovascular Procedures, Ischemic Stroke, Cerebrovascular Disease/Stroke, Thrombosis, Computerized Tomography (CT)
INTRODUCTION
While the effect of reperfusion does not seem to be modified by sex, there may be differences in stroke outcomes between men and women.1,2 Studies of the intervention arms of randomized controlled trials (RCT) on endovascular treatment (EVT) for large vessel occlusion (LVO) ischemic stroke and studies on ischemic stroke registries have shown conflicting results, but suggest that functional outcome after EVT may differ between men and women.3–11 In addition, a subanalysis of the DEFUSE 3 (Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke) study as well as other endovascular studies have found differences in collateral perfusion between men and women.4,5,12 These studies raise the question whether sex differences in outcome exist and can be attributed to differences in cerebral perfusion characteristics.
AIMS
In this study we examine the effect of sex on imaging characteristics and functional outcome in ischemic stroke patients treated with EVT in the CRISP (Computed tomographic perfusion to Predict Response to Recanalization in ischemic stroke) study.
METHODS
Data supporting the results of this study are available from the senior author (MGL) on reasonable request.
Study design and patient population
The design of the CRISP study has previously been described.13 In short, CRISP is a multi-center prospective cohort study which enrolled 201 ischemic stroke patients with documented LVO in the anterior circulation treated with endovascular therapy within 18 hours from last-known well time. All patients underwent non-contrast CT, CT angiography (CTA) and CT perfusion (CTP) imaging prior to EVT. EVT was initiated within 90 minutes following baseline imaging. Investigators were not blinded to CTP images, but were instructed not to use these images to guide their decision to offer endovascular therapy to eligible patients. Patients with a National Institutes of Health Stroke Scale (NIHSS) score lower than 5 or premorbid modified Rankin Score (mRS) of 2 or higher were excluded. Informed consent was obtained from all patients or their proxy if the patient was unable to consent. The institutional review board at each study site approved the study.
Imaging analysis
CTP ischemic core volumes and mismatch ratio were calculated with RAPID software (iSchemaView, Menlo Park, CA). A relative cerebral blood flow (rCBF) threshold of < 30% was used to define the ischemic core. Critically hypoperfused tissue (i.e. the perfusion lesion) was identified as the brain volume with a time-to-maximum of the tissue residue function (Tmax) of more than 6 seconds. Mismatch ratio was calculated as the ratio of the perfusion lesion volume over the volume of the ischemic core. Penumbra volume was calculated as the difference between the Tmax > 6s lesion volume and the ischemic core volume and the target mismatch ratio (TMM) profile was defined as 1) core volume <70mL, 2) mismatch ratio ≥ 1.8, 3) a volume difference between Tmax >6s lesion and core lesion ≥15 mL and 4) a Tmax >10s lesion <100mL. Collateral perfusion was assessed using the hypoperfusion intensity ratio (HIR; the proportion of tissue with a Tmax >10s within the Tmax > 6s volume).14 All RAPID outputs were manually reviewed by Stanford’s core imaging laboratory and artifacts were removed if needed. Alberta Stroke Program Early CT Score (ASPECTS) was independently assessed by four raters blinded to clinical data except for affected hemisphere.15 A common ASPECTS score for each patient was achieved by reviewing regions of disagreement between at least 2 raters during a joint consensus meeting. The Stanford core imaging laboratory analyzed digital subtraction angiography images for degree of recanalization using the modified Thrombolysis In Cerebral Infarction (mTICI) scale. Final infarct volume was visually outlined on the diffusion-weighted images (DWI) of the 24h magnetic resonance imaging (MRI). Ischemic core growth was calculated as the volume difference between the 24h DWI lesion and the baseline ischemic core lesion.
Outcomes
The primary outcome measure was the score on the modified Rankin Scale (mRS) at 90 days. Secondary outcome measures were the proportion of patients who achieved independence at 90 days, defined as an mRS 0–2, poor outcome (defined as mRS 5–6) and mortality at 90 days. Imaging outcomes were day 1 DWI lesion volume and ischemic core growth between baseline and day 1.
Statistical analysis
The primary analysis tested the association between sex and 90-day functional outcome using ordinal regression analysis with and without adjustment for confounders. We used a test of parallel lines to assess the assumption of proportional odds. Clinical and imaging characteristics were compared between men and women with chi square test for categorical variables and the Mann-Whitney U test for continuous and ordinal variables. We used a Shapiro-Wilk test to examine variables for normal distribution. We used univariable and multivariable logistic regression to examine the association between sex and functional independence (mRS 0–2), poor outcome (mRS 5–6) and mortality at 90 days. Clinical or imaging covariates were considered relevant for inclusion in the multivariable model if they were previously described as independent predictors of outcome (age, baseline NIHSS, premorbid mRS, core volume, TMM, ASPECTS, thrombolytic treatment, onset to groin puncture time interval, complete recanalization after EVT). We used the variance inflation factor to test covariables for multicollinearity before inclusion in the multivariable model. Population characteristics are described as mean (± SD) or median (interquartile range, IQR) unless otherwise specified. Odds ratios are described with a 95% confidence interval. All statistical analyses were 2-sided. Unless otherwise specified, an alpha of < 0.05 was considered significant. We used SPSS statistics (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp) for all statistical analyses.
RESULTS
Patient Characteristics
Of the 201 patients included in the CRISP study, 3 patients were excluded because of incomplete 90-day functional outcome data. Ninety-one patients were female (46%). Population characteristics for men and women are shown in Table 1. Women were older than men (mean 68 [±14] vs. 64 [±15]; p = 0.05). With regards to baseline imaging, women had smaller perfusion lesion volumes (median Tmax > 6s lesion volume in women 105 mL [68–160] vs 149 mL [81–210] in men; p < 0.001) and smaller penumbral volumes (median 96 mL [57–132] vs. 129 [72–188] in men, p = 0.001), but there was no difference in ischemic core volume. TMM was more prevalent in women (95% vs. 84%, p = 0.03) and women presented with a more favorable HIR (median 0.39 [0.25–0.56] vs. 0.51 [0.35–0.66], p= 0.02). There were no differences in thrombolytic treatment, onset-recanalization time, procedure duration or recanalization status after EVT (Table 1).
Table 1.
Population characteristics. Italic = significant results at p < 0.05.
| Characteristic | Women (n=91) | Men (n = 107) | p-value |
|---|---|---|---|
| Age (yr), mean (SD) | 68 (14) | 64 (15) | 0.05 |
| Premorbid mRS score, median (IQR) | 0 (0–0) | 0 (0–0) | 1.00 |
| 0, No. (%) | 77 (84.6) | 90 (84.1) | |
| 1, No. (%) | 12 (13.2) | 15 (14) | |
| 2, No. (%) | 1 (1.1) | 1 (0.9) | |
| 3, No. (%) | 1 (1.1) | 1 (0.9) | |
| Cardiovascular risk factors | |||
| Hypertension, No. (%) | 63 (69.2) | 71 (66.4) | 0.67 |
| Hyperlipidemia, No. (%) | 32 (35.2) | 46 (43) | 0.26 |
| Diabetes, No. (%) | 15 (16.5) | 27 (25.2) | 0.13 |
| History of stroke, No. (%) | 8 (8.8) | 13 (12.1)1 | 0.43 |
| History of myocardial ischemia, No. (%) | 8 (8.8)2 | 10 (9.3) | 0.91 |
| History of atrial fibrillation, No. (%) | 29 (31.9) | 35 (32.7) | 0.90 |
| Median admission NIHSS score (IQR) | 15 (12–20) | 17 (12–22) | 0.47 |
| Left-sided lesion, No. (%) | 47 (51.6) | 59 (55.1) | 0.59 |
| Baseline imaging characteristics | |||
| Ischemic core volume (mL), Median (IQR) | 6 (0–17)2 | 6 (0–27)3 | 0.49 |
| Tmax > 6s lesion volume (mL), Median (IQR) | 105 (68–160)2 | 149 (81–210)3 | < 0.001 |
| Penumbra volume (mL), Median (IQR) | 96 (57–132)2 | 129 (72–188)3 | 0.001 |
| Mismatch ratio, Median (IQR) | 13.7 (6.7 –inf)2 | 24.1 (6.1 – inf)4 | 0.74 |
| Target mismatch, No. (%) | 86 (94.5)2 | 90 (84.1)4 | 0.03 |
| Hypoperfusion Intensity Ratio, Median (IQR) | 0.39 (0.25–0.56)2 | 0.51 (0.35–0.66)5 | 0.02 |
| ASPECTS, Median (IQR) | 7 (5–9)6 | 7 (5–8)7 | 0.90 |
| Treated with intravenous thrombolysis, No. (%) | 44 (48.4) | 44 (41.1) | 0.31 |
| Procedural characteristics | |||
| Time Onset-Perfusion Imaging (min), Median (IQR) | 271 (170–415)2 | 255 (152–387) | 0.62 |
| Time Onset-Groin puncture (min), Median (IQR) | 328 (237–511)2 | 305 (221–444)8 | 0.31 |
| Time Onset-Procedure end (min), Median (IQR) | 427 (300–569)9 | 396 (315–549)10 | 0.54 |
| Complete recanalization (mTICI 2b-3), No. (%) | 76 (83.5)2 | 93 (86.9)4 | 0.30 |
data available for 106 patients;
data available for 90 patients;
data available for 102 patients;
data available for 104 patients;
data available for 101 patients:
data available for 86 patients;
data available for 101 patients;
data available for 105 patients;
data available for 89 patients;
data available for 103 patients.
Abbreviations: SD = standard deviation; IQR = interquartile range; mRS = modified Rankin Scale; NIHSS = National Institutes of Health Stroke Scale; Tmax = Time-to-maximum; inf = infinity; ASPECTS = Alberta Stroke Program Early Computed Tomography Score; mTICI = modified Thrombolysis in Cerebral Infarction.
Association of sex with functional outcome after EVT
Female sex was associated with a more favorable outcome on the full ordinal mRS after adjustment for age, premorbid mRS, admission NIHSS, ASPECTS, baseline ischemic core volume, the presence of target mismatch, thrombolysis, onset to groin puncture time interval and recanalization status (adjusted common OR 1.79 [1.04–3.08; p = 0.04])) (unadjusted analysis: common OR 1.57 [0.96 – 2.57; p = 0.08]) (Figure 1). Good functional outcome (mRS 0–2) did not differ between men and women (adjusted p = 0.35; Table 2 and supplemental Table I and III). However, women were less likely to have severe disability or death (mRS 5–6), adjusted confounders (11% vs 22%; OR 0.29 [0.10 – 0.81], adjusted p = 0.02), Table 2 and supplemental Table II and IV).
Figure 1.
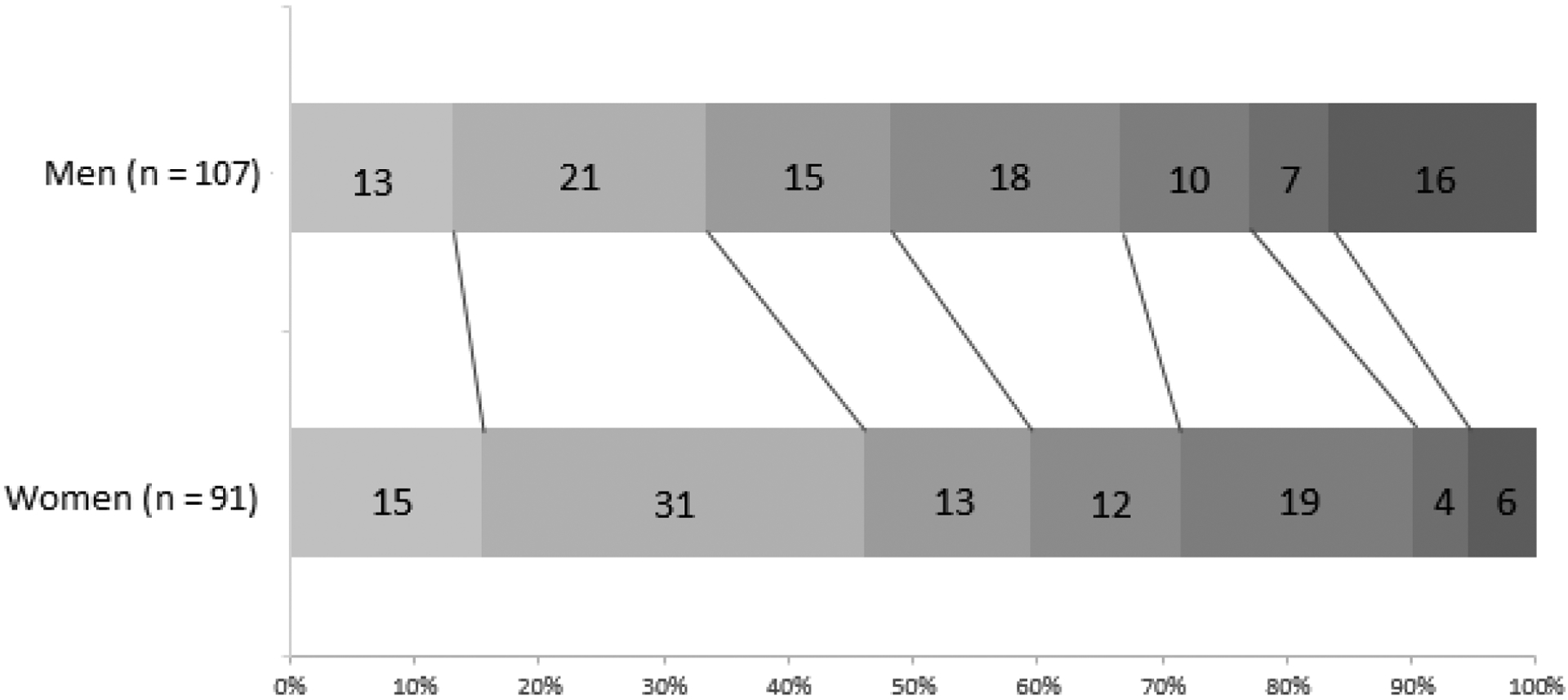
Ninety-day modified Rankin Scores (mRS) for men and women. Proportions for each mRS are given. Adjusted common odds ratio for shift on the mRS in women vs. men = 1.79 (1.04 – 3.08), p = 0.04.
Test of parallel lines p = 0.72
Table 2.
Functional outcomes for women and men at 90 days after treatment. Italic = significant results at p < 0.05. Multivariable analysis was adjusted for age, baseline NIHSS, premorbid mRS, admission ischemic core volume, target mismatch ratio, ASPECTS, thrombolytic treatment, onset to groin puncture time interval and complete recanalization after endovascular treatment.
| Functional outcome day 90 | Women (n=91) | Men (n = 107) | Unadjusted OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|---|---|
| Day 90 mRS, Median (IQR) | 2 (1–4) | 3 (1–4) | 1.57 (0.63 – 2.57) | 1.79 (1.04 – 3.08) |
| mRS 0–2, No (%) | 54 (59.3) | 52 (48.6) | 1.54 (0.88 – 2.72) | 1.40 (0.69 – 2.86) |
| mRS 5–6, No (%) | 9 (9.9) | 24 (22.4) | 0.38 (0.17 – 0.87) | 0.29 (0.10 – 0.81) |
Abbreviations: OR = odds ratio; CI = confidence interval; IQR = interquartile range; mRS = modified Rankin Scale, NIHSS = National institutes of Health Stroke Scale; ASPECTS = Alberta Stroke Program Early CT score.
Association of sex with follow-up imaging after EVT
Day 1 DWI volumes were available for 81% of women and 76% of men. There was less infarct growth between baseline and day 1 in women (median 15 mL [2–38]) than in men (29 mL [15–68]; p < 0.01), resulting in smaller day 1 DWI infarct volumes in women (median 26 mL [10–46]) than in men (50 mL [18–90]; p<0.01). Additional analyses in patient subsets, categorized according to recanalization status, demonstrated that the sex difference in infarct growth was present in patients with complete recanalization (mTICI 2b-3), but not in patients with incomplete recanalization (mTICI 1–2a). (Supplemental Table V).
DISCUSSION
We assessed the difference in outcomes between sexes among patients who received EVT and found that female sex was associated with better functional outcomes and lower mortality and severe disability in a population of unselected ischemic stroke patients who underwent EVT within 18 hours from last seen well. This is in contrast to the Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands (MR CLEAN) trial, which showed higher rates of severe disability and mortality among women in the intervention arm3, the DEFUSE 3 trial, which showed lower rates of good functional outcome (mRS 0–2) in women (38%) compared to men (67%, p = 0.02), and a single-center cohort study of 279 patients, which reported lower rates of independence for women at 90 days adjusted for clinical confounders, recanalization rate and baseline ASPECTS (adjusted OR 0.37 [0.16–0.87]).7 Several other studies have shown similar outcomes between sexes after endovascular treatment, including the Highly Effective Reperfusion evaluated in Multiple Endovascular Stroke Trials (HERMES) meta-analysis (mRS 0–2 45% in women vs 47% in men, unadjusted for clinical and imaging variables)4, a large prospective Japanese registry (adjusted OR 0.83 [0.63–1.09]),6 a post-hoc analysis of the non-randomized Mechanical Embolus Removal in Cerebral Ischemia (MERCI) and Multi MERCI trials,10 a consecutive Swiss registry of EVT treated patients11 and two other single-center cohort studies, adjusted for clinical confounders, baseline ASPECTS and reperfusion rate.8,9 In summary, our results which show better outcomes among women, differ from prior studies that mostly show equal outcomes between men and women, except for the MR CLEAN and DEFUSE 3 studies which showed worse outcomes among women.
Although most abovementioned studies adjusted for a range of confounders, perfusion imaging profile or collateral status was rarely studied. In this study, women had better collateral perfusion assessed on baseline CTP and, likely as a result, less ischemic core growth and smaller final infarcts after successful recanalization. These sex differences were restricted to patients with complete recanalization, which suggests that women better preserved the admission core volume due to better collateral perfusion. The differences in final infarct volume may explain the superior functional outcomes and lower chance of severe disability and death among women.16 The sex differences in perfusion and collateral imaging in this study are in agreement with findings from several prior studies: DEFUSE 3, which showed more favorable collaterals on baseline CTP and a trend towards less ischemic core growth in women;5 the HERMES meta-analysis, which showed better baseline collaterals and smaller final infarct volumes among women;2 and the MR CLEAN registry, where an association between female sex and favorable collateral circulation on CTA was found.12 In contrast, post-hoc analyses of the IMS III (Interventional Management of Stroke III), SWIFT (Solitaire with the Intention for Thrombectomy), and STAR (Solitaire FR Thrombectomy for Acute Revascularisation) trials showed no sex difference in angiography-scored collaterals and SWIFT-PRIME (Solitaire with the Intention for Thrombectomy as Primary Endovascular Treatment) showed no association between collateral grade and sex.9,17 Given that the results of our study as well as prior studies indicate that women may have better collaterals than men when assessed with CTA or CTP and, likely as a result, less infarct growth, it is surprising that only our study showed better functional outcomes in women. Although failure of collateral perfusion could theoretically explain inter-study differences, imaging-to-femoral puncture times in DEFUSE 3 and CRISP were very similar (median imaging-to-femoral puncture time of 59 min for both women [IQR 39–89] and men [IQR 41–86] in DEFUSE 3 and median CTP-femoral puncture time of 60 min [IQR 40–84] in CRISP), and thus likely do not explain the paradoxical finding that women had better collaterals but worse functional outcomes in DEFUSE 3.
Since all patients in the CRISP study underwent EVT we can only analyze sex differences in outcome after EVT treatment but could not study the interaction between sex and the treatment effect of EVT. Several studies have examined the association between female sex and the effect of EVT. A sub-analysis of the MR CLEAN trial revealed higher rates of severe disability and mortality and a reduced benefit of EVT for women.3 This analysis was, however, not prespecified and was uncontrolled for pretreatment imaging characteristics. A subsequent meta-analysis of individual patient data from 7 randomized controlled EVT trials, including the MR CLEAN trial, found no interaction of sex by treatment after adjustment for confounders (p = 0.93).2 The late treatment window DEFUSE 3 study also found no significant sex by treatment interaction.5 Available data thus suggests that the benefit of EVT is similar for men and women.
This study has limitations. Assessment of sex differences on functional outcome after EVT was not a prespecified analysis of the CRISP study. Second, follow-up imaging was not available in all patients. Third, although perfusion imaging was not a selection criterion for CRISP, investigators were not blinded from advanced imaging results and may therefore have introduced a selection bias towards women with better baseline perfusion status. The observed differences in collateral status in this study may therefore not necessarily reflect a biological difference between men and women. Fourth, this study lacks data on sex differences in therapy selection in the days following the endovascular procedure, which may explain differences if, for example, women were more likely transitioned to comfort care measures. Finally, admission ischemic core volumes, perfusion lesion volumes and 24-hour DWI volumes were not adjusted for sexual dimorphism.
In conclusion, in patients who underwent EVT up to 18 hours after last seen well, female sex was associated with better collateral perfusion, less infarct growth and smaller final infarct volumes, which is in accordance with other large clinical studies using perfusion imaging. In this study, women also had better functional outcomes following EVT.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank the CRISP investigators and patients who participated in the study.
SOURCES OF FUNDING
The CT Perfusion to predict Response in Ischemic Stroke Project (CRISP) was funded by a grant from the National Institute of Neurological Disorders and Stroke (principal investigator, M.G.L.).
Jelle Demeestere is funded by a research grant of Scientific Research Fund Flanders (Fonds Wetenschappelijk onderzoek, FWO).
Robin Lemmens is a Senior Clinical Investigator of Scientific Research Fund Flanders (Fonds Wetenschappelijk onderzoek, FWO).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
Sören Christensen and Gregory Albers have equity interest in and are consultants for iSchemaView. Gregory Albers is a consultant for Medtronic. RL reports institutional fees from Bayer, Boehringer-Ingelheim, Genentech, Ischemiaview, Medtronic and Occlutec.
REFERENCES
- 1.Bushnell C, Howard VJ, Lisabeth L, Caso V, Gall S, Kleindorfer D, et al. Sex differences in the evaluation and treatment of acute ischaemic stroke. Lancet Neurol. 2018;17:641–650. [DOI] [PubMed] [Google Scholar]
- 2.Chalos V, de Ridder IR, Lingsma HF, Brown S, van Oostenbrugge RJ, Goyal M, et al. Does Sex Modify the Effect of Endovascular Treatment for Ischemic Stroke? Stroke. 2019;50:2413–2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Ridder IR, Fransen PS, Beumer D, Berkhemer OA, van den Berg LA, Wermer MJ, et al. Is Intra-Arterial Treatment for Acute Ischemic Stroke Less Effective in Women than in Men? Interv Neurol. 2016;5:174–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goyal M, Menon BK, van Zwam WH, Dippel DWJ, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723–1731. [DOI] [PubMed] [Google Scholar]
- 5.Dula AN, Mlynash M, Zuck ND, Albers GW, Warach SJ. Neuroimaging in Ischemic Stroke Is Different Between Men and Women in the DEFUSE 3 Cohort. Stroke. 2020;51:481–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uchida K, Yoshimura S, Sakai N, Yamagami H, Morimoto T. Sex Differences in Management and Outcomes of Acute Ischemic Stroke With Large Vessel Occlusion. Stroke. 2019;50:1915–1918. [DOI] [PubMed] [Google Scholar]
- 7.Madsen TE, DeCroce-Movson E, Hemendinger M, McTaggart RA, Yaghi S, Cutting S, et al. Sex differences in 90-day outcomes after mechanical thrombectomy for acute ischemic stroke. Journal of neurointerventional surgery. 2019;11:221–225. [DOI] [PubMed] [Google Scholar]
- 8.Carvalho A, Cunha A, Gregório T, Paredes L, Costa H, Veloso M, et al. Is the Efficacy of Endovascular Treatment for Acute Ischemic Stroke Sex-Related. Interv Neurol. 2018;7:42–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheth SA, Lee S, Warach SJ, Gralla J, Jahan R, Goyal M, et al. Sex Differences in Outcome After Endovascular Stroke Therapy for Acute Ischemic Stroke. Stroke. 2019;50:2420–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lutsep HL, Hill MD. Effects of sex on mechanical embolectomy outcome. J Stroke Cerebrovasc Dis. 2012;21:240–242. [DOI] [PubMed] [Google Scholar]
- 11.Arnold M, Kappeler L, Nedeltchev K, Brekenfeld C, Fisher U, Keserue B, et al. Recanalization and outcome after intra-arterial thrombolysis in middle cerebral artery and internal carotid artery occlusion: does sex matter? Stroke. 2007;38:1281–1285. [DOI] [PubMed] [Google Scholar]
- 12.Wiegers EJA, Mulder MJHL, Jansen IGH, Venema E, Compagne KCJ, Berkhemer OA, et al. Clinical and Imaging Determinants of Collateral Status in Patients With Acute Ischemic Stroke in MR CLEAN Trial and Registry. Stroke. 2020;51:1493–1502. [DOI] [PubMed] [Google Scholar]
- 13.Lansberg MG, Christensen S, Kemp S, Mlynash M, Mishra N, Federau C, et al. Computed tomographic perfusion to Predict Response to Recanalization in ischemic stroke. Ann Neurol. 2017;81:849–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guenego A, Fahed R, Albers GW, Kuraitis G, Sussman ES, Martin BW, et al. Hypoperfusion intensity ratio correlates with angiographic collaterals in acute ischaemic stroke with M1 occlusion. Eur J Neurol. 2020;27:864–870. [DOI] [PubMed] [Google Scholar]
- 15.Demeestere JSL, Cornelissen SA, Heye S, Wouters A, Dupont P, Christensen S, et al. Alberta Stroke Program Early CT Score Versus Computed Tomographic Perfusion to Predict Functional Outcome After Succesful Reperfusion in Acute Ischemic Stroke. Stroke. 2018;49:2361–2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boers AMM, Jansen IGH, Beenen LFM, Devlin TG, San roman L, Heo JH, et al. Association of follow-up infarct volume with functional outcome in acute ischemic stroke: a pooled analysis of seven randomized trials. J Neurointerv Surg. 2018;10:1137–1142. [DOI] [PubMed] [Google Scholar]
- 17.Liebeskind DS, Tomsick TA, Foster LD, Yeats SD, Carozzella J, Demchuck AM, et al. Collaterals at angiography and outcomes in the Interventional Management of Stroke (IMS) III trial. Stroke. 2014;45:759–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


