Abstract
Introduction:
Hysterectomy is a commonly performed procedure with widely variable costs. As gynecologists divert from invasive to minimally invasive approaches, many factors come into play in determining hysterectomy cost and efforts should be sought to minimize it. Our objective was to identify the predictors of hysterectomy cost.
Materials and Methods:
This was a retrospective cohort study where women who underwent hysterectomy for benign conditions at the University of Texas Medical Branch from 2009 to 2016 were identified. We obtained and analyzed demographic, operative, and financial data from electronic medical records and the hospital finance department.
Results:
We identified 1,847 women. Open hysterectomy was the most frequently practiced (35.8%), followed by vaginal (23.7%), laparoscopic (23.6%), and robotic (16.9%) approaches. Multivariate regression demonstrated that hysterectomy charges can be significantly predicted from surgical approach, patient’s age, operating room (OR) time, length of stay (LOS), estimated blood loss, insurance type, fiscal year, and concomitant procedures. Charges increased by $3,723.57 for each day increase in LOS (P <0.001), by $76.02 for each minute increase in OR time (P <0.001), and by $48.21 for each one-year increase in age (P 0.037). Adjusting for LOS and OR time remarkably decreased the cost of open and robotic hysterectomy, respectively when compared with the vaginal approach.
Conclusion:
Multiple demographic and operative factors can predict the cost of hysterectomy. Healthcare providers, including gynecologists, are required to pursue additional roles in proper resource management and be acquainted with the cost drivers of therapeutic interventions. Future efforts and policies should target modifiable factors to minimize cost and promote value-based practices.
Keywords: Hysterectomy, Laparoscopy, Length of stay, Minimally invasive surgery, Robotic-assisted surgery
INTRODUCTION
Hysterectomy is the most common major gynecologic surgery in the United States (US) with about 600,000 performed annually, mostly for benign indications, such as uterine fibroids, abnormal bleeding, and endometriosis [1]. For several advantages, including less pain and blood loss, shorter hospital stay, and faster recovery, there is a clear trend towards utilizing minimally invasive approaches [2–5]. This is in line with the American College of Obstetricians and Gynecologists recommendations for utilizing minimally invasive approaches, particularly vaginal hysterectomy [6]. Nevertheless, statistical data show that US institutions fall short of these recommendations in that vaginal hysterectomy is performed in only 22% of cases and laparoscopic hysterectomy in 12% of cases, whereas open hysterectomy is performed in 66% of cases [7]. Further evidence suggests that among minimally invasive approaches, the robotic approach is associated with higher costs [8].
Healthcare spending in the U.S. has been surging to potentially unsustainable levels. National Healthcare Expenditure (NHE) soared from $721.4 billion in 1990 to $3.6 trillion in 2018 and is projected to reach $6.2 trillion by 2028. The proportion of NHE to Gross Domestic Product increased from 5% in 1960 to 17.7% in 2018. The largest NHE contributor is hospital costs ($1.2 trillion in 2018, 33.1%) rather than physician salaries (7.3%) [9].
Based on these data, there is an obvious need to increase minimally invasive surgery utilization to accomplish its benefits while containing the costs of the more expensive approaches, a goal we strongly believe is achievable. To this end, the cost drivers of hysterectomy need to be identified and strategically addressed. This allows high value care that combines better outcomes with sustainable costs. Of note, higher cost is not necessarily associated with better surgical outcomes nor is lower spending a predictor of poor care [10].
Evidence-based knowledge of the precise cost predictors of hysterectomy is largely lacking. Specifically, previous studies focused on the route and largely ignored other important predictors, such as length of stay (LOS)1 or operating room (OR)2 time [8]. Therefore, we examined in this report the trend and interplay of the different predictors of hysterectomy cost.
MATERIALS AND METHODS
Study population
This is a retrospective cohort study. Using electronic medical records (EMR), we included all women who underwent hysterectomy from 2009 through 2016 at the University of Texas Medical Branch, a tertiary-care center in Galveston, Texas. Cases were identified using the Current Procedural Terminology (CPT) codes. We excluded cases with a pre- or postoperative diagnosis of gynecologic malignancy, hysterectomy with any concomitant non-gynecologic surgery to avoid cost inflation, and records with incomplete procedure or charge data. We restricted this study to benign indications of hysterectomy as cancer cases possess a different set of characteristics.
Variables studied
We collected demographic and perioperative data from EMR, including the patient’s age at the time of hysterectomy, race, BMI, fiscal year of surgery (2009–2016), approach, estimated blood loss, uterine weight, OR time, LOS, concomitant gynecologic procedures, and need for blood transfusion. Hysterectomy was categorized according to approach into open, laparoscopic, vaginal, and robotic hysterectomy. We maintained an intention-to-treat groups; for example, laparoscopic hysterectomies that were converted to open were still analyzed and included in the laparoscopic category. The concomitant procedures commonly performed at the time of hysterectomy were also identified using CPT codes and included unilateral/bilateral salpingectomy and/or oophorectomy, pelvic-vaginal reconstruction, incontinence repair, adhesolysis, and cystoscopy and/or cystourethroscopy. Concomitant pelvic-vaginal reconstruction procedures included anterior and posterior colporrhaphy, uterosacral and sacrospinous ligament suspension, modified McCall culdoplasty, and sacrocolpopexy. OR time (wheels in-wheels out) was obtained for each patient instead of procedure time to include OR utilization time preceding skin incision and following skin closure. Pre-skin incision OR time (anesthesia, positioning, and prepping) and post-skin closure OR time (wake up and wrap up) may vary and can contribute to variation in charges [11].
In addition, hospital charges data were obtained from the hospital finance management department and included total charges for each individual hospitalization and the primary insurance payer (commercial/managed care, employee, Medicare, Medicaid, correctional, unsponsored, and others). Total charges were analyzed after adjusting for annual inflation at the hospital region and reported in constant 2009 U.S. dollars as provided by the Consumer Price Index (CPI) report from U.S. Bureau of Labor Statistics [12]. Values were then rounded to the nearest dollar. Zero charges were noted as missing. Inflation-adjusted charge data were carefully checked and subjects with missing or spurious charges (less than $500) were excluded from the analyses [8]. We used fiscal (September 1st through August 31st) instead of calendar years as this is how accounting at the hospital finance management department is performed.
Statistical analysis
The primary outcome of our analysis was charges. A multivariate linear regression was conducted to examine the effects of covariates on hysterectomy cost. Assumptions of linear regression were checked. Linearity between predictors and outcome was assessed by partial regression plots and a plot of studentized residuals against the predicted values. Independence of residuals was present as assessed by a Durbin-Watson statistic of 1.844. Multicollinearity and normality were also tested by tolerance values and Q-Q plot, respectively. We run the regression analysis before and after adjusting for particular predictor variables to evaluate their contribution to charges of different approaches to hysterectomy. We also evaluated the annual trend of number of cases, total charges, LOS, and OR time per hysterectomy approach throughout the study period. For more visually clear comparisons, we grouped and labeled laparoscopic, vaginal, and robotic approach categories as minimally invasive. Descriptive statistics were reported as means and standard deviations. Statistical significance was set at P <0.05. All statistical analyses were performed using SPSS statistics v21 (IBM Corporation, Armonk, NY).
Ethical Approval
The study was approved by the Institutional Review Board at the University of Texas Medical Branch on March 20th, 2013. The IRB approval reference number is IRB #13–084.
RESULTS:
This study included 1,847 women. Patient characteristics, perioperative data, payer type, and fiscal year of surgery are listed in Table 1 and stratified by hysterectomy approach (open vs. minimally invasive). Mean age of women was similar between open and minimally invasive groups (45.7 vs. 47.4). Mean estimated blood loss (378.8 vs. 181 mL) and uterine weight (600.5 vs. 152.3 g) as well as proportion of transfused women (12.3% vs. 3.2%) were higher among the open group. Of note, the two groups in table 1 are meant only to describe rather than compare the study population as differences in surgical characteristics, such as uterine weight and additional procedures, would hinder the comparison between groups. Table 2 further stratifies hysterectomy approaches and demonstrates the variation in total charges, OR time, LOS, and the percentages of concomitant incontinence repair and pelvic-vaginal reconstruction procedures. The open approach was the most frequent (35.8%), followed by the vaginal (23.7%), laparoscopic (23.6%), and robotic (16.9%) approaches. The robotic approach was the most expensive at $42,816 while the vaginal approach was the least expensive at $25,535. Similarly, OR time was greatest for robotic hysterectomy (337.5 min) and least for vaginal hysterectomy (232 min). When compared with women who received open hysterectomy, women who underwent robotic, vaginal, and laparoscopic hysterectomy had a shorter hospital stay, 0.9–1.4 days vs. 3.4 days.
Table 1.
Demographic and perioperative characteristics of the study population by approach of hysterectomy.*
| Characteristic | Open (n=662) | Minimally Invasive (n=1,185) |
|---|---|---|
| Age (years) | 45.7 (±10.1) | 47.4 (±13.3) |
| Operating room time | 236.3 (±78.1) | 274.1 (±90.4) |
| Length of stay (days) | 3.4 (±3) | 1.1 (±1.5) |
| Estimated blood loss (mL) | 378.8 (±504.2) | 181 (±211.3) |
| Uterine weight (g) | 600.5 (±899.6) | 152.3 (±147.2) |
| BMI (Kg/m2) | 31.8 (±7.7) | 31.2 (±7.3) |
| Race | ||
| Caucasian | 149 (22.5%) | 410 (34.6%) |
| African American | 177 (26.7%) | 166 (14%) |
| Hispanic | 111 (16.8%) | 232 (19.6%) |
| Additional procedure | ||
| Unilateral salpingectomy ± oophorectomy | 67 (10.1%) | 84 (7.1%) |
| Bilateral salpingectomy ± oophorectomy | 412 (62.2%) | 610 (51.5%) |
| Pelvic-vaginal reconstruction | 26 (3.9%) | 339 (28.6%) |
| Incontinence repair | 29 (4.4%) | 215 (18.1%) |
| Adhesolysis | 226 (34.1%) | 204 (17.2%) |
| Cystoscopy/cystourethroscopy | 150 (22.7%) | 697 (58.8%) |
| Blood transfusion | 80 (12.1%) | 38 (3.2%) |
| Insurance | ||
| Commercial/Managed care | 163 (24.6%) | 482 (40.7%) |
| Correctional | 196 (29.6%) | 242 (20.4%) |
| Employee | 49 (7.4%) | 157 (13.2%) |
| Medicare | 47 (7.1%) | 124 (10.5%) |
| Medicaid | 108 (16.3%) | 132 (11.1%) |
| Unsponsored | 78 (11.8%) | 30 (2.5%) |
| Other | 21 (3.2%) | 18 (1.5%) |
| Year | ||
| 2009 | 56 (8.5%) | 58 (4.9%) |
| 2010 | 102 (15.4%) | 117 (9.9%) |
| 2011 | 119 (18%) | 122 (10.3%) |
| 2012 | 80 (12.1%) | 167 (14.1%) |
| 2013 | 67 (10.1%) | 160 (13.5%) |
| 2014 | 81 (12.2%) | 170 (14.3%) |
| 2015 | 86 (13%) | 224 (18.9%) |
| 2016 | 71 (10.7%) | 167 (14.1%) |
Data are represented as mean (± standard deviation) or n (%). For year of surgery and insurance type, percentages are additive within each hysterectomy approach stratum.
Table 2.
Average cost, operating room time, and length of stay and percentages of concomitant incontinence repair and pelvic-vaginal reconstruction stratified by approach of hysterectomy.
| Characteristic | Approach of Hysterectomy | |||
|---|---|---|---|---|
| Open (n=662) |
Laparoscopic (n=435) |
Robotic (n=312) |
Vaginal (n=438) |
|
| Total charges* (US Dollars) | $28,449 (±18,878) | $36,518 (±14,043) | $42,816 (±12,441) | $25,535 (±10,259) |
| Operating room time* (min) | 236.3 (±78.1) | 271 (±78) | 337.5 (±89.8) | 232 (±75) |
| Length of stay* (days) | 3.4 (±3) | 1.4 (±1.8) | 0.9 (±1.1) | 1 (±1) |
| Incontinence repair | 29 (4.4%) | 19 (4.4%) | 32 (10.3%) | 164 (37.4%) |
| Pelvic-vaginal reconstruction | 26 (3.9%) | 16 (3.7%) | 50 (16%) | 273 (62.3%) |
Data represented as mean (± standard deviation) or n (%).
Figure 1A displays the trend in percentage of cases per hysterectomy approach per fiscal year throughout the study period. Percentage of open cases remarkably decreased from 2009 to 2016 (49.1% vs. 29.8%). On the contrary, proportion of minimally invasive cases increased from 50.9% in 2009 to 70.2% in 2016. Figure 1B shows a consistent increase in total charges per hospitalization among all approaches throughout the study period, whereas figures 1C and 1D depict the trend in LOS and OR time, respectively, per hysterectomy approach for the same time period. The mean LOS showed a congruent decrease from 2009 to 2016 for all approaches (figure 1C).
Figure 1.
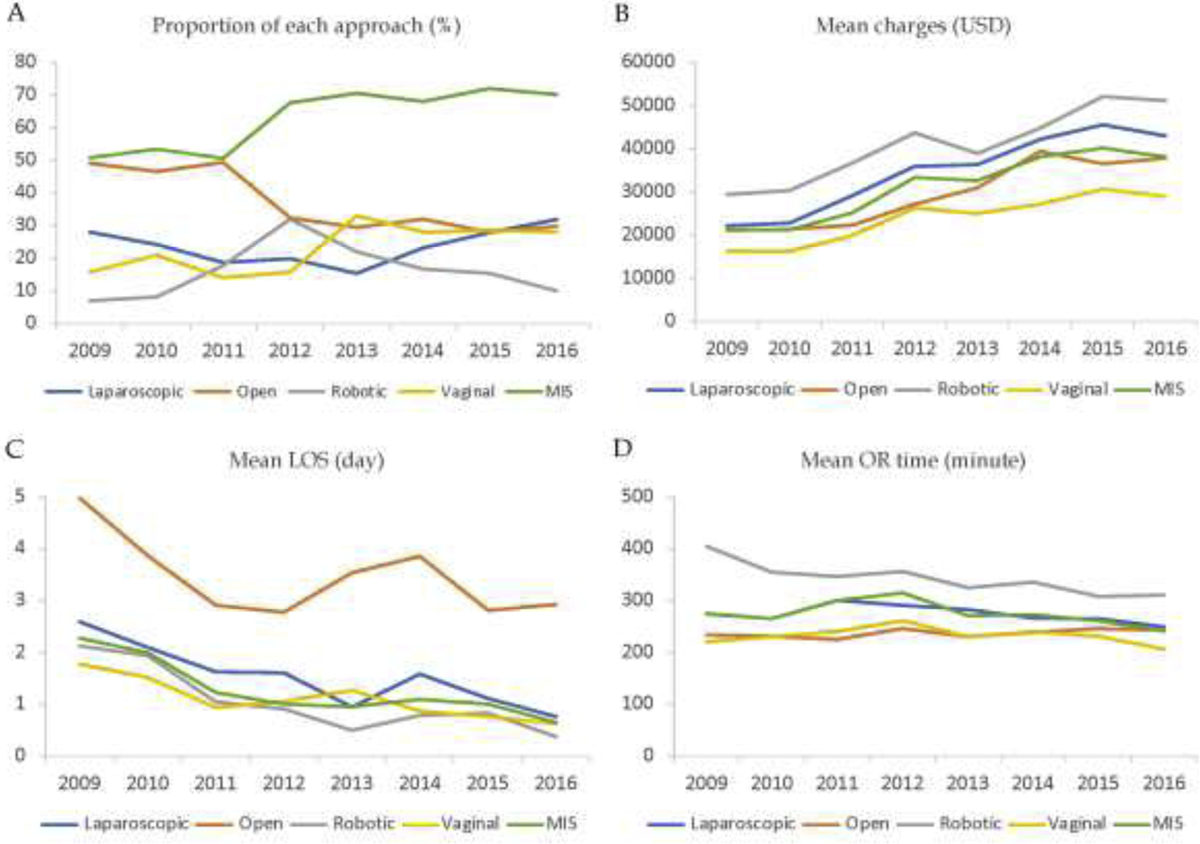
A. Proportion of hysterectomies per approach per fiscal year. B. Mean total charges per approach per fiscal year. All charges were adjusted for inflation to 2009 U.S. Dollars. C. Mean length of stay per approach per fiscal year. D. Mean OR time per approach per fiscal year.
A multivariate regression analysis was run to predict hysterectomy charges from demographic, perioperative, and financial factors. The multivariate regression model significantly predicted hysterectomy charges (P <0.001), R2 = 0.75. Patient’s age, OR time, LOS, estimated blood loss, procedure approach, some payer types, fiscal year of surgery, and some concomitant procedures were significant predictors of hysterectomy charges. Regression coefficients and standard errors are presented in Table 3.
Table 3.
Multivariate linear regression model for hysterectomy cost predictors.
| Predictor |
B (Slope coefficient) |
SEB (Standard error) |
P value | 95% CI |
|---|---|---|---|---|
| Hysterectomy approach | ||||
| Vaginal* | — | — | — | — |
| Laparoscopic | 8,416.547 | 741.645 | 0.000 | 6,961.9 – 9,871.1 |
| Robotic | 11,630.898 | 827.860 | 0.000 | 10,007.2 – 13,254.6 |
| Open | −2,150.618 | 731.669 | 0.003 | −3,585.6 to −715.6 |
| Age | 48.214 | 17.841 | 0.037 | 13.2 – 83.2 |
| Operating room time | 76.021 | 2.964 | 0.000 | 70.2 – 81.8 |
| Length of stay | 3,723.572 | 96.903 | 0.000 | 3,533.5 – 3,913.6 |
| Estimated blood loss | 2.437 | 0.649 | 0.000 | 1.1 – 3.7 |
| Uterine weight | −0.438 | 0.360 | 0.223 | −1.1 – 0.267 |
| BMI | 11.360 | 27.102 | 0.675 | −41.8 – 64.5 |
| Race | ||||
| Caucasian* | — | — | — | — |
| African American | 382.467 | 514.051 | 0.457 | −625.7 – 1,390.7 |
| Hispanic | 163.097 | 514.080 | 0.751 | −845.2 – 1,171.3 |
| Adnexal procedure | ||||
| Unilateral salpingectomy ± oophorectomy* | — | — | — | — |
| Bilateral salpingectomy ± oophorectomy | 881.855 | 432.854 | 0.042 | 32.9 – 1,730.8 |
| Pelvic-vaginal reconstruction | 852.733 | 723.950 | 0.239 | −567.1 – 2,272.6 |
| Incontinence repair | 1,514.353 | 707.724 | 0.033 | 126.3 – 2,902.4 |
| Adhesolysis | 811.583 | 492.919 | 0.100 | −155.2 – 1,778.3 |
| Cystoscopy/cystourethroscopy | 316.721 | 483.670 | 0.513 | −631.9 – 1,265.3 |
| Blood transfusion | 2,225.659 | 863.725 | 0.010 | 531.6 – 3,919.7 |
| Insurance | ||||
| Commercial/Managed care* | — | — | — | — |
| Correctional | −1,312.748 | 572.744 | 0.022 | −2436.1 to −189.4 |
| Employee | −1,287.560 | 672.110 | 0.056 | −2,605.8 – 30.6 |
| Medicare | 1,797.973 | 746.186 | 0.016 | 334.5 – 3,261.5 |
| Medicaid | 394.517 | 647.615 | 0.542 | −875.6 – 1,664.7 |
| Unsponsored | 114.664 | 924.213 | 0.901 | −1,698 – 1,927.3 |
| Other | −149.682 | 1,384.729 | 0.914 | −2,865.6 – 2,566.2 |
| Year | ||||
| 2009* | — | — | — | — |
| 2010 | 2,949.644 | 991.042 | 0.003 | 1,005.9 – 4,893.4 |
| 2011 | 8,445.984 | 985.161 | 0.000 | 6,513.8 – 10,378.2 |
| 2012 | 14,026.409 | 940.977 | 0.000 | 11,676.8 – 15,554.4 |
| 2013 | 15,871.620 | 952.073 | 0.000 | 12,919.8 – 16,836.6 |
| 2014 | 20,124.092 | 928.048 | 0.000 | 17,238.5 – 21,111.9 |
| 2015 | 24,984.105 | 904.063 | 0.000 | 21,887.2 – 25,707.5 |
| 2016 | 26,285.830 | 937.845 | 0.000 | 22,490.7 – 26,456.4 |
Reference group
Predicted hysterectomy charges increased by $48.21 for each one-year increase in age (P 0.037), by $76.02 for each minute increase in OR time (P <0.001), and by $3,723.57 for each day increase in LOS (P <0.001). Compared with vaginal hysterectomy, predicted procedure charges increased by $8,417 for the laparoscopic approach (P <0.001) and by $11,631 for the robotic approach (P <0.001), while it decreased by $2,151 for the open approach (P 0.003). An estimated blood loss of 100 mL predicted a $243.7 increase in hysterectomy charges (P <0.001), whereas transfusing a patient increased charge by $2,225.6 (P 0.010). Women who underwent concomitant bilateral salpingectomy and/or oophorectomy had their procedure cost increased by $881.8 compared with unilateral resection (P 0.042), while anti-incontinence repair increased it by $1,514.3 (P 0.033). With regard to payer type, Medicare increased hysterectomy charges by $1,788 (P 0.016), whereas correctional insurance decreased charges by $1,312 (P 0.022), when compared with commercial/managed care insurance. Fiscal year of surgery showed a consistent predicted increase in hysterectomy charges from 2009 to 2016 that ranged from $2,950 for 2010 to $26,286 for 2016, when compared with fiscal year 2009 (P <0.001).
Multivariate regression analysis was re-run without adjusting for LOS, OR time and uterine weight, one at a time, to quantify their contribution to hysterectomy charges for individual approaches and is presented in Table 4. Before adjusting for LOS, open hysterectomy increased procedure charges by $4,023 as opposed to a charge reduction of $2,151 after adjustment, when compared with vaginal hysterectomy. On the other hand, robotic and laparoscopic hysterectomies predicted a charge increase of $20,639 and $14,330, respectively, before adjusting for OR time compared with a charge increase of only $11,631 and $8,417 after adjustment, when using the vaginal approach as a reference group. Uterine weight was not a significant predictor for hysterectomy charges in our analysis (P 0.223). Adjusting for uterine weight slightly decreased the cost of open and laparoscopic hysterectomy from $1,977 to $2,151 less than vaginal hysterectomy, and from $8,562 to $8,417 more than vaginal hysterectomy, respectively.
Table 4.
Slope coefficient of total charges before and after adjusting for particular predictors.
| Approach | Slope coefficient (B), including all covariates except for LOS | B after adjusting for LOS |
|---|---|---|
| Open | 4,023.039 | −2,150.618 |
| Laparoscopic | 8,109.515 | 8,416.547 |
| Robotic | 10,566.023 | 11,630.898 |
| Slope coefficient (B), including all covariates except for OR time | B after adjusting for OR time | |
| Open | 618.443 | −2,150.618 |
| Laparoscopic | 14,330.477 | 8,416.547 |
| Robotic | 20,639.419 | 11,630.898 |
| Slope coefficient (B), including all covariates except for uterine weight | B after adjusting for uterine weight | |
| Open | −1,977.102 | −2,150.618 |
| Laparoscopic | 8,562.184 | 8,416.547 |
| Robotic | 11,364.341 | 11,630.898 |
Vaginal approach is used as the reference group. LOS, Length of Stay; OR, Operating Room.
DISCUSSION
The results of our study demonstrated the contribution of different predictor variables, such as the approach, LOS, and OR time, to hysterectomy cost and their temporal trends.
In this study, the rate of minimally invasive hysterectomy has shown an increase throughout the study period. Importantly, the increase in robotic and laparoscopic hysterectomy use has notably been at the expense of open not vaginal cases, observations also noted in nationwide studies [1, 8]. In fact, there was a concomitant increase in the vaginal hysterectomy rate, which is a welcome trend as this minimally invasive approach should be encouraged whenever feasible [6]. The goal of the study is not to compare the open and minimally invasive approaches but to analyze the cost drivers in different approaches. The open approach had a remarkably higher uterine weight compared with the minimally invasive approach since larger uteri increase the likelihood of resorting to open hysterectomy [13]. Although the mean uterine weight in the minimally invasive approach is comparable to other studies [14], pathologies in relatively smaller uteri should be evaluated for possible hysteroscopic management to avoid more invasive surgeries and their associated morbidities and costs.
Similar to other studies [4, 8], the robotic approach in this cohort was the most expensive, primarily due to the capital cost of the da Vinci system and longer OR time. Of note, the cost accounting systems, including the da Vinci system capital cost, differ among hospitals. Some hospitals, including the University of Texas Medical Branch, assign different OR time complexity levels according to technical characteristics such as the used equipment. In these cases, the OR time is charged per minute at different rates according to complexity level and robotic cases are included in the highest (most expensive) level [11]. Other hospitals assign a fixed dollar amount to robotic cases whereas a third group of hospitals combine the capital costs into one pool and divide them as indirect costs on all cases, regardless of which equipment is used in each individual case. In these accounting systems, it may be difficult to accurately analyze costs of individual procedures. The remarkable increase in the cost of hysterectomy over the study period is most likely due to the medical cost inflation and the surge in US healthcare costs [9]. This increase persisted after adjusting for the annual general cost inflation.
In our cohort, the charges were lowest for vaginal and open hysterectomy. Our findings mirror published research showing vaginal hysterectomy costs to be the least [15] and robotic hysterectomy the most [11, 15]. This has been similarly reported for other robotically performed gynecologic procedures, such as myomectomy [16]. On the contrary, when performed for oncologic indications, a study found that abdominal hysterectomy was the most costly among alternate approaches [17]. The poorer health status and longer hospital stay of women undergoing hysterectomy for malignant conditions may underlie this finding, leading to higher procedure costs [15]. Of note, as some surgeons started to use energy devices such as LigaSure in open and vaginal hysterectomy [18], the cost of these routes may accordingly increase. Women who underwent concomitant anti-incontinence surgery had a significantly higher hysterectomy charge by $1,514.3. As these procedures are typically performed in older women, increase OR time beyond that required for a hysterectomy, may involve a second billable surgeon, and require utilization of slings, total charges are expected to rise. However, physicians in some surgical specialties, including gynecology, seem to underestimate the costs of certain equipment [19]. One possible strategy hospitals can seek to reduce costs is to standardize surgical instruments or provide cost feedback to surgeons [20]. Unlike other studies [21], the uterine weight was not a significant direct predictor of charges as its impact on cost is probably mediated by other predictors, such as the OR time and operative approach. A patient with a large uterus is more likely to require longer OR time and undergo an open approach and adjusting for these variables accounted for the impact of uterine weight on charges.
In addition to hysterectomy approach, the LOS and OR time were significant predictors of charges. To illustrate their contribution to hysterectomy charges, we built the regression model twice, once including and another excluding each LOS and OR time. We concluded that LOS alone increased predicted open hysterectomy charges by more than $6,000 (from $2,151 less to $4,023 more than vaginal hysterectomy), which signifies that a substantial portion of open hysterectomy cost is due to its longer LOS. Incorporating the recently introduced enhanced recovery after surgery (ERAS) protocols that minimize narcotic requirements, improve bowel function, and encourage early ambulation and discharge was shown to decrease LOS after open hysterectomy [22]. In addition, practicing same-day discharges after minimally invasive approaches, including vaginal hysterectomy, has gained popularity and was shown to decrease costs as well [4, 23]. However, adjusting for LOS in our study did not decrease the cost of the laparoscopic approach, which is rather attributed to the increased OR time and use of disposable instruments in laparoscopic hysterectomy. Of importance, we noted a general trend towards shorter LOS across different routes, which is particularly considered a welcome trend.
Similarly, OR time alone increased predicted charges for robotic and laparoscopic approaches by $9,000 (from $11,631 to $20,639) and almost $6,000 (from $8,417 to $14,330), respectively, compared with vaginal hysterectomy. This OR time-related cost increase, particularly for robotic hysterectomy, may be explained by its relatively recent introduction into gynecologic practice since 2005 [24]. Robotic cases in many studies included those performed during the surgeons’ learning curve, which may have contributed to longer OR times and hence, costs [8, 25, 26]. As our findings suggest, the OR time of robotic hysterectomy showed a general declining trend from 2009 to 2016 (Figure 1D). Enhancements in the Xi da Vinci system may allow for simpler docking and possibly shorter OR time [27]. In addition, many hospitals, including ours, provide training programs for minimally invasive hysterectomy. Improving the residents’ training in vaginal hysterectomy and incorporating different simulation programs are encouraged to increase the utilization of the vaginal approach, improve outcomes, and shorten the OR time [28, 29]. However, the relatively higher OR time of vaginal hysterectomy in our institution compared with some other institutions can be explained by the residents’ involvement in performing hysterectomies. Early in the training process, involving residents in minimally invasive procedures may initially increase OR time [30], which also corresponds to their learning curve.
We recognized some limitations of this study, including those inherent to a retrospective study design. Lack of randomization of women with similar surgical indications to different approaches and choosing hysterectomy route based on the gynecologist’s perspective can lead to selection bias. Additionally, our study did not evaluate for the role of surgical indication due to the large overlap between diagnostic terminology in our data and the big number of women with more than one indication, which would be difficult to account for in regression analysis. We additionally did not assess for post-discharge outcomes or re-admissions, which technically would increase overall costs. Lastly, our study population was recruited from a single institution, which may impact the generalizability of the results.
In summary, this study suggests that several operative factors can significantly drive the cost dynamics of hysterectomy. While cost should not be the leading factor in patient care decisions, physicians should be, nonetheless, conscientious of their financial impact. Efforts should focus on potentially modifiable factors, such as the surgical approach, OR time, and LOS, to promote value-based care. Whenever feasible, vaginal hysterectomy should be encouraged, for example, through improved residents’ training to take precedence over the more costly approaches.
Funding Statement:
This work was supported, in part, by NIH grant 1R01HD094380-01 to Mostafa A. Borahay.
GLOSSARY
- Hysterectomy
A surgical procedure to remove the uterus
- Laparoscopy
A surgical procedure that involves inserting a viewing tube, the laparoscope, through a small incision
- Robotic-assisted surgery
A technique that involves the use of robotic devices to perform surgical procedures
- Length of stay
Length of patient stay from admission to discharge during an inpatient care encounter
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest Statement: Abdelrahman AlAshqar has no conflict of interest to declare. Metin E. Goktepe has no conflict of interest to declare. Gokhan S. Kilic has no conflict of interest to declare. Mostafa A. Borahay has no conflict of interest to declare.
LOS, Length of Stay
OR, Operating Room
REFERENCES
- [1].Wright JD, Herzog TJ, Tsui J, Ananth CV, Lewin SN, Lu YS, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol 2013;122(2 Pt 1):233–41. doi: 10.1097/AOG.0b013e318299a6cf [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lee J, Jennings K, Borahay MA, Rodriguez AM, Kilic GS, Snyder RR, et al. Trends in the national distribution of laparoscopic hysterectomies from 2003 to 2010. J Minim Invasive Gynecol 2014;21(4):656–61. doi: 10.1016/j.jmig.2014.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Smith AJB, AlAshqar A, Chaves KF, Borahay MA. Association of demographic, clinical, and hospital-related factors with use of robotic hysterectomy for benign indications: A national database study. Int J Med Robot 2020:e2107. doi: 10.1002/rcs.2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Borahay MA, Patel PR, Kilic CH, Kilic GS. Outpatient robotic hysterectomy: clinical outcomes and financial analysis of initial experience. Int J Med Robot 2014;10(2):244–50. doi: 10.1002/rcs.1565 [DOI] [PubMed] [Google Scholar]
- [5].Kilic GS, Moore G, Elbatanony A, Radecki C, Phelps JY, Borahay MA. Comparison of Perioperative Outcomes of Total Laparoscopic and Robotically Assisted Hysterectomy for Benign Pathology during Introduction of a Robotic Program. Obstet Gynecol Int 2011;2011:683703. doi: 10.1155/2011/683703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Committee on Gynecologic P. Committee Opinion No 701: Choosing the Route of Hysterectomy for Benign Disease. Obstet Gynecol 2017;129(6):e155–e9. doi: 10.1097/AOG.0000000000002112 [DOI] [PubMed] [Google Scholar]
- [7].Wu JM, Wechter ME, Geller EJ, Nguyen TV, Visco AG. Hysterectomy rates in the United States, 2003. Obstet Gynecol 2007;110(5):1091–5. doi: 10.1097/01.AOG.0000285997.38553.4b [DOI] [PubMed] [Google Scholar]
- [8].Wright JD, Ananth CV, Lewin SN, Burke WM, Lu YS, Neugut AI, et al. Robotically assisted vs laparoscopic hysterectomy among women with benign gynecologic disease. Jama 2013;309(7):689–98. doi: 10.1001/jama.2013.186 [DOI] [PubMed] [Google Scholar]
- [9].Centers for Medicare and Medicaid Services. National Health Expenditure Fact Sheet, https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NHE-Fact-Sheet; 2020. [accessed April 29, 2020.
- [10].Gordon TA, Burleyson GP, Tielsch JM, Cameron JL. The effects of regionalization on cost and outcome for one general high-risk surgical procedure. Ann Surg 1995;221(1):43–9. doi: 10.1097/00000658-199501000-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zeybek B, Oge T, Kilic CH, Borahay MA, Kilic GS. A financial analysis of operating room charges for robot-assisted gynaecologic surgery: Efficiency strategies in the operating room for reducing the costs. Journal of the Turkish German Gynecological Association 2014;15(1):25–9. doi: 10.5152/jtgga.jtgga.2014.79989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].U.S. Bureau of Labor Statistics. Consumer Price Index, https://www.bls.gov/regions/southwest/news-release/consumerpriceindex_houston.htm; 2020. [accessed April 29, 2020.
- [13].Mohan Y, Chiu VY, Lonky NM. Size matters in planning hysterectomy approach. Womens Health (Lond) 2016;12(4):400–3. doi: 10.1177/1745505716653692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Muller A, Thiel FC, Renner SP, Winkler M, Haberle L, Beckmann MW. Hysterectomy-a comparison of approaches. Dtsch Arztebl Int 2010;107(20):353–9. doi: 10.3238/arztebl.2010.0353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wright KN, Jonsdottir GM, Jorgensen S, Shah N, Einarsson JI. Costs and outcomes of abdominal, vaginal, laparoscopic and robotic hysterectomies. JSLS 2012;16(4):519–24. doi: 10.4293/108680812X13462882736736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Behera MA, Likes CE 3rd, Judd JP, Barnett JC, Havrilesky LJ, Wu JM. Cost analysis of abdominal, laparoscopic, and robotic-assisted myomectomies. J Minim Invasive Gynecol 2012;19(1):52–7. doi: 10.1016/j.jmig.2011.09.007 [DOI] [PubMed] [Google Scholar]
- [17].Shah NT, Wright KN, Jonsdottir GM, Jorgensen S, Einarsson JI, Muto MG. The Feasibility of Societal Cost Equivalence between Robotic Hysterectomy and Alternate Hysterectomy Methods for Endometrial Cancer. Obstet Gynecol Int 2011;2011:570464. doi: 10.1155/2011/570464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Aydin C, Yildiz A, Kasap B, Yetimalar H, Kucuk I, Soylu F. Efficacy of electrosurgical bipolar vessel sealing for abdominal hysterectomy with uterine myomas more than 14 weeks in size: a randomized controlled trial. Gynecol Obstet Invest 2012;73(4):326–9. doi: 10.1159/000336400 [DOI] [PubMed] [Google Scholar]
- [19].Jackson CR, Eavey RD, Francis DO. Surgeon Awareness of Operating Room Supply Costs. Ann Otol Rhinol Laryngol 2016;125(5):369–77. doi: 10.1177/0003489415614864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Childers CP, Showen A, Nuckols T, Maggard-Gibbons M. Interventions to Reduce Intraoperative Costs: A Systematic Review. Ann Surg 2018;268(1):48–57. doi: 10.1097/SLA.0000000000002712 [DOI] [PubMed] [Google Scholar]
- [21].Winter ML, Leu SY, Lagrew DC Jr., Bustillo G Cost comparison of robotic-assisted laparoscopic hysterectomy versus standard laparoscopic hysterectomy. J Robot Surg 2015;9(4):269–75. doi: 10.1007/s11701-015-0526-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wijk L, Franzen K, Ljungqvist O, Nilsson K. Implementing a structured Enhanced Recovery After Surgery (ERAS) protocol reduces length of stay after abdominal hysterectomy. Acta Obstet Gynecol Scand 2014;93(8):749–56. doi: 10.1111/aogs.12423 [DOI] [PubMed] [Google Scholar]
- [23].Schiavone MB, Herzog TJ, Ananth CV, Wilde ET, Lewin SN, Burke WM, et al. Feasibility and economic impact of same-day discharge for women who undergo laparoscopic hysterectomy. Am J Obstet Gynecol 2012;207(5):382 e1–9. doi: 10.1016/j.ajog.2012.09.014 [DOI] [PubMed] [Google Scholar]
- [24].Sinha R, Sanjay M, Rupa B, Kumari S. Robotic surgery in gynecology. J Minim Access Surg 2015;11(1):50–9. doi: 10.4103/0972-9941.147690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sarlos D, Kots L, Stevanovic N, Schaer G. Robotic hysterectomy versus conventional laparoscopic hysterectomy: outcome and cost analyses of a matched case-control study. Eur J Obstet Gynecol Reprod Biol 2010;150(1):92–6. doi: 10.1016/j.ejogrb.2010.02.012 [DOI] [PubMed] [Google Scholar]
- [26].Pasic RP, Rizzo JA, Fang H, Ross S, Moore M, Gunnarsson C. Comparing robot-assisted with conventional laparoscopic hysterectomy: impact on cost and clinical outcomes. J Minim Invasive Gynecol 2010;17(6):730–8. doi: 10.1016/j.jmig.2010.06.009 [DOI] [PubMed] [Google Scholar]
- [27].van der Schans EM, Hiep MAJ, Consten ECJ, Broeders I. From Da Vinci Si to Da Vinci Xi: realistic times in draping and docking the robot. J Robot Surg 2020. doi: 10.1007/s11701-020-01057-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Asoglu MR, Achjian T, Akbilgic O, Borahay MA, Kilic GS. The impact of a simulation-based training lab on outcomes of hysterectomy. Journal of the Turkish German Gynecological Association 2016;17(2):60–4. doi: 10.5152/jtgga.2016.16053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Borahay MA, Haver MC, Eastham B, Patel PR, Kilic GS. Modular comparison of laparoscopic and robotic simulation platforms in residency training: a randomized trial. J Minim Invasive Gynecol 2013;20(6):871–9. doi: 10.1016/j.jmig.2013.06.005 [DOI] [PubMed] [Google Scholar]
- [30].Freeman AH, Barrie A, Lyon L, Conell C, Garcia C, Littell RD, et al. Does Surgical Teaching Take Time? Resident Participation in Minimally Invasive Hysterectomy for Endometrial Cancer. J Minim Invasive Gynecol 2017;24(5):783–9. doi: 10.1016/j.jmig.2017.03.012 [DOI] [PubMed] [Google Scholar]


