Abstract
Recent animal research on substance-use disorders (SUDs) has emphasized learning models and the identification of ‘addiction-prone’ animals. Meanwhile, basic neuroscientific research has elucidated molecular, cellular, and circuit functions with increasing sophistication. However, SUD-related research is hampered by continued arguments over which animal models are more ‘addiction like’, as well as the facile assignment of behaviors to a given brain region and vice versa. We argue that SUD-related research would benefit from a ‘bottom-up’ approach including: (i) the characterization of different brain circuits to understand their normal function as well as how they respond to drugs and contribute to SUDs; and (ii) a focus on the use patterns and neurobiological effects of different substances to understand the range of critical SUD-related in vivo phenotypes.
Moving Forward in SUD Research
SUDs (see Glossary) constitute major public health problems, including the effects of excessive intoxication and chronic substance abuse (https://www.niaaa.nih.gov/alcohols-effects-health; https://www.drugabuse.gov/publications/finder/t/160/drugfacts). There are few effective treatments for SUDs and thus further research is needed. Important recent advances have been made in SUD research on human volunteers, including brain imaging, sophisticated behavioral testing, monitoring of drug use and associated behavior, and new pharmaceutical and non-pharmaceutical treatments (e.g., transcranial magnetic stimulation) (https://www.niaaa.nih.gov/alcohol-health/support-treatment; https://www.drugabuse.gov/related-topics/treatmentREF). In SUD-related research using animal models, new behavioral paradigms examine the motivations for drug seeking and taking, as well as decision making affected by and related to abused substances. Models of ‘compulsive’ drug seeking and taking were developed to study individual animals most vulnerable to disordered use [1,2]. The rapid development of new tools for neuronal and circuit measurement and manipulation has resulted in a better appreciation of the roles of different cells, brain regions, and circuits in behavioral control, including SUD-related behaviors. A natural next step is to combine the new information about these behavioral and neurobiological changes with the goal of developing new therapies for SUDs. This effort is timely and laudable but requires careful consideration of how brain circuitry is related to behavior.
In the healthy waking animal, all parts of the brain receive information and are either active or poised to be active as needed to drive behaviors and adapt to changing circumstances. It is difficult to determine how global brain activity is related to behavior and thus neurobiologists usually attempt to assess which neurons, brain regions, and circuits dominate behavioral control (and cognition or affect in the human brain) at any given time. This approach often leads investigators to associate particular brain regions with specific behaviors, and the SUD field is not immune from this trap. Initially, such research begins with tasks designed and operationally defined to provide information about psychological constructs such as fear, anxiety, or substance craving. This approach generates ideas about the neural underpinnings of specific behaviors and explains these underpinnings in ways that are easy to grasp for those familiar with the behaviors.
However, with time the findings are often interpreted as providing information about the neural basis of the entire psychological construct rather than the specific behaviors examined.
Studies of the amygdala and fear provide an excellent case in point. Beautiful work in the 1990s by LeDoux and others [3,4] established that the activity and synaptic efficacy of amygdala neurons was altered during fear learning and extinction, while manipulation of amygdala function provided evidence that this brain region controls fear-related learning. These studies withstood the test of time and are being updated with valuable new ideas [5]. This work also provided important information about amygdala complex functions. However, due in part to this line of research, there was a time when many neurobiologists equated the amygdala with fear and fear with the amygdala. Despite the usefulness of the research, this line of thinking had the dual negative effect of de-emphasizing other brain regions with important roles in fear-related behaviors and the larger role of the amygdala in value/valence coding for positive as well as negative outcomes [6,7]. The idea that the amygdala may be a general arousal-based modulator of learning was also underappreciated [8]. Clearly this was not the intent of the outstanding investigators studying fear conditioning circuitry, but largely the interpretation of neuroscientists from outside the field.
For SUD-related research to avoid such overgeneralization, it is important to realize that brain regions, as part of complex circuits, are involved in multiple functions. It is also necessary to remember that psychological constructs arise from human verbal reports and are operationally defined in animal behavior studies, but do not necessarily correspond to the ways in which neural circuits control behavior. Thus, we would argue, it is important that neurobiologists attempt to understand brain activity, plasticity, and neuroadaptations independent of, and related to, in vivo measurements when evaluating the effects of abused substances.
In the remainder of this review, we consider how this neurobiology-centric information approach can help investigators gain a better understanding of the effects of acute and chronic drug exposure in the context of the corticothalamo-basal ganglia (CTBG) circuitry. We suggest that focused ‘bottom-up’ approaches detailing how molecular and cellular functions contribute to circuit output in relation to defined in vivo phenotypes will provide a better understanding of how SUDs alter behavior. We also emphasize that consideration of divergent actions of different substances on neural function and behavior are important, and the relationship to the human condition must always be considered.
The CTBG Circuitry
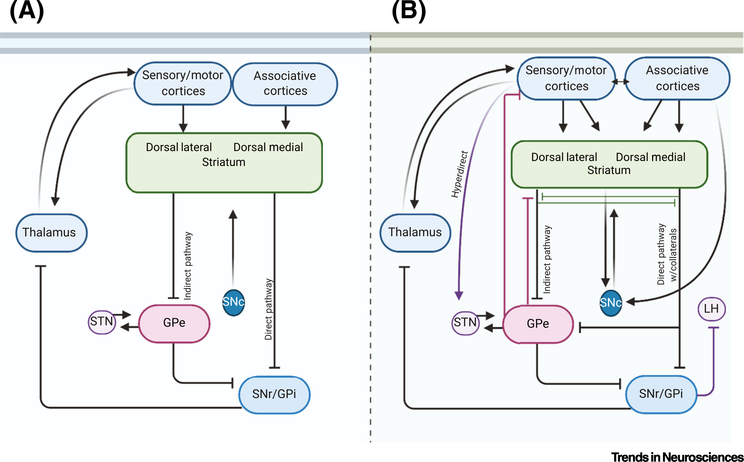
The circuits involving the cortex, the thalamus, the basal ganglia, and the resultant outputs to the brainstem and back to the cortex are predominant anatomical and physiological motifs of the forebrain that have been reviewed extensively (Figure 1A) [9,10]. Several such circuits exist, each with cortical and thalamic glutamatergic inputs to the striatum that drive the activity of medium spiny striatal projection neurons (MSNs) [11]. While it has been tempting to view the CTBG circuits as providing a linear information flow with a binary net effect on the cortex and action production, this limited view of the circuits falls apart with only slightly deeper probing (Figure 1B). Furthermore, the linear concept of CTBG information flow does not capture the continuous processing and ongoing integration occurring within the circuits [12]. Clearly, there is much more to learn about CTBG circuits. Nonetheless, progress has been made in understanding how cortical input, striatal output, and net BG output influence behavior [11,13].
Figure 1. Linear and Nonlinear Conceptualizations of Cortico-Thalamo-Basal Ganglia (CTBG) Circuitry.
(A) Schematic diagram of ‘linear’ information flow in the sensorimotor CTBG circuit. Cortical glutamatergic activation of striatal GABAergic projection neurons leads to output via parallel direct and indirect pathways that ultimately feeds back through the thalamus to gate cortical output. Medium spiny striatal projection neurons (MSNs) project to tonically active GABAergic neurons in the globus pallidus internal segment (GPi) and substantia nigra pars reticulata (SNr) (the ‘direct pathway’) and to the globus pallidus external segment (GPe) (the ‘indirect pathway’) [11]. The tonically active GPe GABAergic neurons are in constant communication with glutamatergic neurons in the subthalamic nucleus (STN). The SNr output inhibits thalamocortical neurons and thus the net effect of the direct pathway MSNs is the disinhibition of cortical output [11]. In the indirect pathway, the GPe projects to the SNr (and other brain regions), providing another inhibitory synapse that can produce cortical inhibition [11]. The striatum is also innervated by midbrain dopaminergic neurons that modulate the function of MSNs and other striatal neurons [11,112]. (B) Schematic diagram showing additional neuronal connections and activity patterns that indicate nonlinear CTBG function. Cortex to STN afferents and glutamatergic projections from the STN to other striatal nuclei (a.k.a. the hyperdirect pathway) bypass the striatum and suppress movement [113]. GABAergic collaterals between striatal MSNs influence striatal output. GABAergic GPe-striatal afferents allow feedback regulation of MSN output, collaterals from direct pathway MSNs regulate GPe and ventral pallidum function [114–116]. These connections can interrupt the linearity of the circuitry. Some MSNs that express D1 dopamine receptors (normally classified as direct pathway MSNs) send collateral projections to the GPe or ventral pallidum [117,118]. Some GPe neurons project to the cortex [119], whereas some GPi neurons project to the lateral habenula [11,120]. Furthermore, neuromodulation arising from interneuron and projection neuron sources is incompletely understood.
Several CTBG loops are proposed to run in parallel, including large associative, limbic, and sensorimotor circuits, with smaller oculomotor and intra-amygdala loops [14,15]. The associative loop is thought to include cortical projections arising from frontal areas such as the medial prefrontal cortex (mPFC), regions of the orbitofrontal, prelimbic, and cingulate cortices and the insula, entorhinal, posterior parietal, retrosplenial, and other temporal lobe cortical areas, and midline thalamic nuclei, as well as the basolateral amygdala (BLA) projecting into the caudate/dorsomedial striatum (DMS) [16–18]. These cortical areas in turn are innervated by a variety of thalamic nuclei [19,20]. The caudate also receives prominent dopaminergic input from the substantia nigra pars compacta (SNc) that provides both movement and stimulus salience/ positive/negative valence-related information [21]. By contrast, the sensorimotor loop motor and primary sensory cortical areas (excluding the olfactory cortex) send projections into the putamen/dorsolateral striatum (DLS), where there is also converging thalamic input [18]. This loop also includes nigral input and thalamocortical projections. In the limbic loop, cortical regions like the infralimbic, the insula, and portions of the prelimbic, cingulate, and orbital cortices as well as the hippocampus and BLA send projections into the ventral striatum [22]. Thalamic input from the paraventricular, paratenial, and rhomboid nuclei also provides strong glutamatergic afferent input to this striatal subregion [23,24]. The dopaminergic input to the limbic circuit comes from the ventral tegmental area (VTA) [25].
Mapping of cortical inputs supports the existence of such loops, while also highlighting the density and diffusion of cortical inputs across numerous loops [18]. Thus, while there is general segregation of cortical inputs, it is by no means complete. While the parallel circuit motif is useful given the predominance of intraloop projections, there is increasing evidence for interloop crosstalk. This includes corticocortical communication supporting information flow across the regions that provide input to the different basal ganglia subnuclei [26]. Direct pathway MSNs from the limbic nucleus accumbens project to SNc dopaminergic neurons that innervate the dorsal striatum [25,27]. This striatal-midbrain-striatal loop has been implicated in shifting the circuit control of drug-related responses [28]. Projections from the central amygdala to the lateral SNr may help to coordinate intercircuit communication [29] and are involved in action learning [30]. Additional mechanisms of communication across CTBG circuits are likely to be discovered, raising the possibility that such circuit interactions contribute to altered in vivo phenotypes following the experience of and exposure to abused substances.
Why It Is Necessary to Take a Circuit Information-Based Approach in SUD Research
One example that highlights the need to supplement psychological construct-based approaches with a circuit information-based approach comes from the focus in the SUD field on CTBG circuit contributions to habitual drug seeking and taking [31,32]. Studies in the learning field suggest that the associative circuit processes information necessary for goal-directed control, where actions are controlled by the immediate contingency and expected outcome value [10,33]. By contrast, the sensorimotor circuit is involved in parallel action learning, where environmental stimuli/context information is combined with internal states and outcome history to produce a stimulus–response (S-R) association that can produce habits [10]. Goal-directed and habitual action strategies are differentiated based on the sensitivity of action production to changes in the outcome value or contingency [10]. In reward/reinforcement learning theory, these processes generally overlap with model-based and model-free strategies, respectively [34], with two systems capable of action control. The limbic circuit is also involved in action control, particularly in tasks involving reward-predictive information and its influence over actions [10,35,36]. These designations helped investigators to understand learning and design experiments to determine how circuit function affects such learning.
SUD researchers have examined drug effects on in vivo phenotypes indicative of goal-directed and habitual action control [31,32]. The concept that chronic drug use/exposure shifts drug seeking and taking from goal-directed to habitual and compulsive control has drawn considerable attention and criticism [28,36–41]. Evidence from both animal and human studies indicates shifting control of drug-related responses from associative or limbic to sensorimotor circuits [31,32,36,42,43]. However, animal studies also indicate that, while disruption of habitual processes impairs drug seeking, the effects of devaluation on drug taking are not affected to the same extent as drug seeking [44–48]. Alterations of the cells, circuitry, and function of PFC areas in the associative CTBG circuitry have also been implicated in these behavioral disruptions, and drugs of abuse, including ethanol, produce aberrant decision making and cognitive/executive function [32,49,50]. However, these findings have not generally been integrated into the larger scenario of drug effects on CTBG circuits and the hypothesized bias toward habitual drug seeking and taking. Finally, there is considerable evidence that drug seeking and taking involve other processes, such as increased incentive salience [51] and allostasis/negative reinforcement [52], that are goal-directed. Thus, the action learning theoretical framework does not capture the entirety of abused substance effects on information represented within or behavior influenced by CTBG circuits. These discrepancies also highlight the need for caution when interpreting findings solely from a theory-based perspective, as it may obscure other possibilities as detailed in the following text.
Anatomical and connectivity studies can reveal much about the information processed by CTBG circuits. For example, based on the connectivity previously discussed, the caudate nucleus and other associative circuit components can respond to a combination of highly processed information about the environment combined with information about the likelihood, importance, and valence of sensory events and outcomes following actions. However, this information can be integrated with the sensorimotor circuit through thalamocortical, corticothalamic, and corticostriatal projections that cross the ‘boundaries’ of the traditional associative and sensorimotor loops [18,29,53]. Indeed, the primary motor cortex is involved in action learning even at early stages where goal-directed strategies are used [54]. Areas in the DMS also communicate with regions of the SNr that are implicated in sensory processing and convulsions during alcohol withdrawal [55,56]. Thus, the caudate/DMS and the associative network are not purely involved in goal-directed action generation. Clinical findings and human research indicate that the caudate, putamen, pallidum, and other BG subregions are implicated in many in vivo and behavioral outcomes, including compulsion, dementia, depression, mania, psychosis and seizures [57–59]. Additional research in animal models is needed to determine the mechanisms through which the associative striatal circuitry contributes to these brain functions and behaviors (Box 1).
Box 1. Initial Characterization of CTBG Circuitry in Abused Substance Actions and SUD-Related In Vivo Phenotypes.
It is widely appreciated that the striatonigral and mesolimbic dopaminergic systems are activated by all drugs of abuse, with more compelling evidence for the VTA/mesolimbic system [31]. These dopaminergic projections and their striatal targets are implicated in drug-induced reward and reinforcement that drives drug-related learning [31]. Another potential role is in salience signaling that contributes to heightened sensitivity to drug-related environmental stimuli and contexts. Locomotor activation and sensitization by drugs of abuse also involves these dopaminergic systems and their action in the striatum, but this effect differs with different types and doses of abused substances and the link to abuse itself is unclear [51].
The associative circuit has been implicated in the rewarding effects of drugs of abuse as well as the goal-directed behaviors discussed previously [31]. There is increasing evidence that associative cortical and striatal regions contribute to drug seeking and taking, especially at early stages of use [31]. This circuitry is also implicated in cognition and decision making, as well as the impairment of these processes produced by some drugs of abuse (e.g., alcohol). Drug craving is thought to recruit activity in the prefrontal cortex and likely in other parts of the associative circuit [121].
The limbic circuit roles in the rewarding effects of drugs of abuse have been well researched [51]. This includes learning associations between environmental stimuli and rewarding drug actions. The limbic circuit is involved in the control of affect [3] and is implicated in affective changes induced by drugs of abuse and withdrawal [52]. Negative affective states associated with drug withdrawal involve changes in the function of the amygdala and associated limbic areas [52]. Hippocampal changes induced by abused substances contribute to impaired learning and memory [100].
Sensorimotor circuitry plays a role in reinforcement produced by drugs of abuse leading to habitual/automatized action control related to drug seeking and taking [31,32,36]. This may include the association of environmental features with drug seeking/taking. Overall, drug-induced activation/disinhibition in sensorimotor circuits may lead to automatized actions during drug taking. However, comparatively little is known about the roles of sensorimotor circuitry in other aspects of substance use and abuse, and neurobiological studies have focused mainly on the DLS and SNc.
It is imperative, we would argue, to think beyond equating behaviors with particular brain regions when examining the effects of abused substances. Instead, examining the potential for integration of the different information streams and their ability to access downstream networks allows a more comprehensive view of circuit function. It is also necessary to understand the circuit function and control of non-drug-related behaviors to fully appreciate drug effects on circuit function and the resultant drug-related in vivo phenotypes. This is not to say that experiments involving drug exposure are not valuable at this time, but rather that these studies will be limited by what we know about basic circuit function and behavioral control. This knowledge will come from comprehensive descriptions of cellular functions contributing to activity patterns and plasticity and how these processes are disrupted in SUDs. This information can then be combined with a deeper understanding of the roles of the different cells, regions, and circuits in multiple behaviors along with the investigation of how circuits work in parallel and through crosstalk and their disruption in SUD-related behaviors.
Circuit Information-Based Approach in SUD Research
It is important to understand how information is represented and processed in circuits and how that information processing is altered in SUD-related in vivo phenotypes (Box 2). With the powerful tools available to modern experimental neuroscience, like multiple electrophysiological and optical techniques [60], real-time measurement of neurotransmitter levels and intracellular signaling [61–63], the adaptation of functional MRI (fMRI) and optoacoustic (OA) techniques to rodent brains [64,65], and tools for optical and pharmacological manipulation of cell activity, intracellular signaling, and gene expression [66–69], the ability to examine circuit connectivity function and contributions to in vivo phenotypes is stronger than ever. Recent advances in transcriptomics combined with other techniques now make it possible to identify and image hundreds of marker genes in their native cell/spatial context [70]. Limitations of these approaches must be considered when interpreting findings. For example, optogenetic activation can produce supranatural levels and synchronization of neuronal activity. Optogenetic inhibition of presynaptic terminals is often problematic, with biophysical constraints limiting its period of effectiveness [71]. Nonetheless, these and other techniques are now finding greater use in the large-scale examination and interrogation of CTBG cells and circuits.
Box 2. In vivo Phenotypes Related to SUDs.
The term in vivo phenotype was chosen to describe the physiological and behavioral measures that are indicative of one aspect of a disorder. This term is similar to the term ‘behavioral phenotype’ [99], with the difference that we include in vivo physiological measures. Our use of the in vivo phenotype concept also encompasses the idea of endophenotypes, but we do not restrict the measures to those for which there are clear genetic underpinnings. Instead, the emphasis is on identifying how the nervous system controls in vivo physiology and carefully proscribed behaviors rather than trying to relate the function of a particular brain region or circuit to an entire behavioral repertoire or psychiatric disorder (e.g., AUD, other SUDs). Of course, particular in vivo phenotypes are chosen with a psychological construct or disorder in mind, but they are thought to represent only a small subset of the entire construct. In Table I we list examples of different SUD-related constructs and the in vivo phenotypic measures used to evaluate them. This list is by no means exhaustive but is meant to give the reader an idea of the range of physiological and a few of the behavioral measures available to researchers.
Table I.
SUD-Related Constructs and In Vivo Phenotypic Measures
| Sleep | Polysomnography, actigraphy |
|---|---|
| Reward processing | Threshold for intracranial self-stimulation, conditioned place preference/aversion |
| Impulsivity | Go/no-go task, delay discounting task |
| Compulsive drug seeking/taking | Punishment for drug taking, adulteration with quinine (for oral intake), preference for drug over alternative reward (e.g., sucrose, social interaction) |
| Pain | Allodynia tests, thermosensitivity and temperature preference tests, grimace measurements, tests of inflammation-related pain |
| Withdrawal hyperexcitability/seizures | EEG, handling- or auditorily induced convulsions |
| Relapse | Forced abstinence/reinstatement, voluntary (reward choice based) abstinence, incubation |
| Stress | Hormonal measurements, autonomic physiology measurements, stress-induction paradigms |
| Anxiety | Elevated mazes, light/dark box test |
| Depression | Porsolt swim test, anhedonia tests (e.g., sucrose preference) |
| Sociability | Social interaction tests |
| Aggression | Resident–intruder test, tube-test for social dominance |
| Cognition/decision making | Pavlovian and instrumental conditioning, reinforcement learning, working memory tests, spatial memory tests, tests of flexibility |
A great example of this synergy between basic circuit understanding and SUD research involves the direct and indirect striatal output pathways in CTBG circuits [72]. The discovery by Gerfen and colleagues of the molecular constituents and targets of the different pathways allowed researchers to develop and test hypotheses about how these pathways are involved in movement initiation and selection [73]. New and ever more detailed information about direct and indirect pathway activity patterns and coordination is being generated rapidly [12,74,75].
Investigators in the SUD area also realized that direct and indirect pathways might control drug-related behaviors and the actions of dopamine (a neuromodulator implicated in many drug actions) [76,77]. This realization led to intensive investigation into how the two pathways are altered by abused substances and how they contribute to changes in SUD-associated circuit function. These studies led to the idea that direct pathway activity in the limbic circuit is strengthened while indirect pathway function is weakened following drug exposure. Enhancing direct pathway function generally enhances drug reward and promotes seeking, as does reducing indirect pathway function. Further, stimulation of the indirect pathway inhibits seeking [76,77]. This approach must be extended to the many other newly appreciated components of CTBG circuitry, including specific corticostriatal and thalamostriatal projections, the function and connections of the GPe, SNr, STN, and other basal ganglia regions, and feedback projections within the CTBG circuits. However, care must be taken to avoid premature interpretations without a full consideration of known technical caveats. Still, when approached with care, this bottom-up approach can complement research that works backward from behavioral changes, by providing basic characterization of brain function and drug effects on brain function with and without ties to particular behaviors.
In addition, the examination of the effects of abused substances on the function of molecules, synapses, cells, and circuits has long been a part of SUD-related research. While a great deal is known about the primary molecular targets of most drugs of abuse, knowledge gaps remain with regard to how drug actions at these targets set in motion immediate and longer-lasting changes in neuronal and circuit function. By examining mechanisms that induce altered transmission and patterns of activity, one can gain insights that go beyond the identification of a given circuit (e.g., Area X projects to Area Y). This research can lead to hypotheses about how molecules, cells, and circuits contribute to behavior and drug actions with less bias about brain–behavior relationships or preconceived ideas based on past work with particular behavioral paradigms.
The cannabis type 1 (CB1) cannabinoid receptor provides an excellent example of how the identification of a drug target, the normal mechanisms engaged by this target, and the involvement of the target in circuit function and behavior has provided a better basis for the examination of drug actions. The discovery that Δ−9-THC produces induces intoxication through activation of the CB1 receptor, which is itself activated by endogenous arachidonic acid-containing lipid metabolites called endocannnabinoids (eCBs) [78,79] opened the door to fundamental discoveries. The predominant role of eCB/CB1 neuromodulation is the inhibition of GABA and glutamate release. This basic information has allowed investigators to examine how CB1 presynaptic modulation contributes to CTBG function and its alteration in SUDs.
One CB1 effect thought to be directly related to drug reward is the suppression of GABAergic inhibition onto VTA dopaminergic neurons [80]. In addition, CB1 receptors on cortical presynaptic terminals in the striatum have been shown to have physiological roles that affect both behavior and neuroadaptation to THC and other drugs of abuse. For example, in the associative striatum, CB1 receptors are expressed on afferent terminals from several cortical inputs including the orbitofrontal cortex. Using chemogenetic, optogenetic, and targeted CB1 knockout strategies, Gremel and coworkers showed that eCB/CB1 actions on OFC-DMS inputs aid decreases in goal-directed decision making [81]. Subsequent research indicated that alcohol dependence can reduce OFC transmission selectively onto the direct pathway [50], identifying one way in which drug dependence may disrupt goal-directed control. Furthermore, CB1 receptors on cortical terminals in DLS have been linked to development of habitual strategies following chronic Δ9-THC [82]. Hence, initial basic research on the eCB/CB1 system provided a bottom-up approach that utilized new functional information to interrogate the molecular, cellular, and subcircuit/circuit contributions to information processing and its disruption in SUDs. There are several similar stories involving other molecules, but much work remains to be done in this area.
A given drug affects more than one, and often several, brain circuits. When adopting this circuit-based approach, multiple circuit elements and the corresponding activity patterns must be examined. An example comes from investigations of how impaired PFC function in CTBG circuits contributes to SUD-related behaviors. It is often stated that PFC damage removes behavioral ‘inhibition’ (i.e., suppression of actions) [32]. This concept must be disentangled from neuronal inhibition per se and a circuit-based explanation of action suppression must be further developed [83]. The PFC efferents are mostly glutamatergic/excitatory. Thus, what is mainly lost following PFC impairment is the pattern and level of activation of associative and limbic circuits [84]. Hence, impaired function of these circuits does not reduce inhibition per se. It may allow greater control by other circuits (with their own cortical elements) or recruit different plasticity mechanisms given the altered level/pattern of input activity. As there is dense crosstalk between cortical areas [26], changes in activity and output profiles may manifest in circuit-specific changes, with synapse-specific plasticity gating the influence of a given input. Behavior arises from information accrued from numerous and varied inputs, and SUD-related changes in PFC function may manifest differently depending on the behavioral computation performed.
While many drugs affect the PFC, much less is known about changes in other cortical areas and their communication with the basal ganglia. If there is indeed a decrease in PFC function and responsiveness to substance-related sensory information, other cortical regions and circuits may have enhanced, or at least unaltered, function that would allow them to control behavior. The emphasis on the enhanced role of the DLS in action control following drug exposure is useful in this context. However, the DLS is not capable of generating actions on its own, as MSN activity must be driven by glutamatergic input from cortex or thalamus [85]. There is little information to date about how abused substances affect key parts of the sensorimotor circuit, such as the primary sensory and motor cortices, STN, pallidum, and midline thalamus. While plasticity is likely to occur in the DLS, rendering the resident MSNs more or less responsive to glutamatergic input [86], altered input from one or more of the regions may drive activity in these neurons. The pattern of changes is likely to differ depending on the substance to which the individual is exposed and the striatal subregion. Finding whether, which, and when these excitatory inputs are collaborators in drug-induced enhancement of sensorimotor circuit function will provide a better picture of drug actions related to SUDs.
Different In Vivo Phenotypes and Circuit Changes Associated with Different Abused Substances
To maximize the use of knowledge gained through the circuit information approach, one needs to investigate the actions of different abused substances across multiple in vivo phenotypes. Almost all SUDs have common features, including intoxication, rewarding actions, social impairment, use despite negative consequences, withdrawal symptoms and negative affect, tolerance, disordered sleep, craving, and the tendency to relapse [87]. However, there are also significant differences among the neural actions of the different drugs, as well as the neuroadaptations and behavioral changes associated with the abuse of different substances. For example, the Diagnostic and Statistical Manual 5th Edition (DSM-5) indicates that Neurocognitive Disorders are associated with alcohol, inhalant and sedative abuse, but not with the other major drugs of abuse [87].
In this context, the emphasis of the SUD research field on assessment of the rewarding/reinforcing effects of abused substances, as well as self-administration of the substances, captures an incomplete picture (Box 3). These phenotypes are important aspects of SUDs given the goal of reducing maladaptive substance use. However, dysfunctional cognition indicates additional behavioral and neural control alterations that must be considered. It is true that some behavioral and neurobiological alterations are observed with self-administration that do not occur with forced drug administration [88,89]. However, other phenotypic changes that can be produced by forced administration contribute to SUDs and should not be ignored. For example, the preceding discussion indicates that impaired cognition/decision making is induced by abused substances when delivered by either exposure paradigm. This impairment almost certainly contributes to relapse and excessive drug use [90,91] and poor environmental coping strategies and aberrant behavior in contexts that do not involve the drug per se. Thus, it is important to understand how both acute and chronic drug exposure alter these phenotypes independent of the assessment of drug intake itself, especially for drugs such as alcohol that alter cognitive performance and decision making [32,49,50,92].
Box 3. Addiction Models in Animal Research.
Investigator-induced drug administration and self-administration procedures have made important contributions to our understanding of abused substance actions and substance seeking and taking. The focus on animal models that mimic compulsive substance use and addiction in humans is laudable but has only face validity. For example, ‘compulsive’ alcohol drinking resistant to altered circumstances or aversive outcomes in rodents usually involves low levels of intake. Excessive alcohol intake is a key phenotype of AUD (DSM-5) and an important factor in organ damage. It is important to examine the pharmacological effects of abused substances, and at least in the case of alcohol the amount consumed matters. Unfortunately, it is difficult to get rodents to consume the levels of alcohol consumed by many humans. Thus, it is important to continue to use forced as well as self-administration paradigms. Some effective models combine this strategy with assays of drinking, tolerance, and dependence [101–104]. Furthermore, it has been demonstrated that most rodents will forego taking abused substances if given the choice of other, natural rewards [105,106]. This is even the case for rodents that show compulsive or ‘addiction-like’ intake [107], although exceptions are emerging [108,109]. Modeling complex human social interactions that promote or discourage SUDs is also difficult in animal models. Given the multiple genetic and phenotypic differences between humans and experimental animals, it is unlikely that one could develop a perfect model of human SUDs. This is not to say that such models should not be pursued, but a focus on in vivo phenotypes related to the different aspects of human SUDs, combined with powerful neurobiological investigation, will reveal much about SUDs and aid in therapeutic development. It is also important to look for common SUD-related neuroadaptations in different organisms. For example, reduced striatal GABAergic inhibition has been observed in mouse and monkey models of alcohol self-administration [110,111]. This synaptic change may be a key component of sensorimotor circuit disinhibition contributing to increased alcohol intake across organisms. By taking full advantage of all animal models, the field can make rapid progress in understanding SUDs without spending precious time arguing about which is the ‘best’ of the many imperfect models.
Withdrawal symptoms associated with cessation of substance abuse range from mild (e.g., with cannabis) to very severe (stimulants and opioids). It is also possible that habitual use is more pronounced for some drugs than for others. For example, the near-automaticity of sustained alcohol drinking may become independent of conscious control. Thus, while it is important to investigate the underlying mechanisms that are common to SUDs, understanding the neural basis of the distinct characteristics of disorders involving different substances is important. These differences should be reflected in the scope of the animal models used to study each drug. Identifying the in vivo phenotypes associated with a particular drug is an important first step in this process [32,93]. For example, investigators interested in alcohol-use disorder (AUD) need to understand pretty much all of the facets of use and withdrawal mentioned in the previous text, while those studying cannabis may not be as focused on the more severe withdrawal symptoms (e.g., seizures).
The unique patterns of use for different drugs are also important to consider in animal studies. In general, tobacco use comprises periodic short intake episodes separated by longer use-free periods (although near-continuous use is sometimes seen). Opiate intake is also periodic and often driven by the cessation of drug effects and anticipation/experience of withdrawal symptoms [94]. By contrast, alcohol abuse often comprises sustained, continuous intake for hours, and the amount of alcohol taken is part of the diagnostic criteria for AUD [87].
The question of why different individuals with SUD abuse different substances (or different combinations of multiple substances) is often not given strong consideration in SUD research. Availability and economic considerations contribute to individuals’ patterns of substance use, as do other factors such as route of administration. These factors may be difficult to incorporate into animal experiments. However, the factors that dispose an animal to prefer one drug over another should at least be considered.
In addition, genetic variations underlying individual differences in responses to abused substances and SUD-related behaviors must continue to be explored. In general, this line of research has focused on behavioral differences in animals with naturally occurring or experimenter-induced genetic differences, with some work on molecular and cellular changes directly related to the function of the protein produced by the gene of interest [95,96]. As genetic variation affects the organism over a lifetime, there is ample opportunity for circuit adaptations that predispose organisms to a greater likelihood of or resistance to drug actions and use disorder liability. The contribution of epigenetic changes to the molecular basis of substance abuse is also an emerging topic [97] that must be integrated with circuit information-based approaches.
Concluding Remarks and Future Perspectives
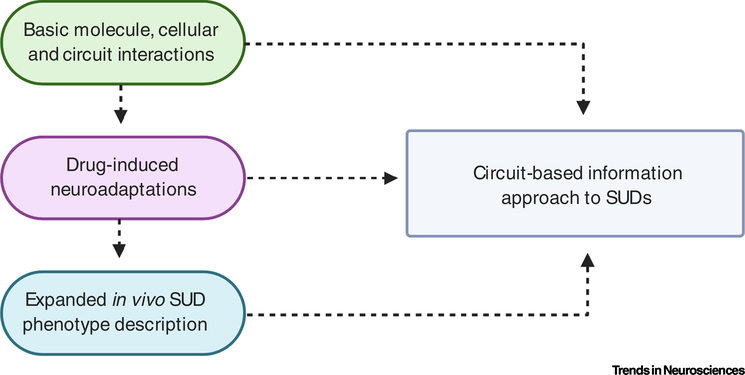
The ability of investigators to assess the effects of abused substances on brain circuitry and behavior depends on the understanding of basic molecule–cell–circuitry–behavior relationships. More work is needed in this area (Figure 2). Neuroscientific studies are still determining the structural and functional connectivity within CTBG circuits as well as the roles of neuropeptides and other neuromodulators in these circuits. Basic studies of this type must not be abandoned in a rush to conduct more ‘applied’ research. Understanding of the basic molecular, cellular, and circuit functions continues to be supplemented by examining the roles of these mechanisms in non-SUD-related behaviors. Clearly, this line of research is actively pursued at present and it would behoove SUD researchers to glean information from these studies to design better experiments examining effects of abused substances. Widespread examination of brain region/circuit roles in behavior runs the risk of implicating some regions in all behaviors. This risk is especially high given the central CTBG circuit roles in movement control, as many tasks require movement. While there is no simple solution to this problem, the use of multiple independent in vivo phenotypes and monitoring of movement and other potential confounding aspects of physiology (e.g., autonomic and endocrine function) will help investigators drill down to those behaviors that are most directly related to circuit function.
Figure 2. Summary Diagram of Bottom-Up Circuit Information Approach.
Continued studies of molecules, cells, and circuits will generate new information useful for understanding the neural basis of behavior and examining drug effects on circuits. This information will, in turn, help to explain a wide range of in vivo phenotypes gathered using traditional and newly developed neuroscientific techniques. Findings are integrated at all three levels of analysis to provide new information about how neural circuits contribute to substance-use disorders (SUDs).
There is also need for more research on the consequences of abused substance actions. The highest priority, in our view, should be neuroadaptations that can be linked to SUD-related in vivo phenotypes. However, describing adaptations even without linking them to in vivo phenotypes provides basic information that can be explored for relationships to SUDs in later studies. Better information on the time courses of neuroadaptations to abused substances is also needed. Too often, studies provide only a snapshot of neural changes at a given time point related to drug exposure. Revealing the temporal pattern of changes can help to determine what in vivo phenotypes might be impacted by these changes. The neuroadaptations will differ for different abused substances and thus it is also important to develop substance-specific profiles.
The range of in vivo phenotypes to be examined should include not only those directly related to rewarding drug effects and self-administration, but also those that encompass all deleterious effects of SUDs. Often, these in vivo phenotypes contribute to enhanced substance use and/or relapse in humans, and it is important to glean information about them even if drug intake is not directly measured. For example, sleep disruption is a common feature of SUDs but has received relatively little attention in basic research [98]. Other in vivo phenotypes, including affective indices, withdrawal seizures (for drugs such as alcohol), pain, fear-, and PTSD-related phenotypes, and autonomic function are all important in their own right and in relation to abuse, so full characterization of the neural basis of these changes is needed. Not all of these aspects of SUDs and the related in vivo phenotypes involve CTBG circuitry, but changes in these circuits will impact many of them. In all of these areas, we have an incomplete understanding of CTBG circuitry roles.
There is a pressing need for a concerted and comprehensive bottom-up strategy aimed at understanding changes in the different brain regions and circuits that are correlated with the range of phenotypic alterations associated with substance use and abuse (see Outstanding Questions). We hope this review has made the case for more research in this area, from the basic to the applied level, bringing to bear the powerful resources of neuroscience to fully understand the neural basis of SUDs.
Outstanding Questions.
How does brain circuitry control complex behaviors related to abused substance actions and substance seeking and taking?
A number of criteria are included in the clinical definition of SUDs that extend beyond rewarding drug effects and relapse to drug use. What endophenotypic-based in vivo/behavioral assays can be used and developed to model these criteria?
How can the investigation of circuit changes associated with these in vivo/ behavioral endophenotypes best inform SUD-related research?
What valuable information about basic circuit function and drug-related behavior can be gained using investigator-administration and self-administration procedures?
What are the neural responses that control the divergent acute and chronic effects of different abused substances?
How should animal models be adjusted to examine the effects that diverge across drug types?
Highlights.
Behavioral models of compulsive drug use and progress in techniques for cell and circuit measurement and manipulation are advancing substance abuse research.
A circuit information-based approach to substance use disorder (SUD)-related research can take full advantage of these developments.
This approach moves beyond the assignment of behavior to a particular brain region towards understanding how an area can contribute to the computations performed.
We discuss examples from research on the corticothalamic–basal ganglia circuit that highlight the usefulness of circuit information-based research on SUDs.
Consideration should also be given to divergent actions of different drugs of abuse in animal model-based research on circuit roles in SUDs.
Acknowledgments
The work was supported by the Division of Intramural Clinical and Biological Research of the National Institute on Alcohol Abuse and Alcoholism, project ZIA AA000416 (D.M.L., PI) and NIH AA026077-01A1 (C.M.G., PI).
Glossary
- Instrumental conditioning
learning paradigm in which an animal is trained to perform a new action (e.g., pressing a lever) by the consequence of that action – namely, the delivery of a desired commodity (e.g., palatable food)
- In vivo phenotype
measurable behaviors or biomarkers that provide information about one aspect of a disorder, in this case SUDs; similar to the concepts of behavioral phenotype and endophenotype but not limited to behavioral measures and without the necessity for shared underlying genetic influences, but also not excluding such influences (Box 2)
- Self-administration
procedures in which an animal consumes a substance or performs an action such as lever pressing to receive a substance (as opposed to the investigator administering the substance)
- Substance-use disorders (SUDs)
a range of symptoms associated with problematic use of legal and illicit drugs, as defined (at least in the USA) by the DSM of the American Psychiatric Association
References
- 1.Deroche-Gamonet V et al. (2004) Evidence for addiction-like behavior in the rat. Science 305, 1014–1017 [DOI] [PubMed] [Google Scholar]
- 2.Belin-Rauscent A et al. (2015) How preclinical models evolved to resemble the diagnostic criteria of drug addiction. Biol. Psychiatry 79, 39–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ledoux JE (2000) Emotion circuits in the brain. Annu. Rev. Neurosci. 23, 155–184 [DOI] [PubMed] [Google Scholar]
- 4.Maren S and Fanselow MS (1996) The amygdala and fear conditioning: has the nut been cracked? Neuron 16, 237–240 [DOI] [PubMed] [Google Scholar]
- 5.Ledoux JE and Pine DS (2016) Using neuroscience to help understand fear and anxiety: a two-system framework. Am. J. Psychiatry 173, 1083–1093 [DOI] [PubMed] [Google Scholar]
- 6.Fernando ABP et al. (2013) The amygdala: securing pleasure and avoiding pain. Front. Behav. Neurosci. 7, 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tye KM (2018) Neural circuit motifs in valence processing. Neuron 100, 436–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGaugh JL (2004) The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu. Rev. Neurosci. 27, 1–28 [DOI] [PubMed] [Google Scholar]
- 9.Reiner AM et al. (2018) Photophysics of diphenyl-pyrazole compounds in solutions and α-synuclein aggregates. Biochim. Biophys. Acta Gen. Subj. 1862, 800–807 [DOI] [PubMed] [Google Scholar]
- 10.Yin HH and Knowlton BJ (2006) The role of the basal ganglia in habit formation. Nat. Rev. Neurosci. 7, 464–476 [DOI] [PubMed] [Google Scholar]
- 11.Gerfen CR and Bolam JP (2010) The neuroanatomical organization of the basal ganglia. Handb. Behav. Neurosci. 20, 3–28 [Google Scholar]
- 12.Klaus A et al. (2019) What, if, and when to move: basal ganglia circuits and self-paced action initiation. Annu. Rev. Neurosci. 42, 459–483 [DOI] [PubMed] [Google Scholar]
- 13.Sano H et al. (2013) Signals through the striatopallidal indirect pathway stop movements by phasic excitation in the substantia nigra. J. Neurosci. 33, 7583–7594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alexander GE and Crutcher MD (1990) Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 13, 266–271 [DOI] [PubMed] [Google Scholar]
- 15.Provost J-S et al. (2015) Neuroimaging studies of the striatum in cognition, part I: healthy individuals. Front. Syst. Neurosci. 9, 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haber SN et al. (2006) Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive-based learning. J. Neurosci. 26, 8368–8376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo Q et al. (2015) Whole-brain mapping of inputs to projection neurons and cholinergic interneurons in the dorsal striatum. PLoS One 10, e0123381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunnicutt BJ et al. (2016) A comprehensive excitatory input map of the striatum reveals novel functional organization. Elife 5, e19103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hunnicutt BJ et al. (2014) A comprehensive thalamocortical projection map at the mesoscopic level. Nat. Neurosci. 17, 1276–1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collins DP et al. (2018) Reciprocal circuits linking the prefrontal cortex with dorsal and ventral thalamic nuclei. Neuron 98, 366–379 e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Subramaniam M and Roeper J (2016) Subtypes of midbrain dopamine neurons. Handb. Behav. Neurosci. 24, 317–334 [Google Scholar]
- 22.Groenewegen HJ et al. (2016) Organization of prefrontal–striatal connections. Handb. Behav. Neurosci. 24, 423–438 [Google Scholar]
- 23.Vertes RP et al. (2015) Limbic circuitry of the midline thalamus. Neurosci. Biobehav. Rev. 54, 89–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Donnell P et al. (1997) Interconnected parallel circuits between rat nucleus accumbens and thalamus revealed by retrograde transynaptic transport of pseudorabies virus. J. Neurosci. 17, 2143–2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ikemoto S (2007) Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens–olfactory tubercle complex. Brain Res. Rev. 56, 27–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris KD and Shepherd GMG (2015) The neocortical circuit: themes and variations. Nat. Neurosci. 18, 170–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haber SN et al. (2000) Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J. Neurosci. 20, 2369–2382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Everitt BJ and Robbins TW (2005) Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat. Neurosci. 8, 1481–1489 [DOI] [PubMed] [Google Scholar]
- 29.Haber SN (2010) Integrative networks across basal ganglia circuits. Handb. Behav. Neurosci. 20, 409–427 [Google Scholar]
- 30.Steinberg EE et al. (2013) A causal link between prediction errors, dopamine neurons and learning. Nat. Neurosci. 16, 966–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gremel CM and Lovinger DM (2017) Associative and sensorimotor cortico-basal ganglia circuit roles in effects of abused drugs. Genes Brain Behav. 16, 71–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Everitt BJ and Robbins TW (2016) Drug addiction: updating actions to habits to compulsions ten years on. Annu. Rev. Psychol. 67, 23–50 [DOI] [PubMed] [Google Scholar]
- 33.Balleine BW and Dickinson A (1998) Goal-directed instrumental action: contingency and incentive learning and their cortical substrates. Neuropharmacology 37, 407–419 [DOI] [PubMed] [Google Scholar]
- 34.Dayan P and Niv Y (2008) Reinforcement learning: the good, the bad and the ugly. Curr. Opin. Neurobiol. 18, 185–196 [DOI] [PubMed] [Google Scholar]
- 35.de Wit S et al. (2007) Stimulus–outcome interactions during instrumental discrimination learning by rats and humans. J. Exp. Psychol. Anim. Behav. Process. 33, 1–11 [DOI] [PubMed] [Google Scholar]
- 36.Lüscher C et al. (2020) The transition to compulsion in addiction. Nat. Rev. Neurosci. 21, 247–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dickinson A et al. (2002) Alcohol seeking by rats: action or habit? Q. J. Exp. Psychol. B 55, 331–348 [DOI] [PubMed] [Google Scholar]
- 38.Gerdeman GL et al. (2003) It could be habit forming: drugs of abuse and striatal synaptic plasticity. Trends Neurosci. 26, 184–192 [DOI] [PubMed] [Google Scholar]
- 39.Hogarth L et al. (2013) Associative learning mechanisms underpinning the transition from recreational drug use to addiction. Ann. N. Y. Acad. Sci. 1282, 12–24 [DOI] [PubMed] [Google Scholar]
- 40.Hogarth L (2020) Addiction is driven by excessive goal-directed drug choice under negative affect: translational critique of habit and compulsion theory. Neuropsychopharmacology 45, 720–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vandaele Y and Janak PH (2018) Defining the place of habit in substance use disorders. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 87, 22–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gremel CM and Lovinger DM (2016) Associative and sensorimotor cortico-basal ganglia circuit roles in effects of abused drugs. Genes Brain Behav. 16, 71–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giuliano C et al. (2019) Compulsive alcohol seeking results from a failure to disengage dorsolateral striatal control over behavior. J. Neurosci. 39, 1744–1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mangieri RA et al. (2012) Ethanol seeking by Long Evans rats is not always a goal-directed behavior. PLoS One 7, e42886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morisot N et al. (2019) mTORC1 in the orbitofrontal cortex promotes habitual alcohol seeking. Elife 8, e51333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Serlin H et al. (2015) Adolescent rats are resistant to forming ethanol seeking habits. Dev. Cogn. Neurosci. 16, 183–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Samson HH et al. (2004) Devaluation of ethanol reinforcement. Alcohol 32, 203–212 [DOI] [PubMed] [Google Scholar]
- 48.Renteria R et al. (2020) Habitual ethanol seeking and licking microstructure of enhanced ethanol self-administration in ethanol dependent mice. Alcohol. Clin. Exp. Res. 44, 880–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gutwinski S et al. (2018) Drink and think: impact of alcohol on cognitive functions and dementia – evidence of dose-related effects. Pharmacopsychiatry 51, 136–143 [DOI] [PubMed] [Google Scholar]
- 50.Renteria R et al. (2018) Chronic alcohol exposure disrupts top-down control over basal ganglia action selection to produce habits. Nat. Commun. 9, 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Robinson TE and Berridge KC (2000) The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction 95, 91–117 [DOI] [PubMed] [Google Scholar]
- 52.Koob GF (2013) Negative reinforcement in drug addiction: the darkness within. Curr. Opin. Neurobiol. 23, 559–563 [DOI] [PubMed] [Google Scholar]
- 53.McFarland NR and Haber SN (2002) Thalamic relay nuclei of the basal ganglia form both reciprocal and nonreciprocal cortical connections, linking multiple frontal cortical areas. J. Neurosci. 22, 8117–8132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kawai R et al. (2015) Motor cortex is required for learning but not for executing a motor skill. Neuron 86, 800–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen G and Buck KJ (2010) Rostroventral caudate putamen involvement in ethanol withdrawal is influenced by a chromosome 4 locus. Genes Brain Behav. 9, 768–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen G et al. (2011) Substantia nigra pars reticulata is crucially involved in barbiturate and ethanol withdrawal in mice. Behav. Brain Res. 218, 152–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Galvan A et al. (2014) The thalamostriatal systems in normal and disease states. Front. Syst. Neurosci. 8, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lauterbach EC et al. (1998) Neuropsychiatric correlates and treatment of lenticulostriatal diseases: a review of the literature and overview of research opportunities in Huntington’s, Wilson’s, and Fahr’s diseases. A report of the ANPA Committee on Research. American Neuropsychiatric Association. J. Neuropsychiatry Clin. Neurosci. 10, 249–266 [DOI] [PubMed] [Google Scholar]
- 59.Vuong J and Devergnas A (2018) The role of the basal ganglia in the control of seizure. J. Neural Transm. (Vienna) 125, 531–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Siegle JH et al. (2015) Neural ensemble communities: open-source approaches to hardware for large-scale electrophysiology. Curr. Opin. Neurobiol. 32, 53–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liang R et al. (2015) Imaging chemical neurotransmission with genetically encoded fluorescent sensors. ACS Chem. Neurosci. 6, 84–93 [DOI] [PubMed] [Google Scholar]
- 62.Tantama M et al. (2012) Optogenetic reporters: fluorescent protein-based genetically encoded indicators of signaling and metabolism in the brain. Prog. Brain Res. 196, 235–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang H et al. (2018) Lighting up the brain: genetically encoded fluorescent sensors for imaging neurotransmitters and neuromodulators. Curr. Opin. Neurobiol. 50, 171–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mandino F et al. (2019) Animal functional magnetic resonance imaging: trends and path toward standardization. Front. Neuroinform. 13, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gottschalk S et al. (2019) Rapid volumetric optoacoustic imaging of neural dynamics across the mouse brain. Nat. Biomed. Eng. 3, 392–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Adamantidis A et al. (2015) Optogenetics: 10 years after ChR2 in neurons – views from the community. Nat. Neurosci. 18, 1202–1212 [DOI] [PubMed] [Google Scholar]
- 67.Roth BL (2016) DREADDs for neuroscientists. Neuron 89, 683–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Konermann S et al. (2013) Optical control of mammalian endogenous transcription and epigenetic states. Nature 500, 472–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yamada M et al. (2018) Light control of the Tet gene expression system in mammalian cells. Cell Rep. 25, 487–500 e6 [DOI] [PubMed] [Google Scholar]
- 70.Moffitt JR et al. (2018) Molecular, spatial, and functional single-cell profiling of the hypothalamic preoptic region. Science 362, eaau5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mahn M et al. (2016) Biophysical constraints of optogenetic inhibition at presynaptic terminals. Nat. Neurosci. 19, 554–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Albin RL et al. (1989) The functional anatomy of basal ganglia disorders. Trends Neurosci. 12, 366–375 [DOI] [PubMed] [Google Scholar]
- 73.Gerfen CR and Surmeier DJ (2011) Modulation of striatal projection systems by dopamine. Annu. Rev. Neurosci. 34, 441–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jin X and Costa RM (2015) Shaping action sequences in basal ganglia circuits. Curr. Opin. Neurobiol. 33, 188–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bariselli S et al. (2019) A competitive model for striatal action selection. Brain Res. 1713, 70–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dobbs LK et al. (2017) Restructuring of basal ganglia circuitry and associated behaviors triggered by low striatal D2 receptor expression: implications for substance use disorders. Genes Brain Behav. 16, 56–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yager LM et al. (2015) The ins and outs of the striatum: role in drug addiction. Neuroscience 301, 529–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Devane WA et al. (1988) Determination and characterization of a cannabinoid receptor in rat brain. Mol. Pharmacol. 34, 605–613 [PubMed] [Google Scholar]
- 79.Matsuda LA et al. (1990) Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346, 561–564 [DOI] [PubMed] [Google Scholar]
- 80.Cheer JF et al. (2007) Phasic dopamine release evoked by abused substances requires cannabinoid receptor activation. J. Neurosci. 27, 791–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gremel CM et al. (2016) Endocannabinoid modulation of orbitostriatal circuits gates habit formation. Neuron 90, 1312–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nazzaro C et al. (2012) SK channel modulation rescues striatal plasticity and control over habit in cannabinoid tolerance. Nat. Neurosci. 15, 284–293 [DOI] [PubMed] [Google Scholar]
- 83.Aron AR et al. (2016) Frontosubthalamic circuits for control of action and cognition. J. Neurosci. 36, 11489–11495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Figee M et al. (2016) Compulsivity in obsessive–compulsive disorder and addictions. Eur. Neuropsychopharmacol. 26, 856–868 [DOI] [PubMed] [Google Scholar]
- 85.Plenz D et al. (2016) The striatal skeleton: medium spiny projection neurons and their lateral connections. Handb. Behav. Neurosci. 24, 121–136 [Google Scholar]
- 86.Johnson KA and Lovinger DM (2016) Presynaptic G protein-coupled receptors: gatekeepers of addiction? Front. Cell. Neurosci. 10, 264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.American Psychiatric Association (2013) Diagnostic and Statistical Manual of Mental Disorders: DSM-5, American Psychiatric Association [Google Scholar]
- 88.Ahmed SH et al. (2020) Non-pharmacological factors that determine drug use and addiction. Neurosci. Biobehav. Rev. 110, 3–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Moolten M and Kornetsky C (1990) Oral self-administration of ethanol and not experimenter-administered ethanol facilitates rewarding electrical brain stimulation. Alcohol 7, 221–225 [DOI] [PubMed] [Google Scholar]
- 90.Bickel WK et al. (2018) 21st Century neurobehavioral theories of decision making in addiction: review and evaluation. Pharmacol. Biochem. Behav. 164, 4–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sebold M et al. (2017) When habits are dangerous: alcohol expectancies and habitual decision making predict relapse in alcohol dependence. Biol. Psychiatry 82, 847–856 [DOI] [PubMed] [Google Scholar]
- 92.Stephens DN and Duka T (2008) Review. Cognitive and emotional consequences of binge drinking: role of amygdala and prefrontal cortex. Philos. Trans. R. Soc. Lond. B Biol. Sci. 363, 3169–3179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lamontagne SJ and Olmstead MC (2019) Animal models in addiction research: a dimensional approach. Neurosci. Biobehav. Rev. 106, 91–101 [DOI] [PubMed] [Google Scholar]
- 94.Schulteis G and Koob GF (1996) Reinforcement processes in opiate addiction: a homeostatic model. Neurochem. Res. 21, 1437–1454 [DOI] [PubMed] [Google Scholar]
- 95.Crabbe JC (2016) Progress with nonhuman animal models of addiction. J. Stud. Alcohol Drugs 77, 696–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yu C and McClellan J (2016) Genetics of substance use disorders. Child Adolesc. Psychiatr. Clin. N. Am. 25, 377–385 [DOI] [PubMed] [Google Scholar]
- 97.Hamilton PJ and Nestler EJ (2019) Epigenetics and addiction. Curr. Opin. Neurobiol. 59, 128–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ahrens AM and Ahmed OJ (2020) Neural circuits linking sleep and addiction: animal models to understand why select individuals are more vulnerable to substance use disorders after sleep deprivation. Neurosci. Biobehav. Rev. 108, 435–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Geyer MA and Moghaddam B (2002) Animal models relevant to schizophrenia disorders In Neuropsychopharmacology: The Fifth Generation of Progress (Davis KL et al., eds), pp. 689–701, American College of Neuropsychopharmacology [Google Scholar]
- 100.Kutlu MG and Gould TJ (2016) Effects of drugs of abuse on hippocampal plasticity and hippocampus-dependent learning and memory: contributions to development and maintenance of addiction. Learn. Mem. 23, 515–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gilpin NW et al. (2008) Vapor inhalation of alcohol in rats. Curr. Protoc. Neurosci. 44, 9.29.1–9.29.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Becker HC and Lopez MF (2016) An animal model of alcohol dependence to screen medications for treating alcoholism. Int. Rev. Neurobiol. 126, 157–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Simms JA et al. (2008) Intermittent access to 20% ethanol induces high ethanol consumption in Long–Evans and Wistar rats. Alcohol. Clin. Exp. Res. 32, 1816–1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fidler TL et al. (2012) Dependence induced increases in intragastric alcohol consumption in mice. Addict. Biol. 17, 13–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cantin L et al. (2010) Cocaine is low on the value ladder of rats: possible evidence for resilience to addiction. PLoS One 5, e11592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Venniro M et al. (2019) Operant social reward decreases incubation of heroin craving in male and female rats. Biol. Psychiatry 86, 848–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Venniro M et al. (2018) Volitional social interaction prevents drug addiction in rat models. Nat. Neurosci. 21, 1520–1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Augier E et al. (2018) A molecular mechanism for choosing alcohol over an alternative reward. Science 360, 1321–1326 [DOI] [PubMed] [Google Scholar]
- 109.Freese L et al. (2018) Pre-trial cocaine biases choice toward cocaine through suppression of the nondrug option. Pharmacol. Biochem. Behav. 173, 65–73 [DOI] [PubMed] [Google Scholar]
- 110.Cuzon Carlson VC et al. (2011) Synaptic and morphological neuroadaptations in the putamen associated with long-term, relapsing alcohol drinking in primates. Neuropsychopharmacology 36, 2513–2528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wilcox MV et al. (2014) Repeated binge-like ethanol drinking alters ethanol drinking patterns and depresses striatal GABAergic transmission. Neuropsychopharmacology 39, 579–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nolan SO et al. (2020) Direct dopamine terminal regulation by local striatal microcircuitry. J. Neurochem. 53, 453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bevan MD (2016) The subthalamic nucleus. Handb. Behav. Neurosci. 24, 277–291 [Google Scholar]
- 114.Corbit VL et al. (2016) Pallidostriatal projections promote β oscillations in a dopamine-depleted biophysical network model. J. Neurosci. 36, 5556–5571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Glajch KE et al. (2016) Npas1+ pallidal neurons target striatal projection neurons. J. Neurosci. 36, 5472–5488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dodson PD et al. (2015) Distinct developmental origins manifest in the specialized encoding of movement by adult neurons of the external globus pallidus. Neuron 86, 501–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cazorla M et al. (2014) Dopamine D2 receptors regulate the anatomical and functional balance of basal ganglia circuitry. Neuron 81, 153–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kupchik YM and Kalivas PW (2017) The direct and indirect pathways of the nucleus accumbens are not what you think. Neuropsychopharmacology 42, 369–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Saunders A et al. (2015) A direct GABAergic output from the basal ganglia to frontal cortex. Nature 521, 85–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Stephenson-Jones M et al. (2016) A basal ganglia circuit for evaluating action outcomes. Nature 539, 289–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Koob GF and Volkow ND (2016) Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry 3, 760–773 [DOI] [PMC free article] [PubMed] [Google Scholar]